
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
RESEARCH SNAPSHOT article
Front. Pharmacol., 12 February 2018
Sec. Experimental Pharmacology and Drug Discovery
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00098
A commentary has been posted on this article:
Commentary: Usage of Mitogen-Activated Protein Kinase Small Molecule Inhibitors: More Than Just Inhibition!
We have identified a phenomenon occurring in the usage of proposed “specific” Mitogen-activated protein kinase (MAPK) inhibitors. We found that especially inhibitors of p38 potentiate the activation of other MAPKs in various cell types. This finding will have tremendous impact on the interpretation of all former studies using MAPK inhibitors.
Most of the Mitogen-activated protein kinase (MAPK) inhibitors have highly different structures (Figure 1A). The p38 MAP kinase inhibitors SB203580 and SB242235 (Lee et al., 1994; Ward et al., 2002) as well as SP600125 targeting JNK1, JNK2, and JNK3 (Bennett et al., 2001) are commonly used. In addition, the MEK inhibitors UO126 selective for MEK1 and MEK2 (Favata et al., 1998), and PD98059 primarily targeting MEK1 and MEK2 with a more than 10-fold lower affinity (Dudley et al., 1995) are established compounds which have been tested extensively (Davies et al., 2000; Bain et al., 2003). In hepatology, these inhibitors have significantly contributed to the knowledge in the field in which MAPKs contribute to inflammation, fibrogenesis, and hepatocellular carcinoma (Borkham-Kamphorst and Weiskirchen, 2016).
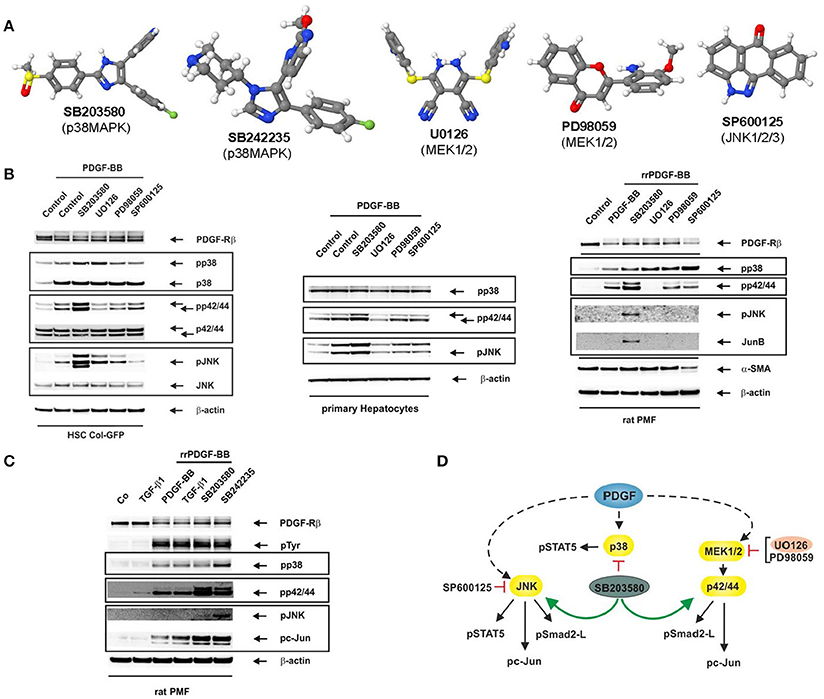
Figure 1. Reciprocal activation of MAPK signalling by MAPK inhibitors. (A) Images of inhibitors used in this study were generated with software Jmol (version 14.2.15). (B) The reporter cell line HSC Col-GFP (left), primary hepatocytes (middle) and (activated) PMF (right) were stimulated for 10 min with PDGF-BB (25 ng/ml) after pre-incubation of cells with the indicated inhibitors (each 10 μM) for 1 h. Thereafter, proteins were extracted and subjected to Western blot analysis with the depicted antibodies. The inhibition of MAP kinases impacts PDGF responses as PD98059 and UO126 reduce pp42/44 phosphorylation. In addition, SP600125 blunts c-Jun activation, while SB203580 and SB242235 reduce STAT5 phosphorylation (data not shown). (C) Rat PMF were stimulated for 10 min with TGF-β1 (1 ng/ml) or PDGF-BB (25 ng/ml) after pre-incubation of cells with the depicted p38 inhibitors (each 10 μM) for 1 h. (D) Deduced impact of inhibitors on MAP kinase activity in cultured HSC Col-GFP. Antibodies used are from Santa Cruz (PDGF-Rβ, sc-432), Cell Signaling (pp42/pp44, CS-9101; p42/p44, CS-4696; pSAPK/JNK, CS-9251; SAPK/JNK, CS-9252; pc-Jun, CS-9261; JunB, CS-3753), BD Biosciences (pp38, 612288; p38, 612168), Millipore (pTyr, 05-321), Cymbus Biotechnology (α-SMA, CBL 171), and Sigma (β-actin, A5441), respectively. In this scheme, PDGF stands for PDGF-BB.
PDGF-BB is a potent mitogen for hepatic stellate cells (HSC) (Borkham-Kamphorst and Weiskirchen, 2016), and stimulation of HSC Col-GFP with PDGF-BB leads to activation of the three major MAP kinases (Figure 1B). As expected, the pre-treatment of cells with the MEK1/MEK2 inhibitors resulted in a direct reduction in ERK1/ERK2 MAPK phosphorylation, while SB203580 and SP600125 blunted MAPK activity as demonstrated by a reduction in substrate phosphorylation of STAT5 (p38, JNK) and c-Jun (JNK) (not shown).
Unexpectedly, blockade of p38 by SB203580 resulted in a significant increase in both ERK1/ERK2 and JNK phosphorylation. Likewise, the MEK1/2 inhibitors UO126 and PD98059 provoked increased phosphorylation of JNK and p38 (only UO126). Most sensitive to the application of small-molecule inhibitors was JNK that became activated by inhibitors targeting the p38 (SB203580) or ERK1/2 pathways. These results suggest that blocking of a MAP kinase by the corresponding inhibitor leads to a simultaneous activation of other MAPK-pathways driven by the same ligand. We found similar results in primary hepatocytes and primary (activated) portal myofibroblasts (PMF). In particular, these experiments revealed a strong stimulation of JNK and ERK phosphorylation in the presence of the p38 inhibitor SB203580. Moreover, the mutual “induction by inhibition” is also evident in PMF when the alternative p38 inhibitor SB242235 is used indicating that the finding is not an artefact of an individual inhibitor (Figure 1C). All experiments were highly reproducible (Supplementary Figure 1). In addition, we could show that not only MAPK phosphorylation itself but also substrate phosphorylation is increased which demonstrates a higher activity of non-targeted MAPKs (Supplementary Figure 2).
Isolation of primary cells (hepatocytes, PMF) and establishment of cell line HSC Col-GFP were done as described previously (Meurer et al., 2011, 2013; Borkham-Kamphorst et al., 2016). SDS-PAGE and Western blot analysis were done as reported (Borkham-Kamphorst et al., 2016).
The observation that a mutually “selective” MAPK-inhibitor becomes an activator of another MAPK-pathway physiologically stimulated by the same trigger has fundamental impact. Numerous reports have more or less uncritically applied MAPK inhibitors and concluded that a pathway targeted by a “specific” inhibitor is responsible for a biological effect. However, considering effects provoked by reciprocal activation loops challenge some of these studies. In our experimental setting, the influence of different small-molecule inhibitors resulted in dependencies depicted in Figure 1D.
It is obvious that the mutual “activation by inhibition” is not limited to straight forward MAPK-signaling network. Although we don't know if the phenomenon of cross-activation can be generalized when blocking one pathway, we think our observations must be critically kept in mind when interpreting experimental results mediated by a “specific” inhibitor.
Potential mechanisms of MAPK crosstalk and regulation by dual-specificity phosphatases under different conditions are discussed elsewhere (Birkenkamp et al., 2000; Shen et al., 2003; Junttila et al., 2008; Ríos et al., 2014).
This study was carried out in accordance with the recommendation of the Landesamt für Umwelt und Naturschutz (LANUV, Recklinghausen, Germany). The protocols for isolation of primary cells were approved by the LANUV.
RW and SM designed the study and drafted manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
RW is financially supported by the German Research Foundation (projects SFB/TRR57 P13 and Q3) and the Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital Aachen (Project O3-1).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00098/full#supplementary-material
Bain, J., McLauchlan, H., Elliott, M., and Cohen, P. (2003). The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204. doi: 10.1042/bj20021535
Bennett, B. L., Sasaki, D. T., Murray, B. W., O'Leary, E. C., Sakata, S. T., Xu, W., et al. (2001). SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686. doi: 10.1073/pnas.251194298
Birkenkamp, K. U., Tuyt, L. M., Lummen, C., Wierenga, A. T., Kruijer, W., and Vellenga, E. (2000). The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br. J. Pharmacol. 131, 99–107. doi: 10.1038/sj.bjp.0703534
Borkham-Kamphorst, E., Steffen, B. T., Van de Leur, E., Tihaa, L., Haas, U., Woitok, M. M., et al. (2016). Adenoviral CCN gene transfers induce in vitro and in vivo endoplasmic reticulum stress and unfolded protein response. Biochim. Biophys. Acta. 1863, 2604–2612. doi: 10.1016/j.bbamcr.2016.07.006
Borkham-Kamphorst, E., and Weiskirchen, R. (2016). The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 28, 53–61. doi: 10.1016/j.cytogfr.2015.10.002.
Davies, S. P., Reddy, H., Caivano, M., and Cohen, P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 351, 95–105. doi: 10.1042/bj3510095
Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995). A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 92, 7686–7689. doi: 10.1073/pnas.92.17.7686
Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632. doi: 10.1074/jbc.273.29.18623
Junttila, M. R., Li, S. P., and Westermarck, J. (2008). Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 22, 954–965. doi: 10.1096/fj.06-7859rev
Lee, J. C., Laydon, J. T., McDonnell, P. C., Gallagher, T. F., Kumar, S., Green, D., et al. (1994). A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746. doi: 10.1038/372739a0
Meurer, S. K., Alsamman, M., Sahin, H., Wasmuth, H. E., Kisseleva, T., Brenner, D. A., et al. (2013). Overexpression of endoglin modulates TGF-β1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS ONE 8:e56116. doi: 10.1371/journal.pone.0056116
Meurer, S. K., Tihaa, L., Borkham-Kamphorst, E., and Weiskirchen, R. (2011). Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell. Signal. 23, 683–699. doi: 10.1016/j.cellsig.2010.12.002
Ríos, P., Nunes-Xavier, C. E., Tabernero, L., Köhn, M., and Pulido, R. (2014). Dual-specificity phosphatases as molecular targets for inhibition in human disease. Antioxid. Redox Signal. 20, 2251–2273. doi: 10.1089/ars.2013.5709
Shen, Y. H., Godlewski, J., Zhu, J., Sathyanarayana, P., Leaner, V., Birrer, M. J., et al. (2003). Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J. Biol. Chem. 278, 26715–26721. doi: 10.1074/jbc.M303264200
Keywords: inhibitors, signal transduction, PDGF-BB, mitogen-activated protein kinases, SB203580, SP600125, PD98059, UO126
Citation: Meurer SK and Weiskirchen R (2018) Usage of Mitogen-Activated Protein Kinase Small Molecule Inhibitors: More Than Just Inhibition! Front. Pharmacol. 9:98. doi: 10.3389/fphar.2018.00098
Received: 24 November 2017; Accepted: 29 January 2018;
Published: 12 February 2018.
Edited by:
Dagmar Meyer zu Heringdorf, Goethe University Frankfurt, GermanyReviewed by:
Michael Kracht, Justus Liebig Universität Gießen, GermanyCopyright © 2018 Meurer and Weiskirchen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffen K. Meurer, c21ldXJlckB1a2FhY2hlbi5kZQ==
Ralf Weiskirchen, cndlaXNraXJjaGVuQHVrYWFjaGVuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.