
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 03 November 2021
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.732496
This article is part of the Research TopicInflammation and Fibrosis in the Gastrointestinal Tract and Liver: Mechanisms and TargetsView all 31 articles
 Shahla Rezaei1,2
Shahla Rezaei1,2 Reza Tabrizi3
Reza Tabrizi3 Peyman Nowrouzi-Sohrabi2,4
Peyman Nowrouzi-Sohrabi2,4 Mohammad Jalali5
Mohammad Jalali5 Mojtaba Shabani-Borujeni6
Mojtaba Shabani-Borujeni6 Shayan Modaresi2
Shayan Modaresi2 Maryam Gholamalizadeh7
Maryam Gholamalizadeh7 Saeid Doaei8*
Saeid Doaei8*Background: Vitamin D was reported to be associated with non−alcoholic fatty liver disease (NAFLD). This systematic review and meta−analysis aimed to investigate the effects of the vitamin D supplementation on anthropometric and biochemical indices in patient with NAFLD.
Methods: PubMed, Web of science, Scopus, and Embase databases were explored to identify all randomized controlled trial (RCT) investigating the effects of vitamin D supplementation on anthropometric and biochemical indices in patients with NAFLD. A random−effects model was used to pool weighted mean difference (WMD) and corresponding 95% confidence intervals (CIs). The statistical heterogeneity among the studies was assessed using I2 statistic (high ≥ 50%, low < 50%) and Cochran’s Q−test.
Results: Sixteen RCTs were included in this meta−analysis. The results identified that high−density lipoprotein−cholesterol (HDL−C) level significantly increased following vitamin D supplementation (P = 0.008). Vitamin D reduced body weight (P = 0.007), body mass index (P = 0.002), waist circumstance (WC) (P = 0.02), serum alanine transaminase (ALT) (P = 0.01), fasting blood sugar (FBS) (P = 0.01), homeostatic model assessment for insulin resistance (HOMA−IR) (P = 0.004), and calcium (P = 0.01). No significant changes were found on body fat, triglyceride (TG), total cholesterol, low−density lipoprotein−cholesterol (LDL−C), aspartate transaminase, alkaline phosphatase, gamma−glutamyl transferase, and adiponectin following vitamin D supplementation.
Conclusion: Vitamin D had significant effects on anthropometric and biochemical indices including HDL−C, body weight, BMI, WC, serum ALT, serum FBS, HOMA−IR, and calcium. Vitamin D supplementation can be considered as an effective strategy in management of patients with NAFLD.
Systematic Review Registration: [website], identifier [registration number]
Non-alcoholic fatty liver disease (NAFLD) is considered to be the most common cause of chronic liver disorders (Younossi, 2019). NAFLD includes a wide range of liver diseases such as fibrosis, cirrhosis and non−alcoholic hepatitis (Petta et al., 2016; Morvaridzadeh et al., 2021). The prevalence of NAFLD is about 20% in general population and about 70–90% in patients with type 2 diabetes (Tolman et al., 2007; Chalasani et al., 2012). The main causes of NAFLD are obesity, excessive dietary fat intake, insulin resistance, and dyslipidemia (Ludwig et al., 1980). Over the past few decades, various pharmacological and nutritional interventions have been assessed to treat NAFLD, but none indicated significant improvements. Currently, no medically approved drugs are available for NAFLD (Del Ben et al., 2014). Weight loss and nutritional interventions are considered standard treatments for NAFLD. Insulin resistance increases the rate of adipose tissue lipolysis and the flow of free fatty acids to the liver cell. Hyperglycemia also causes lipid−related changes in the liver cells by increasing lipogenesis while blocking fatty acid oxidation (FAO) and lipid transport in the liver (Kleiner et al., 2005; Schwimmer et al., 2005).
Vitamin D plays an important role in reducing insulin resistance, obesity, cardiovascular risk, prediabetes, metabolic syndrome, cancer, and cardiovascular diseases (CVDs) (Sepidarkish et al., 2019; Gholamalizadeh et al., 2020). Low serum level of 25-hydroxyvitamin D [25 (OH) D] is reported to be a risk factor for NAFLD disease (Forouhi et al., 2008; Sepidarkish et al., 2019). Vitamin D hypovitaminosis is associated with the severity and incidence of NAFLD in patients with normal liver enzymes. Vitamin D can affect the liver function through the vitamin D receptor (VDR). VDR is naturally present in the liver cells and its higher expression can reduce inflammation in chronic liver diseases (Benetti et al., 2018). Vitamin D also has anti−fibrotic, proliferative, and inflammatory effects on the liver. Some studies on the effects of vitamin D on anthropometric and biochemical indices reported that vitamin D was associated with body weight, fasting blood sugar (FBS), homeostatic model assessment for insulin resistance (HOMA−IR), glucose homeostasis, insulin resistance, high−density lipoprotein−cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), total cholesterol (TC), liver enzyme, and adiponectin (Sarathy et al., 2015; Foroughi et al., 2016; Doaei et al., 2019). A recent systematic review also reported that vitamin D improves the level of inflammatory mediators in patients with nonalcoholic fatty liver disease, but has no effect on anthropometric and glycemic indexes (Hariri and Zohdi, 2019; Mehrdad et al., 2020). However, the findings on the effects of vitamin D on NAFLD were contradictory (Sharifi et al., 2014; Sarathy et al., 2015). Therefore, this study aimed to investigate the effect of Vitamin D supplementation on anthropometric and biochemical indices in patient with NAFLD.
Preferred reporting items for systematic reviews and meta−analyses (PRISMA) (Moher et al., 2009) was used to demonstrate the process of study selection (Figure 1).
Two independent researchers performed the process of the systematic search using the online databases such as PubMed, embase, Scopus, Web of Science, and Cochrane Library for publications from 1980 up to 2021 on the effects of vitamin D supplementation on anthropometric and biochemical indices in patients with NAFLD, without considering any restriction on the language to detect the relevant citations. The PubMed search strategy was improved by consulting with an epidemiologist and was designed as a combination of the following search terms: “Vitamin D OR Cholecalciferol OR Calciol OR “Vitamin D 3” OR “Vitamin D3” OR Cholecalciferols OR Hydroxycholecalciferols OR “Hydroxyvitamins D″ OR Hydroxycholecalciferol OR Calcifediol OR “25−Hydroxyvitamin D 3” OR “25 Hydroxyvitamin D 3” OR “25−Hydroxycholecalciferol” OR Calcidiol OR Hydroxycholecalciferol OR Dedrogyl OR Hidroferol OR Calderol OR Dihydroxycholecalciferols OR “Dihydroxyvitamins D” OR “24,25−Dihydroxyvitamin D 3” OR Dihydroxyvitamin OR “24,25-Dihydroxyvitamin” OR “24,25-Dihydroxycholecalciferol” OR Dihydroxyvitamin OR Calcitriol OR “1 alpha,25-Dihydroxyvitamin” OR “1,25-Dihydroxyvitamin” OR “1,25-Dihydroxyvitamin” OR “1 alpha,25-Dihydroxycholecalciferol” OR “1,25−Dihydroxycholecalciferol” OR Dihydroxycholecalciferol OR Bocatriol OR Calcijex OR Decostriol OR MC1288 OR “MC−1288” OR “MC 1288” OR Osteotriol OR Renatriol OR Rocaltrol OR Silkis OR Sitriol OR Soltriol OR Tirocal” AND “Fatty Liver” OR “Fatty Liver” OR Steatohepatitis OR Steatohepatitides OR “Steatosis of Liver” OR “Visceral Steatosis” OR Steatoses OR Steatosis OR “Visceral Steatoses” OR “Liver Steatosis” OR “Liver Steatoses” OR “Non−alcoholic Fatty Liver Disease” OR “Non−alcoholic Fatty Liver Disease” OR “Nonalcoholic Fatty Liver Disease” OR NAFLD OR “Nonalcoholic Fatty Liver Disease” OR Nonalcoholic OR “Nonalcoholic” OR “Nonalcoholic Fatty” OR “Non−alcoholic Fatty” OR “Nonalcoholic Fatty Liver” OR “Nonalcoholic Fatty Livers” OR NASH OR “Nonalcoholic Steatohepatitis” OR “Nonalcoholic Steatohepatitides” OR Steatohepatitis OR “liver cirrhosis” OR cirrhosis OR cirrhosis. To improve the search strategy, the wild−card term “*” was implicated and the reference lists of relevant articles were hand−scanned. Any doubts were resolved through discussing with the corresponding author.
Based on PICOS criteria, the aim of the present review study on patients with NAFLD (as population) was to evaluate the effect of vitamin D supplementation (as intervention) compared with placebo (as comparator) on anthropometric and biochemical outcomes (as outcome) in randomized interventional trials (as study design). Two independent reviewers initiated the assessment process of the collected papers and following inclusion criteria were considered (Younossi, 2019): randomized controlled trials (RCTs) with parallel design (Petta et al., 2016), studies that assessed the effects of vitamin D supplementation on patients with NAFLD. The exclusion criteria were (Younossi, 2019) being experimental study (Petta et al., 2016), lacking the essential data, for example, non−extractable or unconvertable data (Morvaridzadeh et al., 2021), studies without a suitable control group (Chalasani et al., 2012), trials with combined supplementation of nutrients or/and medicines besides vitamin D and also (Tolman et al., 2007) unpublished data, grey literature, conference abstracts, book chapters, and brief reports. The corresponding author was involved to solve disagreements.
Following data were extracted from the included trials by two independent investigators: first authors’ last name, publication date, country of study, study design, participants’ characteristics, dosage of the supplement, intervention duration, quality of trials, mean changes and standard deviations (SDs) for each outcomes in pre−treatment and post−treatment. The third reviewer solved disagreements.
Two independent reviewers were assigned to qualify the included RCTs and explore the potential risk of bias for the following domains using the Cochrane Risk of Bias Tool: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting and other biases. “low”, “high” or “unclear” terms were used to grade each item (Higgins et al., 2011).
Each question was answered as low risk of bias (score = 1), high risk of bias (score = −1), or unclear (score = 0). Disagreements was resolved by the third author. Scores were summed and studies with scores −6−0, 1−3 and 4−6 considered as low, medium and high quality, respectively (Table 3).
A meta−analysis was performed using STATA software v13 (StataCorp, Texas) and a random−effects model was used to pool weighted mean difference (WMD) and corresponding 95% confidence intervals (CIs). The statistical heterogeneity among the studies was assessed using I2 statistic (high ≥50%, low <50%) and Cochran’s Q−test (Higgins et al., 2019). In presence of high inter−study heterogeneity, a random−effects meta−regression was performed to find its potential sources. Subgroup analysis was done based on country (Iran vs. other), duration of treatment (>12 weeks, ≤ 12 weeks), and dosage (>25,000 IU/day, ≤ 25,000 IU/day). Mean changes of the interested outcomes and the relevant SDs were obtained executing following formulas, respectively: (mean at post intervention–mean at baseline), SD = √([SD2−baseline + SD2−post] − [2r × SD−baseline × SD−post]), assuming correlation coefficient (r) as 0.5 (Higgins et al., 2019). Sensitivity analysis was planned to assess the impact of each study on the pooled results by removing one study in a turn (Higgins et al., 2019). Potential publication bias was evaluated applying Egger regression and Begg’s tests. If there was a significant publication bias, “trim and fills” analysis was done to check the possible change of the significance of the result (Duval and Tweedie, 2000). A P−value < 0.05 was considered as statistically significant.
A total of 3,916 potentially relevant articles were collected in the primary search (Figure 1). No additional article was detected via the hand searching. Among all of the references, 1840 were removed as duplicates. After title and abstract screening, 48 articles remained and after full-text review, 32 articles were excluded from the current study for different reasons. Thus, the final systematic review and meta-analysis was done on 16 papers (Table 1) (Sharifi et al., 2014; Foroughi et al., 2015; Barchetta et al., 2016; Foroughi et al., 2016; Nadjarzadeh et al., 2016; Sharifi et al., 2016; Lorvand Amiri et al., 2017; Sakpal et al., 2017; Dabbaghmanesh et al., 2018; Geier et al., 2018; Mansourian Hosseini et al., 2018; MOUODI et al., 2018; Hajiaghamohammadi et al., 2019; Hussain et al., 2019; Shidfar et al., 2019; Foroughi et al., 2014).
A significant improvement of serum HDL−C [WMD = 1.60, 95% CI = (0.41, 2.78), p = 0.008, I2 = 0.0%] was found following vitamin D intake compared to the controls. Serum levels of TG [WMD = 0.65, 95% CI = (−12.60, 13.91), p = 0.92, I2 = 40.9%], TC [WMD = 5.62, 95% CI = (−4.20, 15.45), p = 0.26, I2 = 69.0%] and LDL−C [WMD = 3.53, 95% CI = (−0.91, 7.97), p = 0.11, I2 = 12.8%] were not significantly affected in the group receiving vitamin D (Figure 2).
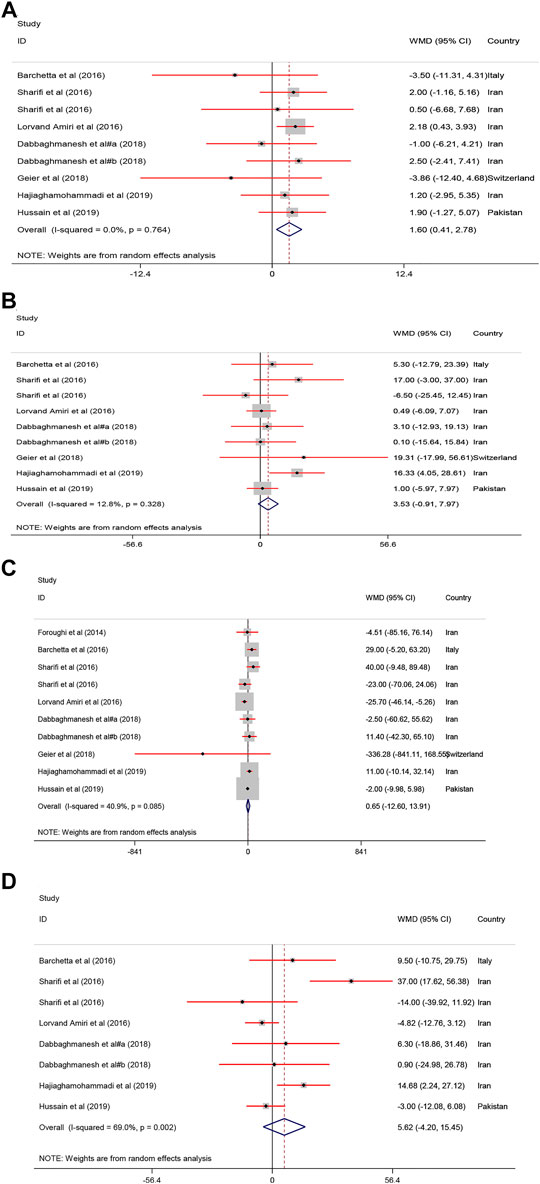
FIGURE 2. The lipid profile standardized mean differences estimates for (A) HDL−C, (B) LDL−C, (C) TG and (D) TC between intervention group (receiving vitamin D supplementation) and placebo groups.
Pooled results of the meta−analysis revealed a significant reduction in body weight [WMD = −0.88, 95% CI = (−1.52, −0.24), p = 0.007, I2 = 29.7%], BMI [WMD = −0.33, 95% CI = (−0.54, −0.12), p = 0.002, I2 = 21.5%], and WC [WMD = −1.04, 95% CI = (−1.97, −0.10), p = 0.02, I2 = 64.0%] in the treatment groups compared with the controls. In contrast, body fat [WMD = 0.46, 95% CI = (−0.47, 1.40), p = 0.33, I2 = 60.5%] was not changed following vitamin D supplementation (Figure 3).
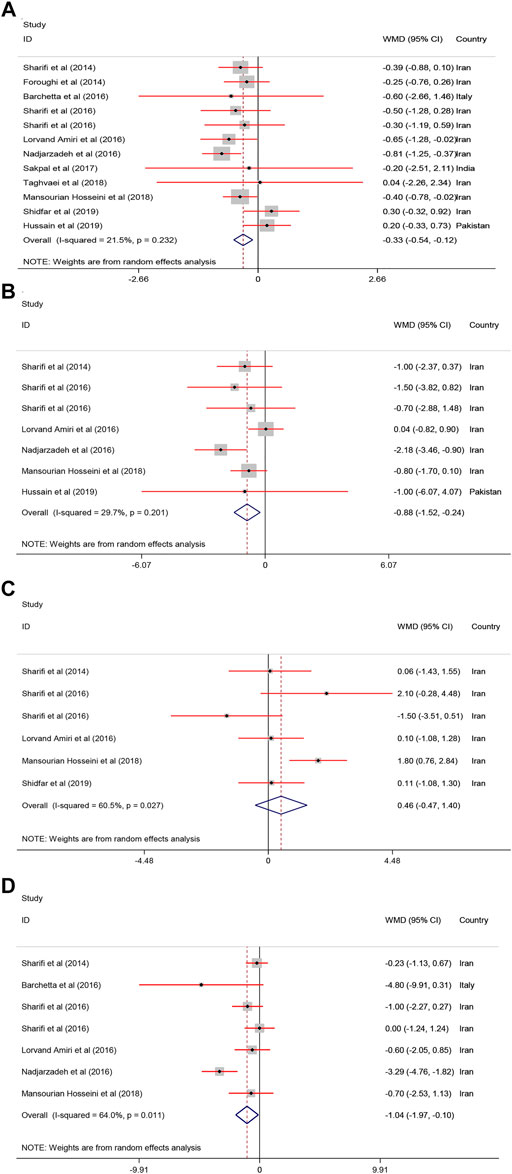
FIGURE 3. The anthropometric parameters standardized mean differences estimates for (A) BMI, (B) weight, and (C) body fat and (D) WC between intervention group (receiving vitamin D supplementation) and placebo groups.
Pooled estimate of 16 papers indicated a significant reduction of serum ALT [WMD = −4.03, 95% CI = (−7.41, −0.66), p = 0.01, I2 = 78.5%] following vitamin D supplementation. However, serum AST [WMD = −1.05, 95% CI = (−3.50, 1.39), p = 0.39, I2 = 78.2%], ALP [WMD = −5.43, 95% CI = (−18.08, 7.22), p = 0.40, I2 = 62.4%], and GGT [WMD = −2.24, 95% CI = (−9.66, 5.18), p = 0.54, I2 = 0.0%] were not affected by vitamin D supplementation (Figure 4).
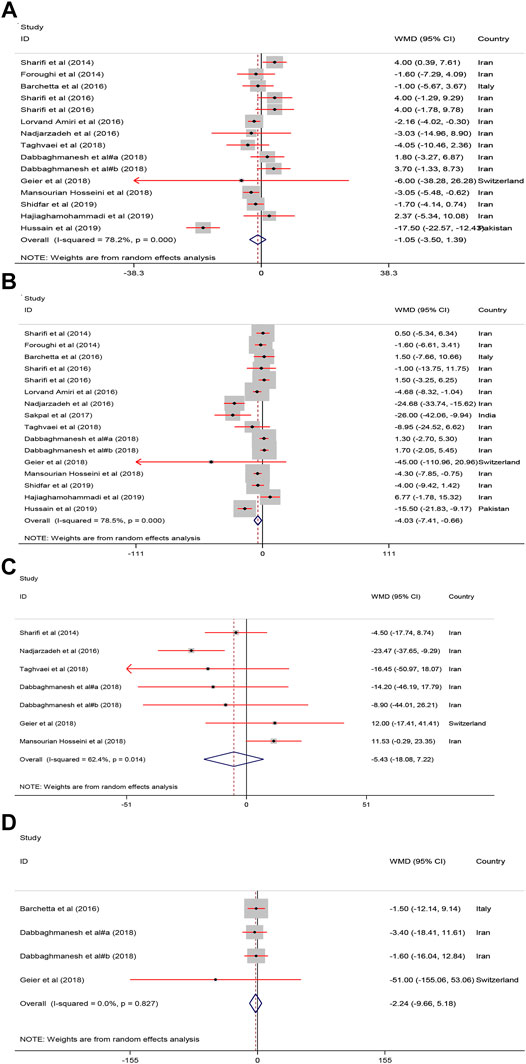
FIGURE 4. The Liver enzymes standardized mean differences estimates for (A) AST (B) ALT, (C) ALP and (D) GGT between intervention group (receiving vitamin D supplementation) and placebo groups.
As shown in Figure 5, receiving vitamin D significantly improved serum FBS [WMD = −5.02, 95% CI = (−8.95, −1.09), p = 0.01, I2 = 81.6%] and HOMA−IR [WMD = −0.79, 95% CI = (−1.33, −0.25), p = 0.004, I2 = 89.0%] compared with to the controls.
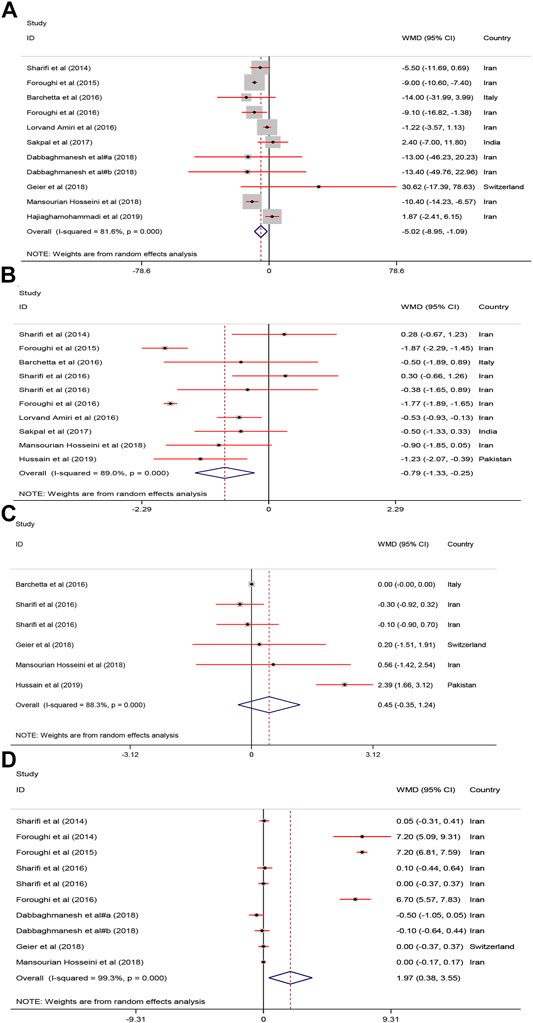
FIGURE 5. The FBS, HOMA, Adiponectin and calcium mean differences estimates for (A) FBG, (B) HOMA−IR, (C) Adiponectin and (D) calcium FBS: fasting plasma glucose; homeostasis model assessment of insulin resistance; RCTs, randomized controlled trials.
As shown in Figure 5, serum calcium was significantly increased after vitamin D supplementation [WMD = 1.97, 95% CI = (0.38, 3.55), p = 0.01, I2 = 99.3%]. Adiponectin was not significantly affected [WMD = 0.45, 95% CI = (−0.35, 1.24), p = 0.26, I2 = 88.3%] by vitamin D supplementation.
As shown in Table 2, serum HDL−C was significantly affected by vitamin D supplementation when subgroup analysis conducted by country (Iran), duration (≤12 weeks), and dose (≤25,000 IU). BMI was changed in country (Iran), duration (>12 weeks), and dose (≤25,000 IU) subgroups. Body weight was changed in studies conducted in Iran and also when used >25,000 IU doses of when which interventions last >12 weeks, serum FBS was significantly affected when studies performed in Iran, last ≤12 weeks, and used doses more than 25,000 IU vitamin D. HOMA−IR was significantly improved all subgroups, except in trials last more than 12 weeks.
The results of meta−regression and sensitivity analysis indicated that total sample size was regarded as a source of inter−study heterogeneity for AST [Coefficient: −0.19, 95% CI = (−0.29, −0.08), p = 0.001]. Sensitivity analyses showed that pooled results of interested outcomes are not sensitive to removing any of included trials.
A significant publication bias was found for HOMA-IR, HDL-C and GGT. Hence, trim and fill analysis was done to detect the potential change of their results. Only the significance of HDL−C was changed and turned into non−significant.
To the best of the authors’ knowledge, this is the first study to systematically review and meta analyze the findings of intervention studies on the effect of vitamin D on a wide range of anthropometric and biochemical indices in patient with NAFLD. The results of the present study indicated that vitamin D supplementation significantly improved the body weight, BMI, WC, HDL-C, ALT, FBS, and HOMA-IR in the intervention group compared to the control group. However, no significant differences were found on body fat, TG, LDL-C, AST, and GGT. Subgroup analyses also showed that the effect of vitamin D on most indices was affected by study location, duration of study and dose used for vitamin D.
Some studies investigated the association between vitamin D supplementation with lipid profile in NAFLD. In line with this study, Dabaghmanesh et al. indicated that 12 weeks of vitamin D supplementation significantly increased HDL-C (Dabbaghmanesh et al., 2018). Another study found that supplementation with calcium combined with vitamin D improved serum ALT and HDL-C levels (Lorvand Amiri et al., 2017).
However, the results regarding the effect of vitamin D on lipid profile have been contradictory. Sharifi et al. found that vitamin D intake supplementation for 4 months significantly decreed LDL-C and total cholesterol in woman (Sharifi et al., 2016). A meta-analysis indicated that supplementation with vitamin D significantly increased LDL-C and had not significant effect on TG and HDL-C in patients with CVDs (Wang et al., 2012). One study found that vitamin D supplementation reduced LDL-C but no significant change was found on HDL-C (Hajiaghamohammadi et al., 2019). In another study supplementation with vitamin D3 as 50,000 IU per week for 12 weeks in NAFLD had no significant effect on lipid profile and body composition (Hussain et al., 2019). The effect of vitamin D on lipid profile in people with NAFLD may be different from its effect in healthy people or in people with other chronic diseases.
Some effects of vitamin D are exerted through its effect on increasing calcium absorption. Therefore, dietary calcium intake can also affect the role of vitamin D in determining serum lipid profile. Shidfar et al. found that vitamin D combined with calcium for 12 weeks reduced ALT, AST, LDL-C/HDL-C, TC/HDL-C, and increased non−HDL−C compared with group revived only vitamin D (Shidfar et al., 2019). Moreover, some studies suggested an inverse association between serum 25(OH)-D levels and TG (Chon et al., 2014). The underlying mechanisms of the effects of vitamin D on lipid profile has not been completely yet understood. The low levels of vitamin D can lead to hyperparathyroidism which can lower serum TG. On the other hand, lower vitamin D level can activate microsomal TG-transfer protein, which leads to increased TG. Vitamin D also regulates macrophage function in reverse cholesterol transportation and reduces inflammation and insulin resistance (Shidfar et al., 2019).
Regarding to anthropometric measurements, this meta−analysis found that vitamin D reduced body weight, BMI, and WC but no significant change was found in body fat. Hussain et al. investigated the association between vitamin D intake and BMI, body fat, and WC in NAFLD and reported that 50,000 IU vitamin D for 12 weeks had no significant effect on anthropometric indices (Hussain et al., 2019). Another study found that the injection of cholecalciferol (600,000 IU) reduced weight, WC, and BMI (Mansourian Hosseini et al., 2018). Sharifi et al. reported that the intervention group with 50,000 IU vitamin D3 for 4 month had lower weight, WC, and BMI compared with the controls (Sharifi et al., 2016). The reason for these differences may be the differences in doses of vitamin D supplementation and also differences in seum level of vitamin D. Doaei et al. reported that the serum level of vitamin D is inversely associated with obesity. However, short term changes of serum vitamin D was not related to changes in weight (Doaei et al., 2020).
The present study identified that ALT increased and some liver enzymes such as AST, ALP, GGT were not affected following vitamin D supplementation. Vitamin D receptor (VDR) is abundantly expressed on liver cells and this vitamin was reported to have an anti−inflammatory effect on liver (Bozic et al., 2016). One study found that 50,000 IU/wk vitamin D supplementation for 10 weeks reduced AST and ALT in the intervention group compared with the control group (Hajiaghamohammadi et al., 2019). The vitamin D supplementation for 48 weeks decreased liver enzyme in patients with NAFLD (Geier et al., 2018). The difference in the results of the effect of vitamin D on the serum level of liver enzymes may be due to the fact that the levels of liver enzymes is greatly affected by other lifestyle and dietary factors. Sakpal et al. indicated that vitamin D intake along with lifestyle modifications improved serum ALT levels (Jamali et al., 2013). Another placebo−controlled study found that 12−weeks hypocaloric diet combined with calcitriol improved ALT and AST in the intervention group compared with the control group (Amiri et al., 2016).
The levels of FBS and HOMA-IR were improved following vitamin D intake. In line with this study, one study indicated that calcitriol intake (25 mg) for 12 weeks improved HOMA−IR in patients with NAFLD (Amiri et al., 2016). A recent study reported that vitamin D intake increased insulin sensitivity (Inzucchi et al., 1998). Another study found a negative association between vitamin D supplementation and FBS (Pittas et al., 2007). In another study, calcitriol supplementation for a short term increased insulin sensitivity (Foroughi et al., 2016). One meta−analysis suggested that vitamin D administration could improve FBS and insulin resistance in patents with impaired glucose tolerance or HOMA−IR (George et al., 2012). However, the results regarding the effect of vitamin D on serum glucose indices were contradictory. One study reported that vitamin D had no association with FBS (Kitson and Roberts, 2012). Interestingly, Lind et al. found that long−term calcitriol supplementation for 2 years decreased HOMA−IR but had no effect on insulin (Lind et al., 1989). The short−term vitamin D intake was associated with lower FBS in renal disease patients while had no effect on fasting insulin levels (Sarathy et al., 2015). The underlying mechanism of the effect of vitamin D on blood glucose is not yet clear. Vitamin D deficiency increase serum parathyroid hormone levels (PTH) and the PTH plays an important role in NAFLD through increasing insulin resistance (IR) (Foss, 2009).
The results of the present study indicated that the level of adiponectin was not significantly affected by vitamin D supplementation. Some studies reported that patients with NAFLD had lower adiponectin concentration compared with the others and adiponectin can be considered as a predictor of the severity of hepatic steatosis (Targher et al., 2006; Nakano et al., 2011). In line with our study, two previous studies reported that vitamin D intake had no effect on serum adiponectin in people with type 2 diabetes and obese children (Patel et al., 2010; Belenchia et al., 2013). On the other hand, Nakano et al. reported that phototherapy and vitamin D increased adiponectin concentration in rats with NAFLD (Kitson and Roberts, 2012). Adiponectin is secreted from adipose tissue and could influence on metabolism of fat and glucose. Adiponectin increased liver insulin sensitivity and decreased glucose excretion from the liver which can lead to prevent of high blood sugar. It has been suggested that vitamin D regulates adiponectin gene expression in adipose tissue (Hajiaghamohammadi et al., 2019). It seems that many factors may influence the effects of vitamin D on serum adiponectin such as differences in doses of vitamin D supplementation, follow−up duration, and VDR gene polymorphisms. Moreover, the association between vitamin D intake and adiponectin may be influenced by other factors such as calcium intake and body weight. The results of a recent meta−analysis study indicated that vitamin D may promote secretion of adiponectin in subjects with diabetes and this effect may be potentiated if vitamin D intake is on daily basis and in combination with calcium but can be weakened by increasing BMI (Nikooyeh and Neyestani, 2021).
The present study found that vitamin D supplementation improved the level of serum calcium. In contrast with this result, Geier et al. found that adiponectin and calcium levels were not significantly different after vitamin D supplementation (Geier et al., 2018). Sharifi et al. showed that vitamin D supplementation has no effect on serum level of calcium (Sharifi et al., 2016). A recent study of more than 5,000 people found that taking a monthly vitamin D supplement did not alter serum calcium (Malihi et al., 2019). Serum calcium levels play an important role in the cardiovascular and muscular system and are maintained at a certain level by complex mechanisms (Fleet, 2017). Further studies on the effect of vitamin D on serum calcium levels and considering the interaction of various factors on serum calcium levels are needed.
The present study showed that Vitamin D supplementation had significant effect on HDL-C, body weight, BMI, WC, serum ALT, serum FBS, HOMA-IR, calcium and no effect on TG, TC, LDL-C, AST, ALP, GGT, and adiponectin. Further studies are needed to identify the underlying mechanisms of the effects of vitamin D supplementation on the anthropometric and biochemical indices in patient with NAFLD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data may be made available upon request.
SR and RT designed the study, and were involved in the data collection, analysis, and drafting of the manuscript. SR, PN-S, MJ, MS-B, SM, SD, and MG were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding for this study was provided by Shiraz University of Medical Sciences, Shiraz, Iran.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was conducted at the school of Nutrition and Food Sciences and shahid beheshti University of Medical Sciences, Tehran, Iran.
NAFLD, with non-alcoholic fatty liver disease; BMI, body mass index; WC, waist circumstance; ALT, alanine transaminase; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance; TG: triglyceride.
Amiri, H. L., Agah, S., Mousavi, S. N., Hosseini, A. F., and Shidfar, F. (2016). Regression of Non−alcoholic Fatty Liver by Vitamin D Supplement: a Double−Blind Randomized Controlled Clinical Trial. Arch. Iranian Med. 19 (9), 0.
Barchetta, I., Del Ben, M., Angelico, F., Di Martino, M., Fraioli, A., La Torre, G., et al. (2016). No Effects of Oral Vitamin D Supplementation on Non−alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: a Randomized, Double−Blind, Placebo−Controlled Trial. BMC Med. 14 (1), 92–10. doi:10.1186/s12916−016−0638−y
Belenchia, A. M., Tosh, A. K., Hillman, L. S., and Peterson, C. A. (2013). Correcting Vitamin D Insufficiency Improves Insulin Sensitivity in Obese Adolescents: a Randomized Controlled Trial. Am. J. Clin. Nutr. 97 (4), 774–781. doi:10.3945/ajcn.112.050013
Benetti, E., Mastrocola, R., Chiazza, F., Nigro, D., D'Antona, G., Bordano, V., et al. (2018). Effects of Vitamin D on Insulin Resistance and Myosteatosis in Diet−Induced Obese Mice. PLoS One 13 (1), e0189707. doi:10.1371/journal.pone.0189707
Bozic, M., Guzmán, C., Benet, M., Sánchez−Campos, S., García−Monzón, C., Gari, E., et al. (2016). Hepatocyte Vitamin D Receptor Regulates Lipid Metabolism and Mediates Experimental Diet−Induced Steatosis. J. Hepatol. 65 (4), 748–757. doi:10.1016/j.jhep.2016.05.031
Chalasani, N., Younossi, Z., Lavine, J. E., Diehl, A. M., Brunt, E. M., Cusi, K., et al. (2012). The Diagnosis and Management of Non−alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55 (6), 2005–2023. doi:10.1002/hep.25762
Chon, S. J., Yun, B. H., Jung, Y. S., Cho, S. H., Choi, Y. S., Kim, S. Y., et al. (2014). Association between Vitamin D Status and Risk of Metabolic Syndrome Among Korean Postmenopausal Women. PloS one 9 (2), e89721. doi:10.1371/journal.pone.0089721
Dabbaghmanesh, M. H., Danafar, F., Eshraghian, A., and Omrani, G. R. (2018). Vitamin D Supplementation for the Treatment of Non−alcoholic Fatty Liver Disease: A Randomized Double Blind Placebo Controlled Trial. Diabetes Metab. Syndr. 12 (4), 513–517. doi:10.1016/j.dsx.2018.03.006
Del Ben, M., Polimeni, L., Baratta, F., Pastori, D., Loffredo, L., and Angelico, F. (2014). Modern Approach to the Clinical Management of Non−alcoholic Fatty Liver Disease. World J. Gastroenterol. 20 (26), 8341–8350. doi:10.3748/wjg.v20.i26.8341
Doaei, S., Jarrahi, S., Torki, S., Haghshenas, R., Jamshidi, Z., Rezaei, S., et al. (2020). Serum Vitamin D Level May Be Associated with Body Weight and Body Composition in Male Adolescents; a Longitudinal Study. Pediatr. Endocrinol. Diabetes Metab. 26 (3), 125–131. doi:10.5114/pedm.2020.97466
Doaei, S., Kalantari, N., Izadi, P., Salonurmi, T., Mosavi Jarrahi, A., Rafieifar, S., et al. (2019). Changes in FTO and IRX3 Gene Expression in Obese and Overweight Male Adolescents Undergoing an Intensive Lifestyle Intervention and the Role of FTO Genotype in This Interaction. J. Transl Med. 17 (1), 176–178. doi:10.1186/s12967−019−1921−4
Duval, S., and Tweedie, R. (2000). Trim and Fill: A Simple Funnel−Plot−Based Method of Testing and Adjusting for Publication Bias in Meta−Analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006−341x.2000.00455.x
Fleet, J. C. (2017). The Role of Vitamin D in the Endocrinology Controlling Calcium Homeostasis. Mol. Cel Endocrinol 453, 36–45. doi:10.1016/j.mce.2017.04.008
Foroughi, M., Maghsoudi, Z., and Askari, G. (2016). The Effect of Vitamin D Supplementation on Blood Sugar and Different Indices of Insulin Resistance in Patients with Non−alcoholic Fatty Liver Disease (NAFLD). Iran J. Nurs. Midwifery Res. 21 (1), 100–104. doi:10.4103/1735−9066.174759
Foroughi, M., Maghsoudi, Z., Ghiasvand, R., Iraj, B., and Askari, G. (2014). Effect of Vitamin D Supplementation on C−Reactive Protein in Patients with Nonalcoholic Fatty Liver. Int. J. Prev. Med. 5 (8), 969–975.
Foroughi, M., Maghsoudi, Z., Ghiasvand, R., Iraj, B., and Askari, G. (2015). The Effect of Vitamin D Supplementation on Insulin Resistance in Patients with Nonalcoholic Fatty Liver. J. Isfahan Med. Sch. 33 (342), 1076–1085.
Forouhi, N. G., Luan, J., Cooper, A., Boucher, B. J., and Wareham, N. J. (2008). Baseline Serum 25−hydroxy Vitamin D Is Predictive of Future Glycemic Status and Insulin Resistance: the Medical Research Council Ely Prospective Study 1990−2000. Diabetes 57 (10), 2619–2625. doi:10.2337/db08−0593
Foss, Y. J. (2009). Vitamin D Deficiency Is the Cause of Common Obesity. Med. Hypotheses 72 (3), 314–321. doi:10.1016/j.mehy.2008.10.005
Geier, A., Eichinger, M., Stirnimann, G., Semela, D., Tay, F., Seifert, B., et al. (2018). Treatment of Non−alcoholic Steatohepatitis Patients with Vitamin D: a Double−Blinded, Randomized, Placebo−Controlled Pilot Study. Scand. J. Gastroenterol. 53 (9), 1114–1120. doi:10.1080/00365521.2018.1501091
George, P. S., Pearson, E. R., and Witham, M. D. (2012). Effect of Vitamin D Supplementation on Glycaemic Control and Insulin Resistance: a Systematic Review and Meta−Analysis. Diabet Med. 29 (8), e142–50. doi:10.1111/j.1464−5491.2012.03672.x
Gholamalizadeh, M., Jarrahi, A. M., Akbari, M. E., Bourbour, F., Mokhtari, Z., Salahshoornezhad, S., et al. (2020). Association between FTO Gene Polymorphisms and Breast Cancer: the Role of Estrogen. Expert Rev. Endocrinol. Metab. 15 (2), 115–121. doi:10.1080/17446651.2020.1730176
Hajiaghamohammadi, A., Shafikhani, A. A., Akbar Shafikhani, A., Bastani, A., and Gaemi, N. (2019). Effect of Vitamin D Replacement on Liver Enzymes in Patients with Non−alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. Res. 8 (3), 2907–2910. doi:10.17554/j.issn.2224−3992.2019.08.819
Hariri, M., and Zohdi, S. (2019). Effect of Vitamin D on Non−alcoholic Fatty Liver Disease: a Systematic Review of Randomized Controlled Clinical Trials. Int. J. Prev. Med. 10, 14. doi:10.4103/ijpvm.IJPVM_499_17
Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. Wiley, NJ, USA, version 6.0(updated July 2019), .
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hussain, M., Iqbal, J., Malik, S. A., Waheed, A., Shabnum, S., Akhtar, L., et al. (2019). Effect of Vitamin D Supplementation on Various Parameters in Non−alcoholic Fatty Liver Disease Patients. Pak J. Pharm. Sci. 32, 1343–1348.
Inzucchi, S. E., Maggs, D. G., Spollett, G. R., Page, S. L., Rife, F. S., Walton, V., et al. (1998). Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. N. Engl. J. Med. 338 (13), 867–872. doi:10.1056/NEJM199803263381303
Jamali, R., Mofid, A., Vahedi, H., Farzaneh, R., and Dowlatshahi, S. (2013). The Effect of helicobacter Pylori Eradication on Liver Fat Content in Subjects with Non−alcoholic Fatty Liver Disease: a Randomized Open−Label Clinical Trial. Hepat. Mon 13 (12), e14679. doi:10.5812/hepatmon.14679
Kitson, M. T., and Roberts, S. K. (2012). D−livering the Message: the Importance of Vitamin D Status in Chronic Liver Disease. J. Hepatol. 57 (4), 897–909. doi:10.1016/j.jhep.2012.04.033
Kleiner, D. E., Brunt, E. M., Van Natta, M., Behling, C., Contos, M. J., Cummings, O. W., et al. (2005). Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 41 (6), 1313–1321. doi:10.1002/hep.20701
Lind, L., Pollare, T., Hvarfner, A., Lithell, H., Sørensen, O. H., and Ljunghall, S. (1989). Long−term Treatment with Active Vitamin D (Alphacalcidol) in Middle−Aged Men with Impaired Glucose Tolerance. Effects on Insulin Secretion and Sensitivity, Glucose Tolerance and Blood Pressure. Diabetes Res. 11 (3), 141–147.
Lorvand Amiri, H., Agah, S., Tolouei Azar, J., Hosseini, S., Shidfar, F., and Mousavi, S. N. (2017). Effect of Daily Calcitriol Supplementation with and without Calcium on Disease Regression in Non−alcoholic Fatty Liver Patients Following an Energy−Restricted Diet: Randomized, Controlled, Double−Blind Trial. Clin. Nutr. 36 (6), 1490–1497. doi:10.1016/j.clnu.2016.09.020
Ludwig, J., Viggiano, T R., Mcgill, D. B., and Oh, B. J. (1980). “Nonalcoholic Steatohepatitis: Mayo Clinic Experiences with a Hitherto Unnamed Disease,” in Mayo Clinic Proceedings, 55, 7, 434−438, .
Malihi, Z., Lawes, C. M. M., Wu, Z., Huang, Y., Waayer, D., Toop, L., et al. (2019). Monthly High−Dose Vitamin D Supplementation Does Not Increase Kidney Stone Risk or Serum Calcium: Results from a Randomized Controlled Trial. Am. J. Clin. Nutr. 109 (6), 1578–1587. doi:10.1093/ajcn/nqy378
Mansourian, S. H., Aliashrafi, S., and Ebrahimi, M. M. (2018). The Effect of a Single Intramuscular Injection of Cholecalciferol on the Serum Levels of Vitamin D, Adiponectin, Insulin Resistance, and Liver Function in Women with Non−alcoholic Fatty Liver Disease (NAFLD): A Randomized, Controlled Clinical Trial. Iranian Red Crescent Med. J. 20 (10). doi:10.5812/ircmj.60746
Mehrdad, M., Doaei, S., Gholamalizadeh, M., Fardaei, M., Fararouei, M., and Eftekhari, M. H. (2020). Association of FTO Rs9939609 Polymorphism with Serum Leptin, Insulin, Adiponectin, and Lipid Profile in Overweight Adults. Adipocyte 9 (1), 51–56. doi:10.1080/21623945.2020.1722550
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta−Analyses: the PRISMA Statement. BMJ 339 (4), b2535–9. doi:10.1136/bmj.b2535
Morvaridzadeh, M., Nachvak, S. M., Mohammadi, R., Moradi, S., Mostafai, R., Pizarro, A. B., et al. (2021). Probiotic Yogurt Fortified with Vitamin D Can Improve Glycemic Status in Non−alcoholic Fatty Liver Disease Patients: a Randomized Clinical Trial. Clin. Nutr. Res. 10 (1), 36–47. doi:10.7762/cnr.2021.10.1.36
Mouodi, M., Fakheri, H. T., Kashi, Z., Maleki, I., and Mohammadpour, R. (2018). Effects of Vitamin D Supplementation on Patients with Non−alcoholic Fatty Liver Disease (NAFLD). Acta Med. 34, 415.
Nadjarzadeh, A., Jani, N., Khoshnevisan, M., Molajaafari, A., Fallahzadeh, H., Khabiri, F., et al. (2016). Effects of Low Caloric Diet with and without Vitamin D Supplementation on Anthropometric Parameters in Patients with Non−alcoholic Fatty Liver. Tolooebehdasht 14 (6), 410–422.
Nakano, T., Cheng, Y. F., Lai, C. Y., Hsu, L. W., Chang, Y. C., Deng, J. Y., et al. (2011). Impact of Artificial Sunlight Therapy on the Progress of Non−alcoholic Fatty Liver Disease in Rats. J. Hepatol. 55 (2), 415–425. doi:10.1016/j.jhep.2010.11.028
Nikooyeh, B., and Neyestani, T. R. (2021). Can Vitamin D Be Considered an Adiponectin Secretagogue? A Systematic Review and Meta−Analysis. J. Steroid Biochem. Mol. Biol. 212, 105925. doi:10.1016/j.jsbmb.2021.105925
Patel, P., Poretsky, L., and Liao, E. (2010). Lack of Effect of Subtherapeutic Vitamin D Treatment on Glycemic and Lipid Parameters in Type 2 Diabetes: a Pilot Prospective Randomized Trial. J. Diabetes 2 (1), 36–40. doi:10.1111/j.1753−0407.2009.00057.x
Petta, S., Gastaldelli, A., Rebelos, E., Bugianesi, E., Messa, P., Miele, L., et al. (2016). Pathophysiology of Non Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 17 (12), 2082. doi:10.3390/ijms17122082
Pittas, A. G., Harris, S. S., Stark, P. C., and Dawson, B. H. (2007). The Effects of Calcium and Vitamin D Supplementation on Blood Glucose and Markers of Inflammation in Nondiabetic Adults. Diabetes care 30 (4), 980–986. doi:10.2337/dc06−1994
Sakpal, M., Satsangi, S., Mehta, M., Duseja, A., Bhadada, S., Das, A., et al. (2017). Vitamin D Supplementation in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. JGH Open 1 (2), 62–67. doi:10.1002/jgh3.12010
Sarathy, H., Pramanik, V., Kahn, J., Abramowitz, M. K., Meier, K., Kishore, P., et al. (2015). The Effects of Short−Term Vitamin D Supplementation on Glucose Metabolism in Dialysis Patients: a Systematic Review and Meta−Analysis. Int. Urol. Nephrol. 47 (3), 537–549. doi:10.1007/s11255−015−0909−0
Schwimmer, J. B., Behling, C., Newbury, R., Deutsch, R., Nievergelt, C., Schork, N. J., et al. (2005). Histopathology of Pediatric Nonalcoholic Fatty Liver Disease. Hepatology 42 (3), 641–649. doi:10.1002/hep.20842
Sepidarkish, M., Farsi, F., Akbari, M. F., Namazi, N., Almasi, A. H., Maleki, A. H., et al. (2019). The Effect of Vitamin D Supplementation on Oxidative Stress Parameters: a Systematic Review and Meta−Analysis of Clinical Trials. Pharmacol. Res. 139, 141–152. doi:10.1016/j.phrs.2018.11.011
Sharifi, N., Amani, R., Hajiani, E., and Cheraghian, B. (2014). Does Vitamin D Improve Liver Enzymes, Oxidative Stress, and Inflammatory Biomarkers in Adults with Non−alcoholic Fatty Liver Disease? A Randomized Clinical Trial. Endocrine 47 (1), 70–80. doi:10.1007/s12020−014−0336−5
Sharifi, N., Amani, R., Hajiani, E., and Cheraghian, B. (2016). Women May Respond Different from Men to Vitamin D Supplementation Regarding Cardiometabolic Biomarkers. Exp. Biol. Med. (Maywood) 241 (8), 830–838. doi:10.1177/1535370216629009
Shidfar, F., Mousavi, S. N., Lorvand, H. A., Agah, S., Hoseini, S., and Hajimiresmail, S. J. (2019). Reduction of Some Atherogenic Indices in Patients with Non−alcoholic Fatty Liver by Vitamin D and Calcium Co−supplementation: a Double Blind Randomized Controlled Clinical Trial. Iran J. Pharm. Res. 18 (1), 496–505.
Targher, G., Bertolini, L., Rodella, S., Zoppini, G., Scala, L., Zenari, L., et al. (2006). Associations between Plasma Adiponectin Concentrations and Liver Histology in Patients with Nonalcoholic Fatty Liver Disease. Clin. Endocrinol. (Oxf) 64 (6), 679–683. doi:10.1111/j.1365−2265.2006.02527.x
Taghvaei, T., Akha, O., Mouodi, M., Fakheri, H. T., Kashi, Z., Maleki, I., Mohammadpour, R., et al. (2018). Effects of Vitamin D Supplementation on Patients with Non-Alcoholic Fatty Liver Disease (NAFLD). Acta Medica. 34 (415). doi:10.19193/0393-6384_2018_2_66
Tolman, K. G., Fonseca, V., Dalpiaz, A., and Tan, M. H. (2007). Spectrum of Liver Disease in Type 2 Diabetes and Management of Patients with Diabetes and Liver Disease. Diabetes care 30 (3), 734–743. doi:10.2337/dc06−1539
Wang, H., Xia, N., Yang, Y., and Peng, D. Q. (2012). Influence of Vitamin D Supplementation on Plasma Lipid Profiles: a Meta−Analysis of Randomized Controlled Trials. Lipids Health Dis. 11 (1), 42–49. doi:10.1186/1476−511X−11−42
Keywords: vitamin D, NAFLD, liver enzyme, anthropometry, biochemical indices
Citation: Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Shabani-Borujeni M, Modaresi S, Gholamalizadeh M and Doaei S (2021) The Effects of Vitamin D Supplementation on Anthropometric and Biochemical Indices in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Pharmacol. 12:732496. doi: 10.3389/fphar.2021.732496
Received: 29 June 2021; Accepted: 06 October 2021;
Published: 03 November 2021.
Edited by:
Wolfgang Richard Stremmel, Medical Center Baden-Baden, GermanyReviewed by:
Yu Zhao, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2021 Rezaei, Tabrizi, Nowrouzi-Sohrabi, Jalali, Shabani-Borujeni, Modaresi, Gholamalizadeh and Doaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeid Doaei, c2RvYWVlQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.