
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 25 March 2021
Sec. Neuropharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.621691
Objective: Antipsychotic compounds are known to induce sedation somnolence and have expanded clinical indications beyond schizophrenia to regulatory approval in bipolar disorder, treatment-resistant depression, and is being repurposed in infectious diseases and oncology. However, the medical sciences literature lacks a comprehensive association between sedation and somnolence among a wide-range of antipsychotic compounds. The objective of this study is to assess the disproportionality of sedation and somnolence among thirty-seven typical and atypical antipsychotics.
Materials and Methods: Patient adverse drug reactions (ADR) cases were obtained from the United States Food and Drug Administration Adverse Events Reporting System (FAERS) between January 01, 2004 and September 30, 2020 for a wide-array of clinical indications and off-label use of antipsychotics. An assessment of disproportionality were based on cases of sedation and somnolence and calculated using the case/non-case methodology. Statistical analysis resulting in the reporting odds-ratio (ROR) with corresponding 95% confidence intervals (95% CI) were conducted using the R statistical programming language.
Results: Throughout the reporting period, there were a total of 9,373,236 cases with 99,251 specific ADRs reporting sedation and somnolence. Zuclopenthixol (n = 224) ROR = 13.3 (95% CI, 11.6–15.3) was most strongly associated of sedation and somnolence and haloperidol decanoate long-acting injection (LAI) was not statistically associated sedation and somnolence. Further, among atypical antipsychotic compounds, tiapride and asenapine were the top two compounds most strongly associated with sedation and somnolence. Comprehensively, the typical antipsychotics ROR = 5.05 (95%CI, 4.97–5.12) had a stronger association with sedation and somnolence when compared to atypical antipsychotics ROR = 4.65 (95%CI, 4.47–4.84).
Conclusion: We conducted a head-to-head comparison of thirty-seven antipsychotics and ranked the compounds based on the association of sedation and somnolence from ADR data collected throughout 16 years from the FAERS. The results are informative and with recent interests in repurposing phenothiazine antipsychotics in infectious disease and oncology provides an informative assessment of the compounds during repurposing and in psychopharmacology.
Antipsychotics were originally termed major tranquilizers as these compounds are effective in abating psychosis, mania, agitation, and aggression with also having an underlying pharmacodynamic outcome of inducing sedation and somnolence (Welsh, 1964). Major tranquilizers modulate monoaminergic targets including: adrenergic, noradrenergic, dopaminergic, histaminergic, and serotonergic receptors to induce sedation and somnolence (Welsh, 1964). Among the other terms used to describe antipsychotics, such as neuroleptics and ataraxic, the major tranquilizers were then re-classified to typical and atypical antipsychotics. Typical antipsychotics are ligands which predominately bind to dopamine D2-receptors, whereas, atypical antipsychotics are ligands with higher binding affinities towards the various subtypes of serotonin 5-HT receptors and may be dichotomized using the ratio of 5-HT2 to dopamine D2 binding affinities (Meltzer et al., 1989). Despite the re-classification of antipsychotics, one common theme is the clinical outcome of producing sedation and somnolence.
In psychiatry, sleep dysregulation is common in patients experiencing psychosis, mania, depression, delirium, suicidal ideation, irritability, delusions, agitation, and other disruptions in mental health. Sedation and somnolence leading to proper sleep is neuroprotective and with increasing interest in repurposing antipsychotics in oncology and infectious diseases meets an unmet clinical need in the medical sciences and patient care (Koyanagi and Stickley, 2015; Laskemoen et al., 2019). Research has shown that with sufficient sleep, one experiences enhanced cognition coupled with efficient memory recall leading to improved academic performance; while in contrast, when sleep is chronically deficient, one may experience impaired memory, depression, anxiety, persecutory ideation, cognitive disorganization, paranoia, and hallucinations (Kelly et al., 2001; Alhola and Polo-Kantola, 2007; Freeman et al., 2010; Reeve et al., 2018; Eugene, 2019; Eugene, 2020a).
Antipsychotic compounds have expanded regulatory approval beyond schizophrenia as demonstrated by clinical studies showing benefit in augmenting antidepressants in treatment-resistant depression, as well as is indicated in patients with bipolar mania, bipolar depression, and mixed features in bipolar disorder (Eugene and Masiak, 2017; Eli Lilly and Company, 2019; AstraZeneca Pharmaceuticals, 2020; America Pharmaceutical, 2020a; Otsuka America Pharmaceutical, 2020a; Otsuka America Pharmaceutical, 2020b). However, despite the wide-spread use of major tranquilizers, there is a fundamental gap in psychopharmacology with ranking a substantive number of antipsychotics based on association of sedation and somnolence.
The most comprehensive study, to date, specifically assessing somnolence in antipsychotics was conducted by Fang and colleagues and includes 12 compounds (Fang et al., 2016). In that study, which evaluated the absolute risk increase of somnolence showed that clozapine being classified as high somnolence, while olanzapine, perphenazine, quetiapine, risperidone, and ziprasidone classified as moderate somnolence, and lastly, asenapine, aripiprazole, cariprazine, lurasiodone, haloperidol, and paliperidone were categorized as low somnolence compounds (Fang et al., 2016). In a post-hoc analysis by Gao and colleagues evaluating the incidence of somnolence in ten clinical trials (n = 4,786) of four total antipsychotics, reported that out of asenapine, olanzapine, risperidone, and haloperidol, only olanzapine and asenapine showed significantly increased rates of somnolence relative to placebo (Gao et al., 2013). The most comprehensive study evaluating sedation, to date, is a network meta-analysis by Huhn and colleagues, which showed out of thirty-two antipsychotics, zuclopenthixol had the highest risk of sedation (Huhn et al., 2019). Taken together, these findings suggest that the wide inter-individual variability in outcomes of sedation and somnolence and may be due to drug-gene influences, drug-drug interactions (DDI), or combination of both influencing drug pharmacokinetics – via DNA sequence variants influencing cytochrome P450 (CYP) enzymes – and pharmacodynamics.
Studies using electroencephalography brain-mapping demonstrate that antipsychotics are associated with the delta frequency band and slow wave sleep via the serotonin 5-HT2A and 5-HT2C receptors (Sharpley et al., 1994; Eugene et al., 2014; Eugene and Masiak, 2014). Whereas, other translational biomarker studies, using prepulse inhibition, reports that sleep deprivation induces perceptual distortion (e.g. auditory hallucinations), cognitive disorganization, and anhedonia among healthy adults (Petrovsky et al., 2014). With this information as a background, the objective of this study is to assess the disproportionality in reporting of sedation and somnolence among thirty-seven typical and atypical antipsychotic drugs using post-marketing adverse drug events reported to the United States Food and Drug Administration.
Patient adverse drug reaction (ADR) cases were obtained from the United States Food and Drug Administration Adverse Events Reporting System (FAERS) and assessed retrospectively in an observational study approach. The first recorded case using the term ‘somnolence’, in the FAERS, was in 1993 and the first record of ‘sedation’ in the FAERS was in 1969. This study includes all cases of either sedation or somnolence from the January 01, 2004 to the September 30, 2020 contained no identifiable patient information in the FAERS reports. The Medical Dictionary for Regulatory Activities (MedDRA) coded preferred terms used in this study are either sedation or somnolence and no other ADR was considered for analysis (Brown et al., 1999). For the thirty-seven antipsychotic compounds included in this study, the primary outcome variable is the reporting odds-ratio and this method has been previously reported in studies from the first author (Eugene and Eugene, 2018; Eugene, 2019; Eugene, 2020a).
A complete list of clinical indications corresponding to the study data presented may be seen in the supplementary files. A partial list is as follows: schizophrenia, bipolar disorder, depression, anxiety, hallucination, sleep disorder, attention deficit/hyperactivity disorder, agitation, aggression, parkinson's disease psychosis, insomnia, mania, suicide attempt, paranoia, autism spectrum disorder, dementia, oppositional defiant disorder, delirium, post-traumatic stress disorder, tourette's disorder, adjuvant therapy, generalised anxiety disorder, impulsive behavior, sedation, psychomotor hyperactivity, intellectual disability, asperger's disorder, sleep disorder therapy, self injurious behavior, substance-induced psychotic disorder, vomiting, sedative therapy, off label use, prophylaxis of nausea and vomiting, huntington's disease, hyperkinesia, developmental delay, postpartum depression, renal cancer, antisocial behaviour, creutzfeldt-jakob disease, tic tourette's disorder, and traumatic brain injury.
The following antipsychotic compound generic names were included in the study: amisulpride, aripiprazole, aripiprazole lauroxil, asenapine, blonanserin, brexpiprazole, cariprazine, chlorpromazine, chlorprothixene, clozapine, cyamemazine, droperidol, fluphenazine, haloperidol, haloperidol decanoate, iloperidone, loxapine, lurasidone, melperone, olanzapine, paliperidone, paliperidone palmitate, periciazine, perphenazine, pimavanserin, pimozide, pipamperone, prochlorperazine, promazine, quetiapine, risperidone, thioridazine, thiothixene, tiapride, trifluoperazine, ziprasidone, and zuclopenthixol.
Disproportionality signal analysis of antipsychotic cases reporting somnolence was calculated using the reporting odds-ratio (ROR) with the corresponding 95% confidence interval (CI). The ROR is the odds of a certain event occurring for a medicinal product, compared to the odds of the same event occurring with all other medicinal products in the database. This case/non-case method, similar to a case-control study, via a two-by-two contingency table is calculated by the fisher.test command in R. For study inclusion, a minimum of one adverse event case was required. All computations and the figure illustration were performed using R (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) (Team, 2013). A signal is considered statistically significant when the lower limit of the 95% CI of the ROR is greater than one.
From January 2004 to September 30, 2020, there were a total of 9,373,236 overall cases reported to the FAERS and 99,251 cases specifically were reported as sedation and somnolence. Among the thirty-seven antipsychotic compounds, quetiapine (n = 96,985), an atypical antipsychotic, has the highest number of reported cases; whereas, haloperidol decanoate (n = 6), a typical antipsychotic in a long-acting injection formulation, had the least number of cases. The top ranked antipsychotics with the strongest association of sedation and somnolence were: zuclopenthixol (n = 224) ROR = 13.3 (95% CI, 11.6–15.3), tiapride (n = 76) ROR = 11.8 (95% CI, 9.3–15.0), and cyamemazine (n = 245) ROR = 10.7 (95% CI, 9.4–12.2). The long-acting injection formulation, haloperidol decanoate (n = 6) ROR = 1.7 (95% CI, 0.8–3.8) was not associated with sedation or somnolence; while, in contrast the pill version of haloperidol (n = 1158) had a strong association with a ROR = 5.6 (95% CI, 5.3–6.0).
The following three antipsychotic compounds were least associated with sedation and somnolence (ROR crosses 2): prochlorperazine (n = 202) ROR = 1.4 (95% CI, 1.2–1.6), paliperidone (n = 641) ROR = 1.9 (95% CI, 1.8–2.0), and aripiprazole lauroxil (n = 36) ROR = 2.1 (95% CI, 1.5–3.0). Of note, as with the pill formulation of haloperidol, the aripiprazole pill formulation had a stronger association with sedation and somnolence (n = 2,647) ROR = 3.7 (95% CI, 3.6–3.9), when compared to the long-acting injection formulations of the two compounds. Overall, the typical (ROR = 5.05 [95% CI, 4.97–5.12]) antipsychotics had a stronger association with sedation and somnolence when compared to atypical (ROR = 4.65 [95% CI, 4.47–4.84]) antipsychotics.
Figure 1 illustrates the forest plots illustrating RORs with corresponding 95% CIs for antipsychotic compounds, sorted from the highest to lowest of sedation and somnolence. In Figure 1, the dashed line at 1 represents the reference point for statistical significance for sedation and somnolence. A list of the thirty-seven antipsychotic compounds sorted from highest to lowest reporting odds of sedation and somnolence are provided as a Supplementary File S2.
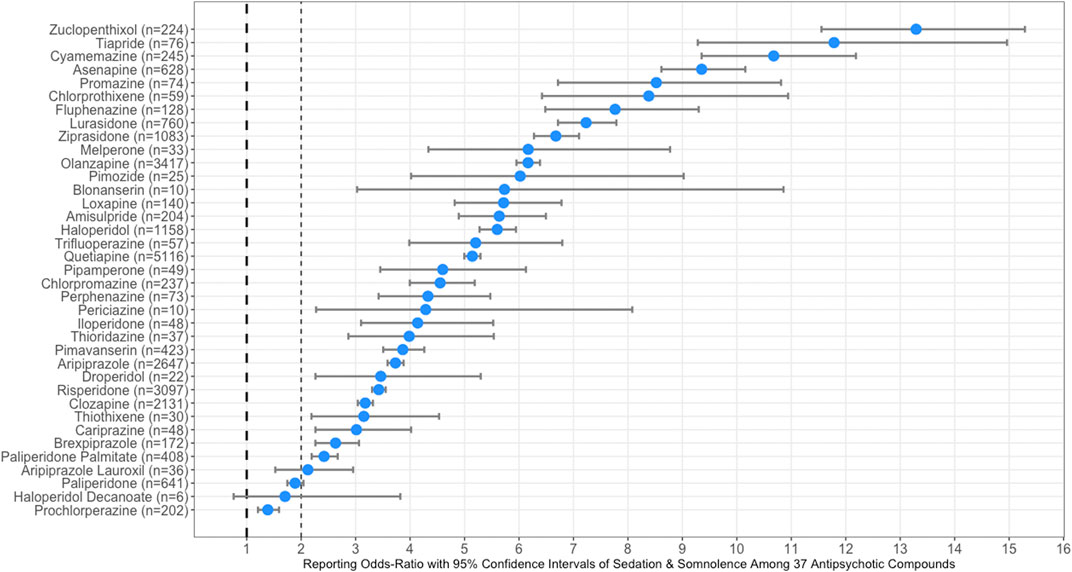
FIGURE 1. Forest plots illustrating reporting odds-ratios (RORs) with 95% confidence intervals (CI) of sedation and somnolence among all thirty-seven typical an atypical antipsychotic compounds. The dashed line at one represents the reference point for statistical significance of sedation and somnolence.
In this pharmacovigilance study with adverse drug reactions spanning sixteen-years of reports to the United States Food and Drug Administration, thirty-five of the thirty-seven typical and atypical antipsychotic formulations showed statistical significance with sedation and somnolence. As mentioned, the typical antipsychotics had a stronger association with sedation and somnolence as compared to the atypical antipsychotics. We found zuclopenthixol ranked highest in association with sedation and somnolence and our findings are consistent with a large-scale network meta-analysis which reported zuclopenthixol also being ranked first among 32 antipsychotic drugs (Huhn et al., 2019). The methodology used in this study for a direct head-to-head comparison of drugs reported to the FAERS provides a pragmatic approach in clinical pharmacology to assess signal strength of ADRs beyond traditional studies using meta-analyses.
Compound metabolites are of increasing importance to clinical pharmacologists due to an increasing interests in pharmacogenomics and drug-interactions associated CYP enzymes, as well as polymorphisms of receptors, transporters, and also transcription factors influencing CYP enzymes (Qin et al., 2019). Risperidone is metabolized via CYP2D6 to paliperidone and both compounds are represented here as independent compounds with the paliperidone pill formulation having a weaker association with sedation and somnolence when compared to the parent compound risperidone. Another compound with a well-known metabolite is loxapine, and a previous study showed amoxapine, a metabolite of loxapine, ranked number one among thirty antidepressants in reporting odds of somnolence (Eugene, 2020a). The current study results are informative, especially in patients who experience acute agitation and aggression associated with mania and psychosis. For example, the most recent approved inhaled formulation of loxapine has a median time to the maximum plasma concentration (Tmax) of 2 min and reported to be effective in acute agitation in bipolar I disorder and schizophrenia (Galen, 2019). The capsule formulation of loxapine is reported to cause sedation within 20 to 30 min (Actavis Pharma, 2016).
Zuclopenthixol, the neuroleptic with the strongest association with sedation and somnolence, has emerged as a compound that shows in vitro inhibition of the severe acute respiratory coronavirus 2 (SARS-CoV-2) pathogen which causes the novel coronavirus disease 2019 (COVID-19) (Bocci et al., 2020). A recent review article reported thioridazine, fluphenazine, perphenazine, chlorpromazine, prochlorperazine inhibits RNA viruses as these phenothiazines inhibit cell-cell fusion, viral replication, clathrin-dependent endocytosis, and entry into cells (Otręba et al., 2020). Dyall and colleagues, reported triflupromazine inhibits the middle east respiratory syndrome coronavirus (MERS-CoV) and the SARS-CoV-1 (Dyall et al., 2014). Another study, found trifluoperazine inhibits lung metastasis, bone metastasis, and brain metastasis of melanoma via the mechanism of cell cycle arrest at the G0/G1 phase and mitochondrial-dependent intrinsic apoptosis (Xia et al., 2020). Via the same mechanism, another study described fluphenazine suppresses growth of triple negative breast cancer, as well as lung and brain metastasis in a subcutaneous xenograft model (Xu et al., 2019). Another phenothiazine, perphenazine, was shown to inhibit growth of progesterone-receptor resistant endometrial cancer (Chen et al., 2020). The anticancer properties of antipsychotics, more particularly, the typical antipsychotics are described being related to the dopamine D2-receptor antagonist properties which disrupt critical metabolic processes in tumors and cancer cells (Weissenrieder et al., 2019).
Researchers in France reported that chlorpromazine, a derivative of methylene blue, inhibits SARS-CoV-2 in vitro and other studies have shown clinical benefit of chlorpromazine in the most invasive glial tumor, the grade IV astrocytoma, glioblastoma multiforme (Amaral et al., 2004; Kamarudin and Parhar, 2019; Abbruzzese et al., 2020; Plaze et al., 2020). Methylene blue, the first synthetic compound in medicine has antimalarial properties, anti-SARS-Cov-2 properties, and a recent study reported the compound to be neuroprotective (López-Muñoz et al., 2005; Poteet et al., 2012; Gendrot et al., 2020). Other drug repurposing efforts identified haloperidol, a typical antipsychotic that is a ligand of the sigma-1 and sigma-2 receptors, also inhibits SARS-Cov-2 in vitro (Gordon et al., 2020). With the aforementioned efforts in repurposing neuroleptics in oncology and infectious disease, the results of this study will be informative to clinical research teams.
Antipsychotic drugs are increasingly being used to augment antidepressants. A classic example is the olanzapine/fluoxetine combination approved for treatment-resistant depression and bipolar depression (Eli Lilly and Company, 2018). Fluoxetine is strong inhibitor of CYP2C19 and CYP2D6 and will increase the area-under-the-concentration-time curve (AUC) of sensitive CYP2C19 or CYP2D6 substrates by greater than 5-fold drug exposure (United States Food and Drug Administration, 2020). Moreover, fluoxetine’s enantiomer metabolites (R)-norfluoxetine and (S)-norfluoxetine produce time-dependent inhibition of CYP3A4 (Ki = 8 µM) and CYP2C19 (Ki = 7 µM), respectively and has shown to inhibit SARS-Cov-2 (Lutz et al., 2013; Eugene, 2020b). Olanzapine is a substrate of CYP1A2 and should not be administered with strong CYP1A2 inhibitors to avoid excessive increases in AUC and Cmax. For example, fluvoxamine is a strong CYP1A2 and CYP2C19 inhibitor and would result in a drug-interaction increasing the AUC of asenapine, chlorpromazine, clozapine, olanzapine, and thiothixene. Among antipsychotics: aripiprazole, brexpiprazole, and quetiapine are also used in augmenting antidepressants. Both aripiprazole and brexpiprazole are CYP2D6 substrates and would result in a drug-drug-interaction (DDI) if combined with fluoxetine, paroxetine, sertraline, bupoprion, and duloxetine due to the antidepressants being CYP2D6 inhibitors. The pharmacokinetic outcomes of increased an AUC and Cmax would be even further exacerbated if the patient is a CYP2D6 Intermediate Metabolizer or CYP2D6 Poor Metabolizer (Hefner, 2018; Eugene, 2019).
Aside from being indicated in patients diagnosed with schizophrenia, quetiapine is indicated in patients diagnosed with manic episodes bipolar I disorder, as well as monotherapy in bipolar depression (AstraZeneca Pharmaceuticals, 2020). Further, quetiapine is biotransformed by CYP3A4 to norquetiapine, which has moderate binding affinity to the norepinephrine transporter (Ki = 12 nM) as is found in some traditional antidepressant compounds (Besnard et al., 2012). It is of benefit to recognize that strong CYP3A4 inhibitors include: boceprevir, cobicistat, clarithromycin, danoprevir and ritonavir, elvitegravir and ritonavir, grapefruit juice, indinavir and ritonavir, itraconazole, ketoconazole, lopinavir and ritonavir, paritaprevir and ritonavir and (ombitasvir and/or dasabuvir), posaconazole, ritonavir, saquinavir and ritonavir, telaprevir, tipranavir and ritonavir, telithromycin, troleandomycin, voriconazole, idelalisib, nefazodone, and nelfinavir (United States Food and Drug Administration, 2020). Moreover, a list of known strong and moderate CYP3A4 inducers are: apalutamide, carbamazepine, enzalutamide, mitotane, phenytoin, rifampin, St. John’s Wort, bosentan, efavirenz, etravirine, phenobarbital, and primidone (United States Food and Drug Administration, 2020).
The limitations of this observational study are that the cases are based on voluntary reporting of adverse drug events and are likely under-reported, resulting in the total number of cases not complete in patients treated with the tested compounds. Further, the results are indicative of pharmacovigilance signal strength and not causation. Our work does not factor in a potential dose-related propensity to induce sedation or somnolence as well as, does not factor in age, gender, or body-mass index. Nevertheless, the results are well catalogued, maintained, and available for public access within the U.S. FDA Adverse Events Reporting System. This also means that not all marketed antipsychotic compounds are included in this study, but the thirty-seven antipsychotics that are included provide an informative reference in clinical pharmacology, research, and medical education. Overall, the FAERS patient cases are a broad representative sample of the population and is reported by clinicians, pharmaceutical companies, and consumers.
The main study findings, from this population-wide head-to-head comparison of thirty-seven antipsychotics, is that zuclopenthixol showed the strongest association with sedation and somnolence while prochloperazine resulted in the weakest association. Taken together, this study reports adverse drug reaction data collected throughout 16 years from the FDA Adverse Events Reporting System serving as a clinically relevant reference in psychiatry and in efforts of drug repurposing of antipsychotic compounds in other fields of medicine.
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Study conception and design: AE, BE. Acquisition of data: AE, BE. Analysis and interpretation of data: AE, BE, MM, JM. Drafting of manuscript: AE, BE. Critical revision: AE, BE, MM, JM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the FDA Adverse Events Reporting System.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.621691/full#supplementary-material.
Abbruzzese, C., Matteoni, S., Persico, M., Villani, V., and Paggi, M. G. (2020). Repurposing chlorpromazine in the treatment of glioblastoma multiforme: Analysis of literature and forthcoming steps. J. Exp. Clin. Cancer Res. 39, 26. doi:10.1186/s13046-020-1534-z
Actavis Pharma (2016). DailyMed - LOXAPINE- loxapine capsule. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a6107bb1-3e23-4c5d-bbf4-5e29deff6728#LINK_eae82f04-bb2d-47ad-9127-b4e2d52f5f7a [Accessed March 14, 2020].
Alhola, P., and Polo-Kantola, P. (2007). Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 3, 553–567.
Amaral, L., Viveiros, M., and Molnar, J. (2004). Antimicrobial activity of phenothiazines. In Vivo 18 (6), 725–732.
AstraZeneca Pharmaceuticals (2020). DailyMed - SEROQUEL XR- quetiapine fumarate tablet, extended release. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=473a3ac4-67f4-4782-baa9-7f9bdd8761f4 [Accessed March 14, 2020].
Besnard, J., Ruda, G. F., Setola, V., Abecassis, K., Rodriguiz, R. M., Huang, X. P., et al. (2012). Automated design of ligands to polypharmacological profiles. Nature 492, 215. doi:10.1038/nature11691
Bocci, G., Bradfute, S. B., Ye, C., Garcia, M. J., Parvathareddy, J., Reichard, W., et al. (2020). Virtual and in Vitro antiviral screening revive therapeutic drugs for COVID-19. ACS Pharmacol. Transl. Sci. 3, 1278. doi:10.1021/acsptsci.0c00131
Brown, E. G., Wood, L., and Wood, S. (1999). The medical dictionary for regulatory Activities (MedDRA). Drug Saf. 20, 109. doi:10.2165/00002018-199920020-00002
Chen, Y., Cui, Y., Sun, X., Wu, H., Ou, M., Tang, Y., et al. (2020). Repurposing of antipsychotics perphenazine for the treatment of endometrial cancer. Bioorg. Med. Chem. Lett. 30, 127239. doi:10.1016/j.bmcl.2020.127239
Dyall, J., Coleman, C. M., Hart, B. J., Venkataraman, T., Holbrook, M. R., Kindrachuk, J., et al. (2014). Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 58, 4885. doi:10.1128/AAC.03036-14
Eli Lilly and Company (2018). DailyMed - ZYPREXA - olanzapine tablet ZYPREXA ZYDIS - olanzapine tablet, orally disintegrating ZYPREXA INTRAMUSCULAR - olanzapine injection, powder, for solution. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d5051fbc-846b-4946-82df-341fb1216341 [Accessed January 29, 2019].
Eli Lilly and Company (2019). SYMBYAX (olanzapine and fluoxetine hydrochloride) capsule. Dailymed - U.S. Natl. Libr. Med.
Eugene, A. R., and Masiak, J. (2014). Electrophysiological neuroimaging using sLORETA comparing 100 schizophrenia patients to 48 patients with major depression. Brain (Bacau) 5, 16–25. doi:10.5281/zenodo.1044125
Eugene, A. R., and Masiak, J. (2017). A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord. J. Psychiatry 71, 417–424. doi:10.1080/08039488.2017.1314011
Eugene, A. R., and Eugene, B. (2018). An opportunity for clinical pharmacology trained physicians to improve patient drug safety: A retrospective analysis of adverse drug reactions in teenagers. F1000Res 7, 677. doi:10.12688/F1000RESEARCH.14970.2
Eugene, A. R., Masiak, J., Masiak, M., Kapica, J., and Weinshilboum, R. M. (2014). Isolating the norepinephrine pathway comparing lithium in bipolar patients to SSRIs in depressive patients. Brain (Bacau) 5, 5–15. doi:10.5281/zenodo.1044128
Eugene, A. R. (2019). Optimizing drug selection in psychopharmacology based on 40 significant CYP2C19- And CYP2D6-biased adverse drug reactions of selective serotonin reuptake inhibitors. PeerJ 7, e7860. doi:10.7717/peerj.7860
Eugene, A. R. (2020a). Association of sleep among 30 antidepressants: a population-wide adverse drug reaction study. PeerJ 8, e8748, doi:10.7717/peerj.8748
Eugene, A. R. (2020b). Fluoxetine pharmacokinetics and tissue distribution suggest a possible role in reducing SARS-CoV-2 titers. medRxiv 12 (17), 20248442. doi:10.1101/2020.12.17.20248442
Fang, F., Sun, H., Wang, Z., Ren, M., Calabrese, J. R., and Gao, K. (2016). Antipsychotic drug-induced somnolence: incidence, mechanisms, and management. CNS Drugs, 30, 845. doi:10.1007/s40263-016-0352-5
Freeman, D., Brugha, T., Meltzer, H., Jenkins, R., Stahl, D., and Bebbington, P. (2010). Persecutory ideation and insomnia: findings from the second British national survey of psychiatric morbidity. J. Psychiatr. Res. 44, 1021. doi:10.1016/j.jpsychires.2010.03.018
Galen, U. S. (2019). DailyMed - ADASUVE- loxapine aerosol, powder. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=257148b5-869e-4a06-89ca-8b8686e8e528 [Accessed March 14, 2020].
Gao, K., Mackle, M., Cazorla, P., Zhao, J., and Szegedi, A. (2013). Comparison of somnolence associated with asenapine, olanzapine, risperidone, and haloperidol relative to placebo in patients with schizophrenia or bipolar disorder. Neuropsychiatr. Dis. Treat. 9, 1145. doi:10.2147/NDT.S41333
Gendrot, M., Andreani, J., Duflot, I., Boxberger, M., Le Bideau, M., Mosnier, J., et al. (2020). Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents 56, 106202. doi:10.1016/j.ijantimicag.2020.106202
Gordon, D. E., Jang, G. M., Bouhaddou, M., Xu, J., Obernier, K., White, K. M., et al. (2020). A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583 (7816), 459–468. doi:10.1038/s41586-020-2286-9
Hefner, G. (2018). Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Psychopharmakotherapie 51 (1–2), 9–62. doi:10.1055/s-0043-116492
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., et al. (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 (10202), 3. doi:10.1016/s0140-6736(19)31135-3
Kamarudin, M. N. A., and Parhar, I. (2019). Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: A comprehensive review. Oncotarget 10, 3952. doi:10.18632/oncotarget.26994
Kelly, W. E., Kelly, K. E., and Clanton, R. C. (2001). The relationship between sleep length and grade-point average among college students. Coll. Stud. J. 35 (1), 84–86.
Koyanagi, A., and Stickley, A. (2015). The Association between sleep problems and psychotic symptoms in the general population: A global perspective. Sleep 38, 1875. doi:10.5665/sleep.5232
López-Muñoz, F., Alamo, C., Cuenca, E., Shen, W., Clervoy, P., and Rubio, G. (2005). History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry 17, 113. doi:10.1080/10401230591002002
Laskemoen, J. F., Simonsen, C., Büchmann, C., Barrett, E. A., Bjella, T., Lagerberg, T. V., et al. (2019). Sleep disturbances in schizophrenia spectrum and bipolar disorders - a transdiagnostic perspective. Compr. Psychiatry 91, 6. doi:10.1016/j.comppsych.2019.02.006
Lutz, J. D., VandenBrink, B. M., Babu, K. N., Nelson, W. L., Kunze, K. L., and Isoherranen, N. (2013). Stereoselective inhibition of CYP2C19 and CYP3A4 by fluoxetine and its metabolite: Implications for risk assessment of multiple time-dependent inhibitor systems. Drug Metab. Dispos. 41, 2056. doi:10.1124/dmd.113.052639
Meltzer, H. Y., Matsubara, S., and Lee, J. C. (1989). The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol. Bull. 25, 390.
Otręba, M., Kośmider, L., and Rzepecka-Stojko, A. (2020). Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 887, 173553. doi:10.1016/j.ejphar.2020.173553
Otsuka America Pharmaceutical (2020a). DailyMed - ABILIFY- aripiprazole tablet ABILIFY- aripiprazole solution ABILIFY- aripiprazole tablet, orally disintegrating ABILIFY- aripiprazole injection, solution. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac [Accessed March 14, 2020].
Otsuka America Pharmaceutical (2020b). DailyMed - REXULTI- brexpiprazole tablet. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2d301358-6291-4ec1-bd87-37b4ad9bd850 [Accessed March 14, 2020].
Petrovsky, N., Ettinger, U., Hill, A., Frenzel, L., Meyhöfer, I., Wagner, M., et al. (2014). Sleep deprivation disrupts prepulse inhibition and induces psychosis-like symptoms in healthy humans. J. Neurosci. 34, 9134. doi:10.1523/JNEUROSCI.0904-14.2014
Plaze, M., Attali, D., Prot, M., Petit, A.-C., Blatzer, M., Vinckier, F., et al. (2020). Inhibition of the replication of SARS-CoV-2 in human cells by the FDA-approved drug chlorpromazine. Int. J. Antimicrob. Agents, 106274. doi:10.1016/j.ijantimicag.2020.106274
Poteet, E., Winters, A., Yan, L. J., Shufelt, K., Green, K. N., Simpkins, J. W., et al. (2012). Neuroprotective actions of methylene blue and its derivatives. PLoS One 7, e48279. doi:10.1371/journal.pone.0048279
Qin, S., Eugene, A. R., Liu, D., Zhang, L., Neavin, D., Biernacka, J. M., et al. (2020). Dual roles for the TSPYL family in mediating serotonin transport and the metabolism of selective serotonin reuptake inhibitors in patients with major depressive disorder. Clin. Pharmacol. Ther. 107, 662–670. doi:10.1002/cpt.1692
Reeve, S., Emsley, R., Sheaves, B., and Freeman, D. (2018). Disrupting sleep: the effects of sleep loss on psychotic experiences tested in an experimental study with mediation Analysis. Schizophr. Bull. 44 (3), 662–671. doi:10.1093/schbul/sbx103
Sharpley, A. L., Elliott, J. M., Attenburrow, M. J., and Cowen, P. J. (1994). Slow wave sleep in humans: role of 5-HT2A and 5-HT2C receptors. Neuropharmacology 33, 467–471. doi:10.1016/0028-3908(94)90077-9
Team (2013). R development core team. R A lang. Environ. Stat. Comput. Available at: http://www.mendeley.com/research/r-language-environment-statistical-computing-96/%5Cnpapers2://publication/uuid/A1207DAB-22D3-4A04-82FB-D4DD5AD57C28.
United States Food and Drug Administration (2020). Drug development and drug interactions: Table of substrates, Inhibitors and Inducers | FDA. Available at: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers [Accessed February 23, 2020].
Weissenrieder, J. S., Neighbors, J. D., Mailman, R. B., and Hohl, R. J. (2019). Cancer and the dopamine D2Receptor: A pharmacological perspective. J. Pharmacol. Exp. Ther. 370, 111. doi:10.1124/jpet.119.256818
Welsh, A. L. (1964). The newer tranquilizing drugs. Med. Clin. North Am. 48 (2), 459–481. doi:10.1016/S0025-7125(16)33476-9
Xia, Y., Xu, F., Xiong, M., Yang, H., Lin, W., Xie, Y., et al. (2021). Repurposing of antipsychotic trifluoperazine for treating brain metastasis, lung metastasis and bone metastasis of melanoma by disrupting autophagy flux. Pharmacol. Res. 163, 105295. doi:10.1016/j.phrs.2020.105295
Keywords: sedation, sleep, pharmacogenomics, treatment resistant, major tranquilizers, COVID-19, SARS-CoV-2, psychiatry
Citation: Eugene AR, Eugene B, Masiak M and Masiak JS (2021) Head-to-Head Comparison of Sedation and Somnolence Among 37 Antipsychotics in Schizophrenia, Bipolar Disorder, Major Depression, Autism Spectrum Disorders, Delirium, and Repurposed in COVID-19, Infectious Diseases, and Oncology From the FAERS, 2004–2020. Front. Pharmacol. 12:621691. doi: 10.3389/fphar.2021.621691
Received: 26 October 2020; Accepted: 02 February 2021;
Published: 25 March 2021.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Tianmei Si, Peking University Sixth Hospital, ChinaCopyright © 2021 Eugene, Eugene, Masiak and Masiak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andy R. Eugene, YW5keWV1Z2VuZS5tZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.