
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 29 April 2024
Sec. Inflammation Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1389786
This article is part of the Research Topic Epigenetics of inflammatory reactions and pharmacological modulation View all 5 articles
Osteoarthritis (OA) is a common chronic disease characterized by progressive cartilage degeneration and secondary synovial inflammation. Bergamottin (Ber) is an important natural derivative of the furanocoumarin compound, extracted from natural foods, such as the pulp of grapefruits and pomelos. Ber exhibits several characteristicsthat are beneficial to human health, such as anti-inflammation, antioxidant, and anti-cancer effects. However, the role of Ber in the treatment of OA has not been elucidated to date. Therefore, in the present study, in vitro experiments were conducted, which demonstrated that Ber reduces the secretion of inducible nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and prostaglandin E2 (PGE2) under the stimulation of interleukin-1β (IL-1β). Ber also reversed the IL-1 β-mediated aggrecan and type II collagen degradation within the extracellular matrix (ECM). In addition, in vivo experiments were conducted, in which Ber ameliorated the progression of OA in mice. It was revealed that Ber exerted its cellular effect by activating the Sirt1/NF-kB pathways. In conclusion, the present study demonstrated the therapeutic potential of Ber in the context of OA.
Osteoarthritis (OA) is a common bone disease, which is generally accompanied by the loss of articular cartilage, fibrosis, and damage to the entire articular surface (Pereira et al., 2015; Abramoff and Caldera, 2020). With the increase in human life expectancy, both the incidence and prevalence of osteoarthritis are on a rapid rise (Hunter et al., 2014). The main symptoms of OA include swelling around the affected joints, stiffness, reduced range of motion at the joints, joint pain, and limited mobility. OA is more prevalent among middle-aged and elderly individuals, and females gender is affected (Loeser, 2009). The traditional treatment of OA includes pain management and articular prosthesis for end-stage diseases. While the specific pathogenesis of OA remains to be elucidated to date, several factors reportedly influence the progression of osteoarthritis, including obesity, aging, trauma, inflammation, osteoporosis, and joint deformities (Millerand et al., 2019; Katz et al., 2021). Among these factors, inflammation is considered to play a crucial role in the occurrence and development of osteoarthritis (Arra and Abu-Amer, 2023). Interleukin-1β (IL-1β) is one of several inflammatory factors that can lead to the occurrence of osteoarthritis by inducing the overexpression of related metabolic enzymes and inflammatory cytokines, such as NO, PGE2, ADAMTS, and MMPs (Lin et al., 2020a). This ultimately resulting in the degradation of the extracellular matrix. The joint cartilage tissue comprises type II collagen, glycosaminoglycans, and chondroitin sulfate, all of which together form the extracellular matrix (Lin et al., 2020b). Inflammatory factors mediate the production of a large number of several metabolic enzymes and inflammatory cytokines, it ultimately leading to the degradation of the extracellular matrix and resulting in the occurrence of osteoarthritis (Theeuwes et al., 2021).
Several studies have reported a significant increase in the expression of IL-1β in the serum and synovial fluid of osteoarthritis patients (Au et al., 2007; Santangelo et al., 2012). Therefore, inhibiting the expression of IL-1β could be used as a potential target in the treatment of osteoarthritis.
NF-κB is a highly classical molecular regulatory pathway that can promotes the occurrence and development of inflammation (Barnes and Karin, 1997). The release of various pro-inflammatory factors facilitates the expression and nuclear translocation of nuclear factor-kappa B (NF-κB) can be facilitated (Kumar et al., 2004). The activation of NF-κB then induces expressions of MMPs and the degradation of the cartilage matrix. Silent Information Regulator Factor 1 (SIRT1) is a member of the mammalian sirtuin family of proteins. It is a conserved nicotinamide adenine dinucleotide (NAD)+-dependent deacetylase (Xie et al., 2013). It plays a regulatory role in various biological processes, including cellular metabolism, gene silencing, cell survival, and DNA repair. SIRT1 is a homolog of Sir2 in mammals, the latter belonging to the class III of histone deacetylases (Shah et al., 2017). SIRT1 reportedly deacetylates various non-histone substrates and histones. Research has demonstrated that SIRT1 could reduce the expressions of inflammatory factors by inhibiting NF-κB (Jia et al., 2022). Therefore, the SIRT1/NF-κB pathway could be an important signaling pathway involved in the regulation of both the normal development and pathological destruction of cartilage.
Current therapeutic approaches are limited to the use of hyaluronic acid, opioids or corticosteroids. However, these only relieve articular pain and swelling, with overuse leading to deleterious side effects. Therefore, an effective and safe drug that relieves clinical symptoms of OA while also decelerating its progression has to be developed. Ber is a natural furanocoumarin compound with anti-inflammatory, antioxidant, and anti-aging properties (Wang et al., 2022). Studies have revealed that Ber inhibits the invasive activity of human glioma cells’ matrix metalloproteinase 9 in human glioma cells by regulating the activity of Rac1(Hwang et al., 2010). Additionally, Ko et al. reported Ber can suppressed the epithelial-mesenchymal transition and blocks the downregulation of fibronectin through the PI3K/AKT/mTOR pathway (Ko et al., 2018). Research by Kim et al. suggests that Ber can inhibit the NF-κB pathway, thereby exerting anti-cancer effects (Kim et al., 2016). While the above findings suggest that Ber exhibits significant anti-inflammatory therapeutic effects, its role in osteoarthritis has not been reported so far.
Therefore, the present study aimed to elucidate the role of Ber on the anti-inflammatory activity and ECM of chondrocytes and cartilage in OA. The study involved the use of in vitro and in vivo experimental models to investigate the anti-inflammatory properties of Ber and unravel the mechanisms underlying the effects of Ber on the pathogenesis of OA.
Bergamottin (Ber) (purity >98%) and Phosphate buffered saline (PBS) was obtained from ChemeGen BioTechnology (Shanghai, China). Anti-Lamin B1 and anti-IκB were acquired from Proteintech (Wuhan, China). Anti-iNOS, anti-Sirt1, anti-P65, anti-GAPDH, Anti-Sirt1, and anti-COX-2 were purchased from Bioss (Beijing, China). EX-527 and MTT were purchased from Sigma-Aldrich (CA, USA).
The chondrocyte extraction and culture were performed according to the previous studies (Xian et al., 2022). Chondrocytes were isolated from the 5-day-old mice. Knee joint cartilage was exposed under sterile conditions and collected into a PBS solution. Then, treat cartilage tissue with type II collagenase. Finally, chondrocytes were cultured in DMEM/F12 medium (10% FBS and 1% penicillin/streptomycin) in an incubator with 5% CO2 at 37°C.
C57BL/6 mice were obtained from Wenzhou Medical University. The model of OA was established by meniscus injury surgery. Mice were randomly separated into three groups (n = 6 in each): a control group (sham-operated), OA group (OA), and OA treated with Ber group (Ber). Mice were intraperitoneally injected with Ber (20 mg/kg) once a day for eight consecutive weeks. The mice were reared in specific pathogen-free cages, given unrestricted movement, and provided autoclaved food and water. All animal experiments were carried out and approved by the Animal Administration Committee of Wenzhou Medical University.
The Cell counting Kit 8 (CCK-8) reagent is a commonly used method for measuring cell viability. The chondrocytes were seeded into 96-well plates and treated with concentrations of IL-1β (10 ng/mL) and Ber (0–40 μM) for 24 or 48 h, respectively. The effect of Ber on cells was measured by using a spectrophotometer (Synergy HT, Bio-Tek, United States).
Chondrocytes (3 × 105 cells per mL) were seeded in 6-well plates and treated with Ber(5, 10, or 20 μM) 24 h prior to the addition of IL-1β (10 ng mL−1). They were incubated for 24 h and then the concentration of NO was measured using the Griess reaction as previously described (Au et al., 2007). The optical density of each sample was measured at a wavelength of 543 nm. The culture medium supernatant from each sample was collected, the levels of PGE2, Aggrecan, ADAMTS-5, and IL-6 in the culture medium were measured with an ELISA kit (R&D Systems, Minneapolis, MN, United States) according to the manufacturer’s instructions.
The cell proteins were isolated using RIPA lysis. The concentration of proteins was measured by the BCA Assay Kit. Protein samples (20 μM) were separated by SDS-PAGE electrophoresis and electrotransferred to the PVDF membrane (Millipore, MA, United States). The protein sample was transferred to a membrane and sealed in 5% nonfat milk for 1 h. The primary antibody (1:1000) was incubated overnight at 4°C. After being washed with TBST, the membranes were incubated with secondary antibodies for 60 min. Odyssey Infrared Imaging System (LI-COR, United States) was used to obtain images. The blot images were detected by SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher)
First, we mapped the molecular structure of SA using ChemBioDraw and minimized its energy using ChemBio3D. Based on the requirements of current molecular docking experiments, we downloaded the corresponding Sirt1 from the PDB website (https://www.rcsb.org/). After being processed in PyMoL (version 1.7.6), the lowest energy conformations for docking were decided based on default parameters. When the molecular structures of Ber and Sirt1 required for docking analysis were finalized, subsequent molecular docking analysis was performed by AutoDockTools (version 1.5.6). The final 3D views of the images were obtained via PyMoL.
The chondrocytes were seeded into 6-well plates and treated with Ber(20 μM). Then chondrocytes were fixed with 4% paraformaldehyde (PFA, Servicebio, Wuhan, China) for 10 min and permeabilized with 0.1% Triton X-100 (BioFroxx, Germany) for 15 min. The sections were blocked with 5% bovine serum albumin. The samples were incubated with the primary antibody overnight at 4°C, followed by incubation with the fluorescent secondary antibody for 60 min. The next day, samples were treated with secondary antibodies for 45 min and stained with DAPI for 15 min. Finally, the samples were examined using an inverted fluorescence microscope (Leica DMIL, Germany).
The cartilage tissues of mice were fixed with 10% paraformaldehyde, then dehydrated, embedded, sectioned, and stained with hematoxylin-Eosin and Safranin O/Fast Green staining. The bright-field images were captured using a microscope (Thermo Fisher) and processed with ImageJ software (v1.8.0).
GraphPad Prism 7.0 software was used to execute statistical analyses and create the statistical charts. One-way ANOVA followed by Tukey’s t-tests multiple comparisons was used to compare the groups. p < 0.05 indicates a statistically significant difference.
Figure 1A illustrates the molecular structure of Ber. The Safranin O staining results for cellular proteoglycans are presented in Figure 1B. The cytotoxic effect of Ber on chondrocytes was assessed using the CCK-8 assay. First, the chondrocytes were treated with different concentrations of Ber (0, 2.5, 5, 10, 20, 30, and 40 μM) for 24 and 48 h. Subsequently, the toxicity of Ber was assessed using the CCK-8 reagent. As presented in the results in Figures 1C,D, Ber exerted no toxic effects on chondrocytes within the concentration range of 5–20 μM, while the Ber concentrations 30 μM resulted in evident cytotoxicity. Therefore, Ber concentrations of 5, 10, and 20 μM were selected for the subsequent cell experiments to be conducted in the present study.
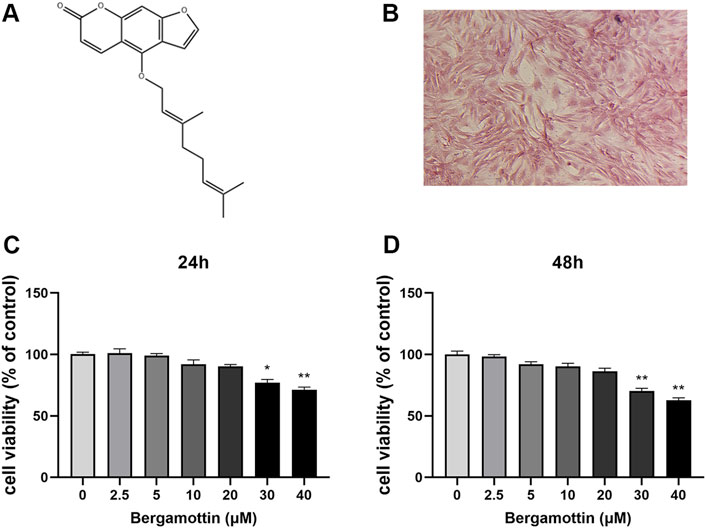
Figure 1. Cytotoxic analysis of Ber. (A) Chemical structure of SA. (B) The Safranin O staining of chondrocytes. (C, D) Cells were cultured with different concentrations of Ber (0, 2.5, 5, 10, 20, 30, and 40 μM) for 24 h and 48 h. The cell viability was determined by CCK-8 assay. p < 0.05 vs. control group; **p < 0.01 vs. control group, n = 5.
IL-1β induction leads to the production of various inflammatory cytokines in chondrocytes, ultimately causing inflammation in these cells. Whether Ber could inhibit IL-1β-induced inflammation in chondrocytes was assessed in the present study. ELISA and Western blotting were performed to detect the expression levels of various inflammatory cytokines, and the results are presented in Figures 2A–C. In comparison to the control group, the IL-1β group presented significantly increased expression levels of COX-2 and iNOS, while the Ber-treatment group exhibited reduced expression levels of IL-1β-induced COX-2 and iNOS in a dose-dependent manner. The ELISA results revealed a significant upregulation in IL-6, PGE2, TNF-α, and NO expression levels in the IL-1β-induced group. In contrast, the Ber group exhibited an inhibitory effect on the expression levels of IL-1β-induced NO, TNF-α, IL-6, and PGE2 (Figures 2D–G). The above results indicated that Ber could significantly inhibit IL-1β-induced inflammation in chondrocytes.
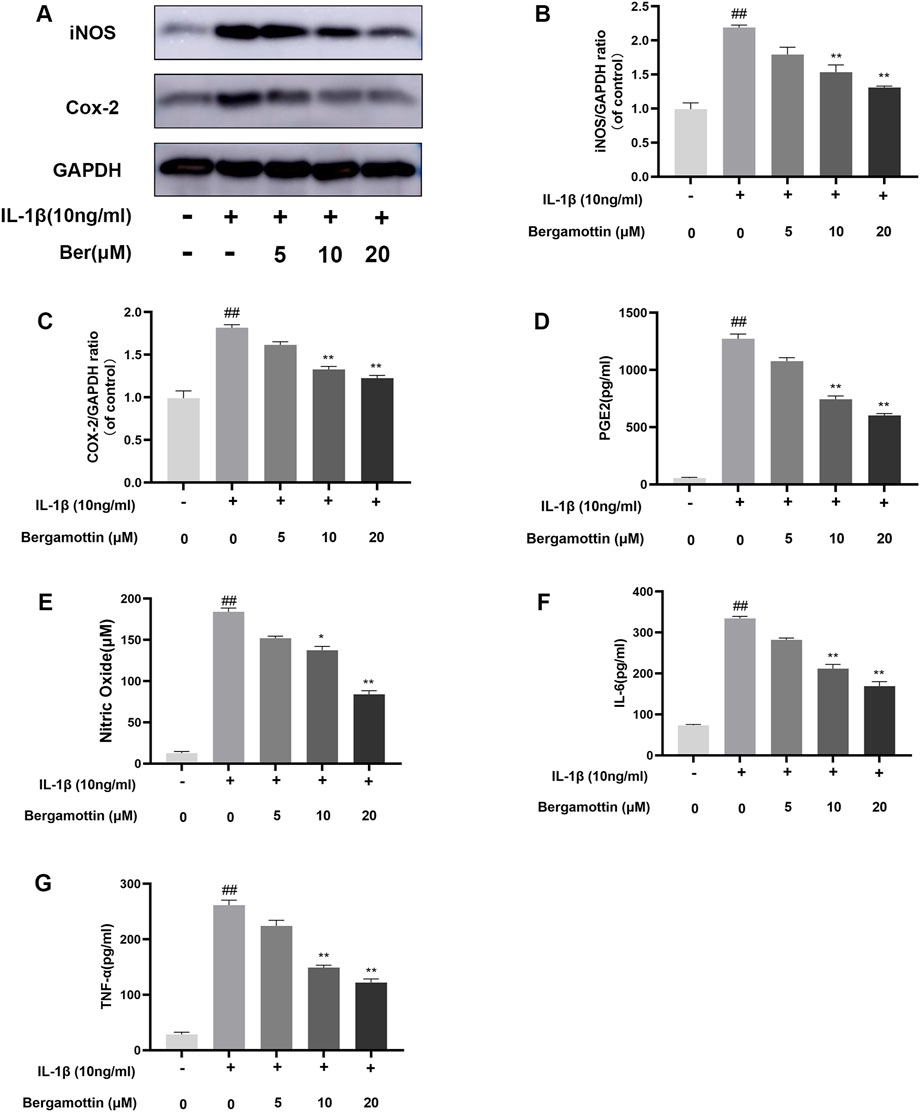
Figure 2. Ber inhibits IL-1β-induced PGE2, NO, IL-6, TNF-α, COX-2 and iNOS production. (A–C): The level of INOS and COX-2 in the chondrocytes. (D–G): The levels of NO, PGE2, and IL-6 in the chondrocytes were measured by ELISA. ##p ≤ 0.01 vs. control group; **p < 0.01 vs. IL-1β group, n = 5.
Type II collagen and proteoglycans are the major components within chondrocytes, and the expressions of these reflect the functionality of chondrocytes. IL-1β induction leads to the production of metalloproteinases, which could lead to in the degradation of the extracellular matrix of chondrocytes. Therefore, the impact of Ber on IL-1β-induced degradation of the extracellular matrix of chondrocytes, was investigated in the present study using Western blotting to determine the expressions of metalloproteinases. The results are presented in Figures 3A–D, IL-1β induction led to abnormal expressions of MMP-13 and ADAMTS-5, while Ber significantly inhibited their expressions of these metalloproteinases. Collagen II fluorescence staining results revealed that Ber effectively inhibited the IL-1β-induced degradation. These findings suggested that Ber could significantly inhibit the IL-1β-induced degradation of the extracellular matrix (Figures 3E,F).
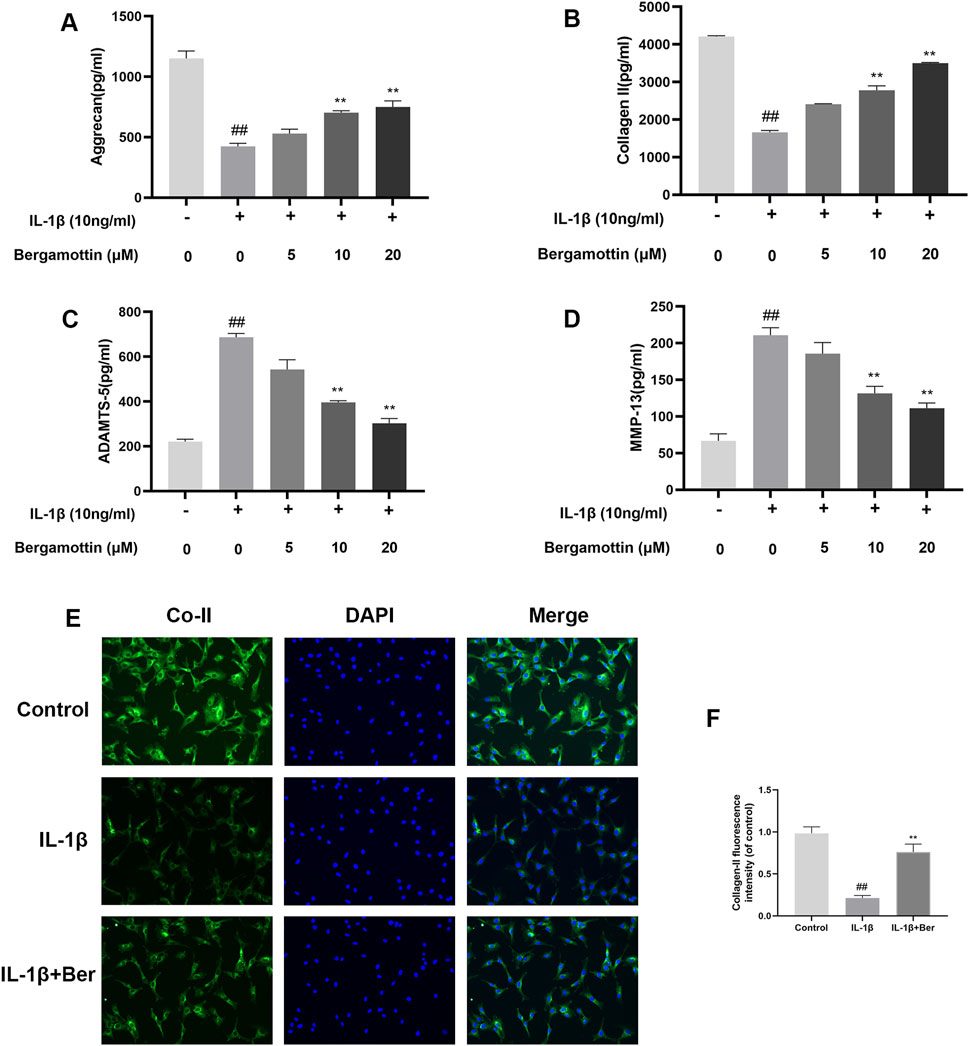
Figure 3. Ber inhibits ECM degradation of chondrocytes. (A–D): The level of ADAMTS5 and Collagen II in the chondrocytes was evaluated by ELISA. (E, F): Immunofluorescence staining for Collagen II and the nucleus was performed. P##p ≤ 0.01 vs. control group; **p < 0.01 vs. IL-1β group, n = 5.
NF-kB plays a significant regulatory role in inflammatory diseases, and its activation could further exacerbate inflammation. Therefore, the impact of Ber on NF-kB in IL-1β-induced chondrocytes was investigated in the present study. Western bloting was conducted to evaluate the expressions of IκBα and P65, and the results are presented in Figures 4A–D. It was observed that under normal conditions, P65 expression was low, and IκBα expression was high. IL-1β then induced an increase in P65 expression, and Ber inhibited the expression of P65 in a dose-dependent manner. The chondrocytes were subjected to p65 cellular immunofluorescence staining.The results revealed that IL-1β could cause P65 to translocate to the nucleus of the cell., and Ber could inhibit this phenomenon (Figure 4E). The above results suggested that Ber inhibited the activation of NF-kB within chondrocytes, thereby exerting an inhibitory effect on IL-1β-induced inflammation in chondrocytes.
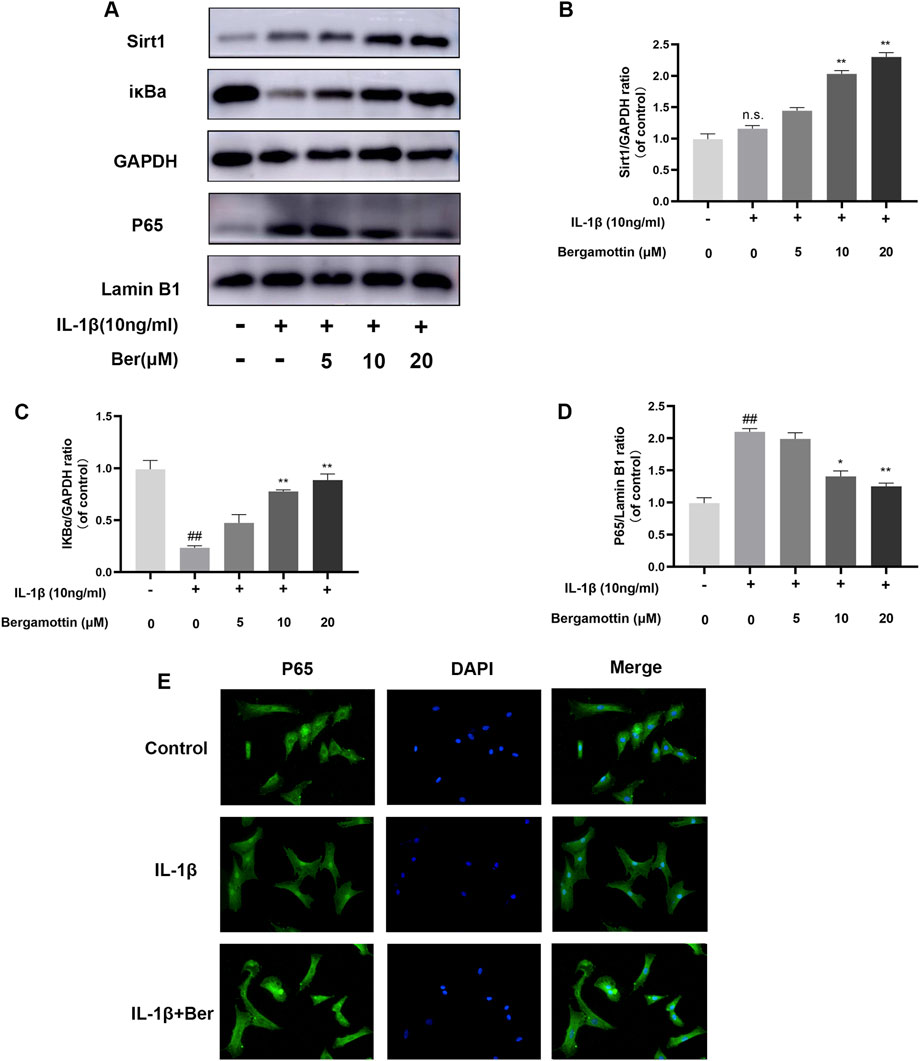
Figure 4. Ber inhibits IL-1β-induced NF-κB activation. (A–D): The production of P65 and IκBα in chondrocytes. (E): The p65 was observed by immunofluorescence. ##p ≤ 0.01 vs. control group; **p < 0.01 vs. IL-1β group, n = 5.
Sirt1 effectively inhibits the activation of NF-kB. In the molecular docking analyses conducted in the present study, Ber could bind to Sirt1 (Figures 5A–D). The small molecule Ber could bind to the SIRT1 protein through hydrophobic interactions with three residues: PHE273 at the distances of 3.5Å, 3.7Å, and 3.8Å; ARG274 at the distances of 3.2Å and 3.9Å; and ILE411 at a distance of 3.9Å. In addition, Ber exhibited hydrophobic interactions with four residues: PHE297 at distances of 3.5Å, 3.5Å, 3.8Å, and 3.8Å. Accordingly, it was inferred that presence of these interactions allowed the small molecule Ber to stably bind to the receptor protein SIRT1. Subsequently, the impact of Ber on IL-1β-induced Sirt1 in chondrocytes was investigated by assessing the expression levels of Sirt1 using Western blotting. Ber was observed to increase the expression levels of Sirt1 in a dose-dependent manner (Figures 4A,B). This finding suggested that the inhibition of IL-1β-induced NF-kB activation caused by Ber could have occurred through the activation of Sirt1.
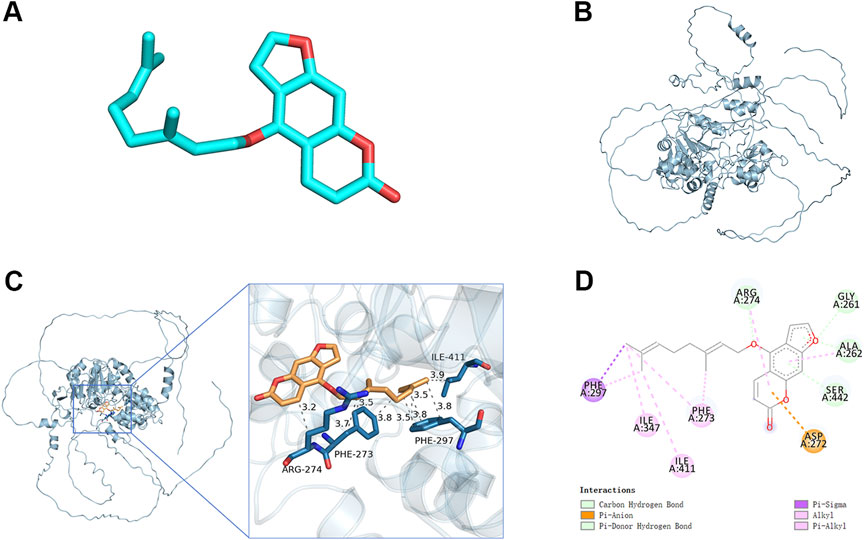
Figure 5. Molecular docking study of Ber to Sirt1. (A) 2D structure of Ber. (B) 2D structure of Sirt1. (C, D) The general view of Ber in the domain of Sirt1 structure according to the Space-filling model and Ribbon model. ##p ≤ 0.01 vs. control group; **p < 0.01 vs. IL-1β group, n = 5.
The potential mechanism underlying the activation of Sirt1 by Ber was investigated using EX-527 (an inhibitor of Sirt1). In the Western blotting analysis, EX-527 could significantly inhibits the expression of Sirt1, while the nuclear expression of p65 in chondrocytes was observed to increases (Figures 6A–C). In addition, EX-527 markedly eliminated the IL-1β-induced upregulation of COL-II expression in chondrocytes (Figures 6D–F). Furthermore, EX-527 eliminated the downregulation of MMP-13, NO, PGE2, TNF-α, and IL-6 in chondrocytes caused by treatment with Ber and IL-1β (Figures 6G–J).
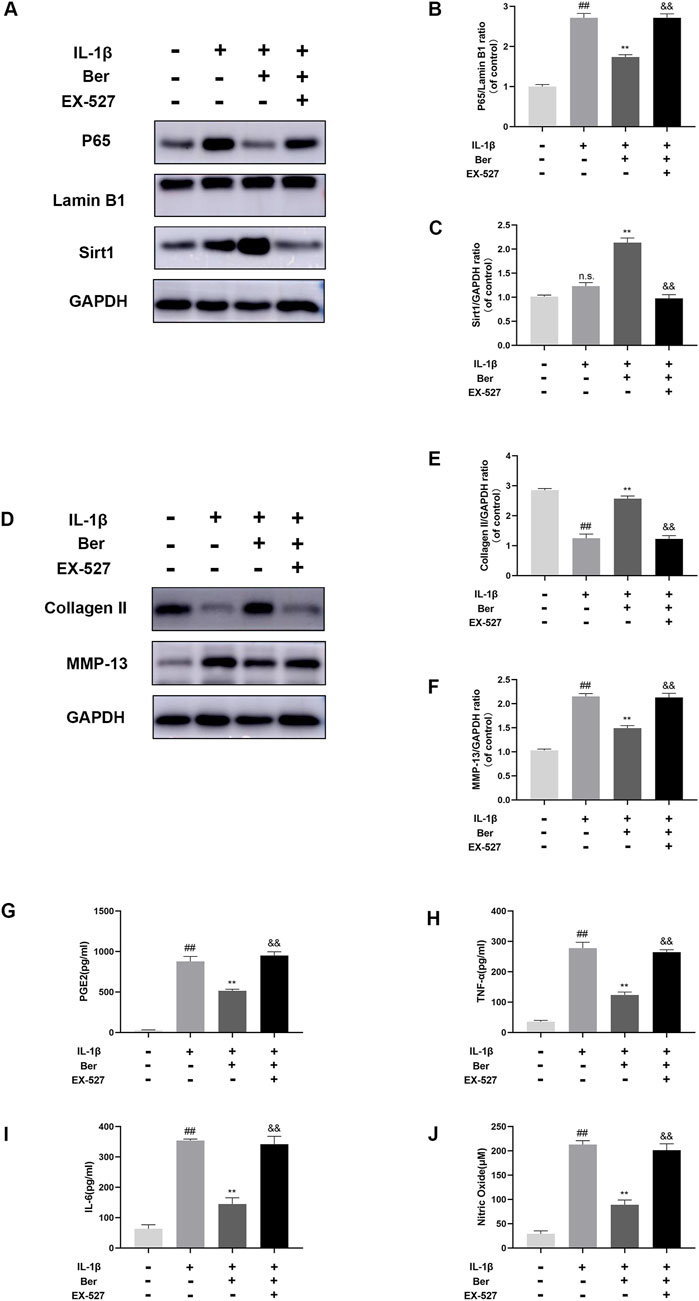
Figure 6. Ex-527 inhibits the effects of Ber in IL-1β-induced chondrocytes. (A–C) The level of P65 and Sirt1 in the chondrocytes. (D–F) The level of COL-Ⅱ and MMP-13 in the chondrocytes. (G–J) ELISA assay of IL-6 TNF-α, PGE2, AND NO in the chondrocytes. ##p ≤ 0.01 vs. control group; **p < 0.01 vs. IL-1β group, &&p < 0.01 vs. IL-1β+Ber group n = 5.
The effects of Ber on osteoarthritis (OA) were also investigated in vivo, The OA mouse model was established through DMM surgery. Afterward, these mice were administered with 20 mg/kg of Ber orally every day for the next 8 weeks. Safranin O staining was performed to assess the severity of OA in these mice. The results revealed that compared to the sham surgery group, the surgery group exhibited significant cartilage degradation, loss of extracellular matrix glycosaminoglycans, and cellular degeneration (Figure 7A). On the other hand, the group treated with Ber exhibited evident cartilage repair. H&E staining also revealed similar results and indicated that Ber had significantly improved osteoarthritis (Figure 7B). The subsequent immunohistochemical staining revealed a marked upregulation of Sirt 1 in the Ber group (Figures 7C,D). The changes in OARSI scores were also consistent with the results of H&E and Safranin O staining analyses, with the Ber group presenting lower than scores compared to the DMM group (Figure 7E). In summary, the above results provided evidence that Ber mitigated the progression of OA by limiting cartilage degradation, cartilage sclerosis, and the abnormal formation of bone spurs.
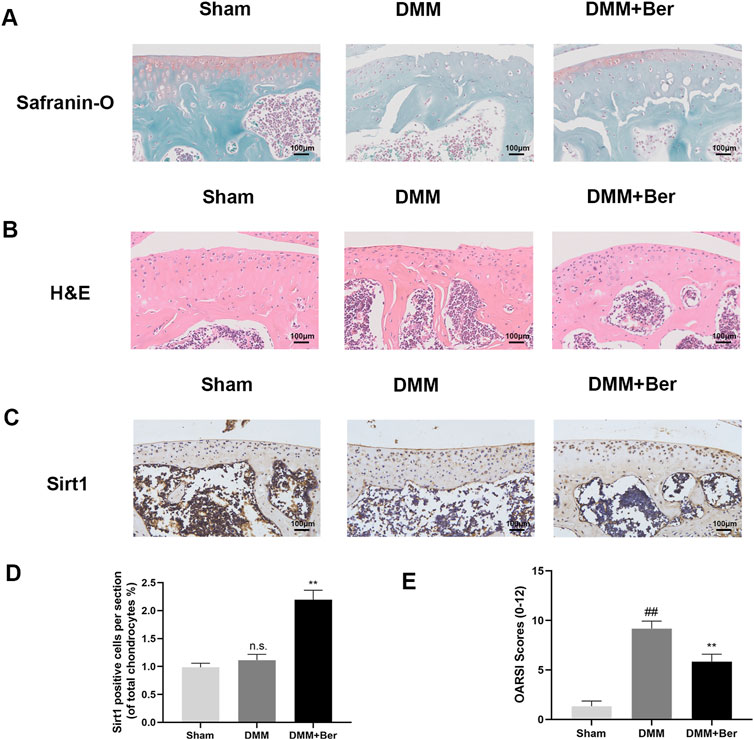
Figure 7. Ber inhibits the development of OA in the DMM model. (A) Safranin-O staining of cartilage. (B) H&E staining of cartilage. (C, E) Immunohistochemistry images of Sirt1 in the cartilage. (D) OARSI scores of cartilage. ##p ≤ 0.01 vs. Sham group; **p < 0.01 vs. DMM group, n = 6.
OA is a common disease that poses a significant threat to human wellbeing and vitality. OA has emerged as the primary cause of disability among older individuals (Silverwood et al., 2015). Over 300 million people worldwide have their daily lives significantly impacted by the long-term suffering due to arthritis (Thysen et al., 2015). OA causes pain to the affected individual and also places a significant economic burden on the entire family of the patient. The current treatments for osteoarthritis are primarily focused on symptom relief; although the medications used often lead to serious side effects (Sun et al., 2017). The primary drugs used for relieving osteoarthritis are non-steroidal anti-inflammatory drugs, although the etiology of arthritis remains poorly understood to date (da Costa et al., 2017). Therefore, it is imperative to develop novel drugs targeting the specific molecular points in OA to achieve effective treatment of this disease.
Bergamottin is a common biologically active furanocoumarin, abundantly produced abundantly in grapefruits (Li et al., 2022; Zhang et al., 2022). Bergamottin has been drawing increasing interest recently owing to its anti-inflammatory properties. It is reported that Bergamottin alleviates LPS-induced acute lung injury by inducing SIRT1 and inhibiting NF-κB (An et al., 2021). Research also suggests that Bergamottin prevents LPS-induced endotoxic shock by modulating the NF-κB signaling pathway (Liu et al., 2022). Importantly, Bergamottin blocks the activation of NF-κB signaling (Mizuno et al., 2017). These findings suggest that Bergamottin might have anti-inflammatory effects on patients with osteoarthritis.
Substantial research indicates that the NF-κB pathway plays a crucial role in the regulation of inflammatory mediators during the development of osteoarthritis (Liacini et al., 2002). Under normal conditions, the subunit protein p65 of NF-κB is bound to IκBα in the cytoplasm (Ozawa et al., 2015). However, upon IL-1β induction, IκBα undergoes phosphorylation, followed by degradation, leading to the translocation of activated p65 from cell cytoplasm to the nucleus (W. Li et al., 2016). The translocation of p65 into the nucleus, it promotes the expression of the related inflammatory factors such as NO and IL-6 (Sasaki et al., 1998). The inflammatory factor iNOS elevates the expressions of NO and MMPs, leading to the degradation of the extracellular matrix (ECM) (Goldring and Goldring, 2004). The degradation of the extracellular matrix (ECM) and/or the upregulation of matrix-degrading enzymes in chondrocytes are crucial factors for cartilage destruction (Goldring et al., 1994). The present study demonstrated that Ber could reduce the levels of matrix metalloproteinase 13 (MMP13) and ADAMTS-5 proteins in LPS-induced chondrocytes while increasing the levels of type II collagen and Aggrecan.
SIRTUIN 1 is the mammalian ortholog of yeast SIR2 (silencing information regulator). It belongs to the sirtuin family and is associated with the modulation of cell survival and transcriptional silencing (Shi et al., 2021; Wu et al., 2021). SIRTUIN 1 interacts with the NF- κB RelA/p65 subunit and also deacetylates RelA/p65 at the lysine 31,011 residue to suppress transcription, thereby affecting the transcription of various apoptosis-related genes such as ARGs, BAX, Bcl-2, Cyto-c, or inflammation-related genes (IL-6 and IL-1β) and catabolic genes (MMP3, MMP13, and ADAMTSs) (Feng et al., 2019; Liu et al., 2021).
In the present study, Ber was demonstrated to activate Sirt1, which then inhibited the activation of the NF-κB pathways. In order to investigate the association between Sirt 1 and NF- κB activation, an inhibitor of Sirt 1 was employed to suppress the expression of Sirt 1 in chondrocytes. As depicted in Figure 6, Ber alleviated inflammation in IL-1β-induced chondrocytes through the activation of Sirt1/NF-κB, and EX-527 (an inhibitor of Sirt1) could significantly eliminate this effect of Ber. These results demonstrated that Ber increased the IL-1β-mediated ECM deterioration and inflammation within chondrocytes through the Sirt 1/NF-κB signal transduction pathway.
Collectively, the results of the present study demonstrated that Ber inhibited the IL-1β-induced inflammation via the Sirt1/NF-κB pathway. DMM, as a classic animal OA model, is used widely to simulate human OA diseases. In the present study, we found that the DMM group mice exhibited cartilage degradation, extracellular matrix reduction, and matrix proteoglycan loss. However, all of these phenomena were improved dramatically upon treatment with Ber.
Further, the findings of the present study suggested that Ber inhibits the activation of NF-κB by activating the Sirt1 pathway in chondrocytes, thereby significantly ameliorating the IL-1β-induced inflammation in these cells (Figure 8). Moreover, Ber also suppressed the degradation of ECM in the in vivo models. Therefore, the in vivo and in vitro data were consistent, demonstrating the value of Ber in the treatment of OA.
Ber inhibits the inflammatory conditions induced by IL-1β induction, including the reduced expressions of MMP-13, COX-2, INOS, and ADAMTS-5 in the OA chondrocytes. Moreover, Ber inhibits IL-1β-induced inflammation by activating the Sirt1/NF-κB pathway. Accordingly, the addition of Ber to the cell culture may protect against OA in an OA mouse model. Therefore, Ber may be used as an alternative therapeutic agent for the treatment of OA in the future.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
The animal study was approved by the Animal Research Committee of Wenzhou Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
FS: Data curation, Formal Analysis, Project administration, Resources, Writing–original draft. WZ: Investigation, Methodology, Software, Validation, Visualization, Writing–original draft. QT: Conceptualization, Data curation, Formal Analysis, Project administration, Resources, Software, Writing–review and editing. JW: Funding acquisition, Investigation, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Wenzhou Municipal Sci-Tech Bureau and Rui’an Science and Technology Innovation Special Fund (MS2023053).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abramoff, B., and Caldera, F. E. (2020). Osteoarthritis: pathology, diagnosis, and treatment options. Med. Clin. North Am. 104 (2), 293–311. doi:10.1016/j.mcna.2019.10.007
An, N., Yang, T., Zhang, X. X., and Xu, M. X. (2021). Bergamottin alleviates LPS-induced acute lung injury by inducing SIRT1 and suppressing NF-κB. Innate Immun. 27 (7-8), 543–552. doi:10.1177/17534259211062553
Arra, M., and Abu-Amer, Y. (2023). Cross-talk of inflammation and chondrocyte intracellular metabolism in osteoarthritis. Osteoarthr. Cartil. 31 (8), 1012–1021. doi:10.1016/j.joca.2023.04.003
Au, R. Y., Al-Talib, T. K., Au, A. Y., Phan, P. V., and Frondoza, C. G. (2007). Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 15 (11), 1249–1255. doi:10.1016/j.joca.2007.07.009
Barnes, P. J., and Karin, M. (1997). Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336 (15), 1066–1071. doi:10.1056/NEJM199704103361506
da Costa, B. R., Reichenbach, S., Keller, N., Nartey, L., Wandel, S., Jüni, P., et al. (2017). Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 390 (10090), e21–e33. doi:10.1016/s0140-6736(17)31744-0
Feng, K., Chen, Z., Pengcheng, L., Zhang, S., and Wang, X. (2019). Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell Physiol. 234 (10), 18192–18205. doi:10.1002/jcp.28452
Goldring, M. B., Fukuo, K., Birkhead, J. R., Dudek, E., and Sandell, L. J. (1994). Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J. Cell Biochem. 54 (1), 85–99. doi:10.1002/jcb.240540110
Goldring, S. R., and Goldring, M. B. (2004). The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 427 (Suppl. l), S27–S36. doi:10.1097/01.blo.0000144854.66565.8f
Hunter, D. J., Schofield, D., and Callander, E. (2014). The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 10 (7), 437–441. doi:10.1038/nrrheum.2014.44
Hwang, Y. P., Yun, H. J., Choi, J. H., Kang, K. W., and Jeong, H. G. (2010). Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by bergamottin via the inhibition of protein kinase Cdelta/p38 mitogen-activated protein kinase and JNK/nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol. Nutr. Food Res. 54 (7), 977–990. doi:10.1002/mnfr.200900283
Jia, C., Li, X., Pan, J., Ma, H., Wu, D., Lu, H., et al. (2022). Silencing of angiopoietin-like protein 4 (Angptl4) decreases inflammation, extracellular matrix degradation, and apoptosis in osteoarthritis via the sirtuin 1/NF-κB pathway. Oxid. Med. Cell Longev. 2022, 1135827. doi:10.1155/2022/1135827
Katz, J. N., Arant, K. R., and Loeser, R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325 (6), 568–578. doi:10.1001/jama.2020.22171
Kim, S. M., Lee, E. J., Lee, J. H., Yang, W. M., Nam, D., Lee, J. H., et al. (2016). Simvastatin in combination with bergamottin potentiates TNF-induced apoptosis through modulation of NF-κB signalling pathway in human chronic myelogenous leukaemia. Pharm. Biol. 54 (10), 2050–2060. doi:10.3109/13880209.2016.1141221
Ko, J. H., Nam, D., Um, J. Y., Jung, S. H., Sethi, G., and Ahn, K. S. (2018). Bergamottin suppresses metastasis of lung cancer cells through abrogation of diverse oncogenic signaling cascades and epithelial-to-mesenchymal transition. Molecules 23 (7), 1601. doi:10.3390/molecules23071601
Kumar, A., Takada, Y., Boriek, A. M., and Aggarwal, B. B. (2004). Nuclear factor-kappaB: its role in health and disease. J. Mol. Med. Berl. 82 (7), 434–448. doi:10.1007/s00109-004-0555-y
Li, M., Zhang, Y., Zeb, A., Wu, Y., and Cheng, L. (2022). Bergamottin and PAP-1 induced ACE2 degradation to alleviate infection of SARS-CoV-2. Int. J. Mol. Sci. 23 (20), 12565. doi:10.3390/ijms232012565
Li, W., Wang, X., Niu, X., Zhang, H., He, Z., Wang, Y., et al. (2016). Protective effects of nobiletin against endotoxic shock in mice through inhibiting TNF-α, IL-6, and HMGB1 and regulating NF-κB pathway. Inflammation 39 (2), 786–797. doi:10.1007/s10753-016-0307-5
Liacini, A., Sylvester, J., Li, W. Q., and Zafarullah, M. (2002). Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 21 (3), 251–262. doi:10.1016/s0945-053x(02)00007-0
Lin, Z., Fu, C., Yan, Z., Wu, Y., Zhan, J., Lou, Z., et al. (2020a). The protective effect of hesperetin in osteoarthritis: an in vitro and in vivo study. Food Funct. 11 (3), 2654–2666. doi:10.1039/c9fo02552a
Lin, Z., Lin, C., Fu, C., Lu, H., Jin, H., Chen, Q., et al. (2020b). The protective effect of Ellagic acid (EA) in osteoarthritis: an in vitro and in vivo study. Biomed. Pharmacother. 125, 109845. doi:10.1016/j.biopha.2020.109845
Liu, A., Zhang, B., Zhao, W., Tu, Y., Wang, Q., and Li, J. (2021). Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated suppression of NF-κB and MAPKs signaling pathways. Bioengineered 12 (1), 183–195. doi:10.1080/21655979.2020.1863015
Liu, S., Song, D., and Yuan, D. (2022). Bergamottin protects against LPS-induced endotoxic shock by regulating the NF-κB signaling pathway. Immunol. Res. 70 (1), 33–43. doi:10.1007/s12026-021-09235-y
Loeser, R. F. (2009). Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 17 (8), 971–979. doi:10.1016/j.joca.2009.03.002
Millerand, M., Berenbaum, F., and Jacques, C. (2019). Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 37 (5), 48–56.
Mizuno, H., Hatano, T., Taketomi, A., Kawabata, M., and Nakabayashi, T. (2017). Bergamottin promotes adipocyte differentiation and inhibits tumor necrosis factor-α-induced inflammatory cytokines induction in 3T3-L1 cells. Yakugaku Zasshi 137 (6), 775–781. doi:10.1248/yakushi.16-00269
Ozawa, M., Nishida, K., Yoshida, A., Saito, T., Harada, R., Machida, T., et al. (2015). Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1β production and subsequent NF-κB activation. Inflamm. Res. 64 (3-4), 243–252. doi:10.1007/s00011-015-0804-2
Pereira, D., Ramos, E., and Branco, J. (2015). Osteoarthritis. Acta Med. Port. 28 (1), 99–106. doi:10.20344/amp.5477
Santangelo, K. S., Nuovo, G. J., and Bertone, A. L. (2012). In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr. Cartil. 20 (12), 1610–1618. doi:10.1016/j.joca.2012.08.011
Sasaki, K., Hattori, T., Fujisawa, T., Takahashi, K., Inoue, H., and Takigawa, M. (1998). Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J. Biochem. 123 (3), 431–439. doi:10.1093/oxfordjournals.jbchem.a021955
Shah, S. A., Khan, M., Jo, M. H., Jo, M. G., Amin, F. U., and Kim, M. O. (2017). Melatonin stimulates the SIRT1/nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-Induced oxidative stress to rescue postnatal rat brain. CNS Neurosci. Ther. 23 (1), 33–44. doi:10.1111/cns.12588
Shi, J., Cao, F., Chang, Y., Xin, C., Jiang, X., Xu, J., et al. (2021). Long non-coding RNA MCM3AP-AS1 protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced inflammation via regulating miR-138-5p/SIRT1. Bioengineered 12 (1), 1445–1456. doi:10.1080/21655979.2021.1905247
Silverwood, V., Blagojevic-Bucknall, M., Jinks, C., Jordan, J. L., Protheroe, J., and Jordan, K. P. (2015). Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr. Cartil. 23 (4), 507–515. doi:10.1016/j.joca.2014.11.019
Sun, M. M., Beier, F., and Pest, M. A. (2017). Recent developments in emerging therapeutic targets of osteoarthritis. Curr. Opin. Rheumatol. 29 (1), 96–102. doi:10.1097/BOR.0000000000000351
Theeuwes, W. F., van den Bosch, M. H. J., Thurlings, R. M., Blom, A. B., and van Lent, P. (2021). The role of inflammation in mesenchymal stromal cell therapy in osteoarthritis, perspectives for post-traumatic osteoarthritis: a review. Rheumatol. Oxf. 60 (3), 1042–1053. doi:10.1093/rheumatology/keaa910
Thysen, S., Luyten, F. P., and Lories, R. J. (2015). Targets, models and challenges in osteoarthritis research. Dis. Model Mech. 8 (1), 17–30. doi:10.1242/dmm.016881
Wang, X., Tian, Y., Liang, X., Yin, C., Huai, Y., Zhao, Y., et al. (2022). Bergamottin promotes osteoblast differentiation and bone formation via activating the Wnt/β-catenin signaling pathway. Food Funct. 13 (5), 2913–2924. doi:10.1039/d1fo02755g
Wu, B. W., Wu, M. S., Liu, Y., Lu, M., Guo, J. D., Meng, Y. H., et al. (2021). SIRT1-mediated deacetylation of NF-κB inhibits the MLCK/MLC2 pathway and the expression of ET-1 thus alleviating the development of coronary artery spasm. Am. J. Physiol. Heart Circ. Physiol. 320 (1), H458–H468. doi:10.1152/ajpheart.00366.2020
Xian, S., Lin, Z., Zhou, C., and Wu, X. (2022). The protective effect of evodiamine in osteoarthritis: an in vitro and in vivo study in mice model. Front. Pharmacol. 13, 899108. doi:10.3389/fphar.2022.899108
Xie, J., Zhang, X., and Zhang, L. (2013). Negative regulation of inflammation by SIRT1. Pharmacol. Res. 67 (1), 60–67. doi:10.1016/j.phrs.2012.10.010
Keywords: osteoarthritis, Sirt1/NF-κB, Bergamottin, chondrocytes, anti-inflammation
Citation: Shen G, Zhang W, Tu Q and Wang J (2024) Bergamottin (Ber) ameliorates the progression of osteoarthritis via the Sirt1/NF-κB pathway. Front. Pharmacol. 15:1389786. doi: 10.3389/fphar.2024.1389786
Received: 22 February 2024; Accepted: 12 April 2024;
Published: 29 April 2024.
Edited by:
Opeyemi Joshua Olatunji, Prince of Songkla University, ThailandReviewed by:
Abdul Basit, Prince of Songkla University, ThailandCopyright © 2024 Shen, Zhang, Tu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juncheng Wang, MTcxODI4NjMzQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.