- 1Department of Burn Surgery, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang, China
- 2Postgraduate Department, First Affiliated Hospital of Gannan Medical College, Ganzhou, China
- 3Department of Orthopedics, Taizhou Municipal Hospital, Taizhou, Zhejiang, China
Keloid scars (keloids), a prototypical form of aberrant scar tissue formation, continue to pose a significant therapeutic challenge within dermatology and plastic surgery due to suboptimal treatment outcomes. Gelatinases are a subgroup of matrix metalloproteinases (MMPs), a family of enzymes that play an important role in the degradation and remodeling of the ECM (a pivotal factor for keloids development). Gelatinases include gelatinase A (MMP-2) and gelatinase B (MMP-9). Since accumulating evidence has shown that gelatinases played a crucial role in the process of keloid formation, we summarized the current knowledge on the association between MMP-2 and MMP-9 expression and the pathological process of keloids through a comprehensive review. This review demonstrated that the interplay between MMP-2, MMP-9, and their regulators, such as TGF-β1/Smad, PI3K/AKT, and LncRNA-ZNF252P-AS1/miR-15b-5p/BTF3 signaling cascades, involved in the intricate balance governing ECM homeostasis, collectively driving the excessive collagen deposition and altered tissue architecture observed in keloids. In summary, this review consolidates the current understanding of MMP-2 and MMP-9 in keloid pathogenesis, shedding light on their intricate involvement in the dysregulated keloids processes. The potential for targeted therapeutic interventions presents promising opportunities for advancing keloid management strategies.
1 Introduction
Keloid scars (keloids), a type of abnormal scar tissue formation, remain a therapeutic challenge in the field of dermatology and plastic surgery. The characteristic of keloids is the dysregulated fibroproliferation, excessive production of extracellular matrix (ECM), and extension beyond the initial wound (Khattab et al., 2022). Patients suffering from keloids often feel pruritus and pain, which cause immense physical and mental problems and profoundly impair the quality of life (Tian et al., 2023). Epidemiological studies have demonstrated a higher prevalence of keloids among females. Furthermore, individuals of African and Asian descent, particularly those with darker skin complexions, exhibit a greater incidence of keloid formation. The estimated prevalence of excessive scarring was 2.4%, 1.1% and 0.4% in Black, Asians and Caucasians, respectively (Lee et al., 2023). Therefore, an in-depth study on keloids is of profound significance. Currently, the pathogenesis of keloids remains unclear. Although there are various clinical treatment methods available, none of them can fundamentally cure keloids. In addition, keloids are highly susceptible to recurrence, and the keloids continue to grow and invade surrounding normal tissues.
The pathogenesis of keloids is complex, which is a confluence of multiple contributing factors. A lack of animal models has limited investigational studies into exact pathological mechanism of keloid formation (Ekstein et al., 2021). Scar formation and tissue regeneration are essential processes of organism repair injury (Wei et al., 2020). The wound healing processes leading to tissue repair and regeneration are generally divided into four phases: hemostasis, inflammation, proliferation, and remodeling (Sathiyaseelan et al., 2023). The recruitment of inflammatory cells and fibroblasts contribute to scar remodeling in the early phase of wound healing (Perez et al., 2017). Specifically, fibroblasts create a collagen-containing ECM that is balance of synthesis and degradation (Li et al., 2023; Van Haaften et al., 2020). Therefore, an imbalance between collagen production and ECM degradation contributes to scar formation. Many researchers have found that the excessive ECM production is closely associated with a decrease or increase of matrix metalloproteinases (MMPs), especially gelatinases (Panichakul et al., 2022; Seyed et al., 2023).
MMPs are a family of zinc-dependent endopeptidases, and they share a common structural motif, known as the catalytic domain (Anchan et al., 2022). This domain contains a catalytic zinc ion required for their enzymatic activity (Bonvicini et al., 2014). MMPs are divided into different subgroups based on their substrate specificity and domain structure. Some common subgroups include collagenases, gelatinases, stromelysins, and membrane-type MMPs (Li et al., 2022). MMPs play a crucial role in the degradation and remodeling of the ECM. The ECM is a complex network of proteins and carbohydrates that provides structural and biochemical support to cells (Caon et al., 2020). MMPs are responsible for breaking down various components of the ECM, allowing for tissue remodeling, wound healing, and other physiological processes (Kim et al., 2022; Zhang et al., 2023). Recently, MMPs have been shown to participated in the pathogenesis of many diseases, including keloids, idiopathic pulmonary fibrosis and various tumors (Chen et al., 2022; de Almeida et al., 2022; Herzog et al., 2019). Meanwhile, MMPs have been proposed as appropriate therapeutic targets for many diseases (Craig et al., 2015; Liu et al., 2023). Luo et al. (2023) reported that oleanolic acid significantly suppressed keloid fibroblast proliferation and reduced ECM deposition by increasing the level of MMP-1, suggesting that oleanolic acid might be a potent drug for treatment of keloids. Similarly, Jeon et al. (2016) also found that hepatocyte growth factor can be used to treat keloids by increasing MMP-1 expression. Further study showed that rats were treated with MMP-1 by intraperitoneal injection significantly reduced scar formation (Keskin et al., 2021). Some studies showed that gelatinases were also involved in the progression of keloids. In recent years, the role of gelatinases in keloids have received increasing attention of researchers. In this review, we mainly summarize the current knowledge about gelatinases in the progress of keloids.
2 The overview of gelatinases
Gelatinases are a subgroup of MMPs, a family of enzymes that play an important role in the degradation and remodeling of the ECM (Kalev-Altman et al., 2022). Gelatinases specifically degrade both gelatins and collagens, and they are involved in various physiological and pathological processes (Sukhonthasilakun et al., 2023). Gelatinases include gelatinase A (MMP-2) and gelatinase B (MMP-9). MMP-2 is a key enzyme involved in the degradation of gelatin, collagen, and other ECM components (Ekstein et al., 2021). MMP-2 is produced in a latent form and needs to be activated to perform its enzymatic function (Ekstein et al., 2021). MMP-9 is another enzyme that specifically targets gelatin and other ECM proteins (Hur et al., 2022). Like MMP-2, MMP-9 is secreted in an inactive form (Hur et al., 2022). The activation of MMP-2 and MMP-9 typically involves the removal of a propeptide domain (Jang et al., 2022). Several factors, including tissue inhibitors of metalloproteinases (TIMPs) and other proteases, are involved in this activation process (Eiro et al., 2023). TIMPs are a specific endogenous inhibitor and block access to ECM substrates by binding to the active site of MMPs (Robert et al., 2016). As a receptor of MMPs, TIMP-2 connects MMPs with membrane-type matrix metalloproteinase-1 (MT1-MMP) (Sato and Takino, 2010). TIMP-2 can promote the activation of MMPs proenzyme when MT1-MMP is removed from the binding of TIMP-2 (Sato and Takino, 2010). Gelatinases play essential roles in tissue remodeling, wound healing, and organ homeostasis by effecting angiogenesis, tissue repair, and cell migration (Aksoy et al., 2019; Ravanti and Kahari, 2000). Dysregulation of gelatinases is associated with various diseases, including keloids (Hao et al., 2018; Ulrich et al., 2010). For example, overactivity of gelatinases has been linked to excessive tissue degradation in diseases such as cancer metastasis, arthritis, and tissue fibrosis (Orsolic et al., 2020). On the other hand, insufficient gelatinase activity can lead to abnormal tissue repair and chronic inflammation (Brilha et al., 2018).
The regulation mechanism of gelatinases involves a complex interplay of various factors. The expression of gelatinases, like other MMPs, is modulated by transcriptional and posttranscriptional regulation. It was reported that inflammatory signals could increase MMP-2 and MMP-9 gene expression by activating transcription factors like AP-1, NF-κB, and SP-1 (Aljada et al., 2001; Shen et al., 2022; Yang et al., 2022). Fan et al. (2022) found that Kangfuxiaoyanshuan, a Traditional Chinese Medicine formulation, alleviated inflammation by inhibiting the NF-κB activation through decreasing phosphorylation of p65, resulting in reduced expression of TGF-β and MMP-2. In addition, growth factors and cytokines, such as TGF-β, EGF, and TNF-α, were also reported to modulate the expression and activity of gelatinases (Kondapaka et al., 1997; Rajashekhar et al., 2014; Tian et al., 2007). They can stimulate or inhibit MMP-2 and MMP-9 production through various signaling pathways, depending on the context and cell type (Kondapaka et al., 1997; Rajashekhar et al., 2014; Tian et al., 2007). For example, TNF-α has been proven to play an essential role in herpes simplex keratitis by stimulating MMP-2 and MMP-9 activities through the activation of FAK/ERK signaling in human corneal epithelial cells (Yang et al., 2012). It has also been shown that epigenetic modifications, such as DNA methylation and histone acetylation, affect gelatinase expression by influencing the accessibility of the MMPs gene promoter regions to transcription factors (Duraisamy et al., 2017; Liang et al., 2022). Studies showed that MMP-2 and MMP-9 can influence the overall MMPs activity by cleaving and activating latent forms of other MMPs, which indicated that gelatinases themselves, along with other MMPs, can participate in feedback loops (Kim et al., 2014).
Numerous studies have demonstrated that MMP-2 and MMP-9 are critical in cell proliferation, differentiation, apoptosis and angiogenesis, and are extensively implicated in the pathogenesis of various diseases, including neurological diseases, diverse tumors, and inflammatory conditions. Studies suggested that level of MMP-2 and MMP-9 significantly increases in the brain after stroke (Kumar and Patnaik, 2018). However, inhibition of MMP-2 and MMP-9 confers neuroprotection in stroke (Kumar and Patnaik, 2018). Lambert et al. (2003) reported that the expression of MMP-2 and MMP-9 were increased in human choroidal neovascularization occurring during the exudative most aggressive form of age-related macular degeneration. Additionally, inhibition of MMP-2 and MMP-9 could reduce angiogenesis (Lambert et al., 2003). Further, Hwang et al. (2021) demonstrated that salinomycin suppressed TGF-β1-induced EMT by inhibiting MMP-2 and MMP-9 via AMPK/SIRT pathway, thereby inhibiting the cell migration and invasion of lung cancer. Consequently, MMP-2 and MMP-9 may influence the progression of keloids through multiple signaling pathways, either promoting or inhibiting their development. Figure 1 shows the interactions between MMP-2 and MMP-9 and their targeted gene/proteins and signal pathways.
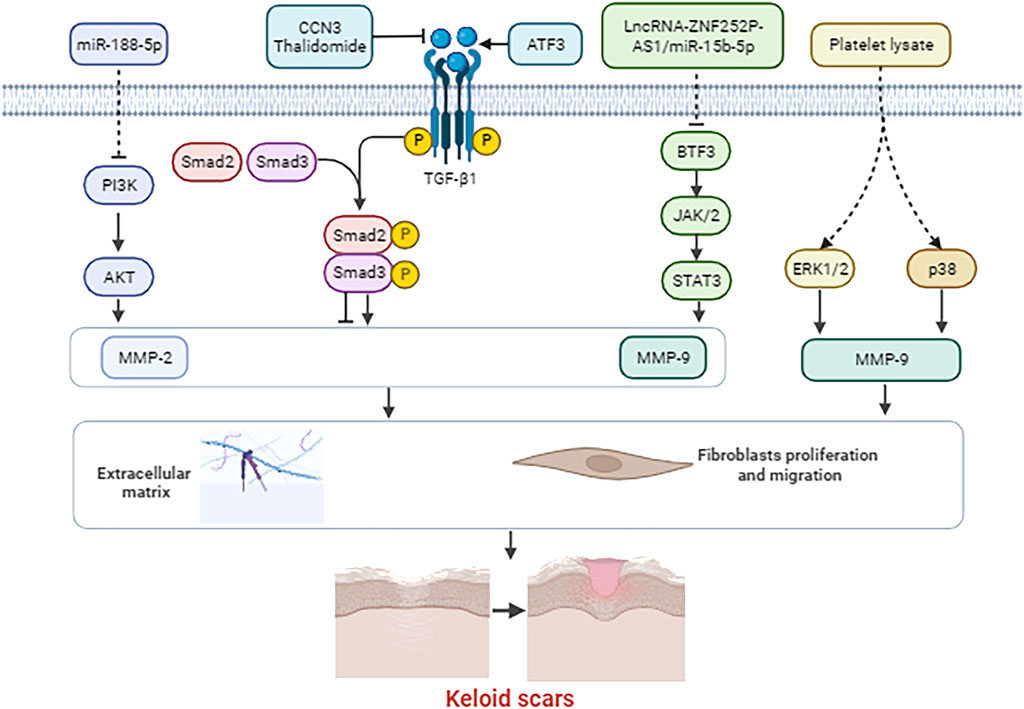
Figure 1. The interactions between MMP-2 and MMP-9 and their targeted gene/proteins and signal pathways.
3 The roles of gelatinases in the progress of keloids
3.1 Regulation of extracellular matrix (ECM) and collagen
3.1.1 Positive association between TGF-β1 and MMP-2/MMP-9
The ECM plays an important role in the development of keloids for its functions of structural support, cell adhesion, regulation of cell signaling, mechanical stress response, and impaired remodeling (Kim and Kim, 2024). Collagen plays multiple crucial roles in the ECM through tensile strength, shape and integrity maintenance, and regulation of cell adhesion and cellular signaling. Currently, mounting studies have identified the potential effects of MMP-2 and MMP-9 and their targeted proteins/signal pathways on the interactions of ECM or collagen molecules. TGF-β1 plays a significant role in the development and progression of keloids. TGF-β1 is a cytokine that is involved in various cellular processes, including tissue repair and fibrosis. TGF-β1 is a potent stimulator of fibroblast proliferation and collagen production. As we all known, the overactivation of fibroblasts can lead to excessive collagen deposition. TGF-β1 has been proved to a key factor in promoting this abnormal fibroblast activity. Additionally, TGF-β1 induces the synthesis of collagen, particularly type I and type III collagen, which are major components of the ECM. Studies showed that TGF-β1 is involved in the remodeling of the ECM by upregulating the expression of MMPs and TIMPs. Zhang et al. (2017) demonstrated that activation of the TGF-β1 by either mechanical stress significantly attenuated fibroblasts cell proliferation and ECM components by increasing the MMP2/TIMP2 mRNA ratio. Increasing evidence suggests that CCN3 is a negative regulator of the ECM (Ren et al., 2014; Yin et al., 2023). Liu et al. (2018) reported that TGF-β1 significantly decreased the expression of MMP-2 and MMP-9, and increased the expression of TIMP-1 in human mesangial cells. Furthermore, TGF-β1 significantly increased the accumulation of ECM (Liu et al., 2018). Importantly, overexpression of CCN3 attenuated TGF-β1-induced changes in MMP-2, MMP-9 and TIMP-1 (Liu et al., 2018). These results indicated that CCN3 inhibits accumulation of ECM by regulating the expression of MMP-2, MMP-9 and TIMP-1 via the regulation of TGF-β1. A recent study reported that TGF-β1 inhibitor significantly inhibited the development of cardiac fibrosis in mutant mice by blocking the expression of SMAD proteins, MMP-2 and MMP-9 (Subramanian et al., 2022). Sadick et al. (2008) demonstrated an increased expression of TGF-β1 and MMP-2 and MMP-9 in tissue samples from keloids. Further study found that antisense TGF-β1 oligonucleotide treatment significantly decreased MMP-9 secretion, but had no effect on MMP-2 in vitro (Sadick et al., 2008). Activating transcription factor 3 (ATF3) is the ATF/CREB family and plays critical roles in modulating cellular behaviors by activating or repressing downstream genes (Wang et al., 2022). Wang et al. (2021) demonstrated the expression of ATF3 was upregulated in human keloid tissues. ATF3 has also been showed to suppress apoptosis and promote invasion of keloid fibroblast cells (Wang et al., 2021). In addition, upregulation of ATF3 significantly elevated the level of TGF-β1 and the phosphorylation of Smad2 and Smad3, while inhibition of ATF3 decreased TGF-β1 level and the phosphorylation of Smad2 and Smad3 in keloid fibroblast cells (Wang et al., 2021). Meanwhile, the mRNA and protein levels of MMP2 and MMP9 were elevated in ATF3-overexpressing cells, and ATF3 knockdown significantly downregulated MMP2 and MMP9 expression (Wang et al., 2021). Consistently, Jiang et al. (2016) also reported that growth differentiation factor-9 (GDF-9), a member of the TGF-β family, promoted the proliferation and migration of keloid fibroblasts by upregulating MMP-2 and MMP-9 expression, and enhancing Smad2 and Smad3 phosphorylation. Also, other studies reach similar conclusions (Bran et al., 2010; Lee et al., 2013; Zhao et al., 2019). Study showed that TGF-β1 controlled cell migration and invasion by regulating MMP-2 and MMP-9 activities (Muscella et al., 2020). These findings suggest that the TGF-β1/Smad signaling pathway may facilitate keloid growth by upregulating the expression of MMP-2 and MMP-9.
3.1.2 Negative association between TGF-β1 and MMP-2/MMP-9
Conversely, the upregulation of MMP-2 and MMP-9 have also been reported to inhibit keloid formation. Silibinin, a natural polyphenolic flavonoid, has been reported to possess anti-inflammatory, antioxidant, antiapoptotic and anti-fibrotic properties (Tuli et al., 2021). Cho et al. (2013) demonstrated that silibinin induced the downregulation of type I collagen and inhibited the activation of Smad2/3. Meanwhile, silibinin significantly promoted the expression of MMP-2 (Cho et al., 2013). Therefore, silibinin may prevent fibrotic skin changes by downregulating type I collagen expression through the upregulation of MMP-2 and the inhibition of the Smad2/3 signaling pathway. Accumulating evidences have indicated that thalidomide, a-N-phthalimidoglutarimide, is important in fibrotic diseases, mainly due to its anti-fibrotic properties (Bajwah et al., 2013; Bose and Verstovsek, 2018). Liang et al. (2013) found that TGF-β1 could induce fibronectin expression in keloid fibroblasts and the effect was suppressed by pretreatment with thalidomide. In addition, pretreatment with thalidomide suppressed the TGF-β1-induced phosphorylation of Smad3 (Liang et al., 2013). Furthermore, thalidomide increased the activity of MMP-9, leading to fibronectin degradation (Liang et al., 2013). The findings are in consist with results obtained from results from Spiekman et al. (2014). These results indicated that thalidomide might inhibit keloids formation by upregulating MMP-9 expression through the inhibition of the TGF-β1/Smad3 signaling pathway.
In the early stage of skin wound healing, the expression of MMP-9 is important for the removal of ECM components from damaged tissues, which can help to create an environment conducive to cell migration and proliferation (Banerjee et al., 2024). TGF - β1 is also involved in the regulation of cell proliferation, differentiation, and migration during this time period. TGF - β1 can regulate the activity and expression of MMP-9 to a certain degree, so as to bring the remodeling of extracellular matrix into a dynamic balance, neither excessive degradation nor excessive deposition, and promote the normal repair of tissues (Li et al., 2022). TGF-β1 can regulate the activity and expression of MMP-9 to a certain extent, so that the remodeling of the ECM is in a state of dynamic equilibrium, neither over-degraded nor over-deposited, and the normal repair of tissues is promoted. MMP-2 is involved in the degradation of ECM, which is necessary for cell migration and tissue remodeling (D’Abadia et al., 2020). TGF-β1 can indirectly inhibit MMP-2 activity by upregulating the expression of TIMPs. A fine balance exists between TGF-β1 and MMP-2/MMP-9 to ensure that tissue repair occurs properly. Therefore, the relationship between them is not a simple positive or negative correlation, but a relationship of mutual cooperation and mutual constraint during keloid formation.
3.1.3 Roles of TIMPs in keloid formation
TIMPs are the endogenous inhibitors of MMPs. Downregulation of TIMPs in keloid fibroblasts is found to elevate degradation of the excessive collagen bundles in keloid ECM. Aoki et al. found that the expression of MMP-2 was increased in keloids expressing small interfering RNA of TIMP-1 or TIMP-2, regulating ECM degradation and remodeling through the Collagen types I and III (Aoki et al., 2014). Fujiwara et al. (2005) revealed that keloid-derived fibroblasts exhibited an increased secretion of factors associated with collagen turnover and relied on matrix metalloproteinase (i.e., MMP-1 and MMP-2) for migration. Monocyte chemoattractant protein - 1 (MCP - 1), which is a C–C chemokine, has been demonstrated to prompt the recruitment of monocytes to the injured tissue and to play a crucial role in wound healing. Yeh et al. (2009) showed that IL-1β could induce a significant increase in MCP-1 and MMP-2 production in keloid-derived fibroblasts, which contributed to an imbalance in ECM formation and excess deposition of collagen in keloid. Imaizumi et al. (2009) reported that MMP-2 activity cooperated with TIMP-2 and MT1-MMP might contribute to the remodeling of collagen bundle areas and the invasion of fibroblasts into the surrounding normal regions via the promoted degradation of the ECM. Hepatocyte growth factor (HGF) functions to suppresses collagen synthesis. Lee et al. (2011) indicated that the enzymatic activities of MMP-2 was positively associated with HGF protein in the pathologic keloids, which was mediated by the regulation of type I and III collagen. Therefore, one of the main molecular mechanisms underlying the effects of MMP-2 and MMP-9 might attributed to their regulation on the ECM and collagens.
3.2 miR-188-5p inhibits keloids formation by suppressing MMP-2 and MMP-9 through inhibition of PI3K/AKT signaling pathway
Previous studies have shown that miRNAs may be involved in the development of keloids (Yu et al., 2015). Recent data have shown that miR-188-5p plays a crucial role in keloid formation. Vascular endothelial growth factor (VEGF), a specific provascular endothelial growth factor, is involved in keloids formation by modulating angiogenesis (Song et al., 2018). Zhou et al. (2022) reported that the inhibition of miR-188-5p promoted the proliferation, migration and cell cycle process, and inhibited the apoptosis of keloid fibroblasts. Furthermore, miR-188-5p inhibitor positively regulate VEGFA expression (Zhou et al., 2022). In addition, downregulation of VEGFA also abolished the promotive effect of miR-188-5p inhibitor (Zhou et al., 2022). Therefore, miR-188-5p may inhibit keloids formation by repressing the expression of VEGFA. It was reported miR-188-5p promoted tumor growth of pediatric acute promyelocytic leukemia by activating the PI3K/AKT signaling pathway (Wang et al., 2020). Yao et al. (2019) demonstrated that luteolin, a naturally occurring flavonoid, induced the apoptosis and inhibited the proliferation of human melanoma cells by decreasing the expressions of MMP-2 and MMP-9 via the PI3K/AKT pathway. A recent study from Zhu et al. (2019) indicated that miR-188-5p was significantly downregulated in keloid tissue compared with normal skin tissues. Upregulated expression of miR-188-5p inhibited keloids fibroblasts proliferation, migration, and invasion (Zhu et al., 2019). Furthermore, miR-188-5p mimics repressed the expression levels of MMP- 2, MMP-9, PI3K, and p-AKT in keloids fibroblasts (Zhu et al., 2019). In contrast, miR-188-5p inhibitor significantly increased the expression of MMP-2, MMP-9, PI3K, and p-AKT. Importantly, PI3K/AKT inhibitor reversed the promotive effect of miR-188-5p on MMP-2 and MMP-9 in keloids fibroblasts (Zhu et al., 2019). These findings together demonstrated that miR-188-5p inhibited keloids formation by suppressing PI3K/AKT/MMP-2/9 signaling pathway.
3.3 Platelets (PL) may promote keloid formation by upregulating MMP-9 expression through the regulation of p38 and ERK1/2 pathway
As is well known, growth factors play essential roles in the tissue neoformation and healing process (Miricescu et al., 2021). Growth factors are involved in many of the processes to tissue repair, including angiogenesis and cell proliferation, while they also influence l the synthesis and degradation of ECM proteins (Nagarkoti et al., 2023; Pot et al., 2023). Platelets contain different growth factors and cytokines, contributing to the formation of clot at sites of vascular injury by preventing blood loss (Schmidt et al., 2019). In the past several decades, the research on the physiological characteristics of platelets gradually deepened in tissue injury, which made it possible to treat keloids with platelets. A recent meta-analysis showed that platelet-rich plasma has a 23% response rate in the management of scars, and it were 22% and 23% in patients with laser or micro-needling, respectively (Ebrahimi et al., 2022). This suggests that platelet-rich plasma seems to be a safe and effective treatment for keloids. Tsai et al. (2023) reported that type A platelet-derived growth factor (PDGF-AA), an important growth factors in regulating cell growth and function, inhibits Leydig cell growth, migration, and invasion by activating ERK. In addition, PDGF has also been shown to facilitate the invasion and metastasis of cholangiocarcinoma cells by upregulating the expression of MMP-2/MMP-9 and inducing epithelial-mesenchymal transition (EMT) through activating the p38/MAPK signaling pathway (Pan et al., 2020). Scopelliti et al. (2020) demonstrated that platelet lysate (PL) promoted wound healing by increasing fibroblast production of ECM components and keratinocyte migration. However, whether PL is involved in the development of keloids remains unknown. Ranzato et al. (2011) showed that PL upregulated the expression of MMP-9 rather than MMP-2 in human keratinocyte cell line. Furthermore, both inhibitor of ERK1⁄2 pathway and inhibitor of p38 significantly inhibited MMP-9 activity induced by PL (Ranzato et al., 2011). As is well known, collagen type I is a major component of ECM and skin connective tissue, while collagen type III is secreted in the granulation tissue that is formed during wound healing (Li et al., 2023). PL has been reported to increase the production of collagen type III, but has no effect on the production of collagen type I (Ranzato et al., 2011). Taken together, PL may promote keratinocyte epithelialization and enhancing fibroblast matrix deposition by upregulating MMP-9 expression through p38 and ERK1/2 pathway, leading to keloid formation. Platelets initiate a cascade of events that lead to fibroblast activation and ECM production, while MMP - 2 and MMP - 9 play a role in the abnormal ECM remodeling and cell-related processes that are characteristic of keloid formation. The interplay between these factors is complex. At present, however, the available relevant studies were limited, which needs further validation in future.
3.4 LncRNA-ZNF252P-AS1/miR-15b-5p/BTF3 promotes keloid progression by up-regulating MMP2 and MMP9 through inhibiting JAK2/STAT3 signaling pathway
Keloids is highly heterogeneous and its cells display Warburg metabolism (Sun, 2022). Warburg metabolism was firstly found in neoplastic cells by Dr. Otto H (Li et al., 2020). Warburg and this discovery led to the awarding of the Nobel Prize (Li et al., 2020). Recently, JAK/STAT signaling pathways has been reported to be an inducer of Warburg metabolism. Chen et al. (2023) reported that tofacitinib decreased the volume and dermis thickness of the keloid by inhibiting fibroblast proliferation and collagen I synthesis through the suppression of STAT3. In addition, IL-6 (interleukin-6) and sIL-6r (soluble IL-6 receptor) are involved in joint cartilage destruction by stimulating the production of MMPs via JAK/STAT signaling pathway in chondrocytes (Aida et al., 2012). Zhou et al. (2020) found that JAK/STAT signaling pathway inhibitor inhibited the invasion and progression of keloid fibroblasts by downregulating the expression of MMP-2 and upregulating the expression of TIMP-2. Mounting studies have shown that microRNAs play an important role in the mechanism of keloid formation. Kuai and Jian (2022) demonstrated that miR-23b-3p was upregulated in keloid fibroblasts. Further study found that inhibition of miR-23b-3p significantly inhibited keloids by facilitating A20 expression (Kuai and Jian, 2022). The basic transcription factor 3 (BTF3) has been reported to be closely associated with cell proliferation and apoptosis. Wu et al. (2020) showed that the BTF3 promoted the migratory and invasive abilities of cervical cancer cells via interaction with MMP-2 and MMP-9. Recently, the expression of lncRNA-ZNF252P-AS1, pJAK2, p-STAT3, BTF3 MMP-2 and MMP-9 were found to be upregulated, whereas miR-15b-5p expression is downregulated in keloid tissue and keloid fibroblasts (Guo et al., 2022). Furthermore, miR-15b-5p overexpression significantly downregulated proliferation and migration ability of KFs, while this phenomenon was reversed by BTF3 overexpression (Guo et al., 2022). In addition, miR-15b-5p overexpression downregulated MMP-2, MMP-9 and collagen I protein levels, while the overexpression of BTF3 upregulated these proteins levels (Guo et al., 2022). Luciferase reporting experiments confirmed that BFT3 was targeted by miR-15b-5p and negatively modulated in keloid fibroblasts (Guo et al., 2022). Moreover, BTF3 knockdown has been reported to inhibit the JAK/STAT3 signaling pathway (Guo et al., 2022). It was reported that lncRNA-ZNF252P-AS1 overexpression significantly downregulated miR-15b-5p level and upregulated BTF3 level (Guo et al., 2022). Furthermore, lncRNA-ZNF252P-AS1 overexpression significantly increased the proliferation and migration ability of keloid fibroblasts and upregulated MMP-2 and MMP-9 levels (Guo et al., 2022). Importantly, silencing lncRNA-ZNF252P-AS1 inhibited keloid progression and decreased p-JAK2 and p-STAT3 expression (Guo et al., 2022). These studies suggested that lncRNA-ZNF252P-AS1/miR-15b-5p/BTF3 might promote keloid progression by up-regulating MMP2, MMP9 and collagen I protein levels through inhibiting JAK2/STAT3 signaling pathway. Therefore, inactivation of lncRNA-ZNF252P-AS1 may be a potential therapeutic target for keloid.
Figure 2 shows the underlying molecular mechanisms of MMP-2 and MMP-9 and their associated genes and signal cascades in the pathological process of keloids.
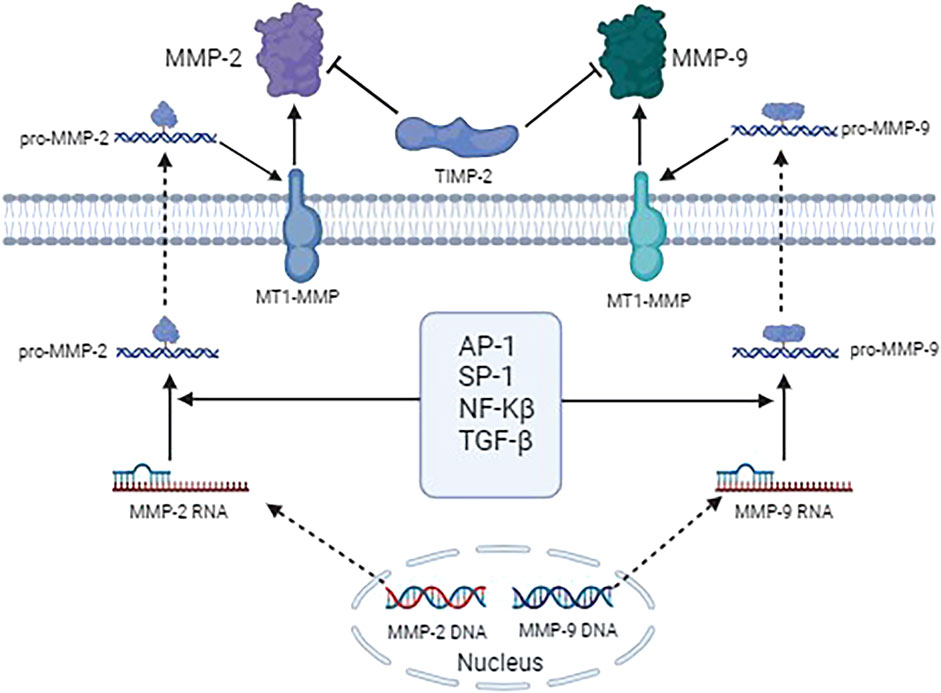
Figure 2. Molecular mechanisms of MMP-2 and MMP-9 and their associated genes and signal cascades in the pathological process of keloids.
3.5 Roles of MMP-2 and MMP-9 in some treatment modalities on keloids
Since both MMP-2 and MMP-9 have been found to be involved in the pathogenesis of keloids development, several potential drugs or substances that targeted the two genes are found to be effective on the treatments or preventions of keloids. Intralesional steroid injection (i.e., triamcinolone) is a widely used treatment for keloids. Besides, 5-fluorouracil (5-FU) has also been found to be one of the promising drugs for treating keloids. Huang et al. (2013) reported that a combination of triamcinolone and 5-FU could improve the scar regression and declined the recurrence of keloids by modulating keloid fibroblasts through the regulation of MMP-2 expression. Starfish hatching enzyme was reported to have diverse functions, including hydrolyze type I collagen. Li et al. (2014) demonstrated that the starfish hatching enzyme treatment could improve the scar and keloid by decreasing the proliferation of fibroblasts. Mechanistically, the starfish hatching enzyme exerted the anti-keloid effects by regulating the fibroblast-populated collagen gel conditions via the interaction of MMP-2 and MMP-9 and the inflammatory genes. Cryotherapy is also one of the promising therapeutic methods to treat keloid scars. Based on the observations of that CD163+ M2 macrophages and MMP-9 were dramatically elevated in cryotherapy-treated tissue, Lee et al. (2020) concluded that cryotherapy improved keloids by recruiting tissue re-modeling M2 macrophages with accompanying MMP-9. Dispel-Scar Ointment (DSO), a common-used in the traditional Chinese medicine, has been found to effectively treat keloids. Li et al. (2024) explored the molecular mechanisms underlying the influence of DSO on keloid by performing a network pharmacology, molecular docking analysis, and experiment validations. They found that MMP2-flavoxanthin, MMP9-luteolin, and MMP-9-kaempferol bound best to DSO, which might be associated with the reduction of TGF-β1, pSMAD2, and CoL1a1 expression. Tranilast, an anti-allergic agent, has been found to inhibit keloid and hypertrophic scar formation. Shimizu et al. (2006) implied that tranilast could suppress the formation of keloid scarring by inhibiting the expression of MMPs (i.e., MMP-7, MMP-8, and MMP-9) and TIMP (i.e., TIMP-1) in neutrophils. Clinical data showed that botulinum toxin type A (BTXA) could inhibit the development of hypertrophic scarring, while the potential mechanisms were unclear. Hao et al. (2018) found that BTXA promoted the healing of scars by suppressing the proliferation of keloid fibroblasts as well as regulating the expression of TGF-β1 and MMP-2. These studies demonstrated that the therapeutic effect of existing therapies for keloids might be partially attributed to the regulation of both MMP-2 and MMP-9. All the drugs and substances mentioned above were marketing approval. The researchers found that these drugs can alleviate keloids formation by elaborately regulating MMP-2 and MMP-9 expressions. However, the inhibitors of MMP-2 and MMP-9 for treating keloids are not yet available in human trials. Nevertheless, the present study revealed that developing more efficient drug delivery systems on MMP-2 and MMP-9 may be one of the successful treatments for managing keloids. Liposomes or polymeric nanoparticles can be designed to encapsulate anti-MMP2/9 drugs and deliver them directly to the fibroblasts or ECM in the keloid tissue. On the other hand, by analyzing the expression patterns of MMP-2, MMP-9 in a patient’s keloid tissue, it may be possible to predict the response to different pharmacological interventions and tailor the treatment accordingly.
4 Clinical research
In addition to the above experimental research, the roles of MMP-2 and MMP-9 have also been explored in clinical tissue specimens of keloids. A previous pilot study demonstrated that the medians levels of both MMP-2 and MMP-9 were increased in the hypertrophic scar and keloid groups as compared to the donor skin (Tanriverdi-Akhisaroglu et al., 2009). Paltatzidou et al. (2017) assessed the expression of MMP-9 in the lesional skin biopsies taken from patients who received 5-fluorouracil treatment with skin keloids. They found that MMP - 9 was strongly expressed in the multinuclear giant cells of keloid biopsies, while it was significantly decreased after adding cryotherapy (P < 0.05). The above results revealed that both MMP-2 and MMP-9 was validation by the clinical settings, which made it possible to achieve clinical transformation by targeting the two genes. Up to date, only two eligible studies belonging to clinical researches implied the roles of MMP-2/MMP-9 on keloid formation. Therefore, more future clinical studies are still warranted to better evaluate the association between MMP-2/MMP-9 and keloid formation.
5 Limitations
It is important to acknowledge certain limitations in the current body of literature, including variations in study methodologies and potential gaps in our understanding of the intricate signaling networks involved. Based on the above findings, MMP-9 expression may have contrasting effects on keloid formation. According to the experimental and clinical data, MMP-9 expression levels are usually elevated in keloid tissue. High level of MMP-9 is found to degrade collagen, elastin, and other components of the extracellular matrix and promotes the migration and proliferation of fibroblasts, which leads to the continuous expansion of keloid tissue. The expression levels of some inflammatory cytokines and growth factors are also often elevated in keloid tissues, and these factors may stimulate fibroblasts to secrete MMP-9, further exacerbating keloid fibrosis. Therefore, downregulating MMP-9 expression may significantly inhibit keloid development. For example, Zhou et al. (2022) found that miR-188-5p inhibited keloids formation by suppressing MMP-9 expression. However, a previous study conducted by Liang et al. (2013) demonstrated that thalidomide might inhibit keloids formation by upregulating MMP-9 expression. The inconsistent results from different studies might be attributed to different stages and progress of the disease, or the complexity of regulatory networks. Keloids formation is usually a dynamic process and different stages may involve different pathophysiologic mechanisms. In addition, MMP-9 may be regulated by multiple transcription factors, and different transcription factors may have different activities at different stages of keloids formation, causing the MMP-9 expression to exhibit opposite effects in this disease. As a result, more studies are still warranted to confirm the exact role of MMP-9 in the development and progression of keloid formation.
6 Conclusion
This comprehensive review extensively illustrates intricate roles of MMP-2 and MMP-9 in the regulation of keloids. The reviewed studies demonstrate elevated expression levels of MMP-2 and MMP-9 in keloid tissue compared to normal skin, suggesting their pivotal role in driving the excessive collagen deposition and altered tissue architecture observed in keloids. Furthermore, the interplay between MMP-2, MMP-9, and their regulators, such as TGF-β1/Smad, PI3K/AKT and LncRNA-ZNF252P-AS1/miR-15b-5p/BTF3 signaling pathways, highlights the intricate balance governing ECM homeostasis. Dysregulation of this balance not only underscores the significance of MMP-2 and MMP-9 but also opens new avenues for exploring targeted therapies for keloids. In summary, this review consolidates our current understanding of MMP-2 and MMP-9 in keloid pathogenesis, shedding light on their intricate involvement in the dysregulated keloids processes. The potential for targeted therapeutic interventions offers promising avenues for advancing keloid management strategies.
Author contributions
YW: Conceptualization, Data curation, Writing–original draft. LiZ: Validation, Writing–review and editing. LaZ: Visualization, Writing–review and editing. YT: Methodology, Writing–review and editing. XL: Conceptualization, Investigation, Writing–review and editing. ZC: Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aida, Y., Honda, K., Tanigawa, S., Nakayama, G., Matsumura, H., Suzuki, N., et al. (2012). Il-6 and soluble il-6 receptor stimulate the production of mmps and their inhibitors via jak-stat and erk-mapk signalling in human chondrocytes. Cell. Biol. Int. 36 (4), 367–376. doi:10.1042/CBI20110150
Aksoy, H., Cevik, O., Sen, A., Goger, F., Sekerler, T., and Sener, A. (2019). Effect of horse-chestnut seed extract on matrix metalloproteinase-1 and -9 during diabetic wound healing. J. Food Biochem. 43 (3), e12758. doi:10.1111/jfbc.12758
Aljada, A., Ghanim, H., Mohanty, P., Hofmeyer, D., Tripathy, D., and Dandona, P. (2001). Hydrocortisone suppresses intranuclear activator-protein-1 (ap-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (mmp-2 and mmp-9). J. Clin. Endocrinol. Metab. 86 (12), 5988–5991. doi:10.1210/jcem.86.12.8212
Anchan, A., Finlay, G., Angel, C. E., Hucklesby, J., and Graham, S. E. (2022). Melanoma mediated disruption of brain endothelial barrier integrity is not prevented by the inhibition of matrix metalloproteinases and proteases. Biosens. (Basel). 12 (8), 660. doi:10.3390/bios12080660
Aoki, M., Miyake, K., Ogawa, R., Dohi, T., Akaishi, S., Hyakusoku, H., et al. (2014). Sirna knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type i. J. Investig. Dermatol. 134 (3), 818–826. doi:10.1038/jid.2013.396
Bajwah, S., Ross, J. R., Peacock, J. L., Higginson, I. J., Wells, A. U., Patel, A. S., et al. (2013). Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax 68 (9), 867–879. doi:10.1136/thoraxjnl-2012-202040
Banerjee, A., Singh, P., Sheikh, P. A., Kumar, A., Koul, V., and Bhattacharyya, J. (2024). Simultaneous regulation of age/rage signaling and mmp-9 expression by an immunomodulating hydrogel accelerates healing in diabetic wounds. Biomater. Adv. 163, 213937. doi:10.1016/j.bioadv.2024.213937
Bonvicini, F., Antognoni, F., Iannello, C., Maxia, A., Poli, F., and Gentilomi, G. A. (2014). Relevant and selective activity of pancratium illyricum l. Against candida albicans clinical isolates: a combined effect on yeast growth and virulence. BMC Complement. Altern. Med. 14, 409. doi:10.1186/1472-6882-14-409
Bose, P., and Verstovsek, S. (2018). Management of myelofibrosis-related cytopenias. Curr. Hematol. Malig. Rep. 13 (3), 164–172. doi:10.1007/s11899-018-0447-9
Bran, G. M., Goessler, U. R., Baftiri, A., Hormann, K., Riedel, F., and Sadick, H. (2010). Effect of transforming growth factor-beta1 antisense oligonucleotides on matrix metalloproteinases and their inhibitors in keloid fibroblasts. Otolaryngol. Head. Neck Surg. 143 (1), 66–71. doi:10.1016/j.otohns.2010.03.029
Brilha, S., Chong, D., Khawaja, A. A., Ong, C., Guppy, N. J., Porter, J. C., et al. (2018). Integrin α2β1 expression regulates matrix metalloproteinase-1-dependent bronchial epithelial repair in pulmonary tuberculosis. Front. Immunol. 9, 1348. doi:10.3389/fimmu.2018.01348
Caon, I., Bartolini, B., Parnigoni, A., Carava, E., Moretto, P., Viola, M., et al. (2020). Revisiting the hallmarks of cancer: the role of hyaluronan. Semin. Cancer Biol. 62, 9–19. doi:10.1016/j.semcancer.2019.07.007
Chen, I. C., Liu, Y. C., Wu, Y. H., Lo, S. H., Dai, Z. K., Hsu, J. H., et al. (2022). Evaluation of proteasome inhibitors in the treatment of idiopathic pulmonary fibrosis. Cells 11 (9), 1543. doi:10.3390/cells11091543
Chen, J. Y., Feng, Q. L., Pan, H. H., Zhu, D. H., He, R. L., Deng, C. C., et al. (2023). An open-label, uncontrolled, single-arm clinical trial of tofacitinib, an oral jak1 and jak3 kinase inhibitor, in Chinese patients with keloid. Dermatology 239 (5), 818–827. doi:10.1159/000532064
Cho, J. W., Il, K. J., and Lee, K. S. (2013). Downregulation of type i collagen expression in silibinin-treated human skin fibroblasts by blocking the activation of smad2/3-dependent signaling pathways: potential therapeutic use in the chemoprevention of keloids. Int. J. Mol. Med. 31 (5), 1148–1152. doi:10.3892/ijmm.2013.1303
Craig, V. J., Zhang, L., Hagood, J. S., and Owen, C. A. (2015). Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 53 (5), 585–600. doi:10.1165/rcmb.2015-0020TR
D’Abadia, P. L., BailAo, E., Lino, J. R., Oliveira, M. G., Silva, V. B., Oliveira, L., et al. (2020). Hancornia speciosa serum fraction latex stimulates the angiogenesis and extracellular matrix remodeling processes. An. Acad. Bras. Cienc. 92 (2), e20190107. doi:10.1590/0001-3765202020190107
de Almeida, L., Thode, H., Eslambolchi, Y., Chopra, S., Young, D., Gill, S., et al. (2022). Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 74 (3), 712–768. doi:10.1124/pharmrev.121.000349
Duraisamy, A. J., Mishra, M., and Kowluru, R. A. (2017). Crosstalk between histone and dna methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Investig. Ophthalmol. Vis. Sci. 58 (14), 6440–6448. doi:10.1167/iovs.17-22706
Ebrahimi, Z., Alimohamadi, Y., Janani, M., Hejazi, P., Kamali, M., and Goodarzi, A. (2022). Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 16 (10), 875–899. doi:10.1002/term.3338
Eiro, N., Barreiro-Alonso, E., Fraile, M., Gonzalez, L. O., Altadill, A., and Vizoso, F. J. (2023). Expression of mmp-2, mmp-7, mmp-9, and timp-1 by inflamed mucosa in the initial diagnosis of ulcerative colitis as a response marker for conventional medical treatment. Pathobiology 90 (2), 81–93. doi:10.1159/000524978
Ekstein, S. F., Wyles, S. P., Moran, S. L., and Meves, A. (2021). Keloids: a review of therapeutic management. Int. J. Dermatol. 60 (6), 661–671. doi:10.1111/ijd.15159
Fan, L., Liu, Z., Zhang, Z., Li, T., Zong, X., and Bai, H. (2022). Kangfuxiaoyanshuan alleviates uterine inflammation and adhesion via inhibiting NF-κB p65 and TGF-β/MMP-2 signaling pathway in pelvic inflammatory disease rats. Front. Pharmacol. 13, 894149. doi:10.3389/fphar.2022.894149
Fujiwara, M., Muragaki, Y., and Ooshima, A. (2005). Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br. J. Dermatol 153 (2), 295–300. doi:10.1111/j.1365-2133.2005.06698.x
Guo, Y., Li, M., Long, J., Fan, P., Zuo, C., and Wang, Y. (2022). Lncrna-znf252p-as1/mir-15b-5p promotes the proliferation of keloid fibroblast by regulating the btf3-stat3 signaling pathway. J. Dermatol. Sci. 108 (3), 146–156. doi:10.1016/j.jdermsci.2022.12.010
Hao, R., Li, Z., Chen, X., and Ye, W. (2018). Efficacy and possible mechanisms of botulinum toxin type a on hypertrophic scarring. J. Cosmet. Dermatol 17 (3), 340–346. doi:10.1111/jocd.12534
Herzog, C., Haun, R. S., and Kaushal, G. P. (2019). Role of meprin metalloproteinases in cytokine processing and inflammation. Cytokine 114, 18–25. doi:10.1016/j.cyto.2018.11.032
Huang, L., Cai, Y. J., Lung, I., Leung, B. C., and Burd, A. (2013). A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J. Plast. Reconstr. Aesthet. Surg. 66 (9), e251–e259. doi:10.1016/j.bjps.2013.06.004
Hur, G. H., Ryu, A. R., Kim, Y. W., and Lee, M. Y. (2022). The potential anti-photoaging effect of photodynamic therapy using chlorin e6-curcumin conjugate in uvb-irradiated fibroblasts and hairless mice. Pharmaceutics 14 (5), 968. doi:10.3390/pharmaceutics14050968
Hwang, K. E., Kim, H. J., Song, I. S., Park, C., Jung, J. W., Park, D. S., et al. (2021). Salinomycin suppresses TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the AMPK/SIRT1 pathway in non-small cell lung cancer. Int. J. Med. Sci. 18 (3), 715–726. doi:10.7150/ijms.50080
Imaizumi, R., Akasaka, Y., Inomata, N., Okada, E., Ito, K., Ishikawa, Y., et al. (2009). Promoted activation of matrix metalloproteinase (mmp)-2 in keloid fibroblasts and increased expression of mmp-2 in collagen bundle regions: implications for mechanisms of keloid progression. Histopathology 54 (6), 722–730. doi:10.1111/j.1365-2559.2009.03287.x
Jang, B., Kim, A., Lee, Y., Hwang, J., Sung, J. Y., Jang, E. J., et al. (2022). Substituted syndecan-2-derived mimetic peptides show improved antitumor activity over the parent syndecan-2-derived peptide. Int. J. Mol. Sci. 23 (11), 5888. doi:10.3390/ijms23115888
Jeon, Y. R., Ahn, H. M., Choi, I. K., Yun, C. O., Rah, D. K., Lew, D. H., et al. (2016). Hepatocyte growth factor-expressing adenovirus upregulates matrix metalloproteinase-1 expression in keloid fibroblasts. Int. J. Dermatol. 55 (3), 356–361. doi:10.1111/ijd.12965
Jiang, Z., Yu, Q., Xia, L., Zhang, Y., Wang, X., Wu, X., et al. (2016). Growth differentiation factor-9 promotes fibroblast proliferation and migration in keloids through the smad2/3 pathway. Cell. Physiol. Biochem. 40 (1-2), 207–218. doi:10.1159/000452538
Kalev-Altman, R., Janssen, J. N., Ben-Haim, N., Levy, T., Shitrit-Tovli, A., Milgram, J., et al. (2022). The gelatinases, matrix metalloproteinases 2 and 9, play individual roles in skeleton development. Matrix Biol. 113, 100–121. doi:10.1016/j.matbio.2022.10.002
Keskin, E. S., Keskin, E. R., Ozturk, M. B., and Cakan, D. (2021). The effect of mmp-1 on wound healing and scar formation. Aesthetic Plast. Surg. 45 (6), 2973–2979. doi:10.1007/s00266-021-02369-2
Khattab, F. M., Bessar, H., and Khater, E. M. (2022). Keloid therapy: a neoteric comparative study. J. Cosmet. Dermatol 21 (9), 3962–3969. doi:10.1111/jocd.15247
Kim, B., Abdel-Rahman, M. H., Wang, T., Pouly, S., Mahmoud, A. M., and Cebulla, C. M. (2014). Retinal mmp-12, mmp-13, timp-1, and timp-2 expression in murine experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 55 (4), 2031–2040. doi:10.1167/iovs.13-13374
Kim, H. J., and Kim, Y. H. (2024). Comprehensive insights into keloid pathogenesis and advanced therapeutic strategies. Int. J. Mol. Sci. 25 (16), 8776. doi:10.3390/ijms25168776
Kim, H. S., Hwang, H. J., Kim, H. J., Choi, Y., Lee, D., Jung, H. H., et al. (2022). Effect of decellularized extracellular matrix bioscaffolds derived from fibroblasts on skin wound healing and remodeling. Front. Bioeng. Biotechnol. 10, 865545. doi:10.3389/fbioe.2022.865545
Kondapaka, S. B., Fridman, R., and Reddy, K. B. (1997). Epidermal growth factor and amphiregulin up-regulate matrix metalloproteinase-9 (mmp-9) in human breast cancer cells. Int. J. Cancer. 70 (6), 722–726. doi:10.1002/(sici)1097-0215(19970317)70:6<722::aid-ijc15>3.0.co;2-b
Kuai, Q., and Jian, X. (2022). Inhibition of mir-23b-3p ameliorates scar-like phenotypes of keloid fibroblasts by facilitating a20 expression. Clin. Cosmet. Investig. Dermatol 15, 1549–1559. doi:10.2147/CCID.S367347
Kumar, G., and Patnaik, R. (2018). Inhibition of gelatinases (mmp-2 and mmp-9) by withania somnifera phytochemicals confers neuroprotection in stroke: an in silico analysis. Interdiscip. Sci. 10 (4), 722–733. doi:10.1007/s12539-017-0231-x
Lambert, V., Wielockx, B., Munaut, C., Galopin, C., Jost, M., Itoh, T., et al. (2003). Mmp-2 and mmp-9 synergize in promoting choroidal neovascularization. FASEB J. 17 (15), 2290–2292. doi:10.1096/fj.03-0113fje
Lee, C. C., Tsai, C. H., Chen, C. H., Yeh, Y. C., Chung, W. H., and Chen, C. B. (2023). An updated review of the immunological mechanisms of keloid scars. Front. Immunol. 14, 1117630. doi:10.3389/fimmu.2023.1117630
Lee, W. J., Choi, I. K., Lee, J. H., Kim, Y. O., and Yun, C. O. (2013). A novel three-dimensional model system for keloid study: organotypic multicellular scar model. Wound Repair Regen. 21 (1), 155–165. doi:10.1111/j.1524-475X.2012.00869.x
Lee, W. J., Park, S. E., and Rah, D. K. (2011). Effects of hepatocyte growth factor on collagen synthesis and matrix metalloproteinase production in keloids. J. Korean Med. Sci. 26 (8), 1081–1086. doi:10.3346/jkms.2011.26.8.1081
Lee, Y. I., Kim, S. M., Kim, J., Kim, J., Song, S. Y., Lee, W. J., et al. (2020). Tissue-remodelling m2 macrophages recruits matrix metallo-proteinase-9 for cryotherapy-induced fibrotic resolution during keloid treatment. Acta Derm. Venereol. 100 (17), adv00306. doi:10.2340/00015555-3665
Li, H., Zhou, J., Sun, H., Qiu, Z., Gao, X., and Xu, Y. (2020). Camere: a novel tool for inference of cancer metabolic reprogramming. Front. Oncol. 10, 207. doi:10.3389/fonc.2020.00207
Li, L., Ma, Y., He, G., Ma, S., Wang, Y., and Sun, Y. (2023). Pilose antler extract restores type i and iii collagen to accelerate wound healing. Biomed. Pharmacother. 161, 114510. doi:10.1016/j.biopha.2023.114510
Li, R. L., Duan, H. X., Liang, Q., Huang, Y. L., Wang, L. Y., Zhang, Q., et al. (2022). Targeting matrix metalloproteases: a promising strategy for herbal medicines to treat rheumatoid arthritis. Front. Immunol. 13, 1046810. doi:10.3389/fimmu.2022.1046810
Li, Y., Zhang, X., He, D., Ma, Z., Xue, K., and Li, H. (2022). 45S5 Bioglass® works synergistically with siRNA to downregulate the expression of matrix metalloproteinase-9 in diabetic wounds. Acta Biomater. 145, 372–389. doi:10.1016/j.actbio.2022.04.010
Li, Z., Cao, Y., Li, H., Le, S., and Yin, L. (2024). Network pharmacology, molecular docking analysis and experiment validations on molecular targets and mechanisms of the dispel-scar ointment in scar treatment. Comb. Chem. High. Throughput Screen 27. doi:10.2174/0113862073335953240820075044
Liang, C. J., Yen, Y. H., Hung, L. Y., Wang, S. H., Pu, C. M., Chien, H. F., et al. (2013). Thalidomide inhibits fibronectin production in TGF-β1-treated normal and keloid fibroblasts via inhibition of the p38/Smad3 pathway. Biochem. Pharmacol. 85 (11), 1594–1602. doi:10.1016/j.bcp.2013.02.038
Liang, L., Cen, H., Huang, J., Qin, A., Xu, W., Wang, S., et al. (2022). The reversion of dna methylation-induced mirna silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol. Cancer. 21 (1), 186. doi:10.1186/s12943-022-01651-4
Liu, H. F., Liu, H., Lv, L. L., Ma, K. L., Wen, Y., Chen, L., et al. (2018). CCN3 suppresses TGF-β1-induced extracellular matrix accumulation in human mesangial cells in vitro. Acta Pharmacol. Sin. 39 (2), 222–229. doi:10.1038/aps.2017.87
Liu, M., Huang, L., Liu, Y., Yang, S., Rao, Y., Chen, X., et al. (2023). Identification of the mmp family as therapeutic targets and prognostic biomarkers in the microenvironment of head and neck squamous cell carcinoma. J. Transl. Med. 21 (1), 208. doi:10.1186/s12967-023-04052-3
Luo, Y., Wang, D., Yuan, X., Jin, Z., and Pi, L. (2023). Oleanolic acid regulates the proliferation and extracellular matrix of keloid fibroblasts by mediating the TGF-β1/SMAD signaling pathway. J. Cosmet. Dermatol 22 (7), 2083–2089. doi:10.1111/jocd.15673
Miricescu, D., Badoiu, S. C., Stanescu-Spinu, I. I., Totan, A. R., Stefani, C., and Greabu, M. (2021). Growth factors, reactive oxygen species, and metformin-promoters of the wound healing process in burns? Int. J. Mol. Sci. 22 (17), 9512. doi:10.3390/ijms22179512
Muscella, A., Vetrugno, C., Cossa, L. G., and Marsigliante, S. (2020). TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 153 (4), 525–538. doi:10.1111/jnc.14913
Nagarkoti, S., Kim, Y. M., Ash, D., Das, A., Vitriol, E., Read, T. A., et al. (2023). Protein disulfide isomerase a1 as a novel redox sensor in vegfr2 signaling and angiogenesis. Angiogenesis 26 (1), 77–96. doi:10.1007/s10456-022-09852-7
Orsolic, N., Kunstic, M., Kukolj, M., Odeh, D., and Ancic, D. (2020). Natural phenolic acid, product of the honey bee, for the control of oxidative stress, peritoneal angiogenesis, and tumor growth in mice. Molecules 25 (23), 5583. doi:10.3390/molecules25235583
Paltatzidou, K., Xenos, K., Panagiotopoulos, A., Pouliou, E., Katsika-Chatziolou, E., Stavropoulos, P., et al. (2017). Localization of mmp-9 in multinuclear giant cells in keloids after treatment with 5-fluorouracil with or without combination of cryotherapy and cryotherapy alone. J. Eur. Acad. Dermatol Venereol. 31 (2), e121–e123. doi:10.1111/jdv.13869
Pan, S., Hu, Y., Hu, M., Jian, H., Chen, M., Gan, L., et al. (2020). Platelet-derived pdgf promotes the invasion and metastasis of cholangiocarcinoma by upregulating mmp2/mmp9 expression and inducing emt via the p38/mapk signalling pathway. Am. J. Transl. Res. 12 (7), 3577–3595.
Panichakul, T., Ponnikorn, S., Tupchiangmai, W., Haritakun, W., and Srisanga, K. (2022). Skin anti-aging potential of ipomoea pes-caprae ethanolic extracts on promoting cell proliferation and collagen production in human fibroblasts (ccd-986sk cells). Pharm. (Basel) 15 (8), 969. doi:10.3390/ph15080969
Perez, J. R., Lee, S., Ybarra, N., Maria, O., Serban, M., Jeyaseelan, K., et al. (2017). A comparative analysis of longitudinal computed tomography and histopathology for evaluating the potential of mesenchymal stem cells in mitigating radiation-induced pulmonary fibrosis. Sci. Rep. 7 (1), 9056. doi:10.1038/s41598-017-09021-7
Pot, S. A., Lin, Z., Shiu, J., Benn, M. C., and Vogel, V. (2023). Growth factors and mechano-regulated reciprocal crosstalk with extracellular matrix tune the keratocyte-fibroblast/myofibroblast transition. Sci. Rep. 13 (1), 11350. doi:10.1038/s41598-023-37776-9
Rajashekhar, G., Shivanna, M., Kompella, U. B., Wang, Y., and Srinivas, S. P. (2014). Role of MMP-9 in the breakdown of barrier integrity of the corneal endothelium in response to TNF-α. Exp. Eye Res. 122, 77–85. doi:10.1016/j.exer.2014.03.004
Ranzato, E., Martinotti, S., Volante, A., Mazzucco, L., and Burlando, B. (2011). Platelet lysate modulates mmp-2 and mmp-9 expression, matrix deposition and cell-to-matrix adhesion in keratinocytes and fibroblasts. Exp. Dermatol. 20 (4), 308–313. doi:10.1111/j.1600-0625.2010.01173.x
Ravanti, L., and Kahari, V. M. (2000). Matrix metalloproteinases in wound repair (review). Int. J. Mol. Med. 6 (4), 391–407. doi:10.3892/ijmm.6.4.391
Ren, Z., Hou, Y., Ma, S., Tao, Y., Li, J., Cao, H., et al. (2014). Effects of ccn3 on fibroblast proliferation, apoptosis and extracellular matrix production. Int. J. Mol. Med. 33 (6), 1607–1612. doi:10.3892/ijmm.2014.1735
Robert, S., Gicquel, T., Victoni, T., Valenca, S., Barreto, E., Bailly-Maitre, B., et al. (2016). Involvement of matrix metalloproteinases (mmps) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 36 (4), e00360. doi:10.1042/BSR20160107
Sadick, H., Herberger, A., Riedel, K., Bran, G., Goessler, U., Hoermann, K., et al. (2008). Tgf-beta1 antisense therapy modulates expression of matrix metalloproteinases in keloid-derived fibroblasts. Int. J. Mol. Med. 22 (1), 55–60. doi:10.3892/ijmm.22.1.55
Sathiyaseelan, A., Zhang, X., and Wang, M. H. (2023). Enhancing the antioxidant, antibacterial, and wound healing effects of melaleuca alternifolia oil by microencapsulating it in chitosan-sodium alginate microspheres. Nutrients 15 (6), 1319. doi:10.3390/nu15061319
Sato, H., and Takino, T. (2010). Coordinate action of membrane-type matrix metalloproteinase-1 (mt1-mmp) and mmp-2 enhances pericellular proteolysis and invasion. Cancer Sci. 101 (4), 843–847. doi:10.1111/j.1349-7006.2010.01498.x
Schmidt, G. J., Reumiller, C. M., Ercan, H., Resch, U., Butt, E., Heber, S., et al. (2019). Comparative proteomics reveals unexpected quantitative phosphorylation differences linked to platelet activation state. Sci. Rep. 9 (1), 19009. doi:10.1038/s41598-019-55391-5
Scopelliti, F., Cattani, C., Dimartino, V., Scarponi, C., Madonna, S., Albanesi, C., et al. (2020). Platelet lysate promotes the expansion of t regulatory cells that favours in vitro wound healing by increasing keratinocyte migration and fibroblast production of extracellular matrix components. Eur. J. Dermatol. 30 (1), 3–11. doi:10.1684/ejd.2020.3711
Seyed, H. E., Alizadeh, Z. M., Tarrahimofrad, H., Zamani, J., Haddad, K. H., Ahmad, E., et al. (2023). Synergistic effects of dendrosomal nanocurcumin and oxaliplatin on oncogenic properties of ovarian cancer cell lines by down-expression of mmps. Biol. Res. 56 (1), 3. doi:10.1186/s40659-023-00412-x
Shen, Y., Teng, L., Qu, Y., Liu, J., Zhu, X., Chen, S., et al. (2022). Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 284, 114791. doi:10.1016/j.jep.2021.114791
Shimizu, T., Kanai, K., Kyo, Y., Asano, K., Hisamitsu, T., and Suzaki, H. (2006). Effect of tranilast on matrix metalloproteinase production from neutrophils in-vitro. J. Pharm. Pharmacol. 58 (1), 91–99. doi:10.1211/jpp.58.1.0011
Song, K. X., Liu, S., Zhang, M. Z., Liang, W. Z., Liu, H., Dong, X. H., et al. (2018). Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J. Zhejiang Univ. Sci. B 19 (11), 853–862. doi:10.1631/jzus.B1800132
Spiekman, M., Przybyt, E., Plantinga, J. A., Gibbs, S., van der Lei, B., and Harmsen, M. C. (2014). Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast. Reconstr. Surg. 134 (4), 699–712. doi:10.1097/PRS.0000000000000504
Subramanian, U., Ramasamy, C., Ramachandran, S., Oakes, J. M., Gardner, J. D., and Pandey, K. N. (2022). Genetic disruption of guanylyl cyclase/natriuretic peptide receptor-A triggers differential cardiac fibrosis and disorders in male and female mutant mice: role of TGF-β1/SMAD signaling pathway. Int. J. Mol. Sci. 23 (19), 11487. doi:10.3390/ijms231911487
Sukhonthasilakun, S., Mahakunakorn, P., Naladta, A., Nuankaew, K., Nualkaew, S., Yenjai, C., et al. (2023). Anti-inflammatory effects of derris scandens extract on narrowband-ultraviolet b exposed hacat human keratinocytes. J. Ayurveda Integr. Med. 14 (2), 100693. doi:10.1016/j.jaim.2023.100693
Sun, H. (2022). Metabolic reprogramming and warburg effect in keloids. Burns 48 (5), 1266–1267. doi:10.1016/j.burns.2022.04.021
Tanriverdi-Akhisaroglu, S., Menderes, A., and Oktay, G. (2009). Matrix metalloproteinase-2 and -9 activities in human keloids, hypertrophic and atrophic scars: a pilot study. Cell. Biochem. Funct. 27 (2), 81–87. doi:10.1002/cbf.1537
Tian, F., Jiang, Q., Chen, J., and Liu, Z. (2023). Silicone gel sheeting for treating keloid scars. Cochrane Database Syst. Rev. 1 (1), D13878. doi:10.1002/14651858.CD013878.pub2
Tian, Y. C., Chen, Y. C., Chang, C. T., Hung, C. C., Wu, M. S., Phillips, A., et al. (2007). Epidermal growth factor and transforming growth factor-beta1 enhance hk-2 cell migration through a synergistic increase of matrix metalloproteinase and sustained activation of erk signaling pathway. Exp. Cell. Res. 313 (11), 2367–2377. doi:10.1016/j.yexcr.2007.03.022
Tsai, Y. C., Kuo, T. N., Chao, Y. Y., Lee, P. R., Lin, R. C., Xiao, X. Y., et al. (2023). Pdgf-aa activates akt and erk signaling for testicular interstitial leydig cell growth via primary cilia. J. Cell. Biochem. 124 (1), 89–102. doi:10.1002/jcb.30345
Tuli, H. S., Mittal, S., Aggarwal, D., Parashar, G., Parashar, N. C., Upadhyay, S. K., et al. (2021). Path of silibinin from diet to medicine: a dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin. Cancer Biol. 73, 196–218. doi:10.1016/j.semcancer.2020.09.014
Ulrich, D., Ulrich, F., Unglaub, F., Piatkowski, A., and Pallua, N. (2010). Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J. Plast. Reconstr. Aesthet. Surg. 63 (6), 1015–1021. doi:10.1016/j.bjps.2009.04.021
van Haaften, W. T., Blokzijl, T., Hofker, H. S., Olinga, P., Dijkstra, G., Bank, R. A., et al. (2020). Intestinal stenosis in crohn's disease shows a generalized upregulation of genes involved in collagen metabolism and recognition that could serve as novel anti-fibrotic drug targets. Ther. Adv. Gastroenterol. 13, 1756284820952578. doi:10.1177/1756284820952578
Wang, B., Yang, X., Sun, X., Liu, J., Fu, Y., Liu, B., et al. (2022). Atf3 in atherosclerosis: a controversial transcription factor. J. Mol. Med. Berl. 100 (11), 1557–1568. doi:10.1007/s00109-022-02263-7
Wang, D., Chen, J., Ding, Y., Kong, H., You, H., Zhao, Y., et al. (2020). Mir-188-5p promotes tumor growth by targeting cd2ap through pi3k/akt/mtor signaling in children with acute promyelocytic leukemia. Onco Targets Ther. 13, 6681–6697. doi:10.2147/OTT.S244813
Wang, X. M., Liu, X. M., Wang, Y., and Chen, Z. Y. (2021). Activating transcription factor 3 (atf3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (tgf-beta)/smad signaling pathway. Bioengineered 12 (1), 117–126. doi:10.1080/21655979.2020.1860491
Wei, J. J., Kim, H. S., Spencer, C. A., Brennan-Crispi, D., Zheng, Y., Johnson, N. M., et al. (2020). Activation of trpa1 nociceptor promotes systemic adult mammalian skin regeneration. Sci. Immunol. 5 (50), eaba5683. doi:10.1126/sciimmunol.aba5683
Wu, X., Liu, L., and Zhang, H. (2020). Mir-802 inhibits the epithelial-mesenchymal transition, migration and invasion of cervical cancer by regulating btf3. Mol. Med. Rep. 22 (3), 1883–1891. doi:10.3892/mmr.2020.11267
Yang, C. C., Hsiao, L. D., Shih, Y. F., Su, M. H., and Yang, C. M. (2022). Sphingosine 1-phosphate-upregulated cox-2/pge(2) system contributes to human cardiac fibroblast apoptosis: involvement of mmp-9-dependent transactivation of egfr cascade. Oxid. Med. Cell. Longev. 2022, 7664290. doi:10.1155/2022/7664290
Yang, Y. N., Wang, F., Zhou, W., Wu, Z. Q., and Xing, Y. Q. (2012). TNF-α stimulates MMP-2 and MMP-9 activities in human corneal epithelial cells via the activation of FAK/ERK signaling. Ophthalmic Res. 48 (4), 165–170. doi:10.1159/000338819
Yao, X., Jiang, W., Yu, D., and Yan, Z. (2019). Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing mmp-2 and mmp-9 through the pi3k/akt pathway. Food Funct. 10 (2), 703–712. doi:10.1039/c8fo02013b
Yeh, F. L., Shen, H. D., and Tai, H. Y. (2009). Decreased production of mcp-1 and mmp-2 by keloid-derived fibroblasts. Burns 35 (3), 348–351. doi:10.1016/j.burns.2008.06.018
Yin, H., Liu, N., Zhou, X., Chen, J., and Duan, L. (2023). The advance of ccn3 in fibrosis. J. Cell. Commun. Signal 17, 1219–1227. doi:10.1007/s12079-023-00778-3
Yu, X., Li, Z., Chan, M. T., and Wu, W. K. (2015). Microrna deregulation in keloids: an opportunity for clinical intervention? Cell. Prolif. 48 (6), 626–630. doi:10.1111/cpr.12225
Zhang, C., Jiang, G., and Gao, X. (2023). Matrix metalloproteinase-responsive drug delivery systems. Bioconjug Chem. 34 (8), 1349–1365. doi:10.1021/acs.bioconjchem.3c00266
Zhang, Q., Liu, C., Hong, S., Min, J., Yang, Q., Hu, M., et al. (2017). Excess mechanical stress and hydrogen peroxide remodel extracellular matrix of cultured human uterosacral ligament fibroblasts by disturbing the balance of MMPs/TIMPs via the regulation of TGF‑β1 signaling pathway. Mol. Med. Rep. 15 (1), 423–430. doi:10.3892/mmr.2016.5994
Zhao, B., Liu, J. Q., Yang, C., Zheng, Z., Zhou, Q., Guan, H., et al. (2019). Erratum to Human amniotic epithelial cells conditioned medium attenuates TGF-β1-induced human dermal fibroblasts transformation to myofibroblasts via TGF-β1/Smad3 pathway. Cytotherapy 21 (9), 1004–1005. doi:10.1016/j.jcyt.2019.07.003
Zhou, X., Lu, J., Wu, B., and Guo, Z. (2022). Hoxa11-as facilitates the proliferation, cell cycle process and migration of keloid fibroblasts through sponging mir-188-5p to regulate vegfa. J. Dermatol. Sci. 106 (2), 111–118. doi:10.1016/j.jdermsci.2022.04.004
Zhou, Y., Sun, Y., Hou, W., Ma, L., Tao, Y., Li, D., et al. (2020). The jak2/stat3 pathway inhibitor, ag490, suppresses the abnormal behavior of keloid fibroblasts in vitro. Int. J. Mol. Med. 46 (1), 191–200. doi:10.3892/ijmm.2020.4592
Keywords: keloids, gelatinases, MMP-2, MMP-9, molecular mechanism
Citation: Wang Y, Zheng L, Zhang L, Tai Y, Lin X and Cai Z (2024) Roles of MMP-2 and MMP-9 and their associated molecules in the pathogenesis of keloids: a comprehensive review. Front. Pharmacol. 15:1444653. doi: 10.3389/fphar.2024.1444653
Received: 06 June 2024; Accepted: 12 November 2024;
Published: 25 November 2024.
Edited by:
Udhaya Kumar, Baylor College of Medicine, United StatesReviewed by:
Grzegorz Młynarczyk, Medical University of Białystok, PolandCopyright © 2024 Wang, Zheng, Zhang, Tai, Lin and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhencheng Cai, emhlbmNoZW5nY2FpMjAyNEAxMjYuY29t
†ORCID: Zhencheng Cai, orcid.org/0009-0005-2140-1684
‡These authors have contributed equally to this work
 Yajie Wang1‡
Yajie Wang1‡ Zhencheng Cai
Zhencheng Cai