- 1Unit of Clinical Pharmacology, Department of Biomedical and Clinical Sciences L. Sacco, "Luigi Sacco" University Hospital, Università di Milano, Milan, Italy
- 2Department of Biomedical and Clinical Sciences, Università di Milano, Fatebenefratelli Hospital, Milan, Italy
- 3Internal Medicine, Fatebefratelli Hospital, Milan, Italy
The published experience with biologics in childbearing age with autoimmune and inflammatory diseases mainly deals with the use of TNFα inhibitors (TNFα-i). Limited data are available for biologics targeting other cytokines or immunocompetent cells, especially for the inflammasome targeted therapy including IL-1 inhibitors and colchicine. We conducted a nested case-control study by using the US Food and Drug Administration Adverse Event Reporting System database aimed at quantifying the association between the use of IL-1 inhibitors/colchicine in pregnant women and the occurrence of maternal/fetal adverse effects. The reporting odds ratio was used as a measure of disproportional reporting. From the total cohort (40,033 pregnant women), we retrieved 7,620 reports related to neonatal AEs, 2,889 to fetal disorders, 8,364 to abortion, 8,787 to congenital disorders, and 7,937 to labor/delivery complications. Inflammasome-targeted drugs did not present any disproportionate reporting for all these clusters of AEs. TNFα-i confirmed their safety during pregnancy with aROR < 1 for all clusters of AEs except for labor complications. Finally, we performed a systematic review of the current literature. Data from the eligible studies (12 observational studies and 6 case reports; yielding a total of 2,075 patients) were reassuring. We found no major safety issues on malformations risk of inflammasome targeted therapies in pregnancy. However, due to limited data, the routine use of these agents should be considered in pregnancy only if risk benefit assessment justifies the potential risk to the fetus.
Introduction
In the past 2 decades, the number of women in childbearing age with autoimmune and inflammatory diseases has significantly increased, in parallel with some concerns on the safety of biological drugs used to treat these clinical conditions. A huge number of therapeutic options have become available, which may ensure optimal control of disease activity and markedly ameliorate patients’ quality of life. Despite the therapeutic armamentarium to manage pregnant women with systemic autoimmune rheumatic diseases (SARDs) includes a variety of biological agents, scant data are available concerning their use during reproduction and pregnancy, especially in long-term studies (Østensen et al., 2008; Østensen et al., 2015).
Biologics have firstly become available about 20 years ago. Since then, the published experience with biologics during pregnancy mainly deals with the use of TNFα inhibitors (TNFα-i) (Flint et al., 2016; Skorpen et al., 2016; Østensen, 2017); data from a growing number of publications on the use of TNFα-i pregnant women with most autoimmune diseases is reassuring (Smeele and Dolhain, 2019).
In contrast, pregnancy experience of exposure to other monoclonal antibodies targeting cytokines or immunocompetent including CD20 (rituximab), interleukin (IL)-6 receptors (tocilizumab), IL-17A (secukinumab) and nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome, has been limited to conference abstracts or simple case reports (Komaki et al., 2017). With regard to therapy of NLRP3-associated diseases, three biologic agents blocking IL-1 are available such as the neutralizing IL-1β antibody canakinumab, the recombinant IL-1 receptor antagonist anakinra, and the soluble decoy IL-1 receptor rilonacept (Dinarello and van der Meer, 2013; Zahid et al., 2019; Klein et al., 2020).
These agents have been used during pregnancy only recently, starting from mothers that required maintenance of biologic therapy to control the undelaying condition. However, the need of a prolonged drug administration during gestation raises many controversial issues about the effects of in utero exposure to these agents on the fetus/maternal health. Biologic agents are increasingly used and thus long-term data on the effect of these therapies on fetus development and maternal outcomes would be highly necessary. Prospective registries, some of which are already in place, are intended to serve at the purpose but their data are not available yet. Alternatively, spontaneous reporting systems represent a valuable source of information in frail populations, such as pregnant women. Despite the intrinsic limitations, data-mining of adverse drug reaction (ADR) reports allow to obtain real word data about the safety/efficacy profile of specific drugs, to compare therapeutic options, and gain insight on potential mechanisms of ADRs (Gentili et al., 2018; Perrotta et al., 2019).
In the past decades, no pharmacovigilance studies using spontaneous reporting system database specifically address the inflammasome targeted therapy potential risk of harm in pregnant women or the outcomes in their infants. We thus conducted a case-control study by using the US Food and Drug Administration Adverse Event Reporting System (FAERS) database aimed at quantifying the association between the use of inhibitors of the NLRP3 inflammasome in pregnant women and the occurrence of maternal and fetal adverse effects. Finally, in view of the growing number of studies on this issue, we performed a systematic review of the current literature to include and discuss real-world data, thus contributing to fill the knowledge gaps regarding safe and effective use of new biological drugs in pregnancy.
Materials and Methods
US Food and Drug Administration Adverse Event Reporting System Analysis: Data Source and Study Design
In order to appropriately estimate the incidence of pregnancy and neonatal adverse events (AEs) after the administration of inflammasome-targeted drugs, an analysis of the FAERS® has been conducted. FAERS® is an online database maintained by FDA that collects every adverse drug reaction (ADR) report submitted in the US territory and every serious ADR report filed in all 150 countries enrolled in the Program for International Drug Monitoring (PIDM) established by the World Health Organization (WHO).
In FAERS®, healthcare professionals, patients and marketing authorization holders may submit ADR reports in the form of Individual Case Safety Reports (ICSRs). FAERS® employs the latest version of the Medical Dictionary for Regulatory Activities (MedDRA®) in order to properly encode every ADR and WHO Anatomical Therapeutic Chemical (ATC) codes to standardize drug nomenclature.
ICSRs provide administrative information (country, type of report, qualification of the reporter), patient demographics (gender, age, weight), adverse events (seriousness of the ADR, date of onset reaction, outcome), information about drug therapy (drug name, drug start and stop dates, time to onset, dose, indication, dechallenge and rechallenge), but the level of completeness of information varies from case to case (Sakaeda et al., 2013). As the number of safety reports sent to the FDA annually is continuously expanding, the database is largely used to detect novel drug-related safety events (Carnovale et al., 2019a; Carnovale et al., 2019b; Mazhar et al., 2019; Pozzi et al., 2019).
Data Acquisition and Data Processing
This study was designed as a nested case-control study and data were downloaded from the FAERS Public Dashboard (FDA Public Dashboard, 2020). The base cohort consisted of all cases involving any AEs occurred during pregnancy (search terms: “complication of pregnancy”; “Exposure during pregnancy”; “Fetal exposure during pregnancy”; “Maternal exposure during pregnancy”).
The study period covered the first quarter of 2010 to the first quarter of 2020.
Duplicate records were detected and deleted accordingly as previously described (Carnovale et al., 2019; Mazhar et al., 2019). In order to reduce the risk of a misleading relationship between drugs and AEs, only reports entered by healthcare professionals were eligible for the final cohort.
Definition of Cases and Controls
Cases were defined as all Individual Case Safety Reports (ICSRs) where inflammasome-targeted drugs were reported as suspect drug. We then used the Standardized MedDRA Queries (SMQ) related to the group “Pregnancy and neonatal topics” to identify the risk of particular clusters of AEs in our cases. To find ICSRs of interest (i.e., cases reporting any maternal and/or fetal outcome following the use anti-IL drugs/colchicine) in FAERS database, we used the following SMQs: congenital, familial and genetic disorders; fetal disorders; neonatal disorders; termination of pregnancy and risk of abortion; pregnancy, labor and delivery complications and risk factors (excl. abortions and stillbirth) (Introductory Guide for Standardised MedDRA Queries, 2020).
Statistical Analyses
Descriptive analysis was performed for cases and non-cases, in terms of age, male sex (new-borns’ ICSR) and the presence of other co-suspect drugs.
To identify signals of disproportionate reporting (SDR) for pregnancy and neonatal outcomes in association with inflammasome-targeted drugs, we used the reporting odds ratio (ROR) as a measure of disproportional reporting, which estimates the frequency of an event of interest with the tested drugs compared with the other drugs. SDR were detected when the number of reports was higher than three and ROR–95% CI was greater than one. We calculated the adjusted ROR (aROR) by using multivariate logistic regression analysis adjusted for the presence of co-suspects, that was considered a potential confounding factor.
Sub-analysis was then performed to compare anti-IL1 agents and colchicine with TNFα-i, a well-known class of drugs used during pregnancy chronically and safely. We used R software for data tiding and statistical analysis (R core team, 2019).
Systematic Review
Literature Search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Moher et al., 2009) (Supplementary Table S1). We searched PubMed/MEDLINE, EMBASE databases up to June 23rd, 2020 with no language restriction. We provided our search strategy for PubMed in the Supplementary material (Supplementary Material S1); the search strategy was adapted as needed for each database. In brief, we used two terms: Interleukin 1 Receptor Antagonist/colchicine, and Pregnancy. We combined terms with the Boolean operator “AND”.
Eligibility Criteria
Inclusion criteria for the qualitative analysis were: studies enrolling human subjects; studies enrolling pregnant women affected by any clinical conditions treated with any anti-IL drugs/colchicine; studies reporting any maternal and/or fetal outcome following the use anti-IL drugs/colchicine; any type of observational studies and clinical trials; case report/case series.
We excluded: literature reviews; conference proceedings/abstracts and unpublished thesis; studies that did not report outcomes of interest; studies that reported the effect of pregnancy on IL-1 Inhibitor therapies/colchicine rather than vice versa.
We identified additional articles potentially eligible through the reference lists of articles included in our systematic review. We did not contact authors for missing data.
Study Selection
We imported into EndNote all the retrieved references from the final search in order to eliminate duplicate records. We screened the titles and abstracts of potentially eligible papers; whereas, we disregarded the papers deemed highly unlikely to be relevant. Finally, we assessed for eligibility all full-text versions of the remaining articles based on our pre-specified eligibility criteria as previously described. Two independent reviewers (CC and VB) conducted the entire search process. Discrepancies between review authors were resolved by discussion with a third review author (ET) to achieve a decision.
Data Extraction and Synthesis
Data from all included studies were extracted using pre-specified forms. The following relevant information was extracted: study type, study duration, number of subjects, number of women treated with biological agents, number of pregnancies, maternal diagnosis, mean age at conception, drug of interest, dose/scheme, concomitant treatment, any maternal and/or fetal outcome following the use of Interleukin 1 Receptor Antagonists/colchicine (including live birth, miscarriages, early/late/voluntary abortion, fetal death, small for gestation age-SGA, congenital malformation and stillbirth) in pregnant women, type of delivery, week at delivery, APGAR score, and duration of follow-up.
Results
Pharmacovigilance Study
Study Population
Of 11,789,929 ICSRs we retrieved from the FAERS in our period of observation, 40,033 involved maternal, fetal and neonatal reactions. Of these, only 84 (0.2%) reported the drugs of interest as “suspect,” i.e., 69 for Anti-IL1 and 15 for colchicine; no reports involved both drugs as “suspect.” The mean age between cases and non-cases was similar in the pregnant women (29 ± 6 vs. 31 ± 7 years old) with a range between 15 and 68 years old. Among the 84 cases, 24 ICSRs reported AEs for newborns and the maternal diagnoses mostly reported were “Familial Mediterranean Fever” (11 ICSRs) and “Rheumatological diseases” (14 ICSRs). In 59.5% of cases, more than one suspect drug was reported; 48.5% of non-cases reported co-suspect drugs.
Disproportionality Analysis
The number of cases, the crude and adjusted ROR for each cluster of AEs are presented in Table 1. From the total cohort, we retrieved 7,620 reports related to neonatal AEs, 2,889 to fetal disorders, 8,364 to abortion, 8,787 to congenital disorders, and 7,937 to labor/delivery complications.
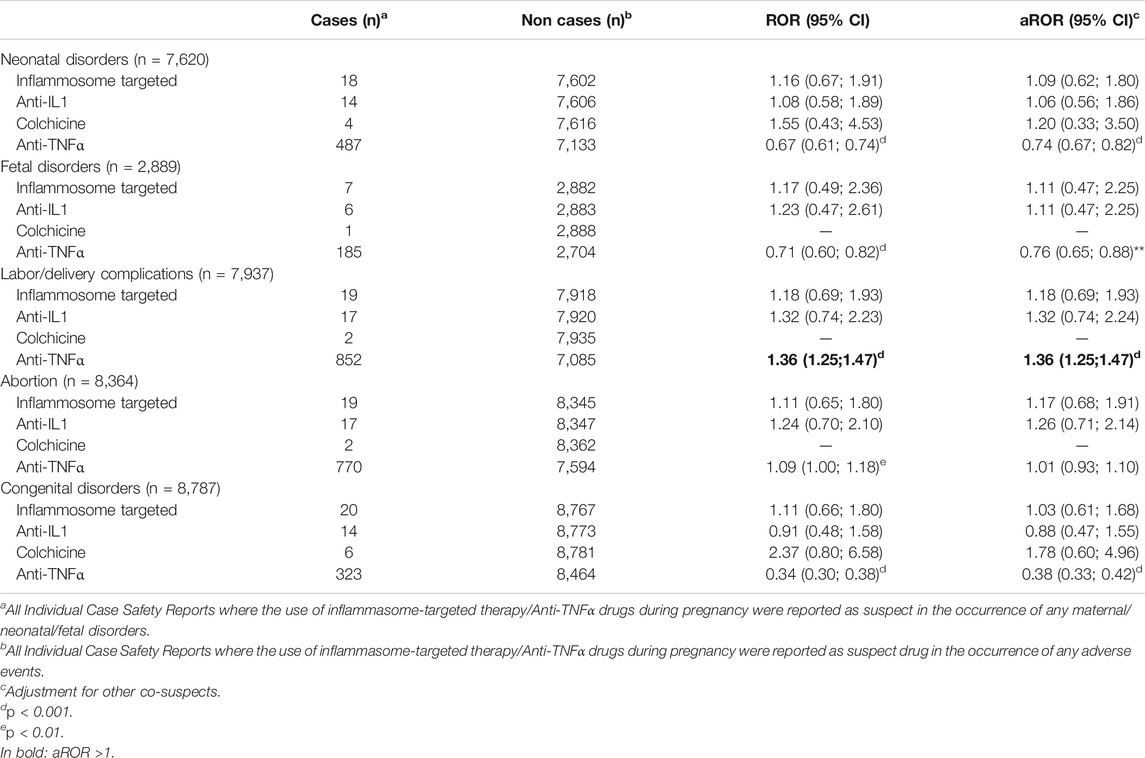
TABLE 1. Disproportionality analysis of adverse drug reactions spontaneous reporting database FAERS for the identification of Pregnancy and neonatal disorders.
According to the aROR and 95% CI, inflammasome-targeted drugs did not present any disproportionate reporting for all these clusters of AEs. Next, we performed a sub-analysis for individual drugs, including IL-1 inhibitors and colchicine and using TNFα-i as a better-characterized reference. No disproportionate reporting was identified for IL-1 inhibitors and colchicine. TNFα-inhibitors confirmed their safety during pregnancy with aROR <1 for all clusters of AEs except for labor complications.
Systematic Review
Characteristics of the Reviewed Studies
The study selection and screening are presented in the PRISMA flow-chart (Figure 1). Of the 1,419 articles retrieved (624 results were from PubMed, and 795 from EMBASE), 18 met the inclusion criteria.
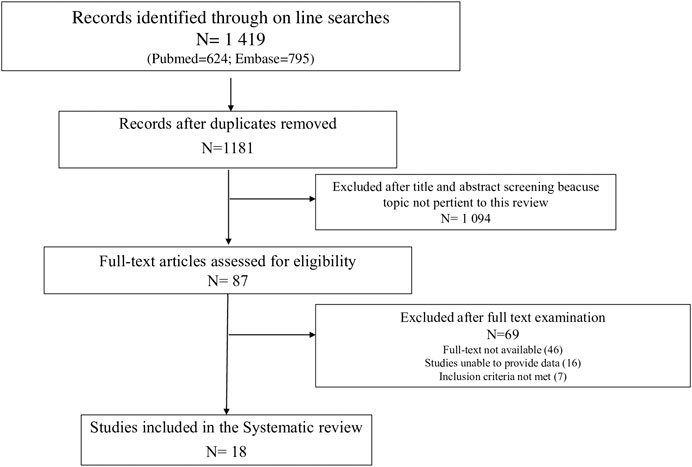
FIGURE 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of process of study selection.
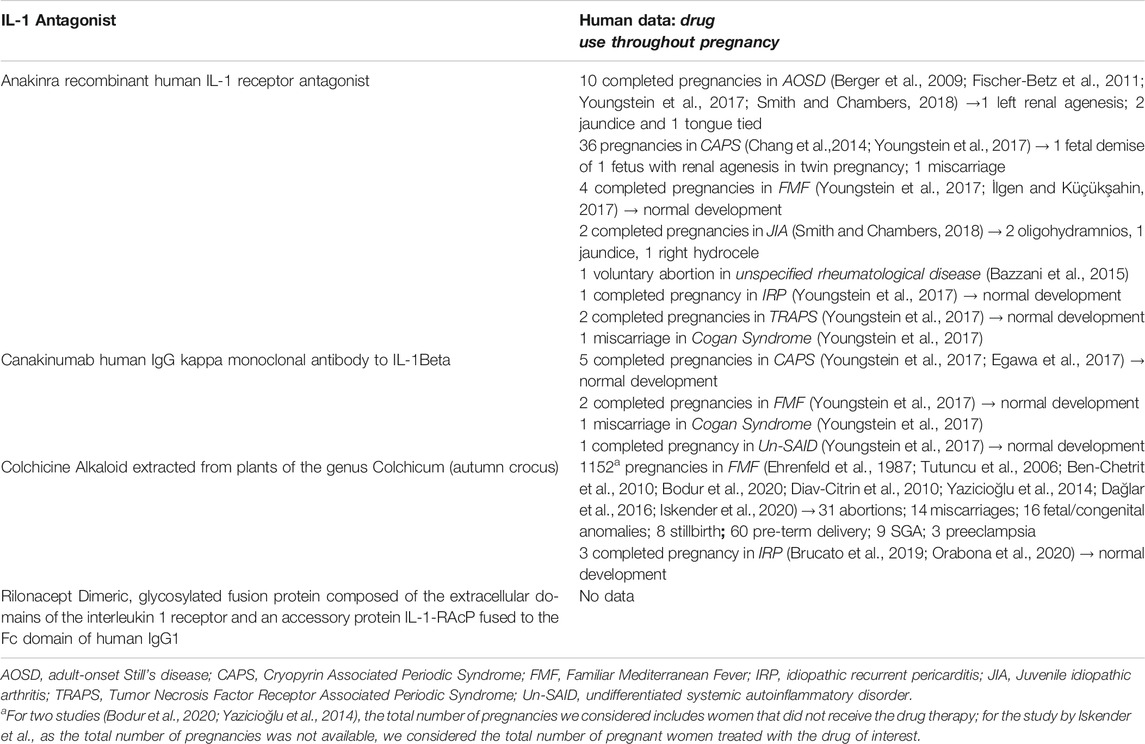
Twelve observational studies and 6 case report/series were eligible for the inclusion in our systematic review (Table 2), yielding a total of 2,075 patients. No clinical trials reporting maternal and/or fetal outcome following the use of Interleukin 1 Receptor Antagonists/colchicine in pregnant women were found.
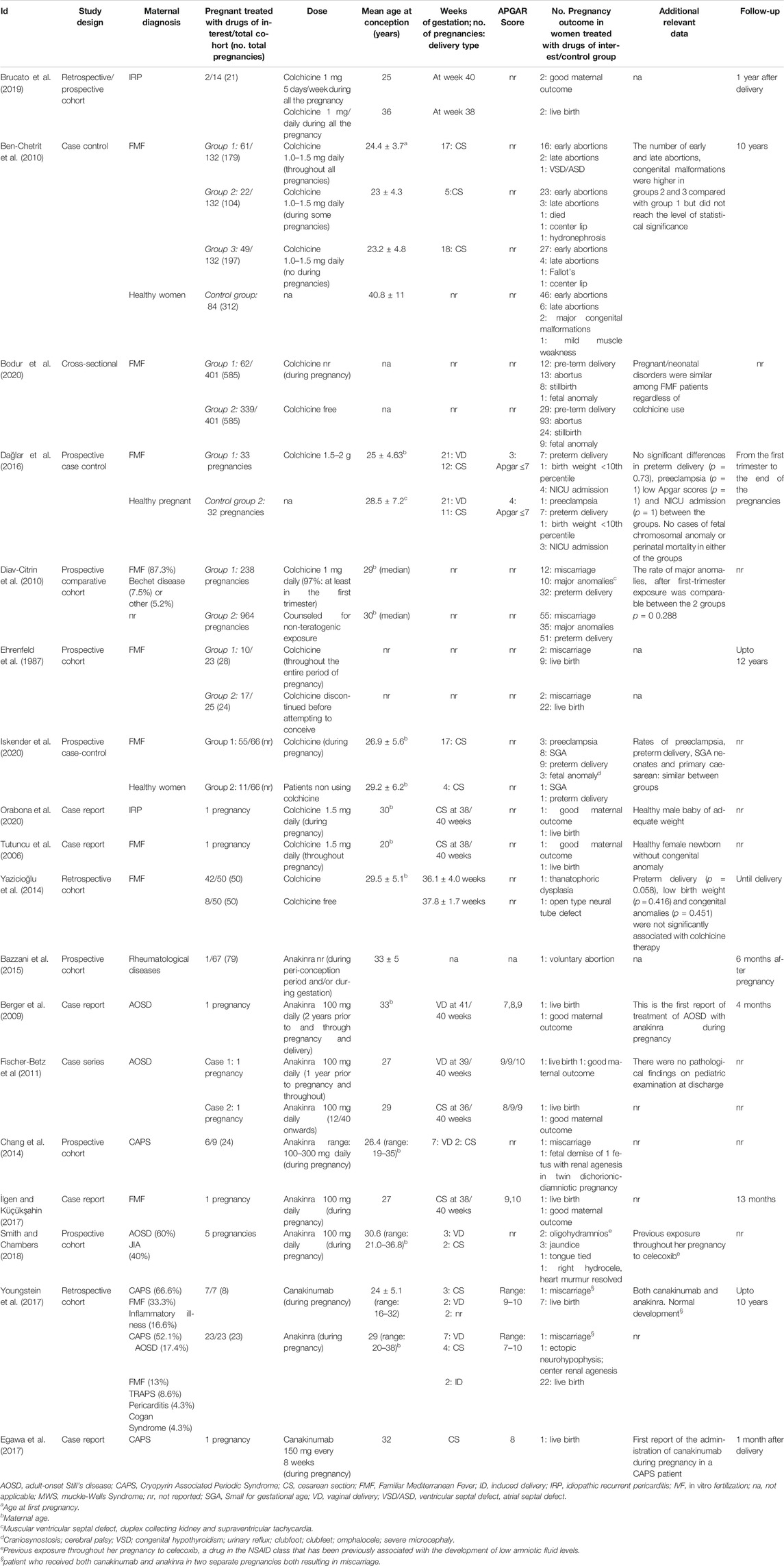
TABLE 2. Detailed overview of published data on Inflammosome target therapies and evidence for their use during pregnancy.
The average maternal age at conception ranges from 20 to 36 years. Of the 553 women treated with drugs of interest during pregnancy, 236 (42.6%) had a diagnosis of Familiar Mediterranean Fever (FMF); 3 (0.5%) of adult-onset Still’s disease (AOSD), 7 (1.2%) of Cryopyrin Associated Periodic Syndrome (CAPS), 3 (0.5%) of idiopathic recurrent pericarditis (IRP), and in the remaining cases a mixed cohort of women had various rheumatological diseases (FMF, CAPS, AOSD, Behcet’s disease, pericarditis, Cogan Syndrome, Juvenile idiopathic arthritis).
With respect to the type of medication used, 10 studies reported data on colchicine, 6 on anakinra, and 2 on canakinumab. The period from the baseline to the last follow-up varied considerably among the studies with a range from the first trimester to 10 years.
Data on the Use of anti-IL1 Drugs During Pregnancy
No studies reporting maternal and/or fetal outcome following the use of rilonacept were found.
Only a retrospective cohort study (Youngstein et al., 2017) and a case report (Egawa et al., 2017) provided data on the use of canakinumab during pregnancy (Table 2); in total, data were available for nine pregnancies; of these, eight completed pregnancies resulted in the normal development of the fetus; only 1 miscarriage in Cogan Syndrome patient was found.
Seven studies reported data on the use of anakinra in pregnant women (in total, data were available for 57 pregnancies).
In the prospective cohort study by Bazzani et al. (2015), only one woman took anakinra during a peri-conception period and gestation; the pregnancy resulted in a voluntary abortion. Data on case report/series (Fischer-Betz et al., 2011; Berger et al., 2009; İlgen and Küçükşahin, 2017) reported no pregnancy complications or fetal disorders in mothers with AOSD and FMF treated with anakinra (100 mg daily; 1–2 years prior to and through pregnancy and delivery). All pregnancies resulted in the normal development of a fetus.
Five pregnancies with anakinra exposure identified in the study by Smith and Chambers (2018) resulted in full-term singleton live births with no major or long-term complications. Two subjects developed oligohydramnios for unclear reasons.
Of twenty-three anakinra-exposed pregnancies in the retrospective study by Youngstein et al. (2017), 21 resulted in the birth of healthy infants (with follow-up of up to 10 years); one baby with unilateral renal agenesis and ectopic neurohypophysis was also reported. There were no serious neonatal infections.
In the prospective study by Chang et al. (2014) 6 women with CAPS were treated with anakinra throughout the pregnancy; no preterm births or serious pregnancy complications were reported and the miscarriage rates for pregnancies without and with anakinra treatment were 27% and 11%, respectively. One fetus of the twin pregnancy had renal agenesis and died in utero.
Despite reassuring findings, the lack of well-controlled studies make data on the use of anakinra in pregnancy difficult to interpret (Table 3).
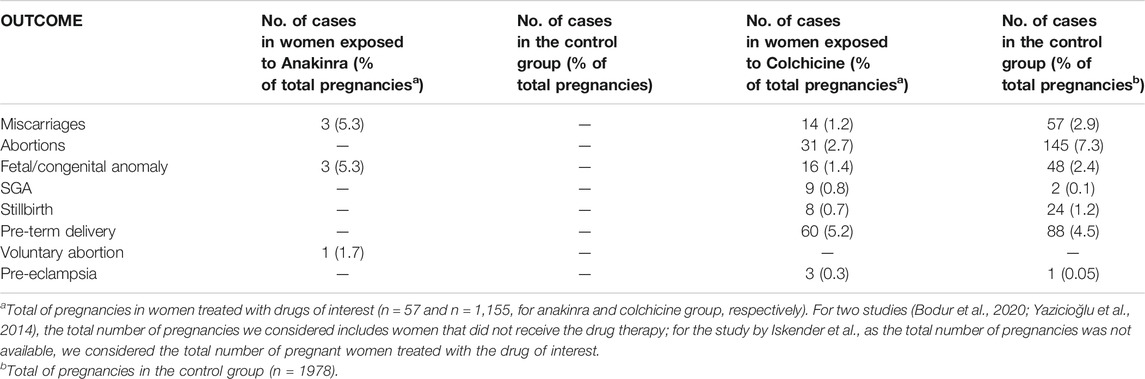
TABLE 3. Findings of systematic reviews on anakinra and colchicine for each outcome included in the analysis.
Data on the Use of Colchicine
Of 10 studies involving patients exposed to colchicine for any duration during pregnancy, eight were observational [of these, four were cohort studies (Ehrenfeld et al., 1987; Diav-Citrin et al., 2010; Yazicioğlu et al., 2014; Brucato et al., 2019); 3 case-control (Ben-Chetrit et al., 2010; Dağlar et al., 2016; Iskender et al., 2020); and 1 cross-sectional (Bodur et al., 2020)] and 2 were case report (Tutuncu et al., 2006; Orabona et al., 2020). Except for the study by Ehrenfeld et al, the remaining studies have been published recently (range: 2020–2006). Colchicine doses between 1 and 2 mg daily were reported. Two studies included healthy patients in their control groups (n = 116 women) (Ben-Chetrit et al., 2010; Dağlar et al., 2016).
In total, data were available for 1,155 pregnancies exposed to colchicine for any duration during pregnancy.
Of 1,152 pregnancies in women with FMF, 31% resulted in abortions, 14% in miscarriages, 16% in fetal/congenital anomalies, eight in stillbirth, 60 pre-term delivery; nine SGA and 3 preeclampsia.
There were three completed pregnancies in IRP patients, with good maternal/fetal outcomes (Orabona et al., 2020; Brucato et al., 2019). As reported in Table 3, findings from studies with control or comparator group reported no difference between the group of women treated with colchicine throughout all pregnancies and healthy women, in terms of miscarriages, early/late abortions, still-birth, fetal/congenital malformations, SGA, pre-term deliveries, and preeclampsia (Ehrenfeld et al., 1987; Ben-Chetrit et al., 2010; Diav-Citrin et al., 2010; Yazicioğlu et al., 2014; Dağlar et al., 2016; Iskender et al., 2020; Bodur et al., 2020). However, the robustness of uncontrolled evidence was poor in the reviewed studies.
Table 4 reported a summary of evidence on the use of Inflammasome target therapies in pregnancy.
Discussion
Available data assessed from the published literature shows that the rates of major congenital deformities and miscarriages for investigated inflammasome target therapies do not differ considerably from those rates in the general population. It should be stressed that from the published evidence, despite their heterogeneousness, no new safety concerns were identified from a total of 18 reports regarding the use of inflammasomes inhibitors during pregnancy. This has been further supported by the analysis of one of the largest spontaneous reporting system databases where we systematically examined the association for all currently clinically approved IL-1 inhibitors with an enormously higher number of reports representing pregnant population (40,033), strengthening the statistical power of the analyses.
Although there is little definitive evidence for the safety of IL-1 targeted therapies in pregnancy, emerging data from prospective birth cohort studies should help inform their use in pregnancy when no other suitable alternative available that can effectively control the maternal disease. Small scale clinical studies are not robust enough to answer safety concerns. In real-world, pregnant women take drugs either intentionally or unintentionally. Intentionally because some conditions require treatment during pregnancy, whilst a large proportion of pregnancies are unplanned entails unintentional drugs (Sheffield et al., 2014). Therefore, a constant vigilance of spontaneous reporting systems is essential to guarantee the safety profile of novel or repurposed treatment options. Findings obtained from case/non-case analyses of FAERS showed no potential safety signals for maternal and fetal AEs. These results are in accordance with the emerging evidence from small scale studies assessed in the present review. In FAERS, AEs related to pregnancy and neonatal outcomes are reported more frequently for anti-TNFα than treatments targeting IL-1. This could be explained by the fact that anti-TNFα drugs are the most prescribed treatment in pregnancy because of their safety evidence has already been translated into clinical practice (Flint et al., 2016; Skorpen et al., 2016). Like any epidemiology studies, valid results from case/non-case analyses depends on the classification of events and exposure. Underreporting of ADRs due to “misclassification bias” may explain a smaller number of reports for the comparator drug in case/non-case analyses, and henceforth direction and magnitude of the effect (Carnovale et al., 2019; Mazhar et al., 2019).
Our findings expand on Nguyen et al. (Nguyen at al., 2020) systematic review of 11 studies that investigated the risk of adverse pregnancy and neonatal outcomes associated with non-TNFα inhibitors and disease-modifying anti-rheumatic drugs (DMARDs) in pregnancy. Their findings do not suggest an increased risk associated with non-TNF α inhibitors and DMARDs in pregnancy. Nevertheless, the methods in the two studies are different. We have investigated a range of studies focused on IL-1 inhibitors only and have summed up data on congenital malformation/miscarriages. We have also summarized the pattern of reported major congenital malformations. In the present review, each IL-1 inhibitor was considered separately that can be of additional value if one is interested in specific IL-1 inhibitor drug. Moreover, data on maternal baseline characteristics are also detailed as the underlying maternal conditions are essential factors to be considered as a potential confounder for the pregnancy-related outcomes.
According to EMA guidelines, to rule out a ≥2-fold or ≥10-fold risk of major congenital malformations for medicine use during pregnancy, there is a need of moderate amount of data on pregnant women (between 300 and 1,000 pregnancy outcomes) collected prospectively (EMEA, 2008). This information should be collected from exposed pregnancies to that particular drug during the first trimester. Under this recommendation, the ascertainment of risk on summed up data remains unclear for most of the IL-1 antagonist because of the limited total number of exposures. Although for colchicine it can be concluded that it does not carry a ≥2-fold increased risk of combined major congenital malformations as compared to this risk in the general population which was estimated as 2–5.5% (Egbe et al., 2015). Over the past few years, the approach toward the use of colchicine throughout pregnancy has changed. Older reports and the 2015 ESC Guidelines (Adler et al., 2015) recommended against the use of colchicine during pregnancy due to limited information, while available data suggest that did not raise any concern about the teratogenicity of the drug. No association between an increased rate of adverse maternal/fetal outcomes with the colchicine throughout pregnancy was found in a recent systematic review of the literature (Indraratna et al., 2018).
A recent retrospective review of 34 exposed pregnancies to colchicine (Duman et al., 2019) reported the birth of 23 healthy infants and seven with several problems. Of seven infants with problems, four had congenital heart disease, one had nephrolithiasis, one had an inguinal hernia and one had mortality secondary to respiratory distress. The investigator acknowledged that the causality between colchicine use and the reported malformations was not adequately ascertained. These findings indicate that the influence of colchicine cannot be ruled out completely and should be beard in mind in cases of colchicine use for indications other than FMF or Behcet’s disease.
Safety data on IL-1 inhibitors anakinra and canakinumab remain very limited. Reviewed real-word data appear reassuring. In a retrospective study by the International Society for Systemic Auto-inflammatory diseases, 43 pregnancies exposed to IL-1 therapies (canakinumab, n = 14 and anakinra n = 29) from seven countries (Youngstein et al., 2017), were identified. There were no developmental abnormalities with a median follow-up of 18 months. They also reported the outcome of 14 neonates breastfed by mothers taking anakinra (n = 10) or canakinumab (n = 4) for up to 10 months, with no reported serious infections.
Largely reassuring outcomes was also reported in a small case series of seven pregnant women with AOSD treated with anakinra (Fischer-Betz et al., 2011; Smith and Chambers, 2018). Of seven pregnant women, two developed oligo-hydramnios, and one developed pregnancy-induced hypertension while taking anakinra. All the infants were born full-term (range: 36–40 weeks of gestation) and there were no adverse outcomes or major complications. Of the seven mothers, three breastfed their newborns successfully, where two mothers chose to breastfeed while continuing anakinra treatment with no reported adverse outcomes. Chang et al. (2014) described 24 pregnancies in patients with CAPS, reporting a lower miscarriage rate in women on anakinra than in those treated with other drugs (10% vs. 30%) suggesting anakinra use may have a protective effect on pregnancy outcomes for patients with IL-1-driven disease. Due to the scantiness of available data on the safety of IL-1 antagonists during conception, until additional information is available, these agents are not currently recommended during pregnancy and their discontinuation should be considered once pregnancy is confirmed (Sammaritano et al., 2020).
For several reasons, it is difficult to determine the impact of medications on obstetric and neonatal outcomes. In prospective studies, early miscarriages are missed because women are only included when they are pregnant and not in the pre-conception period. Whilst retrospective studies are endangered by publication bias because only women with an abnormal pregnancy are reported (Mitchell et al., 2011; Thorpe et al., 2013). Prospective studies that enroll the women from the phase they actively try to become pregnant would cover the pre-conception phase and provide reliable data. Furthermore, confounding by indication (some diseases cause more miscarriages) and by concomitant drugs can also affect the results.
Large population-based cohort studies from routine healthcare settings are crucial for improving the evidence for the safety of IL-1 treatments during pregnancy. Use of growing number of available electronic health care databases may be valuable strategy to capture information on medicine utilization patterns, and medicine safety when used during pregnancy. However, for future non-RCT studies there is a need to overcome important methodological issues related to methods for quantifying exposures to these drugs and other confounders during the whole of pregnancy and for adjusting for the latter in analyses.
Limitations
The robustness of uncontrolled evidence was poor in the reviewed studies. IL-1/colchicine-exposed women are likely to have other factors at some point in pregnancy as poor pregnancy outcomes are multifactorial, generally, this was not measured and so, could not be adjusted for in uncontrolled studies, making interpretation of these studies’ findings difficult. Type of disease and disease activity during pregnancy, co-morbid conditions, and concomitant drug treatments could also have contributed to negative outcomes. In some cases, the information on other outcomes (such as previous births, previous miscarriages, preterm births, ADRs, etc.) was not available. Finally, the use of a pharmacovigilance database has some intrinsic limitations; reporting might be influenced by factors including the notoriety bias, selection bias and under-reporting, which precludes making causal inferences except in unusual circumstances (Faillie 2019).
Conclusion
Despite limitations, no major safety issues were reported and no increasing trend could be identified in the reported malformations risk of biological IL-1 therapies in pregnancy. Colchicine appears to be compatible with pregnancy. Though these provide no suggestion that biological IL-1 treatments might be harmful, however, due to limited total number of exposed pregnancies the use of these agents in the clinical practice should be considered in pregnancy only if the risk benefit assessment of maintaining disease control justifies the potential risk to the fetus. These findings contribute additional evidence to inform treatment decision making for patients with IRD during pregnancy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made substantial, direct and intellectual contribution to the work and approved it for publication. CC, and ET conceptualized and designed the study, interpreted the data drafted the manuscript, revised and approved the final manuscript as submitted. VB participated in the conceptualization and design of the study, carried out the initial analyses, revised the manuscript and approved the final manuscript as submitted. FM, SR, MN, EN, ST participated in the interpretation of the data, revised the article, and approved the final article as submitted. AB conceptualized and designed the study, interpreted the data, coordinated and supervised data collection, critically reviewed the manuscript and approved the final manuscript as submitted.
Funding
The Authors received financial support for the publication of this article from the Swedish Orphan Biovitrum (Sobi™).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
FM is enrolled in the PhD in Experimental and Clinical Pharmacological Sciences, University of Milano, which supports his fellowship”.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.612259/full#supplementary-material.
References
Adler, Y., Charron, P., Imazio, M., Badano, L., Barón-Esquivias, G., Bogaert, J., et al. (2015). 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European association for cardio-thoracic surgery (EACTS). Eur. Heart J. 36 (42), 2921–2964. doi:10.1093/eurheartj/ehv318
Bazzani, C., Scrivo, R., Andreoli, L., Baldissera, E., Biggioggero, M., Canti, V., et al. (2015). Prospectively-followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre study. Clin. Exp. Rheumatol. 33 (5), 688–693.
Ben-Chetrit, E., Ben-Chetrit, A., Berkun, Y., and Ben-Chetrit, E. (2010). Pregnancy outcomes in women with familial Mediterranean fever receiving colchicine: is amniocentesis justified? Arthritis Care Res. 62 (2), 143–148. doi:10.1002/acr.20061
Berger, C. T., Recher, M., Steiner, U., and Hauser, T. M. (2009). A patient's wish: anakinra in pregnancy. Ann. Rheum. Dis. 68 (11), 1794–1795. doi:10.1136/ard.2008.105833
Bodur, H., Yurdakul, F. G., Çay, H. F., Uçar, Ü., Keskin, Y., and Sargın, B. (2020). Familial Mediterranean fever: assessment of clinical manifestations, pregnancy, genetic mutational analyses, and disease severity in a national cohort. Rheumatol. Int. 40 (1), 29–40. doi:10.1007/s00296-019-04443-0
Brucato, A., Pluymaekers, N., Tombetti, E., Rampello, S., Maestroni, S., Lucianetti, M., et al. (2019). Management of idiopathic recurrent pericarditis during pregnancy. Int. J. Cardiol. 282, 60–65. doi:10.1016/j.ijcard.2019.02.003
Carnovale, C., Mazhar, F., Arzenton, E., Moretti, U., Pozzi, M., Mosini, G., et al. (2019a). Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase®. Expet Opin. Drug Saf. 18 (11), 1099–1108. doi:10.1080/14740338.2019.1668373
Carnovale, C., Mosini, G., Gringeri, M., Battini, V., Mazhar, F., Pozzi, M., et al. (2019b). Interaction between paracetamol and lamotrigine: new insights from the FDA Adverse Event Reporting System (FAERS) database. Eur. J. Clin. Pharmacol. 75 (9), 1323–1325. doi:10.1007/s00228-019-02691-4
Chang, Z., Spong, C. Y., Jesus, A. A., Davis, M. A., Plass, N., Stone, D. L., et al. 2014). Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS). Arthritis Rheum. 66 (11), 3227–3232. doi:10.1002/art.38811
Dağlar, K., Kirbaş, A., Timur, H., Kara, O., Inal, H., Gencosmanoglu, G., et al. (2016). Subclinical inflammation and simple blood parameters in pregnant with familial mediterranean fever. J. Clin. Anal. Med. 7 (5), 634–638. doi:10.4328/JCAM.4158
Diav-Citrin, O., Shechtman, S., Schwartz, V., Avgil-Tsadok, M., Finkel-Pekarsky, V., Wajnberg, R., et al. (2010). Pregnancy outcome after in utero exposure to colchicine. Am. J. Obstet. Gynecol. 203 (2), 144–146. doi:10.1016/j.ajog.2010.02.063
Dinarello, C. A., and van der Meer, J. W. M. (2013). Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 25, 469–484. doi:10.1016/j.smim.2013.10.008
Duman, N. C., Karabacak, M., Oglu, M. G. I., Inanc, N., Asik, Z. N. T., Atagunduz, P., et al. (2019). “Colchicine use during pregnancy: case reports,” in Annual European Congress of Rheumatology (EULAR), Madrid, Spain, 2019, Vol. 78, 2082–2083. doi:10.1136/annrheumdis-2019-eular.3999
Egawa, M., Imai, K., Mori, M., Miyasaka, N., and Kubota, T. (2017). Placental transfer of canakinumab in a patient with muckle-wells syndrome. J. Clin. Immunol. 37 (4), 339–341. doi:10.1007/s10875-017-0389-3
Egbe, A., Uppu, S., Lee, S., Stroustrup, A., Ho, D., and Srivastava, S. (2015). Congenital malformations in the newborn population: a population study and analysis of the effect of sex and prematurity. Pediatr. Neonatol. 56 (1), 25–30. doi:10.1016/j.pedneo.2014.03.010
Ehrenfeld, M., Brzezinski, A., Levy, M., and Eliakim, M. (1987). Fertility and obstetric history in patients with familial Mediterranean fever on long-term colchicine therapy. Br. J. Obstet. Gynaecol. 94 (12), 1186–1191. doi:10.1111/j.1471-0528.1987.tb02320.x
EMEA (2008). Guideline on risk assessment of medicinal products on human reproduction and lactation: from data to labelling, 24. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-assessment-medicinal-products-human-reproduction-lactation-data-labelling_en.pdf (Accessed June 20, 2020). July 2008 EMEA/CHMP/203927/2005.
Faillie, J. (2019). Case-non-case studies: principle, methods, bias and interpretation. Therapie 74 (2), 225–232. doi:10.1016/j.therap.2019.01.006
FDA Public Dashboard (2020). Available at: https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis (Accessed June 30, 2020).
Fischer-Betz, R., Specker, C., and Schneider, M. (2011). Successful outcome of two pregnancies in patients with adult-onset Still’s disease treated with IL-1 receptor antagonist (anakinra). Clin. Exp. Rheumatol. 29 (6), 1021–1023.
Flint, J., Panchal, S., Hurrell, A., van de Venne, M., Gayed, M., Schreiber, K., et al. (2016). BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology. 55 (9), 1693–1697. doi:10.1093/rheumatology/kev404
Gentili, M., Pozzi, M., Peeters, G., Radice, S., and Carnovale, C. (2018). Review of the methods to obtain paediatric drug safety information: spontaneous reporting and healthcare databases, active surveillance programmes, systematic reviews and meta-analyses. Curr. Clin. Pharmacol. 13 (1), 28–39. doi:10.2174/1574884713666180206164634
Götestam Skorpen, C., Hoeltzenbein, M., Tincani, A., Fischer-Betz, R., Elefant, E., Chambers, C., et al. (2016). The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 75 (5), 795–810. doi:10.1136/annrheumdis-2015-208840
Indraratna, P. L., Virk, S., Gurram, D., and Day, R. O. (2018). Use of colchicine in pregnancy: a systematic review and meta-analysis. Rheumatology. 57, 382–387. doi:10.1093/rheumatology/kex353
Introductory Guide for Standardised MedDRA Queries (2020). Introductory Guide for Standardised MedDRA Queries (SMQs) version 23.0. Available at: https://admin.new.meddra.org/sites/default/files/guidance/file/SMQ_intguide_23_0_English.pdf (Accessed June 20, 2020).
Iskender, D., Kara, O., Ozturk Kaymak, A., Daglar, H. K., Kirbas, A., Iskender, C. T., et al. (2020). Association between basal proteinuria levels and pregnancy outcomes in familial Mediterranean fever. J. Obstet. Gynaecol. 40, 1102–1105. doi:10.1080/01443615.2019.1700944
İlgen, U., and Küçükşahin, O. (2017). Anakinra use during pregnancy: report of a case with familial Mediterranean fever and infertility. Eur. J. Rheumatol. 4 (1), 66–67. doi:10.5152/eurjrheum.2017.16075
Klein, A. L., Imazio, M., Cremer, P., Brucato, A., Abbate, A., Fang, F., et al. (2020). RHAPSODY investigators. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N. Engl. J. Med. (Online ahead of print). doi:10.1056/NEJMoa2027892
Komaki, F., Komaki, Y., Micic, D., Ido, A., and Sakuraba, A. (2017). Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J. Autoimmun. 76, 38–52. doi:10.1016/j.jaut.2016.11.004
Mazhar, F., Pozzi, M., Gentili, M., Scatigna, M., Clementi, E., Radice, S., et al. (2019). Association of hyponatraemia and antidepressant drugs: a pharmacovigilance-pharmacodynamic assessment through an analysis of the US Food and drug administration adverse event reporting system (FAERS) database. CNS Drugs. 33, 581–592. doi:10.1007/s40263-019-00631-5
Mitchell, A. A., Gilboa, S. M., Werler, M. M., Kelley, K. E., Louik, C., and Hernández-Díaz, S. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obstet. Gynecol. 205 (1), 51–58. doi:10.1016/j.ajog.2011.02.029
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.The PRISMA Group (2009). Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 3 (7), e1000097. doi:10.1371/journal.pmed1000097
Nguyen, A. H., Ahmed, K., Flint, G., and Giles, I. (2020). Systematic review of the safety of non-tumour necrosis factor inhibitor and targeted synthetic drugs in rheumatic disease in pregnancy. Rheumatology. 59 (2). doi:10.1093/rheumatology/keaa111.114
Orabona, R., Zanardini, C., Zatti, S., Lojacono, A., Procopio, R., Vizzardi, E., et al. (2020). Onset of idiopathic recurrent pericarditis during pregnancy: successful management with colchicine and intravenous immunoglobulins. J. Cardiovasc. Med. 21 (5), 393–395. doi:10.2459/JCM.0000000000000904
Østensen, M., Andreoli, L., Brucato, A., Cetin, I., Chambers, C., Clowse, M. E., et al. (2015). State of the art: reproduction and pregnancy in rheumatic diseases. Autoimmun. Rev. 14 (5), 376–386. doi:10.1016/j.autrev.2014.12.011
Østensen, M., Lockshin, M., Doria, A., Valesini, G., Meroni, P., Gordon, C., et al. (2008). Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology. 47 (Suppl 3), 28–31. doi:10.1093/rheumatology/ken168
Østensen, M. (2017). The use of biologics in pregnant patients with rheumatic disease. Expet Rev. Clin. Pharmacol. 10, 661–669. doi:10.1080/17512433.2017.1305268
Perrotta, C., Giordano, F., Colombo, A., Carnovale, C., Castiglioni, M., Di Bernardo, I., et al. (2019). Postpartum bleeding in pregnant women receiving SSRIs/SNRIs: new insights from a descriptive observational study and an analysis of data from the FAERS database. Clin. Ther. 41 (9), 1755–1766. doi:10.1016/j.clinthera.2019.06.008
Pozzi, M., Carnovale, C., Mazhar, F., Peeters, G. G. A. M., Gentili, M., Nobile, M., et al. (2019). Adverse drug reactions related to mood and emotion in pediatric patients treated for attention deficit/hyperactivity disorder: a comparative analysis of the US Food and drug administration adverse event reporting system database. J. Clin. Psychopharmacol. 39 (4), 386–392. doi:10.1097/JCP.0000000000001058.PMID:31205193
R Core Team (2019). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/ (Accessed June 20, 2020).
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci 10, 796–803. doi:10.7150/ijms.6048
Sammaritano, L. R., Bermas, B. L., Chakravarty, E. E., Chambers, C., Clowse, M. E. B., Lockshin, M. D., et al. (2020). 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheum. 72 (4), 529–556. doi:10.1002/art.41191
Sheffield, J. S., Siegel, D., Mirochnick, M., Heine, R. P., Nguyen, C., Bergman, K. L., et al. (2014). Designing drug trials: considerations for pregnant women. Clin. Infect. Dis. 59 (Suppl 7), S437–S444. doi:10.1093/cid/ciu709
Smeele, H. T. W., and Dolhain, R. J. E. M. (2019). Current perspectives on fertility, pregnancy and childbirth in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 49 (Suppl 3), S32–S35. doi:10.1016/j.semarthrit.2019.09.010
Smith, C. J. F., and Chambers, C. D. (2018). Five successful pregnancies with antenatal anakinra exposure. Rheumatology. 57 (7), 1271–1275. doi:10.1093/rheumatology/key093
Thorpe, P. G., Gilboa, S. M., Hernandez-Diaz, S., Lind, J., Cragan, J. D., Briggs, G., et al. (2013). Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol. Drug Saf. 22 (9), 1013–1018. doi:10.1002/pds.3495
Tutuncu, L., Atasoyu, E. M., Evrenkaya, R., and Mungen, E. (2006). Familial Mediterranean fever-related nephrotic syndrome and successful full-term pregnancy. Arch. Med. Res. 37 (1), 178–180. doi:10.1016/j.arcmed.2005.04.013
Yazicioğlu, A., Turgal, M., Yücel, O., Özyüncü, Ö., and Beksaç, M. S. (2014). Pregnancy outcome in women with familial Mediterranean fever: a retrospective analysis of 50 cases with a 10-year experience. Arch. Rheumatol. 29 (2), 94–98. doi:10.5606/ArchRheumatol.2014.3707
Youngstein, T., Hoffmann, P., Gül, A., Lane, T., Williams, R., Rowczenio, D. M., et al. (2017). International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology. 56 (12), 2102–2108. doi:10.1093/rheumatology/kex305
Keywords: pregnancy, pharmacovigilance, inflammasome targeted therapy, IL-inhibitors, colchicine, pharmacovgilance
Citation: Carnovale C, Tombetti E, Battini V, Mazhar F, Radice S, Nivuori M, Negro E, Tamanini S and Brucato A (2021) Inflammasome Targeted Therapy in Pregnancy: New Insights From an Analysis of Real-World Data From the FAERS Database and a Systematic Review. Front. Pharmacol. 11:612259. doi: 10.3389/fphar.2020.612259
Received: 30 September 2020; Accepted: 16 December 2020;
Published: 20 January 2021.
Edited by:
Cecilia Beatrice Chighizola, Istituto Auxologico Italiano (IRCCS), ItalyReviewed by:
Ariela Hoxha, San Bortolo Hospital, ItalyGabriele Simonini, University of Florence, Italy
Copyright © 2021 Carnovale, Tombetti, Battini, Mazhar, Radice, Nivuori, Negro, Tamanini and Brucato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Carnovale, carla.carnovale@unimi.it
†These authors have contributed equally to this work.
 Carla Carnovale
Carla Carnovale