- National Tobacco Cultivation, Physiology and Biochemistry Research Center, Flavors and Fragrance Engineering and Technology Research Center of Henan Province, College of Tobacco Science, Henan Agricultural University, Zhengzhou, Henan, China
Introduction
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder characterized by cognitive decline and memory impairment. With increasing global prevalence, the need for effective therapeutic interventions is critical. Among the currently approved treatments, acetylcholinesterase inhibitors (AChEIs) like huperzine A and galantamine stand out due to their neuroprotective roles. These plant-derived alkaloids have demonstrated significant efficacy in alleviating symptoms by increasing acetylcholine levels in the brain.
While numerous other plant alkaloids exhibit varying degrees of neuroactive properties, huperzine A and galantamine remain the only plant-derived alkaloids currently approved and marketed as specific treatments for AD and other neurodegenerative diseases. For example, alkaloids such as berberine (from Berberis species) and rhynchophylline (from Uncaria rhynchophylla) have shown potential in targeting amyloid-beta (Aβ) aggregation, oxidative stress, and tau hyperphosphorylation. Similarly, harmine has demonstrated the ability to inhibit tau hyperphosphorylation through dual inhibition of glycogen synthase kinase-3 beta (GSK-3β) and dual specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A). However, these metabolites have not yet advanced to clinical applications (Ng et al., 2015; Rezaul Islam et al., 2024).
The elucidation of the biosynthetic pathways of huperzine A and galantamine marks a significant advancement in understanding plant biochemistry and specialized metabolism. It not only advances our understanding of plant-derived neuroactive metabolites but also provides opportunities for sustainable and scalable production through synthetic biology approaches. By leveraging this approach, researchers can reconstruct the biosynthetic pathways of plant-derived natural products in microbial or plant systems, facilitating efficient production and reducing the reliance on native plant sources for these valuable compounds (Liu et al., 2023; Zhang et al., 2023; Bai et al., 2024; Teng et al., 2024). This opinion highlights the implications of these discoveries for future research and application in neurodegenerative disease treatment.
Huperzine A: a lycopodium alkaloid
Huperzine A, derived from Huperzia serrata (Lycopodiaceae), is a well-known AChEI that has been widely used in traditional Chinese medicine (Ma and Gang, 2004; Yang et al., 2017; Wang et al., 2020; Zhang et al., 2024). The elucidation of the biosynthetic pathway of huperzine A has provided crucial insights into the formation of Lycopodium alkaloids and uncovered numerous enzymes with novel functions (Li et al., 2022; Ushimaru and Abe, 2023; Cheng et al., 2024). Recent studies have identified three novel neofunctionalized α-carbonic anhydrase-like (CAL) enzymes responsible for the key Mannich-like condensations that form core carbon–carbon bonds in Lycopodium alkaloids, key steps in the construction of their polycyclic skeletons. Through transcriptome analysis and enzyme characterization, Nett et al. identified key enzymes such as CAL-1 and CAL-2, which promote crucial annulation reactions (Nett et al., 2023; Liu F. et al., 2024; Zamar et al., 2024). The pathway proceeds through stereospecific modifications and scaffold tailoring, involving additional enzymes like Fe(II)-dependent dioxygenases, which introduce oxidation steps crucial for the final bioactive form of huperzine A (Figure 1) (Nett et al., 2021; Nett et al., 2023; Ushimaru and Abe, 2023). These findings shed light on the complex evolution of neuroactive alkaloids in Lycopodium species, suggesting that such enzymes have evolved for specialized metabolite production as a defense mechanism.
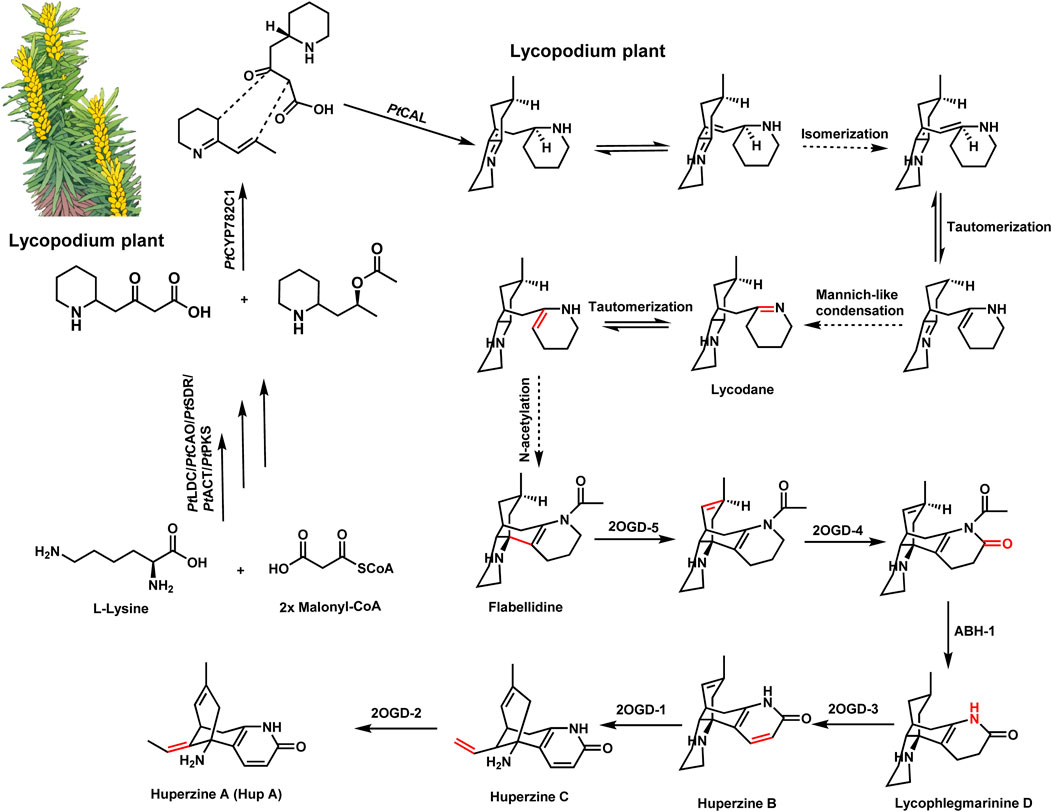
Figure 1. Schematic illustration of the biosynthesis of lycopodium alkaloids. Biosynthetic pathway of huperzine A (HupA). PtLDC, lycine decarboxylase; PtCAO, copper amine oxidase; PtPKS, piperidyl ketide synthase; PtSDR, short-chain dehydrogenase/reductase; PtCAT, acetyltransferase; PtCAL, alpha-carbonic anhydrases-like; 2OGD-1, 2-oxoglutarate-dependent dioxygenase 1; 2OGD-2, 2-oxoglutarate-dependent dioxygenase 2; 2OGD-3, 2-oxoglutarate-dependent dioxygenase 3; 2OGD-4, 2-oxoglutarate-dependent dioxygenase 4; 2OGD-5, 2-oxoglutarate-dependent dioxygenase 5.
Moreover, transient expression of huperzine A biosynthetic genes in Nicotiana benthamiana allowed for the successful production of Lycopodium alkaloid congeners, underscoring the potential for scalable biosynthesis through heterologous platforms. This breakthrough not only deepens our understanding of plant-derived alkaloids but also opens the door to bioengineering huperzine A production in microbial or plant chassis, reducing reliance on natural resources (Zhang et al., 2022; Gao et al., 2023; Liu et al., 2023; Bai et al., 2024; Golubova et al., 2024).
Galantamine: an amaryllidaceae alkaloid
Galantamine, an alkaloid derived from plants in the Amaryllidaceae family, particularly daffodils (Narcissus spp.), is another crucial AChEI used in AD treatment (Prvulovic et al., 2010). Similar to huperzine A, the biosynthetic pathway of galantamine was recently elucidated, providing invaluable insights into its production (Kilgore et al., 2014; Li et al., 2018; Li et al., 2019; Hu et al., 2021; Mehta et al., 2024). The discovery began with identifying the key precursor, 4′-O-methylnorbelladine (4OMN), followed by oxidative coupling catalyzed by cytochrome P450 enzymes such as NtCYP96T6. This enzyme facilitates the para-ortho (p-o’) oxidative coupling necessary to produce the galantamine skeleton. Subsequent methylation and reduction steps, catalyzed by NtNMT1 and NtAKR1 respectively, complete the biosynthesis of galantamine (Figure 2) (Mehta et al., 2024).
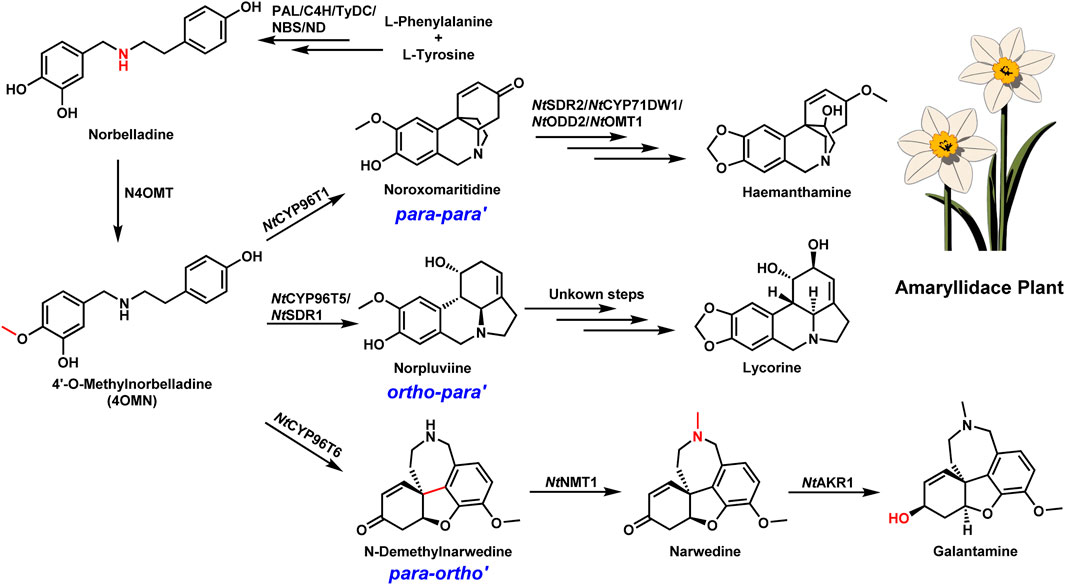
Figure 2. Schematic illustration of the biosynthesis of amaryllidaceae alkaloids. Biosynthetic pathway of galantamine. PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; TyDC, tyrosine decarboxylase; NBS, norbelladine synthase; ND, norcraugsodine reductase; NtSDR2, short-chain dehydrogenase/reductase 2; NtCYP71DW1, cytochrome P450 71DW1; NtODD2, 2-oxoglutarate-dependent dioxygenase 2; NtOMT1, O-Methyltransferase 1; NtCYP 96T1, cytochrome P450 96T1; NtCYP 96T5, cytochrome P450 96T5; NtCYP 96T6, cytochrome P450 96T6; NtSDR1, short-chain dehydrogenase/reductase 1; NtNMT1, N-demethylnarwedine methyltransferase 1; NtAKR1, aldo-keto reductase 1. Dashed arrows represent steps that are hypothesized to occur spontaneously or without enzymatic catalysis.
This discovery has profound implications for synthetic biology and metabolic engineering. With galantamine currently sourced primarily from natural populations of daffodils, the ability to biosynthesize it through engineered microbial systems holds significant promise for sustainable and scalable production (Zhang et al., 2022; Gao et al., 2023). Additionally, the elucidation of galantamine’s pathway helps to understand how plants generate chemical diversity from simple precursors, providing a foundation for engineering other related alkaloids with potential therapeutic value.
Challenges and future directions
The elucidation of huperzine A and galantamine biosynthetic pathways underscores the complexity and elegance of plant specialized metabolism. Both alkaloids share the common feature of acting as acetylcholinesterase inhibitors, though their evolutionary and biosynthetic origins differ significantly. The Lycopodium and Amaryllidaceae families, through distinct evolutionary pressures, have developed highly specialized enzymes that allow these plants to synthesize neuroactive metabolites with intricate polycyclic structures. While the elucidation of these biosynthetic pathways represents a significant advancement, several challenges remain.
First, the in vivo functional roles of these alkaloids in plants are not fully understood. It is speculated that they serve as defense metabolites against herbivores, but the regulatory mechanisms governing their production remain elusive (Chavez et al., 2024). Further research into the ecological roles of these alkaloids could provide important insights into the evolution of medicinal plants, the evolution of biosynthetic pathways, and their interactions with the environment (Szypuła and Pietrosiuk, 2023; Zhang et al., 2024).
Second, the scalability of producing huperzine A and galantamine through heterologous systems remains a key challenge. While transient expression in N. benthamiana has demonstrated proof-of-concept for biosynthesis, translating these findings into industrial-scale production will require optimization of gene expression, precursor supply, and enzymatic activity in microbial or plant-based platforms (Liu J. C. et al., 2024; Yang et al., 2024). Optimizing precursor supply, enhancing enzyme activity, and achieving high-yield production in heterologous systems are critical bottlenecks. Microbial synthetic biology platforms, such as Saccharomyces cerevisiae and Pichia pastoris, offer promising avenues for large-scale production due to their scalability and ease of genetic manipulation (Zhang et al., 2022; Gao et al., 2023; Yang et al., 2024). On the other hand, plant chassis like N. benthamiana provide unique advantages, including natural metabolic environments and compartmentalized cells conducive to complex biosynthesis (Liu et al., 2023; Zhang et al., 2023; Golubova et al., 2024; Liu J. C. et al., 2024). Advances in CRISPR-based genome editing, multi-gene pathway assembly, and metabolic flux optimization are pivotal for overcoming current limitations (Liao et al., 2023; Xie et al., 2023; Teng et al., 2024). By leveraging these tools, researchers can create efficient production platforms not only for huperzine A and galantamine but also for other plant-derived neuroactive alkaloids, paving the way for accessible and sustainable therapeutics for Alzheimer’s disease.
Finally, the potential for discovering new neuroactive alkaloids in related plant species should not be overlooked. The pathways for huperzine A and galantamine likely represent only a fraction of the neuroactive metabolites that plants produce. Systematic exploration of the metabolic pathways in related species could yield novel AChE inhibitors or other metabolites targeting neurodegenerative diseases.
Conclusion
The elucidation of the biosynthetic pathways of huperzine A and galantamine marks a pivotal moment in plant biochemistry and neuropharmacology. These discoveries not only deepen our understanding of plant metabolism but also offer practical pathways for the sustainable production of crucial AD treatments. As the global population ages and the burden of neurodegenerative diseases grows, plant-derived neuroactive alkaloids like huperzine A and galantamine will continue to play an essential role in treatment. The future of this research lies in the intersection of synthetic biology, metabolic engineering, and traditional plant sciences, paving the way for innovative solutions to Alzheimer’s disease and other neurological disorders.
Author contributions
QY: Conceptualization, Writing–review and editing. ZZ: Conceptualization, Writing–review and editing. MY: Conceptualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (32400218), the Key Technology R&D Program of Henan Province (242102110240, 232102110053), the Special Support Fund for High-level Talents and skills improvement of Henan Agricultural University (30501474).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, Y., Liu, X., and Baldwin, I. T. (2024). Using synthetic biology to understand the function of plant specialized metabolites. Annu. Rev. Plant Biol. 75, 629–653. doi:10.1146/annurev-arplant-060223-013842
Chavez, B. G., Leite Dias, S., and D'auria, J. C. (2024). The evolution of tropane alkaloids: coca does it differently. Curr. Opin. Plant Biol. 81, 102606. doi:10.1016/j.pbi.2024.102606
Cheng, B., Song, L., and Chen, F. (2024). Huperzine alkaloids: forty years of total syntheses. Nat. Prod. Rep. 41, 59–84. doi:10.1039/d3np00029j
Gao, J., Zuo, Y., Xiao, F., Wang, Y., Li, D., Xu, J., et al. (2023). Biosynthesis of catharanthine in engineered Pichia pastoris. Nat. Synth. 2, 231–242. doi:10.1038/s44160-022-00205-2
Golubova, D., Tansley, C., Su, H., and Patron, N. J. (2024). Engineering Nicotiana benthamiana as a platform for natural product biosynthesis. Curr. Opin. Plant Biol. 81, 102611. doi:10.1016/j.pbi.2024.102611
Hu, J., Li, W., Liu, Z., Zhang, G., and Luo, Y. (2021). Molecular cloning and functional characterization of tyrosine decarboxylases from galanthamine-producing Lycoris radiata. Acta Physiol. Plant. 43, 84. doi:10.1007/s11738-021-03258-6
Kilgore, M. B., Augustin, M. M., Starks, C. M., O'neil-Johnson, M., May, G. D., Crow, J. A., et al. (2014). Cloning and characterization of a norbelladine 4'-O-methyltransferase involved in the biosynthesis of the Alzheimer's drug galanthamine in Narcissus sp. aff. pseudonarcissus. PLoS One 9, e103223. doi:10.1371/journal.pone.0103223
Li, W., Qiao, C., Pang, J., Zhang, G., and Luo, Y. (2019). The versatile O-methyltransferase LrOMT catalyzes multiple O-methylation reactions in amaryllidaceae alkaloids biosynthesis. Int. J. Biol. Macromol. 141, 680–692. doi:10.1016/j.ijbiomac.2019.09.011
Li, W., Yang, Y., Qiao, C., Zhang, G., and Luo, Y. (2018). Functional characterization of phenylalanine ammonia-lyase- and cinnamate 4-hydroxylase-encoding genes from Lycoris radiata, a galanthamine-producing plant. Int. J. Biol. Macromol. 117, 1264–1279. doi:10.1016/j.ijbiomac.2018.06.046
Li, X., Li, W., Tian, P., and Tan, T. (2022). Delineating biosynthesis of Huperzine A, A plant-derived medicine for the treatment of Alzheimer's disease. Biotechnol. Adv. 60, 108026. doi:10.1016/j.biotechadv.2022.108026
Liao, J., Liu, T., Xie, L., Mo, C., Qiao, J., Huang, X., et al. (2023). Heterologous mogrosides biosynthesis in cucumber and tomato by genetic manipulation. Commun. Biol. 6, 191. doi:10.1038/s42003-023-04553-3
Liu, F., Fernie, A. R., and Zhang, Y. (2024). Plant gene co-expression defines the biosynthetic pathway of neuroactive alkaloids. Mol. Plant 17, 372–374. doi:10.1016/j.molp.2024.02.002
Liu, J. C., De La Pena, R., Tocol, C., and Sattely, E. S. (2024). Reconstitution of early paclitaxel biosynthetic network. Nat. Commun. 15, 1419. doi:10.1038/s41467-024-45574-8
Liu, X., Zhang, P., Zhao, Q., and Huang, A. C. (2023). Making small molecules in plants: a chassis for synthetic biology-based production of plant natural products. J. Integr. Plant Biol. 65, 417–443. doi:10.1111/jipb.13330
Ma, X., and Gang, D. R. (2004). The Lycopodium alkaloids. Nat. Product. Rep. 21, 752–772. doi:10.1039/b409720n
Mehta, N., Meng, Y., Zare, R., Kamenetsky-Goldstein, R., and Sattely, E. (2024). A developmental gradient reveals biosynthetic pathways to eukaryotic toxins in monocot geophytes. Cell 187, 5620–5637.e10. doi:10.1016/j.cell.2024.08.027
Nett, R. S., Dho, Y., Low, Y. Y., and Sattely, E. S. (2021). A metabolic regulon reveals early and late acting enzymes in neuroactive Lycopodium alkaloid biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 118, e2102949118. doi:10.1073/pnas.2102949118
Nett, R. S., Dho, Y., Tsai, C., Passow, D., Martinez Grundman, J., Low, Y. Y., et al. (2023). Plant carbonic anhydrase-like enzymes in neuroactive alkaloid biosynthesis. Nature 624, 182–191. doi:10.1038/s41586-023-06716-y
Ng, Y. P., Or, T. C. T., and Ip, N. Y. (2015). Plant alkaloids as drug leads for Alzheimer's disease. Neurochem. Int. 89, 260–270. doi:10.1016/j.neuint.2015.07.018
Prvulovic, D., Hampel, H., and Pantel, J. (2010). Galantamine for Alzheimer's disease. Expert Opin. Drug Metabolism and Toxicol. 6, 345–354. doi:10.1517/17425251003592137
Rezaul Islam, M., Akash, S., Murshedul Islam, M., Sarkar, N., Kumer, A., Chakraborty, S., et al. (2024). Alkaloids as drug leads in Alzheimer's treatment: mechanistic and therapeutic insights. Brain Res. 1834, 148886. doi:10.1016/j.brainres.2024.148886
Szypuła, W. J., and Pietrosiuk, A. (2023). “Biological and ecological roles of club mosses (lycopodiaceae) alkaloids,” in Plant specialized metabolites: phytochemistry, ecology and biotechnology. Editors J.-M. Mérillon, and K. G. Ramawat (Cham: Springer Nature Switzerland), 1–25.
Teng, Y., Jiang, T., and Yan, Y. (2024). The expanded CRISPR toolbox for constructing microbial cell factories. Trends Biotechnol. 42, 104–118. doi:10.1016/j.tibtech.2023.06.012
Ushimaru, R., and Abe, I. (2023). Assembling neuroactive alkaloids. Nat. Plants 9, 1946–1947. doi:10.1038/s41477-023-01586-8
Wang, J., Zhang, Z. K., Jiang, F. F., Qi, B. W., Ding, N., Hnin, S. Y. Y., et al. (2020). Deciphering the biosynthetic mechanism of pelletierine in lycopodium alkaloid biosynthesis. Org. Lett. 22, 8725–8729. doi:10.1021/acs.orglett.0c03339
Xie, L., Luo, Z., Jia, X., Mo, C., Huang, X., Suo, Y., et al. (2023). Synthesis of crocin I and crocin II by multigene stacking in Nicotiana benthamiana. Int. J. Mol. Sci. 24, 14139. doi:10.3390/ijms241814139
Yang, C., Wang, Y., Su, Z., Xiong, L., Wang, P., Lei, W., et al. (2024). Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol. Nat. Commun. 15, 2339. doi:10.1038/s41467-024-46583-3
Yang, M., You, W., Wu, S., Fan, Z., Xu, B., Zhu, M., et al. (2017). Global transcriptome analysis of Huperzia serrata and identification of critical genes involved in the biosynthesis of huperzine A. BMC Genomics 18, 245. doi:10.1186/s12864-017-3615-8
Zamar, D. L., Papon, N., and Courdavault, V. (2024). SPOTLIGHT: alpha carbonic anhydrases join the club of alkaloid biosynthetic enzymes. J. Plant Physiol. 293, 154169. doi:10.1016/j.jplph.2023.154169
Zhang, J., Hansen, L. G., Gudich, O., Viehrig, K., Lassen, L. M. M., Schrubbers, L., et al. (2022). A microbial supply chain for production of the anti-cancer drug vinblastine. Nature. 609, 341–347. doi:10.1038/s41586-022-05157-3
Zhang, Y., Wiese, L., Fang, H., Alseekh, S., Perez De Souza, L., Scossa, F., et al. (2023). Synthetic biology identifies the minimal gene set required for paclitaxel biosynthesis in a plant chassis. Mol. Plant 16, 1951–1961. doi:10.1016/j.molp.2023.10.016
Keywords: Alzheimer’s disease, biosynthesis, alkaloid, huperzine A, galantamine
Citation: Yin Q, Zhu Z and Yang M (2025) Biosynthesis of plant neuroactive alkaloids treating Alzheimer’s disease. Front. Pharmacol. 16:1500955. doi: 10.3389/fphar.2025.1500955
Received: 24 September 2024; Accepted: 20 January 2025;
Published: 05 February 2025.
Edited by:
Qi Liang, Air Force Medical University, ChinaReviewed by:
Yogan Khatri, Cayman Chemical, United StatesCopyright © 2025 Yin, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengquan Yang, bXF5YW5nQHNpYnMuYWMuY24=
 Quanyu Yin
Quanyu Yin Mengquan Yang
Mengquan Yang