
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 17 February 2025
Sec. Drug Metabolism and Transport
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1466877
This article is part of the Research TopicADME of Drugs to Treat Infectious DiseasesView all 8 articles
Renal cell carcinoma (RCC) is a common substantive tumor. According to incomplete statistics, RCC incidence accounts for approximately 90% of renal malignant tumors, and is the second most prevalent major malignant tumor in the genitourinary system, following bladder cancer. Only 10%–15% of chemotherapy regimens for metastatic renal cell carcinoma (mRCC) are effective, and mRCC has a high mortality. Drug transporters are proteins located on the cell membrane that are responsible for the absorption, distribution, and excretion of drugs. Lots of drug transporters are expressed in the kidneys. Changes in carrier function weaken balance, cause disease, or modify the effectiveness of drug treatment. The changes in expression of these transporters during cancer pathology results in multi-drug resistance to cancer chemotherapy. In the treatment of RCC, the study of drug transporters helps to optimize treatment regimens, improve therapeutic effects, and reduce drug side effects. In this review, we summarize advances in the role of renal drug transporters in the genesis, progression, and treatment of RCC.
Kidney cancer is a relatively common type of cancer, accounting for approximately 3%–5% of all malignancies (Rose and Kim, 2024). Renal cell carcinoma (RCC) is a type of cancer that originates from the tubular epithelial cells of the kidney, and it has three histological subtypes: clear cell RCC (ccRCC), papillary RCC (pRCC), and chromophobe RCC (chRCC) (Hosseiniyan Khatibi et al., 2022). RCC represents over 90% of all kidney cancer (Hsieh et al., 2017). Moreover, RCC incidence is increasing worldwide, with higher rates observed in developed than in developing countries (Padala and Kallam, 2023). Although overall RCC incidence has increased over the last three decades, the death rate of RCC has declined rapidly because of early diagnosis and treatment (Medina-Rico et al., 2018). Even with progress in disease control, some patients may still develop locally advanced diseases and distant metastases (Vasudev et al., 2020; Ciarimboli et al., 2021). The field of RCC treatment has significantly changed over the past three decades. Indeed, the treatment landscape for RCC has undergone significant transformation in recent years owing to steady progress in the development of targeted therapeutics (Pérez-Herrero and Fernández-Medarde, 2015) and immunotherapy (Szeto and Finley, 2019). Immune checkpoint inhibitors (ICI) in combination with vascular endothelial growth factor tyrosine kinase inhibitors (TKI) have become the standard primary therapy for many advanced RCC (Chen et al., 2023).
Drug transporters are responsible for the absorption, distribution, metabolism, and excretion of drugs from the human body. The relationship between RCC and various transporters involves many aspects such as drug metabolism, nutrient transport, and cell survival. Therefore, transporters play a crucial role in sustaining the physiological balance of the body and in administering drugs. Changes in the function of transporters can impair homeostasis, cause disease, or modify the efficacy of the drugs (Ciarimboli, 2023). Although many renal drug transporters have been characterized in detail with respect to the significance for proper kidney function, their role in kidney cancer progression is less known. Drug transporter expression may reflect resistance to systemic therapy in RCC, and can be used to predict prognosis. This review summarizes progress in the significance of renal drug transporters in the genesis, progression and treatment of RCC.
Drug transporters are typically classified into two major families: the solute carrier (SLC) family and the adenosine triphosphate-binding cassette (ABC) family (Liu, 2019a).
The largest transporter family is the ABC family. The ABC transporter family is one of the most diverse groups of transmembrane proteins involved in active transport processes. The ABC proteins have numerous functions to list in detail. However, they mainly transport a diverse range of substrates, from simple ions to polar, amphiphilic, and hydrophobic organic molecules, peptides, complex lipids, and even small proteins (Theodoulou and Kerr, 2015). Over 40 ABC transporters have been discovered in humans and partitioned into seven subfamilies (ABCA to ABCG) based on various criteria such as gene structure and amino acid sequence. At least 11 ABC transporters have been implicated in multi-drug resistance, including P-glycoproteins (P-gp/ABCB1), multi-drug resistance proteins (MRP/ABCC), and breast cancer resistance proteins (BCRP/ABCG2). They actively remove anti-tumor drugs from cancer cells, reduce their intracellular concentrations, thereby conferring resistance to chemotherapy (Liu, 2019b). These ABC transporters have significant effects on many cell types, including renal tubular cells. The reabsorption and secretion functions of the nephron are mediated by a variety of transporters located in the basolateral and luminal membranes of the tubular cells. Many studies indicated that transporters play important roles in drug pharmacokinetics and demonstrated the impact of renal transporters on the disposition of drugs, drug-drug interactions (DDI), and nephron toxicities (Yang and Han, 2019).
The SLC transporters include the SLC-21A gene subfamily (organic anion transporter polypeptide, OATP), SLC-22A gene subfamily (organic anion transporter, OAT; organic cation transporter, OCT; organic cation/carnitine transporter, OCTN), SLC-15A gene subfamily (peptide transporter, PEPT), and SLC-47A gene subfamily (multi-drug and toxin excretion, MATE) (Liu, 2019c). OCTs and OCTNs are responsible for transporting organic cations, and are involved in the transport of several drugs in the body. OATP-4C1 is the major OATP transporter in kidney, mainly located on the basolateral membrane of proximal renal tubular cells (Sato et al., 2017).
The kidney performs the critical task of maintaining homeostasis through the coordinated action of multiple transport systems specifically expressed in different parts of the kidney’s functional unit, the nephrons (Lee et al., 2015). Exogenous substances secreted by the kidney mainly occur in the proximal renal tubules, which have specific transport mechanisms that facilitate the passage of foreign substances from the blood into the tubular cells (uptake) and from these cells into the tubular fluid (excretion).
Transporters in renal proximal tubule epithelial cells (RPTEC) contribute to drug disposition. In the proximal renal tubules, epithelial cells have two distinct membrane domains, the basal-lateral membrane and the apical (or lumen) membrane, both of which have transporters. Basolateral transporters are responsible for absorbing solutes from the blood into the epithelium, whereas apical transporters are responsible for excreting solutes from the cell into tubular fluid (Ivanyuk et al., 2017). Drug transporters are divided into uptake and efflux transporters, according to the direction of transmembrane transport of the substrates (Figure 1).
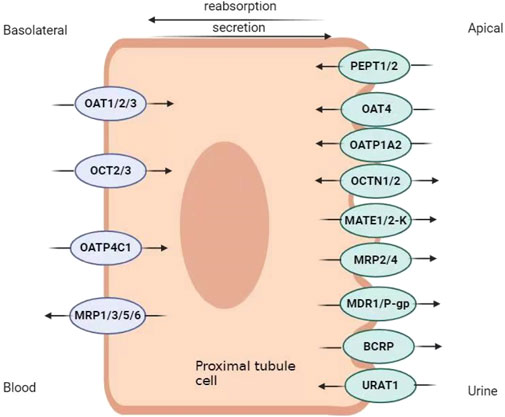
Figure 1. Renal transporter distribution map. OAT, organic anion transporter; OCT, organic cation transporter; OATP, organic anion transporter polypeptide; MRP, multi-drug resistance protein; OCTN, organic cation/carnitine transporter; PEPT, peptide transporter; MATE, multi-drug and toxin excretion; URAT, urate transporter.
The basolateral uptake of drugs by transporters OAT1, OAT3, OATP-4C1, OCT2, and OCT3 is critical for the kidneys to process a variety of drugs and exogenous substances and, ingested substrates at target sites for efficacy. OATs are instrumental in the tubular secretion of numerous drugs, specifically antibiotics, antiviral therapeutics, diuretics, and non-steroidal anti-inflammatory drugs (Momper et al., 2019). OAT1 and OAT3 are the most studied SLC families (Nigam, 2018). OAT1 is located mainly in the basolateral membrane of proximal tubular cells (Nigam, 2015). OAT2 binds specifically to antiviral medications (Nigam, 2015), and OAT3 is the most abundantly expressed transporter in the proximal tubules of the human kidney (Bunprajun et al., 2019). OAT1 and OAT3 transport penicillin and non-steroidal anti-inflammatory drugs (Koepsell et al., 2007; Dudley et al., 2000; Bourdet et al., 2005; Jung et al., 2008; Sato et al., 2008; Tahara et al., 2005). OAT substrates include anti-tumor drugs methotrexate and ubenimex (Zhu et al., 2014). In addition, OAT4 and urate transporter (URAT) 1, both of which belong to the solute vector family, are expressed in the apical membranes of proximal renal tubular cells. OAT4 and URAT1 facilitate the reabsorption of uric acid from proximal tubular cells into the blood (Xu et al., 2017). Probenecid and benzbromarone are commonly used to treat hyperuricemia. They block the reabsorption of uric acid by inhibiting URAT1, and promote urate excretion, thereby reducing blood uric acid levels (Shin et al., 2011). Moreover, the angiotensin II receptor blocker losartan also binds to URAT1, increases uric acid excretion, and reduces blood uric acid levels (Vanwert et al., 2010).
OCTs are members of the SLC22 family (Döring and Petzinger, 2014), and OCT1, OCT2 and OCT3 are expressed in humans. The main substrates of OCTs are fampridine, cisplatin, oxaliplatin, metformin, lamivudine and adolol (Cheung et al., 2017). Metformin reabsorption is influenced by OCT1, which is located on the apical membrane of both the proximal and distal tubules in the kidney (Ivanyuk et al., 2017). OCT2 mainly transports metformin, cisplatin, lamivudine and atenolol (George et al., 2017; Jung et al., 2013). OCT3 is also expressed in the kidney (George et al., 2017). OATP-4C1 is the primary carrier for the transport of digoxin, methotrexate and sitagliptin (Sato et al., 2017; Ivanyuk et al., 2017; Klaassen and Lu, 2008). OCTNs includes OCTN1 and OCTN2 (Pochini et al., 2019). OCTN1 can transport some important drugs such as verapamil, quinidine and gabapentin (Ivanyuk et al., 2017; Nigam, 2015). OCTN2 transports cefepime (Ivanyuk et al., 2017).
PEPT1 is mainly expressed in the small intestine, and its role in intestinal inflammation and inflammatory bowel disease has been previously elucidated (Ingersoll et al., 2012). PEPT2, as an apically expressed transporter, mediates the reabsorption of small anionic peptides (dipeptides and tripeptides) coupled with H+ uptake, and may thus influence the pharmacokinetics of various peptide-like compounds (Ivanyuk et al., 2017; Sala-Rabanal et al., 2008). It has been shown to recognize some ß-lactam antibiotics (ampicillin, amoxicillin, cephalexin, cefaclor, cefadroxil), valacyclovir, and bestatin, and is likely to mediate their reabsorption from the primitive urine, thus potentially slowing down their elimination (El-Sheikh et al., 2008; Li et al., 2006; Ganapathy et al., 1998; Tomita et al., 1990).
Efflux transporters pump the substrate out of the cell to reduce the cellular substrate concentration. They are ABC transporters such as MPR2, MPR4, P-gp and BCRP. Although MATE proteins belong to the SLC superfamily, they also function as efflux transporters. MATE proteins include MATE 1 and MATE 2K (Veiga-Matos et al., 2020). The MATE proteins facilitate the translocation of norfloxacin, ciprofloxacin, levofloxacin, cephalexin, cefradine, dofetilide, cisplatin, oxiliplatin, nadolol, emtricitabine, metformin and cimetidine (Ivanyuk et al., 2017; Nies et al., 2016; Misaka et al., 2016; Uddin et al., 2022; Waissbluth et al., 2023; Miyamae et al., 2001; Reznicek et al., 2017; He et al., 2011). MRPs transport various substrates, including anions formed when drugs (such as methotrexate and cisplatin) conjugated with sulfate, gluconate, or glutathione (Borst et al., 2000). Urine removal is regulated by OCTN1, OCTN2, MATE1, MATE 2K, P-gp, MPR2, MPR4, and BCRP (Morrissey et al., 2013). P-gp has been extensively studied. P-gp transports a variety of anti-tumor drugs, such as paclitaxel and vincristine (Hlavata et al., 2012; Waghray and Zhang, 2018). P-gp also transports various anti-infective drugs, such as macrolides (azithromycin, erythromycin, clarithromycin) and tetracycline (Akamine et al., 2019). BCRP can actively remove anti-tumor drugs such as imatinib, methotrexate (Fletcher et al., 2010). MRP4 also affects uric acid secretion in proximal tubules (Yang et al., 2010). Methotrexate is a MRP4 substrate, which is secreted into the tubule lumen by MRP4 (Hoque et al., 2009). Multi-drug efflux pumps from different families expel antimicrobial agents from the bacteria, thereby leading to drug resistance (Chitsaz and Brown, 2017). When RCC patients require the concurrent use of antimicrobial agents, attention should be paid to transporter-mediated DDI, with a focus on the efficacy and adverse reactions of both anti-tumor drugs and antimicrobial agents. The main substrates of some drug transporters are listed in Table 1.
Under normal physiological conditions, the expression and regulation of drug transporters can help maintain kidney homeostasis (Caetano-pinto et al., 2022). The activity and the expression of drug transporters plays a key role in renal secretion and reabsorption function in RPTEC. Moreover, the expression of drug transporters in RCC cells often differ from those in normal renal cells (Table 2). In RCC, changes in transporter expression affect uptake and efflux processes of anti-tumor drugs, thus affect the therapeutic effects of anti-tumor drugs. The expression and function of drug transporters are influenced by many factors, such as microbiota influence, post-translational modification, transcriptional regulation, enriched epigenetic regulations and exogenous modulations (Yin et al., 2024). Two cellular regulatory processes contribute to the pathophysiology of RCC: DNA methylation (Herman et al., 1994) and epidermal growth factor receptor (EGFR) signaling (Minner et al., 2012; Muroni et al., 2021). A hypermethylated state is associated with the loss of the Von Hippel-Lindau tumor suppressor protein (Clifford et al., 1998) and deregulation of enzymes and carrier proteins responsible for drug metabolism and disposition, including drug transporters (Winter et al., 2016). Epigenetic changes in drug transporter genes are associated with drug response in cancer (Ivanov et al., 2012), and multiple types of epithelial cancers are associated with defective, overexpressed, or constitutive activation of EGFR (Uribe et al., 2021).
The urea transporter encoded by SLC14A1 (UT-B) plays a key role in the kidney, where it transports urea and maintains normal kidney function. Mutations or abnormal expression of SLC14A1 may be associated with the occurrence and development of kidney cancer. SLC14A1 is expressed at lower levels in kidney cancer tissues than in normal kidney tissues (Wan et al., 2023). Moreover, the higher the SLC14A1 expression levels, the lower the kidney cancer differentiation grade and the higher the overall patient survival rate. This indicates that SLC14A1 inhibits the occurrence and development of kidney cancer (Wan et al., 2023). Therefore, SLC14A1 is a potential target for the treatment of kidney cancer. However, these hypotheses are still preliminary and further research is needed to confirm the exact relationship between SLC14A1 and kidney cancer. In addition, the occurrence and development of kidney cancer is complex processes involving the interaction of multiple genes and factors; therefore, SLC14A1 cannot be regarded as the sole cause of kidney cancer.
Sodium-coupled dicarboxylate transporter (NaDC1) encoded by SLC13A2 plays an important role in regulating the acid-base balance, preventing calcium kidney stones, regulating sodium-chloride transport in the collecting duct, and regulating blood pressure (Osis et al., 2019). Apical NaDC1 immunomarker is present throughout the proximal convoluted tubule but is not detected in kidney tumors, including ccRCC and pRCC, that presumably originate in the proximal convoluted tubule, as well as in tumors of non-proximal convoluted tubule origin (Lee et al., 2017). This suggests that NaDC1 expression may is downregulated in RCC.
The relationship between the expression of SLC22 genes and survival in patients with kidney cancer was assessed. In the Cancer Genome Analysis (TCGA) project, two RCC RNA-seq datasets, namely ccRCC and pRCC, were found to have multiple differentially expressed (DE) SLC22 transporter genes compared with those in normal kidney tissue. These included SLC22A6, SLC22A7, SLC22A8, SLC22A12, and SLC22A13. The patients with disease had an association between overall survival and expression of most of these DE genes. Many important SLC22 genes, including those of the OAT and OAT-related groups, had decreased expression over the continuum of stages of RCC from well-functioning, healthy kidneys to advanced metastatic disease. Alternatively, analysis of patients with different classifications of tumor size/progression, lymph node involvement, and presence of metastasis identified multiple SLC22 transporters as significantly changed, often decreasing with severity. A number of the identified transporters (e.g., URAT1/SLC22A12, OAT1/SLC22A6, OAT3/SLC22A8, BCRP/ABCG2) are well-established uric acid transporters. This may be clinically important since, a number of studies indicate that altered uric acid levels and kidney cancer are associated (Whisenant and Nigam, 2022).
According to the Oncomine cancer transcriptome database, most uptake transporters except OCTN2 and PEPT1 are transcriptionally repressed in RCC (Rhodes et al., 2004), and the kidneys contain various SLC22 transporters (Rosenthal et al., 2019; Nigam et al., 2015). In RCC, most of the genes for SLC22 are downregulated, which affects the uptake of some anti-tumor drugs in the kidneys, thereby impacting the therapeutic efficacy of these drugs and potentially leading to the progression of the cancer. Winter et al. (2016) found that, OCT2 expression in RCC cells was below the limit of quantification. Western blot analysis revealed no OCT2 protein expression and OCT expression was restored by inhibiting its methylation. Oxaliplatin, a platinum-based anticancer drug, covalently binds to DNA to form DNA adducts, which trigger various signal transduction pathways. Platinum resistance is caused by insufficient DNA-binding; thus, cellular accumulation of the drug is an important determinant of oxaliplatin’s cytotoxicity (Kelland, 2007). Early clinical trials have shown that oxaliplatin is ineffective against advanced RCC (Chaouche et al., 2000; Porta et al., 2004). Furthermore, OCT2 is a major transporter that enhances cellular uptake and cytotoxicity of oxaliplatin in vitro (Tatsumi et al., 2014). Most proteins showing reduced expression have not yet been characterized; however, studies strongly suggest that reduction in uptake transporters contributes to multidrug resistance in RCC (Puris et al., 2023).
The expression of OCT2 in RCC is has been relatively well studied. Caetano-pinto et al. (2022) (Lee et al., 2017) characterized the activity, expression, and potential regulatory mechanisms of renal drug transporters in RCC in vitro using different cell lines and a non-malignant RPTEC. They found that the expression of OCT2 was absent in the RCC cell line, CAKI-1. Moreover, a limited amount of OCT2 expression was recovered by the inhibition of methylation in CAKI-1 cells. Hence, both OCT2 and MATE 2K are repressed in RCC cells, resulting in insufficient accumulation of oxaliplatin and subsequent therapy failure. In RCC cell lines, decitabine (DAC) was used to inhibit DNA methylation by blocking DNA methyltransferases. OCT2 but not MATE-2K expression was restored in RCC cells after DAC treatment, resulting in high oxaliplatin uptake and low oxaliplatin efflux, high oxaliplatin accumulation, and increased oxaliplatin cytotoxicity (Liu et al., 2016). Therefore, sequential combination of DAC and oxaliplatin is a promising treatment option to sensitize RCC cells to oxaliplatin by activating OCT2-mediated transport.
The effect of microRNAs on transporters has also been studied in RCC. MicroRNAs (miRNA) are a set of endogenous single-stranded small RNAs with a length of approximately 21–23 nt, they modify gene expression post-transcriptionally, participate in the mediation of over 60% of protein-coding gene expression, and participate in almost all intracellular biological processes. Hence, miRNAs not only affect normal cell growth, differentiation, and other aspects, but also play a role in cancer, heart disease, inflammation, and more (Shen et al., 2012; Lu et al., 2017). With the growth of miRNA research and the development of molecular biology technology, an increasing number of studies have shown that abnormal expression of miRNAs can affect the tumor formation and growth. The miRNA expression profiles in RCC tissue samples have been screened and have revealed that the formation and metastasis of renal cancer are strongly correlated with some miRNAs (Miranda-Poma et al., 2022; Petillo et al., 2009). Moreover, miRNAs belonging to the Let-7 family are significantly downregulated in patients with nephroblastoma (Huo et al., 2010). Most miRNAs in RCC tissues exhibited a downward trend. These abnormally expressed miRNAs can be used as targets or targeted drug components to inhibit their downstream regulation to curb tumor proliferation and progression. They can also be used as biomarkers for the diagnosis of RCC before radioactive examination. High miR-630 levels inhibit the expression of OCT2 mRNA, thereby inhibiting its protein expression levels and weakening its uptake of classical substrates and the anticancer drug oxaliplatin (Chen et al., 2019a). This suggests that the inhibition of OCT2 as a result of high miR-630 expression is one of the mechanisms of oxaliplatin resistance in RCC.
OCT2 was also differentially expressed in primary and metastatic tissues of kidney cancer. Interestingly, a significant decrease in OCT2 mRNA expression was found in primary RCC but not in metastatic RCC (Visentin et al., 2018). Moreover, the main choline transporter in the kidney, OCT2, recognizes fluorocholine as a substrate (Visentin et al., 2017). Furthermore, a high likelihood exists for the dominant role of OCT2 in [18F] fluorocholine renal uptake, and changes in OCT2 expression levels during renal carcinogenesis may affect [18F] fluorocholine accumulation. Compared with that in surrounding normal tissues, metastatic RCCs may accumulate abnormal amounts of [18F] fluorocholine due to OCT2 modulation. The use of [18F] fluorocholine positron emission tomography/computed tomography may improve sensitivity for the detection of early-stage metastatic disease, which is a major clinical challenge during the initial staging of RCC (Chaouche et al., 2000).
SLC22A3 (human OCT3) is highly expressed in two of the five RCC cell lines (A498 and 786-O) (Walsh et al., 2009). In A498 cells, [3H]MPP (the model substrate of OCT3) accumulation was >10 fold higher than in ACHN cells. Irinotecan, vincristine, and melphalan inhibited uptake of [3H]MPP into these cells and also into hOCT3 stably transfected Chinese hamster ovary (CHO) cells. The growth of CHO-hOCT3 was inhibited by 20% more with irinotecan and by 50% more with vincristine compared with non-transfected CHO cells. Melphalan produced 20%–30% more inhibition in hOCT3-expressing cells compared with non-expressing control cells. Expression of hOCT3 in kidney carcinoma cell lines increases chemosensitivity to melphalan, irinotecan, and vincristine. That supports the hypothesis that the sensitivity of tumor cells to chemotherapeutic treatment depends on the expression of transporter proteins mediating specific drug accumulation into target cells. This fact renders OCT3 an appropriate candidate for individualized kidney tumor therapy (Shnitsar et al., 2009). Along these lines, it is worthwhile considering to test for OCT3 expression and to tailor the cytostatic therapy.
Little was known about the expression of OATs in kidney tumors and their interactions with cytostatics. The expression of SLC transporters in the kidney tumor cell lines 786-O, RCCNG1, A498, LN78, and ACHN, and their interactions with chemotherapeutics have been investigated (Walsh et al., 2009). An mRNA level analysis in kidney cancer cell lines revealed the presence of OAT1. However, the uptake of PAH was relatively low, and it was not inhibited by 500 μmol/L probenecid (a standard blocker of OATs). OATs are, therefore, unsuitable as targets for anionic cytostatic chemotherapy for RCC.
Overexpression of ABC transporters in cancer cells is a well-documented multi-drug resistance mechanism. The transport of drugs from the intracellular to the extracellular is facilitated by various transporters, which the expression and function of those transporters are highly regulated (König et al., 2013). In addition, hyperexpression of these transporters has been reported in untreated solid tumors and various types of leukemia (Nakanishi and Ross, 2012). However, the development of such inhibitors has been challenging owing to the specificity and complexity of the function of ABC transporters. Therefore, the evolution of resistance to multiple drugs in cancer cells is a significant barrier for the successful treatment of the disease (Wu et al., 2023).
Several diseases, including cancer, are affected by the inter-individual variability in BCRP/ABCG2. BCRP expression may be reduced by the minor alleles of two ABCG2 variants, rs2231137 G34A (V12M) and rs2231142 C421A (Q141K). Gene variants rs2231137 G34A and rs2231142 C421A have been reported to be associated with disease risk, reduced efficacy of drug treatments, and increased adverse reactions in different human diseases (Chen et al., 2019b). Relationship between carcinogenesis and common ABCG2 variants is controversial in population-based association studies in various types of cancer. Association of ABCG2 rs2231142 C421A with the development of breast cancer was examined in 100 Kurdish patients and 200 healthy controls (Ghafouri et al., 2016). Patients with AA genotype of rs2231142 were at a higher risk of breast cancer. A meta-analysis found that the rs2231142 A allele is associated with a lower risk for the development of multiple cancer types, including leukemia and colorectal cancer. The relationship between the common ABCG2 variants and cancer risk is complex and may be different in divergent human populations and variable across cancer types. Further studies are needed to clarify the impacts of BCRP transporter genotypes upon carcinogenesis. However, the common ABCG2 variants may also be related to severe drug-induced adverse reactions to chemotherapy. In a study of 219 Japanese patients with RCC, the rs2231142 C421A genetic variant was associated with severe thrombocytopenia following sunitinib therapy (Low et al., 2016). Therefore, sunitinib doses must be adjusted in patients with the rs2231142 A variant.
ABCC2 belongs to the ABC transporter family and induced chemotherapy resistance; hence, it was named MRP2 (Jeong et al., 2015). Reportedly, the MRP 2 and other ABC transporters influence the anti-tumor therapeutic effects of TKIs (Kathawala et al., 2015; Shibayama et al., 2011). As a TKI, sunitinib disrupts signaling pathways that lead to tumor proliferation and angiogenesis in cancer cells and is often considered the frontline treatment for pRCC. Moreover, as a drug transporter, MRP2 may influence the effect of sunitinib on cancer cells (Warta et al., 2014; Zhang et al., 2014). The development of drug resistance is a common obstacle for TKI treatment. One hypothesized resistance mechanism is the active expulsion of intracellular substances by ABC transporter proteins (He and Wei, 2012). Therefore, a combination of sunitinib and MRP2 blockers for the treatment of pRCC2 may enhance anticancer efficacy of Sunitinib. Saleeb et al. conducted experiments using AKI-2 cells in vitro and mouse models in vivo. Five groups were tested: anti-vascular endothelial growth factor (sunitinib), MRP2 blocker (MK 571), mammalian target of rapamycin inhibitor (everolimus), and sunitinib + MK 571. Compared with that of the other treatment groups, the sunitinib + MRP2 blocker group produced a marked therapeutic reaction in vitro and in vivo. The MRP2 blocking results of both in vitro and in vivo experiments showed elevated sunitinib uptake levels. This suggests that the combination of sunitinib and MRP2 blockers targeting pRCC has a therapeutic potential (Saleeb et al., 2018).
Many clinical studies have evaluated the role of P-gp in the development of RCC. P-gp is an important membrane transporter that effluxes drugs from cells, and affects cellular drug concentrations, and exerts antitumor effects (Pilotto Heming et al., 2022). Lee and Thevenod (2019) found that oncogenic pituitary homeobox 2, a de facto master regulator of developmental organ asymmetry, upregulates the expression of P-gp in A498 RCC cells. Many anticancer drugs are the substrate of P-gp (Dei et al., 2019). Therefore, exploring the role of P-gp in RCC progression is important for improving RCC treatment outcomes. Elevated P-gp expression in RCC cells expels anticancer medications from cells, thereby resulting in decreased intracellular drug levels and subsequent diminished efficacy against tumors (Walsh et al., 2009). ABCB1 methylation is associated with P-gp expression in RCC (especially ccRCC) (Yan et al., 2019), and the P-gp mRNA expression levels in ccRCC is higher than that in healthy kidney tissues (Yamaguchi et al., 2010). P-gp inhibition increases the anti-tumor effects of sunitinib in RCC treated with elacridar (Sato et al., 2015). In addition, both P-gp and BCRP expression were increased in patients with ccRCC compared with that in patients with normal kidney tissue or function (Reustle et al., 2018). Higher BCRP inhibition was associated with better results when sunitinib was used for cancer treatment (Reustle et al., 2018).
Studies on the effects of drug transporters on RCC are relatively scarce, yet hold significant importance. As mentioned above, transporters are closely related to the occurrence, development, and drug efficacy of RCC. Timely RCC diagnosis and inhibition of disease progression can be achieved by exploring the expression of relevant transporters. However, RCC sometimes develop multidrug resistance to drugs, and transporters may have a vital impact on this process. Drug resistance and metastasis of malignant tumors are a key cause of death in patients with cancer and are a major challenge for cancer treatment (Jolly et al., 2019). The vast majority of cancer deaths can be attributed to the development of drug resistance (Bukowski et al., 2020); hence, drug resistance remains a major barrier to achieving a successful cure for cancer (Vasan et al., 2019). By leveraging the DDI mechanisms mediated by drug transporters and combining the use of efflux transporter inhibitors such as P-gp, BCRP, and MRP2 inhibitors. The multi-drug resistance in RCC can be reversed in some situations, because of that higher expression of efflux transporters is one of the multi-drug resistance mechanisms, there are also other mechanisms causing multi-drug resistance. Therefore, more studies on the genetic polymorphisms of drug transporters should be investigated to reveal the impact of these differences on kidney cancer treatments. However, insufficient research on the genetic polymorphism of drug transporters has been conducted. Overall, research on drug transport and kidney cancer aims to improve drug efficacy, reduce side effects, and promote personalized therapy.
Transporters may directly and indirectly affect the development and progression of RCC. The expression and function of these drug transporters has an important effect on drug concentrations, and the alteration of drug exposure. That may affect the efficacy and toxicity of anti-tumor drugs. Thus, current information indicates that the changes of transporters have indirect affected disease occurrence or progression. Understanding the role of these drug transporters in RCC will provide more information about specific treatments. Researchers and clinicians can consider these factors in order to choose a suitable therapeutic drug or a combination drug strategy to maximize the concentration of the drug in the tumor, improve the efficiency of treatment and possibly increase drug resistance through combination drugs and other measures. The directly relationship between RCC and drug transporters is known less and is still worth being studied further. Hence, this review summarizes the existing literature, aims to provide support for clinical work and basic scientific research, and encourages the scientific community to focus on changes in drug transport expression to ensure the effectiveness and safety of patient medications.
YZu: Writing–original draft. TL: Writing–original draft. SY: Conceptualization, Writing–review and editing. XC: Formal analysis, writing–review and editing. XT: Formal analysis, writing–review and editing. DD: Supervision, Writing–review and editing. FL: Supervision, Writing–review and editing. YZh: Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by a grant from the National Natural Science Foundation of China (No.82003837).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RCC, renal cell carcinoma; ccRCC, clear cell RCC; pRCC, papillary RCC; ICI, checkpoint inhibitors; TKI, tyrosine kinase inhibitor; SLC, solute carrier; ABC, adenosine triphosphate-binding cassette; P-gp, P-glycoproteins; MRP, multi-drug resistance protein; OATP, organic anion transporter polypeptide; OAT, organic anion transporter; OCT, organic cation transporter; OCTN, organic cation/carnitine transporter; PEPT, peptide transporter; MATE, multi-drug and toxin excretion; BCRP, breast cancer resistance protein; RPTEC, renal proximal tubule epithelial cells; URAT, urate transporter; EGFR, epidermal growth factor receptor; NaDC1, sodium-coupled dicarboxylate transporter; DAC, decitabine; miRNA, microRNA; DDI, drug-drug interactions.
Akamine, Y., Yasui-Furukori, N., and Uno, T. (2019). Drug-drug interactions of P-gp substrates unrelated to CYP metabolism. Curr. drug Metab. 20 (2), 124–129. doi:10.2174/1389200219666181003142036
Alves, R., Gonçalves, A. C., Jorge, J., Almeida, A. M., and Sarmento-Ribeiro, A. B. (2022). Combination of elacridar with imatinib modulates resistance associated with drug efflux transporters in chronic myeloid leukemia. Biomedicines 10 (5), 1158. doi:10.3390/biomedicines10051158
Babu, E., Takeda, M., Narikawa, S., Kobayashi, Y., Yamamoto, T., Cha, S. H., et al. (2002). Human organic anion transporters mediate the transport of tetracycline. Jpn. J. Pharmacol. 88 (1), 69–76. doi:10.1254/jjp.88.69
Banerjee, S. K., Jagannath, C., Hunter, R. L., and Dasgupta, A. (2000). Bioavailability of tobramycin after oral delivery in FVB mice using CRL-1605 copolymer, an inhibitor of P-glycoprotein. Life Sci. 67 (16), 2011–2016. doi:10.1016/s0024-3205(00)00786-4
Borst, P., Evers, R., Kool, M., and Wijnholds, J. (2000). A family of drug transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 92 (16), 1295–1302. doi:10.1093/jnci/92.16.1295
Bourdet, D. L., Pritchard, J. B., and Thakker, D. R. (2005). Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J. Pharmacol. Exp. Ther. 315 (3), 1288–1297. doi:10.1124/jpet.105.091223
Bukowski, K., Kciuk, M., and Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21 (9), 3233. doi:10.3390/ijms21093233
Bunprajun, T., Yuajit, C., Noitem, R., and Chatsudthipong, V. (2019). Exhaustive exercise decreases renal organic anion transporter 3 function. J. physiological Sci. JPS 69 (2), 245–251. doi:10.1007/s12576-018-0641-5
Burckhardt, G., and Burckhardt, B. C. (2011). In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb. Exp. Pharmacol. (201), 29–104. doi:10.1007/978-3-642-14541-4_2
Caetano-Pinto, P., Justian, N., Dib, M., Fischer, J., Somova, M., Burchardt, M., et al. (2022). In vitro characterization of renal drug transporter activity in kidney cancer. Int. J. Mol. Sci. 23 (17), 10177. doi:10.3390/ijms231710177
Chaouche, M., Pasturaud, A. L., Kamioner, D., Grandjean, M., Franiatte, J., and Tourani, J. M. (2000). Oxaliplatin, 5-fluorouracil, and folinic acid (Folfox) in patients with metastatic renal cell carcinoma: results of a pilot study. Am. J. Clin. Oncol. 23 (3), 288–289. doi:10.1097/00000421-200006000-00016
Chen, Y. W., Wang, L., Panian, J., Dhanji, S., Derweesh, I., Rose, B., et al. (2023). Treatment landscape of renal cell carcinoma. Curr. Treat. options Oncol. 24 (12), 1889–1916. doi:10.1007/s11864-023-01161-5
Chen, L., Chen, L., Qin, Z., Lei, J., Ye, S., Zeng, K., et al. (2019a). Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm. Sin. B 9 (5), 1008–1020. doi:10.1016/j.apsb.2019.01.002
Chen, L., Manautou, J. E., Rasmussen, T. P., and Zhong, X. B. (2019b). Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 9 (4), 659–674. doi:10.1016/j.apsb.2019.01.007
Cheung, K. W. K., Hsueh, C. H., Zhao, P., Meyer, T. W., Zhang, L., Huang, S. M., et al. (2017). The effect of uremic solutes on the organic cation transporter 2. J. Pharm. Sci. 106 (9), 2551–2557. doi:10.1016/j.xphs.2017.04.076
Chitsaz, M., and Brown, M. H. (2017). The role played by drug efflux pumps in bacterial multidrug resistance. Essays Biochem. 61 (1), 127–139. doi:10.1042/EBC20160064
Ciarimboli, G. (2023). Overcoming biological barriers: importance of membrane transporters in homeostasis, disease and disease treatment. Int. J. Mol. Sci. 24 (8), 7212. doi:10.3390/ijms24087212
Ciarimboli, G., Theil, G., Bialek, J., and Edemir, B. (2021). Contribution and expression of organic cation transporters and aquaporin water channels in renal cancer. Rev. physiology, Biochem. Pharmacol. 181, 81–104. doi:10.1007/112_2020_34
Clifford, S. C., Prowse, A. H., Affara, N. A., Buys, C. H., and Maher, E. R. (1998). Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosom. Cancer 22 (3), 200–209. doi:10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#
Dash, R. P., Jayachandra Babu, R., and Srinivas, N. R. (2017). Therapeutic potential and utility of elacridar with respect to P-glycoprotein inhibition: an insight from the published in vitro, preclinical and clinical studies. Eur. J. drug metabolism Pharmacokinet. 42 (6), 915–933. doi:10.1007/s13318-017-0411-4
Dei, S., Braconi, L., Romanelli, M. N., and Teodori, E. (2019). Recent advances in the search of BCRP- and dual P-gp/BCRP-based multidrug resistance modulators. Cancer drug Resist. Alhambra, Calif. 2 (3), 710–743. doi:10.20517/cdr.2019.31
Döring, B., and Petzinger, E. (2014). Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug metab. Rev. 46 (3), 261–282. doi:10.3109/03602532.2014.882353
Dudley, A. J., Bleasby, K., and Brown, C. D. (2000). The organic cation transporter OCT2 mediates the uptake of beta-adrenoceptor antagonists across the apical membrane of renal LLC-PK(1) cell monolayers. Br. J. Pharmacol. 131 (1), 71–79. doi:10.1038/sj.bjp.0703518
EL-Mahdy, H. A., EL-Husseiny, A. A., Kandil, Y. I., and Gamal El-Din, A. M. (2020). Diltiazem potentiates the cytotoxicity of gemcitabine and 5-fluorouracil in PANC-1 human pancreatic cancer cells through inhibition of P-glycoprotein. Life Sci. 262, 118518. doi:10.1016/j.lfs.2020.118518
EL-Sheikh, A. A., Greupink, R., Wortelboer, H. M., van den Heuvel, J. J. M. W., Schreurs, M., Koenderink, J. B., et al. (2013). Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl. Res. J. laboratory Clin. Med. 162 (6), 398–409. doi:10.1016/j.trsl.2013.08.003
EL-Sheikh, A. A., Masereeuw, R., and Russel, F. G. (2008). Mechanisms of renal anionic drug transport. Eur. J. Pharmacol. 585 (2-3), 245–255. doi:10.1016/j.ejphar.2008.02.085
Fletcher, J. I., Haber, M., Henderson, M. J., and Norris, M. D. (2010). ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer 10 (2), 147–156. doi:10.1038/nrc2789
Ganapathy, M. E., Huang, W., Wang, H., Ganapathy, V., and Leibach, F. H. (1998). Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem. biophysical Res. Commun. 246 (2), 470–475. doi:10.1006/bbrc.1998.8628
George, B., You, D., Joy, M. S., and Aleksunes, L. M. (2017). Xenobiotic transporters and kidney injury. Adv. drug Deliv. Rev. 116, 73–91. doi:10.1016/j.addr.2017.01.005
Ghafouri, H., Ghaderi, B., Amini, S., Nikkhoo, B., Abdi, M., and Hoseini, A. (2016). Association of ABCB1 and ABCG2 single nucleotide polymorphisms with clinical findings and response to chemotherapy treatments in Kurdish patients with breast cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 37 (6), 7901–7906. doi:10.1007/s13277-015-4679-1
Green, B. R., and Bain, L. J. (2013). Mrp2 is involved in the efflux and disposition of fosinopril. J. Appl. Toxicol. JAT 33 (6), 458–465. doi:10.1002/jat.1767
He, G. X., Thorpe, C., Walsh, D., Crow, R., Chen, H., Kumar, S., et al. (2011). EmmdR, a new member of the MATE family of multidrug transporters, extrudes quinolones from Enterobacter cloacae. Archives Microbiol. 193 (10), 759–765. doi:10.1007/s00203-011-0738-1
He, M., and Wei, M. J. (2012). Reversing multidrug resistance by tyrosine kinase inhibitors. Chin. J. cancer 31 (3), 126–133. doi:10.5732/cjc.011.10315
Herman, J. G., Latif, F., Weng, Y., Lerman, M. I., Zbar, B., Liu, S., et al. (1994). Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc. Natl. Acad. Sci. U. S. A. 91 (21), 9700–9704. doi:10.1073/pnas.91.21.9700
Hlavata, I., Mohelnikova-Duchonova, B., Vaclavikova, R., Liska, V., Pitule, P., Novak, P., et al. (2012). The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 27 (2), 187–196. doi:10.1093/mutage/ger075
Hoque, M. T., Conseil, G., and Cole, S. P. (2009). Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem. biophysical Res. Commun. 379 (1), 60–64. doi:10.1016/j.bbrc.2008.12.014
Hosseiniyan Khatibi, S. M., Ardalan, M., Teshnehlab, M., Vahed, S. Z., and Pirmoradi, S. (2022). Panels of mRNAs and miRNAs for decoding molecular mechanisms of Renal Cell Carcinoma (RCC) subtypes utilizing Artificial Intelligence approaches. Sci. Rep. 12 (1), 16393. doi:10.1038/s41598-022-20783-7
Hsieh, J. J., Purdue, M. P., Signoretti, S., Swanton, C., Albiges, L., Schmidinger, M., et al. (2017). Renal cell carcinoma. Nat. Rev. Dis. Prim. 3 (1), 17009. doi:10.1038/nrdp.2017.9
Hu, C., Lancaster, C. S., Zuo, Z., Hu, S., Chen, Z., Rubnitz, J. E., et al. (2012). Inhibition of OCTN2-mediated transport of carnitine by etoposide. Mol. cancer Ther. 11 (4), 921–929. doi:10.1158/1535-7163.MCT-11-0980
Huo, H., Magro, P. G., Pietsch, E. C., Patel, B. B., and Scotto, K. W. (2010). Histone methyltransferase MLL1 regulates MDR1 transcription and chemoresistance. Cancer Res. 70 (21), 8726–8735. doi:10.1158/0008-5472.CAN-10-0755
Hu, S., Franke, R. M., Filipski, K. K., Hu, C., Orwick, S. J., de Bruijn, E. A., et al. (2008). Interaction of imatinib with human organic ion carriers. Clin. cancer Res. 14 (10), 3141–3148. doi:10.1158/1078-0432.CCR-07-4913
Ingersoll, S. A., Ayyadurai, S., Charania, M. A., Laroui, H., Yan, Y., and Merlin, D. (2012). The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am. J. physiology Gastrointest. liver physiology 302 (5), G484–G492. doi:10.1152/ajpgi.00477.2011
Inui, K., Terada, T., Masuda, S., and Saito, H. (2000). Physiological and pharmacological implications of peptide transporters, PEPT1 and PEPT2. Nephrol. Dial. Transplant. 15 (Suppl. 6), 11–13. doi:10.1093/ndt/15.suppl_6.11
Ivanov, M., Kacevska, M., and Ingelman-Sundberg, M. (2012). Epigenomics and interindividual differences in drug response. Clin. Pharmacol. Ther. 92 (6), 727–736. doi:10.1038/clpt.2012.152
Ivanyuk, A., Livio, F., Biollaz, J., and Buclin, T. (2017). Renal drug transporters and drug interactions. Clin. Pharmacokinet. 56 (8), 825–892. doi:10.1007/s40262-017-0506-8
Jeong, H. S., Ryoo, I. G., and Kwak, M. K. (2015). Regulation of the expression of renal drug transporters in KEAP1-knockdown human tubular cells. Toxicol. vitro Int. J. Publ. Assoc. BIBRA 29 (5), 884–892. doi:10.1016/j.tiv.2015.03.013
Jolly, M. K., Somarelli, J. A., Sheth, M., Biddle, A., Tripathi, S. C., Armstrong, A. J., et al. (2019). Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. and Ther. 194, 161–184. doi:10.1016/j.pharmthera.2018.09.007
Jong, N. N., Nakanishi, T., Liu, J. J., Tamai, I., and McKeage, M. J. (2011). Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 338 (2), 537–547. doi:10.1124/jpet.111.181297
Jung, N., Lehmann, C., Rubbert, A., Knispel, M., Hartmann, P., van Lunzen, J., et al. (2008). Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug metabolism Dispos. Biol. fate Chem. 36 (8), 1616–1623. doi:10.1124/dmd.108.020826
Jung, N., Lehmann, C., Rubbert, A., Schömig, E., Fätkenheuer, G., Hartmann, P., et al. (2013). Organic cation transporters OCT1 and OCT2 determine the accumulation of lamivudine in CD4 cells of HIV-infected patients. Infection 41 (2), 379–385. doi:10.1007/s15010-012-0308-8
Kathawala, R. J., Gupta, P., Ashby, C. R., and Chen, Z. S. (2015). The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist. Updat. Rev. Comment. Antimicrob. anticancer Chemother. 18, 1–17. doi:10.1016/j.drup.2014.11.002
Kelland, L. (2007). The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7 (8), 573–584. doi:10.1038/nrc2167
Khamdang, S., Takeda, M., Noshiro, R., Narikawa, S., Enomoto, A., Anzai, N., et al. (2002). Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 303 (2), 534–539. doi:10.1124/jpet.102.037580
Klaassen, C. D., and Lu, H. (2008). Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol. Sci. official J. Soc. Toxicol. 101 (2), 186–196. doi:10.1093/toxsci/kfm214
Koepsell, H. (2013). The SLC22 family with transporters of organic cations, anions and zwitterions. Mol. aspects Med. 34 (2-3), 413–435. doi:10.1016/j.mam.2012.10.010
Koepsell, H., Lips, K., and Volk, C. (2007). Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 24 (7), 1227–1251. doi:10.1007/s11095-007-9254-z
KöNIG, J., MüLLER, F., and Fromm, M. F. (2013). Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol. Rev. 65 (3), 944–966. doi:10.1124/pr.113.007518
Lee, H. W., Handlogten, M. E., Osis, G., Clapp, W. L., Wakefield, D. N., Verlander, J. W., et al. (2017). Expression of sodium-dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) in normal and neoplastic human kidney. Am. J. physiology Ren. physiology 312 (3), F427–F435. doi:10.1152/ajprenal.00559.2016
Lee, J. W., Chou, C. L., and Knepper, M. A. (2015). Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. JASN 26 (11), 2669–2677. doi:10.1681/ASN.2014111067
Lee, W. K., and Thevenod, F. (2019). Oncogenic PITX2 facilitates tumor cell drug resistance by inverse regulation of hOCT3/SLC22A3 and ABC drug transporters in colon and kidney cancers. Cancer Lett. 449, 237–251. doi:10.1016/j.canlet.2019.01.044
Liao, X. Y., Deng, Q. Q., Han, L., Wu, Z. T., Peng, Z. L., Xie, Y., et al. (2020). Leflunomide increased the renal exposure of acyclovir by inhibiting OAT1/3 and MRP2. Acta Pharmacol. Sin. 41 (1), 129–137. doi:10.1038/s41401-019-0283-z
Li, M., Anderson, G. D., Phillips, B. R., Kong, W., Shen, D. D., and Wang, J. (2006). Interactions of amoxicillin and cefaclor with human renal organic anion and peptide transporters. Drug metabolism Dispos. Biol. fate Chem. 34 (4), 547–555. doi:10.1124/dmd.105.006791
Liu, X. (2019a). Overview: role of drug transporters in drug disposition and its clinical significance. Adv. Exp. Med. Biol. 1141, 1–12. doi:10.1007/978-981-13-7647-4_1
Liu, X. (2019b). ABC family transporters. Adv. Exp. Med. Biol. 1141, 13–100. doi:10.1007/978-981-13-7647-4_2
Liu, X. (2019c). SLC family transporters. Adv. Exp. Med. Biol. 1141, 101–202. doi:10.1007/978-981-13-7647-4_3
Liu, Y., Zheng, X., Yu, Q., Wang, H., Tan, F., Zhu, Q., et al. (2016). Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Sci. Transl. Med. 8 (348), 348ra97. doi:10.1126/scitranslmed.aaf3124
Low, S. K., Fukunaga, K., Takahashi, A., Matsuda, K., Hongo, F., Nakanishi, H., et al. (2016). Association study of a functional variant on ABCG2 gene with sunitinib-induced severe adverse drug reaction. PloS one 11 (2), e0148177. doi:10.1371/journal.pone.0148177
Lu, C., Shan, Z., Li, C., and Yang, L. (2017). MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed. and Pharmacother. = Biomedecine and Pharmacother. 86, 450–456. doi:10.1016/j.biopha.2016.11.139
Mandal, A. K., Mercado, A., Foster, A., Zandi-Nejad, K., and Mount, D. B. (2017). Uricosuric targets of tranilast. Pharmacol. Res. and Perspect. 5 (2), e00291. doi:10.1002/prp2.291
Medina-Rico, M., Ramos, H. L., Lobo, M., Romo, J., and Prada, J. G. (2018). Epidemiology of renal cancer in developing countries: review of the literature. Can. Urological Assoc. J. = J. de l'Association des urologues du Can. 12 (3), E154–E162. doi:10.5489/cuaj.4464
Mikkaichi, T., Suzuki, T., Onogawa, T., Tanemoto, M., Mizutamari, H., Okada, M., et al. (2004). Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc. Natl. Acad. Sci. U. S. A. 101 (10), 3569–3574. doi:10.1073/pnas.0304987101
Milane, A., Fernandez, C., Vautier, S., Bensimon, G., Meininger, V., and Farinotti, R. (2007). Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J. Neurochem. 103 (1), 164–173. doi:10.1111/j.1471-4159.2007.04772.x
Minner, S., Rump, D., Tennstedt, P., Simon, R., Burandt, E., Terracciano, L., et al. (2012). Epidermal growth factor receptor protein expression and genomic alterations in renal cell carcinoma. Cancer 118 (5), 1268–1275. doi:10.1002/cncr.26436
Miranda-Poma, J., Trilla-Fuertes, L., LóPEZ-Camacho, E., Zapater-Moros, A., López-Vacas, R., Lumbreras-Herrera, M. I., et al. (2022). MiRNAs in renal cell carcinoma. Clin. and Transl. Oncol. 24 (11), 2055–2063. doi:10.1007/s12094-022-02866-z
Misaka, S., Knop, J., Singer, K., Hoier, E., Keiser, M., Müller, F., et al. (2016). The nonmetabolized β-blocker nadolol is a substrate of OCT1, OCT2, MATE1, MATE2-K, and P-glycoprotein, but not of OATP1B1 and OATP1B3. Mol. Pharm. 13 (2), 512–519. doi:10.1021/acs.molpharmaceut.5b00733
Miyamae, S., Ueda, O., Yoshimura, F., Hwang, J., Tanaka, Y., and Nikaido, H. (2001). A MATE family multidrug efflux transporter pumps out fluoroquinolones in Bacteroides thetaiotaomicron. Antimicrob. agents Chemother. 45 (12), 3341–3346. doi:10.1128/AAC.45.12.3341-3346.2001
Momper, J. D., Yang, J., Gockenbach, M., Vaida, F., and Nigam, S. K. (2019). Dynamics of organic anion transporter-mediated tubular secretion during postnatal human kidney development and maturation. Clin. J. Am. Soc. Nephrol. CJASN 14 (4), 540–548. doi:10.2215/CJN.10350818
Morrissey, K. M., Stocker, S. L., Wittwer, M. B., Xu, L., and Giacomini, K. M. (2013). Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 53, 503–529. doi:10.1146/annurev-pharmtox-011112-140317
Mulgaonkar, A., Venitz, J., Gründemann, D., and Sweet, D. H. (2013). Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob. agents Chemother. 57 (6), 2705–2711. doi:10.1128/AAC.02289-12
Muroni, M. R., Ribback, S., Sotgiu, G., Kroeger, N., Saderi, L., Angius, A., et al. (2021). Prognostic impact of membranous/nuclear epidermal growth factor receptor localization in clear cell renal cell carcinoma. Int. J. Mol. Sci. 22 (16), 8747. doi:10.3390/ijms22168747
Nakanishi, T., and Ross, D. D. (2012). Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin. J. cancer 31 (2), 73–99. doi:10.5732/cjc.011.10320
Nepal, M. R., Taheri, H., Li, Y., Talebi, Z., Uddin, M. E., Jin, Y., et al. (2022). Targeting OCT2 with duloxetine to prevent oxaliplatin-induced peripheral neurotoxicity. Cancer Res. Commun. 2 (11), 1334–1343. doi:10.1158/2767-9764.crc-22-0172
Nies, A. T., Damme, K., Kruck, S., Schaeffeler, E., and Schwab, M. (2016). Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Archives Toxicol. 90 (7), 1555–1584. doi:10.1007/s00204-016-1728-5
Nigam, S. K. (2015). What do drug transporters really do? Nat. Rev. Drug Discov. 14 (1), 29–44. doi:10.1038/nrd4461
Nigam, S. K. (2018). The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 58, 663–687. doi:10.1146/annurev-pharmtox-010617-052713
Nigam, S. K., Bush, K. T., Martovetsky, G., Ahn, S. Y., Liu, H. C., Richard, E., et al. (2015). The organic anion transporter (OAT) family: a systems biology perspective. Physiol. Rev. 95 (1), 83–123. doi:10.1152/physrev.00025.2013
Noguchi, S., Nishimura, T., Mukaida, S., Benet, L. Z., Nakashima, E., and Tomi, M. (2017). Cellular uptake of levocetirizine by organic anion transporter 4. J. Pharm. Sci. 106 (9), 2895–2898. doi:10.1016/j.xphs.2017.03.026
Noguchi, S., Okochi, M., Atsuta, H., Kimura, R., Fukumoto, A., Takahashi, K., et al. (2021). Substrate recognition of renally eliminated angiotensin II receptor blockers by organic anion transporter 4. Drug metabolism Pharmacokinet. 36, 100363. doi:10.1016/j.dmpk.2020.10.002
Osis, G., Webster, K. L., Harris, A. N., Lee, H. W., Chen, C., Fang, L., et al. (2019). Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A. Am. J. physiology Ren. physiology 317 (2), F489-–F501. doi:10.1152/ajprenal.00015.2019
Padala, S. A., and Kallam, A. (2023). Clear cell renal carcinoma [M]. StatPearls. Treasure island (FL) ineligible companies. Disclosure: avyakta kallam declares no relevant financial relationships with ineligible companies.; StatPearls publishing copyright © 2023. Treasure Island (FL): StatPearls Publishing LLC.
Pedersen, J. M., Khan, E. K., BergströM, C. A. S., Palm, J., Hoogstraate, J., and Artursson, P. (2017). Substrate and method dependent inhibition of three ABC-transporters (MDR1, BCRP, and MRP2). Eur. J. Pharm. Sci. official J. Eur. Fed. Pharm. Sci. 103, 70–76. doi:10.1016/j.ejps.2017.03.002
Pérez-Herrero, E., and Fernández-Medarde, A. (2015). Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 93, 52–79. doi:10.1016/j.ejpb.2015.03.018
Perez-Tomas, R. (2006). Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 13 (16), 1859–1876. doi:10.2174/092986706777585077
Petillo, D., Kort, E. J., Anema, J., Furge, K. A., Yang, X. J., and Teh, B. T. (2009). MicroRNA profiling of human kidney cancer subtypes. Int. J. Oncol. 35 (1), 109–114. doi:10.3892/ijo_00000318
Pilotto Heming, C., Muriithi, W., Wanjiku Macharia, L., Niemeyer Filho, P., Moura-Neto, V., and Aran, V. (2022). P-glycoprotein and cancer: what do we currently know? Heliyon 8 (10), e11171. doi:10.1016/j.heliyon.2022.e11171
Pochini, L., Galluccio, M., Scalise, M., Console, L., and Indiveri, C. (2019). OCTN: a small transporter subfamily with great relevance to human pathophysiology, drug discovery, and diagnostics. SLAS Discov. Adv. life Sci. R and D 24 (2), 89–110. doi:10.1177/2472555218812821
Porta, C., Zimatore, M., Imarisio, I., Natalizi, A., Sartore-Bianchi, A., Danova, M., et al. (2004). Gemcitabine and oxaliplatin in the treatment of patients with immunotherapy-resistant advanced renal cell carcinoma: final results of a single-institution Phase II study. Cancer 100 (10), 2132–2138. doi:10.1002/cncr.20226
Puris, E., Fricker, G., and Gynther, M. (2023). The role of solute carrier transporters in efficient anticancer drug delivery and therapy. Pharmaceutics 15 (2), 364. doi:10.3390/pharmaceutics15020364
Rengelshausen, J., Göggelmann, C., Burhenne, J., Riedel, K. D., Ludwig, J., Weiss, J., et al. (2003). Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction. Br. J. Clin. Pharmacol. 56 (1), 32–38. doi:10.1046/j.1365-2125.2003.01824.x
Reustle, A., Fisel, P., Renner, O., Büttner, F., Winter, S., Rausch, S., et al. (2018). Characterization of the breast cancer resistance protein (BCRP/ABCG2) in clear cell renal cell carcinoma. Int. J. cancer 143 (12), 3181–3193. doi:10.1002/ijc.31741
Reznicek, J., Ceckova, M., Cerveny, L., Müller, F., and Staud, F. (2017). Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica; fate foreign Compd. Biol. Syst. 47 (1), 77–85. doi:10.3109/00498254.2016.1158886
Rhodes, D. R., Yu, J., Shanker, K., Deshpande, N., Varambally, R., Ghosh, D., et al. (2004). ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia (New York, NY) 6 (1), 1–6. doi:10.1016/s1476-5586(04)80047-2
Rose, T. L., and Kim, W. Y. (2024). Renal cell carcinoma: a review. Jama 332, 1001–1010. doi:10.1001/jama.2024.12848
Rosenthal, S. B., Bush, K. T., and Nigam, S. K. (2019). A network of SLC and ABC transporter and DME genes involved in remote sensing and signaling in the gut-liver-kidney Axis. Sci. Rep. 9 (1), 11879. doi:10.1038/s41598-019-47798-x
Safar, Z., Kis, E., Erdo, F., Zolnerciks, J. K., and Krajcsi, P. (2019). ABCG2/BCRP: variants, transporter interaction profile of substrates and inhibitors. Expert Opin. drug metabolism and Toxicol. 15 (4), 313–328. doi:10.1080/17425255.2019.1591373
Sala-Rabanal, M., Loo, D. D., Hirayama, B. A., and Wright, E. M. (2008). Molecular mechanism of dipeptide and drug transport by the human renal H+/oligopeptide cotransporter hPEPT2. Am. J. physiology Ren. physiology 294 (6), F1422–F1432. doi:10.1152/ajprenal.00030.2008
Saleeb, R. M., Farag, M., Lichner, Z., Brimo, F., Bartlett, J., Bjarnason, G., et al. (2018). Modulating ATP binding cassette transporters in papillary renal cell carcinoma type 2 enhances its response to targeted molecular therapy. Mol. Oncol. 12 (10), 1673–1688. doi:10.1002/1878-0261.12346
Sato, H., Siddig, S., Uzu, M., Suzuki, S., Nomura, Y., Kashiba, T., et al. (2015). Elacridar enhances the cytotoxic effects of sunitinib and prevents multidrug resistance in renal carcinoma cells. Eur. J. Pharmacol. 746, 258–266. doi:10.1016/j.ejphar.2014.11.021
Sato, T., Maekawa, M., Mano, N., Abe, T., and Yamaguchi, H. (2021). Role of OATP4C1 in renal handling of remdesivir and its nucleoside analog GS-441524: the first approved drug for patients with COVID-19. J. Pharm. and Pharm. Sci. a Publ. Can. Soc. Pharm. Sci. Soc. Can. des Sci. Pharm. 24, 227–236. doi:10.18433/jpps31813
Sato, T., Masuda, S., Yonezawa, A., Tanihara, Y., Katsura, T., and Inui, K. I. (2008). Transcellular transport of organic cations in double-transfected MDCK cells expressing human organic cation transporters hOCT1/hMATE1 and hOCT2/hMATE1. Biochem. Pharmacol. 76 (7), 894–903. doi:10.1016/j.bcp.2008.07.005
Sato, T., Mishima, E., Mano, N., Abe, T., and Yamaguchi, H. (2017). Potential drug interactions mediated by renal organic anion transporter OATP4C1. J. Pharmacol. Exp. Ther. 362 (2), 271–277. doi:10.1124/jpet.117.241703
Schaub, T. P., Kartenbeck, J., könig, J., Spring, H., Dörsam, J., Staehler, G., et al. (1999). Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J. Am. Soc. Nephrol. JASN 10 (6), 1159–1169. doi:10.1681/ASN.V1061159
Shen, D. W., Pouliot, L. M., Hall, M. D., and Gottesman, M. M. (2012). Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 64 (3), 706–721. doi:10.1124/pr.111.005637
Shibayama, Y., Nakano, K., Maeda, H., Taguchi, M., Ikeda, R., Sugawara, M., et al. (2011). Multidrug resistance protein 2 implicates anticancer drug-resistance to sorafenib. Biol. and Pharm. Bull. 34 (3), 433–435. doi:10.1248/bpb.34.433
Shin, H. J., Takeda, M., Enomoto, A., Fujimura, M., Miyazaki, H., Anzai, N., et al. (2011). Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrol. Carlt. Vic. 16 (2), 156–162. doi:10.1111/j.1440-1797.2010.01368.x
Shnitsar, V., Eckardt, R., Gupta, S., Grottker, J., Müller, G. A., Koepsell, H., et al. (2009). Expression of human organic cation transporter 3 in kidney carcinoma cell lines increases chemosensitivity to melphalan, irinotecan, and vincristine. Cancer Res. 69 (4), 1494–1501. doi:10.1158/0008-5472.CAN-08-2483
Smeets, N. J. L., Litjens, C. H. C., VAN Den Heuvel, J., van Hove, H., van den Broek, P., Russel, F. G. M., et al. (2020). Completing the enalaprilat excretion pathway-renal handling by the proximal tubule. Pharmaceutics 12 (10), 935. doi:10.3390/pharmaceutics12100935
Sun, D., Liu, J., Wang, Y., and Dong, J. (2022). Co-administration of MDR1 and BCRP or EGFR/PI3K inhibitors overcomes lenvatinib resistance in hepatocellular carcinoma. Front. Oncol. 12, 944537. doi:10.3389/fonc.2022.944537
Szeto, G. L., and Finley, S. D. (2019). Integrative approaches to cancer immunotherapy. Trends cancer 5 (7), 400–410. doi:10.1016/j.trecan.2019.05.010
Tahara, H., Kusuhara, H., Endou, H., Koepsell, H., Imaoka, T., Fuse, E., et al. (2005). A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 315 (1), 337–345. doi:10.1124/jpet.105.088104
Tatsumi, S., Matsuoka, H., Hashimoto, Y., Hatta, K., Maeda, K., and Kamoshida, S. (2014). Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int. J. Clin. Exp. Pathol. 7 (1), 204–212.
Theodoulou, F. L., and Kerr, I. D. (2015). ABC transporter research: going strong 40 years on. Biochem. Soc. Trans. 43 (5), 1033–1040. doi:10.1042/BST20150139
Tomita, Y., Katsura, T., Okano, T., Inui, K., and Hori, R. (1990). Transport mechanisms of bestatin in rabbit intestinal brush-border membranes: role of H+/dipeptide cotransport system. J. Pharmacol. Exp. Ther. 252 (2), 859–862.
Uddin, M. E., Eisenmann, E. D., Li, Y., Huang, K. M., Garrison, D. A., Talebi, Z., et al. (2022). MATE1 deficiency exacerbates dofetilide-induced proarrhythmia. Int. J. Mol. Sci. 23 (15), 8607. doi:10.3390/ijms23158607
Uribe, M. L., Marrocco, I., and Yarden, Y. (2021). EGFR in cancer: signaling mechanisms, drugs, and acquired resistance. Cancers 13 (11), 2748. doi:10.3390/cancers13112748
Vanwert, A. L., Gionfriddo, M. R., and Sweet, D. H. (2010). Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm. and drug Dispos. 31 (1), 1–71. doi:10.1002/bdd.693
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575 (7782), 299–309. doi:10.1038/s41586-019-1730-1
Vasudev, N. S., Wilson, M., Stewart, G. D., Adeyoju, A., Cartledge, J., Kimuli, M., et al. (2020). Challenges of early renal cancer detection: symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ open 10 (5), e035938. doi:10.1136/bmjopen-2019-035938
Veiga-Matos, J., RemiãO, F., and Motales, A. (2020). Pharmacokinetics and toxicokinetics roles of membrane transporters at kidney level. J. Pharm. and Pharm. Sci. a Publ. Can. Soc. Pharm. Sci. Soc. Can. des Sci. Pharm. 23, 333–356. doi:10.18433/jpps30865
Visentin, M., Torozi, A., Gai, Z., Häusler, S., Li, C., Hiller, C., et al. (2018). Fluorocholine transport mediated by the organic cation transporter 2 (OCT2, SLC22A2): implication for imaging of kidney tumors. Drug metabolism Dispos. Biol. fate Chem. 46 (8), 1129–1136. doi:10.1124/dmd.118.081091
Visentin, M., VAN Rosmalen, B. V., Hiller, C., Bieze, M., Hofstetter, L., Verheij, J., et al. (2017). Impact of organic cation transporters (OCT-SLC22A) on differential diagnosis of intrahepatic lesions. Drug metabolism Dispos. Biol. fate Chem. 45 (2), 166–173. doi:10.1124/dmd.116.072371
Waghray, D., and Zhang, Q. (2018). Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment. J. Med. Chem. 61 (12), 5108–5121. doi:10.1021/acs.jmedchem.7b01457
Waissbluth, S., Martínez, A. D., Figueroa-Cares, C., Sánchez, H. A., and Maass, J. C. (2023). MATE1 expression in the cochlea and its potential involvement in cisplatin cellular uptake and ototoxicity. Acta oto-laryngologica 143 (3), 242–249. doi:10.1080/00016489.2023.2184864
Walsh, N., Larkin, A., Kennedy, S., Connolly, L., Ballot, J., Ooi, W., et al. (2009). Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 9, 6. doi:10.1186/1471-2490-9-6
Wan, Z., Wang, Y., Li, C., and Zheng, D. (2023). SLC14A1 is a new biomarker in renal cancer. Clin. and Transl. Oncol. 25 (8), 2607–2623. doi:10.1007/s12094-023-03140-6
Warta, R., Theile, D., Mogler, C., Herpel, E., Grabe, N., Lahrmann, B., et al. (2014). Association of drug transporter expression with mortality and progression-free survival in stage IV head and neck squamous cell carcinoma. PloS one 9 (9), e108908. doi:10.1371/journal.pone.0108908
Whisenant, T. C., and Nigam, S. K. (2022). Organic anion transporters (OAT) and other SLC22 transporters in progression of renal cell carcinoma. Cancers 14 (19), 4772. doi:10.3390/cancers14194772
Winter, S., Fisel, P., Buttner, F., Rausch, S., D'Amico, D., Hennenlotter, J., et al. (2016). Methylomes of renal cell lines and tumors or metastases differ significantly with impact on pharmacogenes. Sci. Rep. 6, 29930. doi:10.1038/srep29930
Wu, C. P., Hsiao, S. H., and Wu, Y. S. (2023). Perspectives on drug repurposing to overcome cancer multidrug resistance mediated by ABCB1 and ABCG2. Drug Resist. Updat. Rev. Comment. Antimicrob. anticancer Chemother. 71, 101011. doi:10.1016/j.drup.2023.101011
Xiao, G., Rowbottom, C., Boiselle, C., and Gan, L. S. (2018). Fampridine is a substrate and inhibitor of human OCT2, but not of human MATE1, or MATE2K. Pharm. Res. 35 (8), 159. doi:10.1007/s11095-018-2445-y
Xu, L., Shi, Y., Zhuang, S., and Liu, N. (2017). Recent advances on uric acid transporters. Oncotarget 8 (59), 100852–100862. doi:10.18632/oncotarget.20135
Yamaguchi, H., Sugie, M., Okada, M., Mikkaichi, T., Toyohara, T., Abe, T., et al. (2010). Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug metabolism Pharmacokinet. 25 (3), 314–317. doi:10.2133/dmpk.25.314
Yamashita, F., Ohtani, H., Koyabu, N., Ushigome, F., Satoh, H., Murakami, H., et al. (2006). Inhibitory effects of angiotensin II receptor antagonists and leukotriene receptor antagonists on the transport of human organic anion transporter 4. J. Pharm. Pharmacol. 58 (11), 1499–1505. doi:10.1211/jpp.58.11.0011
Yanase, K., Tsukahara, S., Asada, S., Ishikawa, E., Imai, Y., and Sugimoto, Y. (2004). Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Mol. cancer Ther. 3 (9), 1119–1125. doi:10.1158/1535-7163.1119.3.9
Yang, X., and Han, L. (2019). Roles of renal drug transporter in drug disposition and renal toxicity. Adv. Exp. Med. Biol. 1141, 341–360. doi:10.1007/978-981-13-7647-4_7
Yang, Z., Xiaohua, W., Lei, J., Ruoyun, T., Mingxia, X., Weichun, H., et al. (2010). Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am. J. physiology Ren. physiology 299 (2), F336–F346. doi:10.1152/ajprenal.00053.2010
Yan, L., Ding, B., Liu, H., Zhang, Y., Zeng, J., Hu, J., et al. (2019). Inhibition of SMYD2 suppresses tumor progression by down-regulating microRNA-125b and attenuates multi-drug resistance in renal cell carcinoma. Theranostics 9 (26), 8377–8391. doi:10.7150/thno.37628
Yin, J., Chen, Z., You, N., Li, F., Zhang, H., Xue, J., et al. (2024). VARIDT 3.0: the phenotypic and regulatory variability of drug transporter. Nucleic acids Res. 52 (D1), D1490–D1502. doi:10.1093/nar/gkad818
Zhang, H., Zhang, Y. K., Wang, Y. J., Kathawala, R. J., Patel, A., Zhu, H., et al. (2014). WHI-P154 enhances the chemotherapeutic effect of anticancer agents in ABCG2-overexpressing cells. Cancer Sci. 105 (8), 1071–1078. doi:10.1111/cas.12462
Keywords: renal cell carcinoma, renal tubular epithelial cell, drug transporters, therapeutic effect, adverse reaction
Citation: Zuo Y, Li T, Yang S, Chen X, Tao X, Dong D, Liu F and Zhu Y (2025) Contribution and expression of renal drug transporters in renal cell carcinoma. Front. Pharmacol. 15:1466877. doi: 10.3389/fphar.2024.1466877
Received: 18 July 2024; Accepted: 23 December 2024;
Published: 17 February 2025.
Edited by:
Cyprian Onyeji, University of Nigeria, Nsukka, NigeriaReviewed by:
Xinning Yang, United States Food and Drug Administration, United StatesCopyright © 2025 Zuo, Li, Yang, Chen, Tao, Dong, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanna Zhu, emh1eWFubmEuMTk4NkAxNjMuY29t; Fang Liu, bGl1ZmFuZ19keUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.