- 1Institute of Sports and Exercise Biology, School of Physical Education, Shaanxi Normal University, Xi’an, China
- 2Department of Cardiology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Liverpool Centre for Cardiovascular Science, Liverpool John Moores University, Liverpool, United Kingdom
- 4Cardiovascular Prevention and Rehabilitation (EPIC) Center, Montreal Heart Institute, Montreal, QC, Canada
- 5School of Kinesiology and Exercise Science, Faculty of Medicine, Université de Montréal, Montreal, QC, Canada
- 6Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart and Chest Hospital, Liverpool, United Kingdom
- 7Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom
- 8Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom
Background: Regular exercise is an effective non-pharmacological therapy for treatment and prevention of cardiovascular disease (CVD). The therapeutic benefits of exercise are mediated partly through improved vascular and increase in metabolic health. Release of exercise-responsive myokines, including irisin, is associated with beneficial effects of exercise in CVD patients.
Observations: The present review provides an overview of the role of exercise in cardiac rehabilitation of patients with myocardial infarction (MI). Further, the role of irisin as a motion-responsive molecule in improving vascular and metabolic health is explored. Possible mechanism of cardioprotective effect of irisin-mediated exercise on myocardial infarction are also summarized in this review.
Conclusion and significance of the review: Irisin is associated with reduced inflammation, antioxidant properties, and anti-apoptotic effect, implying that it is a potential key mediator of the beneficial effects of exercise on vascular and metabolic health. The findings show that irisin is a promising therapeutic target for treatment of patients with cardiovascular disease, particularly post-MI. Further research should be conducted to elucidate the potential mechanisms of cardioprotective effects of irisin and explored whether irisin induced by exercise exerts rehabilitation effects post-MI.
1 Introduction
Exercise is an effective non-pharmacological intervention that improves cardiovascular health and function. Moreover, exercise alleviates cardiovascular disease (CVD) risk factors, and reduces all-cause mortality in CVD patients (Lear et al., 2017; Lin et al., 2020). Due to the dose-response relationship between exercise intensity and/or duration and overall cardiovascular benefit, the correct choice of exercise rehabilitation mode is particularly important for patients with CVD (Aengevaeren et al., 2017; Franklin et al., 2020). Exercise has been found to improve left ventricular function after myocardial infarction (MI) (Maessen et al., 2017; Alhumaid et al., 2022), which beneficial effects are partly mediated by promotion of cardiovascular function, mitochondrial biogenesis, and through stimulation of skeletal muscle to release myokines (Rosenkilde et al., 2018; Pinckard et al., 2019). However, its molecular mechanisms are not clear completely.
Irisin is mainly secreted in skeletal (Boström et al., 2012) and cardiac muscle tissue (Aydin et al., 2014), and is implicated in modulation of mitochondrial function and energy balance (Ouyang et al., 2020; Xin et al., 2020) lipid and glucose metabolism (Perakakis et al., 2017; Wang et al., 2020), and amelioration of impaired cardiac function in some metabolic disorders (Wang et al., 2017; Jiang et al., 2021). Previous findings show that circulating irisin level is lower in patients with chronic heart failure (Silvestrini et al., 2019), middle cerebral artery occlusion (MCAO) patients (Li et al., 2017), and subjects with Alzheimer disease (AD) (Kim et al., 2018) compared with normal subjects. Low irisin levels are associated with increase in levels of circulating inflammatory cytokines and/or angiotensin Ⅱ observed in various diseases. Exercise is the main inducer of irisin secretion both in healthy and dysregulated metabolism individuals (Huh et al., 2015). Studies have confirmed that exercise-induced irisin is correlated with improvement of cardiac function in general (Seo et al., 2020), which partly by modulating autophagy and mitochondrial function (Li et al., 2021a; He et al., 2021). Further previous animal studies report that irisin is implicated in cerebrovascular protective effects of exercise by alleviating ischemic neuron injury (Li et al., 2017; Lourenco et al., 2019). Irisin has a significant endothelial protective effect (determined by flow-mediated arterial dilation) (Wang et al., 2015) and inhibits progression of atherosclerosis (determined by flow-mediated arterial dilation (Lu et al., 2015; Chen et al., 2022). These findings indicate that irisin is a potential factor that mediates the protective effects of exercise in patients with cardio-cerebrovascular disease.
In the present review, cardioprotective effect of exercise on MI was explored. Moreover, the role of irisin, as an important exercise effector molecule in cardioprotective effects of exercise training was summarized. In addition, the possible cardiac protective mechanism of irisin reported in clinical studies and animal experiments was reviewed. The findings of the present review indicate that irisin plays an important role in exercise rehabilitation of MI.
2 Role of Exercise in Cardiac Rehabilitation of Patients With Acute Myocardial Infarction
2.1 Exercise-Based Cardiac Rehabilitation Conducted Immediately After Acute Myocardial Infarction Achieves Optimal Results
Cardiac rehabilitation is recommended in all patients with ACS, recent myocardial revascularization, stable angina pectoris, and stable coronary artery disease (CAD) (Taylor et al., 2022). Cardiac rehabilitation reduces risk factors, and increases the aerobic fitness, medication adherence, and survival after percutaneous coronary intervention and coronary artery bypass graft surgery (Colantonio et al., 2017; Rosenson et al., 2017). Moreover, it improves survival and reduces risk of recurrent MI in patients with acute MI (AMI) (Novaković et al., 2022). A previous meta-analysis comprising 63 trials and 14,486 patients assigned to exercise-based cardiac rehabilitation or no referral following MI or revascularization, reported that cardiac rehabilitation was associated with a lower risk of cardiovascular death [relative risk (RR) 0.74, 95% CI 0.64–0.86] and hospital readmission (RR 0.82, 95% CI 0.70–0.96) at 12-months (Anderson et al., 2016).
The rehabilitation is conducted to play an important role in achieving positive outcomes. Exercise training interventions had significantly higher beneficial effects on left-ventricular remodelling when exercise training is initiated immediately after AMI (from 1 week). Notably, when exercise was delayed for more than a week, the patients required an additional month of training to achieve the same level of benefit on cardiac remodelling as those who began exercise immediately after AMI (Haykowsky et al., 2011). A randomised controlled trial (RCT) comprising patients with AMI reported that early training intervention (<2 weeks post-MI) significantly improved health-related quality of life and functional capacity (through 6-min walk test) compared with the control (only usual care) (Peixoto et al., 2015). A previous clinical analysis was conducted using regression model to explore the recovery time and health of patients. The results showed that each 1-day increase in cardiac rehabilitation wait time led to a 1% reduction in the likelihood of improvement across all fitness-related measures including exercise level and Dartmouth quality of life physical fitness scale (Fell et al., 2016). Further, the findings showed that a shorter waiting period for cardiac rehabilitation after clinical coronary revascularization increases the health benefits of patients with coronary heart disease, such as optimizing cardiopulmonary function (peak oxygen uptake VO2peak; β = −0.165, p < 0.001) (Marzolini et al., 2015). This implies that individuals with AMI should begin a cardiac rehabilitation exercise immediately after hospital discharge to minimise risks of recurrence of cardiac events, reduce mortality risk, and improve quality of life (Parker et al., 2011).
2.2 Modality of Exercise Training in Cardiac Rehabilitation After AMI
Aerobic exercise training (AET) is the main exercise type in cardiac rehabilitation. AET improves health-related quality of life and several physiological parameters including cardiorespiratory fitness, cardiac function, handgrip strength, and knee extension strength (Izawa et al., 2004). The Exercise in Left Ventricular Dysfunction (ELVD) trial evaluated efficacy of exercise in patients with a first Q wave MI and a left ventricular ejection fraction (LVEF) below 40% and the results indicated that AET improved the quality of life of patients (Giannuzzi et al., 1997). Previous findings showed that 6-months AET markedly increased aerobic fitness and LVEF which are independent predictors of mortality in CVD patients (Keteyian et al., 2008; Kalam et al., 2014).
Studies have reported resistance training (RT) and AET result in a similar reduction (10%) in mortality rate in MI mice (Barboza et al., 2016). To increase the additional benefits of RT for patients with CVD, including those with MI and AMI, such as improved glucose metabolism, body composition, bone mineral density, muscle strength, and endurance (Williams et al., 2007; Kirkman et al., 2022), RT is mostly recommended in combination with AET (Williams et al., 2007; Price et al., 2016; Ambrosetti et al., 2020). Several studies have explored the benefits of combination of RT with traditional AET. A previous RCT of 26 MI patients was conducted with patients randomly assigned to AET with high-intensity group or to combined AET and RT group. The results showed that LVEF (Farheen et al., 2019), peak V̇O2 and quality of life (Khalid et al., 2019) were significantly improved in the combined group compared with the values in the AET group. Moreover, a Cochrane meta-analysis comprising 10,794 CAD patients showed that combination of AET and RT was associated with a 13% and 26% reduction in all-cause and cardiovascular mortality, respectively, and a 31% reduction in hospital readmission (Heran et al., 2011).
2.3 Dose of Exercise in Cardiac Rehabilitation After AMI
High-intensity interval (HIIT) and moderate-intensity continuous (MICT) training are complementary training modalities recommended by most exercise prescription guidelines for CAD patients (Vanhees et al., 2012; Mezzani et al., 2013; Price et al., 2016). Several meta-analyses have demonstrated that HIIT has similar or even higher benefits compared with MICT in improving peak VO2 (Conraads et al., 2015; Gomes-Neto et al., 2017). A previous RCT (Boidin et al., 2019) compared the effect of a 12-weeks HIIT to MICT in post-ACS patients (<6 weeks, 89% with MI) on the risk factors for arrhythmic death (i.e., heart rate recovery, heart rate variability, occurrence of ventricular arrhythmias, and QT dispersion (Wei et al., 1995; Freeman et al., 2006). The findings demonstrated that the two training interventions had no effects on these risk factors for arrhythmic death. Notably, a higher volume of exercise with MICT is required to achieve the same degree of reduction in all-cause and cardiovascular mortality observed with HIIT (Eijsvogels et al., 2016).
These findings indicate that an exercise training program should include moderate-to-high intensity AET and RT to markedly improve survival and quality of life in patients following MI. HIIT is a promising modality of exercise, owing to the lower dose of training needed to achieve the same magnitude of benefit as AET. Although HIIT is considered safe and effective in low-risk post-acute coronary syndrome patients, further research should be conducted to determine the safety in patients with AMI patients.
3 Irisin: Relation With Cardiac Rehabilitation-Mediated Protection
The novel myokine, irisin is a peptide obtained from hydrolysis of the transmembrane protein fibronectin type III domain protein 5 (FNDC5). Irisin has been found in both mouse and human serum following hydrolysis of FNDC5, and can be secreted in multiple tissues (Huh et al., 2012). Studies report that irisin is highly expressed after exercise, with plasma irisin levels peaking at 6 h after exercise, and returning to pre-exercise levels within 24 h, thereby mediating the beneficial effect of exercise (Pang et al., 2018; Maak et al., 2021). The effects of irisin in different tissues is dependent on metabolic phenotype. For instance, irisin levels in adipose tissue are significantly higher following HIIT compared with the levels after MICT in rats with dysregulated metabolic profile. However, the profile of irisin in skeletal muscle is not different after HIIT or MICT intervention. This implies that HIIT has protective effects against obesity and promotes metabolic dysfunction-induced reductions in adipose irisin levels (Tine Kartinah et al., 2018).
3.1 Relationship Between Irisin and Acute and Chronic Physical Activity Levels
3.1.1 In Animals
Animal experiments have been conducted in the last 5-years to evaluate the effects of exercise patterns on irisin concentrations (Table 1). The experimental animal models included healthy, obesity (most), diabetic, aging, Alzheimer’s, and AMI. Three studies (Bell et al., 2016; Pang et al., 2018; Nadermann and Volkoff, 2020a) focused on the impact of acute exercise, and the results showed that irisin acts as an acute exercise effector and high expression of irisin was mainly detected in blood or muscle. Further, the effector of chronic exercise training was irisin or FNDC5 (Zhang et al., 2017; Kazeminasab et al., 2018; Lourenco et al., 2019), both detected in most tissues and organs. Notably, the expression patterns of irisin or FNDC5 were independent of the type of exercise (MICT, AET, and RT) and intensity or duration of the exercise. Findings on the profile of FNDC5 after exercise are not consistent. Training leads to a higher concentration of FNDC5 in hippocampus of Alzheimer’s mice (Lourenco et al., 2019). In addition, training upregulates expression of FNDC5 at protein and/or mRNA level in bone or muscle tissue in obese or normal members (Reisi et al., 2016; Rocha-Rodrigues et al., 2016; Zhang et al., 2017). However, some studies report that exercise training only upregulated FNDC5 protein content in skeletal muscles, but not its mRNA expression (Kazeminasab et al., 2018).
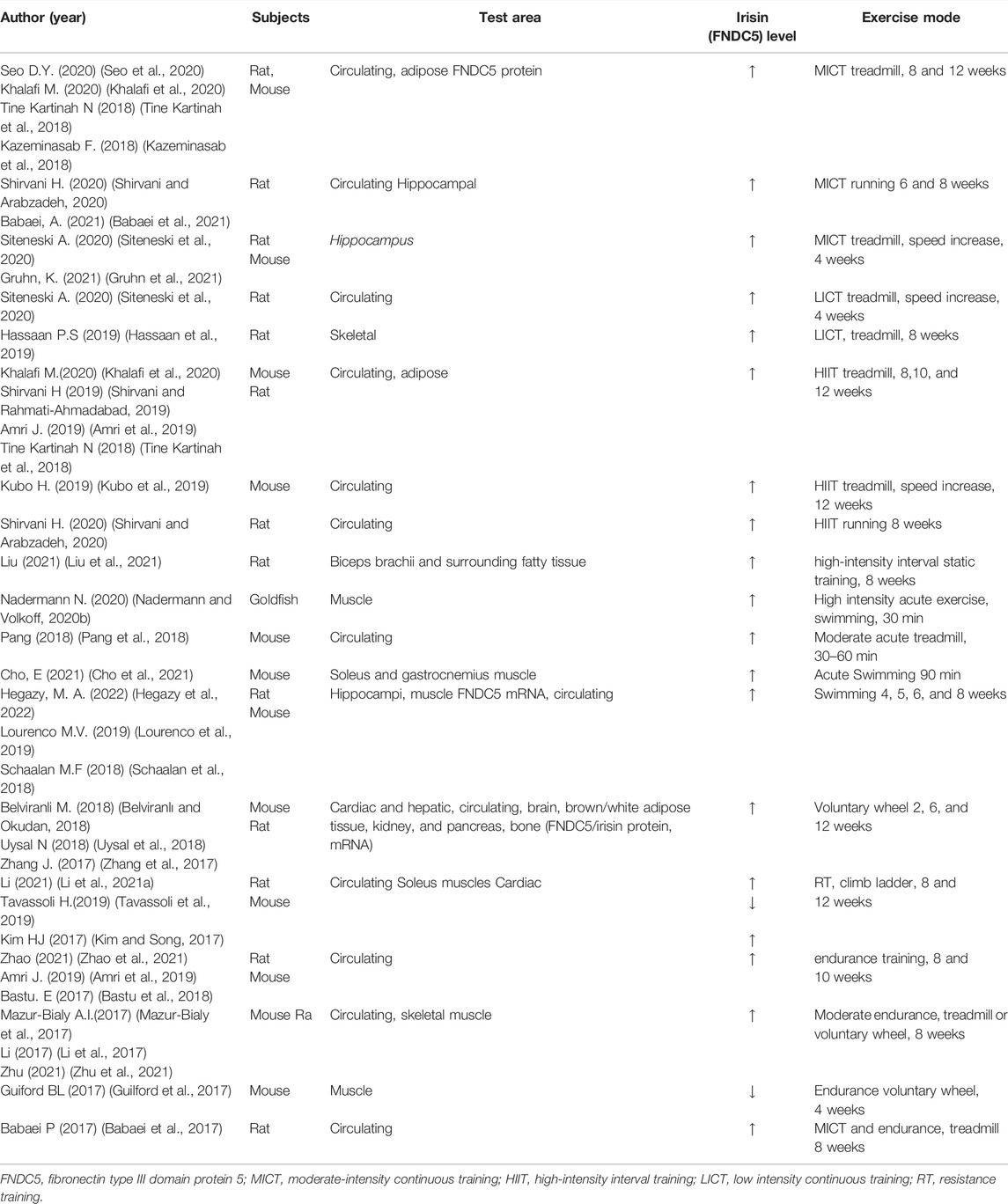
TABLE 1. Study characteristics of animal experiments that explored the effects of exercise on circulating irisin concentrations.
3.1.2 Clinical Studies
The ABCD study conducted in a general population demonstrated that irisin concentration is positively correlated with daily levels of physical activity (Buscemi et al., 2018). In addition, irisin concentration was correlated with gender (Zügel et al., 2016). Studies report that resting irisin concentration is higher in females compared with the level in males (Anastasilakis et al., 2014). Only few studies have explored response of irisin levels in patients with metabolic diseases undertaking different types of exercise. Findings from small sample clinical study showed no difference in exercise-induced (including high-intensity interval exercise, continuous moderate-intensity exercise, and resistance exercise) circulating irisin levels between healthy individuals and subjects with metabolic syndrome. This finding implied that the beneficial effects of exercise on glucolipid metabolism in patients with metabolic syndrome may be partly achieved by upregulation of irisin expression (Huh et al., 2015).
Six RCTs conducted from 2016 to 2021 explored the effect of various exercise modes on irisin expression, mainly in age-related, metabolic disease obesity (Weber-Rajek et al., 2019), progressive multiple sclerosis (Briken et al., 2016), non-alcoholic fatty liver disease models (Jia et al., 2018), and healthy young individuals (Qiu et al., 2018). The findings from these studies are summarized in Table 2. The findings from RCT studies indicated that exercise significantly upregulated expression of irisin (Kim et al., 2015; Briken et al., 2016; Bonfante et al., 2017; Jia et al., 2018; Weber-Rajek et al., 2019). Although further studies are required to verify these findings, these effects are possibly independent of exercise mode and duration of exercise. Notably, RT and combined exercise (CT) showed a higher increase in irisin levels relative to the levels in the aerobic exercise group (Jia et al., 2018). Moreover, RT and CT significantly improved metabolism and anthropometric indexes compared with the control (Amanat et al., 2020). These findings further confirm that exercise upregulates irisin level, implying that irisin is an effector molecule of exercise.
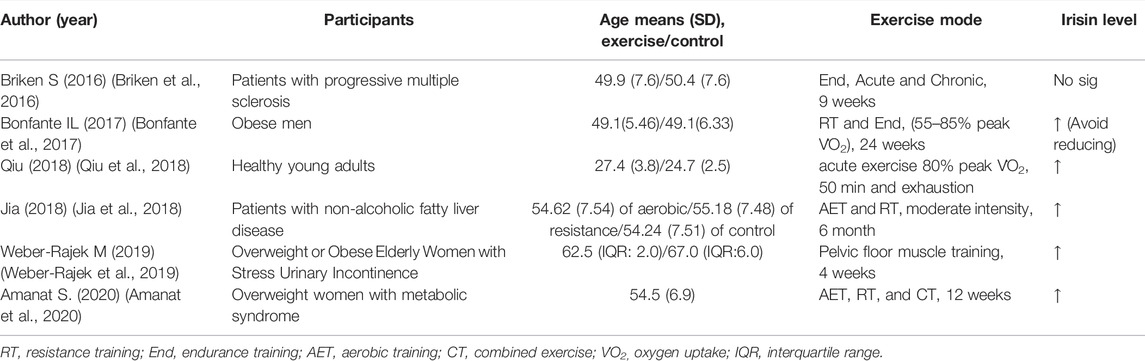
TABLE 2. Characteristics of randomized-controlled trials that explored the effects of exercise on circulating irisin concentrations in adults.
3.2 Relationship Between Irisin Concentration and Cardiovascular Disease
Findings from animal experiments indicate that irisin is highly secreted in the myocardium (Aydin et al., 2014). Reduction of irisin concentration following AMI was first explored through animal experiments, even within 2 h post-AMI (Kuloglu et al., 2014). The findings showed that low irisin content is correlated with high expression level of markers representing myocardial damage (such as troponin and creatine phosphokinase-myocardial band isoenzyme). Several studies report similar findings in human trials (Emanuele et al., 2014; Anastasilakis et al., 2017). A cross-sectional study reported that the level of serum irisin in patients with coronary artery disease (CAD) was significantly lower relative to the level in the control, indicating that it is a potential independent predictor for CAD (Deng, 2016). Moreover, findings from a non-randomized, interventional study showed a significant negative correlation between circulating irisin and the degree of stenosis (Anastasilakis et al., 2017).
Myocardial hypoxia occurs after infarction. Compensatory reduction of irisin level induces reduction in ATP utilization and improves energy supply of ischemic myocardium (Kuloglu et al., 2014). However, reduction in irisin levels is exacerbated by aggravation of myocardial ischemia and hypoxia due to significant loss in myocardium, which induces ventricular remodeling and ultimately leads to heart failure (Matsuo et al., 2015; Zhang et al., 2020a). It is therefore inferred that moderate supplementation with irisin may help to improve post-infarction cardiac function.
3.3 Irisin Plays a Protective Role in CVD
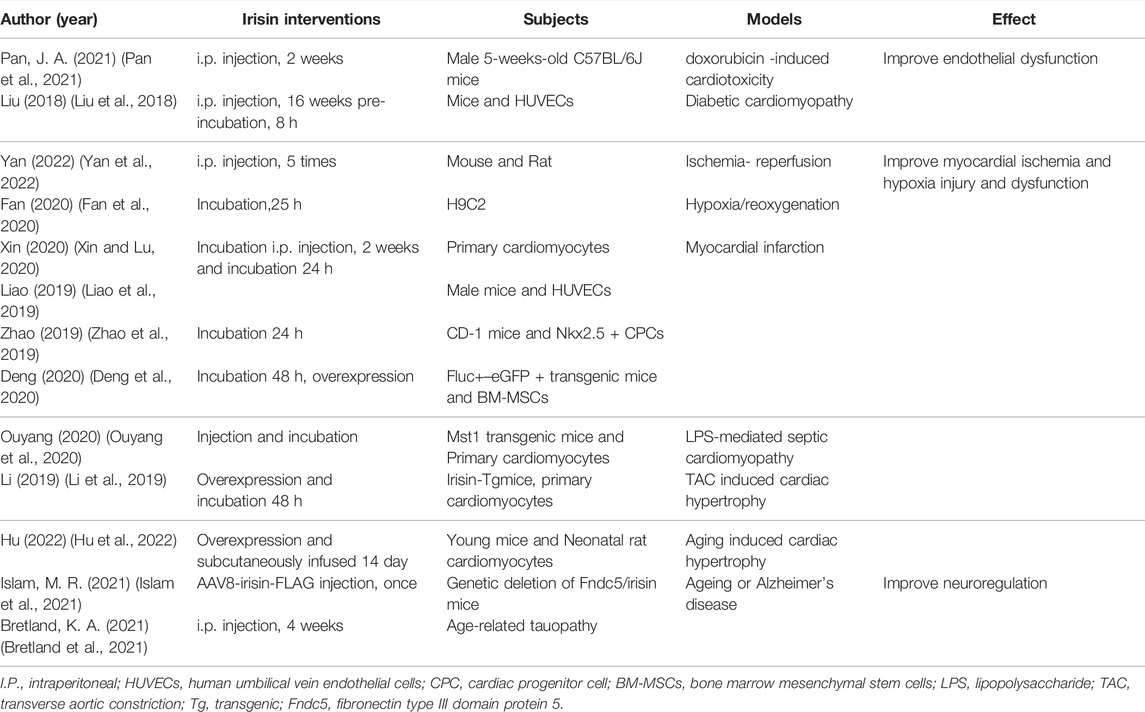
revious findings indicate that expression of irisin is downregulated in some metabolic diseases, such as diabetes (Du et al., 2016). Stimulation of irisin may be an important molecular mechanism of metformin, a conventional drug for diabetes (Li et al., 2015). Irisin plays an important role in reduction of CVD risk factors and maintenance of cardiac function (Polyzos et al., 2018; Calan and Demirpence, 2019). However, it has also been suggested that abnormally high values of circulating irisin may be associated with increased risk factors for cardiovascular disease (Calan and Demirpence, 2019) and may predict major adverse cardiovascular events in patients with acute coronary syndrome (ACS) (Aronis et al., 2015). In contrast, most recent animal studies and studies using myocardial cell culture reported that exogenous irisin intervention effectively improved vascular endothelial function and impaired cardiac function under pathological conditions. To compare the recent studies on myocardial protection with irisin, the author lists them in Table 3.
Administration of irisin (0.5 μg/g body weight/day) in models of endothelial structural and functional abnormalities significantly reduces atherosclerosis in apolipoprotein E-deficient mice by reducing levels of inflammation and apoptosis (Zhang et al., 2016). Moreover, acute intravenous injection of irisin (10 μg/kg) reduces blood pressure in spontaneously hypertensive rats by promoting NO production and endothelial NO synthase (eNOS) phosphorylation in endothelial cells (Fu et al., 2016). Furthermore, determination of endothelium-dependent vasodilation shows that intraperitoneal injection irisin (0.5 μg g−1·day−1) daily in the morning for 8 weeks improves impaired endothelial function of the aorta caused by obesity (Han et al., 2015). In addition, irisin treatment (microinjected into the nucleus ambiguous) promotes neuronal depolarization through the blood-brain barrier and reduces abnormal heart rate response through central cardiovascular regulation (Brailoiu et al., 2015).
Findings from animal and cell studies indicate that administration of exogenous irisin alleviates function injury of various organs such as the liver (Bi et al., 2019), intestines (Du et al., 2019), pulmonary (Chen et al., 2017), cerebral (Jin et al., 2019), kidney (Zhang et al., 2020b), and heart (Wang et al., 2017; Gul-Kahraman et al., 2019) caused by ischemia and reperfusion. Previous findings indicate that the protective effect of irisin is attributed to its anti-inflammatory effect (Matsuo et al., 2015; Deng et al., 2018; Xiong et al., 2018), anti-oxidative stress activity (Zhang et al., 2020c) and effect on reducing endothelial injury (Ye et al., 2018). Recent studies reported that irisin plays an important role in alleviating tissue fibrosis (Zhou et al., 2018; Chen et al., 2019), improving mitochondrial function (Xie et al., 2015; Bi et al., 2019) and promoting angiogenesis (Song et al., 2014; Wu et al., 2015). Further, treatment of in the MI model with irisin (intraperitoneal injection or extracellular incubation) significantly reduces the level of cardiomyocyte apoptosis and myocardial infarct size, as well as significantly improves mitochondrial function, thus promoting recovery of ventricular function (Zhao et al., 2016; Wang et al., 2017; Wang et al., 2018; Li et al., 2019; Liao et al., 2019; Zhao et al., 2019; Deng et al., 2020; Fan et al., 2020; Ouyang et al., 2020; Xin and Lu, 2020). NKX2.5 + CPCs isolated from mouse embryonic stem cells were pre-treated with irisin and implanted into a myocardial infarction tissue. The findings showed significant increase in cardiac remodeling in post-MI hearts treated with irisin compared with controls (NKX2.5 + CPCs without irisin) (Zhao et al., 2019). The cardiac function and the related pathological indicators of AMI in mice treated with or without irisin were compared and the results showed that irisin reduced the area of MI and improved the cardiac function by activating ERK signaling pathways and promoting angiogenesis (Liao et al., 2019). Exercise upregulates the low expression of cardiac irisin caused by MI and further improves impaired cardiac function and renal function in animal MI rehabilitation model (Wu et al., 2020; Li et al., 2021a).
4 Possible Mechanisms of the Role of Irisin in AMI Rehabilitation
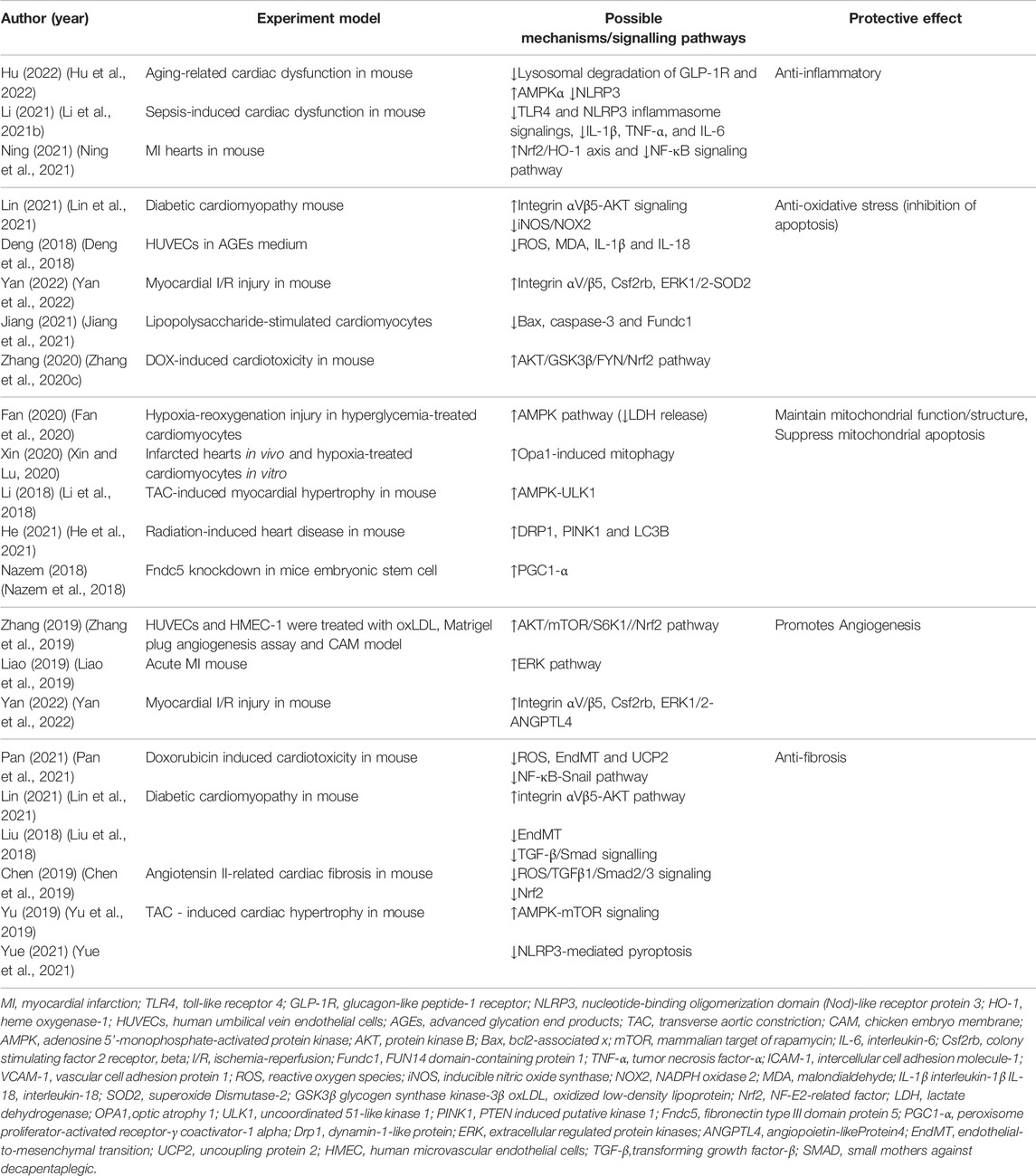
A previous study reported that exogenous irisin had therapeutic potential for pathologies associated with inflammation, oxidative stress, and apoptosis (Askari et al., 2018), but its receptor had not been identified. Recent studies have reported that simultaneous knockdown of integrins αV and β5 significantly attenuates the antioxidant/nitrosative stress and anti-apoptotic effects of irisin on H9C2, while pretreatment of adipose tissue-derived mesenchymal stromal cells (ADSCs) with irisin can promote ischemic cardioprotective effects by binding to cardiac integrins αV/β5 to induce the release of chemokine CSF2 from ischemic hearts and promotes the cardiac homing of ADSCs. It is therefore speculated that integrin αV/β5 may mediate the cardioprotective effects of irisin. However, the specific mechanisms involved in cardioprotection by irisin and its associated receptors still need to be verified by numerous studies. Mechanisms of irisin in cardiac protection reported in the past 5 years are summarized in Table 4.
4.1 Inflammation
Exercise-induced irisin expression is implicated in regulation of several cardiovascular and metabolic conditions, and the therapeutic effect is partly attributed to the anti-inflammatory effects of irisin (Díaz et al., 2018). Findings from a human study confirmed that exercise-induced high irisin secretion is implicated in reduction in arterial stiffness and improvement of endothelial function through activation of the arterial AMPK/Akt/eNOS pathway in obesity (Inoue et al., 2020). Further animal experiments showed that irisin treatment significantly alleviates endothelial dysfunction in diabetic mice and downregulates mRNA expression of macrophages and T lymphocytes in atherosclerotic plaques as well as expression of inflammatory cytokines (IL-6, TNF-α) in aortic tissue, which further abrogates development of atherosclerosis, and analysis showed that these anti-inflammatory effects are correlated with activation of the AMPK/PI3K/PKB/eNOS pathway by irisin (Lu et al., 2015). Moreover, exogenous irisin supplementation in animal models has a direct therapeutic effect on atherosclerotic diseases by suppressing ox-LDL-induced cell inflammation and apoptosis. This therapeutic effect is attributed to inhibition of ROS/p38MAPK/NF-κB signaling (Zhang et al., 2016), and/or ROS/NLRP3 inflammasome signaling (Deng et al., 2018).
4.2 Antioxidation (Inhibition of Necrosis and Apoptosis)
Irisin plays a protect role against cardiomyocytes and vascular endothelial cells by reducing oxidative stress through AMPK-PI3K-Akt-eNOS-ROS pathway (Askari et al., 2018). A study using myocardial ischemia-reperfusion mice model showed that irisin treatment significantly increased activity of antioxidant factors such as SOD-1 and p38 and markedly reduces of myocardial infarct size (Wang et al., 2017) Moreover, irisin overexpression or irisin treatment exhibited cardioprotective effect by inhibiting ROS and upregulating expression of antioxidant molecules such as GSH and total SOD in acute and chronic cardiotoxicity models. The results showed that therapeutic activity of irisin was mediated through the AKT/GSK3β/FYN/Nrf2 axis (Zhang et al., 2020c). A recent study revealed that aerobic exercise alleviated the levels of oxidative stress and apoptosis in Type II cardiorenal syndrome (CRS II) model, which was partially mediated by increase in irisin secretion (Wu et al., 2020).
4.3 Irisin Maintains Mitochondrial Function/Structure
Abnormal structure and function of mitochondria and the resulting energy metabolism disorder plays a key role in cellular energy stress and apoptosis (Ma et al., 2017). Studies report that the protective effect of irisin on the injured myocardium due to cardiotoxicity or abnormal oxygen supply is attributed to improved mitochondrial function, autophagy regulation, and reduced apoptosis (Zhao et al., 2016; Zhang et al., 2020c). Exercise-induced irisin activated mitophagy and reduced MI area in a MI mice model and exhibited protective effects against cardiac function (Li et al., 2021a). These findings indicate that mitophagy is a potential mechanism through which exercise rehabilitation alleviates infarction. This finding was confirmed in other ischemia/reperfusion injury models, whereby irisin treatment restored integrity of the structure of mitochondria (by suppressing the opening of mitochondrial permeability transition pore and mitochondrial swelling) and restored mitochondrial respiration function (Wang et al., 2017; Bi et al., 2020). Recent studies report exogenous irisin administration alleviates pressure overload-induced cardiac hypertrophy by activating ULK1 autophagy pathway, whereas endogenous irisin knockout disrupts mitochondrial homeostasis and significantly decreases cardiac differentiation in mouse embryonic stem cells (Li et al., 2018; Nazem et al., 2018).
4.4 Angiogenesis
A previous study treated human microvascular endothelial cells (HUVEC) and transgenic TG (fil1: GFP) zebrafish with human recombinant irisin. The findings showed that administration of exogenous irisin upregulated expression of MMP-2 and MMP-9 (interstitial metalloproteinases) in vascular endothelial cells. This finding indicated that irisin modulates vascular growth of endothelial cells by regulates ERK pathway (Wu et al., 2015). Similarly, irisin could inhibit oxidized low-density lipoprotein (oxLDL) impaired angiogenesis by modulating ERK signaling pathways (Zhang et al., 2019). A recent animal study reported that administration of irisin after AMI reduces myocardial infarction size and improves cardiac function after MI (Liao et al., 2019). The therapeutic effect of irisin was attributed to the angiogenic effect mediated by HUVEC migration, which may be dependent on ERK pathway activation. However, studies have not fully explored the mechanisms underlying the effect of exercise-induced irisin vascular endothelial function.
4.5 Anti-fibrosis
The phenotype study found that irisin administration can significantly ameliorates fibrotic remodeling in post-MI hearts and alleviated injured cardiac function (Deng et al., 2020). Mechanistic studies reported that both myocardial FNDC5 overexpression and exogenous irisin administration attenuated cardiac adverse structural remodeling due to diabetes, including myocardial fibrosis, and that its protective effects were closely associated with activation of integrin αVβ5-AKT signaling and attenuation of oxidative/nitrosative stress (Lin et al., 2021). Further study found irisin treatment inhibited TGF‐β/Smad signaling and high glucose‐induced cardiac endothelial‐to‐mesenchymal transition (EndMT), which contribute to cardiac fibrosis and heart failure (Liu et al., 2018). It has also been suggested that this protection mechanism may be the result of Nrf2 mediated inhibition of oxidative stress in angiotensin Ⅱ related myocardial fibrosis model (Chen et al., 2019). Mice transverse aortic constriction (TAC)-induced cardiac hypertrophy model reported irisin treatment attenuates pressure overload-induced cardiac hypertrophy and fibrosis mainly through regulating AMPK-mTOR signaling or inhibiting NOD-like receptor protein 3 (NLRP3) -mediated pyroptosis activation (Yu et al., 2019; Yue et al., 2021). A recent study revealed that irisin as a mediator of the beneficial effects of exercise in cardioprotection like ameliorate EndMT through inhibiting activation of NF-κB-Snail pathway due to excessive accumulation of UCP2 and ROS and regulating the autophagy disorders (Pan et al., 2021).
In summary, these findings show that irisin (including exercise-induced irisin secretion) exerts a myocardial protective role through its anti-inflammation activity, antioxidant stress effect, and anti-apoptosis properties, as well as improving mitochondrial function, promoting angiogenesis and fibrotic remodeling. This indicates that irisin has high therapeutic and rehabilitation potential for treatment of patients post-MI. Further studies should explore the mechanism through which exercise prevents and alleviates heart disease and the role of irisin induced by exercise in these mechanisms.
5 Conclusion and Prospect
Exercise-based cardiac rehabilitation is an effective cardioprotective intervention strategy for patients with CVD (Anderson et al., 2016; Bennett et al., 2017). The protective effects of exercise on ischemic heart are partly mediated by vascular adaptations, mitochondrial biogenesis, as well as stimulation of skeletal and cardiac muscle tissue to release myokines including irisin (Fiuza-Luces et al., 2018). Irisin treatment improves outcomes of CVD, which is associated with its properties of reversing inflammation, oxidative stress and excessive apoptosis, implying that irisin is a promising therapeutic target for treatment of CVD. Notably, exercise can improve cardiac function following MI by upregulating myocardial irisin expression (irrespective of exercise mode). However, the exact mechanism has not been elucidated, and multiple clinical and animal studies should be conducted to explore the role of irisin in MI rehabilitation (Figure 1). In addition, studies should evaluate whether Irisin-related agents can be supplemented to improve clinical benefits in patients who are intolerant to exercise after MI. This review provides a possible reference for a therapeutic target for exercise rehabilitation in MI patients.
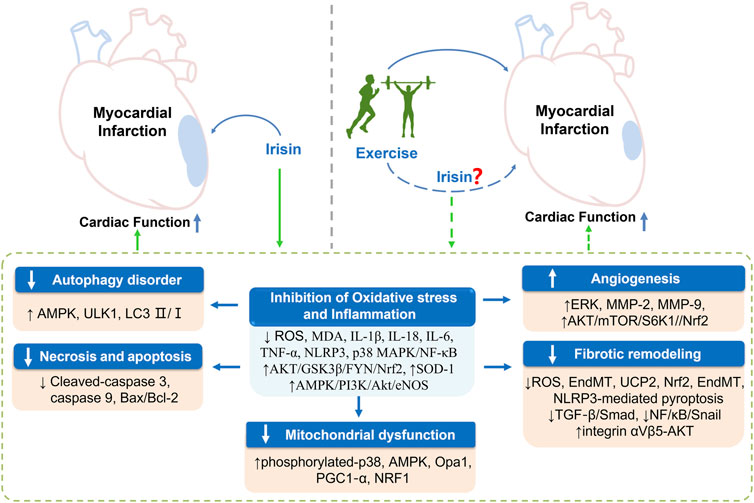
FIGURE 1. Exercise exhibits cardioprotective effect against post-myocardial infarction by mediating irisin expression and the potential mechanisms. Exercise and exogenous intervention with irisin can improve impaired cardiac function after MI by inhibiting inflammation and oxidative stress, and further improving the abnormalities of autophagy, apoptosis, and mitochondrial function, promoting angiogenesis, and inhibiting fibrotic remodeling caused by infarction. Meanwhile, exercise is an effective stimulus for upregulating irisin expression, however, whether exercise exerts the above beneficial effects through mediating irisin still needs to be verified by numerous studies. AMPK, adenosine 5′-monophosphate-activated protein kinase; PI3k, phosphoinositide 3-kinase; AKT, protein kinase B; eNOS, endothelial nitric oxide synthase; Bax, bcl2-associated x; mTOR, mammalian target of rapamycin; IL-1β, interleukin-1β; IL-18, interleukin-18; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-B; NLRP3, nucleotide-binding oligomerization domain (Nod)-like receptor protein 3; MDA, malondialdehyde; SOD-1, superoxide Dismutase-1; GSK3β, glycogen synthase kinase-3β; Nrf2, NF-E2-related factor; OPA1, optic atrophy 1; ULK1, uncoordinated 51-like kinase 1; NRF1, nuclear respiratory factor; PGC1‐α, peroxisome proliferator-activated receptor-γ coactivator-1 alpha; ERK, extracellular regulated protein kinases; MMP-2, matrix metallo-proteinase-2; MMP-9, matrix metallo-proteinase-9; EndMT, endothelial-to-mesenchymal transition.
Author Contributions
SQ and ZT contributed to the conception or design of the work. SQ contributed to writing the original draft, reviewing and editing. MB and BB contributed to writing the original draft and reviewing. GL, DT, and ZT critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
National Natural Science Foundation of China, Grant/Award Numbers: 32171128
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aengevaeren V. L., Mosterd A., Braber T. L., Prakken N. H. J., Doevendans P. A., Grobbee D. E., et al. (2017). Relationship between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 136 (2), 138–148. doi:10.1161/circulationaha.117.027834
Alhumaid W., Small S. D., Kirkham A. A., Becher H., Pituskin E., Prado C. M., et al. (2022). A Contemporary Review of the Effects of Exercise Training on Cardiac Structure and Function and Cardiovascular Risk Profile: Insights from Imaging. Front. Cardiovasc. Med. 9, 753652. doi:10.3389/fcvm.2022.753652
Amanat S., Sinaei E., Panji M., MohammadporHodki R., Bagheri-Hosseinabadi Z., Asadimehr H., et al. (2020). A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front. Physiol. 11, 562895. doi:10.3389/fphys.2020.562895
Ambrosetti M., Abreu A., Corrà U., Davos C. H., Hansen D., Frederix I., et al. (2020). Secondary Prevention through Comprehensive Cardiovascular Rehabilitation: From Knowledge to Implementation Update. A Position Paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2020, 2047487320913379.
Amri J., Parastesh M., Sadegh M., Latifi S., Alaee M. (2019). High-intensity Interval Training Improved Fasting Blood Glucose and Lipid Profiles in Type 2 Diabetic Rats More Than Endurance Training; Possible Involvement of Irisin and Betatrophin. Physiol. Int. 106 (3), 213–224. doi:10.1556/2060.106.2019.24
Anastasilakis A. D., Koulaxis D., Kefala N., Polyzos S. A., Upadhyay J., Pagkalidou E., et al. (2017). Circulating Irisin Levels Are Lower in Patients with Either Stable Coronary Artery Disease (CAD) or Myocardial Infarction (MI) versus Healthy Controls, whereas Follistatin and Activin A Levels Are Higher and Can Discriminate MI from CAD with Similar to CK-MB Accuracy. Metabolism 73, 1–8. doi:10.1016/j.metabol.2017.05.002
Anastasilakis A. D., Polyzos S. A., Saridakis Z. G., Kynigopoulos G., Skouvaklidou E. C., Molyvas D., et al. (2014). Circulating Irisin in Healthy, Young Individuals: Day-Night Rhythm, Effects of Food Intake and Exercise, and Associations with Gender, Physical Activity, Diet, and Body Composition. J. Clin. Endocrinol. Metab. 99 (9), 3247–3255. doi:10.1210/jc.2014-1367
Anderson L., Oldridge N., Thompson D. R., Zwisler A.-D., Rees K., Martin N., et al. (2016). Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease. J. Am. Coll. Cardiol. 67 (1), 1–12. doi:10.1016/j.jacc.2015.10.044
Aronis K. N., Moreno M., Polyzos S. A., Moreno-Navarrete J. M., Ricart W., Delgado E., et al. (2015). Circulating Irisin Levels and Coronary Heart Disease: Association with Future Acute Coronary Syndrome and Major Adverse Cardiovascular Events. Int. J. Obes. 39 (1), 156–161. doi:10.1038/ijo.2014.101
Askari H., Rajani S. F., Poorebrahim M., Haghi-Aminjan H., Raeis-Abdollahi E., Abdollahi M. (2018). A Glance at the Therapeutic Potential of Irisin against Diseases Involving Inflammation, Oxidative Stress, and Apoptosis: An Introductory Review. Pharmacol. Res. 129, 44–55. doi:10.1016/j.phrs.2018.01.012
Aydin S., Kuloglu T., Aydin S., Eren M. N., Celik A., Yilmaz M., et al. (2014). Cardiac, Skeletal Muscle and Serum Irisin Responses to with or without Water Exercise in Young and Old Male Rats: Cardiac Muscle Produces More Irisin Than Skeletal Muscle. Peptides 52, 68–73. doi:10.1016/j.peptides.2013.11.024
Babaei A., Nourshahi M., Fani M., Entezari Z., Jameie S. B., Haghparast A. (2021). The Effectiveness of Continuous and Interval Exercise Preconditioning against Chronic Unpredictable Stress: Involvement of Hippocampal PGC-1α/FNDC5/BDNF Pathway. J. Psychiatric Res. 136, 173–183. doi:10.1016/j.jpsychires.2021.02.006
Babaei P., Shirkouhi S. G., Hosseini R., Soltani Tehrani B. (2017). Vitamin D Is Associated with Metabotropic but Not Neurotrophic Effects of Exercise in Ovariectomized Rats. Diabetol. Metab. Syndr. 9, 91. doi:10.1186/s13098-017-0288-z
Barboza C. A., Souza G. I., Oliveira J. C., Silva L. M., Mostarda C. T., Dourado P. M., et al. (2016). Cardioprotective Properties of Aerobic and Resistance Training against Myocardial Infarction. Int. J. Sports Med. 37 (6), 421–430. doi:10.1055/s-0035-1565136
Bastu E., Zeybek U., Gurel Gurevin E., Yüksel Ozgor B., Celik F., Okumus N., et al. (2018). Effects of Irisin and Exercise on Metabolic Parameters and Reproductive Hormone Levels in High-Fat Diet-Induced Obese Female Mice. Reprod. Sci. 25 (2), 281–291. doi:10.1177/1933719117711264
Bell M., Levine C., Downey R., Griffitts C., Mann S., Frye C., et al. (2016). Influence of Endurance and Sprinting Exercise on Plasma Adiponectin, Leptin and Irisin Concentrations in Racing Greyhounds and Sled Dogs. Aust. Vet. J. 94 (5), 154–159. doi:10.1111/avj.12436
Belviranlı M., Okudan N. (2018). Exercise Training Increases Cardiac, Hepatic and Circulating Levels of Brain-Derived Neurotrophic Factor and Irisin in Young and Aged Rats. Horm. Mol. Biol. Clin. Investig. 36 (3). doi:10.1515/hmbci-2018-0053
Bennett D. A., Du H., Clarke R., Guo Y., Yang L., Bian Z., et al. (2017). Association of Physical Activity with Risk of Major Cardiovascular Disease in Chinese Men and Women. JAMA Cardiol. 2 (12), 1349–1358. doi:10.1001/jamacardio.2017.4069
Bi J., Zhang J., Ren Y., Du Z., Li Q., Wang Y., et al. (2019). Irisin Alleviates Liver Ischemia-Reperfusion Injury by Inhibiting Excessive Mitochondrial Fission, Promoting Mitochondrial Biogenesis and Decreasing Oxidative Stress. Redox Biol. 20, 296–306. doi:10.1016/j.redox.2018.10.019
Bi J., Zhang J., Ren Y., Du Z., Li T., Wang T., et al. (2020). Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin αVβ5 Receptor. J. Cell. Mol. Medi 24 (1), 996–1009. doi:10.1111/jcmm.14811
Boidin M., Gayda M., Henri C., Hayami D., Trachsel L. D., Besnier F., et al. (2019). Effects of Interval Training on Risk Markers for Arrhythmic Death: a Randomized Controlled Trial. Clin. Rehabil. 33 (8), 1320–1330. doi:10.1177/0269215519840388
Bonfante I. L. P., Chacon-Mikahil M. P. T., Brunelli D. T., Gáspari A. F., Duft R. G., Lopes W. A., et al. (2017). Combined Training, FNDC5/irisin Levels and Metabolic Markers in Obese Men: A Randomised Controlled Trial. Eur. J. Sport Sci. 17 (5), 629–637. doi:10.1080/17461391.2017.1296025
Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., et al. (2012). A PGC1-α-dependent Myokine that Drives Brown-fat-like Development of White Fat and Thermogenesis. Nature 481 (7382), 463–468. doi:10.1038/nature10777
Brailoiu E., Deliu E., Sporici R. A., Brailoiu G. C. (2015). Irisin Evokes Bradycardia by Activating Cardiac-Projecting Neurons of Nucleus Ambiguus. Physiol. Rep. 3 (6), e12419. doi:10.14814/phy2.12419
Bretland K. A., Lin L., Bretland K. M., Smith M. A., Fleming S. M., Dengler‐Crish C. M. (2021). Irisin Treatment Lowers Levels of Phosphorylated Tau in the hippocampus of Pre‐symptomatic Female but Not Male Htau Mice. Neuropathol. Appl. Neurobiol. 47 (7), 967–978. doi:10.1111/nan.12711
Briken S., Rosenkranz S. C., Keminer O., Patra S., Ketels G., Heesen C., et al. (2016). Effects of Exercise on Irisin, BDNF and IL-6 Serum Levels in Patients with Progressive Multiple Sclerosis. J. Neuroimmunol. 299, 53–58. doi:10.1016/j.jneuroim.2016.08.007
Buscemi S., Corleo D., Vasto S., Buscemi C., Massenti M. F., Nuzzo D., et al. (2018). Factors Associated with Circulating Concentrations of Irisin in the General Population Cohort of the ABCD Study. Int. J. Obes. 42 (3), 398–404. doi:10.1038/ijo.2017.255
Calan M., Demirpence M. (2019). Increased Circulating Levels of Irisin Are Associated with Cardiovascular Risk Factors in Subjects with Acromegaly. Hormones 18 (4), 435–442. doi:10.1007/s42000-019-00151-3
Chen J., Li K., Shao J., Lai Z., Gao R., Wang C., et al. (2022). Irisin Suppresses Nicotine-Mediated Atherosclerosis by Attenuating Endothelial Cell Migration, Proliferation, Cell Cycle Arrest, and Cell Senescence. Front. Cardiovasc. Med. 9, 851603. doi:10.3389/fcvm.2022.851603
Chen K., Xu Z., Liu Y., Wang Z., Li Y., Xu X., et al. (2017). Irisin Protects Mitochondria Function during Pulmonary Ischemia/reperfusion Injury. Sci. Transl. Med. 9 (418), eaao6298. doi:10.1126/scitranslmed.aao6298
Chen R.-R., Fan X.-H., Chen G., Zeng G.-W., Xue Y.-G., Liu X.-T., et al. (2019). Irisin Attenuates Angiotensin II-Induced Cardiac Fibrosis via Nrf2 Mediated Inhibition of ROS/TGFβ1/Smad2/3 Signaling axis. Chemico-Biological Interact. 302, 11–21. doi:10.1016/j.cbi.2019.01.031
Cho E., Jeong D. Y., Kim J. G., Lee S. (2021). The Acute Effects of Swimming Exercise on PGC-1α-FNDC5/Irisin-UCP1 Expression in Male C57BL/6J Mice. Metabolites 11 (2), 111. doi:10.3390/metabo11020111
Colantonio L. D., Huang L., Monda K. L., Bittner V., Serban M.-C., Taylor B., et al. (2017). Adherence to High-Intensity Statins Following a Myocardial Infarction Hospitalization Among Medicare Beneficiaries. JAMA Cardiol. 2 (8), 890–895. doi:10.1001/jamacardio.2017.0911
Conraads V. M., Pattyn N., De Maeyer C., Beckers P. J., Coeckelberghs E., Cornelissen V. A., et al. (2015). Aerobic Interval Training and Continuous Training Equally Improve Aerobic Exercise Capacity in Patients with Coronary Artery Disease: the SAINTEX-CAD Study. Int. J. Cardiol. 179, 203–210. doi:10.1016/j.ijcard.2014.10.155
Deng J., Zhang N., Wang Y., Yang C., Wang Y., Xin C., et al. (2020). FNDC5/irisin Improves the Therapeutic Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells for Myocardial Infarction. Stem Cell Res. Ther. 11 (1), 228. doi:10.1186/s13287-020-01746-z
Deng W. (2016). Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med. Sci. Monit. 22, 4193–4197. doi:10.12659/msm.897376
Deng X., Huang W., Peng J., Zhu T.-T., Sun X.-L., Zhou X.-Y., et al. (2018). Irisin Alleviates Advanced Glycation End Products-Induced Inflammation and Endothelial Dysfunction via Inhibiting ROS-NLRP3 Inflammasome Signaling. Inflammation 41 (1), 260–275. doi:10.1007/s10753-017-0685-3
Díaz B. B., González D. A., Gannar F., Pérez M. C. R., de León A. C. (2018). Myokines, Physical Activity, Insulin Resistance and Autoimmune Diseases. Immunol. Lett. 203, 1–5. doi:10.1016/j.imlet.2018.09.002
Du J., Fan X., Yang B., Chen Y., Liu K.-X., Zhou J. (2019). Irisin Pretreatment Ameliorates Intestinal Ischemia/reperfusion Injury in Mice through Activation of the Nrf2 Pathway. Int. Immunopharmacol. 73, 225–235. doi:10.1016/j.intimp.2019.05.011
Du X.-l., Jiang W.-x., Lv Z.-t. (2016). Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 48 (10), 644–652. doi:10.1055/s-0042-108730
Eijsvogels T. M. H., Molossi S., Lee D.-c., Emery M. S., Thompson P. D. (2016). Exercise at the Extremes. J. Am. Coll. Cardiol. 67 (3), 316–329. doi:10.1016/j.jacc.2015.11.034
Emanuele E., Minoretti P., Pareja-Galeano H., Sanchis-Gomar F., Garatachea N., Lucia A. (2014). Serum Irisin Levels, Precocious Myocardial Infarction, and Healthy Exceptional Longevity. Am. J. Med. 127 (9), 888–890. doi:10.1016/j.amjmed.2014.04.025
Fan J., Zhu Q., Wu Z., Ding J., Qin S., Liu H., et al. (2020). Protective Effects of Irisin on Hypoxia‐reoxygenation Injury in Hyperglycemia‐treated Cardiomyocytes: Role of AMPK Pathway and Mitochondrial Protection. J. Cell. Physiology 235 (2), 1165–1174. doi:10.1002/jcp.29030
Farheen H., Khalid Z., Tariq M., Sadiq T., Amjad I., Ramzan T. (2019). Combined Effect of Aerobic and Resistance Interval Training on Ejection Fraction in Myocardial Infarction. J. Coll. Physicians Surg. Pak 29 (3), 290–292. doi:10.29271/jcpsp.2019.03.290
Fell J., Dale V., Doherty P. (2016). Does the Timing of Cardiac Rehabilitation Impact Fitness Outcomes? an Observational Analysis. Open Heart 3 (1), e000369. doi:10.1136/openhrt-2015-000369
Fiuza-Luces C., Santos-Lozano A., Joyner M., Carrera-Bastos P., Picazo O., Zugaza J. L., et al. (2018). Exercise Benefits in Cardiovascular Disease: beyond Attenuation of Traditional Risk Factors. Nat. Rev. Cardiol. 15 (12), 731–743. doi:10.1038/s41569-018-0065-1
Franklin B. A., Thompson P. D., Al-Zaiti S. S., Albert C. M., Hivert M. F., Levine B. D., et al. (2020). Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks into Perspective-An Update: A Scientific Statement from the American Heart Association. Circulation 141 (13), e705–e36. doi:10.1161/CIR.0000000000000749
Freeman J. V., Dewey F. E., Hadley D. M., Myers J., Froelicher V. F. (2006). Autonomic Nervous System Interaction with the Cardiovascular System during Exercise. Prog. Cardiovasc. Dis. 48 (5), 342–362. doi:10.1016/j.pcad.2005.11.003
Fu J., Han Y., Wang J., Liu Y., Zheng S., Zhou L., et al. (2016). Irisin Lowers Blood Pressure by Improvement of Endothelial Dysfunction via AMPK-Akt-eNOS-NO Pathway in the Spontaneously Hypertensive Rat. J. Am. Heart Assoc. 5 (11), e003433. doi:10.1161/JAHA.116.003433
Giannuzzi P., Temporelli P. L., Corrà U., Gattone M., Giordano A., Tavazzi L., et al. (1997). Attenuation of Unfavorable Remodeling by Exercise Training in Postinfarction Patients with Left Ventricular Dysfunction. Circulation 96 (6), 1790–1797. doi:10.1161/01.cir.96.6.1790
Gomes-Neto M., Durães A. R., Reis H. F. C. d., Neves V. R., Martinez B. P., Carvalho V. O. (2017). High-intensity Interval Training versus Moderate-Intensity Continuous Training on Exercise Capacity and Quality of Life in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 24 (16), 1696–1707. doi:10.1177/2047487317728370
Gruhn K., Siteneski A., Camargo A., Freitas A. E., Olescowicz G., Brocardo P. S., et al. (2021). Physical Exercise Stimulates Hippocampal mTORC1 and FNDC5/irisin Signaling Pathway in Mice: Possible Implication for its Antidepressant Effect. Behav. Brain Res. 400, 113040. doi:10.1016/j.bbr.2020.113040
Guilford B. L., Parson J. C., Grote C. W., Vick S. N., Ryals J. M., Wright D. E. (2017). Increased FNDC5 Is Associated with Insulin Resistance in High Fat-Fed Mice. Physiol. Rep. 5 (13), e13319. doi:10.14814/phy2.13319
Gul-Kahraman K., Yilmaz-Bozoglan M., Sahna E. (2019). Physiological and Pharmacological Effects of Melatonin on Remote Ischemic Perconditioning after Myocardial Ischemia-Reperfusion Injury in Rats: Role of Cybb, Fas, NfκB, Irisin Signaling Pathway. J. Pineal Res. 67 (2), e12589. doi:10.1111/jpi.12589
Han F., Zhang S., Hou N., Wang D., Sun X. (2015). Irisin Improves Endothelial Function in Obese Mice through the AMPK-eNOS Pathway. Am. J. Physiology-Heart Circulatory Physiology 309 (9), H1501–H1508. doi:10.1152/ajpheart.00443.2015
Hassaan P. S., Nassar S. Z., Issa Y., Zahran N. (2019). Irisin vs. Treadmill Exercise in Post Myocardial Infarction Cardiac Rehabilitation in Rats. Archives Med. Res. 50 (2), 44–54. doi:10.1016/j.arcmed.2019.05.009
Haykowsky M., Scott J., Esch B., Schopflocher D., Myers J., Paterson I., et al. (2011). A Meta-Analysis of the Effects of Exercise Training on Left Ventricular Remodeling Following Myocardial Infarction: Start Early and Go Longer for Greatest Exercise Benefits on Remodeling. Trials 12, 92. doi:10.1186/1745-6215-12-92
He W., Tang Y., Li C., Zhang X., Huang S., Tan B., et al. (2021). Exercise Enhanced Cardiac Function in Mice with Radiation-Induced Heart Disease via the FNDC5/Irisin-dependent Mitochondrial Turnover Pathway. Front. Physiol. 12, 739485. doi:10.3389/fphys.2021.739485
Hegazy M. A., Abdelmonsif D. A., Zeitoun T. M., El-Sayed N. S., Samy D. M. (2022). Swimming Exercise versus L-Carnosine Supplementation for Alzheimer's Dementia in Rats: Implication of Circulating and Hippocampal FNDC5/irisin. J. Physiol. Biochem. 78 (1), 109–124. doi:10.1007/s13105-021-00845-6
Heran B. S., Chen J. M., Ebrahim S., Moxham T., Oldridge N., Rees K., et al. (2011). Exercise-based Cardiac Rehabilitation for Coronary Heart Disease. Cochrane Database Syst. Rev. (7), Cd001800. doi:10.1002/14651858.CD001800.pub2
Hu C., Zhang X., Hu M., Teng T., Yuan Y. P., Song P., et al. (2022). Fibronectin Type III Domain-Containing 5 Improves Aging-Related Cardiac Dysfunction in Mice. Aging Cell 21 (3), e13556. doi:10.1111/acel.13556
Huh J. Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M. T., Schneider B. E., et al. (2012). FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. mRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 61 (12), 1725–1738. doi:10.1016/j.metabol.2012.09.002
Huh J. Y., Siopi A., Mougios V., Park K. H., Mantzoros C. S. (2015). Irisin in Response to Exercise in Humans with and without Metabolic Syndrome. J. Clin. Endocrinol. Metabolism 100 (3), E453–E457. doi:10.1210/jc.2014-2416
Inoue K., Fujie S., Hasegawa N., Horii N., Uchida M., Iemitsu K., et al. (2020). Aerobic Exercise Training-Induced Irisin Secretion Is Associated with the Reduction of Arterial Stiffness via Nitric Oxide Production in Adults with Obesity. Appl. Physiol. Nutr. Metab. 45 (7), 715–722. doi:10.1139/apnm-2019-0602
Islam M. R., Valaris S., Young M. F., Haley E. B., Luo R., Bond S. F., et al. (2021). Exercise Hormone Irisin Is a Critical Regulator of Cognitive Function. Nat. Metab. 3 (8), 1058–1070. doi:10.1038/s42255-021-00438-z
Izawa K., Hirano Y., Yamada S., Oka K., Omiya K., Iijima S. (2004). Improvement in Physiological Outcomes and Health-Related Quality of Life Following Cardiac Rehabilitation in Patients with Acute Myocardial Infarction. Circ. J. 68 (4), 315–320. doi:10.1253/circj.68.315
Jia G. Y., Han T., Gao L., Wang L., Wang S. C., Yang L., et al. (2018). Effect of Aerobic Exercise and Resistance Exercise in Improving Non-alcoholic Fatty Liver Disease: a Randomized Controlled Trial. Zhonghua Gan Zang Bing Za Zhi 26 (1), 34–41. doi:10.3760/cma.j.issn.1007-3418.2018.01.009
Jiang X., Cai S., Jin Y., Wu F., He J., Wu X., et al. (2021). Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-dependent Mitophagy. Oxid. Med. Cell Longev. 2021, 2989974. doi:10.1155/2021/2989974
Jin Z., Guo P., Li X., Ke J., Wang Y., Wu H. (2019). Neuroprotective Effects of Irisin against Cerebral Ischemia/Reperfusion Injury via Notch Signaling Pathway. Biomed. Pharmacother. 120, 109452. doi:10.1016/j.biopha.2019.109452
Kalam K., Otahal P., Marwick T. H. (2014). Prognostic Implications of Global LV Dysfunction: a Systematic Review and Meta-Analysis of Global Longitudinal Strain and Ejection Fraction. Heart 100 (21), 1673–1680. doi:10.1136/heartjnl-2014-305538
Kazeminasab F., Marandi S. M., Ghaedi K., Safaeinejad Z., Esfarjani F., Nasr-Esfahani M. H. (2018). A Comparative Study on the Effects of High-Fat Diet and Endurance Training on the PGC-1α-FNDC5/irisin Pathway in Obese and Nonobese Male C57BL/6 Mice. Appl. Physiol. Nutr. Metab. 43 (7), 651–662. doi:10.1139/apnm-2017-0614
Keteyian S. J., Brawner C. A., Savage P. D., Ehrman J. K., Schairer J., Divine G., et al. (2008). Peak Aerobic Capacity Predicts Prognosis in Patients with Coronary Heart Disease. Am. Heart J. 156 (2), 292–300. doi:10.1016/j.ahj.2008.03.017
Khalafi M., Mohebbi H., Symonds M. E., Karimi P., Akbari A., Tabari E., et al. (2020). The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients 12 (4), 925. doi:10.3390/nu12040925
Khalid Z., Farheen H., Tariq M. I., Amjad I. (2019). Effectiveness of Resistance Interval Training versus Aerobic Interval Training on Peak Oxygen Uptake in Patients with Myocardial Infarction. J. Pak Med. Assoc. 69 (8), 1194–1198.
Kim H.-j., So B., Choi M., Kang D., Song W. (2015). Resistance Exercise Training Increases the Expression of Irisin Concomitant with Improvement of Muscle Function in Aging Mice and Humans. Exp. Gerontol. 70, 11–17. doi:10.1016/j.exger.2015.07.006
Kim H.-j., Song W. (2017). Resistance Training Increases Fibroblast Growth Factor-21 and Irisin Levels in the Skeletal Muscle of Zucker Diabetic Fatty Rats. Jenb 21 (3), 50–54. doi:10.20463/jenb.2017.0008
Kim H., Wrann C. D., Jedrychowski M., Vidoni S., Kitase Y., Nagano K., et al. (2018). Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 175 (7), 1756–1768. e17. doi:10.1016/j.cell.2018.10.025
Kirkman D. L., Lee D.-c., Carbone S. (2022). Resistance Exercise for Cardiac Rehabilitation. Prog. Cardiovasc. Dis. 70, 66–72. doi:10.1016/j.pcad.2022.01.004
Kubo H., Asai K., Kojima K., Sugitani A., Kyomoto Y., Okamoto A., et al. (2019). Exercise Ameliorates Emphysema of Cigarette Smoke-Induced COPD in Mice through the Exercise-Irisin-Nrf2 Axis. Copd 14, 2507–2516. doi:10.2147/copd.s226623
Kuloglu T., Aydin S., Eren M. N., Yilmaz M., Sahin İ., Kalayci M., et al. (2014). Irisin: A Potentially Candidate Marker for Myocardial Infarction. Peptides 55, 85–91. doi:10.1016/j.peptides.2014.02.008
Lear S. A., Hu W., Rangarajan S., Gasevic D., Leong D., Iqbal R., et al. (2017). The Effect of Physical Activity on Mortality and Cardiovascular Disease in 130 000 People from 17 High-Income, Middle-Income, and Low-Income Countries: the PURE Study. Lancet 390 (10113), 2643–2654. doi:10.1016/s0140-6736(17)31634-3
Li D.-J., Huang F., Lu W.-J., Jiang G.-J., Deng Y.-P., Shen F.-M. (2015). Metformin Promotes Irisin Release from Murine Skeletal Muscle Independently of AMP-Activated Protein Kinase Activation. Acta Physiol. 213 (3), 711–721. doi:10.1111/apha.12421
Li D.-J., Li Y.-H., Yuan H.-B., Qu L.-F., Wang P. (2017). The Novel Exercise-Induced Hormone Irisin Protects against Neuronal Injury via Activation of the Akt and ERK1/2 Signaling Pathways and Contributes to the Neuroprotection of Physical Exercise in Cerebral Ischemia. Metabolism 68, 31–42. doi:10.1016/j.metabol.2016.12.003
Li H., Qin S., Liang Q., Xi Y., Bo W., Cai M., et al. (2021). Exercise Training Enhances Myocardial Mitophagy and Improves Cardiac Function via Irisin/FNDC5-PINK1/Parkin Pathway in MI Mice. Biomedicines 9 (6), 701. doi:10.3390/biomedicines9060701
Li Q., Zhang M., Zhao Y., Dong M. (2021). Irisin Protects against LPS-Stressed Cardiac Damage through Inhibiting Inflammation, Apoptosis, and Pyroptosis. Shock 56 (6), 1009–1018. doi:10.1097/shk.0000000000001775
Li R.-L., Wu S.-S., Wu Y., Wang X.-X., Chen H.-Y., Xin J.-j., et al. (2018). Irisin Alleviates Pressure Overload-Induced Cardiac Hypertrophy by Inducing Protective Autophagy via mTOR-independent Activation of the AMPK-ULK1 Pathway. J. Mol. Cell. Cardiol. 121, 242–255. doi:10.1016/j.yjmcc.2018.07.250
Li R., Wang X., Wu S., Wu Y., Chen H., Xin J., et al. (2019). Irisin Ameliorates Angiotensin II‐induced Cardiomyocyte Apoptosis through Autophagy. J. Cell. Physiology 234 (10), 17578–17588. doi:10.1002/jcp.28382
Liao Q., Qu S., Tang L.-x., Li L.-p., He D.-f., Zeng C.-y., et al. (2019). Irisin Exerts a Therapeutic Effect against Myocardial Infarction via Promoting Angiogenesis. Acta Pharmacol. Sin. 40 (10), 1314–1321. doi:10.1038/s41401-019-0230-z
Lin C., Guo Y., Xia Y., Li C., Xu X., Qi T., et al. (2021). FNDC5/Irisin Attenuates Diabetic Cardiomyopathy in a Type 2 Diabetes Mouse Model by Activation of Integrin αV/β5-AKT Signaling and Reduction of Oxidative/nitrosative Stress. J. Mol. Cell. Cardiol. 160, 27–41. doi:10.1016/j.yjmcc.2021.06.013
Lin H., Sardana M., Zhang Y., Liu C., Trinquart L., Benjamin E. J., et al. (2020). Association of Habitual Physical Activity with Cardiovascular Disease Risk. Circ. Res. 127 (10), 1253–1260. doi:10.1161/circresaha.120.317578
Liu X., Mujahid H., Rong B., Lu Q. H., Zhang W., Li P., et al. (2018). Irisin Inhibits High Glucose-Induced Endothelial-To-Mesenchymal Transition and Exerts a Dose-dependent Bidirectional Effect on Diabetic Cardiomyopathy. J. Cell Mol. Med. 22 (2), 808–822. doi:10.1111/jcmm.13360
Liu Y., Guo C., Liu S., Zhang S., Mao Y., Fang L. (2021). Eight Weeks of High-Intensity Interval Static Strength Training Improves Skeletal Muscle Atrophy and Motor Function in Aged Rats via the PGC-1α/FNDC5/UCP1 Pathway. Cia 16, 811–821. doi:10.2147/cia.s308893
Lourenco M. V., Frozza R. L., de Freitas G. B., Zhang H., Kincheski G. C., Ribeiro F. C., et al. (2019). Exercise-linked FNDC5/irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer's Models. Nat. Med. 25 (1), 165–175. doi:10.1038/s41591-018-0275-4
Lu J., Xiang G., Liu M., Mei W., Xiang L., Dong J. (2015). Irisin Protects against Endothelial Injury and Ameliorates Atherosclerosis in Apolipoprotein E-Null Diabetic Mice. Atherosclerosis 243 (2), 438–448. doi:10.1016/j.atherosclerosis.2015.10.020
Ma Z., Xin Z., Di W., Yan X., Li X., Reiter R. J., et al. (2017). Melatonin and Mitochondrial Function during Ischemia/reperfusion Injury. Cell. Mol. Life Sci. 74 (21), 3989–3998. doi:10.1007/s00018-017-2618-6
Maak S., Norheim F., Drevon C. A., Erickson H. P. (2021). Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 42 (4), 436–456. doi:10.1210/endrev/bnab003
Maessen M. F., Eijsvogels T. M., Stevens G., van Dijk A. P., Hopman M. T. (2017). Benefits of Lifelong Exercise Training on Left Ventricular Function after Myocardial Infarction. Eur. J. Prev. Cardiol. 24 (17), 1856–1866. doi:10.1177/2047487317728765
Marzolini S., Blanchard C., Alter D. A., Grace S. L., Oh P. I. (2015). Delays in Referral and Enrolment Are Associated with Mitigated Benefits of Cardiac Rehabilitation after Coronary Artery Bypass Surgery. Circ Cardiovasc. Qual. Outcomes 8 (6), 608–620. doi:10.1161/circoutcomes.115.001751
Matsuo Y., Gleitsmann K., Mangner N., Werner S., Fischer T., Bowen T. S., et al. (2015). Fibronectin Type III Domain Containing 5 Expression in Skeletal Muscle in Chronic Heart Failure-Relevance of Inflammatory Cytokines. J. Cachexia Sarcopenia Muscle 6 (1), 62–72. doi:10.1002/jcsm.12006
Mazur-Bialy A. I., Bilski J., Wojcik D., Brzozowski B., Surmiak M., Hubalewska-Mazgaj M., et al. (2017). Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 9 (4), 410. doi:10.3390/nu9040410
Mezzani A., Hamm L. F., Jones A. M., McBride P. E., Moholdt T., Stone J. A., et al. (2013). Aerobic Exercise Intensity Assessment and Prescription in Cardiac Rehabilitation: a Joint Position Statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 20 (3), 442–467. doi:10.1177/2047487312460484
Nadermann N., Volkoff H. (2020). Effects of Short-Term Exercise on Food Intake and the Expression of Appetite-Regulating Factors in Goldfish. Peptides. doi:10.1016/j.peptides.2019.170182
Nadermann N., Volkoff H. (2020). Effects of Short-Term Exercise on Food Intake and the Expression of Appetite-Regulating Factors in Goldfish. Peptides 123, 170182. doi:10.1016/j.peptides.2019.170182
Nazem S., Rabiee F., Ghaedi K., Babashah S., Sadeghizadeh M., Nasr‐Esfahani M. H. (2018). Fndc5 Knockdown Induced Suppression of Mitochondrial Integrity and Significantly Decreased Cardiac Differentiation of Mouse Embryonic Stem Cells. J. Cell. Biochem. 119 (6), 4528–4539. doi:10.1002/jcb.26590
Ning H., Chen H., Deng J., Xiao C., Xu M., Shan L., et al. (2021). Exosomes Secreted by FNDC5-BMMSCs Protect Myocardial Infarction by Anti-inflammation and Macrophage Polarization via NF-κB Signaling Pathway and Nrf2/HO-1 axis. Stem Cell Res. Ther. 12 (1), 519. doi:10.1186/s13287-021-02591-4
Novaković M., Novak T., Vižintin Cuderman T., Krevel B., Tasič J., Rajkovič U., et al. (2022). Exercise Capacity Improvement after Cardiac Rehabilitation Following Myocardial Infarction and its Association with Long-Term Cardiovascular Events. Eur. J. Cardiovasc Nurs. 21 (1), 76–84. doi:10.1093/eurjcn/zvab015
Ouyang H., Li Q., Zhong J., Xia F., Zheng S., Lu J., et al. (2020). Combination of Melatonin and Irisin Ameliorates Lipopolysaccharide‐induced Cardiac Dysfunction through Suppressing the Mst1-JNK Pathways. J. Cell. Physiology 235 (10), 6647–6659. doi:10.1002/jcp.29561
Pan J.-a., Zhang H., Lin H., Gao L., Zhang H.-l., Zhang J.-f., et al. (2021). Irisin Ameliorates Doxorubicin-Induced Cardiac Perivascular Fibrosis through Inhibiting Endothelial-To-Mesenchymal Transition by Regulating ROS Accumulation and Autophagy Disorder in Endothelial Cells. Redox Biol. 46, 102120. doi:10.1016/j.redox.2021.102120
Pang M., Yang J., Rao J., Wang H., Zhang J., Wang S., et al. (2018). Time-Dependent Changes in Increased Levels of Plasma Irisin and Muscle PGC-1α and FNDC5 after Exercise in Mice. Tohoku J. Exp. Med. 244 (2), 93–103. doi:10.1620/tjem.244.93
Parker K., Stone J. A., Arena R., Lundberg D., Aggarwal S., Goodhart D., et al. (2011). An Early Cardiac Access Clinic Significantly Improves Cardiac Rehabilitation Participation and Completion Rates in Low-Risk ST-Elevation Myocardial Infarction Patients. Can. J. Cardiol. 27 (5), 619–627. doi:10.1016/j.cjca.2010.12.076
Peixoto T. C. A., Begot I., Bolzan D. W., Machado L., Reis M. S., Papa V., et al. (2015). Early Exercise-Based Rehabilitation Improves Health-Related Quality of Life and Functional Capacity after Acute Myocardial Infarction: a Randomized Controlled Trial. Can. J. Cardiol. 31 (3), 308–313. doi:10.1016/j.cjca.2014.11.014
Perakakis N., Triantafyllou G. A., Fernández-Real J. M., Huh J. Y., Park K. H., Seufert J., et al. (2017). Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 13 (6), 324–337. doi:10.1038/nrendo.2016.221
Pinckard K., Baskin K. K., Stanford K. I. (2019). Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 6, 69. doi:10.3389/fcvm.2019.00069
Polyzos S. A., Anastasilakis A. D., Efstathiadou Z. A., Makras P., Perakakis N., Kountouras J., et al. (2018). Irisin in Metabolic Diseases. Endocrine 59 (2), 260–274. doi:10.1007/s12020-017-1476-1
Price K. J., Gordon B. A., Bird S. R., Benson A. C. (2016). A Review of Guidelines for Cardiac Rehabilitation Exercise Programmes: Is There an International Consensus? Eur. J. Prev. Cardiol. 23 (16), 1715–1733. doi:10.1177/2047487316657669
Qiu S., Bosnyák E., Treff G., Steinacker J. M., Nieß A. M., Krüger K., et al. (2018). Acute Exercise-Induced Irisin Release in Healthy Adults: Associations with Training Status and Exercise Mode. Eur. J. Sport Sci. 18 (9), 1226–1233. doi:10.1080/17461391.2018.1478452
Reisi J., Ghaedi K., Rajabi H., Marandi S. M. (2016). Can Resistance Exercise Alter Irisin Levels and Expression Profiles of FNDC5 and UCP1 in Rats? Asian J. Sports Med. 7 (4), e35205. doi:10.5812/asjsm.35205
Rocha-Rodrigues S., Rodríguez A., Gouveia A. M., Gonçalves I. O., Becerril S., Ramírez B., et al. (2016). Effects of Physical Exercise on Myokines Expression and Brown Adipose-like Phenotype Modulation in Rats Fed a High-Fat Diet. Life Sci. 165, 100–108. doi:10.1016/j.lfs.2016.09.023
Rosenkilde M., Rygaard L., Nordby P., Nielsen L. B., Stallknecht B. (2018). Exercise and Weight Loss Effects on Cardiovascular Risk Factors in Overweight Men. J. Appl. Physiol. (1985) 125 (3), 901–908. doi:10.1152/japplphysiol.01092.2017
Rosenson R. S., Farkouh M. E., Mefford M., Bittner V., Brown T. M., Taylor B., et al. (2017). Trends in Use of High-Intensity Statin Therapy after Myocardial Infarction, 2011 to 2014. J. Am. Coll. Cardiol. 69 (22), 2696–2706. doi:10.1016/j.jacc.2017.03.585
Schaalan M. F., Ramadan B. K., Abd Elwahab A. H. (2018). Synergistic Effect of Carnosine on Browning of Adipose Tissue in Exercised Obese Rats; a Focus on Circulating Irisin Levels. J. Cell Physiol. 233 (6), 5044–5057. doi:10.1002/jcp.26370
Seo D. Y., Bae J. H., Kim T. N., Kwak H. B., Kha P. T., Han J. (2020). Exercise-induced Circulating Irisin Level Is Correlated with Improved Cardiac Function in Rats. Int. J. Environ. Res. Public Health 17, 3863. doi:10.3390/ijerph17113863
Shirvani H., Arabzadeh E. (2020). Metabolic Cross-Talk between Skeletal Muscle and Adipose Tissue in High-Intensity Interval Training vs. Moderate-Intensity Continuous Training by Regulation of PGC-1α. Eating and Weight Disorders, 25(1), 17–24. doi:10.1007/s40519-018-0491-4
Shirvani H., Rahmati-Ahmadabad S. (2019). Irisin Interaction with Adipose Tissue Secretions by Exercise Training and Flaxseed Oil Supplement. Lipids Health Dis. 18 (1), 15. doi:10.1186/s12944-019-0960-4
Silvestrini A., Bruno C., Vergani E., Venuti A., Favuzzi A. M. R., Guidi F., et al. (2019). Circulating Irisin Levels in Heart Failure with Preserved or Reduced Ejection Fraction: A Pilot Study. PLoS One 14 (1), e0210320. doi:10.1371/journal.pone.0210320
Siteneski A., Olescowicz G., Pazini F. L., Camargo A., Fraga D. B., Brocardo P. S., et al. (2020). Antidepressant-like and Pro-neurogenic Effects of Physical Exercise: the Putative Role of FNDC5/irisin Pathway. J. Neural Transm. 127 (3), 355–370. doi:10.1007/s00702-020-02143-9
Song H., Wu F., Zhang Y., Zhang Y., Wang F., Jiang M., et al. (2014). Irisin Promotes Human Umbilical Vein Endothelial Cell Proliferation through the ERK Signaling Pathway and Partly Suppresses High Glucose-Induced Apoptosis. PLoS One 9 (10), e110273. doi:10.1371/journal.pone.0110273
Tavassoli H., Heidarianpour A., Hedayati M. (2019). The Effects of Resistance Exercise Training Followed by De-training on Irisin and Some Metabolic Parameters in Type 2 Diabetic Rat Model. Arch. Physiol. Biochem., 1–8. doi:10.1080/13813455.2019.1673432
Taylor R. S., Dalal H. M., McDonagh S. T. J. (2022). The Role of Cardiac Rehabilitation in Improving Cardiovascular Outcomes. Nat. Rev. Cardiol. 19 (3), 180–194. doi:10.1038/s41569-021-00611-7
Tine Kartinah N., Rosalyn Sianipar I., Nafi'ah , Rabia (2018). The Effects of Exercise Regimens on Irisin Levels in Obese Rats Model: Comparing High-Intensity Intermittent with Continuous Moderate-Intensity Training. Biomed. Res. Int. 2018, 4708287. doi:10.1155/2018/4708287
Uysal N., Yuksel O., Kizildag S., Yuce Z., Gumus H., Karakilic A., et al. (2018). Regular Aerobic Exercise Correlates with Reduced Anxiety and Incresed Levels of Irisin in Brain and White Adipose Tissue. Neurosci. Lett. 676, 92–97. doi:10.1016/j.neulet.2018.04.023
Vanhees L., Geladas N., Hansen D., Kouidi E., Niebauer J., Reiner Ž., et al. (2012). Importance of Characteristics and Modalities of Physical Activity and Exercise in the Management of Cardiovascular Health in Individuals with Cardiovascular Risk Factors: Recommendations from the EACPR (Part II). Eur. J. Prev. Cardiol. 19 (5), 1005–1033. doi:10.1177/1741826711430926
Wang H.-h., Zhang X.-w., Chen W.-k., Huang Q.-x., Chen Q.-q. (2015). Relationship between Serum Irisin Levels and Urinary Albumin Excretion in Patients with Type 2 Diabetes. J. Diabetes its Complicat. 29 (3), 384–389. doi:10.1016/j.jdiacomp.2015.01.001
Wang H., Zhao Y. T., Zhang S., Dubielecka P. M., Du J., Yano N., et al. (2017). Irisin Plays a Pivotal Role to Protect the Heart against Ischemia and Reperfusion Injury. J. Cell Physiol. 232 (12), 3775–3785. doi:10.1002/jcp.25857
Wang J., Zhao Y. T., Zhang L., Dubielecka P. M., Zhuang S., Qin G., et al. (2020). Irisin Improves Myocardial Performance and Attenuates Insulin Resistance in Spontaneous Mutation (Leprdb) Mice. Front. Pharmacol. 11, 769. doi:10.3389/fphar.2020.00769
Wang Z., Chen K., Han Y., Zhu H., Zhou X., Tan T., et al. (2018). Irisin Protects Heart against Ischemia-Reperfusion Injury through a SOD2-dependent Mitochondria Mechanism. J. Cardiovasc. Pharmacol. 72 (6), 259–269. doi:10.1097/fjc.0000000000000608
Weber-Rajek M., Radzimińska A., Strączyńska A., Strojek K., Piekorz Z., Kozakiewicz M., et al. (2019). A Randomized-Controlled Trial Pilot Study Examining the Effect of Pelvic Floor Muscle Training on the Irisin Concentration in Overweight or Obese Elderly Women with Stress Urinary Incontinence. Biomed. Res. Int. 2019, 7356187. doi:10.1155/2019/7356187
Wei K., Dorian P., Newman D., Langer A. (1995). Association between QT Dispersion and Autonomic Dysfunction in Patients with Diabetes Mellitus. J. Am. Coll. Cardiol. 26 (4), 859–863. doi:10.1016/0735-1097(95)00279-8
Williams M. A., Haskell W. L., Ades P. A., Amsterdam E. A., Bittner V., Franklin B. A., et al. (2007). Resistance Exercise in Individuals with and without Cardiovascular Disease: 2007 Update. Circulation 116 (5), 572–584. doi:10.1161/circulationaha.107.185214
Wu F., Li Z., Cai M., Xi Y., Xu Z., Zhang Z., et al. (2020). Aerobic Exercise Alleviates Oxidative Stress-Induced Apoptosis in Kidneys of Myocardial Infarction Mice by Inhibiting ALCAT1 and Activating FNDC5/Irisin Signaling Pathway. Free Radic. Biol. Med. 158, 171–180. doi:10.1016/j.freeradbiomed.2020.06.038
Wu F., Song H., Zhang Y., Zhang Y., Mu Q., Jiang M., et al. (2015). Irisin Induces Angiogenesis in Human Umbilical Vein Endothelial Cells In Vitro and in Zebrafish Embryos In Vivo via Activation of the ERK Signaling Pathway. PLoS One 10 (8), e0134662. doi:10.1371/journal.pone.0134662
Xie C., Zhang Y., Tran T. D. N., Wang H., Li S., George E. V., et al. (2015). Irisin Controls Growth, Intracellular Ca2+ Signals, and Mitochondrial Thermogenesis in Cardiomyoblasts. PLoS One 10 (8), e0136816. doi:10.1371/journal.pone.0136816
Xin C., Zhang Z., Gao G., Ding L., Yang C., Wang C., et al. (2020). Irisin Attenuates Myocardial Ischemia/Reperfusion Injury and Improves Mitochondrial Function through AMPK Pathway in Diabetic Mice. Front. Pharmacol. 11, 565160. doi:10.3389/fphar.2020.565160
Xin T., Lu C. (2020). Irisin Activates Opa1-Induced Mitophagy to Protect Cardiomyocytes against Apoptosis Following Myocardial Infarction. Aging 12 (5), 4474–4488. doi:10.18632/aging.102899
Xiong X.-Q., Geng Z., Zhou B., Zhang F., Han Y., Zhou Y.-B., et al. (2018). FNDC5 Attenuates Adipose Tissue Inflammation and Insulin Resistance via AMPK-Mediated Macrophage Polarization in Obesity. Metabolism 83, 31–41. doi:10.1016/j.metabol.2018.01.013
Yan W., Chen Y., Guo Y., Xia Y., Li C., Du Y., et al. (2022). Irisin Promotes Cardiac Homing of Intravenously Delivered MSCs and Protects against Ischemic Heart Injury. Adv. Sci. (Weinh). 9 (7), e2103697. doi:10.1002/advs.202103697
Ye L., Xu M., Hu M., Zhang H., Tan X., Li Q., et al. (2018). TRPV4 Is Involved in Irisin-Induced Endothelium-dependent Vasodilation. Biochem. Biophysical Res. Commun. 495 (1), 41–45. doi:10.1016/j.bbrc.2017.10.160
Yu Q., Kou W., Xu X., Zhou S., Luan P., Xu X., et al. (2019). FNDC5/Irisin Inhibits Pathological Cardiac Hypertrophy. Clin. Sci. (Lond). 133 (5), 611–627. doi:10.1042/cs20190016
Yue R., Zheng Z., Luo Y., Wang X., Lv M., Qin D., et al. (2021). NLRP3-mediated Pyroptosis Aggravates Pressure Overload-Induced Cardiac Hypertrophy, Fibrosis, and Dysfunction in Mice: Cardioprotective Role of Irisin. Cell Death Discov. 7 (1), 50. doi:10.1038/s41420-021-00434-y
Zhang J., Bi J., Ren Y., Du Z., Li T., Wang T., et al. (2020). Involvement of GPX4 in Irisin's Protection against Ischemia Reperfusion-Induced Acute Kidney Injury. J. Cell Physiol. 236, 931–945. doi:10.1002/jcp.29903
Zhang J., Valverde P., Zhu X., Murray D., Wu Y., Yu L., et al. (2017). Exercise-induced Irisin in Bone and Systemic Irisin Administration Reveal New Regulatory Mechanisms of Bone Metabolism. Bone Res. 5, 16056. doi:10.1038/boneres.2016.56
Zhang M., Xu Y., Jiang L. (2019). Irisin Attenuates Oxidized Low‐density Lipoprotein Impaired Angiogenesis through AKT/mTOR/S6K1/Nrf2 Pathway. J. Cell. Physiology 234 (10), 18951–18962. doi:10.1002/jcp.28535
Zhang X., Hu C., Wu H. M., Ma Z. G., Tang Q. Z. (2020). Fibronectin Type III Domain-Containing 5 in Cardiovascular and Metabolic Diseases: a Promising Biomarker and Therapeutic Target. Acta Pharmacol. Sin. 42, 1390–1400. doi:10.1038/s41401-020-00557-5
Zhang X., Hu C., Kong C.-Y., Song P., Wu H.-M., Xu S.-C., et al. (2020). FNDC5 Alleviates Oxidative Stress and Cardiomyocyte Apoptosis in Doxorubicin-Induced Cardiotoxicity via Activating AKT. Cell Death Differ. 27 (2), 540–555. doi:10.1038/s41418-019-0372-z
Zhang Y., Mu Q., Zhou Z., Song H., Zhang Y., Wu F., et al. (2016). Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction. PLoS One 11 (6), e0158038. doi:10.1371/journal.pone.0158038
Zhao R., Zhou Y., Li J., Lin J., Cui W., Peng Y., et al. (2021). Irisin Regulating Skeletal Response to Endurance Exercise in Ovariectomized Mice by Promoting Akt/β-Catenin Pathway. Front. Physiol. 12, 639066. doi:10.3389/fphys.2021.639066
Zhao Y. T., Wang H., Zhang S., Du J., Zhuang S., Zhao T. C. (2016). Irisin Ameliorates Hypoxia/Reoxygenation-Induced Injury through Modulation of Histone Deacetylase 4. PLoS One 11 (11), e0166182. doi:10.1371/journal.pone.0166182
Zhao Y. T., Wang J., Yano N., Zhang L. X., Wang H., Zhang S., et al. (2019). Irisin Promotes Cardiac Progenitor Cell‐induced Myocardial Repair and Functional Improvement in Infarcted Heart. J. Cell Physiol. 234 (2), 1671–1681. doi:10.1002/jcp.27037
Zhou B., Ling L., Zhang F., Liu T.-Y., Zhou H., Qi X.-H., et al. (2018). Fibronectin Type III Domain-Containing 5 Attenuates Liver Fibrosis via Inhibition of Hepatic Stellate Cell Activation. Cell Physiol. Biochem. 48 (1), 227–236. doi:10.1159/000491722
Zhu W., Sahar N. E., Javaid H. M. A., Pak E. S., Liang G., Wang Y., et al. (2021). Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 10 (12), 3360. doi:10.3390/cells10123306
Keywords: irisin, exercise, myocardial infarction, cardiac rehabilitation, cardioprotection
Citation: Qin S, Tian Z, Boidin M, Buckley BJR, Thijssen DHJ and Lip GYH (2022) Irisin is an Effector Molecule in Exercise Rehabilitation Following Myocardial Infarction (Review). Front. Physiol. 13:935772. doi: 10.3389/fphys.2022.935772
Received: 04 May 2022; Accepted: 01 June 2022;
Published: 29 June 2022.
Edited by:
Ning Chen, Wuhan Sports University, ChinaReviewed by:
Michael Kirberger, Georgia Gwinnett College, United StatesJunhao Huang, Guangzhou Sport University, China
Copyright © 2022 Qin, Tian, Boidin, Buckley, Thijssen and Lip. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenjun Tian, dGlhbnpoakBzbm51LmVkdS5jbg==
 Shuguang Qin
Shuguang Qin Zhenjun Tian
Zhenjun Tian Maxime Boidin
Maxime Boidin Benjamin J. R. Buckley
Benjamin J. R. Buckley Dick H. J. Thijssen3,8
Dick H. J. Thijssen3,8 Gregory Y. H. Lip
Gregory Y. H. Lip