- 1Department of Clinical Pharmacology and Therapeutic, College of Medicine, Al-Mustansiriyah University, Baghdad, Iraq
- 2Department of Anesthesia, Al-Nukaba University College, Baghdad, Iraq
- 3Pharmacognosy Department, Faculty of Pharmacy, Tanta University, Tanta, Egypt
- 4Pharmacology and Toxicology Department, Faculty of Pharmacy, Tanta University, Tanta, Egypt
- 5Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 6Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
- 7Pharmacology Department & Health Research Unit, Medical College, Jouf University, Sakakah, Saudi Arabia
- 8Pharmacology Department, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
- 9Laboratory Medicine Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia
- 10Center for Food Analysis (NAL), Technological Development Support Laboratory (LADETEC), Federal University of Rio de Janeiro (UFRJ), Cidade Universitária, Rio de Janeiro, Brazil
Doxorubicin (DOX) is an anticancer agent for treating solid and soft tissue malignancies. However, the clinical use of DOX is restricted by cumulative, dose-dependent cardiotoxicity. Therefore, the present study aimed to assess the cardioprotective effects of P. ginseng C. A. Mey, febuxostat, and their combination against DOX-induced cardiotoxicity. Thirty-five Sprague Dawley male rats were used in this study. The animals were randomly divided into five groups, with seven rats per group. The control group received normal saline, the induced group received DOX only, and the treated group received P. ginseng, febuxostat, and their combination before DOX treatment. Biomarkers of acute cardiac toxicity were assessed in each group. Results showed that treatment with the combination of febuxostat and P. ginseng before DOX led to a significant improvement in the biomarkers of acute DOX-induced cardiotoxicity. In conclusion, the combination of P. ginseng and febuxostat produced more significant cardioprotective effects against DOX-induced cardiotoxicity when compared to either P. ginseng or febuxostat when used alone. The potential mechanism of this combination was mainly mediated by the anti-inflammatory and antioxidant effects of P. ginseng and febuxostat.
Introduction
Doxorubicin (DOX) belongs to the anthracycline antibiotic family, considered the most effective anticancer agent for treating malignancies. However, the clinical use of DOX is restricted by its cumulative, dose-dependent cardiotoxicity, which may lead to irreversible heart failure or reduce the quality of life (Al-Kuraishy et al., 2015). In addition, DOX-induced cardiotoxicity is presented with acute heart failure, arrhythmias, and progressive cardiomyopathy (Al-Kuraishy and Al-Gareeb, 2016).
DOX-induced cardiotoxicity is mainly due to oxidative stress development, mitochondrial damage, and lipid peroxidation (Alkuraishy et al., 2017). DOX produces a variety of reactive oxygen species (ROS), which cause endoplasmic reticulum calcium leakage, DNA damage, and autophagy flux suppression, ultimately resulting in ferroptosis and lipid peroxidation (Abushouk et al., 2019; Podyacheva et al., 2021). The heart is sensitive to oxidative damage due to low antioxidant enzymes, large mitochondrial density/volume, and a higher oxygen consumption rate (Onohuean et al., 2021). Oxidative stress due to DOX-induced cardiotoxicity also develops due to the reduction of endogenous antioxidant capacity. Of interest, nuclear factor erythroid 2 (Nrf2), which acts as a sensor to regulate adaptive responses during oxidative stress, can potentially increase the expression of antioxidant enzymes. Nrf2 has been inhibited in DOX-induced cardiotoxicity (Xu et al., 2020).
Different herbal medicines and drugs alone or in combination have been tried to attenuate or prevent DOX-induced cardiotoxicity (Amin et al., 2021; Mu et al., 2021). Mainly, anti-inflammatory and antioxidant agents may play a crucial role in preventing DOX-induced cardiotoxicity (Sahasrabudhe et al., 2018). Several natural products demonstrated high potency against DOX-induced cardiotoxicity (El-Kharrag et al., 2017; Nurtay et al., 2021; Xie et al., 2021). Induced cardiotoxicity has been prevented by natural product nanoparticles (Al Shamsi et al., 2004; Amin and Mahmoud-Ghoneim 2011; Baig et al., 2019). For example, (Al Fatease et al., 2019) found that combinational polymeric micelles for delivery of resveratrol and quercetin in ovarian cancer were shown to be effective.
P. ginseng Family Araliaceae is found in eastern Asia and North America. P. ginseng contains more than 40 isolated active ingredients, including ginsenosides, sesquiterpenes, polyacetylenes, polysaccharides, and peptidoglycans (Farooqui et al., 2017). P. ginseng is increasingly used as alternative medicine or complementary medicine in treating different diseases, including cancer, neurodegenerative, cardiovascular, and chronic inflammation (Choi et al., 2015). P. ginseng reduces oxidative stress and restores antioxidant capacity in rats (Zhao et al., 2015). Its significant cardioprotective effects may lead to the synthesis of daily supplements that protect the heart from DOX-induced cardiotoxicity.
Febuxostat is a xanthine oxidase inhibitor indicated in patients with gout suffering from hyperuricemia, and it is used chiefly in the management of chronic gout (Zhang et al., 2021). With minimal adverse effects, Febuxostat is more effective than allopurinol at its standard doses (Wang et al., 2021).
The focus on xanthine oxidase inhibitors has increased due to their anti-inflammatory, antioxidant, and immune-modulatory effects, which might be beneficial in the treatment of different inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and ulcerative colitis (El-Mahdy et al., 2020) and thus, it may be beneficial in alleviating oxidative stress and inflammation associated with DOX-induced cardiotoxicity. Therefore, the present study aimed to evaluate the possible cardioprotective effects of P. ginseng and febuxostat alone or in combination against DOX-induced cardiotoxicity.
Materials and Methods
Drugs and Chemicals
Doxorubicin (Adricin®) was obtained from Hikma Pharmaceuticals (Cairo, Egypt). Febuxostat 120 mg (Feburic®) from Alhikma Co. (Jordan) was used. The commercially used P. ginseng was a well-prepared capsule (P. ginseng capsule, Euro Herbs, California Gold Nutrition, United States). It was prepared from the plant’s roots with the following botanical characteristics (phylum: Embryophyta Siphonogama, subphylum: Angiospermae, class: Dicotyledoneae, subclass: Archichlamydeae, order: Umbelliflorae, family: Araliaceae, genus: Panax). All the other chemicals were of high analytical grade and were commercially obtained (Global Medical Instrumentation, Ohaos, United States). DOX, P. ginseng, and febuxostat were dissolved in normal saline separately. The dose of each drug was calculated according to previous experimental studies in which these doses exhibited the best results (Ammar et al., 2011; Shamim and Khan, 2018; Abdel-Aziz et al., 2020).
Animals
Thirty-five Sprague Dawley male rats were used in this study. The animals were obtained from the Iraqi Center for Cancer and Medical Genetic Research (Mustansiriyah University, Iraq). Their body weights ranged from 150 to 250 g. The rats were housed in sterile cages and kept at 25°C with a 12/12 light-dark cycle. The rats were allowed to chow pellets and drink tap water ad libitum (standard pellet, Purina, United States). They were left for 2 weeks without interference to acclimatize. All cages and materials used to prepare food were sterile; all rats were free of any illness during the observation period. The used animal procedures were held according to the guide for the care of laboratory animals (LaFollette et al., 2020). The experiment was carried out in accordance with the criteria for the care and use of laboratory animals, which were authorized by the Research Ethical Committee (Faculty of Pharmacy, Tanta University, Egypt), Approval No. (PO-2021-00126-E).
Study Design
After 2 weeks of acclimatization, one diseased rat was excluded. The animals were randomly divided into five groups, with seven rats in each group. On day ten, all groups were sacrificed. Control group: Received normal saline per oral (2.5 ml/kg)/day) for 10 days (n = 7), Doxorubicin group: Received normal saline per oral (2.5 ml/kg/day) for 10 days, followed by a single dose of DOX (15 mg/kg) intraperitoneally (IP) (Ammar et al., 2011) and on day eight, serving as a DOX group (n = 7). DOX + P. ginseng group: Received P. ginseng per oral (100 mg/kg) (Shamim and Khan, 2018) daily for ten successive days, and on day eight, 1 h after drug administration, a single dose of DOX (15 mg/kg) IP, was given (n = 7). DOX + febuxostat group: Received febuxostat (10 mg/kg) (Abdel-Aziz et al., 2020) per oral daily for ten successive days, and on day eight, 1 h after drug administration, a single dose of DOX (15 mg/kg) IP was given (n = 7). DOX + combination group: Received P. ginseng (100 mg/kg) and febuxostat (10 mg/kg) per oral, 1 h apart for successful separation and to avoid physical drug interaction, daily for 10 days, and on day eight, 1 h after drug administration, a single dose of DOX (15 mg/kg) IP was given (n = 7). On the 11th day of the study, animals were sacrificed, and hearts were taken for histopathological observations. Blood samples were taken for biochemical analysis.
Samples Collection
At room temperature, 25°C, the rats were anesthetized using chloroform. Blood samples were collected by intracardiac puncture in sterile, labeled tubes and then centrifuged for 10 min at 3,000 rpm. The samples were stored at −20°C to be assessed later. The rats were sacrificed to obtain the hearts immediately immersed in normal iced saline to prevent ischemic heart injury caused by further beating. Hearts were fixed in neutral buffered formalin (10%) to harden the tissue and avoid structural changes due to autolysis by the tissue lysosomal enzymes.
Assessment of Biochemical Variables
Serum levels of brain natriuretic peptide (BNP), cardiac troponin-I (cTn-I), caspase-3, glutathione peroxidase (GP), malondialdehyde (MDA), lipid peroxidase (LPO) and tumor necrosis factor-alpha (TNF-α) were determined using ELISA kit methods (MyBioSource, San Diego, CA, United States).
Histopathological Studies
Animals’ hearts were fixed in a formaldehyde solution (10%) to harden the tissue. Cross-sectional cuts were made to obtain the ventricles. Dehydration was done gradually to prevent shrinkage of the tissue. Then an infiltration process was done to support the tissue during the sectioning step by filling the tissue with paraffin. They were embedded in the following sequence: distilled water for washing, 70% alcohol for 2 h, 80% alcohol for 2 h, 90% alcohol for 2 h, 95% alcohol for 2 h, 100% alcohol for 2 h, xylene for 1 h, and finally paraffin for 2 h. The tissue is embedded and solidified into a hard paraffin cube at room temperature and then sectioned by a microtome to produce a thin tissue section of a known thickness. The sections were placed on the slides and left to dry for about 24 h. Finally, the slides were stained with two different dyes (eosin and hematoxylin).
Statistical Analysis
Data are presented as mean ± S.D. (standard deviation) Multiple comparisons among different groups were performed by one-way analysis of variance (ANOVA), followed by Tukey-Kramer as a posthoc test using GraphPad Prism version 9 (GraphPad Software, Inc. San Diego, CA, United States). Results were considered statistically significant at p < 0.05.
Results
Biochemical Parameters
DOX-induced cardiotoxicity was evidenced by the significant reduction in GP serum level and the increase in LPO and MDA levels compared to the control (p < 0.05). Febuxostat administration before DOX led to a significant elevation of GP serum levels (p < 0.05). However, when compared to the DOX group, the suppressive effect of febuxostat on LPO and MDA was insignificant (p > 0.05). Also, P. ginseng administration before DOX significantly elevated the GP and suppressed the MDA levels (p < 0.05) but the suppressive effect on LPO was insignificant (p > 0.05). Moreover, the combination of febuxostat and P. ginseng demonstrated a significant improvement in GP, LPO, and MDA (p < 0.05) (Table 1).
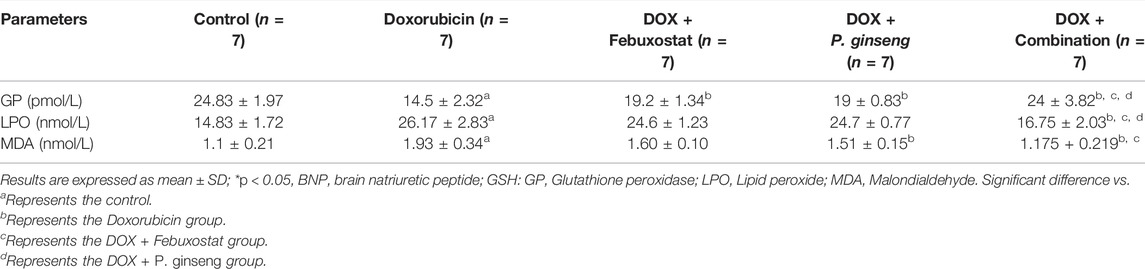
TABLE 1. Effects of P. ginseng and Febuxostat on oxidative stress biomarkers in DOX-induced cardiotoxicity.
DOX administration significantly increased cTnI, BNP, caspase-3, and TNF-α levels (p < 0.05). Febuxostat administration before DOX led to a significant decrease in cTnI and caspase-3 (p < 0.05), but the effect on BNP and TNF-α was insignificant (p > 0.05) when compared to the DOX group. Treatment with P. ginseng led to a significant decrease in cTnI, BNP, caspase-3, and TNF-α (p < 0.05). Furthermore, the combination of febuxostat and P. ginseng illustrated a significant suppressive effect on TNF-α and cTnI as compared to each single-drug therapy (p < 0.05) (Figure 1).
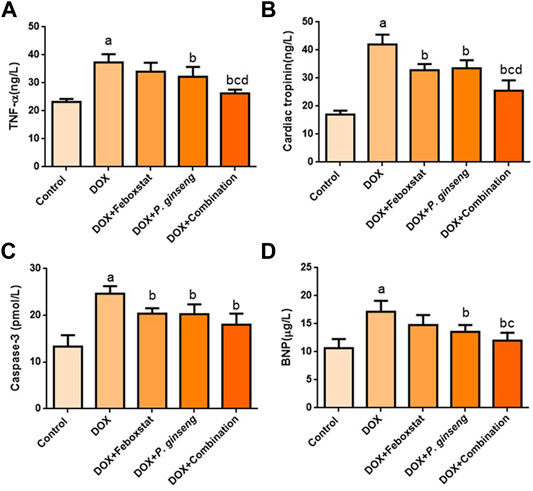
FIGURE 1. Effects of drug treatments on (A) Serum TNF-α level, (B) Cardiac Tropinin level (C) Serum Caspase-3 level, and (D) Serum BNP level.
Histopathological Changes
The control group sections showed a normal myocardial tissue structure with a peripherally located normal oval nucleus and branching striated muscle fibers. While the sections of the DOX group showed many congested vessels with extravasation of red blood cells, edema, cytoplasmic vacuolations, decreased nuclei, loss of muscle fiber striation, and fragmentation with necrosis. While sections of the febuxostat group showed improved myocardial damage with preserved nuclei and without fragmentation of muscle fibers, congested and dilated, blood vessels were still present. Also, edema and extravasation of red blood cells were still present. The P. ginseng group myocardial tissue section showed improved myocardial damage apart from edema and vacuolations. In addition, sections of the combination group showed nearly normal-looking cardiac muscle tissue. Light microscopic magnification was done using two powers, ×40 and ×100 (Figure 2).
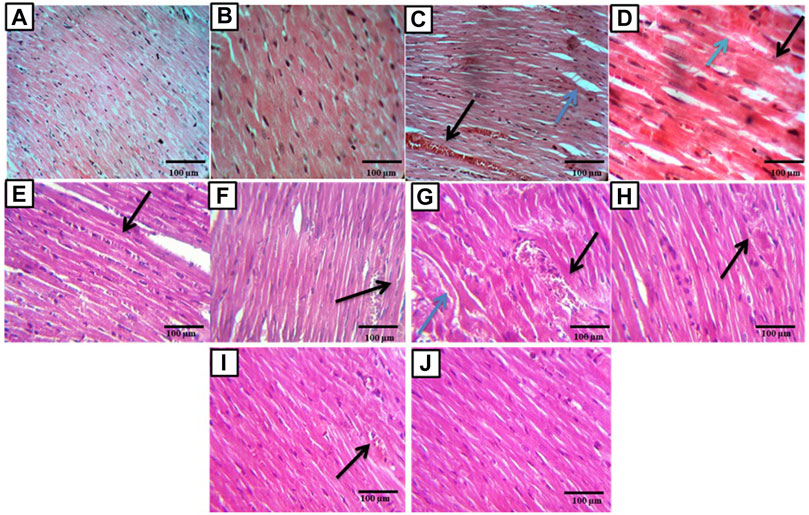
FIGURE 2. Histopathological examination of cardiac sections: (A,B) sections showed normal rat myocardial tissue, magnification ×40, ×100 respectively. (C) Section of the DOX affected myocardial tissue showed congested and dilated blood vessel (black arrow) with edema (blue arrow) (×40). (D) Section of the DOX affected myocardial tissue showed fragmented muscle fibers (black arrow), decreased number of nuclei, and extravasation of R.B.C.s (blue arrow) (×100). (E,F) Sections of the DOX + Febuoxatat group showed improved DOX-induced myocardial damage with preserved nuclei and no muscle fibers fragmentation but congested and dilated blood vessels are still present (arrow) (×40), no muscles fibers fragmentation but odema and extravasation of R.B.C.s are still present (black arrow) (×100). (G,H) Sections of the DOX + P.Ginseng group showed improved DOX-induced myocardial damage apart from odema and vaculations (blue arrows) (×40) and showed area of coagulative necrosis, vascular congestion and chronic inflammation cell (black arrow) (×100). (I,J) sections of the DOX + combination group showed nearly normal-looking cardiac muscle tissue (×40) apart from a congested blood vessel (black arrow) (×100).
Discussion
Cardiac toxicity is the principal dose-limiting factor for DOX use as an anticancer treatment (Yin and Shen, 2021). DOX-induced cardiotoxicity develops due to complex molecular mechanisms including mitochondrial dysfunction, apoptosis, necrosis, and oxidative stress (Cardinale et al., 2020). cTn-I has been regarded as the gold standard biomarker for myocardial injury and cardiotoxicity (Murabito et al., 2020; Abdalla et al., 2021). Of note, cTn-I is released into the plasma when cardiac myocytes are injured (Varricchi et al., 2018).
The present study clearly showed that DOX-induced myocardial injury led to significant elevations in the cTn-I and LPO serum levels, as consistent with recent findings (Kumari et al., 2020; Lan et al., 2020). The increase in LPO levels could be attributed to the toxic effects of DOX on the cardiomyocytes through oxidative degradation. Febuxostat reduced cTn-I and LPO serum levels, as documented by recent studies (Ayza et al., 2020; Tanaka et al., 2021). For this reason, febuxostat may exert a cardioprotective effect against DOX-induced cardiotoxicity.
Regarding the effect on oxidative stress, DOX showed a significant decline in the endogenous antioxidant capacity, as evident by a significant reduction in GP serum levels and a remarkable increase in MDA levels, confirming the well-known hypothesis that free radicals play a significant role in DOX-induced cardiotoxicity, and these results were in line with previous reports (Gammella et al., 2014; Lenihan, 2014; Nebigil and Désaubry, 2018). Moreover, the pretreatment with febuxostat led to a significant increase in GP levels and a substantial decrease in MDA serum, coinciding with different studies (Shim et al., 2017; Ahmed et al., 2021).
Myocardial TNF-α was an autocrine contributor to myocardial dysfunction and cardiomyocyte death in ischemia-reperfusion injury, sepsis, and chronic heart failure (Sheppard et al., 2013). In the present study, DOX significantly elevated the TNF-α serum level. However, febuxostat lowered the TNF-α serum level. This outcome corresponds with different studies that disclosed a potential effect of febuxostat in reducing pro-inflammatory cytokines (El-mahdy et al., 2020; Ahmed et al., 2021). Likewise, BNP was significantly increased with DOX treatment. The results of the present study are in agreement with previous results (Sheppard et al., 2013). On the other hand, another study reported that low BNP serum levels during acute DOX-induced cardiotoxicity might be due to inhibiting the expression of the BNP gene (Murabito et al., 2020).
In the present study, febuxostat showed a reasonable but non-significant decrease in BNP serum levels, as shown previously (Ahmed et al., 2021). This was due to the antioxidant, anti-inflammatory, and anti-apoptotic effects of febuxostat (Al-Kuraishy et al., 2019). Furthermore, DOX may induce myocardial cell apoptosis by activating mitochondrial caspase-3 (Fidale et al., 2018). This study clearly showed a significant elevation in the plasma level of caspase-3 with DOX treatment, as revealed previously (Shim et al., 2017). However, febuxostat pretreatment significantly decreased caspase-3 serum levels, coinciding with the findings of a previous study (Krishnamurthy et al., 2015).
Our observations demonstrated that febuxostat could limit the infarct size of acute myocardial infarction. This reduction is accompanied by changes in the level of matrix metalloproteinase enzymes, biomarkers of cardiac cell injury (Al-Kuraishy et al., 2019).
The present study also showed that cTn-I was reduced significantly with P. ginseng pretreatment, coinciding with a previous experimental study (Al Shamsi et al., 2006), which showed that the active ingredient of the P. ginseng extract, ginsenoside-Rg1, enhanced angiogenesis and reduced ventricular remodeling in a rat model of myocardial infarction. Similarly, P. ginseng pretreatment showed a significant decrease in serum BNP and caspase-3 levels, coinciding with various studies (Mohan et al., 2018; Hamza et al., 2021; Murali et al., 2021).
Moreover, P. ginseng significantly elevated the GP serum level. Several studies (Al-Dabbagh et al., 2018; Li et al., 2020; Nazarbek et al., 2021) confirmed this finding. As well, the MDA serum level showed a significant reduction after the administration of P. ginseng, coinciding with a previous report (Wang et al., 2019). Furthermore, P. ginseng reduced the TNF-α serum level, which might be attributed to TNF-α inhibition in the myocardium (Parlakpinar et al., 2019).
The oxidative degradation of lipids was also ameliorated by the antioxidant effect of P. ginseng. In this study, P. ginseng pretreatment showed a decline in the LPO level. This result corresponds with previous studies confirming the antioxidant effect of P. ginseng (Zhang et al., 2018; Benassi et al., 2021). Indeed, ginsenoside-Rg1 enhanced angiogenesis by suppressing the progression of cardiac fibrosis (Geng et al., 2020; Abdalla et al., 2021). Thus, the remarkable cardioprotective effect of P. ginseng in the present study may be produced by its anti-proliferative action, as evident by the amelioration of DOX-induced histopathological changes (Yu et al., 2021).
The potential protective mechanism of P. ginseng against DOX-induced cardiotoxicity is related to the antioxidant, anti-apoptotic, and anti-inflammatory effects of P. ginseng constituents. An experimental study demonstrated that P. ginseng mitigated the electrocardiographic and histopathological changes induced by DOX and restored the antioxidant capacity (Chen et al., 2021). Another study reported that P. ginseng constituents might be a novel candidate for improving DOX-induced cardiotoxicity (Wan et al., 2021).
Of interest, the pretreatment with a combination of P. ginseng and febuxostat significantly reduced the biomarkers of DOX-induced cardiotoxicity more than either P. ginseng or febuxostat when used alone. Previous studies declared that both P. ginseng and febuxostat had antioxidant and anti-inflammatory properties that were interrelated at the molecular level. Both P. ginseng and febuxostat exerted antioxidant properties partially by interfering with NADPH oxidase activity (Lu et al., 2018; Juaid et al., 2021). Additionally, both P. ginseng and febuxostat had anti-inflammatory and antioxidant properties that significantly reduced oxidative stress and inflammatory reactions (Sung et al., 2021; Xu et al., 2021).
Taken together, the present study confirmed the protective effects of the febuxostat and P. ginseng combination against DOX-induced cardiotoxicity as manifested by the considerable improvement in toxicity biomarkers and histological damage.
Applying the current findings on clinical studies could be of a great importance in the reduction of DOX-induced cardiotoxicity in DOX-treated patients as in leukemia, lymphoma, and solid tumors (Kalyanaraman, 2020). Anti-inflammatory and antioxidant agents could be a therapeutic potential strategy in the mitigation of cardiotoxicity in patients treated by DOX (Bruynzeel et al., 2007). Therefore, P. ginseng and febuxostat in virtue of their antioxidant and anti-inflammatory properties could be a novel combination in reducing DOX-induced cardiotoxicity in high-risk patients.
Conclusion
The current study indicated that the combination of P. ginseng and febuxostat confers better cardioprotective effects against DOX-induced cardiotoxicity than single-drug therapy. The anti-inflammatory and anti-apoptotic activities may mediate the potential cardioprotective effects and antioxidant effects of P. ginseng and febuxostat. Therefore, preclinical and clinical studies are warranted to confirm the clinical benefit of P. ginseng or febuxostat in patients at high risk of developing DOX-induced cardiotoxicity.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors upon reasonable request.
Ethics Statement
The animal study was reviewed and approved by The Research Ethical Committee (Faculty of Pharmacy, Tanta University, Egypt), Approval No. (PO-2021-00126-E).
Author Contributions
HA-K, AA-G, and HA-H: conceptualization and performed the experiment. WN, AE-K, GB, and NN performed data analysis, writing, and editing. GM-H and AQ cooperated in performing the experiment. CC-J: supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla, A., Murali, C., and Amin, A. (2021). Safranal Inhibits Angiogenesis via Targeting HIF-1α/VEGF Machinery: In Vitro and Ex Vivo Insights. Front. Oncol. 11, 172. doi:10.3389/fonc.2021.789172
Abdel-Aziz, A. M., Gamal El-Tahawy, N. F., Salah Abdel Haleem, M. A., Mohammed, M. M., Ali, A. I., and Ibrahim, Y. F. (2020). Amelioration of Testosterone-Induced Benign Prostatic Hyperplasia Using Febuxostat in Rats: The Role of VEGF/TGFβ and iNOS/COX-2. Eur. J. Pharmacol. 889, 173631. doi:10.1016/j.ejphar.2020.173631
Abushouk, A. I., Salem, A. M. A., Saad, A., Afifi, A. M., Afify, A. Y., Afify, H., et al. (2019). Mesenchymal Stem Cell Therapy for Doxorubicin-Induced Cardiomyopathy: Potential Mechanisms, Governing Factors, and Implications of the Heart Stem Cell Debate. Front. Pharmacol. 10, 635. doi:10.3389/fphar.2019.00635
Ahmed, A. Z., Mumbrekar, K. D., Satyam, S. M., Shetty, P., D'Souza, M. R., and Singh, V. K. (2021). Chia Seed Oil Ameliorates Doxorubicin-Induced Cardiotoxicity in Female Wistar Rats: an Electrocardiographic, Biochemical and Histopathological Approach. Cardiovasc Toxicol. 21 (7), 533–542. doi:10.1007/s12012-021-09644-3
Al Shamsi, M., Amin, A., and Adeghate, E. (2006). Vitamin E Ameliorates Some Biochemical Parameters in Normal and Diabetic Rats. Ann. N. Y. Acad. Sci. 1084, 411–431. doi:10.1196/annals.1372.033
Al Shamsi, M. S., Amin, A., and Adeghate, E. (2004). Beneficial Effect of Vitamin E on the Metabolic Parameters of Diabetic Rats. Mol. Cell Biochem. 261, 35–42. doi:10.1023/B:MCBI.0000028735.79172.9b
Al-Dabbagh, B., Elhaty, I. A., Murali, C., Madhoon, A. A., and Amin, A. (2018). <i>Salvadora Persica</i> (Miswak): Antioxidant and Promising Antiangiogenic Insights. Ajps 09 (6), 1228–1244. doi:10.4236/ajps.2018.96091
Al-Kuraishy, H. M., and Al-Gareeb, A. I. (2016). Potential Effects of Pomegranate on Lipid Peroxidation and Pro-inflammatory Changes in Daunorubicin-Induced Cardiotoxicity in Rats. Int. J. Prev. Med. 7, 85. doi:10.4103/2008-7802.184314
Al-Shamsi, M., Amin, A., and Adeghate, E. (2006). Effect of Vitamin C on Liver and Kidney Functions in Normal and Diabetic Rats. Ann. N. Y. Acad. Sci. 1084, 371–390. doi:10.1196/annals.1372.031
Alkuraishy, H., Khaleel, K., and Mohammed, M. (2015). Significant Attenuation and Amelioration Effects of Labetalol in Doxorubicin Induced Cardiotoxicity: An Animal Model Study. Journal-CVS 3 (2), 25–29. doi:10.5455/jcvs.2015321
Alkuraishy, H. M., Al-Gareeb, A. I., and Al-hussaniy, H. A. (2017). Doxorubicin-induced Cardiotoxicity: Molecular Mechanism and Protection by Conventional Drugs and Natural Products. Int. J. Clin. Oncol. Cancer Res. 2 (2), 31–44. doi:10.11648/j.ijcocr.20170202.12
Amin, A., Farrukh, A., Murali, C., Soleimani, A., Praz, F., Graziani, G., et al. (2021). Saffron and its Major Ingredients' Effect on Colon Cancer Cells with Mismatch Repair Deficiency and Microsatellite Instability. Molecules 26 (13), 3855. doi:10.3390/molecules26133855
Amin, A., and Mahmoud-Ghoneim, D. (2011). Texture Analysis of Liver Fibrosis Microscopic Images: a Study on the Effect of Biomarkers. Acta Biochim. Biophys. Sin. (Shanghai) 43 (3), 193–203. doi:10.1093/abbs/gmq129
Ammar, el-S. M., Said, S. A., Suddek, G. M., and El-Damarawy, S. L. (2011). Amelioration of Doxorubicin-Induced Cardiotoxicity by Deferiprone in Rats. Can. J. Physiol. Pharmacol. 89 (4), 269–276. doi:10.1139/y11-020
Ayza, M. A., Zewdie, K. A., Tesfaye, B. A., Wondafrash, D. Z., and Berhe, A. H. (2020). The Role of Antioxidants in Ameliorating Cyclophosphamide-Induced Cardiotoxicity. Oxidative Med. Cell. Longev. 2020, 1–14. doi:10.1155/2020/4965171
Baig, B., Halim, S. A., Farrukh, A., Greish, Y., and Amin, A. (2019). Current Status of Nanomaterial-Based Treatment for Hepatocellular Carcinoma. Biomed. Pharmacother. 116, 108852. doi:10.1016/j.biopha.2019.108852
Benassi, E., Fan, H., Sun, Q., Dukenbayev, K., Wang, Q., Shaimoldina, A., et al. (2021). Generation of Particle Assemblies Mimicking Enzymatic Activity by Processing of Herbal Food: the Case of Rhizoma Polygonati and Other Natural Ingredients in Traditional Chinese Medicine. Nanoscale Adv. 3 (8), 2222–2235. doi:10.1039/d0na00958j
Bruynzeel, A. M., Niessen, H. W., Bronzwaer, J. G., van der Hoeven, J. J., Berkhof, J., Bast, A., et al. (2007). The Effect of Monohydroxyethylrutoside on Doxorubicin-Induced Cardiotoxicity in Patients Treated for Metastatic Cancer in a Phase II Study. Br. J. Cancer 97 (8), 1084–1089. doi:10.1038/sj.bjc.6603994
Cardinale, D., Iacopo, F., and Cipolla, C. M. (2020). Cardiotoxicity of Anthracyclines. Front. Cardiovasc Med. 7, 26. doi:10.3389/fcvm.2020.00026
Chen, Y., Wang, L., Liu, T., Qiu, Z., Qiu, Y., and Liu, D. (2021). Inhibitory Effects of Panax Ginseng Glycoproteins in Models of Doxorubicin-Induced Cardiac Toxicity In Vivo and In Vitro. Food Funct. 12 (21), 10862–10874. doi:10.1039/d1fo01307f
Choi, S. H., Jung, S. W., Lee, B. H., Kim, H. J., Hwang, S. H., Kim, H. K., et al. (2015). Ginseng Pharmacology: a New Paradigm Based on Gintonin-Lysophosphatidic Acid Receptor Interactions. Front. Pharmacol. 6, 245. doi:10.3389/fphar.2015.00245
El-Kharrag, R., Amin, A., Hisaindee, S., Greish, Y., and Karam, S. M. (2017). Development of a Therapeutic Model of Precancerous Liver Using Crocin-Coated Magnetite Nanoparticles. Int. J. Oncol. 50 (1), 212–222. doi:10.3892/ijo.2016.3769
El-Mahdy, N. A., Saleh, D. A., Amer, M. S., and Abu-Risha, S. E. (2020). Role of Allopurinol and Febuxostat in the Amelioration of Dextran-Induced Colitis in Rats. Eur. J. Pharm. Sci. 141, 105116. doi:10.1016/j.ejps.2019.105116
Farooqui, Z., Ahmed, F., Rizwan, S., Shahid, F., Khan, A. A., and Khan, F. (2017). Protective Effect of Nigella Sativa Oil on Cisplatin Induced Nephrotoxicity and Oxidative Damage in Rat Kidney. Biomed. Pharmacother. 85, 7–15. doi:10.1016/j.biopha.2016.11.110
Fatease, A. A., Shah, V., Nguyen, D. X., Cote, B., LeBlanc, N., Rao, D. A., et al. (2019). Chemosensitization and Mitigation of Adriamycin-Induced Cardiotoxicity Using Combinational Polymeric Micelles for Co-delivery of Quercetin/resveratrol and Resveratrol/curcumin in Ovarian Cancer. Nanomedicine 19, 39–48. doi:10.1016/j.nano.2019.03.011
Fidale, T. M., Antunes, H. K. M., Alex dos Santos, L., Rodrigues de Souza, F., Deconte, S. R., Borges Rosa de Moura, F., et al. (2018). Increased Dietary Leucine Reduces Doxorubicin-Associated Cardiac Dysfunction in Rats. Front. Physiol. 8, 1042. doi:10.3389/fphys.2017.01042
Gammella, E., Maccarinelli, F., Buratti, P., Recalcati, S., and Cairo, G. (2014). The Role of Iron in Anthracycline Cardiotoxicity. Front. Pharmacol. 5, 25. doi:10.3389/fphar.2014.00025
Geng, J., Fu, W., Yu, X., Lu, Z., Liu, Y., Sun, M., et al. (2020). Ginsenoside Rg3 Alleviates Ox-LDL Induced Endothelial Dysfunction and Prevents Atherosclerosis in ApoE-/- Mice by Regulating PPARγ/FAK Signaling Pathway. Front. Pharmacol. 11, 500. doi:10.3389/fphar.2020.00500
Hamza, A. A., Heeba, G. H., Hamza, S., Abdalla, A., and Amin, A. (2021). Standardized Extract of Ginger Ameliorates Liver Cancer by Reducing Proliferation and Inducing Apoptosis through Inhibition Oxidative Stress/Inflammation Pathway. Biomed. Pharmacother. 134, 111102. doi:10.1016/j.biopha.2020.111102
Hayder M Al-kuraishy, H. M., Ali I Al-gareeb, A., and Hany Akeel Naji, H. A. (2019). Febuxostat Modulates Oxidative and Apoptotic Pathways in Acute Doxorubicin-induced Cardiotoxicity: an Experimental Animal Model Study. Asian J. Pharm. Clin. Res. 12 (4), 73–76. doi:10.22159/ajpcr.2019.v12i4.31162
Juaid, N., Amin, A., Abdalla, A., Reese, K., Alamri, Z., Moulay, M., et al. (2021). Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights. Int. J. Mol. Sci. 22 (19), 10774. doi:10.3390/ijms221910774
Kalyanaraman, B. (2020). Teaching the Basics of the Mechanism of Doxorubicin-Induced Cardiotoxicity: Have We Been Barking up the Wrong Tree? Redox Biol. 29 (29), 101394. doi:10.1016/j.redox.2019.101394
Krishnamurthy, B., Rani, N., Bharti, S., Golechha, M., Bhatia, J., Nag, T. C., et al. (2015). Febuxostat Ameliorates Doxorubicin-Induced Cardiotoxicity in Rats. Chem. Biol. Interact. 237, 96–103. doi:10.1016/j.cbi.2015.05.013
Kumari, H., Huang, W. H., and Chan, M. W. Y. (2020). Review on the Role of Epigenetic Modifications in Doxorubicin-Induced Cardiotoxicity. Front. Cardiovasc Med. 7, 56. doi:10.3389/fcvm.2020.00056
LaFollette, M. R., Riley, M. C., Cloutier, S., Brady, C. M., O'Haire, M. E., and Gaskill, B. N. (2020). Laboratory Animal Welfare Meets Human Welfare: a Cross-Sectional Study of Professional Quality of Life, Including Compassion Fatigue in Laboratory Animal Personnel. Front. Vet. Sci. 7, 114. doi:10.3389/fvets.2020.00114
Lan, Y., Wang, Y., Huang, K., and Zeng, Q. (2020). Heat Shock Protein 22 Attenuates Doxorubicin-Induced Cardiotoxicity via Regulating Inflammation and Apoptosis. Front. Pharmacol. 11, 257. doi:10.3389/fphar.2020.00257
Lenihan, D. J. (2014). Cardiac Biomarkers, Cardiotoxicity, and Active Collaboration. J. Am. Coll. Cardiol. 63, 817. doi:10.1016/j.jacc.2013.10.060
Li, L., Li, J., Wang, Q., Zhao, X., Yang, D., Niu, L., et al. (2020). Shenmai Injection Protects against Doxorubicin-Induced Cardiotoxicity via Maintaining Mitochondrial Homeostasis. Front. Pharmacol. 11, 815. doi:10.3389/fphar.2020.00815
Lu, L., Sun, X., Chen, C., Qin, Y., and Guo, X. (2018). Shexiang Baoxin Pill, Derived from the Traditional Chinese Medicine, Provides Protective Roles against Cardiovascular Diseases. Front. Pharmacol. 9, 1161. doi:10.3389/fphar.2018.01161
Mohan, N., Jiang, J., Dokmanovic, M., and Wu, W. J. (2018). Trastuzumab-mediated Cardiotoxicity: Current Understanding, Challenges, and Frontiers. Antib. Ther. 1, 13–17. doi:10.1093/abt/tby003
Mu, C., Sheng, Y., Wang, Q., Amin, A., Li, X., and Xie, Y. (2021). Potential Compound from Herbal Food of Rhizoma Polygonati for Treatment of COVID-19 Analyzed by Network Pharmacology: Viral and Cancer Signaling Mechanisms. J. Funct. Foods 77, 104149. doi:10.1016/j.jff.2020.104149
Murabito, A., Hirsch, E., and Ghigo, A. (2020). Mechanisms of Anthracycline-Induced Cardiotoxicity: Is Mitochondrial Dysfunction the Answer? Front. Cardiovasc Med. 7, 35. doi:10.3389/fcvm.2020.00035
Murali, C., Mudgil, P., Gan, C. Y., Tarazi, H., El-Awady, R., Abdalla, Y., et al. (2021). Camel Whey Protein Hydrolysates Induced G2/M Cellcycle Arrest in Human Colorectal Carcinoma. Sci. Rep. 11, 7062–7114. doi:10.1038/s41598-021-86391-z
Nazarbek, G., Kutzhanova, A., Nurtay, L., Mu, C., Kazybay, B., Li, X., et al. (2021). Nano-evolution and Protein-Based Enzymatic Evolution Predicts Novel Types of Natural Product Nanozymes of Traditional Chinese Medicine: Cases of Herbzymes of Taishan-Huangjing (Rhizoma Polygonati) and Goji (Lycium Chinense). Nanoscale Adv. 3, 6728–6738. doi:10.1039/d1na00475a
Nebigil, C. G., and Désaubry, L. (2018). Updates in Anthracycline-Mediated Cardiotoxicity. Front. Pharmacol. 9, 1262. doi:10.3389/fphar.2018.01262
Nurtay, L., Sun, Q., Mu, C., Cao, Z., Wang, Q., Liang, Z., et al. (2021). Rhizoma Polygonati from Mount Tai: Nutritional Value and Usefulness as a Traditional Chinese Medicine, Source of Herbzyme, and Potential Remediating Agent for COVID-19 and Chronic and Hidden Hunger. Acupunct. Herb. Med. 1, 31–38. doi:10.1097/HM9.0000000000000008
Onohuean, H., Al-Kuraishy, H. M., Al-Gareeb, A. I., Qusti, S., Alshammari, E. M., and Batiha, G. E. (2021). Covid-19 and Development of Heart Failure: Mystery and Truth. Naunyn Schmiedeb. Arch. Pharmacol. 394, 2013–2021. doi:10.1007/s00210-021-02147-6
Parlakpinar, H., Ozhan, O., Ermis, N., Vardi, N., Cigremis, Y., Tanriverdi, L. H., et al. (2019). Acute and Subacute Effects of Low versus High Doses of Standardized Panax Ginseng Extract on the Heart: an Experimental Study. Cardiovasc Toxicol. 19, 306–320. doi:10.1007/s12012-019-09512-1
Podyacheva, E. Y., Kushnareva, E. A., Karpov, A. A., and Toropova, Y. G. (2021). Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View from the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 12, 1398. doi:10.3389/fphar.2021.670479
Sahasrabudhe, N. M., Beukema, M., Tian, L., Troost, B., Scholte, J., Bruininx, E., et al. (2018). Dietary Fiber Pectin Directly Blocks Toll-like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 9, 383. doi:10.3389/fimmu.2018.00383
Shamim, M., and Khan, N. I. (2018). PReemptive Effect of Panax Ginseng Extract on Sensorimotor Dysfunction in Experimentally Induced Middle Cerebral Artery Occlusion-Reperfusion Injury Model of Ischemic Stroke. Fuuast. J. Biol. 8, 271. https://fuuastjb.org/index.php/fuuastjb/article/view/194.
Sheppard, R. J., Berger, J., and Sebag, I. A. (2013). Cardiotoxicity of Cancer Therapeutics: Current Issues in Screening, Prevention, and Therapy. Front. Pharmacol. 4, 19. doi:10.3389/fphar.2013.00019
Shim, J. V., Chun, B., Van Hasselt, J. G. C., Birtwistle, M. R., Saucerman, J. J., and Sobie, E. A. (2017). Mechanistic Systems Modeling to Improve Understanding and Prediction of Cardiotoxicity Caused by Targeted Cancer Therapeutics. Front. Physiol. 8, 651. doi:10.3389/fphys.2017.00651
Sung, Y. Y., Yuk, H. J., and Kim, D. S. (2021). Saengmaeksan, a Traditional Herbal Formulation Consisting of Panax Ginseng, Ameliorates Hyperuricemia by Inhibiting Xanthine Oxidase Activity and Enhancing Urate Excretion in Rats. J. Ginseng Res. 45, 565–574. doi:10.1016/j.jgr.2021.01.001
Tanaka, Y., Nagoshi, T., Yoshii, A., Oi, Y., Takahashi, H., Kimura, H., et al. (2021). Xanthine Oxidase Inhibition Attenuates Doxorubicin-Induced Cardiotoxicity in Mice. Free Radic. Biol. Med. 162, 298–308. doi:10.1016/j.freeradbiomed.2020.10.303
Varricchi, G., Ameri, P., Cadeddu, C., Ghigo, A., Madonna, R., Marone, G., et al. (2018). Antineoplastic Drug-Induced Cardiotoxicity: a Redox Perspective. Front. Physiol. 9, 167. doi:10.3389/fphys.2018.00167
Wan, Y., Wang, J., Xu, J. F., Tang, F., Chen, L., Tan, Y. Z., et al. (2021). Panax Ginseng and its Ginsenosides: Potential Candidates for the Prevention and Treatment of Chemotherapy-Induced Side Effects. J. Ginseng Res. 45 (6), 617–630. doi:10.1016/j.jgr.2021.03.001
Wang, G., Lei, C., Tian, Y., Wang, Y., Zhang, L., and Zhang, R. (2019). Rb1, the Primary Active Ingredient in Panax Ginseng CA Meyer, Exerts Antidepressant-like Effects via the BDNF–TrkB–CREB Pathway. Front. Pharmacol. 1034. doi:10.3389/fphar.2019.01034
Xie, Y., Mu, C., Kazybay, B., Sun, Q., Kutzhanova, A., Nazarbek, G., et al. (2021). Network Pharmacology and Experimental Investigation of Rhizoma Polygonati Extract Targeted Kinase with Herbzyme Activity for Potent Drug Delivery. Drug Deliv. 28, 2187–2197. doi:10.1080/10717544.2021.1977422
Xu, H., Yu, W., Sun, S., Li, C., Zhang, Y., and Ren, J. (2020). Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity through Promoting Mitochondrial Autophagy. Front. Physiol. 11, 113. doi:10.3389/fphys.2020.00113
Xu, X. Y., Yi, E. S., Kang, C. H., Liu, Y., Lee, Y. G., Choi, H. S., et al. (2021). Whitening and Inhibiting NF-Κb-Mediated Inflammation Properties of the Biotransformed Green Ginseng Berry of New Cultivar K1, Ginsenoside Rg2 Enriched, on B16 and LPS-Stimulated RAW 264.7 Cells. J. Ginseng Res. 45, 631–641. doi:10.1016/j.jgr.2021.02.007
Yin, Y., and Shen, H. (2021). Advances in Cardiotoxicity Induced by Altered Mitochondrial Dynamics and Mitophagy. Front. Cardiovasc. Med. 8, 95. doi:10.3389/fcvm.2021.739095
Yu, P., Li, Y., Fu, W., Li, X., Liu, Y., Wang, Y., et al. (2021). Panax Quinquefolius L. Saponins Protect Myocardial Ischemia Reperfusion No-Reflow through Inhibiting the Activation of NLRP3 Inflammasome via TLR4/MyD88/NF-Κb Signaling Pathway. Front. Pharmacol. 11, 813. doi:10.3389/fphar.2020.607813
Zhang, Q. Y., Wang, F. X., Jia, K. K., and Kong, L. D. (2018). Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 9, 1253. doi:10.3389/fphar.2018.01253
Zhang, S, S., Xu, T., Shi, Q., Li, S., Wang, L., An, Z., et al. (2021). Cardiovascular Safety of Febuxostat and Allopurinol in Hyperuricemic Patients with or without Gout: A Network Meta-Analysis. Front. Med. 8, 891. doi:10.3389/fmed.2021.698437
Keywords: doxorubicin, ginseng, febuxostat, cardiac tropinin, BNP, TNF-α, glutathione peroxidase
Citation: Al-Kuraishy HM, Al-Hussaniy HA, Al-Gareeb AI, Negm WA, El-Kadem AH, Batiha GE-S, N. Welson N, Mostafa-Hedeab G, Qasem AH and Conte-Junior CA (2022) Combination of Panax ginseng C. A. Mey and Febuxostat Boasted Cardioprotective Effects Against Doxorubicin-Induced Acute Cardiotoxicity in Rats. Front. Pharmacol. 13:905828. doi: 10.3389/fphar.2022.905828
Received: 27 March 2022; Accepted: 01 June 2022;
Published: 22 June 2022.
Edited by:
Yusof Kamisah, Universiti Kebangaan Malaysia, MalaysiaReviewed by:
Amr Amin, The University of Chicago, United StatesAmany Mohammed Gad, Sinai University, Egypt
Copyright © 2022 Al-Kuraishy, Al-Hussaniy, Al-Gareeb, Negm, El-Kadem, Batiha, N. Welson, Mostafa-Hedeab, Qasem and Conte-Junior. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaber El-Saber Batiha, Z2FiZXJiYXRpaGFAZ21haWwuY29t; Nermeen N. Welson, bmVybWVlbm5lbXJAeWFob28uY29t
 Hayder M. Al-Kuraishy1
Hayder M. Al-Kuraishy1 Walaa A. Negm
Walaa A. Negm Aya H. El-Kadem
Aya H. El-Kadem Gaber El-Saber Batiha
Gaber El-Saber Batiha Nermeen N. Welson
Nermeen N. Welson Gomaa Mostafa-Hedeab
Gomaa Mostafa-Hedeab Ahmed H Qasem
Ahmed H Qasem Carlos Adam Conte-Junior
Carlos Adam Conte-Junior