
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 06 July 2021
Sec. Neuropharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.705254
This article is part of the Research Topic Stimulant Use and Addictive Disorder View all 22 articles
 Jason Yuen1,2
Jason Yuen1,2 Abhinav Goyal1,3
Abhinav Goyal1,3 Aaron E. Rusheen1,3
Aaron E. Rusheen1,3 Abbas Z. Kouzani4
Abbas Z. Kouzani4 Michael Berk2
Michael Berk2 Jee Hyun Kim2
Jee Hyun Kim2 Susannah J. Tye5
Susannah J. Tye5 Charles D. Blaha1
Charles D. Blaha1 Kevin E. Bennet1,6
Kevin E. Bennet1,6 Dong-Pyo Jang7
Dong-Pyo Jang7 Kendall H. Lee1,8
Kendall H. Lee1,8 Hojin Shin1
Hojin Shin1 Yoonbae Oh1,8*
Yoonbae Oh1,8*For over 40 years, in vivo microdialysis techniques have been at the forefront in measuring the effects of illicit substances on brain tonic extracellular levels of dopamine that underlie many aspects of drug addiction. However, the size of microdialysis probes and sampling rate may limit this technique’s ability to provide an accurate assessment of drug effects in microneural environments. A novel electrochemical method known as multiple-cyclic square wave voltammetry (M-CSWV), was recently developed to measure second-to-second changes in tonic dopamine levels at microelectrodes, providing spatiotemporal resolution superior to microdialysis. Here, we utilized M-CSWV and fast-scan cyclic voltammetry (FSCV) to measure changes in tonic or phasic dopamine release in the nucleus accumbens core (NAcc) after acute cocaine administration. Carbon-fiber microelectrodes (CFM) and stimulating electrodes were implanted into the NAcc and medial forebrain bundle (MFB) of urethane anesthetized (1.5 g/kg i.p.) Sprague-Dawley rats, respectively. Using FSCV, depths of each electrode were optimized by determining maximal MFB electrical stimulation-evoked phasic dopamine release. Changes in phasic responses were measured after a single dose of intravenous saline or cocaine hydrochloride (3 mg/kg; n = 4). In a separate group, changes in tonic dopamine levels were measured using M-CSWV after intravenous saline and after cocaine hydrochloride (3 mg/kg; n = 5). Both the phasic and tonic dopamine responses in the NAcc were augmented by the injection of cocaine compared to saline control. The phasic and tonic levels changed by approximately x2.4 and x1.9, respectively. These increases were largely consistent with previous studies using FSCV and microdialysis. However, the minimal disruption/disturbance of neuronal tissue by the CFM may explain why the baseline tonic dopamine values (134 ± 32 nM) measured by M-CSWV were found to be 10-fold higher when compared to conventional microdialysis. In this study, we demonstrated phasic dopamine dynamics in the NAcc with acute cocaine administration. M-CSWV was able to record rapid changes in tonic levels of dopamine, which cannot be achieved with other current voltammetric techniques. Taken together, M-CSWV has the potential to provide an unprecedented level of physiologic insight into dopamine signaling, both in vitro and in vivo, which will significantly enhance our understanding of neurochemical mechanisms underlying psychiatric conditions.
Substance dependence is a global public health problem. A 2019 survey revealed 20.4 million people aged 12 or older in the United States suffered from substance use disorder (Substance Abuse and Mental Health Services Administration, 2020). Around 40–60% of patients experience relapse within one year of treatment discharge (McLellan et al., 2000), which is hypothesized to be a result of long-term neuroplastic changes after chronic drug use (Kalivas and O'Brien, 2008). Therefore, it is important to understand the neurobiology of addiction in order to improve our treatment strategies.
Dopamine is widely implicated in addiction. Its functions include determining the incentive value of naturally occurring positive rewarding stimuli (e.g., food, water, and conspecific mates) (Blaha and Phillips, 1996). Previous studies have also demonstrated that dopamine release in the nucleus accumbens, dorsal striatum, and the prefrontal cortex is a cardinal feature in models of addiction, with dopamine receptor blockade in these areas disrupting drug-seeking behaviors (Berke and Hyman, 2000; Ito et al., 2002; Vanderschuren et al., 2005; Murray et al., 2012; Zbukvic et al., 2016; Hodebourg et al., 2019). However, measuring dopamine with high temporal and spatial resolution in vivo is a major challenge.
There are generally two distinct patterns of spike firing exhibited by neuronal dopamine-containing cells in the mammalian midbrain: tonic activity and phasic burst activity (Grace, 1991; Grace, 2016). Phasic activity causes a transient and robust release of dopamine in the synapse that serves as a learning signal for neural plasticity (Schultz, 2007). Tonic activity refers to continuous spontaneous extra-synaptic dopamine release driven by pacemaker-like firing of dopamine neurons, providing a relatively homeostatic extracellular level of dopamine (i.e., tonic concentration) in the striatum thought to modulate behavioral flexibility (Goto et al., 2007).
Tonic concentrations of dopamine in the brain have been typically quantified in the sub-nM range using microdialysis (Watson et al., 2006; Gu et al., 2015). Microdialysis has been utilized for sampling neurochemical substances, such as dopamine with high selectivity and sensitivity. However, it has several drawbacks when compared to electrochemical techniques (Robinson et al., 2003; Heien et al., 2004; Chefer et al., 2009; Rodeberg et al., 2017; Kim et al., 2021). These include limited temporal resolution ( ≥ 1 min) in comparison to voltammetry (milliseconds to seconds), and the relatively large dimensions of dialysis probes (typically > 200 μm), resulting in variable physicochemical characteristics, tissue damage, and relatively low spatiotemporal resolution (Morelli et al., 1992; Di Chiara et al., 1993; Blaha et al., 1996; Chefer et al., 2009; Oh et al., 2018; Rusheen et al., 2020). For these reasons, and the fact that it requires continuous sampling from the brain and laboratory analysis, its application in the human nervous system is limited.
In contrast, electrochemical methods, such as fast-scan cyclic voltammetry (FSCV), have features that are well-suited to quantitatively measure changes in extracellular dopamine concentrations (Millar, 1997; Robinson et al., 2003; Huffman and Venton, 2009; Lama et al., 2012). FSCV provides excellent temporal resolution (milliseconds time response) and detection sensitivity ( <5 nM). In this technique, a carbon-fiber microelectrode (CFM, diameter typically <10 μm) is held at a resting potential and then ramped to an electric potential sufficient to oxidize and reduce the electroactive species before the potential is returned to the resting potential (Robinson et al., 2003). This results in a measured current, which yields a cyclic voltammogram. The electrical scan takes less than 10 ms and is repeated every 100 ms (corresponding to a rate of 10 Hz). The voltammogram gives a chemical signature, which can be used to identify the chemical species of interest and quantify phasic changes in extracellular concentration. However, because of a large capacitive current which must be subtracted out to resolve the faradaic current, the application of conventional FSCV provides only measurements of phasic changes in neurochemical concentrations (Howell et al., 1986). FSCV cannot measure dysregulation in tonic concentrations of neurotransmitters (minutes to hours) that are likely to be important characteristics of many neurologic and psychiatric conditions (Dreher and Burnod, 2002; Berke, 2018).
For the measurement of tonic dopamine levels in the brain in real time, several electrochemical techniques were developed such as fast-scan controlled-adsorption voltammetry (FSCAV) (Atcherley et al., 2013), square wave voltammetry (Taylor et al., 2019), and convolution-based current removal technique (Johnson et al., 2017). Among these techniques, FSCAV from Heien and colleagues has been applied to study tonic dopamine levels in various experiment setups (Atcherley et al., 2015; Burrell et al., 2015; Abdalla et al., 2017). FSCAV utilizes adsorption properties of dopamine to the carbon microelectrode using multiple conventional FSCV waveforms. We have previously developed a technique that uses cyclic square wave voltammetric waveforms, called Multiple-Cyclic Square Wave Voltammetry (M-CSWV). The time resolution is 10 s, which is slower than FSCV but is well-matched to the timescale of changes in tonic concentrations of dopamine relevant to the pathologies of interest (Schultz, 2007). Since M-CSWV utilizes square waveforms, M-CSWV is able to harvest higher dimensional data for analysis that leads to higher sensitivity and selectivity than other tonic level measurement techniques (Kim et al., 2019; Kim et al., 2021).
In this study, we aim to elucidate the acute effects of cocaine administration on phasic dopamine release by using FSCV and tonic dopamine levels by using M-CSWV in the nucleus accumbens core (NAcc). Cocaine is one of the most common illicit drugs with an increasing prevalence of use and dependence (John and Wu, 2017).
Nine male Sprague-Dawley rats (Envigo, United States) were used for this study. Rats were kept in social housing in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited vivarium following a standard 12-h light/dark cycle at constant temperature (21°C) and humidity (45%) with ad libitum food and water. The present studies were approved by the Institutional Animal Care and Use Committee (IACUC), Mayo Clinic, Rochester, MN. The NIH Guide for the Care and Use of Laboratory Animals guidelines (Department of Health and Human Services, NIH publication No. 86-23, revised 1985) were followed for all aspects of animal care.
CFMs were fabricated using an established standardized CFM design at Mayo Clinic. (Chang et al., 2013; Oh et al., 2016). Briefly, each microelectrode involved isolating and inserting a single carbon fiber (AS4, diameter = 7 μm; Hexel, Dublin, CA) into a silica tube (20 µM ID, 90 µM OD, 10 µM coat with polyimide; Polymicro Technologies, Phoenix, AZ). The connection between the carbon fiber and the silica tubing was covered with epoxy resin. The silica tubing was then attached to a nitinol (Nitinol #1, an alloy of nickel and titanium; Fort Wayne Metals, IN) extension wire with a silver-based conductive paste (Chang et al., 2013). The carbon fiber attached nitinol wire was insulated with polyimide tubing (0.0089″ID, 0.0134″OD, 0.00225″ WT; Vention Medical, Salem, NH) up to the carbon fiber sensing part. The exposed carbon fiber was then trimmed under a dissecting microscope to a length of 50 µm. Teflon-coated silver (Ag) wire (A-M systems, Inc., Sequim, WA) was prepared as an Ag/AgCl counter-reference electrode by chlorinating the exposed tip in saline with a 9 V dry cell battery. CFMs were pretested in a flow cell prior to coating deposition with a PEDOT:Nafion deposition solution (Vreeland et al., 2015), which minimized the effect of in vivo biofouling.
Each rat was anesthetized with urethane (1.5 g/kg i.p.; Sigma-Aldrich, St Louis, MO) and administered buprenorphine (0.05–0.1 mg/kg s.c, Par Pharmaceutical, Chestnut Ridge, NY, United States) for analgesia. Following anesthesia, they were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Respiratory rate (RespiRAT, Intuitive Measurement Systems), hind-paw and tail pinch were used to monitor the physiological state and depth of anesthesia. Using a standard rat atlas (Paxinos and Watson, 2007), three trephine holes were drilled, the first for placement of a CFM into the NAcc (AP 1.2 mm, ML 2.0 mm, DV 6–7 mm from dura), the second for a stimulating electrode into the medial forebrain bundle (MFB) (twisted bipolar stimulating electrode—Plastics One, MS 303/2, Roanoke, VA, with the tips separated by 1 mm; AP −4.6 mm, ML 1.3 mm, DV 8–9 mm from dura), and a third for an Ag/AgCl into the contralateral cortex (Clark et al., 2010) (Figure 1A).
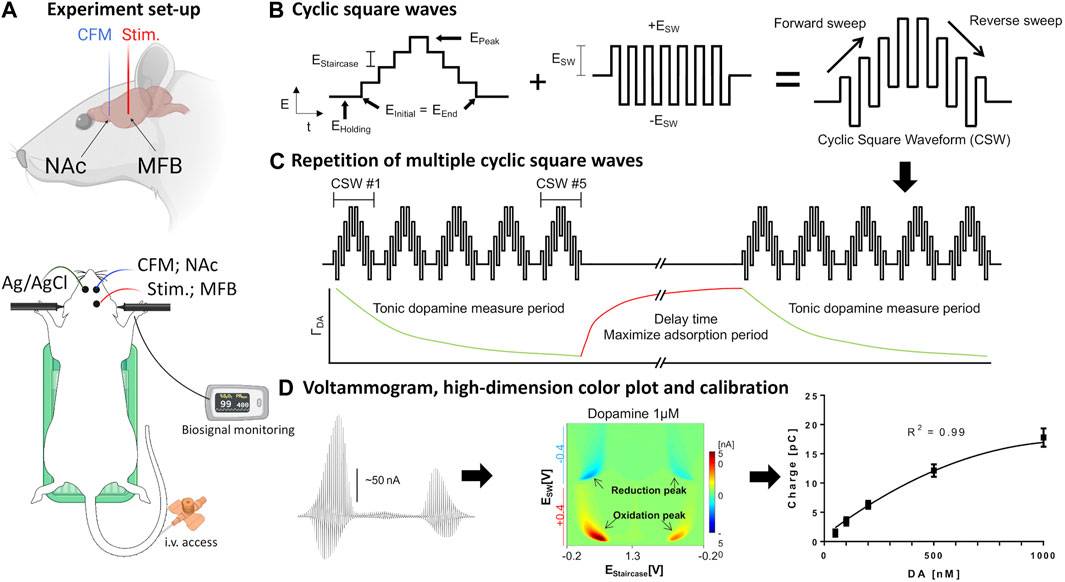
FIGURE 1. Set-up of in vivo voltammetry experiment. (A) Rat surgery set-up. Recording and stimulating electrodes were inserted unilaterally into the core of the nucleus accumbens and medial forebrain bundle, respectively. The counter-reference Ag/AgCl electrode was inserted contralaterally into cortical tissue. The rat is placed in a stereotactic frame with tail vein access, heating pad, and pulse oximetry monitoring. (B,C) Schematic design of waveform-CSWV applied to the CFM and its response. (D) Left-to-right: Raw voltammogram after removal of background currents, high-dimensional pseudo-color plot, M-CSWV signal calibration with tonic dopamine experiment (n = 4 electrodes). Figures adopted from (Oh et al., 2018) with permission. Ag/AgCl, silver chloride reference electrode; CFM, carbon-fiber electrode; CSW, cyclic square wave; MFB, medial forebrain bundle; NAc, nucleus accumbens; stim., bipolar stimulating electrode. Parts of the Figure were created with Biorender.com.
The stimulating electrode in the MFB and CFM in the NAcc were first adjusted to obtain a robust stimulation-evoked dopamine signal via FSCV (−0.4–1.3 V sweep; 10 Hz). MFB was chosen as it is known to induce dopamine release in the NAcc (Ng et al., 1991; Shu et al., 2013). For the phasic dopamine group, evoked responses (60 Hz, 0.2 mA, 2 ms pulse width, 2 s duration) were recorded at pre-, 5, 10, and 20, 30 40, 50, 60 min post-injection. This was performed using WINCS Harmoni system (Lee et al., 2017), a wireless neurochemical sensing system. Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) at a single bolus of 3 mg/kg i.v., was used. Cocaine hydrochloride was given for 5-min duration in all cases.
For the tonic dopamine group, the system was switched to the M-CSWV sensing technique with electrodes at the same position after identification of an optimal CFM position in the NAcc using FSCV. Cocaine at the same dose and route as above was administered after baseline and post-saline recording. Further recordings were performed for another 90 min to monitor the potentially lasting effects of cocaine on tonic dopamine levels. Dynamic background subtraction and capacitive background current modeling was used to eliminate large capacitive background currents, allowing tonic dopamine concentrations to be measured every 10 s (Oh et al., 2018). Because of the uniqueness of the waveform, the voltammetric outcome of M-CSWV provides a wealth of electrochemical information beyond that provided by conventional FSCV (Figures 1B–D).
After experimentation, changes in dopamine release for phasic studies were determined by calibration of CFMs using a flow cell injection apparatus; whereas for tonic dopamine levels, calibration with dopamine solutions were used (Oh et al., 2018). The media used consisted of TRIS buffer (15 mM tris, 3.25 mM KCl, 140 mM NaCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2, and 2.0 mM Na2SO4, with the pH adjusted to 7.4) (Oh et al., 2018).
Measurement of phasic dopamine release using FSCV offers many important applications, including modeling dopamine release and reuptake kinetics, and modeling the effects of pharmacologic agents on these processes. To quantitatively characterize the effects of cocaine administration on synaptic dopamine release, we used the restricted diffusion model of Walters et al. (Walters et al., 2015). This model proposes that the synapse-electrode system consists of two anatomically separated compartments and allows for restricted diffusion from the synaptic compartment to the electrode compartment. This allows for more accurate fitting of in vivo data compared to previous models such as the diffusion gap model. We applied the restricted diffusion model to our data to extract best-fit estimates for the parameters Rp, kR, kU, and kT (see Table 1 for more information). Best fit was evaluated with root mean square error (RMSE). The model was fit to five sets of stimulation-induced phasic dopamine releases with saline onboard, and five sets with cocaine on board, 5 min after administration.
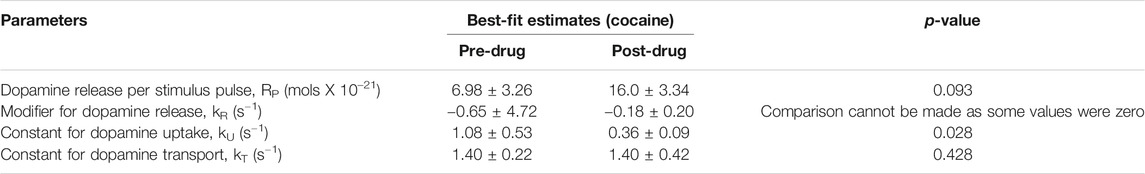
TABLE 1. Parameters calculated for the FSCV response pre-cocaine and 10 min post-cocaine, based on the model by Walters et al. (Walters et al., 2015). N = 4 rats. S.E.M. values provided. One-tailed paired t-test was performed. A range is provided in the reference values to account for the slow and fast dopamine domains (dorsal striatal measurements based on medial forebrain bundle stimulation).
Statistical analysis was performed using ratio two-tailed paired t-tests (PRISM 8, GraphPad). Significance was set at p < 0.05. In the phasic experiments, three planned paired t-tests were performed (response at 5 min after cocaine vs baseline, saline, 60 min post-cocaine). In the tonic experiments, the peak level after cocaine injection was compared to baseline and saline levels.
Cocaine administration consistently led to enhancement of stimulation-evoked dopamine responses (Figure 2). The evoked phasic response at 5 min after cocaine injection was significantly higher than saline control (ratio two-tailed paired t-test, p = 0.0124, n = 4 rats), baseline (ratio two-tailed paired t-test, p = 0.0326, n = 4 rats) and 60 min after cocaine administration (ratio two-tailed paired t-test, p = 0.0225, n = 4 rats). With repeated stimulation performed for 60 min after injection, the peak level dropped to the pre-injection baseline level (Figures 2B,D).
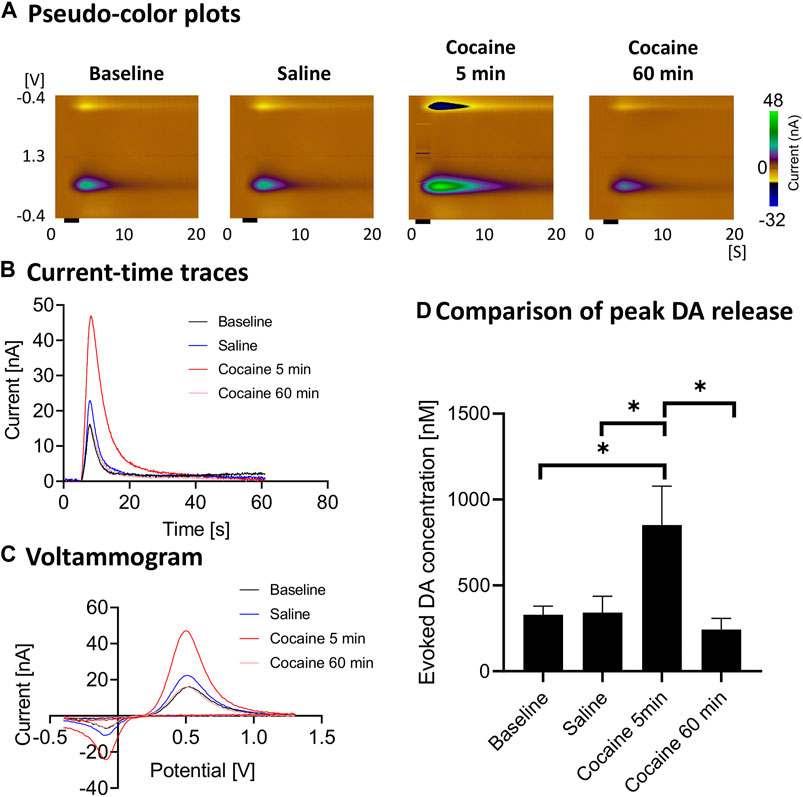
FIGURE 2. Peak and gradual decay of cocaine-induced changes in stimulation-evoked dopamine release. In vivo FSCV measurements in the nucleus accumbens core showing augmented dopamine responses to cocaine injection (3 mg/kg, i.v.). Responses were measured following medial forebrain bundle stimulation (2 s, biphasic, 300 μA, 2 ms pulse width). (A) Representative pseudo-color plots pre- and post-cocaine injection, (B) Oxidative current-time traces, (C) Voltammograms (at the peak) and (D) Maximum change in dopamine concentration with medial forebrain bundle stimulation at different time points (n = 4 rats). Black bar represents electrical stimulation (2 s). *denotes p < 0.05. n = 4 rats. DA, dopamine.
Using the restricted diffusion model discussed in the Methods section, the reuptake parameters for our experiments were calculated both before and after cocaine administration (Table 1). This model includes dopamine release per stimulus pulse and kinetic terms for dopamine reuptake, transport, and release. Cocaine, a dopamine reuptake inhibitor, would be expected to decrease the kinetic parameter for reuptake. Indeed, this is what was found (p = 0.028) (Table 1). Cocaine administration did not significantly influence the values of the other kinetic parameters.
As measured by M-CSWV, a representative example of the temporal changes in tonic dopamine levels in response to cocaine administration is shown in Figure 3. There were variations in the temporal pattern and time to peak changes in concentration (Figure 3A). Baseline recordings were taken for 30 min (Figure 3B), and saline was injected as a control (Figure 3C). Cocaine was injected 90 min later and showed a significant increase in tonic dopamine levels (Figures 3D–F). The tonic dopamine levels were measured for 60–70 min post cocaine injection. Overall, cocaine injections significantly increased dopamine levels in NAcc from 134 ± 32 nM to 281 ± 60 nM (ratio two-tailed paired t-test, p = 0.002, n = 5 rats) (Figure 3F).
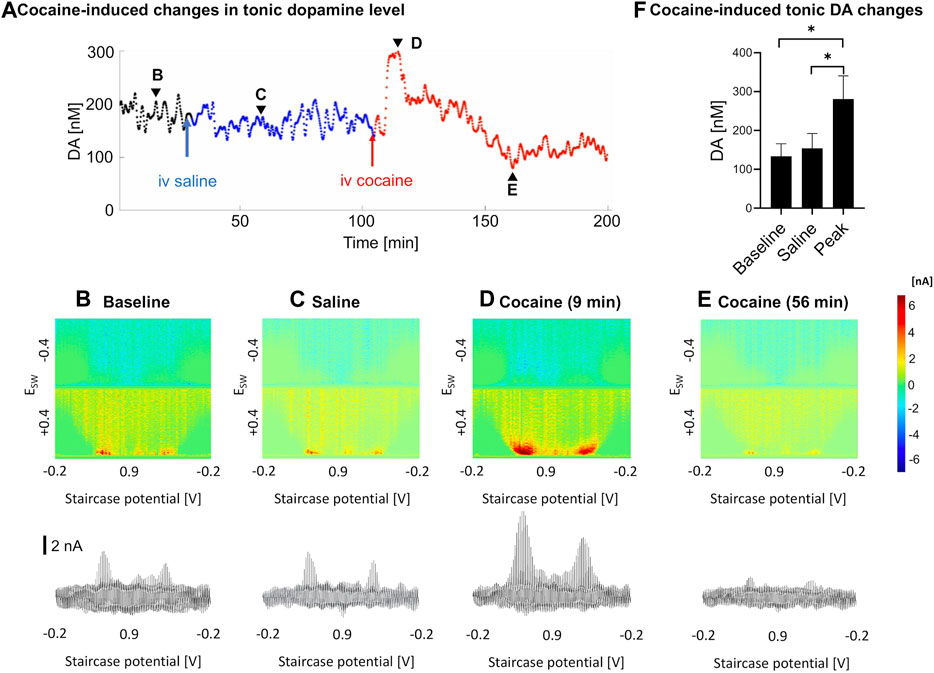
FIGURE 3. An example of tonic dopamine measurements obtained from the nucleus accumbens core in a single rat with saline then cocaine i.v. injections. (A). Changes in tonic dopamine concentration over time, where the black line denotes the stabilization period, blue line denotes control (saline) and red line denotes post-cocaine measurements. (B–E). High-dimension color plots and voltammograms pre- and post-injections, corresponding to the time points (arrowheads) in (A), respectively, (F) Comparison of tonic dopamine concentrations pre- and post-(peak) injection. Ratio two-tailed paired t-test, p = 0.002, n = 5 rats. See supplementary information for a video of the experiment. DA, dopamine.
This is the first study to characterize changes in tonic dopamine levels in the NAcc in the presence of cocaine in near real-time with an electrochemical technique in vivo. By utilizing the M-CSWV technique with its unrivaled temporal resolution and high spatial resolution provided by CFMs, we have demonstrated a more precise picture of how tonic dopamine dynamics change in response to acute cocaine administration.
The main findings of our study (see Figures 2A–D) are consistent with the literature which has shown that cocaine (and opioids) leads to increases in dopamine responses in the core of the nucleus accumbens as measured using FSCV (Aragona et al., 2008; Vander Weele et al., 2014). Aside from the robust increase in stimulation-evoked dopamine release after cocaine administration, dopamine release appeared to drop to below pre-cocaine levels (see Figure 2D). However, this did not reach statistical significance with this sample size. This may be due to blockade of dopamine reuptake by cocaine leading to decreased releasable vesicular pool over the course of the cocaine effect, as well as the effect of continuous stimulation. However, the latter is less likely to be the main contributor, given the synapses were provided at least 10 min to recover between stimulations. Further experiments would help to confirm this phenomenon. Also, the reuptake constant, kU was lowered after cocaine administration, which is expected since cocaine is a competitive antagonist of the dopamine transporter and kU is directly proportional to reuptake rate (see Table 1). Cocaine administration would not be expected to influence kinetic parameters for transport between the synapse and the electrode. Consistent with these expectations, these other parameters were not significantly influenced by cocaine administration.
From previous studies, it was found that the NAcc appears to consist of a patchwork of domains that show distinct dopamine kinetics, each demonstrating slow and/or fast evoked responses when the MFB is stimulated (Shu et al., 2013). The dopamine phasic response within the core is also heterogenous in response to cocaine self-administration (Owesson-White et al., 2009). This may explain why there are differences in our values, compared to values published by Walters et al. (2015), as well as the fact that we used different pharmacological agents. The differences may be accounted for by different stimulation parameters, especially the duration of stimulation. However, they do have similar orders of magnitude, which is expected, as both cocaine and nomifensine act by limiting the reuptake of dopamine. As far as we are aware, no studies have used this model to evaluate the evoked response of cocaine, therefore, our results could provide a benchmark for future studies.
In previous microdialysis studies where cocaine was given acutely (Bradberry et al., 1993; Pontieri et al., 1995), intravenous cocaine led to rapid rise in nucleus accumbens tonic dopamine levels, which peaked at 10–20 min (where dopamine was measured at 10–20 min intervals). Peak levels of dopamine varied between 150 to 400% of baseline. In addition, in pharmacokinetic studies, cocaine was eliminated in a biexponential manner after i.v. administration with mean elimination half-lives of 4.4 and 24.8 min, with a rapidly decaying serum concentration (Ma et al., 1999; Sun and Lau, 2001). In our study, a trough was observed in a subset of samples after the peak but not in all (see Figure 3A). This may be due to dopamine depletion after the sharp increase. This may be analogous to the small drop in the mean phasic response at ∼60 min compared to pre-cocaine baseline. Further experiments are needed to confirm this phenomenon.
Previous studies have shown that dopamine release varies widely among test subjects and within dopaminergic structures (Verheij et al., 2008; Owesson-White et al., 2009; Shu et al., 2013), necessitating a trial-and-error approach where the depths of CFM and stimulating electrodes are continually adjusted until the so-called “hotspot” is found. This hotspot is thought to occur when the CFM is close to a site of large synaptic release of dopamine. Variations in the location and behavior of these hotspots may explain the variable effects we see after cocaine administration in our study.
Overall, the present results suggest that M-CSWV can measure drug-induced changes in tonic dopamine levels with high temporal and spatial resolution when compared to microdialysis. Most of these studies used microdialysis with a temporal resolution of 10–20 min (Bradberry et al., 1993; Pontieri et al., 1995; Cadoni et al., 2000). Despite recent developments to reduce the resolution from 20 min down to 1 min (Gu et al., 2015; Ngo et al., 2017), M-CSWV still provides a much higher time resolution with the added benefit of spatial resolution and minimal tissue disruption when used with CFMs.
It is important to note however, that there are two major differences in our M-CSWV results in comparison to microdialysis. First, the tonic dopamine concentrations are very different in magnitude. Our baseline dopamine levels, determined by post-in vivo calibration, were at around 100–200 nM; whereas microdialysis studies commonly report values between 10 to 20 nM (Bradberry et al., 1993; Cadoni et al., 2000). Although the order of magnitude differs by a factor of ten, our values are broadly consistent with previous accumbal and striatal dopamine concentrations measured by various electrochemical techniques (Blaha, 1996; Atcherley et al., 2015; Johnson et al., 2017; Oh et al., 2018; Taylor et al., 2019; Barath et al., 2020). The possibility that other interferents such as norepinephrine, which has similar electrochemical properties as dopamine, may be a contributing factor in the differences is unlikely since other studies have demonstrated that the amount of norepinephrine and serotonin in the NAcc is comparatively low (Andrews and Lucki, 2001; McKittrick and Abercrombie, 2007; Zhang et al., 2020). Therefore, the disparities likely represent the fundamental differences between microdialysis and electrochemical techniques. Previous studies have suggested that the traumatized layer of tissue of the order of 200 µm caused by the relatively large diameter of the microdialysis probe may lead to a reduction in dopamine extraction, although relative changes could still be measured (Peters and Michael, 1998; Bungay et al., 2003; Borland et al., 2005). This is minimized by the relatively small diameter of the carbon fibers used in voltammetry. Being able to identify tonic values may help to quantify differences between subjects, as well as the diagnosis of different pathologies, especially given some neuropathological diseases are known to be driven by degeneration and depletion of neurotransmitters such as dopamine (Denys et al., 2004; Beitz, 2014; Oliva and Wanat, 2016; Maia and Conceicao, 2018). With these advantages, there is a strong argument that dopamine levels measured by M-CSWV can serve as important biomarkers for monitoring treatments with rapid bioavailability such as deep brain stimulation.
The other major difference is the relative change in magnitude. Rather than a 400% increase as in the described literature, in our study the dopamine signal mostly doubled. This may again be attributed to the underestimation of baseline in microdialysis, as well as the possibility that cocaine-induced increases in synaptic dopamine may be affected by factors related to the physical presence of the microdialysis probe, such as the formation of a layer of traumatized tissue (Di Chiara et al., 1993; Blaha et al., 1996). The rate and dose of drug administration may also have an impact (Minogianis et al., 2019). As mentioned in the Methods section, the rate was controlled at 5-min duration to avoid overdosing. The use of different anesthetic agents in other studies, such as chloral hydrate, may also lead to discrepancies in results (Bradberry et al., 1993).
Our study focused on the NAcc, so the results should not be generalized to the nucleus accumbens shell, since they are distinct subdivisions of the accumbens or other regions. For example, in one in vitro FSCV study, dopamine uptake in the shell was approximately one-third of that measured in the core, and the former was less sensitive to both cocaine and nomifensine (dopamine reuptake inhibitor) (Jones et al., 1996). Also, intravenous cocaine increased extracellular dopamine in the shell more markedly than in the core of the rat nucleus accumbens. Another study utilized immunochemistry to demonstrate that dopaminergic axons in the shell contained lower densities of dopamine transporter than those in the core (Nirenberg et al., 1997). This suggests a more tightly regulated phasic dopamine transmission in this subregion and highlights the value of both phasic and tonic measurements across both regions for future work. In addition, Dreyer et al. utilized a computer model to interpret in vivo FSCV data from the NAcc and shell after rodents were administered cocaine (Dreyer et al., 2016). After studying the dynamics involved in presynaptic terminal autoreceptor feedback, they concluded that extracellular dopamine concentrations in the core resulted from constant dopamine firing, whereas the shell concentrations reflected dynamic firing patterns. This supported our decision to record from the NAcc using this new tonic dopamine measurement method.
While our technique focuses on a single analyte, dopamine, and therefore may not be as comprehensive as microdialysis studies, precise measurement of dopamine alone is still highly important. Dopamine has a major role in the reward circuit and is one of the key neurotransmitters in the pathophysiology of addiction (Berke and Hyman, 2000). Newer techniques have also been devised to voltammetrically measure serotonin with high sensitivity and selectivity, which can be incorporated in future study designs (Shin et al., 2020). DLight, which is a new technique that uses genetically encoded indicators based on fluorescent proteins with microscopy also allows measurements of neurochemicals with high temporal resolution (Patriarchi et al., 2018). However, the need for a viral vector currently limits its potential use in human subjects.
Another notable characteristic of this study is that the animal experiments were performed under anesthesia in an acute setting using a single dose of cocaine. While we appreciate that addiction is often secondary to chronic drug use in humans, acute experiments offer insight into the first step of the pathophysiological process. Additionally, this study paves the way for future chronic experiments by proving the feasibility of this technique to study the effects of other drugs of abuse. In the future, it is our intention to apply the M-CSWV intraoperatively, particularly in the context of neurological (e.g., Parkinson’s disease) and psychiatric (e.g., addiction) disorders.
Although cocaine is known to enhance dopamine transmission in the nucleus accumbens, this is the first study that utilized M-CSWV to measure accumbal tonic dopamine levels, and to characterize the effect of cocaine on these levels in near real-time. Overall, this technique provides unprecedented insight into the temporal changes in dopamine dynamics, and it will likely be of much value in future addiction studies.
Supplementary video. An example of tonic dopamine measurements obtained from the NAcc in a single rat with saline then cocaine i.v. injections. Upper panel shows the color plot and the lower panel shows the changes in tonic dopamine concentration over time, where the black line denotes the stabilization period, blue line denotes control (saline) and red line denotes post-cocaine measurements.
The data presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Mayo Clinic, Rochester, MN.
KL, D-PJ, and YO conceptualized the study. JY, AR, and HS conducted experiments and collected the data. JY, YO, and HS designed the analyses. JY and HS conducted the analyses. AG and HS assisted in software and conducting the analysis on phasic responses. JY drafted the first manuscript. AK, MB, JK, ST, CB, KB, KL, D-PJ, and YO critically reviewed and revised the manuscript. KL, HS, and YO supervised all aspects of this work. JY drafted the figures. All authors accepted the final version of the article.
This research was supported by the NIH R01NS112176 award and Minnesota Partnership for Biotechnology and Medical Genomics Grant MNP #19.13. Training grant funding for AR was supported by NIH F31NS115202-01A1, NIH R25GM055252-23, NIH TL1TR002380-03, and NIH T32GM065841-17. MB is supported by a NHMRC Senior Principal Research Fellowship (1156072).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.705254/full#supplementary-material
Abdalla, A., Atcherley, C. W., Pathirathna, P., Samaranayake, S., Qiang, B., Peña, E., et al. (2017). In Vivo Ambient Serotonin Measurements at Carbon-Fiber Microelectrodes. Anal. Chem. 89 (18), 9703–9711. doi:10.1021/acs.analchem.7b01257
Andrews, C., and Lucki, I. (2001). Effects of Cocaine on Extracellular Dopamine and Serotonin Levels in the Nucleus Accumbens. Psychopharmacology 155 (3), 221–229. doi:10.1007/s002130100704
Aragona, B. J., Cleaveland, N. A., Stuber, G. D., Day, J. J., Carelli, R. M., and Wightman, R. M. (2008). Preferential Enhancement of Dopamine Transmission within the Nucleus Accumbens Shell by Cocaine Is Attributable to a Direct Increase in Phasic Dopamine Release Events. J. Neurosci. 28 (35), 8821–8831. doi:10.1523/JNEUROSCI.2225-08.2008
Atcherley, C. W., Laude, N. D., Parent, K. L., and Heien, M. L. (2013). Fast-scan Controlled-Adsorption Voltammetry for the Quantification of Absolute Concentrations and Adsorption Dynamics. Langmuir 29 (48), 14885–14892. doi:10.1021/la402686s
Atcherley, C. W., Wood, K. M., Parent, K. L., Hashemi, P., and Heien, M. L. (2015). The Coaction of Tonic and Phasic Dopamine Dynamics. Chem. Commun. 51 (12), 2235–2238. doi:10.1039/c4cc06165a
Barath, A. S., Rusheen, A. E., Rojas Cabrera, J. M., Price, J. B., Owen, R. L., Shin, H., et al. (2020). Hypoxia-Associated Changes in Striatal Tonic Dopamine Release: Real-Time In Vivo Measurements with a Novel Voltammetry Technique. Front. Neurosci. 14, 869. doi:10.3389/fnins.2020.00869
Berke, J. D., and Hyman, S. E. (2000). Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron 25 (3), 515–532. doi:10.1016/s0896-6273(00)81056-9
Berke, J. D. (2018). What Does Dopamine Mean?. Nat. Neurosci. 21 (6), 787–793. doi:10.1038/s41593-018-0152-y
Blaha, C. D., and Phillips, A. G. (1996). A Critical Assessment of Electrochemical Procedures Applied to the Measurement of Dopamine and its Metabolites during Drug-Induced and Species-Typical Behaviours. Behav. Pharmacol. 7 (7), 675–708. doi:10.1097/00008877-199611000-00014
Blaha, C. D., Coury, A., and Phillips, A. G. (1996). Does Monoamine Oxidase Inhibition by Pargyline Increase Extracellular Dopamine Concentrations in the Striatum?. Neuroscience 75 (2), 543–550. doi:10.1016/0306-4522(96)00289-8
Blaha, C. D. (1996). Evaluation of Stearate-Graphite Paste Electrodes for Chronic Measurement of Extracellular Dopamine Concentrations in the Mammalian Brain. Pharmacol. Biochem. Behav. 55 (3), 351–364. doi:10.1016/s0091-3057(96)00104-9
Borland, L. M., Shi, G., Yang, H., and Michael, A. C. (2005). Voltammetric Study of Extracellular Dopamine Near Microdialysis Probes Acutely Implanted in the Striatum of the Anesthetized Rat. J. Neurosci. Methods 146 (2), 149–158. doi:10.1016/j.jneumeth.2005.02.002
Bradberry, C. W., Nobiletti, J. B., Elsworth, J. D., Murphy, B., Jatlow, P., and Roth, R. H. (1993). Cocaine and Cocaethylene: Microdialysis Comparison of Brain Drug Levels and Effects on Dopamine and Serotonin. J. Neurochem. 60 (4), 1429–1435. doi:10.1111/j.1471-4159.1993.tb03305.x
Bungay, P. M., Newton-Vinson, P., Isele, W., Garris, P. A., and Justice, J. B. (2003). Microdialysis of Dopamine Interpreted with Quantitative Model Incorporating Probe Implantation Trauma. J. Neurochem. 86 (4), 932–946. doi:10.1046/j.1471-4159.2003.01904.x
Burrell, M. H., Atcherley, C. W., Heien, M. L., and Lipski, J. (2015). A Novel Electrochemical Approach for Prolonged Measurement of Absolute Levels of Extracellular Dopamine in Brain Slices. ACS Chem. Neurosci. 6 (11), 1802–1812. doi:10.1021/acschemneuro.5b00120
Cadoni, C., Solinas, M., and Di Chiara, G. (2000). Psychostimulant Sensitization: Differential Changes in Accumbal Shell and Core Dopamine. Eur. J. Pharmacol. 388 (1), 69–76. doi:10.1016/s0014-2999(99)00824-9
Chang, S.-Y., Kimble, C. J., Kim, I., Paek, S. B., Kressin, K. R., Boesche, J. B., et al. (2013). Development of the Mayo Investigational Neuromodulation Control System: toward a Closed-Loop Electrochemical Feedback System for Deep Brain Stimulation. Jns 119 (6), 1556–1565. doi:10.3171/2013.8.JNS122142
Chefer, V. I., Thompson, A. C., Zapata, A., and Shippenberg, T. S. (2009). Overview of Brain Microdialysis. Curr. Protoc. Neurosci. 47Chapter 7, Unit7.1. doi:10.1002/0471142301.ns0701s47
Clark, J. J., Sandberg, S. G., Wanat, M. J., Gan, J. O., Horne, E. A., Hart, A. S., et al. (2010). Chronic Microsensors for Longitudinal, Subsecond Dopamine Detection in Behaving Animals. Nat. Methods 7 (2), 126–129. doi:10.1038/nmeth.1412
Denys, D., Zohar, J., and Westenberg, H. G. (2004). The Role of Dopamine in Obsessive-Compulsive Disorder: Preclinical and Clinical Evidence. J. Clin. Psychiatry 65 (Suppl. 14), 11–17. doi:10.4088/jcp.v65n0803
Di Chiara, G., Carboni, E., Morelli, M., Cozzolino, A., Tanda, G. L., Pinna, A., et al. (1993). Stimulation of Dopamine Transmission in the Dorsal Caudate Nucleus by Pargyline as Demonstrated by Dopamine and Acetylcholine Microdialysis and Fos Immunohistochemistry. Neuroscience 55 (2), 451–456. doi:10.1016/0306-4522(93)90514-g
Dreher, J. C., and Burnod, Y. (2002). An Integrative Theory of the Phasic and Tonic Modes of Dopamine Modulation in the Prefrontal Cortex. Neural Netw. 15 (4-6), 583–602. doi:10.1016/s0893-6080(02)00051-5
Dreyer, J. K., Vander Weele, C. M., Lovic, V., and Aragona, B. J. (2016). Functionally Distinct Dopamine Signals in Nucleus Accumbens Core and Shell in the Freely Moving Rat. J. Neurosci. 36 (1), 98–112. doi:10.1523/jneurosci.2326-15.2016
Goto, Y., Otani, S., and Grace, A. (2007). The Yin and Yang of Dopamine Release: a New Perspective. Neuropharmacology 53 (5), 583–587. doi:10.1016/j.neuropharm.2007.07.007
Grace, A. A. (1991). Phasic versus Tonic Dopamine Release and the Modulation of Dopamine System Responsivity: a Hypothesis for the Etiology of Schizophrenia. Neuroscience 41 (1), 1–24. doi:10.1016/0306-4522(91)90196-u
Grace, A. A. (2016). Dysregulation of the Dopamine System in the Pathophysiology of Schizophrenia and Depression. Nat. Rev. Neurosci. 17 (8), 524–532. doi:10.1038/nrn.2016.57
Gu, H., Varner, E. L., Groskreutz, S. R., Michael, A. C., and Weber, S. G. (2015). In Vivo Monitoring of Dopamine by Microdialysis with 1 Min Temporal Resolution Using Online Capillary Liquid Chromatography with Electrochemical Detection. Anal. Chem. 87 (12), 6088–6094. doi:10.1021/acs.analchem.5b00633
Heien, M. L., Johnson, M. A., and Wightman, R. M. (2004). Resolving Neurotransmitters Detected by Fast-Scan Cyclic Voltammetry. Anal. Chem. 76 (19), 5697–5704. doi:10.1021/ac0491509
Hodebourg, R., Murray, J. E., Fouyssac, M., Puaud, M., Everitt, B. J., and Belin, D. (2019). Heroin Seeking Becomes Dependent on Dorsal Striatal Dopaminergic Mechanisms and Can Be Decreased by N‐acetylcysteine. Eur. J. Neurosci. 50 (3), 2036–2044. doi:10.1111/ejn.13894
Howell, J. O., Kuhr, W. G., Ensman, R. E., and Mark Wightman, R. (1986). Background Subtraction for Rapid Scan Voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 209(1), 77–90. doi:10.1016/0022-0728(86)80187-5
Huffman, M. L., and Venton, B. J. (2009). Carbon-fiber Microelectrodes for In Vivo Applications. Analyst 134 (1), 18–24. doi:10.1039/b807563h
Ito, R., Dalley, J. W., Robbins, T. W., and Everitt, B. J. (2002). Dopamine Release in the Dorsal Striatum during Cocaine-Seeking Behavior under the Control of a Drug-Associated Cue. J. Neurosci. 22 (14), 6247–6253. doi:10.1523/jneurosci.22-14-06247.2002
John, W. S., and Wu, L.-T. (2017). Trends and Correlates of Cocaine Use and Cocaine Use Disorder in the United States from 2011 to 2015. Drug and Alcohol Depend. 180, 376–384. doi:10.1016/j.drugalcdep.2017.08.031
Johnson, J. A., Hobbs, C. N., and Wightman, R. M. (2017). Removal of Differential Capacitive Interferences in Fast-Scan Cyclic Voltammetry. Anal. Chem. 89 (11), 6166–6174. doi:10.1021/acs.analchem.7b01005
Jones, S. R., O'Dell, S. J., Marshall, J. F., and Wightman, R. M. (1996). Functional and Anatomical Evidence for Different Dopamine Dynamics in the Core and Shell of the Nucleus Accumbens in Slices of Rat Brain. Synapse 23 (3), 224–231. doi:10.1002/(sici)1098-2396(199607)23:3<224::aid-syn12>3.0.co;2-z
Kalivas, P. W., and O'Brien, C. (2008). Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacol 33 (1), 166–180. doi:10.1038/sj.npp.1301564
Kim, J., Oh, Y., Park, C., Kang, Y. M., Shin, H., Kim, I. Y., et al. (2019). Comparison Study of Partial Least Squares Regression Analysis and Principal Component Analysis in Fast-Scan Cyclic Voltammetry. Int. J. Electrochem. Sci. 14 (7), 5924–5937. doi:10.20964/2019.07.03
Kim, J., Barath, A. S., Rusheen, A. E., Rojas Cabrera, J. M., Price, J. B., Shin, H., et al. (2021). Automatic and Reliable Quantification of Tonic Dopamine Concentrations In Vivo Using a Novel Probabilistic Inference Method. ACS Omega 6 (10), 6607–6613. doi:10.1021/acsomega.0c05217
Lama, R. D., Charlson, K., Anantharam, A., and Hashemi, P. (2012). Ultrafast Detection and Quantification of Brain Signaling Molecules with Carbon Fiber Microelectrodes. Anal. Chem. 84 (19), 8096–8101. doi:10.1021/ac301670h
Lee, K. H., Lujan, J. L., Trevathan, J. K., Ross, E. K., Bartoletta, J. J., Park, H. O., et al. (2017). WINCS Harmoni: Closed-Loop Dynamic Neurochemical Control of Therapeutic Interventions. Sci. Rep. 7, 46675. doi:10.1038/srep46675
Ma, F., Falk, J. L., and Lau, C. E. (1999). Cocaine Pharmacodynamics after Intravenous and Oral Administration in Rats: Relation to Pharmacokinetics. Psychopharmacology 144 (4), 323–332. doi:10.1007/s002130051014
Maia, T. V., and Conceição, V. A. (2018). Dopaminergic Disturbances in Tourette Syndrome: An Integrative Account. Biol. Psychiatry 84 (5), 332–344. doi:10.1016/j.biopsych.2018.02.1172
McKittrick, C. R., and Abercrombie, E. D. (2007). Catecholamine Mapping within Nucleus Accumbens: Differences in Basal and Amphetamine-Stimulated Efflux of Norepinephrine and Dopamine in Shell and Core. J. Neurochem. 100 (5), 1247–1256. doi:10.1111/j.1471-4159.2006.04300.x
McLellan, A. T., Lewis, D. C., O'Brien, C. P., and Kleber, H. D. (2000). Drug Dependence, a Chronic Medical Illness. JAMA 284 (13), 1689–1695. doi:10.1001/jama.284.13.1689
Millar, J. (1997). In Vivo detection of Neurotransmitters with Fast Cyclic Voltammetry. Methods Mol. Biol. 72, 251–266. doi:10.1385/0-89603-394-5:251
Minogianis, E. A., Shams, W. M., Mabrouk, O. S., Wong, J. M. T., Brake, W. G., Kennedy, R. T., et al. (2019). Varying the Rate of Intravenous Cocaine Infusion Influences the Temporal Dynamics of Both Drug and Dopamine Concentrations in the Striatum. Eur. J. Neurosci. 50 (3), 2054–2064. doi:10.1111/ejn.13941
Morelli, M., Carboni, E., Cozzolino, A., Tanda, G. L., Pinna, A., and Chiara, G. (1992). Combined Microdialysis and Fos Immunohistochemistry for the Estimation of Dopamine Neurotransmission in the Rat Caudate-Putamen. J. Neurochem. 59 (3), 1158–1160. doi:10.1111/j.1471-4159.1992.tb08359.x
Murray, J. E., Belin, D., and Everitt, B. J. (2012). Double Dissociation of the Dorsomedial and Dorsolateral Striatal Control over the Acquisition and Performance of Cocaine Seeking. Neuropsychopharmacol 37 (11), 2456–2466. doi:10.1038/npp.2012.104
Ng, J. P., Hubert, G. W., and Justice, J. B. (1991). Increased Stimulated Release and Uptake of Dopamine in Nucleus Accumbens after Repeated Cocaine Administration as Measured by In Vivo Voltammetry. J. Neurochem. 56 (5), 1485–1492. doi:10.1111/j.1471-4159.1991.tb02042.x
Ngo, K. T., Varner, E. L., Michael, A. C., and Weber, S. G. (2017). Monitoring Dopamine Responses to Potassium Ion and Nomifensine by In Vivo Microdialysis with Online Liquid Chromatography at One-Minute Resolution. ACS Chem. Neurosci. 8 (2), 329–338. doi:10.1021/acschemneuro.6b00383
Nirenberg, M. J., Chan, J., Pohorille, A., Vaughan, R. A., Uhl, G. R., Kuhar, M. J., et al. (1997). The Dopamine Transporter: Comparative Ultrastructure of Dopaminergic Axons in Limbic and Motor Compartments of the Nucleus Accumbens. J. Neurosci. 17 (18), 6899–6907. doi:10.1523/jneurosci.17-18-06899.1997
Oh, Y., Park, C., Kim, D. H., Shin, H., Kang, Y. M., DeWaele, M., et al. (2016). Monitoring In Vivo Changes in Tonic Extracellular Dopamine Level by Charge-Balancing Multiple Waveform Fast-Scan Cyclic Voltammetry. Anal. Chem. 88 (22), 10962–10970. doi:10.1021/acs.analchem.6b02605
Oh, Y., Heien, M. L., Park, C., Kang, Y. M., Kim, J., Boschen, S. L., et al. (2018). Tracking Tonic Dopamine Levels In Vivo Using Multiple Cyclic Square Wave Voltammetry. Biosens. Bioelectron. 121, 174–182. doi:10.1016/j.bios.2018.08.034
Oliva, I., and Wanat, M. J. (2016). Ventral Tegmental Area Afferents and Drug-dependent Behaviors. Front. Psychiatry 7, 30. doi:10.3389/fpsyt.2016.00030
Owesson-White, C. A., Ariansen, J., Stuber, G. D., Cleaveland, N. A., Cheer, J. F., Mark Wightman, R., et al. (2009). Neural Encoding of Cocaine-Seeking Behavior Is Coincident with Phasic Dopamine Release in the Accumbens Core and Shell. Eur. J. Neurosci. 30 (6), 1117–1127. doi:10.1111/j.1460-9568.2009.06916.x
Patriarchi, T., Cho, J. R., Merten, K., Howe, M. W., Marley, A., Xiong, W.-H., et al. (2018). Ultrafast Neuronal Imaging of Dopamine Dynamics with Designed Genetically Encoded Sensors. Science 360 (6396), eaat4422. doi:10.1126/science.aat4422
Paxinos, G., and Watson, C. (2007). The Rat Brain in Stereotaxic Coordinates. Amsterdam; Boston: Academic Press/Elsevier.
Peters, J. L., and Michael, A. C. (1998). Modeling Voltammetry and Microdialysis of Striatal Extracellular Dopamine: the Impact of Dopamine Uptake on Extraction and Recovery Ratios. J. Neurochem. 70 (2), 594–603. doi:10.1046/j.1471-4159.1998.70020594.x
Pontieri, F. E., Tanda, G., and Di Chiara, G. (1995). Intravenous Cocaine, Morphine, and Amphetamine Preferentially Increase Extracellular Dopamine in the "shell" as Compared with the "core" of the Rat Nucleus Accumbens. Proc. Natl. Acad. Sci. 92 (26), 12304–12308. doi:10.1073/pnas.92.26.12304
Robinson, D. L., Venton, B. J., Heien, M. L. A. V., and Wightman, R. M. (2003). Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry In Vivo. Clin. Chem. 49 (10), 1763–1773. doi:10.1373/49.10.1763
Rodeberg, N. T., Sandberg, S. G., Johnson, J. A., Phillips, P. E. M., and Wightman, R. M. (2017). Hitchhiker's Guide to Voltammetry: Acute and Chronic Electrodes for In Vivo Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 8 (2), 221–234. doi:10.1021/acschemneuro.6b00393
Rusheen, A. E., Gee, T. A., Jang, D. P., Blaha, C. D., Bennet, K. E., Lee, K. H., et al. (2020). Evaluation of Electrochemical Methods for Tonic Dopamine Detection In Vivo. Trac Trends Anal. Chem. 132, 116049. doi:10.1016/j.trac.2020.116049
Schultz, W. (2007). Behavioral Dopamine Signals. Trends Neurosciences 30 (5), 203–210. doi:10.1016/j.tins.2007.03.007
Shin, H., Oh, Y., Park, C., Kang, Y., Cho, H. U., Blaha, C. D., et al. (2020). Sensitive and Selective Measurement of Serotoninin VivoUsing Fast Cyclic Square-Wave Voltammetry. Anal. Chem. 92 (1), 774–781. doi:10.1021/acs.analchem.9b03164
Shu, Z., Taylor, I. M., and Michael, A. C. (2013). The Dopamine Patchwork of the Rat Nucleus Accumbens Core. Eur. J. Neurosci. 38 (8), 3221–3229. doi:10.1111/ejn.12319
Substance Abuse and Mental Health Services Administration (2020). Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services AdministrationAvailable at: https://www.samhsa.gov/data/ (Accessed March 20, 2021).
Sun, L., and Lau, C. E. (2001). Simultaneous Pharmacokinetic Modeling of Cocaine and its Metabolites, Norcocaine and Benzoylecgonine, after Intravenous and Oral Administration in Rats. Drug Metab. Dispos 29 (9), 1183–1189.
Taylor, I. M., Patel, N. A., Freedman, N. C., Castagnola, E., and Cui, X. T. (2019). Direct In Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 91 (20), 12917–12927. doi:10.1021/acs.analchem.9b02904
Vander Weele, C. M., Porter-Stransky, K. A., Mabrouk, O. S., Lovic, V., Singer, B. F., Kennedy, R. T., et al. (2014). Rapid Dopamine Transmission within the Nucleus Accumbens: Dramatic Difference between Morphine and Oxycodone Delivery. Eur. J. Neurosci. 40 (7), 3041–3054. doi:10.1111/ejn.12709
Vanderschuren, L. J. M. J., Di Ciano, P., and Everitt, B. J. (2005). Involvement of the Dorsal Striatum in Cue-Controlled Cocaine Seeking. J. Neurosci. 25 (38), 8665–8670. doi:10.1523/JNEUROSCI.0925-05.2005
Verheij, M. M. M., de Mulder, E. L. W., De Leonibus, E., van Loo, K. M. J., and Cools, A. R. (2008). Rats that Differentially Respond to Cocaine Differ in Their Dopaminergic Storage Capacity of the Nucleus Accumbens. J. Neurochem. 105 (6), 2122–2133. doi:10.1111/j.1471-4159.2008.05323.x
Vreeland, R. F., Atcherley, C. W., Russell, W. S., Xie, J. Y., Lu, D., Laude, N. D., et al. (2015). Biocompatible PEDOT:Nafion Composite Electrode Coatings for Selective Detection of Neurotransmitters In Vivo. Anal. Chem. 87 (5), 2600–2607. doi:10.1021/ac502165f
Walters, S. H., Robbins, E. M., and Michael, A. C. (2015). Modeling the Kinetic Diversity of Dopamine in the Dorsal Striatum. ACS Chem. Neurosci. 6 (8), 1468–1475. doi:10.1021/acschemneuro.5b00128
Watson, C. J., Venton, B. J., and Kennedy, R. T. (2006). In Vivo measurements of Neurotransmitters by Microdialysis Sampling. Anal. Chem. 78 (5), 1391–1399. doi:10.1021/ac0693722
Zbukvic, I. C., Ganella, D. E., Perry, C. J., Madsen, H. B., Bye, C. R., Lawrence, A. J., et al. (2016). Role of Dopamine 2 Receptor in Impaired Drug-Cue Extinction in Adolescent Rats. Cereb. Cortex 26 (6), 2895–2904. doi:10.1093/cercor/bhw051
Keywords: cocaine, tonic dopamine, addiction, voltammetry, nucleus accumbens, neuroscience, psychiatry, mental disorders
Citation: Yuen J, Goyal A, Rusheen AE, Kouzani AZ, Berk M, Kim JH, Tye SJ, Blaha CD, Bennet KE, Jang D-P, Lee KH, Shin H and Oh Y (2021) Cocaine-Induced Changes in Tonic Dopamine Concentrations Measured Using Multiple-Cyclic Square Wave Voltammetry in vivo. Front. Pharmacol. 12:705254. doi: 10.3389/fphar.2021.705254
Received: 05 May 2021; Accepted: 24 June 2021;
Published: 06 July 2021.
Edited by:
Jianfeng Liu, Texas A&M University, United StatesReviewed by:
Alexander G. Zestos, American University, United StatesCopyright © 2021 Yuen, Goyal, Rusheen, Kouzani, Berk, Kim, Tye, Blaha, Bennet, Jang, Lee, Shin and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoonbae Oh, oh.yoonbae@mayo.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.