
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 June 2020
Sec. Drug Metabolism and Transport
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00711
Background: Amomi fructus is a famous traditional Chinese medicine (TCM) that can exert beneficial effects during the treatment of gastrointestinal diseases and is used widely in China and other countries in Southeast Asia. However, the nonvolatile active ingredients that are present in the water extractions from A. fructus used to treat gastrointestinal diseases have yet to be elucidated. The goal of this study was to identify the nonvolatile active ingredients of A. fructus.
Methods: We used an in situ single-pass intestinal perfusion (SPIP) model to identify the active ingredients of A. fructus that play significant roles in gastrointestinal absorption. In addition, we developed a high-performance liquid chromatography (HPLC) method to identify key fractions in intestinal outflow perfusate.
Results: Nineteen components were identified in a water extraction from A. fructus; these exhibited different absorption capabilities in different intestinal segments. Of these, six components were determined by the newly developed HPLC method: catechin, vanillic acid, epicatechin, polydatin, isoquercitrin, and quercitrin.
Conclusions: The current study aimed to identify the active ingredients present in water extractions prepared from A. fructus in a single-intestinal perfusate from rats. Our findings provide an experimental basis to explain the pharmacodynamic actions of A. fructus.
Globally, many patients are required to undergo surgery for benign or malignant gastrointestinal lesions every year. The mortality and complication rates associated with patients undergoing gastrointestinal surgery have decreased over recent years, mostly due to the development of modern medicine and surgical technology. However, gastrointestinal surgery always results in disorders associated with gastrointestinal motility. These disorders vary in severity and are known to be influenced by a wide range of factors, including anesthesia, surgical emergency, trauma, inflammation, and pain, but particularly by radical gastrectomy and other highly traumatic surgeries (Nusrat and Bielefeldt, 2013; Merkow et al., 2015; Reddy et al., 2018).
Conventional forms of western medicine are commonly used to facilitate the treatment of gastrointestinal disease, including fasting, gastric decompression, nutritional support, gastro-kinetic agents (e.g., domperidone, cisapride, and duphalac), and anal preparations for promoting defecation (e.g., glycerin and mannitol preparations). However, while these methods may alleviate clinical symptoms to some extent, they are also associated with adverse drug reactions and high costs.
In recent years, practitioners and researchers of traditional Chinese medicine (TCM) and integrated traditional Chinese medicine (ITCM) have made many valuable clinical observations and carried out numerous experimental studies with regards to the recovery of gastrointestinal function after surgery. It is clearly evident from the existing literature that compound prescriptions of TCM that contain Amomi fructus as the predominant medicine are widely used in the treatment of gastrointestinal diseases (Lee et al., 2016). Around 60 different Chinese formulas containing A. fructus as the major component are officially documented in Chinese Pharmacopeia (2015 version) (Commission of Chinese Pharmacopoeia, 2015).
Previous studies have reported that the water extraction of A. fructus can significantly enhance the amplitude of the basic electrical rhythm on electrogastrograms and can also promote intestinal peristalsis. Furthermore, there are clear differences in the resumption of gurgling sounds, along with exhaust and defecation times, when compared between patients receiving water extraction of A. fructus and patients receiving conventional forms of modern medicine, such as enteral nutrition solution via nasal feeding (Huang et al., 2007; Chen et al., 2018; Suo et al., 2018).
However, previous studies relating to the active components of A. fructus are limited to descriptions of volatile oils and other volatile components, such as bornyl acetate, camphor, and limonene (Xu et al., 2018; Ao et al., 2019). In order to fully understand the specific intervental effects of A. fructus on the gastrointestinal tract and explore the active ingredients more comprehensively, we cannot ignore other nonvolatile components in the water extraction of A. fructus, which may also be important, such as Zn, Mn, other trace elements, vanillic acid, quercetin, piceid, and other water-soluble components (Xue et al., 2016).
In situ intestinal perfusion has been used widely to study the absorption of drugs in the intestine (Yerasi et al., 2015; Li et al., 2016; Li et al., 2018; Zhang et al., 2019; Wang et al., 2020). The basic principle of this method is the hypothesis that the reduction of drugs is equivalent to the absorption of drugs and to evaluate the absorption of drugs in the small intestine by reducing drug components in the perfusion solution. Using this method, it is possible to express the level of absorption by the rate of absorption and the amount of drugs absorbed. Moreover, a number of kinetic parameters can be calculated to reflect the absorption of drugs, thus allowing us to analyze the metabolic profiles underlying the permeation and absorption of drugs, such as the effective permeability coefficient (Peff) and the absorption rate constant (KA). In this study, we used SPIP as it is an established and effective model for in situ drug absorption studies. This model is characterized by a low flow rate (0.2–0.3 ml/min), a stable absorption rate, and causes minimal damage to the mucosa of the intestinal wall (Huo et al., 2014; Lin et al., 2015).
Collectively, the available evidence indicates that conventional western medicine is not fully effective for the treatment of gastrointestinal motility disorders following gastric surgery and hypothesized that TCM and ITCM may provide good therapeutic effects for such patients, particularly considering the widespread application of compound prescriptions of TCM containing A. fructus as the main medicine. However, it is highly evident that existing studies relating to the active components of A. fructus are limited to descriptions of volatile components; nonvolatile components have yet to be investigated, even though these components may exhibit beneficial pharmacological activity. In the present study, we investigated promising nonvolatile components in water extractions prepared from A. fructus in the intestine of rats using the SPIP model and an efficient quantitation method based on high-performance liquid chromatography (HPLC).
Samples of A. fructus (Figure 1) were purchased from Anguo Traditional Chinese Medicine Factory (Anguo, China), produced in Guangdong Province. Samples were acquired from the dried ripe fruit of Amomum villosum Lour. by Professor Yonghong Yan (Beijing University of Chinese Medicine, Beijing, China). Voucher specimens were then deposited in our laboratory.
We purchased standards for quercetin (purity=98.0%) and vanillic acid (purity=98.0%) from Shanghai Shidande Standard Technical Service Co., Ltd. (Shanghai, China). HPLC-grade methanol (purity≥99.9%) was purchased from Fisher Chemical (Waltham, USA). All water used in this research was double distilled. Other reagents and chemicals were all analytical grade. MgCl2·6H2O (purity≥98.0%), KCl (purity≥99.5%), NaCl (purity≥99.5%), CaCl2 (purity≥96.0%), D-Glucose ([α]20D: +52.5° - +53.0°), NaHCO3 (purity≥99.5%), NaH2PO4 (purity≥99.0%), concentrated hydrochloric acid (ω%36.0–38.0%), and acetic acid (purity≥99.5%) were supplied by Beijing Chemical Works (Beijing, China). Ethyl carbamate (trade name: urethane) was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Finally, 0.9% sodium chloride injection solution was obtained from Shijiazhuang Pharmaceutical Siyao Co., Ltd. (Shijiazhuang, China).
Male SD rats (clean grade, with a body weight of approximately 250 g) were provided by Sbeifu Biotechnology Co., Ltd. (Beijing, China; License number: SCXK-20160002). The condition of all rats was examined by the Experimental Animal Center of the Academy of Military Medical Sciences (Beijing, China).
Krebs-Ringer’s (K-R) nutrient solution was prepared by fully dissolving 0.084 g MgCl2·6H2O, 0.35 g KCl, 7.80 g NaCl, 0.37 g CaCl2, 1.48 g Dextrose, 1.37 g NaHCO3, and 0.02 g NaH2PO4 in 1,000 ml double distilled water. The pH of the solution was then adjusted to 7.0 using 1 mol·L-1 HCL. The CaCl2 was dissolved separately prior to addition to the nutrient solution. This solution was prepared according to a previously described method with some modifications (Wang et al., 2017).
A. Fructus was homogenized for 4 seconds using a pulverizer. Traditionally, A. fructus is first crushed with or without other medicines. Then, 150 ml of double distilled water was measured into a flat-bottomed 500 ml flask and heated in an induction cooker (power: 2,200 W). Then, the finely homogenized samples (10 g) were weighed and added into the flat-bottomed flask while the water boiled continuously. The heat was then reduced to 400 W for 2 min with the solution continuing to boil gently. Next, the water extraction of A. fructus was filtered with a double-layer gauze and the filtrate was centrifuged at 3,000 rpm/s for 20 min to remove A. fructus residue. The water extraction of A. fructus was then concentrated to 0.5 g/ml by rotary evaporation.
In order to reduce the adsorption of drugs by tubes during the experiment, the tubing of the peristaltic pump was saturated with the perfusion solution until the concentration of the outflow solution was equal to that of the perfusion solution. The rats were fasted for 24 h, during which they were allowed to drink water ad libitum. Next, the rats were given intraperitoneal injections of 25% urethane at a dose of 0.007 ml/g. The rats were placed in a supine position on the operating table. Their abdominal cavity were opened along the midline of the abdomen, and the duodenum, jejunum, and ileum were separated from the intestinal segments. Tubes were carefully inserted at either end of this segment and ligated with a sterile surgical line. The intestinal contents were then washed with K-R solution which had been preheated to 37°C. The intestines were then balanced with K-R solution for 15 min and air was pumped into the intestines to drain the residual K-R solution. The intestinal segments were then perfused with the water extraction of A. fructus from the peristaltic pump inlet. The small bottles containing the perfusion solution had been accurately weighed in advance. The volume flow was set as 0.2 ml/min, and the absorption was maintained for approximately 30 min. The outflow perfusate was collected with another small bottle which had also been weighed in advance. The bottles containing the perfusion solution and the outflow perfusate bottles were changed quickly every 15 min and weighed. Sampling and weighing were carried out at 45 and 180 min, thus allowing us to calculate the mass of the solution perfused and collected. In situ single-pass intestinal perfusion was implemented in accordance with the literatures with some modifications (Li et al., 2012; Zhou et al., 2012; Jang et al., 2013; Wang and Li, 2018; Xu et al., 2019). At the end of the experiment, the corresponding intestinal segments (duodenum, jejunum, and ileum) were dissected and their length (L) and perimeter (s) were measured. Then, 1 ml of outflow perfusate was carefully transferred and concentrated until they were dry by rotary evaporation. Finally, the concentrate was dissolved in 80% methanol solution and the resulting sample solution was filtered through a 0.22-µm filter before being analyzed by HPLC.
All HPLC analyses were performed on a Waters 2695 HPLC system (Waters Corporation, Milford, MA, USA). Chromatographic separation was carried out with a Waters Sunfire C18 column (5 µm, 4.6 × 250 mm). Elution was carried out by a gradient with a mobile phase consisting of methanol (C) and 0.05% formic acid in water (D) at a flow rate of 1 ml/min. The gradient elution was conducted as follows: 5% C (0.0–10.0 min), 12% C (10.0–24.0 min), 19% C (24.0–37.0 min), 36.6% C (37.0–47.0 min), 40% C (47.0–67.0 min), 36.6% C (67.0–70.0 min), and 70% C (37.0–47.0 min). The column temperature was maintained at 35°C throughout the entire process. The sample size for injection into the HPLC system was set at 20 µl. The detection wavelength was set to 298 nm.
Analytical standards for quercetin (1.78 mg) and vanillic acid (3.04 mg) were finely weighed into separate volumetric flasks. These were then adjusted to 10 ml by the addition of 80% methanol. The resultant solutions were shaken up-and-down until well mixed.
A stock solution of mixed standards was prepared by precisely aliquoting 3 ml of each of the quercetin and vanillic acid standard into an 10 ml Ep tube and shaking up-and-down until the solution was mixed well. A range of other standards were prepared by diluting this stock solution in the following proportions: 1, ½, ¼,1/8, 1/16, and 1/32. All solutions (20 µl in volume) were then filtered through 0.22-µm filters and analyzed by HPLC.
A stock solution of mixed standards of vanillic acid (600 μl) and quercetin (600 μl) was injected 6 times continuously according to the chromatographic conditions under “2.7”. The peak areas of vanillic acid and quercetin were recorded and the relative standard deviation (RSD) values of vanillic acid and quercetin were calculated.
Krebs-Ringer’s (K-R) nutrient solution was mixed with the medication solution and allowed to pass through the duodenum, jejunum, and ileum of experimental rats. A blank K-R nutrient solution was also passed through these anatomical structures. Following passage through the intestines of the experimental animals, the test and blank solutions were treated as described in In Situ Single-Pass Intestinal Perfusion. These samples were then analyzed by HPLC as described in Conditions for HPLC Analysis.
All data are presented as a mean ± standard deviation (SD). The effective permeability coefficient (Peff), as determined by gravimetry, was calculated using Equation (1) in which Vin and Vout are the intestinal perfusate input and output volumes (ml); these were calculated by gravimetric methods, assuming that the density of the perfusion solution at the inlet and outlet was the same. Q represents the perfusion rate (0.2 ml/min), Cin and Cout represent the mass concentrations of the enteric importer and exporter perfusate (μM/L), and 2πrL is the area of the mass transfer surface (cm2).
Standard curves for quercetin and vanillic acid were performed for peak area (Y) and the quantity of standard solution injected (X), respectively:
The linearities for quercetin and vanillic acid in the working standard solutions (0.0028–0.0890 mg/ml, 0.0048–0.1520 mg/ml) was good, as demonstrated by the fact that their correlation coefficients (r) exceeded 0.9999.
The RSD values for peak area response were 0.31% (vanillic acid) and 0.17% (quercetin). These figures indicated that the HPLC method showed very good levels of precision for our analysis under the specified conditions.
Chromatograms showed that the blank K-R nutrient solution that had passed through the duodenum, jejunum, and ileum did not interfere with the chromatographic peaks of the tested samples (Figure 2).
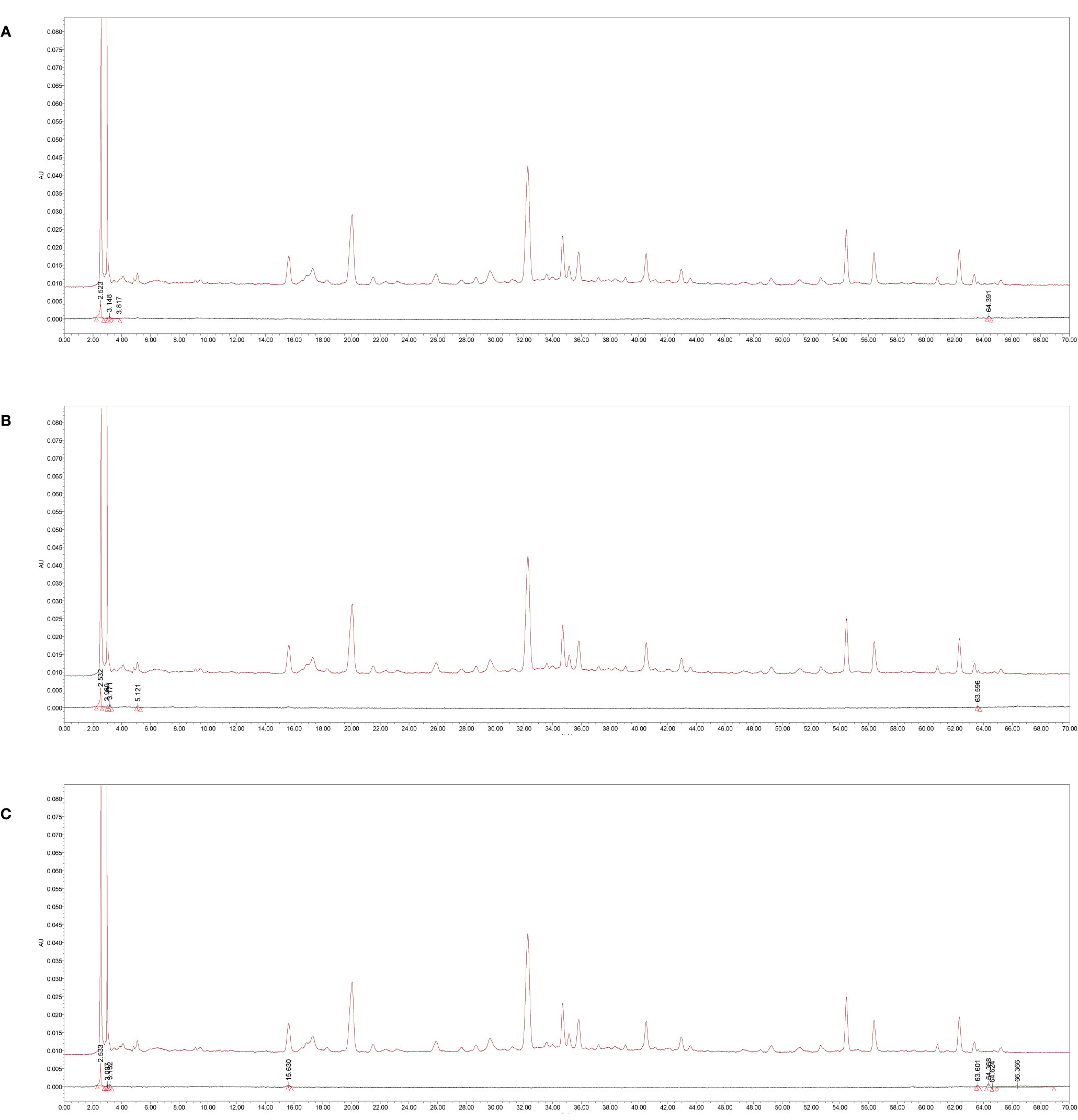
Figure 2 The specificity high-performance liquid chromatography (HPLC) chromatograms of absorption samples of water extraction from Amomi fructus in single-pass intestinal perfusion (SPIP). (A) Blank Krebs-Ringer’s (K-R) nutrient solution and K-R nutrient solution mixed with medicine solution via duodenum, (B) blank K-R nutrient solution and K-R nutrient solution mixed with medicine solution via jejunum, (C) blank K-R nutrient solution and K-R nutrient solution mixed with medicine solution via ileum.
We wanted to investigate the metabolic conditions of the K-R solution containing water extraction from A. fructus in different intestinal segments within 3 h of passage. In order to do this, we first needed to produce HPLC chromatograms of the water extraction of A. fructus. As shown in Figure 3, we observed 19 characteristic peaks in the HPLC chromatograms produced by A. fructus water extractions. A total of six compounds (C4, C7, C8, C11, C13, and C14) were identified on the HPLC chromatograms by the use of analytical standard solutions (Figure 4): these compounds were identified as catechin, vanillic acid, epicatechin, polygonin, isoquercitrin, and quercitrin, respectively.
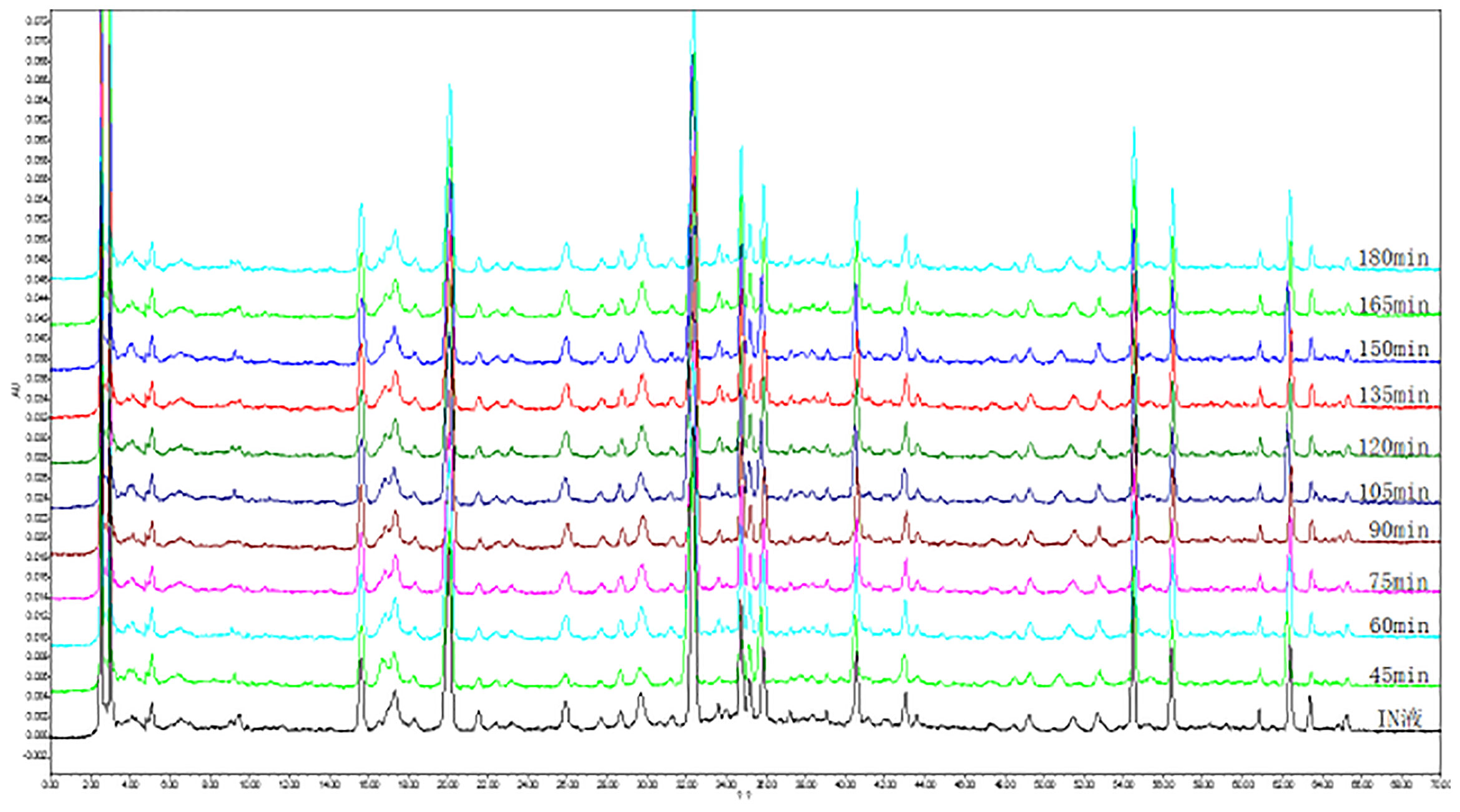
Figure 3 High-performance liquid chromatography (HPLC) chromatogram of the water extraction of A. fructus at different time via jejunum.
First, we used standard curves to calculate the concentrations of quercetin and vanillic acid. The contents of the other unknown components were calculated by using the concentration of vanillic acid as a reference. In this study, we used the weight analysis method to correct the inflow and outflow volume of the perfusion solution and eliminate the influence of volume change; we then calculated the Peff of each component over 180 min (Table 1).
The principle of single-pass intestinal perfusion is that the measured reduction in drug concentration is equal to amount that has been absorbed. Generally, absorption performance can be classified into three groups by calculating Peff. When Peff < 3×10-6 cm/s the drug absorption is said to be poor, while a Peff > 2×10-5 cm/s indicates good drug absorption; between these two values, drug absorption is said to be moderate (Fagerholm et al., 1996). Based on these criteria, we found that the 19 components in A. fructus water extraction showed different absorption capabilities in different segments of the intestine (Figure 5). Components Cl, C2, C3, C4, C5, C6, C7, C8, C11, C12, C13, C14, C16, C17, C18, and C19 showed strong absorption in the duodenum. Component C10 showed moderate absorption in the duodenum, while components C9 and C15 showed poor absorption in the duodenum. The absorption characteristics of the 19 components in the jejunum are shown in Table 1; components Cl, C2, C3, C5, C6, C7, C8, C11, C12, C13, C16, and C17 showed strong absorption, while C4 and C14 showed moderate absorption. It is worth highlighting that negative Peff values were obtained for C9, C10, C15, C18, and C19, suggesting that these five components were enriched in the jejunum. We also discovered that all of the 19 components could be absorbed in the ileum, except for the C10 component which showed moderate absorption. Other components showed absorption in the ileum. Generally, most components were absorbed in the ileum; this was followed by the duodenum and then the jejunum. The absorption capacity of the three intestinal segments to the C3, C5, C7, C11, and C17 components was highest in the duodenum, followed by the jejunum and then the ileum. The absorption capacity of the C2, C4, C10, C13, C14, C16, C18, and C19 components was highest in ileum, followed by the duodenum and then the jejunum. The absorption capacity of the C1 and C8 components was highest in the duodenum, followed by the ileum and the jejunum while the absorption capacity of the C6 component was strongest in the jejunum, followed by the ileum and then the duodenum. Finally, the absorption capacity for component C12 was highest in the jejunum, followed by the duodenum and the ileum. Components C9 and C15 were absorbed only in the duodenum; absorption as strong in in this segment for these components.
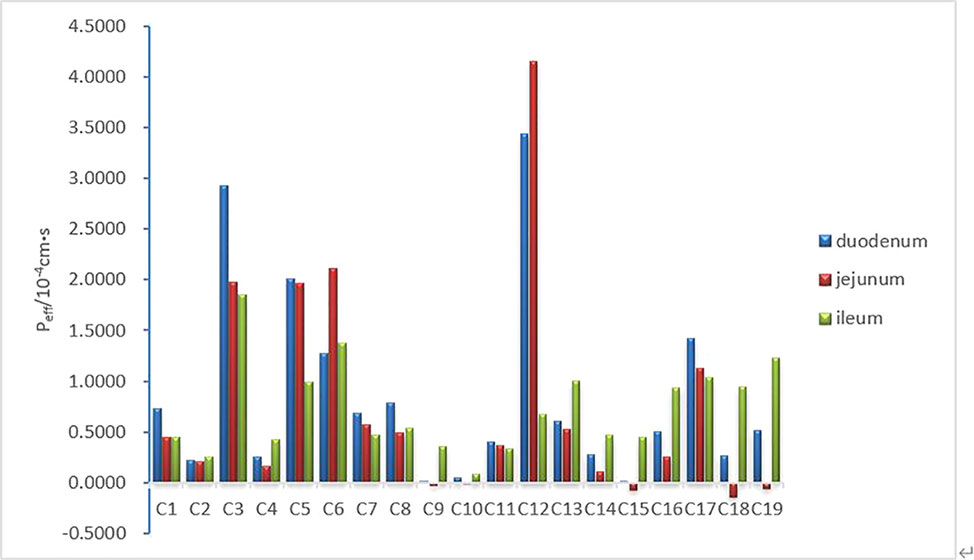
Figure 5 Compare the Peff of 19 components obtained from single-pass intestinal perfusion (SPIP) perfusion.
A. fructus is used as a common medicine in TCM and also as a supplement, dietary agent, food additive, and spice in China and other Southeast Asian countries, such as Thailand, Vietnam, Burma, and Indonesia. A. fructus is generally boiled in water; the decoction in TCM arose from the Tang Dynasty over 1,300 years ago. This highlights the fact that A. fructus contains nonvolatile components that can be dissolved in water and absorbed through the gastrointestinal tract in order to exert their medicinal effect. A number of studies have proven that the water extraction from A. fructus exerts beneficial effects in the treatment of inflammation, cancer, and is also able to maintain the balance of the intestinal microecology (Nagare et al., 2010; Choi et al., 2015; Wu et al., 2019). However, previous studies of A. fructus only involved descriptions of volatile components, thus restricting its further development as a medicinal agent. Studies on the in vivo metabolism of A. fructus have not been reported previously. It is also uncertain which of the water-soluble components of A. fructus can be absorbed by the intestine. Therefore, this study aimed to explore the absorption characteristics of the nonvolatile components contained within A. fructus using in situ single-pass perfusion methodology.
Our results revealed there were 19 water-soluble components of A. fructus which showed different levels of absorption in different segments of the intestine. interestingly, components C9, C10, C15, C18, and C19 showed negative Peff values in the jejunum. We hypothesize that there were two reasons for this. Firstly, that some large polar molecules in the A. fructus water extraction had become degraded, such as glycosides, or that the polymers of these five components had been hydrolyzed to form smaller molecular compounds, thus increasing the contents of these components. Secondly, it is possible that the 5 components with negative values had not been metabolized or absorbed by the jejunum.
Most oral preparations are usually absorbed from the small intestine and exert effect before even reaching the blood stream (Kuang et al., 2017). In the pre-experiment, we investigated the stability of components in the water extraction of A. fructus using an artificial gastrointestinal solution. We found that these components were stable in the artificial gastrointestinal solution and did not metabolize or transform; collectively, these findings allowed us to establish the single-pass intestinal perfusion experiment described herein. In addition, our present results showed that quercetin and vanillic acid were present in the water extraction of A. fructus. Vanillic acid and quercetin were also shown to exist in the perfusion solution, as verified by a pre-experiment. The perfusion solution featured relatively high concentrations of these two components. Therefore, this study aimed to determine the concentrations of quercetin and vanillic acid using standard curves. The relative concentrations of the other components in the perfusion solution at the different segments were based on the concentration of vanillic acid.
In the current study, we used an integrated approach based on SPIP and HPLC to identify the absorption characteristics of nonvolatile components in A. fructus. We successfully identified six chemical constituents using this approach. Collectively, our data indicate that the use of SPIP in a rat model provided a good estimation of the effective permeability and site of intestinal absorption for the nonvolatile components in A. fructus. Our results provide further insight into the therapeutic composition and absorption characteristics of A. fructus and provide a foundation from which to perform research and development to promote its wider clinical application.
All datasets generated for this study are included in the article/supplementary material.
Animal experiments were conducted in accordance with the guidelines for animal experiments. The animal study was reviewed and approved the Animal Ethics Committee, Beijing University of Chinese Medicine.
YuY analyzed the data and prepared the paper. WM and GC carried out the pharmacological experiments. WM arranged all of the experiments. RY conducted physical and chemical analyses. HC processed the samples. YL designed the study and provided theoretical guidance. HZ revised the manuscript and read and approved the final manuscript. YoY administered the project.
This study was financially supported by the National Natural Science Foundation of China (Grants nos. 81573542, and 81403054) and Beijing University of Chinese Medicine (Grants nos. 2019-JYB-JS-006).
WM was employed by company Yangtze River Pharmaceutical Group Jiangsu Longfeng Tang Traditional Chinese Medicine Co., Ltd. RY was employed by company Beijing Increase Innovative Medicine Co., Ltd. HC was employed by company Beijing Highthink Pharmaceutical Technology Service Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ao, H., Wang, J., Chen, L., Li, S., Dai, C. (2019). Comparison of Volatile Oil between the Fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules 24 (9), 1663. doi: 10.3390/molecules24091663
Chen, Z., Ni, W., Yang, C., Zhang, T., Lu, S., Zhao, R., et al. (2018). Therapeutic Effect of Amomum villosum on Inflammatory Bowel Disease in Rats. Front. Pharmacol. 9, 639. doi: 10.3389/fphar.2018.00639
Choi, H. G., Je, I. G., Kim, G. J., Choi, H., Kim, S. H., Kim, J. A., et al. (2015). Anti-allergic inflammatory activities of compounds of amomi fructus. Nat. Prod. Commun. 10 (4), 631–632. doi: 10.1177/1934578X1501000425
Commission of Chinese Pharmacopoeia (2015). Chinese Pharmacopoeia, China Medico-Pharmaceutical, Vol. 1 (Beijing: Science & Technology Publishing House).
Fagerholm, U., Jahansson, M., Lennernas, H. (1996). Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 13 (9), 1336. doi: 10.1023/a:1016065715308
Huang, Y. L., Yen, G. C., Sheu, F., Lin, J. Y., Chau, C. F. (2007). Dose effects of the food spice cardamom on aspects of hamster gut physiology. Mol. Nutr. Food Res. 51 (5), 602–608. doi: 10.1002/mnfr.200600249
Huo, Y., Huang, Y., Hou, X., Han, L., Wang, L., Liu, E., et al. (2014). Effects of fructus psoraleae extract on the intestinal absorption kinetics of geniposide and geniposidic acid in rat. Molecules 19 (6), 7557–7567. doi: 10.3390/molecules19067557
Jang, S. B., Kim, D., Kim, S. Y., Park, C., Jeong, J. H., Kuh, H. J., et al. (2013). Impact of Micellar Vehicles on in situ Intestinal Absorption Properties of Beta-Lapachone in Rats. Korean J. Physiol. Pharmacol.: Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 17 (1), 9–13. doi: 10.4196/kjpp.2013.17.1.9
Kuang, G., Yi, H., Zhu, M., Zhou, J., Shang, X., Zhao, Z., et al. (2017). Study of Absorption Characteristics of the Total Saponins from Radix Ilicis Pubescentis in an In Situ Single-Pass Intestinal Perfusion (SPIP) Rat Model by Using Ultra Performance Liquid Chromatography (UPLC). Molecules 22 (11), 1867. doi: 10.3390/molecules22111867
Lee, Y. G., Park, J. H., Jeon, E. S., Kim, J. H., Lim, B. K. (2016). Fructus Amomi Cardamomi Extract Inhibit Coxsackievirus-B3 Induced Myocarditis in Murine Myocarditis Model. J. Microbiol. Biotechnol. 26 (11), 2012–2018. doi: 10.4014/jmb.1605.05056
Li, C., Zhang, L., Zhou, L., Wo, S. K., Lin, G., Zuo, Z. (2012). Comparison of intestinal absorption and disposition of structurally similar bioactive flavones in Radix Scutellariae. AAPS J. 14 (1), 23–34. doi: 10.1208/s12248-011-9310-9
Li, S., Wang, Y., Jiang, T., Wang, H., Yang, S., Lv, Z. (2016). Absorption and Transport of Sea Cucumber Saponins from Apostichopus japonicus. Mar. Drugs 14 (6), 114. doi: 10.3390/md14060114
Li, Y., Zhang, B., Liu, M., Zhang, X., Shi, D., Guo, L., et al. (2018). Further Study of Influence of Panax notoginseng on Intestinal Absorption Characteristics of Triptolide and Tripterine in Rats with Tripterygium wilfordii. Pharmacogn. Mag. 14 (53), 95–102. doi: 10.4103/pm.pm_67_17
Lin, Q., Ling, L. Q., Guo, L., Gong, T., Sun, X., Zhang, Z. R. (2015). Intestinal absorption characteristics of imperialine: in vitro and in situ assessments. Acta Pharmacol. Sin. 36 (7), 863–873. doi: 10.1038/aps.2015.27
Merkow, R. P., Ju, M. H., Chung, J. W. (2015). Underlying Reasons Associated With Hospital Readmission Following Surgery in the United States. J. Vasc. Surg. 62 (1), 265. doi: 10.1016/j.jvs.2015.05.023
Nagare, N., Damre, A., Singh, K. S., Mallurwar, S. R., Iyer, S., Naik, A., et al. (2010). Determination of site of absorption of propranolol in rat gut using in situ single-pass intestinal perfusion. Indian J. Pharm. Sci. 72 (5), 625–633. doi: 10.4103/0250-474X.78533
Nusrat, S., Bielefeldt, K. (2013). Gastroparesis on the rise: incidence vs awareness? Neurogastroenterol. Motil. 25 (1), 16–22. doi: 10.1111/j.1365-2982.2012.02002.x
Reddy, K., Patrick, C., Liaquat, H., Rodriquez, E., Stocker, A., Cave, B., et al. (2018). Differences in Referral Access to Care Between Gastrointestinal Subspecialty Patients: Barriers and Opportunities. Health Equity 2 (1), 103–108. doi: 10.1089/heq.2018.0001
Suo, S., Lai, Y., Li, M., Song, Q., Cai, J., Zhao, J., et al. (2018). Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. Int. J. Published Br. Ind. Biol. Res. Assoc. 119, 31–36. doi: 10.1016/j.fct.2018.05.051
Wang, Z., Li, Y. (2018). Raloxifene/SBE-beta-CD Inclusion Complexes Formulated into Nanoparticles with Chitosan to Overcome the Absorption Barrier for Bioavailability Enhancement. Pharmaceutics 10 (3), 76. doi: 10.3390/pharmaceutics10030076
Wang, Y. H., Ke, X. M., Zhang, C. H., Yang, R. P. (2017). Absorption mechanism of three curcumin constituents through in situ intestinal perfusion method. Braz. J. Med. Biol. Res. Rev. Bras. Pesquisas Medicas Miologicas 50 (11), e6353. doi: 10.1590/1414-431X20176353
Wang, C., Zhou, Y., Gong, X., Zheng, L., Li, Y. (2020). In vitro and in situ study on characterization and mechanism of the intestinal absorption of 2,3,5,4′-tetrahydroxy-stilbene-2-O-beta-D-glucoside. BMC Pharmacol. Toxicol. 21 (1), 7. doi: 10.1186/s40360-020-0384-9
Wu, L., Zhao, L., Su, X., Zhang, P., Ling, G. (2019). Repaglinide-loaded nanostructured lipid carriers with different particle sizes for improving oral absorption: preparation, characterization, pharmacokinetics, and in situ intestinal perfusion. Drug Delivery 26 (1), 1–10. doi: 10.1080/10717544.2019.1689313
Xu, D., Lin, Y., Bauer, R., Chen, H. R., Yang, R. Q., Zou, H. Q., et al. (2018). Organoleptic Evaluation of Amomi Fructus and Its Further Background Verified via Morphological Measurement and GC Coupled with E-Nose. Evid, Based Complement. Alternat. Med. 2018, 4689767. doi: 10.1155/2018/4689767
Xu, Y., Wu, S., Wu, Y., Gong, M., Wang, Z. (2019). Recognition and Optimization of Ingredients Treating Nitroglycerin-Induced Migraine Rats from Wuzhuyu Decoction. Evidence-Based Complement. Altern. Med.: eCAM 2019, 6156754. doi: 10.1155/2019/6156754
Xue, X., Zhao, Z., Li, Q., Wang, D., Xu, X., Zhu, L., et al. (2016). Determination of Flavonoids by Solidification of Floating Organic Drop Liquid-Phase Microextraction and High-Performance Liquid Chromatography. Anal. Lett. 49 (15), 2384–2396. doi: 10.1080/00032719.2016.1149859
Yerasi, N., Vurimindi, H., Devarakonda, K. (2015). Frog intestinal perfusion to evaluate drug permeability: application to p-gp and cyp3a4 substrates. Front. Pharmacol. 6, 141. doi: 10.3389/fphar.2015.00141
Zhang, X., Cheng, X., Wu, Y., Feng, D., Qian, Y., Chen, L., et al. (2019). In Vitro and In Situ Characterization of the Intestinal Absorption of Capilliposide B and Capilliposide C from Lysimachia capillipes Hemsl. Molecules 24 (7), 1227. doi: 10.3390/molecules24071227
Keywords: Amomi fructus, gastrointestinal diseases, water extraction, in situ single-pass intestinal perfusion, high-performance liquid chromatography
Citation: Yao Y, Mi W, Cao G, Yang R, Chen H, Liu Y, Zou H and Yan Y (2020) The Absorption Characteristics of Nonvolatile Components in a Water Extraction From Amomi fructus as Determined by In Situ Single-Pass Intestinal Perfusion and High-Performance Liquid Chromatography. Front. Pharmacol. 11:711. doi: 10.3389/fphar.2020.00711
Received: 21 February 2020; Accepted: 30 April 2020;
Published: 05 June 2020.
Edited by:
Sabina Passamonti, University of Trieste, ItalyReviewed by:
Tao Feng, Shanghai Institute of Technology, ChinaCopyright © 2020 Yao, Mi, Cao, Yang, Chen, Liu, Zou and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqin Zou, em91aHVpcWluX2J1Y21Ac2luYS5jbg==; Yonghong Yan, bHhkeXloQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.