- 1Key Laboratory of Major Diseases in Children, Ministry of Education, National Clinical Research Center for Respiratory Diseases, National Key Discipline of Pediatrics, Laboratory of Infection and Microbiology, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Department of Preventive Health, First Affiliated Hospital of Tsinghua University, Beijing, China
- 3Division of Infectious Diseases, Shenzhen Children’s Hospital, Shenzhen, Guangdong, China
- 4Shenzhen Nanshan Medical Group Headquarters, Taohua Yuan Community Health Service Center, Shenzhen, Guangdong, China
- 5Department of Preventive Health, Beijing Children’s Hospital, Capital Medical University, Beijing, China
- 6Shenzhen Nanshan Medical Group Headquarters, Chiwan Community Health Service Center, Shenzhen, Guangdong, China
- 7Shenzhen Nanshan Medical Group Headquarters, Haiwan Community Health Service Center, Shenzhen, Guangdong, China
- 8Vaccines/Antivirals & Evidence Generation, Pfizer, Inc., Collegeville, PA, United States
- 9Vaccines & Antivirals, Emerging Markets, Pfizer, Inc., Paris, France
- 10Vaccines, Medical Affairs, Emerging Markets, Pfizer, Inc., New York, NY, United States
Objectives: To describe the carriage rate, serotype distribution, and antimicrobial susceptibility patterns of Streptococcus pneumoniae (S. pneumoniae) nasopharyngeal (NP) isolates among healthy children aged 30 days to <60 months in the cities of Beijing and Shenzhen during 2018–2021.
Methods: A NP swab sample was collected among four annual cohorts of healthy children at routine well-child visits. S. pneumoniae was identified by culture, optochin sensitivity and bile solubility, serotypes determined by latex agglutination and Quellung, and antimicrobial susceptibility testing performed using E-test strips.
Results: S. pneumoniae NP carriage was 13.1% (645/4,911), with the highest S. pneumoniae carriage prevalence (15.3%) observed in 25 to <60 months. The carriage prevalence was 15.1% in children 13–24 months, 13.2% in children 7–12 months, and 8.2% in children 30 days to 6 months (P < 0.01). Living with siblings [20.0% vs. 9.4%: OR: 2.42 (95% CI: 2.05–2.87)] or attending day-care [31.8% vs. 11.3%: OR: 3.67 (95% CI: 2.94–4.57)] increased the risk (P < 0.01). During the period (January 2020–April 2021) of strict non-pharmaceutical interventions to prevent and control the COVID-19 pandemic, the proportion of children with S. pneumoniae colonization declined from 16.0% (94/587) to 5.8% (108/1,848) in Beijing while increasing from 14.5% (64/443) to 18.6% (379/2,033) in Shenzhen. Among S. pneumoniae isolates, 36.7% (237/645) belonged to 13-valent pneumococcal conjugate vaccine (PCV13) serotypes, 64.3% (408/645) were non-PCV13 serotypes, including 20.8% (134/645) non-serotypeable S. pneumoniae (NST). A total of 158/644 isolates (24.5%) were MDR. For the PCV13 isolates, MDR was detected in 36.3% (86/237) of isolates; in comparison, 17.6% (72/407) of non-PCV13 serotypes, including NST, were MDR (P < 0.01). S. pneumoniae NP carriage was detected in 10.7% of children with previous pneumococcal vaccination (PCV7 or PCV13 only) compared with 14.9% in children without previous pneumococcal vaccination.
Conclusions: The highest S. pneumoniae carriage prevalence were found in the oldest age group (25 to <60 months) and in children living with siblings or attending day-care. Vaccination with PCV7 or PCV13 was associated with lower PCV13-serotype colonization. In Beijing, S. pneumoniae carriage significantly declined during the COVID-19 pandemic.
1 Introduction
Streptococcus pneumoniae (S. pneumoniae), which can cause invasive and non-invasive diseases, is the main bacterial pathogen for community–acquired pneumonia (CAP) and mucosal diseases such as otitis media (1). S. pneumoniae frequently colonizes the human nasopharynx and is transmitted through respiratory droplets. The cross-sectional point prevalence of nasopharyngeal (NP) carriage in infants and young children, who are the main reservoir of this organism, ranges from 27% to 85% (2). NP carriage rates are particularly high among children in low- and middle-income countries (LMICs), as well as among children in high-income countries who belong to indigenous populations (1, 3). In China, there are limited data regarding S. pneumoniae carriage in healthy children; however, previous studies, based on pediatric inpatients with pneumococcal infections, indicated geographical differences in serotype distribution between northern and southern China (4). Therefore, the present cross-sectional study in China, designed to investigate the S. pneumoniae NP carriage rate, serotype distribution, and antimicrobial susceptibility patterns among healthy children aged 30 days to <60 months, involved one northern city and one southern city.
Until the 13-valent pneumococcal conjugate vaccine (PCV13) licensure in China in October 2016, PCV7 was available in the private market for children less than 5 years of age, although uptake was <10% (5). Likewise, PCV13 has been used in the private market, and it is not included in the national immunization program (NIP). PCV13 administration in children in China is based on the principle of “informed consent and voluntary payment”: from 2016, it was only for children less than 15 months of age (6); after 2023, the indication was expanded to the age of 5 years (7). Recent data show that the vaccination rate of PCV13 was increasing year by year in economically developed cities. For example, in Huangpu District in Shanghai, a study reported that the PCV13-Pfizer (full series) uptake increased from 2016 to 2019, ranging from 1.9% (2016), 15.6% (2017), 33.3% (2018), and 42.7% (2019) (8).
Currently, there are 21.5 million people living in Beijing, of whom about 13.5 million are classified as “local residents.” The annual birth cohort is approximately 170 thousand (9). In Beijing, the uptake of non-national immunization program (non-NIP) vaccines in the private sector, including PCV, varies significantly between years and districts. Data from one survey showed that non-NIP vaccine uptake ranged from 3.2% (influenza) to 95.8% (varicella) among children born between 2001 and 2006 (10). Another survey reported that the uptake of PCV7 in Beijing was approximately 18% among children born between 2011 and 2013 (11).
Shenzhen is a developed city with a population of 11 million, of whom 3.5 million are “local residents.” The annual birth cohort is approximately 71 thousand (12). The uptake for non-NIP vaccines is greater in Shenzhen than in other cities. For example, within one Shenzhen district, pneumococcal vaccine uptake [including PCV7 or the 23-valent pneumococcal polysaccharide vaccine (PPSV23)] among children younger than 7 years of age was estimated at 51% (13).
The present study was performed from January 2018 to November 2021, which covered the early stage of PCV13 availability for children <15 months of age, and the study extended into the first 2 years of the COVID-19 pandemic. From February 2020, measures were enforced which included mandatory self-isolation for individuals presenting with respiratory symptoms or fever, requirements for physical distancing maintaining a separation of over 1.5 m, and restrictions limiting the size of social gatherings.
2 Methods
2.1 Study design
This was a cross-sectional study designed to collect NP swabs among children aged 1–60 months living in Beijing (included two enrolling sites: Beijing Children's Hospital affiliated with Capital Medical University and the First Affiliated Hospital of Tsinghua University) and Shenzhen (included four enrolling sites: Shenzhen Children's Hospital and three Community Health Service Centers). The planned number of enrolled children was close to 5,000. Enrolled children were stratified into four age groups: 30 days to 6 months, 7–12 months, 13–24 months, and 25 to less than 60 months. The planned distribution aimed for approximately 25% of participants in each age group.
2.2 Enrollment and study procedures
Children aged 30 days to <60 months who resided in Beijing or Shenzhen were enrolled if determined to be healthy by medical history and the judgment of the investigator. Children with a major congenital malformation or serious chronic disorder were excluded, as were children who participated previously in this study. Children with any of the following conditions were temporarily excluded: (1) current upper or lower respiratory illness or a febrile episode (axillary temperature of ≥38.0°C) within the last 24 h; (2) using antibiotics within the previous 10 days; or (3) history of hospitalization or medical consultation for any type of illness within the previous 15 days. Informed consent was signed by the parents or legal guardians prior to any research procedures. A brief medical history was done, and details were obtained on demographics, previous antimicrobial use within the previous 10–30 days, possible risk factors (i.e., living in a residence with a smoker, living with one or more siblings, attending day-care, parent/guardian working in a medical institution, etc.), and the pneumococcal vaccination history (via the “Child Immunization Record Booklet”). Children with an incomplete, missing, or unclear “Child Immunization Record Booklet” were classified as having an “uncertain vaccination” status. During this visit, a single NP swab was collected by a medically qualified health care professional for the detection of S. pneumoniae.
2.3 Laboratory methods
NP swabs were taken using the World Health Organization (WHO)-recommended methodology. The specimens were processed based on the WHO recommendations for characterizing S. pneumoniae (14). Broth enrichment for 4 h was an approach used to increase the sensitivity of cultures (15). On the same day as swabbing, NP samples were inoculated onto Columbia agar (with 5% sheep blood and 5.0 μg/ml gentamicin) and were incubated aerobically at 37°C in 5% CO2 for 48 h. The suspected strains were identified using the optochin sensitivity (>14 mm with a 6-mm optochin disk incubated overnight in 5% CO2) and bile lysis tests. Strains that were positive for both tests were identified as S. pneumoniae and included in the study.
Serogroups were tested using the Pneumotest-Latex kit (Statens Serum Institute, Copenhagen, Denmark), and serotypes were determined by the Quellung reactions using factor antisera (Statens Serum Institute, Copenhagen, Denmark) as previously described (16). Non-serotypeable S. pneumoniae was defined as an optochin and bile solubility positive isolate with no reaction detectable under the microscope both to the Pneumotest-Latex kit for serogroup and to the Omni antiserum for serotype (Statens Serum Institute, Copenhagen, Denmark). Minimum inhibitory concentration (MIC) values to amoxicillin, penicillin (meningitis and non–meningitis), ceftriaxone, erythromycin, imipenem, meropenem, levofloxacin, linezolid, vancomycin, and sulfamethoxazole/trimethoprim were obtained by means of the E-test strips (PDM Epsilometer, AB Biodisk, Solma, Sweden) following Clinical and Laboratory Standards Institute 2019 criteria (17). Penicillin was interpreted according to the criteria for meningitis (intravenous) and non-meningitis (intravenous or oral) (18). Ceftriaxone was interpreted according to non–meningitis. S. pneumoniae American Type Culture Collection 49619 (ATCC49619) was used as the quality control strain and included in each set of tests. Multi-drug resistant (MDR) S. pneumoniae were defined as being resistant to three or more antimicrobials classes evaluated in this study. For the Penicillin-I class, the penicillin (oral) resistance rate was used to calculate the MDR.
2.4 Statistical analysis
Descriptive statistics were used to summarize the subjects' baseline characteristics. The confidence intervals (CIs) for the proportions were computed using the F distribution as described in the Collett method (19) and implemented in SAS PROC FREQ. Odds ratios (OR) and 95% CIs with the Wald method (20) were used to compare the carriage prevalence of S. pneumoniae and of PCV13 serotypes between age groups. The χ2 test and Fisher's exact test were used for significance comparison between groups via SPSS 26.0 (SPSS Inc., Cary, NC). P < 0.05 was deemed to indicate statistical significance.
3 Results
3.1 Characteristics of the study population
In total, 4,911 healthy children were involved in this analysis (Supplementary Figure 1), evenly distributed across the four age groups, which included 1,103 (22.5%) children aged 30 days to 6 months, 1,184 (24.1%) children aged 7–12 months, 1,240 (25.2%) children aged 13–24 months, and 1,384 (28.2%) children aged 25 to <60 months. Male subjects accounted for 54.6% (n = 2,683) of the enrolled children. The vaccination information of study participants is shown in Supplementary Table 1. There were 3 and 166 children previously vaccinated with 3 doses of PCV7 or a single dose of PPSV23, respectively. Among study participants, the proportion of children vaccinated with PCV13 in Shenzhen was 8.4% (208/2,476, 1 dose PCV13), 8.4% (208/2,476, 2 doses PCV13) and 20.4% (505/2,476, 3 doses PCV13), respectively, compared with 4.3% (105/2,435, 1 dose of PCV13), 4.0% (98/2,476, 2 doses of PCV13) and 16.3% (396/2,476, 3 doses of PCV13) in Beijing. Moreover, the proportion of children who completed 4 doses of PCV13 vaccination in Beijing was 13.2%, and it was 12.8% in Shenzhen.
3.2 Carriage rate and risk factors
The overall S. pneumoniae carriage prevalence was 13.1% (n = 645), with different carriage rates between Beijing and Shenzhen (8.3% vs. 17.9%, respectively) (P < 0.01). Carriage prevalence increased by age group. Overall, a 15.3% S. pneumoniae carriage prevalence was found in the oldest age group of 25 to <60 months, while the carriage prevalence was 15.1% in children 13–24 months, 13.2% in children 7–12 months, and 8.2% in children 30 days to 6 months (P < 0.01, Table 1). This pattern of increasing S. pneumoniae carriage prevalence with age was observed in both Beijing and Shenzhen. In Beijing, it was 5.6% in children aged 30 days to 6 months, 6.4% in children aged 7–12 months, 9.6% in children aged 13–24 months, and 10.7% in children aged 25 to <60 months; in Shenzhen, it was 11.3%, 18.1%, 19.2%, and 21.6%, respectively.
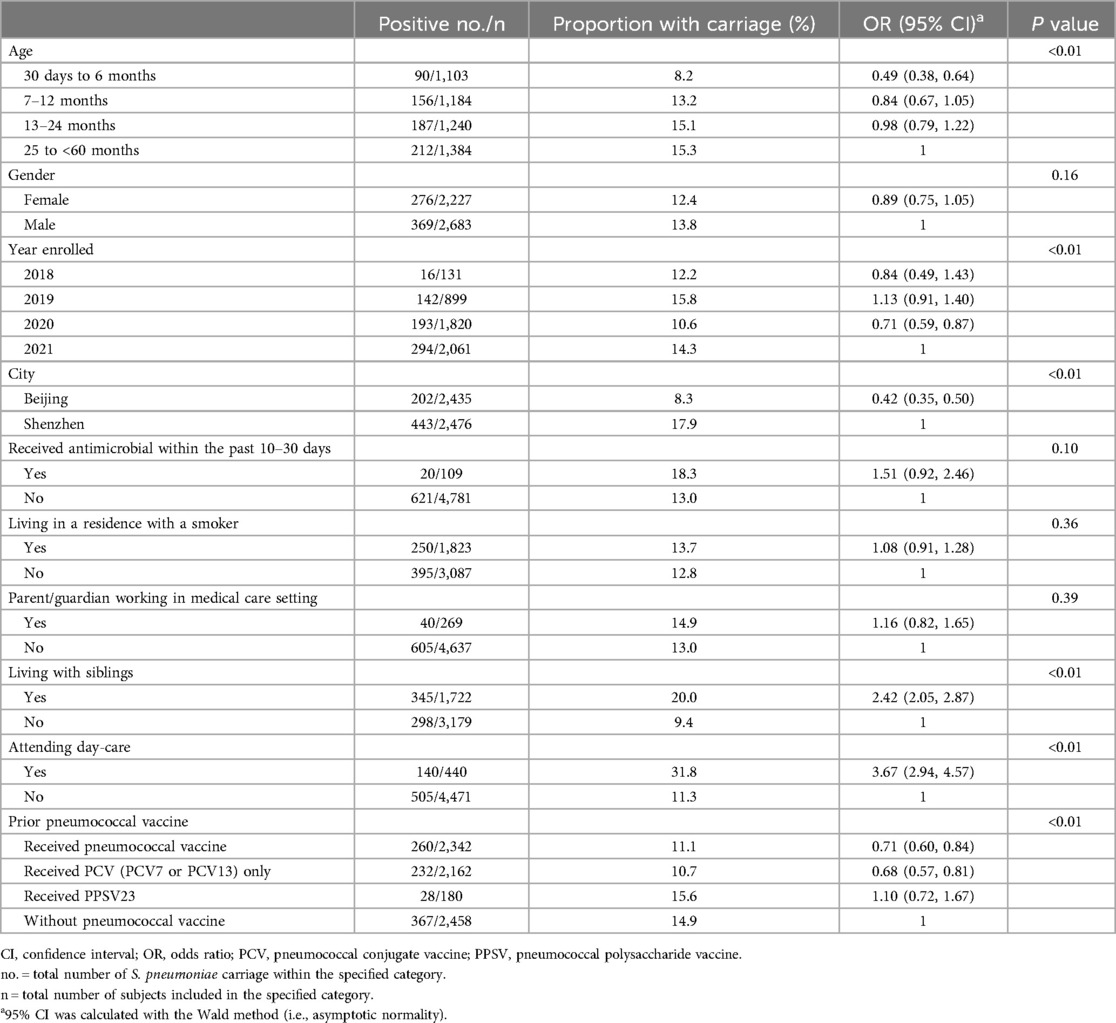
Table 1. Epidemiological factors associated with pneumococcal carriage in children aged 30 days to <60 months.
In Beijing, the strict administration of COVID-19 prevention and control measures began in January 2020. By study year, the highest S. pneumoniae carriage prevalence was found in 2019 (15.8%), in both study centers, and the lowest carriage prevalence occurred in 2020 (10.6%) (P < 0.01, Table 1). The carriage rate of S. pneumoniae in older children (25 to <60 months) in Beijing decreased from 22.2% (53/239) in 2018–2019 to 5.8% (32/557) in 2020–2021 (P < 0.01), which had a more marked change than in Shenzhen where the carriage rates were 22.0% in 2018–2019 and 21.4% in 2020–2021 (Supplementary Table S2).
The S. pneumoniae carriage rate was 20.0% in children living with siblings vs. 9.4% without siblings, OR: 2.42 [95% CI: 2.05–2.87]. The carriage rate of S. pneumoniae in children attending day-care was 31.8% vs. 11.3% for those not attending day care centers, OR: 3.67 [95% CI: 2.94—4.57. S. pneumoniae carriage prevalence associated with the history of PCV7 or PCV13 vaccination, which was 14.9% in children without pneumococcal vaccine, 11.4% in those who received 1, 2, or 3 doses of PCV7 or PCV13, and 9.4% in those children who received 4 doses of PCV13 (Supplementary Figure S2), while there was no effect of PPSV23 (15.6%). Other tested predictors of S. pneumoniae carriage were not statistically significant, including gender, receiving an antimicrobial within the past 10–30 days, living in a residence with a smoker, or parent/guardian working in a medical institution.
3.3 Serotype distribution
In this study, there were 645 S. pneumoniae isolates obtained from all participants included in the study, and among the 511 encapsulated S. pneumoniae isolates, 51 different serotypes were identified. Among all S. pneumoniae isolates, the most frequent serotypes were 19F (72/645, 11.2%), 6B (45/645, 7.0%), 23F (41/645, 6.4%), and 6C (34/645, 5.3%). The spectrum of carriage serotypes differed between Beijing and Shenzhen (Figure 1). Among isolates collected in Beijing, the most frequent serotypes were 19F (29/202, 14.4%), 23F (23/202, 11.4%), 6C (14/202, 6.9%), and 15A (10/202, 5.0%). Among isolates collected in Shenzhen, most frequent serotypes were 19F (43/443, 9.7%), 6B (36/443, 8.1%), 23A (23/443, 5.2%), 6C (20/443, 4.5%), and 15A (20/443, 4.5%).
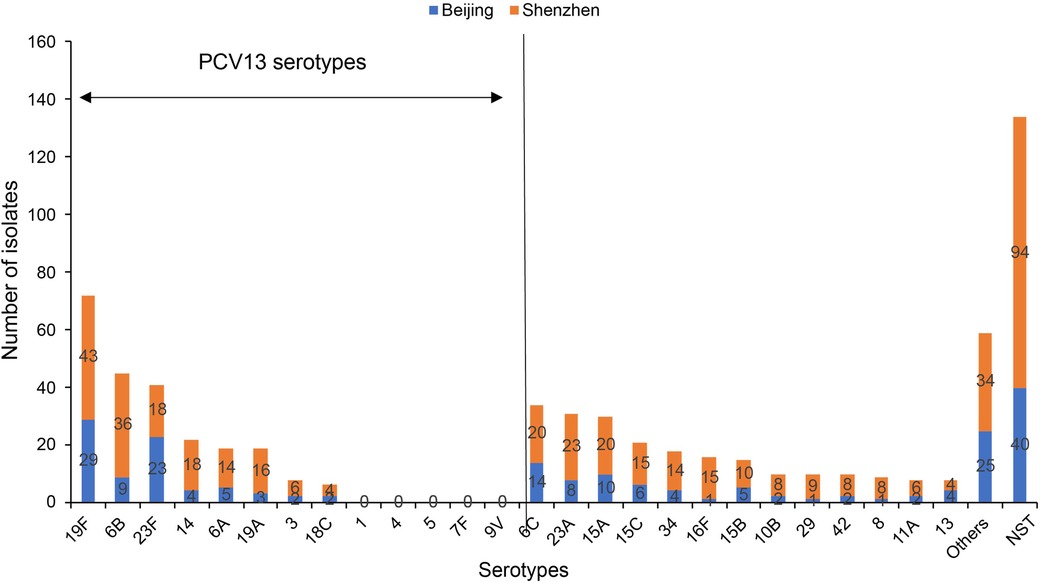
Figure 1. The most common pneumococcal serotypes identified in nasopharyngeal swabs collected from Chinese children aged 30 days to <60 months, shown by region (Beijing and Shenzhen). NST, non-serotypeable S. pneumoniae; PCV, pneumococcal conjugate vaccine. Others includes four isolates each for type 20,6D and 7C; three isolates each for type 9V, 10A,17F, 23B and 24F; two isolates each for type 1, 9N, 7A, 9A, 11B, 11C, 11F, 18B, 28F and 40; one isolate each for type 22F, 33F, 7B, 16A, 19B, 19C, 24B, 28A, 35F, 36, 37 and 43.
Among the 645 S. pneumoniae isolates (511 encapsulated and 134 non-serotypeable), the estimated serotype coverage for PCV7, PCV13, PCV15, PCV20, and PPSV23 was 29.3% (n = 189), 36.7% (n = 237), 37.1% (n = 239), 42.5% (n = 274), and 40.9% (n = 264), respectively. The serotype distribution and pneumococcal vaccine coverage are shown according to age group in Supplementary Figure S3. The vaccine coverage increased by age group. By contrast, the non-PCV13 serotypes coverage rates decreased significantly from 74.4% for 30 days to <6 months, 66.7% for 7–12 months, 64.7% for 13–24 months, and 54.7% for 25 to <60 months (χ2 = 12.448, P = 0.006). The most frequent non-PCV13 serotypes included: 6C, 23A, 15A and 15C. However, non-PCV13 serotype order differed slightly with PCV13 history: the top non-PCV13 serotypes were 15C, 15A and 23A among those fully vaccinated with PCV13; 15A, 23A and 6C among those partially vaccinated with PCV13; and 6C, 15A and 23A among those receiving no PCV13 (Figure 2).
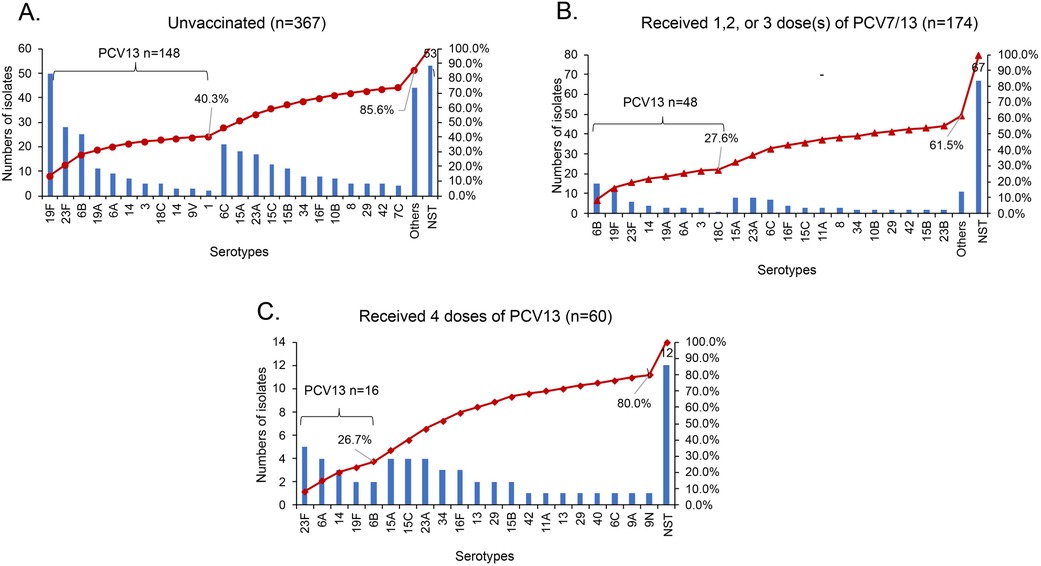
Figure 2. The serotype distribution of S. pneumoniae in nasopharynx of children aged 30 days to <60 months with different vaccination status. NST, non-serotypeable S. pneumoniae; PCV: pneumococcal conjugate vaccine. (A) Other includes three isolates each for type 11A, 17F, 20, 24F, 34 and 6D; two isolates each for type 10A, 11C, 11F, 13 and 42; one isolate each for type 11B, 16A, 18B, 19B, 19C, 22F, 24B, 28A, 28F, 7A, 7B, 9A, 13, 36, 37 and 43. (B) Other includes one isolate each for type 10A, 11B, 18B, 28F, 33F, 35F, 6D, 7A, 9N, 13 and 40.
The serotype distribution of S. pneumoniae in the nasopharynx of children aged 30 days to 60 months by vaccination status is shown in Figure 2. In unvaccinated children, PCV13 serotypes accounted for 40.3% of the total serotypes overall, and the most prevalent serotypes were 19F, 23F, and 6B (Figure 2A). Among the 174 children vaccinated with ≤3 doses of PCV13, PCV13 serotypes account for 27.6% of all isolates, and serotype 6B (8.6%) accounted for the highest proportion, followed by 19F (7.5%) and 23F (3.4%). In this group of 174 children vaccinated with ≤3 doses of PCV13, only 61.5% of the S. pneumoniae isolates were serotypeable S. pneumoniae (Figure 2B). Among the 60 children who received the 4-dose PCV13 series, the PCV13 serotypes accounted for 26.7% of all serotypes. The most common PCV13 serotypes included 23F (8.3%) and 6A (6.7%), and the predominant non-PCV13 serotypes were 15A (6.7%), 15C (6.7%), and 23A (6.7%). The serotypeable S. pneumoniae proportion was 80.0% (Figure 2C).
3.4 Antimicrobial susceptibility
The susceptibility pattern and MIC distribution to 10 antimicrobials of 644 S. pneumoniae isolates (1 strain isolated in Beijing failed to survive for drug susceptibility testing) are listed in Table 2. Most isolates were susceptible to amoxicillin (93.5%), penicillin (non-meningitis) (92.1%), ceftriaxone (88.8%), imipenem (75.0%), meropenem (68.5%), levofloxacin (98.9%), or linezolid (99.2%). Furthermore, all isolates evaluated were susceptible to vancomycin (100%). In contrast, an elevated proportion of resistant isolates was observed for penicillin (meningitis) (72.4%), erythromycin (96.3%), and trimethoprim/sulfamethoxazole (68.2%).
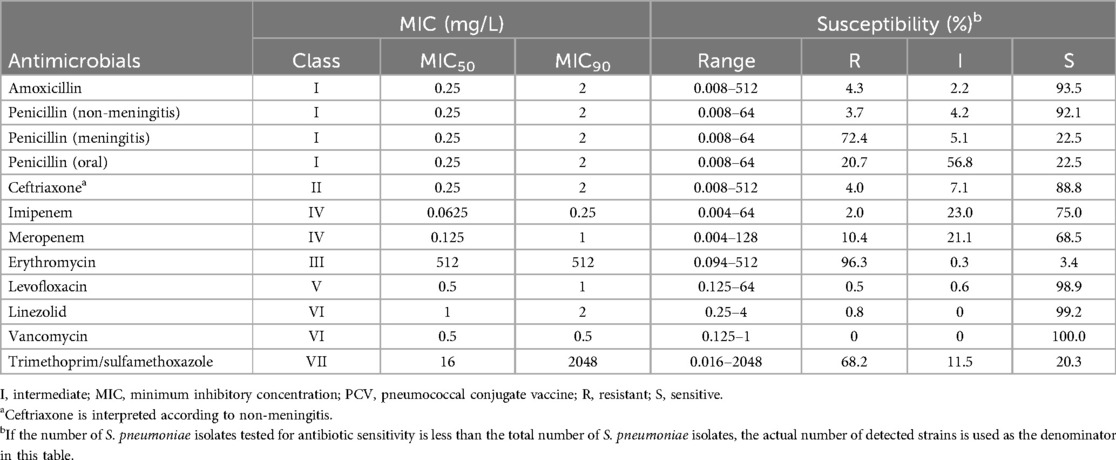
Table 2. Antimicrobial susceptibility test results of 644 isolates of S. pneumoniae isolated from Chinese children aged 30 days to <60 months, 2018–2021.
From a total of 158/644 isolates (24.5%), 21 serotypes (included 3, 6A, 6B, 6C, 6D, 7C, 8, 10B, 11A, 14, 15A, 15B, 15C, 16F, 18B, 19A, 19F, 23A, 23F, 24F, 34) were MDR. For the PCV13 covered isolates, MDR was detected in 36.3% (86/237) of isolates; in comparison, 17.1% (25/274) of non-PCV13 serotypes, including NST isolates, were MDR. In particular, PCV13 serotypes had a statistically significantly higher resistance rates to penicillin (meningitis) (87.3% vs. 66.3%; P < 0.01), penicillin (oral) (31.6% vs. 14.3%; P < 0.01), and meropenem (20.7% vs. 4.4%; P < 0.01) (Table 3). For PCV15, PCV20, and PPSV23 covered isolates, MDR was detected in 87/239 isolates (36.4%), 98/274 isolates (35.8%), and 91/264 isolates (34.5%), respectively.
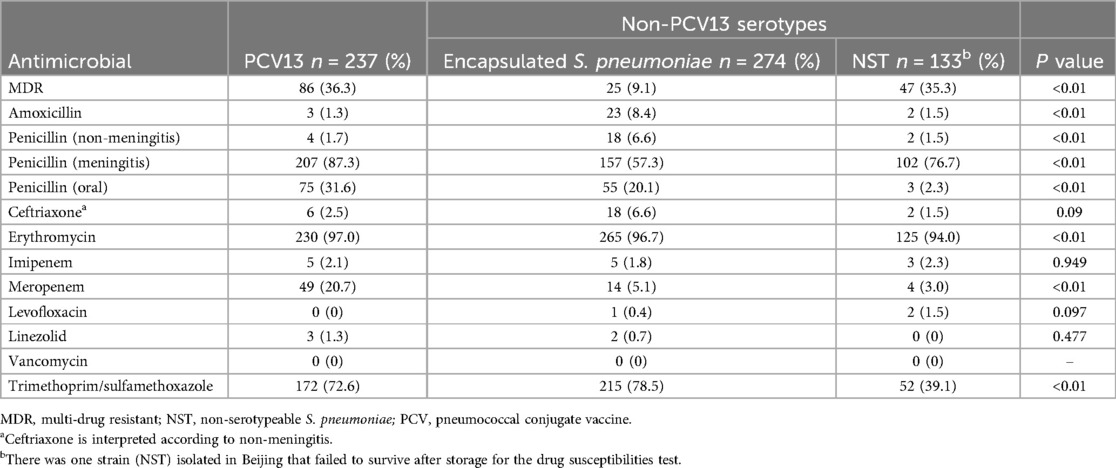
Table 3. Comparison of resistance rates between PCV13 and non-PCV13 isolates in Beijing and Shenzhen.
4 Discussion
In this study conducted among Chinese children 30 days to <60 months of age, overall S. pneumoniae NP carriage was 13.1%. These results were similar to the carriage prevalence reported from children younger than 5 years in Taiwan (12.0%, 60/500) (21), but lower than the NP carriage observed from other studies in Ghana (32.6%, 63/193) (22), Ethiopia (43.8%, 311/710) (23), or Turkey (17.8%, 103/580) (24). In a previous meta-analysis performed in China prior to the introduction of PCV7 that included children younger than 5 years of age, NP carriage was 24.4% (25). In an earlier study conducted between 2012 and 2014 by the Beijing Center for Disease Control and Prevention (CDC) among unvaccinated children 2–5 years old, it was reported that among 3,281 children aged 3.1 (±0.8) years, pneumococcal NP carriage was detected in 22% of the samples (25, 26). Potential explanations for the lower NP pneumococcal carriage observed among Chinese children in the current study could be related to: the increasing uptake in PCV13 among Chinese children (27); the fact that the study was conducted during COVID-19 pandemic with all the implemented recommendations in China to prevent viral respiratory transmission (28, 29); and the low number of children per family and the custom that children under 3 years of age are cared for at home generally by grandparents or nannies instead of at day care centers, as the entry for public day nursery is 3 years of age (30, 31).
We found that vaccination with PCV13 reduced pneumococcal carriage. While an indirect protection with PCV13 against PCV13 serotypes is well-established when PCV13 is included in the national immunization program, the results of the current study also suggest that even in the setting of private market uptake, there may have been an indirect benefit of carriage. As reported previously (32), we found that vaccination with PPSV23 did not influence NP carriage, reflecting an inherent limitation of plain polysaccharide vaccine.
Dominant serotypes differed slightly with PCV13 vaccination history: the top serotypes among unvaccinated children were 19F, 23F, 6B, and 6C, whereas serotypes 15A, 6B, 19F, 15A, 23A, and 6C were the most frequent serotypes among children partially vaccinated with PCV7 (3 children) or PCV13; and 23F, 6A, 15C, 15A, and 23A were the most frequent serotypes observed among children fully vaccinated with PCV13. The association between PCV vaccination and lower vaccine serotype NP carriage has been previously described (33). In Greece, for instance, where PCVs have been in the NIP since 2006, initially with PCV7 and since 2010 with PCV13 at an estimated four-dose uptake rate of about 80%, molecular surveillance of pneumococcal carriage following completion of immunization with the PCV13 (3 + 1 schedule) revealed that non-PCV13 serotypes represented 83.8% of total isolates. Serotypes 19A and 3 were the only two PCV13 serotypes that increased in proportion over the time of the study (P < 0.001 and P = 0.012, respectively) (33).
The percentage of non-serotypeable S. pneumoniae (20.7%) isolates observed in our study is consistent with results from studies conducted in other geographical locations (34–36), which helps to support the representativeness of the samples and the validity of the research results.
In the present study, children enrolled in Beijing had lower S. pneumoniae NP carriage compared with children enrolled in Shenzhen, which became most apparent in the 2020–2021 COVID-19 period. As noted, the carriage rate of S. pneumoniae in Beijing decreased significantly in the post-COVID period, but the carriage rate in Shenzhen did not. The decreasing carriage rate of S. pneumoniae in children in Beijing during the COVID-19 pandemic could be related to the continuous and strict prevention and control measures (such as universal masks, distancing, and hand washing). For instance, in Beijing closed kindergartens from February 2020 until the end of this study while Shenzhen did not. These measures contributed to limiting the transmission of COVID-19, but also reduced the spread of other pathogens. From January 2020 to February 2020, the spread of the epidemic in China had been first curbed. Most of the declared infectious disease incidences showed a downward trend in 2020 in China (37). Other settings outside of China did not observe such a COVID-19 pandemic effect. For instance, in a prospective cohort study in Israel, the mean proportion of children younger than 3 years old carrying pneumococcus, which was 44.3 ± 1.9% during 2016–2019, was somewhat reduced in October–December 2020, although the rates during January–February 2021 were not significantly different from those in the pre-COVID period (38). Nonetheless, unexamined interregional factors (e.g., climate, population characteristics, and economic levels) might have contributed to the observed differences in colonization in China during the COVID-19 pandemic and modified S. pneumoniae transmission.
To our knowledge, this present study (2018–2021) is the largest pneumococcal carriage study conducted in healthy children in China. Serotype 19F (11.2%) was the most prevalent S. pneumoniae carriage serotype, followed by 6B (7.0%), 23F (6.4%), 6C (5.3%), and 23A (4.8%). A meta-analysis among Chinese children in the post-PCV7 era (from October 2008 to 2016) reported that serotypes 19F, 6A, and 23F were most often isolated (24). A systematic review and meta-analysis of studies of carriage of S. pneumoniae and other respiratory bacterial pathogens in LMICs reported that serotypes 6A, 6B, 19A, 19F, and 23F were the serotypes most often isolated (35). In our study, the top five circulating serotypes included two non-PCV13 serotypes (6C and 23A). Researchers from Germany (39), Canada (40), Gambia (41) and Israel (42) suggested that increases in the carriage of non-PCV13 serotypes had offset reductions in the carriage prevalence of vaccine-targeted serotypes. Furthermore, the present study focuses on the children living in only two Chinese cities, where 47.7% of children had received at least one dose of PCV13. The present results demonstrated that 63.3% (408/645) of isolates obtained were non-PCV13 serotypes (e.g., 6C, 23A, 15A, 15C, 34, 16F, and 15B). Between 2014 and 2019, Xu et al. (43) reported that 12.5% (2/16) of fatal IPDs were caused by non-PCV13 serotypes (15A and 15B) that were not identified before the introduction of PCV13. Therefore, it is particularly important to monitor the serotype of S. pneumoniae in the nasopharynx of healthy children.
The highest antimicrobial resistance rates were observed for erythromycin (96.6%) or trimethoprim/sulfamethoxazole (68.2%), while resistance to penicillin (based on the breakpoints for non-meningitis isolates) and other beta-lactam agents was low (<5%). The MIC50 and MIC90 against erythromycin was >256 mg/L, which could reflect macrolide overuse in China (44). The high macrolide resistance is consistent with data reported in other Asian countries (45).
A total of 158 (24.5%) isolates were MDR. Vaccine-serotype pneumococcal isolates possessed more MDR than non-vaccine serotypes, which emphasizes the role for PCV immunization in the control of pneumococcal disease associated with antibiotic resistance. National vaccination using PCVs prevent episodes of both S. pneumoniae diseases and carriage, the latter which can inhibit the spread of antibiotic resistance (46). As non-PCV13 serotypes such as 11A and 24F are associated with antimicrobial resistance, further surveillance is needed (47). Of note, the MDR isolates of non-vaccine serotypes in this study increased year by year during the study period. We found that the rate of MDR non-PCV13 serotypes (including NST) in 2018 was 0.0%, followed by an increase towards 9.6% in 2019, 18.1% in 2020, and 18.7% in 2021. These MDR non-PCV13 strains can evade, at the same time, the pressures of vaccine-induced immunity and antimicrobial selection. The potential relationship between antimicrobial selective pressure and serotype replacement is a reminder that antimicrobials should be used properly to ensure that PCVs remains a powerful tool in the fight against antimicrobial resistance in S. pneumoniae.
One limitation of the present study is that the study was conducted in only in two cities with strong economies and wide availability of medical care, which does not reflect the diversity of China. Further investigations could provide more information about S. pneumoniae NP carriage. Other limitations of this study were a relatively modest pre-NIPs sample size. To compensate, we drew upon the pneumococcal NP swab carriage study conducted previous as an additional reference point (25, 26).
This investigation revealed that one-seventh or one-eighth of children <60 months of age carried S. pneumoniae in their nasopharynx in Beijing and Shenzhen, and like previous studies, S. pneumoniae carriage prevalence increased for subjects of older age (25 to <60 months), those living with siblings, attending day nursery, or who were unvaccinated with PCV13. The strict administration of measures to control COVID-19 led to a decrease in S. pneumoniae carriage rates, which was particularly evident in Beijing. Although PCV immunization uptake was limited, it showed a preventive effect (individual protection) on vaccine serotype S. pneumoniae carriage among vaccinated healthy young children in the community, providing evidence that control of S. pneumoniae carriage through vaccination could have a positive effect on antibiotic resistance.
Data availability statement
The original data contributions presented in the study are included in the article or supplementary material. Further inquiries can be directed to the corresponding author(s).
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
QD, AA: Writing – review & editing, Writing – original draft, Investigation, Data curation and Formal Analysis. ZL, SY: Writing – review & editing, Writing – original draft, Investigation, Data curation, Project administration. HW, YW, LL, XW, SY, QR, KP: Writing – review & editing, Project administration. EG, MF: Writing – review & editing, Supervision, Formal Analysis. GDCM: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was conducted as a collaboration between Beijing Children's Hospital Capital Medical University and Pfizer. Beijing Children’s Hospital Capital Medical University is the study sponsor. Pfizer provided funding for this study.
Acknowledgments
The authors thank the study participants and their guardians. We thank graduated students Ju Jia and Dandan Liu for data and single nasopharyngeal swab collection. Our thanks also extend to the respective hospital directors and to the clinicians from the seven participating health centers for facilitating the research and data collection. Editorial support for this manuscript was provided by Gandhali Deshpande, Sudipta Chatterjee, Melissa Furtado and Qi Yan at Pfizer and funded by Pfizer.
Conflict of interest
EG, AA, MF, and KP are employees of Pfizer and may hold stock or stock options.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1382165/full#supplementary-material
Abbreviations
ATCC, American type culture collection; CDC, Center for Disease Control and Prevention; CLSI, Clinical & Laboratory Standards Institute; LMICs, low- and middle-income countries; MDR, multi-drug resistant; MIC, minimum inhibitory concentration; NST, non-serotypeable S. pneumoniae; NP, nasopharyngeal; OR, odds ratio; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; SAS, statistical analysis system; STGG, skim milk–tryptone–glucose–glycerin; WHO, World Health Organization.
References
1. Francois Watkins LK, Milucky JL, McGee L, Siné St-Surin F, Liu P, Tran T, et al. Nasopharyngeal carriage of Streptococcus pneumoniae among young children in Haiti before pneumococcal conjugate vaccine Introduction. J Infect Dis. (2021) 224(12 Suppl 2):S248–57. doi: 10.1093/infdis/jiab119
2. Daningrat WOD, Amalia H, Ayu IM, Satzke C, Safari D. Carriage of Streptococcus pneumoniae in children under five years of age prior to pneumococcal vaccine introduction in Southeast Asia: a systematic review and meta-analysis (2001–2019). J Microbiol Immunol Infect. (2022) 55(1):6–17. doi: 10.1016/j.jmii.2021.08.002
3. Chan J, Nguyen CD, Dunne EM, Kim Mulholland E, Mungun T, Pomat WS, et al. Using pneumococcal carriage studies to monitor vaccine impact in low- and middle-income countries. Vaccine. (2019) 37(43):6299–309. doi: 10.1016/j.vaccine.2019.08.073
4. Lyu S, Hu HL, Yang YH, Yao KH. A systematic review about Streptococcus Pneumoniae serotype distribution in children in mainland of China before the PCV13 was licensed. Expert Rev Vaccines. (2017) 16(10):997–1006. doi: 10.1080/14760584.2017.1360771
5. Chenyan Y, Wei N, Zhu X, Wang QH, An ZJ. Investigation and analysis of pneumococcal vaccination in children in China. Zhong Guo Gong Gong Wei Sheng Za Zhi. (2018) 34(11):1468–70.
6. 13-Valent Pneumococcal Conjugate Vaccine Label Information. Prevenar 13. USA: Pfizer Inc. (2016). Available online at: pfizer.com (Accessed December 28, 2023).
7. 13-Valent Pneumococcal Conjugate Vaccine Label Information. Prevenar 13. USA: Pfizer Inc. (2023). Available online at: pfizer.com (Accessed December 28, 2023).
8. Wang J, Wu Q-S, Lu J, Ni Y-H, Zhou F. Low vaccination coverage of pneumococcal conjugate vaccines (PCVs) in Shanghai, China: a database analysis based on birth cohorts from 2012 to 2020. Vaccine. (2021) 39(42):6189–94. doi: 10.1016/j.vaccine.2021.09.011
9. Statistics. Available at Beijing Statistical Yearbook-2016. Beijing: Beijing Municipal Bureau of Statistics (2016). Available online at: https://nj.tjj.beijing.gov.cn/nj/main/2016-tjnj/zk/e/indexeh.htm (Accessed December 28, 2023).
10. Fu W, Chen ZC, Wang GB, Fu DP, Feng XL. Analysis of vaccine coverage situation of children aged from 0 to 6 in Zhongguancun area in Beijing from 2011 to 2016. Zhong Guo Wei Sheng Chan Ye Za Zhi. (2017) 14(16):3–10. doi: 10.16659/j.cnki.1672-5654.2017.16.003
11. Zhang WP. The category II vaccines: uses and current status among 0–3 years old children in Puhuangyu, Beijing. Shou Du Gong Gong Wei Sheng Za Zhi. (2017) 11(3):130–3. doi: 10.16760/j.cnki.sdggws.2017.03.013
12. Statistics. Shenzhen Statistical Yearbook. Shenzhen: Shenzhen Statistical Yearbook (2016). Available online at: http://www.sz.gov.cn/cn/xxgk/zfxxgj/tjsj/tjnj/content/post_1347277.html (accessed December 15, 2018).
13. Fang Q, Wang YG, Cai L. A cross sectional survey of the vaccination rate of NIP vaccine and non-NIP vaccine among local children and migrant children in Futian, Shenzhen in 2013. Pract Prev Med. (2015) 22(3):322–3.
14. Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the world health organization pneumococcal carriage working group. Vaccine. (2013) 32(1):165–79. doi: 10.1016/j.vaccine.2013.08.062
15. da Gloria Carvalho M, Pimenta FC, Jackson D, Roundtree A, Ahmad Y, Millar EV, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. (2010) 48(5):1611–8. doi: 10.1128/JCM.02243-09
16. Sørensen UB. Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol. (1993) 31(8):2097–100. doi: 10.1128/jcm.31.8.2097-2100.1993
17. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. In: 29th edition of Performance Standards for Antimicrobial Susceptibility Testing published by CLSI. Clinical and Laboratory Standards Institute. (2019). M100. Available online at: m100ed29_sample.pdf clsi.org (Accessed December 15, 2023).
18. Centers for Disease Control and Prevention (CDC). Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae–United States, 2006–2007. MMWR Morb Mortal Wkly Rep. (2008) 57(50):1353–5. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5750a2.htm (Accessed February 6, 2024).19092758
19. Collet D. Modelling Binary Data. London: Chapman & Hall (1991). 369. doi: 10.1007/978-1-4899-4475-7
20. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. (1998) 52:119–26. doi: 10.1080/00031305.1998.10480550
21. Janapatla RP, Su LH, Chen HH, Chang HJ, Tsai TC, Chen PY, et al. Epidemiology of culture-confirmed infections of Streptococcus pneumoniae (2012–2015) and nasopharyngeal carriage in children and households in Taiwan (2014–2015). J Med Microbiol. (2017) 66(6):729–36. doi: 10.1099/jmm.0.000488
22. Narwortey DK, Owusu-Ofori A, Slotved H-C, Donkor ES, Ansah PO, Welaga P, et al. Nasopharyngeal carriage of Streptococcus pneumoniae among healthy children in Kassena-Nankana districts of northern Ghana. BMC Infect Dis. (2021) 21:1–10. doi: 10.1186/s12879-021-06302-5
23. Wada FW, Tufa EG, Berheto TM, Solomon FB. Nasopharyngeal carriage of Streptococcus pneumoniae and antimicrobial susceptibility pattern among school children in south Ethiopia: post-vaccination era. BMC Res Notes. (2019) 12(1):306. doi: 10.1186/s13104-019-4330-0
24. Ceyhan M, Karadag-Oncel E, Hascelik G, Ustundag G, Gurbuz V, Samlioglu P, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children aged less than five years. Vaccine. (2021) 39(15):2041–7. doi: 10.1016/j.vaccine.2021.03.028
25. Wang L, Fu J, Liang Z, Chen J. Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae in China: a meta-analysis. BMC Infect Dis. (2017) 17(1):765. doi: 10.1186/s12879-017-2816-8
26. Hadjipanayis A, Efstathiou E, Alexandrou M, Panayiotou L, Zachariadou C, Petrou P, et al. Nasopharyngeal pneumococcal carriage among healthy children in Cyprus post widespread simultaneous implementation of PCV10 and PCV13 vaccines. PLoS One. (2016) 11(10):e0163269. doi: 10.1371/journal.pone.0163269
27. Liu L, Zhang Z, Zhang X, Xu C, Song Y, Li L, et al. Coverage of 13-valent pneumococcal conjugate vaccine among children 0–15 months of age—9 provinces, China, 2019–2021. China CDC Wkly. (2023) 5(17):379–84. doi: 10.46234/ccdcw2023.072
28. Rybak A, Levy C, Angoulvant F, Auvrignon A, Gembara P, Danis K, et al. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open. (2022) 5(6):e2218959. doi: 10.1001/jamanetworkopen.2022.18959
29. Petrović V, Milosavljević B, Djilas M, Marković M, Vuković V, Andrijević I, et al. Pneumococcal nasopharyngeal carriage in children under 5 years of age at an outpatient healthcare facility in Novi Sad, Serbia during the COVID-19 pandemic. IJID Reg. (2022) 4:88–96. doi: 10.1016/j.ijregi.2022.07.001
30. Hussen S, Asnake S, Wachamo D, Tadesse BT. Pneumococcal nasopharyngeal carriage and antimicrobial susceptibility profile in children under five in southern Ethiopia. F1000Res. (2020) 9:1466. doi: 10.12688/f1000research.27583.3
31. Lv M, Bai S, Sun Y, Zhang T, Li A, Wu J. Impact of the pneumococcal heptavalent conjugated vaccine on Streptococcus pneumoniae nasopharyngeal carriage and antimicrobial susceptibility in children 2–5 year old in Beijing, China. World J Vaccines. (2017) 7(03):27. doi: 10.4236/wjv.2017.73003
32. Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. (1996) 174(6):1271–8. doi: 10.1093/infdis/174.6.1271
33. Syrogiannopoulos GA, Grivea IN, Moriondo M, Nieddu F, Michoula AN, Calabrese MR, et al. Molecular surveillance of pneumococcal carriage following completion of immunization with the 13-valent pneumococcal conjugate vaccine administered in a 3+1 schedule. Sci Rep. (2021) 11(1):24534. doi: 10.1038/s41598-021-03720-y
34. Keller LE, Robinson DA, McDaniel LS. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. mBio. (2016) 22 7(2):e01792. doi: 10.1128/mBio.01792-15
35. Langereis JD, de Jonge MI. Non-encapsulated streptococcus pneumoniae, vaccination as a measure to interfere with horizontal gene transfer. Virulence. (2017) 8(6):637–9. doi: 10.1080/21505594.2017.1309492
36. Danino D, Ben-Shimol S, van der Beek BA, Givon-Lavi N, Avni YS, Greenberg D, et al. Decline in pneumococcal disease in young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis Off Pub Infect Dis Soc Am. (2022) 75(1):e1154–64. doi: 10.1093/cid/ciab1014
37. Chen B, Wang M, Huang X, Xie M, Pan L, Liu H, et al. Changes in incidence of notifiable infectious diseases in China under the prevention and control measures of COVID-19. Front Public Health. (2021) 9:728768. doi: 10.3389/fpubh.2021.728768
38. Adegbola RA, DeAntonio R, Hill PC, Roca A, Usuf E, Hoet B, et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS One. (2014) 9(8):e103293. doi: 10.1371/journal.pone.0103293
39. Weinberger R, von Kries R, van der Linden M, Rieck T, Siedler A, Falkenhorst G. Invasive pneumococcal disease in children under 16 years of age: incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine. (2018) 36(4):572–7. doi: 10.1016/j.vaccine.2017.11.085
40. Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007–2017. PLoS One. (2019) 14(12):e0226353. doi: 10.1371/journal.pone.0226353
41. Mackenzie GA, Hill PC, Jeffries DJ, Hossain I, Uchendu U, Ameh D, et al. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in the Gambia: a population-based surveillance study. Lancet Infect Dis. (2016) 16(6):703–11. doi: 10.1016/s1473-3099(16)00054-2
42. Regev-Yochay G, Reisenberg K, Katzir M, Wiener-Well Y, Rahav G, Strahilevitz J, et al. Pneumococcal meningitis in adults after introduction of PCV7 and PCV13, Israel, July 2009–June 2015. Emerg Infect Dis. (2018) 24(7):1275–84. doi: 10.3201/eid2407.170721
43. Xu Y, Wang Q, Yao K, Dong F, Song W, Liu G, et al. Clinical characteristics and serotype distribution of invasive pneumococcal disease in pediatric patients from Beijing, China. Eur J Clin Microbiol Infect Dis. (2021) 40(9):1833–42. doi: 10.1007/s10096-021-04238-x
44. Zhao C, Li Z, Zhang F, Zhang X, Ji P, Zeng J, et al. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates from 17 Chinese cities from 2011 to 2016. BMC Infect Dis. (2017) 17(1):804. doi: 10.1186/s12879-017-2880-0
45. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian network for surveillance of resistant pathogens (ANSORP) study. Antimicrob Agents Chemother. (2012) 56(3):1418–26. doi: 10.1128/aac.05658-11
46. Al-Lahham A. Multicenter study of pneumococcal carriage in children 2 to 4 years of age in the winter seasons of 2017–2019 in Irbid and Madaba governorates of Jordan. PLoS One. (2020) 15(8):e0237247. doi: 10.1371/journal.pone.0237247
Keywords: Streptococcus pneumoniae, nasopharyngeal carriage, pneumococcal conjugate vaccine, serotypes, antimicrobial resistance
Citation: Du Q, Liu Z, Wang H, Wang Y, Liu L, Wen X, Yu S, Ren Q, Gonzalez E, Arguedas A, Fletcher MA, Pan K, Morales GDC, Deng J and Yao K (2024) Nasopharyngeal carriage of Streptococcus pneumoniae among children aged 30 days to <60 months in Beijing and Shenzhen, China (2018–2021) during pneumococcal conjugate vaccine introduction and the coronavirus disease (COVID-19) pandemic. Front. Pediatr. 12:1382165. doi: 10.3389/fped.2024.1382165
Received: 7 February 2024; Accepted: 2 August 2024;
Published: 3 September 2024.
Edited by:
George A. Syrogiannopoulos, University of Thessaly, GreeceReviewed by:
Sónia Almeida, Universidade Nova de Lisboa, PortugalHelena C. Maltezou, National Public Health Organization (EHEA), Greece
Willem René Miellet, University Medical Center Utrecht, Netherlands
Copyright: © 2024 Du, Liu, Wang, Wang, Liu, Wen, Yu, Ren, Gonzalez, Arguedas, Fletcher, Pan, Morales, Deng and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jikui Deng, c3pzZXR5eWRlbmdAc2luYS5jb20=; Kaihu Yao, eWFva2FpaHVAYmNoLmNvbS5jbg==
†These authors share first authorship
‡ORCID:
Qianqian Du
orcid.org/0000-0002-5833-4527
Zhaoqiu Liu
orcid.org/0009-0002-5649-4049
Yani Wang
orcid.org/0009-0000-9579-498X
Li Liu
orcid.org/0009-0002-6537-6407
Xuexia Wen
orcid.org/0009-0002-1709-8473
Sangjie Yu
orcid.org/0009-0002-4995-2224
Qingqing Ren
orcid.org/0009-0007-7366-0463
Adriano Arguedas
orcid.org/0000-0002-1659-8225
Mark A. Fletcher
orcid.org/0000-0002-2660-8730
Kaihu Yao
orcid.org/0000-0003-1548-8670
 Qianqian Du
Qianqian Du Zhaoqiu Liu2,†,‡
Zhaoqiu Liu2,†,‡ Hongmei Wang
Hongmei Wang Adriano Arguedas
Adriano Arguedas Mark A. Fletcher
Mark A. Fletcher Jikui Deng
Jikui Deng Kaihu Yao
Kaihu Yao