- 1Department of Physical Education, College of Physical Education, Shenzhen University, Shenzhen, China
- 2Department of Sport and Exercise Science, College of Education, Zhejiang University, Hangzhou, China
Objective: Metabolic disorders are common among children and adolescents with obesity and are associated with insulin resistance, hyperlipidemia, hypertension, and other cardiovascular risk factors. High-intensity interval training (HIIT) is a time-efficient method to improve cardiometabolic health. We performed a meta-analysis to determine the effects of HIIT on glycolipid metabolism in children with metabolic disorders.
Methods: Meta-analyses were conducted to determine the effect of HIIT on glycolipid metabolism markers. Subgroup analysis with potential moderators was explored [i.e., training intensity standard and work/rest time ratio (WRR)].
Results: Eighteen trials involving 538 participants were included. HIIT showed positive effects on glycolipid metabolism, such as triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), blood glucose (BG), blood insulin (BI), and homeostasis model assessment (HOMA)-IR, when compared to the non-training control group (CON); in addition to BG (p = 0.257), the combined results of other indicators have high heterogeneity (p = 0.000). HIIT showed no superior effects when compared to moderate-intensity training (MIT). Subgroup analysis demonstrated that HIIT protocol with a WRR of 1:1 was superior to MIT for reducing TG and LDL-C and used %maximal aerobic speed (MAS) as the exercise intensity was superior to MIT for reducing TG. HIIT protocol used %heart rate (HR) as the exercise intensity was superior to MIT for increasing HDL-C, decreasing BI, and HOMA-IR.
Conclusion: HIIT improved glycolipid metabolism in children with metabolic disorders. WRR and training intensity can affect the intervention effects of HIIT.
Systematic Review Registration: [https://www.crd.york.ac.uk/], identifier [CRD42021291473]
Introduction
Obesity is the excessive accumulation of adipose tissue (1). The current evidence showed that obesity could induce various harmful health consequences, such as metabolic syndrome (MetS) (2). Depending on the diagnostic criteria, combined with the high incidence of childhood obesity, the global prevalence of MetS in childhood and adolescence has been estimated to differ between 6 and 39% (2). Metabolic disorders often coexist with other MetS factors, such as obesity, dyslipidemia, and type 2 diabetes mellitus (T2D), and are associated with cardiovascular disease (CVD) risk (3, 4).
Physical activity (PA) is essential for children and adolescents’ normal growth and development and plays a vital role in reducing disease risk and promoting health (5). Recent PA guidelines for children and adolescents aged 5–17 years recommend an average of 60 min of moderate- to vigorous-intensity PA per day to maintain and improve metabolic health (6). Improvement effects of glycolipid metabolism have been established in some randomized controlled trials, including participants with overweight/obesity, T2D, and other chronic diseases (7–9). Unfortunately, extensive international data showed that over 80% of children and adolescents do not meet the recommended levels of PA (10). In addition, lack of time and poor long-term adherence may be the main obstacles to perform physical exercise (11, 12). The benefits of high-intensity exercise have been supported by many evidence in adults, such as decreasing body fat and improving dyslipidemia (13). Some studies have focused on its feasibility in children. Considering children’s interval and burst exercise pattern in their natural state, high-intensity interval training (HIIT) seems more feasible (14). HIIT as an enhancement pattern of interval training including burst high-intensity exercise (ranging from 85 to 250% VO2max for 6 s to 4 min) interspersed by brief bouts of low-intensity recovery (ranging from 20 to 40% VO2max for 10 s to 5 min) or rest (15). Recent studies demonstrated that HIIT might improve dyslipidemia, insulin level, and blood glucose (BG) parameters of children and adolescents with obesity or metabolic disorders (16). Meanwhile, compared to traditional long-time moderate-intensity continuous training (MICT), HIIT has more time-efficiency and higher adherence (13, 15). However, the improvement of HIIT on glycolipid metabolism is controversial. Some acute (single session) and long-term (≥2 weeks) interventions have shown that HIIT can reduce blood lipid profiles, postprandial BG, and fasting BG, and can improve peripheral insulin sensitivity (17, 18); others did not find effective improvement in glycolipid metabolism parameters (19, 20). In addition, a recent systematic review of 823 subjects from 29 studies showed that HIIT did not significantly improve blood lipid indicators (21).
Therefore, the main aim was to examine a meta-analysis comparing the effects of HIIT on glycolipid metabolism parameters of children with metabolic disorders. The secondary purpose was to explore the impact of HIIT components on the intervention effect according to subgroup analysis. We hypothesized that HIIT could improve some glycolipid metabolism indicators, and the HIIT details may affect the size of the effects.
Methodology
Inclusion and Exclusion Criteria
Studies were considered to be eligible according to the following criteria: (1) participants with metabolic disorders, including overweight/obesity, type 1 diabetes (T1D), T2D, MetS, or non-alcoholic fatty liver disease (NAFLD); (2) participants were randomly assigned to an HIIT group and other forms of exercise group (moderate-intensity training [MIT]); (3) high intensity classified as “maximal velocity,” “ ≥ 85% VO2max” (22), “ ≥ 80% maximal heart rate,” (23) or “ ≥ 100% maximal aerobic speed (MAS) (24); (4) outcomes included glycolipid parameters [e.g., triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), BG, blood insulin (BI), or homeostasis model assessment (HOMA)-IR]; and (5) available in English or Chinese. Conference abstracts, case studies, dissertations, books, reviews, theses, and articles published in non-peer-reviewed journals were not included for consideration.
Search Strategy
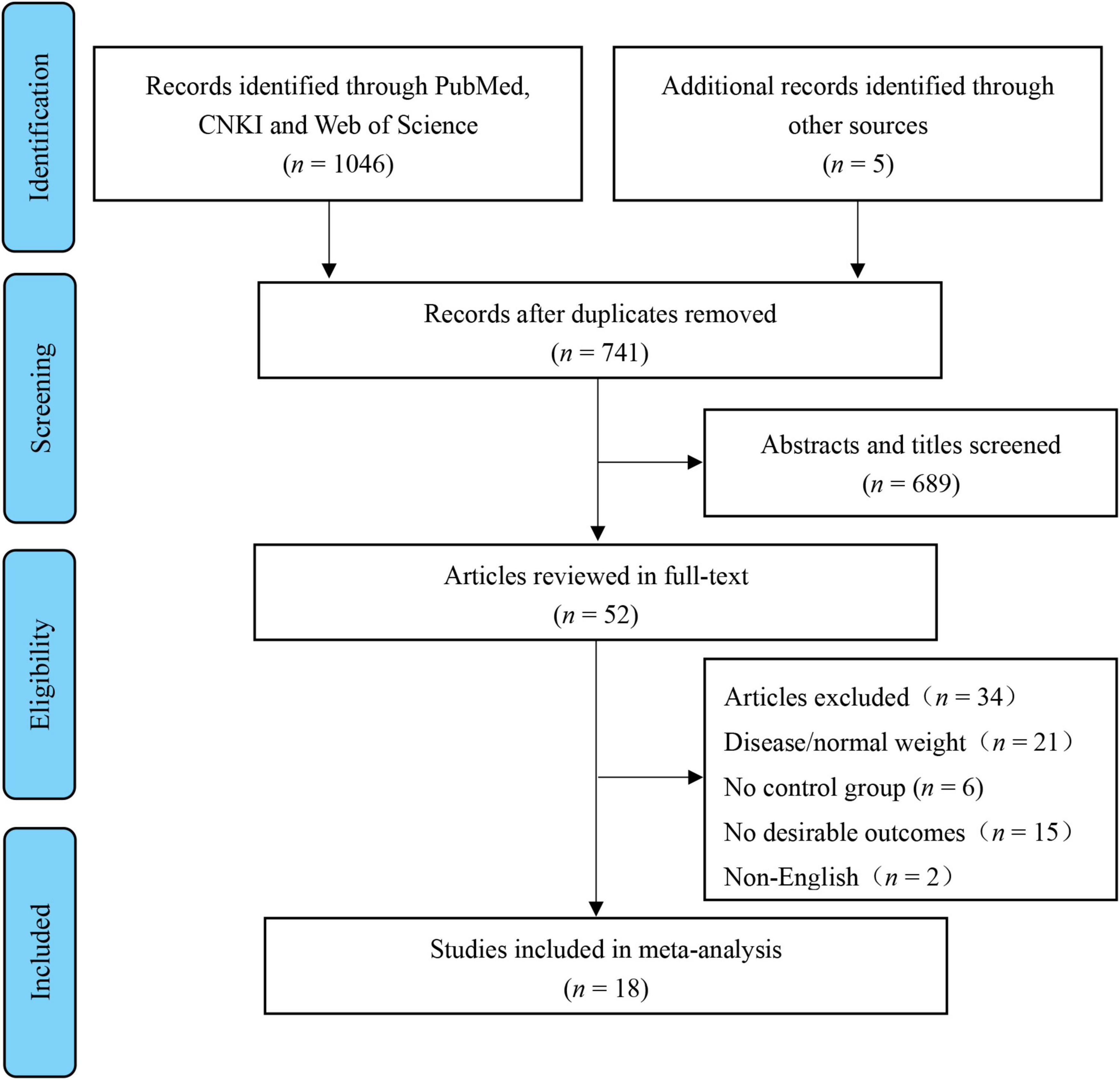
This review’s registry is on PROSPERO (ID: CRD420183694). Preferred Reporting Items performed a systematic search for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25). The retrieval date of the electronic databases was searched until November 2021, with no restriction on the year of publication. Two independent researchers (C.M. and Z.Y.) searched the relevant studies through Chinese (CNKI) and English-language (PubMed, Web of Science, and SPORTDiscus) electronic databases using the following terms: high-intensity interval OR high intensity intermittent OR sprint interval OR HIIT OR HIIE OR SIT OR interval training AND child* OR youth OR adolescen* OR girl* OR boy* OR kid* OR student* OR preadolescen* OR childhood. In addition, more references were searched through all retrieved studies to ensure that no relevant articles were missed. Figure 1 shows the study selection process.
Data Extraction
Two authors (C.M. and L.S.) performed the data extraction, which allowed characteristics, including (1) author, study design, and public year; (2) subject characteristics; (3) exercise intervention and control protocols; and (4) values of glycolipid metabolism parameters at baseline after the intervention. Data were expressed as mean (M) and SD, using the formula (SD = × SE) to convert SE into SD.
Risk of Bias Assessment
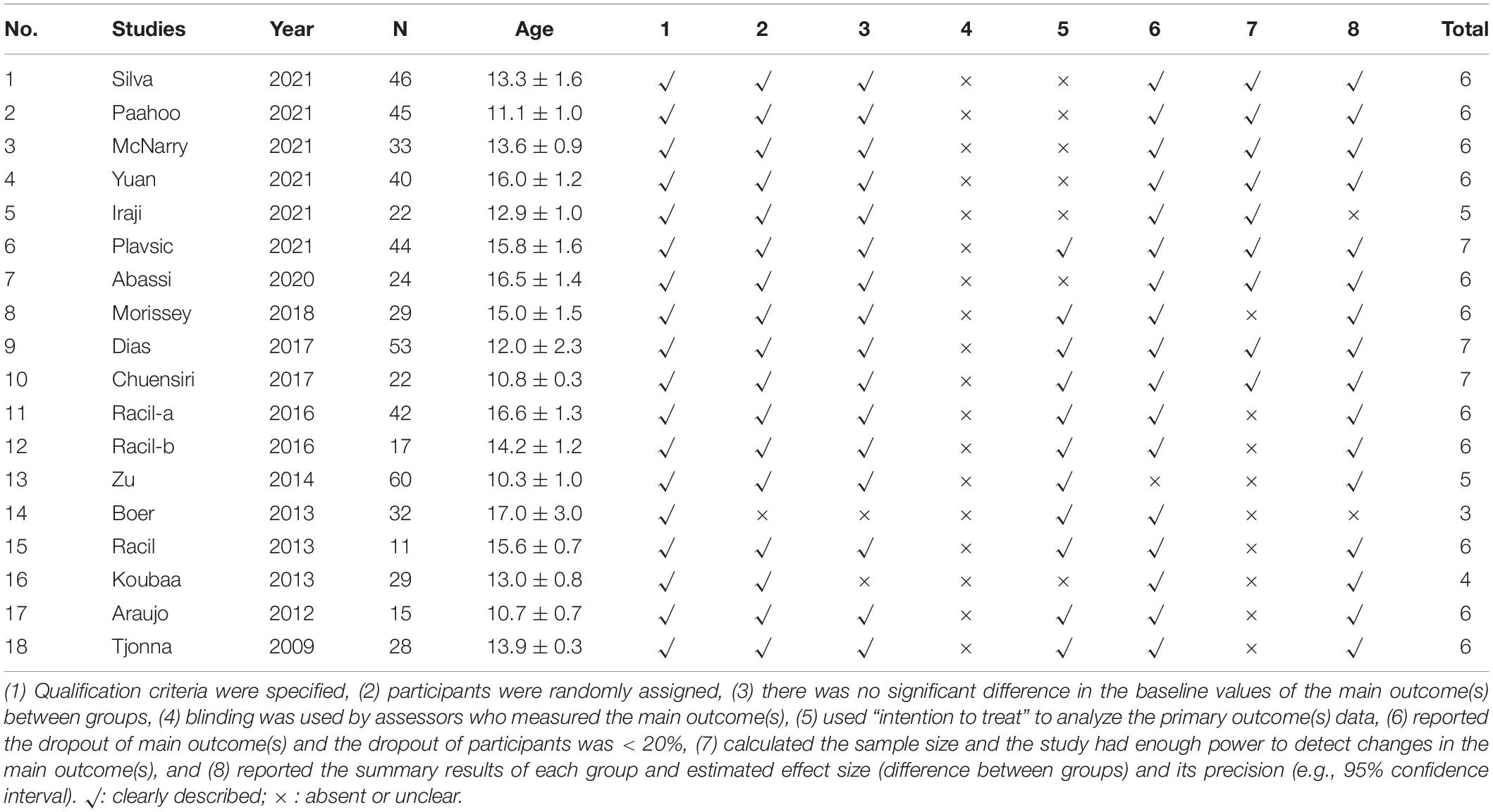
The publication bias was assessed using Egger’s and Begg’s tests; if the test result has p ≤ 0.05, it has existing bias (42). A funnel plot for visual interpretation was created, and then Egger’s test was used to confirm or refute the publication bias. Egger’s test (p > 0.05) showed no publication bias. If there was a significant publication bias, the stability of the results was evaluated using a trim-and-fill method and the leave-one-out sensitivity analysis to assess the impact of the overall effect size of the pooled data (43) (Table 1).
Statistical Analyses
Meta-analyses were conducted to determine the effect of HIIT on glycolipid metabolism parameters when compared to the MIT or control group (CON). We used the STATA software 14.0 for Windows (STATA 14.0, Stata Corp., United States) to examine the mean values or change score and standard deviations in the meta-analysis. The meta-analysis results with random effects are represented in the figures (the mixed effects are reported in the text). Heterogeneity was quantified using Cochrane’s Q test and Higgins I (2), where < 25, 25–75, and > 75% represent low, moderate, and high heterogeneities, respectively (44). The effect size of the standardized mean difference (SMD) in glycolipid metabolism parameters was calculated, and the 95% confidence intervals (95%CIs) were reported. The significance level was set at p < 0.05. Subgroup moderator analyses were conducted to determine whether HIIT effects differed according to training intensity standard [i.e., %MAS or %heart rate (HR)] and work/rest time ratio (WRR, = 1:1 or ≠1:1).
Results
The search identified 1,051 articles published before 30 November 2021. After removing 741 duplicate records, 689 not relevant articles were excluded. Of the remaining 52 articles, 18 met the inclusion criteria and were included in the review (Figure 1).
As a result, 538 participants from 18 studies were included in the final analysis. Eight to ten studies compared the effects of HIIT vs. CON, and six to eight studies compared the effects of HIIT vs. MIT on TG, TC, HDL-C, LDL-C, BG, BI, and HOMA-IR (19, 20, 26–41). Table 2 shows the characteristics of HIIT and MIT in included studies. The intervention duration ranged from 8 to 24 weeks. Training sessions were performed on a treadmill, cycling, and playing game 2 or 3 times per week. The total training time of HIIT ranged from 6.7 to 45 min.
High-Intensity Interval Training and Blood Lipid Outcomes
Table 3 shows the pooled analyses results. HIIT has significant effects when compared to CON in terms of reducing TG (SMD: −1.30, 95%CI: −2.01 to −0.58; I2 = 88.0%, p = 0.000), TC (SMD: −1.24, 95%CI: −1.84 to −0.64; I2 = 77.8%, p = 0.000), LDL-C (SMD: −1.13, 95%CI: −1.71 to −0.55; I2 = 79.3%, p = 0.000), and increasing HDL-C (SMD: 1.21, 95%CI: 0.43 to 1.99; I2 = 89.9%, p = 0.000) in children with metabolic disorders. However, there was no significant difference between HIIT and MIT on TG (SMD: −0.21, 95%CI: −0.52–0.09; I2 = 39.1%, p = 0.119), TC (SMD: −0.18, 95%CI: −0.73–0.36; I2 = 79.9%, p = 0.000), LDL-C (SMD: −0.38, 95%CI: −1.00–0.25; I2 = 83.0%, p = 0.000), and HDL-C (SMD: 0.30, 95%CI: −0.47–1.06; I2 = 88.1%, p = 0.000).
The results of Egger’s and Begg’s tests showed that there was a significant publication bias when compared to CON on TG (p-value for Egger: 0.001; p-value for Begg: 0.016), TC (p-value for Egger: 0.001; p-value for Begg: 0.001), and LDL-C (p-value for Egger: 0.001; p-value for Begg: 0.007). The conclusion did not change when the potential publication bias was adjusted using the trim-and-fill method. The funnel plot after shearing and supplementation showed no apparent asymmetry, suggesting no publication bias.
High-Intensity Interval Training and Glucose Outcomes
The results of meta-analysis showed that HIIT was superior to CON in terms of decreasing BG (SMD: −0.37, 95%CI: −0.64 to −0.09; I2 = 21.6%, p = 0.257), BI (SMD: −2.30, 95%CI: −3.47 to −1.12; I2 = 92.7%, p = 0.000), and HOMA-IR (SMD: −1.79, 95%CI: −2.95 to −0.62; I2 = 94.1%, p = 0.000). In addition, when compared with MIT, HIIT was not superior to BG (SMD: −1.02, 95%CI: −2.23–0.19; I2 = 94.2%, p = 0.000), BI (SMD: −0.58, 95%CI: −1.30–0.15; I2 = 83.8%, p = 0.000), and HOMA-IR (SMD: −1.16, 95%CI: −2.38–0.06; I2 = 94.1%, p = 0.000).
The results of Egger’s and Begg’s tests showed that there was no significant publication bias when compared to CON on BG (p-value for Egger: 0.781; p-value for Begg: 0.805), but have a significant bias on BI (p-value for Egger: 0.007; p-value for Begg: 0.026) and HOMA-IR (p-value for Egger: 0.001; p-value for Begg: 0.061). There was no significant publication bias when compared to MIT on BG (p-value for Egger: 0.019; p-value for Begg: 0.176), BI (p-value for Egger: 0.521; p-value for Begg: 0.851), and HOMA-IR (p-value for Egger: 0.083; p-value for Begg: 0.293).
Subgroup Analysis
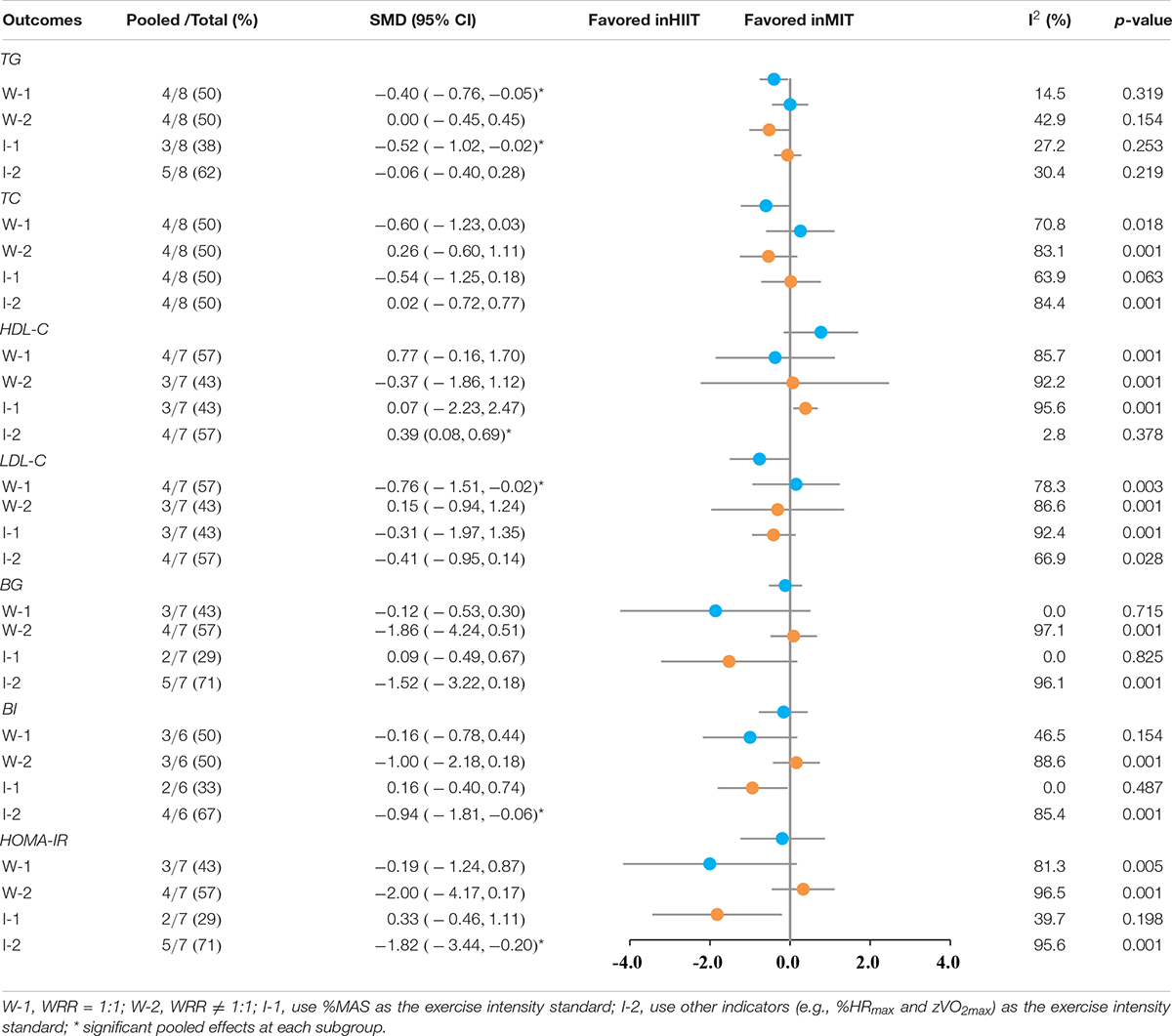
According to our previous study (45), a subgroup analysis of training elements that may affect the effects of HIIT intervention was performed. The results of subgroup analyses are shown in Table 4. HIIT protocol with W-1 (WRR = 1) was superior to MIT for reducing TG (SMD: −0.40, 95%CI: −0.76 to −0.05; I2 = 14.5%, p = 0.319) and LDL-C (SMD: −0.76, 95%CI: −1.51 to −0.02; I2 = 78.3%, p = 0.003). HIIT protocol with I-1 (used %MAS as the exercise intensity standard) was superior to MIT for reducing TG (SMD: −0.06, 95%CI: −1.02 to −0.02; I2 = 27.2%, p = 0.253). HIIT protocol with I-2 (used %HR as the exercise intensity standard) was superior to MIT for increasing HDL-C (SMD: 0.39, 95%CI: 0.08–0.69; I2 = 2.8%, p = 0.378), decreasing BI (SMD: −0.94, 95%CI: −1.81 to −0.06; I2 = 85.4%, p = 0.001), and HOMA-IR (SMD: −1.82, 95%CI: −3.44 to −0.20; I2 = 95.6%, p = 0.001).
Discussion
This study aimed to compare the effects of HIIT and CON or MIT on glycolipid metabolism parameters in children with metabolic disorders and to examine whether one protocol was superior to the other. First, results demonstrated that HIIT is an effective intervention to improve glycolipid metabolism parameters in children with metabolic disorders. Second, HIIT and MIT appear to be similarly effective on these measures, but HIIT seems to be more time-efficient. Third, the WRR and exercise intensity standard selection played an important role in intervention results.
The MetS is not a disease but a group of risk factors, such as high hypertension, high BG, hyperlipidemia, and abdominal fat (2). It was often accompanied by obesity (46). Management of childhood obesity and improvements of obesity-induced metabolic disorders, such as hypertension, hyperlipidemia, and insulin resistance, are effective ways to prevent and treat MetS (47). Evidence from our study suggested that HIIT can improve blood lipids in children with metabolic disorders, but there was no significant difference when compared to MIT. Our results were consistent with the previous meta-analysis, which compared the effects of HIIT and MIT on blood lipids in adults (21). However, subgroup analysis showed that WRR and exercise intensity might impact the intervention effect; HIIT protocol with WRR equal to 1 may favor the reduction of TG and LDL-C (Table 4). The effect of HIIT on blood lipids is controversial. Some studies have shown that HIIT has no significant impact on TC, TG, HDL-C, or LDL-C in children with obesity (19, 26), and a systematic review is also in line with this conclusion (13). In contrast, the study by Racil et al. demonstrated that 12-week HIIT significantly improved the blood lipid of obese children (37), and Chuensiri’s study further supports this result (33). Meanwhile, the metabolism of lipid profile is dependent on training intensity and duration (37). Animal experiments have showed that HIIT improves lipid metabolism, possibly regulating mitochondrial biosynthesis.
Childhood obesity is often accompanied by BG and insulin abnormalities, even developing insulin resistance or MetS (48). With the increasing incidence of obesity in children, 6–39% of obese children and adolescents already present with metabolism syndrome (49). Fasting glucose is predominantly a marker of hepatic insulin sensitivity (2). Therefore, strategies to improve glucose metabolism in children with obesity play an important role in disease prevention. Our results demonstrated that HIIT could decrease the BG, BI, and HOMA-IR of children with metabolic disorders, but not superior to MIT. Studies evaluating the effects of glucose metabolism markers by HIIT were inconsistent; some report reduced BG and BI (30, 34, 36, 41), while others report no change (19, 20). In line with our results, where a decrease in BG or BI was observed, the decline appeared to be like that after MIT (34). It was followed in animal experiments that the improvement of BG and BI in T2D mice after 8-week HIIT accompanied by the increase of glycogen content in skeletal muscle (48). Some studies have shown that upregulation of GLUT4, increased aerobic enzyme activity, and mitochondrial biogenesis may be a potential mechanism of HIIT promoting glucose uptake and improving insulin sensitivity (17, 50–52).
To the best of our knowledge, there are few reports on HIIT improving glycolipid metabolism in children with metabolic disorders. Therefore, our results provide strong evidence for the metabolic health of children and adolescents. For children, the benefits of exercise are apparent, but their PA is still in a downward trend (6). This study has shown that HIIT can improve the glycolipid metabolism of children with metabolic disorders. Considering that HIIT is more in line with children’s exercise mode and higher exercise compliance if HIIT is the recommended form of children’s PA, it may better affect their health promotion (14). In the future, relevant exercise intervention experiments should be carried out in schools further to verify the impact of HIIT on relevant indicators in children.
There are some limitations to this meta-analysis. The first one was the high heterogeneity of pooled effects that may be due to methodological differences, study design, exercise protocols, and quality of a study. It may have weakened results, but the robust result after the trim-and-fill method suggested no significant publication bias. However, we have carried out a subgroup analysis of the training protocol components, which has enhanced the strength of evidence. A relatively small number of included studies were another limitation of our review. Larger sample sizes and more diverse studies are needed to address these limitations.
Conclusion
Our findings indicated that HIIT might constitute an effective training protocol for improving glycolipid metabolism markers in children with metabolic disorders. The secondary result demonstrated that HIIT does not have superior improvements in glycolipid metabolism markers over MIT. Still, the components of HIIT, such as exercise intensity and WRR, may play an essential role in the effect of the intervention. However, whether these metabolic adaptations follow HIIT in children and adolescents needs further examination.
Perspective on Sports Medicine
To the best of our knowledge, this is the first meta-analysis to investigate the effects of HIIT on glycolipid markers in children with metabolic disorders. HIIT decreases the levels of lipid profiles and increases HDL-C, but did not superior to MIT. Thus, our findings indicated that HIIT might be a feasible and time-dependent intervention to improve glycolipid metabolism in children with metabolic disorders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MC participated in the study design data analysis and drafted and critically revised the manuscript. YT, SL, and YZ were responsible for selecting articles for inclusion and conducting the risk of bias assessment. YZ was responsible for the data extraction and helped to revise the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Guangdong Planning Office of Philosophy and Social Science (ID: GD20YTY02), the Humanities and Social Science Fund of the Ministry of Education of China (ID: 16YJC890021), and the Shenzhen Science and Technology Innovation Commission supports projects (20200810135056001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful for the support of the Normal College of Shenzhen University and all authors who responded to our requests for additional information.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.887852/full#supplementary-material
References
1. Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. (2015) 45:1209–17. doi: 10.1111/eci.12519
2. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
3. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218
4. Bianco A, Pomara F, Thomas E, Paoli A, Battaglia G, Petrucci M, et al. Type 2 diabetes family histories, body composition and fasting glucose levels: a cross-section analysis in healthy sedentary male and female. Iranian J Public Health. (2013) 42:681–90.
5. Hills AP, Andersen LB, Byrne NM. Physical activity and obesity in children. Br J Sports Med. (2011) 45:866–70.
6. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
7. Chang C, Liu W, Zhao X, Li S, Yu C. Effect of supervised exercise intervention on metabolic risk factors and physical fitness in Chinese obese children in early puberty. Obes Rev. (2008) 9(Suppl. 1):135–41. doi: 10.1111/j.1467-789X.2007.00455.x
8. Tolfrey K, Jones AM, Campbell IG. The effect of aerobic exercise training on the lipid-lipoprotein profile of children and adolescents. Sports Med. (2000) 29:99–112. doi: 10.2165/00007256-200029020-00003
9. Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med. (2016) 93:211–8. doi: 10.1016/j.ypmed.2016.10.020
10. Rhodes RE, Guerrero MD, Vanderloo LM, Barbeau K, Birken CS, Chaput JP, et al. Development of a consensus statement on the role of the family in the physical activity, sedentary, and sleep behaviors of children and youth. Int J Behav Nutr Phys Act. (2020) 17:74. doi: 10.1186/s12966-020-00973-0
11. Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Med. (2013) 43:313–38. doi: 10.1007/s40279-013-0029-x
12. Malik AA, Williams CA, Weston KL, Barker AR. Perceptual responses to high- and moderate-intensity interval exercise in adolescents. Med Sci Sports Exerc. (2018) 50:1021–30. doi: 10.1249/MSS.0000000000001508
13. Paoli A, Pacelli QF, Moro T, Marcolin G, Neri M, Battaglia G, et al. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis. (2013) 12:131–131. doi: 10.1186/1476-511X-12-131
14. Bailey RC, Olson J, Pepper SL, Porszasz J, Barstow TJ, Cooper DM. The level and tempo of children’s physical activities – an observational study. Med Sci Sports Exerc. (1995) 27:1033–41. doi: 10.1249/00005768-199507000-00012
15. Batacan RB Jr., Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. (2017) 51:494–503. doi: 10.1136/bjsports-2015-095841
16. Cockcroft EJ, Bond B, Williams CA, Harris S, Jackman SR, Armstrong N. The effects of two weeks high-intensity interval training on fasting glucose, glucose tolerance and insulin resistance in adolescent boys: a pilot study. BMC Sports Sci Med Rehabil. (2019) 11:29. doi: 10.1186/s13102-019-0141-9
17. Cassidy S, Thoma C, Houghton D, Trenell MI. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia. (2017) 60:7–23. doi: 10.1007/s00125-016-4106-1
18. Martin-Smith R, Buchan DS, Baker JS, Macdonald MJ, Sculthorpe NF, Easton C, et al. sprint interval training and the school curriculum: benefits upon cardiorespiratory fitness, physical activity profiles, and cardiometabolic risk profiles of healthy adolescents. Pediatr Exerc Sci. (2019) 31:296–305. doi: 10.1123/pes.2018-0155
19. Dias KA, Ingul CB, Tjønna AE, Keating SE, Gomersall SR, Follestad T, et al. Effect of high-intensity interval training on fitness, fat mass and cardiometabolic biomarkers in children with obesity: a randomised controlled trial. Sports Med. (2018) 48:733–46. doi: 10.1007/s40279-017-0777-0
20. Plavsic L, Knezevic OM, Sovtic A, Minic P, Rakonjac Z, Colmenero I. Effects of high-intensity interval training and nutrition advice on cardiometabolic markers and aerobic fitness in adolescent girls with obesity. Appl Physiol Nutr Metab. (2020) 45:294–300. doi: 10.1139/apnm-2019-0137
21. Wood G, Murrell A, van der Touw T, Smart N. HIIT is not superior to MICT in altering blood lipids: a systematic review and meta-analysis. BMJ Open Sport Exerc Med. (2019) 5:e000647. doi: 10.1136/bmjsem-2019-000647
22. Gibala MJ, Mcgee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev. (2008) 36:58–63. doi: 10.1097/JES.0b013e318168ec1f
23. Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. (2014) 48:1227–34. doi: 10.1136/bjsports-2013-092576
24. Abassi W, Ouerghi N, Nikolaidis PT, Hill L, Metcalf HE, Sims EL, et al. Interval training with different intensities in overweight/obese adolescent females. Int J Sports Med. (2021). 1–10 doi: 10.1055/a-1648-4653 [Epub ahead of print].
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
26. da Silva MR, Waclawovsky G, Perin L, Camboim I, Eibel B, Lehnen AM. Effects of high-intensity interval training on endothelial function, lipid profile, body composition and physical fitness in normal-weight and overweight-obese adolescents: a clinical trial. Physiol Behav. (2020) 213:112728. doi: 10.1016/j.physbeh.2019.112728
27. Paahoo A, Tadibi V, Behpoor N. Effectiveness of continuous aerobic vs. high-intensity interval training on atherosclerotic and inflammatory markers in boys with overweight/obesity. Pediatr Exerc Sci. (2021) 33:132–8. doi: 10.1123/pes.2020-0138
28. McNarry MA, Lester L, Ellins EA, Halcox JP, Davies G, Winn CON, et al. Asthma and high-intensity interval training have no effect on clustered cardiometabolic risk or arterial stiffness in adolescents. Eur J Appl Physiol. (2021) 121:1967–78. doi: 10.1007/s00421-020-04590-4
29. Lingling Y. Effects of high-intensity interval training on cardiorespiratory fitness, body composition and blood lipid level of overweight/obese male adolescents. Chin J Phys Med Rehabil. (2021) 43:251–3. doi: 10.3760/cma.j.issn.0254-1424.2021.03.014
30. Iraji H, Minasian V, Kelishadi R. Changes in liver enzymes and metabolic profile in adolescents with fatty liver following exercise interventions. Pediatr Gastroenterol Hepatol Nutr. (2021) 24:54–64. doi: 10.5223/pghn.2021.24.1.54
31. Abassi W, Ouerghi N, Ghouili H, Haouami S, Bouassida A. Greater effects of high– compared with moderate-intensity interval training on thyroid hormones in overweight/obese adolescent girls. Horm Mol Biol Clin Invest. (2020) 41:20200031. doi: 10.1515/hmbci-2020-0031
32. Morrissey C, Montero D, Raverdy C, Masson D, Amiot MJ, Vinet A. Effects of exercise intensity on microvascular function in obese adolescents. Int J Sports Med. (2018) 39:450–5. doi: 10.1055/a-0577-4280
33. Chuensiri N, Suksom D, Tanaka H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child Obes. (2018) 14:41–9. doi: 10.1089/chi.2017.0024
34. Racil G, Coquart JB, Elmontassar W, Haddad M, Goebel R, Chaouachi A, et al. Greater effects of high- compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol Sport. (2016) 33:145–52. doi: 10.5604/20831862.1198633
35. Racil G, Zouhal H, Elmontassar W, Ben Abderrahmane A, De Sousa MV, Chamari K, et al. Plyometric exercise combined with high-intensity interval training improves metabolic abnormalities in young obese females more so than interval training alone. Appl Physiol Nutr Metab. (2016) 41:103–9. doi: 10.1139/apnm-2015-0384
36. Zu X. Effects of endurance training and high-intensity interval training on health-related index of obese children. Med J Nat Defen Forc Southw Chin. (2014) 24, 408–411.
37. Racil G, Ounis OB, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. (2013) 113:2531–40. doi: 10.1007/s00421-013-2689-5
38. Koubaa A. Effect of intermittent and continuous training on body composition cardiorespiratory fitness and lipid profile in obese adolescents. IOSR. (2013) 3:31–7. doi: 10.9790/3013-32103137
39. Boer PH, Meeus M, Terblanche E, Rombaut L, Wandele ID, Hermans L, et al. The influence of sprint interval training on body composition, physical and metabolic fitness in adolescents and young adults with intellectual disability: a randomized controlled trial. Clin Rehabil. (2014) 28:221–31. doi: 10.1177/0269215513498609
40. de Araujo ACC, Roschel H, Picanço AR, do Prado DM, Villares SM, de Sá Pinto AL, et al. Similar health benefits of endurance and high-intensity interval training in obese children. PLoS One. (2012) 7:e42747. doi: 10.1371/journal.pone.0042747
41. Tjønna AE, Stølen TO, Bye A, Volden M, Slørdahl SA, Odegård R, et al. Aerobic interval training reduces cardiovascular risk factors more than a multi-treatment approach in overweight adolescents. Clin Sci (Lond). (2009) 116:317–26. doi: 10.1042/CS20080249
42. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
43. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
44. Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomized trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
45. Cao M, Tang Y, Li S, Zou Y. Effects of high-intensity interval training and moderate-intensity continuous training on cardiometabolic risk factors in overweight and obesity children and adolescents: a meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2021) 18:11905. doi: 10.3390/ijerph182211905
46. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999-2016. Pediatrics. (2018) 141:e20173459. doi: 10.1542/peds.2017-3459
47. Wilfley DE, Staiano AE, Altman M, Lindros J, Lima A, Hassink SG, et al. Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity. (2017) 25:16–29. doi: 10.1002/oby.21712
48. DeBoer MD. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients. (2019) 11:1788. doi: 10.3390/nu11081788
49. Weihe P, Weihrauch-Blüher S. Metabolic syndrome in children and adolescents: diagnostic criteria, therapeutic options and perspectives. Curr Obes Rep. (2019) 8:472–9. doi: 10.1007/s13679-019-00357-x
50. Zheng L, Rao Z, Guo Y, Chen P, Xiao W. High-intensity interval training restores glycolipid metabolism and mitochondrial function in skeletal muscle of mice with type 2 diabetes. Front Endocrinol (Lausanne). (2020) 11:561. doi: 10.3389/fendo.2020.00561
51. Zorzano A, Palacin M, Guma A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. (2005) 183:43–58. doi: 10.1111/j.1365-201X.2004.01380.x
52. Chavanelle V, Boisseau N, Otero YF, Combaret L, Dardevet D, Montaurier C, et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci Rep. (2017) 7:204. doi: 10.1038/s41598-017-00276-8
Keywords: high-intensity interval training, glycolipids, metabolism, obesity, children
Citation: Cao M, Li S, Tang Y and Zou Y (2022) A Meta-Analysis of High-Intensity Interval Training on Glycolipid Metabolism in Children With Metabolic Disorders. Front. Pediatr. 10:887852. doi: 10.3389/fped.2022.887852
Received: 02 March 2022; Accepted: 04 April 2022;
Published: 12 May 2022.
Edited by:
Jared Tucker, Helen DeVos Children’s Hospital, United StatesReviewed by:
Gary Liguori, University of Rhode Island, United StatesGiuseppe Battaglia, University of Palermo, Italy
Copyright © 2022 Cao, Li, Tang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Cao, Y2FvbWVuZzEyM0BzenUuZWR1LmNu
 Meng Cao
Meng Cao Shu Li1
Shu Li1 Yu Zou
Yu Zou