
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 27 March 2025
Sec. Pediatric Pulmonology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1343870
This article is part of the Research Topic Pediatric Respiratory Critical Illness: Etiology, Diagnosis, and Treatment View all 8 articles
With the global rise in preterm birth rates, bronchopulmonary dysplasia (BPD) continues to be a significant problem, affecting morbidity and mortality in surviving preterm infants. Preterm infants are particularly susceptible to oxidative stress induced by sudden increases in oxygen concentration, which plays a crucial role in the pathogenesis of BPD. Herein, we addressed the pathophysiologic mechanisms, clinical treatment, and predictive biomarkers of BPD from an oxidative stress perspective. We first review the importance of oxygen in preterm infants and point out that sustained exposure to hyperoxia exacerbates the susceptibility of the immature lung to free radicals. The antioxidant properties of clinical therapies for BPD in preterm infants are then summarized. Subsequently, based on lipid, protein, and DNA damage mechanisms, we obtained the most comprehensive, accurate, and representative oxidative stress biomarkers. A total of 37 research papers on oxidative stress in BPD were collected. We conclude that 8-OHdG is the most promising biomarker for early prediction of BPD pathogenesis compared to lipid and protein oxidative stress biomarkers.
Preterm birth, defined as birth before 37 weeks of gestation, is a significant global health issue for infants (1). According to the World Health Organization's report, an estimated 15 million premature births occur annually worldwide, with preterm rates varying from 5% to 18% across countries in 2022. In 2019, preterm mortality accounted for approximately 47% of all under-5 deaths globally. The increasing incidence of preterm births has attracted considerable attention, becoming a focal point in medical research (1, 2). Several factors contribute to this trend, including changes in maternal age, with both very young and older mothers being at risk, as well as multiple pregnancies and high cesarean rates due to medical interventions. China, the second-most populous country in the world, contributes the second-highest number of preterm births, with more than 1 million births annually (3). Data from the Lancet Global Health indicates that the increasing preterm birth rate in China has accelerated since the adjustment of child policy. Two primary factors contributing to this surge include the rising maternal age at childbearing and the greater use of assisted reproductive technology, both of which elevate the multiple pregnancy rate and the proportion of late preterm births (4). Furthermore, infections caused by pathogens, chronic health conditions, inadequate prenatal care, socioeconomic disparities, and lifestyle factors such as smoking and substance abuse all play significant roles (5). Numerous clinical studies (6–8) and systematic reviews (9–12) have demonstrated the role of COVID-19 infection during pregnancy as a significant risk factor for adverse maternal and fetal outcomes, particularly preterm birth (5). These events indicate the urgent need for global attention to address the rising rates of preterm birth (13).
Premature neonates often struggle to adapt to the extrauterine environment due to their underdeveloped organs, making them vulnerable to various diseases, with BPD being particularly notable (14–16). It is noteworthy that approximately 80% of preterm infants aged 22–24 weeks and around 20% of those aged 28 weeks are affected by BPD (17). Despite advancements in perinatal medicine that have improved survival rates among premature infants, the incidence of BPD remains steady or even increases due to the rise in preterm births with underdeveloped lungs (13, 18, 19). Additionally, BPD, being a systemic condition, significantly impacts health and quality of life, leading to poor pulmonary function outcomes, an increased risk of requiring home oxygen therapy, and hospitalization for respiratory infections (20). Patients with BPD often experience airflow limitations, reduced gas transfer, and decreased lung density, which heighten their susceptibility to long-term chronic obstructive pulmonary disease (21, 22). Due to its high morbidity rates, diverse phenotypic variations, and substantial medical and economic burdens on healthcare systems, BPD has emerged as a prevalent and complex concern in perinatal medicine (17, 23–26).
Oxidative stress has long been recognized as a significant contributor to the development of numerous neonatal diseases, including BPD. Factors contributing to developing BPD-related oxidative stress include abrupt changes in postnatal oxygen tension, additional exposure to high oxygen levels due to respiratory insufficiency, deficient antioxidant mechanisms, infection, and inflammation. These factors increase oxidative burden, leading to lung injury and developmental abnormalities (27–31). Multiple studies have demonstrated elevated levels of oxidative stress biomarkers in newborns who develop BPD compared to those unaffected by the condition (32–36).
Clinical interventions for BPD encompass protective ventilation, pulmonary surfactants, steroids, caffeine, vitamin A, nitric oxide, and nutritional optimization (28). However, the efficacy and safety of some of these approaches remain controversial (31). Considering the theoretical potential of antioxidant therapy in mitigating oxidative stress and pulmonary injury in BPD (28, 31), this review aims to analyze these interventions from an antioxidant perspective to enhance their clinical applicability. Furthermore, research indicates an elevation in oxidative stress biomarkers even in the early stages of BPD, suggesting exposure to the detrimental effects of oxidative stress in immature lungs before developing BPD (37–40). Thus, early oxidative stress levels are critical for predicting BPD, aiding in disease severity assessment, and providing personalized precision therapies for affected preterm infants (27). Consequently, this review synthesizes the most accurate and representative oxidative stress biomarkers based on lipid, protein, and DNA damage mechanisms, discussing their utility in predicting BPD development.
Reactive oxygen species (ROS) are highly reactive oxidative molecules or ions primarily generated by endogenous oxidative metabolism processes. Moderate ROS production is crucial in cellular signaling transduction, apoptosis, proliferation, and inflammation within biological processes. However, excessive ROS levels or compromised antioxidant defense mechanisms can lead to oxidative stress, resulting in cellular structural and functional damage, consequently contributing to various diseases (41–43).
Postnatal risk factors such as high oxygen levels, hypoxia, ventilation, infections, and inflammation dramatically increase ROS production and contribute to BPD (28, 29, 41–43). Mechanical ventilation leads to alveolar overinflation and damage and triggers the activation of inflammatory signaling pathways and pulmonary fibrosis, a critical factor in developing classical BPD (27, 44). With the introduction of antenatal steroids, exogenous surfactant therapy, and protective ventilation strategies such as reduced tidal volumes and decreased invasive ventilation, these clinical interventions have somewhat reduced the incidence and mortality rates of oxidative stress and BPD (31, 45).
Advancements in healthcare have shifted the gestational age criteria for BPD diagnosis from extremely premature infants (gestational age less than 32 weeks) to very premature infants (gestational age less than 28 weeks) (44), exposing the lungs to high oxygen levels during the late canalicular or early saccular stages (17, 46). Premature birth transitions the lungs from the low-oxygen environment of the uterus to the high-oxygen environment of indoor air, exposing them to relatively high oxygen concentrations (30). Fetuses in utero only need to cope with blood oxygen tensions of 25–30 mmHg and benefit from maternal antioxidant protection, thereby avoiding oxidative stress (47). In comparison, preterm infants must cope with oxygen tensions of 60–100 mmHg for an extended period with immature lungs. Furthermore, the alveolar gas exchange surface area at these stages is incompletely developed, unable to provide sufficient oxygen for metabolism (48, 49). Oxygen therapy has become a necessary standard treatment to prevent newborns from dying due to respiratory failure (13). These factors make infants prone to oxidative stress due to exposure to high oxygen concentrations, leading to the development of BPD. Notably, the endogenous antioxidant system within immature lungs fully matures shortly before full-term delivery (41). Consequently, the excess ROS cannot be effectively eliminated, leading to ROS accumulation and oxidative stress. Excessive ROS induces apoptosis and dysfunction of alveolar epithelial type II cells responsible for synthesizing and secreting pulmonary surfactant (29), impairing lung vascular and alveolar development (28), thus contributing to the emergence of the new phenotype of BPD (50, 51).
The treatment landscape for BPD continues to evolve, yet achieving satisfactory outcomes remains elusive (17, 52, 53). Both basic researchers and clinicians are challenged by the iatrogenic injuries associated with improving the survival of preterm infants (54). Given the central role of oxidative stress in BPD pathogenesis, strategies targeting antioxidants hold promise for prevention and treatment (42, 55). Numerous studies on antioxidant therapy and reviews summarize the clinical treatment methods (56–60). However, the antioxidant aspects of the clinical treatment of BPD remain essential. Therefore, this article reviews current clinical approaches to BPD treatment from an antioxidant perspective.
We searched the PubMed database for published clinical studies covering the neonatal (birth-1 month) up to 2023. The search strategy included medical subject headings (MeSH headings) and free text terms related to antioxidants, pulmonary surfactants, vitamin A, vitamin E, vitamin D, caffeine, nutritional interventions, and BPD.
Since the certified importance of pulmonary surfactants in enhancing the survival of preterm infants (61), the administration of prenatal corticosteroids to boost endogenous surfactant production before birth and the introduction of exogenous surfactants after birth have been pivotal milestones in neonatal medicine (62). These developments have altered the original definition of BPD proposed by Northway in 1967 and launched the post-surfactant era (20, 63).
Pulmonary surfactants coat the alveolar surface and are complex mixtures of phospholipids and proteins (64, 65). The alveoli of preterm infants are at the stage of the late canalicular or early saccular periods during which type II alveolar cells have not fully developed, leading to alterations in the quantity, quality, or composition of surfactant secretion (66). Additionally, surfactant proteins are influenced by hyperoxia, potentially prolonging the need for ventilator support and increasing the risk of BPD (67–69). Studies in preterm infants have demonstrated that natural surfactant treatment reduces oxidative stress parameters in tracheal aspirates from ventilated infants (25, 70–72). Firstly, exogenous surfactant therapy can improve neonatal adverse outcomes by reducing inhaled oxygen concentration and exogenous ROS formation during oxygen therapy (68, 73). Secondly, surfactant proteins inhibit inflammatory processes and enhance microbial clearance (66), which also play a vital role in reducing the production of endogenous ROS to some extent (74). Thirdly, natural surfactants contain polyunsaturated phospholipids along with enzymatic antioxidants like SOD and CAT (75–77), thereby shielding the cell membrane from the assault and damage caused by ROS. These properties also underscore the susceptibility of lungs with an insufficient antioxidant system to oxidative damage under hyperoxia (78).
Since the beginning of the last century, natural surfactants have been regarded as superior to synthetic surfactants in enhancing respiration, reducing mortality, and lowering the incidence of BPD (79, 80). Treatment with poractant alfa may offer more advantages than calfactant and other animal-derived surfactants in preventing BPD (81, 82), potentially due to its lower dipalmitoyl phosphatidylcholine value and higher activity of antioxidant components both in natural surfactants and poractants (83). Additionally, the differentiation of epithelial type II alveolar cells and the surfactant production rate may be regulated by endogenous glucocorticoids and accelerated by exogenous glucocorticoids, primarily through the regulation of gene expression associated with increased surfactant protein synthesis, which has been extensively reported in animal experiments (75, 84–87). Although antenatal corticosteroids are commonly regarded as a routine approach to promoting fetal lung maturation in preterm birth, there remains controversy surrounding their postnatal use for preventing BPD, including issues related to the timing of administration, choice of agents, and routes of administration (88, 89).
Vitamin A, the best-studied non-enzyme antioxidant in BPD, plays a crucial role in regulating fetal lung development and maturation, maintaining the integrity of respiratory epithelial cells, influencing pulmonary vessel development, reducing the need for supplemental oxygen in premature infants, and ultimately decreasing premature infant mortality (25, 90). Many investigations suggest that vitamin A deficiency is prevalent in very-low-birth-weight infants from birth to term (91, 92). The deficiency in preterm infants may be related to the maternal vitamin A level or inefficient placental transmission, which leaves them malnourished (93, 94). Vitamin A deficiency is also associated with BPD, and there has been considerable evidence that supplementation can reduce the mortality of BPD and infant mortality (95–97).
In terms of sources, the two primary forms of vitamin A in our bodies are animal-derived (including retinol and its derivatives) and plant-derived carotenoids (such as α-carotene, β-carotene, and β-cryptoxanthin) (25, 98). As retinol is the main active form of vitamin A in the human body and has high absorption, the types of vitamin A used in clinical practice are retinol and its derivatives (98). Animal-derived vitamin A can act as a chain-breaking antioxidant, preventing cell damage by inhibiting the interaction of peroxyl radicals with lipids to produce hydroperoxides (99, 100). Despite the lower absorption and conversion rates compared to retinol, it is still worth exploring whether carotenoids can reduce the incidence of BPD, primarily owing to their direct antioxidant activities in scavenging singlet oxygen and peroxide-free radicals (101, 102), as well as increasing the production of enzymatic antioxidants (103, 104).
Currently, research on the delivery route of vitamin A primarily focuses on intramuscular and oral administration. While intramuscular administration can effectively reduce BPD and infant mortality rates, it is costly and associated with painful side effects (105, 106). The efficacy of oral administration in reducing BPD incidence remains debated. Additionally, intratracheal administration shows promise due to its ability to increase retinol concentration in tissues like serum, liver, and lungs in animal models, indicating its feasibility and warranting further investigation (107–109). Although the preventive effect of vitamin A on BPD is still controversial, it has become more widely used as a result of the increasing rate of very-low-birth-weight infants (18, 110–112).
Like vitamin A, vitamin E is also a crucial fat-soluble non-enzymatic antioxidant with potent anti-inflammatory and antioxidant properties, playing a significant role in embryonic lung development (113, 114). Research demonstrates that the fetus primarily obtains vitamin E from the mother through the placenta (115). Fetal levels of vitamin E are closely correlated with maternal levels during the same period and increase with gestational age (116, 117). Given our inability to synthesize this nutrient, premature and low birth weight infants commonly experience vitamin E deficiency, increasing the risk of adverse outcomes such as BPD (115, 118, 119). Moreover, premature infants have an increased demand for non-enzymatic antioxidants compared to full-term infants. Hence, additional vitamin supplementation may lower the incidence of BPD (117, 120, 121). However, research on the effectiveness of vitamin E is limited in duration, quantity, and depth when compared to vitamin A. Most studies on oral and intravenous administration of vitamin E have failed to demonstrate a reduction in BPD incidence and have even led to adverse outcomes such as infant death and necrotizing enterocolitis (122–124). Consequently, vitamin E supplementation was temporarily suspended in the late 1990s.
More recently, Ogihara and Mino discussed the research findings on BPD conducted during the pre-surfactant era in the 1980s and early 1990s (115). They suggested that these findings may not apply to modern neonatal care due to significant differences in the definition of prematurity between the pre-surfactant and post-surfactant eras (25, 55, 115). Since 2000, studies have re-confirmed the relationship between vitamin E deficiency and the severity of BPD (125, 126), although progress remains slow and the outcomes are preliminary (127–129). Since vitamin E is an essential component of pulmonary surfactant and plays a crucial role in the post-surfactant era (119, 130, 131), it is unsurprising that vitamin E possesses anti-inflammatory and antioxidant properties that can mitigate the incidence of BPD. In addition, high levels of vitamin E have been detected in breast milk, indicating its essentiality for newborns with specific nutritional needs, especially in low birth weight infants (118, 132, 133). Although various isomers of vitamin E have similar antioxidant functions in preventing lipid peroxidation (114, 134), high doses of α-tocopherol were widely used due to its availability in the human body during the pre-surfactant era (117, 135). Data on the effectiveness of specific subtypes and different routes of administration for vitamin E supplementation trials on lung health are necessary to determine whether re-challenging in modern neonatal intensive care units is worthwhile (114, 115, 130), which may improve lung development and can reduce the adverse outcomes caused by the injection and oral administration.
Since 2000, there has been an increase in studies examining the relationship between vitamin D deficiency and BPD. Apart from its various immunomodulatory and anti-inflammatory functions (136), it possesses indirect antioxidant properties that may alleviate conditions induced by oxidative stress, such as diabetic retinopathy (137), endothelial dysfunction (138), skin aging (139), and mood disorders (140). The potential mechanism for reducing oxidative stress involves vitamin D enhancing the expression of SOD2, glutathione, and nuclear factor NRF2, which are responsible for antioxidant enzyme expression (137–139, 141).
The vitamin D level of early neonates is closely linked to that of their mothers and gestational age, as the fetus obtains vitamin D from the mother via the placenta (142). As a result of modern lifestyles, vitamin D deficiency is prevalent in pregnant women (143), impacting serum vitamin D levels in preterm infants and subsequently impairing lung development (144). Several clinical studies and reviews have established an association between neonatal BPD and low vitamin D levels, with a higher incidence observed in the vitamin D deficiency group (127, 144–146). A low level of 25-hydroxyvitamin D in the bloodstream is a valuable predictor for the prediction of BPD (147–149). Although early vitamin D supplementation has shown promise in significantly increasing serum levels of 25(OH)D3, reducing inflammatory responses, and decreasing the incidence of BPD in preterm infants, more extensive clinical trials with varying doses are still necessary (150–154).
Recent research has demonstrated that vitamin D administration improves alveolar structural simplification induced by hyperoxia and elucidated underlying mechanisms from the perspective of reducing inflammation (155–161). Vitamin D administration reduced the expression of proinflammatory cytokines IL-6, IFN-γ, TNF-α and IFN-γ (156–158), regulated the balance of M1 and M2 macrophages by decreasing the expression of IL-10 and Arg-1 (155), protected neonatal rats from hyperoxia-induced BPD by regulating the vitamin D-VDR signaling pathway (158), antagonized the activation of TLR4 (159), contributed to the recovery of mitochondrial morphology (156), reduced cell apoptosis (156, 159), and promoted the growth of vascular structures (157). In addition, low doses of vitamin D improve the formation of alveolar and pulmonary vascularization in BPD by inhibiting neutrophil extracellular traps under hyperoxia, whereas higher doses may lead to more severe outcomes (157). Moreover, inhaled vitamin D is crucial for promoting surfactant phospholipid synthesis, which is vital in reducing oxidative stress caused by hyperoxia exposure (161). Overall, due to its potent antioxidant properties and the potential benefits of inhalation over oral administration in promoting neonatal lung development (137, 161), further studies on vitamin D supplementation from an antioxidant perspective are warranted to reduce the incidence of BPD.
Caffeine, a methylxanthine drug, has been widely used to treat apnea of prematurity for decades and is one of the few pharmacological interventions that has been shown to significantly reduce the risk of BPD in preterm infants (162). Caffeine treatment primarily enhances diaphragmatic contractility, increases minute ventilation, and stimulates the central nervous system, effectively improving respiratory function in preterm infants. These mechanisms help reduce the need for mechanical ventilation, improve lung function, and facilitate successful extubation (163). Therefore, caffeine therapy plays a crucial role in lowering the incidence of BPD. Multiple clinical trials have supported the clinical benefits of caffeine in treating and preventing BPD (163). As a result, current research on caffeine therapy mainly focuses on the timing of treatment and dosage. Although increasing evidence supports the early use of caffeine in preterm neonates, formal guidelines specifying the exact timing to start treatment have yet to be established. Clinical trials are needed to determine the optimal timing for caffeine administration and to identify the infant population that would benefit most from early caffeine therapy. Further studies are also necessary to validate and elucidate the precise impact of early caffeine treatment on complications in preterm infants.
Yan et al. found that early administration of caffeine can reduce the severity of BPD by approximately 60% (164). Similarly, Chen et al. reported that caffeine administration within the first three days of life shows promising results in preventing severe BPD and mortality in extremely preterm infants (165). Other studies have indicated that early preventive use of caffeine citrate not only significantly reduces the incidence of BPD in preterm infants but also decreases the occurrence of other complications. Ye et al. found that early preventive administration of caffeine citrate reduced the risk of later free radical disease in preterm infants, including BPD (166). Jiang et al. found that early preventive use of caffeine citrate is more effective than standard caffeine treatment in reducing the incidence of BPD in preterm infants (167). Szatkowski et al. reported that an increased proportion of early preventive caffeine use is associated with a reduced risk of BPD and brain injury in preterm infants (168). By comparing early preventive use of caffeine citrate (within 72 h after birth) and standard caffeine treatment, Elmowafi et al. found that the former reduces the duration of oxygen therapy, ventilation needs, and the incidence of mild to moderate BPD in preterm infants (169). Lamba et al. found that early high-dose caffeine therapy (10 mg/kg/day) lowers the risk of moderate to severe BPD without increasing the incidence of measured complications (170). Rauf et al. observed that early initiation of high-dose caffeine can prevent apnea and extubation failure in preterm neonates (171). Additionally, some studies suggest that early caffeine treatment may lead to complications and even increase mortality rates. Taha et al. found that early preventive use of caffeine citrate improves survival rates in preterm infants without BPD. However, it also increases the risk of fatal necrotizing enterocolitis (172). Similarly, research by Dobson (173) and Yun (174) indicates that while early oral caffeine treatment reduces the incidence of BPD, it is accompanied by an increased mortality rate.
Evidence suggests that caffeine and its methylxanthine metabolites may reduce oxidative stress by modulating inflammation-related pathways. Caffeine inhibits oxidative stress by suppressing the activation of IRE1 and PERK induced by endoplasmic reticulum stress to prevent skin senescence (175) or by activating A2AR/SIRT3/AMPK-mediated autophagy in the leptin-induced phosphorylation of STAT3 (176). Caffeine treatment has also been shown to reduce oxidative stress by enhancing the activity of antioxidant defense enzymes, mitigating DNA damage, and modulating transcription, indicating its antioxidant function to some extent (23). Recent clinical evidence indicates that caffeine's protective effect on neonatal lung health might involve reducing the expression of genes such as MMP9, TNF-α and TLR4, thus alleviating pulmonary inflammation which may be a mechanism behind the significant reduction in BPD incidence observed in the caffeine treatment group (167). Furthermore, although there is no statistically significant difference in BPD incidence between preventive and treatment groups, the preventive group showed significantly lower levels of IL-6 and IL-8 than the treatment group (177). This reduction in cytokine levels may contribute to a lower incidence of BPD.
Although clinical studies have not yet clarified how caffeine reduces the incidence of BPD through mediating redox pathways (178), a series of cellular and animal experiments indicate that caffeine has antioxidant properties. In a cellular model of BPD induced by hyperoxia, caffeine may reduce apoptosis, promote proliferation, and alleviate oxidative stress by inhibiting the A2AR/cAMP/PKA/Src/ERK1/p38MAPK signaling pathway, thus preventing lung damage (179). In the animal model of BPD, caffeine treatment significantly mitigates cell death and changes in apoptosis-related factors induced by hyperoxia (180) and protects murine lungs from oxidative damage by inhibiting the NLRP3 inflammasome and NF-κB pathways, which reduces apoptosis in type II alveolar epithelial cells (181). Additionally, caffeine treatment may protect developing lungs from injury induced by hyperoxia by alleviating endoplasmic reticulum stress (182).
Given caffeine's important role in the prevention and treatment of BPD, there is still a lack of comprehensive understanding of its molecular mechanisms, particularly regarding whether caffeine treatment can reduce lung injury by alleviating oxidative stress. Therefore, further research in this area is essential.
Premature infants fail to regulate inflammatory immune responses, resulting in sustained lung injury and chronic pulmonary inflammation (183). However, specific functional nutrients with antioxidant properties may play a role in reducing pulmonary inflammation (184). The primary consideration here is the potential mechanism of breast milk as an antioxidant therapy, as single antioxidant therapy for BPD has not yielded the expected clinical outcomes (28). Yang et al. indicated that breast milk is the safest, most natural, and most comprehensive infant nutrition (28). It provides all the necessary calories, proteins, and lipids for newborn growth and development, along with various antioxidants such as unsaturated fatty acids, vitamins, trace elements, glutathione (GSH), SOD, glutathione peroxidase (GSH-Px), melatonin, probiotics, short-chain fatty acids, and lactoferrin, offering robust antioxidant capabilities to newborns (28, 185).
In clinical practice, breastfeeding has been shown to reduce the incidence of BPD in premature infants significantly. Compared to formula feeding, both exclusive breastfeeding and pasteurized donor human milk feeding have resulted in a lower incidence of BPD in premature infants (186, 187). A higher incidence of BPD was also found in preterm infants who received pasteurized or frozen breastfeeding compared to exclusive breastfeeding (188, 189). The possible reasons are as follows. Although carbohydrates remain relatively intact, many components in breast milk change during freezing, pasteurization, and subsequent reheating. Freezing breast milk increases the formation of lipid peroxides, which can damage cell membranes, increase oxidative stress, and potentially lead to cellular injury. Furthermore, freezing decreases the concentration of bioactive proteins, such as secretory immunoglobulin A, lactoperoxidase, and lysozyme in breast milk, which is crucial in combating oxidative stress and maintaining immune balance. Moreover, the processes may reduce the content of antioxidants in breast milk and lead to the loss of activity of immune cells and stem cells, which are vital for protecting infants from oxidative damage and promoting tissue repair (186–189).
The amount of breast milk intake in premature infants is negatively correlated with the incidence of BPD (190). For premature infants breastfed from birth to 36 weeks postmenstrual age, each 10% increase in breastfeeding was accompanied by a 9.5% reduction in the risk of developing BPD (191). Based on these studies, breast milk seems beneficial in preventing and treating BPD. Given the various antioxidants in breast milk, we believe this is a primary mechanism by which breast milk helps reduce BPD (28).
Currently, it remains uncertain whether individual components of breast milk can function independently as antioxidants due to inconsistent findings in this area (28). For instance, taking Omega-3 polyunsaturated fatty acids as an example, the risk of BPD increases with decreased DHA levels and increased LA (192). The incidence of BPD was reduced in infants whose mothers received a DHA diet compared to those who did not (184). However, some studies have found that Omega-3 polyunsaturated fatty acid interventions do not affect the incidence of BPD (193, 194). Similar results are seen with trace elements, vitamins, and lactoferrin (28, 185). Possible reasons for this inconsistency include the limited efficacy of single-ingredient antioxidants for treating BPD.
Among all antioxidant enzyme replacement therapies for preclinical strategies, recombinant human SOD has shown the most promising outcomes (25). Though no positive effect on mortality and morbidity was observed at 36 weeks postmenstrual age in the treatment of intratracheal recombinant human SOD, the number of infants with respiratory sequelae or requiring pulmonary resuscitation decreased at 1-year corrected age (195). A clinical trial has also investigated the role of recombinant human SOD therapy in alleviating ROP, a form of oxygen radical disease. The incidence of ROP was significantly reduced in infants younger than 25 weeks (196). Despite these advancements, progress in recombinant human SOD therapy has been slow over the years. In animal trials, overexpression of SOD at the transcriptional level (59, 60), oral supplementation of coenzyme Q10 (57), caffeine (95), and chrysin treatment (197) could alleviate lipid oxidative stress, decrease alveolar damage, and improve lung function. Research has shown that glucosinolate and quercetin can reduce lung inflammation by regulating transcription factors or antioxidant-related proteins (56, 58). Although antioxidant treatments have shown efficacy through histopathological assessment of target organs in many animal models, translating basic research findings into clinical practice remains challenging (198, 199).
The previous chapter described possible mechanisms for reducing oxidative stress through treating BPD in the clinic. Currently, there is a lack of studies linking oxidative damage to outcomes. However, there is a strong need to identify validated biomarkers of oxidative stress, which would provide a theoretical basis for clinicians to develop preventive and immediate adjustments to therapeutic strategies, improve the prognosis of preterm infants, and reduce the burden of this condition on the preterm infant. Although biomarkers of oxidative stress have been identified in different tissues or body fluids, many of these biomarkers do not correlate well with BPD, do not reflect the state of oxidative stress, or lack specificity. Therefore, the focus should be on understanding which markers can be practically applied in the clinic to predict the occurrence of BPD.
Overall, oxidative stress can be categorized into four broad categories: “antioxidant defenses”, “lipid peroxidation”, “nucleic acid oxidative damage”, and “oxidative damage to proteins”. The antioxidant defense system consists of enzymatic and non-enzymatic categories. The former mainly include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), thioredoxin reductase (TRX), peroxiredoxin (PRX), and glutathione S-transferase (GST), while the latter mainly includes metal-binding proteins (MBP), ascorbate (AA), Vitamin E, Vitamin C, uric acid, and GSH (200).
Structural lipids, also known as membrane lipids, are essential components of cells, such as the plasma membrane, Golgi apparatus, and endoplasmic reticulum. They are composed of more than 70% phospholipids and 10%–20% cholesterol (Ch). Each phospholipid consists of two esterified fatty acyl chains, one saturated (sn-1 chain) and the other unsaturated (sn-2 chain). The unsaturated chain includes linoleic acid (LA, C18:2, omega6), arachidonic acid (AA, C20:4, omega-6), conjugated linoleic acid (CLA, C18:2, omega6), eicosapentaenoic acid (EPA, C20:5, omega-3), docosahexaenoic acid (DHA, C22:6, omega-3), and alpha-linolenic acid (ALA, C18:3, w-6). Among these, LA, AA, DHA, and Ch are the most abundant unsaturated fatty acids in mammalian cell membranes, and their double bonds are susceptible to oxidation under oxidative stress, leading to lipid peroxidation (201).
Lipid peroxidation, driven by a free radical chain reaction, consists of three reaction phases: initiation, propagation, and termination (202, 203). In the initial phase, hydrogen atoms in the methylene groups of the unsaturated fatty acids side chains are captured by pro-oxidants such as hydroxyl radicals (OH•) to produce lipid radicals (L•). During the propagation phase, L• reacts with O2 to form lipid peroxyl radicals (LOO•). LOO• can further extract hydrogen atoms from neighboring PUFA residues to generate new L• and lipid peroxyl radicals (LOOH), and the resulting new L• drives lipid peroxidation similarly. In the termination phase, the LOO• species reacts with hydrogen atoms from the antioxidant to generate lipid hydroxide (LOH) or combines with another LOO• to form non-radical product, as shown in Figure 1.
Unsaturated fatty acids with more than three double bonds generate intracyclic peroxide intermediate isomers (LOOH), which are subsequently reduced to prostaglandin-like compounds (LOH) and their isomers. This process involves the formation of F2-IsoPs from AA, F3-IsoPs from EPA, and F4-neuroprostanes (NPs) from DHA. These primary products are further metabolized by Hock rearrangement or β-breakage reactions to form toxic reactive carbonyl species (RCS), including malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), formaldehyde (FA), acrolein, methylglyoxal (MGO) (202–204). The RCS readily triggers irreversible modification and cross-linking with proteins, nucleic acids, and other biological macromolecules, resulting in physical dysfunction, as shown in Figure 2.
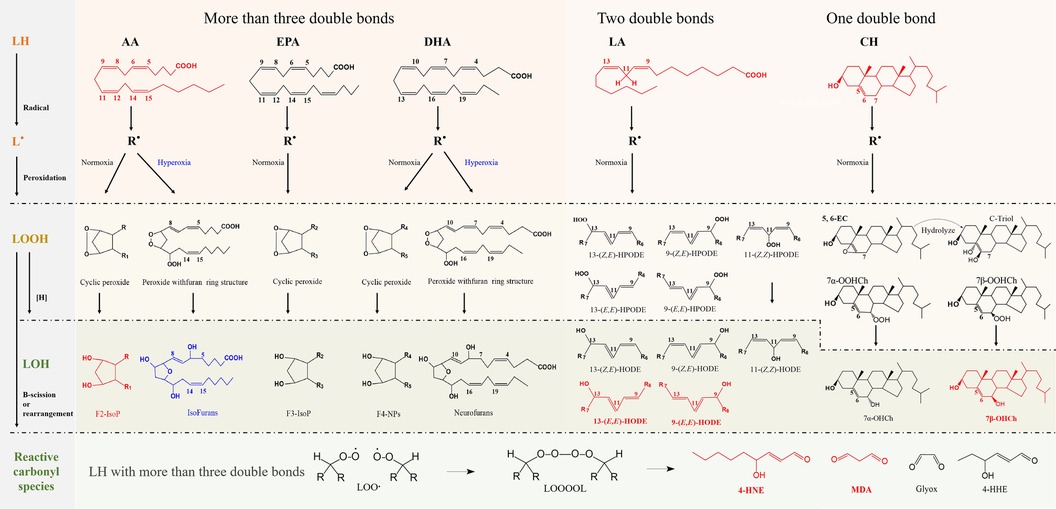
Figure 2. Schematic representation of the free radical-driven oxidative stress process for lipid. The processes in generating biomarkers for lipid peroxidation and the abbreviations of representative biomarkers are highlighted in red.
In contrast, fatty acids containing fewer double bonds are less susceptible to oxidation than substances containing polyunsaturated fatty acids. Linoleates (containing two double bonds), the body's most abundant polyunsaturated fatty acids, and Ch (monounsaturated lipids) generate oxidized products only through free radical chain reactions. LA generates hydroperoxyoctadecadienoic acid (HPODE) and its isomers, and these hydroperoxides are subsequently reduced to hydroxyoctadecadienoic acid (HODE) and its isomers (204, 205). Ch generates 7α- and 7β-hydroperoxycholesterol (7α-OOHCh and 7β-OOHCh) as well as 5α, 6α- and 5β, 6β-epoxycholesterol. The first two are subsequently reduced to 7α- and 7β-hydroxycholesterol (7α-OHCh, 7β-OHCh), and the latter can be produced as Cholestane-3β,5α,6β-Triol (C-Triol) in the presence of hydrolases (206). However, 7α-OHCh can be generated by enzymatic reactions, and thus, 7β-OHCh is considered the primary product of Ch under free radical mediation, as shown in Figure 2.
Lipid hydroxides may serve as more suitable biomarkers than hydroperoxides, and trans-hydroxides are formed only due to free radical-mediated peroxidation (200, 202). Considering the abundance of unsaturated fatty acids, it is generally accepted that F2-IsoPs, HODE, and 7β-OHCh are the primary oxidative stress markers (highlighted in red), as shown in Figure 2. In addition, with increasing oxygen tension, isofurans (IsoFs) and neurofurans (NFs) can be formed by peroxidation of AA and DHA, respectively (204). The formation mechanism of IsoFs and NFs compounds is similar to that of F2-IsoPs, as shown in Figure 2.
Free radicals attack protein molecules and mainly affect amino acid side chains, which causes them to undergo oxidative modifications. These modifications include oxidation of sulfur-containing amino acids, oxidation of aromatic amino acids, carbonylation, sugar oxidation, and nitration formation (207).
Although all amino acids can be modified by ROS, sulfur-containing amino acids (methionine, cysteine) are particularly susceptible to oxidative reactions due to the higher sensitivity of the sulfur group. Cysteine thiol undergoes two oxidation pathways (207). One pathway involves the reversible formation of a disulfide bond with another thiol, regulated by several intracellular enzymes. The second pathway involves the gradual oxidation of thiol, resulting in the reversible formation of sulfenic acid (-SOH) and sulfinic acid (-SO2H) or the irreversible formation of sulfonic acid (-SO3H) (208). The selenol of selenocysteine can be regarded as a specialized thiol as it catalyzes thiol/disulfide exchange reactions (209). Like thiols, selenols can undergo oxidation to diselenides, selenoxides, selenenic acids, seleninic acids, and selenonic acids (210, 211). Selenium's higher nucleophilicity and electrophilicity enable it to efficiently cycle between its reduced state (selenols) and oxidized state (diselenides), making it an effective redox regulator (212). Notably, five glutathione peroxidases (GPx), three thioredoxin reductases (TrxR), and methionine sulfoxide reductase 2 (MsrB) are selenium enzymes involved in redox reactions (213).
Methionine is highly susceptible to ROS oxidation, a reaction prevalent in almost all living organisms (214). Under more intense experimental conditions, such as higher concentrations of N-bromosuccinimide, methionine sulfoxide can undergo irreversible oxidation to methionine sulfone (215). However, relatively few studies have investigated the physiological conditions of methionine sulfone. In aging mice, methionine sulfoxide and methionine sulfone have been identified as the most abundant amino acid oxidation modifications. The levels of methionine sulfone in histones and the cytoplasm of aging mice are significantly higher than those in young mice (216). In contrast to the well-known susceptibility of cysteine to oxidation, methionine oxidation has been largely overlooked (217). This oversight can be attributed to its hydrophobic and relatively weak nucleophilicity in the thioether group of methionine (218). Additionally, the reversible catalysis of methionine sulfoxide by methionine sulfoxide reductases and its identification as methionine in traditional Edman sequencing procedures further contributes to the neglect of methionine oxidation (219). The levels of methionine sulfoxide indicate the overall cellular redox status, making it a promising clinical biomarker (207, 215).
The oxidation of other amino acids requires more stringent conditions than that of sulfur-containing amino acids, and aromatic amino acids are secondarily susceptible to oxidation because their molecular structure contains multiple conjugated double bonds, and their high electron density properties make them susceptible to oxidation, generating various stable oxidative modification products (220). Tyrosine generates an intermediate tyrosyl radical followed by dihydroxyphenylalanine or bis-tyrosine. Tryptophan is oxidized to hydroxytryptophan by hydroxyl radicals, followed by oxygen cleavage of hydroxytryptophan to form N-formyl kynurenine. Hydroxyl radicals oxidize phenylalanine and histidine to form ortho-tyrosine and 2-oxohistidine, respectively (207).
The oxidative modification of amino acids formed by the combined action of glycosylation and oxidation, known as glycoxidation, is an irreversible process (221). Reducing sugars first react non-enzymatically with free amino groups in amino acids or proteins (usually lysine or arginine) to form compounds with sugar groups called basal glycation products. The basal glycation products are susceptible to oxidative stress, which triggers oxidation and condensation reactions. These reactions lead to further structural changes in the basal glycation products to form more complex advanced glycation end products (AGEs). The most abundant AGEs in the body are carboxymethyl lysine and pentosidine, produced from lysine and formed by cross-linking between lysine and arginine residues, respectively (207).
Nitrification modifications are also formed under conditions of oxidative stress. Nitric oxide reacts with superoxide anion to form the reactive nitrogen species—peroxynitrite, which subsequently undergoes nitration with phenolic groups in the tyrosine molecule, irreversibly adding nitro substituents to the amino acid side chain of tyrosine to form 3-nitrotyrosine (207, 222).
The process of introducing reactive carbonyl groups such as aldehydes, ketones, and lactams into the amino acid side chains of proteins is called “protein carbonylation” (223). Protein carbonylation is usually defined as an irreversible post-translational modification that causes conformational changes in the polypeptide chain and results in loss of protein function. Carbonylation is relatively challenging to induce compared to other oxidative modifications and is considered a significant marker of oxidative damage to proteins. The protein carbonylation generation pathways in which ROS are directly involved fall into two categories. ROS can directly attack lysine, arginine, proline, and threonine side chains, introducing carbonyl groups and generating α-aminoadipic acid semialdehydes (AAS) derived from lysine and γ-glutamic acid semialdehydes derived from arginine and proline. In addition, the active carbonyls formed by ROS-attacking lipids can also be added to nucleophilic amino acids (i.e., cysteine, histidine, and lysine) by Michael addition or by generation of Schiff bases (207, 224).
These modifications also irreversibly cause proteins to produce cross-links, altering the composition and folding of proteins and affecting their function as receptors, enzymes, carriers, or structural proteins (207). Advanced oxidized protein products (AOPPs) are cross-linked protein-containing products containing dityrosine and carbonyl groups formed by the reaction of plasma proteins with oxidants and are also considered markers of oxidant-mediated protein damage (225).
Due to the reactivity of nitrogen and oxygen atoms in nucleic bases, nucleic acids are highly susceptible to damage induced by oxidative stress. While all four bases are impacted by ROS, guanine (G) exhibits the lowest redox potential relative to the other bases (G: −3.0 V, A: −2.71 V, C: −2.56 V, and T: −2.32 V) (226). As a result, guanine nucleotides and deoxyguanine nucleotides are more prone to oxidation, resulting in the formation of 8-oxoGuo and 8-oxo-dG, respectively. Furthermore, 8-oxoGuo and 8-oxodG can be released into the bloodstream and excreted in urine, allowing their detection in human serum/plasma and urine samples. They serve as well-established biomarkers for oxidative damage to DNA and RNA (227, 228).
However, the oversight of RNA oxidative damage primarily stems from the relatively short half-life of RNA, and the initial research focused on DNA oxidation, leading previous studies to concentrate predominantly on DNA. In fact, RNA molecules are more susceptible to the influence of reactive oxygen species (ROS) than DNA. This susceptibility is mainly due to ribonucleotides being more abundant than deoxyribonucleotides, and RNA lacks protective and repair mechanisms, making its bases more prone to oxidation. Although previous research has linked 8-oxoGuo to various diseases, including Alzheimer's disease (229, 230), Parkinson's disease (231), type 2 diabetes (232), obesity (233), atherosclerosis (227), heart failure (234), and tumors (235, 236), studies on RNA oxidative stress products related to BPD, are yet to be reported. Hence, this section primarily discusses DNA oxidation.
In the free nucleotide pool, guanine is oxidized to 8-oxo-dGTP and involved in DNA; in the DNA molecule, guanine is oxidized to 8-oxo-7,8-dihydroguanine (8-oxoG). The 8-oxo-dGTP in the nucleotide pool is first hydrolyzed to 8-oxodGMP by MutT homolog 1 (MTH1), followed by 8-oxo-2′-deoxyguanosine (8-oxodG or 8-OHdG) formation by 5′-Nucleotidase (237, 238). The base excision repair (BER) system recognizes and eliminates 8-oxoG from DNA strands. When the BER system fails to recognize larger or more complex damages, nucleotide excision repair (NER) is initiated to excise 8-oxodG-containing DNA fragments (237). In addition, cells with the most severe DNA damage (necrosis or apoptosis) will also release 8-oxodG-containing DNA fragments. The excision of 8-oxoG by the BER system also generates apurinic (AP) sites, which are highly reactive and susceptible to 3′ phosphate bond breaks, resulting in single-strand breaks. In the presence of oxygen, 8-oxoG readily produces a synconformation and pairs with adenine (226). Although DNA repair enzymes continuously monitor and repair chromosomes, 8-oxoG readily accumulates and induces deleterious mutations through free radical overload, causing guanine to thymine mutations and cytosine to adenine transversions during DNA replication, as shown in Figure 3. Thus, the major types of DNA damage following oxidation by ROS are oxidative base modifications (8-oxoG), AP sites, single-strand breaks, and mutations (G: C-T: A) (226). Whereas 8-oxoG, 8-oxodG, and 8-oxodG-containing DNA fragments are released into the bloodstream and are taken up by the kidneys and excreted into the urine, 8-oxoG and 8-oxodG are the most critical types of DNA damage, which have been extensively studied in tissues and body fluids (239).
We thoroughly searched the PubMed database for published clinical studies encompassing the neonatal (birth-1 month) up to 2023. Our search strategy incorporated medical subject headings (MeSH headings) and free-text terms associated with “BPD”, “lipid peroxidation”, “protein oxidative damage”, “nucleic acid oxidative damage”, and representative biomarkers. Our analysis was centered on identifying biomarkers applicable in clinical settings to forecast the progression of BPD. In total, we reviewed and summarized 37 clinical studies, which are detailed in Supplementary Table S1.
The publication dates of the articles ranged from 1988 to 2023 and involved 15 countries. Predominantly, studies were conducted in the USA (n = 11) and Finland (n = 4). The study subjects included premature and full-term infants, with sample sizes ranging from 19 to 253. The most common sample types used in the research were urine-derived (30.77%), blood-derived (42.31%), and respiratory-derived (26.92%) samples, including BALF, exhaled breath condensate (EBC), tracheal aspirates (TA), erythrocytes, cord, serum, blood, plasma, and urine. Among these, urine (30.77%), plasma (17.31%), and TA (17.31%) were the most frequently studied sample types, as shown in Figure 4A.
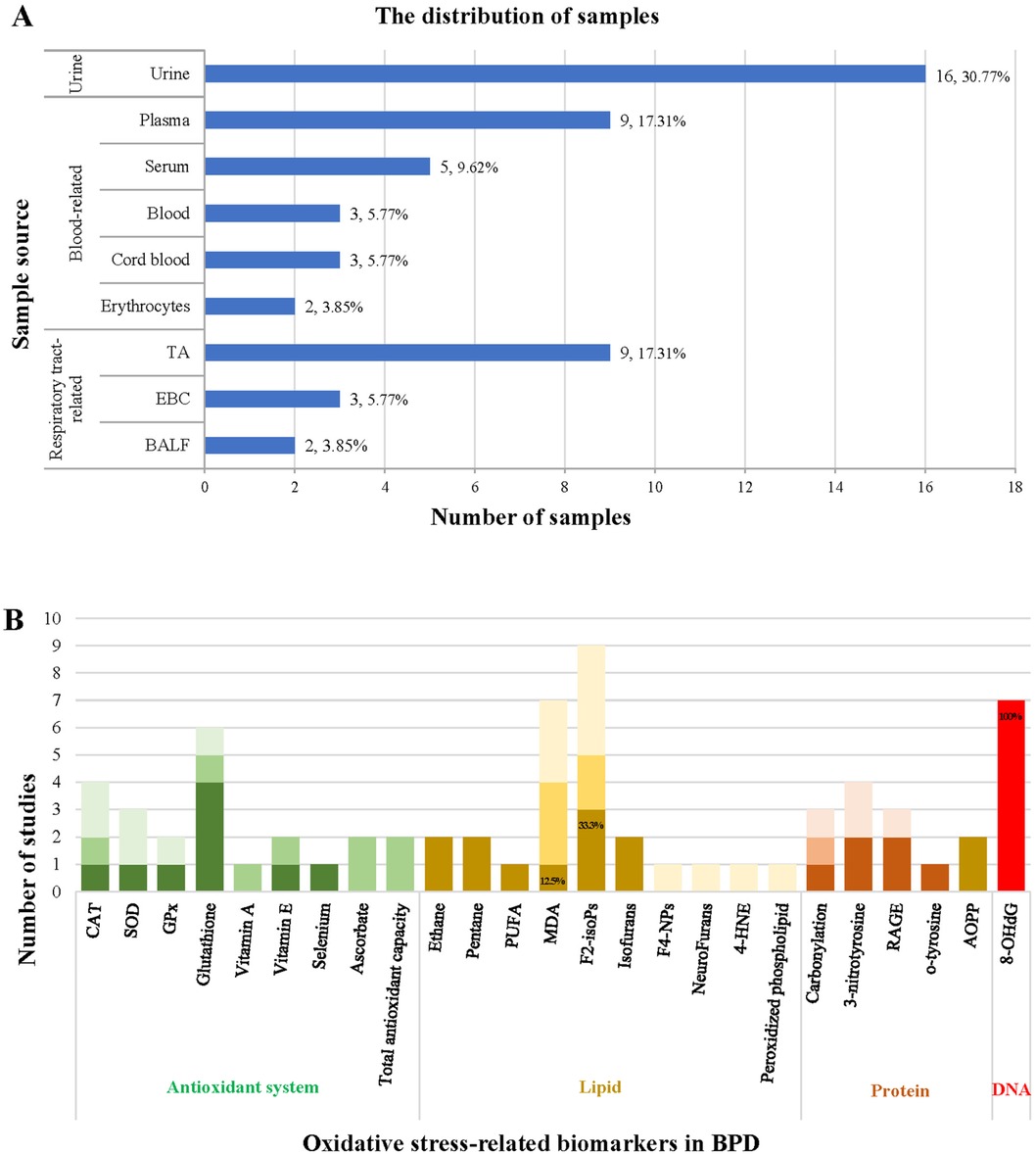
Figure 4. Summary of the source of samples (A) and the correlation of oxidative stress-related biomarkers with BPD (B).
Our analysis reveals that biomarkers of lipid peroxidation were the most investigated assays, followed by antioxidant systems, protein oxidation, and nucleic acid oxidation. Notably, F2-isoPs (n = 9), MDA (n = 7), and 8-OHdG (n = 7) emerged as the most studied biomarkers, as illustrated in Figure 4B. We also elucidated the correlations between the antioxidant system, lipid peroxidation, protein oxidative damage, nucleic acid oxidative damage, and the development of BPD in Supplementary Table S1. Additionally, the accuracy of biomarkers associated with the antioxidant system, lipid peroxidation, and oxidative damage to proteins is depicted in Figure 4B. However, despite F2-isoPs and MDA being the two most extensively studied biomarkers of oxidative stress in these articles, only 33.3% and 12.5% of articles reported their predictive capability for BPD, respectively.
The darkest color blocks in Figure 4B indicate that the biomarkers can predict the development of BPD; darker color blocks indicate that the biomarkers show differences between premature infants and control groups or between different treatment groups but cannot predict the development of BPD; the lightest color blocks indicate that the biomarkers cannot predict the development of BPD.
Among the nine studies investigating F2-isoPs levels and their association with BPD, only two suggested that elevated plasma F2-isoPs levels can predict adverse outcomes in preterm infants (240, 241). One study indicated a correlation between increased F2-isoPs levels and a more minor gestational age (36). However, the remaining six reports concluded that F2-isoPs levels cannot predict the development of BPD in preterm infants (40, 242–246). Notably, two studies also examined the levels of other lipid peroxidation products, such as isofurans. They indicated that isofurans may exhibit more robust predictive capabilities, potentially emerging as the optimal biomarker for lipid peroxidation (40, 245). Considering the mechanism of lipid peroxidation, IsoFs may be more suitable than F2-isoPs as a lipid peroxidation metabolite for predicting BPD due to their specificity as a biomarker generated in the presence of increased oxygen concentration. Nonetheless, a limited number of studies investigating isofuran levels and their correlation with BPD emphasize the need for further research in this area (40, 245).
Seven studies have assessed MDA levels as a secondary product of lipid peroxidation. Among them, three reported that MDA levels could not predict BPD (135, 247, 248) and the other three showed that higher MDA concentrations are associated with gestational age (33, 35) or lower body weights (32). Only Weinberger et al. reported a correlation between elevated urinary MDA measurements and the risk for oxidative respiratory distress (249).
In contrast, elevated levels of 8-OHdG have been consistently associated with the clinical outcome of BPD across studies, suggesting that 8-OHdG could serve as a reliable biomarker for predicting BPD (37–40, 245, 250–252). Joung et al. demonstrated that the 8-OHdG values in “classic” BPD on the third day were higher than those of “atypical” BPD. Furthermore, 8-OHdG levels on the seventh day were an independent risk factor for developing moderate/severe BPD (250). Hsiao et al. reported higher 8-OHdG levels in serum and tracheal aspirates (TA) in the BPD group on the 1st day after birth (p < 0.05) and persistently 8-OHdG levels increased in TA fluid on the 28th day of life in the BPD group (p < 0.05) compared to the non-BPD group (37). Moreover, Hsiao et al. also suggested that urine 8-OHdG concentrations from days 14 to 28 may be practical non-invasive predictors of BPD development in preterm infants (39).
In a prospective cohort study, Vento et al. found that extremely preterm neonates receiving antenatal steroids had decreased 8-OHdG levels accompanied by increased antioxidant enzyme activity, lower ortho-tyrosine levels, and a lower incidence of BPD compared to those not receiving steroids (252). Tokuriki et al. demonstrated that urinary levels of 8-OHdG during the early postnatal period correlated with the subsequent development of BPD. In contrast, urinary levels of advanced oxidative protein products (AOPP) and Nε-(hexanoyl) lysine showed no such correlation (251), suggesting that 8-OHdG may be preferable to protein oxidation products as a predictor of oxidative stress biomarkers for BPD.
DNA oxidative stress biomarkers may offer greater accuracy in predicting the development of BPD for several reasons. (i) Location: Lipids and proteins in the cytoplasm or cell membrane are usually more susceptible to OS, significantly exacerbated by oxygen therapy (83, 201). In contrast, DNA, located in the nucleus and protected by the nuclear membrane, is less vulnerable to the effects of ROS. (ii) Biochemical properties: Lipids and proteins contain a large number of easily oxidizable groups, such as unsaturated fatty acids (201, 207, 253) and amino acids (207, 220), making them more prone to oxidation under hyperoxia. (iii) Metabolic activity: Lipids and proteins participate in metabolic processes that generate ROS and other oxidative substances, leading to oxidative damage. In contrast, DNA does not directly engage in metabolic reactions, reducing its exposure to oxidation (201). (iv) Biological importance: DNA integrity is crucial for proper cellular function and overall health, as it stores and transmits genetic information. Cells have evolved defense mechanisms, including enzymes and antioxidant systems, to repair and protect DNA from oxidative damage, whereas lipids and proteins lack comparable protective mechanisms (254). (v) Reflecting oxidative damage directly: DNA molecules have a more straightforward structure than lipids and proteins, making them more directly reflective of oxidative damage (201). (vi) Wide range of applications: 8-OHdG emerges as a pivotal biomarker with promising applications in disease research, clinical diagnostics, and environmental health assessments (255–257). However, its lack of tissue and diagnostic specificity poses challenges, necessitating consideration of other relevant factors when interpreting experimental results to accurately assess the extent of oxidative damage (243).
Based on our comprehensive review of clinical studies, it is evident that the relationship between many reported biomarkers and the outcome of BPD is ambiguous. Only 8-OHdG shows the most promise as an oxidative damage biomarker for predicting BPD and complications related to prematurity, and the relevant information is described in Table 1. However, its integration into clinical practice has been hindered by several factors, including the need for standardized methods and reference ranges and the absence of validation through prospective trials (258). Selecting sample sources and assays is crucial in obtaining convenient and reliable biomarkers for early BPD prognostic prediction in clinical settings. Five of the seven studies on 8-OHdG utilized urine samples (39, 40, 250–252), while the remaining two collected serum and tracheal aspirates (37, 38). The collection and processing of urine samples are relatively straightforward, usually involving minimal pre-processing steps. In contrast, collecting blood and BALF may compromise the integrity of the skin and mucosa, thereby raising the risk of pain and infection in premature infants (39). Consequently, urine samples are convenient for large-scale research and clinical monitoring purposes. Urinary 8-OHdG is generally regarded as a reflection of systemic oxidative stress, thereby serving as an indicator for assessing overall oxidative stress status (256). Rigorous analytical methods and quality control measures are imperative to ensure a reliable assessment of 8-OHdG levels, providing researchers and clinicians with a basis for optimizing neonatal care. Five of the seven studies investigating 8-OHdG utilized ELISA assay kits for measurement (37–39, 250, 251), while the remaining two employed mass spectrometry (40, 252). Although ELISA assays have been calibrated for measurements and proven helpful in assessing the impact on BPD development and progression, there remains a necessity for more sensitive, specific, and clinically validated laboratory detection methods. Techniques like HPLC-MS and LC-MS/MS hold promise in obtaining urinary 8-OHdG biomarkers from urine samples, thereby enhancing the accuracy and reliability of BPD prediction (256, 259).
Since the levels of oxidative stress-induced biomarkers can reflect the severity of BPD, their levels can guide clinical management decisions for premature infants, thereby reducing oxidative stress-related diseases and providing valuable insights into the treatment of oxidative stress-related neonatal diseases (260). Currently, various strategies have been proposed for treating neonatal BPD, including protective ventilation, surfactant therapy, corticosteroids, caffeine, vitamin A, nitric oxide, and nutritional interventions (261). Therefore, assessing the relationship between these strategies and oxidative stress levels or BPD incidence is critical. Ten studies have described the changes in oxidative stress biomarkers following drug administration (252, 262), inhaled nitric oxide therapy (34, 263), exposure to different oxygen concentrations (40, 245, 246), and nutritional interventions (243, 248, 264), and their relationship with the outcome of BPD.
Reports suggest that using corticosteroids improves the oxidative-reductive balance, resulting in increased antioxidant enzyme activity, decreased 8-OHdG levels, lower ortho-tyrosine, and a reduced incidence of BPD (252). Although no significant differences in clinical outcomes were observed between control and beclomethasone-treated infants, bronchoalveolar lining fluid analysis revealed evidence of phospholipid peroxidation in control infants compared to beclomethasone-treated infants on day 2 of life (262).
There were no significant differences in concentrations of 3-nitrotyrosine and carbonylation between control and inhaled nitric oxide-treated infants (34, 263).
A study conducted in Spain in 2009 showed that urinary markers of oxidative stress were significantly elevated in infants receiving 90% oxygen compared to those receiving 30% oxygen in the first week after birth. Additionally, GSSG levels on day three and urinary isofuran, o-tyrosine, and 8-oxodG levels on day seven significantly correlated with chronic lung disease development. However, the groups had no differences in urinary F2-isoPs levels (40). Furthermore, another study from Spain in 2015 reported no differences in oxidative stress biomarkers, mortality, or major perinatal morbidities between infants receiving 30% oxygen and those receiving 60%–65% oxygen. Nonetheless, isofurans detected in the first 4 days after birth were correlated with the later development of BPD compared to F2-isoPs, F4-NPs, and NeuroFurans (245). However, a recent study from India indicated that in premature infants receiving room air vs. 100% oxygen therapy within 4 h after birth, F2-isoPs levels showed no significant difference in mortality or BPD (246).
Unfortunately, nutritional supplementation does not reduce the incidence of BPD significantly. A study from Canada in 2010 suggested that while the LIP + MVP TPN modality may help protect against the oxidant load associated with oxygen supplementation, its effectiveness in reducing the incidence of BPD remains unclear (243). Another study from Turkey in 2019 demonstrated that total antioxidant capacity was higher in the SMOFlipid group compared with the ClinOleic group on day 7. Although BPD was lower in the SMOFlipid group than in the ClinOleic group, this finding was non-significant (264). Similarly, another study from Turkey in 2019 indicated that FMOS and OO/SO lipid emulsions had similar effects on lipid peroxidation on the 28th day of life and short-term morbidities such as BPD (248).
We have been grappling with BPD since Northway first outlined it in 1967, spanning over 60 years. BPD stands out as the most prevalent chronic lung disease affecting premature infants, with a solid correlation to significant morbidity and mortality. The pathogenesis of BPD is complex, primarily characterized by increased exposure to oxidative stress and immature antioxidant systems, ultimately resulting in abnormal pulmonary and vascular growth. Oxidative stress triggers oxidative damage to lipids, proteins, and nucleic acids. Numerous studies have shown elevated levels of oxidative stress markers in newborns who develop BPD. Thus, monitoring these markers in premature infants helps predict the severity of BPD and evaluate the effectiveness of therapeutic interventions.
With advancements in medical care, various approaches, including pulmonary surfactants, vitamin A, vitamin E, vitamin D, caffeine, and nutritional interventions, have been utilized in the clinical management of BPD. However, the efficacy and safety of some of these methods remain contentious and necessitate further evaluation. Considering the pivotal role of oxidative stress in BPD pathogenesis, interpreting clinical treatment strategies from an antioxidant perspective is justifiable.
Furthermore, we analyzed 37 clinical studies on oxidative stress markers to investigate the relationship between lipid peroxidation, protein oxidation, oxidative DNA damage, and the outcome of BPD in newborns. 8-OHdG is the most informative among these biomarkers, elucidating the disease severity and enabling personalized precision treatment for affected infants.
Overall, our review contributes to a deeper understanding of BPD pathogenesis stemming from continuous hyperoxia exposure during treatment. It sheds light on the mechanisms underlying the antioxidant aspects of clinical BPD management. As pathogenesis is not fully understood, it is promising to explore new evidence related to oxidative stress and the pathogenesis of BPD from the perspective of microbiomics, metabolomics, and proteomics (265).
ML: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. W-XC: Writing – original draft, Writing – review & editing. SL: Conceptualization, Writing – original draft. JW: Writing – review & editing. Y-RC: Writing – review & editing. LL: Conceptualization, Writing – review & editing. GY: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by China Postdoctoral Science Foundation, grant number 2022M712183.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1343870/full#supplementary-material
1. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):e37–46. doi: 10.1016/S2214-109X(18)30451-0
2. Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. (2022) 176(8):787–96. doi: 10.1001/jamapediatrics.2022.1622
3. Zhang J, Sun K, Zhang Y. The rising preterm birth rate in China: a cause for concern. Lancet Glob Health. (2021) 9(9):e1179–80. doi: 10.1016/S2214-109X(21)00337-5
4. Deng K, Liang J, Mu Y, Liu Z, Wang Y, Li M, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. (2021) 9(9):e1226–41. doi: 10.1016/S2214-109X(21)00298-9
5. Kumar VHS, Prasath A, Blanco C, Kenney PO, Ostwald CM, Meyer TS, et al. Respiratory failure in an extremely premature neonate with COVID-19. Children (Basel). (2021) 8(6):477. doi: 10.3390/children8060477
6. Piersigilli F, Carkeek K, Hocq C, van Grambezen B, Hubinont C, Chatzis O, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health. (2020) 4(6):476–8. doi: 10.1016/S2352-4642(20)30140-1
7. Marchand G, Patil AS, Masoud AT, Ware K, King A, Ruther S, et al. Systematic review and meta-analysis of COVID-19 maternal and neonatal clinical features and pregnancy outcomes up to June 3, 2021. AJOG Glob Rep. (2022) 2(1):100049. doi: 10.1016/j.xagr.2021.100049
8. Ng DC, Chin L, Choo PPL, Paramasivam U. COVID-19 in a premature infant. BMJ Case Rep. (2021) 14(5):e243783. doi: 10.1136/bcr-2021-243783
9. de Medeiros KS, Sarmento ACA, Costa APF, Macedo LTA, da Silva LAS, de Freitas CL, et al. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: a comprehensive systematic review and meta-analysis. Int J Gynaecol Obstet. (2022) 156(3):394–405. doi: 10.1002/ijgo.14015
10. Gosdin L, Wallace B, Lanzieri TM, Olsen EO, Lewis EL, Chang DJ, et al. Six-month outcomes of infants born to people with sars-cov-2 in pregnancy. Pediatrics. (2022) 150(6):e2022059009. doi: 10.1542/peds.2022-059009
11. Maslin K, McKeon-Carter R, Hosking J, Stockley L, Southby C, Shawe J, et al. Preterm births in South-West England before and during the COVID-19 pandemic: an audit of retrospective data. Eur J Pediatr. (2022) 181(2):859–63. doi: 10.1007/s00431-021-04265-y
12. Perez-Lopez FR, Saviron-Cornudella R, Chedraui P, Lopez-Baena MT, Perez-Roncero G, Sanz-Arenal A, et al. Obstetric and perinatal outcomes of pregnancies with COVID 19: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2022) 35(25):9742–58. doi: 10.1080/14767058.2022.2051008
13. Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. Br Med J. (2021) 375:n1974. doi: 10.1136/bmj.n1974
14. Socha-Banasiak A, Pawlowska M, Czkwianianc E, Pierzynowska K. From intrauterine to extrauterine life-the role of endogenous and exogenous factors in the regulation of the intestinal microbiota community and gut maturation in early life. Front Nutr. (2021) 8:696966. doi: 10.3389/fnut.2021.696966
15. Flake AW. A supportive physiologic environment for the extreme premature infant: improving life outside the womb. J Pediatr Surg. (2022) 57(2):167–71. doi: 10.1016/j.jpedsurg.2021.10.025
16. Salimi U, Dummula K, Tucker MH, Dela Cruz CS, Sampath V. Postnatal sepsis and bronchopulmonary dysplasia in premature infants: mechanistic insights into “new bpd”. Am J Respir Cell Mol Biol. (2022) 66(2):137–45. doi: 10.1165/rcmb.2021-0353PS
17. Thebaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. (2019) 5(1):78. doi: 10.1038/s41572-019-0127-7
18. Abiramalatha T, Ramaswamy VV, Bandyopadhyay T, Somanath SH, Shaik NB, Pullattayil AK, et al. Interventions to prevent bronchopulmonary dysplasia in preterm neonates: an umbrella review of systematic reviews and meta-analyses. JAMA Pediatr. (2022) 176(5):502–16. doi: 10.1001/jamapediatrics.2021.6619
19. Muehlbacher T, Bassler D, Bryant MB. Evidence for the management of bronchopulmonary dysplasia in very preterm infants. Children (Basel). (2021) 8(4):298. doi: 10.3390/children8040298
20. Haggie S, Robinson P, Selvadurai H, Fitzgerald DA. Bronchopulmonary dysplasia: a review of the pulmonary sequelae in the post-surfactant era. J Paediatr Child Health. (2020) 56(5):680–9. doi: 10.1111/jpc.14878
21. William DL, Sarath R, Jeanie C. Bronchopulmonary dysplasia and expiratory airflow at 8 years in children born extremely preterm in the post-surfactant era. Thorax. (2023) 78(5):484. doi: 10.1136/thoraxjnl-2022-218792
22. Lowe J, Kotecha SJ, Kotecha S. Long term effects following extreme prematurity: respiratory problems. In: Boyle EM, Cusack J, editors. Emerging Topics and Controversies in Neonatology. Cham: Springer International Publishing (2020). p. 351–66.
23. Yuan Y, Yang Y, Lei X, Dong W. Caffeine and bronchopulmonary dysplasia: clinical benefits and the mechanisms involved. Pediatr Pulmonol. (2022) 57(6):1392–400. doi: 10.1002/ppul.25898
24. Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: a meta-analysis. Pediatrics. (2016) 138(6):e20162511. doi: 10.1542/peds.2016-2511
25. Behnke J, Dippel CM, Choi Y, Rekers L, Schmidt A, Lauer T, et al. Oxygen toxicity to the immature lung-part II: the unmet clinical need for causal therapy. Int J Mol Sci. (2021) 22(19):10694. doi: 10.3390/ijms221910694
26. Santema HY, Stolk J, Los M, Stoel BC, Tsonaka R, Merth IT. Prediction of lung function and lung density of young adults who had bronchopulmonary dysplasia. ERJ Open Res. (2020) 6(4):00157–2020. doi: 10.1183/23120541.00157-2020
27. Kimble A, Robbins ME, Perez M. Pathogenesis of bronchopulmonary dysplasia: role of oxidative stress from “omics” studies. Antioxidants (Basel). (2022) 11(12):2380. doi: 10.3390/antiox11122380
28. Yang X, Jiang S, Deng X, Luo Z, Chen A, Yu R. Effects of antioxidants in human milk on bronchopulmonary dysplasia prevention and treatment: a review. Front Nutr. (2022) 9:924036. doi: 10.3389/fnut.2022.924036
29. Deng X, Bao Z, Yang X, Mei Y, Zhou Q, Chen A, et al. Molecular mechanisms of cell death in bronchopulmonary dysplasia. Apoptosis. (2023) 28(1-2):39–54. doi: 10.1007/s10495-022-01791-4
30. Martini S, Aceti A, Della Gatta AN, Beghetti I, Marsico C, Pilu G, et al. Antenatal and postnatal sequelae of oxidative stress in preterm infants: a narrative review targeting pathophysiological mechanisms. Antioxidants (Basel). (2023) 12(2):422. doi: 10.3390/antiox12020422
31. Ferrante G, Montante C, Notarbartolo V, Giuffre M. Antioxidants: role the in prevention and treatment of bronchopulmonary dysplasia. Paediatr Respir Rev. (2022) 42:53–8. doi: 10.1016/j.prrv.2022.01.003
32. Buss IH, Darlow BA, Winterbourn CC. Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr Res. (2000) 47(5):640–5. doi: 10.1203/00006450-200005000-00014
33. Collard KJ, Godeck S, Holley JE, Quinn MW. Pulmonary antioxidant concentrations and oxidative damage in ventilated premature babies. Arch Dis Child Fetal Neonatal Ed. (2004) 89(5):F412–6. doi: 10.1136/adc.2002.016717
34. Ballard PL, Truog WE, Merrill JD, Gow A, Posencheg M, Golombek SG, et al. Plasma biomarkers of oxidative stress: relationship to lung disease and inhaled nitric oxide therapy in premature infants. Pediatrics. (2008) 121(3):555–61. doi: 10.1542/peds.2007-2479
35. Abdel Ghany EA, Alsharany W, Ali AA, Youness ER, Hussein JS. Anti-Oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr Int Child Health. (2016) 36(2):134–40. doi: 10.1179/2046905515Y.0000000017
36. Urs R, Ni Chin R, Hemy N, Wilson AC, Pillow JJ, Hall GL, et al. Elevated leukotriene B4 and 8-isoprostane in exhaled breath condensate from preterm-born infants. BMC Pediatr. (2023) 23(1):386. doi: 10.1186/s12887-023-04210-y
37. Hsiao CC, Chang JC, Tsao LY, Yang RC, Chen HN, Lee CH, et al. Correlates of elevated interleukin-6 and 8-hydroxy-2'-deoxyguanosine levels in tracheal aspirates from very low birth weight infants who develop bronchopulmonary dysplasia. Pediatr Neonatol. (2017) 58(1):63–9. doi: 10.1016/j.pedneo.2016.01.004
38. Hsiao CC, Lee CH, Yang RC, Chen JY, Su TC, Chang YJ, et al. Heat shock protein-70 levels are associated with a state of oxidative damage in the development of bronchopulmonary dysplasia. Front Pediatr. (2021) 9:616452. doi: 10.3389/fped.2021.616452
39. Cui X, Fu J. Urinary biomarkers for the early prediction of bronchopulmonary dysplasia in preterm infants: a pilot study. Front Pediatr. (2022) 10:959513. doi: 10.3389/fped.2022.959513
40. Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. (2009) 124(3):e439–49. doi: 10.1542/peds.2009-0434
41. Sies H, Jones DP. Reactive oxygen species (ros) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. (2020) 21(7):363–83. doi: 10.1038/s41580-020-0230-3
42. Schieber M, Chandel NS. Ros function in redox signaling and oxidative stress. Curr Biol. (2014) 24(10):R453–62. doi: 10.1016/j.cub.2014.03.034
43. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. (2002) 82(1):47–95. doi: 10.1152/physrev.00018.2001
44. Voynow JA. “New” bronchopulmonary dysplasia and chronic lung disease. Paediatr Respir Rev. (2017) 24:17–8. doi: 10.1016/j.prrv.2017.06.006
45. Schulzke SM, Stoecklin B. Update on ventilatory management of extremely preterm infants-a neonatal intensive care unit perspective. Paediatr Anaesth. (2022) 32(2):363–71. doi: 10.1111/pan.14369
46. Bancalari E, Jain D. Bronchopulmonary dysplasia: 50 years after the original description. Neonatology. (2019) 115(4):384–91. doi: 10.1159/000497422
47. Matta BF, Lam AM, Mayberg TS. The influence of arterial oxygenation on cerebral venous oxygen saturation during hyperventilation. Can J Anaesth. (1994) 41(11):1041–6. doi: 10.1007/BF03015651
48. Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol. (1984) 46:617–28. doi: 10.1146/annurev.ph.46.030184.003153
49. Schittny JC. Development of the lung. Cell Tissue Res. (2017) 367(3):427–44. doi: 10.1007/s00441-016-2545-0
50. Lara-Canton I, Solaz A, Parra-Llorca A, Garcia-Robles A, Millan I, Torres-Cuevas I, et al. Oxygen supplementation during preterm stabilization and the relevance of the first 5 min after birth. Front Pediatr. (2020) 8:12. doi: 10.3389/fped.2020.00012
51. Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. (2018) 678:177–83. doi: 10.1016/j.gene.2018.08.031
52. Bancalari E, Claure N. Importance and challenges associated with oxygen control in premature infants. J Pediatr. (2022) 247:8–9. doi: 10.1016/j.jpeds.2022.05.042
53. Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. (2013) 131(4):716–23. doi: 10.1542/peds.2012-1835
54. Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. (2018) 172(4):361–7. doi: 10.1001/jamapediatrics.2017.5323
55. Perez M, Robbins ME, Revhaug C, Saugstad OD. Oxygen radical disease in the newborn, revisited: oxidative stress and disease in the newborn period. Free Radic Biol Med. (2019) 142:61–72. doi: 10.1016/j.freeradbiomed.2019.03.035
56. Li Q, Wall SB, Ren C, Velten M, Hill CL, Locy ML, et al. Thioredoxin reductase inhibition attenuates neonatal hyperoxic lung injury and enhances nuclear factor E2-related factor 2 activation. Am J Respir Cell Mol Biol. (2016) 55(3):419–28. doi: 10.1165/rcmb.2015-0228OC
57. D'Agrosa C, Cai CL, Siddiqui F, Deslouches K, Wadowski S, Aranda JV, et al. Comparison of coenzyme Q10 or fish oil for prevention of intermittent hypoxia-induced oxidative injury in neonatal rat lungs. Respir Res. (2021) 22(1):196. doi: 10.1186/s12931-021-01786-w
58. Wu Q, Chong L, Shao Y, Chen S, Li C. Lipoxin A4 reduces hyperoxia-induced lung injury in neonatal rats through Pink1 signaling pathway. Int Immunopharmacol. (2019) 73:414–23. doi: 10.1016/j.intimp.2019.05.046
59. Ahmed MN, Codipilly C, Hogg N, Auten RL. The protective effect of overexpression of extracellular superoxide dismutase on nitric oxide bioavailability in the lung after exposure to hyperoxia stress. Exp Lung Res. (2011) 37(1):10–7. doi: 10.3109/01902148.2010.497893
60. Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, et al. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol. (2001) 24(4):436–41. doi: 10.1165/ajrcmb.24.4.4240
61. Bonadies L, Zaramella P, Porzionato A, Perilongo G, Muraca M, Baraldi E. Present and future of bronchopulmonary dysplasia. J Clin Med. (2020) 9(5):1539. doi: 10.3390/jcm9051539
62. Speer CP, Sweet DG, Halliday HL. Surfactant therapy: past, present and future. Early Hum Dev. (2013) 89 Suppl 1:S22–4. doi: 10.1016/S0378-3782(13)70008-2
63. Northway WH J, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. (1967) 276(7):357–68. doi: 10.1056/NEJM196702162760701
64. Morgan TE. Pulmonary surfactant. N Engl J Med. (1971) 284(21):1185–93. doi: 10.1056/NEJM197105272842105
65. Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta. (1998) 1408(2–3):79–89. doi: 10.1016/s0925-4439(98)00060-x
66. Milad N, Morissette MC. Revisiting the role of pulmonary surfactant in chronic inflammatory lung diseases and environmental exposure. Eur Respir Rev. (2021) 30(162):210077. doi: 10.1183/16000617.0077-2021
67. Jin Y, Peng LQ, Zhao AL. Hyperoxia induces the apoptosis of alveolar epithelial cells and changes of pulmonary surfactant proteins. Eur Rev Med Pharmacol Sci. (2018) 22(2):492–7. doi: 10.26355/eurrev_201801_14200
68. Dargaville PA, Kamlin COF, Orsini F, Wang X, De Paoli AG, Kanmaz Kutman HG, et al. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the optimist-a randomized clinical trial. JAMA. (2021) 326(24):2478–87. doi: 10.1001/jama.2021.21892
69. Zenri H, Rodriquez-Capote K, McCaig L, Yao LJ, Brackenbury A, Possmayer F, et al. Hyperoxia exposure impairs surfactant function and metabolism. Crit Care Med. (2004) 32(5):1155–60. doi: 10.1097/01.ccm.0000126264.00551.c8
70. Boda D, Nemeth I, Pinter S. Surface tension, glutathione content and redox ratio of the tracheal aspirate fluid of premature infants with IRDS. Biol Neonate. (1998) 74(4):281–8. doi: 10.1159/000014035
71. Dumpa V, Bhandari V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. (2018) 42(7):444–52. doi: 10.1053/j.semperi.2018.09.006
72. Verder H, Bohlin K, Kamper J, Lindwall R, Jonsson B. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr. (2009) 98(9):1400–8. doi: 10.1111/j.1651-2227.2009.01413.x
73. Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. (1999) 13(6):1455–76. doi: 10.1183/09031936.99.13614779
74. Caruso G, Fresta CG, Costantino A, Lazzarino G, Amorini AM, Lazzarino G, et al. Lung surfactant decreases biochemical alterations and oxidative stress induced by a sub-toxic concentration of carbon nanoparticles in alveolar epithelial and microglial cells. Int J Mol Sci. (2021) 22(5):2694. doi: 10.3390/ijms22052694
75. Rooney SA. Lung surfactant. Environ Health Perspect. (1984) 55::205–26. doi: 10.1289/ehp.8455205
76. Fridovich I, Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. (1986) 48:693–702. doi: 10.1146/annurev.ph.48.030186.003401
77. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. (2021) 20(9):689–709. doi: 10.1038/s41573-021-00233-1
78. Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. (2016) 26(3):165–76. doi: 10.1016/j.tcb.2015.10.014
79. Halliday HL. Natural vs synthetic surfactants in neonatal respiratory distress syndrome. Drugs. (1996) 51(2):226–37. doi: 10.2165/00003495-199651020-00004
80. Halliday HL. Controversies: synthetic or natural surfactant. The case for natural surfactant. J Perinat Med. (1996) 24(5):417–26. doi: 10.1515/jpme.1996.24.5.417
81. Lemyre B, Fusch C, Schmolzer GM, Rouvinez Bouali N, Reddy D, Barrowman N, et al. Poractant alfa versus bovine lipid extract surfactant for infants 24+0 to 31+6 weeks gestational age: a randomized controlled trial. PLoS One. (2017) 12(5):e0175922. doi: 10.1371/journal.pone.0175922
82. Malloy CA, Nicoski P, Muraskas JK. A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr. (2005) 94(6):779–84. doi: 10.1111/j.1651-2227.2005.tb01984.x
83. Veldhuizen EJ, Haagsman HP. Role of pulmonary surfactant components in surface film formation and dynamics. Biochim Biophys Acta. (2000) 1467(2):255–70. doi: 10.1016/s0005-2736(00)00256-x
84. Whitsett JA, Weaver TE, Clark JC, Sawtell N, Glasser SW, Korfhagen TR, et al. Glucocorticoid enhances surfactant proteolipid Phe and pVal synthesis and RNA in fetal lung. J Biol Chem. (1987) 262(32):15618–23. doi: 10.1016/S0021-9258(18)47771-X
85. Gerber AN. Glucocorticoids and the lung. Adv Exp Med Biol. (2015) 872:279–98. doi: 10.1007/978-1-4939-2895-8_12
86. Bry K, Lappalainen U, Hallman M. Cytokines and production of surfactant components. Semin Perinatol. (1996) 20(3):194–205. doi: 10.1016/s0146-0005(96)80048-6
87. Tillis CC, Huang HW, Bi W, Pan S, Bruce SR, Alcorn JL. Glucocorticoid regulation of human pulmonary surfactant protein-B (Sp-B) mrna stability is independent of activated glucocorticoid receptor. Am J Physiol Lung Cell Mol Physiol. (2011) 300(6):L940–50. doi: 10.1152/ajplung.00420.2010
88. Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update. (2016) 22(2):240–59. doi: 10.1093/humupd/dmv047
89. Sotiriadis A, McGoldrick E, Makrydimas G, Papatheodorou S, Ioannidis JP, Stewart F, et al. Antenatal corticosteroids prior to planned caesarean at term for improving neonatal outcomes. Cochrane Database Syst Rev. (2021) 12(12):CD006614. doi: 10.1002/14651858.CD006614.pub4
90. Wiseman EM, Bar-El Dadon S, Reifen R. The vicious cycle of vitamin a deficiency: a review. Crit Rev Food Sci Nutr. (2017) 57(17):3703–14. doi: 10.1080/10408398.2016.1160362
91. Huang L, Zhu D, Pang G. The effects of early vitamin a supplementation on the prevention and treatment of bronchopulmonary dysplasia in premature infants: a systematic review and meta-analysis. Transl Pediatr. (2021) 10(12):3218–29. doi: 10.21037/tp-21-496
92. Timoneda J, Rodriguez-Fernandez L, Zaragoza R, Marin MP, Cabezuelo MT, Torres L, et al. Vitamin a deficiency and the lung. Nutrients. (2018) 10(9):1132. doi: 10.3390/nu10091132
93. Inder TE, Carr AC, Winterbourn CC, Austin NC, Darlow BA. Vitamin a and E status in very low birth weight infants: development of an improved parenteral delivery system. J Pediatr. (1995) 126(1):128–31. doi: 10.1016/s0022-3476(95)70515-5
94. Haider BA, Sharma R, Bhutta ZA. Neonatal vitamin a supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst Rev. (2017) 2(2):CD006980. doi: 10.1002/14651858.CD006980.pub3
95. Endesfelder S, Strauss E, Scheuer T, Schmitz T, Buhrer C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir Res. (2019) 20(1):88. doi: 10.1186/s12931-019-1063-5
96. Blaner WS. Vitamin a signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. (2019) 197:153–78. doi: 10.1016/j.pharmthera.2019.01.006
97. Friel JK, Diehl-Jones WL, Suh M, Tsopmo A, Shirwadkar VP. Impact of iron and vitamin C-containing supplements on preterm human milk: in vitro. Free Radic Biol Med. (2007) 42(10):1591–8. doi: 10.1016/j.freeradbiomed.2007.02.022
98. Carazo A, Macakova K, Matousova K, Krcmova LK, Protti M, Mladenka P. Vitamin a update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. (2021) 13(5):1703. doi: 10.3390/nu13051703
99. Sotler R, Poljsak B, Dahmane R, Jukic T, Pavan Jukic D, Rotim C, et al. Prooxidant activities of antioxidants and their impact on health. Acta Clin Croat. (2019) 58(4):726–36. doi: 10.20471/acc.2019.58.04.20
100. Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin a and carotenoids and their relevance to heart disease. Free Radic Biol Med. (1999) 26(5–6):746–61. doi: 10.1016/s0891-5849(98)00266-4
101. Kabir MT, Rahman MH, Shah M, Jamiruddin MR, Basak D, Al-Harrasi A, et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother. (2022) 146:112610. doi: 10.1016/j.biopha.2021.112610
102. Islam F, Muni M, Mitra S, Emran TB, Chandran D, Das R, et al. Recent advances in respiratory diseases: dietary carotenoids as choice of therapeutics. Biomed Pharmacother. (2022) 155:113786. doi: 10.1016/j.biopha.2022.113786
103. Lu LW, Gao Y, Quek SY, Foster M, Eason CT, Liu M, et al. The landscape of potential health benefits of carotenoids as natural supportive therapeutics in protecting against coronavirus infection. Biomed Pharmacother. (2022) 154:113625. doi: 10.1016/j.biopha.2022.113625
104. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. (2014) 34(11):907–29. doi: 10.1016/j.nutres.2014.07.010
105. Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonatal Ed. (2018) 103(3):F285–91. doi: 10.1136/archdischild-2017-314264
106. Giridhar S, Kumar J, Attri SV, Dutta S, Kumar P. Intramuscular followed by oral vitamin a supplementation in neonates with birth weight from 750 to 1250 g: a randomized controlled trial. Indian J Clin Biochem. (2020) 35(2):197–204. doi: 10.1007/s12291-018-0807-1
107. Wahl HB, Hutten MC, Monz D, Tutdibi E, Ophelders D, Nikiforou M, et al. Vitamin a supplementation by endotracheal application of a nano-encapsulated preparation is feasible in ventilated preterm lambs. J Aerosol Med Pulm Drug Deliv. (2018) 31(6):323–30. doi: 10.1089/jamp.2017.1438
108. Biesalski HK. Effects of intra-tracheal application of vitamin a on concentrations of retinol derivatives in plasma, lungs and selected tissues of rats. Int J Vitam Nutr Res. (1996) 66(2):106–12.8843984
109. Singh AJ, Bronshtein V, Khashu M, Lee K, Potts JE, Friel J, et al. Vitamin a is systemically bioavailable after intratracheal administration with surfactant in an animal model of newborn respiratory distress. Pediatr Res. (2010) 67(6):619–23. doi: 10.1203/PDR.0b013e3181da8fe8
110. Mactier H, Mokaya MM, Farrell L, Edwards CA. Vitamin a provision for preterm infants: are we meeting current guidelines? Arch Dis Child Fetal Neonatal Ed. (2011) 96(4):F286–9. doi: 10.1136/adc.2010.190017
111. Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr. (2010) 50(1):85–91. doi: 10.1097/MPG.0b013e3181adaee0
112. Rakshasbhuvankar AA, Simmer K, Patole SK, Stoecklin B, Nathan EA, Clarke MW, et al. Enteral vitamin a for reducing severity of bronchopulmonary dysplasia: a randomized trial. Pediatrics. (2021) 147(1):e2020009985. doi: 10.1542/peds.2020-009985
113. Blaner WS, Shmarakov IO, Traber MG. Vitamin a and vitamin E: will the real antioxidant please stand up? Annu Rev Nutr. (2021) 41:105–31. doi: 10.1146/annurev-nutr-082018-124228
114. Stone CA J, McEvoy CT, Aschner JL, Kirk A, Rosas-Salazar C, Cook-Mills JM, et al. Update on vitamin E and its potential role in preventing or treating bronchopulmonary dysplasia. Neonatology. (2018) 113(4):366–78. doi: 10.1159/000487388
115. Ogihara T, Mino M. Vitamin E and preterm infants. Free Radic Biol Med. (2022) 180:13–32. doi: 10.1016/j.freeradbiomed.2021.11.037
116. da Silva A, de Sousa Reboucas A, Mendonca BMA, Silva D, Dimenstein R, Ribeiro K. Relationship between the dietary intake, Serum, and breast milk concentrations of vitamin a and vitamin E in a cohort of women over the course of lactation. Matern Child Nutr. (2019) 15(3):e12772. doi: 10.1111/mcn.12772
117. Bortolotti A, Traina GL, Barzago MM, Celardo A, Bonati M. Placental transfer and tissue distribution of vitamin E in pregnant rabbits. Biopharm Drug Dispos. (1990) 11(8):679–88. doi: 10.1002/bdd.2510110804
118. Xi Y, Wang X, Liu K, Zhang H, Ren X, Zhao A, et al. Vitamin E concentration in breast milk in different periods of lactation: meta-analysis. Front Nutr. (2022) 9:1050011. doi: 10.3389/fnut.2022.1050011
119. Kolleck I, Sinha P, Rustow B. Vitamin E as an antioxidant of the lung: mechanisms of vitamin E delivery to alveolar type ii cells. Am J Respir Crit Care Med. (2002) 166(12 Pt 2):S62–6. doi: 10.1164/rccm.2206019
120. Ehrenkranz RA, Bonta BW, Ablow RC, Warshaw JB. Amelioration of bronchopulmonary dysplasia after vitamin E administration. A preliminary report. N Engl J Med. (1978) 299(11):564–9. doi: 10.1056/NEJM197809142991102
121. Coleman M, Thompson TR. A possible role of vitamin E in the prevention or amelioration of bronchopulmonary dysplasia. Am J Pediatr Hematol Oncol. (1979) 1(2):175–8.543512
122. Mino M. Use and safety of elevated dosages of vitamin E in infants and children. Int J Vitam Nutr Res Suppl. (1989) 30:69–80.2507708
123. Watts JL, Milner R, Zipursky A, Paes B, Ling E, Gill G, et al. Failure of supplementation with vitamin E to prevent bronchopulmonary dysplasia in infants less than 1,500 g birth weight. Eur Respir J. (1991) 4(2):188–90. doi: 10.1183/09031936.93.04020188
124. Bell EF, Filer LJ Jr. The role of vitamin E in the nutrition of premature infants. Am J Clin Nutr. (1981) 34(3):414–22. doi: 10.1093/ajcn/34.3.414
125. Zasada M, Suski M, Bokiniec R, Szwarc-Duma M, Borszewska-Kornacka MK, Madej J, et al. Comparative two time-point proteome analysis of the plasma from preterm infants with and without bronchopulmonary dysplasia. Ital J Pediatr. (2019) 45(1):112. doi: 10.1186/s13052-019-0676-0
126. Stone CA Jr., Cook-Mills J, Gebretsadik T, Rosas-Salazar C, Turi K, Brunwasser SM, et al. Delineation of the individual effects of vitamin E isoforms on early life incident wheezing. J Pediatr. (2019) 206:156–63.e3. doi: 10.1016/j.jpeds.2018.10.045
127. Ge H, Liu W, Li H, Zhang M, Zhang M, Liu C, et al. The association of vitamin D and vitamin E levels at birth with bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. (2021) 56(7):2108–13. doi: 10.1002/ppul.25414
128. Deger I, Ertugrul S, Yilmaz ST, Ozbey ZK, Yolbas I, Kaplan I. The relationship between vitamin a and vitamin E levels and neonatal morbidities. Eur Rev Med Pharmacol Sci. (2022) 26(6):1963–9. doi: 10.26355/eurrev_202203_28344
129. Amin SB, Laroia N, Sinkin RA, Kendig JW. Effect of dexamethasone therapy on Serum vitamin E concentrations in premature infants with bronchopulmonary dysplasia. J Perinatol. (2003) 23(7):552–5. doi: 10.1038/sj.jp.7210984
130. Guthmann F, Kolleck I, Schachtrup C, Schlame M, Spener F, Rustow B. Vitamin E deficiency reduces surfactant lipid biosynthesis in alveolar type II cells. Free Radic Biol Med. (2003) 34(6):663–73. doi: 10.1016/s0891-5849(02)01376-x
131. Guthmann F, Haupt R, Schlame M, Stevens PA, Rustow B. Alveolar surfactant subfractions differ in their lipid composition. Int J Biochem Cell Biol. (1995) 27(10):1021–6. doi: 10.1016/1357-2725(95)00078-4
132. Nguyen MTT, Kim J, Lee H, Won S, Kim Y, Jung JA, et al. A comparison of vitamin and lutein concentrations in breast milk from four Asian countries. Nutrients. (2020) 12(6):1794. doi: 10.3390/nu12061794
133. Calvo-Lerma J, Selma-Royo M, Hervas D, Yang B, Intonen L, Gonzalez S, et al. Breast milk lipidome is associated with maternal diet and Infants’ growth. Front Nutr. (2022) 9:854786. doi: 10.3389/fnut.2022.854786
134. Saito Y. Diverse cytoprotective actions of vitamin E isoforms- role as peroxyl radical scavengers and complementary functions with selenoproteins. Free Radic Biol Med. (2021) 175:121–9. doi: 10.1016/j.freeradbiomed.2021.08.234
135. Falciglia HS, Johnson JR, Sullivan J, Hall CF, Miller JD, Riechmann GC, et al. Role of antioxidant nutrients and lipid peroxidation in premature infants with respiratory distress syndrome and bronchopulmonary dysplasia. Am J Perinatol. (2003) 20(2):97–107. doi: 10.1055/s-2003-38315
136. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. (2019) 18(9):102350. doi: 10.1016/j.autrev.2019.102350
137. Valle MS, Russo C, Malaguarnera L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab Res Rev. (2021) 37(8):e3447. doi: 10.1002/dmrr.3447
138. Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and endothelial function. Nutrients. (2020) 12(2):575. doi: 10.3390/nu12020575
139. Bocheva G, Slominski RM, Slominski AT. The impact of vitamin D on skin aging. Int J Mol Sci. (2021) 22(16):9097. doi: 10.3390/ijms22169097
140. Akpinar S, Karadag MG. Is vitamin D important in anxiety or depression? What is the truth? Curr Nutr Rep. (2022) 11(4):675–81. doi: 10.1007/s13668-022-00441-0
141. Szymczak-Pajor I, Drzewoski J, Sliwinska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. (2020) 21(18):6644. doi: 10.3390/ijms21186644
142. Cetinkaya M, Cekmez F, Erener-Ercan T, Buyukkale G, Demirhan A, Aydemir G, et al. Maternal/neonatal vitamin D deficiency: a risk factor for bronchopulmonary dysplasia in preterms? J Perinatol. (2015) 35(10):813–7. doi: 10.1038/jp.2015.88
143. Kanatani KT, Nakayama T, Adachi Y, Hamazaki K, Onishi K, Konishi Y, et al. High frequency of vitamin D deficiency in current pregnant Japanese women associated with UV avoidance and hypo-vitamin D diet. PLoS One. (2019) 14(3):e0213264. doi: 10.1371/journal.pone.0213264
144. Lu T, Liang B, Jia Y, Cai J, Wang D, Liu M, et al. Relationship between bronchopulmonary dysplasia, long-term lung function, and vitamin D level at birth in preterm infants. Transl Pediatr. (2021) 10(11):3075–81. doi: 10.21037/tp-21-494
145. Park HW, Lim G, Park YM, Chang M, Son JS, Lee R. Association between vitamin D level and bronchopulmonary dysplasia: a systematic review and meta-analysis. PLoS One. (2020) 15(7):e0235332. doi: 10.1371/journal.pone.0235332
146. Mao X, Qiu J, Zhao L, Xu J, Yin J, Yang Y, et al. Vitamin D and il-10 deficiency in preterm neonates with bronchopulmonary dysplasia. Front Pediatr. (2018) 6:246. doi: 10.3389/fped.2018.00246
147. Papalia H, Samonini A, Buffat C, Gras E, des Robert C, Landrier JF, et al. Low vitamin D levels at birth and early respiratory outcome in infants with gestational age less than 29 weeks. Front Pediatr. (2021) 9:790839. doi: 10.3389/fped.2021.790839
148. Zhang X, Luo K, He X, Chen P. Association of vitamin D status at birth with pulmonary disease morbidity in very preterm infants. Pediatr Pulmonol. (2021) 56(5):1215–20. doi: 10.1002/ppul.25233
149. Yu H, Fu J, Feng Y. Utility of umbilical cord blood 25-hydroxyvitamin D levels for predicting bronchopulmonary dysplasia in preterm infants with very low and extremely low birth weight. Front Pediatr. (2022) 10:956952. doi: 10.3389/fped.2022.956952
150. Byun SY, Bae MH, Lee NR, Han YM, Park KH. Association between vitamin D deficiency at one month of age and bronchopulmonary dysplasia. Medicine (Baltimore). (2021) 100(48):e27966. doi: 10.1097/MD.0000000000027966
151. Ge H, Qiao Y, Ge J, Li J, Hu K, Chen X, et al. Effects of early vitamin D supplementation on the prevention of bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. (2022) 57(4):1015–21. doi: 10.1002/ppul.25813
152. Yang Y, Li Z, Yan G, Jie Q, Rui C. Effect of different doses of vitamin D supplementation on preterm infants—an updated meta-analysis. J Matern Fetal Neonatal Med. (2018) 31(22):3065–74. doi: 10.1080/14767058.2017.1363731
153. Aristizabal N, Holder MP, Durham L, Ashraf AP, Taylor S, Salas AA. Safety and efficacy of early vitamin D supplementation in critically ill extremely preterm infants: an ancillary study of a randomized trial. J Acad Nutr Diet. (2023) 123(1):87–94. doi: 10.1016/j.jand.2022.06.012
154. Fort P, Salas AA, Nicola T, Craig CM, Carlo WA, Ambalavanan N. A comparison of 3 vitamin D dosing regimens in extremely preterm infants: a randomized controlled trial. J Pediatr. (2016) 174:132–8.e1. doi: 10.1016/j.jpeds.2016.03.028
155. Zhen H, Hu H, Rong G, Huang X, Tan C, Yu X. Vita or VitD ameliorates bronchopulmonary dysplasia by regulating the balance between M1 and M2 macrophages. Biomed Pharmacother. (2021) 141:111836. doi: 10.1016/j.biopha.2021.111836
156. Hu J, Wu Z, Wang H, Geng H, Huo J, Zhu X, et al. Vitamin D ameliorates apoptosis and inflammation by targeting the mitochondrial and Mek1/2-Erk1/2 pathways in hyperoxia-induced bronchopulmonary dysplasia. J Inflamm Res. (2022) 15:4891–906. doi: 10.2147/JIR.S371906
157. Chen C, Weng H, Zhang X, Wang S, Lu C, Jin H, et al. Low-dose vitamin D protects hyperoxia-induced bronchopulmonary dysplasia by inhibiting neutrophil extracellular traps. Front Pediatr. (2020) 8:335. doi: 10.3389/fped.2020.00335
158. Wang Y, Jiang L. Role of vitamin D-vitamin D receptor signaling on hyperoxia-induced bronchopulmonary dysplasia in neonatal rats. Pediatr Pulmonol. (2021) 56(7):2335–44. doi: 10.1002/ppul.25418
159. Yao L, Shi Y, Zhao X, Hou A, Xing Y, Fu J, et al. Vitamin D attenuates hyperoxia-induced lung injury through downregulation of toll-like receptor 4. Int J Mol Med. (2017) 39(6):1403–8. doi: 10.3892/ijmm.2017.2961
160. Liu C, Chen Z, Li W, Huang L, Zhang Y. Vitamin D enhances alveolar development in antenatal lipopolysaccharide-treated rats through the suppression of interferon-gamma production. Front Immunol. (2017) 8:1923. doi: 10.3389/fimmu.2017.01923
161. Taylor SK, Sakurai R, Sakurai T, Rehan VK. Inhaled vitamin D: a novel strategy to enhance neonatal lung maturation. Lung. (2016) 194(6):931–43. doi: 10.1007/s00408-016-9939-3
162. Moschino L, Zivanovic S, Hartley C, Trevisanuto D, Baraldi E, Roehr CC. Caffeine in preterm infants: where are we in 2020? ERJ Open Res. (2020) 6(1):00330–2019. doi: 10.1183/23120541.00330-2019
163. Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. (2018) 16(1):36. doi: 10.1186/s12967-018-1417-7
164. Yan B, Li Y, Sun M, Meng Y, Li X. Variables related to bronchopulmonary dysplasia severity: a six-year retrospective study. J Matern Fetal Neonatal Med. (2023) 36(2):2248335. doi: 10.1080/14767058.2023.2248335
165. Chen X, Yuan L, Jiang S, Gu X, Lei X, Hu L, et al. Synergistic effects of achieving perinatal interventions on bronchopulmonary dysplasia in preterm infants. Eur J Pediatr. (2024) 183(4):1711–21. doi: 10.1007/s00431-023-05355-9
166. Ye H, Bai L, Yang M, Yang X, Zheng M, Zhong X, et al. A two-center retrospective study: association of early caffeine administration and oxygen radical diseases in neonatology in Chinese preterm neonates. Front Pediatr. (2023) 11:1158286. doi: 10.3389/fped.2023.1158286
167. Jiang Q, Wu X. Effect of early preventive use of caffeine citrate on prevention together with treatment of BPD within premature infants and its influence on inflammatory factors. Biotechnol Genet Eng Rev. (2023) 40(3):2730–44. doi: 10.1080/02648725.2023.2202517
168. Szatkowski L, Fateh S, Abramson J, Kwok TC, Sharkey D, Budge H, et al. Observational cohort study of use of caffeine in preterm infants and association between early caffeine use and neonatal outcomes. Arch Dis Child Fetal Neonatal Ed. (2023) 108(5):505–10. doi: 10.1136/archdischild-2022-324919
169. Elmowafi M, Mohsen N, Nour I, Nasef N. Prophylactic versus therapeutic caffeine for apnea of prematurity: a randomized controlled trial. J Matern Fetal Neonatal Med. (2022) 35(25):6053–61. doi: 10.1080/14767058.2021.1904873
170. Lamba V, Winners O, Fort P. Early high-dose caffeine improves respiratory outcomes in preterm infants. Children (Basel). (2021) 8(6):501. doi: 10.3390/children8060501
171. Rauf S, Shah S, Bibi Z, Munir R, Jiskani H, Ahmad S, et al. Association of caffeine daily dose with respiratory outcomes in preterm neonates: a retrospective cohort study. Inquiry. (2024) 61:469580241248098. doi: 10.1177/00469580241248098
172. Taha D, Kirkby S, Nawab U, Dysart KC, Genen L, Greenspan JS, et al. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J Matern Fetal Neonatal Med. (2014) 27(16):1698–702. doi: 10.3109/14767058.2014.885941
173. Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. (2014) 164(5):992–8.e3. doi: 10.1016/j.jpeds.2013.12.025
174. Yun WZ, Kassab YW, Yao LM, Khairuddin N, Ming LC, Hadi MA. Effectiveness and safety of early versus late caffeine therapy in managing apnoea of prematurity among preterm infants: a retrospective cohort study. Int J Clin Pharm. (2022) 44(5):1140–8. doi: 10.1007/s11096-022-01437-0
175. Li YF, Ouyang SH, Tu LF, Wang X, Yuan WL, Wang GE, et al. Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics. (2018) 8(20):5713–30. doi: 10.7150/thno.28778
176. Hosoi T, Toyoda K, Nakatsu K, Ozawa K. Caffeine attenuated ER stress-induced leptin resistance in neurons. Neurosci Lett. (2014) 569:23–6. doi: 10.1016/j.neulet.2014.03.053
177. Kou C, Han D, Li Z, Wu W, Liu Z, Zhang Y, et al. Influence of prevention of caffeine citrate on cytokine profile and bronchopulmonary dysplasia in preterm infants with apnea. Minerva Pediatr. (2020) 72(2):95–100. doi: 10.23736/S0026-4946.19.05428-8
178. Tian C, Li D, Fu J. Molecular mechanism of caffeine in preventing bronchopulmonary dysplasia in premature infants. Front Pediatr. (2022) 10:902437. doi: 10.3389/fped.2022.902437
179. Wang X, Lv S, Sun J, Zhang M, Zhang L, Sun Y, et al. Caffeine reduces oxidative stress to protect against hyperoxia-induced lung injury via the adenosine A2a receptor/cAMP/PKA/Src/ERK1/2/p38MAPK pathway. Redox Rep. (2022) 27(1):270–8. doi: 10.1080/13510002.2022.2143114
180. Endesfelder S, Strauss E, Bendix I, Schmitz T, Buhrer C. Prevention of oxygen-induced inflammatory lung injury by caffeine in neonatal rats. Oxid Med Cell Longev. (2020) 2020:3840124. doi: 10.1155/2020/3840124
181. Chen S, Wu Q, Zhong D, Li C, Du L. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through Nlrp3 inflammasome and nf-kappab pathway. Respir Res. (2020) 21(1):140. doi: 10.1186/s12931-020-01403-2
182. Teng RJ, Jing X, Michalkiewicz T, Afolayan AJ, Wu TJ, Konduri GG. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. (2017) 312(5):L586–98. doi: 10.1152/ajplung.00405.2016
183. Shahzad T, Radajewski S, Chao CM, Bellusci S, Ehrhardt H. Pathogenesis of bronchopulmonary dysplasia: when inflammation meets organ development. Mol Cell Pediatr. (2016) 3(1):23. doi: 10.1186/s40348-016-0051-9
184. Manley BJ, Makrides M, Collins CT, McPhee AJ, Gibson RA, Ryan P, et al. High-Dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics. (2011) 128(1):e71–7. doi: 10.1542/peds.2010-2405
185. Rocha G, Guimaraes H, Pereira-da-Silva L. The role of nutrition in the prevention and management of bronchopulmonary dysplasia: a literature review and clinical approach. Int J Environ Res Public Health. (2021) 18(12):6245. doi: 10.3390/ijerph18126245
186. Hanford J, Mannebach K, Ohler A, Patten M, Pardalos J. Rates of comorbidities in very low birth weight infants fed an exclusive human milk diet versus a bovine supplemented diet. Breastfeed Med. (2021) 16(10):814–20. doi: 10.1089/bfm.2020.0345
187. Lund AM, Domellof M, Pivodic A, Hellstrom A, Stoltz Sjostrom E, Hansen-Pupp I. Mother’s own milk and its relationship to growth and morbidity in a population-based cohort of extremely preterm infants. J Pediatr Gastroenterol Nutr. (2022) 74(2):292–300. doi: 10.1097/MPG.0000000000003352
188. Sun H, Cao Y, Han S, Cheng R, Liu L, Liu J, et al. A randomized controlled trial protocol comparing the feeds of fresh versus frozen mother’s own milk for preterm infants in the nicu. Trials. (2020) 21(1):170. doi: 10.1186/s13063-019-3981-4
189. Uberos J, Sanchez-Ruiz I, Fernandez-Marin E, Ruiz-Lopez A, Cubero-Millan I, Campos-Martinez A. Breast-Feeding as protective factor against bronchopulmonary dysplasia in preterm infants. Br J Nutr. (2024) 131(8):1405–12. doi: 10.1017/S0007114523002982
190. Xu Y, Yu Z, Li Q, Zhou J, Yin X, Ma Y, et al. Dose-dependent effect of human milk on bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatr. (2020) 20(1):522. doi: 10.1186/s12887-020-02394-1
191. Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed. (2017) 102(3):F256–61. doi: 10.1136/archdischild-2016-310898
192. Wendel K, Aas MF, Gunnarsdottir G, Rossholt ME, Bratlie M, Nordvik T, et al. Effect of arachidonic and docosahexaenoic acid supplementation on respiratory outcomes and neonatal morbidities in preterm infants. Clin Nutr. (2023) 42(1):22–8. doi: 10.1016/j.clnu.2022.11.012
193. Marc I, Boutin A, Pronovost E, Perez Herrera NM, Guillot M, Bergeron F, et al. Association between enteral supplementation with high-dose docosahexaenoic acid and risk of bronchopulmonary dysplasia in preterm infants: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6(3):e233934. doi: 10.1001/jamanetworkopen.2023.3934
194. Sullivan TR, Gould JF, Bednarz JM, McPhee AJ, Gibson R, Anderson PJ, et al. Mediation analysis to untangle opposing associations of high-dose docosahexaenoic acid with iq and bronchopulmonary dysplasia in children born preterm. JAMA Netw Open. (2023) 6(6):e2317870. doi: 10.1001/jamanetworkopen.2023.17870
195. Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, et al. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. (2003) 111(3):469–76. doi: 10.1542/peds.111.3.469
196. Parad RB, Allred EN, Rosenfeld WN, Davis JM. Reduction of retinopathy of prematurity in extremely low gestational age newborns treated with recombinant human Cu/Zn superoxide dismutase. Neonatology. (2012) 102(2):139–44. doi: 10.1159/000336639
197. Ozdemir R, Gokce IK, Taslidere AC, Tanbek K, Gul CC, Sandal S, et al. Does chrysin prevent severe lung damage in hyperoxia-induced lung injury model? Int Immunopharmacol. (2021) 99:108033. doi: 10.1016/j.intimp.2021.108033
198. Hsia CC, Hyde DM, Ochs M, Weibel ER, Structure AEJTFoQAoL. An official research policy statement of the American thoracic society/European respiratory society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. (2010) 181(4):394–418. doi: 10.1164/rccm.200809-1522ST
199. Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. (2008) 1(2–3):94–8. doi: 10.1242/dmm.000315
200. Demirci-Cekic S, Ozkan G, Avan AN, Uzunboy S, Capanoglu E, Apak R. Biomarkers of oxidative stress and antioxidant defense. J Pharm Biomed Anal. (2022) 209:114477. doi: 10.1016/j.jpba.2021.114477
201. Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. (2018) 19(5):281–96. doi: 10.1038/nrm.2017.138
202. Li J, Pan L, Pan W, Li N, Tang B. Recent progress of oxidative stress associated biomarker detection. Chem Commun (Camb). (2023) 59(48):7361–74. doi: 10.1039/d3cc00878a
203. Wolk M, Prabutzki P, Fedorova M. Analytical toolbox to unlock the diversity of oxidized lipids. Acc Chem Res. (2023) 56(7):835–45. doi: 10.1021/acs.accounts.2c00842
204. Weinberger B, Hirsch D, Yin K, Spur BW. Chapter 21—lipid mediators and lung function. In: Parent RA, editor. Comparative Biology of the Normal Lung. 2nd ed. San Diego: Academic Press (2015). p. 403–21.
205. Ito J, Shimizu N, Kobayashi E, Hanzawa Y, Otoki Y, Kato S, et al. A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils. Sci Rep. (2017) 7(1):10026. doi: 10.1038/s41598-017-10536-2
206. Eckl PM, Bresgen N. Genotoxicity of lipid oxidation compounds. Free Radic Biol Med. (2017) 111:244–52. doi: 10.1016/j.freeradbiomed.2017.02.002
207. Kehm R, Baldensperger T, Raupbach J, Hohn A. Protein oxidation—formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. (2021) 42:101901. doi: 10.1016/j.redox.2021.101901
208. Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. (2004) 164(3):341–6. doi: 10.1083/jcb.200311055
209. Hondal RJ, Marino SM, Gladyshev VN. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid Redox Signal. (2013) 18(13):1675–89. doi: 10.1089/ars.2012.5013
210. Palla T, Mirzahosseini A, Noszal B. Properties of selenolate-diselenide redox equilibria in view of their thiolate-disulfide counterparts. Antioxidants (Basel). (2023) 12(4):822. doi: 10.3390/antiox12040822
211. Orian L, Flohe L. Selenium-catalyzed reduction of hydroperoxides in chemistry and biology. Antioxidants (Basel). (2021) 10(10):1560. doi: 10.3390/antiox10101560
212. Guo L, Yang W, Huang Q, Qiang J, Hart JR, Wang W, et al. Selenocysteine-specific mass spectrometry reveals tissue-distinct selenoproteomes and candidate selenoproteins. Cell Chem Biol. (2018) 25(11):1380–8.e4. doi: 10.1016/j.chembiol.2018.08.006
213. Steinbrenner H, Speckmann B, Klotz LO. Selenoproteins: antioxidant selenoenzymes and beyond. Arch Biochem Biophys. (2016) 595:113–9. doi: 10.1016/j.abb.2015.06.024
214. Hoshi T, Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol. (2001) 531(Pt 1):1–11. doi: 10.1111/j.1469-7793.2001.0001j.x
215. Suzuki S, Kodera Y, Saito T, Fujimoto K, Momozono A, Hayashi A, et al. Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci Rep. (2016) 6:38299. doi: 10.1038/srep38299
216. Baldensperger T, Eggen M, Kappen J, Winterhalter PR, Pfirrmann T, Glomb MA. Comprehensive analysis of posttranslational protein modifications in aging of subcellular compartments. Sci Rep. (2020) 10(1):7596. doi: 10.1038/s41598-020-64265-0
217. Lim JM, Kim G, Levine RL. Methionine in proteins: it’s not just for protein initiation anymore. Neurochem Res. (2019) 44(1):247–57. doi: 10.1007/s11064-017-2460-0
218. Kim J, Li BX, Huang RY, Qiao JX, Ewing WR, MacMillan DWC. Site-selective functionalization of methionine residues via photoredox catalysis. J Am Chem Soc. (2020) 142(51):21260–6. doi: 10.1021/jacs.0c09926
219. Guan Z, Yates NA, Bakhtiar R. Detection and characterization of methionine oxidation in peptides by collision-induced dissociation and electron capture dissociation. J Am Soc Mass Spectrom. (2003) 14(6):605–13. doi: 10.1016/S1044-0305(03)00201-0
220. Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. (2003) 25(3–4):207–18. doi: 10.1007/s00726-003-0011-2
221. Reddy VP, Aryal P, Darkwah EK. Advanced glycation end products in health and disease. Microorganisms. (2022) 10(9):1848. doi: 10.3390/microorganisms10091848
222. Campolo N, Issoglio FM, Estrin DA, Bartesaghi S, Radi R. 3-Nitrotyrosine and related derivatives in proteins: precursors, radical intermediates and impact in function. Essays Biochem. (2020) 64(1):111–33. doi: 10.1042/EBC20190052
223. Akagawa M. Protein carbonylation: molecular mechanisms, biological implications, and analytical approaches. Free Radic Res. (2021) 55(4):307–20. doi: 10.1080/10715762.2020.1851027
224. Hawkins CL, Davies MJ. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem. (2019) 294(51):19683–708. doi: 10.1074/jbc.REV119.006217
225. Zhao Y, Zhang L, Ouyang X, Jiang Z, Xie Z, Fan L, et al. Advanced oxidation protein products play critical roles in liver diseases. Eur J Clin Invest. (2019) 49(6):e13098. doi: 10.1111/eci.13098
226. Hahm JY, Park J, Jang ES, Chi SW. 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp Mol Med. (2022) 54(10):1626–42. doi: 10.1038/s12276-022-00822-z
227. Zou Y, Ma X, Tang Y, Lin L, Yu J, Zhong J, et al. A robust LC-MS/MS method to measure 8-oxoguo, 8-oxodg, and nmn in human serum and urine. Anal Biochem. (2023) 660:114970. doi: 10.1016/j.ab.2022.114970
228. Chao MR, Evans MD, Hu CW, Ji Y, Moller P, Rossner P, et al. Biomarkers of nucleic acid oxidation—a summary state-of-the-art. Redox Biol. (2021) 42:101872. doi: 10.1016/j.redox.2021.101872
229. Cioffi F, Adam RHI, Bansal R, Broersen K. A review of oxidative stress products and related genes in early Alzheimer’s disease. J Alzheimers Dis. (2021) 83(3):977–1001. doi: 10.3233/JAD-210497
230. Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. (2009) 118(1):151–66. doi: 10.1007/s00401-009-0508-1
231. Gmitterova K, Gawinecka J, Heinemann U, Valkovic P, Zerr I. DNA versus rna oxidation in Parkinson’s disease: which is more important? Neurosci Lett. (2018) 662:22–8. doi: 10.1016/j.neulet.2017.09.048
232. Legaard GE, Feineis CS, Johansen MY, Hansen KB, Vaag AA, Larsen EL, et al. Effects of an exercise-based lifestyle intervention on systemic markers of oxidative stress and advanced glycation endproducts in persons with type 2 diabetes: secondary analysis of a randomised clinical trial. Free Radic Biol Med. (2022) 188:328–36. doi: 10.1016/j.freeradbiomed.2022.06.013
233. Jors A, Lund MAV, Jespersen T, Hansen T, Poulsen HE, Holm JC. Urinary markers of nucleic acid oxidation increase with age, obesity and insulin resistance in Danish children and adolescents. Free Radic Biol Med. (2020) 155:81–6. doi: 10.1016/j.freeradbiomed.2020.05.009
234. Liu T, Cai JP, Zhang LQ, Sun N, Cui J, Wang H, et al. The mechanism of RNA oxidation involved in the development of heart failure. Free Radic Res. (2019) 53(8):910–21. doi: 10.1080/10715762.2019.1646424
235. Shih YM, Chang YJ, Cooke MS, Pan CH, Hu CH, Chao MR, et al. Alkylating and oxidative stresses in smoking and non-smoking patients with COPD: implications for lung carcinogenesis. Free Radic Biol Med. (2021) 164:99–106. doi: 10.1016/j.freeradbiomed.2020.12.442
236. Hong X, Hu Y, Yuan Z, Fang Z, Zhang X, Yuan Y, et al. Oxidatively damaged nucleic acid: linking diabetes and cancer. Antioxid Redox Signal. (2022) 37(16–18):1153–67. doi: 10.1089/ars.2022.0096
237. Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol. (2012) 18(4):302–8. doi: 10.3748/wjg.v18.i4.302
238. Mundt JM, Hah SS, Sumbad RA, Schramm V, Henderson PT. Incorporation of extracellular 8-oxodg into DNA and RNA requires purine nucleoside phosphorylase in mcf-7 cells. Nucleic Acids Res. (2008) 36(1):228–36. doi: 10.1093/nar/gkm1032
239. Stoddard S, Riggleman A, Carpenter A, Baranova A. The detection of 8-oxo-7,8-dihydro-2'-deoxyguanosine in circulating cell-free DNA: a step towards longitudinal monitoring of health. Adv Exp Med Biol. (2020) 1241:125–38. doi: 10.1007/978-3-030-41283-8_8
240. Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R. Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr Res. (2004) 56(1):88–93. doi: 10.1203/01.PDR.0000130478.05324.9D
241. Matthews MA, Aschner JL, Stark AR, Moore PE, Slaughter JC, Steele S, et al. Increasing F2-isoprostanes in the first month after birth predicts poor respiratory and neurodevelopmental outcomes in very preterm infants. J Perinatol. (2016) 36(9):779–83. doi: 10.1038/jp.2016.74
242. Reuter SD, O'Donovan DJ, Hegemier SE, Smith EO, Heird WC, Fernandes CJ. Urinary F2-isoprostanes are poor prognostic indicators for the development of bronchopulmonary dysplasia. J Perinatol. (2007) 27(5):303–6. doi: 10.1038/sj.jp.7211684
243. Chessex P, Watson C, Kaczala GW, Rouleau T, Lavoie ME, Friel J, et al. Determinants of oxidant stress in extremely low birth weight premature infants. Free Radic Biol Med. (2010) 49(9):1380–6. doi: 10.1016/j.freeradbiomed.2010.07.018
244. Vera KB, Moore D, Flack E, Liske M, Summar M. Significant differences in markers of oxidant injury between idiopathic and bronchopulmonary-dysplasia-associated pulmonary hypertension in children. Pulm Med. (2012) 2012:301475. doi: 10.1155/2012/301475
245. Kuligowski J, Aguar M, Rook D, Lliso I, Torres-Cuevas I, Escobar J, et al. Urinary lipid peroxidation byproducts: are they relevant for predicting neonatal morbidity in preterm infants? Antioxid Redox Signal. (2015) 23(2):178–84. doi: 10.1089/ars.2015.6262
246. Liyakat NA, Kumar P, Sundaram V. Room air versus 100% oxygen for delivery room resuscitation of preterm neonates in low resource settings: a randomised, blinded, controlled trial. J Paediatr Child Health. (2023) 59(6):794–801. doi: 10.1111/jpc.16391
247. Bhunwal S, Mukhopadhyay K, Bhattacharya S, Dey P, Dhaliwal LK. Bronchopulmonary dysplasia in preterm neonates in a level iii neonatal unit in India. Indian Pediatr. (2018) 55(3):211–5. doi: 10.1007/s13312-018-1319-z
248. Yildizdas HY, Poyraz B, Atli G, Sertdemir Y, Mert K, Ozlu F, et al. Effects of two different lipid emulsions on antioxidant Status, lipid peroxidation and parenteral nutrition- related cholestasis in premature babies, a randomized-controlled study. Pediatr Neonatol. (2019) 60(4):359–67. doi: 10.1016/j.pedneo.2018.07.012
249. Weinberger B, Anwar M, Henien S, Sosnovsky A, Hiatt M, Jochnowitz N, et al. Association of lipid peroxidation with antenatal betamethasone and oxygen radial disorders in preterm infants. Biol Neonate. (2004) 85(2):121–7. doi: 10.1159/000074968
250. Joung KE, Kim HS, Lee J, Shim GH, Choi CW, Kim EK, et al. Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radic Res. (2011) 45(9):1024–32. doi: 10.3109/10715762.2011.588229
251. Tokuriki S, Okuno T, Ohta G, Ohshima Y. Carboxyhemoglobin formation in preterm infants is related to the subsequent development of bronchopulmonary dysplasia. Dis Markers. (2015) 2015:620921. doi: 10.1155/2015/620921
252. Vento M, Aguar M, Escobar J, Arduini A, Escrig R, Brugada M, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. (2009) 11(12):2945–55. doi: 10.1089/ars.2009.2671
253. Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans. (2003) 31(Pt 6):1417–22. doi: 10.1042/bst0311417
254. Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. (2017) 58(5):235–63. doi: 10.1002/em.22087
255. Rafiee A, Ospina MB, Pitt TM, Quemerais B. Oxidative stress and DNA damage resulting from welding fumes exposure among professional welders: a systematic review and meta-analysis. Environ Res. (2022) 214(Pt 4):114152. doi: 10.1016/j.envres.2022.114152
256. Goriuc A, Cojocaru KA, Luchian I, Ursu RG, Butnaru O, Foia L. Using 8-hydroxy-2'-deoxiguanosine (8-ohdg) as a reliable biomarker for assessing periodontal disease associated with diabetes. Int J Mol Sci. (2024) 25(3):1425. doi: 10.3390/ijms25031425
257. Wang D, Liang Q, Tai D, Wang Y, Hao H, Liu Z, et al. Association of urinary arsenic with the oxidative DNA damage marker 8-hydroxy-2 deoxyguanosine: a meta-analysis. Sci Total Environ. (2023) 904:166600. doi: 10.1016/j.scitotenv.2023.166600
258. Bigagli E, Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev. (2019) 2019:5953685. doi: 10.1155/2019/5953685
259. Blahova L, Janos T, Mustieles V, Rodriguez-Carrillo A, Fernandez MF, Blaha L. Rapid extraction and analysis of oxidative stress and DNA damage biomarker 8-hydroxy-2'-deoxyguanosine (8-ohdg) in urine: application to a study with pregnant women. Int J Hyg Environ Health. (2023) 250:114175. doi: 10.1016/j.ijheh.2023.114175
260. Poulsen HE, Weimann A, Henriksen T, Kjaer LK, Larsen EL, Carlsson ER, et al. Oxidatively generated modifications to nucleic acids in vivo: measurement in urine and plasma. Free Radic Biol Med. (2019) 145:336–41. doi: 10.1016/j.freeradbiomed.2019.10.001
261. Zayegh AM, Davis PG. Bpd treatments: the never-ending smorgasbord. Semin Fetal Neonatal Med. (2021) 26(2):101223. doi: 10.1016/j.siny.2021.101223
262. Zimmerman JJ, Gabbert D, Shivpuri C, Kayata S, Ciesielski W, Miller J, et al. Meter-dosed, inhaled beclomethasone attenuates bronchoalveolar oxyradical inflammation in premature infants at risk for bronchopulmonary dysplasia. Am J Perinatol. (1998) 15(10):567–76. doi: 10.1055/s-2007-994062
263. Laube M, Amann E, Uhlig U, Yang Y, Fuchs HW, Zemlin M, et al. Inflammatory mediators in tracheal aspirates of preterm infants participating in a randomized trial of inhaled nitric oxide. PLoS One. (2017) 12(1):e0169352. doi: 10.1371/journal.pone.0169352
264. Ozkan H, Koksal N, Dorum BA, Kocael F, Ozarda Y, Bozyigit C, et al. New-generation fish oil and olive oil lipid for prevention of oxidative damage in preterm infants: single center clinical trial at university hospital in Turkey. Pediatr Int. (2019) 61(4):388–92. doi: 10.1111/ped.13798
Keywords: bronchopulmonary dysplasia, hyperoxia exposure, oxidative stress, antioxidant treatments, predictive biomarkers
Citation: Li M, Cheng W-X, Li S, Wang J, Chen Y-R, Li L and Yang G (2025) Research progress on pathophysiologic mechanisms, clinical treatment and predictive biomarkers in bronchopulmonary dysplasia: from the perspective of oxidative stress. Front. Pediatr. 12:1343870. doi: 10.3389/fped.2024.1343870
Received: 24 November 2023; Accepted: 9 September 2024;
Published: 27 March 2025.
Edited by:
Hongxing Dang, Children's Hospital of Chongqing Medical University, ChinaReviewed by:
Renqiang Yu, Jiangnan University, ChinaCopyright: © 2025 Li, Cheng, Li, Wang, Chen, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: LiangTGlsaUBzdXN0ZWNoLmVkdS5jbg==Gui Yang, eWFuZ2d1aTAwMTdAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.