- 1School of Biological Sciences and Technology, University of Jinan, Jinan, China
- 2School of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan, China
Tanshinone IIA (Tan IIA) is a pharmacologically lipophilic active constituent isolated from the roots and rhizomes of the Chinese medicinal herb Salvia miltiorrhiza Bunge (Danshen). Tan IIA is currently used in China and other neighboring countries to treat patients with cardiovascular system, diabetes, apoplexy, arthritis, sepsis, and other diseases. Recently, it was reported that tan IIA could have a wide range of antitumor effects on several human tumor cell lines, but the research of the mechanism of tan IIA is relatively scattered in cancer. This review aimed to summarize the recent advances in the anticancer effects of tan IIA and to provide a novel perspective on clinical use of tan IIA.
Introduction
Salviae miltiorrhiza (Danshen) is the dried root and rhizome of Salvia miltiorrhiza Bge (Lamiaceae), which is a traditional Chinese medicine herb (Figure 1) (Tseng et al., 2014). It is mainly distributed in Anhui, Shanxi, Hebei, Sichuan, Shandong, Jiangsu, and other provinces in China and considered to have the action of relieving pain, activating blood circulation and removing blood stasis, clearing the heart and removing annoyance, cooling blood, and eliminating carbuncle, according to the mechanism of traditional Chinese medicine (TCM) (Li et al., 2016a; Zhou et al., 2019). There are two main active ingredients in S. miltiorrhiza. One is the hydrophilic component, which belongs to water-soluble substances, such as tanshinol, and the other is the lipophilic component, which belongs to fat soluble substances, such as tanshinone I and tan IIA (Kwak et al., 2008; Shang et al., 2012; Lin et al., 2019).
Tan IIA (C19H18O3, 14,16-epoxy-20-nor-5(10),6,8,13,15-abietapentaene-11,12- dione) (Wei et al., 2012), a natural diterpene quinone in S. miltiorrhiza, possesses miscellaneous biological activities such as anti-inflammatory (Dong et al., 2009; Li et al., 2015a; Fan et al., 2016), antiviral (Xiao et al., 2013; Zhang et al., 2014a), antioxidant (Gong et al., 2019), neuron-protective (Xia et al., 2005; Weng et al., 2018), antiatherosclerotic (Chang et al., 2014a; Tan et al., 2019), antiallergic (Li et al., 2018a; Heo and Im, 2019), anticonvulsant (Olivia et al., 2013), antifatigue (Lin et al., 2017), anti-Alzheimer’s disease (Jiang et al., 2014; Li et al., 2015b), and antiangiogenic activities (Fan et al., 2011), reducing organ damage (Ma et al., 2018a), and protection from angina pectoris and myocardial infarction (Lv et al., 2018) (its structure is shown in Figure 1). Newly, it was said that tan IIA could have a wide range of antitumor effects in multiple human tumor cell lines by inhibiting tumor growth, inducing apoptosis, regulating cell cycle, regulating signaling pathways, and reversing the multidrug resistance in various human tumor cells (Kim et al., 2015). However, because the mechanism of tan IIA cell is relatively scattered in cancer, this paper provides the research progress of the antitumor effect of tan IIA on leukemia, lung cancer, hepatocellular carcinoma, gastric carcinoma, colorectal cancer, glioma, osteosarcoma, cervical cancer, ovarian cancer, breast cancer, and prostate cancer, and its antitumor mechanism was also discussed.
Antitumor Effects
Tan IIA could exhibit antitumor activity in many cancer cells such as leukemia, lung cancer, hepatocellular carcinoma, gastric carcinoma, colorectal cancer, glioma, osteosarcoma, cervical cancer, ovarian cancer, breast cancer, and prostate cancer. Tan IIA could induce autophagy and apoptosis and inhibit cell growth and migration, by activating AMPK and inhibiting PI3K/Akt/mTOR signaling pathway, and so on.
Leukemia
Leukemia, including chronic myeloid leukemia (CML), acute myeloid leukemia (AML), and acute promyelocytic leukemia (APL), is one of the blood or bone marrow cancers. Some viruses, petrochemical products, ionizing radiation, and alkylating chemotherapy drugs are considered as major reasons of leukemia (Liu et al., 2012a). Around 100 million children and adults worldwide suffer from some forms of leukemia every year. At present, the treatment of leukemia remains a top research priority. In recent years, TCM has attracted wide attention as a clinical alternative to the treatment of leukemia because of its anti-inflammatory, antivirus, antioxidation, antitumor, apoptosis inducing effect (Boon and Wong, 2004). Among them, Tan IIA played an important role.
CML is a myeloproliferative disease. The translocation of chromosomes 9 and 22 leads to the clonal inflation of transformed hemopoietic stem cells, which may lead to resistance in tumor treatment (Yun et al., 2013a). Yun, et al. observed that tan IIA induced mitochondria dependent apoptosis through excitation of JNK in KBM 5 leukemia cells (Yun et al., 2013a). Tan IIA could raise sub-G1 apoptotic portion, activate Caspases 9 and 3, release cytochrome c from mitochondria into cytoplasm, and downregulate Survivin, Bcl-2, Bcl-xL, and c-IAP2 (Yun et al., 2013a). Then, they also discovered that tan IIA could induce autophagy via AMPK and ERK and restraint of mTOR and p70 S6K (Yun et al., 2013b).
AML is characterized by unlimited proliferation of myeloid cells (Liu et al., 2012a), with five-year mortality rate of more than 70% (Zhang et al., 2019). Therefore, we need to find more effective therapeutic strategies to treat AML. Zhang et al. revealed that tan IIA may induce apoptosis and autophagy in U937 cells via inhibiting PI3K/Akt/mTOR signaling pathway (Zhang et al., 2019). Tan IIA induced apoptosis in U937 cells via upregulating the levels of active Caspase 3 and Bax and downregulating Bcl-2. In addition, tan IIA inhibited the capacity of migration and invasion in U937 cells. Liu et al. discovered that tan IIA activated P × R (Pregnane × receptor), which inhibited nuclear factor-κB (NF-κB) activity, leading to significantly downregulating the expression of CCL2 by about ten times (Liu et al., 2012a).
APL is a seldom seen disease accounting for about 10% of AML. Zhang et al. indicated that C/EBPβ and CHOP participate in tan IIA induced variation and apoptosis of APL cells (Zhang et al., 2010). Tan IIA may upregulate C/EBPβ and CHOP; the C/EBPβ was very important (Zhang et al., 2010). Liu et al. suggested that tan IIA could induce apoptosis by excitation of Caspase 3, downregulation and upregulation of Bcl-2 and Bax, respectively, and the disruption of mitochondrial membrane potential (Liu et al., 2006). Moreover, the treatment by tan IIA may weaken adhesion and invasion of NB4 cells through the extracellular matrix (ECM). Yoon et al. demonstrated that induced apoptosis by tan IIA was accompanied by the PARP specific proteolytic cleavage and Caspase 3 activation (Yoon et al., 2000).
Liu et al. indicated that tan IIA has available antiproliferation effect on THP-1 cells by apoptosis; it is basically related to the destruction of Δψm (the mitochondrial membrane potential), activation of Caspase 3, and downregulation and upregulation of Survivin and Bax, respectively (Liu et al., 2009).
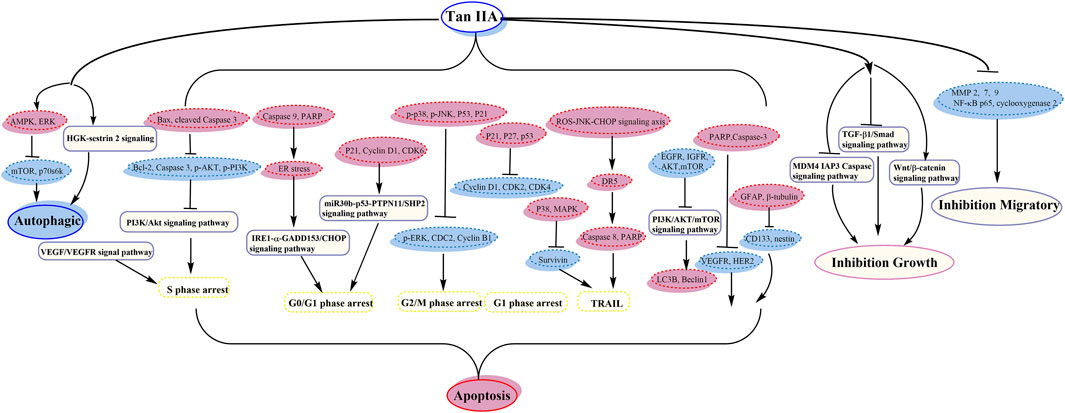
Guo et al. demonstrated that nutlin-3 and tan IIA meaningfully potentiated the apoptotic effect of imatinib by downregulating AKT/mTOR pathway (Guo et al., 2017). Next year, they showed that the association of nutlin-3 and tan IIA may synergistically induce apoptosis, cytotoxicity, cell cycle arrest, and autophagy; thus the antileukemia effect was through effective activation of p53, inhibition of the AKT/mTOR pathway, and activation of the RAF/MEK pathway (Guo et al., 2018) (its anticancer pathway is shown in Figure 2).
Lung Cancer
Lung cancer is a usual respiratory harmful tumor in the clinic; its morbidity is increasing with the change of modern circumstance and lifestyle (Li et al., 2016b). It has become the main cause of tumor related death in Taiwan and Western countries and has been one of the diseases which can strictly threaten the human life and fitness (Zhang et al., 2014b). With the consecutive ripeness of science and technology, the curative effect by pharmacon of lung cancer is greatly modified, but lung cancer remains fatal, widespread, and expensive to patients and society (Zhang et al., 2016). Therefore, how to efficiently treat lung cancer has been the focus of clinical investigation.
Xie et al. showed that tan IIA could restrain cell proliferation, induce apoptosis, and arrest cell cycle at the S phase (Xie et al., 2015). It may block VEGF/VEGFR signal pathway, cause cell cycle arrest, and indirectly inhibit downstream signal pathway and then upregulate the expression of apoptosis genes, downregulate antiapoptosis genes, then inhibit the development, and promote the apoptosis of tumor cells (Xie et al., 2015). Zhang et al. showed that tan IIA may induce cytochrome c-mediated caspase cascade apoptosis via the JNK pathway (Zhang et al., 2014b). It induced apoptosis through cytochrome c release from mitochondria and Bax migration to mitochondria (Zhang et al., 2014b). Wang et al. confirmed that the mechanism of tan IIA inhibiting cell proliferation and epithelial-mesenchymal transition (EMT) might be through the TGF-β1/Smad signaling pathway (Wang et al., 2018). Cheng et al. detected that tan IIA could inhibit H146 cells by upregulating Bax/Bcl-2 and diminishing mitochondrial membrane potential (Cheng and Su, 2010). The inhibition of tan IIA might be through endoplasmic reticulum (ER) stress caused through the release of Ca2+ and the increased expression of GADD153 protein. It induced the increase of Bax/Bcl-2 and Caspase 3 and the decrease in matrix metalloproteinases (MMP), leading to the suppression of the proliferation in H146 cells (Cheng and Su, 2010). Kim et al. have proven that tan IIA induced TRAIL sensitization of lung cancer cells by selective ER stress induction (Kim et al., 2016). So, tan IIA may induce apoptosis of TRAIL via upregulating DR5 and downregulating Survivin via selective activation of PERK/ATF4 and inhibition of STAT3, respectively.
Chiu et al. showed that tan IIA induced apoptosis by the abduction of ROS and diminishing the mitochondrial membrane potential in A549 cells (Chiu and Su, 2010). Tan IIA might decrease the expression of Bcl-2 and increase Bax, p53, and Cyto-c and may work via the abduction of ROS and a higher scale of Bax/Bcl-2. Liu et al. suggested that the apoptosis pathway by NQO1-activated and p53-independent mechanism determines the antitumor function of tan IIA against non-small cell lung cancer (Liu et al., 2012b). Tan IIA may activate ROS detonated, p53-independent, and caspase-dependent mitochondria apoptotic mechanism by increased Bax/Bcl-xL, disruption of mitochondrial membrane potential, release of cytochrome c, and caspase excitation and PARP-1 cleavage (Liu et al., 2012b).
Zu et al. indicated that tan IIA could inhibit the activity of p53 deficient H1299 cell practicability via the MDM4-IAP3-caspase signaling pathway and increase sensitivity to doxorubicin (DOX) (Zu et al., 2018). Liao et al. demonstrated that tan IIA together with cisplatin restrains non‐small cell lung cancer by downregulating the phosphatidylinositol 3-kinase/Akt signaling pathway (Liao et al., 2019). It could destroy migration and invasion, prevent the cell cycle in the S phase, and induce apoptosis (Liao et al., 2019). The expression of cleaved Caspase 3 and Bax was upregulated; nevertheless the expression of Caspase 3, p-PI3K, p-Akt, and Bcl-2 proteins was downregulated (Liao et al., 2019). Li et al. showed that tan IIA together with cyclophosphamide (CTX) could downregulate and upregulate Bcl-2 and Bax, respectively, inhibit the neovascularization of cancer organizations, and raise the immunological action, with a remarkable antitumor activity (Li et al., 2016b) (its anticancer pathway is shown in Figure 2).
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a common hepatic malignancy in the world and the occurrence rate of liver cancer has been sustained to increase over the last few decades (Chiu et al., 2018). The intricacy of the molecular etiopathogenesis and drug resistance of HCC brings great impediments in cure (Ren et al., 2017). Therefore, tremendous efforts have been devoted to developing effective antitumor drugs with less side effects. The ingredients isolated from traditional medicinal plants have attracted a wide range of interest (Ma et al., 2013; Chang et al., 2014b).
Dai et al. indicated that tan IIA showed anticancer effect on BEL 7402 cells through apoptosis and G0/G1 arrest (Dai et al., 2011). It induced apoptosis through excitation of calcium dependent apoptosis signaling pathways and upregulation of MT 1A (Dai et al., 2011). Lin et al. indicated that tan IIA contemporaneously induced both Nec-1 restraint and FLIPS regulation reconciled apoptosis/necroptosis in HepG2 cells (Lin et al., 2016). Chien et al. showed that tan IIA may inhibit J5 cell growth through increasing and decreasing Caspase 3 and CD31, respectively (Chien et al., 2012). Jeon et al. revealed that direct suppression of cytochrome P450 2J2 by tan IIA induced apoptosis (Jeon et al., 2015). The CYP2J2 inhibits carcinoma cell apoptosis via upregulation and downregulation of Bcl-2 and Bax, respectively (Jeon et al., 2015). Tan IIA has been said to produce cytotoxicity by apoptosis without generating mutations in the GSH/GSSG ratio (Lee et al., 2008). Wang et al. found that tan IIA could restrain the raised metastasis induced by PR of hepatocellular carcinoma and drag on survival in part through VEGFR1/PDGFR-related vascular normalization (Wang et al., 2012). It immediately heightened tube formation of TECs, associated with VEGFR1/PDGFR upregulation. Ren et al. found that the multiple mechanisms involved in tan IIA induced death formed from miR30b-p53 pathway and PTPN11/SHP2 pathway (Ren et al., 2017). The transsituation of p53 may be the original signal, and miR30b-p53-PTPN11/SHP2 may be a fresh signaling pathway concerned in tan IIA induced cell death (Ren et al., 2017). Tan IIA could change Bax/Bcl2, p21, Caspase 3, cyclin D1, and CDK6, and it induced apoptosis through arresting cell cycle at G1/G0 phase (Ren et al., 2017).
Chiu et al. detected that tan IIA and sorafenib or SC-1 have collaborative cytotoxicity (Chiu et al., 2018). Tan IIA could restrain HCC proliferation via downregulation of pSTAT3 induced by sorafenib/SC-1. Chang et al. investigated that combination of tan IIA and trans-resveratrol (Resv) could raise the effect of apoptosis, sub-G1 arrest, and DNA fragmentation (Chang et al., 2014b). Tan IIA blocked the cells at sub-G1 phase, while Resv induced S and G2/M phase arrest, and tan IIA provoked a marked feature of thanatosis, while Resv mainly induced apoptosis (Chang et al., 2014b). Kan et al. suggested the accession of DOX cytotoxicity by tan IIA (Kan et al., 2014) (its anticancer pathway is shown in Figure 2).
Gastric Carcinoma
Gastric carcinoma is an ordinary malignancy around the world and is a great threat to public health worldwide, with the second central consideration of tumor related death (Chen et al., 2012). It has the characteristics of high incidence rate, invasion, and incidence rate and poor prognosis (Dong et al., 2008). The familiar treatment of gastric cancer is gastrectomy together with chemotherapy and chemotherapy which has multidrug resistance and cytotoxicity to regular cells (Chen et al., 2012). Heretofore, many methods have been applied by the scientists to surmount the drug resistance in cancer (Xu et al., 2018). The effective chemical components from herbal medicine may contribute to improving the therapeutic effect of gastric cancer patients (Zhang et al., 2018a). Natural products are the leading compounds in the development of anticancer drugs and show versatile anticancer actions and have attracted more and more attention (Xu et al., 2018). Therefore, it is an emergency work in clinical practice to find a new treatment (Dong et al., 2008).
Dong et al. indicated that tan IIA could induce apoptosis of MKN 45 cells, and it may happen in G2/M phase; the possible molecular mechanisms are downregulation and upregulation of Bcl-2 and p53, respectively (Dong et al., 2008). Yu et al. found that tan IIA suppresses SGC 7901 cell proliferation and transplantation by downregulation of FOXM1 (Yu et al., 2017). Chen et al. indicated that tan IIA may cause cycle arrest in the G2/M phase and produce intrinsic apoptotic signaling pathway (Chen et al., 2012). Zhang et al. revealed that tan IIA inhibited cell proliferation and tumor growth via downregulating STAT3 (Zhang et al., 2018a). The treatment of tan IIA might induce apoptosis; it may increase cleaved Caspase 3 and decrease Bcl-2 (Zhang et al., 2018a).
Su et al. showed that tan IIA could inhibit AGS cell germination via decreasing Mcl-1, TCTP, BiP, and Bcl-xL and increasing Bax and CHOP (Su, 2014a). The same year, they suggested that tan IIA suppressed AGS cells by increasing the expression of p53, p-p38, and p-JNK and reducing the expression of p-ERK, CDC2, and cyclin B1 (Su, 2014b). One of the molecular mechanisms might be to increase p-p38 and p-JNK and decrease p-ERK to induce the activation of p53 and increase the expression of p21 to downregulate CDC2 and cyclin B1, which then induces G2/M phase arrest (Su, 2014b). Another way may be to improve the expression of TNF-α, FAS, and Caspases 3 and 8 to induce apoptosis (Su, 2014b). Second year, they reported that tan IIA decreased the migratory ability via decreasing the expression of MMP-2, MMP-7, MMP-9, NF-κB-p65, and cyclooxygenase-2 (Su, 2015). Third year, they documented that tan IIA may restrain AGS cells by decreasing EGFR, IGFR, AKT, and mTOR and blocking the PI3K/Akt/mTOR pathway (Su and Chiu, 2016). Then, they showed that tan IIA may induce AGS cells apoptosis by decreasing VEGFR and HER2, blocking the Ras/Raf/MEK/ERK pathway, and inducing the excitation of PARP and Caspase 3 (Su, 2018).
Xu et al. demonstrated that tan IIA might raise the antitumor effect of DOX in drug-resistant gastric cancer cells, by inhibiting MRP1 function, enhancing cell cycle arrest, and increasing apoptosis and autophagy (Xu et al., 2018) (its anticancer pathway is shown in Figure 2).
Colorectal Cancer
Colorectal cancer is the third most ordinary type of human tumor because it is closely related to a range of factors according to the World Health Organization (Ma et al., 2018b). With the development of medical technology, great advancement has been made in the diagnosis and treatment of this disease, but recent chemotherapeutic plans are ungratified and the recurrence and death rate of colon cancer are still high (Su et al., 2008). Accordingly, the development of new treatment methods is particularly important. Among others, the phylactic and remedial capabilities of natural products of restraining or overturning cells associated with cancer conception, advancement, and succession are accepting much attention (Su et al., 2008).
Su et al. investigated that tan IIA may induce apoptosis via downregulating ErbB-2 (erythroblastosis oncogene B; HER-2/neu) and upregulating TNF-α in colon cancer cells (Su and Lin, 2008a). And then, they suggested that tan IIA triggered apoptosis by activating both inherent pathways concerning mitochondrial release of cytochrome c and outside pathways concerning excitation of Fas-caspase cascades (Su et al., 2008). Finally, they supported that tan IIA may build up the effectiveness of 5-FU in a colon cancer nude SCID mouse model by downregulating expression of NF-κB-p65, VEGF, P-gp, MMP-7, and LC3-II (Su, 2012).
Ma et al. showed that tan IIA may restrain COX-2 and activate Wnt/β-catenin signaling pathway, downregulate VEGF, and result in inhibition of colon cancer cells (Ma et al., 2018b). COX-2 is an important rate-limiting enzyme in the synthesis of prostaglandins; it could produce prostaglandin PEG2 after metabolism, which could increase the proliferation of cells and reduce the cell death. Tan IIA could also cause a β-catenin decrease, and Wnt/β-catenin signaling pathway is a highly conserved cell signaling system (Ma et al., 2018b).
Tu et al. supported that tan IIA might ameliorate inflammatory microenvironment through inhibiting of microRNA-155 and repressing the proliferation of Hct116 and Ht29 cells (Tu et al., 2012). Bai et al. revealed that tan IIA may induce cell death and enhance sensitizing to 5-FU cure by restraining excitation of NF-κB (Bai et al., 2016) (its anticancer pathway is shown in Figure 2).
Glioma
Glioma is the most common primary central nervous system cancer, which has the characteristics of high invasiveness, high recurrence rate, and poor prognosis (Ding et al., 2017). So far, the treatment options of malignant glioma patients were still limited, which has an important impact on human health (Wang et al., 2007). Due to the limitations of current treatment methods, it is necessary to develop novel therapeutic strategies according to the specific biological characteristics of this tumor (Wang et al., 2007). It is very considerable to find more valid therapy to further antitumor effect and drag on the survival of patients (Ding et al., 2017).
Ding et al. verified that tan IIA could induce apoptosis and autophagy and raise LC3B and Beclin 1 and play an antitumor role by restraining the PI3K/Akt/mTOR pathway (Ding et al., 2017). It could decrease p-PI3K, p-Akt, and Bcl-2, increase Bax, restrain viability of cells, and facilitate apoptosis. Tang et al. showed that tan IIA efficiently inhibited the STAT3 pathway and downregulated Bcl-xL and cyclin D1 which were targets of STAT3, induced apoptosis, and inhibited tumor cell growth in C6 glioma cells (Tang et al., 2010). Wang et al. suggested that the cells treated by tan IIA showed astrocyte or nerve fiber-like modalism, and tan IIA increased GFAP mRNA, decreased nestin mRNA meaningfully, increased apoptotic cells expressively, increased cells in G0/G1 phase and decreased cells in S phase, and increased expression of ADPRTL1 and CYP1A1 mRNA (Wang et al., 2007).
Yang et al. suggested that tan IIA might increase variation and neural lineage flags including GFAP and β-tubulin, decrease glioma stem cells (GSCs) flags including CD133 and nestin, and then induce GSC apoptosis (Yang et al., 2014). Tan IIA inhibited the growth by interrupting IL6/STAT3 signaling pathways, not only reducing expression of IL6, but also reducing activated STAT3 (Yang et al., 2014) (its anticancer pathway is shown in Figure 2).
Osteosarcoma
Osteosarcoma is a highly invasive tumor, which is the most common elementary malignant cancer in adolescents and young people, and it mainly occurs in the areas of positive bone growth and renovation (Yen et al., 2018). The characteristics of osteosarcoma are high metastatic expanding liability, poor prognosis, and low patient survival rate (Ma et al., 2016). Present treatment strategies include chemotherapy and aggressive surgical resection, but the five-year survival rate remains 5–20% (Ma et al., 2016). In recent years, more and more evidence has shown that TCM could apply potential drugs to prevent or treat various cancers (Zhang et al., 2012a). Therefore, it is of great practical significance and urgency to develop new plan to restrain recrudescent and intractable osteosarcoma and research the mechanism of antitumor effect (Huang et al., 2017).
Ma et al. confirmed that interaction between Beclin-1-dependent autophagy and caspase-dependent apoptosis is induced by tan IIA. ROS play a central part in adjusting the cytotoxicity of tan IIA (Ma et al., 2016). Yen et al. discovered that HGK-sestrin 2 signaling-mediated autophagy is advantageous to anticancer effectiveness of tan IIA (Yen et al., 2018). They defined the activation of HGK/SAPK/JNK1/Jun kinase pathways in upregulating transcription of SESN2, in which tan IIA invited HGK/JNK1-dependent Jun excitation and gave rise to increasing Jun recruitment to AP-1-binding site in the SESN2 promoter region (Yen et al., 2018). Zhang et al. showed that tan IIA induced apoptosis and inhibited the diffusion, transference, and aggression in MG-63 cells (Zhang et al., 2012a). It could inhibit mRNA, MMP-2, and MMP-9, restrain cell aggression though Matrigel, and reduce MG-63 migration activity (Zhang et al., 2012a). Huang et al. demonstrated that tan IIA could induce apoptosis through inherent pathways and result in mitochondrial lesion and suppress vasculogenesis (Huang et al., 2017). The balance of mitochondrial fission/fusion and the adjustment of cancer vasculogenesis were also relevant in the new antitumor effect of tan IIA (Huang et al., 2017) (its anticancer pathway is shown in Figure 2).
Cervical Cancer
Cervical cancer (CC) is one of the most ordinary gynecological harmful cancers that severely threaten women health and is one of the major causes of female death all over the world (Qin et al., 2018). High risk human papilloma virus (HPV) infection plays a crucial role in the multifactor etiology (Munagala et al., 2015). New therapeutic drugs and effective antitumor cure hinge on developments in investigation. In recent years, the investigation has shown that certain TCM has showed antiviral and tumor apoptosis properties (Pan et al., 2010).
Munagala et al. suggested that tan IIA strongly restrained diffusion of C33a, CaSki, HeLa, and SiHa cells (Munagala et al., 2015). Tan IIA was found to downregulate HPV E6 and E7; regulate associated E6AP and E2F1; create S phase cell cycle arrest; attract accumulation of p53 and alter p53-dependent targets; modulate pRb; cause p53-mediated apoptosis by moderating Caspase 3, Bcl-2, Bax, and PARP cleavage in HPV positive CaSki cells. It could repress HPV E6 and E7 and then result in breeding of p53-dependent cancer allayer liveness resulting in growth inhibition (Munagala et al., 2015).
Zhou et al. concluded that tan IIA might prevent cancer cells in mitosis via disorganizing the mitotic spindle and after that trigger cells to enter apoptosis via the mitochondria dependent apoptotic pathway in HeLa cells (Zhou et al., 2008). It may selectively kill mitotic cells over interphase cells and destroy only the mitotic spindle during the M phase but not the microtubule structure in interphase cells (Zhou et al., 2008). Pan et al. demonstrated that it may firmly bind to the β-subunit of the microtubule protein, and it strongly inhibits the growth by interfering with the process of microtubule assembly, then resulting in G2/M phase arrest and sequent apoptosis (Pan et al., 2010).
Pan et al. evidenced that tan IIA could reveal tough growth prohibitive effect on CaSki cells by accelerating caspase cascades with concomitant upregulation of the phosphorylation of p38 and JNK signaling (Pan et al., 2013). Qin et al. showed that tan IIA restrained the migration and invasion of cervix carcinoma stem cells via inhibiting YAP transcriptional activity (Qin et al., 2018) (its anticancer pathway is shown in Figure 2).
Ovarian Cancer
Ovarian cancer is one of the most ordinary human malignancies and results in death from gynecological malignancies (Huang et al., 2015). Due to the lack of sensitive and specific early detection methods, the diagnosis of ovarian cancer is usually late, and the treatment plan is limited (Li et al., 2018b). So, the development of antiapoptotic TCM monomers has been the center of surveys in the remedy of cancer (Zhang et al., 2018b).
Zhang et al. showed that tan IIA could induce apoptosis via attenuation of PI3K/AKT/JNK signaling pathways (Zhang et al., 2018b). It meaningfully increased the apoptosis by cleavage excitation of Caspases 3, 8, and 9 (Zhang et al., 2018b). Huang et al. investigated that it induced arrest of cell cycle at the G2/M phase, decreased Bcl-2, increased Bax, promoted SKOV3 cell apoptosis, and inhibited cell proliferation and viability (Huang et al., 2015). Li et al. demonstrated that it may induce apoptosis in TOV-21G cells via direct upregulation of miR-205 and in turn downregulation of Survivin (Li et al., 2018b). Chang et al. confirmed that tan IIA enhanced tumor necrosis TRAIL-induced apoptosis by upregulating DR5 acceptor by the ROS-JNK-CHOP signaling axis (Chang et al., 2015). Lin et al. illustrated that tan IIA enhanced the effect of TRAIL by downregulating Survivin in ovarian carcinoma cells (Lin et al., 2015). The downregulation of Survivin induced via tan IIA requires p38 MAPK activation and is regulated by both transcription process and proteasome degradation.
Jiao et al. revealed that tan IIA has remarkable antiproliferative effect on COC1/DDP cells by generating apoptosis and downregulating cisplatin-resistance genes (Jiao and Wen, 2011). The apoptosis was mainly related to the reduction of Survivin, and lessened cisplatin resistance was invited by the reduction of ERCC1 and LRP (Jiao and Wen, 2011) (its anticancer pathway is shown in Figure 2).
Breast Cancer
Breast cancer is one of the most ordinary malignancies and the main reason of cancer death for women all over the world (Yu et al., 2014). It has the characteristics of high recurrence rate, high metastasis rate, and high mortality (Lin et al., 2013). For the past few years, with the increasing incidence rate and mortality of breast cancer, more and more attention has been paid to research of chemotherapy and new anticancer drugs; it has prompted people to seek more prevention and treatment methods (Li et al., 2015c).
Yan et al. found that tan IIA inhibited BT 20 cells by increasing Caspase 12, GADD153, Caspase 3, phospho-JNK, phospho-p38, and Bax and decreasing Bcl-xL and phospho-ER (Yan et al., 2012). Its molecular mechanism might be by generating ER stress and the MAPK pathway (Yan et al., 2012). Wang et al. confirmed tan IIA inhibition of proliferation and apoptosis through upregulation (CDKN1A, ARHC, CYP1A1, CLU, and ADPRTL1) and downregulation (MAP3K1, CEACAM6 and MMP-7) of many genes containing cell cycle management, signal transduction, cell propagation, apoptosis, vasculogenesis, invasion, and metastasis of cancer cells (Wang et al., 2005). Lu et al. suggested that tan IIA proved a stable prohibitive effect on the diffusion of ER-positive and ER-negative breast cancer cells by reducing P53 and Bcl-2 (Lu et al., 2009). Su et al. showed that tan IIA restrained MDA-MB-231 cells via increased Bax/Bcl-xL (Su and Lin, 2008b). Tan IIA increased p21 and Caspase 8. p21 (WAF1 and Cip-1) has the feasibility to induce arrest of G1 and apoptosis. Then, they indicated that tan IIA restrained cells by reducing LC3-II, Erb-B2, and NF-κB-p65 (Su et al., 2012). Li et al. showed that tan IIA restrained the vasculogenesis and growth via the suppression of hypoxia-inducible factor 1α (HIF-1α) synthesis and VEGF, in which the mTOR/p70S6K/4E-BP1 signaling pathway was participating (Li et al., 2015c).
Lin et al. showed that tan IIA restrained breast cancer stem cells growth via attenuation of IL-6/STAT3/NF-κB signaling pathways (Lin et al., 2013). After tan IIA treatment, the expression of IL-6, NF-κB-p65, phospho-STAT3 (Tyr705), and STAT3 in nucleus and cyclin D1 were reduced meaningfully (Lin et al., 2013).
Fu et al. reported that it may ameliorate hypoxia induced iatrochemistry obstruction to DOX and EMT, which may be attributed to the downregulation of HIF-1α (Fu et al., 2014). Li et al. demonstrated that tan IIA might boost the susceptivity to DOX by restraining the PTEN/AKT pathway and downregulating efflux ABC transporters incorporating MRP1, BCRP, and P-gp (Li et al., 2019). Li et al. suggested that it enhanced the chemosensitivity to DOX via reducing MDR-related ABC transporters (Li and Lai, 2017). It could facilitate endocellular DOX cumulation of MCF-7 cell and increase the sensitivity to DOX (Li and Lai, 2017). Lin et al. showed that tan IIA may shorten the taxol resistance via inhibition of the tau expression in MCF‐7 cells (Lin et al., 2018) (its anticancer pathway is shown in Figure 2).
Prostate Cancer
Prostate cancer is the most ordinary aggressive cancer and the second major consideration of death in men (Chiu et al., 2013). At present, the remedial formations of prostate cancer generally have drug resistance and high toxicity (Zhang et al., 2012b). Therefore, it is still the priority of prostate cancer investigation to find a more effective chemical prevention plan with the least side effects (Gong et al., 2011).
Won et al. observed that tan IIA may induce mitochondria dependent apoptosis by inhibiting PI3K/AKT survival pathway (Won et al., 2010). It may reduce PI3K, p85 subunit, and the phosphorylation of AKT and mTOR (Won et al., 2010). Tan IIA induced p53 excitation and mitochondrial lesion, resulting in Caspase 9/ 3 reconciled apoptosis (Won et al., 2010). Then, they demonstrated that it induced arrest of G1 through activation of p53 signaling and restraint of androgen receptor (AR) in LNCaP prostate cancer cells (Won et al., 2012). Tan IIA could induce arrest of cell cycle at G1 phase and reduce cyclin D1, CDK2, and CDK4 and activate the phosphorylation of p53 at Ser 15 residue and its downstream p21 and p27 (Won et al., 2012). Chiu et al. suggested that it restrained growth by reaction of ER stress (Chiu et al., 2013). It could arrest cell cycle G0/G1 and the underlying mechanism for apoptosis via the excitation of PARP, Caspases 9/3, and the reaction of ER stress through the IRE1-α-GADD153/CHOP pathway (Chiu et al., 2013).
Li et al. showed that the apoptosis and autophagy were dependent on the ROS abducted via tan IIA in PC-3 cells (Li et al., 2015d) (its anticancer pathway is shown in Figure 2).
Conclusion
Recently, TCM has played more and more important roles in health conservation, the prevention and treatment of diseases, and plant drug detection. The use of TCM to prevent the occurrence and revolution of multiple malignant diseases has become an important choice for the cure of malignant disease. There has been great effort not only to develop new drugs, but also to conclude how the consisting ingredients exhibit their activities. S. miltiorrhiza has been widely used in eastern countries, especially in China, to treat miscellaneous diseases for its extraordinary pharmacological actions, including free radical scavenging, anticoagulation, and vasodilatation.
Tan IIA is an effective component in the extract of S. miltiorrhiza Bunge, which has been diffusely used in TCM exercise for more than thousand years to treat diverse diseases. It significantly induced apoptosis on a panel of cancer cells, such as leukemia, lung cancer, hepatocellular carcinoma, gastric carcinoma, colorectal cancer, glioma, osteosarcoma, cervical cancer, ovarian cancer, breast cancer, and prostate cancer.
Overall, Tan IIA has remarkable prohibitive effect on a variety of tumor cells and its possible mechanism involves regulating cell cycle, inhibiting cell diffusion, inducing apoptosis and differentiation, inhibiting tumor aggression and diversion, inhibiting angiogenesis and reversing tumor MDR, and so on. Tan IIA, as a sort of medicine possessing multiple pharmacological actions, has the characteristics of high efficiency, low toxicity, and natural source and possessed considerable potential value clinically. The combination of tan IIA and other clinical commonly chemotherapeutic drugs could enhance the therapeutic effect of chemotherapeutic drugs, which makes tan IIA have a good application prospect in tumor therapy and adjuvant therapy and also provides a new idea for various cancer treatment.
Author Contributions
Z-YF collected the documentations and wrote the original manuscript; MZ, J-nL, and XZ classified the pharmacological literatures; Y-qZ and LF proposed amendments and modified the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development project (2017YFC1702702) and Shandong Provincial Technological Innovation and Guidance Project (2017YFC1702702).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bai, Y. Q., Zhang, L. D., Fang, X. H., and Yang, Y. (2016). Tanshinone IIA enhances chemosensitivity of colon cancer cells by suppressing nuclear factor-κB. Exp. Ther. Med. 11, 1085–1089. doi:10.3892/etm.2016.2984
Boon, H., and Wong, J. (2004). Botanical medicine and cancer: a review of the safety and efficacy. Expet Opin. Pharmacother. 5, 2485–2501. doi:10.1517/14656566.5.12.2485
Chang, C. C., Chu, C. F., Wang, C. N., Wu, H. T., Bi, K. W., Pang, J. H., et al. (2014a). The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 21, 207–216. doi:10.1016/j.phymed.2013.09.012
Chang, T. W., Lin, C. Y., Tzeng, Y. J., and Lur, H. S. (2014b). Synergistic combinations of tanshinone IIA and trans-resveratrol toward cisplatin-comparable cytotoxicity in HepG2 human hepatocellular carcinoma cells. Anticancer Res. 34, 5473–5480. doi:10.1016/0378-1097(93)90022-T
Chang, C. C., Kuan, C. P., Lin, J. Y., Lai, J. S., and Ho, T. F. (2015). Tanshinone IIA facilitates TRAIL sensitization by up-regulating DR5 through the ROS-JNK-CHOP signaling axis in human ovarian carcinoma cell lines. Chem. Res. Toxicol. 28, 1574–1583. doi:10.1021/acs.chemrestox.5b00150
Chen, J., Shi, D. Y., Liu, S. L., and Zhong, L. (2012). Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol. Rep. 27, 523–528. doi:10.3892/or.2011.1524
Cheng, C. Y., and Su, C. C. (2010). Tanshinone IIA may inhibit the growth of small cell lung cancer H146 cells by up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial membrane potential. Mol. Med. Rep. 3, 645–650. doi:10.3892/mmr_00000310
Chien, S. Y., Kuo, S. J., Chen, Y. L., Chen, D. R., Cheng, C. Y., and Su, C. C. (2012). Tanshinone IIA inhibits human hepatocellular carcinoma J5 cell growth by increasing bax and caspase 3 and decreasing CD31 expression in vivo. Mol. Med. Rep. 5, 282–286. doi:10.3892/mmr.2011.631
Chiu, T. L., and Su, C. C. (2010). Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential. Int. J. Mol. Med. 25, 231–236. doi:10.3892/ijmm_00000335
Chiu, S. C., Huang, S. Y., Chen, S. P., Su, C. C., Chiu, T. L., and Pang, C. Y. (2013). Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 16, 315–322. doi:10.1038/pcan.2013.38
Chiu, C. M., Huang, S. Y., Chang, S. F., Liao, K. F., and Chiu, S. C. (2018). Synergistic antitumor effects of tanshinone IIA and sorafenib or its derivative SC-1 in hepatocellular carcinoma cells. Onco.Targets Ther. 11, 1777–1785. doi:10.2147/OTT.S161534
Dai, Z. K., Qin, J. K., Huang, J. E., Luo, Y., Xu, Q., and Zhao, H. L. (2011). Tanshinone IIA activates calcium-dependent apoptosis signaling pathway in human hepatoma cells. J. Nat. Med. 66, 192–201. doi:10.1007/s11418-011-0576-0
Ding, L. J., Wang, S. D., Wang, W. Y., Wang, S., Wang, W., Lv, P., et al. (2017). Tanshinone IIA affects autophagy and apoptosis of glioma cells by inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway. Pharmacology 99, 188–195. doi:10.1159/000452340
Dong, X. R., Dong, J. H., and Peng, G. (2008). Growth-inhibiting and apoptosis-inducing effects of tanshinone II A on human gastric carcinoma cells. J. Huazhong Univ. Sci. Technol.—Med. Sci. 27, 706–709. doi:10.1007/s11596-007-0623-y
Dong, X. R., Dong, J. H., Zhang, R. G., Fan, L., Liu, L., and Wu, G. (2009). Anti-inflammatory effects of tanshinone IIA on radiation-induced microglia BV-2 cells inflammatory response. Cancer Biother. Radiopharm. 24, 681–687. doi:10.1089/cbr.2009.0640
Fan, G. W., Zhu, Y., Guo, H., Wang, X., Wang, H., and Gao, X. (2011). Direct vasorelaxation by a novel phytoestrogen tanshinone IIA is mediated by nongenomic action of estrogen receptor through endothelial nitric oxide synthase activation and calcium mobilization. J. Cardiovasc. Pharmacol. 57, 340–347. doi:10.1097/FJC.0b013e31820a0da1
Fan, G. W., Jiang, X. R., Wu, X. Y., Fordjour, P. A., Miao, L., Zhang, H., et al. (2016). Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-κB pathway. Inflammation 39, 375–384. doi:10.1007/s10753-015-0259-1
Fu, P., Du, F. Y., Chen, W., Yao, M., Lv, K., and Liu, Y. (2014). Tanshinone IIA blocks epithelial-mesenchymal transition through HIF-1α downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines. Oncol. Rep. 31, 2561–2568. doi:10.3892/or.2014.3140
Gong, Y., Li, Y. L., Lu, Y., Li, L., Abdolmaleky, H., Blackburn, G. L., et al. (2011). Bioactive tanshinones in Salvia Miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Canc. 129, 1042–1052. doi:10.1002/ijc.25678
Gong, G., Gu, Y. Q., Zhang, Y. F., Liu, W., Li, L., and Li, J. (2019). RETRACTED: tanshinone IIA alleviates oxidative damage after spinal cord injury in vitro and in vivo through up-regulating miR-124. Life Sci. 216, 147–155. doi:10.1016/j.lfs.2018.11.046
Guo, Y., Li, Y., Xiang, B., Huang, X. O., Ma, H. B., Wang, F. F., et al. (2017). Nutlin-3 plus tanshinone IIA exhibits synergetic anti-leukemia effect with imatinib by reactivating p53 and inhibiting AKT/mTOR pathway in Ph+ ALL. Biochem. J. 474, 4153–4170. doi:10.1042/BCJ20170386
Guo, Y., Li, Y., Wang, F. F., Xiang, B., Huang, X. O., and Ma, H. B. (2018). The combination of nutlin-3 and tanshinone IIA promotes synergistic cytotoxicity in acute leukemic cells expressing wild-type p53 by co-regulating MDM2-P53 and the AKT/mTOR pathway. Int. J. Biochem. Cell Biol. 106, 8–20. doi:10.1016/j.biocel.2018.10.008
Heo, J. Y., and Im, D. S. (2019). Anti-allergic effects of salvianolic acid A and tanshinone IIA from Salvia miltiorrhiza determined using in vivo and in vitro experiments. Int. Immunopharm. 67, 69–77. doi:10.1016/j.intimp.2018.12.010
Huang, J., Lin, H., and Hong, Y. K. (2015). In vitro anti-tumor activity of the tanshinone IIA against SKOV3 cells. Nat. Prod. Res. 30, 1844–1846. doi:10.1080/14786419.2015.1068774
Huang, S. T., Huang, C. C., Huang, W. L., Lin, T. K., Liao, P. L., Wang, P. W., et al. (2017). Tanshinone IIA induces intrinsic apoptosis in osteosarcoma cells both in vivo and in vitro associated with mitochondrial dysfunction. Sci. Rep. 7, 40382. doi:10.1038/srep40382
Jeon, Y. J., Kim, J. S., Hwang, G. H., Wu, Z., Han, H. J., Park, S. H., et al. (2015). Inhibition of cytochrome P450 2J2 by tanshinone IIA induces apoptotic cell death in hepatocellular carcinoma HepG2 cells. Eur. J. Pharmacol. 764, 480–488. doi:10.1016/j.ejphar.2015.07.047
Jiang, P., Li, C., Xiang, Z. H., and Jiao, B. (2014). Tanshinone IIA reduces the risk of alzheimer’s disease by inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation in the temporal lobes of rat models of alzheimer’s disease. Mol. Med. Rep. 10, 689–694. doi:10.3892/mmr.2014.2254
Jiao, J. W., and Wen, F. (2011). Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol. Rep. 25, 781–788. doi:10.3892/or.2010.1107
Kan, S. D., Cheung, W. M., Zhou, Y. L., and Ho, W. S. (2014). Enhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2 human hepatoma cells. Planta Med. 80, 70–76. doi:10.1055/s-0033-1360126
Kim, J. Y., Song, J. J., Kwon, B. M., and Lee, J. D. (2015). Tanshinone IIA exerts antitumor activity against vestibular schwannoma cells by inhibiting the expression of hypoxia-inducible factor-1α. Mol. Med. Rep. 12, 4604–4609. doi:10.3892/mmr.2015.3932
Kim, E. O., Kang, S. E., Im, C. R., Lee, J. H., Ahn, K. S., Yang, W. M., et al. (2016). Tanshinone IIA induces TRAIL sensitization of human lung cancer cells through selective ER stress induction. Int. J. Oncol. 48, 2205–2212. doi:10.3892/ijo.2016.3441
Kwak, H. B., Sun, H. M., Ha, H., Kim, H. N., Lee, J. H., Kim, H. H., et al. (2008). Tanshinone IIA suppresses inflammatory bone loss by inhibiting the synthesis of prostaglandin E2 in osteoblasts. Eur. J. Pharmacol. 601, 30–37. doi:10.1016/j.ejphar.2008.10.034
Lee, W. Y. W., Chiu, L. C. M., and Yeung, J. H. K. (2008). Cytotoxicity of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on HepG2 cells in relation to glutathione perturbation. Food Chem. Toxicol. 46, 328–338. doi:10.1016/j.fct.2007.08.013
Li, K., and Lai, H. (2017). TanshinoneIIA enhances the chemosensitivity of breast cancer cells to doxorubicin through down-regulating the expression of MDR-related ABC transporters. Biomed. Pharmacother. 96, 371–377. doi:10.1016/j.biopha.2017.10.016
Li, W., Zhang, Y., Xing, C. Y., and Zhang, M. (2015a). Tanshinone IIA represses inflammatory response and reduces radiculopathic pain by inhibiting IRAK-1 and NF-κB/p38/JNK signaling. Int. Immunopharm. 28, 382–389. doi:10.1016/j.intimp.2015.06.032
Li, J., Wen, P. Y., Li, W. W., and Zhou, J. (2015b). Upregulation effects of tanshinone IIA on the expressions of NeuN, Nissl body, and IκB and downregulation effects on the expressions of GFAP and NF-κB in the brain tissues of rat models of alzheimer’s disease. Neuroreport 26, 758–766. doi:10.1097/WNR.0000000000000419
Li, G. B., Shan, C. Y., Liu, L., Zhou, T., Zhou, J., Hu, X., et al. (2015c). Tanshinone IIA inhibits HIF-1α and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PloS One 10, e0117440–14. doi:10.1371/journal.pone.0117440
Li, C. L., Han, X. C., Zhang, H., Wu, J., and Li, B. (2015d). The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells. Tumour Biol 37, 7667–7674. doi:10.1007/s13277-015-4602-9
Li, F. L., Han, G. S., and Wu, K. X. (2016a). Tanshinone IIA alleviates the AD phenotypes in APP and PS1 transgenic mice. BioMed Res. Int. 2016, 7631801. doi:10.1155/2016/7631801
Li, Q., Hu, K., Tang, S., Xu, L. F., and Luo, Y. C. (2016b). Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer. Asian Pac. J. Trop. Med. 9, 1084–1088. doi:10.1016/j.apjtm.2016.09.003
Li, X., Park, S. J., Jin, F. S., Deng, Y., Yang, J. H., Chang, J. H., et al. (2018a). Tanshinone IIA suppresses FcεRI-mediated mast cell signaling and anaphylaxis by activation of the Sirt1/LKB1/AMPK Pathway. Biochem. Pharmacol. 152, 362–372. doi:10.1016/j.bcp.2018.04.015
Li, N., Yang, L., Zhang, B. L., and Chen, S. (2018b). Tanshinone IIA effects on ovarian cancer cell line. J. Pharm. Pharmacol. 70, 1369–1377. doi:10.1111/jphp.12961
Li, K., Liu, W. S., Zhao, Q., Fan, C., Lai, H., et al. (2019). Combination of tanshinone IIA and doxorubicin possesses synergism and attenuation effects on doxorubicin in the treatment of breast cancer. Phytother. Res. 33, 1658–1669. doi:10.1002/ptr.6353
Liao, X. Z., Gao, Y., Huang, S., Chen, Z. Z., Sun, L. L., Liu, J. H., et al. (2019). Tanshinone IIA combined with cisplatin synergistically inhibits non-small-cell lung cancer in vitro and in vivo via down-regulating the phosphatidylinositol 3-kinase/Akt signalling pathway. Phytother. Res. 33, 2298–2309. doi:10.1002/ptr.6392
Lin, C. Y., Wang, L., Wang, H., Yang, L., Guo, H., and Wang, X. (2013). Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J. Cell. Biochem. 114, 2061–2070. doi:10.1002/jcb.24553
Lin, J. Y., Ke, Y. M., Lai, J. S., and Ho, T. F. (2015). Tanshinone IIA enhances the effects of TRAIL by downregulating survivin in human ovarian carcinoma cells. Phytomedicine 22 (10), 929–938. doi:10.1016/j.phymed.2015.06.012
Lin, C. Y., Chang, T. W., Hsieh, W. H., Hung, M. C., Lin, I. H., Lai, S. C., et al. (2016). Simultaneous induction of apoptosis and necroptosis by tanshinone IIA in human hepatocellular carcinoma HepG2 cells. Cell Death Dis. 2, 16065. doi:10.1038/cddiscovery.2016.65
Lin, C. Y., Jhang, Y. S., Lai, S. C., Chen, E. L., Lin, I. H., Chang, T. W., et al. (2017). Antifatigue properties of tanshinone IIA in mice subjected to the forced swimming test. Pharm. Biol. 55, 2264–2269. doi:10.1080/13880209.2017.1401648
Lin, H., Zheng, L. Y., Li, S. X., Xie, B., Cui, B., Xia, A., et al. (2018). Cytotoxicity of tanshinone IIA combined with taxol on drug-resist breast cancer cells MCF-7 through inhibition of tau. Phytother. Res. 32, 667–671. doi:10.1002/ptr.6014
Lin, L., Jadoon, S. S., Liu, S. Z., Zhang, R. Y., Li, F., Zhang, M. Y., et al. (2019). Tanshinone IIA ameliorates spatial learning and memory deficits by inhibiting the activity of ERK and GSK-3β. J. Geriatr. Psychiatr. Neurol. 32, 152–163. doi:10.1177/0891988719837373
Liu, J. J., Lin, D. J., Liu, P. Q., Huang, M., Li, X. D., and Huang, R. W. (2006). Induction of apoptosis and inhibition of cell adhesive and invasive effects by tanshinone IIA in acute promyelocytic leukemia cells in vitro. J. Biomed. Sci. 13, 813–823. doi:10.1007/s11373-006-9110-x
Liu, J. J., Zhang, Y., Lin, D. J., and Xiao, R. Z. (2009). Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol. Rep. 21, 1075–1081. doi:10.3892/or_00000326
Liu, C., Li, J. Q., Wang, L. J., Wu, F., Huang, L., Xu, Y., et al. (2012a). Analysis of tanshinone IIA induced cellular apoptosis in leukemia cells by genome-wide expression profiling. BMC Compl. Alternative Med. 12, 5–10. doi:10.1186/1472-6882-12-5
Liu, F., Yu, G., Wang, G. J., Liu, H., Wu, X., Wang, Q., et al. (2012b). An NQO1-initiated and p53-independent apoptotic pathway determines the anti-tumor effect of tanshinone IIA against non-small cell lung cancer. PloS One 7, e42138. doi:10.1371/journal.pone.0042138
Lu, Q., Zhang, P. R., Zhang, X., and Chen, J. (2009). Experimental study of the anti-cancer mechanism of tanshinone IIA against human breast cancer. Int. J. Mol. Med. 24, 773–780. doi:10.3892/ijmm_00000291
Lv, C., Zeng, H. W., Wang, J. X., Yuan, X., Zhang, C., Fang, T., et al. (2018). The antitumor natural product tanshinone IIA inhibits protein kinase C and acts synergistically with 17-AAG. Cell Death Dis. 9, 165–178. doi:10.1038/s41419-017-0247-5
Ma, H., Fan, Q., Yu, J., Xin, J., and Zhang, C. (2013). Novel microemulsion of tanshinone IIA, isolated from Salvia miltiorrhiza Bunge, exerts anticancer activity through inducing apoptosis in hepatoma cells. Am. J. Chin. Med. 41, 197–210. doi:10.1142/S0192415X13500146
Ma, K., Zhang, C., Huang, M. Y., Guo, Y. X., and Hu, G. Q. (2016). Crosstalk between Beclin-1-dependent autophagy and caspase dependent-apoptosis induced by tanshinone IIA in human osteosarcoma MG-63 cells. Oncol. Rep. 36, 1807–1818. doi:10.3892/or.2016.5003
Ma, S. L., Wang, X., Wang, Y. J., and Zuo, X. (2018a). Sodium tanshinone IIA sulfonate improves hemodynamic parameters, cytokine release, and multi-organ damage in endotoxemia rabbits. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 24, 2975–2982. doi:10.12659/MSM.909996
Ma, S. Y., Lei, Y. L., Zhang, L., and Wang, J. (2018b). Research on the inhibiting effect of tanshinone IIA on colon cancer cell growth via COX-2-Wnt/β-catenin signaling pathway. J. BUON. 23, 1337–1342.
Munagala, R., Aqil, F., Jeyabalan, J., and Gupta, R. C. (2015). Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Canc. Lett. 356, 536–546. doi:10.1016/j.canlet.2014.09.037
Olivia, E. B., Adriana, O. P., Jan, M., Huang, H., Ying, X., De Borggraeve, W., et al. (2013). Tanshinone IIA exhibits anticonvulsant activity in zebrafish and mouse seizure models. ACS Chem. Neurosci. 4, 1479–1487. doi:10.1021/cn400140e
Pan, T. L., Hung, Y. C., Wang, P. W., Chen, S. T., Hsu, T. K., Sintupisut, N., et al. (2010). Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics 10, 914–929. doi:10.1002/pmic.200900178
Pan, T. L., Wang, P. W., Hung, Y. C., Huang, C. H., and Rau, K. M. (2013). Proteomic analysis reveals tanshinone IIA enhances apoptosis of advanced cervix carcinoma CaSki cells through mitochondria intrinsic and endoplasmic reticulum stress pathways. Proteomics 13, 3411–3423. doi:10.1002/pmic.201300274
Qin, J. H., Shi, H. B., Xu, Y. J., Zhao, F., and Wang, Q. (2018). Tanshinone IIA inhibits cervix carcinoma stem cells migration and invasion via inhibiting YAP transcriptional activity. Biomed. Pharmacother. 105, 758–765. doi:10.1016/j.biopha.2018.06.028
Ren, X. Q., Wang, C., Xie, B. B., Hu, L., Chai, H., Ding, L., et al. (2017). Tanshinone IIA induced cell death via miR30b-p53-PTPN11/SHP2 signaling pathway in human hepatocellular carcinoma cells. Eur. J. Pharmacol. 796, 233–241. doi:10.1016/j.ejphar.2016.11.046
Shang, Q. H., Xu, H., and Huang, L. (2012). Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012, 716459. doi:10.1155/2012/716459
Su, C. C. (2012). Tanshinone IIA potentiates the efficacy of 5-FU in Colo205 colon cancer cells in vivo through downregulation of P-gp and LC3-II. Exp. Ther. Med. 3, 555–559. doi:10.3892/etm.2011.441
Su, C. C. (2014a). Tanshinone IIA inhibits human gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl-1 and Bcl-xL and increasing Bax and CHOP protein expression. Int. J. Mol. Med. 34, 1661–1668. doi:10.3892/ijmm.2014.1949
Su, C. C. (2014b). Tanshinone IIA inhibits gastric carcinoma AGS cells through increasing p-p38, p-JNK and p53 but reducing p-ERK, CDC2 and cyclin B1 expression. Anticancer Res. 34, 7097–7110.
Su, C. C. (2015). Tanshinone IIA decreases the migratory ability of AGS cells by decreasing the protein expression of matrix metalloproteinases, nuclear factor κB-p65 and cyclooxygenase-2. Mol. Med. Rep. 13, 1263–1268. doi:10.3892/mmr.2015.4658
Su, C. C. (2018). Tanshinone IIA inhibits gastric carcinoma AGS cells by decreasing the protein expression of VEGFR and blocking Ras/Raf/MEK/ERK pathway. Int. J. Mol. Med. 41, 2389–2396. doi:10.3892/ijmm.2018.3407
Su, C. C., and Chiu, T. L. (2016). Tanshinone IIA decreases the protein expression of EGFR, and IGFR blocking the PI3K/Akt/mTOR pathway in gastric carcinoma AGS cells both in vitro and in vivo. Oncol. Rep. 36, 1173–1179. doi:10.3892/or.2016.4857
Su, C. C., and Lin, Y. H. (2008a). Tanshinone IIA down-regulates the protein expression of ErbB-2 and up-regulates TNF-alpha in colon cancer cells in vitro and in vivo. Int. J. Mol. Med. 22, 847–851. doi:10.3892/ijmm_00000094
Su, C. C., and Lin, Y. H. (2008b). Tanshinone IIA inhibits human breast cancer cells through increased Bax to Bcl-xL ratios. Int. J. Mol. Med. 22, 357–361. doi:10.3892/ijmm_00000030
Su, C. C., Chen, G. W., Kang, J. C., and Chan, M. H. (2008). Growth inhibition and apoptosis induction by tanshinone IIA in human colon adenocarcinoma cells. Planta Med. 74, 1357–1362. doi:10.1055/s-2008-1081299
Su, C. C., Chien, S. Y., Kuo, S. J., Chen, Y. L., Cheng, C. Y., and Chen, D. R. (2012). Tanshinone IIA inhibits human breast cancer MDA-MB-231 cells by decreasing LC3-II, Erb-B2 and NF-κBp65. Mol. Med. Rep. 5, 1019–1022. doi:10.3892/mmr.2012.756
Tan, Y. L., Ou, H. X., Zhang, M., Gong, D., Zhao, Z. W., Chen, L. Y., et al. (2019). Tanshinone IIA promotes macrophage cholesterol efflux and attenuates atherosclerosis of apoE-/- mice by omentin-1/ABCA1 pathway. Curr. Pharmaceut. Biotechnol. 20, 422–432. doi:10.2174/1389201020666190404125213
Tang, C., Xue, H. L., Huang, H. B., and Wang, X. G. (2010). Tanshinone IIA inhibits constitutive STAT3 activation, suppresses proliferation, and induces apoptosis in rat C6 glioma cells. Neurosci. Lett. 470, 126–129. doi:10.1016/j.neulet.2009.12.069
Tseng, P. Y., Lu, W. C., Hsieh, M. J., Chien, S. Y., and Chen, M. K. (2014). Tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway. BioMed Res. Int. 2014, 540516. doi:10.1155/2014/540516
Tu, J. J., Xing, Y. Y., Guo, Y. J., Tang, F., Guo, L., and Xi, T. (2012). TanshinoneIIA ameliorates inflammatory microenvironment of colon cancer cells via repression of microRNA-155. Int. Immunopharm. 14, 353–361. doi:10.1016/j.intimp.2012.08.015
Wang, X. J., Wei, Y. Q., Yuan, S. L., Liu, G., Lu, Y., Zhang, J., et al. (2005). Potential anticancer activity of tanshinone IIA against human breast cancer. Int. J. Canc. 116, 799–807. doi:10.1002/ijc.20880
Wang, J., Wang, X. J., Jiang, S., Yuan, S., Lin, P., Zhang, J., et al. (2007). Growth inhibition and induction of apoptosis and differentiation of tanshinone IIA in human glioma cells. J. Neuro Oncol. 82, 11–21. doi:10.1007/s11060-006-9242-x
Wang, W. Q., Liu, L., Sun, H. C., Fu, Y. L., Xu, H. X., Chai, Z. T., et al. (2012). Tanshinone IIA inhibits metastasis after palliative resection of hepatocellular carcinoma and prolongs survival in part via vascular normalization. J. Hematol. Oncol. 5, 69–80. doi:10.1186/1756-8722-5-69
Wang, B. Y., Jin, Y., Zhang, C. L., and Zhang, J. X. (2018). Mechanism of the effect of tanshinone IIA epithelial-mesenchymal transition via the TGF-β1/smad pathway in human alveolar epithelial cell line A549. Biomed. Res. 29, 2570–2577. doi:10.4066/biomedicalresearch.29-17-4034
Wei, X. L., Zhou, L. B., Hu, L. Y., and Huang, Y. (2012). Tanshinone IIA arrests cell cycle and induces apoptosis in 786-O human renal cell carcinoma cells. Oncol. Lett. 3, 1144–1148. doi:10.3892/ol.2012.626
Weng, Y. F., Lin, J. X., Liu, H., Wu, H., Yan, Z., and Zhao, J. (2018). AMPK activation by tanshinone IIA protects neuronal cells from oxygen-glucose deprivation. Oncotarget 9, 4511–4521. doi:10.18632/oncotarget.23391
Won, S. H., Lee, H. J., Jeong, S. J., Lee, H. J., Lee, E. O., Jung, D. B., et al. (2010). Tanshinone IIA induces mitochondria dependent apoptosis in prostate cancer cells in association with an inhibition of phosphoinositide 3-kinase/AKT pathway. Biol. Pharm. Bull. 33, 1828–1834. doi:10.1248/bpb.33.1828
Won, S. H., Lee, H. J., Jeong, S. J., Lü, J., and Kim, S. H. (2012). Activation of p53 signaling and inhibition of androgen receptor mediate tanshinone IIA induced G1 arrest in LNCaP prostate cancer cells. Phytother. Res. 26, 669–674. doi:10.1002/ptr.3616
Xia, W. J., Yang, M., Fok, T. F., Li, K., Chan, W. Y., Ng, P. C., et al. (2005). Partial neuroprotective effect of pretreatment with tanshinone IIA on neonatal hypoxia-ischemia brain damage. Pediatr. Res. 58, 784–790. doi:10.1203/01.PDR.0000180550.99162.BC
Xiao, J. Y., Zhang, G. X., Qiu, P. X., Liu, X., Wu, Y., Du, B., et al. (2013). Tanshinone IIA increases the bystander effect of herpes simplex virus thymidine kinase/ganciclovir gene therapy via enhanced gap junctional intercellular communication. PloS One 8, e67662. doi:10.1371/journal.pone.0067662
Xie, J., Liu, J. H., Liu, H., Liang, S., Lin, M., Gu, Y., et al. (2015). The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm. Sin. B. 5, 554–563. doi:10.1016/j.apsb.2015.07.008
Xu, Z. Y., Chen, L., Xiao, Z. G., Zhu, Y., Jiang, H., Jin, Y., et al. (2018). Potentiation of the anticancer effect of doxorubicinin drug-resistant gastric cancer cells by tanshinone IIA. Phytomedicine 51, 58–67. doi:10.1016/j.phymed.2018.05.012
Yan, M. Y., Chien, S. Y., Kuo, S. J., Chen, D. R., and Su, C. C. (2012). Tanshinone IIA inhibits BT-20 human breast cancer cell proliferation through increasing caspase 12, GADD153 and phospho-p38 protein expression. Int. J. Mol. Med. 29, 855–863. doi:10.3892/ijmm.2012.908
Yang, L. Q., Guo, H. J., Dong, L. H., Wang, L., Liu, C., and Wang, X. (2014). Tanshinone IIA inhibits the growth, attenuates the stemness and induces the apoptosis of human glioma stem cells. Oncol. Rep. 32, 1303–1311. doi:10.3892/or.2014.3293
Yen, J. H., Huang, S. T., Huang, H. S., Fong, Y. C., Wu, Y. Y., Chiang, J. H., et al. (2018). HGK-sestrin 2 signaling-mediated autophagy contributes to antitumor efficacy of tanshinone IIA in human osteosarcoma cells. Cell Death Dis. 9, 1003–1017. doi:10.1038/s41419-018-1016-9
Yoon, Y., Kim, Y. O., Jeon, W. K., Park, H. J., and Sung, H. J. (2000). Tanshinone IIA isolated from Salvia miltiorrhiza Bunge induced apoptosis in HL60 human premyelocytic leukemia cell line. J. Ethnopharmacol. 68, 121–127. doi:10.1016/s0378-8741(99)00059-8
Yu, T., Zhou, Z. C., Mu, Y. G., de Lima Lopes, G., and Luo, K. Q. (2014). A novel anti-cancer agent, acetyltanshinone IIA, inhibits oestrogen receptor positive breast cancer cell growth by down-regulating the oestrogen receptor. Canc. Lett. 346, 94–103. doi:10.1016/j.canlet.2013.12.023
Yu, J., Wang, X. X., Li, Y. H., and Tang, B. (2017). Tanshinone IIA suppresses gastric cancer cell proliferation and migration by downregulation of FOXM1. Oncol. Rep. 37, 1394–1400. doi:10.3892/or.2017.5408
Yun, S. M., Jeong, S. J., Kim, J. H., Jung, J. H., Lee, H. J., Sohn, E. J., et al. (2013a). Activation of c-jun n-terminal kinase mediates tanshinone IIA-induced apoptosis in KBM-5 chronic myeloid leukemia cells. Biol. Pharm. Bull. 36, 208–214. doi:10.1248/bpb.b12-00537
Yun, S. M., Jung, J. H., Jeong, S. J., Sohn, E. J., Kim, B., and Kim, S. H. (2013b). Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cells. Phytother. Res. 28, 458–464. doi:10.1002/ptr.5015
Zhang, K. J., Li, J., Meng, W. T., Xing, H., and Yang, Y. (2010). C/EBPβ and CHOP participate in tanshinone IIA-induced differentiation and apoptosis of acute promyelocytic leukemia cells in vitro. Int. J. Hematol. 92, 571–578. doi:10.1007/s12185-010-0686-6
Zhang, Y., Wei, R. X., Zhu, X. B., Cai, L., Jin, W., and Hu, H. (2012a). Tanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG-63 cell line in vitro. Anti Canc. Drugs. 23, 212–219. doi:10.1097/CAD.0b013e32834e5592
Zhang, Y., Won, S. H., Jiang, C., Lee, H. J., Jeong, S. J., Lee, E. O., et al. (2012b). Tanshinones from Chinese medicinal herb danshen (Salvia Miltiorrhiza bunge) suppress prostate cancer growth and androgen receptor signaling. Pharm. Res. 29, 1595–1608. doi:10.1007/s11095-012-0670-3
Zhang, H. S., Chen, X. Y., Wu, T. C., and Zhang, F. J. (2014a). Tanshinone II A inhibits tat-induced HIV-1 transactivation through redox-regulated AMPK/Nampt pathway. J. Cell. Physiol. 229, 1193–1201. doi:10.1002/jcp.24552
Zhang, J., Wang, J., Jiang, J. Y., Liu, S. D., Fu, K., and Liu, H. Y. (2014b). Tanshinone IIA induces cytochrome c-mediated caspase cascade apoptosis in A549 human lung cancer cells via the JNK pathway. Int. J. Oncol. 45, 683–690. doi:10.3892/ijo.2014.2471
Zhang, B. J., Ma, Z. L., Li, X., Zhang, C., Shao, Y., Liu, Z., et al. (2016). Tanshinones suppress non-small cell lung cancer through up-regulating miR-137. Acta Biochim. Biophys. Sin. 48, 768–770. doi:10.1093/abbs/gmw053
Zhang, Y. J., Guo, S. G., Fang, J., Peng, B., Zhang, Y., and Cao, T. (2018a). Tanshinone IIA inhibits cell proliferation and tumor growth by downregulating STAT3 in human gastric cancer. Exp. Ther. Med. 16, 2931–2937. doi:10.3892/etm.2018.6562
Zhang, X., Zhou, Y., and Gu, Y. E. (2018b). Tanshinone IIA induces apoptosis of ovarian cancer cells in vitro and in vivo through attenuation of PI3K/AKT/JNK signaling pathways. Oncol. Lett. 17, 1896–1902. doi:10.3892/ol.2018.9744
Zhang, Y. P., Geng, Y., He, J. T., Wu, D., Zhang, T., Xue, L., et al. (2019). Tanshinone IIA induces apoptosis and autophagy in acute monocytic leukemia via downregulation of PI3K/Akt pathway. Am. J. Transl. Res. 11, 2995–3006. .
Zhou, L., Chan, W. K., Xu, N., Xiao, K., Luo, H., Luo, K. Q., et al. (2008). Tanshinone IIA, an isolated compound from Salvia miltiorrhiza Bunge, induces apoptosis in HeLa cells through mitotic arrest. Life Sci. 83, 394–403. doi:10.1016/j.lfs.2008.07.011
Zhou, Y. Q., Liu, X. Q., Zhang, X. D., Wen, J., Cheng, J., Li, P., et al. (2019). Decreased vasodilatory effect of Tanshinone ⅡA sodium sulfonate on mesenteric artery in hypertension. Eur. J. Pharmacol. 854, 365–371. doi:10.1016/j.ejphar.2019.04.049
Keywords: Salvia miltiorrhiza, tanshinone IIA, anticancer, mechanism, traditional Chinese medicine
Citation: Fang Z, Zhang M, Liu J-n, Zhao X, Zhang Y-q and Fang L (2021) Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 11:611087. doi: 10.3389/fphar.2020.611087
Received: 28 September 2020; Accepted: 26 November 2020;
Published: 14 January 2021.
Edited by:
Haifan Wu, Wichita State University, United StatesReviewed by:
Changchun Yuan, North University of China, ChinaMassimo Libra, University of Catania, Italy
Copyright © 2021 Fang, Zhang, Liu, Zhao, Zhang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-qing Zhang, enlxNjIyMDAzQDEyNi5jb20=; Lei Fang, ZmxlaXZAMTYzLmNvbQ==
 Zhong‐ying Fang
Zhong‐ying Fang Miao Zhang1
Miao Zhang1 Lei Fang
Lei Fang