- 1The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Department of Cardiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Shandong Key Laboratory of Cardiovascular Proteomics, Department of Geriatric Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 3Department of Cardiology, Zibo Central Hospital, Zibo, Shandong, China
- 4Department of General Practice, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
Background: The (R)-CDOP combination regimen, based on pegylated liposomal doxorubicin, is increasingly used for elderly patients with non-Hodgkin’s lymphoma. However, the cardiotoxicity and efficacy of the (R)-CDOP regimen compared with conventional anthracyclines have not been demonstrated in the general population. Therefore, this systematic review and meta-analysis evaluated the risk of cardiotoxicity and efficacy associated with the (R)-CDOP regimen in patients with non-Hodgkin’s lymphoma.
Methods: PubMed, Embase, Cochrane Library, CNKI, WanFang Database, and VIP were searched. The search covered the period from the start of the clinical use of (R)-CDOP to April 2022. We searched the literature for cardiovascular adverse events associated with (R)-CDOP in non-Hodgkin’s lymphoma. The data were analyzed using R 4.2.0 and Stata 12.0.
Results: From the included studies, the important findings were as follows: total cardiovascular event rate, 7.45% (95% confidence interval [CI] = 4.86%–10.44%); non-serious cardiovascular adverse event rate, 6.48% (95% CI = 3.70%–9.8%); serious cardiovascular adverse event rate, 0.67% (95% CI = 0.00%–2.12%); heart failure rate, 0.55% (95% CI = 0.00%–1.93%); rate of treatment discontinuation attributable to left ventricular dysfunction or heart failure, 0.02% (95% CI = 0.00%–0.57%); and cardiovascular death rate, 0.00% (95% CI = 0.00%–0.37%). Compared with the (R)-CHOP regimen, the (R)-CDOP regimen reduced the risk of cardiovascular events, including total cardiovascular adverse events (odds ratio [OR] = 0.161, 95% CI = 0.103–0.251, p < 0.001, and NNT = 3.7), non-serious cardiovascular adverse events (OR = 0.171, 95% CI = 0.093–0.314, p < 0.001, and NNT = 3.6), serious cardiovascular adverse events (OR = 0.252, 95% CI = 0.119–0.535, p < 0.001, and NNT = 6.8), and heart failure (OR = 0.294, 95% CI = 0.128–0.674, p = 0.004, and NNT = 9.5). To evaluate the survival benefits, we compared (R)-CDOP and (R)-CHOP regimens. We found that the (R)-CDOP regimen was no less efficacious, including complete remission (CR) (OR = 1.398, 95% CI = 0.997–1.960, and p = 0.052), partial response (PR) (OR = 1.631, 95% CI = 1.162–2.289, and p = 0.005), objective response rate (ORR) (OR = 2.236, 95% CI = 1.594–3.135, and p < 0.001), stable disease (SD) (OR = 0.526, 95% CI = 0.356–0.776, and p = 0.001), and progressive disease (PD) (OR = 0.537, 95% CI = 0.323–0.894, and p = 0.017).
Conclusion: Our findings suggested that the (R)-CDOP regimen had a lower risk of cardiovascular adverse events in non-Hodgkin’s lymphoma than the (R)-CHOP regimen, demonstrating its safety with regard to cardiotoxicity. In addition, this study found the (R)-CDOP regimen was no less efficacious than the (R)-CHOP regimen in the treatment of non-Hodgkin’s lymphoma. These findings need to be validated by higher-quality research because of the limited number and quality of included studies.
Introduction
Non-Hodgkin’s lymphoma (NHL) is a lymphoid malignancy with a variety of biological and clinical behaviors, usually involving lymphatic and hematopoietic tissues but also other organs (Ganapathi, Brown, Prakash, & Bhargava, 2021). Most patients usually have persistent painless lymphadenopathy, but some patients also develop systemic symptoms such as night sweats, persistent fever, and unexplained weight loss (Bowzyk Al-Naeeb, Ajithkumar, Behan, & Hodson, 2018). NHL is the most common hematological malignancy worldwide, accounting for nearly 3% of all cancer diagnoses, and its average age of diagnosis is 67 years (Thandra et al., 2021). Patients with NHL are generally elderly, and they often have cardiovascular risk factors or a history of heart disease, making them more susceptible to cardiotoxicity caused by chemotherapeutic drugs (Abuamsha, Kadri, & Hernandez, 2019). In addition to being a reason for treatment discontinuation, severe cardiotoxicity may be a reason for serious or even life-threatening events.
The (R)-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone) regimen, an anthracycline-based regimen, is often the first-line treatment for NHL, particularly for diffuse large B-cell lymphoma (Sehn & Salles, 2021). Cardiotoxicity is a particular complication of anthracyclines. The cumulative dose of anthracyclines has been reported consistently as an important factor causing cardiotoxicity. Swain SM et al. found that if the dosage of anthracyclines was greater than 550 mg/m2, it would cause relevant cardiomyopathy in 26% of patients (Swain, Whaley, & Ewer, 2003). At present, the main measure to prevent related cardiotoxicity is to reduce the cumulative dose to less than 450 mg/m2, but this also limits efficacy and cannot prevent all cardiovascular adverse events (Jones et al., 2021). Due to the uncertainty of the predictors of cardiotoxicity, the optimal monitoring strategy has not been agreed upon. Therefore, the individual management of chemotherapy drugs is still challenging. Doxorubicin liposome is a nanoparticle-based anti-tumor drug approved by the FDA and has been widely used to treat a variety of tumors (Herrmann et al., 2022). However, their clinical application has been limited by their structural instability, drug leakage, short shelf life, and poor tissue targeting. Pegylated liposomal doxorubicin (PLD) is a new type of liposome, in which macromolecular polyethylene glycol (PEG) is coated with doxorubicin on the surface, which makes the release rate of doxorubicin from the heart much lower than that from other tissues, effectively reducing the cardiotoxicity of doxorubicin (O'Brien et al., 2004; Theodoulou & Hudis, 2004). PLD is increasingly used to treat elderly patients with NHL, providing a new strategy for elderly patients. Despite the good safety of PLD and the widespread use of (R)-CDOP in the population, it is unknown whether the use of multiple drugs increases the risk of cardiotoxicity compared with that of PLD alone.
To obtain better scientific evidence, we conducted a meta-analysis to assess the risk of cardiovascular adverse events associated with the (R)-CDOP regimen. Furthermore, we explored the cardiovascular adverse event risk and efficacy of the (R)-CDOP regimen compared with the (R)-CHOP regimen.
Methods
Search strategy and study selection
PubMed, Embase, Cochrane Library, CNKI, WanFang Database, and VIP were searched. The search covered the period from the start of the clinical use of the (R)-CDOP regimen to April 2022. English databases were searched using combinations of the following keywords: “lymphoma,” “pegylated liposomal doxorubicin,” and “liposome.” The search terms for Chinese databases included “lymphoma” and “liposome.” The complete search string is provided in the Supplementary Appendix S1 (p 1). There was no language restriction. Literature tracing was performed using the references in the included studies. The complete inclusion and exclusion criteria are provided in the Supplementary Appendix S1 (p 2).
Outcomes
The outcomes included non-serious and serious cardiovascular adverse events. Non-serious cardiovascular adverse events included grade 1–2 cardiovascular adverse events (preferably using a well-established toxicity grading system to quantify severity) and grade 1–2 cardiovascular adverse events according to the CTCAE 5.0 (if the cardiovascular adverse events were not graded). Cardiovascular adverse events were considered serious if at least one of the following outcomes could be extracted: grade 3–4 cardiovascular adverse events (preferably using a well-established toxicity grading system to quantify severity), grade 3–4 cardiovascular adverse events according to the CTCAE 5.0 (if the cardiovascular adverse events were not graded), heart failure (10% decrease in the LVEF from the baseline to <53% (Herrmann, 2020)), cardiac function (grade III + IV), interruption of therapy because of the left ventricular dysfunction or heart failure, and treatment-related cardiovascular death.
Data extraction, evaluation, and synthesis
Two reviewers independently completed the literature search and literature screening using EndNote X9 literature management software. The reviewers used a pre-designed Excel sheet for literature extraction. Additional data were collected from the eligible studies, including first author, publication time, study region, study type, median age, the proportion of female patients, disease subtype, treatment period, treatment interval, median follow-up time, cardiotoxicity grading system, and outcome indicators. Disagreements throughout the process were resolved with a third reviewer.
Quality assessment in individual studies
The methodological index for non-randomized controlled studies (MINORS) was used to evaluate the quality of the included studies (Slim et al., 2003). For this index, studies with scores of 0–8 points were graded C, those with scores of 9–16 points were graded B, and those with scores of 17–24 points were graded A. The classification indices are described in detail in the Supplementary Appendix S1. The complete evaluation is detailed in the Supplementary Appendix S1 (p 3).
Risk of bias
Since the included studies primarily assessed the effectiveness of chemotherapy regimens rather than the occurrence of adverse effects, incomplete outcome data and selective reporting were considered important potential sources of bias. Therefore, we did not use the traditional Cochrane risk-of-bias tool to assess bias in the studies. Since the outcomes of this study were cardiovascular adverse events, we performed a quality assessment for cardiovascular adverse events (Linschoten et al., 2020). Specific information on the risk of bias is presented in the Supplementary Appendix S1 (p 4). The funnel plots of the publication bias have been included, which allows a better evaluation of the homogeneity of its distribution. Specific information on the risk of publication bias is presented in the appendix (p 5).
Statistical analyses
All statistical analyses were conducted using R (version 4.2.0) and Stata (version 12.0). To estimate the incidence of cardiovascular adverse events in patients treated with (R)-CDOP, a double-arcsine transformation was used to obtain a normal distribution appropriate for pooling, with the proportion estimates expressed as 95% confidence intervals (CIs). The (R)-CDOP regimen was compared with the (R)-CHOP regimen. Dichotomous variables were expressed as odds ratios (ORs) and 95% CIs. The study heterogeneity was assessed using the Q-test, with I2 > 50% indicating high heterogeneity and I2 < 50% indicating low heterogeneity.
Results
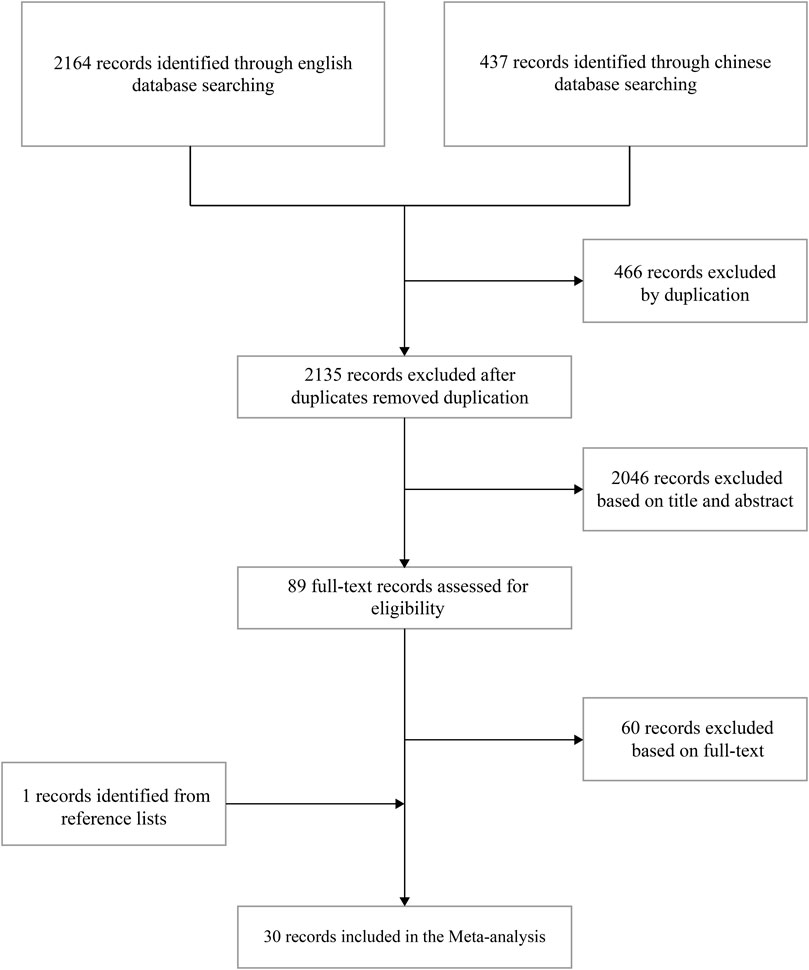
In total, 2,601 studies were retrieved (PubMed, n = 30; Embase, n = 599; Cochrane Library, n = 1,535; CNKI, n = 181; WanFang Database, n = 181; and VIP, n = 75). Through literature screening, 30 studies were evaluated for eligibility. The specific literature screening process is presented in Figure 1.
General characteristics of studies included in the meta-analysis
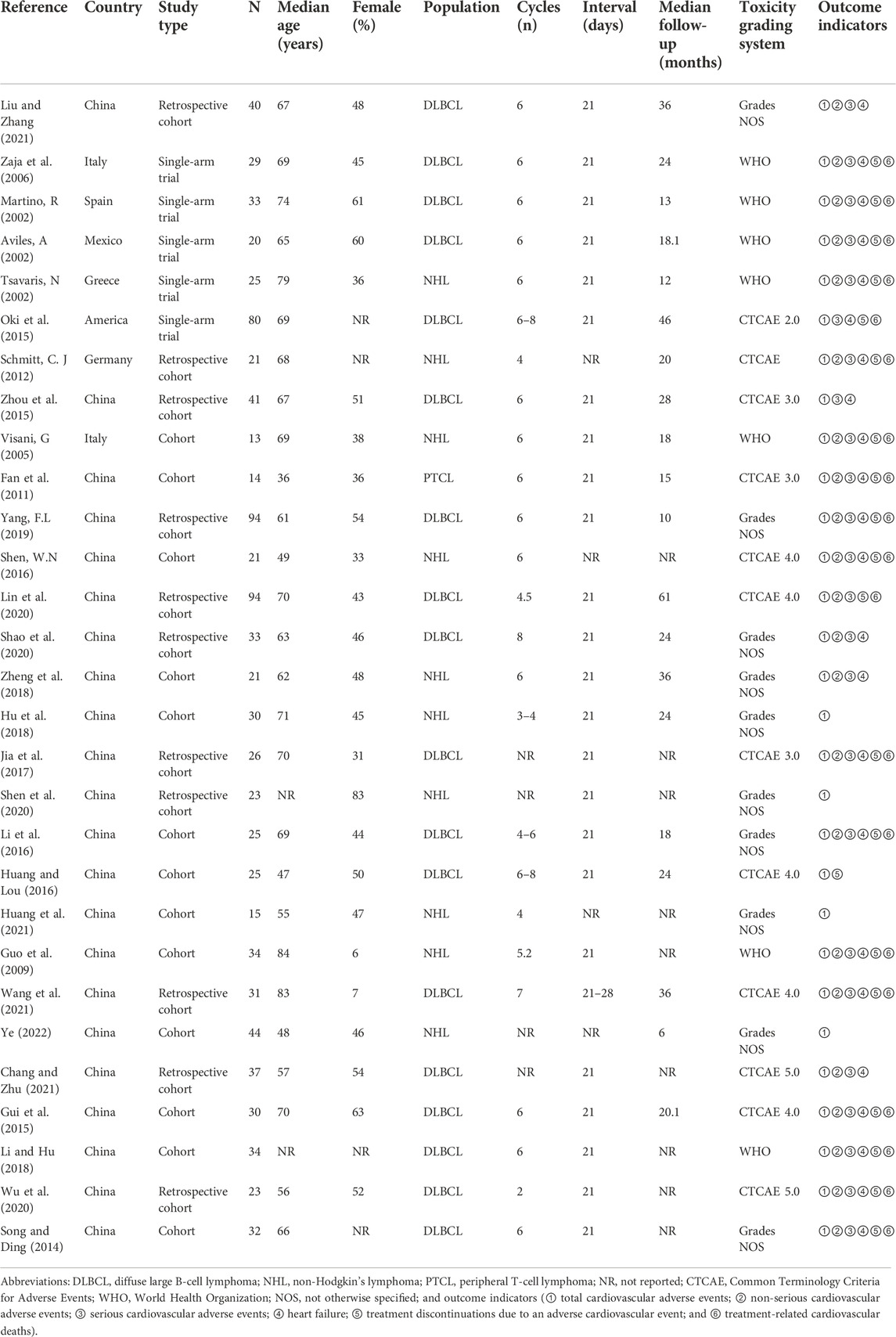
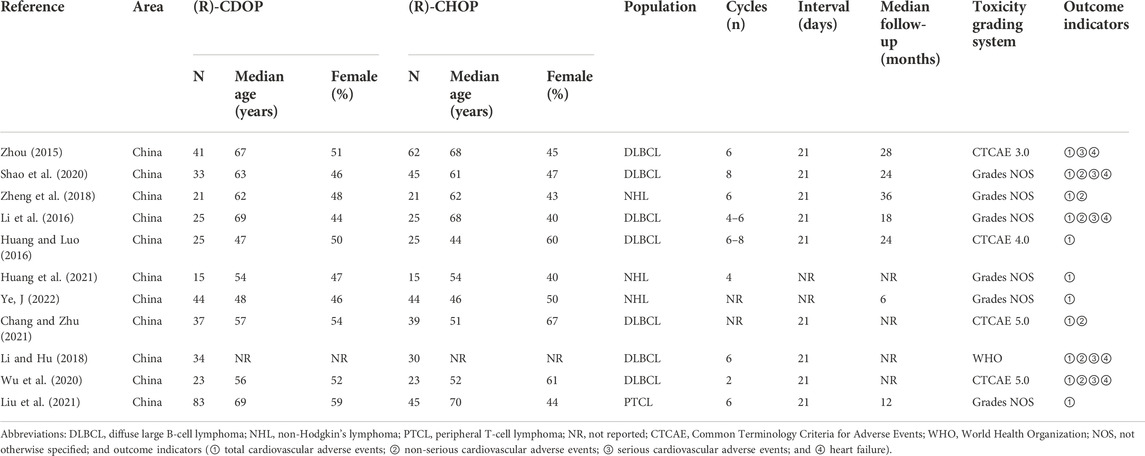
We conducted a meta-analysis to examine the incidence of cardiovascular adverse events associated with the (R)-CDOP regimen. Thirty publications involving 1,071 patients were included. The basic characteristics of the study population are presented in Table 1. To further compare cardiovascular adverse event rates between the (R)-CDOP and (R)-CHOP regimens, 11 publications with a total of 381 patients in the (R)-CDOP group and 374 patients in the (R)-CHOP group were included. The main population characteristics are presented in Table 2.
Literature evaluation
We assessed the quality of 30 studies using the MINORS scale. Sixteen studies were single-arm studies without a control group, and 14 studies had a control group. In total, 9, 19, and 2 studies were graded A, B, and C, respectively. The results of the quality assessment are presented in the Supplementary Appendix S1 (p 6). The results of the risk of bias are also presented in the Supplementary Appendix S1 (p 7 and p 8).
Meta-analysis for cardiovascular adverse events
Incidence of cardiovascular adverse events with the (R)-CDOP combination regimen
In the analysis of total cardiovascular adverse events, 30 studies with 1,071 patients were included in the analysis. The study heterogeneity was high (τ2 = 0.0099, I2 = 59.2%, and p < 0.0001). The total cardiovascular adverse event rate was 7.45% (95% CI = 4.86%–10.44%; Figure 2).
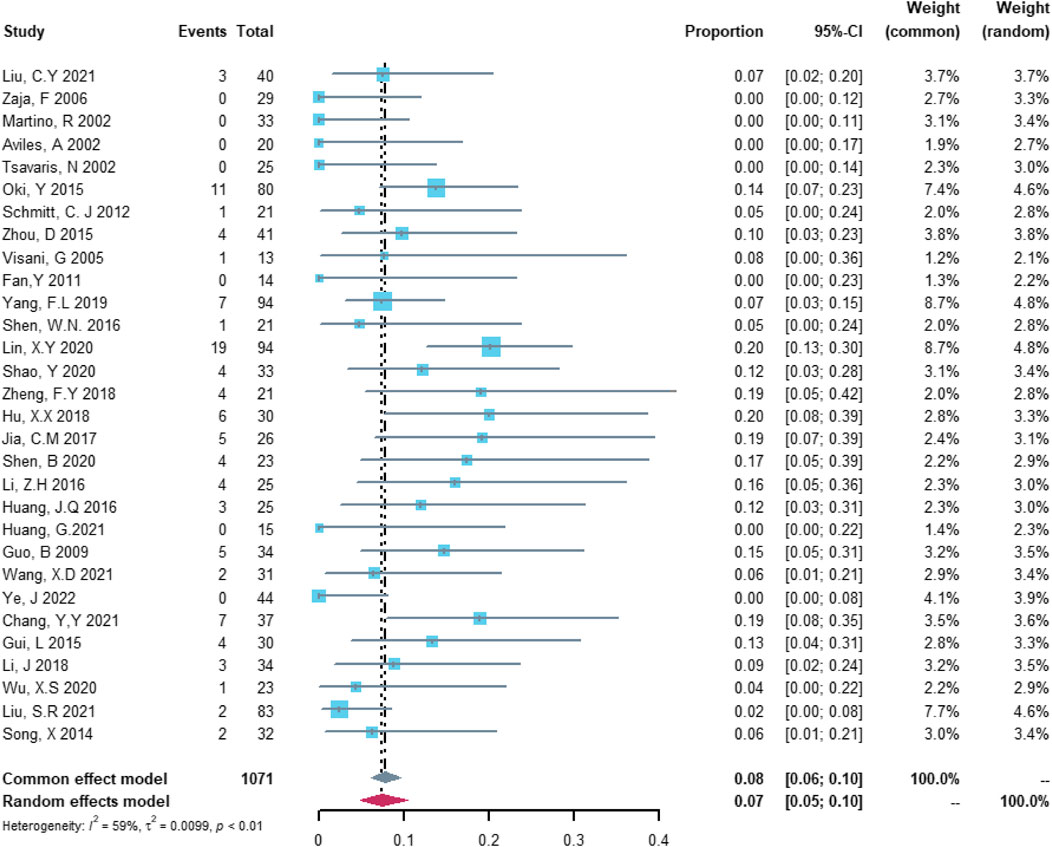
FIGURE 2. Summary pooled proportion analysis of total cardiovascular adverse events associated with the (R)-CDOP combination regimen.
In the analysis of non-serious cardiovascular adverse events, 22 studies with 730 patients were included. The study heterogeneity was high (τ2 = 0.0091, I2 = 55.5%, and p = 0.0009). The non-serious cardiovascular adverse event rate was 6.48% (95% CI = 3.70%–9.8%; Figure 3).
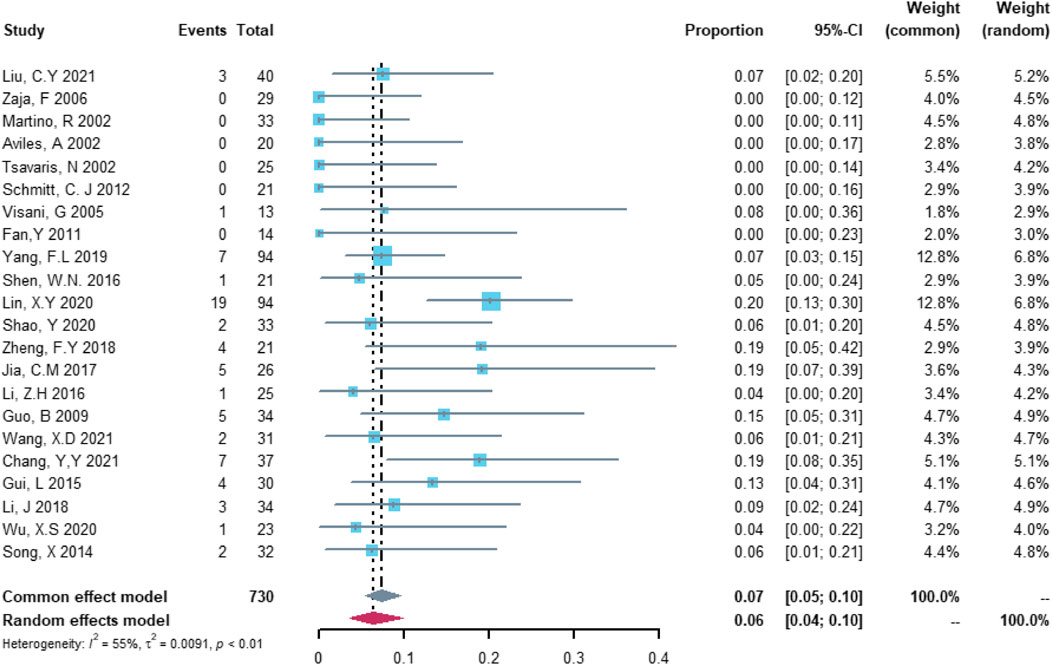
FIGURE 3. Summary pooled proportion analysis of non-serious cardiovascular adverse events associated with the (R)-CDOP combination regimen.
Twenty-four studies with 851 patients were included in the analysis of serious cardiovascular adverse events. The study heterogeneity was low (τ2 = 0.0065, I2 = 49.2%, and p = 0.0037). The serious cardiovascular adverse event rate was 0.67% (95% CI = 0.00%–2.12%; Figure 4).
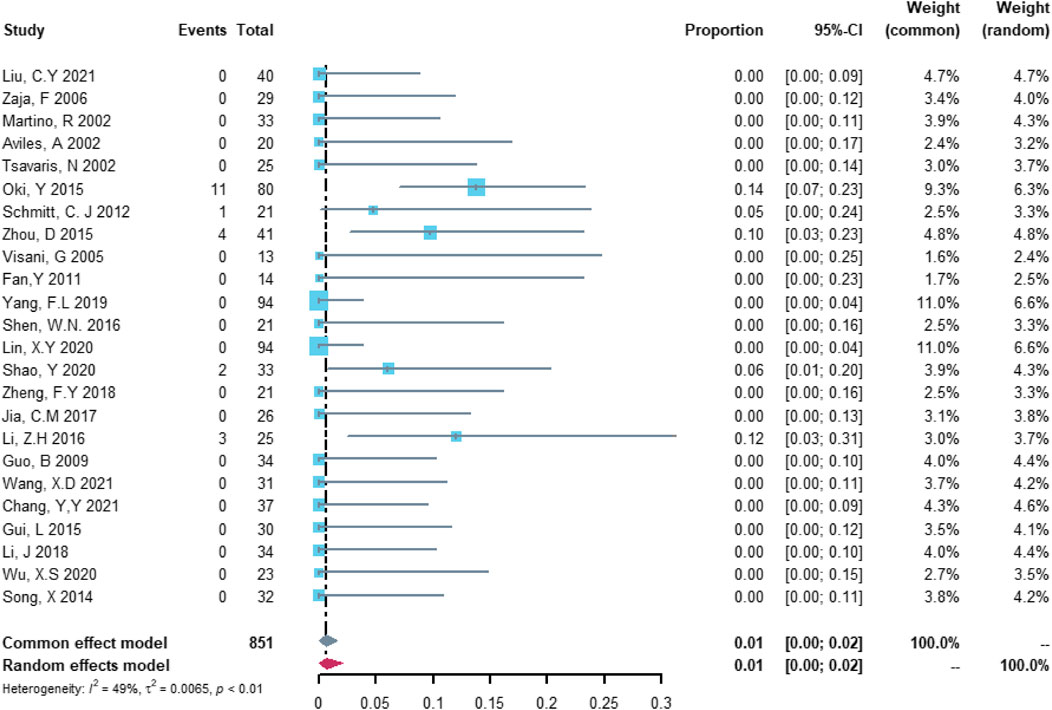
FIGURE 4. Summary pooled proportion analysis of serious cardiovascular adverse events associated with the (R)-CDOP combination regimen.
Twenty-three studies with 757 patients were included in the analysis of heart failure. The study heterogeneity was low (τ2 = 0.0054, I2 = 40.2%, and p = 0.0249). The heart failure rate was 0.55% (95% CI = 0.00%–1.93%; Figure 5).
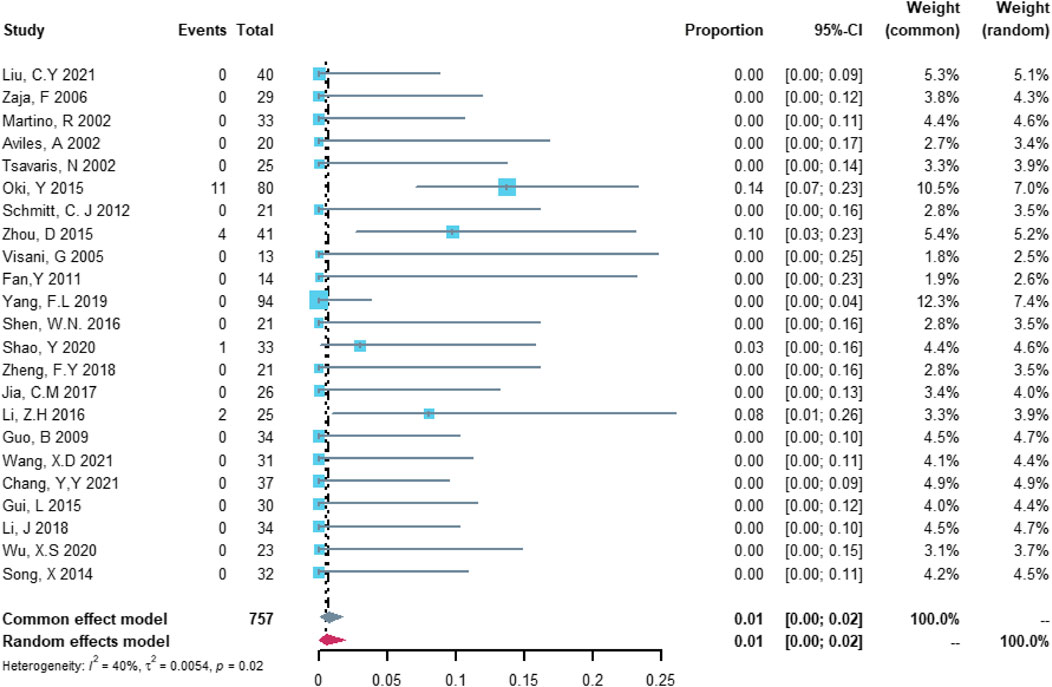
FIGURE 5. Summary pooled proportion analysis of heart failure cardiovascular adverse events associated with the (R)-CDOP combination regimen.
Twenty studies with 704 patients were included in the analysis of treatment discontinuation attributable to left ventricular dysfunction or heart failure. The study heterogeneity was low (τ2 = 0.0000, I2 = 0.0%, and p = 0.9999). The treatment discontinuation rate was 0.02% (95% CI = 0.00%–0.57%; Figure 6).
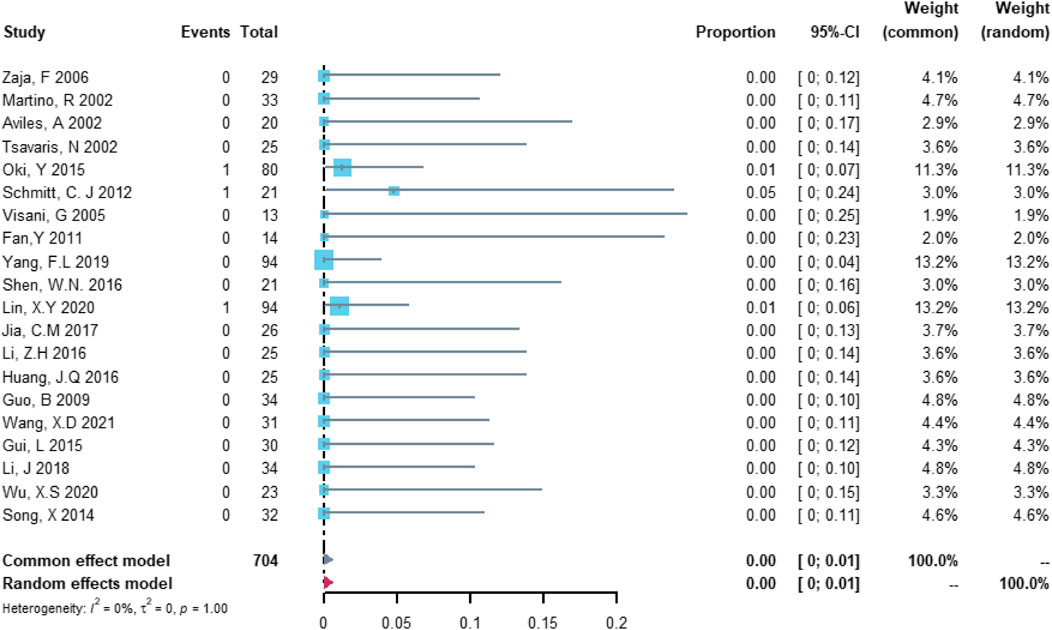
FIGURE 6. Summary pooled proportion analysis of treatment discontinuation events due to the left ventricular dysfunction or heart failure associated with the (R)-CDOP combination regimen.
Twenty studies with 704 patients were included in the analysis of cardiovascular death. The study heterogeneity was low (τ2 = 0.0000, I2 = 0.0%, and p = 0.9997). The cardiovascular death rate was 0.00% (95% CI = 0.00%–0.37%; Figure 7).
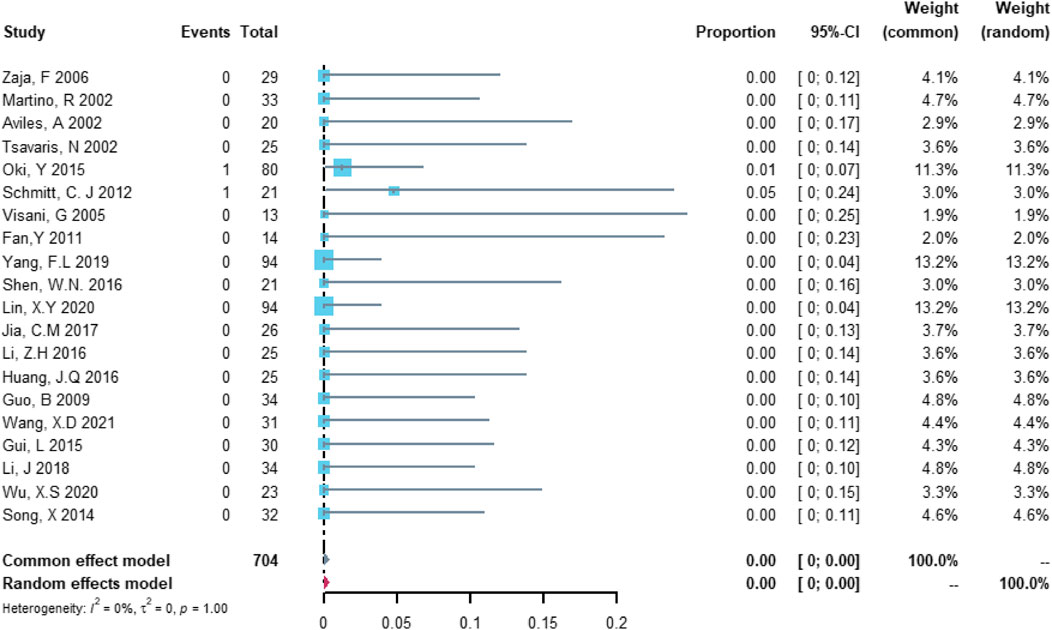
FIGURE 7. Summary pooled proportion analysis of cardiovascular death events associated with the (R)-CDOP combination regimen.
Comparison of the risk of cardiovascular adverse events between the (R)-CDOP and (R)-CHOP regimens
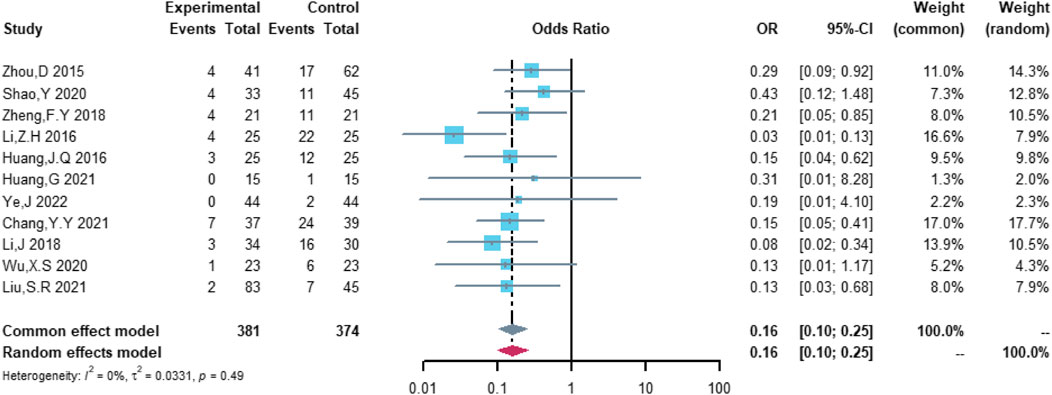
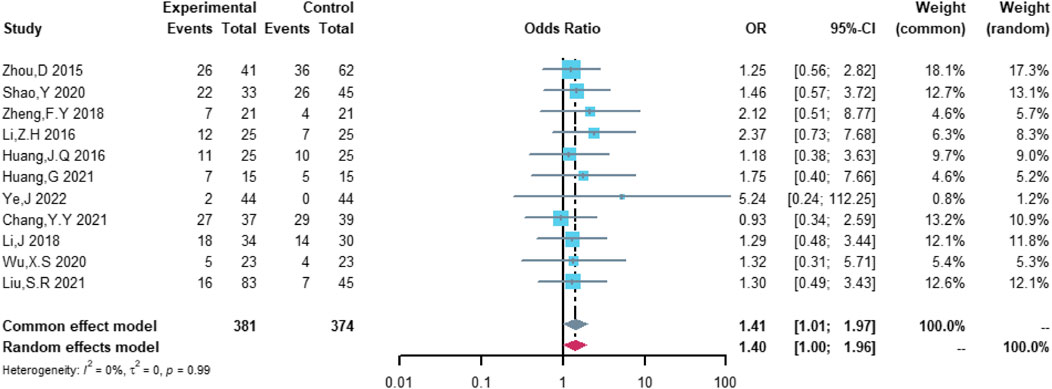
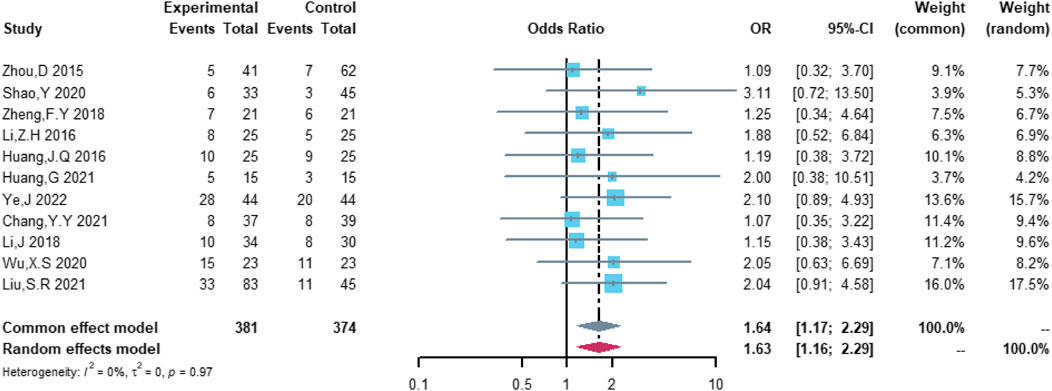
Eleven studies were included in the analysis of total cardiovascular adverse events, including 381 patients in the (R)-CDOP group and 374 patients in the (R)-CHOP group. The study heterogeneity was low (τ2 = 0.0331, I2 = 0.0%, and p = 0.487). (R)-CDOP was linked to a lower risk of total cardiovascular adverse events than (R)-CHOP (OR = 0.161, 95% CI = 0.103–0.251, p = 0.000, and NNT = 3.7; Figure 8).
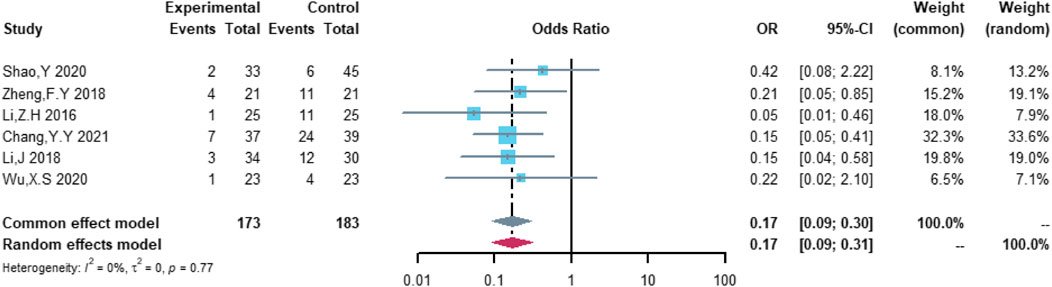
A total of six studies were included in the analysis of non-serious cardiovascular adverse events, including 173 patients in the (R)-CDOP group and 183 patients in the (R)-CHOP group. The study heterogeneity was low (τ2 = 0.0000, I2 = 0.0%, and p = 0.770). (R)-CDOP reduced the risk of non-serious cardiovascular adverse events vs. (R)-CHOP (OR = 0.171, 95% CI = 0.093–0.314, p = 0.000, and NNT = 3.6; Figure 9).
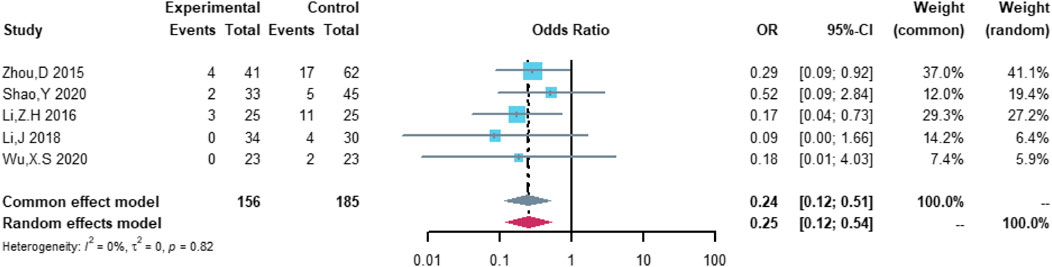
Five studies were included in the analysis of serious cardiovascular adverse events, including 156 patients in the (R)-CDOP group and 185 patients in the (R)-CHOP group. The study heterogeneity was low (τ2 = 0.0000, I2 = 0.0%, and p = 0.819). (R)-CDOP reduced the risk of serious cardiovascular adverse events vs. (R)-CHOP (OR = 0.252, 95% CI = 0.119–0.535, p = 0.000, and NNT = 6.8; Figure 10).
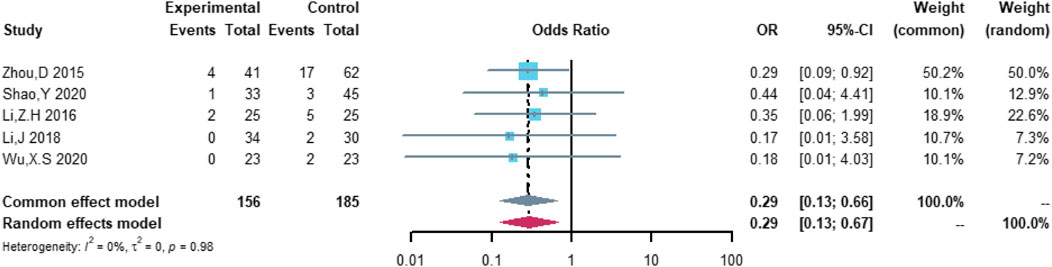
Five studies were included in the analysis of heart failure, including 156 patients in the (R)-CDOP group and 185 patients in the (R)-CHOP group. The study heterogeneity was low (τ2 = 0.0000, I2 = 0.0%, and p = 0.984). (R)-CDOP was linked to a lower risk of heart failure than (R)-CHOP (OR = 0.294, 95% CI = 0.128–0.674, p = 0.004, and NNT = 9.5; Figure 11).
Comparison of the efficacy between the (R)-CDOP and (R)-CHOP regimens
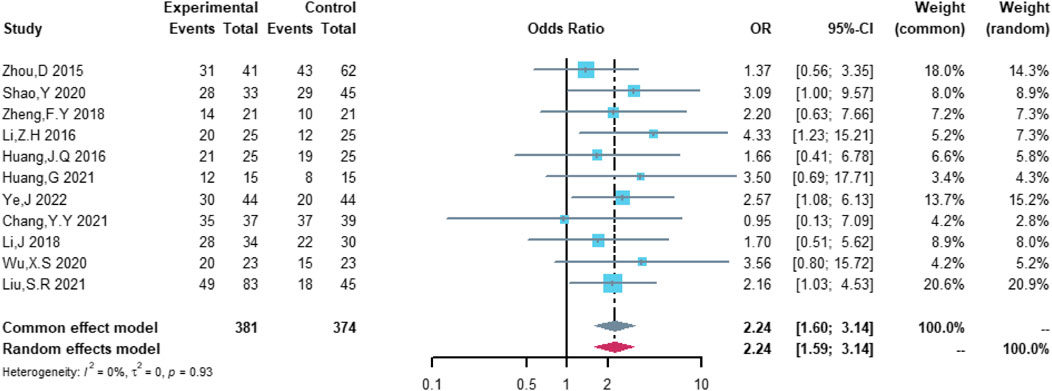
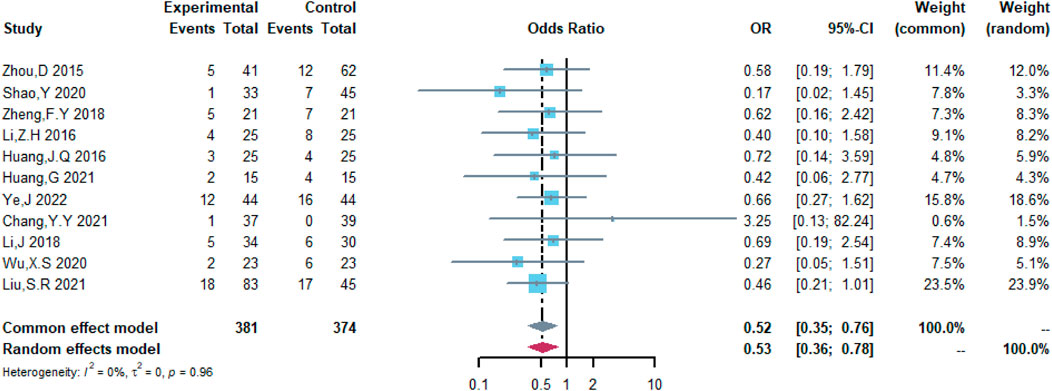
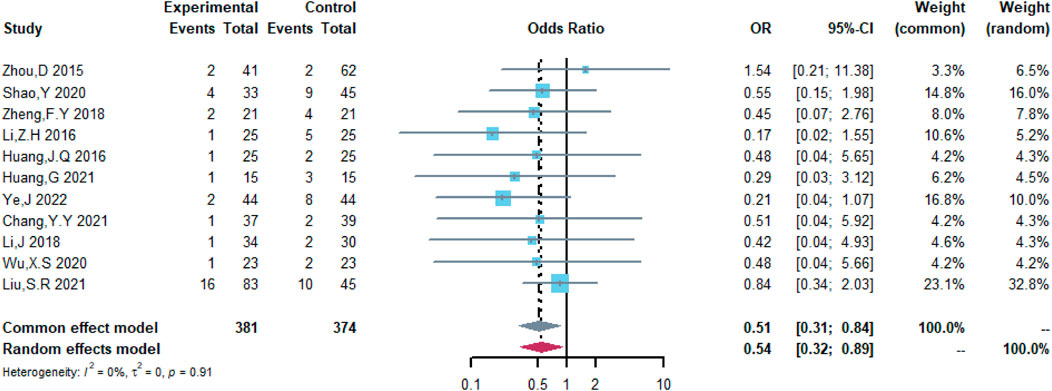
Therefore, the study was designed to compare (R)-CDOP and (R)-CHOP regimens on clinical outcomes by systematically calculating the survival benefits. Eleven studies were included in the analysis of efficacy, including 381 patients in the (R)-CDOP group and 374 patients in the (R)-CHOP group. We found that the (R)-CDOP regimen is better than the (R)-CHOP regimen, but there was no significant difference in terms of CR (OR = 1.398, 95% CI = 0.997–1.960, and p = 0.052; Figure 12). The (R)-CDOP regimen had a better OR than the (R)-CHOP regimen (OR = 1.631, 95% CI = 1.162–2.289, and p = 0.005; Figure 13). We also found that the ORR had similar changes (OR = 2.236, 95% CI = 1.594–3.135, and p < 0.001; Figure 14). In addition, we evaluated the SD and PD, and then, we found that the (R)-CDOP regimen had a lower risk than the (R)-CHOP regimen in terms of SD and PD (OR = 0.526, 95% CI = 0.356–0.776, and p = 0.001, Figure 15; OR = 0.537, 95% CI = 0.323–0.894, and p = 0.017, Figure 16, respectively). These findings indicated that the (R)-CDOP regimen might maintain efficacy and could be considered for patients with NHL.
Discussion
This study investigated the rate of cardiovascular adverse events with the (R)-CDOP regimen in patients with NHL. The study revealed that the (R)-CDOP combination carried a lower risk of cardiovascular adverse events than the (R)-CHOP regimen, indicating the cardiovascular safety of (R)-CDOP. In addition, the (R)-CDOP regimen might improve the prognosis compared with the (R)-CHOP regimen, providing more options for patients in the future.
Anthracyclines apply to the treatment of various types of cancers alone or in combination with other anti-tumor drugs. Mitochondrial dysfunction (Varricchi et al., 2018), disruption of calcium homeostasis (Tscheschner et al., 2019), and apoptosis-related protein production (Merten, Jiang, Feng, & Kang, 2006; Saleme et al., 2019) are important mechanisms of the cardiotoxic effects of anthracyclines. The cardiotoxicity caused by cumulative doses of these drugs is a major limiting factor in their application (Martins-Teixeira & Carvalho, 2020). A study showed that the incidence of left ventricular dysfunction was 10%, 16%, 32%, and 65% at the cumulative doses of doxorubicin of 250, 300, 400, and 550 mg/m2, respectively (Bernstein, 2018). According to the guidelines of the American Society of Clinical Oncology (ASCO), the cumulative dose of doxorubicin ≥ 250 mg/m2 is considered a high dose (Armenian et al., 2017). To mitigate this cardiotoxicity, novel anthracyclines are increasingly used in the treatment of cancer. Although the long-term cardiotoxicity of these drugs is unclear, studies revealed that anthracycline liposomes, particularly PLD, demonstrated cardiac safety for patients (X. R. Li, Cheng, Zhang, Wang, & Huang, 2022). Yamaguchi et al. revealed that doxorubicin liposomes were less cardiotoxic than conventional doxorubicin in a meta-analysis (Yamaguchi et al., 2015). L Ansari et al. also showed lower cardiotoxicity than traditional doxorubicin in women with metastatic breast cancer treated with pegylated liposomal doxorubicin alone (Ansari et al., 2017). The rate of cardiovascular adverse events in patients who received PLD-based combination therapy for NHL has not been studied adequately. The majority of people in this study were older than 60 years. The overall cardiovascular adverse event rate was 7.45%, indicating the safety of the (R)-CDOP regimen in the elderly population.
In the clinical use of chemotherapeutic agents, potentially lethal toxicity can lead to serious cardiovascular adverse events such as myocardial infarction, heart failure, cardiac arrest, and cardiogenic death. Therefore, we need to further investigate the severity of cardiovascular adverse events associated with (R)-CDOP (Caspani et al., 2021). Due to the differences in the incidence and severity of cardiotoxicity, a general tool is required to assess their severity, which includes the World Health Organization (WHO) and the Common Terminology Criteria for Adverse Events (CTCAE). Grade 3 and higher cardiovascular events often require immediate treatment (Atkinson et al., 2016). We classified cardiovascular events of grade 3 or higher, treatment discontinuation attributable to cardiovascular events, and death caused by cardiovascular events as serious cardiovascular adverse events. We found that the serious adverse cardiovascular event rate was 0.67%, including a heart failure rate of 0.55%, indicating that serious cardiovascular events are not likely to occur in clinical practice. Our study further found that the (R)-CDOP regimen had a 0.02% rate of treatment interruption attributable to left ventricular dysfunction or heart failure, and no deaths attributable to cardiovascular events occurred. This suggests that the (R)-CDOP regimen can significantly prolong patient survival by avoiding treatment interruption or death attributable to cardiotoxicity, thus providing greater benefit to patients.
The (R)-CDOP regimen is often used as an alternative to the (R)-CHOP regimen. However, the cardiac safety of the former regimen is unclear in the overall NHL treatment population. Further studies are still needed to determine whether the multidrug combinations increase cardiotoxicity. This study revealed that (R)-CDOP carried lower risks of total (OR = 0.161) and non-serious cardiovascular adverse events (OR = 0.171) than the (R)-CHOP group, implying a reduced risk of non-fatal cardiotoxicities, such as ECG abnormalities that do not require treatment, asymptomatic cardiac insufficiency, and minor abnormal cardiac laboratory results, for (R)-CDOP. Serious cardiovascular adverse events such as refractory heart failure or other difficult-to-control cardiac symptoms place a large burden on families and the entire healthcare system, which requires us to further explore the severity of cardiovascular adverse events. This study recorded lower risks of serious cardiovascular adverse events (OR = 0.252) and heart failure (OR = 0.294) for (R)-CDOP, providing evidence of its cardiovascular safety in the treatment of NHL. In addition, the meta-analysis of the efficacy suggested that R-CDOP might provide another option for patients with NHL.
To mitigate the occurrence of serious cardiovascular adverse events, cardioprotective agents have been used in combination with anthracyclines. Dexrazoxane is the only drug approved by the FDA for the prevention of cardiotoxicity (Yu et al., 2020). Some clinical trials are currently investigating cardioprotective agents for anthracycline-induced cardiotoxicities, such as beta-blockers and angiotensin-converting enzyme inhibitors. Caspani et al. noted that RAAS blockers, beta-blockers, and aldosterone antagonists provided a statistically significant benefit in the prevention of reduced left ventricular ejection fraction, but this positive effect remains to be confirmed (Caspani et al., 2021). The OVERCOME trial showed that patients with hematological tumors who took enalapril or carvedilol had a lower frequency of heart failure, LVEF <45%, or sudden cardiac death compared with the placebo group (Bosch et al., 2013). Therefore, the use of combination chemotherapy and prevention of chemotherapy-related cardiotoxicity will require a concerted effort by cardiologists and hematologists.
This meta-analysis provided evidence for the cardiac safety of (R)-CDOP combination for NHL, but several limitations must be noted. First, the definition of cardiovascular adverse events was not uniform in some of the studies, which may have affected the incidence of the reported adverse events. Second, single-arm studies include some heterogeneity, which may have affected the accuracy of the results. Finally, some of the included studies had small sample sizes, and the studies did not provide detailed classifications of cardiovascular adverse events.
Conclusion
The (R)-CDOP regimen had a lower cardiovascular risk and was no less efficacious than (R)-CHOP in the treatment of NHL. This analysis suggested that (R)-CDOP therapy is useful in clinical practice and clinical reference. Due to the limitations regarding the number and quality of the included studies, these findings must be validated by additional high-quality studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
BL, JQ, and YL contributed to the literature review and preparation of the manuscript. MZ, LH, and ZW contributed to the study conception and design. BL, LS, and YM drafted the manuscript, revised it critically for important intellectual content, and gave approval for the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1060668/full#supplementary-material
References
Abuamsha, H., Kadri, A. N., and Hernandez, A. V. (2019). Cardiovascular mortality among patients with non-Hodgkin lymphoma: Differences according to lymphoma subtype. Hematol. Oncol. 37 (3), 261–269. doi:10.1002/hon.2607
Ansari, L., Shiehzadeh, F., Taherzadeh, Z., Nikoofal-Sahlabadi, S., Momtazi-Borojeni, A. A., Sahebkar, A., et al. (2017). The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: A systematic review of clinical trials. Cancer Gene Ther. 24 (5), 189–193. doi:10.1038/cgt.2017.9
Armenian, S. H., Lacchetti, C., Barac, A., Carver, J., Constine, L. S., Denduluri, N., et al. (2017). Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical Oncology clinical practice guideline. J. Clin. Oncol. 35 (8), 893–911. doi:10.1200/jco.2016.70.5400
Atkinson, T. M., Ryan, S. J., Bennett, A. V., Stover, A. M., Saracino, R. M., Rogak, L. J., et al. (2016). The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support. Care Cancer 24 (8), 3669–3676. doi:10.1007/s00520-016-3297-9
Avilés, A., Neri, N., Castañeda, C., Talavera, A., Huerta-Guzmán, J., and González, M. (2002). Pegylated liposomal doxorubicin in combination chemotherapy in the treatment of previously untreated aggressive diffuse large-B-cell lymphoma. Med. Oncol. 19 (1), 55–58. doi:10.1385/mo:19:1:55
Bernstein, D. (2018). Anthracycline cardiotoxicity: Worrisome enough to have you quaking? Circ. Res. 122 (2), 188–190. doi:10.1161/circresaha.117.312395
Bosch, X., Rovira, M., Sitges, M., Domènech, A., Ortiz-Pérez, J. T., de Caralt, T. M., et al. (2013). Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left ventricular dysfunction with enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of malignant hEmopathies). J. Am. Coll. Cardiol. 61 (23), 2355–2362. doi:10.1016/j.jacc.2013.02.072
Bowzyk Al-Naeeb, A., Ajithkumar, T., Behan, S., and Hodson, D. J. (2018). Non-Hodgkin lymphoma. Bmj 362, k3204. doi:10.1136/bmj.k3204
Caspani, F., Tralongo, A. C., Campiotti, L., Asteggiano, R., Guasti, L., and Squizzato, A. (2021). Prevention of anthracycline-induced cardiotoxicity: A systematic review and meta-analysis. Intern. Emerg. Med. 16 (2), 477–486. doi:10.1007/s11739-020-02508-8
Chang, Y. Y., and Zhu, B. (2021). Clinical evaluation of R-CDOP regimen containing pegylated liposomal doxorubicin in treatment of diffuse large B-cell lymphoma. J. Pract. Oncol. 36 (05), 429–434. doi:10.13267/j.cnki.syzlzz.2021.086
Fan, Y., Lin, N. M., Luo, L. H., Fang, L., Huang, Z. Y., Yu, H. F., et al. (2011). Pharmacodynamic and pharmacokinetic study of pegylated liposomal doxorubicin combination (CCOP) chemotherapy in patients with peripheral T-cell lymphomas. Acta Pharmacol. Sin. 32 (3), 408–414. doi:10.1038/aps.2010.217
Ganapathi, K. A., Brown, L. E., Prakash, S., and Bhargava, P. (2021). New developments in non-Hodgkin lymphoid malignancies. Pathology 53 (3), 349–366. doi:10.1016/j.pathol.2021.01.002
Gui, L., Shi, Y., K., Yang, J. L., Liu, P., and Qin, Y. (2015). Pegylated liposomal doxorubicin in CHOP regimen for untreated elderly patients with advanced diffuse large B-cell lymphoma: Results from a prospective phase II study. Chin. J. Clin. Oncol. 42 (03), 162–166. doi:10.3969/j.issn.1000-8179.20141732
Guo, G., Zhu, H. L., Li, S. X., Lu, X. C., and fan, H. (2009). Clinical study of doxorybicin hydrochloride liposome-containing regimens on elderly patients with non-Hodgkin’s lymphoma. Chin. J. Heal Care Med. 11 (04), 272–274.
Herrmann, J. (2020). Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 17 (8), 474–502. doi:10.1038/s41569-020-0348-1
Herrmann, J., Lenihan, D., Armenian, S., Barac, A., Blaes, A., Cardinale, D., et al. (2022). Defining cardiovascular toxicities of cancer therapies: An international cardio-oncology society (IC-OS) consensus statement. Eur. Heart J. 43 (4), 280–299. doi:10.1093/eurheartj/ehab674
Hu, X. X., Cheng, Y. X., and Ding, X. L. (2018). Clinical efficacy and safety of the first line of doxorubicin liposome (PLD) in elderly patients with lymphoma. Chin. Comm. Doc. 34 (10), 53–54.
Huang, G., Liao, J. J., and Huang, J., X. (2021). Evaluation of the clinical efficacy and safety of liposomal adriamycin in the treatment of malignant lymphoma. Contemp. Med. 27 (33), 150–151. doi:10.3969/j.issn.1009-4393.2021.33.063
Huang, J. Q., and Luo, B. H. (2016). A comparative study of liposomal doxorubicin and doxorubicin in the treatment of diffuse large B-cell lymphoma. Strait Pharm. J. Strait Pharm J 28 (07), 118–121. doi:10.3969/j.issn.1006-3765.2016.07.054
Jia, C. M., Bai, L. Y., Kong, L. Z., Han, J. C., and Liu, A. C. (2017). Therapeutic effects of liposomal doxorubicin in elderly patients with B-cell lymphoma. J. Harbin Med. Univ. J Harbin Med Univ 51 (04), 345–347.
Jones, R. L., Wagner, A. J., Kawai, A., Tamura, K., Shahir, A., Van Tine, B. A., et al. (2021). Prospective evaluation of doxorubicin cardiotoxicity in patients with advanced soft-tissue sarcoma treated in the ANNOUNCE phase III randomized trial. Clin. Cancer Res. 27 (14), 3861–3866. doi:10.1158/1078-0432.Ccr-20-4592
Li, J., and Hu, R. (2018). Efficacy and safety of R-CHOP regimen containing pegylated liposomal doxorubicin in the treatment of elderly patients with primary diffuse large B-cell lymphoma. Mod. Oncol. 26 (01), 0102–0105. doi:10.3969/j.issn.1672-4992.2018.01.027
Li, X. R., Cheng, X. H., Zhang, G. N., Wang, X. X., and Huang, J. M. (2022). Cardiac safety analysis of first-line chemotherapy drug pegylated liposomal doxorubicin in ovarian cancer. J. Ovarian Res. 15 (1), 96. doi:10.1186/s13048-022-01029-6
Li, Z. H., Xing, M. T., Zhang, Y. P., Wang, Y., and Zhan, X. R. (2016). Clinical efficiency and safety of the first-line CHOP regimen containing PLD applied to treat aged patients with advanced DLBCL. Zhongguo Shi Yan Xue Ye Xue Za Zhi 24 (3), 744–748. doi:10.7534/j.issn.1009-2137.2016.03.020
Lin, X., Y., Qi, F., Gui, L., Zhang, C., G., and Dong, M. (2020). The efficacy and safety profile of first- line R-CDOP like regimen in diffuse large B-cell lymphoma with cardiovascular diseases or high risk factor. Chin. Pharm. J. 55 (13), 1122–1127.
Linschoten, M., Kamphuis, J. A. M., van Rhenen, A., Bosman, L. P., Cramer, M. J., Doevendans, P. A., et al. (2020). Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (RCHOP): A systematic review and meta-analysis. The Lancet Haematology 7 (4), e295–e308. doi:10.1016/S2352-3026(20)30031-4
Liu, C., and Zhang, M. (2021). Analysis and evaluation of DRCOP scheme based on polyethylene glycol liposome doxorubicin in patients with diffuse large B-cell lymphoma. Am. J. Transl. Res. 13 (5), 5362–5367.
Liu, S. R., Zhao, J. L., Huang, J., and Luo, Y. Q. (2021). Analysis of the efficacy and adverse reactions of R- CHOP chemotherapy scheme containing pegylated liposomal doxorubicin in the treatment of peripheral T- cell lymphoma in elderly patients. Geriatr. Health Care 27 (05), 921–924. doi:10.3969/j.issn.1008-8296.2021.05.007
Martino, R., Perea, G., Caballero, M. D., Mateos, M. V., Ribera, J. M., de Oteyza, J. P., et al. (2002). Cyclophosphamide, pegylated liposomal doxorubicin (caelyx), vincristine and prednisone (CCOP) in elderly patients with diffuse large B-cell lymphoma: Results from a prospective phase II study. Haematologica 87 (8), 822–827.
Martins-Teixeira, M. B., and Carvalho, I. (2020). Antitumour anthracyclines: Progress and perspectives. ChemMedChem 15 (11), 933–948. doi:10.1002/cmdc.202000131
Merten, K. E., Jiang, Y., Feng, W., and Kang, Y. J. (2006). Calcineurin activation is not necessary for doxorubicin-induced hypertrophy in H9c2 embryonic rat cardiac cells: Involvement of the phosphoinositide 3-kinase-Akt pathway. J. Pharmacol. Exp. Ther. 319 (2), 934–940. doi:10.1124/jpet.106.108845
O'Brien, M. E., Wigler, N., Inbar, M., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 15 (3), 440–449. doi:10.1093/annonc/mdh097
Oki, Y., Ewer, M. S., Lenihan, D. J., Fisch, M. J., Hagemeister, F. B., Fanale, M., et al. (2015). Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: An open label, single arm, phase II trial. Clin. Lymphoma Myeloma Leuk. 15 (3), 152–158. doi:10.1016/j.clml.2014.09.001
Saleme, B., Gurtu, V., Zhang, Y., Kinnaird, A., Boukouris, A. E., Gopal, K., et al. (2019). Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci. Transl. Med. 11 (478), eaau8866. doi:10.1126/scitranslmed.aau8866
Schmitt, C. J., Dietrich, S., Ho, A. D., and Witzens-Harig, M. (2012). Replacement of conventional doxorubicin by pegylated liposomal doxorubicin is a safe and effective alternative in the treatment of non-Hodgkin's lymphoma patients with cardiac risk factors. Ann. Hematol. 91 (3), 391–397. doi:10.1007/s00277-011-1308-y
Sehn, L. H., and Salles, G. (2021). Diffuse large B-cell lymphoma. N. Engl. J. Med. 384 (9), 842–858. doi:10.1056/NEJMra2027612
Shao, Y., Tang, S. H., Lu, Y., Zhang, P. S., Liu, X. H., Chen, D., et al. (2020). The efficacy and safety of R-CDOP regimen in the treatment of large mass and/or multi-site extranodal of diffuse large B-cell lymphomain 2 years. J. Clin. Hematol. 33 (07), 481–485+492. doi:10.13201/j.issn.1004-2806.2020.07.009
Shen, B., Li, Y., Lu, X. L., Wu, H. B., and Zhao, X. Y. (2020). Pharmacoeconomic study of anthracycline in the treatment of non-Hodgkin's lymphoma. Clin. Med. J. 18 (10), 20–23. doi:10.3969/j.issn.1672-3384.2020.10.005
Shen, W. N., Ji, D. M., Xue, K., Zhang, Q. L., Lyu, F. F., Hong, X. N., et al. (2016). A phase Ⅰ dose-escalating trial of pegylated liposomal doxorubicin in combination with cyclophosphamide, vincristine and prednisone for aggressive non-Hodgkin lymphoma. Zhonghua Xue Ye Xue Za Zhi 37 (12), 1044–1048. doi:10.3760/cma.j.issn.0253-2727.2016.12.007
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 73 (9), 712–716. doi:10.1046/j.1445-2197.2003.02748.x
Song, X., and Ding, L. F. (2014). Efficacy of R-OHOP regimen containing pegylated liposomal doxorubicin in the treatment of elderly patients with diffuse large B-cell lymphoma. Chin. foreign Women Health 9 (0), 170.
Swain, S. M., Whaley, F. S., and Ewer, M. S. (2003). Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 97 (11), 2869–2879. doi:10.1002/cncr.11407
Thandra, K. C., Barsouk, A., Saginala, K., Padala, S. A., Barsouk, A., and Rawla, P. (2021). Epidemiology of non-hodgkin's lymphoma. Med. Sci. 9 (1), 5. doi:10.3390/medsci9010005
Theodoulou, M., and Hudis, C. (2004). Cardiac profiles of liposomal anthracyclines: Greater cardiac safety versus conventional doxorubicin? Cancer 100 (10), 2052–2063. doi:10.1002/cncr.20207
Tsavaris, N., Kosmas, C., Vadiaka, M., Giannouli, S., Siakantaris, M. P., Vassilakopoulos, T., et al. (2002). Pegylated liposomal doxorubicin in the CHOP regimen for older patients with aggressive (stages III/IV) non-Hodgkin's lymphoma. Anticancer Res. 22 (3), 1845–1848.
Tscheschner, H., Meinhardt, E., Schlegel, P., Jungmann, A., Lehmann, L. H., Müller, O. J., et al. (2019). CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS One 14 (4), e0215992. doi:10.1371/journal.pone.0215992
Varricchi, G., Ameri, P., Cadeddu, C., Ghigo, A., Madonna, R., Marone, G., et al. (2018). Antineoplastic drug-induced cardiotoxicity: A redox perspective. Front. Physiol. 9, 167. doi:10.3389/fphys.2018.00167
Visani, G., Guiducci, B., D'Adamo, F., Mele, A., Nicolini, G., Leopardi, G., et al. (2005). Cyclophosphamide, pegylated liposomal doxorubicin, vincristine and prednisone (CDOP) plus rituximab is effective and well tolerated in poor performance status elderly patients with non-Hodgkin's lymphoma. Leuk. Lymphoma 46 (3), 477–479. doi:10.1080/10428190400013688
Wang, X. D., Guo, B., Zhai, B., Yang, B., Fan, H., Lu, X. C., et al. (2021). Doxorubicin hydrochloride liposome-based CHOP regimen in the initial treatment of elderly patients with diffuse large B-cell lymphoma: A retrospective study. Zhongguo Shi Yan Xue Ye Xue Za Zhi 29 (4), 1136–1140. doi:10.19746/j.cnki.issn1009-2137.2021.04.018
Wu, X. S., Chen, Q. J., and Zhang, X. D. (2020). Clinical efficacy and safety of R-CDOP regimen with pegylated liposomal doxorubicin in first-line treatment of diffuse large B-cell lymphoma. Pract. Pharm. Clin. Remed 23 (11), 992–997. doi:10.14053/j.cnki.ppcr.202011006
Yamaguchi, N., Fujii, T., Aoi, S., Kozuch, P. S., Hortobagyi, G. N., and Blum, R. H. (2015). Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: A bayesian network meta-analysis. Eur. J. Cancer 51 (16), 2314–2320. doi:10.1016/j.ejca.2015.07.031
Yang, F. L., Shi, M. L., Wu, X. F., and Qi, J. D. (2019). Clinical efficacy and safety of R-CDOP regimen for treatment of newly treated DLBCL patients with adverse prognostic factors. Zhongguo Shi Yan Xue Ye Xue Za Zhi 27 (4), 1143–1148. doi:10.19746/j.cnki.issn.1009-2137.2019.04.024
Ye, J. (2022). Value of pegylated liposomal doxorubicin in the adjuvant treatment of patients with lymphoma and its effect on body's immune function. Acta Med. Sin. 35 (02), 66–70. doi:10.19296/j.cnk.1008-2409.2022-02-016
Yu, X., Ruan, Y., Huang, X., Dou, L., Lan, M., Cui, J., et al. (2020). Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun. 523 (1), 140–146. doi:10.1016/j.bbrc.2019.12.027
Zaja, F., Tomadini, V., Zaccaria, A., Lenoci, M., Battista, M., Molinari, A. L., et al. (2006). CHOP-rituximab with pegylated liposomal doxorubicin for the treatment of elderly patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 47 (10), 2174–2180. doi:10.1080/10428190600799946
Zheng, F. Y., Zhang, X. L., and Wang, C. L. (2018). Clinical effect of liposomal doxorubicin compared with adriamycin in the treatment of lymphoma. J. Trop. Med. 18 (04), 501–503+518. doi:10.3969/j.issn.1672-3619.2018.04.020
Keywords: non-Hodgkin’s lymphoma, liposomes, (R)-CDOP combination regimen, cardiotoxicity, adverse cardiovascular events
Citation: Lu B, Shen L, Ma Y, Qi J, Li Y, Wang Z, Han L and Zhong M (2022) Cardiovascular adverse events associated with cyclophosphamide, pegylated liposomal doxorubicin, vincristine, and prednisone with or without rituximab ((R)-CDOP) in non-Hodgkin’s lymphoma: A systematic review and meta-analysis. Front. Pharmacol. 13:1060668. doi: 10.3389/fphar.2022.1060668
Received: 03 October 2022; Accepted: 16 November 2022;
Published: 01 December 2022.
Edited by:
Fanfan Zhou, The University of Sydney, AustraliaReviewed by:
Salvador F Aliño, University of Valencia, SpainLuigi Rigacci, Policlinico Universitario Campus Bio-Medico, Italy
Copyright © 2022 Lu, Shen, Ma, Qi, Li, Wang, Han and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Zhong, emhvbmdtaW5nMnNkdUAxNjMuY29t; Lu Han, Jinan, bHVoYW5fc2R1QDE2My5jb20=
 Bin Lu1
Bin Lu1 Zhihao Wang
Zhihao Wang Lu Han
Lu Han Ming Zhong
Ming Zhong