
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 17 February 2025
Sec. Neonatology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1550115
 Wenqain Chen1,2
Wenqain Chen1,2 Supasek Kongsomros2
Supasek Kongsomros2 Alexander Thorman2
Alexander Thorman2 Leyla Esfandiari3,4
Leyla Esfandiari3,4 Ardythe L. Morrow2,4,5
Ardythe L. Morrow2,4,5 Somchai Chutipongtanate2,4*
Somchai Chutipongtanate2,4* David S. Newburg2*
David S. Newburg2*
With the continuous improvement in perinatal care, the number of viable preterm infants is gradually increasing, along with the rise in preterm-related diseases such as necrotizing enterocolitis, bronchopulmonary dysplasia, perinatal brain injury, retinopathy of prematurity, and sepsis. Due to the unique pathophysiology of preterm infants, diagnosing and treating these diseases has become particularly challenging, significantly affecting their survival rate and long-term quality of life. Extracellular vesicles (EVs), as key mediators of intercellular communication, play an important regulatory role in the pathophysiology of these diseases. Because of their biological characteristics, EVs could serve as biomarkers and potential therapeutic agents for preterm-related diseases. This review summarizes the biological properties of EVs, their relationship with preterm-related diseases, and their prospects for diagnosis and treatment. EVs face unique challenges and opportunities for clinical applications.
Globally, approximately 15 million premature births occur annually, representing an estimated 11% of all deliveries (1). Advances in perinatal care and neonatal resuscitation techniques have increased the prevalence of preterm births, leading to a rise in associated complications among preterm infants, including necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), perinatal brain injury (PBI), retinopathy of prematurity (ROP), and sepsis (2–5). Over the past decade, these complications have remained a significant cause of neonatal mortality and emerged as a leading cause of death among children under five years old (6). These complications not only profoundly impact the survival rate and long-term quality of life of preterm infants, but also impose psychological stress on families and incur substantial economic costs (7).
Early detection and treatment of these diseases have become urgent priorities to reduce the incidence of preterm birth and enhance the survival quality and long-term prognosis of preterm infants. As these diseases occur in immature and developing organs and involve complex underlying pathophysiological mechanisms, reliable diagnostic tools and therapeutic interventions are currently lacking for many of them.
Extracellular vesicles (EVs) are mediators of intercellular signaling and play regulatory roles in the pathophysiological processes of preterm-related diseases. The lipid bilayer of EVs protects their cargoes from degradation, giving EVs unique characteristics that hold promise as biomarkers for diagnosing preterm infant-related diseases and as therapeutic tools (Figure 1). This review summarizes the biological characteristics of EVs and their relationship with preterm delivery, focuses on the role of EVs in complications associated with preterm infants, and discusses their potential as diagnostic and therapeutic tools. Finally, it highlights key issues that need to be addressed to allow the clinical application of EVs.
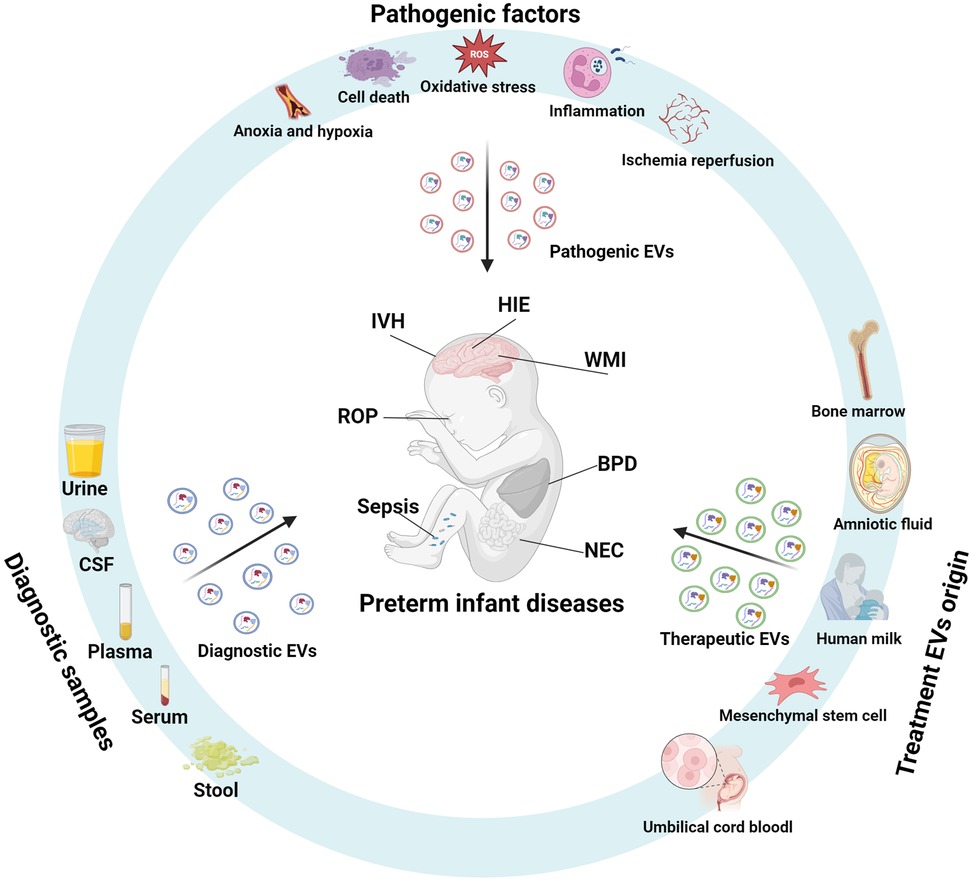
Figure 1. Extracellular vesicles in the pathophysiology, diagnosis, and therapy of preterm-related diseases. During pathological processes such as inflammation, ischemia-hypoxia, cell death, oxidative stress, and ischemia-reperfusion, extracellular vesicles (EVs) act as mediators of intercellular signaling, playing a role in the pathophysiology of preterm-related diseases. These diseases affect the quantity and composition of EV cargo, and analyzing the EVs in body fluids or tissue samples can aid in disease diagnosis. EVs derived from mesenchymal stem cells, breast milk, amniotic fluid, umbilical cord blood, and bone marrow exhibit anti-inflammatory, antioxidant, and cell regeneration-promoting properties, making them promising therapeutic agents for preterm-related diseases. (Created with BioRender.com).
Extracellular vesicles (EVs) are non-replicating, lipid bilayer-bound vesicles released from cells into the extracellular space (8). They are classified into exosomes, microvesicles, and apoptotic bodies based on their biogenesis, cellular origin, and biophysical properties (9). Exosomes, typically 40–150 nm in diameter, originate from the inward budding of the endosomal membrane to form intraluminal vesicles within multivesicular bodies. These intraluminal vesicles are released into the extracellular space as exosomes upon the fusion of multivesicular bodies with the plasma membrane (10, 11). The exosome biogenesis is regulated by the endosomal sorting complex required for transport (ESCRT)-dependent or ESCRT-independent pathways (12), involving specific sorting and packaging of cargo into exosomes (13). Microvesicles, approximately 100–1,000 nm in diameter, bud directly from the plasma membrane, enclosing cytoplasmic contents, and are typically released during cellular stress and activation (10, 11). Apoptotic bodies, with diameters ranging from 500–2000 nm, form during cell apoptosis, characterized by membrane shrinkage and invagination, leading to the packaging of cytoplasmic material, including DNA and organelles (14).
EVs carry diverse cargoes, which vary by cell type and cellular status, affecting their function and fate (15, 16). EVs have pivotal roles in physiology (17), immunology (18), and metabolism (19). EVs function as cell-to-cell messengers by transferring mRNA that, upon entering cells, are translated into specific proteins with unique biological effects (20). Besides mRNA, EVs transport various molecules between cells, including proteins, lipids, DNA, and non-coding RNA, making them vital regulators of cellular communication (20, 21).They are detectable in body fluids such as blood, saliva, and urine, thereby offering a convenient means for disease detection (22). As the cargo of EVs is cell-specific, reflecting their cells of origin (15, 16), EVs can be used as biomarkers for studying specific cell types involved in various diseases. EV concentration can indicate disease progression, with studies achieving high accuracy in distinguishing the severity of bronchopulmonary dysplasia (BPD) based on EV levels (23).
EVs also hold therapeutic promise (24). They carry molecules capable of modifying cell signaling and gene expression, thereby exerting therapeutic effects (25, 26). Compared to traditional drug delivery methods, EVs offer advantages such as enhanced cargo protection and tissue penetration (27). Derived from benign sources, therapeutic EVs are less likely to provoke adverse reactions, which can be further improved by reducing surface proteins (28, 29). Furthermore, they can be engineered for targeted delivery, thereby enhancing their efficacy (30).
As interest in EVs as potential biomarkers and therapeutics grows and EV research has significantly increased, the International Society for Extracellular Vesicles introduced the Minimal information for studies of extracellular vesicles guidelines to standardize protocols and reporting (8, 31). These guidelines cover nomenclature, separation techniques, characterization, functional studies, and sample collection. However, they discourage using exosomes or microvesicles unless their subcellular origin is confirmed but recommend using EV with terms based on size, density, molecular composition, or cellular origin (8).
From a clinical viewpoint, there are three major roles of EVs in preterm infant diseases: pathogenic EVs, diagnostic EVs, and therapeutic EVs. Pathogenic EVs typically originate from damaged cells or diseased tissues and are enriched with pro-inflammatory factors and damage-related molecules, directly contributing to disease progression (32, 33). Diagnostic EVs, derived from body fluids, can be obtained non-invasively and carry disease-specific biomarkers, making them suitable for early diagnosis and real-time monitoring (34, 35). Therapeutic EVs generally come from stem cells or plant/animal extracts and are modified to enhance their drug or gene delivery capabilities, intervening in pathological processes and promoting tissue repair (36, 37). In terms of composition and function, pathogenic EVs carry inflammatory factors and pathological mediators that drive disease progression, while diagnostic EVs contain highly specific biomarkers that aid in disease detection (38, 39). Therapeutic EVs deliver drugs, RNA, or targeted molecules for therapeutic intervention (36). While these three types of EVs differ in their origin, composition and function, they all demonstrate significant clinical translational potential and complement each other, advancing the application of extracellular vesicles in neonatal disease research and therapy.
Childbirth represents a complex interplay between the fetus and the mother, where factors such as fetal endocrine signals, maternal endocrine signals, other signaling, and immune changes play crucial roles in maintaining pregnancy (40, 41). Disruptions in the balance of endocrine and immune systems can lead to an overload of inflammation, ultimately culminating in spontaneous preterm birth (42). This process shares similarities with full-term delivery, involving heightened uterine contractions, cervical dilation, and rupture of fetal membranes, all triggered by a transition in the uterine muscle layer from a quiescent state to intermittent contractions (43). Progesterone plays a key role in inhibiting the expression of pro-inflammatory factors to maintain the quiescent state of the uterine muscle layer (44). Fetal inflammatory signals can lead to functional progesterone withdrawal, increased intrauterine inflammatory factors, immune cell activation, disruption of maternal inflammatory balance, and ultimately preterm delivery (42).
EVs are significant players in the pathophysiological processes of spontaneous preterm birth. In a mouse model, EVs carrying inflammatory mediators increase gradually from day 5–19 of pregnancy. Late pregnancy EVs, when injected into mice at day 15 of pregnancy, induce preterm birth and related inflammation (45). This suggests that EVs regulate parturition through paracrine signaling. Menon et al. (46) discovered decreased placental-derived EVs in maternal plasma of preterm birth compared to term birth mothers, with significant differences in protein composition associated with inflammation, epithelial-mesenchymal transition, coagulation/complement activation, and cell death. Another study compared content in maternal plasma between different preterm birth causes, revealing variations in total circulating EV protein mainly related to inflammation and metabolic signaling (47). Gray et al. (48) observed dysregulation of circulating miRNAs in plasma of spontaneous preterm birth compared to normal pregnancies. Analysis of EV miRNA characteristics between term and preterm deliveries identified differences in miRNAs targeting signaling pathways such as TGF-β, p53, and glucocorticoid receptor signaling, implicating circulating EV miRNAs in preterm birth mechanisms (49). McElrath et al. (50) explored the potential of EVs isolated from maternal plasma in the first trimester of singleton pregnancies as biomarkers for spontaneous preterm birth before 35 weeks, identifying 5 EV proteins as predictive markers with promising diagnostic performance. Zhao et al. (51) analyzed EV lipids in maternal plasma during mid-pregnancy, identifying microvesicle phosphatidyl serine (34:0) as a potential predictor for preterm birth.
EVs also hold therapeutic potential in spontaneous premature birth research. Sheller-Miller et al. (52) designed EVs containing NF-κB inhibitors, demonstrating their ability to prolong gestation and reduce maternal inflammation, suggesting EVs could serve as stable and specific interventions to mitigate inflammation associated with preterm birth.
Necrotizing enterocolitis (NEC) poses a significant threat to preterm infants, representing a common and often life-threatening gastrointestinal emergency. Global incidence rates have seen a troubling increase over the past decade, particularly among premature infants weighing less than 1,000 grams at birth (53). Due to the challenges in early diagnosis and the lack of effective treatments, NEC often progresses rapidly, with mortality rates estimated around 25% and reaching up to 80% in severe cases of fulminant NEC (54).
The pathogenesis of NEC is multifaceted, closely intertwined with intestinal epithelial damage, mucosal repair mechanisms, and inflammatory responses; each can be regulated by EVs. EVs derived from intestinal epithelial cells activate wound repair pathways (55) and contribute to maintaining intestinal immune balance (38). Post-injury, intestinal epithelial cells release EVs into the mesenteric lymph, and these EVs have immunomodulatory effects that suppress post-injury inflammatory signaling and NEC progression (33, 38). Additionally, polymorphonuclear neutrophils release EVs during NEC, triggering acute remodeling of epithelial junctions, enhancing neutrophil recruitment, and exacerbating epithelial damage (56). Moreover, adherent-invasive Escherichia coli (AIEC) infection can boost EV secretion from intestinal epithelial cells, with these EVs promoting AIEC replication and inducing pro-inflammatory responses (57). This evidence underscores the influence of EVs on NEC occurrence and development through intercellular communication.
Early diagnosis of NEC is paramount for reducing morbidity and mortality rates, yet reliable biomarkers for early diagnosis remain elusive (58). EVs have potential for biomarkers for early NEC diagnosis (Table 1). Significant changes in urinary EV-derived miRNA (including miR-376a, miR-518a-3p, and miR-604) in NEC cases relative to non-NEC sepsis and healthy controls suggest urinary EV-miRNA as potential specific biomarkers for NEC (35).
EVs also offer promise as a novel therapeutic approach for NEC (Table 2). EVs derived from bone marrow mesenchymal stem cells (MSC) have shown potential in restoring intestinal barrier function, akin to bone marrow mesenchymal stem cells infusion alone, indicating their potential as cell-free therapy for neonatal NEC (59). MSC-EVs containing miRNAs specific to Snail/Claudin signaling pathways have induced improvements in intestinal barrier function (36). EVs from other stem cell sources, such as amniotic fluid-derived MSC, amniotic fluid-derived neural stem cells, bone marrow-derived MSC, and neonatal intestinal neural stem cells, have similarly exhibited therapeutic effects in reducing experimentally induced NEC incidence (60).
Human milk-derived EVs (HMEVs) have potential to prevent NEC in premature infants (37). HMEVs contribute to intestinal development, maintain barrier function, and offer protective effects against NEC. HMEVs have been shown to enhance cell migration, protect against oxidative stress-induced damage, and promote intestinal stem cell survival through various signaling pathways, thereby preventing and treating NEC (61–63). Additionally, HMEVs contain miRNAs and other bioactive molecules that regulate immune responses and inflammation, further mitigating NEC severity (64, 65). Proteomic analysis has identified lactoferrin as a key cargo of HMEVs with protective properties against NEC (66). Furthermore, the omega-3 fatty acids present in HMEVs contribute to intestinal epithelial reformation, fibrosis alleviation, and immune response regulation (67).
Bronchopulmonary dysplasia (BPD) is a multifactorial chronic lung disease commonly associated with prematurity and a leading cause of respiratory disease-related mortality in premature infants (68). The global incidence of BPD is estimated to range from 11% to 50% (69). With advances in perinatal medicine, the survival rate of extremely premature infants has notably increased, with a parallel increase in BPD incidence (70–72). Long-term complications that follow preterm BPD, such as neurodevelopmental impairment and cardiopulmonary dysfunction, result in a significant social burden (73).
BPD is associated with abnormal prenatal repair and repetitive postnatal lung injuries, characterized by pulmonary airway and vascular system inflammation and destruction, leading to alveolar simplification, pulmonary fibrosis, and pulmonary hypertension (69). EVs are implicated in these pathological processes. Genschmer et al. (32) demonstrated that EVs derived from infants with severe BPD could induce lung parenchymal simplification, increased airway resistance, and right ventricular hypertrophy in newborn mice, whereas those from non-BPD infants did not cause lung injury. Premature infants, due to incomplete lung development and inadequate surfactant production, often require high-concentration oxygen therapy and mechanical ventilation, both of which contribute to lung injury (74). EVs isolated from rats exposed to high oxygen levels exacerbate lung injury associated with BPD, and concentrations of EV particles are elevated in tracheal aspirates of infants with severe BPD, suggesting a role of EVs in BPD pathogenesis (75).
EVs carry specific proteins or RNA molecules relevant to lung diseases and can serve as biomarkers for predicting BPD (Table 1). Lal et al. (23) identified EV-derived miR876-3p as a potential biomarker for severe BPD in premature infants, with reduced expression at birth predicting future development of severe BPD. Likewise, increases in EV specific surface proteins (CD24 and CD14) during lung development are associated with elevated BPD risk (76). Serum EV-miRNA-21 was upregulated in premature infants with BPD, suggesting its potential as an early biomarker for BPD development (34).
Considerable research has explored the use of EVs in BPD treatment (Table 2). Among various delivery methods, intratracheal administration is considered the preferred approach for treating severe lung diseases due to its ability to directly target the affected area, provide high local drug delivery, and minimize systemic toxicity. Additionally, it offers a needle-free route with rapid onset, low metabolism, and high bioavailability (77). This method is already widely used in clinical treatments for lung diseases in preterm infants (78). Intratracheal administration of MSC-EVs improves lung function, promotes vascularization, and reduces inflammation in BPD animal models (79, 80). This therapeutic effect is associated with changes in molecular pathways, such as PTEN/Akt and miRNAs, involved in BPD pathogenesis (81–84).
Moreover, human milk feeding has been linked to a reduced incidence of BPD in infants, with HMEVs playing a protective role in lung epithelial cells in rats (85–87). Circulating RNA molecules, such as circDNAJB6 and circABPD1 derived from HMEVs, have shown potential in alleviating BPD pathology (88, 89).
Premature infants face a heightened risk of perinatal brain injury, with the likelihood of long-term neurological impairment increasing as gestational age decreases, reaching a lifetime disability rate of up to 5.2% among extremely premature infants (90). The pathophysiological mechanisms underlying brain injury in premature infants are multifaceted, involving prenatal factors such as intrauterine infections and chorioamnionitis, perinatal factors such as birth asphyxia, and postnatal factors including hemorrhage, infection, and mechanical ventilation. EVs serving as intercellular messengers significantly influence the pathophysiology, diagnosis, and treatment of perinatal brain injury in premature infants (Tables 1, 2). The roles of EVs in several types of perinatal brain injury are summarized as follows.
Hypoxic-ischemic encephalopathy (HIE) in newborns is a primary cause of perinatal brain injury, arising from hypoxia-ischemia during the perinatal period, culminating in devastating consequences. In developed nations, HIE's incidence is estimated at 1–6 cases per 1,000 live births, constituting 15%–35% of all neonatal brain disorders (91, 92), with a mortality rate accounting for 23% of global neonatal deaths (93). Therapeutic hypothermia is currently the most effective method for treating HIE. However, even with hypothermia therapy, approximately 30% of survivors endure long-term severe neurodevelopmental disorders, including sensory, cognitive, and neuropsychological deficits (94, 95).
Throughout the pathophysiological cascade of HIE, involving ischemia-hypoxia and subsequent ischemia-reperfusion, neuronal cell damage particularly affects oligodendrocytes. Neuronal EVs likely exert regulatory roles in HIE pathogenesis. Chiang et al. (96) observed significant differences in expression levels of 45 EV-derived miRNAs between normoxic and ischemic/reperfused neuronal models. Functional analysis of these differentially expressed EV-miRNAs implicated their involvement in various pathways related to cell survival and death, neuronal signaling, and dendritic growth, underscoring a pivotal role of EVs in HIE pathogenesis (96).
Research on the use of EVs as diagnostic biomarkers for neonatal HIE is limited. Pineles et al. (39) purified central nervous system-derived EVs from serum of term and near-term infants treated with hypothermia. The protein levels of EVs at different time points significantly correlated with the severity of HIE, with decreased levels of synaptic proteins between 0 and 12 h after birth and increased levels of lipocalin-2 between 12 and 48 h after birth (39). The negative predictive values for increased synaptic proteins was 70% and decreased lipocalin-2 was 91%, suggesting that the content of central nervous system EVs in peripheral blood can serve as a biomarker for the severity of HIE and response to hypothermia therapy (39).
Currently, the only proven effective therapy for HIE is therapeutic hypothermia, but due to the short treatment window (within 6 h after birth) and unsuitability for premature infants with gestational age <35 weeks (97), researchers are exploring the combined use of EVs to improve HIE treatment and outcomes. In an HIE sheep model, human MSC-EVs ameliorated brain function impairment, reduced seizure frequency and duration, and restored subcortical white matter myelination (98). Intranasally administered EVs derived from immortalized mesenchymal stromal cells mitigate neuronal damage in neonatal HIE by suppressing neuroinflammation and fostering neuroregeneration, thereby attenuating long-term cognitive deficits and behavioral abnormalities (99, 100). These protective effects are mediated through EV modulation of the PI3K/AKT signaling pathway, which inhibits calcium overload and neuronal cell death (101), prevention of HIE-induced blood-brain barrier leakage via targeting the membrane-associated protein A1/formylpeptide receptor axis (102), and immunomodulation (103). Additionally, miRNAs encapsulated within EVs are potent mediators of neuroprotection against HIE-induced neuronal damage. EVs derived from astrocytes deliver miR-124-3p to inhibit abnormal activation of hippocampal immune cells in HIE (104). MiR-410 from bone marrow MSC EVs inhibits neuronal apoptosis induced by HIE (105). EVs containing miR-21a-5p exert anti-inflammatory and anti-apoptotic effects (106). Human MSC-EV cargo miR-let-7-5p has neuroprotective and anti-inflammatory effects; pretreatment with hydrogen sulfide enhances their neuroprotective capabilities (105, 107). Astrocyte-derived EVs containing miR-17-5p alleviate neuronal apoptosis and inflammation in HIE neonatal rats (108). Additionally, miR-93 in MSC-EVs inhibits HIE-induced neuronal damage through the JMJD3-dependent p53/KLF2 signaling axis, while miR-150-3p in neural stem cell-derived EVs protects the central nervous system from ischemia-reperfusion injury (109, 110). EVs derived from neural stem cells promote neuronal survival, inhibit apoptosis, enhance Nrf2 nuclear translocation to counter oxidative stress, and foster axonal growth and angiogenesis (111).
Intraventricular Hemorrhage (IVH) is one of the most common neurological complications in premature infants, occurring in an estimated 25%–30% of VLBW infants (112). The pathophysiology of IVH is related to the inherent fragility of the germinal matrix in premature infants and disruption of cerebral blood flow (113). Increased severity of intraventricular hemorrhage (IVH) increases risk of adverse neurodevelopmental outcomes. The most common complications after IVH are post-hemorrhagic hydrocephalus (PHH) and periventricular leukomalacia (PVL). Analysis of EVs from cerebrospinal fluid (CSF) in patients with PHH found enrichment of miRNAs such as miR-9, miR-17, miR-26a, miR-124, and miR-1911, suggesting that miRNAs in EVs from CSF of IVH patients could be used as biomarkers for predicting PHH (114). Studies have found that EVs also have neuroprotective effects against IVH. Brain-derived neurotrophic factor in MSC-EVs can mitigate IVH-induced neuroinflammation and cell apoptosis, and prevent the progression of post-hemorrhagic hydrocephalus, improving prognosis (115). After intracerebral hemorrhage, MiR-146a-5p in MSC-EVs can inhibit neuronal apoptosis and provide neuroprotection and functional improvement by suppressing the expression of IRAK1 and NFAT5, thus inhibiting inflammation associated with M1 polarization of microglia (116).
Various perinatal insults culminate in focal cystic necrosis and/or diffuse white matter injury (WMI) in the central nervous system, with astrocyte hypertrophy (gliosis), microglial activation, decreased white matter volume, and impaired myelination (117). The incidence of WMI in premature infants is 33%, which increases with decreasing gestational age (118). WMI correlates with adverse cognitive, language, and behavioral outcomes in premature infants (119) and is a major contributor to cerebral palsy (120).
The etiology of WMI in premature infants is multifactorial, with inflammation playing a pivotal role in its pathogenesis (121). Systemic inflammation activates microglia and astrocytes with production of pro-inflammatory mediators disrupting the blood-brain barrier, allowing systemic proinflammatory molecules to further exacerbate brain injury (122). During WMI, EVs from astrocytes enter the peripheral circulation (123), promoting leukocyte migration into the brain by inhibiting peroxisome proliferator-activated receptor α, thereby inducing inflammation in brain. EVs from microglia containing abundant TNF-α can induce reactive astrocyte transformation and demyelination (124).
Fetal central nervous system EVs can traverse the blood-brain barrier and placental barrier to enter the maternal circulation, rendering them potential early biomarkers for perinatal brain injury (125). After ethanol exposure in early pregnancy, such EVs derived from the fetal central nervous system and isolated from maternal plasma predicted adverse fetal neurological outcomes (126). Similarly, a biomarker for acute brain injury can be levels of synaptotagmin in neuron-derived EVs purified from peripheral blood samples (127).
Current treatments for WMI primarily focus on promoting neural recovery, but EVs exhibit neuroprotective effects against WMI. In response to lipopolysaccharide activation of microglia in vitro, MSC-EVs reduce the production of pro-inflammatory cytokines (128). In an animal model of inflammation-induced WMI, MSC-EVs reduced inflammation-induced neuronal cell degeneration, reduced microglial proliferation, and prevented reactive astrocyte proliferation (129). MSC-EV administration restored short-term myelination defects and long-term microstructural abnormalities in white matter, thereby improving persistent cognitive function (129). In a model of hypoxia combined with inflammation-induced white matter injury, MSC-EVs promoted normal myelination of damaged neurons, facilitated oligodendrocyte maturation, and supported regeneration of neuronal cells, significantly enhancing learning ability in animals with WMI (130).
Perinatal arterial ischemic stroke, with an incidence of approximately 1 in 2,300, is associated with severe long-term neurological and cognitive deficits, including cerebral palsy and developmental disorders (131). Arterial ischemic stroke is an occlusive cerebrovascular event, usually thrombotic in nature, with an unclear pathogenesis. One study reported that MSC-EVs administered via intraventricular or intranasal routes accumulate in the ipsilateral hemisphere of occluded neonatal stroke, preventing perinatal arterial ischemic stroke through interactions with microglia (132).
Retinopathy of Prematurity (ROP), a potentially blinding vascular proliferative retinal disease, is the second leading cause of blindness in children in the United States (133). Two main factors contributing to the pathogenesis of ROP are immaturity of retinal vasculature and oxidative damage caused by hyperbaric oxygen exposure (134). Prematurity can include retinal vascular immaturity, making it susceptible to retinal damage when exposed to high oxygen levels, sometimes even in ambient air. Hypoxia-inducible factor 1α is reduced by elevated oxygen levels, reducing levels of VEGF and IGF-1, thereby inhibiting retinal vascular growth. Impaired retinal vascular growth decreases retinal oxygenation and increases vascular signaling, promoting leakage and dysregulated proliferation of immature retinal vessels, which can result in vitreoretinal traction and retinal detachment (135).
Current treatment options for ROP include laser photocoagulation, VEGF inhibitors, and, in severe cases, scleral buckling and/or vitrectomy. All of these carry risks of vision-threatening complications. Less invasive and more effective therapies for ROP are needed. In an oxygen-induced retinopathy model, MSC-EV treatment preserved retinal blood flow, attenuated neovascularization, reduced retinal thinning, and exhibited good tolerability without requiring immunosuppression (136). Intravitreal injection of MSC-EVs alleviates neuroinflammation and cell apoptosis induced by retinal ischemia-reperfusion injury. MSC-EV proteomic analysis detected survival-promoting proteins, such as those involved in the cAMP response element-binding protein pathway (137). Insufficient cAMP response element-binding protein signaling is associated with retinal ischemia and alterations in retinal neurotrophic and inflammatory systems (138). In a preterm retinopathy animal model, EVs derived from microglia can alleviate photoreceptor damage, promoting normal vascular formation, perhaps mediated by miR-24-3p (139). Lymphocyte microparticles attenuate oxygen-induced retinopathy by reducing retinal neovascularization and macrophage infiltration. Lymphocyte microparticle miR-181a may play a regulatory role in retinal vascular neogenesis (140).
Neonatal sepsis, an invasion of pathogenic microorganisms such as bacteria, triggers a systemic inflammatory response syndrome in the body, leading to potentially severe sequelae and multi-organ damage. Neonatal sepsis is categorized into early-onset sepsis (EOS) and late-onset sepsis based on the time of onset. About 16% of the 2.8 million newborn deaths worldwide are attributed to sepsis (141). Early-onset sepsis accounts for 8% of deaths within the first 7 days of life, while late-onset sepsis is responsible for 37% of deaths occurring after 7 days (141).
EVs feature prominently in sepsis. Bacteria, the primary infectious agents, release bacterial outer membrane vesicles carrying endotoxins into septic patients’ circulatory systems, exacerbating inflammatory responses (142). Increased quantities of host-derived EVs upon bacterial stimulation correlate with sepsis severity (143). In septic mouse serum, EVs encapsulate numerous cytokines and chemokines, and EV inhibitors reduce EV formation and inflammatory cytokine release (144). EVs contribute to multi-organ damage in sepsis, with miR-1262 from septic patients’ EVs inhibiting glycolysis and promoting cardiomyocyte apoptosis (145). Acute lung injury and acute respiratory distress syndrome have upregulated bronchoalveolar lavage fluid and circulating EVs (146). LPS injection in mice increases pulmonary alveolar macrophage EV release, activating NLRP3 inflammasomes and exacerbating sepsis-induced inflammation (147). After LPS stimulation, choroid plexus epithelial cells secrete EVs containing inflammatory proteins and miRNAs, which effect the central nervous system (148).
Diagnosis of early neonatal sepsis requires sensitive and specific biomarkers due to its atypical clinical presentation. Plasma EV levels correlate with organ failure severity and patient outcomes (149). In sepsis, EV-miRNA expression correlates with risk, severity, and prognosis (150). In septic patients’ serum, upregulated circRNA-104484 and circRNA-104670 EVs have diagnostic potential (151). Elevated miRNA-34a and decreased miR-15 and miR-27a in EVs predicts septic shock occurrence (152).
Inhibiting EV generation reduces inflammation and improves the prognosis of septic patient survival (144). Modifying miRNAs in cell derived EVs can modulate the sepsis cytokine storm (153). MSC-EVs carrying anti-inflammatory miRNAs such as miR-17 mitigate LPS-induced inflammation and apoptosis (154). miR30b-3p in MSC-EVs inhibits LPS-induced pulmonary inflammation and enhances cell proliferation (155). MSC-EVs alleviate systemic inflammatory response, improve mouse survival, and protect lung tissues in septic mice (156). LPS-activated macrophages engulf adipose-derived MSC exosomes, inhibiting IL-27 secretion (157).
To enable the increased use of EVs for their widespread clinical application in the diagnosis and treatment of diseases in premature infants, some key areas that warrant further research include the following:
The complexity of sample physicochemical properties presents significant challenges for the isolation of EVs (158). Current methods for EV isolation include centrifugation, ultrafiltration, chromatography, immunoseparation, and some commercial kits (159). Alternative isolation methods for EVs have limitations and may affect EV purity and biological activity. For example, the most used differential centrifugation method may not effectively purify EVs from viscous fluids (160), and high-speed centrifugation may lead to co-precipitation of EVs with protein aggregates and apoptotic bodies, resulting in decreased EV purity (161). Ultrafiltration may cause a decrease in EVs yield due to entrapment of exosomes in the pores of the filter membrane, and the force applied to the sample passing through the filter membrane may damage, deform, and rupture large vesicles (162). Immunoseparation is expensive, and it is generally used for isolating cell-free samples because cells or tissues may express similar exosomal membrane markers (163). Therefore, understanding the influence of different isolation methods on the biological activity of EVs is crucial. Developing a unified, efficient, and low-cost method for purifying and scaling up EVs from various samples is crucial.
The therapeutic effect of EVs is dose dependent (164), so quantification of EVs is needed to accurately assess the side effects and therapeutic effects of EV administration. Current quantitative methods include concentrations of reporter proteins, dynamic light scattering, tunable resistive pulse sensing, and nanoparticle tracking analysis; each of these has its advantages and limitations (21). There is currently a lack of uniformity in the quantification of EVs because different researchers often use different parameters to calculate EV doses. Furthermore, subtle variations in tissue culture conditions not only affect the quantity of EVs but also their composition. EVs may be confused with fragments, aggregates, and contaminants, leading to difficulties in quantification. Therefore, rigorous and effective analysis of pre-isolation EVs is needed for accurate quantification (165).
The distribution pattern of EVs in the body depends on the route of administration. Relative to intravenous injection, intraperitoneal and subcutaneous injections have less accumulation of EVs in the liver and spleen but more accumulation in the pancreas and gastrointestinal tract (166). Therefore, defining the optimal administration route for different diseases in premature infants would maximize therapeutic efficacy.
Different sources of EVs and differences in the stimuli experienced by a cell type may cause major differences in EV contents, affecting their diagnostic and therapeutic value. For example, preconditioning rat bone marrow MSC under high oxygen conditions in vitro has stronger therapeutic effects on lung injury than untreated MSCs (167). Therefore, it is necessary to further compare the therapeutic differences of EVs from different sources and the changes in the contents of EVs under different treatment conditions of the same cells, as well as their effects on the therapeutic efficacy of diseases in premature infants.
Assessing the long-term effects of EVs on immunocompromised premature infants is essential. Large-sample cohort studies and randomized controlled trials are needed to evaluate the long-term effects of EVs on immune function and neurological development.
By addressing these and other unresolved issues, we can maximize our ability to use EVs toward improving the health outcomes of premature infants.
As the field of EVs in preterm-related diseases grows, key areas of research are essential to bridge the gap between laboratory findings and clinical applications. Developing efficient, reproducible, and cost-effective methods for isolating and characterizing EVs is critical (168). Current techniques often compromise purity or yield, requiring advancements in high-throughput technologies to address these challenges (161). Disease-specific EV-derived molecules hold promise for early and non-invasive diagnosis. However, it is not uncommon for specific biomarkers identified in the preclinical phase to fail miserably during clinical validation. Large-scale prospective or multi-cohort studies are warranted to validate diagnostic EV performance and to determine the context of use before integrating it into routine diagnostic workflows (169). For therapeutic purposes, better understanding the molecular mechanisms of selective cargo sorting for miRNAs and proteins is essential for designing tailored EV therapies and optimizing their composition for enhanced functionality (13). Moreover, research should focus on combining EVs with emerging technologies to enhance cargo loading, tissue targeting, and stability, while evaluating their safety, immunogenicity, and long-term effects in preterm infants (170).
With the recent profound advances in neonatal medicine, including improved survival of very low birthweight premature infants, our Neonatal Intensive Care Unit populations have expanded dramatically. The consequent related morbidity has surged annually, emerging as the leading cause of child mortality and impacting long-term prognoses. EVs are pivotal in intercellular signal transduction, which is a key component in early development. Thus, EVs exert crucial regulatory roles in the pathophysiological processes of spontaneous preterm birth and associated conditions in premature infants. Their unique biological characteristics render EVs promising in disease diagnosis and treatment. However, their widespread clinical application is limited by the current dearth of information regarding composition of various EVs, scaled up EV production, the dose-response relationship of EVs, specifics of treatment modalities, and safety and efficacy of EVs and their components. In the future, we will focus on optimizing EV isolation and characterization techniques, uncovering their biogenesis and cargo sorting mechanisms, developing EV-based non-invasive diagnostic biomarkers, and advancing their therapeutic applications in preterm-related diseases.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
WC: Conceptualization, Writing – original draft, Writing – review & editing. SK: Writing – review & editing. AT: Writing – review & editing. LE: Writing – review & editing. AM: Writing – review & editing. SC: Conceptualization, Writing – review & editing. DN: Writing – review & editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MSC, mesenchymal stem cells; AF-MSCs, amniotic fluid-derived mesenchymal stem cells; AF-NSCs, amniotic fluid-derived neural stem cells; BM-MSCs, bone marrow-derived mesenchymal stem cells; E-NSCs, enteric neural stem cells; NSC, neural stem cells; EVs, extracellular vesicles; CNS, central nervous system; NEC, necrotizing enterocolitis; BPD, bronchopulmonary dysplasia; HIE, hypoxic-ischemic encephalopathy; IVH, intraventricular hemorrhage; WMI, white matter injury; PAIS, perinatal arterial ischemic stroke; ROP, retinopathy of prematurity.
1. Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. (2016) 21(2):74–9. doi: 10.1016/j.siny.2015.12.007
2. Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. (2018) 42(7):478–84. doi: 10.1053/j.semperi.2018.09.013
3. Jones IH, Hall NJ. Contemporary outcomes for infants with necrotizing enterocolitis-A systematic review. J Pediatr. (2020) 220:86–92.e3. doi: 10.1016/j.jpeds.2019.11.011
4. Waitzman NJ, Jalali A, Grosse SD. Preterm birth lifetime costs in the United States in 2016: an update. Semin Perinatol. (2021) 45(3):151390. doi: 10.1016/j.semperi.2021.151390
5. Finder M, Boylan GB, Twomey D, Ahearne C, Murray DM, Hallberg B. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. (2020) 174(1):48–55. doi: 10.1001/jamapediatrics.2019.4011
6. Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. The Lancet Child & Adolescent Health. (2022) 6(2):106–15. doi: 10.1016/S2352-4642(21)00311-4
7. Purdy IB, Craig JW, Zeanah P. NICU discharge planning and beyond: recommendations for parent psychosocial support. J Perinatol. (2015) 35(Suppl 1):S24–8. doi: 10.1038/jp.2015.146
8. Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. (2024) 13(2):e12404. doi: 10.1002/jev2.12404
9. Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, et al. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr. (2017) 147(1):3–10. doi: 10.3945/jn.116.238949
10. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. (2017) 66:30–41. doi: 10.1016/j.plipres.2017.03.001
11. McVey MJ, Kuebler WM. Extracellular vesicles: biomarkers and regulators of vascular function during extracorporeal circulation. Oncotarget. (2018) 9(98):37229–51. doi: 10.18632/oncotarget.26433
12. Shields SB, Oestreich AJ, Winistorfer S, Nguyen D, Payne JA, Katzmann DJ, et al. ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J Cell Biol. (2009) 185(2):213–24. doi: 10.1083/jcb.200811130
13. Lee YJ, Shin KJ, Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp Mol Med. (2024) 56(4):877–89. doi: 10.1038/s12276-024-01209-y
14. Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. (2016) 17(2):170. doi: 10.3390/ijms17020170
15. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19(4):213–28. doi: 10.1038/nrm.2017.125
16. Takahashi Y, Takakura Y. Extracellular vesicle-based therapeutics: extracellular vesicles as therapeutic targets and agents. Pharmacol Ther. (2023) 242:108352. doi: 10.1016/j.pharmthera.2023.108352
17. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. (2013) 10(3):301–12. doi: 10.1016/j.scr.2013.01.002
18. Brown M, Johnson LA, Leone DA, Majek P, Vaahtomeri K, Senfter D, et al. Lymphatic exosomes promote dendritic cell migration along guidance cues. J Cell Biol. (2018) 217(6):2205–21. doi: 10.1083/jcb.201612051
19. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. (2018) 67(2):235–47. doi: 10.2337/db17-0356
20. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9(6):654–9. doi: 10.1038/ncb1596
21. Farrelly R, Kennedy MG, Spencer R, Forbes K. Extracellular vesicles as markers and mediators of pregnancy complications: gestational diabetes, pre-eclampsia, preterm birth and fetal growth restriction. J Physiol (Lond). (2023) 601(22):4973–88. doi: 10.1113/JP282849
22. Liu J, Sun W, Liu C, Na Q. Umbilical cord blood-derived exosomes in maternal-fetal disease: a review. Reproductive Sciences (Thousand Oaks, Calif). (2023) 30(1):54–61. doi: 10.1007/s43032-022-00879-1
23. Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI insight. (2018) 3(5):e93994. doi: 10.1172/jci.insight.93994
24. Pezzana C, Agnely F, Bochot A, Siepmann J, Menasché P. Extracellular vesicles and biomaterial design: new therapies for cardiac repair. Trends Mol Med. (2021) 27(3):231–47. doi: 10.1016/j.molmed.2020.10.006
25. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (New York NY). (2020) 367(6478):eaau6977. doi: 10.1126/science.aau6977
26. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomed. (2021) 16:1281–312. doi: 10.2147/IJN.S291956
27. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. (2021) 16(7):748–59. doi: 10.1038/s41565-021-00931-2
28. Wu S, Benny M, Duara J, Williams K, Tan A, Schmidt A, et al. Extracellular vesicles: pathogenic messengers and potential therapy for neonatal lung diseases. Front Pediatr. (2023) 11:1205882. doi: 10.3389/fped.2023.1205882
29. Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. (2017) 8(5):1214–25. doi: 10.1016/j.stemcr.2017.04.008
30. Susa F, Limongi T, Dumontel B, Vighetto V, Cauda V. Engineered extracellular vesicles as a reliable tool in cancer nanomedicine. Cancers (Basel). (2019) 11(12):1979. doi: 10.3390/cancers11121979
31. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750
32. Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. (2019) 176(1-2):113–126.e15. doi: 10.1016/j.cell.2018.12.002
33. Chen W, Wang X, Yan X, Yu Z, Zhang J, Han S. The emerging role of exosomes in the pathogenesis, prognosis and treatment of necrotizing enterocolitis. Am J Transl Res. (2020) 12(11):7020–33.
34. Go H, Maeda H, Miyazaki K, Maeda R, Kume Y, Namba F, et al. Extracellular vesicle miRNA-21 is a potential biomarker for predicting chronic lung disease in premature infants. Am J Physiol Lung Cell Mol Physiol. (2020) 318(5):L845–l51. doi: 10.1152/ajplung.00166.2019
35. Galley JD, Mar P, Wang Y, Han R, Rajab A, Besner GE. Urine-derived extracellular vesicle miRNAs as possible biomarkers for and mediators of necrotizing enterocolitis: a proof of concept study. J Pediatr Surg. (2021) 56(11):1966–75. doi: 10.1016/j.jpedsurg.2021.02.016
36. Li YY, Xu QW, Xu PY, Li WM. MSC-derived exosomal miR-34a/c-5p and miR-29b-3p improve intestinal barrier function by targeting the snail/claudins signaling pathway. Life Sci. (2020) 257:118017. doi: 10.1016/j.lfs.2020.118017
37. Madden JW. Human breast milk exosomes may protect against necrotizing enterocolitis in preterm infants. Pediatr Res. (2021) 90(2):244–5. doi: 10.1038/s41390-021-01580-w
38. Jiang L, Shen Y, Guo D, Yang D, Liu J, Fei X, et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. (2016) 7:13045. doi: 10.1038/ncomms13045
39. Pineles B, Mani A, Sura L, Rossignol C, Albayram M, Weiss MD, et al. Neuronal exosome proteins: novel biomarkers for predicting neonatal response to therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. (2022) 107(1):60–4. doi: 10.1136/archdischild-2020-321096
40. Challis JR, Smith SK. Fetal endocrine signals and preterm labor. Biol Neonate. (2001) 79(3-4):163–7. doi: 10.1159/000047085
41. Fuchs AR, Fields MJ, Freidman S, Shemesh M, Ivell R. Oxytocin and the timing of parturition. Influence of oxytocin receptor gene expression, oxytocin secretion, and oxytocin-induced prostaglandin F2 alpha and E2 release. Adv Exp Med Biol. (1995) 395:405–20.
42. Ghafourian M, Mahdavi R, Akbari Jonoush Z, Sadeghi M, Ghadiri N, Farzaneh M, et al. The implications of exosomes in pregnancy: emerging as new diagnostic markers and therapeutics targets. Cell Commun Signal. (2022) 20(1):51. doi: 10.1186/s12964-022-00853-z
43. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2
44. Condrat CE, Varlas VN, Duică F, Antoniadis P, Danila CA, Cretoiu D, et al. Pregnancy-Related extracellular vesicles revisited. Int J Mol Sci. (2021) 22(8):3904. doi: 10.3390/ijms22083904
45. Sheller-Miller S, Trivedi J, Yellon SM, Menon R. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci Rep. (2019) 9(1):608. doi: 10.1038/s41598-018-37002-x
46. Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal P, et al. Protein profile changes in circulating placental extracellular vesicles in term and preterm births: a longitudinal study. Endocrinology. (2020) 161(4):bqaa009. doi: 10.1210/endocr/bqaa009
47. Menon R, Dixon CL, Sheller-Miller S, Fortunato SJ, Saade GR, Palma C, et al. Quantitative proteomics by SWATH-MS of maternal plasma exosomes determine pathways associated with term and preterm birth. Endocrinology. (2019) 160(3):639–50. doi: 10.1210/en.2018-00820
48. Gray C, McCowan LM, Patel R, Taylor RS, Vickers MH. Maternal plasma miRNAs as biomarkers during mid-pregnancy to predict later spontaneous preterm birth: a pilot study. Sci Rep. (2017) 7(1):815. doi: 10.1038/s41598-017-00713-8
49. Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal PK, et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. (2019) 160(2):249–75. doi: 10.1210/en.2018-00836
50. McElrath TF, Cantonwine DE, Jeyabalan A, Doss RC, Page G, Roberts JM, et al. Circulating microparticle proteins obtained in the late first trimester predict spontaneous preterm birth at less than 35 weeks’ gestation: a panel validation with specific characterization by parity. Am J Obstet Gynecol. (2019) 220(5):488.e1–.e11. doi: 10.1016/j.ajog.2019.01.220
51. Zhao Q, Ma Z, Wang X, Liang M, Wang W, Su F, et al. Lipidomic biomarkers of extracellular vesicles for the prediction of preterm birth in the early second trimester. J Proteome Res. (2020) 19(10):4104–13. doi: 10.1021/acs.jproteome.0c00525
52. Sheller-Miller S, Radnaa E, Yoo JK, Kim E, Choi K, Kim Y, et al. Exosomal delivery of NF-κB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models. Sci Adv. (2021) 7(4):eabd3865. doi: 10.1126/sciadv.abd3865
53. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F182–f9. doi: 10.1136/archdischild-2017-313880
54. Garg PM, O'Connor A, Ansari MAY, Vu B, Hobart H, Paschal JL, et al. Hematological predictors of mortality in neonates with fulminant necrotizing enterocolitis. J Perinatol. (2021) 41(5):1110–21. doi: 10.1038/s41372-021-01044-3
55. Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. (2015) 125(3):1215–27. doi: 10.1172/JCI76693
56. Butin-Israeli V, Houser MC, Feng M, Thorp EB, Nusrat A, Parkos CA, et al. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. (2016) 30(12):4007–20. doi: 10.1096/fj.201600734R
57. Larabi A, Dalmasso G, Delmas J, Barnich N, Nguyen HTT. Exosomes transfer miRNAs from cell-to-cell to inhibit autophagy during infection with Crohn’s disease-associated adherent-invasive E. coli. Gut Microbes. (2020) 11(6):1677–94. doi: 10.1080/19490976.2020.1771985
58. Gunasekaran A, Devette C, Levin S, Chaaban H. Biomarkers of necrotizing enterocolitis: the search continues. Clin Perinatol. (2022) 49(1):181–94. doi: 10.1016/j.clp.2021.11.011
59. Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. (2016) 51(6):942–7. doi: 10.1016/j.jpedsurg.2016.02.061
60. McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. (2018) 53(6):1215–20. doi: 10.1016/j.jpedsurg.2018.02.086
61. Zonneveld MI, van Herwijnen MJC, Fernandez-Gutierrez MM, Giovanazzi A, de Groot AM, Kleinjan M, et al. Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J Extracell Vesicles. (2021) 10(5):e12071. doi: 10.1002/jev2.12071
62. Martin C, Patel M, Williams S, Arora H, Brawner K, Sims B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. (2018) 24(5):278–84. doi: 10.1177/1753425918785715
63. Dong P, Zhang Y, Yan DY, Wang Y, Xu X, Zhao YC, et al. Protective effects of human milk-derived exosomes on intestinal stem cells damaged by oxidative stress. Cell Transplant. (2020) 29:963689720912690. doi: 10.1177/0963689720912690
64. Guo MM, Zhang K, Zhang JH. Human breast milk-derived exosomal miR-148a-3p protects against necrotizing enterocolitis by regulating p53 and sirtuin 1. Inflammation. (2022) 45(3):1254–68. doi: 10.1007/s10753-021-01618-5
65. Yan X, Liu L, Yao S, Chen Y, Yu Q, Jiang C, et al. LncRNA and mRNA profiles of human milk-derived exosomes and their possible roles in protecting against necrotizing enterocolitis. Food Funct. (2022) 13(24):12953–65. doi: 10.1039/D2FO01866G
66. Yang M, Song D, Cao X, Wu R, Liu B, Ye W, et al. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food research international (Ottawa. Ont. (2017) 92:17–25. doi: 10.1016/j.foodres.2016.11.041
67. Gómez-Ferrer M, Amaro-Prellezo E, Albiach-Delgado A, Ten-Domenech I, Kuligowski J, Sepúlveda P. Identification of omega-3 oxylipins in human milk-derived extracellular vesicles with pro-resolutive actions in gastrointestinal inflammation. Front Immunol. (2023) 14:1293737. doi: 10.3389/fimmu.2023.1293737
68. Shukla VV, Ambalavanan N. Recent advances in bronchopulmonary dysplasia. Indian J Pediatr. (2021) 88(7):690–5. doi: 10.1007/s12098-021-03766-w
69. Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. (2019) 5(1):78. doi: 10.1038/s41572-019-0127-7
70. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. (2015) 314(10):1039–51. doi: 10.1001/jama.2015.10244
71. Nakashima T, Inoue H, Sakemi Y, Ochiai M, Yamashita H, Ohga S. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J Pediatr. (2021) 230:119–125.e7. doi: 10.1016/j.jpeds.2020.11.041
72. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA network Open. (2021) 4(5):e219382. doi: 10.1001/jamanetworkopen.2021.9382
73. Álvarez-Fuente M, Arruza L, Muro M, Zozaya C, Avila A, López-Ortego P, et al. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr. (2017) 176(12):1587–93. doi: 10.1007/s00431-017-3009-6
74. Carvalho CG, Procianoy RS, Neto EC, Silveira RC. Preterm neonates with respiratory distress syndrome: ventilator-induced lung injury and oxidative stress. J Immunol Res. (2018) 2018:6963754. doi: 10.1155/2018/6963754
75. Ali A, Zambrano R, Duncan MR, Chen S, Luo S, Yuan H, et al. Hyperoxia-activated circulating extracellular vesicles induce lung and brain injury in neonatal rats. Sci Rep. (2021) 11(1):8791. doi: 10.1038/s41598-021-87706-w
76. Ransom MA, Bunn KE, Negretti NM, Jetter CS, Bressman ZJ, Sucre JMS, et al. Developmental trajectory of extracellular vesicle characteristics from the lungs of preterm infants. Am J Physiol Lung Cell Mol Physiol. (2023) 324(3):L385–l92. doi: 10.1152/ajplung.00389.2022
77. Costabile G, Conte G, Brusco S, Savadi P, Miro A, Quaglia F, et al. State-of-the-Art review on inhalable lipid and polymer nanocarriers: design and development perspectives. Pharmaceutics. (2024) 16(3):347. doi: 10.3390/pharmaceutics16030347
78. Manley BJ, Kamlin COF, Donath SM, Francis KL, Cheong JLY, Dargaville PA, et al. Intratracheal budesonide mixed with surfactant for extremely preterm infants: the PLUSS randomized clinical trial. JAMA. (2024) 332(22):1889–99. doi: 10.1001/jama.2024.17380
79. Porzionato A, Zaramella P, Dedja A, Guidolin D, Bonadies L, Macchi V, et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2021) 320(5):L688–l704. doi: 10.1152/ajplung.00148.2020
80. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. (2018) 197(1):104–16. doi: 10.1164/rccm.201705-0925OC
81. You J, Zhou O, Liu J, Zou W, Zhang L, Tian D, et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles alleviate lung injury in rat model of bronchopulmonary dysplasia by affecting cell survival and angiogenesis. Stem Cells Dev. (2020) 29(23):1520–32. doi: 10.1089/scd.2020.0156
82. Wu Y, Li J, Yuan R, Deng Z, Wu X. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425. Arch Biochem Biophys. (2021) 697:108712. doi: 10.1016/j.abb.2020.108712
83. Wu Y, Zhang Z, Li J, Zhong H, Yuan R, Deng Z, et al. Mechanism of adipose-derived mesenchymal stem cell-derived extracellular vesicles carrying miR-21-5p in hyperoxia-induced lung injury. Stem Cell Rev Rep. (2022) 18(3):1007–24. doi: 10.1007/s12015-021-10311-x
84. He X, Kuang J, Wang Y, Lan G, Shi X. Bone marrow stromal cell-secreted extracellular vesicles containing miR-34c-5p alleviate lung injury and inflammation in bronchopulmonary dysplasia through promotion of PTEN degradation by targeting OTUD3. Immunol Investig. (2023) 52(6):681–702. doi: 10.1080/08820139.2023.2217854
85. Uberos J, Sanchez-Ruiz I, Fernández-Marin E, Ruiz-López A, Cubero-Millan I, Campos-Martínez A. Breast-feeding as protective factor against bronchopulmonary dysplasia in preterm infants. Br J Nutr. (2024) 131(8):1405–12. doi: 10.1017/S0007114523002982
86. Lu X, Gao Y, Liu C, Pan M, Chen X. Effect of breast milk on the frequency of bronchopulmonary dysplasia in very low birth weight premature infants: a meta-analysis. Breastfeed Med. (2023) 18(9):636–44. doi: 10.1089/bfm.2023.0093
87. Zhou Y, Liu Y, Xu G, Liu L, Li H, Li Y, et al. Human breast milk-derived exosomes through inhibiting AT II cell apoptosis to prevent bronchopulmonary dysplasia in rat lung. J Cell Mol Med. (2022) 26(15):4169–82. doi: 10.1111/jcmm.17334
88. Li Y, Yu B, Li H, Hou W, Yin J, Zhou Y, et al. Human milk exosome-derived circDNAJB6 improves bronchopulmonary dysplasia model by promoting DNAJB6 gene transcription. J Bioenerg Biomembr. (2024) 56(2):171–80. doi: 10.1007/s10863-024-10002-5
89. Li H, Ma K, Dou H, Liu L, Qian Y, Li S, et al. CircABPD1 alleviates oxidative lung injury of bronchopulmonary dysplasia through regulating miR-330-3p/HIF1α axis. Int J Biochem Cell Biol. (2023) 163:106464. doi: 10.1016/j.biocel.2023.106464
90. Gamage T, Fraser M. The role of extracellular vesicles in the developing brain: current perspective and promising source of biomarkers and therapy for perinatal brain injury. Front Neurosci. (2021) 15:744840. doi: 10.3389/fnins.2021.744840
91. Shankaran S. Neonatal encephalopathy: treatment with hypothermia. J Neurotrauma. (2009) 26(3):437–43. doi: 10.1089/neu.2008.0678
92. Tung S, Delavogia E, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Harnessing the therapeutic potential of the stem cell secretome in neonatal diseases. Semin Perinatol. (2023) 47(3):151730. doi: 10.1016/j.semperi.2023.151730
93. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet (London, England). (2010) 375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1
94. Marlow N, Shankaran S, Rogers EE, Maitre NL, Smyser CD. Neurological and developmental outcomes following neonatal encephalopathy treated with therapeutic hypothermia. Semin Fetal Neonatal Med. (2021) 26(5):101274. doi: 10.1016/j.siny.2021.101274
95. Cainelli E, Vedovelli L, Mastretta E, Gregori D, Suppiej A, Bisiacchi PS. Long-Term outcomes after neonatal hypoxic-ischemic encephalopathy in the era of therapeutic hypothermia: a longitudinal, prospective, multicenter case-control study in children without overt brain damage. Children (Basel, Switzerland). (2021) 8(11):1076. doi: 10.3390/children8111076
96. Chiang CS, Fu SJ, Hsu CL, Jeng CJ, Tang CY, Huang YS, et al. Neuronal exosomes secreted under oxygen-glucose deprivation/reperfusion presenting differentially expressed miRNAs and affecting neuronal survival and neurite outgrowth. NeuroMol Med. (2021) 23(3):404–15. doi: 10.1007/s12017-020-08641-z
97. Walsh W. Report of a pilot study of cooling four preterm infants 32-35 weeks gestation with HIE. J Neonatal Perinatal Med. (2015) 8(1):47–51. doi: 10.3233/npm-15814078
98. Jellema RK, Wolfs TG, Lima Passos V, Zwanenburg A, Ophelders DR, Kuypers E, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. (2013) 8(8):e73031. doi: 10.1371/journal.pone.0073031
99. Labusek N, Mouloud Y, Köster C, Diesterbeck E, Tertel T, Wiek C, et al. Extracellular vesicles from immortalized mesenchymal stromal cells protect against neonatal hypoxic-ischemic brain injury. Inflamm Regen. (2023) 43(1):24. doi: 10.1186/s41232-023-00274-6
100. Labusek N, Ghari P, Mouloud Y, Köster C, Diesterbeck E, Hadamitzky M, et al. Hypothermia combined with extracellular vesicles from clonally expanded immortalized mesenchymal stromal cells improves neurodevelopmental impairment in neonatal hypoxic-ischemic brain injury. J Neuroinflammation. (2023) 20(1):280. doi: 10.1186/s12974-023-02961-0
101. Turovsky EA, Golovicheva VV, Varlamova EG, Danilina TI, Goryunov KV, Shevtsova YA, et al. Mesenchymal stromal cell-derived extracellular vesicles afford neuroprotection by modulating PI3K/AKT pathway and calcium oscillations. Int J Biol Sci. (2022) 18(14):5345–68. doi: 10.7150/ijbs.73747
102. Gussenhoven R, Klein L, Ophelders D, Habets DHJ, Giebel B, Kramer BW, et al. Annexin A1 as neuroprotective determinant for blood-brain barrier integrity in neonatal hypoxic-ischemic encephalopathy. J Clin Med. (2019) 8(2):137. doi: 10.3390/jcm8020137
103. Shu J, Jiang L, Wang M, Wang R, Wang X, Gao C, et al. Human bone marrow mesenchymal stem cells-derived exosomes protect against nerve injury via regulating immune microenvironment in neonatal hypoxic-ischemic brain damage model. Immunobiology. (2022) 227(3):152178. doi: 10.1016/j.imbio.2022.152178
104. Li L, Li M. Astrocyte-derived extracellular vesicles inhibit the abnormal activation of immune function in neonatal mice with hypoxic-ischemic brain damage by carrying miR-124-3p. Neurol Res. (2023) 45(12):1079–90. doi: 10.1080/01616412.2023.2257416
105. Joerger-Messerli MS, Oppliger B, Spinelli M, Thomi G, di Salvo I, Schneider P, et al. Extracellular vesicles derived from wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-ischemia in neuronal cells. Cell Transplant. (2018) 27(1):168–80. doi: 10.1177/0963689717738256
106. Xin D, Li T, Chu X, Ke H, Yu Z, Cao L, et al. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of miR-21a-5p. Acta Biomater. (2020) 113:597–613. doi: 10.1016/j.actbio.2020.06.037
107. Chu X, Liu D, Li T, Ke H, Xin D, Wang S, et al. Hydrogen sulfide-modified extracellular vesicles from mesenchymal stem cells for treatment of hypoxic-ischemic brain injury. J Controlled Release. (2020) 328:13–27. doi: 10.1016/j.jconrel.2020.08.037
108. Du L, Jiang Y, Sun Y. Astrocyte-derived exosomes carry microRNA-17-5p to protect neonatal rats from hypoxic-ischemic brain damage via inhibiting BNIP-2 expression. Neurotoxicology. (2021) 83:28–39. doi: 10.1016/j.neuro.2020.12.006
109. Luo H, Huang F, Huang Z, Huang H, Liu C, Feng Y, et al. microRNA-93 packaged in extracellular vesicles from mesenchymal stem cells reduce neonatal hypoxic-ischemic brain injury. Brain Res. (2022) 1794:148042. doi: 10.1016/j.brainres.2022.148042
110. Luo H, Ye G, Liu Y, Huang D, Luo Q, Chen W, et al. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci Lett. (2022) 779:136635. doi: 10.1016/j.neulet.2022.136635
111. Liu Q, Tan Y, Qu T, Zhang J, Duan X, Xu H, et al. Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro. Life Sci. (2020) 254:117772. doi: 10.1016/j.lfs.2020.117772
112. Christian EA, Jin DL, Attenello F, Wen T, Cen S, Mack WJ, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J Neurosurg Pediatr. (2016) 17(3):260–9. doi: 10.3171/2015.7.PEDS15140
113. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. (2010) 67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176
114. Spaull R, McPherson B, Gialeli A, Clayton A, Uney J, Heep A, et al. Exosomes populate the cerebrospinal fluid of preterm infants with post-haemorrhagic hydrocephalus. Int J Dev Neurosci. (2019) 73:59–65. doi: 10.1016/j.ijdevneu.2019.01.004
115. Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med. (2021) 10(3):374–84. doi: 10.1002/sctm.20-0301
116. Duan S, Wang F, Cao J, Wang C. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. (2020) 14:3143–58. doi: 10.2147/DDDT.S255828
117. van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, et al. Origin and dynamics of oligodendrocytes in the developing brain: implications for perinatal white matter injury. Glia. (2018) 66(2):221–38. doi: 10.1002/glia.23256
118. Romero-Guzman GJ, Lopez-Munoz F. Prevalence and risk factors for periventricular leukomalacia in preterm infants. A systematic review. Rev Neurol. (2017) 65(2):57–62. doi: 10.33588/rn.6502.2017002
119. Cayam-Rand D, Guo T, Grunau RE, Benavente-Fernández I, Synnes A, Chau V, et al. Predicting developmental outcomes in preterm infants: a simple white matter injury imaging rule. Neurology. (2019) 93(13):e1231–e40. doi: 10.1212/WNL.0000000000008172
120. Drougia A, Giapros V, Krallis N, Theocharis P, Nikaki A, Tzoufi M, et al. Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15-year review. Early Hum Dev. (2007) 83(8):541–7. doi: 10.1016/j.earlhumdev.2006.10.004
121. Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol. (2015) 11(4):192–208. doi: 10.1038/nrneurol.2015.13
122. Baburamani AA, Supramaniam VG, Hagberg H, Mallard C. Microglia toxicity in preterm brain injury. Reprod Toxicol. (2014) 48:106–12. doi: 10.1016/j.reprotox.2014.04.002
123. Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. (2017) 10(473):eaai7696. doi: 10.1126/scisignal.aai7696
124. Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. (2019) 138(6):987–1012. doi: 10.1007/s00401-019-02049-1
125. Goetzl L, Darbinian N, Goetzl EJ. Novel window on early human neurodevelopment via fetal exosomes in maternal blood. Ann Clin Transl Neurol. (2016) 3(5):381–5. doi: 10.1002/acn3.296
126. Goetzl L, Darbinian N, Merabova N. Noninvasive assessment of fetal central nervous system insult: potential application to prenatal diagnosis. Prenat Diagn. (2019) 39(8):609–15. doi: 10.1002/pd.5474
127. Goetzl L, Merabova N, Darbinian N, Martirosyan D, Poletto E, Fugarolas K, et al. Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol. (2018) 5(1):4–10. doi: 10.1002/acn3.499
128. Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. (2019) 10(1):105. doi: 10.1186/s13287-019-1207-z
129. Drommelschmidt K, Serdar M, Bendix I, Herz J, Bertling F, Prager S, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. (2017) 60:220–32. doi: 10.1016/j.bbi.2016.11.011
130. Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells. (2019) 8(8):855. doi: 10.3390/cells8080855
131. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. (2004) 3(3):150–8. doi: 10.1016/S1474-4422(04)00679-9
132. Pathipati P, Lecuyer M, Faustino J, Strivelli J, Phinney DG, Vexler ZS. Mesenchymal stem cell (MSC)-derived extracellular vesicles protect from neonatal stroke by interacting with microglial cells. Neurotherapeutics. (2021) 18(3):1939–52. doi: 10.1007/s13311-021-01076-9
133. Bashinsky AL. Retinopathy of prematurity. N C Med J. (2017)78(2):124–8. doi: 10.18043/ncm.78.2.124
134. Hellström A, Hård AL. Screening and novel therapies for retinopathy of prematurity - A review. Early Hum Dev. (2019) 138:104846. doi: 10.1016/j.earlhumdev.2019.104846
135. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. (2012) 367(26):2515–26. doi: 10.1056/NEJMra1208129
136. Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, et al. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res. (2017) 42(10):1358–67. doi: 10.1080/02713683.2017.1319491
137. Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. (2019) 197:146–60. doi: 10.1016/j.biomaterials.2019.01.016
138. Guo XJ, Tian XS, Ruan Z, Chen YT, Wu L, Gong Q, et al. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res. (2014) 125:156–63. doi: 10.1016/j.exer.2014.06.003
139. Xu W, Wu Y, Hu Z, Sun L, Dou G, Zhang Z, et al. Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Molecular Therapy Nucleic Acids. (2019) 16:778–90. doi: 10.1016/j.omtn.2019.04.029
140. Yang C, Tahiri H, Cai C, Gu M, Gagnon C, Hardy P. microRNA-181a inhibits ocular neovascularization by interfering with vascular endothelial growth factor expression. Cardiovasc Ther. (2018) 36(3):e12329. doi: 10.1111/1755-5922.12329
141. Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000–2013. Bull W H O. (2015) 93(1):19–28. doi: 10.2471/BLT.14.139790
142. Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. (2009) 5(4):e1000382. doi: 10.1371/journal.ppat.1000382
143. Dakhlallah DA, Wisler J, Gencheva M, Brown CM, Leatherman ER, Singh K, et al. Circulating extracellular vesicle content reveals de novo DNA methyltransferase expression as a molecular method to predict septic shock. J Extracell Vesicles. (2019) 8(1):1669881. doi: 10.1080/20013078.2019.1669881
144. Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. (2015) 1852(11):2362–71. doi: 10.1016/j.bbadis.2015.08.010
145. Sun F, Geng H, Sun Y, Feng W, Tian T, Ye L, et al. Exosomes derived from the blood of patients with sepsis regulate apoptosis and aerobic glycolysis in human myocardial cells via the hsa-miR-1262/SLC2A1 signaling pathway. Mol Med Rep. (2022) 25(4):119. doi: 10.3892/mmr.2022.12635
146. Letsiou E, Sammani S, Zhang W, Zhou T, Quijada H, Moreno-Vinasco L, et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol. (2015) 52(2):193–204. doi: 10.1165/rcmb.2013-0347OC
147. Soni S, Wilson MR, O'Dea KP, Yoshida M, Katbeh U, Woods SJ, et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. (2016) 71(11):1020–9. doi: 10.1136/thoraxjnl-2015-208032
148. Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. (2016) 8(10):1162–83. doi: 10.15252/emmm.201606271
149. Im Y, Yoo H, Lee JY, Park J, Suh GY, Jeon K. Association of plasma exosomes with severity of organ failure and mortality in patients with sepsis. J Cell Mol Med. (2020) 24(16):9439–45. doi: 10.1111/jcmm.15606
150. Hermann S, Brandes F, Kirchner B, Buschmann D, Borrmann M, Klein M, et al. Diagnostic potential of circulating cell-free microRNAs for community-acquired pneumonia and pneumonia-related sepsis. J Cell Mol Med. (2020) 24(20):12054–64. doi: 10.1111/jcmm.15837
151. Tian C, Liu J, Di X, Cong S, Zhao M, Wang K. Exosomal hsa_circRNA_104484 and hsa_circRNA_104670 may serve as potential novel biomarkers and therapeutic targets for sepsis. Sci Rep. (2021) 11(1):14141. doi: 10.1038/s41598-021-93246-0
152. Goodwin AJ, Guo C, Cook JA, Wolf B, Halushka PV, Fan H. Plasma levels of microRNA are altered with the development of shock in human sepsis: an observational study. Critical care (London. England. (2015) 19:440. doi: 10.1186/s13054-015-1162-8
153. Li Y, Zhang H, Chen C, Qiao K, Li Z, Han J, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of sepsis. Adv Mater. (2022) 34(19):e2108476. doi: 10.1002/adma.202108476
154. Su Y, Song X, Teng J, Zhou X, Dong Z, Li P, et al. Mesenchymal stem cells-derived extracellular vesicles carrying microRNA-17 inhibits macrophage apoptosis in lipopolysaccharide-induced sepsis. Int Immunopharmacol. (2021) 95:107408. doi: 10.1016/j.intimp.2021.107408
155. Yi X, Wei X, Lv H, An Y, Li L, Lu P, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. (2019) 383(2):111454. doi: 10.1016/j.yexcr.2019.05.035
156. Deng H, Zhu L, Zhang Y, Zheng L, Hu S, Zhou W, et al. Differential lung protective capacity of exosomes derived from human adipose tissue, bone marrow, and umbilical cord mesenchymal stem cells in sepsis-induced acute lung injury. Oxid Med Cell Longevity. (2022) 2022:7837837. doi: 10.1155/2022/7837837
157. Wang X, Liu D, Zhang X, Yang L, Xia Z, Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. (2022) 8(1):18. doi: 10.1038/s41420-021-00785-6
158. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. (2014) 3:26913. doi: 10.3402/jev.v3.26913
159. Bano R, Ahmad F, Mohsin M. A perspective on the isolation and characterization of extracellular vesicles from different biofluids. RSC Adv. (2021) 11(32):19598–615. doi: 10.1039/D1RA01576A
160. Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. (2011) 117(11):3172–80. doi: 10.1182/blood-2010-06-290460
161. Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. (2015) 4:29509. doi: 10.3402/jev.v4.29509
162. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Controlled Release. (2015) 219:396–405. doi: 10.1016/j.jconrel.2015.07.030
163. Boriachek K, Islam MN, Möller A, Salomon C, Nguyen NT, Hossain MSA, et al. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. (2018) 14(6):1702153. doi: 10.1002/smll.201702153
164. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. (2015) 55:439–64. doi: 10.1146/annurev-pharmtox-010814-124630
165. Willis GR, Kourembanas S, Mitsialis SA. Therapeutic applications of extracellular vesicles: perspectives from newborn medicine. Methods Mol Biol. (2017) 1660:409–32. doi: 10.1007/978-1-4939-7253-1_34
166. Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. (2015) 4:26316. doi: 10.3402/jev.v4.26316
167. Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thébaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. (2012) 21(15):2789–97. doi: 10.1089/scd.2010.0566
168. Adamo G, Picciotto S, Gargano P, Paterna A, Raccosta S, Rao E, et al. DetectEV: a functional enzymatic assay to assess integrity and bioactivity of extracellular vesicles. J Extracell Vesicles. (2025) 14(1):e70030. doi: 10.1002/jev2.70030
169. Urabe F, Tamura T, Sakamoto S, Kimura T, Ochiya T. Extracellular vesicles as novel uro-oncology biomarkers: insights toward clinical applications. Curr Opin Urol. (2025) 35(1):13–8. doi: 10.1097/MOU.0000000000001194
Keywords: preterm infants, extracellular vesicles, diseases, biomarkers, therapeutics
Citation: Chen W, Kongsomros S, Thorman A, Esfandiari L, Morrow AL, Chutipongtanate S and Newburg DS (2025) Extracellular vesicles and preterm infant diseases. Front. Pediatr. 13:1550115. doi: 10.3389/fped.2025.1550115
Received: 22 December 2024; Accepted: 6 February 2025;
Published: 17 February 2025.
Edited by:
Paolo Montaldo, Imperial College London, United KingdomReviewed by:
Defne Engür, University of Health Sciences, TürkiyeCopyright: © 2025 Chen, Kongsomros, Thorman, Esfandiari, Morrow, Chutipongtanate and Newburg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Somchai Chutipongtanate, Y2h1dGlwc2lAdWNtYWlsLnVjLmVkdQ==; c2NodXRpLnJhbWFAZ21haWwuY29t; David S. Newburg, bmV3YnVyZGRAdWNtYWlsLnVjLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.