
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 29 January 2025
Sec. Experimental Pharmacology and Drug Discovery
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1500511
 Penghai Sun1
Penghai Sun1 Ziyuan Wang1
Ziyuan Wang1 Yinchao Ma1
Yinchao Ma1 Yuan Liu1
Yuan Liu1 Yintong Xue1
Yintong Xue1 Yan Li1
Yan Li1 Xiang Gao1
Xiang Gao1 Yuedan Wang1
Yuedan Wang1 Ming Chu1,2,3,4*
Ming Chu1,2,3,4*Berberine is an isoquinoline alkaloid, which has demonstrated significant therapeutic potential in the treatment of various diseases, including tumors, acute and chronic infections, autoimmune disorders, and diabetes. Studies have demonstrated that berberine exhibits polypharmacological effects, including antibacterial, anti-inflammatory, antioxidant, and hypoglycemic activities. To further elucidate the multifaceted pharmacological mechanisms of berberine, we reviewed 7 targets of berberine identified through co-crystal structure analysis, including filamentous temperature-sensitive protein Z (FtsZ), QacR, BmrR, phospholipase A2 (PLA2), RamR, NIMA-related kinase 7 (NEK7), and mesenchymal-epithelial transition (MET). Through target fishing, molecular docking, and surface plasmon resonance (SPR) analyses, combined with cellular and molecular experiments, we further identified 6 targets of berberine. These findings provide a comprehensive summary of berberine’s direct molecular targets, offering a theoretical foundation for further exploration of its diverse pharmacological activities.
Berberine, an isoquinoline alkaloid found in Berberidaceae, Ranunculaceae, and Papaveraceae, was initially utilized for the treatment of diarrhea (Singh and Mahajan, 2013). Notably, accumulating evidence has demonstrated that berberine plays a significant role in managing diverse conditions, including diabetes, hyperlipidemia, gastrointestinal infections, cancer, and Alzheimer’s disease (Wang et al., 2022; Shen et al., 2020; Xiong et al., 2022; Sun C. et al., 2024; Goel, 2023). These therapeutic effects are attributed to its polypharmacological effects, including antimicrobial, anti-inflammatory, antioxidant, and hypoglycemic activities (Ehteshamfar et al., 2020; Fatahian et al., 2020; Chu et al., 2014; Mombeini et al., 2022; Ilyas et al., 2020). Mechanistically, berberine primarily regulates key signaling pathways, including nuclear factor-κB (NF-κB), janus kinases (JAK)/Signal transducer and activator of transcriptions (STAT), mitogen-activated protein kinases (MAPK), adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR), phosphatidylinositol 3-kinase (PI3K)/AKT, and other signaling pathways, to exert these diverse pharmacological effects (Chen et al., 2017; Haftcheshmeh et al., 2022; Sun A. et al., 2024). Focusing on identified targets of berberine, we chose seven targets that met the inclusion criteria based on co-crystal structure analyses. These targets include QacR, BmrR, the d (CGTACG)2 DNA sequence, PLA2, RamR, NEK7, and MET [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. In 2008, Prerna N. Domadia et al. identified that berberine targets FtsZ by binding to its hydrophobic pocket, thereby disrupting the formation of the Z-ring (Domadia et al., 2008). Kate M. Peters et al. proposed that the multiple drug-binding pockets of QacR exhibit multifunctionality, allowing interactions with various cationic drugs, including berberine, through multiple binding modes in 2008 [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. Similarly, Newberry et al. identified berberine as a natural activator of BmrR, offering critical insights into its interaction with BmrR and its role in regulating bacterial resistance (Newberry et al., 2008). In 2011, Ferraroni et al. first reported the crystal structure of berberine in complex with the d (CGTACG)2 DNA sequence (Ferraroni et al., 2011). Subsequently, in 2012, D. Naveen et al. demonstrated through SPR analysis that berberine binds to phospholipase A2(PLA2) in a concentration-dependent manner (Chandra et al., 2012). In 2013, Yamasaki et al. resolved the crystal structure of the RamR-berberine complex, highlighting its relevance to bacterial resistance (Yamasaki et al., 2013). In 2020, Zeng et al. showed that berberine directly binds to NEK7, inhibiting the NEK7- nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) interaction and thereby exerting anti-inflammatory effects (Zeng et al., 2021). Furthermore, in 2022, Chen et al. found that berberine acts as a direct MET inhibitor, playing a pivotal role in the treatment of non-small cell lung cancer (NSCLC) (Chen et al., 2022).
In 2018, we proposed the Drug-Target Space (DTS) model, establishing the foundation for AI-based drug-target screening (Chu et al., 2018). Building on this framework, we identified candidate targets of berberine. Using SPR, molecular docking, along with cellular and animal experiments, we confirmed that beta-site amyloid precursor protein cleaving enzyme (BACE1) and amyloid beta1-42 (Aβ1-42) are direct targets of berberine, elucidating its pharmacological basis in the treatment of Alzheimer’s disease. Subsequently, we identified additional berberine targets, including myeloid differentiation 2 (MD-2), phenol-soluble modulins alpha 2(PSMα2), transforming growth factor-beta receptor 1 (TGFBR1), and Janus kinase 2 (JAK2). These findings have unveiled novel mechanisms underlying berberine’s polypharmacological actions, particularly in the context of its antimicrobial and anti-inflammatory effects, as well as its therapeutic potential in Alzheimer’s disease, pancreatic cancer, lung metastasis, and myasthenia gravis (Chu et al., 2014; Song et al., 2022; Chu et al., 2018; Chu et al., 2016; Tian et al., 2022).
FtsZ (filamentous temperature-sensitive protein Z) is a key protein involved in cell division in bacteria. In 2008, Prerna N. Domadia and colleagues demonstrated that berberine directly targets Escherichia coli FtsZ, inhibiting the dynamics of Z-ring assembly and disrupting the process of cell division in bacteria. In their study, berberine exhibited a high binding affinity to FtsZ, with a dissociation constant (KD) of 0.023 μM at an FtsZ concentration of 10 μM. Berberine was found to interact with hydrophobic residues near the GTP-binding pocket of FtsZ, including Pro134, Phe135, Phe182, Leu189, Ile163, and Pro164 [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. Notably, in 2023, Angela Di Somma et al. synthesized a series of berberine derivatives with enhanced antibacterial activity by targeting FtsZ, underscoring the potential of FtsZ-targeting compounds for the development of more effective antimicrobial agents (Di Somma et al., 2023).
The binding interaction between berberine and QacR was first demonstrated. QacR is a protein associated with multidrug resistance and is found in Staphylococcus aureus (Figure 1). The qacA gene encodes QacA, a multidrug efflux protein, which can expel various toxic compounds from bacterial cells, leading to bacterial resistance. QacR regulates the expression of multidrug resistance by inhibiting the transcription of the qacA multidrug transporter gene (Forman et al., 2016). In 2008, Peters and colleagues proposed that QacR primarily interacts with berberine through E57 and E58 glutamic acid residues. Moreover, different cationic drugs binding to the QacR pocket can adopt distinct positions to neutralize charges. When cationic lipophilic drugs bind to QacR, the protein undergoes a conformational change, forming a multidrug-binding pocket (Schumacher et al., 2001). Within this pocket, glutamic acid residues and aromatic residues mediate drug interactions. QacR can interact with various cationic drugs through multiple mechanisms (Peters et al., 2008).
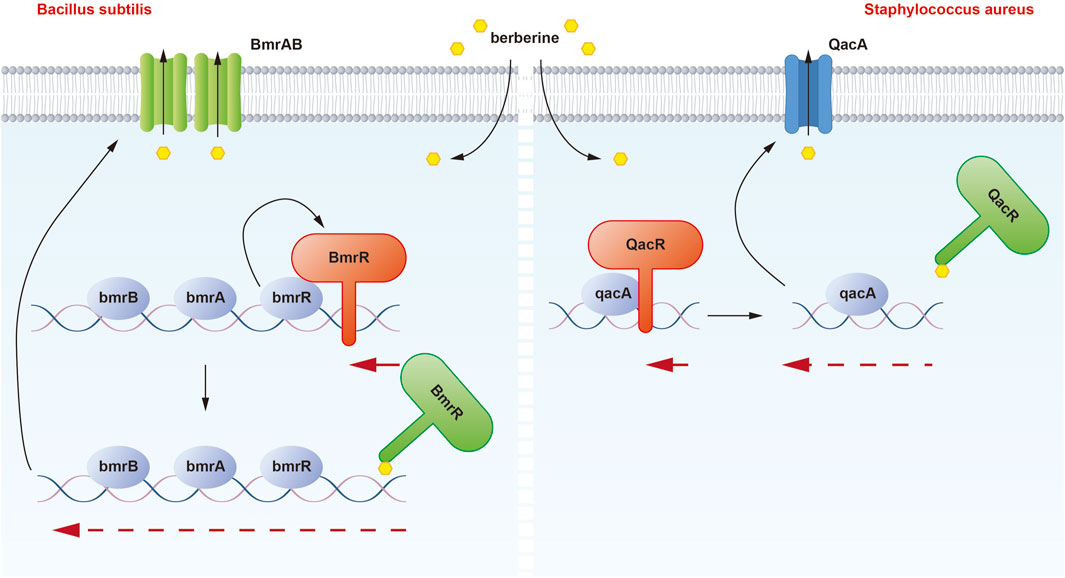
Figure 1. Illustration of the binding mechanism of berberine with QacR in Staphylococcus aureus and BmrR in Bacillus subtilis. In the presence of berberine, the inhibitory effect of QacR on qacA expression is relieved. Under normal conditions, BmrR binds to the bmr box and represses transcription of the BmrRAB operon, which is disrupted when berberine binds to BmrR. These modifications result in the transcription of BmrAB and QacA transporters to pump out berberine.
Berberine can enter the drug-binding pocket of BmrR and bind to it. Berberine may play a significant role in the regulation of bacterial resistance by binding to and activating the BmrR protein (Figure 1). BmrR belongs to the mercuric -responsive transcriptional regulator (MerR) family of multidrug-binding transcription factors and influences the function of the multidrug efflux pump Bmr by regulating the expression of the bmr gene, thereby affecting bacterial resistance (Wade, 2010). In 2008, Newberry et al. discovered that berberine could form a complex with the BmrR protein, and its binding site was similar to that of drugs like R6G. Structural studies of the BmrR-Ber-DNA complex revealed that berberine’s orientation in the drug-binding pocket is such that its acridine system is wedged between Trp61 and Tyr93, while the 1,3-dioxo-6a-azaniumylindole moiety stacks with Tyr123. Additionally, the positive charge center of berberine is situated on the N1 nitrogen of the Be ring and is surrounded by the side chains of Glu57 and Glu58. Berberine forms a hydrogen bond with P144 through its carbonyl oxygen, further contributing to complex formation.
Berberine’s antimicrobial, anti-inflammatory, antioxidant, and anticancer activities have been extensively reported, primarily attributed to its ability to form complexes with DNA [(Ferraroni et al., 2011; Vlavcheski et al., 2022; Devarajan et al., 2021)]. In 2003, Mazzini and colleagues investigated the interactions between berberine and double-stranded oligonucleotides, including d (AAGAATTCTT)2, d (GCGATCGC)2, d (CGTATACG)2, d (CGTACG)2, 5′-d (ACCTTTTTGATGT)-3′/5 (ACATCAAAAAGGT)-3′, as well as single-stranded 5′-d (ACATCAAAAAGGT)-3′, using 1H, 31P NMR, and UV spectroscopic techniques. They found that berberine tended to bind to DNA sequences rich in AT base pairs (Mazzini et al., 2003). In 2011, Ferraroni and colleagues reported the crystal structure of berberine with the d (CGTACG)2 DNA sequence. In 2021, Wickhorst and others discovered that berberine derivatives substituted with 9- and 12-dimethylaminophenyl groups exhibited strong binding affinity to quadruplex DNA. Furthermore, these derivatives exhibited differential binding modes and pH-dependent effects on nucleic acids. Unlike the original berberine, which exhibited enhanced DNA binding at neutral conditions, these derivatives showed stronger binding at pH 5 [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)].
In 2012, Chandra et al. conducted surface plasmon resonance analysis and found that berberine bound to Phospholipase A2 (PLA2) in a concentration-dependent manner, with a measured equilibrium dissociation constant (KD) of 5.55 × 10-8M. Additionally, through molecular docking experiments, Chandra et al. identified the active site residues on ppPLA2 that came into contact with berberine. The most crucial residues involved in this interaction included G32, R53, D49, Y69, Y52, H48, G33, S34, and P68. Furthermore, when berberine was biotransformed by Rhizopus oryzae, the resulting hydroxylated derivatives of berberine exhibited stronger binding affinity and inhibitory effects on PLA2. This altered the way berberine interacted with the active site of PLA2, making it more favorable for berberine to bind to the protein’s active site (Chandra et al., 2012). PLA2 belongs to the class of lipolytic enzymes and hydrolyzes the ester bond at the sn-2 position of phosphatidylcholine. During hydrolysis, PLA2 releases fatty acids such as arachidonic acid (AA), participating in processes that alter cell membrane structure and playing a crucial role in inflammation, cell signal transduction, and carcinogenesis (Khan and Ilies, 2023; Cathcart et al., 2011; Peng et al., 2021) (Figure 2A).
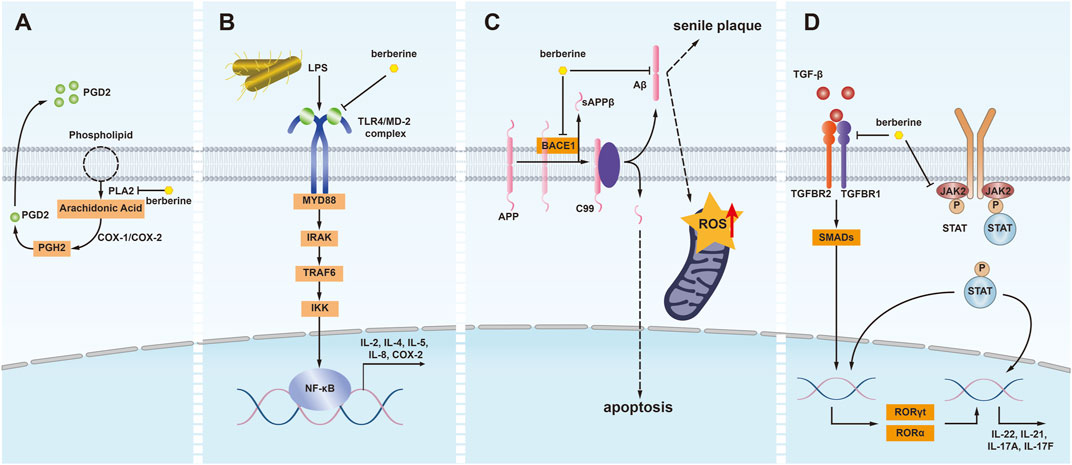
Figure 2. Illustrating of the binding mechanisms of berberine with PLA2, MD2, BACE1, Aβ1-42, TGFBR1, and JAK2 in human cells. (A) Phospholipase A2 (PLA2) converts phospholipids into arachidonic acid, which is then transformed into prostaglandin H2 (PGH2) by cyclooxygenase-1/2 (COX-1/2) and subsequently into prostaglandin D2 (PGD2) by hematopoietic prostaglandin D synthase (HPGDS). Berberine exhibits anti-inflammatory effects by inhibiting PLA2, thereby reducing arachidonic acid production. (B) Lipopolysaccharides (LPS) initiate inflammatory responses via the Toll-like receptor 4 (TLR4)/MD-2 signaling pathway. Berberine disrupts this pathway by binding to MD-2, mitigating the inflammatory response. (C) In the amyloidogenic pathway, amyloid precursor protein is cleaved by beta-secretase 1 (BACE1) to generate amyloid-beta (Aβ) peptides. The accumulation and aggregation of Aβ peptides lead to neurotoxic amyloid plaques, inducing senile plaques, apoptosis, and increased reactive oxygen species (ROS) within mitochondria. Berberine inhibits BACE1 and Aβ, exerting anti-aging and antioxidant effects. (D) Transforming growth factor-beta (TGF-β) binds to heteromeric receptor complexes composed of TGF-β receptor 1 (TGFBR1) and TGF-β receptor 2 (TGFBR2), triggering signal transduction. This leads to the phosphorylation of SMAD family members, which then migrate to the nucleus to regulate gene expression. Berberine inhibits the binding of TGF-β to its receptor complex, reducing the expression of inflammatory genes, including RORγt, RORα, and IL-22. Additionally, berberine inhibits the phosphorylation of STAT proteins by inhibiting Janus kinase 2 (JAK2), further exerting anti-inflammatory effects.
In 2013, Yamasaki et al. reported the crystal structure of the complex formed between RamR and berberine. They determined that the KD value for the binding of berberine to RamR was 17.9 ± 0.03 μM using surface plasmon resonance experiments. After bacterial cells were treated with berberine, the promoter activity of RamR was enhanced. Similar to other drugs, berberine’s Phe155 and RamR’s Phe155 were found to be parallel, indicating that they interacted with RamR through π–π stacking interactions (Yamasaki et al., 2013). In a manner analogous to the mechanisms observed with QacR and BmrR, RamR is a transcriptional repressor of the RamA protein gene, which regulates the expression of the multidrug efflux system genes acrAB-tolC and is an important factor in multidrug resistance. When berberine is used in antibacterial therapy, it activates RamR, resulting in the upregulation of the acrAB-tolC system, which enhances bacterial resistance. In 2022, Jyoti Mehta and colleagues discovered that methanol extracts of Diospyros lotus (a medicinal plant) could inhibit the AcrAB-TolC efflux pump activity in Salmonella enterica serovar Typhimurium, leading to a 2- to 4-fold increase in the antibacterial potency of berberine (Mehta et al., 2022).
In 2020, Zeng et al. reported that berberine directly bind to and target NIMA-related kinase 7 (NEK7) protein to block NEK7-NLRP3 interaction, achieving anti-inflammatory efficacy in a NEK7-dependent manner with an IC50 of 4.2 μM [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. Researchers have conducted numerous significant studies on the mechanisms by which NEK7 regulates the NLRP3 inflammasome signaling pathway. These pathways include potassium efflux, ROS signaling, lysosomal destabilization, and NF-κB signaling (He et al., 2016; Sharif et al., 2019; Gross et al., 2016; Chen et al., 2019; Liu et al., 2020). NEK7 is considered a potential therapeutic target for NLRP3-related diseases, and inhibitors targeting NEK7, such as berberine, may suppress inflammatory responses by modulating NLRP3 [(Zeng et al., 2021; Jin et al., 2023)].
The inhibitory effect of berberine on the MET gene has been demonstrated. MET is a proto-oncogene that encodes the transmembrane receptor for hepatocyte growth factor (HGF). MET exhibits tyrosine kinase (TK) activity, and the MET tyrosine kinase is the only known high-affinity receptor for HGF. The HGF/MET signaling pathway is well characterized and recognized for its essential role in carcinogenesis and tumour progression (Gowda et al., 2024; Kumar et al., 2024). Studies have shown that MET amplification is among the most common mediators of TKI resistance (Peng et al., 2019; Hartmaier et al., 2023). Therefore, the study of MET inhibitors holds significant importance for the treatment of diseases such as non-small cell lung cancer (Yun et al., 2020). In 2022, Chen et al. found that berberine can act as a naturally-existing MET inhibitor to synergize with osimertinib in overcoming osimertinib acquired resistance caused by MET amplification (Chen et al., 2022). Furthermore, they showed that berberine inhibits MET activity in a dose-dependent manner, with an IC50 of 19.64 μM. Further research on berberine derivatives plays a crucial role in the future development of more optimized MET inhibitors.
In 2016, we found that berberine inhibited the formation of amyloid-like fibers in S. aureus, including PSMs. Further molecular dynamics simulations revealed that berberine could bind to the phenyl ring of Phe19 in PSMα2 [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. Phenol-soluble modulins (PSMs) are important virulence factors in S. aureus that can comprise the structural scaffold of S. aureus biofilms through self-assembly into functional amyloids (Zaman and Andreasen, 2020). Functional amyloids enhance bacterial resilience to various environmental stresses, augmenting their persistence within the host while concurrently fostering resistance to antimicrobial agents and the immune system (Torrent et al., 2012; Andreasen et al., 2019). Therefore, berberine inhibits the formation of amyloid-like fibers by affecting the aggregation of PSMs, thereby suppressing the formation of the S. aureus biofilm and enhancing the bactericidal activity of antibiotics (Chu et al., 2016).
In 2014, we investigated the impact of berberine on Salmonella Typhimurium infection. We discovered that berberine could bind to the TLR4/MD-2 receptor complex with higher affinity compared to lipopolysaccharides (LPS) (Chu et al., 2014). MD-2 belongs to the Toll-like receptor (TLR) family and typically forms a complex with the TLR4 protein. This complex is responsible for recognizing and responding to exogenous molecules such as bacterial LPS, leading to the activation of signaling pathways like NF-κB. Consequently, this activation triggers an inflammatory response in immune cells. Excessive activation of TLR4/MD-2 is closely associated with the development of sepsis, endotoxemia, acute lung injury, rheumatoid arthritis, and cardiovascular diseases (Zhang et al., 2022) (Figure 2B).
We investigated the immunological mechanisms and effects of berberine in the treatment of Alzheimer’s disease, revealing that berberine specifically binds to BACE1, one of the key targets in Alzheimer’s disease (Chu et al., 2018). BACE1 is a crucial target in Alzheimer’s disease and holds significance in aging, diabetes, hypertension and cancer (Bao and Shen, 2023). The pathological role of BACE1 in cerebral amyloid angiopathy (CAA) and Alzheimer’s disease has been confirmed in experimental studies. Research has shown that BACE1 expression contributes to the cleavage of amyloid precursor protein (APP) in neurons of APP-overexpressing mice, thereby enhancing the generation of Aβ in neurons (Ihara, 2022). In 2016, Faraco et al. reported that hypertension increases Aβ levels in APP-overexpressing mice by upregulating BACE1 in the brain, although the specific molecular mechanisms, particularly the cell types responsible for the upregulation of BACE1 expression, have not been clarified (Faraco et al., 2016). In 2018, using molecular modeling techniques, we found that berberine was guided into the electronegative binding pocket of BACE1, where the N+ of berberine interacts electrostatically with the crucial anion (Asp80) of BACE1. Additionally, the phenyl group forms a π-π stacking interaction with the Tyr119 active site residue. Furthermore, surface plasmon resonance experiments demonstrated the binding affinity between berberine and BACE1. The equilibrium dissociation constant KD was calculated to be 1.261 μM [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. As a potential drug molecule, berberine has the ability to bind to BACE1, potentially intervening in Aβ production (Figure 2C).
In addition to BACE1, our study utilizing multi-target drug modeling and surface plasmon resonance experiments identified Aβ 1-42 as a high-affinity target of berberine, suggesting its potential in treating Alzheimer’s disease (Chu et al., 2018). Oligomeric Aβ1-42 is closely associated with neurodegenerative diseases, especially Alzheimer’s disease. It induces oxidative stress and mitochondrial damage in neurons (Thammasart et al., 2023). The aggregation and deposition of Aβ1-42 in the brain are one of the primary mechanisms leading to neuronal damage and cognitive decline (Figure 2C).
In 2022, we studied the impact of berberine on lung metastasis in pancreatic cancer and found that berberine can function as a transforming growth factor-beta receptor 1 (TGFBR1) inhibitor, preventing pancreatic cancer cells from breaking through endothelial cells and metastasizing. Through surface plasmon resonance experiments and molecular docking techniques, we determined that the equilibrium dissociation constant (KD) for the binding of berberine to TGFBR1 is 18.0 μM. Berberine interacts with key residues in the active site of TGFBR1, the primary receptor of the TGF-β signaling pathway, including Glu45, Tyr49, Asp81, Tyr82, and His83. It has been demonstrated that when TGF-β molecules bind to the TGFBR2 receptor, TGFBR1 is activated and subsequently transmits the signal into the cell through processes such as phosphorylation, influencing gene expression and cellular behavior. Abnormal activity or mutations in TGFBR1 are associated with various diseases, including cancer, cardiovascular diseases, and immune disorders (Lu et al., 2021; Chen et al., 2020; Xu et al., 2023; Tang et al., 2022). Furthermore, our research showed that berberine inhibits TGFBR1 kinase activity in a dose-dependent manner, with an IC50 of 7.056 μM [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. This suggests that berberine can serve as an inhibitor in the TGF-β signaling pathway, offering therapeutic potential for cancer, cardiovascular diseases, immune disorders, and more (Figure 2D).
In 2022, we used surface plasmon resonance experiments to confirm the ligand-binding interaction between berberine and JAK2, with a measured KD of 15.83 μM. Additionally, molecular modeling by Song et al. revealed interactions between BBR and specific residues of JAK2, including VAL863, LEU855, LYS857, LEU932, LEU983, GLY993, and ASP994 [(Schumacher et al., 2001; Newberry et al., 2008; Yamasaki et al., 2013; Chandra et al., 2012; Ferraroni et al., 2011; Song et al., 2022; Chen et al., 2022; Zeng et al., 2021)]. Earlier studies have also shown that as a member of the protein tyrosine kinase (PTK) family of JAK proteins, the abnormal activation of JAK2 is closely associated with inflammation, hematopoiesis, malignant tumors, and various age-related diseases (Yang et al., 2021; Fidler et al., 2021; Stevens et al., 2023; Cho et al., 2022). Furthermore, we demonstrated that oral berberine can improve the clinical symptoms of experimental autoimmune myasthenia gravis (EAMG) in rats by reducing the frequency of T helper (TH)1, TH17, and TH1/TH17 cell subsets. We also isolated mononuclear cells (MNCs) from the spleens of EAMG rats and treated them with BBR in vitro, finding that the phosphorylation levels of JAK1, JAK2, JAK3, STAT1, and STAT3 were significantly reduced. Similar to JAK2, JAK1 and JAK3 are also likely targets of berberine interaction (Song et al., 2022). In 2023, Huang et al. obtained similar conclusions in a chronic myelogenous leukemia (CML) -like mouse model (Huang et al., 2023) (Figure 2D).
This review highlights the multiple target actions of berberine in cells and its diverse mechanisms of action. In addition to the 13 berberine targets highlighted in this review, other identified targets include RXRα, ABL1, AKR1B10, and TIGAR (Ruan et al., 2017; Yin et al., 2020; Yang et al., 2024; Qi et al., 2024) (Table 1). Berberine has demonstrated a wide range of pharmacological effects, including anti-inflammatory, anti-tumor, and therapeutic potential in inflammatory diseases, acute and chronic infections, autoimmune disorders, and diabetes. Despite significant progress in understanding these effects, further studies are needed to deepen our understanding of berberine’s specific molecular mechanisms and its broader immunopharmacological properties. While berberine shows promise in various therapeutic areas, several limitations need to be addressed. Current research is largely preclinical, and the translation of these findings into clinical applications remains a challenge, requiring rigorous clinical trials for validation. More research should explore new therapeutic avenues where berberine may offer benefits. In addition, the bioavailability of BBR is rather low after it is absorbed by the gastrointestinal tract which restricts the clinical application. There is an urgent need to enhance the bioavailability of berberine, and further pharmacokinetic studies are warranted.
In conclusion, while berberine holds significant potential, its clinical utility is contingent upon further research and validation, offering both challenges and exciting opportunities for the development of future therapeutic strategies.
PS: Writing–original draft, Writing–review and editing, Conceptualization, Visualization. ZW: Conceptualization, Writing–review and editing. YM: Conceptualization, Writing–review and editing. YuL: Funding acquisition, Writing–review and editing. YX: Project administration, Writing–review and editing. YaL: Project administration, Writing–review and editing. XG: Funding acquisition, Writing–review and editing. YW: Writing–review and editing. MC: Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Significant Science and Technology Project of Beijing Life Science Academy [grant number 2024500CB0030, 2023000CA0040]; the National Natural Science Foundation of China [grant number 81603119]; the Natural Science Foundation of Beijing Municipality [grant number 7174316]; the Peking University Medicine Seed Fund for Interdisciplinary Research supported by “the Fundamental Research Funds for the Central Universities” [grant number No. BMU2022MX017, No. BMU2022MX003].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Andreasen, M., Meisl, G., Taylor, J. D., Michaels, T. C. T., Levin, A., Otzen, D. E., et al. (2019). Physical determinants of amyloid assembly in biofilm formation. mBio 10 (1), doi:10.1128/mBio.02279-18
Bao, H., and Shen, Y. (2023). Unmasking BACE1 in aging and age-related diseases. Trends Mol. Med. 29 (2), 99–111. doi:10.1016/j.molmed.2022.11.008
Cathcart, M. C., Lysaght, J., and Pidgeon, G. P. (2011). Eicosanoid signalling pathways in the development and progression of colorectal cancer: novel approaches for prevention/intervention. Cancer Metastasis Rev. 30 (3-4), 363–385. doi:10.1007/s10555-011-9324-x
Chandra, D. N., Abhilash, J., Prasanth, G. K., Sabu, A., Sadasivan, C., and Haridas, M. (2012). Inverted binding due to a minor structural change in berberine enhances its phospholipase A2 inhibitory effect. Int. J. Biol. Macromol. 50 (3), 578–585. doi:10.1016/j.ijbiomac.2012.01.029
Chandra, D. N., Prasanth, G. K., Singh, N., Kumar, S., Jithesh, O., Sadasivan, C., et al. (2011). Identification of a novel and potent inhibitor of phospholipase A(2) in a medicinal plant: crystal structure at 1.93A and Surface Plasmon Resonance analysis of phospholipase A(2) complexed with berberine. Biochim. Biophys. Acta 1814 (5), 657–663. doi:10.1016/j.bbapap.2011.03.002
Chen, H. B., Luo, C. D., Liang, J. L., Zhang, Z. B., Lin, G. S., Wu, J. Z., et al. (2017). Anti-inflammatory activity of coptisine free base in mice through inhibition of NF-κB and MAPK signaling pathways. Eur. J. Pharmacol. 811, 222–231. doi:10.1016/j.ejphar.2017.06.027
Chen, S., Luo, Y., Cui, L., and Yang, Q. (2020). miR-96-5p regulated TGF-β/SMAD signaling pathway and suppressed endometrial cell viability and migration via targeting TGFBR1. Cell Cycle 19 (14), 1740–1753. doi:10.1080/15384101.2020.1777804
Chen, X., Liu, G., Yuan, Y., Wu, G., Wang, S., and Yuan, L. (2019). NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 10 (12), 906. doi:10.1038/s41419-019-2157-1
Chen, Z., Vallega, K. A., Chen, H., Zhou, J., Ramalingam, S. S., and Sun, S. Y. (2022). The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 175, 105998. doi:10.1016/j.phrs.2021.105998
Cho, H. J., Hwang, J. A., Yang, E. J., Kim, E. C., Kim, J. R., Kim, S. Y., et al. (2022). Nintedanib induces senolytic effect via STAT3 inhibition. Cell Death Dis. 13 (9), 760. doi:10.1038/s41419-022-05207-8
Chu, M., Chen, X., Wang, J., Guo, L., Wang, Q., Gao, Z., et al. (2018). Polypharmacology of berberine based on multi-target binding motifs. Front. Pharmacol. 9, 801. doi:10.3389/fphar.2018.00801
Chu, M., Ding, R., Chu, Z. Y., Zhang, M. B., Liu, X. Y., Xie, S. H., et al. (2014). Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement. Altern. Med. 14, 89. doi:10.1186/1472-6882-14-89
Chu, M., Zhang, M. B., Liu, Y. C., Kang, J. R., Chu, Z. Y., Yin, K. L., et al. (2016). Role of berberine in the treatment of methicillin-resistant Staphylococcus aureus infections. Sci. Rep. 6, 24748. doi:10.1038/srep24748
Devarajan, N., Jayaraman, S., Mahendra, J., Venkatratnam, P., Rajagopal, P., Palaniappan, H., et al. (2021). Berberine-A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytother. Res. 35 (6), 3059–3077. doi:10.1002/ptr.7032
Di Somma, A., Cane, C., Rotondo, N. P., Cavalluzzi, M. M., Lentini, G., and Duilio, A. (2023). A comparative study of the inhibitory action of berberine derivatives on the recombinant protein FtsZ of E. coli. Int. J. Mol. Sci. 24 (6), 5674. doi:10.3390/ijms24065674
Domadia, P. N., Bhunia, A., Sivaraman, J., Swarup, S., and Dasgupta, D. (2008). Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 47 (10), 3225–3234. doi:10.1021/bi7018546
Ehteshamfar, S. M., Akhbari, M., Afshari, J. T., Seyedi, M., Nikfar, B., Shapouri-Moghaddam, A., et al. (2020). Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell Mol. Med. 24 (23), 13573–13588. doi:10.1111/jcmm.16049
Faraco, G., Park, L., Zhou, P., Luo, W., Paul, S. M., Anrather, J., et al. (2016). Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow. Metab. 36 (1), 241–252. doi:10.1038/jcbfm.2015.79
Fatahian, A., Haftcheshmeh, S. M., Azhdari, S., Farshchi, H. K., Nikfar, B., and Momtazi-Borojeni, A. A. (2020). Promising anti-atherosclerotic effect of berberine: evidence from in vitro, in vivo, and clinical studies. Rev. Physiol. Biochem. Pharmacol. 178, 83–110. doi:10.1007/112_2020_42
Ferraroni, M., Bazzicalupi, C., Bilia, A. R., and Gratteri, P. (2011). X-Ray diffraction analyses of the natural isoquinoline alkaloids Berberine and Sanguinarine complexed with double helix DNA d(CGTACG). Chem. Commun. (Camb) 47 (17), 4917–4919. doi:10.1039/c1cc10971e
Fidler, T. P., Xue, C., Yalcinkaya, M., Hardaway, B., Abramowicz, S., Xiao, T., et al. (2021). The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592 (7853), 296–301. doi:10.1038/s41586-021-03341-5
Forman, M. E., Fletcher, M. H., Jennings, M. C., Duggan, S. M., Minbiole, K. P., and Wuest, W. M. (2016). Structure-resistance relationships: interrogating antiseptic resistance in bacteria with multicationic quaternary ammonium dyes. ChemMedChem 11 (9), 958–962. doi:10.1002/cmdc.201600095
Goel, A. (2023). Current understanding and future prospects on Berberine for anticancer therapy. Chem. Biol. Drug Des. 102 (1), 177–200. doi:10.1111/cbdd.14231
Gowda, S. V., Kim, N. Y., Harsha, K. B., Gowda, D., Suresh, R. N., Deivasigamani, A., et al. (2024). A new 1,2,3-triazole-indirubin hybrid suppresses tumor growth and pulmonary metastasis by mitigating the HGF/c-MET axis in hepatocellular carcinoma. J. Adv. Res. doi:10.1016/j.jare.2024.08.033
Gross, C. J., Mishra, R., Schneider, K. S., Medard, G., Wettmarshausen, J., Dittlein, D. C., et al. (2016). K(+) efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45 (4), 761–773. doi:10.1016/j.immuni.2016.08.010
Haftcheshmeh, S. M., Abedi, M., Mashayekhi, K., Mousavi, M. J., Navashenaq, J. G., Mohammadi, A., et al. (2022). Berberine as a natural modulator of inflammatory signaling pathways in the immune system: focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 36 (3), 1216–1230. doi:10.1002/ptr.7407
Hartmaier, R. J., Markovets, A. A., Ahn, M. J., Sequist, L. V., Han, J. Y., Cho, B. C., et al. (2023). Osimertinib + savolitinib to overcome acquired MET-mediated resistance in epidermal growth factor receptor-mutated, MET-amplified non-small cell lung cancer: TATTON. Cancer Discov. 13 (1), 98–113. doi:10.1158/2159-8290.CD-22-0586
He, Y., Zeng, M. Y., Yang, D., Motro, B., and Nunez, G. (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530 (7590), 354–357. doi:10.1038/nature16959
Huang, G., Yin, Z., Wang, X., Wen, Z., Su, R., Li, C., et al. (2023). System analysis of Huang-Lian-Jie-Du-Tang and their key active ingredients for overcoming CML resistance by suppression of leukemia stem cells. Phytomedicine 117, 154918. doi:10.1016/j.phymed.2023.154918
Ihara, M. (2022). Endothelial BACE1: bridging the gap between hypertension and Alzheimer's disease. Circ. Res. 130 (9), 1342–1344. doi:10.1161/CIRCRESAHA.122.321078
Ilyas, Z., Perna, S., Al-Thawadi, S., Alalwan, T. A., Riva, A., Petrangolini, G., et al. (2020). The effect of Berberine on weight loss in order to prevent obesity: a systematic review. Biomed. Pharmacother. 127, 110137. doi:10.1016/j.biopha.2020.110137
Jin, X., Liu, D., Zhou, X., Luo, X., Huang, Q., and Huang, Y. (2023). Entrectinib inhibits NLRP3 inflammasome and inflammatory diseases by directly targeting NEK7. Cell Rep. Med. 4 (12), 101310. doi:10.1016/j.xcrm.2023.101310
Khan, S. A., and Ilies, M. A. (2023). The phospholipase A2 superfamily: structure, isozymes, catalysis, physiologic and pathologic roles. Int. J. Mol. Sci. 24 (2), 1353. doi:10.3390/ijms24021353
Kumar, V., Yochum, Z. A., Devadassan, P., Huang, E. H., Miller, E., Baruwal, R., et al. (2024). TWIST1 is a critical downstream target of the HGF/MET pathway and is required for MET driven acquired resistance in oncogene driven lung cancer. Oncogene 43 (19), 1431–1444. doi:10.1038/s41388-024-02987-5
Liu, G., Chen, X., Wang, Q., and Yuan, L. (2020). NEK7: a potential therapy target for NLRP3-related diseases. Biosci. Trends 14 (2), 74–82. doi:10.5582/bst.2020.01029
Lu, X., Xu, C., Xu, Z., Lu, C., Yang, R., Zhang, F., et al. (2021). Piperlongumine inhibits the growth of non-small cell lung cancer cells via the miR-34b-3p/TGFBR1 pathway. BMC Complement. Med. Ther. 21 (1), 15. doi:10.1186/s12906-020-03123-y
Mazzini, S., Bellucci, M. C., and Mondelli, R. (2003). Mode of binding of the cytotoxic alkaloid berberine with the double helix oligonucleotide d(AAGAATTCTT)(2). Bioorg Med. Chem. 11 (4), 505–514. doi:10.1016/s0968-0896(02)00466-2
Mehta, J., Rolta, R., and Dev, K. (2022). Role of medicinal plants from North Western Himalayas as an efflux pump inhibitor against MDR AcrAB-TolC Salmonella enterica serovar typhimurium: in vitro and in silico studies. J. Ethnopharmacol. 282, 114589. doi:10.1016/j.jep.2021.114589
Mombeini, M. A., Kalantar, H., Sadeghi, E., Goudarzi, M., Khalili, H., and Kalantar, M. (2022). Protective effects of berberine as a natural antioxidant and anti-inflammatory agent against nephrotoxicity induced by cyclophosphamide in mice. Naunyn Schmiedeb. Arch. Pharmacol. 395 (2), 187–194. doi:10.1007/s00210-021-02182-3
Newberry, K. J., Huffman, J. L., Miller, M. C., Vazquez-Laslop, N., Neyfakh, A. A., and Brennan, R. G. (2008). Structures of BmrR-drug complexes reveal a rigid multidrug binding pocket and transcription activation through tyrosine expulsion. J. Biol. Chem. 283 (39), 26795–26804. doi:10.1074/jbc.M804191200
Peng, S., Wang, R., Zhang, X., Ma, Y., Zhong, L., Li, K., et al. (2019). EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol. Cancer 18 (1), 165. doi:10.1186/s12943-019-1073-4
Peng, Z., Chang, Y., Fan, J., Ji, W., and Su, C. (2021). Phospholipase A2 superfamily in cancer. Cancer Lett. 497, 165–177. doi:10.1016/j.canlet.2020.10.021
Peters, K. M., Schuman, J. T., Skurray, R. A., Brown, M. H., Brennan, R. G., and Schumacher, M. A. (2008). QacR-cation recognition is mediated by a redundancy of residues capable of charge neutralization. Biochemistry 47 (31), 8122–8129. doi:10.1021/bi8008246
Qi, F., Zhang, M., Yang, G., Wang, W., Hu, Y., Shen, Y., et al. (2024). Identification of TIGAR, a direct proteomic target associated with the hypoglycemic effect of Berberine. Fitoterapia 180, 106332. doi:10.1016/j.fitote.2024.106332
Ruan, H., Zhan, Y. Y., Hou, J., Xu, B., Chen, B., Tian, Y., et al. (2017). Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene 36 (50), 6906–6918. doi:10.1038/onc.2017.296
Schumacher, M. A., Miller, M. C., Grkovic, S., Brown, M. H., Skurray, R. A., and Brennan, R. G. (2001). Structural mechanisms of QacR induction and multidrug recognition. Science 294 (5549), 2158–2163. doi:10.1126/science.1066020
Sharif, H., Wang, L., Wang, W. L., Magupalli, V. G., Andreeva, L., Qiao, Q., et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570 (7761), 338–343. doi:10.1038/s41586-019-1295-z
Shen, Y., Zou, Y., Chen, X., Li, P., Rao, Y., Yang, X., et al. (2020). Antibacterial self-assembled nanodrugs composed of berberine derivatives and rhamnolipids against Helicobacter pylori. J. Control Release 328, 575–586. doi:10.1016/j.jconrel.2020.09.025
Singh, I. P., and Mahajan, S. (2013). Berberine and its derivatives: a patent review (2009 - 2012). Expert Opin. Ther. Pat. 23 (2), 215–231. doi:10.1517/13543776.2013.746314
Song, J., Yang, J., Jing, S., Yan, C., Huan, X., Chen, S., et al. (2022). Berberine attenuates experimental autoimmune myasthenia gravis via rebalancing the T cell subsets. J. Neuroimmunol. 362, 577787. doi:10.1016/j.jneuroim.2021.577787
Stevens, L. E., Peluffo, G., Qiu, X., Temko, D., Fassl, A., Li, Z., et al. (2023). JAK-STAT signaling in inflammatory breast cancer enables chemotherapy-resistant cell States. Cancer Res. 83 (2), 264–284. doi:10.1158/0008-5472.CAN-22-0423
Sun, A., Yang, H., Li, T., Luo, J., Zhou, L., Chen, R., et al. (2024b). Molecular mechanisms, targets and clinical potential of berberine in regulating metabolism: a review focussing on databases and molecular docking studies. Front. Pharmacol. 15, 1368950. doi:10.3389/fphar.2024.1368950
Sun, C., Dong, S., Chen, W., Li, J., Luo, E., and Ji, J. (2024a). Berberine alleviates Alzheimer's disease by regulating the gut microenvironment, restoring the gut barrier and brain-gut axis balance. Phytomedicine 129, 155624. doi:10.1016/j.phymed.2024.155624
Tang, H., Zhu, W., Cao, L., Zhang, J., Li, J., Ma, D., et al. (2022). miR-210-3p protects against osteoarthritis through inhibiting subchondral angiogenesis by targeting the expression of TGFBR1 and ID4. Front. Immunol. 13, 982278. doi:10.3389/fimmu.2022.982278
Thammasart, S., Namchaiw, P., Pasuwat, K., Tonsomboon, K., and Khantachawana, A. (2023). Attenuation Aβ1-42-induced neurotoxicity in neuronal cell by 660nm and 810nm LED light irradiation. PLoS One 18 (7), e0283976. doi:10.1371/journal.pone.0283976
Tian, W., Hao, H., Chu, M., Gong, J., Li, W., Fang, Y., et al. (2022). Berberine suppresses lung metastasis of cancer via inhibiting endothelial transforming growth factor beta receptor 1. Front. Pharmacol. 13, 917827. doi:10.3389/fphar.2022.917827
Torrent, M., Pulido, D., Nogues, M. V., and Boix, E. (2012). Exploring new biological functions of amyloids: bacteria cell agglutination mediated by host protein aggregation. PLoS Pathog. 8 (11), e1003005. doi:10.1371/journal.ppat.1003005
Vlavcheski, F., O'Neill, E. J., Gagacev, F., and Tsiani, E. (2022). Effects of berberine against pancreatitis and pancreatic cancer. Molecules 27 (23), 8630. doi:10.3390/molecules27238630
Wade, H. (2010). MD recognition by MDR gene regulators. Curr. Opin. Struct. Biol. 20 (4), 489–496. doi:10.1016/j.sbi.2010.06.003
Wang, S., Ren, H., Zhong, H., Zhao, X., Li, C., Ma, J., et al. (2022). Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes 14 (1), 2003176. doi:10.1080/19490976.2021.2003176
Xiong, R. G., Huang, S. Y., Wu, S. X., Zhou, D. D., Yang, Z. J., Saimaiti, A., et al. (2022). Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules 27 (14), 4523. doi:10.3390/molecules27144523
Xu, L., Su, Y., Yang, X., Bai, X., Wang, Y., Zhuo, C., et al. (2023). Gramine protects against pressure overload-induced pathological cardiac hypertrophy through Runx1-TGFBR1 signaling. Phytomedicine 114, 154779. doi:10.1016/j.phymed.2023.154779
Yamasaki, S., Nikaido, E., Nakashima, R., Sakurai, K., Fujiwara, D., Fujii, I., et al. (2013). The crystal structure of multidrug-resistance regulator RamR with multiple drugs. Nat. Commun. 4, 2078. doi:10.1038/ncomms3078
Yang, N. N., Yang, J. W., Ye, Y., Huang, J., Wang, L., Wang, Y., et al. (2021). Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics 11 (9), 4078–4089. doi:10.7150/thno.52574
Yang, S., Cao, S. J., Li, C. Y., Zhang, Q., Zhang, B. L., Qiu, F., et al. (2024). Berberine directly targets AKR1B10 protein to modulate lipid and glucose metabolism disorders in NAFLD. J. Ethnopharmacol. 332, 118354. doi:10.1016/j.jep.2024.118354
Yin, Z., Huang, G., Gu, C., Liu, Y., Yang, J., and Fei, J. (2020). Discovery of berberine that targetedly induces autophagic degradation of both BCR-ABL and BCR-ABL T315I through recruiting LRSAM1 for overcoming imatinib resistance. Clin. Cancer Res. 26 (15), 4040–4053. doi:10.1158/1078-0432.CCR-19-2460
Yun, J., Lee, S. H., Kim, S. Y., Jeong, S. Y., Kim, J. H., Pyo, K. H., et al. (2020). Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 10 (8), 1194–1209. doi:10.1158/2159-8290.CD-20-0116
Zaman, M., and Andreasen, M. (2020). Cross-talk between individual phenol-soluble modulins in Staphylococcus aureus biofilm enables rapid and efficient amyloid formation. Elife 9, e59776. doi:10.7554/eLife.59776
Zeng, Q., Deng, H., Li, Y., Fan, T., Liu, Y., Tang, S., et al. (2021). Berberine directly targets the NEK7 protein to block the NEK7-NLRP3 interaction and exert anti-inflammatory activity. J. Med. Chem. 64 (1), 768–781. doi:10.1021/acs.jmedchem.0c01743
Zhang, Y., Liang, X., Bao, X., Xiao, W., and Chen, G. (2022). Toll-like receptor 4 (TLR4) inhibitors: current research and prospective. Eur. J. Med. Chem. 235, 114291. doi:10.1016/j.ejmech.2022.114291
AA arachidonic acid
Aβ1-42 amyloid beta1-42
AKR1B10 aldo-keto reductase 1B10
AMP adenosine 5′-monophosphate
AMPK activated protein kinase
APP amyloid precursor protein
BACE1 Beta-site amyloid precursor protein cleaving enzyme 1
CAA cerebral amyloid angiopathy
CML chronic myelogenous leukemia
DTS Drug-Target Space
EAMG experimental autoimmune myasthenia gravis
EGFR epidermal growth factor receptor
Ftsz filamentous temperature-sensitive protein Z
JAK/STATs janus kinases/Signal transducer and activator of transcriptions
JAK2 janus kinase 2
LPS lipopolysaccharides
MAPK mitogen-activated protein kinases
MBM multi-target binding motifs
MD-2 myeloid differentiation factor 2
MerR mercuric -responsive transcriptional regulator
MET mesenchymal-epithelial transition
MNCs mononuclear cells
mTOR mammalian target of rapamycin
NEK7 NIMA-related kinase 7
NF-κB nuclear factor-κB
NLRP3 nucleotide-binding oligomerization domain-like receptor protein 3
NSCLC non-small cell lung cancer
PI3K phosphatidylinositol 3-kinase
PLA2 phospholipase A2
PSMα2 phenol-soluble modulins alpha2
PSMs phenol-soluble modulins
PTK protein tyrosine kinase
RXRα retinoid X receptor alpha
TGF-β Transforming growth factor β
TGFBR1 transforming growth factor-beta receptor 1
TH T helper
TIGAR TP53-induced glycolysis and apoptosis regulator
TLR Toll-like receptor.
Keywords: berberine, ftsZ, QacR, BmrR, PLA2, ramR, Nek7, met
Citation: Sun P, Wang Z, Ma Y, Liu Y, Xue Y, Li Y, Gao X, Wang Y and Chu M (2025) Advance in identified targets of berberine. Front. Pharmacol. 16:1500511. doi: 10.3389/fphar.2025.1500511
Received: 23 September 2024; Accepted: 06 January 2025;
Published: 29 January 2025.
Edited by:
Giovanni Lentini, University of Bari Aldo Moro, ItalyReviewed by:
Zhen Chen, Emory University, United StatesCopyright © 2025 Sun, Wang, Ma, Liu, Xue, Li, Gao, Wang and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Chu, ZmFtb3VzQGJqbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.