Corrigendum: Effect of a Topical Nonsteroidal Anti-Inflammatory Drug (0.1% Pranoprofen) on VEGF and COX-2 Expression in Primary Pterygium
- 1Department of Ophthalmology, Nanjing Lishui District People’s Hospital, Lishui Branch of Southeast University Affiliated Zhongda Hospital, Nanjing, China
- 2Department of Ophthalmology, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Pathology, Nanjing Lishui District People’s Hospital, Lishui Branch of Southeast University Affiliated Zhongda Hospital, Nanjing, China
Purpose: To evaluate the effect of a topical nonsteroidal anti-inflammatory drug (0.1% pranoprofen) on the expression of VEGF and Cox-2 in primary pterygium.
Methods: This was a prospective, randomized study. Between January 2019 and April 2020, 120 patients diagnosed with primary pterygium were enrolled and randomly divided into three groups before operation: 1) 40 patients in group 1 received topical pranoprofen 0.1% four times daily for 4 weeks, 2) 40 patients in group 2 received topical fluorometholone 0.1% four times daily for 4 weeks, and 3) patients in group 3 did not receive treatment. For each group, the age, sex, eye type, best-corrected visual acuity (BCVA), intraocular pressure (IOP), duration of onset, combined systemic diseases, and the results regarding vascular endothelial growth factor (VEGF) and cyclo-oxygen-ase-2 (COX-2) in postoperative pterygial tissues were evaluated in detail.
Results: There were no significant differences regarding age, sex, eye type, combined systemic diseases, duration of onset, IOP, and BCVA within the three groups (p > 0.05). The reduction of VEGF and CoX-2 expression of pterygial vascular endothelial cells in group 1 were statistically significant compared to group 2 and group 3 (p < 0.05). There were significant correlations between COX-2 and VEGF expression of pterygial tissues within the three groups (p < 0.05).
Conclusion: The present findings suggested that the topical pranoprofen 0.1% could reduce the expression of VEGF and COX-2 in primary pterygium. We confirmed that treatment with pranoprofen offers advantages in early intervention and has therapeutic potential in reducing the postoperative recurrence of primary pterygium patients.
Clinical Trial registration: The study was registered with the Chinese Clinical Trial Registry. (http://www.chictr.org.cn/index.aspx, Registration Number: ChiCTR2100047726).
Introduction
Pterygium is a common, benign conjunctival degenerative disease with a global prevalence, characterized by a proliferative disorder or a neoplastic-like growth lesion in the cornea (Van Acker et al., 2019); its exact pathogenesis is not fully understood (Detorakis and Spandidos, 2009).
There are some existing clinical guidelines for treating primary pterygium. Monitoring is the indicated form of management in the early stages (Guo et al., 2019). Several interventional methods have been proposed in advanced cases, including conjunctival autograft, amniotic membrane, and other adjuvant therapies (Guo et al., 2019). However, no surgical methods can completely prevent the recurrence of pterygium (Clearfield et al., 2017). Therefore, preventing the growth of the early pterygium or reducing its postoperative recurrence is worthy of study.
Recently, some studies have reported that the formation and development of pterygium are associated with vascular endothelial growth factor (VEGF), cyclo-oxygenase (COX), and multiple proinflammatory cytokines (e.g., interleukin-6, interleukin-8) (Adiguzel et al., 2007; Park et al., 2011; Martín-López et al., 2019). Some researchers have used subconjunctival injections of anti-VEGF drugs as adjuvant therapy to treat pterygium and have achieved favourable outcomes (Singh et al., 2015; Mohamed et al., 2018). However, at present, few prospective and comprehensive studies are available on the topical effect of anti-VEGF drugs in patients with primary pterygium.
Pranoprofen is a member of the nonsteroidal anti-inflammatory drug (NSAID) family; it is an inhibitor of the COX enzyme, and has been used to treat pain, fever, and inflammation (Yoshinaga et al., 2011). Previous studies have shown that systemic application of NSAIDs was effective for the treatment of cancer due to its inhibitory effect on VEGF (Ghanghas et al., 2016; Tsoi et al., 2019). Similar outcomes were demonstrated in the treatment of VEGF-related ocular diseases, such as choroidal neovascularization and corneal angiogenesis (Pakneshan et al., 2008; Semeraro et al., 2019a). Recently, topical pranoprofen has been used to treat acute central serous chorioretinopathy and age-related macular degeneration (An and Kwon, 2016; Semeraro et al., 2019b). Fluorometholone is a corticoid, and it can inhibit inflammation by modulating inflammatory mediators such as arachidonic acid metabolites, chemokines, and cytokines, as well as their receptors. Topical fluorometholone has been proven to be safe and effective for patients with primary pterygium (Ozgurhan et al., 2013). However, to the best of our knowledge, the effect of their inhibition of VEGF and Cox-2 on pterygium has not been described in literature. Herein, we evaluated the effects of topical 0.1% pranoprofen, in comparison with fluorometholone, on the expression of VEGF and COX-2 in patients with primary pterygium.
Materials and Methods
Study Design
This study was designed as a prospective, randomized study of 120 adult patients diagnosed with primary pterygium in a tertiary general hospital. The patients were enrolled in this study between January 2019 and April 2020. The study was designed and conducted in accordance with the Declaration of Helsinki and was submitted to the appropriate review board of Lishui District People’s Hospital (2019LX01, 22/11/2018). All participating patients provided informed consent for this study. After obtaining an informed consent, the patients were randomly divided into three groups: 1) 40 patients in group 1 received topical pranoprofen 0.1% (Pranopulin; Senju Pharmaceutical Co., Ltd.) four times daily for 4 weeks, 2) 40 patients in group 2 received topical fluorometholone 0.1% (Flumetholon; Santen Pharmaceutical Co., Ltd.) four times daily for 4 weeks, and 3) 40 patients in group 3 did not receive treatment. Randomization was done at the start of the study with prepared randomized digital tables using SPSS software (Version 22.0. IBM Corp., Armonk, NY). An independent observer generated the random allocation sequence and assigned interventions. The allocation sequence was concealed using numbered and sealed opaque envelopes. All other doctors and participants were blinded during and after the assignment.
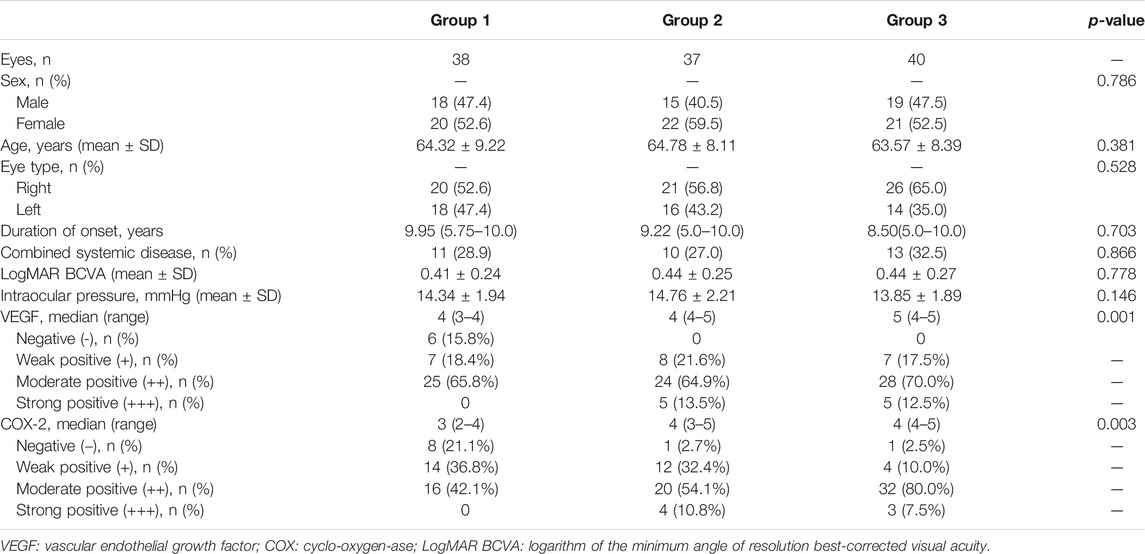
The patients’ age, sex, eye type, best-corrected visual acuity (BCVA), intraocular pressure (IOP), duration of onset, combined systemic diseases, and the results regarding VEGF and COX-2 expression in postoperative pterygial tissues were recorded. BCVA was converted to the logarithm of the minimum angle of resolution (LogMAR) for data analyses.
Patient follow-up was planned on days 7, 14, 21, and 28 after commencing treatment in groups 1 and 2. All patients were evaluated for any ocular adverse effects at each follow-up, such as burning, pain, or ocular hypertension. Patients with poor compliance or those who could not be followed up on time were withdrawn from the study. Pterygium surgeries (pterygium excision and conjunctival autograft harvest from the supero-temporal bulbar conjunctiva) were performed after 4 weeks of treatment in groups 1 and 2. All the pterygium tissue samples were obtained during the operation and sent to the Department of Pathology for evaluation of VEGF and COX-2 expression. Pterygium was confirmed using haematoxylin and eosin staining.
Inclusion Criteria
Patients with primary pterygium tissue invading the cornea between 2 and 3 mm from the nasal conjunctiva were enrolled.
Exclusion Criteria
The exclusion criteria were as follows: 1) pseudopterygium; 2) recurrent pterygium; 3) pterygium with active ocular inflammation; 4) patients who had poor compliance; 5) patients who refused surgery; and 5) the invasion of pterygium tissue was less than 2 mm or more than 3 mm into the cornea.
Haematoxylin and Eosin staining
Samples from each patient initially underwent haematoxylin and eosin staining. The surgical tissues were fixed in 4% neutral formaldehyde solution for 12 h, and routinely embedded in paraffin, then the tissues were cut into 5 μm slices. The slides were stained with hematoxylin for 8 min and washed several times in running tap water. Then the slides were stained in eosin for 1 min. After that, the slides were dehydrated by placing them consecutively in 80% ethanol, 95% ethanol I and 95% ethanol II solutions for 3 min each time, and they were finally soaked in xylene for 3 min. Air dried slides were mounted with neutral resin and covered with coverslip. Slides were examined by light microscopy.
Immunohistochemistry
Immunohistochemical staining for VEGF and COX-2 was performed on 4-μm-thick formalin-fixed and paraffin-embedded sections of surgical specimens from pterygium patients using the Elivision™ plus two-step system (BENCHMARKXT; Roche, Switzerland). VEGF and COX-2 staining was evaluated in the cytoplasm of vascular endothelial cells. Three slices from each patient and five fields in each slice were analyzed.
Scoring of Stained Tissue Sections
The expression of VEGF and COX-2 was classified according to the staining intensity and percentage of positively-stained cells. A 4-point rating scale was used to evaluate the staining intensity (0, no staining; 1, weak; 2, moderate; and 3, strong). A 5-point rating scale was used to represent the percentage of positively-stained cells: (0, ≤10%; 1, 11–25%; 2, 26–50%; 3, 51–75%; and 4, > 75%). Finally, a total score (TS) was calculated from the sum of these scores to reflect the expression level: score 0–1, negative (-); score 2–3, weak positive (+); score 4–5, moderate positive (++); and score 6–7, strong positive (+++). The sections were coded and evaluated by two independent experienced pathologists (Dr. Xiaoli Yue and Dr. Ting Zhang), who were blinded to the patients’ data. If discrepancies occurred, a third investigator (Dr. Hongbing Xiong) evaluated the tissue sections to obtain consistent results and a consensus was reached among the three investigators. The TS and expression levels of VEGF and COX-2 in the tissue sections of the three groups were statistically observed, and the differences between the three groups were analyzed.
Statistical Analysis
Data from the patients’ clinical records were processed with SPSS (Statistical Package for Social Sciences, version 23.0, IBM). Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as counts (%). For categorical variables, Pearson’s χ2 test was used; for continuous variables, the Kruskal–Wallis test was used. Relationships between VEGF and COX-2 expression were assessed using Spearman’s correlation analysis. Two-tailed tests of significance were performed, and p-values <0.05 were considered statistically significant.
Results
Demographics
A total of 120 patients diagnosed with primary pterygium were randomly divided into three groups. There was a loss of two patients in group 1 and three patients in group 2 during the study period because two patients moved to different cities and three patients did not comply with the surgery and follow-up schedule. Therefore, the data of 115 patients with primary pterygium were included in the final analysis.
In the final 115 patients, 52 were male and 63 were female; their average age was 64.21 ± 8.53 years (range 45–85). Primary pterygium occurred in the right eye in 67 patients, and in the left eye in 48 patients. Group 1 (n = 38) comprised 18 (47.4%) men and 20 (52.6%) women, with an average age of 64.32 ± 9.22 years. It included 20 (52.6%) and 18 (47.4%) cases of primary pterygium in the right eye and left eye, respectively. The median duration of onset was 9.95 years, and 11 (28.9%) cases were combined with systemic diseases. Group 2 (n = 37), comprised 15 (40.5%) men and 22 (59.5%) women with an average age of 64.78 ± 8.11 years. It included 21 (56.8%) and 16 (43.2%) cases of primary pterygium in the right eye and left eye, respectively. The median duration of onset was 9.22 years, and 10 cases (27.0%) were combined with systemic diseases. Group 3 (n = 40) comprised 19 (47.5%) men and 21 (52.5%) women with an average age of 63.57 ± 8.39 years. It included 26 (65.0%) and 14 (35.0%) cases of primary pterygium in the right eye and left eye, respectively. The median duration of onset was 8.5 years, and 13 (32.5%) cases were combined with systemic diseases (Table 1). No obvious ocular side effects were observed in our study.
Post-Surgical Evaluation of Clinical Outcomes
The suture was removed at the 7-days follow-up after the operation. The cornea was completely repaired within 10 days, and the conjunctival wound was not cracked or curled. There were no postoperative complications such as graft detachment, corneal ulcer, or formation of granulomas. At the 9-months follow-up, there was recurrence in 2 (5.3%), 2 (5.4%), and 3 (7.5%) cases in groups 1, 2, and 3, respectively (all p > 0.05, not showed in the table). The recurrence was defined by any new fibrovascular tissue crossing the limbus and extending over the cornea.
Combined Systemic Diseases
In group 1, 10 cases were complicated with hypertension, and one was complicated with diabetes mellitus. In group 2, there were eight and two cases complicated with hypertension and diabetes mellitus, respectively. In group 3, there were 11 and two cases complicated with hypertension and diabetes mellitus, respectively (Table 1).
Haematoxylin and Eosin staining
All the haematoxylin and eosin staining slides can be characterized by the atrophy and/or hyperplasia of epithelial cells, with the presence of increased goblet cells, distinct fibroblast proliferation with mild inflammatory cell infiltration were observed under the mucosa, the capillary hyperplasia and vasodilation were also noted (Figures 1A,D,G). These evidences were consistent with the pathological findings of pterygium.
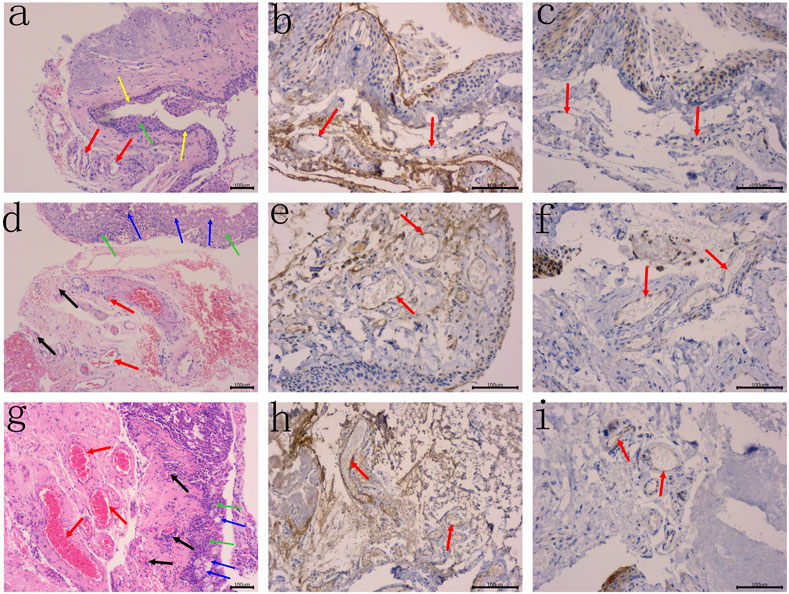
FIGURE 1. The atrophy of epithelial cells (yellow arrows) and/or hyperplasia (green arrows), with the presence of increased goblet cells (blue arrows) were noted, distinct fibroblast proliferation with mild inflammatory cell infiltration were observed under the mucosa. (A) HE staining of human primary pterygium (case 15 in group 1) is demonstrated, the small vessels (red arrows) were observed, without evidence of capillary hyperplasia and vasodilation, original magnification: 100×. (B–C) Primary pterygium specimen with negative VEGF and COX-2 expression, respectively. No staining reaction in the cytoplasm of vascular enothelial cells (red arrows), original magnification: B–C 200×. (D) HE staining of human primary pterygium (case 9 in group 2) is demonstrated, moderate capillary hyperplasia indicating angiogenesis (black arrows), and moderate vasodilation (red arrows) were noted, original magnification: 100×. (E–F) Primary pterygium specimen with moderate VEGF and COX-2 expression, respectively. Note the typical diffuse staining reaction in the cytoplasm of vascular endothelial cells (red arrows), original magnification: E–F 200×. (G) HE staining of human primary pterygium (case 37 in group 3) is demonstrated, capillary cluster hyperplasia indicating severe angiogenesis (black arrows), and severe vasodilation (red arrows) were observed, original magnification: 100×. (H–I) Primary pterygium specimen with high VEGF and COX-2 expression, respectively. Note the typical diffuse staining reaction in the cytoplasm of vascular endothelial cells (red arrows), original magnification: H–I 200×.
VEGF
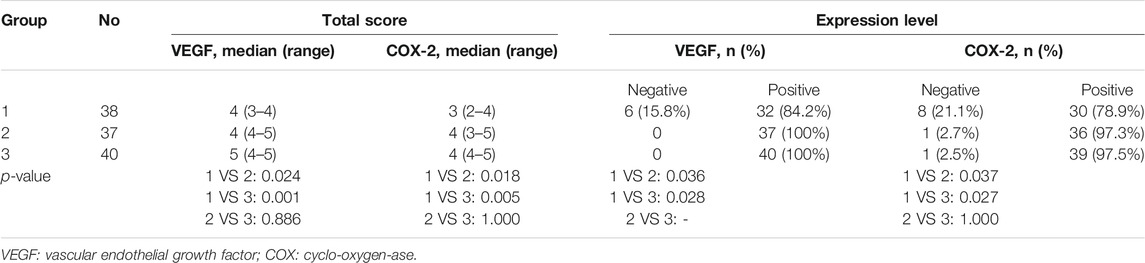
The VEGF expression levels in the pterygium are listed in Tables 1 and 2. In group 1, there were 6, 7, 19, and 6 samples with a TS of 0, 3, 4, and 5, respectively. Six (15.8%), 7 (18.4%), and 25 (65.8%) samples were classified as negative (-), weak positive (+), and moderate positive (++), respectively. No samples were classified as strong positive (+++). In group 2, there were 1, 7, 13, 11, and 4 samples with a TS of 2, 3, 4, 5, and 6, respectively. One sample had a TS of 7. Eight (21.6%), 24 (64.9%), and 5 (13.5%) samples were classified as weak positive (+), moderate positive (++), and strong positive (+++), respectively. No sample was classified as negative (-). In group 3, there were 1, 6, 9, 19, and 3 samples with a TS of 2, 3, 4, 5, and 6, respectively. Two samples had a TS of 7. Seven (17.5%), 28 (70.0%), and 5 (12.5%) samples were classified as weak positive (+), moderate positive (++), and strong positive (+++), respectively. No sample was classified as negative (-) (Figures 1B, E, H) (Tables 1, 2).
COX-2
The COX-2 expression levels in pterygium studied are listed in Tables 1, 2. In group 1, there were 3, 5, 4, 10, 9, and 7 samples with a TS of 0, 1, 2, 3, 4, and 5, respectively. Eight (21.1%), 14 (36.8%), and 16 (42.1%) samples were classified as negative (-), weak positive (+), and moderate positive (++), respectively. No samples were classified as strong positive (+++). In group 2, there were 1, 5, 7, 8, 12, and 4 samples with a TS of 1, 2, 3, 4, 5, and 6, respectively. One (2.7%), 12 (32.4%), 20 (54.1%), and 4 (10.8%) samples were classified as negative (-), weak positive (+), moderate positive (++), and strong positive (+++). In group 3, there were 1, 3, 1, 23, 9, and 3 samples with a TS of 1, 2, 3, 4, 5, and 6, respectively. One (2.5%), 4 (10.0%), 32 (80.0%), and 3 (7.5%) samples were classified as negative (-), weak positive (+), moderate positive (++), and strong positive (+++), respectively (Figure 1) (Tables 1, 2).
Correlations Between TS and VEGF and COX-2 Expression
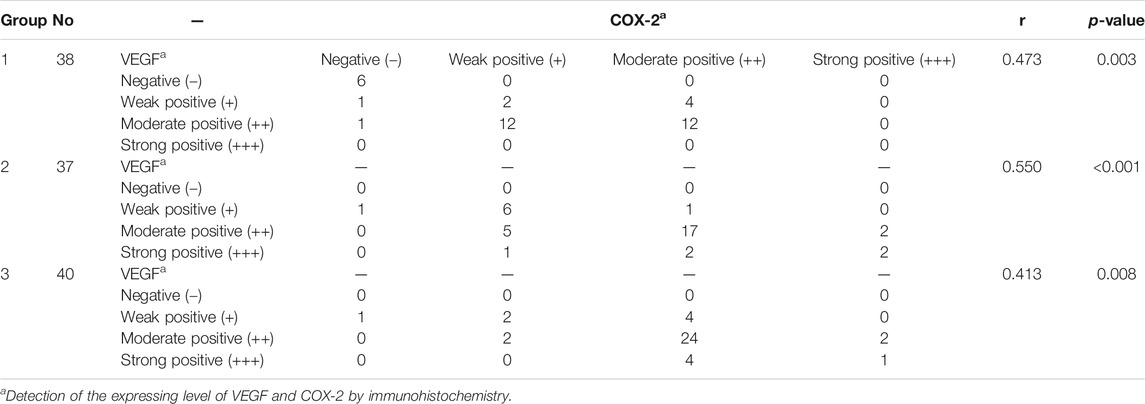
There were significant correlations between the TS and expression of VEGF and COX-2 in pterygial vascular endothelial cells within groups 1, 2, and 3 (all p < 0.05, Spearman’s coefficient of correlation), as shown in Figure 2 and Table 3, respectively.
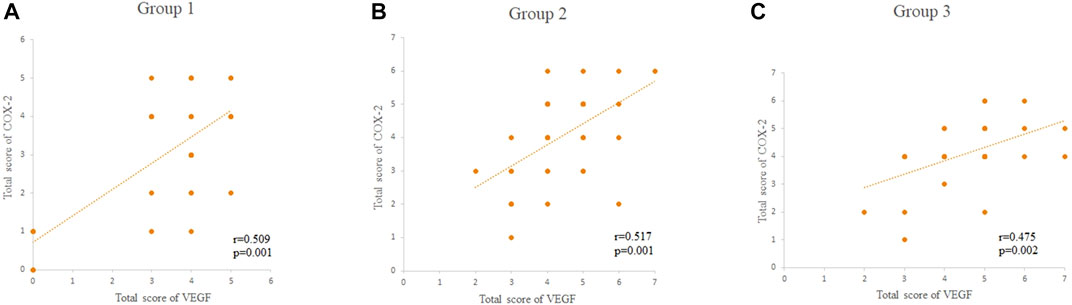
FIGURE 2. (A–C) The correlations between the total score of VEGF and COX-2 within the groups 1, 2, and 3, respectively. The total score of VEGF and COX-2 was scored as 0–7. HE: Hematoxylin and eosin; VEGF: vascular endothelial growth factor; COX-2: cyclo-oxygen-ase-2.
Group Comparisons
There were no significant differences in age, sex, eye type, combined systemic diseases, duration of the disease, IOP, and BCVA among the three groups (all p > 0.05). The TS and expression levels of VEGF and COX-2 in pterygium tissues in group 1 were significantly lower than those in groups 2 and 3 (all p < 0.05). However, there were no significant differences in TS and expression levels of VEGF and COX-2 between groups 3 and 2 (all p > 0.05) (Tables 1, 2).
Discussion
Pterygium is a common, benign conjunctival degenerative disease, which has a widespread incidence (Van Acker et al., 2019). While the underlying mechanism remains unclear, it is generally assumed that it is associated with exposure to ultraviolet irradiation (Detorakis and Spandidos, 2009). In our study, we evaluated the effect of topical 0.1% pranoprofen on the expression of VEGF and Cox-2 in 38 primary pterygium tissues. Our results revealed that VEGF and COX-2 expression was increased in vascular endothelial cells in group 3, which demonstrated that they may be part of the critical pathophysiological mechanism of primary pterygium. Compared to group 2 and group 3, the expression of VEGF and COX-2 in group 1 was significantly decreased, indicating that the application of 0.1% pranoprofen inhibited VEGF and COX-2 expression.
The formation and development of cancer has been shown to be closely associated with the overexpression of VEGF (Padera et al., 2008). Similarly, the pterygium presents tumour-like features (Chui et al., 2011), and recent evidence has indicated that the formation and development of pterygium are probably related to increased expression of VEGF, COX, and multiple proinflammatory cytokines (Martín-López et al., 2019; Adiguzel et al., 2007; Park et al., 2011). Wu et al. (2017) found that long-term exposure to ultraviolet radiation caused the upregulation of these factors, which may result in pterygium. VEGF was demonstrated to be the most potent factor (Detorakis and Spandidos, 2009). Fukuhara et al. (2013) showed that VEGF expression was increased in the vascular endothelial component of pterygium compared with the normal conjunction.
The beneficial effects of anti-VEGF therapies in the treatment of primary pterygium have been widely reported. Sarac et al. (2014), Singh et al. (2015) have reported the regression of pterygial tissues and corneal neovascularization in patients with pterygium who received bevacizumab injections into the pterygial tissue. Nava Castaneda et al. (2014) also reported a lower recurrence rate after adjuvant subconjunctival bevacizumab injection to the conjunctival autograft for primary pterygium. Kasetsuwan et al. (2015) investigated the use of topical bevacizumab after pterygium excision and reported similar beneficial effects. However, Nava-Castaneda et al. (2014) found conjunctival autograft ischemia was a common complication after subconjunctival bevacizumab injections. In addition, anti-VEGF therapies are relatively expensive, invasive, and must be administered frequently.
Recently, NSAIDs have been proven to be potent for the treatment of cancer due to their inhibitory effect on VEGF and COX-2 (Ghanghas et al., 2016; Tsoi et al., 2019). Similarly, reports have demonstrated that NSAIDs are effective in suppressing the retinal and corneal angiogenic response to VEGF (Pakneshan et al., 2008; Semeraro et al., 2019a), and nonselective NSAIDs have been shown to suppress VEGF-induced angiogenesis better than selective COX inhibitors (Singer et al., 2015). However, the exact mechanism by which NSAIDs inhibit VEGF remains unknown. Yoshinaga et al. (2011) proposed that NSAIDs inhibit VEGF-related choroidal neovascularization through the HO-1-dependent pathway, while Pakneshan et al. (2008) proposed that NSAIDs suppress the expression of VEGF by inhibiting the COX enzyme.
Pranopulin is a 0.1% pranoprofen topical ophthalmic agent. Topical pranoprofen non-selectively inhibits the expression of COX-1 and COX-2, suppresses the bio-activity of prostaglandins, and is used to alleviate ocular inflammation, cystoid macular oedema, and postoperative uncomfortable symptoms (Akyol-Salman et al., 2007; Zhu et al., 2018). Recent studies have found that 0.1% pranoprofen was well tolerated and benefited patients with dry eye (Chen et al., 2014). Compared with 0.1% fluorometholone, pranoprofen reduced the IOP level and indicated better anti-inflammatory efficacy and safety (Li et al., 2013). Topical pranoprofen has been proven effective for acute central serous chorioretinopathy and age-related macular degeneration (An and Kwon, 2016; Semeraro et al., 2019b).
Based on the above findings, we assumed that the application of a topical nonsteroidal anti-inflammatory drug (0.1% pranoprofen) may be effective and safe for pterygium. Postoperative recurrence of pterygium patients has been shown to be related to the levels of VEGF and inflammatory factors (Adiguzel et al., 2007). In the present study, we did not observe any difference in postoperative recurrence between the three groups in the 9-months follow-up period, and we concluded that this was due to the following reasons. First, the follow-up duration in our study may not be sufficient to evaluate the efficacy of our interventions with respect to the recurrence. We consider that recurrence would be lower in group 1 than in the two other groups in the long term follow-up. Second, the recurrent and total sample size in each group was small. More data from multiple centers and longer-term follow-up are required to better understand the effect of topical 0.1% pranoprofen on primary pterygium. No obvious ocular side effects were observed in our study, suggesting that the treatments in groups 1 and 2 were safe and well-tolerated.
Glucocorticoids have been shown to suppress the expression of several inflammation-related factors such as arachidonic acid and COX-2, and are used for their anti-inflammatory effect (Lim et al., 2014). In group 2, the patients were treated with topical 0.1% fluorometholone; however, there were no significant differences in the expression of VEGF and COX-2 between groups 2 and 3. This suggests that topical 0.1% fluorometholone cannot downregulate the expression of COX-2. A previous study of nasal polyps by Pujols et al. (2009) has reported similar findings. As a result, we suggest that the true effect of glucocorticoids on COX-2 in the pterygium tissues may be unclear.
Our analysis of the correlations between the TS and VEGF and COX-2 expression within the three groups found that Cox-2 and VEGF expression was closely correlated in the pterygium tissues, which is consistent with the discovery of Liu D et al. (2017). VEGF and COX-2 were frequently found at the basal and middle layers of the endothelial cells of the microvessels. It also demonstrated that the over-expression of COX-2 is associated with increased levels of angiogenic factors, including VEGF in cultured human umbilical vein endothelial cells (Koolwijk et al., 1996). Therefore, we concluded that inflammatory factors (e.g., interleukin-6, interleukin-8, and prostaglandin) may have a significant role in the pathogenesis of vascular permeability, which can induce the expression of VEGF. Similarly, VEGF may stimulate COX-2 expression in pterygial vascular endothelial cells.
There were several limitations to this study. First, it had a small sample size. Second, the follow-up time was relatively short. Third, for ethical reasons, normal conjunctival tissue could not be obtained during the operation. Fourth, it is unknown whether 0.1% pranoprofen is effective for recurrent pterygium.
Conclusion
We obtained data on the effect of 0.1% pranoprofen on the expression of VEGF and COX-2 in primary pterygium tissue, providing an evidence-based basis for the early treatment of pterygium. We found that VEGF and COX-2 expression was closely correlated in the pterygium tissues. In other words, we confirmed that treatment with pranoprofen offers advantages in early intervention and has therapeutic potential in reducing the postoperative recurrence of primary pterygium patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Lishui District People’s Hospital (2019LX01). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BY wrote the manuscript, established the diagnosis and reviewed the manuscript; YD and GL collected the data of the patient, edited the manuscript and analyzed the data; XY evaluate the tissue sections, FW and XZ consulted literatures, BW dealt with the tables. All authors read and approved the final manuscript.
Funding
The current study was supported by Clinical Medical Science and Technology Development Foundation of Jiangsu University (Grant Nos. JLY20180034).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Ting Zhang, and Hongbing Xiong (Lishui District People’s Hospital, Nanjing, Jiangsu Province, China) for their pathological examination and evaluation. We also thank Jie Luan (Southeast University Affiliated Zhongda Hospital, Nanjing, Jiangsu Province, China) and Zhaoyang Dong (Jiangning District Hospital of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China) for their precious suggestions of this study.
Abbreviations
BCVA, best-corrected visual acuity; COX, cyclo-oxygen-ase; IOP, intraocular pressure; LogMAR, logarithm of the minimum angle of resolution; NSAID, nonsteroidal anti-inflammatory drug; TS, total score; VEGF, vascular endothelial growth factor.
References
Adiguzel, U., Karabacak, T., Sari, A., Oz, O., and Cinel, L. (2007). Cyclooxygenase-2 Expression in Primary and Recurrent Pterygium. Eur. J. Ophthalmol. 17, 879–884. doi:10.1177/112067210701700602
Akyol-Salman, I., Leçe-Sertöz, D., and Baykal, O. (2007). Topical Pranoprofen 0.1% Is as Effective Anti-inflammatory and Analgesic Agent as Diclofenac Sodium 0.1% after Strabismus Surgery. J. Ocul. Pharmacol. Ther. 23, 280–283. doi:10.1089/jop.2006.108
An, S. H., and Kwon, Y. H. (2016). Effect of a Topical Nonsteroidal Anti-inflammatory Agent (0.1 % Pranoprofen) on Acute central Serous Chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 254, 1489–1496. doi:10.1007/s00417-015-3215-8
Chen, J., Dong, F., Chen, W., Sun, X., Deng, Y., Hong, J., et al. (2014). Clinical Efficacy of 0.1% Pranoprofen in Treatment of Dry Eye Patients: a Multicenter, Randomized, Controlled Clinical Trial. Chin. Med. J. (Engl) 127, 2407–2412.
Chui, J., Coroneo, M. T., Tat, L. T., Crouch, R., Wakefield, D., and Di Girolamo, N. (2011). Ophthalmic Pterygium. Am. J. Pathol. 178, 817–827. doi:10.1016/j.ajpath.2010.10.037
Clearfield, E., Hawkins, B. S., and Kuo, I. C. (2017). Conjunctival Autograft versus Amniotic Membrane Transplantation for Treatment of Pterygium: Findings from a Cochrane Systematic Review. Am. J. Ophthalmol. 182, 8–17. doi:10.1016/j.ajo.2017.07.004
Detorakis, E. T., and Spandidos, D. A. (2009). Pathogenetic Mechanisms and Treatment Options for Ophthalmic Pterygium: Trends and Perspectives (Review). Int. J. Mol. Med. 23, 439–447. doi:10.3892/ijmm_00000149
Fukuhara, J., Kase, S., Ohashi, T., Ando, R., Dong, Z., Noda, K., et al. (2013). Expression of Vascular Endothelial Growth Factor C in Human Pterygium. Histochem. Cel Biol 139, 381–389. doi:10.1007/s00418-012-1019-z
Ghanghas, P., Jain, S., Rana, C., and Sanyal, S. N. (2016). Chemoprevention of Colon Cancer through Inhibition of Angiogenesis and Induction of Apoptosis by Nonsteroidal Anti-inflammatory Drugs. J. Environ. Pathol. Toxicol. Oncol. 35, 273–289. doi:10.1615/jenvironpatholtoxicoloncol.2016015704
Guo, Q., Li, X., Cui, M. N., Liang, Y., Li, X. P., Zhao, J., et al. (2019). Low-Dose Mitomycin C Decreases the Postoperative Recurrence Rate of Pterygium by Perturbing NLRP3 Inflammatory Signalling Pathway and Suppressing the Expression of Inflammatory Factors. J. Ophthalmol. 15, 2019.9472782
Kasetsuwan, N., Reinprayoon, U., and Satitpitakul, V. (2015). Prevention of Recurrent Pterygium with Topical Bevacizumab 0.05% Eye Drops: a Randomized Controlled Trial. Clin. Ther. 37, 2347–2351. doi:10.1016/j.clinthera.2015.08.023
Koolwijk, P., van Erck, M. G., de Vree, W. J., Vermeer, M. A., Weich, H. A., Hanemaaijer, R., et al. (1996). Cooperative Effect of TNFalpha, bFGF, and VEGF on the Formation of Tubular Structures of Human Microvascular Endothelial Cells in a Fibrin Matrix. Role of Urokinase Activity. J. Cel Biol 132, 1177–1188. doi:10.1083/jcb.132.6.1177
Li, Z., Mu, G., Chen, W., Gao, L., Jhanji, V., and Wang, L. (2013). Comparative Evaluation of Topical Pranoprofen and Fluorometholone in Cases with Chronic Allergic Conjunctivitis. Cornea 32, 579–582. doi:10.1097/ico.0b013e318265684b
Lim, W., Park, C., Shim, M. K., Lee, Y. H., Lee, Y. M., and Lee, Y. (2014). Glucocorticoids Suppress Hypoxia-Induced COX-2 and Hypoxia Inducible Factor-1α Expression through the Induction of Glucocorticoid-Induced Leucine Zipper. Br. J. Pharmacol. 171, 735–745. doi:10.1111/bph.12491
Liu, D., Peng, C., Jiang, Z., and Tao, L. (2017). Relationship between Expression of Cyclooxygenase 2 and Neovascularization in Human Pterygia. Oncotarget 8, 105630–105636. doi:10.18632/oncotarget.22351
Martín-López, J., Pérez-Rico, C., García-Honduvilla, N., Buján, J., and Pascual, G. (2019). Elevated Blood/lymphatic Vessel Ratio in Pterygium and its Relationship with Vascular Endothelial Growth Factor (VEGF) Distribution. Histol. Histopathol 34, 917–929.
Mohamed, T. A., Soliman, W., Fathalla, A. M., and El Refaie, A. (2018). Effect of Single Subconjunctival Injection of Bevacizumab on Primary Pterygium: Clinical, Histopathological and Immunohistochemical Study. Int. J. Ophthalmol. 11, 797–801. doi:10.18240/ijo.2018.05.13
Nava-Castañeda, A., Olvera-Morales, O., Ramos-Castellon, C., Garnica-Hayashi, L., and Garfias, Y. (2014). Randomized, Controlled Trial of Conjunctival Autografting Combined with Subconjunctival Bevacizumab for Primary Pterygium Treatment: 1-year Follow-Up. Clin. Exp. Ophthalmol 42, 235–241. doi:10.1111/ceo.12140
Ozgurhan, E. B., Kara, N., Bozkurt, E., Gencer, B., Agca, A., Alkin, Z., et al. (2013). Effect of Fluorometholone/tetrahydrozoline Fixed Combination on Conjunctival Autograft Morphology after Primary Pterygium Excision. Biomed. Res. Int. 2013, 481843. doi:10.1155/2013/481843
Padera, T. P., Kuo, A. H., Hoshida, T., Liao, S., Lobo, J., Kozak, K. R., et al. (2008). Differential Response of Primary Tumor versus Lymphatic Metastasis to VEGFR-2 and VEGFR-3 Kinase Inhibitors Cediranib and Vandetanib. Mol. Cancer Ther. 7, 2272–2279. doi:10.1158/1535-7163.mct-08-0182
Pakneshan, P., Birsner, A. E., Adini, I., Becker, C. M., and D'Amato, R. J. (2008). Differential Suppression of Vascular Permeability and Corneal Angiogenesis by Nonsteroidal Anti-inflammatory Drugs. Invest. Ophthalmol. Vis. Sci. 49, 3909–3913. doi:10.1167/iovs.07-1527
Park, C. Y., Choi, J. S., Lee, S. J., Hwang, S. W., Kim, E. J., and Chuck, R. S. (2011). Cyclooxygenase-2-expressing Macrophages in Human Pterygium Co-express Vascular Endothelial Growth Factor. Mol. Vis. 17, 3468–3480.
Pujols, L., Benitez, P., Alobid, I., Martinez-Antón, A., Roca-Ferrer, J., Mullol, J., et al. (2009). Glucocorticoid Therapy Increases COX-2 Gene Expression in Nasal Polyps In Vivo. Eur. Respir. J. 33, 502–508. doi:10.1111/bph.12491
Sarac, O., Demirel, S., and Oltulu, R. (2014). Efficacy of Intralesional Bevacizumab Administration in Primary Pterygium. Eye Contact Lens 40, 46–50. doi:10.1097/icl.0000000000000004
Semeraro, F., Gambicordi, E., Cancarini, A., Morescalchi, F., Costagliola, C., and Russo, A. (2019). Treatment of Exudative Age‐related Macular Degeneration with Aflibercept Combined with Pranoprofen Eye Drops or Nutraceutical Support with omega‐3: A Randomized Trial. Br. J. Clin. Pharmacol. 85, 908–913. doi:10.1111/bcp.13871
Semeraro, F., Morescalchi, F., Cancarini, A., Russo, A., Rezzola, S., and Costagliola, C. (2019). Diabetic Retinopathy, a Vascular and Inflammatory Disease: Therapeutic Implications. Diabetes Metab. 45, 517–527. doi:10.1016/j.diabet.2019.04.002
Singer, D. D., Kennedy, J., and Wittpenn, J. R. (2015). Topical NSAIDs Effect on Corneal Sensitivity. Cornea 34, 541–543. doi:10.1097/ico.0000000000000309
Singh, P., Sarkar, L., Sethi, H., and Gupta, V. (2015). A Randomized Controlled Prospective Study to Assess the Role of Subconjunctival Bevacizumab in Primary Pterygium Surgery in Indian Patients. Indian J. Ophthalmol. 63, 779–784. doi:10.4103/0301-4738.171508
Tsoi, K. K. F., Ho, J. M. W., Chan, F. C. H., and Sung, J. J. Y. (2019). Long‐term Use of Low‐dose Aspirin for Cancer Prevention: A 10‐year Population Cohort Study in Hong Kong. Int. J. Cancer 145, 267–273. doi:10.1002/ijc.32083
Van Acker, S. I., Haagdorens, M., Roelant, E., Rozema, J., Possemiers, T., Van Gerwen, V., et al. (2019). Pterygium Pathology: A Prospective Case-Control Study on Tear Film Cytokine Levels. Mediators Inflamm. 12, 9416262.
Wu, M., Wang, J., Zhang, Q., Wang, Y., Niu, L., and Shao, T. (2017). Overexpression of Low-Density Lipoprotein Receptors Stimulated by Vascular Endothelial Growth Factor in Fibroblasts from Pterygium. Biomed. Pharmacother. 93, 609–615. doi:10.1016/j.biopha.2017.06.090
Yoshinaga, N., Arimura, N., Otsuka, H., Kawahara, K.-i., Hashiguchi, T., Maruyama, I., et al. (2011). NSAIDs Inhibit Neovascularization of Choroid through HO-1-dependent Pathway. Lab. Invest. 91, 1277–1290. doi:10.1038/labinvest.2011.101
Keywords: 01% Pranoprofen, vascular endothelial growth factor, cyclo-oxygen-ase-2, pterygium, nonsteroidal anti-inflammatory drug
Citation: Yao B, Wang F, Zhao X, Wang B, Yue X, Ding Y and Liu G (2021) Effect of a Topical Nonsteroidal Anti-Inflammatory Drug (0.1% Pranoprofen) on VEGF and COX-2 Expression in Primary Pterygium. Front. Pharmacol. 12:709251. doi: 10.3389/fphar.2021.709251
Received: 14 May 2021; Accepted: 30 June 2021;
Published: 09 July 2021.
Edited by:
Stefania Tacconelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Peter Anguria, Wits Health Consortium (WHC), South AfricaDaniela Almeida Cabrini, Federal University of Paraná, Brazil
Copyright © 2021 Yao, Wang, Zhao, Wang, Yue, Ding and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangtao Yao, eWFvYmFtdGFvX25qbXVAMTYzLmNvbQ==; Gang Liu, bGcxOTc0MzI5QDEyNi5jb20=; Yuhua Ding, ZGluZ3l1aHVhX25qbXVAMTYzLmNvbQ==; Xiaoli Yue, Y2h5dWVjaDIwMjBAMTYzLmNvbQ==
 Bangtao Yao
Bangtao Yao Fei Wang
Fei Wang Xiaogui Zhao
Xiaogui Zhao Bei Wang
Bei Wang Xiaoli Yue
Xiaoli Yue Yuhua Ding
Yuhua Ding Gang Liu
Gang Liu