- 1Institutes for Systems Genetics, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 2iGlobal Research and Publishing Foundation, New Delhi, India
- 3Chitkara College of Pharmacy, Chitkara University Punjab, Rajpura, India
- 4Department of Health and Family Welfare, Civil Hospital, Rampura Phul, India
- 5Akal College of Pharmacy and Technical Education, Mastuana Sahib, Sangrur, India
- 6Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India
- 7Institute of Scholars, Bengaluru, India
Ethnopharmacological relevance: The genus Alternanthera (Amaranthaceae) comprises 139 species including 14 species used traditionally for the treatment of various ailments such as hypertension, pain, inflammation, diabetes, cancer, microbial and mental disorders.
Aim of the review: To search research gaps through critical assessment of pharmacological activities not performed to validate traditional claims of various species of Alternanthera. This review will aid natural product researchers in identifying Alternanthera species with therapeutic potential for future investigation.
Materials and methods: Scattered raw data on ethnopharmacological, morphological, phytochemical, pharmacological, toxicological, and clinical studies of various species of the genus Alternanthera have been compiled utilizing search engines like SciFinder, Google Scholar, PubMed, Science Direct, and Open J-Gate for 100 years up to April 2021.
Results: Few species of Alternanthera genus have been exhaustively investigated phytochemically, and about 129 chemical constituents related to different classes such as flavonoids, steroids, saponins, alkaloids, triterpenoids, glycosides, and phenolic compounds have been isolated from 9 species. Anticancer, antioxidant, antibacterial, CNS depressive, antidiabetic, analgesic, anti-inflammatory, and immunomodulator effects have been explored in the twelve species of the genus. A toxicity study has been conducted on 3 species and a clinical study on 2 species.
Conclusions: The available literature on pharmacological studies of Alternanthera species reveals that few species have been selected based on ethnobotanical surveys for scientific validation of their traditional claims. But most of these studies have been conducted on uncharacterized and non-standardized crude extracts. A roadmap of research needs to be developed for the isolation of new bioactive compounds from Alternanthera species, which can emerge out as clinically potential medicines.
Introduction
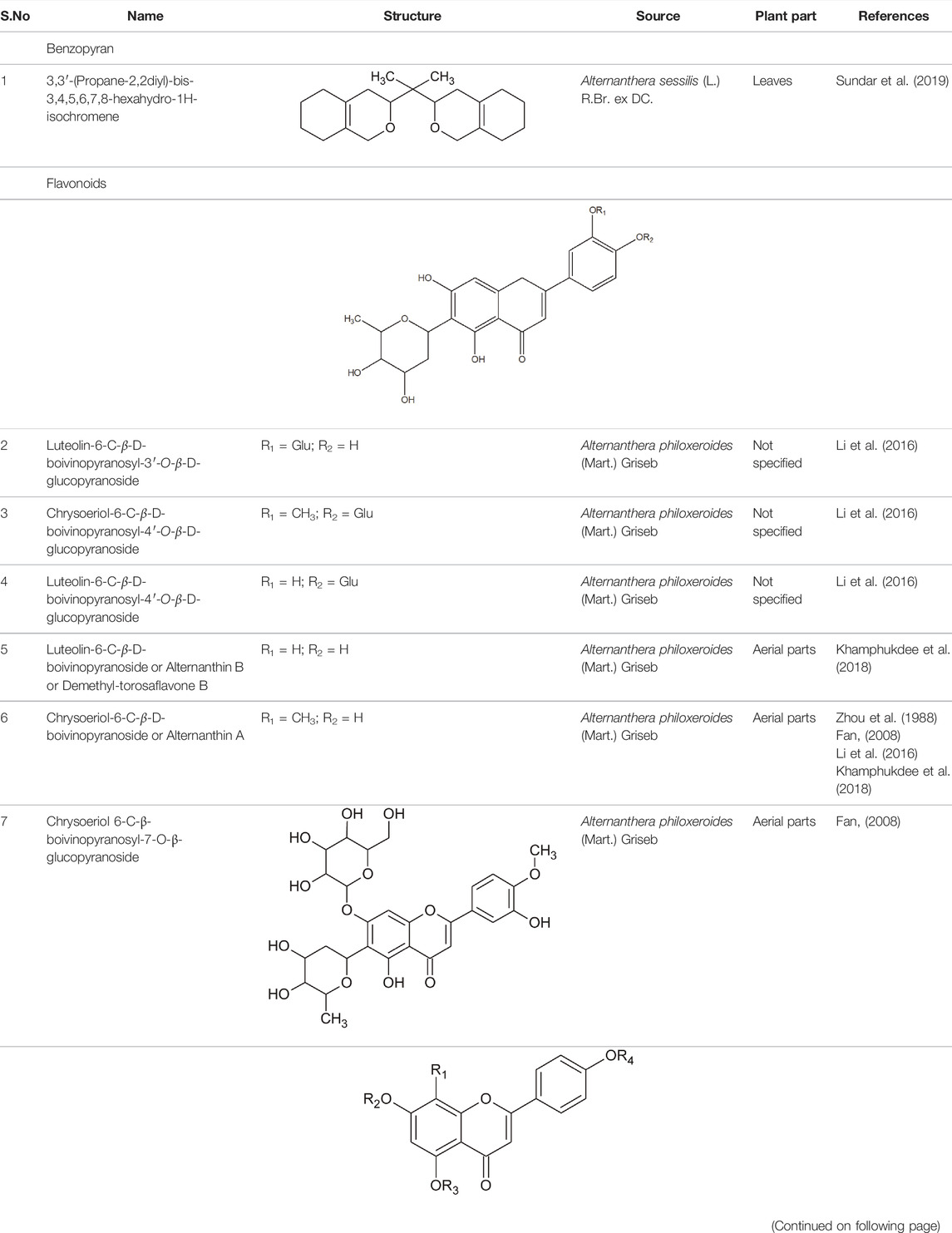
The family Amaranthaceae comprises 65 genera and about 850 species (Hundiwale et al., 2012; Chandrashekhar, 2019). These species are mainly distributed in tropical regions of the United States of America, Africa, and India. Amongst 65 genera and 850 species, only 17 genera and 50 species have been recorded to be found in India. The plants from this family include herbs, shrubs, and universal weeds. The genus Alternanthera, a significant delegate of the family Amaranthaceae was coined by by Forsskal in 1775. The genus Alternanthera comprises roughly 139 species which are distributed in India, China, Sri Lanka, the United States of America, and Africa (Figure 1). Though not complete and exhaustive, but phytochemical characterization was found to be reported that of Alternanthera sessilis (L.) R.Br. ex DC., Alternanthera philoxeroides (Mart.) Griseb., Alternanthera brasiliana (L.) Kuntze, Alternanthera hirtula (Mart.) R.E.Fr., Alternanthera praelonga A.St.-Hil., Alternanthera littoralis P.Beauv., Alternanthera bettzickiana (Regel) G.Nicholson, and Alternanthera pungens Kunth (Table 1 with complete details).
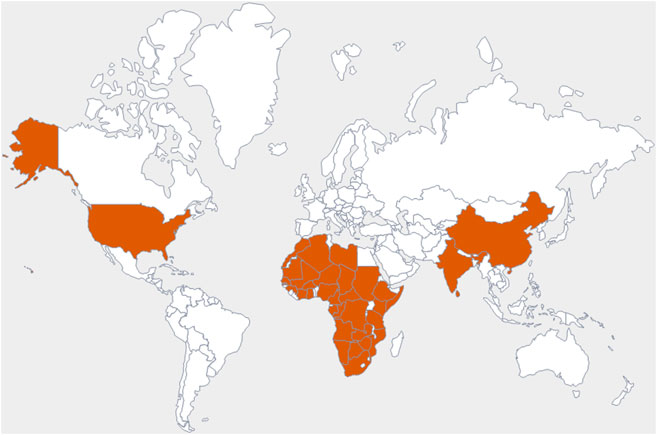
FIGURE 1. Commonly observed geographical distribution of Alternanthera species, indicated in dark orange.
The present review emphasizes traditional uses, chemical constituents, pharmacological actions, clinical potential, and safety profile of Alternanthera species. The current work has been compiled to fulfill the following goals: 1) to explore if traditional claims of Alternanthera species have been scientifically justified by pharmacological and clinical studies, and also to assess critically if their mechanism of actions is established, 2) to explore whether detailed phytochemical investigations have been conducted to detect and isolate main/bioactive constitutes of various species, 3) to reveal whether appropriate analytical methods have been developed for standardization of plant materials based on marker compounds, 4) to analyze whether isolated compounds from Alternanthera species have potential to be developed as lead molecules unaltered or needs derivatization to develop semisynthetic drugs through proper SAR studies and 5) to check if the safety and toxicity profiles of Alternanthera species have been studied. The scattered raw data has been compiled from online databases such as SciFinder, Google Scholar, PubMed, Science Direct, and Open J-Gate for 100 years up to April 2021 and offline databases such as Aromatic Plants Abstract, scientific journals, and books from different libraries of National repute. Keywords selected were based on various species of Alternanthera genus, and different biological activities. The articles which were in English and available with full text were included. Manuscript written in non-English versions were excluded. A total of 156 articles related to Alternanthera genus were finally studied and cited. But the cross-sectional literature review led us to cover a total of around 500 articles in this review article. The review article is categorized into six sections: 1) morphology emphasizes morphological characters of different Alternanthera species; 2) ethnopharmacology covers traditional uses of different Alternanthera species; 3) phytoconstituents includes name and structure of chemicals constituents isolated from various species of the genus; 4) biological activities focus on different pharmacological activities reported in various species and presented in the table; 5) toxicity studies include scientific reports of toxicity studies of different Alternanthera species and 6) clinical studies describe clinical trials conducted on humans.
Morphology
The morphological profile of various species of the genus was found to be similar with some variations. A. brasiliana (L.) Kuntze (a perennial herb mainly distributed in Brazil) is prostrate, 7.5–45 cm long branches, introducing a round stem, long internodes, and swollen nodes, at which inverse leaves connect (Kumar S. et al., 2011). Branches are glabrous, two lines of hair, nodes frequently villous; leaves are 2.5–7.5 cm, considerably longer when developing in watery spots, rather plump, at some point indefinitely denticulate; flowers are white, found in the form of bunches; seeds are 1.25–1.5 mm, sub-orbicular.
A. denticulata R. Br. and A. nahui Heenan and de Lange comprise stem of 100 mm height and located in an upright position (Heenan et al., 2009). The uniform spreading of minute hairs is present on the stems of both plants. The dark green-colored leaves (length—30 mm and breadth—6 mm) of both plants are linear, entire, narrow, elliptic, denticulate margins, and oblong in appearance. The abaxial surface of the tepals (length: 2.0–4.2 mm) is described by keeled, a character that is presented at the base of mature and dried tepals.
A. philoxeroides (Mart.) Griseb., a perennial herb, has stems crawling or gliding rising towards pinnacle, establishing at the lower hubs, branched, empty, with a longitudinal hairy groove score on two inverse sides (Pulipati et al., 2015). The fresh and delicious stems can develop on a level plane and float on the outside of the water, framing pontoons, or structure tangled bunches that develop onto banks. The leaves are inverse two by two, with an unmistakable midrib, and ranges from 5–10 cm. The plant consists of leaf, lanceolate shape, intense pinnacle, whole edge, glabrous surface, graduate base, and short strong petiole.
A. pungens Kunth is a perennial herb with a stem of 10–15 cm long with hair. The leaves are green in color and ovate in a shape of about 0.5–4.5 cm long and 0.3–2 cm in width (Naidu, 2012). It is native to the Southern American continent generally found in South Carolina, Florida, and California spreading around the road sides (Gupta et al., 2012). In 1918 it was first reported in the Southern parts of India (Rao, 2000).
A. sessilis (L.) R.Br. ex DC. is a perennial herb with purple-colored and glabrous branches grown from the root bases about 50 cm in length (Anitha and Kanimozhi, 2012). The fresh leaves are shiny, 1.3–3.0 cm long and 0.5–1.0 cm wide however the leaves are bigger in wet living spaces, direct elliptic, oval or obovate, zenith adjusted and base cuneate. The blossoms are subtle, white, borne in little, axillary heads; bracts are obovate and 1 mm long. The bracteoles are shorter, persevering; subequal, and intense. Utricleare cordi-structure and are unequivocally compacted. The seeds are orbicular. The plant bears blossoms and natural products consistently.
Ethnopharmacology and Traditional Uses
The infusion of inflorescences of A. Brasiliana (L.) Kuntze with water is used in headaches, coughs, colds, and grippe (Hundiwale et al., 2012). The infusion of leaves with a cup of water has been used in the treatment of fever while a decoction of roots is used in diarrhea. Traditionally, the various plant parts (stems, leaves, flowers, roots) of A. caracasana Kunth have been used to treat dysentery, diarrhea, and fever. The infusion of the plant is used as lavage or beverage in the traditional system of medicines (Canales-Martínez et al., 2008). The aerial parts of A. Brasiliana (L.) Kuntze are indicated in the treatment of inflammation, pain, and various infections (Hundiwale et al., 2012). The leaves of A. ficoidea (L.) P.Beauv. has been used in the treatment of heart and cancer problems (Patil and Kore, 2019). A. littoralis P. Beauv. has a long tradition of use in the treatment of infectious and inflammatory diseases (Koolen et al., 2017). The old texts indicated the use of A. littoralis P. Beauv. in the treatment of inflammatory, infectious diseases (de Santana Aquino et al., 2015), viral infections, immunity problems, cancer, malaria, and diarrhea (Hundiwale et al., 2012; Sekar, 2012). A. nodiflora R.Br. has been in the treatment of skin, degenerative and microbial infections (Feka et al., 2014). A. paronychioides A.St.-Hil. has been used in the treatment of hyperuricemia, rheumatic arthritis, uremia, nephritis, gout, cystitis, diabetes, and systemic neuralgia in TCM (Wu et al., 2013). In Ayurveda, the syrup of the whole plant of A. philoxeroides (Mart.) Griseb. has been employed in the treatment of influenza (Hundiwale et al., 2012). The aqueous infusion of leaf and flower of A. porrigens (Jacq.) Kuntze has been recorded in old texts for the treatment of hepatic pain, kidney problems, and influenza. A. pungens Kunth has been employed as folk medicine in Argentina, commonly known as Yerba del pollo, recorded in the Pharmacopeia National Argentina (1978) for various medicinal purposes. It has been traditionally used in the treatment of swelling, nasopharyngeal infections, as a painkiller in labor pain, and also for lactation stimulus in veterinary-related cases (Burkill, 1985). It is also used in the treatment of gonorrhea (Semenya and Potgieter, 2014), menstrual disorder, miscarriage (Lucky and Diame, 2010) and to treat dysentery, cholera, and many parasitic diseases (Grønhaug et al., 2008; Guede et al., 2010). In Sudan, it is used in aqueous form for the treatment of cough. In Brazil, the aerial parts are used against grippe and vermifuge (Agra et al., 2007). It is used for crushing kidney stones or renal calculi in the form of decoction. The whole plant of A. sessilis (L.) R.Br. ex DC. has been used as green vegetable for maintain the nutrient balance in body (Astudillo-Vázquez et al., 2008). The roasted leaves and stems (p.o.) of A. sessilis (L.) R.Br. ex DC. have been in the treatment of stomach pain, ulcer, and gastric problems (Kumar S. M. et al., 2011). The aerial parts of A. sessilis (L.) R.Br. ex DC. have been used as a diuretic in the Ayurvedic system of medicines (Hundiwale et al., 2012). The leaves of A. sessilis (L.) R.Br. ex DC. are used as a diuretic, antipyretic and antiseptic and roots are used as amenorrhea, inflammations, ovarian diseases, and female sterility. The young shoots of A. sessilis (L.) R.Br. ex DC. have been used as lactagogue and febrifuge (Hosamani et al., 2004). Keeping these in mind, the most common traditional uses for the Alternanthera species were recorded for the treatment and management of inflammation, pain, infectious diseases, and gastric problems.
Phytoconstituents Isolated and Identified in Alternanthera Species
GC–MS of n-hexane extract of A. philoxeroides (Mart.) Griseb. leaves showed the presence of 25 compounds. Among this Acetic acid, 2-(2-methoxycarbonylamino-5-nitrophenylthio)-, methyl ester (31.92%); 1,4-Benzenediol, 2,5-bis(1,1-dimethylethyl) (15.06%); 4-Pyridinecarboxamide, 6-bromo-4,5-dicyano-1,2,3,4-tetrahydro-3,3-dimethyl-2-[[(1methylethyamino] oxy] (8.53%); L-Cysteine, N-(trifluoroacetyl)-, butyl ester, trifluoroacetate (ester) (6.59%); Cyclopentaneundecanoic acid, methyl ester (5.4%) and 3-Bromo-N-(2-thiazolyl) benzamide (3.49%) are dominant (Akbar et al., 2021). LC-MS/MS and GC-MS analysis of an ethanolic extract of A. brasiliana (L.) Kuntze aerial parts were performed (Alencar Filho et al., 2019). Five compounds (luteolin-8-C-rhamnosylglucoside, 2″-O-rhamnosylvitexin, 2″-O-rhamnosyl-6-C-glucosyl methyl-luteolin, rutin, and 2″-O-rhamnosylswertisin) were identified by LC-MS/MS whereas twenty-two compounds were identified by GC-MS but major proportions were n-hexadecanoic acid with 16.61% followed by linoleic acid, clionasterol, α-tocopherol, stigmast-7-en-3-ol, and α-amyrin. The GC-MS analysis of volatile oil obtained from leaves of A. pungens Kunth showed the presence of 12 compounds and the major compound was β-ionone (42.18%) (Ogunmoye et al., 2020). Other compounds identified were Hexahydrofarnesyl acetone (15.53%), Methyl palmitate (6.13%), 1-Octadecyne (4.72%), Undecane (3.73%), p-Metha-1,3,8-triene (3.65%), Isophytol (3.21%), δ-Cadinene (3.06%), 1,2-Dimethyl cyclooctene (3.05%), p-Cymene (2.96%), Phytol (2.67%) and Neophytadiene (2.50%).
The phytoconstituents—benzopyran, flavonoids, volatile oil, sterols, triterpenoid/saponins, phenolic compounds, ionone, anthraquinone, hydroxycinnamic acids, alkaloids, etc. have been scientifically reported from 9 species of Alternanthera. The chemical constituents (along with their structure) isolated from different species of the Alternanthera genus are shown in Table 1.
Referring to the data tabulated in Table 1 covering the isolated phytoconstituents from 9 species of Alternanthera genus, we have prepared an interactive mapping (Figure 2) to give some quick insight about it to the readers. Notably, it has also been observed that some of the phytocompounds like kaempferol, stigmasterol, quercetin, vitexin, ferulic acid, caffeic acid, etc have been isolated from various species of Alternanthera genus. This somehow lead us to suggest that these phytocompounds could serve as standardization of these markers could be helpful in identifying Alternanthera species, and avoid adulteration. Some of the compounds isolated from the species of Alternanthera genus are very common and usually been reported from multiple biological sources and well known for many pharmacological activities. For instance, kaempferol has been isolated from various other sources including Euonymus alatus (Thunb.) Siebold (Fang et al., 2008; Singla et al., 2021), Vachellia nilotica (L.) P.J.H.Hurter and Mabb.(Singh et al., 2008), etc, with multiple therapeutic potential, including but not limited to antiproliferative (Park et al., 2021), antiviral (Arabyan et al., 2021), hepatoprotective (Alshehri et al., 2021), antioxidant (Sharma et al., 2021), etc. Similarly, chlorogenic acid had been reported from multiple resources, including Cocos nucifera L. (Bankar et al., 2011), apple fruit (Hulme, 1953), Neolamarckia cadamba (Roxb.) Bosser (Kapil et al., 1995), etc with multiple therapeutic potential like neuroprotective (Hung et al., 2021), antihepatotoxic (Kapil et al., 1995), etc. Since species of Alternanthera genus containing other compounds also along with these common phytomolecules, there could be a possibility of synergistic potential and enhanced activity. Thus, we suggest the researchers to explore the therapeutic potential based on the common bioactive compounds.
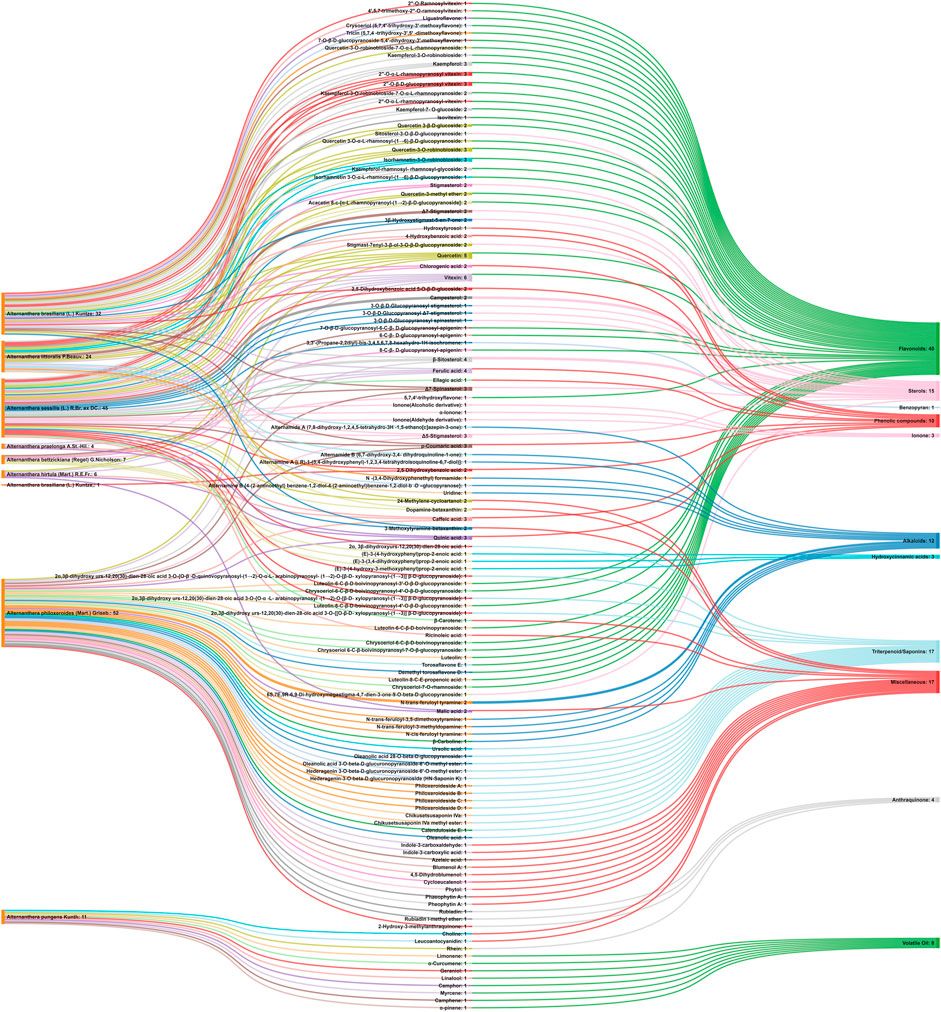
FIGURE 2. Interaction analysis map to express association and relationship between phytochemical classifications, compounds, and biological sources.
Pharmacological Activities
Several scientific investigations were conducted to validate traditional claims of various species of Alternanthera. Uncharacterized/non-standardized crude extracts of various species of Alternanthera were used in most of these scientific pharmacological studies. Alternanthera species have been observed to display analgesic, anticancer, anti-inflammatory, antimicrobial, antioxidant, hepatoprotective, hypotensive, allelopathic, α-glucosidase inhibitory, anthelmintic, anti-allergic, antianxiety, sedative, antiapoptotic, antiarthritic, antiasthmatic, anticataract, anticonvulsant, antidepressant, antidiabetic, antidiarrhoeal, antifungal, antibacterial, anti-HBV, antiparkinsonian, antiprotozoal, antispasmodic, antiviral, gastrointestinal protective, immunomodulatory and wound healing activities. The plant species, extract/fraction/isolate, dose tested/route of administration, bioactive dose, positive control, negative control, In vivo/in vitro models, and mechanism of action have been summarized in Table 2.
Referring to the data tabulated in Table 2, and interactive Figure 3, it is quite evident that the Alternanthera genus is having tremendous potential having polypharmacological effects. 35 different types of pharmacological effects were elicited by different species of Alternanthera genus. While the species like Alternanthera sessilis (L.) R.Br. ex DC., Alternanthera brasiliana (L.) Kuntze, and Alternanthera philoxeroides (Mart.) Griseb. were most widely explored, it opens up the opportunity for the researchers to explore other species of this genus.
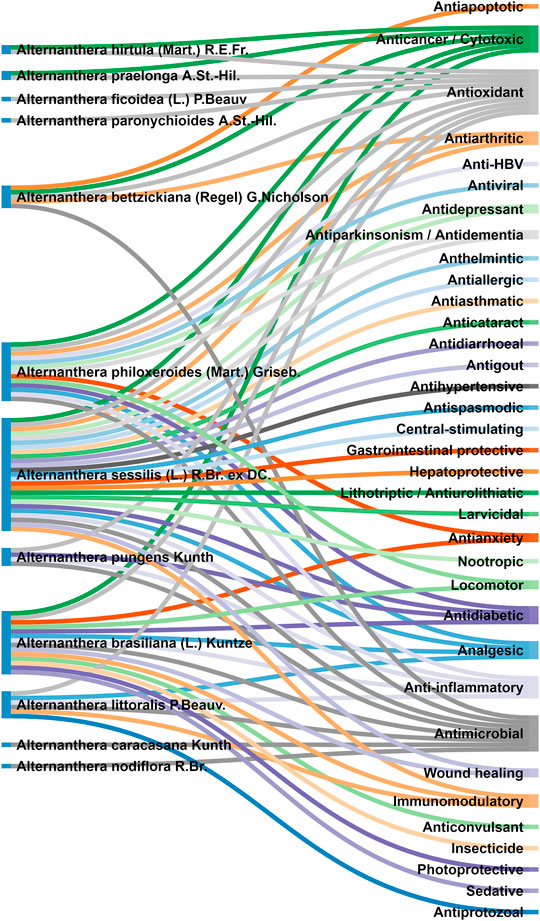
FIGURE 3. Interactive analysis mapping between various species of Alternanthera genus and their elicited pharmacological properties.
Analgesic Activity
Pelisoli Formagio and the team had evaluated the aqueous extract from the aerial parts of Alternanthera brasiliana (L.) Kuntze for its analgesic potential. 90.35% reduction of acetic acid induced contractions were observed in mice, when treated with 25 mg/kg of the aqueous extract (Pelisoli Formagio et al., 2012). Coutinho and the team had performed the formalin test in mice for assessment of analgesic effect of ethanolic extract from the leaves of Alternanthera brasiliana (L.) Kuntze. At 100 mg/kg, ethanolic extract was capable of reducing the edematogenic process by 64.17% (Coutinho et al., 2017). Phytoconstituents like kaempferol (Parveen et al., 2007), quercetin (Anjaneyulu and Chopra, 2003), vitexin (Zhu et al., 2016), etc may be responsible for the analgesic potential of Alternanthera brasiliana (L.) Kuntze.
de Santana Aquino and the team had evaluated ethanolic extract as well as isolated compound, 2″-O-α-L-rhamnopyranosylvitexin from the aerial parts of Alternanthera littoralis P.Beauv. for analgesic potential. Results suggested that the ethanolic extract as well as 2″-O-α-L-rhamnopyranosylvitexin are capable of exerting significant analgesic effect, most probably through the TNF pathway (de Santana Aquino et al., 2015). Since kaempferol, quercetin, and vitexin were also been reported from Alternanthera littoralis P.Beauv. (Figure 2), so these compounds could also attribute in analgesic potential of the extract.
Khatun and the team had prepared the methanolic extract from the whole plant part of Alternanthera philoxeroides (Mart.) Griseb. and evaluated for its analgesic potential in the acetic acid induced mice. They found that 400 mg/kg dose of methanolic extract was capable of reducing constrictions by 44.8%. Phytoconstituents like kaempferol (Parveen et al., 2007), quercetin (Anjaneyulu and Chopra, 2003), vitexin (Zhu et al., 2016), caffeic acid (Gamaro et al., 2011), ursolic acid (Vasconcelos et al., 2006), etc may be responsible for the analgesic potential of Alternanthera philoxeroides (Mart.) Griseb.
Various research teams have independently assessed the analgesic potential of Alternanthera sessilis (L.) R.Br. ex DC.: Mondal and the team used ethanolic extract of the leaves (Mondal et al., 2014); Mohapatra and the team used hydroethanolic extract of leaves (Mohapatra et al., 2018); Hossain and the team used methanolic extract of aerial parts (Hossain et al., 2014); while Mohaimenul and the team used ethanolic extract of aerial parts (Mohaimenul et al., 2020). It is thus quite validated that aerial parts especially leaves of Alternanthera sessilis (L.) R.Br. ex DC. have the analgesic potential. Various mechanisms observed by those researchers for this activity. Some of them are like inhibition of interleukins like IL-4, IL-5, and IL-13, dopaminergic and serotonergic pathways, inhibition of lipoxygenase and cyclooxygenase, etc. Along with kaempferol, vitexin, and quercetin, compounds like stigmasterol (Walker et al., 2017) may also be responsible for such analgesic effect.
Anthelmintic Activity
Vennila and Nivetha had prepared various extracts from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. and performed In vitro—Pheretima Posthuma method for assessment of anthelmintic activity. They observed that methanolic extract was the most potent and active at all the tested concentrations. The possible mechanism proposed by them was membrane lysis which subsequently led to paralysis or death of the worm (Vennila and Nivetha, 2015). On the other hand, Mondal and the team had assessed anthelmintic activity of ethanolic extract of the whole plant as well as the isolated ellagic acid by using In vitro—Adult motility test. They had also indicated the disruption of cell permeability, along with various other pathways and found ellagic acid a key responsible compound (Mondal et al., 2015). Other compounds that may be responsible for this pharmacological effects could be quercetin (Borges et al., 2020), β-sitosterol (Deepak et al., 2002), etc.
Antiallergic Activity
Rayees and the team checked the antiallergic activity of 95% ethanolic extract from aerial parts of Alternanthera sessilis (L.) R.Br. ex DC. Studies were conducted in rat basophilic leukemia (RBL-2H3) cells. They found that the treatment with ethanolic extract resulted in nuclear factor-KB (NF-kB) dependent inhibition of cytokines like IL-6, TNF-α, IL-13, and IL-4, along with the decrease in β-hexosaminidase release (Rayees et al., 2013). Compounds like β-sitosterol (Yuk et al., 2007; Mahajan and Mehta, 2011), kaempferol (Oh et al., 2013), quercetin (Mlcek et al., 2016), vitexin (Venturini et al., 2018), stigmasterol (Antwi et al., 2018), etc may be responsible for the antiallergic activity of Alternanthera sessilis (L.) R.Br. ex DC.
Antianxiety Property
Various research teams have independently assessed the antianxiety potential of Alternanthera brasiliana (L.) Kuntze: Pelisoli Formagio had used the aqueous extract of the leaves (Pelisoli Formagio et al., 2012); Oyemitan and the team had used the ethanolic extract of the leaves (Oyemitan et al., 2015); while Barua and the team had used the methanolic extract of the leaves (Barua et al., 2013). It is thus quite validated that the leaves of Alternanthera brasiliana (L.) Kuntze have the antianxiety potential. Various mechanisms observed by those researchers for this activity. Some of them are like activation of GABA receptor and 5-HT partial agonistic action. Phytomolecules like stigmasterol (Karim et al., 2021), kaempferol (Kaur et al., 2017), quercetin (Singh et al., 2013), p-coumaric acid (He Y. et al., 2021), etc may be responsible for this antianxiety property of Alternanthera brasiliana (L.) Kuntze.
Khamphukdee and the team had assessed ethanolic extract from the leaves of Alternanthera philoxeroides (Mart.) Griseb. for antianxiety potential by performing In vivo—Elevated plus-maze test, Light/Dark transition test, and Locomotor activity test in female mice. They observed that both the test doses i.e. 250 and 500 mg/kg/day of the extract was able to reduce the anxiety, most probably through the esterogenic pathway. Quercetin and kaempferol were detected in this plant also, so may be responsible for such antianxiety behavior.
Antiapoptotic Activity
Wu and the team had studied the antiapoptotic potential of ethanolic extract from the whole plant of Alternanthera bettzickiana (Regel) G.Nicholson. They found that ethanolic extract has strong tendency to reduce apoptosis which was modulated via multiple mechanisms including reduction of reactive oxygen species, inhibition of caspase-3 and caspase-9 activation, etc. They had reported quercetin as the major compound in that extract, and they found same mechanisms when evaluated quercetin for antiapoptotic potential.
Antiarthritic Activity
Manan and the team had studied antiarthritic potential of the ethanolic extract obtained from the aerial parts of Alternanthera bettzickiana (Regel) G.Nicholson using in silico, in vitro and in vivo methodologies. HPLC analysis indicated the presence of catechin, gallic acid, sinapic acid, chlorogenic acid, alpha-tocopherol, gamma-tocopherol, and quercetin. They have found that even the 250 mg/kg/day of the ethanolic extract was able to modulate the parameters suggesting the antiarthritic potential when compared with standard drug and disease control. In silico analysis suggested the strong interaction between the HPLC-analysed phytomolecules and cyclooxygenases (Manan et al., 2020).
Sunmathi and the team had studied the antiarthritic activity of ethanolic extracts obtained from the leaves of Alternanthera philoxeroides (Mart.) Griseb. and Alternanthera sessilis (L.) R.Br. ex DC. using in vitro methodologies. They found that 500 μg/ml of ethanolic extract of Alternanthera philoxeroides (Mart.) Griseb. and Alternanthera sessilis (L.) R.Br. ex DC. were able to stabilize the membrane by 64.92 and 75.43%, respectively. Phytomolecules like vitexin (Yang et al., 2019) and quercetin (Mamani-Matsuda et al., 2006) may be responsible for the antiarthritic activity of Alternanthera philoxeroides (Mart.) Griseb. and Alternanthera sessilis (L.) R.Br. ex DC.
Antiasthmatic Activity
Various research teams have independently assessed the antiasthmatic potential of Alternanthera sessilis (L.) R.Br. ex DC.: Fathima and the team had used ethanolic extract of leaves (Fathima et al., 2016) while Saqib and Janbaz had used 70% Ethanolic extract of the whole plant and its dichloromethane and aqueous fractions (Saqib and Janbaz, 2016). This validates the applicability of Alternanthera sessilis (L.) R.Br. ex DC. in the treatment management of asthma. Ethanolic extract obtained from the leaves was found to reduce the leucocyte count and significantly inhibited the histamine release (Fathima et al., 2016). 70% ethanolic extract of the whole plant was found to act via calcium channel blocking mechanism (Saqib and Janbaz, 2016). Phytomolecules like kaempferol (Gong et al., 2012), vitexin (Venturini et al., 2018), quercetin (Fortunato et al., 2012), stigmasterol (Antwi et al., 2017a), chlorogenic acid (Kim et al., 2010), etc. may be key components for the antiasthamatic activity of Alternanthera sessilis (L.) R.Br. ex DC.
Anticancer/Cytotoxic Property
Various research teams have independently assessed the anticancer property of Alternanthera bettzickiana (Regel) G.Nicholson: M Nagalingam and the team had used aqueous extract of the leaves (Nagalingam et al., 2018) while R Jothi Ramalingam and the team had used aqueous extract of leaves and silver nanoparticles and Ag-mesoporous MnO2 nanocomposite (Jothi Ramalingam et al., 2017). This validates the potential of leaves from Alternanthera bettzickiana (Regel) G.Nicholson and their nanoparticles in colon cancer and lung cancer. Apigenin analogues present in the Alternanthera bettzickiana (Regel) G.Nicholson may be responsible for the anticancer property (Madunić et al., 2018; Imran et al., 2020).
Similarly, various research teams have independently assessed the anticancer property of Alternanthera brasiliana (L.) Kuntze: Brochado and the team had used aqueous fraction of the ethanolic extract from the leaves. They had also isolated 6 bioactive compounds from this fraction viz. robinin, clovin, quercetin 3-O-robinobioside, kaempferol 3-O-robinobioside, kaempferol 3-O-rutinoside-7-O-a-L-rhamnopyranoside, and kaempferol 3-O-rutinoside (Brochado et al., 2003); Samudral and the team had used ethyl acetate extract obtained from the leaves (Samudrala et al., 2015). These pieces of evidence validates the anticancer potential of Alternanthera brasiliana (L.) Kuntze leaves. Brochado and the team found Kaempferol 3-O-robinobioside and kaempferol 3-O-rutinoside as the active phytomolecules (Brochado et al., 2003).
Independently several researches had also been conducted from various labs to assess the potential of Alternanthera philoxeroides (Mart.) Griseb. as anticancer agent: Zhang and the team had used the methanolic extract of the leaves and checked cytotoxicity against H9c2 cell lines. They found that even at 20 mg/ml, the methanolic extract was able to inhibit the doxorubicin induced cardiomyocyte apoptosis by more than 50%. They had also observed the presence of -carboline and quercetin (Zhang et al., 2018). Fang and the team had isolated 5 phytomolecules from the aerial parts of Alternanthera philoxeroides (Mart.) Griseb., and checked their inhibitory activity against Hela and L929 cell lines. While N-trans-feruloyl-3,5-dimethoxytyramine, alternanthin, N-trans-feruloyl-3-methyldopamine, and N-trans-feruloyl tyramine were found to have more than 50% inhibition at 30 μg/ml against Hela cell line, only Alternanthin B, and alternanthin were having more than 50% inhibition at 30 μg/ml against L929 cell line (Fang et al., 2007). Fang and the team had further isolated 4 more compounds from the aerial parts of Alternanthera philoxeroides (Mart.) Griseb. The triterpenoidal saponins, Philoxeroidesides A, B, C, and D were found to inhibit SK-N-SH cell line with an IC50 of 51, 118.69, 60.6, and 37.29 μg/ml, respectively, while inhibited HL60 cell line with an IC50 of 185.29, 185.57, 271.45, and 45.93 μg/ml, respectively. Philoxeroidesides D was found to be quite potential against both the cell lines (Fang J.-B. et al., 2009). In another study performed by Correa and the team where they had used ethanolic extracts obtained from the whole plant of Alternanthera philoxeroides (Mart.) Griseb.; Alternanthera hirtula (Mart.) R.E.Fr., and Alternanthera praelonga A.St.-Hil. They tested the ethanolic extracts against various human cancer cells lines including that from melanoma, breast, kidney, lung, prostate, ovary, colon, leukemia, along with non-cancer cell line from green monkey kidney. Out of all the cancer cell lines, these ethanolic extracts were being able to be found potent only against the leukemia cell line, K562 (Correa et al., 2016).
Several researchers have independently assessed the potential of Alternanthera sessilis (L.) R.Br. ex DC. for the management of cancer: Jain and the team had used the methanolic extract of leaves (Jain et al., 2016); Firdhouse and Lalitha had used silver nanoparticles of the aqueous extract (Firdhouse and Lalitha, 2013); Qian and the team had used gold nanoparticles of the aqueous extract of leaves (Qian et al., 2019); D Suganya and the team had used aqueous extract of leaves and stems (Suganya et al., 2019); Pathak and the team had used n-hexane and methanolic extracts of aerial parts (Pathak et al., 2020); Mohaimenul and the team had used ethanolic extract of aerial parts (Mohaimenul et al., 2020); Yap and the team had used ethanolic, 70% ethanolic, 80% methanolic, ethyl acetate, and aqueous extracts of the whole plant (Yap et al., 2019); Sathishkumar and the team had used silver nanoparticles of the aqueous extract of leaves (Sathishkumar et al., 2016); Arulselvan and the team had used ethanolic extract of aerial parts, stem, and leaves (Arulselvan et al., 2018); while Guerra and the team aqueous extract of aerial parts (Guerra et al., 2003). All these studies indicated the true potential of Alternanthera sessilis (L.) R.Br. ex DC. for the treatment and management of cancer, with leaving no doubt in it. Phytomolecules present in the Alternanthera sessilis (L.) R.Br. ex DC. like kaempferol (Imran et al., 2019), vitexin (Liu et al., 2019; Lee et al., 2020), quercetin (Rauf et al., 2018), stigmasterol (Ali et al., 2015), chlorogenic acid (Barahuie et al., 2017), campesterol (Bae et al., 2021), and β-sitosterol (Pradhan et al., 2016), etc. may be responsible for this anticancer property.
Anticataract Property
Kota and the team had checked the anticataract property of ethyl acetate extract obtained from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. Cataract induced in eye lenses of the chicks were subjected for the treatment with 100, 200, and 400 mg of ethyl acetate extract, followed by analysis of lipid peroxidation and Na+- K+ ATPases. They found that 100 and 200 mg ethyl acetate treatment will lead to decrease in malondialdehyde and increase in the inorganic phosphorous content (Kota et al., 2017). Phytomolecules like quercetin (Lan et al., 2020), chlorogenic acid (Kim et al., 2011), and β-sitosterol (Haroon et al., 2020) may be responsible for this anticataract property of Alternanthera sessilis (L.) R.Br. ex DC.
Anticonvulsant Activity
Independently several researches had also been conducted from various labs to assess the potential of Alternanthera brasiliana (L.) Kuntze as anticonvulsant agent: Oyemitan and the team had used the ethanolic extract of leaves (Oyemitan et al., 2015); Schallenberger and the team had also used the ethanolic extract of leaves (Schallenberger et al., 2017); while Barua and the team had used the methanolic extract of leaves (Barua et al., 2013). This had validated the anticonvulsant potential of the leaves of Alternanthera brasiliana (L.) Kuntze. Various mechanisms elucidated by them are like modulation of GABAergic system, controlling the entry of calcium and sodium ions in the cells, and glycine regulation in spinal cord (Oyemitan et al., 2015). Phytomolecules like vitexin (de Oliveira et al., 2020), quercetin (Nassiri-Asl et al., 2014; Nieoczym et al., 2014), stigmasterol (Karim et al., 2021), chlorogenic acid (Aseervatham et al., 2016), and ferulic acid (Hassanzadeh et al., 2017) may be responsible for the antiepileptic effect of Alternanthera brasiliana (L.) Kuntze.
Antidepressant Activity
Khamphukdee and the team had assessed the antidepressant effect of the ethanolic extract obtained from the leaves of Alternanthera philoxeroides (Mart.) Griseb. They found that the extract was having significant antidepressant effect modulated through the estrogenic pathway (Khamphukdee et al., 2018). Phytomolecules like quercetin (Anjaneyulu and Chopra, 2003), vitexin (Can et al., 2013), β-sitosterol (Zhao et al., 2016), p-coumaric acid (Lee et al., 2018), caffeic acid (Monteiro et al., 2020), ursolic acid (Machado et al., 2012; Singla et al., 2017), and malic acid (Gómez-Moreno et al., 2013) may be responsible for the antidepressant activity of Alternanthera philoxeroides (Mart.) Griseb.
Gupta and K. Singh had evaluated the antidepressant activity of methanolic extract obtained from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. They had observed that the antidepressant effect of the methanolic extract was acting via interaction with adrenergic, dopaminergic serotonergic, and GABAergic system (Gupta and Singh, 2014). Phytomolecules like quercetin, vitexin, and p-coumaric acid had also been reported from Alternanthera sessilis (L.) R.Br. ex DC., along with other antidepressant agents like kaempferol (Park et al., 2010b), ferulic acid (Chen et al., 2014) and chlorogenic acid (Park et al., 2010a). These phytomolecules may be responsible for the antidepressant activity of Alternanthera sessilis (L.) R.Br. ex DC.
Antidiabetic Activity
Reza and the team had assessed the antidiabetic potential of 80% ethanolic extracts obtained from the stem and leaves of Alternanthera brasiliana (L.) Kuntze. They found that the ethanolic extracts were being able to significantly modulate the biochemical parameters like blood glucose, lipid peroxidation, and free radicals in the alloxan-induced diabetic Swiss albino mice (Reza et al., 2019). Phytomolecules like kaempferol (Ibitoye et al., 2018), quercetin (Vessal et al., 2003), stigmasterol (Wang et al., 2017; Singla and Shen, 2020), p-coumaric acid (Amalan et al., 2016), ferulic acid (Narasimhan et al., 2015), and chlorogenic acid (Ong et al., 2013) may be responsible for the antidiabetic potential of Alternanthera brasiliana (L.) Kuntze.
Khatun and the team as well as Bhattacherjee and the team had independently assessed the antidiabetic activity of Alternanthera philoxeroides (Mart.) Griseb. Various important mechanisms had been observed by them including regeneration of the β-cells of the pancreas, alpha-glucosidase inhibition, as well as the inhibition of the glucose absorption from the gut wall (Khatun et al., 2012; Bhattacherjee et al., 2014). Compounds like quercetin and p-coumaric acid had been reported from Alternanthera philoxeroides (Mart.) Griseb., and may be responsible for such antidiabetic effect.
Mourya and the team had used aqueous and ethanolic extracts obtained from the whole plant of Alternanthera pungens Kunth for the assessment of antidiabetic potential. Dose dependent antidiabetic activity was observed by them when studied in alloxan-induced diabetic Wistar rats. Phytocompounds like camphene (Hachlafi et al., 2021), camphor (Drikvandi et al., 2020), geraniol (Babukumar et al., 2017), and limonene (Murali and Saravanan, 2012) may be responsible for such antidiabetic property of Alternanthera pungens Kunth.
Independently several researches had also been conducted from various labs to assess the potential of Alternanthera sessilis (L.) R.Br. ex DC. as antidiabetic agent: Kumar and the team had used aqueous and ethanolic extracts of aerial parts (Kumar S. M. et al., 2011); Tan and Kim had used hexane, ethyl acetate, and aqueous fractions of aerial parts (Tan and Kim, 2013); Hossain and the team had used methanolic extract of aerial parts (Hossain et al., 2014); Sundar and the team had used petroleum ether extract of leaves (Sundar et al., 2019); Das and the team had used 95% ethanolic extract of the whole plant (Das et al., 2015); Rao and the team had used ethanolic extract of the whole plant (Rao et al., 2011); Manalo and the team had used n-hexane, ethyl acetate, and water fractions of the methanolic extract of leaves (Manalo et al., 2020); Mohaimenul and the team had used ethanolic extract of aerial parts (Mohaimenul et al., 2020); Tiwari and the team had used the juice (Tiwari et al., 2013); Chai and the team had used hexane, chloroform, ethyl acetate, butanol, and aqueous fractions of methanolic extracts of leaves and callus (Chai et al., 2016). Plenty of evidences obtained from the above researches leaved no doubt in that fact that Alternanthera sessilis (L.) R.Br. ex DC. possesses antidiabetic properties. Various mechanisms demonstrated by different preparations from Alternanthera sessilis (L.) R.Br. ex DC., including but not limited to modulation of insulin sensitivity, improvement in pancreatic insulin secretion, reduction in blood glucose level, inhibition of α-glucosidase enzyme, etc. Phytomolecules like kaempferol (Ibitoye et al., 2018), quercetin (Vessal et al., 2003), stigmasterol (Wang et al., 2017; Singla and Shen, 2020), 4-hydroxybenzoic acid (Peungvicha et al., 1998), β-sitosterol (Ponnulakshmi et al., 2019), ellagic acid (Fatima et al., 2015), ferulic acid (Narasimhan et al., 2015), and chlorogenic acid (Ong et al., 2013) may be responsible for the antidiabetic potential of Alternanthera sessilis (L.) R.Br. ex DC.
Antidiarrheal Activity
Zavala and the team had evaluated the antidiarrheal property of hexane, chloroform, methanolic, and aqueous extracts obtained from the whole plant of Alternanthera sessilis (L.) R.Br. ex DC. They had observed that out of all extracts, methanolic and aqueous extracts had shown significant inhibition of castor oil-induced diarrhea. Methanolic extract was further found to inhibit normal defecation in mice also. Peristaltic movement was also modulated by the methanolic extract (Zavala et al., 1998). Phytomolecules like quercetin (Lozoya et al., 1994; Song et al., 2011; Shi et al., 2020), β-sitosterol (Ding et al., 2018), ellagic acid (Chen et al., 2020), ferulic acid (Hu et al., 2021), and chlorogenic acid (Zhang et al., 2017; Chen et al., 2018) may be responsible for the antidiarrheal property of Alternanthera sessilis (L.) R.Br. ex DC.
Antigout Activity
Chong and Loh had assessed the antigout potential of methanolic extract obtained from the aerial parts of Alternanthera sessilis (L.) R.Br. ex DC. Methanolic extract was able to inhibit xanthine oxidase enzyme with an IC50 of 557.77 μg/ml (Chong and Loh, 2020). Phytomolecules like kaempferol (Wang et al., 2015d), quercetin (Bindoli et al., 1985), stigmasterol (Chiang and Chen, 2008), ellagic acid (Sun et al., 2021), ferulic acid (Nile et al., 2016), and chlorogenic acid (Wang et al., 2009) may be responsible for the antigout potential of Alternanthera sessilis (L.) R.Br. ex DC.
Anti-Hepatitis B Virus Activity
Li and the team had isolated C-boivinopyranosyl flavones from Alternanthera philoxeroides (Mart.) Griseb. and found that luteolin-6-C-β-d-boivinopyranosyl-3′-O-β-d-glucopyranoside, chrysoeriol-6-C-β-d-Boivinopyranosyl-4′-O-β-d-glucopyranoside, and luteolin-6-C-β-d-boivinopyranosyl-4′-O-β-d-glucopyranoside were strongly inhibiting the viral antigen, HBsAg in HBV-infected HepG2.2.15 with an IC50 of 28.65, 22.20, and 31.54 µM, respectively (Li et al., 2016).
Antihypertensive Activity
Saqib and Janbaz had evaluated the antihypertensive effect of 70% Ethanolic extract of the whole plant and its dichloromethane and aqueous fractions from Alternanthera sessilis (L.) R.Br. ex DC. The in vivo studies suggested that the ethanolic extract was capable to reducing both the systolic and the diastolic pressure. Phytomolecules like kaempferol (Ahmad et al., 1993; Binang and Takuwa, 2021), quercetin (Perez-Vizcaino et al., 2009; Binang and Takuwa, 2021), vitexin (Xue et al., 2020), β-sitosterol (Olaiya et al., 2014), ellagic acid (Berkban et al., 2015), ferulic acid (Li et al., 2020), and chlorogenic acid (Zhao et al., 2011) may be responsible for the antihypertensive potential of Alternanthera sessilis (L.) R.Br. ex DC.
Anti-Inflammatory Activity
Pelisoli Formagio and the team had performed the in vivo studies to assess the anti-inflammatory activity of the aqueous extract obtained from the leaves of Alternanthera brasiliana (L.) Kuntze while P Shivashankar and the team had used the methanolic extract obtained from the leaves. Pelisoli Formagio and the team had observed the significant decrease in the polymorphonuclear cells as well as increase in the mononuclear cells in rat’s exudate after treated with the aqueous extract, while P Shivashankar and the team found the reduction in the colon weight in acetic acid-induced colitis model of adult Wistar albino rats after treatment with the methanolic extract (Pelisoli Formagio et al., 2012; P et al., 2016). Phytomolecules like kaempferol (Devi et al., 2015), quercetin (Lesjak et al., 2018), stigmasterol (Morgan et al., 2021), p-coumaric acid (Pragasam et al., 2012), ferulic acid (Ozaki, 1992), and chlorogenic acid (Hwang et al., 2013) may be responsible for the anti-inflammatory potential of Alternanthera brasiliana (L.) Kuntze.
de Santana Aquino and the team had evaluated anti-inflammatory activity of ethanolic extract of aerial parts and the isolated compound, 2″-O-α-L-rhamnopyranosylvitexin from Alternanthera littoralis P.Beauv. They found that the ethanolic extract was able to reduce the paw edema as well as capable to reducing leukocyte migration. In addition to these, the isolated compound was also able to reduce protein leakage into the pleural cavity (de Santana Aquino et al., 2015). Other phytomolecules that could be responsible for the anti-inflammatory activity of the ethanolic extract will be kaempferol, quercetin, stigmasterol, etc.
Sunmathi and the team had evaluated anti-inflammatory activity of ethanolic extract obtained from the leaves of Alternanthera philoxeroides (Mart.) Griseb. Dose dependent membrane stabilization was observed. Phytomolecules like quercetin (Lesjak et al., 2018), vitexin (Rosa et al., 2016), β-sitosterol (Loizou et al., 2010), p-coumaric acid (Pragasam et al., 2012), caffeic acid (da Cunha et al., 2009), ursolic acid (Baricevic et al., 2001), and malic acid (Obertreis et al., 1996) may be responsible for the anti-inflammatory activity of Alternanthera philoxeroides (Mart.) Griseb.
Franck and the team had evaluated the anti-inflammatory activity of aqueous extract obtained from the leaves of Alternanthera pungens Kunth. They had observed the decreased level of histamine release, serotonin and kinin, prostaglandin, proteases, lysosomes, and protein C-reactive. Phytomolecules like α-pinene (Kim et al., 2015), myrcene (Rufino et al., 2015), limonene (Rufino et al., 2015), choline (Rowley et al., 2010), rhein (Gao et al., 2014), linalool (Peana et al., 2002), geraniol (Ye et al., 2019), and camphor (Ehrnhöfer-Ressler et al., 2013) which were reported earlier in Alternanthera pungens Kunth., may be responsible for this anti-inflammatory effect.
Independently several researches had also been conducted from various labs to assess the potential of Alternanthera sessilis (L.) R.Br. ex DC. as anti-inflammatory agent: Sunmathi and the team had used ethanolic extract obtained from the leaves (Sunmathi et al., 2016); Muniandy and the team had used 90% ethanolic extract of stems (Muniandy et al., 2018a); Sundar and the team had used petroleum ether and methanolic extracts of leaves (Sundar et al., 2019); Kassuya and the team had used Ethanolic extract of whole plant (EEAT) as well as the isolated molecule, 2″-O-β-D-glucopyranosyl-vitexin (Kassuya et al., 2021); Biella and the team had used aqueous extract of the whole plant (Biella et al., 2008). Plenty of evidences obtained from the above researches leaved no doubt in that fact that Alternanthera sessilis (L.) R.Br. ex DC. possesses anti-inflammatory properties. Various mechanisms demonstrated by different preparations from Alternanthera sessilis (L.) R.Br. ex DC., including but not limited to cyclooxygenase -1 and -2 inhibition (Biella et al., 2008), modulating NF- κB pathway (Muniandy et al., 2018a), leukocyte migration (Kassuya et al., 2021), etc. Phytomolecules like kaempferol (Devi et al., 2015; Pizzo et al., 2018), quercetin (Lesjak et al., 2018), vitexin (Rosa et al., 2016), stigmasterol (Morgan et al., 2021), β-sitosterol (Loizou et al., 2010), 4-hydroxybenzoic acid (Winter et al., 2017), ellagic acid (Corbett et al., 2010), ferulic acid (Ozaki, 1992), campesterol (Moreno-Anzúrez et al., 2017), spinasterol (Jeong et al., 2010), β-carotene (Uteshev et al., 2000), p-coumaric acid (Pragasam et al., 2012), ricinoleic acid (Vieira et al., 2001), and chlorogenic acid (Hwang et al., 2013) may be responsible for the anti-inflammatory potential of Alternanthera sessilis (L.) R.Br. ex DC.
Antimicrobial Activity
Independently, several research teams had evaluated the antimicrobial effects of the leaves of Alternanthera bettzickiana (Regel) G.Nicholson: Vidhya and the team had used hexane, chloroform, ethyl acetate, methanolic, and aqueous extracts of leaves (Vidhya et al., 2015); R, Jothi Ramalingam and the team had used aqueous extract of leaves and silver nanoparticles and Ag-mesoporous MnO2 nanocomposite (Jothi Ramalingam et al., 2017); Nagalingam and the team had used the aqueous extract obtained from leaves (Au-NP) (Nagalingam et al., 2018). These research were focused on leaves and somehow validated the antimicrobial property of it. Various mechanisms elucidated were like cell wall lysis, protein synthesis inhibition, and topoisomerase inhibition, etc (Vidhya et al., 2015; Jothi Ramalingam et al., 2017; Nagalingam et al., 2018). Phytocompounds like apigenin analogs (Koo, 2003; Thirukumaran et al., 2019) may be responsible for this antimicrobial property of Alternanthera bettzickiana (Regel) G.Nicholson.
Coutinho and the team had evaluated the antimicrobial property of ethanolic extract obtained from the leaves of Alternanthera brasiliana (L.) Kuntze. They had observed that though the ethanolic extract as such was having insignificant potential, but it elicited significant synergetic potential when combined with gentamycin and tested against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa (Coutinho et al., 2017). Johann and the team had also performed the antimicrobial experiments on the ethanolic extract obtained from the aerial parts of Alternanthera brasiliana (L.) Kuntze, and they had also observed that the extract was inactive against various murine macrophages and fungal strains (Johann et al., 2010). Other research team like that of Akachukwu and Uchegbu had also reported mild activity of the ethanolic extract obtained from its leaves (Akachukwu and Uchegbu, 2016) while Kumar and the team noticed significant activity elicited by the silver nanoparticles obtained from the leaves aqueous extract (Kumar et al., 2014).
Canales-Martínez and the team had evaluated the antimicrobial effect of the hexane, chloroform, methanolic, acetone, and ethyl acetate extracts obtained from the aerial parts of Alternanthera caracasana Kunth and also isolated a bioactive compound, 7-methoxycoumarin. They observed that the ethyl acetate extract as well as 7-methoxycoumarin were active against various Gram-positive and Gram-negative bacterial strains, but inactive against Candida albicans (Canales-Martínez et al., 2008). Phytochemical profiling of Alternanthera caracasana Kunth is still not done, leaving a scope for the researchers.
Gasparetto and the team had used crude hexane and ethanolic extract obtained from the leaves of Alternanthera littoralis P.Beauv., and assessed them for their antimicrobial potential. They noticed that the antifungal activity was exhibited by the crude extracts only when combined with photo-irradiation by a diode laser (Gasparetto et al., 2010). Phytocompounds like kaempferol (del Valle et al., 2016), stigmasterol (Alawode et al., 2021), hydroxytyrosol (Bisignano et al., 1999), quercetin (Gatto et al., 2002), vitexin (Das et al., 2016), and uridine (Wiegmann et al., 2016) which were reported earlier from Alternanthera littoralis P.Beauv., may be responsible for such antimicrobial effects.
Feka and the team had studied the antimicrobial property of the aqueous and methanolic extracts obtained from the whole plant of Alternanthera nodiflora R.Br. They found that the methanolic extract was having significant antimicrobial activity against bacterial and yeast strains, but inactive against mould test strain (Feka et al., 2014). Phytochemical profiling of Alternanthera nodiflora R.Br. is still not done, leaving a scope for the researchers.
Independently several research teams had evaluated the antimicrobial potential of Alternanthera philoxeroides (Mart.) Griseb.: Bhattacherjee and the team had used methanol-soluble fraction obtained from the leaves (Bhattacherjee et al., 2014); Rawani and the team had used aqueous and chloroform: methanol (1:1) extracts of leaves (Rawani et al., 2011); Pulipati and the team had used ethanolic extract obtained from the leaves (Pulipati et al., 2016); Akbar and the team had used methanolic extract of leaves, stem and roots as well as their n-hexane, chloroform and ethyl acetate fractions (Akbar et al., 2021); while Pulipati and Babu had used the methanolic extract of leaves (Pulipati and Babu, 2020). These independent researches left no doubt and validated the antimicrobial feature of Alternanthera philoxeroides (Mart.) Griseb. They had reported multiple mechanisms of actions like bacterial cell wall lysis and protein synthesis inhibition (Bhattacherjee et al., 2014; Pulipati et al., 2016; Pulipati and Babu, 2020). Phytomolecules like quercetin (Gatto et al., 2002), vitexin (Das et al., 2016), β-sitosterol (Ododo et al., 2016), stigmasterol (Alawode et al., 2021), p-coumaric acid (Boz, 2015), caffeic acid (Lima et al., 2016), luteolin analogs (Chiruvella et al., 2007; Qian et al., 2020), chrysoeriol analogs (Jang et al., 2020), malic acid (Raybaudi-Massilia et al., 2009), β-carboline (Arshad et al., 2008; Suzuki et al., 2018), ursolic acid (Collins and Charles, 1987), oleanolic acid (Horiuchi et al., 2007), azelaic acid (Leeming et al., 1986), phytol (Pejin et al., 2014), and rubiadin (Marioni et al., 2016) which were earlier reported from Alternanthera philoxeroides (Mart.) Griseb., may be responsible for this antimicrobial property.
Jakhar and Dahiya had studied the aqueous, acetone, ethanolic, and petroleum ether extracts obtained from the aerial parts of Alternanthera pungens Kunth for assessment of antimicrobial effect against various bacterial and fungal strains. They found that all the extracts were having potential as antibacterial, but the antifungal property was exhibited by only acetone and aqueous extracts. Noticed mechanisms were inhibition of DNA replication as well as blocking of cellular respiration. Phytochemicals like choline (Siopa et al., 2016), rhein (Joung et al., 2012), limonene (Vuuren and Viljoen, 2007), α-curcumene (Santos da Silva et al., 2015), geraniol (Lira et al., 2020), linalool (Park S.-N. et al., 2012), camphor (Masry et al., 2021), myrcene (Chaves-Quirós et al., 2020), and α-pinene (Dhar et al., 2014; Cloeckaert et al., 2015) which were earlier reported from Alternanthera pungens Kunth, may be responsible for such antimicrobial action.
Plenty of independent researches have been extracted from the literature, covering evaluation of antimicrobial activity of Alternanthera sessilis (L.) R.Br. ex DC.: Osuna and the team had used hexane and methanolic extracts obtained from the aerial parts (Osuna et al., 2008); Jalalpure and the team had used petroleum ether (40–60°C), chloroform, acetone, methanolic, and aqueous extracts of leaves (Jalalpure et al., 2008); Monroy and Limsiaco had used aqueous, ethanolic, and acetone extracts obtained from leaves (Monroy and Limsiaco, 2016); Niraimathi and the team had used silver nanoparticles of aqueous extract of leaves (Niraimathi et al., 2013); Rajamurugan and the team had used ethanolic extract obtained from the leaves (Rajamurugan et al., 2013); D Suganya and the team had used aqueous extract of leaves and stems (Suganya et al., 2019); Kota and the team had used petroleum ether, ethyl acetate, chloroform, and methanolic extract obtained from the leaves (Kota et al., 2017); Sundar and the team had used petroleum ether and methanolic extracts of leaves (Sundar et al., 2019); while Salvador and the team had used hexane and ethanolic extracts obtained from the adult plants (Salvador et al., 2009). These studies clearly concluded that Alternanthera sessilis (L.) R.Br. ex DC. possesses antimicrobial properties. Several mechanisms elucidated by them are like cell membrane lysis, prevention of protein synthesis, blocking cellular respiration, inhibition of DNA replication, deprivation of iron for microbial growth, etc (Osuna et al., 2008; Salvador et al., 2009; Rajamurugan et al., 2013; Monroy and Limsiaco, 2016; Kota et al., 2017; Suganya et al., 2019). Phytomolecules like Vitexin (Das et al., 2016), Kaempferol (del Valle et al., 2016), Quercetin (Gatto et al., 2002), Kaempferol-7- O-glucoside (Singh et al., 2011), Stigmasterol (Alawode et al., 2021), β-Sitosterol (Ododo et al., 2016), Ellagic acid (Abuelsaad et al., 2013; De et al., 2018), Ferulic acid (Shi et al., 2016), p-Coumaric acid (Boz, 2015), 4-Hydroxybenzoic acid (Cho J.-Y. et al., 2014), 2,5-Dihydroxybenzoic acid (Kim et al., 2007), Chlorogenic acid (Li et al., 2013; Kabir et al., 2014), Ionone (Mikhlin et al., 1983), β-Carotene (Hayashi et al., 2012), and Ricinoleic acid (Novak et al., 1961) which were earlier reported from Alternanthera sessilis (L.) R.Br. ex DC. may be responsible for its antimicrobial property.
Antioxidant Activity
Petrus and the team had evaluated the antioxidant activity of the 80% aqueous methanolic extract obtained from the flowers of Alternanthera bettzickiana (Regel) G.Nicholson. They had observed that the extract possessed radical scavenging and ferrous ion chelating properties (Petrus A. et al., 2014). On the other hand, Vidhya and the team had evaluated the antioxidant activity of the hexane, chloroform, ethyl acetate, methanolic, and aqueous extracts obtained from the leaves Alternanthera bettzickiana (Regel) G.Nicholson. They observed that out of all, methanolic extract was exhibiting stronger radical scavenging activity (Vidhya et al., 2015). Phytomolecules like apigenin analogs (Prince Vijeya Singh et al., 2004) which were earlier reported from Alternanthera bettzickiana (Regel) G.Nicholson, may be responsible for this antioxidant potential.
Independently, several research teams had investigated the antioxidant potential of Alternanthera brasiliana (L.) Kuntze: Reza and the team had used 80% ethanolic extract of stem and leaves (Reza et al., 2019); Enechi and the team had used ethanolic extract of leaves (Enechi et al., 2013); Chandran R had used methanolic extract of leaves (Chandran, 2017); Attaugwu and Uvere had used ethanolic extract of leaves (Attaugwu and Uvere, 2017); Pereira and the team had used ethanolic extract and its dichloromethane, ethyl acetate, n-butanolic fractions of leaves (Pereira et al., 2013); Araujo and the team had used ethanolic extract of aerial parts and its hexane, chloroform, and ethyl acetate fractions (Araujo et al., 2014); while Akachukwu and Uchegbu had used ethanolic extract of leaves (Akachukwu and Uchegbu, 2016). These pieces of evidence increase the credibility of Alternanthera brasiliana (L.) Kuntze as antioxidant. Phytoconstituents like Ligustroflavone (Kang et al., 2021), Vitexin (An et al., 2012), Kaempferol (Park et al., 2006), Quercetin (Zhang et al., 2011), Tricin (Duarte-Almeida et al., 2007), Quercetin 3-β-D-glucoside (Niranjan Panat et al., 2015), Isorhamnetin-3-O-robinobioside (Boubaker et al., 2012), Stigmasterol (Liang et al., 2020), β-Sitosterol (Gupta et al., 2011), Ferulic acid (Graf, 1992), p-Coumaric acid (Kiliç and Yeşiloğlu, 2013), 4-Hydroxybenzoic acid (Velika and Kron, 2012), 2,5-Dihydroxybenzoic acid (Calderón Guzmán et al., 2007), Chlorogenic acid (Sato et al., 2011), Dopamine-betaxanthin (Cai et al., 2003), and 3-Methoxytyramine-betaxanthin (Cai et al., 2003) which were earlier reported from Alternanthera brasiliana (L.) Kuntze, may be responsible for its antioxidant property.
Patil and Kore had evaluated the antioxidant property of methanolic extracts obtained from different parts viz. leaves, stem, and roots of Alternanthera ficoidea (L.) P.Beauv. They had observed that out of all, the methanolic extract from the roots was having most potent antioxidant activity (Patil and Kore, 2019). To the best of our knowledge, the phytochemial characterization of Alternanthera ficoidea (L.) P.Beauv. was not yet done, leaving an ample scope for the researchers.
Koolen and the team had isolated seven phytoconstituents from the aerial sections of Alternanthera littoralis P.Beauv. and evaluated them for the antioxidant potential using In vitro—ORAC assay. They had observed that out of all compounds, Alternamide B was the most significant one as antioxidant. Researchers had further suggested the catechol scaffold as a pharmacophore for this activity (Koolen et al., 2017).
Two independent research teams had evaluated the antioxidant potential of Alternanthera paronychioides A.St.-Hil.: Wu and the team had used methanolic, ethanolic, and aqueous extracts of the whole plant (Wu et al., 2013) while Tukun and the team had used aqueous extract obtained from the leaves (Tukun et al., 2014). These preliminary studies signifies the role of Alternanthera paronychioides A.St.-Hil. as antioxidant. To the best of our knowledge, the phytochemial characterization of Alternanthera paronychioides A.St.-Hil. was not yet done, leaving an ample scope for the researchers.
Bhattacherjee and the team had evaluated the antioxidant activity of methanol soluble fraction obtained from the leaves of Alternanthera philoxeroides (Mart.) Griseb. (Bhattacherjee et al., 2014). while Correa and the team had used ethanolic extracts of the whole plant (Correa et al., 2016). These preliminary studies suggested that the Alternanthera philoxeroides (Mart.) Griseb. is worthy of further investigation as antioxidant. Phytomolecules like Luteolin and luteolin analogs (Romanova et al., 2001), Chrysoeriol analogs (Mishra et al., 2003), Vitexin (An et al., 2012), Quercetin (Zhang et al., 2011), β-Sitosterol (Gupta et al., 2011), Δ5-Stigmasterol (Liang et al., 2020), Ursolic acid (Bobé et al., 2012; do Nascimento et al., 2014), Oleanolic acid and Oleanolic acid analogs (Wang et al., 2010), Calenduloside E (Tang et al., 2019), Caffeic acid (Gulcin, 2006), Quinic acid (Pero et al., 2009), p-Coumaric acid (Kiliç and Yeşiloğlu, 2013), Rubiadin (Tripathi et al., 1997), β-Carboline (Moura et al., 2007), Malic acid (Jin et al., 2016), Azelaic acid (Muthulakshmi and Saravanan, 2013), Cycloeucalenol (Wang W. et al., 2015), Phytol (Santos et al., 2013), and Pheophytin A (Endo et al., 1985) which were previously been reported from Alternanthera philoxeroides (Mart.) Griseb., may be responsible for this antioxidant property.
Several research teams have independently assessed the antioxidant potential of Alternanthera pungens Kunth: Mourya and the team had used ethanolic and aqueous extracts obtained from the leaves (Mourya et al., 2019); Franck and the team had used aqueous extract of leaves (Franck et al., 2016); while Jakhar and Dahiya had used aqueous, acetone, ethanolic, and petroleum ether extracts of aerial parts (Jakhar and Dahiya, 2017). These studies validated the antioxidant potential of Alternanthera pungens Kunth. Various phytochemicals like Limonene (Roberto et al., 2009), Geraniol (Aytac et al., 2016), Linalool (Duarte et al., 2016), Camphor (Drikvandi et al., 2020), Myrcene (Khalili et al., 2020), Camphene (Tiwari and Kakkar, 2009), and α-pinene (Aydin et al., 2013) which were reported earlier from Alternanthera pungens Kunth, may be responsible for its antioxidant action.
While going through literature, we have found enough pieces of evidences reporting and validating the antioxidant property of Alternanthera sessilis (L.) R.Br. ex DC.: Borah and the team had used 90% methanolic, 70% acetone, 80% ethanolic extracts of leaves and stems (Borah et al., 2011); Chai and the team had used hexane, chloroform, ethyl acetate, butanolic, and aqueous fractions of leaves and callus methanol extracts (Chai et al., 2016); Sharma and the team 30% hydroethanolic extract of the whole plant (Sharma et al., 2013); Khan and the team had used separate Methanolic and hexane extracts of leaves and stems (Khan et al., 2018); Azizah and the team had used ethanolic and aqueous extracts of aerial parts (Azizah et al., 2015); Muniandy and the team had used 90% hydroethanolic extract of stem (Muniandy et al., 2018b); Othman and the team had used ethanolic and aqueous extracts of aerial parts (Othman et al., 2016); Tiwari and the team had used juice (Tiwari et al., 2013); Rajamurugan and the team had used ethanolic extract of leaves (Rajamurugan et al., 2013); Jain and the team had used methanolic extract of leaves (Jain et al., 2016); Suganya and the team had used aqueous extract of leaves and stems (Suganya et al., 2019); Mohd Hazli and the team had used hexane, ethyl acetate, ethanolic, and aqueous extracts of leaves and stem (Mohd Hazli et al., 2019); Niraimathi and the team had used silver nanoparticles from aqueous extract of leaves (Niraimathi et al., 2013); Yap and the team had used 100% ethanolic, 70% ethanolic, 80% methanolic, ethyl acetate, and aqueous extracts of the whole plant (Yap et al., 2019); Kota and the team had used petroleum ether, ethyl acetate, chloroform, and methanolic extract of leaves (Kota et al., 2017); Sundar and the team had used petroleum ether and methanolic extracts of leaves (Sundar et al., 2019); Pathak and the team had used n-hexane and methanolic extracts of aerial parts (Pathak et al., 2020); Khan and the team had used the volatile oil of leaves and flowers (Khan et al., 2016); while Salvador and the team had used ethanolic extract and its four fractions; Acacetin 8-c-[α-L-rhamnopyranoyl-(1→2)-β-D-glucopyranoside]; 2″-O-α-L-rhamnopyranosyl-vitexin; 2″-O-β-D-glucopyranosyl vitexin and Vitexin (Salvador et al., 2006). Results from these researches left no doubt in the credibility and applicability of Alternanthera sessilis (L.) R.Br. ex DC. in reducing oxidative stress. Phytomolecules like Vitexin and vitexin analogs (An et al., 2012), Kaempferol and kaempferol analogs (Park et al., 2006), Quercetin and quercetin analogs (Zhang et al., 2011), Acacetin analogs (Li et al., 2019), Isorhamnetin-3-O-robinobioside (Boubaker et al., 2012), Stigmasterol (Liang et al., 2020), Campesterol (Yoshida and Niki, 2003), β-Sitosterol (Gupta et al., 2011), Spinasterol (Adebiyi et al., 2018), Ellagic acid (Priyadarsini et al., 2002), Ferulic acid (Graf, 1992), p-Coumaric acid (Kiliç and Yeşiloğlu, 2013), 4-Hydroxybenzoic acid (Velika and Kron, 2012), 2,5-Dihydroxybenzoic acid (Calderón Guzmán et al., 2007), Chlorogenic acid (Sato et al., 2011), Ionone (Liu et al., 2009), β-Carotene (Paiva and Russell, 1999), Ricinoleic acid (Park et al., 2020), Dopamine-betaxanthin (Cai et al., 2003), and 3-Methoxytyramine-betaxanthin (Cai et al., 2003) which were earlier been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for its antioxidant action.
Antiparkinsonism/Antidementia Property
Khamphukdee and the team had evaluated the antidementia activity of the ethanolic extract obtained from the whole plant of Alternanthera philoxeroides (Mart.) Griseb. They had noticed various mechanisms behind it like inhibition of lipid peroxidation in the whole brain, downregulation of neuroinflammatory cytokines (IL-1β, IL-6, and TNF-α), etc (Khamphukdee et al., 2021). Phytomolecules like Luteolin and luteolin analogs (Delgado et al., 2021), Vitexin (Malar et al., 2020; Zhang et al., 2021), Quercetin (Yao et al., 2010), Torosaflavone E (Khamphukdee et al., 2021), Demethyl torosaflavone D (Khamphukdee et al., 2021), β-Sitosterol (Kim et al., 2008), Stigmasterol (Park S. J. et al., 2012; Pratiwi et al., 2021), Ursolic acid (Habtemariam, 2019), Oleanolic acid and oleanolic acid analogs (Lin et al., 2021), Caffeic acid (Khan et al., 2013; Deshmukh et al., 2016), Quinic acid (Liu et al., 2020), p-Coumaric acid (Kim H.-B. et al., 2017), β-Carboline (Zhao et al., 2013; Li et al., 2018), Malic acid (Tian et al., 2021), Blumenol A (Emir et al., 2019), Phytol (Sathya et al., 2020), and Pheophytin A (Park et al., 2014) which were earlier reported from Alternanthera philoxeroides (Mart.) Griseb., may be responsible for this antidementia property.
Ittiyavirah and Hameed had evaluated the antiparkinsonian activity of silver nanoparticles and ethanolic extract obtained from the whole plant of Alternanthera sessilis (L.) R.Br. ex DC. They had observed that the silver nanoparticles as well as the ethanolic extract were able to impart neuroprotection with decrease in catalepsy as well as in muscle rigidity, along with locomotion improvement (Ittiyavirah and Hameed, 2015). Phytomolecules like Vitexin and vitexin analogs (Hu et al., 2018), Kaempferol and kaempferol analogs (Filomeni et al., 2012), Quercetin-3-methyl ether (Kim et al., 2009), Quercetin (Lv et al., 2012), Acacetin analogs (Kim S. M. et al., 2017), Stigmasterol (Haque and Moon, 2018), β-Sitosterol (Kim et al., 2008), Spinasterol (Jeong et al., 2010), Ellagic acid (Baluchnejadmojarad et al., 2017), Ferulic acid (Haque et al., 2015), p-Coumaric acid (Vauzour et al., 2010), 4-Hydroxybenzoic acid (Winter et al., 2017), Chlorogenic acid (Singh et al., 2018), and Ionone (Ma et al., 2014) which were previously been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for the antiparkinsonian activity.
Antiprotozoal Activity
Koolen and the team had isolated compounds like Alternamide A-B and Alternamine A-B from the aerial parts of Alternanthera littoralis P.Beauv. and evaluated for their antiprotozoal activity againt protozoal strains viz. Trypanosoma cruzi trypomastigotes and Leishmania amazonensis. They had observed that out of all the tested compounds, Alternamine A was the most efficient one (Koolen et al., 2017).
Antispasmodic Activity
Garín-Aguilar and the team had antispasmodic activity of aqueous, hexane, methanolic extract, and fractions of methanol extract (F1-F6) obtained from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. (Garín-Aguilar et al., 2013). while Saqib and Janbaz had used 70% ethanolic extract of the whole plant and its dichloromethane, aqueous fractions (Saqib and Janbaz, 2016). They had observed that Alternanthera sessilis (L.) R.Br. ex DC. possesses significant antispasmolytic activity. Phytomolecules like Vitexin and vitexin analogs (Ragone et al., 2007), Quercetin and quercetin analogs (Lozoya et al., 1994; Morales et al., 1994), Acacetin analogs (González-Trujano et al., 2012), Stigmasterol (Ammar et al., 2009), β-Sitosterol (Rehman et al., 2012), and Ellagic acid (Krenn et al., 2011) which were previously been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be the contributors towards the antispasmodic activity of the extracts.
Antiviral Activity
Rattanathongkom and the team had isolated Chikusetsusaponin IVa isolated from the whole plant of Alternanthera philoxeroides (Mart.) Griseb. and evaluated antiviral activity against various viral cell lines through in vitro and in vivo assays. They had observed the dose-dependent activity along with the potential of Chikusetsusaponin IVa in inhibiting the viral protein synthesis (Rattanathongkom et al., 2009).
Central-Stimulating Activity
Mondal and the team had evaluated the central stimulating potential of the ethanolic extract obtained from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. Results were quite significant (Mondal et al., 2014). Phytoconstituents acting on GABA receptors like Ricinoleic acid (Witt et al., 2002), Chlorogenic acid (Hara et al., 2014), p-Coumaric acid (Scheepens et al., 2014), Ferulic acid (Cheng et al., 2010; Sonar et al., 2019), Ellagic acid (Girish et al., 2013), Spinasterol (Socała et al., 2015), Stigmasterol (Karim et al., 2021), Acacetin analogs (Gálvez et al., 2015), Vitexin and vitexin analogs (Zhu et al., 2016; de Oliveira et al., 2020), and Quercetin and quercetin analogs (Goutman and Calvo, 2004; Kim et al., 2014) which were previously been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be behind this GABA receptor mediated central-stimulating activity.
Gastrointestinal Protective Activity
Astudillo-Vázquez and the team had evaluated the gastrointestinal protective potential of the aqueous and ethanolic extracts obtained from the whole plant of Alternanthera sessilis (L.) R.Br. ex DC. They noticed that the antidiarrheal property i.e. decreasing the gastrointestinal content is the major factor behind the gastrointestinal protective activity of Alternanthera sessilis (L.) R.Br. ex DC. (Astudillo-Vázquez et al., 2008). Phytomolecules like Vitexin and vitexin analogs (Figer et al., 2017), Kaempferol and kaempferol analogs (Beber et al., 2017; Campos-Vidal et al., 2021), Quercetin and quercetin analogs (de la Lastra et al., 1994), Stigmasterol (Sánchez-Mendoza et al., 2008), β-Sitosterol (Sánchez-Mendoza et al., 2008), Ellagic acid (Beserra et al., 2011), Ferulic acid (Shahid et al., 2018), p-Coumaric acid (Panda and Suresh, 2015), Chlorogenic acid (Ahmed et al., 2021), and β-Carotene (Mózsik et al., 1996) which were earlier reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for this gastrointestinal protective potential.
Hepatoprotective Activity
Lin and the team had evaluated the hepatoprotective activity of the aqueous extract obtained from the whole plant of Alternanthera sessilis (L.) R.Br. ex DC. (Lin et al., 1994). while Bhuyan and the team had evaluated the hepatoprotective potential of the methanolic extract obtained from the whole plant (Bhuyan et al., 2017). Both these independent researches finally concluded that the Alternanthera sessilis (L.) R.Br. ex DC. is hepatoprotective. Phytomolecules like Vitexin and vitexin analogs (Duan et al., 2020), Kaempferol and kaempferol analogs (Wang M. et al., 2015; Wang et al., 2015c), Quercetin-3-methyl ether (Tseng et al., 2012), Quercetin and quercetin analogs (Miltonprabu et al., 2017), Acacetin analogs (Cho H.-I. et al., 2014), Stigmasterol (Carter et al., 2007), β-Sitosterol (Abdou et al., 2019), Ellagic acid (Girish and Pradhan, 2012), Ferulic acid (Rukkumani et al., 2004), p-Coumaric acid (Parvizi et al., 2020), 2,5-Dihydroxybenzoic acid (Pujari and Bandawane, 2021), Chlorogenic acid (Chen et al., 2019), and β-Carotene (Manda and Bhatia, 2003) which were previously reported from Alternanthera sessilis (L.) R.Br. ex DC., may be the contributory constituents towards the elicited hepatoprotective activity.
Immunomodulatory Activity
Several research teams had independently assessed the immunomodulatory potential of Alternanthera sessilis (L.) R.Br. ex DC.: Biella and the team had used aqueous extract of the whole plant (Biella et al., 2008); Guerra and the team had used aqueous extract of aerial parts (Guerra et al., 2003); while Moraes and the team had used aqueous and ethanolic extract of leaves as well as tetrahydrofuran, dichloromethane, aqueous, petroleum ether soluble fraction (Moraes et al., 1994). These studies validated the immunomodulatory property of Alternanthera sessilis (L.) R.Br. ex DC. Phytomolecules like Vitexin and vitexin analogs (Rosa et al., 2016), Kaempferol and kaempferol analogs (Lin et al., 2011; Swarnalatha et al., 2015), Quercetin-3-methyl ether (Martino et al., 2016), Quercetin and quercetin analogs (Manjunath and Thimmulappa, 2021), Acacetin analogs (Zhao et al., 2014), Stigmasterol (Antwi et al., 2017b), β-Sitosterol (Desai et al., 2009), Ellagic acid (Abuelsaad et al., 2013), Ferulic acid (He F. et al., 2021), p-Coumaric acid (Pragasam et al., 2012), Chlorogenic acid (Guo et al., 2021), and β-Carotene (Jyonouchi et al., 2009) which were previously been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for this immunomodulatory potential.
Moraes and the team had also evaluated the immunomodulatory activity of aqueous and ethanolic extract of leaves as well as tetrahydrofuran, dichloromethane, aqueous, petroleum ether soluble fractions obtained from Alternanthera brasiliana (L.) Kuntze and Alternanthera littoralis P.Beauv. (Moraes et al., 1994). Phytomolecules like Vitexin and vitexin analogs (Rosa et al., 2016), Kaempferol and kaempferol analogs (Lin et al., 2011; Swarnalatha et al., 2015), Quercetin and quercetin analogs (Manjunath and Thimmulappa, 2021), Tricin (Santos et al., 2017), Stigmasterol (Antwi et al., 2017b), β-Sitosterol (Desai et al., 2009), Ferulic acid (He F. et al., 2021), p-Coumaric acid (Pragasam et al., 2012), and Chlorogenic acid (Guo et al., 2021) which were previously reported from Alternanthera brasiliana (L.) Kuntze, may be responsible towards its immunomodulatory activity. Phytomolecules like Vitexin and vitexin analogs (Rosa et al., 2016), Kaempferol (Lin et al., 2011; Swarnalatha et al., 2015), Quercetin-3-methyl ether (Martino et al., 2016), Quercetin and quercetin analogs (Manjunath and Thimmulappa, 2021), Acacetin analogs (Zhao et al., 2014), Stigmasterol (Antwi et al., 2017b), and Hydroxytyrosol (Shan and Miao, 2022) which were previously reported from Alternanthera littoralis P.Beauv., may be responsible for its immunomodulatory activity.
Insecticidal Property
Coutinho and the team had evaluated the insecticidal potential of the ethanolic extract obtained from the leaves of Alternanthera brasiliana (L.) Kuntze. against Drosophila melanogaster (Harwich strain). They found that the tested concentrations of the ethanolic extract were having a mild insecticidal effect, and that too after 24–48 h exposure (Coutinho et al., 2017). Phytomolecules like Kaempferol and kaempferol analogs (Zhang et al., 2016), Quercetin and quercetin analogs (Mesbah et al., 2007), Stigmasterol (Gade et al., 2017), β-Sitosterol (Zolotar et al., 2002), Spinasterol (Ahmed et al., 2020), and Ferulic acid (Yang et al., 2017) which were previously isolated from Alternanthera brasiliana (L.) Kuntze., may be responsible for this insecticidal property.
Lithotriptic/Antiurolithiatic Activity
Dhanya and the team had evaluated the antiurolithiatic activity of Kalka—fine paste of macerated fresh plant material of Alternanthera sessilis (L.) R.Br. ex DC. while Babu and the team had used ethanolic extract of the whole plant for the assessment of antiurolithiatic activity (Dhanya et al., 2017; Babu et al., 2021). Results obtained by both these independent studies are quite significant and reflects the potential of Alternanthera sessilis (L.) R.Br. ex DC. as lithotriptic agent. Phytomolecules like Kaempferol and kaempferol analogs (Cechinel-Zanchett et al., 2020), Quercetin and quercetin analogs (Dinnimath et al., 2017), Stigmasterol (Lobine et al., 2020), and Ferulic acid (Zhao et al., 2019) which were previously been reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for this antiurolithiatic activity.
Larvicidal Activity
Babu and the team had also evaluated the larvicidal property of ethanolic extract obtained from the whole plant of Alternanthera sessilis (L.) R.Br. ex DC. They found that the ethanolic extract was having a dose dependent percent mortality against mosquito larvae (Babu et al., 2021). Phytomolecules like Stigmasterol (Gade et al., 2017), β-Sitosterol (Angajala and Subashini, 2018), and Ferulic acid (Pavela, 2011), which were earlier isolated from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible behind this larvicidal activity.
Nootropic Activity
Gupta and Singh had evaluated the nootropic activity of methanolic extract obtained from the leaves of Alternanthera sessilis (L.) R.Br. ex DC. And results were quite promising (Gupta and Singh, 2012b). Phytomolecules like Kaempferol and kaempferol analogs (Das et al., 2018), Quercetin and quercetin analogs (Halder et al., 2015), Ellagic acid (Bansal et al., 2017; Kiasalari et al., 2017), and Ferulic acid (Yang et al., 2016; Mhillaj et al., 2017) which had been previously isolated from Alternanthera sessilis (L.) R.Br. ex DC., may be the contributing phytomolecules towards this nootropic activity.
Photoprotective Activity
Alencar Filho and the team had evaluated the photoprotective effect of the gel prepared from 5% w/w of extract Alternanthera brasiliana (L.) Kuntze enriched in flavonoids. They had observed that the stabilization of the ROS and resonating permission are the mechanisms behind this photoprotective activity of the gel extract (Alencar Filho et al., 2020). Phytomolecules like Kaempferol and kaempferol analogs (Monici et al., 1994), Quercetin and quercetin analogs (Saija, 2003; Gonçalves et al., 2019), Tricin (Moon et al., 2018), Stigmasterol (Bayer et al., 2011), β-Sitosterol (Bayer et al., 2011), Ferulic acid (Lin et al., 2005; Peres et al., 2018), p-Coumaric acid (Biswas et al., 2021), and Chlorogenic acid (Wang et al., 2021) which were earlier reported from Alternanthera brasiliana (L.) Kuntze, may be responsible for this photoprotective property of the gel extract.
Sedative Property
Oyemitan and the team had evaluated the sedative action of the ethanolic extract obtained from the leaves of Alternanthera brasiliana (L.) Kuntze. They had observed that the ethanolic extract was expressing the sedative property by acting on stimulatory or central excitatory channels (Oyemitan et al., 2015). Phytomolecules like Quercetin and quercetin analogs (Nakhaee et al., 2021), β-Sitosterol (Aguirre-Hernández et al., 2007), and Ferulic acid (Tu et al., 2012) which were previously been reported from Alternanthera brasiliana (L.) Kuntze., may be responsible for this sedative action.
Wound Healing Property
Barua and the team had reported several studies validating the wound healing property of Alternanthera brasiliana (L.) Kuntze (Barua et al., 2009; Barua C. et al., 2012; Baru et al., 2012; Barua C. C. et al., 2012). Phytomolecules like Vitexin and vitexin analogs (Bektas et al., 2020), Kaempferol and kaempferol analogs (Petpiroon et al., 2015; Özay et al., 2019), Quercetin and quercetin analogs (Gomathi et al., 2003), Tricin (Han et al., 2016), β-Sitosterol (Abbas et al., 2019), Ferulic acid (Ghaisas et al., 2014), p-Coumaric acid (Kong et al., 2013; Boeing et al., 2020), and Chlorogenic acid (Bagdas et al., 2015) which had been isolated from Alternanthera brasiliana (L.) Kuntze previously, may be responsible for this wound healing property.
Muniandy and the team had evaluated the wound healing action of the 90% hydroethanolic extract obtained from the stem of Alternanthera sessilis (L.) R.Br. ex DC. while Jalalpure and the team had used chloroform extract obtained from the leaves Alternanthera sessilis (L.) R.Br. ex DC. Both these teams had independently ascertained the wound healing property of Alternanthera sessilis (L.) R.Br. ex DC. (Jalalpure et al., 2008; Muniandy et al., 2018b). Phytomolecules like Vitexin and vitexin analogs (Bektas et al., 2020), Kaempferol and kaempferol analogs (Petpiroon et al., 2015; Özay et al., 2019), Quercetin and quercetin analogs (Gomathi et al., 2003), Acacetin analogs (Bhat et al., 2013), β-Sitosterol (Abbas et al., 2019), Ellagic acid (Mo et al., 2014), Ferulic acid (Ghaisas et al., 2014), p-Coumaric acid (Kong et al., 2013; Boeing et al., 2020), and Chlorogenic acid (Bagdas et al., 2015), β-Carotene (Gerber and Erdman, 1982), and Ricinoleic acid (Nada et al., 2018) which had earlier reported from Alternanthera sessilis (L.) R.Br. ex DC., may be responsible for this wound healing property.
After this exhaustive cross-literature review for the bioactive compounds that may be responsible elements behind the potent pharmacological actions elicited by the extracts, we have summarized those in a smart interactive illustration (Figure 4).
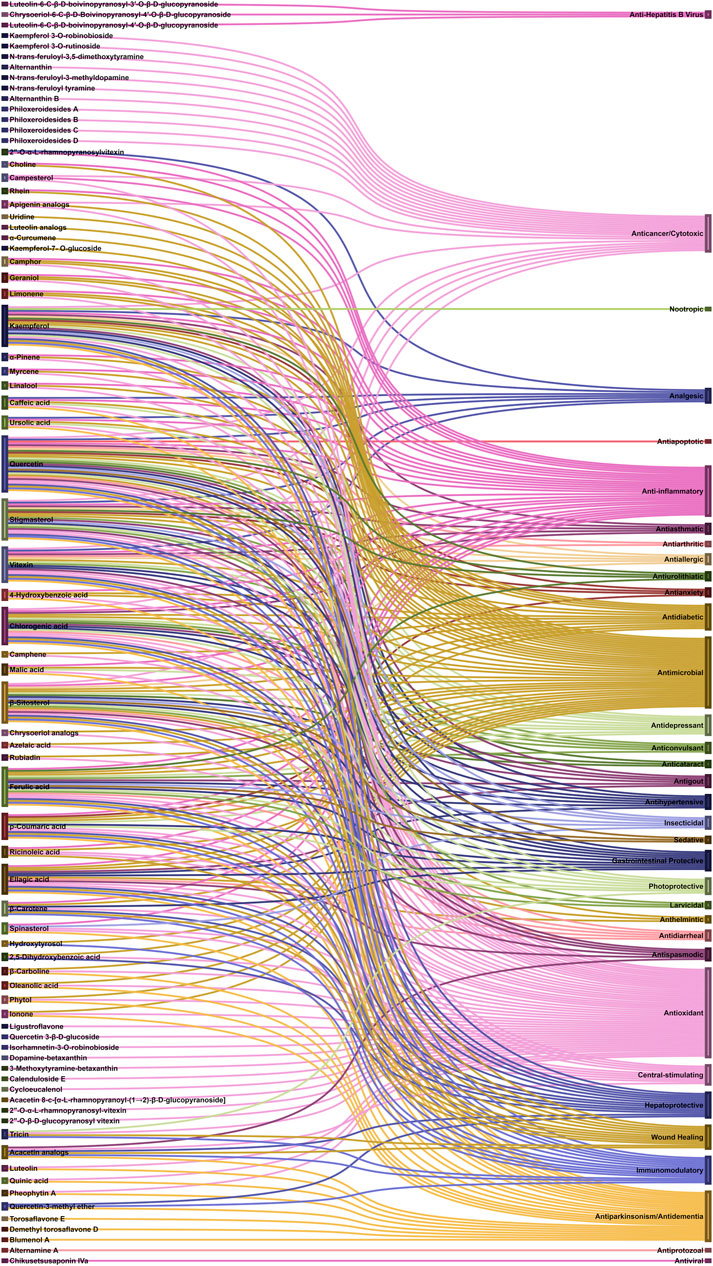
FIGURE 4. Bioactive Molecules and their elicited pharmacological activities. This information was collected as a cross-sectional literature review while exploring the possible bioactive molecules behind the pharmacological activities of the crude extracts obtained from various species of Alternanthera Genus.
It is indispensable to confirm if traditional claims of Alternanthera species have been proven by systematic scientifically designed pharmacological (preclinical or clinical) studies. Traditional claims and reported pharmacological activities of various species are presented in Table 3, and observations are as follows:
a) Traditional claims of some species (Alternanthera brasiliana (L.) Kuntze, Alternanthera caracasana Kunth, A. dentata (now reclaimed as Alternanthera brasiliana (L.) Kuntze), A. ficoides (now reclaimed as Alternanthera sessilis (L.) R.Br. ex DC.), Alternanthera littoralis P.Beauv., A. maritima (now reclaimed as Alternanthera littoralis P.Beauv.), Alternanthera nodiflora R.Br., Alternanthera paronychioides A.St.-Hil., Alternanthera porrigens (Jacq.) Kuntze, Alternanthera pungens Kunth, Alternanthera sessilis (L.) R.Br. ex DC., A. tenella (now reclaimed as Alternanthera sessilis (L.) R.Br. ex DC.), and A. triandra (now reclaimed as Alternanthera sessilis (L.) R.Br. ex DC.)) have not been validated scientifically.
b) Traditionally used species like Alternanthera caracasana Kunth and Alternanthera porrigens (Jacq.) Kuntze have not been investigated for any pharmacological activities. These species hold great potential for future research intending to validate traditional claims.
c) Species (Alternanthera brasiliana (L.) Kuntze, Alternanthera paronychioides A.St.-Hil., Alternanthera philoxeroides (Mart.) Griseb., and Alternanthera sessilis (L.) R.Br. ex DC.) have been screened for those pharmacological actions which are not claimed traditionally. These species may have been chosen following a chemotaxonomical or ecological approach.
d) Literature did not reveal any traditional use of three species (Alternanthera bettzickiana (Regel) G.Nicholson, Alternanthera hirtula (Mart.) R.E.Fr., and Alternanthera praelonga A.St.-Hil.) but evaluated for varied pharmacological activities.
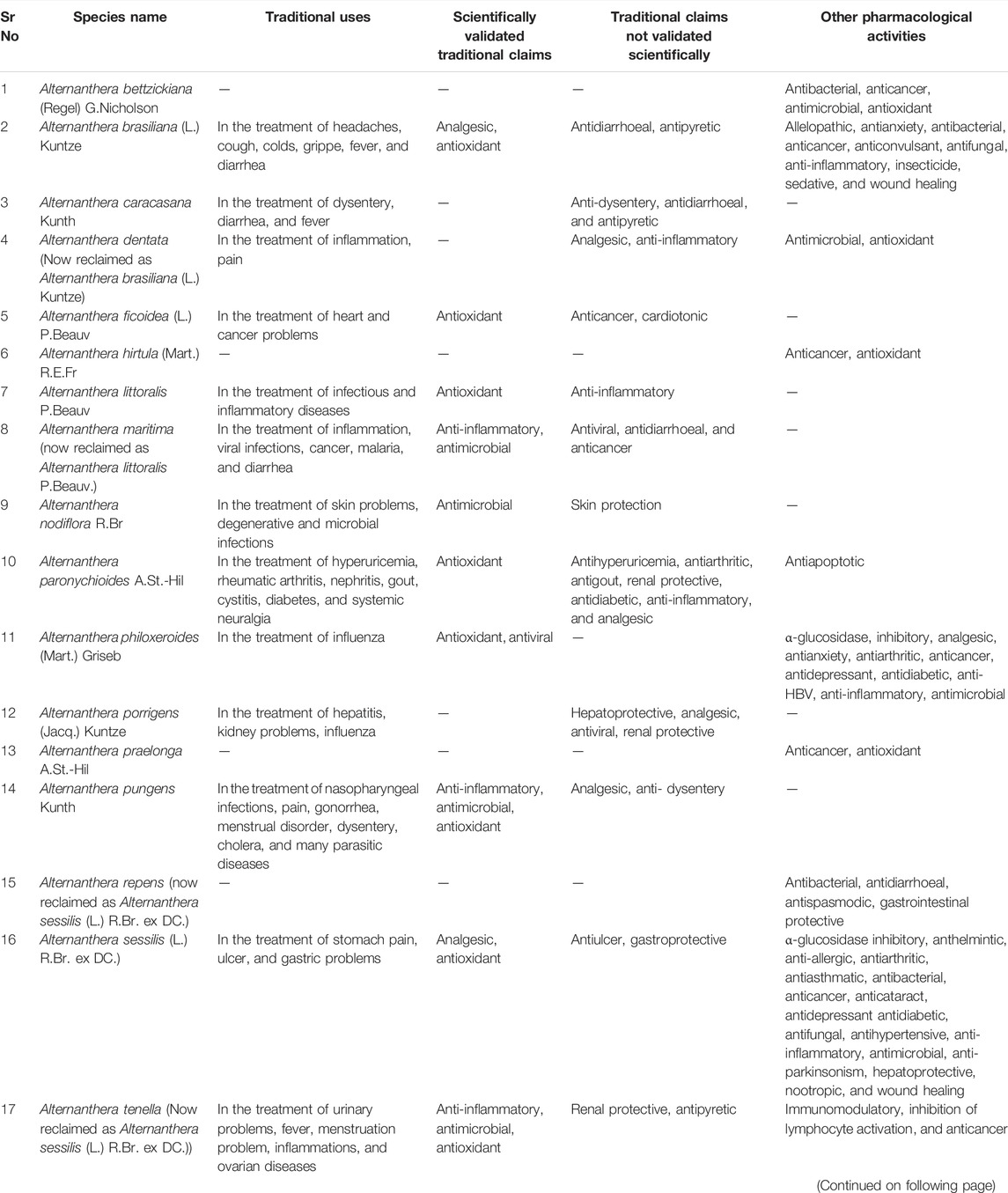
TABLE 3. Relationship between reported scientific pharmacological activities of Alternanthera species and their traditional claims.
Toxicological Studies
Hydroalcoholic extract of Alternanthera brasiliana (L.) Kuntze and Alternanthera bettzickiana (Regel) G.Nicholson leaves was orally administered (200 mg/kg dose) for 14 days in mice to observe any change in behavior of animals (Kasthuri and Ramesh, 2018). Further, hematological and histopathological changes were also observed. Sub-acute toxicity study suggested that both extracts samples did not show any harmful side effects. Hydroethanolic leaf extract of Alternanthera bettzickiana (Regel) G.Nicholson displayed a progressively powerful cytotoxic impact on DLA cell lines than Alternanthera brasiliana (L.) Kuntze extract.
The oral acute toxicity study was conducted on 95% ethanolic extract of Alternanthera philoxeroides (Mart.) Griseb. at the dose of 500 mg/kg in male and female rodents (Thanabhorn et al., 2005). The ethanolic extract did not show mortality and gross morphological alterations in the organs of rodents. Oral administration of 1,000 mg/kg/day for 14 days showed no significant changes in the body and inner organs weights, hematological and clinical parameters.
Clinical Studies
The studies have shown antiretroviral activity of Alternanthera pungens Kunth herbal tea due to antioxidant potential when administered to HIV patients (Djohan et al., 2009). Blood samples were taken from fasted patients who received an Alternanthera pungens Kunth tea for 12 months every day before dinner. The markers of oxidative stress (malondialdehyde and advanced oxidation protein end products), plasma T lymphocytes, transaminases, and creatinine were determined in the blood sample. A significant decrease in concentrations of markers of oxidative stress and an increase in plasma levels of CD4 and CD8 T cells after this period were observed. Further, no signs of hepatic and renal toxicity were seen in HIV patients.
In another case study, the potential of Alternanthera sessilis (L.) R.Br. ex DC., Momordica charantia L., and Colocasia esculenta (L.) Schott were investigated in reducing postprandial blood glucose levels in healthy human subjects and patients with type II diabetes (Bachok et al., 2014). The results of the clinical report suggested that Alternanthera sessilis (L.) R.Br. ex DC. reduced the non-significant glucose level in 3 h in comparison to standard control diet in healthy and diseased subjects. This case study was conducted in India with eight healthy subjects and six diabetic subjects.
Conclusion
Scrutiny of available literature reveals that out of 139 species of the genus Alternanthera:
a) Nine species have been investigated phytochemically,
b) Fifteen species possess strong ethnopharmacological records,
c) Twelve species have been scientifically evaluated in the in vitro or in vivo experimental models for various pharmacological activities,
d) Three species have been subjected to toxicity studies for establishing safety profiles,
e) Two species have been examined for clinical studies.
To date, 129 compounds have been isolated from 9 species of Alternanthera. 129 bioactive compounds were classified in 11 phytochemical classes, covering information about 40 flavonoids, 17 triterpenoid/saponins, 15 sterols, 12 alkaloids, 10 phenolic compounds, 3 ionone, 1 benzopyran, 3 hydroxycinnamic acids, 4 anthraquinone, 8 volatile oils and 17 miscellaneous compounds. Flavonoids (∼32%) constitute the main class of phytoconstituents in the genus Alternanthera followed by triterpenoids (∼13%). The isolated triterpenoids such as oleanolic acid, ursolic acid, and flavonoids such as luteolin, apigenin, vitexin, kaempferol, quercetin aglycones and their glycosides from the genus have proven therapeutic value. In terms of the phytochemical exploration, the most explored species of Alternanthera genus were Alternanthera philoxeroides (Mart.) Griseb. (52 compounds), Alternanthera sessilis (L.). R.Br. ex DC. (45 compounds), Alternanthera brasiliana (L.). Kuntze (32 compounds), and Alternanthera littoralis P.Beauv (24 compounds). Alternanthera sessilis (L.) R.Br. ex DC. has so far yielded a diverse class of compounds, like benzopyran, flavonoids, sterols, triterpenoid/saponin, phenolic compounds, ionone, and miscellaneous compounds. Similarly, Alternanthera philoxeroides (Mart.) Griseb. has also yielded a diverse class of compounds like flavonoids, sterols, triterpenoid/saponins, phenolic compounds, anthraquinone, alkaloids, and miscellaneous compounds.While volatile oil related compounds were extracted only from Alternanthera pungens Kunth, ionone analogues were isolated from Alternanthera sessilis (L.) R.Br. ex DC. only and hydroxycinnamic acids were reported only from Alternanthera bettzickiana (Regel) G.Nicholson. Researchers could explore rest of the species of Alternanthera genus to check if containing ionone analogues, volatile oils, and hydroxycinnamic acids. Further, the species of Alternanthera genus which were least explored in terms of phytochemical characterization is also leading for possible opportunities for the researchers.
To the best of our knowledge, the phytochemial characterization of Alternanthera paronychioides A.St.-Hil., Alternanthera caracasana Kunth, Alternanthera nodiflora R.Br., and Alternanthera ficoidea (L.) P.Beauv. was not yet done, leaving an ample scope for the researchers.
Some phytoconstituents like quercetin, vitexin, chlorogenic acid, kaempferol, ferulic acid, β-sitosterol, p-coumaric acid, caffeic acid, quinic acid, etc had been reported from more than one species of Alternanthera. Probably, we could say that these phytoconstituents may be common secondary metabolites in Alternanthera genus. So, we recommend the researchers to explore the rest of the Alternanthera species for these common metabolites. These metabolites could serve as biomarkers for them.
As twelve species of Alternanthera have been investigated scientifically for pharmacological activities, only 9 species of the genus have been explored phytochemically. Few medicinally promising Alternanthera species have not been taken into consideration for phytochemical studies. The existing literature demonstrates that 5 species of genus Alternanthera such as.
Alternanthera brasiliana (L.) Kuntze, Alternanthera caracasana Kunth, Alternanthera ficoidea (L.) P.Beauv., Alternanthera nodiflora R.Br., and Alternanthera paronychioides A.St.-Hil. have been scientifically reported to exhibit various pharmacological activities, but these species have never been subjected to bioactivity directed fractionation to isolate bioactive phytoconstituents using appropriate chromatographic techniques. Therefore, natural product scientists should expand their research activities on Alternanthera species to isolate more bioactive compounds which can be developed as safer and efficacious lead molecules or potent analogs of bioactive markers. Further, it seems necessary to mention a major research gap in phytochemical studies that no emphasis has been given to standardizing these plants based on marker compounds. Appropriate analytical methods need to be developed using HPLC, HPTLC, or LC-MS for the standardization of Alternanthera species. Molecular docking and QSAR studies on selective bioactive markers of these species are also lacking. It has been observed that crude uncharacterized extracts of Alternanthera species have been used in most pharmacological studies. This observation attracts attention towards the isolation of bioactive compounds from Alternanthera following the bioactivity-guided fractionation approach. Highlighting a mechanistic approach for pharmacological activities is another area of research to be covered. Alternamide A-B and Alternamine A-B were evaluated only for antiprotozoal activity while Chikusetsusaponin IVa was checked for antiviral activity only, leaving a wide scope for the researchers.
Amongst 139 species of Alternanthera, only 12 species have shown medicinal value in preclinical studies, and out of these only Alternanthera pungens Kunth and Alternanthera sessilis (L.) R.Br. ex DC. have been investigated clinically for antiretroviral and antidiabetic activities, respectively. The toxicity studies have been conducted on 3 species such as Alternanthera bettzickiana (Regel) G.Nicholson, Alternanthera brasiliana (L.) Kuntze, and Alternanthera philoxeroides (Mart.) Griseb. to establish their safety profile. Please be noted that as per the latest guidelines and recommendations of the ethnopharmacology team, the scientific names of the plants have been reassessed and considered the name given on https://mpns.science.kew.org/mpns-portal/. So the universally recognized name has been mentioned rather than the synonym indicated in the cited articles.
It is finally concluded that a well-planned roadmap of research activities is needed to be designed on traditionally used and medicinally promising plants of genus Alternanthera, so that their products and preparations may emerge out to be clinically potential and safe medicines in the treatment of various ailments.
Author Contributions
RM and BS contributed to the conception and design of the study. RS, VD, DK, SB, MB, SK, AD, and SS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (32070671), the COVID-19 Research Projects of West China Hospital Sichuan University (Grant no. HX-2019-nCoV-057), and the Regional Innovation Cooperation between Sichuan and Guangxi Provinces (2020YFQ0019).
Author Disclaimer
The scientific name of plants was mentioned as per the universally accepted nomenclature, specified and recommended by the Ethnopharmacology team. So, the names specified in the manuscript will seems to be different from that of cited articles. To cross-check the nomenclature, refer https://mpns.science.kew.org/mpns-portal/.
Conflict of Interest
RS and SS are having honorary based association with iGlobal Research and Publishing Foundation, New Delhi India, who declare that there are no conflicts of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the financial support received from the National Natural Science Foundation of China, the West China Hospital Sichuan University, and the Regional Innovation Cooperation between Sichuan and Guangxi Provinces.
References
Abbas, M. M., Al-Rawi, N., Abbas, M. A., and Al-Khateeb, I. (2019). Naringenin Potentiated β-sitosterol Healing Effect on the Scratch Wound Assay. Res. Pharm. Sci. 14 (6), 566–573. doi:10.4103/1735-5362.272565
Abdou, E. M., Fayed, M. A. A., Helal, D., and Ahmed, K. A. (2019). Assessment of the Hepatoprotective Effect of Developed Lipid-Polymer Hybrid Nanoparticles (LPHNPs) Encapsulating Naturally Extracted β-Sitosterol against CCl4 Induced Hepatotoxicity in Rats. Sci. Rep. 9 (1), 19779. doi:10.1038/s41598-019-56320-2
Abuelsaad, A. S., Mohamed, I., Allam, G., and Al-Solumani, A. A. (2013). Antimicrobial and Immunomodulating Activities of Hesperidin and Ellagic Acid against Diarrheic Aeromonas Hydrophila in a Murine Model. Life Sci. 93 (20), 714–722. doi:10.1016/j.lfs.2013.09.019
Adebiyi, O. E., Olopade, J. O., and Olayemi, F. O. (2018). Sodium Metavanadate Induced Cognitive Decline, Behavioral Impairments, Oxidative Stress and Down Regulation of Myelin Basic Protein in Mice hippocampus: Ameliorative Roles of β-spinasterol, and Stigmasterol. Brain Behav. 8 (7), e01014. doi:10.1002/brb3.1014
Agra, M. F., Baracho, G. S., Nurit, K., Basílio, I. J., and Coelho, V. P. (2007). Medicinal and Poisonous Diversity of the flora of "Cariri Paraibano", Brazil. J. Ethnopharmacol 111 (2), 383–395. doi:10.1016/j.jep.2006.12.007
Aguirre-Hernández, E., Rosas-Acevedo, H., Soto-Hernández, M., Martínez, A. L., Moreno, J., and González-Trujano, M. E. (2007). Bioactivity-guided Isolation of Beta-Sitosterol and Some Fatty Acids as Active Compounds in the Anxiolytic and Sedative Effects of Tilia Americana Var. Mexicana. Planta Med. 73 (11), 1148–1155. doi:10.1055/s-2007-981593
Ahmad, M., Gilani, A.-U. -H., Aftab, K., and Ahmad, V. U. (1993). Effects of Kaempferol-3-O-Rutinoside on Rat Blood Pressure. Phytother. Res. 7 (4), 314–316. doi:10.1002/ptr.2650070411
Ahmed, M., Qin, P., Ji, M., An, R., Guo, H., and Shafi, J. (2020). Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus Colocynthis L. With Aphicidal Activity against Cabbage Aphid Brevicoryne Brassicae L. Molecules 25 (9), 1. doi:10.3390/molecules25092184
Ahmed, M. A. E., Mohanad, M., Ahmed, A. A. E., Aboulhoda, B. E., and El-Awdan, S. A. (2021). Mechanistic Insights into the Protective Effects of Chlorogenic Acid against Indomethacin-Induced Gastric Ulcer in Rats: Modulation of the Cross Talk between Autophagy and Apoptosis Signaling. Life Sci. 275, 119370. doi:10.1016/j.lfs.2021.119370
Akachukwu, D., and Uchegbu, R. (2016). GC-MS, Antimicrobial and In Vitro Antioxidant Assay of the Leaf Extract of Alternanthera Dentata. Jamps 11 (2), 1–7. doi:10.9734/jamps/2016/29855
Akbar, M., Amin, A., Khalil, T., Iqbal, M. S., Nazir, A., and Taswar, A. (2021). Antibacterial Activity of Alternanthera Philoxeroides (Mart.) Griseb. Against Bacterial Phytopathogens: Erwinia Carotovora, Ralstonia Solanacearum and Xanthomonas Axonopodis. Aj 53 (1), 83–92. doi:10.26651/allelo.j/2021-53-1-1329
Alawode, T. T., Lajide, L., Olaleye, M., and Owolabi, B. (2021). Stigmasterol and β-Sitosterol: Antimicrobial Compounds in the Leaves of Icacina Trichantha Identified by GC-MS. Beni-suef Univ. J. Basic Appl. Sci. 10 (1), 1. doi:10.1186/s43088-021-00170-3
Alencar Filho, J. M. T., Teixeira, H. A. P., Sampaio, P. A., Pereira, E. C. V., Amariz, I. A. E., Rolim Neto, P. J., et al. (2019). Phytochemical Analysis in Alternanthera Brasiliana by LC-MS/MS and GC-MS. Nat. Prod. Res. 34 (3), 429–433. doi:10.1080/14786419.2018.1533827
Alencar Filho, J. M. T. d., Sampaio, P. A., Carvalho, I. S. d., Guimarães, A. L., Amariz, I. A. e., Pereira, E. C. V., et al. (2020). Flavonoid Enriched Extract of Alternanthera Brasiliana with Photoprotective Effect: Formulation Development and Evaluation of Quality. Ind. Crops Prod. 149, 112371. doi:10.1016/j.indcrop.2020.112371
Ali, H., Dixit, S., Ali, D., Alqahtani, S. M., Alkahtani, S., and Alarifi, S. (2015). Isolation and Evaluation of Anticancer Efficacy of Stigmasterol in a Mouse Model of DMBA-Induced Skin Carcinoma. Drug Des. Devel Ther. 9, 2793–2800. doi:10.2147/dddt.S83514
Alshehri, A. S., El-Kott, A. F., El-Gerbed, M. S. A., El-Kenawy, A. E., Albadrani, G. M., and Khalifa, H. S. (2021). Kaempferol Prevents Cadmium Chloride-Induced Liver Damage by Upregulating Nrf2 and Suppressing NF-Κb and Keap1. Environ. Sci. Pollut. Res. 29, 13917–13929. doi:10.1007/s11356-021-16711-3
Amalan, V., Vijayakumar, N., Indumathi, D., and Ramakrishnan, A. (2016). Antidiabetic and Antihyperlipidemic Activity of P-Coumaric Acid in Diabetic Rats, Role of Pancreatic GLUT 2: In Vivo Approach. Biomed. Pharmacother. 84, 230–236. doi:10.1016/j.biopha.2016.09.039
Ammar, S., Edziri, H., Mahjoub, M. A., Chatter, R., Bouraoui, A., and Mighri, Z. (2009). Spasmolytic and Anti-inflammatory Effects of Constituents from Hertia Cheirifolia. Phytomedicine 16 (12), 1156–1161. doi:10.1016/j.phymed.2009.03.012
An, F., Yang, G., Tian, J., and Wang, S. (2012). Antioxidant Effects of the Orientin and Vitexin in Trollius Chinensis Bunge in D-Galactose-Aged Mice. Neural Regen. Res. 7 (33), 2565–2575. doi:10.3969/j.issn.1673-5374.2012.33.001
Angajala, G., and Subashini, R. (2018). Evaluation of Larvicidal Potential of β-sitosterol Isolated from Indigenous Aegle Marmelos Correa Crude Leaf Extracts against Blood Feeding Parasites and its Binding Affinity Studies towards Sterol Carrier Protein. Biocatal. Agric. Biotechnol. 16, 586–593. doi:10.1016/j.bcab.2018.10.005
Anitha, R., and Kanimozhi, S. (2012). Pharmacognostic Evaluation of Alternanthera Sessilis (L.) R.Br.Ex.DC. Pharmacognosy J. 4 (28), 31–34. doi:10.5530/pj.2012.28.6
Anjaneyulu, M., and Chopra, K. (2003). Quercetin, a Bioflavonoid, Attenuates thermal Hyperalgesia in a Mouse Model of Diabetic Neuropathic Pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 27 (6), 1001–1005. doi:10.1016/s0278-5846(03)00160-x
Antwi, A. O., Obiri, D. D., Osafo, N., Essel, L. B., Forkuo, A. D., and Atobiga, C. (2018). Stigmasterol Alleviates Cutaneous Allergic Responses in Rodents. Biomed. Res. Int. 2018. doi:10.1155/2018/3984068
Antwi, A. O., Obiri, D. D., and Osafo, N. (2017a). Stigmasterol Modulates Allergic Airway Inflammation in Guinea Pig Model of Ovalbumin-Induced Asthma. Mediators Inflamm. 2017, 2953930–2954011. doi:10.1155/2017/2953930
Antwi, A. O., Obiri, D. D., Osafo, N., Forkuo, A. D., and Essel, L. B. (2017b). Stigmasterol Inhibits Lipopolysaccharide-Induced Innate Immune Responses in Murine Models. Int. Immunopharmacol 53, 105–113. doi:10.1016/j.intimp.2017.10.018
Arabyan, E., Hakobyan, A., Hakobyan, T., Grigoryan, R., Izmailyan, R., Avetisyan, A., et al. (2021). Flavonoid Library Screening Reveals Kaempferol as a Potential Antiviral Agent against African Swine Fever Virus. Front. Microbiol. 12, 736780. doi:10.3389/fmicb.2021.736780
Araujo, E. C. C., Silva, E. E. S., Alencar-Fi, J. M. T., Oliveira, A. P., Guimaraes, A. L., Siqueira-F, J. A., et al. (2014). Identification of Glycosil Flavones and Determination In Vitro of Antioxidant and Photoprotective Activities of Alternanthera Brasiliana L. Kuntze. Res. J. Phytochemistry 8 (4), 148–154. doi:10.3923/rjphyto.2014.148.154
Arshad, N., Zitterl-Eglseer, K., Hasnain, S., and Hess, M. (2008). Effect of Peganum Harmala or its Beta-Carboline Alkaloids on Certain Antibiotic Resistant Strains of Bacteria and Protozoa from Poultry. Phytother Res. 22 (11), 1533–1538. doi:10.1002/ptr.2528
Arulselvan, P., Gothai, S., Muniandy, K., Mohd Esa, N., and Subbiah, S. (2018). Anticancer Potential of Alternanthera Sessilis Extract on HT-29 Human colon Cancer Cells. Asian Pac. J. Trop. Biomed. 8 (8), 394. doi:10.4103/2221-1691.239427
Aseervatham, G. S., Suryakala, U., DoulethunishaSundaram, S., Sundaram, S., Bose, P. C., and Sivasudha, T. (2016). Expression Pattern of NMDA Receptors Reveals Antiepileptic Potential of Apigenin 8-C-Glucoside and Chlorogenic Acid in Pilocarpine Induced Epileptic Mice. Biomed. Pharmacother. 82, 54–64. doi:10.1016/j.biopha.2016.04.066
Astudillo-Vázquez, A., Dávalos Valle, H., De Jesús, L., Herrera, G., and Navarrete, A. (2008). Investigation of Alternanthera Repens and Bidens Odorata on Gastrointestinal Disease. Fitoterapia 79 (7-8), 577–580. doi:10.1016/j.fitote.2008.07.001
Attaugwu, R. N., and Uvere, P. O. (2017). Health Promoting Properties of Alternanthera Brasiliana Leaves and Hibiscus sabdariffa Calyces Used in Fortification of maize-Bambara Groundnut Malt and maize-cowpea Malt Complementary Foods. Food Res. 1 (4), 133–139. doi:10.26656/fr.2017.4.058
Aydin, E., Türkez, H., and Geyikoğlu, F. (2013). Antioxidative, Anticancer and Genotoxic Properties of α-pinene on N2a Neuroblastoma Cells. Biologia 68 (5), 1004–1009. doi:10.2478/s11756-013-0230-2
Aytac, Z., Yildiz, Z. I., Kayaci-Senirmak, F., San Keskin, N. O., Tekinay, T., and Uyar, T. (2016). Electrospinning of Polymer-free Cyclodextrin/geraniol-Inclusion Complex Nanofibers: Enhanced Shelf-Life of Geraniol with Antibacterial and Antioxidant Properties. RSC Adv. 6 (52), 46089–46099. doi:10.1039/c6ra07088d
Azizah, O., Amin, I., and Fouad, A. R. (2015). Antioxidant Properties ofAlternanthera Sessilisred and green. Acta Hortic. 1106, 131–136. doi:10.17660/ActaHortic.2015.1106.20
Babu, M., Joseph, K. H., Sree, A., and Scariya, S. (2021). In-Vitro Evaluation of Anti-urolithiatic and Larvicidal Activity of Alternanthera Sessilis. Biomed. Pharmacol. J. 14 (02), 671–680. doi:10.13005/bpj/2169
Babukumar, S., Vinothkumar, V., Sankaranarayanan, C., and Srinivasan, S. (2017). Geraniol, a Natural Monoterpene, Ameliorates Hyperglycemia by Attenuating the Key Enzymes of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 55 (1), 1442–1449. doi:10.1080/13880209.2017.1301494
Bachok, M. F., Yusof, B. N., Ismail, A., and Hamid, A. A. (2014). Effectiveness of Traditional Malaysian Vegetables (Ulam) in Modulating Blood Glucose Levels. Asia Pac. J. Clin. Nutr. 23 (3), 369–376. doi:10.6133/apjcn.2014.23.3.01
Bae, H., Park, S., Yang, C., Song, G., and Lim, W. (2021). Disruption of Endoplasmic Reticulum and ROS Production in Human Ovarian Cancer by Campesterol. Antioxidants 10 (3), 1. doi:10.3390/antiox10030379
Bagdas, D., Etoz, B. C., Gul, Z., Ziyanok, S., Inan, S., Turacozen, O., et al. (2015). In Vivo systemic Chlorogenic Acid Therapy under Diabetic Conditions: Wound Healing Effects and Cytotoxicity/genotoxicity Profile. Food Chem. Toxicol. 81, 54–61. doi:10.1016/j.fct.2015.04.001
Baluchnejadmojarad, T., Rabiee, N., Zabihnejad, S., and Roghani, M. (2017). Ellagic Acid Exerts Protective Effect in Intrastriatal 6-hydroxydopamine Rat Model of Parkinson’s Disease: Possible Involvement of ERβ/Nrf2/HO-1 Signaling. Brain Res. 1662, 23–30. doi:10.1016/j.brainres.2017.02.021
Bankar, G. R., Nayak, P. G., Bansal, P., Paul, P., Pai, K. S. R., Singla, R. K., et al. (2011). Vasorelaxant and Antihypertensive Effect of Cocos Nucifera Linn. Endocarp on Isolated Rat Thoracic Aorta and DOCA Salt-Induced Hypertensive Rats. J. Ethnopharmacology 134 (1), 50–54. doi:10.1016/j.jep.2010.11.047
Bansal, N., Yadav, P., and Kumar, M. (2017). Ellagic Acid Administration Negated the Development of Streptozotocin-Induced Memory Deficit in Rats. Drug Res. 67 (07), 425–431. doi:10.1055/s-0043-108552
Barahuie, F., Saifullah, B., Dorniani, D., Fakurazi, S., Karthivashan, G., Hussein, M. Z., et al. (2017). Graphene Oxide as a Nanocarrier for Controlled Release and Targeted Delivery of an Anticancer Active Agent, Chlorogenic Acid. Mater. Sci. Eng. C 74, 177–185. doi:10.1016/j.msec.2016.11.114
Baricevic, D., Sosa, S., Della Loggia, R., Tubaro, A., Simonovska, B., Krasna, A., et al. (2001). Topical Anti-inflammatory Activity of Salvia Officinalis L. Leaves: the Relevance of Ursolic Acid. J. Ethnopharmacology 75 (2-3), 125–132. doi:10.1016/s0378-8741(00)00396-2
Baru, C. C., Talukdar, A., Begum, S. A., Buragohain, B., Roy, J. D., Pathak, D. C., et al. (2012). Effect of Alternanthera Brasiliana (L) Kuntze on Healing of Dermal Burn Wound. Indian J. Exp. Biol. 50 (1), 56–60.
Barua, C., Begum, S., Sarma, D., Pathak, D., and Borah, R. (2012a). Healing Efficacy of Methanol Extract of Leaves of Alternanthera Brasiliana Kuntze in Aged Wound Model. J. Basic Clin. Pharm. 3 (4), 1. doi:10.4103/0976-0105.105336
Barua, C. C., Ara Begum, S., Talukdar, A., Datta Roy, J., Buragohain, B., Chandra Pathak, D., et al. (2012b). Influence of Alternanthera Brasiliana (L.) Kuntze on Altered Antioxidant Enzyme Profile during Cutaneous Wound Healing in Immunocompromised Rats. ISRN Pharmacol. 2012, 1–8. doi:10.5402/2012/948792
Barua, C. C., Begum, S. A., Barua, A. G., Borah, R. S., and Lahkar, M. (2013). Anxiolytic and Anticonvulsant Activity of Methanol Extract of Leaves of Alternanthera Brasiliana (L.) Kuntze (Amaranthaceae) in Laboratory Animals. Indian J. Exp. Biol. 51 (6), 450–457.
Barua, C. C., Talukdar, A., Begum, S. A., Sarma, D. K., Fathak, D. C., Barua, A. G., et al. (2009). Wound Healing Activity of Methanolic Extract of Leaves of Alternanthera Brasiliana Kuntz Using In Vivo and In Vitro Model. Indian J. Exp. Biol. 47 (12), 1001–1005.
Bayer, M., Proksch, P., Felsner, I., Brenden, H., Kohne, Z., Walli, R., et al. (2011). Photoprotection against UVAR: Effective Triterpenoids Require a Lipid Raft Stabilizing Chemical Structure. Exp. Dermatol. 20 (11), 955–958. doi:10.1111/j.1600-0625.2011.01350.x
Beber, A. P., de Souza, P., Boeing, T., Somensi, L. B., Mariano, L. N. B., Cury, B. J., et al. (2017). Constituents of Leaves from Bauhinia Curvula Benth. Exert Gastroprotective Activity in Rodents: Role of Quercitrin and Kaempferol. Inflammopharmacology 26 (2), 539–550. doi:10.1007/s10787-017-0313-8
Bektas, N., Şenel, B., Yenilmez, E., Özatik, O., and Arslan, R. (2020). Evaluation of Wound Healing Effect of Chitosan-Based Gel Formulation Containing Vitexin. Saudi Pharm. J. 28 (1), 87–94. doi:10.1016/j.jsps.2019.11.008
Berkban, T., Boonprom, P., Bunbupha, S., Welbat, J., Kukongviriyapan, U., Kukongviriyapan, V., et al. (2015). Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of eNOS and P47phox Expression in Rats. Nutrients 7 (7), 5265–5280. doi:10.3390/nu7075222
Beserra, A. M. S. e. S., Calegari, P. I., Souza, M. d. C., dos Santos, R. A. N., Lima, J. C. d. S., Silva, R. M., et al. (2011). Gastroprotective and Ulcer-Healing Mechanisms of Ellagic Acid in Experimental Rats. J. Agric. Food Chem. 59 (13), 6957–6965. doi:10.1021/jf2003267
Bhat, T. A., Nambiar, D., Tailor, D., Pal, A., Agarwal, R., and Singh, R. P. (2013). Acacetin Inhibits In Vitro and In Vivo Angiogenesis and Downregulates Stat Signaling and VEGF Expression. Cancer Prev. Res. 6 (10), 1128–1139. doi:10.1158/1940-6207.Capr-13-0209
Bhattacherjee, A., Ghosh, T., Sil, R., and Datta, A. (2014). Isolation and Characterisation of Methanol-Soluble Fraction of Alternanthera Philoxeroides (Mart.) – Evaluation of Their Antioxidant, α-glucosidase Inhibitory and Antimicrobial Activity in In Vitro Systems. Nat. Product. Res. 28 (23), 2199–2202. doi:10.1080/14786419.2014.930857
Bhuyan, B., Baishya, K., and Rajak, P. (2017). Effects of Alternanthera Sessilis on Liver Function in Carbon Tetra Chloride Induced Hepatotoxicity in Wister Rat Model. Indian J. Clin. Biochem. 33 (2), 190–195. doi:10.1007/s12291-017-0666-1
Biella, C. d. A., Salvador, M. J., Dias, D. A., Dias-Baruffi, M., and Pereira-Crott, L. S. (2008). Evaluation of Immunomodulatory and Anti-inflammatory Effects and Phytochemical Screening of Alternanthera Tenella Colla (Amaranthaceae) Aqueous Extracts. Memórias do Instituto Oswaldo Cruz 103 (6), 569–577. doi:10.1590/s0074-02762008000600010
Binang, K., and Takuwa, D. T. (2021). Development of Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) Method for Determination of Selected Antihypertensive Active Flavonoids (Rutin, Myricetin, Quercetin, and Kaempferol) in Medicinal Plants Found in Botswana. Phys. Sci. Rev. 0 (0), 1. doi:10.1515/psr-2020-0209
Bindoli, A., Valente, M., and Cavallini, L. (1985). Inhibitory Action of Quercetin on Xanthine Oxidase and Xanthine Dehydrogenase Activity. Pharmacol. Res. Commun. 17 (9), 831–839. doi:10.1016/0031-6989(85)90041-4
Bisignano, G., Tomaino, A., Cascio, R. L., Crisafi, G., Uccella, N., and Saija, A. (1999). On the In-Vitro Antimicrobial Activity of Oleuropein and Hydroxytyrosol. J. Pharm. Pharmacol. 51 (8), 971–974. doi:10.1211/0022357991773258
Biswas, S., Mukherjee, P. K., Kar, A., Bannerjee, S., Jana, S. N., Haldar, P. K., et al. (2021). Enhanced Permeability and Photoprotective Potential of Optimized P-Coumaric Acid-Phospholipid Complex Loaded Gel against UVA Mediated Oxidative Stress. J. Photochem. Photobiol. B: Biol. 221. doi:10.1016/j.jphotobiol.2021.112246
Bobé, P., Checker, R., Sandur, S. K., Sharma, D., Patwardhan, R. S., Jayakumar, S., et al. (2012). Potent Anti-inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-Κb, AP-1 and NF-AT. PLoS ONE 7 (2), 1. doi:10.1371/journal.pone.0031318
Boeing, T., Costa, P., Venzon, L., Meurer, M., Mariano, L. N. B., França, T. C. S., et al. (2020). Gastric Healing Effect of P-Coumaric Acid Isolated from Baccharis Dracunculifolia DC on Animal Model. Naunyn-Schmiedeberg's Arch. Pharmacol. 394 (1), 49–57. doi:10.1007/s00210-020-01928-9
Borah, A., Yadav, R. N. S., and Unni, B. G. (2011). In Vitro antioxidant and Free Radical Scavenging Activity of Alternanthera Sessilis. Int. J. Pharm. Sci. Res. 2 (6), 1502–1506. doi:10.13040/IJPSR.0975-8232.2(6).1502-06
Borges, D. G. L., de Araújo, M. A., Carollo, C. A., Carollo, A. R. H., Lifschitz, A., Conde, M. H., et al. (2020). Combination of Quercetin and Ivermectin: In Vitro and In Vivo Effects against Haemonchus contortus. Acta Tropica 201. doi:10.1016/j.actatropica.2019.105213
Boubaker, J., Sghaier, M. B., Skandrani, I., Ghedira, K., and Chekir-Ghedira, L. (2012). Isorhamnetin 3-O-Robinobioside from Nitraria Retusa Leaves Enhance Antioxidant and Antigenotoxic Activity in Human Chronic Myelogenous Leukemia Cell Line K562. BMC Complement. Altern. Med. 12 (1), 1. doi:10.1186/1472-6882-12-135
Boz, H. (2015). p-Coumaric Acid in Cereals: Presence, Antioxidant and Antimicrobial Effects. Int. J. Food Sci. Tech. 50 (11), 2323–2328. doi:10.1111/ijfs.12898
Brochado, C. d. O., Almeida, A. P. d., Barreto, B. P., Costa, L. P., Ribeiro, L. S., Pereira, R. L. d. C., et al. (2003). Flavonol Robinobiosides and Rutinosides from Alternanthera Brasiliana (Amaranthaceae) and Their Effects on Lymphocyte Proliferation In Vitro. J. Braz. Chem. Soc. 14 (3), 449–451. doi:10.1590/s0103-50532003000300018
Cai, Y., Sun, M., and Corke, H. (2003). Antioxidant Activity of Betalains from Plants of the Amaranthaceae. J. Agric. Food Chem. 51 (8), 2288–2294. doi:10.1021/jf030045u
Calderón Guzmán, D., Trujillo Jiménez, F., Hernández García, E., and Juárez Olguín, H. (2007). Assessment of Antioxidant Effect of 2,5-Dihydroxybenzoic Acid and Vitamin A in Brains of Rats with Induced Hyperoxia. Neurochem. Res. 32 (6), 1036–1040. doi:10.1007/s11064-006-9269-6
Campos-Vidal, Y., Herrera-Ruiz, M., Trejo-Tapia, G., Gonzalez-Cortazar, M., Aparicio, A. J., and Zamilpa, A. (2021). Gastroprotective Activity of Kaempferol Glycosides from Malvaviscus Arboreus Cav. J. Ethnopharmacology 268. doi:10.1016/j.jep.2020.113633
Can, Ö. D., Demir Özkay, Ü., and Üçel, U. İ. (2013). Anti-depressant-like Effect of Vitexin in BALB/c Mice and Evidence for the Involvement of Monoaminergic Mechanisms. Eur. J. Pharmacol. 699 (1-3), 250–257. doi:10.1016/j.ejphar.2012.10.017
Canales-Martínez, M., Hernández-Delgado, T., Flores-Ortiz, C., Durán-Díaz, A., García-Bores, A. M., and Avila-Acevedo, G. (2008). Antimicrobial Activity of Alternanthera Caracasana. Pharm. Biol. 43 (4), 305–307. doi:10.1080/13880200590951685
Carter, B. A., Taylor, O. A., Prendergast, D. R., Zimmerman, T. L., Von Furstenberg, R., Moore, D. D., et al. (2007). Stigmasterol, a Soy Lipid–Derived Phytosterol, Is an Antagonist of the Bile Acid Nuclear Receptor FXR. Pediatr. Res. 62 (3), 301–306. doi:10.1203/PDR.0b013e3181256492
Cechinel-Zanchett, C. C., Bolda Mariano, L. N., Boeing, T., da Costa, J. d. C., Da Silva, L. M., Bastos, J. K., et al. (2020). Diuretic and Renal Protective Effect of Kaempferol 3-O-Alpha-L-Rhamnoside (Afzelin) in Normotensive and Hypertensive Rats. J. Nat. Prod. 83 (6), 1980–1989. doi:10.1021/acs.jnatprod.0c00274
Chai, T. T., Khoo, C. S., Tee, C. S., and Wong, F. C. (2016). Alpha-glucosidase Inhibitory and Antioxidant Potential of Antidiabetic Herb Alternanthera Sessilis: Comparative Analyses of Leaf and Callus Solvent Fractions. Pharmacogn Mag. 12 (48), 253–258. doi:10.4103/0973-1296.192202
Chandran, R. P. (2017). Analysis of Proximate, Phytochemical, Elemental Compositions and Antioxidant Property of Leaf of Alternanthera Brasiliana (L.) Kuntze. MOJ Food Process. Tech. 4 (3), 1. doi:10.15406/mojfpt.2017.04.00090
Chandrashekhar, K. (2019). Ethnobotanical and Phyto-Pharmacological Overview of Matsyakshi (Alternanthera Sessilis R. Br. Ex DC.). J. Ayu. Her. Med. 5 (4), 152–155.
Chaves-Quirós, C., Usuga-Usuga, J., Morales-Uchima, S., Tofiño-Rivera, A., Tobón-Arroyave, S., and Martínez-Pabón, M. (2020). Assessment of Cytotoxic and Antimicrobial Activities of Two Components of Cymbopogon Citratus Essential Oil. J. Clin. Exp. Dentistry 12, e749–e754. doi:10.4317/jced.56863
Chen, J., Li, Y., Yu, B., Chen, D., Mao, X., Zheng, P., et al. (2018). Dietary Chlorogenic Acid Improves Growth Performance of Weaned Pigs through Maintaining Antioxidant Capacity and Intestinal Digestion and Absorption Function. J. Anim. Sci. 96 (3), 1108–1118. doi:10.1093/jas/skx078
Chen, J., Lin, D., Zhang, C., Li, G., Zhang, N., Ruan, L., et al. (2014). Antidepressant-like Effects of Ferulic Acid: Involvement of Serotonergic and Norepinergic Systems. Metab. Brain Dis. 30 (1), 129–136. doi:10.1007/s11011-014-9635-z
Chen, J., Yang, H., and Sheng, Z. (2020). Ellagic Acid Activated PPAR Signaling Pathway to Protect Ileums against Castor Oil-Induced Diarrhea in Mice: Application of Transcriptome Analysis in Drug Screening. Front. Pharmacol. 10. doi:10.3389/fphar.2019.01681
Chen, Z., Yang, Y., Mi, S., Fan, Q., Sun, X., Deng, B., et al. (2019). Hepatoprotective Effect of Chlorogenic Acid against Chronic Liver Injury in Inflammatory Rats. J. Funct. Foods 62. doi:10.1016/j.jff.2019.103540
Cheng, C.-y., Su, S.-y., Tang, N.-y., Ho, T.-y., Lo, W.-y., and Hsieh, C.-l. (2010). Ferulic Acid Inhibits Nitric Oxide-Induced Apoptosis by Enhancing GABAB1 Receptor Expression in Transient Focal Cerebral Ischemia in Rats. Acta Pharmacologica Sinica 31 (8), 889–899. doi:10.1038/aps.2010.66
Chiang, H.-C., and Chen, Y.-Y. (2008). Xanthine Oxidase Inhibitors from the Roots of Eggplant (Solanum MelongenaL.). J. Enzyme Inhib. 7 (3), 225–235. doi:10.3109/14756369309040765
Chiruvella, K. K., Mohammed, A., Dampuri, G., Ghanta, R. G., and Raghavan, S. C. (2007). Phytochemical and Antimicrobial Studies of Methyl Angolensate and Luteolin-7-O-Glucoside Isolated from Callus Cultures of Soymida Febrifuga. Int. J. Biomed. Sci. 3 (4), 269–278.
Cho, H.-I., Park, J.-H., Choi, H.-S., Kwak, J. H., Lee, D.-U., Lee, S. K., et al. (2014a). Protective Mechanisms of Acacetin against D-Galactosamine and Lipopolysaccharide-Induced Fulminant Hepatic Failure in Mice. J. Nat. Prod. 77 (11), 2497–2503. doi:10.1021/np500537x
Cho, J.-Y., Moon, J.-H., Seong, K.-Y., and Park, K.-H. (2014b). Antimicrobial Activity of 4-Hydroxybenzoic Acid Andtrans4-Hydroxycinnamic Acid Isolated and Identified from Rice Hull. Biosci. Biotechnol. Biochem. 62 (11), 2273–2276. doi:10.1271/bbb.62.2273
Chong, S., and Loh, K. E. (2020). Xanthine Oxidase Inhibitory Activity of Methanolic Extract of Alternanthera Sessilis. Sains Malaysiana 49 (2), 405–410. doi:10.17576/jsm-2020-4902-19
Cloeckaert, A., Kovač, J., Šimunović, K., Wu, Z., Klančnik, A., Bucar, F., et al. (2015). Antibiotic Resistance Modulation and Modes of Action of (-)-α-Pinene in Campylobacter Jejuni. Plos One 10 (4), 1. doi:10.1371/journal.pone.0122871
Collett, M. G., and Taylor, S. M. (2019). Photosensitising Toxins in alligator weed (Alternanthera Philoxeroides) Likely to Be Anthraquinones. Toxicon 167, 172–173. doi:10.1016/j.toxicon.2019.06.218
Collins, M. A., and Charles, H. P. (1987). Antimicrobial Activity of Carnosol and Ursolic Acid: Two Anti-oxidant Constituents of Rosmarinus Officinalis L. Food Microbiol. 4 (4), 311–315. doi:10.1016/s0740-0020(87)80005-9
Corbett, S., Daniel, J., Drayton, R., Field, M., Steinhardt, R., and Garrett, N. (2010). Evaluation of the Anti-inflammatory Effects of Ellagic Acid. J. PeriAnesthesia Nurs. 25 (4), 214–220. doi:10.1016/j.jopan.2010.05.011
Correa, W. R., Hernandez Tasco, A. J., and Marinho, J. V. N. (2016). Antioxidant and Cytotoxic Activities and Chemical Profile of Five Amaranthaceae Plants Collected in the South of Brazil. Nat. Prod. Chem. Res. 4 (5), 1–7. doi:10.4172/2329-6836.1000230
Coutinho, H. D. M., de Morais Oliveira-Tintino, C. D., Tintino, S. R., Pereira, R. L. S., de Freitas, T. S., da Silva, M. A. P., et al. (2017). Toxicity against Drosophila melanogaster and Antiedematogenic and Antimicrobial Activities of Alternanthera Brasiliana (L.) Kuntze (Amaranthaceae). Environ. Sci. Pollut. Res. 25 (11), 10353–10361. doi:10.1007/s11356-017-9366-x
da Cunha, F. M., Duma, D., Assreuy, J., Buzzi, F. C., Niero, R., Campos, M. M., et al. (2009). Caffeic Acid Derivatives: In Vitro and In Vivo Anti-inflammatory Properties. Free Radic. Res. 38 (11), 1241–1253. doi:10.1080/10715760400016139
Das, D., Biswal, S., Barhwal, K. K., Chaurasia, O. P., and Hota, S. K. (2018). Kaempferol Inhibits Extra-synaptic NMDAR-Mediated Downregulation of TRkβ in Rat Hippocampus during Hypoxia. Neuroscience 392, 77–91. doi:10.1016/j.neuroscience.2018.09.018
Das, M. C., Sandhu, P., Gupta, P., Rudrapaul, P., De, U. C., Tribedi, P., et al. (2016). Attenuation of Pseudomonas aeruginosa Biofilm Formation by Vitexin: A Combinatorial Study with Azithromycin and Gentamicin. Scientific Rep. 6 (1), 1. doi:10.1038/srep23347
Das, M., Kumar, A. D., Mastanaiah, K., and Das, A. (2015). Evaluation of Anti-diabetic Activity of Ethanolic Extract of Alternanthera Sessilis Linn. In Streptozotocin-Induced Diabetic Rats. Int. J. Pharma Sci. Res. 6 (7), 1027–1032.
de la Lastra, A., oacuteMartin, M. J., and Motilva, V. (1994). Antiulcer and Gastroprotective Effects of Quercetin: A Gross and Histologic Study. Pharmacology 48 (1), 56–62. doi:10.1159/000139162
de Oliveira, D. D., da Silva, C. P., Iglesias, B. B., and Beleboni, R. O. (2020). Vitexin Possesses Anticonvulsant and Anxiolytic-like Effects in Murine Animal Models. Front. Pharmacol. 11. doi:10.3389/fphar.2020.01181
De, R., Sarkar, A., Ghosh, P., Ganguly, M., Karmakar, B. C., Saha, D. R., et al. (2018). Antimicrobial Activity of Ellagic Acid against Helicobacter pylori Isolates from India and during Infections in Mice. J. Antimicrob. Chemother. 73 (6), 1595–1603. doi:10.1093/jac/dky079
De Ruiz, R. E., Fusco, M., and Ruiz, S. O. (1993). Constituents of Alternanthera Pungens. Fitoterapia 64, 95–99.
de Santana Aquino, D. F., Piccinelli, A. C., Soares, F. L. P., Arena, A. C., Salvador, M. J., and Kassuya, C. A. L. (2015). Anti-hyperalgesic and Anti-inflammatory Activity of Alternanthera Maritima Extract and 2″-O-α-L-Rhamnopyranosylvitexin in Mice. Inflammation 38 (6), 2057–2066. doi:10.1007/s10753-015-0187-0
Deepak, M., Dipankar, G., Prashanth, D., Asha, M. K., Amit, A., and Venkataraman, B. V. (2002). Tribulosin and β-sitosterol-D-glucoside, the Anthelmintic Principles of Tribulus Terrestris. Phytomedicine 9 (8), 753–756. doi:10.1078/094471102321621395
del Valle, P., García-Armesto, M. R., de Arriaga, D., González-Donquiles, C., Rodríguez-Fernández, P., and Rúa, J. (2016). Antimicrobial Activity of Kaempferol and Resveratrol in Binary Combinations with Parabens or Propyl Gallate against Enterococcus faecalis. Food Control 61, 213–220. doi:10.1016/j.foodcont.2015.10.001
Deladino, L., Alvarez, I., De Ancos, B., Sánchez-Moreno, C., Molina-García, A. D., and Schneider Teixeira, A. (2017). Betalains and Phenolic Compounds of Leaves and Stems of Alternanthera Brasiliana and Alternanthera Tenella. Food Res. Int. 97, 240–249. doi:10.1016/j.foodres.2017.04.017
Delgado, A., Cholevas, C., and Theoharides, T. C. (2021). Neuroinflammation in Alzheimer's Disease and Beneficial Action of Luteolin. BioFactors 47 (2), 207–217. doi:10.1002/biof.1714
Desai, F., Ramanathan, M., Fink, C. S., Wilding, G. E., Weinstock-Guttman, B., and Awad, A. B. (2009). Comparison of the Immunomodulatory Effects of the Plant Sterol β-sitosterol to Simvastatin in Peripheral Blood Cells from Multiple Sclerosis Patients. Int. Immunopharmacology 9 (1), 153–157. doi:10.1016/j.intimp.2008.10.019
Deshmukh, R., Kaundal, M., Bansal, V., and Samardeep, (2016). Caffeic Acid Attenuates Oxidative Stress, Learning and Memory Deficit in Intra-cerebroventricular Streptozotocin Induced Experimental Dementia in Rats. Biomed. Pharmacother. 81, 56–62. doi:10.1016/j.biopha.2016.03.017
Devi, K. P., Malar, D. S., Nabavi, S. F., Sureda, A., Xiao, J., Nabavi, S. M., et al. (2015). Kaempferol and Inflammation: From Chemistry to Medicine. Pharmacol. Res. 99, 1–10. doi:10.1016/j.phrs.2015.05.002
Dhanya, V., Jollykutty, E., and Premlal, S. (2017). Lithotriptic Effect of Combination of Matsyakshi (Alternanthera Sessilis Linn. R.Br.,) and Tender Coconut Water in Albino Rats. Int. Res. J. Pharm. 8 (9), 56–64. doi:10.7897/2230-8407.089158
Dhar, P., Chan, P., Cohen, D. T., Khawam, F., Gibbons, S., Snyder-Leiby, T., et al. (2014). Synthesis, Antimicrobial Evaluation, and Structure–Activity Relationship of α-Pinene Derivatives. J. Agric. Food Chem. 62 (16), 3548–3552. doi:10.1021/jf403586t
Ding, K., Tan, Y. Y., Ding, Y., Fang, Y., Yang, X., Fang, J., et al. (2018). β‐Sitosterol Improves Experimental Colitis in Mice with a Target against Pathogenic Bacteria. J. Cell Biochem. 120 (4), 5687–5694. doi:10.1002/jcb.27853
Dinnimath, B. M., Jalalpure, S. S., and Patil, U. K. (2017). Antiurolithiatic Activity of Natural Constituents Isolated from Aerva Lanata. J. Ayurveda Integr. Med. 8 (4), 226–232. doi:10.1016/j.jaim.2016.11.006
Djohan, Y. F., Camara, C., Mondé, A. A., Koffi, G., Niamké, G., Déré, L., et al. (2009). Intérêt des antioxydants dans la prise en charge des patients infectés par le VIH: apport de la consommation régulière de tisane d’Alternanthera pungens. Ann. de biologie clinique 67 (5), 563–568. doi:10.1684/abc.2009.0362
do Nascimento, P., Lemos, T., Bizerra, A., Arriaga, Â., Ferreira, D., Santiago, G., et al. (2014). Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 19 (1), 1317–1327. doi:10.3390/molecules19011317
Drikvandi, P., Bahramikia, S., and Alirezaei, M. (2020). Modulation of the Antioxidant Defense System in Liver, Kidney, and Pancreas Tissues of Alloxan‐induced Diabetic Rats by Camphor. J. Food Biochem. 44 (12), 1. doi:10.1111/jfbc.13527
Duan, S., Du, X., Chen, S., Liang, J., Huang, S., Hou, S., et al. (2020). Effect of Vitexin on Alleviating Liver Inflammation in a Dextran Sulfate Sodium (DSS)-induced Colitis Model. Biomed. Pharmacother. 121. doi:10.1016/j.biopha.2019.109683
Duarte, A., Luís, Â., Oleastro, M., and Domingues, F. C. (2016). Antioxidant Properties of Coriander Essential Oil and Linalool and Their Potential to Control Campylobacter Spp. Food Control 61, 115–122. doi:10.1016/j.foodcont.2015.09.033
Duarte-Almeida, J. M., Negri, G., Salatino, A., de Carvalho, J. E., and Lajolo, F. M. (2007). Antiproliferative and Antioxidant Activities of a Tricin Acylated Glycoside from Sugarcane (Saccharum Officinarum) Juice. Phytochemistry 68 (8), 1165–1171. doi:10.1016/j.phytochem.2007.01.015
Ehrnhöfer-Ressler, M. M., Fricke, K., Pignitter, M., Walker, J. M., Walker, J., Rychlik, M., et al. (2013). Identification of 1,8-Cineole, Borneol, Camphor, and Thujone as Anti-inflammatory Compounds in a Salvia Officinalis L. Infusion Using Human Gingival Fibroblasts. J. Agric. Food Chem. 61 (14), 3451–3459. doi:10.1021/jf305472t
Emir, C., Emir, A., Bozkurt, B., and Somer, N. U. (2019). Phytochemical Constituents from Galanthus Alpinus Sosn. Var. Alpinus and Their Anticholinesterase Activities. South Afr. J. Bot. 121, 63–67. doi:10.1016/j.sajb.2018.10.021
Endo, Y., Usuki, R., and Kaneda, T. (1985). Antioxidant Effects of Chlorophyll and Pheophytin on the Autoxidation of Oils in the Dark. I. Comparison of the Inhibitory Effects. J. Am. Oil Chemists' Soc. 62 (9), 1375–1378. doi:10.1007/bf02545962
Enechi, O. C., Odo, C. E., and Wuave, C. P. (2013). Evaluation of the In Vitro Anti-oxidant Activity of Alternanthera Brasiliana Leaves. J. Pharm. Res. 6 (9), 919–924. doi:10.1016/j.jopr.2013.09.006
Facundo, V. A., Azevedo, M. S., Rodrigues, R. V., Nascimento, L. F. d., Militão, J. S. L. T., Silva, G. V. J. d., et al. (2012). Chemical Constituents from Three Medicinal Plants: Piper Renitens, Siparuna Guianensis and Alternanthera Brasiliana. Revista Brasileira de Farmacognosia 22 (5), 1134–1139. doi:10.1590/s0102-695x2012005000040
Fan, W.-Q. (2008). Chemical Constituents of Alternanthera philoxeroides</I>. Chin. J. Nat. Medicines 6 (2), 112–115. doi:10.3724/sp.J.1009.2008.00112
Fang, J.-B., Jia, W., Gao, W.-Y., Yao, Z., Teng, J., Zhao, A.-H., et al. (2007). Antitumor Constituents from Alternanthera Philoxeroides. J. Asian Nat. Prod. Res. 9 (6), 511–515. doi:10.1080/10286020600782231
Fang, J.-B., Yao, Z., Chen, J.-C., Liu, Y.-W., Takaishi, Y., and Duan, H.-Q. (2009a). Cytotoxic Triterpene Saponins fromAlternanthera Philoxeroides. J. Asian Nat. Prod. Res. 11 (3), 261–266. doi:10.1080/10286020802684656
Fang, J. B., Duan, H. Q., Zhang, Y. W., and Yoshihisa, T. (2006). Chemical Constituents from Herb of Alternanthera Philoxeroides. Zhongguo Zhong Yao Za Zhi 31 (13), 1072–1075.
Fang, J., Chen, J., Liu, Y., and Duan, H. (2009b). Constituents from Alternanthera Philoxeroides and Their Antitumor Activity. Zhongguo Zhong Yao Za Zhi 34 (19), 2473–2476.
Fang, X.-K., Gao, J., and Zhu, D.-N. (2008). Kaempferol and Quercetin Isolated from Euonymus Alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 82 (11-12), 615–622. doi:10.1016/j.lfs.2007.12.021
Fathima, S. N., Salwa, A., Anusha, S., and Fatima, S. (2016). Study of Antiasthmatic Activity of Ethanolic Extract of Alternanthera Sessilis Leaves. Int. J. Pharma Res. Health Sci. 4 (6), 1478–1482.
Fatima, N., Hafizur, R. M., Hameed, A., Ahmed, S., Nisar, M., and Kabir, N. (2015). Ellagic Acid in Emblica Officinalis Exerts Anti-diabetic Activity through the Action on β-cells of Pancreas. Eur. J. Nutr. 56 (2), 591–601. doi:10.1007/s00394-015-1103-y
Feka, P. D., Mohammed, S. Y., Shaibu, M. A., and Solomon, R. S. (2014). Phytochemical Screening and Antimicrobial Efficacy of <i>Alternanthera Nodiflora Extracts. Bayero J. Pure Appl. Sci. 6 (2), 1. doi:10.4314/bajopas.v6i2.20
Figer, B., Pissurlenkar, R., Ambre, P., Kalekar, S., Munshi, R., Gatne, M., et al. (2017). Treatment of Gastric Ulcers with Fenugreek Seed Extract; In Vitro, In Vivo and In Silico Approaches. Indian J. Pharm. Sci. 79 (5), 1. doi:10.4172/pharmaceutical-sciences.1000285
Filomeni, G., Graziani, I., De Zio, D., Dini, L., Centonze, D., Rotilio, G., et al. (2012). Neuroprotection of Kaempferol by Autophagy in Models of Rotenone-Mediated Acute Toxicity: Possible Implications for Parkinson's Disease. Neurobiol. Aging 33 (4), 767–785. doi:10.1016/j.neurobiolaging.2010.05.021
Firdhouse, M. J., and Lalitha, P. (2013). Biosynthesis of Silver Nanoparticles Using the Extract of Alternanthera Sessilis—Antiproliferative Effect against Prostate Cancer Cells. Cancer Nanotechnology 4 (6), 137–143. doi:10.1007/s12645-013-0045-4
Fortunato, L. R., Alves, C. d. F., Teixeira, M. M., and Rogerio, A. P. (2012). Quercetin: a Flavonoid with the Potential to Treat Asthma. Braz. J. Pharm. Sci. 48 (4), 589–599. doi:10.1590/s1984-82502012000400002
Franck, A. M., Massara, C. C., Innocent, K. K., Francois, M. G., Gogahy, K., Absalome, M. A., et al. (2016). Phytochemical Screening, Anti-inflammatory and Antioxidant Effects of Aqueous Extract of Alternanthera Pungens (Amaranthaceae) in Rats. AJBBL 5 (1), 1–10.
Gade, S., Rajamanikyam, M., Vadlapudi, V., Nukala, K. M., Aluvala, R., Giddigari, C., et al. (2017). Acetylcholinesterase Inhibitory Activity of Stigmasterol & Hexacosanol Is Responsible for Larvicidal and Repellent Properties of Chromolaena Odorata. Biochim. Biophys. Acta (Bba) - Gen. Subjects 1861 (3), 541–550. doi:10.1016/j.bbagen.2016.11.044
Gálvez, J., Estrada-Reyes, R., Benítez-King, G., Araujo, G., Orozco, S., Fernández-Mas, R., et al. (2015). Involvement of the GABAergic System in the Neuroprotective and Sedative Effects of Acacetin 7-O-Glucoside in Rodents. Restorative Neurol. Neurosci. 33 (5), 683–700. doi:10.3233/rnn-140486
Gamaro, G. D., Suyenaga, E., Borsoi, M., Lermen, J., Pereira, P., and Ardenghi, P. (2011). Effect of Rosmarinic and Caffeic Acids on Inflammatory and Nociception Process in Rats. ISRN Pharmacol. 2011, 1–6. doi:10.5402/2011/451682
Gao, Y., Chen, X., Fang, L., Liu, F., Cai, R., Peng, C., et al. (2014). Rhein Exerts Pro- and Anti-inflammatory Actions by Targeting IKKβ Inhibition in LPS-Activated Macrophages. Free Radic. Biol. Med. 72, 104–112. doi:10.1016/j.freeradbiomed.2014.04.001
Garín-Aguilar, M. E., Benavides-Catalán, D., Segura Cobos, D., Ramírez Sotelo, G., Piña Guzmán, A. B., and Valencia-del Toro, G. (2013). Spasmolytic Effect ofAlternanthera Repenson Isolated Rat Ileum. Pharm. Biol. 52 (4), 479–485. doi:10.3109/13880209.2013.844716
Gasparetto, A., Lapinski, T. F., Zamuner, S. R., Khouri, S., Alves, L. P., Munin, E., et al. (2010). Extracts from Alternanthera Maritima as Natural Photosensitizers in Photodynamic Antimicrobial Chemotherapy (PACT). J. Photochem. Photobiol. B: Biol. 99 (1), 15–20. doi:10.1016/j.jphotobiol.2010.01.009
Gatto, M. T., Falcocchio, S., Grippa, E., Mazzanti, G., Battinelli, L., Nicolosi, G., et al. (2002). Antimicrobial and Anti-lipase Activity of Quercetin and its C2-C16 3-O-Acyl-Esters. Bioorg. Med. Chem. 10 (2), 269–272. doi:10.1016/s0968-0896(01)00275-9
Gerber, L. E., and Erdman, J. W. (1982). Effect of Dietary Retinyl Acetate, β-Carotene and Retinoic Acid on Wound Healing in Rats. J. Nutr. 112 (8), 1555–1564. doi:10.1093/jn/112.8.1555
Ghaisas, M. M., Kshirsagar, S. B., and Sahane, R. S. (2014). Evaluation of Wound Healing Activity of Ferulic Acid in Diabetic Rats. Int. Wound J. 11 (5), 523–532. doi:10.1111/j.1742-481X.2012.01119.x
Girish, C., and Pradhan, S. C. (2012). Hepatoprotective Activities of Picroliv, Curcumin, and Ellagic Acid Compared to Silymarin on Carbon-Tetrachloride-Induced Liver Toxicity in Mice. J. Pharmacol. Pharmacother. 3 (2), 149–155. doi:10.4103/0976-500X.95515
Girish, C., Raj, V., Arya, J., and Balakrishnan, S. (2013). Involvement of the GABAergic System in the Anxiolytic-like Effect of the Flavonoid Ellagic Acid in Mice. Eur. J. Pharmacol. 710 (1-3), 49–58. doi:10.1016/j.ejphar.2013.04.003
Gomathi, K., Gopinath, D., Rafiuddin Ahmed, M., and Jayakumar, R. (2003). Quercetin Incorporated Collagen Matrices for Dermal Wound Healing Processes in Rat. Biomaterials 24 (16), 2767–2772. doi:10.1016/s0142-9612(03)00059-0
Gómez-Moreno, G., Aguilar-Salvatierra, A., Guardia, J., Uribe-Marioni, A., Cabrera-Ayala, M., Delgado-Ruiz, R. A., et al. (2013). The Efficacy of a Topical Sialogogue Spray Containing 1% Malic Acid in Patients with Antidepressant-Induced Dry Mouth: A Double-Blind, Randomized Clinical Trial. Depress. Anxiety 30 (2), 137–142. doi:10.1002/da.22017
Gong, J.-H., Shin, D., Han, S.-Y., Kim, J.-L., and Kang, Y.-H. (2012). Kaempferol Suppresses Eosionphil Infiltration and Airway Inflammation in Airway Epithelial Cells and in Mice with Allergic Asthma. J. Nutr. 142 (1), 47–56. doi:10.3945/jn.111.150748
González-Trujano, M., Ventura-Martínez, R., Chávez, M., Díaz-Reval, I., and Pellicer, F. (2012). Spasmolytic and Antinociceptive Activities of Ursolic Acid and Acacetin Identified in Agastache Mexicana. Planta Med. 78 (08), 793–796. doi:10.1055/s-0031-1298416
Gonçalves, M., Santos, V., Taylor, J., Perasoli, F., Santos, O., Rabelo, A., et al. (2019). Preparation and Characterization of a Quercetin-Tetraethyl Ether-Based Photoprotective Nanoemulsion. Química Nova. doi:10.21577/0100-4042.20170345
Goutman, J. D., and Calvo, D. J. (2004). Studies on the Mechanisms of Action of Picrotoxin, Quercetin and Pregnanolone at the GABAρ1 Receptor. Br. J. Pharmacol. 141 (4), 717–727. doi:10.1038/sj.bjp.0705657
Graf, E. (1992). Antioxidant Potential of Ferulic Acid. Free Radic. Biol. Med. 13 (4), 435–448. doi:10.1016/0891-5849(92)90184-i
Grønhaug, T. E., Glæserud, S., Skogsrud, M., Ballo, N., Bah, S., Diallo, D., et al. (2008). Ethnopharmacological Survey of Six Medicinal Plants from Mali, West-Africa. J. Ethnobiol. Ethnomedicine 4 (1), 1. doi:10.1186/1746-4269-4-26
Guede, N. Z., N’guessan, K., Dibié, T. E., and Grellier, P. (2010). Ethnopharmacological Study of Plants Used to Treat Malaria, in Traditional Medicine, by Bete Populations of Issia. J. Pharm. Sci. Res. 2 (4), 216–227.
Guerra, R. N. M., Pereira, H. A. W., Silveira, L. M. S., and Olea, R. S. G. (2003). Immunomodulatory Properties of Alternanthera Tenella Colla Aqueous Extracts in Mice. Braz. J. Med. Biol. Res. 36 (9), 1215–1219. doi:10.1590/s0100-879x2003000900011
Gulcin, I. (2006). Antioxidant Activity of Caffeic Acid (3,4-dihydroxycinnamic Acid). Toxicology 217 (2-3), 213–220. doi:10.1016/j.tox.2005.09.011
Guo, C., Bi, J., Li, X., Lyu, J., Liu, X., Liu, J., et al. (2021). Effects of Isomerisation and Oxidation on the Immunomodulatory Activity of Chlorogenic Acid in RAW264.7 Macrophages. Int. J. Food Sci. Technol.. doi:10.1111/ijfs.15442
Guo, Q. L., Li, B., Li, J., Li, J. J., Xia, L. Y., and Dong, J. X. (2011). Triterpenoid Saponins of Alternanthera Philoxeroides (Mart.) Griseb. Yao Xue Xue Bao 46 (4), 428–431.
Gupta, H. C., Raj, J., Rathi, A., Sundaram, E. N., and Manchanda, R. K. (2012). Morpho-anatomy of Leaf, Stem and Root of Alternanthera Sessilis (L.) R. Br. Ex DC and Alternanthera Pungens Kunth (Amaranthaceae) and its Significance in Drug Identification. Indian J. Res. Homoeopathy 6, 1–7.
Gupta, R. K., and Saxena, V. K. (1987). Volatile Constituents from the Flowers of Althernanthera Pungens HBK (Amaranthaceae). Indian Perfumer 31, 366–369.
Gupta, R., Sharma, A. K., Dobhal, M. P., Sharma, M. C., and Gupta, R. S. (2011). Antidiabetic and Antioxidant Potential of β-sitosterol in Streptozotocin-Induced Experimental Hyperglycemia. J. Diabetes 3 (1), 29–37. doi:10.1111/j.1753-0407.2010.00107.x
Gupta, R., and Singh, H. K. (2012a). Detection and Quantitation of SS-Sitosterol in Clerodendrum Infortunatum and Alternanthera Sessilis by HPTLC. Pharmacognosy Commun. 2 (1), 31–36. doi:10.5530/pc.2012.1.6
Gupta, R., and Singh, H. K. (2012b). Nootropic Potential of Alternanthera Sessilis and Clerodendrum Infortunatum Leaves on Mice. Asian Pac. J. Trop. Dis. 2, S465–S470. doi:10.1016/s2222-1808(12)60204-7
Gupta, R., and Singh, K. H. (2014). Antidepressant like Effects of Alternanthera Sessilis and Clerodendrum Infortunatum Leaves Extract in Immobility Models. Nat. Prod. J. 4 (1), 33–37. doi:10.2174/2210315504666140515004826
Habtemariam, S. (2019). Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxidative Med. Cell Longevity 2019, 1–18. doi:10.1155/2019/8512048
Hachlafi, N. E. L., Aanniz, T., Menyiy, N. E., Baaboua, A. E., Omari, N. E., Balahbib, A., et al. (2021). In Vitro and In Vivo Biological Investigations of Camphene and its Mechanism Insights: A Review. Food Rev. Int. 28, 1–28. doi:10.1080/87559129.2021.1936007
Halder, S., Kar, R., Galav, V., Mehta, A. K., Bhattacharya, S. K., Mediratta, P. K., et al. (2015). Cadmium Exposure during Lactation Causes Learning and Memory-Impairment in F1 Generation Mice: Amelioration by Quercetin. Drug Chem. Toxicol. 39 (3), 272–278. doi:10.3109/01480545.2015.1092042
Han, J. M., Kwon, H. J., and Jung, H. J. (2016). Tricin, 4′,5,7-Trihydroxy-3′,5′-Dimethoxyflavone, Exhibits Potent Antiangiogenic Activity In Vitro. Int. J. Oncol. 49 (4), 1497–1504. doi:10.3892/ijo.2016.3645
Haque, E., Javed, H., Azimullah, S., Abul Khair, S. B., and Ojha, S. (2015). Neuroprotective Potential of Ferulic Acid in the Rotenone Model of Parkinson’s Disease. Drug Des. Dev. Ther. 9, 5499–5510. doi:10.2147/dddt.S90616
Haque, M. N., and Moon, I. S. (2018). Stigmasterol Upregulates Immediate Early Genes and Promotes Neuronal Cytoarchitecture in Primary Hippocampal Neurons as Revealed by Transcriptome Analysis. Phytomedicine 46, 164–175. doi:10.1016/j.phymed.2018.04.012
Hara, K., Haranishi, Y., Kataoka, K., Takahashi, Y., Terada, T., Nakamura, M., et al. (2014). Chlorogenic Acid Administered Intrathecally Alleviates Mechanical and Cold Hyperalgesia in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 723, 459–464. doi:10.1016/j.ejphar.2013.10.046
Haroon, H. B., Perumalsamy, V., Nair, G., Anand, D. K., Kolli, R., Monichen, J., et al. (2020). Repression of Polyol Pathway Activity by Hemidesmus Indicus Var. Pubescens R.Br. Linn Root Extract, an Aldose Reductase Inhibitor: An In Silico and Ex Vivo Study. Nat. Prod. Bioprospecting 11 (3), 315–324. doi:10.1007/s13659-020-00290-w
Hassanzadeh, P., Arbabi, E., Atyabi, F., and Dinarvand, R. (2017). Ferulic Acid Exhibits Antiepileptogenic Effect and Prevents Oxidative Stress and Cognitive Impairment in the Kindling Model of Epilepsy. Life Sci. 179, 9–14. doi:10.1016/j.lfs.2016.08.011
Hayashi, M., Naknukool, S., Hayakawa, S., Ogawa, M., and Ni’matulah, A.-B. A. (2012). Enhancement of Antimicrobial Activity of a Lactoperoxidase System by Carrot Extract and β-carotene. Food Chem. 130 (3), 541–546. doi:10.1016/j.foodchem.2011.07.067
He, F., Chou, C. J., Scheiner, M., Poeta, E., Yuan Chen, N., Gunesch, S., et al. (2021a). Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects In Vitro and Neuroprotective Effects in a Pharmacological Alzheimer’s Disease Mouse Model. J. Med. Chem. 64 (7), 3794–3812. doi:10.1021/acs.jmedchem.0c01940
He, Y., Chen, S., Tsoi, B., Qi, S., Gu, B., Wang, Z., et al. (2021b). Alpinia Oxyphylla Miq. And its Active Compound P-Coumaric Acid Promote Brain-Derived Neurotrophic Factor Signaling for Inducing Hippocampal Neurogenesis and Improving Post-cerebral Ischemic Spatial Cognitive Functions. Front. Cel Dev. Biol. 8. doi:10.3389/fcell.2020.577790
Heenan, P. B., de Lange, P. J., and Keeling, J. (2009). Alternanthera Nahui, a New Species of Amaranthaceae Indigenous to New Zealand. New Zealand J. Bot. 47 (1), 97–105. doi:10.1080/00288250909509795
Horiuchi, K., Shiota, S., Hatano, T., Yoshida, T., Kuroda, T., and Tsuchiya, T. (2007). Antimicrobial Activity of Oleanolic Acid from Salvia Officinalis and Related Compounds on Vancomycin-Resistant Enterococci (VRE). Biol. Pharm. Bull. 30 (6), 1147–1149. doi:10.1248/bpb.30.1147
Hosamani, K. M., Ganjihal, S. S., and Chavadi, D. V. (2004). Alternanthera Triandra Seed Oil: A Moderate Source of Ricinoleic Acid and its Possible Industrial Utilisation. Ind. Crops Prod. 19 (2), 133–136. doi:10.1016/j.indcrop.2003.07.009
Hossain, A. I., Faisal, M., Rahman, S., Jahan, R., and Rahmatullah, M. (2014). A Preliminary Evaluation of Antihyperglycemic and Analgesic Activity of Alternanthera Sessilis Aerial Parts. BMC Complement. Altern. Med. 14, 169. doi:10.1186/1472-6882-14-169
Hu, M., Li, F., and Wang, W. (2018). Vitexin Protects Dopaminergic Neurons in MPTP-Induced Parkinson’s Disease through PI3K/Akt Signaling Pathway. Drug Des. Dev. Ther. 12, 565–573. doi:10.2147/dddt.S156920
Hu, R., Wu, S., Li, B., Tan, J., Yan, J., Wang, Y., et al. (2021). Dietary Ferulic Acid and Vanillic Acid on Inflammation, Gut Barrier Function and Growth Performance in Lipopolysaccharide-Challenged Piglets. Anim. Nutr.. doi:10.1016/j.aninu.2021.06.009
Hulme, A. C. (1953). The Isolation of Chlorogenic Acid from the Apple Fruit. Biochem. J. 53 (3), 337–340. doi:10.1042/bj0530337
Hundiwale, J. C., Patil, A. V., Kulkarni, M. V., Patil, D. A., and Mali, R. G. (2012). A Current Update on Phytopharmacology of the Genus Alternanthera. J. Pharm. Res. 5 (4), 1924–1929.
Hung, Y. C., Kuo, Y. H., Hsieh, P. W., Hsieh, T. Y., Kuo, J. R., and Wang, S. J. (2021). Chlorogenic Acid Decreases Glutamate Release from Rat Cortical Nerve Terminals by P/Q-Type Ca(2+) Channel Suppression: A Possible Neuroprotective Mechanism. Int. J. Mol. Sci. 22 (21), 1. doi:10.3390/ijms222111447
Hwang, S. J., Kim, Y.-W., Park, Y., Lee, H.-J., and Kim, K.-W. (2013). Anti-inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 63 (1), 81–90. doi:10.1007/s00011-013-0674-4
Ibitoye, O. B., Uwazie, J. N., and Ajiboye, T. O. (2018). Bioactivity-guided Isolation of Kaempferol as the Antidiabetic Principle from Cucumis Sativus L. Fruits. J. Food Biochem. 42 (4), 1. doi:10.1111/jfbc.12479
Imran, M., Aslam Gondal, T., Atif, M., Shahbaz, M., Batool Qaisarani, T., Hanif Mughal, M., et al. (2020). Apigenin as an Anticancer Agent. Phytotherapy Res. 34 (8), 1812–1828. doi:10.1002/ptr.6647
Imran, M., Salehi, B., Sharifi-Rad, J., Aslam Gondal, T., Saeed, F., Imran, A., et al. (2019). Kaempferol: A Key Emphasis to its Anticancer Potential. Molecules 24 (12), 1. doi:10.3390/molecules24122277
Ittiyavirah, S. P., and Hameed, J. (2015). Protective Role of Alternanthera Sessilis (Linn.) Silver Nanoparticles and its Ethanolic Extract against Rotenone Induced Parkinsonism. IOSR J. Pharm. Biol. Sci. 10 (5), 25–32.
Jain, A., Roy, S., Joshi*, A., and Joshi, N. (2016). Evaluation of In-Vitro Cytotoxic and Antioxidant Activity of Methanolic Extracts of Ipomoea Carnea and Alternanthera Sessilis. Int. J. Bioassays 5 (08), 1. doi:10.21746/ijbio.2016.08.008
Jakhar, S., and Dahiya, P. (2017). Antimicrobial, Antioxidant and Phytochemical Potential of Alternanthera Pungens HB&K. J. Pharm. Sci. Res. 9 (8), 1305–1311.
Jalalpure, S. S., Agrawal, N., Patil, M. B., Chimkode, R., and Tripathi, A. (2008). Antimicrobial and Wound Healing Activities of Leaves of Alternanthera Sessilis Linn. Int. J. Green Pharm. 2 (3), 141–144. doi:10.4103/0973-8258.42729
Jang, Y.-H., Park, J.-R., and Kim, K.-M. (2020). Antimicrobial Activity of Chrysoeriol 7 and Chochlioquinone 9, White-Backed Planthopper-Resistant Compounds, against Rice Pathogenic Strains. Biology 9 (11), 1. doi:10.3390/biology9110382
Jeong, G.-S., Li, B., Lee, D.-S., Kim, K. H., Lee, I. K., Lee, K. R., et al. (2010). Cytoprotective and Anti-inflammatory Effects of Spinasterol via the Induction of Heme Oxygenase-1 in Murine Hippocampal and Microglial Cell Lines. Int. Immunopharmacology 10 (12), 1587–1594. doi:10.1016/j.intimp.2010.09.013
Jin, X., Yang, R., Yan, X., Zhou, Y., Wang, X., and Gu, Z. (2016). Malic Acid and Oxalic Acid Spraying Enhances Phytic Acid Degradation and Total Antioxidant Capacity of Mung Bean Sprouts. Int. J. Food Sci. Tech. 51 (2), 370–380. doi:10.1111/ijfs.12941
Johann, S., Cisalpino, P. S., Watanabe, G. A., Cota, B. B., de Siqueira, E. P., Pizzolatti, M. G., et al. (2010). Antifungal Activity of Extracts of Some Plants Used in Brazilian Traditional Medicine against the Pathogenic fungusParacoccidioides Brasiliensis. Pharm. Biol. 48 (4), 388–396. doi:10.3109/13880200903150385
Jothi Ramalingam, R., Vaali-Mohammed, M.-A., Al-Lohedan, H. A., and Appaturi, J. N. (2017). Synthesis and Bio-Physical Characterization of Silver Nanoparticle and Ag-Mesoporous MnO2 Nanocomposite for Anti-microbial and Anti-cancer Activity. J. Mol. Liquids 243, 348–357. doi:10.1016/j.molliq.2017.08.037
Joung, D.-K., Joung, H. E. E., Yang, D.-W., Kwon, D.-Y., Choi, J.-G., Woo, S. E. O., et al. (2012). Synergistic Effect of Rhein in Combination with Ampicillin or Oxacillin against Methicillin-Resistant Staphylococcus aureus. Exp. Ther. Med. 3 (4), 608–612. doi:10.3892/etm.2012.459
Jyonouchi, H., Hill, R. J., Tomita, Y., and Good, R. A. (2009). Studies of Immunomodulating Actions of Carotenoids. I. Effects Ofβ‐carotene and Astaxanthin on Murine Lymphocyte Functions and Cell Surface Marker Expression Inin Vitroculture System. Nutr. Cancer 16 (2), 93–105. doi:10.1080/01635589109514148
Kabir, F., Katayama, S., Tanji, N., and Nakamura, S. (2014). Antimicrobial Effects of Chlorogenic Acid and Related Compounds. J. Korean Soc. Appl. Biol. Chem. 57 (3), 359–365. doi:10.1007/s13765-014-4056-6
Kang, R., Tian, W., Cao, W., Sun, Y., Zhang, H.-N., Feng, Y.-D., et al. (2021). Ligustroflavone Ameliorates CCl4-Induced Liver Fibrosis through Down-Regulating the TGF-β/Smad Signaling Pathway. Chin. J. Nat. Medicines 19 (3), 170–180. doi:10.1016/s1875-5364(21)60018-3
Kapil, A., Koul, I. B., and Suri, O. P. (1995). Antihepatotoxic Effects of Chlorogenic Acid fromAnthocephalus Cadamba. Phytotherapy Res. 9 (3), 189–193. doi:10.1002/ptr.2650090307
Karim, N., Khan, I., Abdelhalim, A., Halim, S. A., Khan, A., and Al-Harrasi, A. (2021). Stigmasterol Can Be New Steroidal Drug for Neurological Disorders: Evidence of the GABAergic Mechanism via Receptor Modulation. Phytomedicine 90. doi:10.1016/j.phymed.2021.153646
Kassuya, R. M., dos Santos, E., Bosso, F. H., Pedroso, T. F., Marinho, J. V. N., Salvador, M. J., et al. (2021). Anti-inflammatory Properties of Ethanolic Extract and 2″-O-β-D-Glucopyranosyl-Vitexin Obtained from Alternanthera Tenella Colla Whole Plant. Inflammation 44 (4), 1540–1552. doi:10.1007/s10753-021-01438-7
Kasthuri, O. R., and Ramesh, B. (2018). Toxicity Studies on Leaf Extracts of Alternanthera Brasiliana (L.) Kuntze and Alternanthera Bettzickiana (Regel) Voss. J. Appl. Pharm. Sci. 8 (10), 82–89. doi:10.7324/japs.2018.81011
Kaur, S., Sharma, A., and Bedi, P. M. S. (2017). Bioactivity Guided Isolation, Characterization and Quantification of an Anxiolytic Constituent - Kaempferol, from Melilotus Officinalis Aerial Parts. J. Biologically Active Prod. Nat. 7 (5), 379–390. doi:10.1080/22311866.2017.1378923
Khalili, M., Attar, M., Amirlatifi, R., Maleki, Z. N., and Hoseini, S. M. (2020). Effects of Dietary Myrcene Administration on Antioxidant Gene Responses in Common Carp (Cyprinus carpio), Exposed to Copper Sulphate. Aquac. Res. 51 (4), 1653–1659. doi:10.1111/are.14511
Khamphukdee, C., Chulikhit, Y., Daodee, S., and Monthakantirat, O. (2017). Potential of Alternanthera Philoxeroides on Improvement of Anxiety-like Behavior Induced by Ovariectomized Mice Model. Indian J. Pharm. Edu. Res. 51 (3s2), s493–s497. doi:10.5530/ijper.51.3s.73
Khamphukdee, C., Monthakantirat, O., Chulikhit, Y., Boonyarat, C., Daodee, S., Aon-im, P., et al. (2021). Antidementia Effects of Alternanthera Philoxeroides in Ovariectomized Mice Supported by NMR-Based Metabolomic Analysis. Molecules 26 (9), 1. doi:10.3390/molecules26092789
Khamphukdee, C., Monthakantirat, O., Chulikhit, Y., Buttachon, S., Lee, M., Silva, A., et al. (2018). Chemical Constituents and Antidepressant-like Effects in Ovariectomized Mice of the Ethanol Extract of Alternanthera Philoxeroides. Molecules 23 (9), 1. doi:10.3390/molecules23092202
Khan, K. A., Kumar, N., Nayak, P. G., Nampoothiri, M., Shenoy, R. R., Krishnadas, N., et al. (2013). Impact of Caffeic Acid on Aluminium Chloride-Induced Dementia in Rats. J. Pharm. Pharmacol. 65 (12), 1745–1752. doi:10.1111/jphp.12126
Khan, M. S., Yusufzai, S. K., Ying, L. Y., and Zulnashriq, W. (2018). Gc-Ms Based Chemical Profiling and Evaluation of Antioxidant Potential of Leaves and Stems of Alternanthera Sessilis Red from Sabah, Malaysia. Int. J. Pharm. Pharm. Sci. 10 (7), 1. doi:10.22159/ijpps.2018v10i7.25204
Khan, M., Yusufzai, S., Kaun, L., Shah, M., and Idris, R. (2016). Chemical Composition and Antioxidant Activity of Essential Oil of Leaves and Flowers of Alternanthera Sessilis Red from Sabah. J. Appl. Pharm. Sci. 6, 157–161. doi:10.7324/japs.2016.601222
Khatun, F., Zaman, F., Mosaiab, T., Mostafa, F., Zaman, M., Rehana, F., et al. (2012). Evaluation of Antinociceptive and Antihyperglycemic Activities in Methanol Extracts of Whole Plants of Alternanthera Philoxeroides (Mart.) Griseb. (Amaranthaceae) in Mice. Pak J. Pharm. Sci. 25 (3), 583–587.
Kiasalari, Z., Heydarifard, R., Khalili, M., Afshin-Majd, S., Baluchnejadmojarad, T., Zahedi, E., et al. (2017). Ellagic Acid Ameliorates Learning and Memory Deficits in a Rat Model of Alzheimer’s Disease: an Exploration of Underlying Mechanisms. Psychopharmacology 234 (12), 1841–1852. doi:10.1007/s00213-017-4589-6
Kiliç, I., and Yeşiloğlu, Y. (2013). Spectroscopic Studies on the Antioxidant Activity of P-Coumaric Acid. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 115, 719–724. doi:10.1016/j.saa.2013.06.110
Kim, C.-S., Kim, J., Lee, Y. M., Sohn, E., Jo, K., and Kim, J. S. (2011). Inhibitory Effects of Chlorogenic Acid on Aldose Reductase Activity In Vitro and Cataractogenesis in Galactose-Fed Rats. Arch. Pharmacal Res. 34 (5), 847–852. doi:10.1007/s12272-011-0519-z
Kim, D.-S., Lee, H.-J., Jeon, Y.-D., Han, Y.-H., Kee, J.-Y., Kim, H.-J., et al. (2015). Alpha-Pinene Exhibits Anti-inflammatory Activity through the Suppression of MAPKs and the NF-Κb Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 43 (04), 731–742. doi:10.1142/s0192415x15500457
Kim, H.-B., Lee, S., Hwang, E.-S., Maeng, S., and Park, J.-H. (2017a). p-Coumaric Acid Enhances Long-Term Potentiation and Recovers Scopolamine-Induced Learning and Memory Impairments. Biochem. Biophysical Res. Commun. 492 (3), 493–499. doi:10.1016/j.bbrc.2017.08.068
Kim, H.-J., Lee, B.-H., Choi, S.-H., Jung, S.-W., Kim, H.-S., Lee, J.-H., et al. (2014). Differential Effects of Quercetin Glycosides on GABAC Receptor Channel Activity. Arch. Pharmacal Res. 38 (1), 108–114. doi:10.1007/s12272-014-0409-2
Kim, H.-R., Lee, D.-M., Lee, S.-H., Seong, A.-R., Gin, D.-W., Hwang, J.-A., et al. (2010). Chlorogenic Acid Suppresses Pulmonary Eosinophilia, IgE Production, and Th2-type Cytokine Production in an Ovalbumin-Induced Allergic Asthma: Activation of STAT-6 and JNK Is Inhibited by Chlorogenic Acid. Int. Immunopharmacology 10 (10), 1242–1248. doi:10.1016/j.intimp.2010.07.005
Kim, H. J., Fan, X., Gabbi, C., Yakimchuk, K., Parini, P., Warner, M., et al. (2008). Liver X Receptor (LXR ): A Link between -sitosterol and Amyotrophic Lateral Sclerosis-Parkinson's Dementia. Proc. Natl. Acad. Sci. 105 (6), 2094–2099. doi:10.1073/pnas.0711599105
Kim, J. H., Campbell, B. C., Mahoney, N., Chan, K. L., Molyneux, R. J., and May, G. S. (2007). Enhancement of Fludioxonil Fungicidal Activity by Disrupting Cellular Glutathione Homeostasis with 2,5-dihydroxybenzoic Acid. FEMS Microbiol. Lett. 270 (2), 284–290. doi:10.1111/j.1574-6968.2007.00682.x
Kim, S. H., Naveen Kumar, C., Kim, H. J., Kim, D. H., Cho, J., Jin, C., et al. (2009). Glucose-containing Flavones—Their Synthesis and Antioxidant and Neuroprotective Activities. Bioorg. Med. Chem. Lett. 19 (21), 6009–6013. doi:10.1016/j.bmcl.2009.09.062
Kim, S. M., Park, Y. J., Shin, M.-S., Kim, H.-R., Kim, M. J., Lee, S. H., et al. (2017b). Acacetin Inhibits Neuronal Cell Death Induced by 6-hydroxydopamine in Cellular Parkinson’s Disease Model. Bioorg. Med. Chem. Lett. 27 (23), 5207–5212. doi:10.1016/j.bmcl.2017.10.048
Kong, C.-S., Jeong, C.-H., Choi, J.-S., Kim, K.-J., and Jeong, J.-W. (2013). Antiangiogenic Effects of P-Coumaric Acid in Human Endothelial Cells. Phytotherapy Res. 27 (3), 317–323. doi:10.1002/ptr.4718
Koo, H. (2003). Inhibition of Streptococcus Mutans Biofilm Accumulation and Polysaccharide Production by Apigenin and Tt-Farnesol. J. Antimicrob. Chemother. 52 (5), 782–789. doi:10.1093/jac/dkg449
Koolen, H. H. F., Pral, E. M. F., Alfieri, S. C., Marinho, J. V. N., Serain, A. F., Hernández-Tasco, A. J., et al. (2017). Antiprotozoal and Antioxidant Alkaloids from Alternanthera Littoralis. Phytochemistry 134, 106–113. doi:10.1016/j.phytochem.2016.11.008
Kota, S., Govada, V. R., Anantha, R. K., and Verma, M. K. (2017). An Investigation into Phytochemical Constituents, Antioxidant, Antibacterial and Anti-cataract Activity of Alternanthera Sessilis, a Predominant Wild Leafy Vegetable of South India. Biocatal. Agric. Biotechnol. 10, 197–203. doi:10.1016/j.bcab.2017.03.008
Krenn, L., Beyer, G., Pertz, H., Karall, E., Kremser, M., Galambosi, B., et al. (2011). In Vitro Antispasmodic and Antiinflammatory Effects of Drosera Rotundifolia. Arzneimittelforschung 54 (07), 402–405. doi:10.1055/s-0031-1296991
Kumar, D. A., Palanichamy, V., and Roopan, S. M. (2014). Green Synthesis of Silver Nanoparticles Using Alternanthera Dentata Leaf Extract at Room Temperature and Their Antimicrobial Activity. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 127, 168–171. doi:10.1016/j.saa.2014.02.058
Kumar, S. M., Rani, S. G., Astalakshmi, N., Manasa, G., Vanaja, P., Sirisha, G., et al. (2011b). Screening of Aqueous and Ethanolic Extracts of Aerial Parts of Alternanthera Sessilis Linn. R.Br.ex.DC (Amaranthaceae) for Antidiabetic Activity. J. Pharm. Sci. Res. 4 (5), 1528–1530.
Kumar, S., Singh, P., Mishra, G., Srivastav, S., Jha, K. K., and Khosa, R. L. (2011a). Phytopharmacological Review of Alternanthera Brasiliana (Amaranthaceae). Asian J. Plant Sci. Res. 1 (1), 41–47.
Kumari, E. V. N., and Krishnan, V. (2016). Antimicrobial Activity of Alternanthera Sessilis (L) R. BR. Ex. DC and Alternanthera Philoxeroides (Mart). Griseb. World J. Res. Rev. 3 (3), 78–81.
Lan, Q., Di, D., Wang, S., Zhao, Q., Gao, Y., Chang, D., et al. (2020). Chitosan-N-acetylcysteine Modified HP-β-CD Inclusion Complex as a Potential Ocular Delivery System for Anti-cataract Drug: Quercetin. J. Drug Deliv. Sci. Tech. 55. doi:10.1016/j.jddst.2019.101407
Lee, J. H., Mohan, C. D., Shanmugam, M. K., Rangappa, S., Sethi, G., Siveen, K. S., et al. (2020). Vitexin Abrogates Invasion and Survival of Hepatocellular Carcinoma Cells through Targeting STAT3 Signaling Pathway. Biochimie 175, 58–68. doi:10.1016/j.biochi.2020.05.006
Lee, S., Kim, H.-B., Hwang, E.-S., Kim, E.-s., Kim, S.-S., Jeon, T.-D., et al. (2018). Antidepressant-like Effects of P-Coumaric Acid on LPS-Induced Depressive and Inflammatory Changes in Rats. Exp. Neurobiol. 27 (3), 189–199. doi:10.5607/en.2018.27.3.189
Leeming, J. P., Holland, K. T., and Bojar, R. A. (1986). The In Vitro Antimicrobial Effect of Azelaic Acid. Br. J. Dermatol. 115 (5), 551–556. doi:10.1111/j.1365-2133.1986.tb05764.x
Lesjak, M., Beara, I., Simin, N., Pintać, D., Majkić, T., Bekvalac, K., et al. (2018). Antioxidant and Anti-inflammatory Activities of Quercetin and its Derivatives. J. Funct. Foods 40, 68–75. doi:10.1016/j.jff.2017.10.047
Li, B., Guo, Q.-L., Tian, Y., Liu, S.-J., Wang, Q., Chen, L., et al. (2016). New Anti-HBV C-Boivinopyranosyl Flavones from Alternanthera Philoxeroides. Molecules 21 (3), 1. doi:10.3390/molecules21030336
Li, G., Wang, X., Xu, Y., Zhang, B., and Xia, X. (2013). Antimicrobial Effect and Mode of Action of Chlorogenic Acid on Staphylococcus aureus. Eur. Food Res. Tech. 238 (4), 589–596. doi:10.1007/s00217-013-2140-5
Li, P., Peng, Y., Ma, Q., Li, Z., and Zhang, X. (2020). p>Study on the Formation of Antihypertensive Twin Drugs by Caffeic Acid and Ferulic Acid with Telmisartan</p>. Drug Des. Dev. Ther. Vol. 14, 977–992. doi:10.2147/dddt.S225705<
Li, S.-P., Wang, Y.-W., Qi, S.-L., Zhang, Y.-P., Deng, G., Ding, W.-Z., et al. (2018). Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 9. doi:10.3389/fphar.2018.00346
Li, X., Ouyang, X., Cai, R., and Chen, D. (2019). 3′,8″-Dimerization Enhances the Antioxidant Capacity of Flavonoids: Evidence from Acacetin and Isoginkgetin. Molecules 24 (11), 1. doi:10.3390/molecules24112039
Liang, Q., Yang, J., He, J., Chen, X., Zhang, H., Jia, M., et al. (2020). Stigmasterol Alleviates Cerebral Ischemia/reperfusion Injury by Attenuating Inflammation and Improving Antioxidant Defenses in Rats. Biosci. Rep. 40 (4), 1. doi:10.1042/bsr20192133
Lima, V. N., Oliveira-Tintino, C. D. M., Santos, E. S., Morais, L. P., Tintino, S. R., Freitas, T. S., et al. (2016). Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathogenesis 99, 56–61. doi:10.1016/j.micpath.2016.08.004
Lin, F.-H., Lin, J.-Y., Gupta, R. D., Tournas, J. A., Burch, J. A., Angelica Selim, M., et al. (2005). Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin. J. Invest. Dermatol. 125 (4), 826–832. doi:10.1111/j.0022-202X.2005.23768.x
Lin, K., Sze, S. C.-W., Liu, B., Zhang, Z., Zhang, Z., Zhu, P., et al. (2021). 20(S)-protopanaxadiol and Oleanolic Acid Ameliorate Cognitive Deficits in APP/PS1 Transgenic Mice by Enhancing Hippocampal Neurogenesis. J. Ginseng Res. 45 (2), 325–333. doi:10.1016/j.jgr.2020.07.003
Lin, M.-K., Yu, Y.-L., Chen, K.-C., Chang, W.-T., Lee, M.-S., Yang, M.-J., et al. (2011). Kaempferol from Semen Cuscutae Attenuates the Immune Function of Dendritic Cells. Immunobiology 216 (10), 1103–1109. doi:10.1016/j.imbio.2011.05.002
Lin, S.-C., Lin, Y.-H., Shyuu, S.-J., and Lin, C.-C. (1994). Hepatoprotective Effects of Taiwan Folk Medicine:Alternanthera Sessilis on Liver Damage Induced by Various Hepatotoxins. Phytotherapy Res. 8 (7), 391–398. doi:10.1002/ptr.2650080703
Lira, M. H. P. d., Andrade Júnior, F. P. d., Moraes, G. F. Q., Macena, G. d. S., Pereira, F. d. O., and Lima, I. O. (2020). Antimicrobial Activity of Geraniol: an Integrative Review. J. Essent. Oil Res. 32 (3), 187–197. doi:10.1080/10412905.2020.1745697
Liu, J.-R., Dong, H.-W., Sun, X.-R., Wang, Q., Sun, W.-G., Parry, J. W., et al. (2009). Effects of β-Ionone on Mammary Carcinogenesis and Antioxidant Status in Rats Treated with DMBA. Nutr. Cancer 62 (1), 58–65. doi:10.1080/01635580903191510
Liu, L., Liu, Y., Zhao, J., Xing, X., Zhang, C., and Meng, H. (2020). Neuroprotective Effects of D-(-)-Quinic Acid on Aluminum Chloride-Induced Dementia in Rats. Evidence-Based Complement. Altern. Med. 2020, 1–10. doi:10.1155/2020/5602597
Liu, X., Jiang, Q., Liu, H., and Luo, S. (2019). Vitexin Induces Apoptosis through Mitochondrial Pathway and PI3K/Akt/mTOR Signaling in Human Non-small Cell Lung Cancer A549 Cells. Biol. Res. 52 (1), 1. doi:10.1186/s40659-019-0214-y
Lobine, D., Ahmed, S., Aschner, M., Khan, H., Mirzaei, H., and Mahomoodally, M. F. (2020). Antiurolithiatic Effects of Pentacyclic Triterpenes: The Distance Traveled from Therapeutic Aspects. Drug Dev. Res. 81 (6), 671–684. doi:10.1002/ddr.21670
Loizou, S., Lekakis, I., Chrousos, G. P., and Moutsatsou, P. (2010). β-Sitosterol Exhibits Anti-inflammatory Activity in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 54 (4), 551–558. doi:10.1002/mnfr.200900012
Lozoya, X., Meckes, M., Abou-Zaid, M., Tortoriello, J., Nozzolillo, C., and Arnason, J. T. (1994). Quercetin Glycosides in Psidium Guajava L. Leaves and Determination of a Spasmolytic Principle. Arch. Med. Res. 25 (1), 11–15.
Lucky, G., and Diame, A. (2010). Ethnobotany and Ecological Studies of Plants Used for Reproductive Health: A Case Study at Bia Biosphere Reserve in the Western Region of Ghana [Online]. France: The Division of Ecological Sciences: UNESCO. Available: http://www.unesco.org/science/doc/mab/MAB_Ghana2008.pdf (Accessed 04 01, 2021).
Lv, C., Hong, T., Yang, Z., Zhang, Y., Wang, L., Dong, M., et al. (2012). Effect of Quercetin in the 1-Methyl-4-Phenyl-1, 2, 3, 6-Tetrahydropyridine-Induced Mouse Model of Parkinson's Disease. Evidence-Based Complement. Altern. Med. 2012, 1–6. doi:10.1155/2012/928643
Ma, B., You, X., and Lu, F. (2014). Inhibitory Effects of β-ionone on Amyloid Fibril Formation of β-lactoglobulin. Int. J. Biol. Macromolecules 64, 162–167. doi:10.1016/j.ijbiomac.2013.12.003
Machado, D. G., Neis, V. B., Balen, G. O., Colla, A., Cunha, M. P., Dalmarco, J. B., et al. (2012). Antidepressant-like Effect of Ursolic Acid Isolated from Rosmarinus Officinalis L. In Mice: Evidence for the Involvement of the Dopaminergic System. Pharmacol. Biochem. Behav. 103 (2), 204–211. doi:10.1016/j.pbb.2012.08.016
Madunić, J., Madunić, I. V., Gajski, G., Popić, J., and Garaj-Vrhovac, V. (2018). Apigenin: A Dietary Flavonoid with Diverse Anticancer Properties. Cancer Lett. 413, 11–22. doi:10.1016/j.canlet.2017.10.041
Mahajan, S. G., and Mehta, A. A. (2011). Suppression of Ovalbumin-Induced Th2-Driven Airway Inflammation by β-sitosterol in a guinea Pig Model of Asthma. Eur. J. Pharmacol. 650 (1), 458–464. doi:10.1016/j.ejphar.2010.09.075
Malar, D. S., Prasanth, M. I., Jeyakumar, M., Balamurugan, K., and Devi, K. P. (2020). Vitexin Prevents Aβ Proteotoxicity in Transgenic Caenorhabditis elegans Model of Alzheimer's Disease by Modulating Unfolded Protein Response. J. Biochem. Mol. Toxicol. 35 (1), 1. doi:10.1002/jbt.22632
Mamani-Matsuda, M., Kauss, T., Al-Kharrat, A., Rambert, J., Fawaz, F., Thiolat, D., et al. (2006). Therapeutic and Preventive Properties of Quercetin in Experimental Arthritis Correlate with Decreased Macrophage Inflammatory Mediators. Biochem. Pharmacol. 72 (10), 1304–1310. doi:10.1016/j.bcp.2006.08.001
Manalo, R. A. M., Arollado, E. C., and Heralde, F. M. (2020). Alternanthera Sessilis Leaf Fractions Possess In Vitro Inhibitory Activities in Mammalian α-amylase and α-glucosidase. Pharm. Sci. Asia 47 (3), 279–286. doi:10.29090/psa.2020.03.019.0076
Manan, M., Saleem, U., Akash, M. S. H., Qasim, M., Hayat, M., Raza, Z., et al. (2020). Antiarthritic Potential of Comprehensively Standardized Extract of Alternanthera Bettzickiana: In Vitro and In Vivo Studies. ACS Omega 5 (31), 19478–19496. doi:10.1021/acsomega.0c01670
Manda, K., and Bhatia, A. L. (2003). Role of β-carotene against Acetaminophen-Induced Hepatotoxicity in Mice. Nutr. Res. 23 (8), 1097–1103. doi:10.1016/s0271-5317(03)00103-9
Manjunath, S. H., and Thimmulappa, R. K. (2021). Antiviral, Immunomodulatory, and Anticoagulant Effects of Quercetin and its Derivatives: Potential Role in Prevention and Management of COVID-19. J. Pharm. Anal.. doi:10.1016/j.jpha.2021.09.009
Marioni, J., da Silva, M. A., Cabrera, J. L., Montoya, S. C. N., and Paraje, M. G. (2016). The Anthraquinones Rubiadin and its 1-methyl Ether Isolated from Heterophyllaea Pustulata Reduces Candida tropicalis Biofilms Formation. Phytomedicine 23 (12), 1321–1328. doi:10.1016/j.phymed.2016.07.008
Martino, R., Arcos, M. L. B., Alonso, R., Sülsen, V., Cremaschi, G., and Anesini, C. (2016). Polyphenol-Rich Fraction fromLarrea Divaricataand its Main Flavonoid Quercetin-3-Methyl Ether Induce Apoptosis in Lymphoma Cells through Nitrosative Stress. Phytotherapy Res. 30 (7), 1128–1136. doi:10.1002/ptr.5615
Masry, S. H. D., Taha, T. H., Botros, W. A., Mahfouz, H., Al-Kahtani, S. N., Ansari, M. J., et al. (2021). Antimicrobial Activity of Camphor Tree Silver Nano-Particles against Foulbrood Diseases and Finding Out New Strain of Serratia marcescens as a Secondary Infection on Honeybee Larvae. Saudi J. Biol. Sci. 28 (4), 2067–2075. doi:10.1016/j.sjbs.2021.02.038
Mesbah, H. A., Saad, A. S., Mourad, A. K., Taman, F. A., and Mohamed, I. B. (2007). Joint Action of Quercetin with Four Insecticides on the Cotton Leaf-Worm Larvae, Spodoptera Littoralis Boisd. (Lep. : Noctuidae) in Egypt. Commun. Agric. Appl. Biol. Sci. 72 (3), 445–457.
Mhillaj, E., Catino, S., Miceli, F. M., Santangelo, R., Trabace, L., Cuomo, V., et al. (2017). Ferulic Acid Improves Cognitive Skills through the Activation of the Heme Oxygenase System in the Rat. Mol. Neurobiol. 55 (2), 905–916. doi:10.1007/s12035-017-0381-1
Mikhlin, E. D., Radina, V. P., Dmitrovskii, A. A., Blinkova, L. P., and Butova, L. G. (1983). Antifungal and Antimicrobial Activity of Beta-Ionone and Vitamin A Derivatives. Prikl Biokhim Mikrobiol 19 (6), 795–803.
Miltonprabu, S., Tomczyk, M., Skalicka-Woźniak, K., Rastrelli, L., Daglia, M., Nabavi, S. F., et al. (2017). Hepatoprotective Effect of Quercetin: From Chemistry to Medicine. Food Chem. Toxicol. 108, 365–374. doi:10.1016/j.fct.2016.08.034
Mishra, B., Priyadarsini, K. I., Kumar, M. S., Unnikrishnan, M. K., and Mohan, H. (2003). Effect of O -glycosilation on the Antioxidant Activity and Free Radical Reactions of a Plant Flavonoid, Chrysoeriol. Bioorg. Med. Chem. 11 (13), 2677–2685. doi:10.1016/s0968-0896(03)00232-3
Mlcek, J., Jurikova, T., Skrovankova, S., and Sochor, J. (2016). Quercetin and its Anti-allergic Immune Response. Molecules 21 (5), 1. doi:10.3390/molecules21050623
Mo, J., Panichayupakaranant, P., Kaewnopparat, N., Nitiruangjaras, A., and Reanmongkol, W. (2014). Wound Healing Activities of Standardized Pomegranate Rind Extract and its Major Antioxidant Ellagic Acid in Rat Dermal Wounds. J. Nat. Medicines 68 (2), 377–386. doi:10.1007/s11418-013-0813-9
Mohaimenul, M. D., Dutta, K., Ferdiousi, N., and Nath Roy, D. (2020). Comparative Studies on Antidiabetic, Analgesic, and Cytotoxic Effect of Ethanolic Extracts of Amaranthus Gangeticus L. And Alternanthera Sessilis L. Asian J. Pharm. Clin. Res. 7, 113–117. doi:10.22159/ajpcr.2020.v13i11.39232
Mohapatra, S. S., Kafle, A., Chatterjee, J., Mohan, P., Roy, R. K., and Reddy, I. (2018). Analgesic Activity of Hydroethanolic Extract of Alternanthera Sessilis in Mice. J. Pharmacognosy Phytochemistry 7 (4), 1836–1839. doi:10.20546/ijcmas.2018.701.120
Mohd Hazli, U. H. A., Abdul-Aziz, A., Mat-Junit, S., Chee, C. F., and Kong, K. W. (2019). Solid-liquid Extraction of Bioactive Compounds with Antioxidant Potential from Alternanthera Sesillis (Red) and Identification of the Polyphenols Using UHPLC-QqQ-MS/MS. Food Res. Int. 115, 241–250. doi:10.1016/j.foodres.2018.08.094
Mondal, H., Hossain, H., Awang, K., Saha, S., Mamun-Ur-Rashid, S., Islam, M. K., et al. (2015). Anthelmintic Activity of Ellagic Acid, a Major Constituent of Alternanthera Sessilis against Haemonchus contortus. Pakistan Vet. J. 35 (1), 58–62.
Mondal, H., Saha, S., Awang, K., Hossain, H., Ablat, A., Islam, M. K., et al. (2014). Central-stimulating and Analgesic Activity of the Ethanolic Extract of Alternanthera Sessilis in Mice. BMC Complement. Altern. Med. 14 (1), 1. doi:10.1186/1472-6882-14-398
Monici, M., Baglioni, P., Mulinacci, N., Baldi, A., and Vincieri, F. F. (1994). A Research Model on Flavonoids as Photoprotectors: Studies on the Photochemistry of Kaempferol and Pelargonidin. Acta Horticulturae 381, 340–347. doi:10.17660/ActaHortic.1994.381.41
Monroy, A. E. M., and Limsiaco, C. L. (2016). Phytochemical and Antimicrobial Analysis of “Lupo” (Alternanthera Sessilis L.R.BR.). West Visayas State. Univ. Res. J. 5 (2), 21–34.
Monteiro, Á. B., Kelly de Souza Rodrigues, C., Petícia do Nascimento, E., Sales, V. d. S., de Araújo Delmondes, G., Nogueira da Costa, M. H., et al. (2020). Anxiolytic and Antidepressant-like Effects of Annona Coriacea (Mart.) and Caffeic Acid in Mice. Food Chem. Toxicol. 136, 1. doi:10.1016/j.fct.2019.111049
Moon, J.-M., Park, S.-H., Jhee, K.-H., and Yang, S.-A. (2018). Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania Latifolia Extract. Molecules 23 (9), 1. doi:10.3390/molecules23092254
Moraes, V. L. G., Santos, L. F. M., Castro, S. B., Loureiro, L. H., Lima, O. A., Souza, M. L. M., et al. (1994). Inhibition of Lymphocyte Activation by Extracts and Fractions of Kalanchoe, Alternanthera, Paullinia and Mikania Species. Phytomedicine 1 (3), 199–204. doi:10.1016/s0944-7113(11)80065-6
Morales, M. A., Tortoriello, J., Meckes, M., Paz, D., and Lozoya, X. (1994). Calcium-antagonist Effect of Quercetin and its Relation with the Spasmolytic Properties of Psidium Guajava L. Arch. Med. Res. 25 (1), 17–21.
Moreno-Anzúrez, N., Marquina, S., Alvarez, L., Zamilpa, A., Castillo-España, P., Perea-Arango, I., et al. (2017). A Cytotoxic and Anti-inflammatory Campesterol Derivative from Genetically Transformed Hairy Roots of Lopezia Racemosa Cav. (Onagraceae). Molecules 22 (1), 1. doi:10.3390/molecules22010118
Morgan, L. V., Petry, F., Scatolin, M., de Oliveira, P. V., Alves, B. O., Zilli, G. A. L., et al. (2021). Investigation of the Anti-inflammatory Effects of Stigmasterol in Mice: Insight into its Mechanism of Action. Behav. Pharmacol. 32 (8), 640–651. doi:10.1097/fbp.0000000000000658
Moura, D. J., Richter, M. F., Boeira, J. M., Pegas Henriques, J. A., and Saffi, J. (2007). Antioxidant Properties of -carboline Alkaloids Are Related to Their Antimutagenic and Antigenotoxic Activities. Mutagenesis 22 (4), 293–302. doi:10.1093/mutage/gem016
Mourya, P., Rohit, S., and Neetesh, K. J. (2020). A Study of Antihyperglycaemic Activity of Alternanthera Pungens Kunth on Alloxan Induced Diabetic Rats. Int. J. Pharm. Life Sci. 11 (7), 44.
Mourya, P., Sharma, N. K., and Gupta, M. K. (2019). Antioxidant Activity of Ethanolic and Aqueous Extracts of Alternanthera Pungens Kunth. Asian J. Pharm. Pharmacol. 5 (6), 1091–1096. doi:10.31024/ajpp.2019.5.6.3
Mózsik, G., Abdel-Salam, O. M. E., Bódis, B., Karádi, O., Király, Á., Sütő, G., et al. (1996). Gastric Mucosal Preventive Effects of Prostacyclin and β-carotene, and Their Biochemical Effects in Rats Treated with Ethanol and HCl at Different Doses and Time Intervals after Administration of Necrotizing Agents. Inflammopharmacology 4 (4), 361–378. doi:10.1007/bf02755789
Muniandy, K., Gothai, S., Badran, K. M. H., Suresh Kumar, S., Esa, N. M., and Arulselvan, P. (2018a). Suppression of Proinflammatory Cytokines and Mediators in LPS-Induced RAW 264.7 Macrophages by Stem Extract of Alternanthera Sessilis via the Inhibition of the NF-Κb Pathway. J. Immunol. Res. 2018, 1–12. doi:10.1155/2018/3430684
Muniandy, K., Gothai, S., Tan, W. S., Kumar, S. S., Mohd Esa, N., Chandramohan, G., et al. (2018b). Vitro Wound Healing Potential of Stem Extract of Alternanthera Sessilis. Evidence-Based Complement. Altern. Med. 2018, 1–13. doi:10.1155/2018/3142073
Murali, R., and Saravanan, R. (2012). Antidiabetic Effect of D-Limonene, a Monoterpene in Streptozotocin-Induced Diabetic Rats. Biomed. Prev. Nutr. 2 (4), 269–275. doi:10.1016/j.bionut.2012.08.008
Muthulakshmi, S., and Saravanan, R. (2013). Protective Effects of Azelaic Acid against High-Fat Diet-Induced Oxidative Stress in Liver, Kidney and Heart of C57BL/6J Mice. Mol. Cell Biochem. 377 (1-2), 23–33. doi:10.1007/s11010-013-1566-1
Nada, A. A., Arul, M. R., Ramos, D. M., Kroneková, Z., Mosnáček, J., Rudraiah, S., et al. (2018). Bioactive Polymeric Formulations for Wound Healing. Polym. Adv. Tech. 29 (6), 1815–1825. doi:10.1002/pat.4288
Nagalingam, M., Kalpana, V. N., Rajeswari, D. R., and Panneerselvam, A. (2018). Biosynthesis, Characterization, and Evaluation of Bioactivities of Leaf Extract-Mediated Biocompatible Gold Nanoparticles from Alternanthera Bettzickiana. Biotechnol. Rep. 19, 1. doi:10.1016/j.btre.2018.e00268
Naidu, V. S. G. R. (2012). Hand Book on Weed Identification. Jaipur, India: Directorate of Weed Science Research.
Nakhaee, S., Farrokhfall, K., Miri-Moghaddam, E., Foadoddini, M., Askari, M., Amirabadizadeh, A., et al. (2021). The Effects of Naloxone, Diazepam, and Quercetin on Seizure and Sedation in Acute on Chronic Tramadol Administration: an Experimental Study. Behav. Brain Functions 17 (1), 1. doi:10.1186/s12993-021-00178-w
Narasimhan, A., Chinnaiyan, M., and Karundevi, B. (2015). Ferulic Acid Exerts its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 40 (8), 769–781. doi:10.1139/apnm-2015-0002
Nassiri-Asl, M., Hajiali, F., Taghiloo, M., Abbasi, E., Mohseni, F., and Yousefi, F. (2014). Comparison between the Effects of Quercetin on Seizure Threshold in Acute and Chronic Seizure Models. Toxicol. Ind. Health 32 (5), 936–944. doi:10.1177/0748233713518603
Nieoczym, D., Socała, K., Raszewski, G., and Wlaź, P. (2014). Effect of Quercetin and Rutin in Some Acute Seizure Models in Mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 54, 50–58. doi:10.1016/j.pnpbp.2014.05.007
Nile, S. H., Ko, E. Y., Kim, D. H., and Keum, Y.-S. (2016). Screening of Ferulic Acid Related Compounds as Inhibitors of Xanthine Oxidase and Cyclooxygenase-2 with Anti-inflammatory Activity. Revista Brasileira de Farmacognosia 26 (1), 50–55. doi:10.1016/j.bjp.2015.08.013
Niraimathi, K. L., Sudha, V., Lavanya, R., and Brindha, P. (2013). Biosynthesis of Silver Nanoparticles Using Alternanthera Sessilis (Linn.) Extract and Their Antimicrobial, Antioxidant Activities. Colloids Surf. B: Biointerfaces 102, 288–291. doi:10.1016/j.colsurfb.2012.08.041
Niranjan Panat, A., Bhushan Amrute, K., Shateesh, B., Santosh Haram, K., Geeta Sharma, K., and Saroj Ghaskadbi, S. (2015). Antioxidant Profiling of C3 Quercetin Glycosides: Quercitrin, Quercetin 3-β-D-Glucoside and Quercetin 3-O-(6”-O-Malonyl)-β-Dglucoside in Cell Free Environment. Free Radicals Antioxid. 5 (2), 90–100. doi:10.5530/fra.2015.2.7
Novak, A. F., Clark, G. C., and Dupuy, H. P. (1961). Antimicrobial Activity of Some Ricinoleic Acid Oleic Acid Derivatives. J. Am. Oil Chemists Soc. 38 (6), 321–324. doi:10.1007/bf02638439
Obertreis, B., Giller, K., Teucher, T., Behnke, B., and Schmitz, H. (1996). Anti-inflammatory Effect of Urtica Dioica Folia Extract in Comparison to Caffeic Malic Acid. Arzneimittelforschung 46 (1), 52–56.
Ododo, M. M., Choudhury, M. K., and Dekebo, A. H. (2016). Structure Elucidation of β-sitosterol with Antibacterial Activity from the Root Bark of Malva Parviflora. SpringerPlus 5 (1), 1. doi:10.1186/s40064-016-2894-x
Ogunmoye, A. O., Atewolara-Odule, O. C., Olubomehin, O. O., Ogundare, S. A., and Yussuf, S. T. (2020). The Chemical Constituents of the Leaves Essential Oil of Alternanthera Pungens (Kunth). Afr. J. Sci. Nat. 10, 123–130. doi:10.46881/ajsn.v10i0.185
Oh, H.-A., Han, N.-R., Kim, M.-J., Kim, H.-M., and Jeong, H.-J. (2013). Evaluation of the Effect of Kaempferol in a Murine Allergic Rhinitis Model. Eur. J. Pharmacol. 718 (1-3), 48–56. doi:10.1016/j.ejphar.2013.08.045
Olaiya, C. O., Esan, A. M., and Alabi, T. D. (2014). Ameliorative Effects of Beta-Sitosterol on Some Biochemical Indices of Hypertension in Wistar Albino Rats. Afr. J. Med. Med. Sci. 43 (Suppl. 1), 157–166.
Ong, K. W., Hsu, A., and Tan, B. K. H. (2013). Anti-diabetic and Anti-lipidemic Effects of Chlorogenic Acid Are Mediated by Ampk Activation. Biochem. Pharmacol. 85 (9), 1341–1351. doi:10.1016/j.bcp.2013.02.008
Osuna, L., Tapia-Pérez, M. E., Jiménez-Ferrer, J. E., Carrillo-Quiróz, B. A., and Silva-Sánchez, J. (2008). Screening ofAlternanthera repens.,Boerhavia coccinea.,Flaveria trinervia.,Tournefortia densiflora., andVitex Mollis. Extracts to Evaluate Their Antibacterial Activity and Effect on Smooth Muscle. I. Pharm. Biol. 43 (9), 749–753. doi:10.1080/13880200500406412
Othman, A., Ismail, A., Hassan, F. A., Yusof, B. N. M., and Khatib, A. (2016). Comparative Evaluation of Nutritional Compositions, Antioxidant Capacities, and Phenolic Compounds of Red and green Sessile Joyweed ( Alternanthera Sessilis ). J. Funct. Foods 21, 263–271. doi:10.1016/j.jff.2015.12.014
Oyemitan, I. A., Bello, O. A., and Akinpelu, L. A. (2015). Neuropharmacological Evaluation of Ethanolic Leaf Extract of Alternanthera Brasiliana (L.) Kuntze (Amaranthaceae) in Mice. Int. J. Pharm. Sci. Res. 6 (9), 3796–3806.
Ozaki, Y. (1992). Antiinflammatory Effects of Tetramethylpyrazine and Ferulic Acid. Chem. Pharm. Bull. 40 (4), 954–956. doi:10.1248/cpb.40.954
Özay, Y., Güzel, S., Yumrutaş, Ö., Pehlivanoğlu, B., Erdoğdu, İ. H., Yildirim, Z., et al. (2019). Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 233, 284–296. doi:10.1016/j.jss.2018.08.009
P, S., K, P., and Lahkar, M. (2016). Effect of Alternanthera Brasiliana in Experimentally Induced Inflammatory Bowel Disease in Albino Rats. Int. J. Basic Clin. Pharmacol. 18, 1809–1815. doi:10.18203/2319-2003.ijbcp20162789
Paiva, S. A. R., and Russell, R. M. (1999). β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 18 (5), 426–433. doi:10.1080/07315724.1999.10718880
Panda, V., and Suresh, S. (2015). Gastro-protective Effects of the Phenolic Acids of Macrotyloma Uniflorum (Horse Gram) on Experimental Gastric Ulcer Models in Rats. Food Biosci. 12, 34–46. doi:10.1016/j.fbio.2015.07.004
Park, C. G., Kim, J. J., and Kim, H. K. (2020). Lipase-mediated Synthesis of Ricinoleic Acid Vanillyl Ester and Evaluation of Antioxidant and Antibacterial Activity. Enzyme Microb. Tech. 133. doi:10.1016/j.enzmictec.2019.109454
Park, J., Lee, G. E., An, H. J., Lee, C. J., Cho, E. S., Kang, H. C., et al. (2021). Kaempferol Sensitizes Cell Proliferation Inhibition in Oxaliplatin-Resistant colon Cancer Cells. Arch. Pharm. Res. 44 (12), 1091–1108. doi:10.1007/s12272-021-01358-y
Park, J. S., Rho, H. S., Kim, D. H., and Chang, I. S. (2006). Enzymatic Preparation of Kaempferol from Green Tea Seed and its Antioxidant Activity. J. Agric. Food Chem. 54 (8), 2951–2956. doi:10.1021/jf052900a
Park, S.-H., Sim, Y.-B., Han, P.-L., Lee, J.-K., and Suh, H.-W. (2010a). Antidepressant-like Effect of Chlorogenic Acid Isolated fromArtemisia capillarisThunb. Anim. Cell Syst. 14 (4), 253–259. doi:10.1080/19768354.2010.528192
Park, S.-H., Sim, Y.-B., Han, P.-L., Lee, J.-K., and Suh, H.-W. (2010b). Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia Ficus-Indica Var. Saboten. Exp. Neurobiol. 19 (1), 30–38. doi:10.5607/en.2010.19.1.30
Park, S.-N., Lim, Y. K., Freire, M. O., Cho, E., Jin, D., and Kook, J.-K. (2012a). Antimicrobial Effect of Linalool and α-terpineol against Periodontopathic and Cariogenic Bacteria. Anaerobe 18 (3), 369–372. doi:10.1016/j.anaerobe.2012.04.001
Park, S., Choi, J. J., Park, B.-K., Yoon, S. J., Choi, J. E., and Jin, M. (2014). Pheophytin a and Chlorophyll a Suppress Neuroinflammatory Responses in Lipopolysaccharide and Interferon-γ-Stimulated BV2 Microglia. Life Sci. 103 (2), 59–67. doi:10.1016/j.lfs.2014.04.003
Park, S. J., Kim, D. H., Jung, J. M., Kim, J. M., Cai, M., Liu, X., et al. (2012b). The Ameliorating Effects of Stigmasterol on Scopolamine-Induced Memory Impairments in Mice. Eur. J. Pharmacol. 676 (1-3), 64–70. doi:10.1016/j.ejphar.2011.11.050
Parveen, Z., Deng, Y., Saeed, M. K., Dai, R., Ahamad, W., and Yu, Y. H. (2007). Antiinflammatory and Analgesic Activities of Thesium Chinense Turcz Extracts and its Major Flavonoids, Kaempferol and Kaempferol-3-O-Glucoside. Yakugaku Zasshi 127 (8), 1275–1279. doi:10.1248/yakushi.127.1275
Parvizi, F., Yaghmaei, P., Haeri Rohani, S. A., and Mard, S. A. (2020). Hepatoprotective Properties of P-Coumaric Acid in a Rat Model of Ischemia-Reperfusion. Avicenna J. Phytomed 10 (6), 633–640.
Pathak, I., Budhathoki, R., Yadav, N., Niraula, M., and Kalauni, S. K. (2020). Phytochemical Screening, Cytotoxic and Antioxidant Activity of Alternathera Sessilis and Moringa Oleifera. Amrit Res. J. 1 (1), 65–71. doi:10.3126/arj.v1i1.32456
Patil, R. B., and Kore, B. A. (2019). Potential of an Invasive weed Alternanthera ficoidea (L.) P. Beauv. As Resource of Antioxidants. Int. J. Scientific Res. Rev. 8 (2), 4041–4046.
Pavela, R. (2011). Antifeedant and Larvicidal Effects of Some Phenolic Components of Essential Oils Lasp Lines of Introduction AgainstSpodoptera littoralis(Boisd.). J. Essent. Oil Bearing Plants 14 (3), 266–273. doi:10.1080/0972060x.2011.10643932
Peana, A. T., D'Aquila, P. S., Panin, F., Serra, G., Pippia, P., and Moretti, M. D. L. (2002). Anti-inflammatory Activity of Linalool and Linalyl Acetate Constituents of Essential Oils. Phytomedicine 9 (8), 721–726. doi:10.1078/094471102321621322
Pejin, B., Savic, A., Sokovic, M., Glamoclija, J., Ciric, A., Nikolic, M., et al. (2014). Furtherin Vitroevaluation of Antiradical and Antimicrobial Activities of Phytol. Nat. Product. Res. 28 (6), 372–376. doi:10.1080/14786419.2013.869692
Pelisoli Formagio, E. L., Mendel, M. T., Fracasso, R., Knobloch, J. G., Teixeira, P. W., Kehl, L., et al. (2012). Evaluation of the Pharmacological Activity of theAlternanthera Brasilianaaqueous Extract. Pharm. Biol. 50 (11), 1442–1447. doi:10.3109/13880209.2012.688058
Pereira, D. F., Zanon, R. B., dos Santos, M., Boligon, A. A., and Athayde, M. L. (2013). Antioxidant Activities and Triterpenoids Isolated fromAlternanthera brasiliana(L.) Kuntze Leaves. Nat. Product. Res. 27 (18), 1660–1663. doi:10.1080/14786419.2012.750313
Peres, D. D. A., Sarruf, F. D., de Oliveira, C. A., Velasco, M. V. R., and Baby, A. R. (2018). Ferulic Acid Photoprotective Properties in Association with UV Filters: Multifunctional Sunscreen with Improved SPF and UVA-PF. J. Photochem. Photobiol. B: Biol. 185, 46–49. doi:10.1016/j.jphotobiol.2018.05.026
Perez-Vizcaino, F., Duarte, J., Jimenez, R., Santos-Buelga, C., and Osuna, A. (2009). Antihypertensive Effects of the Flavonoid Quercetin. Pharmacol. Rep. 61 (1), 67–75. doi:10.1016/s1734-1140(09)70008-8
Pero, R. W., Lund, H., and Leanderson, T. (2009). Antioxidant Metabolism Induced by Quinic Acid. Increased Urinary Excretion of Tryptophan and Nicotinamide. Phytotherapy Res. 23 (3), 335–346. doi:10.1002/ptr.2628
Petpiroon, N., Suktap, C., Pongsamart, S., Chanvorachote, P., and Sukrong, S. (2015). Kaempferol-3-O-rutinoside from Afgekia Mahidoliae Promotes Keratinocyte Migration through FAK and Rac1 Activation. J. Nat. Medicines 69 (3), 340–348. doi:10.1007/s11418-015-0899-3
Petrus, A. A., and Seetharaman, T. R. (2005). Antioxidant Flavone C-Biosides from the Aerial Parts of Alternanthera Pungens. Indian J. Pharm. Sci. 67 (2), 187–192.
Petrus, A. J. A., Kalpana, K., and Devi, A. B. (2014b). Antioxidant Capacity and Lipophilic Constitution of Alternanthera Bettzickiana Flower Extract. Oriental J. Chem. 30 (2), 491–499. doi:10.13005/ojc/300212
Petrus, A., Kalpana, K., and Devi, A. (2014a). Foliar Biophenolic Antioxidant Metabolites of Alternanthera Bettzickiana. Oriental J. Chem. 30 (3), 1197–1203. doi:10.13005/ojc/300334
Peungvicha, P., Temsiririrkkul, R., Prasain, J. K., Tezuka, Y., Kadota, S., Thirawarapan, S. S., et al. (1998). 4-Hydroxybenzoic Acid: a Hypoglycemic Constituent of Aqueous Extract of Pandanus Odorus Root. J. Ethnopharmacology 62 (1), 79–84. doi:10.1016/s0378-8741(98)00061-0
Pizzo, S. V., Wang, J., Fang, X., Ge, L., Cao, F., Zhao, L., et al. (2018). Antitumor, Antioxidant and Anti-inflammatory Activities of Kaempferol and its Corresponding Glycosides and the Enzymatic Preparation of Kaempferol. Plos One 13 (5), 1. doi:10.1371/journal.pone.0197563
Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., and Selvaraj, J. (2019). In Silicoandin Vivoanalysis to Identify the Antidiabetic Activity of Beta Sitosterol in Adipose Tissue of High Fat Diet and Sucrose Induced Type-2 Diabetic Experimental Rats. Toxicol. Mech. Methods 29 (4), 276–290. doi:10.1080/15376516.2018.1545815
Pradhan, M., Suri, C., Choudhary, S., Naik, P. K., and Lopus, M. (2016). Elucidation of the Anticancer Potential and Tubulin Isotype-specific Interactions of β-sitosterol. J. Biomol. Struct. Dyn. 36 (1), 195–208. doi:10.1080/07391102.2016.1271749
Pragasam, S. J., Venkatesan, V., and Rasool, M. (2012). Immunomodulatory and Anti-inflammatory Effect of P-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 36 (1), 169–176. doi:10.1007/s10753-012-9532-8
Pratiwi, R., Nantasenamat, C., Ruankham, W., Suwanjang, W., Prachayasittikul, V., Prachayasittikul, S., et al. (2021). Mechanisms and Neuroprotective Activities of Stigmasterol against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Front. Nutr. 8. doi:10.3389/fnut.2021.648995
Prince Vijeya Singh, J., Selvendiran, K., Mumtaz Banu, S., Padmavathi, R., and Sakthisekaran, D. (2004). Protective Role of Apigenin on the Status of Lipid Peroxidation and Antioxidant Defense against Hepatocarcinogenesis in Wistar Albino Rats. Phytomedicine 11 (4), 309–314. doi:10.1078/0944711041495254
Priyadarsini, K. I., Khopde, S. M., Kumar, S. S., and Mohan, H. (2002). Free Radical Studies of Ellagic Acid, a Natural Phenolic Antioxidant. J. Agric. Food Chem. 50 (7), 2200–2206. doi:10.1021/jf011275g
Pujari, R. R., and Bandawane, D. D. (2021). Hepatoprotective Activity of Gentisic Acid on 5-Fluorouracil-Induced Hepatotoxicity in Wistar Rats. Turkish J. Pharm. Sci. 18 (3), 332–338. doi:10.4274/tjps.galenos.2020.95870
Pulipati, S., and Babu, P. S. (2020). In-vitro Antibacterial Potential of Alternanthera Phyloxeroides (Mart) Griseb. Against Multi-Drug Resistant Uropathogens. Int. J. Pharm. Sci. Res. 11 (8), 3834–3840. doi:10.13040/ijpsr.0975-8232.11(8).3834-40
Pulipati, S., Babu, S. P., Sree, N. B., Kumar, U. E., Shaheela, S., Krishna, J. M., et al. (2016). Phytochemical Analysis and Antimicrobial Investigations of Ethanolic Leaf Extract of Alternanthera Philoxeroides (Mart.) Griseb. World J. Pharm. Pharm. Sci. 5 (2), 1122–1129.
Pulipati, S., Babu, S. P., Sri Devi, B., Rama Devi, G., and Bhanuja, M. (2015). Pharmacognostic Studies of Alternanthera Philoxeroides (Mart.) Griseb. J. Pharmacognosy Phytochemistry 4 (2), 202–204.
Qian, L., Su, W., Wang, Y., Dang, M., Zhang, W., and Wang, C. (2019). Synthesis and Characterization of Gold Nanoparticles from Aqueous Leaf Extract of Alternanthera Sessilis and its Anticancer Activity on Cervical Cancer Cells (HeLa). Artif. Cell Nanomedicine, Biotechnol. 47 (1), 1173–1180. doi:10.1080/21691401.2018.1549064
Qian, W., Liu, M., Fu, Y., Zhang, J., Liu, W., Li, J., et al. (2020). Antimicrobial Mechanism of Luteolin against Staphylococcus aureus and Listeria Monocytogenes and its Antibiofilm Properties. Microb. Pathogenesis 142. doi:10.1016/j.micpath.2020.104056
Ragasa, C. Y., Tremor, N., and Rideout, J. A. (2010). Ionone Derivatives from Alternanthera Sessilis. J. Asian Nat. Prod. Res. 4 (2), 109–115. doi:10.1080/10286020290027380
Ragone, M. I., Sella, M., Conforti, P., Volonté, M. G., and Consolini, A. E. (2007). The Spasmolytic Effect of Aloysia Citriodora, Palau (South American Cedrón) Is Partially Due to its Vitexin but Not Isovitexin on Rat Duodenums. J. Ethnopharmacology 113 (2), 258–266. doi:10.1016/j.jep.2007.06.003
Rajamurugan, R., Deepa, V., Sivashanmugam, M., and Raghavan, C. M. (2013). Phytochemistry, Antioxidant and Antibacterial Activities of Medicinal Plants - A Comparative Study. Int. J. Curr. Res. Rev. 5, 8–19.
Rao, K. V. R., Rao, K. R. S. S., Nelson, R., Nagaiah, K., and Reddy, V. J. S. (2011). Hypoglycaemic and Antidiabetic Effect of Alternanthera Sessilis in normal and Streptozotocin (STZ)-induced Rat. J. Glob. Trends Pharm. Sci. 2 (3), 325–335.
Rao, R. R. (2000). Synoptic Flora of Mysore District. Mysore, India. Today and Tomorrow’s Printer and Publisher.
Rattanathongkom, A., Lee, J.-B., Hayashi, K., Sripanidkulchai, B.-o., Kanchanapoom, T., and Hayashi, T. (2009). Evaluation of Chikusetsusaponin IV a Isolated fromAlternanthera Philoxeroidesfor its Potency against Viral Replication. Planta Med. 75 (08), 829–835. doi:10.1055/s-0029-1185436
Rauf, A., Imran, M., Khan, I. A., ur-Rehman, M., Gilani, S. A., Mehmood, Z., et al. (2018). Anticancer Potential of Quercetin: A Comprehensive Review. Phytotherapy Res. 32 (11), 2109–2130. doi:10.1002/ptr.6155
Rawani, A., Pal, S., and Chandra, G. (2011). Evaluation of Antimicrobial Properties of Four Plant Extracts against Human Pathogens. Asian Pac. J. Trop. Biomed. 1 (1), S71–S75. doi:10.1016/s2221-1691(11)60127-5
Raybaudi-Massilia, R. M., Mosqueda-Melgar, J., and Martín-Belloso, O. (2009). Antimicrobial Activity of Malic Acid against Listeria Monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in Apple, Pear and Melon Juices. Food Control 20 (2), 105–112. doi:10.1016/j.foodcont.2008.02.009
Rayees, S., Kumar, A., Rasool, S., Kaiser, P., Satti, N. K., Sangwan, P. L., et al. (2013). Ethanolic Extract ofAlternanthera sessilis(AS-1) Inhibits IgE-Mediated Allergic Response in RBL-2H3 Cells. Immunological Invest. 42 (6), 470–480. doi:10.3109/08820139.2013.789909
Rehman, N.-u., Mehmood, M. H., Alkharfy, K. M., and Gilani, A.-H. (2012). Studies on Antidiarrheal and Antispasmodic Activities of Lepidium Sativum Crude Extract in Rats. Phytotherapy Res. 26 (1), 136–141. doi:10.1002/ptr.3642
Reza, H. M., Alam, M. A., Sarker, S. D., Nahar, L., Hossain, H., Shill, M. C., et al. (2019). Alternanthera Bicolor Produces Hypoglycemic Effect in Alloxan-Induced Diabetic Mice through its Antioxidant Activity. Dhaka Univ. J. Pharm. Sci. 18 (1), 49–60. doi:10.3329/dujps.v18i1.41431
Roberto, D., Micucci, P., Sebastian, T., Graciela, F., and Anesini, C. (2009). Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 106 (1), 38–44. doi:10.1111/j.1742-7843.2009.00467.x
Romanova, D., Vachalkova, A., Cipak, L., Ovesna, Z., and Rauko, P. (2001). Study of Antioxidant Effect of Apigenin, Luteolin and Quercetin by DNA Protective Method. Neoplasma 48 (2), 104–107.
Rosa, S. I. G., Rios-Santos, F., Balogun, S. O., and Martins, D. T. d. O. (2016). Vitexin Reduces Neutrophil Migration to Inflammatory Focus by Down-Regulating Pro-inflammatory Mediators via Inhibition of P38, ERK1/2 and JNK Pathway. Phytomedicine 23 (1), 9–17. doi:10.1016/j.phymed.2015.11.003
Rowley, T. J., McKinstry, A., Greenidge, E., Smith, W., and Flood, P. (2010). Antinociceptive and Anti-inflammatory Effects of Choline in a Mouse Model of Postoperative Pain. Br. J. Anaesth. 105 (2), 201–207. doi:10.1093/bja/aeq113
Rufino, A. T., Ribeiro, M., Sousa, C., Judas, F., Salgueiro, L., Cavaleiro, C., et al. (2015). Evaluation of the Anti-inflammatory, Anti-catabolic and Pro-anabolic Effects of E-Caryophyllene, Myrcene and Limonene in a Cell Model of Osteoarthritis. Eur. J. Pharmacol. 750, 141–150. doi:10.1016/j.ejphar.2015.01.018
Rukkumani, R., Aruna, K., Suresh Varma, P., and Padmanabhan Menon, V. (2004). Hepatoprotective Role of Ferulic Acid: A Dose-dependent Study. J. Med. Food 7 (4), 456–461. doi:10.1089/jmf.2004.7.456
Saija, A. (2003). In Vitro' Antioxidant and Photoprotective Properties and Interaction with Model Membranes of Three New Quercetin Esters. Eur. J. Pharmaceutics Biopharmaceutics 56 (2), 167–174. doi:10.1016/s0939-6411(03)00101-2
Salvador, M. J., and Dias, D. A. (2004). Flavone C-Glycosides from Alternanthera Maritima (Mart.) St. Hil. (Amaranthaceae). Biochem. Syst. Ecol. 32 (1), 107–110. doi:10.1016/s0305-1978(03)00180-7
Salvador, M. J., Ferreira, E. O., Mertens-Talcott, S. U., De Castro, W. V., Butterweck, V., Derendorf, H., et al. (2006). Isolation and HPLC Quantitative Analysis of Antioxidant Flavonoids from Alternanthera Tenella Colla. Z. für Naturforschung C 61 (1-2), 19–25. doi:10.1515/znc-2006-1-204
Salvador, M. J., Pereira, P. S., França, S. C., Candido, R. C., Ito, I. Y., and Dias, D. A. (2009). Bioactive Chemical Constituents and Comparative Antimicrobial Activity of Callus Culture and Adult Plant Extracts from Alternanthera Tenella. Z. für Naturforschung C 64 (5-6), 373–381. doi:10.1515/znc-2009-5-612
Samudrala, P., Augustine, B., Kasala, E., Bodduluru, L., Barua, C., and Lahkar, M. (2015). Evaluation of Antitumor Activity and Antioxidant Status of Alternanthera Brasiliana against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Pharmacognosy Res. 7 (1), 1. doi:10.4103/0974-8490.147211
Sánchez-Mendoza, M. E., Arrieta, J., and Navarrete, A. (2008). Role of Prostaglandins, Nitric Oxide, Sulfhydryls and Capsaicin-Sensitive Neurons in Gastroprotection of Stigmasterol and β-Sitosterol. Nat. Product. Commun. 3 (4), 1. doi:10.1177/1934578x0800300406
Sanoko, R., Speranza, G., Pizza, C., and Detommasi, N. (1999). Triterpene Saponins from Alternanthera Repens. Phytochemistry 51 (8), 1043–1047. doi:10.1016/s0031-9422(99)00046-1
Santos, A. L., Yamamoto, E. S., Passero, L. F. D., Laurenti, M. D., Martins, L. F., Lima, M. L., et al. (2017). Antileishmanial Activity and Immunomodulatory Effects of Tricin Isolated from Leaves of Casearia Arborea (Salicaceae). Chem. Biodiversity 14 (5), 1. doi:10.1002/cbdv.201600458
Santos, C. C. d. M. P., Salvadori, M. S., Mota, V. G., Costa, L. M., de Almeida, A. A. C., de Oliveira, G. A. L., et al. (2013). Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 1–9. doi:10.1155/2013/949452
Santos da Silva, G. N., Pozzatti, P., Rigatti, F., Hörner, R., Hartz Alves, S., Mallmann, C. A., et al. (2015). Antimicrobial Evaluation of Sesquiterpene Alpha-Curcumene and its Synergism with Imipenem. J. Microbiol. Biotechnol. Food Sci. 04 (05), 434–436. doi:10.15414/jmbfs.2015.4.5.434-436
Saqib, F., and Janbaz, K. H. (2016). Rationalizing Ethnopharmacological Uses of Alternanthera Sessilis: A Folk Medicinal Plant of Pakistan to Manage Diarrhea, Asthma and Hypertension. J. Ethnopharmacology 182, 110–121. doi:10.1016/j.jep.2016.02.017
Sathishkumar, P., Vennila, K., Jayakumar, R., Yusoff, A. R. M., Hadibarata, T., and Palvannan, T. (2016). Phyto-synthesis of Silver Nanoparticles Using Alternanthera Tenella Leaf Extract: an Effective Inhibitor for the Migration of Human Breast Adenocarcinoma (MCF-7) Cells. Bioproc. Biosyst. Eng. 39 (4), 651–659. doi:10.1007/s00449-016-1546-4
Sathya, S., Manogari, B. G., Thamaraiselvi, K., Vaidevi, S., Ruckmani, K., and Devi, K. P. (2020). Phytol Loaded PLGA Nanoparticles Ameliorate Scopolamine-Induced Cognitive Dysfunction by Attenuating Cholinesterase Activity, Oxidative Stress and Apoptosis in Wistar Rat. Nutr. Neurosci. 8, 1–17. doi:10.1080/1028415x.2020.1764290
Sato, Y., Itagaki, S., Kurokawa, T., Ogura, J., Kobayashi, M., Hirano, T., et al. (2011). In Vitro and In Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharmaceutics 403 (1-2), 136–138. doi:10.1016/j.ijpharm.2010.09.035
Schallenberger, C., Vieira, V., Krai, J. S., Morisso, F., Suyenaga, E., Tavares, R. G., et al. (2017). Anticonvulsant Effect of Alternanthera Brasiliana Extract on Pentylenetetrazole-Induced Seizures in Rats. J. Neurosci. Clin. Res. 2 (1), 1–3.
Scheepens, A., Bisson, J.-F., and Skinner, M. (2014). p-Coumaric Acid Activates the GABA-A Receptor In Vitro and Is Orally Anxiolytic In Vivo. Phytotherapy Res. 28 (2), 207–211. doi:10.1002/ptr.4968
Sekar, K. C. (2012). Invasive Alien Plants of Indian Himalayan Region—Diversity and Implication. Am. J. Plant Sci. 03 (02), 177–184. doi:10.4236/ajps.2012.32021
Semenya, S. S., and Potgieter, M. J. (2014). Bapedi Traditional Healers in the Limpopo Province, South Africa: Their Socio-Cultural Profile and Traditional Healing Practice. J. Ethnobiol. Ethnomedicine 10 (1), 1. doi:10.1186/1746-4269-10-4
Shahid, F., Farooqui, Z., and Khan, F. (2018). Cisplatin-induced Gastrointestinal Toxicity: An Update on Possible Mechanisms and on Available Gastroprotective Strategies. Eur. J. Pharmacol. 827, 49–57. doi:10.1016/j.ejphar.2018.03.009
Shan, C., and Miao, F. (2022). Immunomodulatory and Antioxidant Effects of Hydroxytyrosol in Cyclophosphamide-Induced Immunosuppressed Broilers. Poult. Sci. 101 (1), 1. doi:10.1016/j.psj.2021.101516
Sharma, A., Sanadhya, I., Bhot, M., and Varghese, J. (2013). Evaluation of Antioxidant Potential of Alternanthera Sessilis (L.) DC. Res. J. Pharmacognosy Phytochemistry 5 (4), 194–198. doi:10.4103/0974-8490.118767
Sharma, N., Biswas, S., Al-Dayan, N., Alhegaili, A. S., and Sarwat, M. (2021). Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants (Basel) 10 (9), 1. doi:10.3390/antiox10091419
Shi, C., Zhang, X., Sun, Y., Yang, M., Song, K., Zheng, Z., et al. (2016). Antimicrobial Activity of Ferulic Acid AgainstCronobacter Sakazakiiand Possible Mechanism of Action. Foodborne Pathog. Dis. 13 (4), 196–204. doi:10.1089/fpd.2015.1992
Shi, T., Bian, X., Yao, Z., Wang, Y., Gao, W., and Guo, C. (2020). Quercetin Improves Gut Dysbiosis in Antibiotic-Treated Mice. Food Funct. 11 (9), 8003–8013. doi:10.1039/d0fo01439g
Singh, B., Sharma, V., Singh Ishar, M., and Sharma, A. (2013). Bioactivity Guided Isolation of Quercetin as Anxiolytic Compound from Elaeocarpus Ganitrus Beads. Nat. Prod. J. 3 (3), 224–229. doi:10.2174/22103155113039990010
Singh, D., Sharma, S. K., Rani, R., Mishra, S., and Sharma, R. A. (2011). Kaempferol-7-O-Glucoside and Their Antimicrobial Screening Isolate from Cassia Renigera Wall. Int. J. Pharm. Clin. Res. 3 (2), 30–34.
Singh, R., Singh, B., Singh, S., Kumar, N., Kumar, S., and Arora, S. (2008). Anti-free Radical Activities of Kaempferol Isolated from Acacia Nilotica (L.) Willd. Ex. Del. Toxicol. Vitro 22 (8), 1965–1970. doi:10.1016/j.tiv.2008.08.007
Singh, S. S., Rai, S. N., Birla, H., Zahra, W., Kumar, G., Gedda, M. R., et al. (2018). Effect of Chlorogenic Acid Supplementation in MPTP-Intoxicated Mouse. Front. Pharmacol. 9. doi:10.3389/fphar.2018.00757
Singla, R. K., He, X., Chopra, H., Tsagkaris, C., Shen, L., Kamal, M. A., et al. (2021). Natural Products for the Prevention and Control of the COVID-19 Pandemic: Sustainable Bioresources. Front. Pharmacol. 12, 758159. doi:10.3389/fphar.2021.758159
Singla, R. K., Scotti, L., and Dubey, A. K. (2017). In Silico Studies Revealed Multiple Neurological Targets for the Antidepressant Molecule Ursolic Acid. Curr. Neuropharmacology 15 (8), 1. doi:10.2174/1570159x14666161229115508
Singla, R. K., and Shen, B. (2020). In Silico ADMET Evaluation of Natural DPP-IV Inhibitors for Rational Drug Design against Diabetes. Curr. Drug Metab. 21 (10), 768–777. doi:10.2174/1389200221999200901202945
Siopa, F., Figueiredo, T., Frade, R. F. M., Neto, I., Meirinhos, A., Reis, C. P., et al. (2016). Choline-Based Ionic Liquids: Improvement of Antimicrobial Activity. ChemistrySelect 1 (18), 5909–5916. doi:10.1002/slct.201600864
Sivakumar, R., and Sunmathi, D. (2016). Phytochemical Screening and Antimicrobial Activity of Ethanolic Leaf Extract of Alternanthera Sessilis (L.) R.BR. EX DC and Alternanthera Philoxeroides (Mart.) Griseb. Eur. J. Pharm. Med. Res. 3 (3), 409–412.
Socała, K., Nieoczym, D., Pieróg, M., and Wlaź, P. (2015). α-Spinasterol, a TRPV1 Receptor Antagonist, Elevates the Seizure Threshold in Three Acute Seizure Tests in Mice. J. Neural Transm. 122 (9), 1239–1247. doi:10.1007/s00702-015-1391-7
Sonar, V. P., Fois, B., Distinto, S., Maccioni, E., Meleddu, R., Cottiglia, F., et al. (2019). Ferulic Acid Esters and Withanolides: In Search of Withania Somnifera GABAA Receptor Modulators. J. Nat. Prod. 82 (5), 1250–1257. doi:10.1021/acs.jnatprod.8b01023
Song, J. H., Shim, J. K., and Choi, H. J. (2011). Quercetin 7-rhamnoside Reduces Porcine Epidemic Diarrhea Virus Replication via Independent Pathway of Viral Induced Reactive Oxygen Species. Virol. J. 8 (1), 1. doi:10.1186/1743-422x-8-460
Souza, J. G., Tomei, R. R., Kanashiro, A., Kabeya, L. M., Azzolini, A. E. C. S., Dias, D. A., et al. (2007). Ethanolic Crude Extract and Flavonoids Isolated from Alternanthera Maritima: Neutrophil Chemiluminescence Inhibition and Free Radical Scavenging Activity. Z. für Naturforschung C 62 (5-6), 339–347. doi:10.1515/znc-2007-5-604
Suganya, D., Banupriya, R., maheswari A, U., and Elumalai, S. (2019). Studies on Biological Activity of Aqueous Extract of Alternanthera Sessilis (Linn) for Developing Potential Herbal Drug Formulation of Ocular Diseases. Med. Aromatic Plants 08 (01), 1. doi:10.35248/2167-0412.19.8.327
Sun, Z.-R., Liu, H.-R., Hu, D., Fan, M.-S., Wang, M.-Y., An, M.-F., et al. (2021). Ellagic Acid Exerts Beneficial Effects on Hyperuricemia by Inhibiting Xanthine Oxidase and NLRP3 Inflammasome Activation. J. Agric. Food Chem. 69 (43), 12741–12752. doi:10.1021/acs.jafc.1c05239
Sundar, R. D. V., Ravi, L., and Mythili, S. (2019). Discovery of New Anti-fungal Phytochemical PDHC (Propane-diyl-bis-hexahydro-isochromene) Isolated from Alternanthera Sessilis Leaves. Int. J. Pharm. Sci. Res. 10 (3), 1148–1159. doi:10.13040/ijpsr.0975-8232.10(3).1136-47
Sunmathi, D., Sivakumar, R., and Ravikumar, K. (2016). In Vitro anti-inflammatory and Antiarthritic Activity of Ethanolic Leaf Extract of Alternanthera sessilis(L.) R.BR. Ex DC and Alternanthera Philoxeroides (Mart.) Griseb. Int. J. Adv. Pharm. Biol. Chem. 5 (2), 109–115.
Suzuki, K., Nomura, I., Ninomiya, M., Tanaka, K., and Koketsu, M. (2018). Synthesis and Antimicrobial Activity of β-carboline Derivatives with N2-Alkyl Modifications. Bioorg. Med. Chem. Lett. 28 (17), 2976–2978. doi:10.1016/j.bmcl.2018.06.050
Swarnalatha, S., Umamaheswari, A., and Puratchikody, A. (2015). Immunomodulatory Activity of Kaempferol 5-O-β-D-Glucopyranoside from Indigofera Aspalathoides Vahl Ex DC. (Papilionaceae). Med. Chem. Res. 24 (7), 2889–2897. doi:10.1007/s00044-015-1341-9
Tan, K. K., and Kim, K. H. (2013). Alternanthera sessilisRed Ethyl Acetate Fraction Exhibits Antidiabetic Potential on Obese Type 2 Diabetic Rats. Evidence-Based Complement. Altern. Med. 2013, 1–8. doi:10.1155/2013/845172
Tang, T., Wang, S., Cai, T., Cheng, Z., Qi, S., and Qi, Z. (2019). Calenduloside E Inhibits Lipopolysaccharide-Induced Inflammatory Response by Inhibiting Activation of ROS-Mediated JAK1-Stat3 Signaling Pathway in RAW264.7 Cells. Nan Fang Yi Ke Da Xue Xue Bao 39 (8), 904–910. doi:10.12122/j.issn.1673-4254.2019.08.05
Thanabhorn, S., Jaijoy, K., Thamaree, S., Ingkaninan, K., and Panthong, A. (2005). Acute and Subacute Toxicities of the Ethanol Extract from Alternanthera Philoxeroides Griseb. Pharm. Sci. Asia 31 (1-2), 7–14.
Thirukumaran, P., Manoharan, R. K., Parveen, A. S., Atchudan, R., and Kim, S.-C. (2019). Sustainability and Antimicrobial Assessments of Apigenin Based Polybenzoxazine Film. Polymer 172, 100–109. doi:10.1016/j.polymer.2019.03.048
Tian, D., Gao, Q., Lin, J., Chang, Z., Wang, Y., Shi, Y., et al. (2021). Uncovering the Mechanism of the Shenzhi Jiannao Formula against Vascular Dementia Using a Combined Network Pharmacology Approach and Molecular Biology. Phytomedicine 90. doi:10.1016/j.phymed.2021.153637
Tiwari, A., Jyothi, A., Tejeswini, V., Madhusudana, K., Kumar, D., Zehra, A., et al. (2013). Mitigation of Starch and Glucose-Induced Postprandial Glycemic Excursion in Rats by Antioxidant-Rich green-leafy Vegetables′ Juice. Pharmacognosy Mag. 9 (36), 1. doi:10.4103/0973-1296.117872
Tiwari, M., and Kakkar, P. (2009). Plant Derived Antioxidants – Geraniol and Camphene Protect Rat Alveolar Macrophages against T-BHP Induced Oxidative Stress. Toxicol. Vitro 23 (2), 295–301. doi:10.1016/j.tiv.2008.12.014
Tripathi, Y. B., Sharma, M., and Manickam, M. (1997). Rubiadin, a New Antioxidant from Rubia Cordifolia. Indian J. Biochem. Biophys. 34 (3), 302–306.
Tseng, H.-L., Li, C.-J., Huang, L.-H., Chen, C.-Y., Tsai, C.-H., Lin, C.-N., et al. (2012). Quercetin 3-O-Methyl Ether Protects FL83B Cells from Copper Induced Oxidative Stress through the PI3K/Akt and MAPK/Erk Pathway. Toxicol. Appl. Pharmacol. 264 (1), 104–113. doi:10.1016/j.taap.2012.07.022
Tu, Y., Cheng, S.-x., Sun, H.-t., Ma, T.-z., and Zhang, S. (2012). Ferulic Acid Potentiates Pentobarbital-Induced Sleep via the Serotonergic System. Neurosci. Lett. 525 (2), 95–99. doi:10.1016/j.neulet.2012.07.068
Tukun, A. B., Shaheen, N., Banu, C. P., Mohiduzzaman, M., Islam, S., and Begum, M. (2014). Antioxidant Capacity and Total Phenolic Contents in Hydrophilic Extracts of Selected Bangladeshi Medicinal Plants. Asian Pac. J. Trop. Med. 7, S568–S573. doi:10.1016/s1995-7645(14)60291-1
Uteshev, D. B., Kostriukov, E. B., Karabinenko, A. A., Kovaleva, V. L., Makarova, O. V., and Storozhakov, G. I. (2000). The Anti-inflammatory Activity of Intal and Beta-Carotene in a Model of Experimental Granulomatous Lung Inflammation. Patol Fiziol Eksp Ter 2, 19–22.
Vani, M., Rahaman, S. A., and Prameela Rani, A. (2018). Detection and Quantification of Major Phytochemical Markers for Standardization of Talinum Portulacifolium, Gomphrena Serrata, Alternanthera Sessilis and Euphorbia Heterophylla by HPLC. Pharmacognosy J. 10 (3), 439–446. doi:10.5530/pj.2018.3.72
Vasconcelos, M. A. L., Royo, V. A., Ferreira, D. S., Crotti, A. E. M., e Silva, M. L. A., Carvalho, J. C. T., et al. (2006). In Vivo Analgesic and Anti-inflammatory Activities of Ursolic Acid and Oleanoic Acid from Miconia Albicans (Melastomataceae). Z. für Naturforschung C 61 (7-8), 477–482. doi:10.1515/znc-2006-7-803
Vauzour, D., Corona, G., and Spencer, J. P. E. (2010). Caffeic Acid, Tyrosol and P-Coumaric Acid Are Potent Inhibitors of 5-S-Cysteinyl-Dopamine Induced Neurotoxicity. Arch. Biochem. Biophys. 501 (1), 106–111. doi:10.1016/j.abb.2010.03.016
Velika, B., and Kron, I. (2012). Antioxidant Properties of Benzoic Acid Derivatives against Superoxide Radical. Free Radicals Antioxid. 2 (4), 62–67. doi:10.5530/ax.2012.4.11
Vennila, V., and Nivetha, R. (2015). Screening the In Vitro Anthelmintic Activity of Alternanthera Sessilis Leaves. World J. Pharm. Pharm. Sci. 4 (4), 1402–1415.
Venturini, C. L., Macho, A., Arunachalam, K., de Almeida, D. A. T., Rosa, S. I. G., Pavan, E., et al. (2018). Vitexin Inhibits Inflammation in Murine Ovalbumin-Induced Allergic Asthma. Biomed. Pharmacother. 97, 143–151. doi:10.1016/j.biopha.2017.10.073
Vessal, M., Hemmati, M., and Vasei, M. (2003). Antidiabetic Effects of Quercetin in Streptozocin-Induced Diabetic Rats. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 135 (3), 357–364. doi:10.1016/s1532-0456(03)00140-6
Vidhya, T., Suji, T., Dhatchayani, R., Priya, C. L., and Bhaskara Rao, K. V. (2015). Evaluation of In-Vitro Antioxidant, Antimicrobial Activities and GC-MS Analysis of Alternanthera Bettzickiana Linn. Leaf Extracts. Int. J. Pharmacognosy Phytochem. Res. 7 (6), 1072–1079. doi:10.9734/ijbcrr/2015/17241
Vieira, C., Fetzer, S., Sauer, S., Evangelista, S., Averbeck, B., Kress, M., et al. (2001). Pro- and Anti-inflammatory Actions of Ricinoleic Acid: Similarities and Differences with Capsaicin. Naunyn-Schmiedeberg's Arch. Pharmacol. 364 (2), 87–95. doi:10.1007/s002100100427
Vuuren, S. F. v., and Viljoen, A. M. (2007). Antimicrobial Activity of Limonene Enantiomers and 1,8-cineole Alone and in Combination. Flavour Fragrance J. 22 (6), 540–544. doi:10.1002/ffj.1843
Walker, C. I. B., Oliveira, S. M., Tonello, R., Rossato, M. F., da Silva Brum, E., Ferreira, J., et al. (2017). Anti-nociceptive Effect of Stigmasterol in Mouse Models of Acute and Chronic Pain. Naunyn-Schmiedeberg's Arch. Pharmacol. 390 (11), 1163–1172. doi:10.1007/s00210-017-1416-x
Walter, T. M., Merish, S., and Tamizhamuthu, M. (2014). Review of Alternanthera Sessilis with Reference to Traditional Siddha Medicine. Int. J. Pharmacognosy Phytochem. Res. 6 (2), 249–254.
Wang, J., Huang, M., Yang, J., Ma, X., Zheng, S., Deng, S., et al. (2017). Anti-diabetic Activity of Stigmasterol from Soybean Oil by Targeting the GLUT4 Glucose Transporter. Food Nutr. Res. 61 (1), 1. doi:10.1080/16546628.2017.1364117
Wang, M., Shi, Y., Guo, Y., Chen, Y., Zhao, C., Zhou, Y., et al. (2021). Nonadiabatic Dynamics Mechanisms of Natural UV Photoprotection Ompounds Chlorogenic Acid and Isochlorogenic Acid a: Double Conjugated Structures but Single Photoexcited Channel. J. Mol. Liquids 324. doi:10.1016/j.molliq.2020.114725
Wang, M., Sun, J., Jiang, Z., Xie, W., and Zhang, X. (2015a). Hepatoprotective Effect of Kaempferol against Alcoholic Liver Injury in Mice. Am. J. Chin. Med. 43 (02), 241–254. doi:10.1142/s0192415x15500160
Wang, S.-H., Chen, C.-S., Huang, S.-H., Yu, S.-H., Lai, Z.-Y., Huang, S.-T., et al. (2009). Hydrophilic Ester-Bearing Chlorogenic Acid Binds to a Novel Domain to Inhibit Xanthine Oxidase. Planta Med. 75 (11), 1237–1240. doi:10.1055/s-0029-1185521
Wang, W., Guo, J., Zhang, J., Peng, J., Liu, T., and Xin, Z. (2015b). Isolation, Identification and Antioxidant Activity of Bound Phenolic Compounds Present in rice Bran. Food Chem. 171, 40–49. doi:10.1016/j.foodchem.2014.08.095
Wang, X., Ye, X.-l., Liu, R., Chen, H.-L., Bai, H., Liang, X., et al. (2010). Antioxidant Activities of Oleanolic Acid In Vitro: Possible Role of Nrf2 and MAP Kinases. Chemico-Biological Interactions 184 (3), 328–337. doi:10.1016/j.cbi.2010.01.034
Wang, Y., Tang, C., and Zhang, H. (2015c). Hepatoprotective Effects of Kaempferol 3-O-Rutinoside and Kaempferol 3-O-Glucoside from Carthamus tinctorius L. On CCl4-Induced Oxidative Liver Injury in Mice. J. Food Drug Anal. 23 (2), 310–317. doi:10.1016/j.jfda.2014.10.002
Wang, Y., Zhang, G., Pan, J., and Gong, D. (2015d). Novel Insights into the Inhibitory Mechanism of Kaempferol on Xanthine Oxidase. J. Agric. Food Chem. 63 (2), 526–534. doi:10.1021/jf505584m
Wiegmann, D., Koppermann, S., Wirth, M., Niro, G., Leyerer, K., and Ducho, C. (2016). Muraymycin Nucleoside-Peptide Antibiotics: Uridine-Derived Natural Products as lead Structures for the Development of Novel Antibacterial Agents. Beilstein J. Org. Chem. 12, 769–795. doi:10.3762/bjoc.12.77
Winter, A. N., Brenner, M. C., Punessen, N., Snodgrass, M., Byars, C., Arora, Y., et al. (2017). Comparison of the Neuroprotective and Anti-inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxidative Med. Cell Longevity 2017, 1–13. doi:10.1155/2017/6297080
Witt, M. R., Westh-Hansen, S. E., Rasmussen, P. B., Hastrup, S., and Nielsen, M. (2002). Unsaturated Free Fatty Acids Increase Benzodiazepine Receptor Agonist Binding Depending on the Subunit Composition of the GABAA Receptor Complex. J. Neurochem. 67 (5), 2141–2145. doi:10.1046/j.1471-4159.1996.67052141.x
Wu, C.-H., Hsieh, H.-T., Lin, J.-A., and Yen, G.-C. (2013). Alternanthera Paronychioides Protects Pancreatic β-cells from Glucotoxicity by its Antioxidant, Antiapoptotic and Insulin Secretagogue Actions. Food Chem. 139 (1-4), 362–370. doi:10.1016/j.foodchem.2013.01.026
Xue, W., Wang, X., Tang, H., Sun, F., Zhu, H., Huang, D., et al. (2020). Vitexin Attenuates Myocardial Ischemia/reperfusion Injury in Rats by Regulating Mitochondrial Dysfunction Induced by Mitochondrial Dynamics Imbalance. Biomed. Pharmacother. 124. doi:10.1016/j.biopha.2020.109849
Yang, H., Huang, J., Mao, Y., Wang, L., Li, R., and Ha, C. (2019). Vitexin Alleviates Interleukin‐1β‐induced Inflammatory Responses in Chondrocytes from Osteoarthritis Patients: Involvement of HIF‐1α Pathway. Scand. J. Immunol. 90 (2), 1. doi:10.1111/sji.12773
Yang, H., Qu, Z., Zhang, J., Huo, L., Gao, J., and Gao, W. (2016). Ferulic Acid Ameliorates Memory Impairment in D-Galactose-Induced Aging Mouse Model. Int. J. Food Sci. Nutr. 67 (7), 806–817. doi:10.1080/09637486.2016.1198890
Yang, J., Sun, X.-Q., Yan, S.-Y., Pan, W.-J., Zhang, M.-X., and Cai, Q.-N. (2017). Interaction of Ferulic Acid with Glutathione S-Transferase and Carboxylesterase Genes in the Brown Planthopper, Nilaparvata Lugens. J. Chem. Ecol. 43 (7), 693–702. doi:10.1007/s10886-017-0859-3
Yao, Y., Han, D. D., Zhang, T., and Yang, Z. (2010). Quercetin Improves Cognitive Deficits in Rats with Chronic Cerebral Ischemia and Inhibits Voltage-dependent Sodium Channels in Hippocampal CA1 Pyramidal Neurons. Phytotherapy Res. 24 (1), 136–140. doi:10.1002/ptr.2902
Yap, C. H., Mat Junit, S., Abdul Aziz, A., and Kong, K. W. (2019). Multiple Extraction Conditions to Produce Phytochemical- and Antioxidant-Rich Alternanthera Sessilis (Red) Extracts that Attenuate Lipid Accumulation in Steatotic HepG2 Cells. Food Biosci. 32. doi:10.1016/j.fbio.2019.100489
Ye, C.-J., Li, S.-A., Zhang, Y., and Lee, W.-H. (2019). Geraniol Targets KV1.3 Ion Channel and Exhibits Anti-inflammatory Activity In Vitro and In Vivo. Fitoterapia 139. doi:10.1016/j.fitote.2019.104394
Yoshida, Y., and Niki, E. (2003). Antioxidant Effects of Phytosterol and its Components. J. Nutr. Sci. Vitaminology 49 (4), 277–280. doi:10.3177/jnsv.49.277
Yuk, J. E., Woo, J. S., Yun, C.-Y., Lee, J.-S., Kim, J.-H., Song, G.-Y., et al. (2007). Effects of Lactose-β-Sitosterol and β-sitosterol on Ovalbumin-Induced Lung Inflammation in Actively Sensitized Mice. Int. Immunopharmacology 7 (12), 1517–1527. doi:10.1016/j.intimp.2007.07.026
Zavala, M. A., Pérez, S., Pérez, C., Vargas, R., and Pérez, R. M. (1998). Antidiarrhoeal Activity of Waltheria Americana, Commelina Coelestis and Alternanthera Repens. J. Ethnopharmacology 61 (1), 41–47. doi:10.1016/s0378-8741(98)00014-2
Zhang, M., Swarts, S. G., Yin, L., Liu, C., Tian, Y., Cao, Y., et al. (2011). “Antioxidant Properties of Quercetin,” in Oxygen Transport to Tissue XXXII, 283–289. doi:10.1007/978-1-4419-7756-4_38
Zhang, Q., Fan, Z., Xue, W., Sun, F., Zhu, H., Huang, D., et al. (2021). Vitexin Regulates Epac and NLRP3 and Ameliorates Chronic Cerebral Hypoperfusion Injury. Can. J. Physiol. Pharmacol. 99 (10), 1079–1087. doi:10.1139/cjpp-2021-0034
Zhang, X.-Y., Shen, J., Zhou, Y., Wei, Z.-P., and Gao, J.-M. (2016). Insecticidal Constituents from Buddlej Aalbiflora Hemsl. Nat. Product. Res. 31 (12), 1446–1449. doi:10.1080/14786419.2016.1247080
Zhang, X., Li, P., Guo, S., Wang, S., and Liu, D. (2018). Quantitation of β-carboline and Quercetin in alligator weed (Alternanthera Philoxeroides (Mart.) Griseb.) by LC-MS/MS and Evaluation of Cardioprotective Effects of the Methanol Extracts. Drug Discoveries Ther. 12 (6), 341–346. doi:10.5582/ddt.2018.01070
Zhang, Z., Wu, X., Cao, S., Cromie, M., Shen, Y., Feng, Y., et al. (2017). Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice. Nutrients 9 (7), 1. doi:10.3390/nu9070677
Zhao, B., Su, B., Zhang, H., Liu, W., Du, Q., and Li, Y. (2019). Antiurolithiatic Effect of Ferulic Acid on Ethylene Glycolinduced Renal Calculus in Experimental Rats. Trop. J. Pharm. Res. 18 (1), 1. doi:10.4314/tjpr.v18i1.16
Zhao, D., Zheng, L., Qi, L., Wang, S., Guan, L., Xia, Y., et al. (2016). Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum Horneri. Mar. Drugs 14 (7), 1. doi:10.3390/md14070123
Zhao, N., Dong, Q., Fu, X.-X., Du, L.-L., Cheng, X., Du, Y.-M., et al. (2014). Acacetin Blocks Kv1.3 Channels and Inhibits Human T Cell Activation. Cell Physiol. Biochem. 34 (4), 1359–1372. doi:10.1159/000366343
Zhao, T., Ding, K.-m., Zhang, L., Cheng, X.-m., Wang, C.-h., and Wang, Z.-t. (2013). Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities Ofβ-Carboline and Quinoline Alkaloids Derivatives from the Plants of GenusPeganum. J. Chem. 2013, 1–6. doi:10.1155/2013/717232
Zhao, Y., Wang, J., Ballevre, O., Luo, H., and Zhang, W. (2011). Antihypertensive Effects and Mechanisms of Chlorogenic Acids. Hypertens. Res. 35 (4), 370–374. doi:10.1038/hr.2011.195
Zhou, B.-N., Blaskò, G., and Cordell, G. A. (1988). Alternanthin, A C-Glycosylated Flavonoid from Alternanthera Philoxeroides. Phytochemistry 27 (11), 3633–3636. doi:10.1016/0031-9422(88)80781-7
Zhu, Q., Mao, L.-N., Liu, C.-P., Sun, Y.-H., Jiang, B., Zhang, W., et al. (2016). Antinociceptive Effects of Vitexin in a Mouse Model of Postoperative Pain. Scientific Rep. 6 (1), 1. doi:10.1038/srep19266
Keywords: alternanthera, anticancer, antidiabetic, antimicrobial, flavonoids, triterpenoid saponins, natural products (NP)
Citation: Singla RK, Dhir V, Madaan R, Kumar D, Singh Bola S, Bansal M, Kumar S, Dubey AK, Singla S and Shen B (2022) The Genus Alternanthera: Phytochemical and Ethnopharmacological Perspectives. Front. Pharmacol. 13:769111. doi: 10.3389/fphar.2022.769111
Received: 01 September 2021; Accepted: 21 February 2022;
Published: 11 April 2022.
Edited by:
Patrícia Mendonça Rijo, Universidade Lusófona, PortugalReviewed by:
Mohamed L. Ashour, Ain Shams University, EgyptSwapnil Sharma, Banasthali Vidyapith, India
Copyright © 2022 Singla, Dhir, Madaan, Kumar, Singh Bola, Bansal, Kumar, Dubey, Singla and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bairong Shen, YmFpcm9uZy5zaGVuQHNjdS5lZHUuY24=; Reecha Madaan, cmVlY2hhLm1hZGFuQGNoaXRrYXJhLmVkdS5pbg==
†These authors have contributed equally to this work and share first authorship
 Rajeev K. Singla
Rajeev K. Singla Vivek Dhir
Vivek Dhir Reecha Madaan
Reecha Madaan Deepak Kumar
Deepak Kumar Simranjit Singh Bola5
Simranjit Singh Bola5 Suresh Kumar
Suresh Kumar Ankit Kumar Dubey
Ankit Kumar Dubey Shailja Singla
Shailja Singla Bairong Shen
Bairong Shen