
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pain Res., 31 January 2025
Sec. Pharmacological Treatment of Pain
Volume 6 - 2025 | https://doi.org/10.3389/fpain.2025.1537154
Pain management remains a major challenge in the healthcare system. While synthetic analgesics are widely used for pain management, their effectiveness in managing chronic pain is often limited due to low efficacy or side effects. Thus, there is growing interest in exploring alternative pain relief methods, particularly using medicinal plants from traditional Eastern medicine and their phytochemicals. Previous studies have demonstrated the modulatory effects of various phytochemicals derived from herbal medicine on pain-related ion channels, such as voltage-gated sodium channels (Nav), calcium channels (Ca2+), and transient receptor potential (TRP) channels. Since these ion channels are integral to the transmission and modulation of pain signals, the ability of specific phytochemicals to activate or inhibit these channels presents a promising avenue for the development of novel analgesics. The goal of this review is to merge herbal insights with ion channel research to highlight the potential of natural compounds for safe and effective pain management. In this regard, we summarize the discovery and characterization of pain-relieving phytochemicals from herbal medicine, and we discuss their mechanisms of action and their potential to mimic or enhance the effects of conventional analgesics through ion channel modulation.
Pain is a complex and multifaceted experience, serving as both a vital protective mechanism and, in many instances, as a persistent, debilitating condition. Defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, pain is a multidimensional phenomenon that poses significant challenges in effective management (1, 2). While acute pain functions as a critical alert system for injury, chronic pain—often resulting from nerve damage or dysfunction in the nervous system—affects millions globally, severely disrupting physical, emotional, social, and psychological health. It remains one of the leading causes for seeking medical care. It imposes an immense societal and economic burden, reducing work productivity, increasing healthcare costs, and diminishing sufferers' quality of life (3). Despite the availability of numerous pain management options, current treatments for chronic pain are often insufficient, underscoring the urgent need for new therapeutic strategies that offer effective and sustainable relief (4). Pharmacological treatments, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), remain the primary approaches to pain relief. However, these options are associated with significant drawbacks (5–7). Opioids, while highly effective for managing moderate to severe pain, carry high risks of addiction, tolerance, and physical dependence, often requiring escalating doses and increasing the likelihood of overdose, respiratory depression, and opioid use disorder (3, 8). On the other hand, NSAIDs are commonly used for inflammatory and mild to moderate pain but pose serious risks with long-term use, including gastrointestinal complications, cardiovascular events, and kidney damage—particularly in vulnerable populations such as the elderly or those with pre-existing conditions (9, 10). Gabapentin is widely used for pain and generally considered safe, but it has limitations, including the risk of respiratory depression, especially when combined with CNS depressants like opioids (11). These drawbacks emphasize the critical need for safer, more effective, and long-term pain management strategies that minimize harm while improving patients' quality of life (12).
Pain perception and its chronic manifestation are intricately linked to the function and dysregulation of ion channels, which play a pivotal role in transmitting pain signals within the nervous system. These channels, responsible for regulating the flow of ions such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) across cell membranes, are essential for maintaining neuronal excitability and synaptic transmission (13, 14). Dysregulation or sustained activation of these channels, frequently observed in chronic pain states, results in heightened neuronal excitability and abnormal pain signaling. Consequently, targeting ion channels has emerged as a promising therapeutic approach for pain management, with the potential to modulate pain pathways more precisely and with fewer side effects compared to conventional pharmacological treatments (15). Understanding the dynamics of ion channels is, therefore, critical for developing innovative, effective, and safer analgesics for chronic pain relief.
There has been growing interest in alternative pain management therapies, particularly those derived from natural sources such as traditional Eastern medicinal herbs (16). Eastern medicinal herbs have been used for centuries to treat pain and inflammation, offering a plant-based alternative that is gaining recognition for its potential efficacy and safety (17–19). These herbs contain bioactive compounds that can influence pain pathways, making them especially promising for managing chronic conditions (20–22). Recent studies highlight the role of ion channels in pain mechanisms and reveal the molecular pathways mediating the analgesic effects of herbal extracts. These compounds can exert their pain-relieving effects by targeting a single ion channel or simultaneously acting on multiple ion channels, enhancing their analgesic potential.
This review seeks to provide a comprehensive analysis of the efficacy of medicinal herbs in pain management by examining a wide range of scientific studies. It will explore the key ion channels involved in pain transmission and their interactions with specific medicinal herbs that contribute to analgesic effects. Additionally, this review will systematically address how these interactions influence various types of pain. By elucidating the relationship between medicinal herbs and pain-related ion channels, this study aims to identify potential alternatives to conventional pain treatments and pave the way for alternative therapeutic strategies.
Many ion channels including voltage-gated sodium channels (Nav), voltage-gated calcium channels (Cav), ATP-sensitive potassium channels (KATP), voltage-gated potassium channels (Kv), transient receptor potential (TRP) channels, purinergic receptors such as P2X3, P2X4 and P2X7, acid-sensing ion channels (ASICs), play significant roles in pain signaling. Herbal medicines contain multiple bioactive compounds that can simultaneously interact with these ion channels, not only targeting individual channels but also providing a multi-faceted approach to pain management. By modulating multiple ion channels involved in pain transmission, these natural compounds offer a broader and more effective approach to analgesia (23). Understanding these channels paves the way for developing more targeted pain therapies (24–26).
One of the most prominent ion channels involved in pain signaling is the voltage-gated sodium channel (Nav) family, which facilitates the rapid influx of Na+ during the depolarization phase of action potential (AP) (27). Nav channels are divided into tetrodotoxin-sensitive (TTX-S) channels such as Nav1.1, Nav1.2, Nav1.3, Nav1.6, and Nav1.7, and tetrodotoxin-resistant (TTX-R) channels such as Nav1.8 and Nav1.9. While Nav1.1, Nav1.2 are primarily expressed in the central nervous system (CNS) and involved in central sensitization, Nav1.7 is predominantly expressed in the peripheral nervous system (PNS) and is critical for pain transmission (28). Nav1.6 channels are expressed in both the central and peripheral nervous systems, functioning as the major Nav isoform at the nodes of Ranvier while also being present in unmyelinated fibers and at the nerve terminals of certain sensory neuron subsets (29). TTX-R channels, including Nav1.8 and Nav1.9, are predominantly found in PNS, mediating inflammatory and neuropathic pain. Nav1.8 propagates AP under inflammatory conditions, while Nav1.9 modulates chronic pain excitability. Nav1.5, though mainly cardiac, may have a peripheral role requiring further study. Alterations in their expression or function contribute to chronic pain sensitization, with Nav 1.7, Nav 1.8, and Nav 1.9 gene variants linked to pain disorders, making them promising targets for pain management (27, 30, 31). Recent studies aim to develop selective Nav channel inhibitors for effective pain relief with fewer systemic side effects, highlighting their importance in pain signaling and potential for targeted pain management strategies (32).
Voltage-gated calcium channels (Cav) play a pivotal role in the process of pain perception by regulating the release of neurotransmitters at synaptic terminals. These channels facilitate the entry of calcium ions into neurons in response to membrane depolarization, which is essential for the release of neurotransmitters such as glutamate, a key excitatory neurotransmitter in the pain pathway (33). L-type calcium channels, as high-voltage-activated channels, contribute to central sensitization and chronic pain by enhancing synaptic transmission and neuronal excitability. While not directly involved in acute pain, their dysregulation can amplify pain signals in neuropathic and inflammatory pain (34, 35). T-type calcium channels, especially Cav3.2, are low-voltage-activated and play a critical role in nociceptive transmission. Highly expressed in peripheral sensory neurons, they facilitate neuronal hyperexcitability in acute and chronic pain states, making them promising therapeutic targets for pain relief (35–38). Both L-type and T-type channels are crucial in pain signaling, with L-type channels contributing to chronic pain and T-type channels driving nociception and sensitization, offering distinct yet complementary roles in pain modulation (34, 39).
K+ channels are integral in maintaining the resting membrane potential and controlling the excitability of neurons, including those involved in pain transmission. They include various subtypes, such as ATP-sensitive potassium channels (KATP) and voltage-gated potassium channels (Kv) (40). Activation of these channels generally results in an efflux of K+ from neurons, leading to hyperpolarization of the membrane, which makes it less likely for the neuron to reach the threshold required for AP generation. KATP channels, found in both peripheral and central neurons, are modulated by metabolic states and have been shown to play a role in the pain associated with ischemic conditions (41). Kv channels, such as Kv7 (KCNQ), are also critical in stabilizing neuronal membrane potential. Modulation of Kv7 channels has emerged as a promising approach for pain relief, as selective activators of these channels can reduce hyperexcitability in nociceptive neurons (42). Their activation generally reduces neuronal excitability, making them a potential therapeutic target for conditions characterized by hyperexcitability, such as chronic pain. By modulating potassium channel activity, it is possible to reduce the firing of nociceptive neurons, thereby decreasing pain perception.
TRP channels form a diverse family of ion channels that are sensitive to various physical and chemical stimuli, making them key players in pain detection and transmission. Among the TRP family, transient receptor potential vanilloid 1 (TRPV1), transient receptor potential ankyrin 1 (TRPA1), and transient receptor potential melastatin 8 (TRPM8) are particularly relevant to pain research, as they mediate responses to noxious heat, cold, and chemical irritants. TRPV1 is activated by noxious heat (above 43°C), capsaicin, and acidic conditions, which are often associated with tissue injury and inflammation (23). Interestingly, prolonged TRPV1 activation leads to desensitization, reducing pain signaling, which is exploited in treatments like capsaicin cream for neuropathic pain. However, excessive activation can paradoxically cause hyperalgesia or inflammation, highlighting its dual role in pain modulation (43). TRPA1 is typically co-localized with TRPV1 and is activated by irritants, inflammatory mediators, cold, and mechanical stimuli, serving as a molecular integrator for pain and neurogenic inflammation (43, 44). On the other hands, TRPM8 utilizes a distinct analgesic mechanism, being activated by cool temperatures and menthol, which contribute to the sensation of cooling and cold-induced pain relief. Modulating TRPM8 activity has been investigated as a therapeutic approach, particularly for alleviating conditions associated with burning pain or heat hyperalgesia (45–47). Together, these channels represent critical targets for the development of novel analgesic therapies, offering distinct and complementary mechanisms for addressing various types of pain.
ASICs are proton-gated ion channels that are activated by decreases in extracellular pH, which often occur in response to tissue damage or inflammation. These channels are highly expressed in peripheral sensory neurons, where they play a role in detecting pain associated with acidosis, such as that seen in ischemic or inflammatory conditions. When tissue injury leads to a drop in pH, ASICs open, allowing Na+ to enter the neuron, which contributes to the sensation of pain (48–50). The contribution of ASICs to hyperalgesia has made them a target of interest for pain research, as blocking these channels can reduce pain in conditions where tissue acidosis is a major factor. For example, in animal models of inflammatory pain, pharmacological inhibition of ASICs has been shown to alleviate hyperalgesia, indicating their potential as therapeutic targets for treating chronic pain (51).
ATP released from damaged or inflamed tissues activates P2X receptors on primary afferent neurons, leading to depolarization and the initiation of pain signals. These ATP-dependent ligand-gated cation channels are upregulated following nerve injury. P2X3 receptors, expressed in small-diameter sensory neurons, contribute to acute nociception, while P2X2/3 receptors modulate prolonged sensitivity associated with nerve injury or inflammation (52). The P2X7 receptor, expressed in both the nervous and immune systems, plays a critical role in pain development by mediating the release of inflammatory cytokines through ATP activation and intracellular signaling. Its involvement in inflammatory responses and pain modulation has been widely recognized and validated (53). Inhibiting P2X4 receptor function or expression, as well as targeting its regulatory molecules, has shown promise in suppressing neuropathic pain, making P2X4 receptors a critical therapeutic target (54, 55). P2X3 receptors contribute to acute nociception, while P2X2/3 receptors modulate prolonged sensitivity linked to nerve injury or inflammation. In neuropathic pain, P2X4 on microglia maintains nociceptive sensitivity via neuronal-glial interactions, and antagonists targeting these receptors have shown efficacy in reducing pain (56). Further research into the structure, function, and pharmacological inhibitors of P2X4 receptors could advance targeted therapies for chronic pain, addressing a significant challenge in pain medicine.
We classify plants into four main types based on the parts used—roots, stems, leaves, and fruits. This categorization highlights the diverse compounds found in each plant part and their specific interactions with ion channels, which contribute to their analgesic effects (Tables 1–3).
Aconitum which has traditionally been used for pain relief, including rheumatism and neuralgia, due to their potent anti-inflammatory and analgesic effects, making them valuable for managing chronic and inflammatory pain conditions (100, 101). Aconitum species have been used as medicinal herbs, and their various components have been reported to demonstrate analgesic effects. Aconiti Brachypodi Radix, derived from the dried roots of Aconitum brachypodum Diels (Family Ranunculaceae), is particulary renowned for its anti-rheumatic and analgesic properties. Extracts from Aconiti Brachypodi Radix show analgesic effects in vivo, demonstrated through hot-plate, writhing, and formalin tests. In vitro, it suppresses TTX-S sodium currents in rat DRG neurons. These results suggest that the analgesic effect may be linked to the modulation of TTX-S sodium currents in sensory neurons (57, 58). Neoline, an active ingredient, effectively alleviates oxaliplatin-induced neuropathic pain, including mechanical and cold hyperalgesia, by improving neurite elongation in DRG neurons. It modulates pain-related ion channels and relieves pain without causing sedation or motor impairment (102, 103). Bulleyaconitine A, an active ingredient of Aconitum bulleyanum, is known for its long-lasting analgesic effects by modulating voltage-gated sodium channels, particularly Nav1.7 and Nav1.8, and blocking TTX-S sodium channels in DRG neurons through protein kinase C (PKC) inhibition, effectively reducing neuropathic and chronic pain (59). Its action is more potent in neuropathic conditions due to upregulated sodium channels and PKC. Also, as well as Neoline, Bulleyaconitine A attenuates paclitaxel-induced neuropathic pain (104). Additionally, it modulates spinal microglia, enhances morphine's analgesic effects without affecting acute pain, and induces antinociception in rats and mice through alkaloids from Aconitum (105, 106).
Allium macrostemon is traditionally used for thoracic pain and heart-related conditions, known for antioxidant and vasodilatory benefits, though its analgesic effects are unstudied. Recent findings, using HEK293T cells and formalin-induced, acetic acid-induced, and thermal pain models, support its potential for pain relief and development as a Nav1.7-targeted analgesic (60).
Angelica dahurica, a traditional herb from the Apiaceae family, is commonly used for treating headaches, toothaches, and skin issues. Angelica dahurica extract effectively reduces mechanical and thermal hypersensitivity in CFA-induced inflammatory pain in mice. Osthole, an extract from Angelica dahurica, directly inhibits TRPV1 activity in DRG neurons and reduces noxious heat- and capsaicin-induced pain in mice, with calcium imaging studies further demonstrating its potential as a promising analgesic for chronic inflammatory pain (61). Furanocoumarin imperatorin is the main active component that inhibits formalin- and capsaicin-induced pain in rats by acting as a weak agonist of the TRPV1 channel, likely binding near the capsaicin site and delaying desensitization recovery. These findings highlight imperatorin's potential as a lead compound for developing TRPV1-targeted pain treatments (62).
The roots of Angelica sinensis are famous for their use in pain relief, particularly for gynecological conditions and inflammation (107). Sodium ferulate, a major active compound known for its antioxidant and anti-inflammatory properties, has been widely used in the treatment of cardiovascular and cerebrovascular diseases. Recent studies have investigated its effects on hyperalgesia in a chronic constriction injury (CCI) rat model. It significantly increased the mechanical withdrawal threshold and thermal withdrawal latency, indicating reduced pain sensitivity. Moreover, Sodium ferulate's effect on hyperalgesia was mediated through the modulation of the P2X3 receptor in primary sensory afferents. While CCI elevated P2X3 receptor expression in DRG neurons, Sodium ferulate effectively reduced this upregulation, suggesting its potential for alleviating thermal and mechanical hyperalgesia during chronic neuropathic pain (63).
The roots of Angelicae pubescentis have been widely used in traditional medicine to relieve pain associated with arthritis, rheumatism, and muscular discomfort (108). The extracts of Angelicae pubescentis reduce behaviors of acute pain, formalin-induced inflammatory pain, and neuropathic pain in a spared nerve injury (SNI) model and coumarins in the extract are the active anti-nociceptive components (109, 110). The coumarins, key active components derived from roots, are renowned for their anti-inflammatory and analgesic properties. They have been shown to significantly alleviate neuropathic pain and suppress the development of mechanical hypersensitivity induced by SNI. The anti-nociceptive effects of coumarins are linked to their regulation of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, as well as their modulation of TRPV1 and pERK pathways in the peripheral nervous system (64). Among the components of coumarins with demonstrated analgesic effects, columbianadin has been shown to inhibit acute and inflammatory pain behaviors. It also suppresses mechanical and cold hypersensitivity induced by oxaliplatin (109). Additionally, osthole, another coumarin compound, may reduce neuropathic pain behaviors by inhibiting T- and L-type calcium channels in nociceptive DRG neurons in mice (65).
The roots of Asarum sieboldii are used for local anesthetics treat to toothache, headache, and inflammatory diseases. Methyl eugenol (4-allyl-1,2-dimethoxybenzene), a major component extracted from Asarum sieboldii exhibits antinociceptive effects in mice, as shown in the formalin-induced pain test, and reduces NMDA receptor-mediated hyperalgesia through GABA receptors (111). Nav1.7 channels which are TTX-S channels were inhibited by methyl eugenol, demostrated by whole-cell patch clamp experiments in CHO cells (66).
Bupleurum chinense are rich in compounds like saponins, volatile oils, and flavonoids. Its main active ingredient, saikosaponin, has shown various pharmacological effects, including anti-inflammatory, analgesic, and hepatoprotective actions. Saikosaponin, reduces neuropathic pain in CCI rats via p38 MAPK and NF-κB pathways, while saikosaponin A and D alleviate inflammatory pain in carrageenan-induced rats by inhibiting the NF-κB pathway, producing pro-inflammatory mediators (112, 113). Saikosaponin inhibited Nav1.7, reducing thermal pain and decreased pain responses in phase 2 of the formalin-induced pain model in vivo (67).
Cinnamomi Cortex (bark of Cinnamomum cassia Presl) effectively alleviates oxaliplatin-induced cold allodynia in rats. It reduces cold allodynia and suppresses spinal glial and pro-inflammatory cytokine activation, with coumarin contributing to these effects. Cinnamic acid, a major component of Cinnamomum cassia, is particularly effective in reducing cold and mechanical hypersensitivity by inhibiting spinal pain transmission (114, 115). Cinnamomi Cortex has warming properties and influences pain-related pathways by modulating TRP channels. Key compounds like cinnamaldehyde increase TRPV1 and decrease TRPM8 expression in DRG neurons (116), and activate cold-sensitive TRPA1 channels, which raises cytoplasmic Ca2+ levels, enhancing cellular function and warmth effects (68, 117).
Corydalis yanhusuo extracts and its active component, tetrahydropalmatine, alleviate neuropathic pain. Tetrahydropalmatine has antinociceptive effects in acute and chronic pain models, specifically inhibiting the second phase of formalin-induced pain when administered intraperitoneally (118). Corydalis yanhusuo contains active alkaloid components that target Na+ ion channels, particularly Nav1.7 and Nav1.5, which may contribute to its analgesic and anti-arrhythmic effects (69). Molecular docking and patch clamp studies revealed that dihydrosanguinarine and dihydrochelerythrine, other active compounds, inhibit peak currents and modulate activation phases of these channels, supporting potential therapeutic applications in pain (119).
The rhizomes of Curcuma longa (turmeric) are widely used for their potent anti-inflammatory, anti-cancer, antioxidant, and analgesic properties. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], the primary active compound, not only activates KATP channels, contributing to its antinociceptive effects but also specifically modulates the TRPA1 by activating and desensitizing it. This dual action on KATP and TRPA1 channels, both critical in pain perception, underscores curcumin's analgesic potential in managing various chronic pain conditions (70). Curcumin significantly attenuated vincristine-induced neuropathy, likely due to its combined antinociceptive, calcium-inhibitory, and antioxidant effects (120). Also, some studies have shown that curcumin effectively reduces neuroinflammation-driven chronic pain by modulating microglia and astrocytes and suppressing pathways like MAPK, NF-κB, and JAK-STAT. This modulation decreases pro-inflammatory mediators and enhances anti-inflammatory responses, making curcumin effective in treating neuropathic and inflammatory pain (121). Furthermore, nanoparticle-encapsulated curcumin (nano curcumin) has shown efficacy in reducing mechanical and thermal hyperalgesia in HIV-gp120-induced pain models by inhibiting P2X3 receptor activation and decreasing ERK1/2 phosphorylation in rat DRG neurons. This suggests that nano curcumin may be an effective strategy for mitigating neuropathic pain through P2X3-mediated pathways (71).
The methanolic extract of Dioscorea bulbifera var sativa showed significant antinociceptive effects in both inflammatory and neuropathic pain models. It effectively reduced persistent pain induced by CFA and neuropathic pain through partial sciatic nerve ligation (PSNL). The extract also inhibited acute LPS-induced pain, although it had limited effects on thermal hyperalgesia and capsaicin-induced nociception. The antinociceptive effects in the PGE2-induced hyperalgesia model were reversed by L-NAME and glibenclamide, indicating a mechanism involving activation of the NO–cGMP–ATP-sensitive potassium channels pathway. Dioscorea bulbifera may offer therapeutic potential for managing both inflammatory and neuropathic pain (72, 122).
The primary active molecules in ginseng are ginsenosides, also known as ginseng saponins. Ginseng has been shown to modulate pain through its effects on ion channels, specifically by influencing high-voltage-activated Ca2+ channels in rat DRG neurons. Total saponins from ginseng dose-dependently suppressed Ca2+ channel currents, particularly affecting L-, N- channels. Among ginseng's active components, ginsenoside Rg3 was identified as a key inhibitor of Ca2+ channels, likely contributing to ginseng's antinociceptive effects (73). Gintonin, extracted from ginseng root, modulates pain-related ion channels by inhibiting Kv1.2 channel activity in a calcium-dependent manner. This inhibition, which involves phospholipase C and receptor protein tyrosine phosphatase α (RPTPα) pathways, highlights gintonin's potential role in regulating neuronal activity and pain signaling (74).
Licorice, made from roots of Glycyrrhiza uralensis Fisch, is used for its stomach and spleen-protective, pain-relieving, cough-alleviating, and phlegm-reducing effects (123). Licorice contains licochalcone A and licochalcone B, key compounds with potential analgesic effects. In DRG neurons, licochalcone A was found to inhibit Nav1.7 channel, reducing neuronal excitability, whereas licochalcone B, did not affect Nav. In animal models of formalin-induced pain, licochalcone A inhibited pain responses in both phases of the test, while licochalcone B, only reduced pain in phase 2. Licochalcones, particularly licochalcone A, could be promising candidates for developing Nav channel-targeted analgesic drugs (75).
Ligusticum chuanxiong Hort has long been used to treat cardiovascular conditions and related pain, including headaches, chest pain, and neuropathic pain (124). Ligustrazine, a primary active compound from Ligusticum chuanxiong, has demonstrated analgesic effects across various pain types, including angina, neuropathic, inflammatory, and burn pain, and has been shown to alleviate pain hypersensitivity caused by chronic venous disease in mice. Ligustrazine reduced pain responses to mechanical, cold, and thermal stimuli and desensitized TRPA1 channel activity in DRG neurons, thereby decreasing neuronal excitability (76).
The root of Scutellaria baicalensis has been widely used in Asia and the West for its health benefits, traditionally treating cardiovascular diseases, inflammation, and tumors. Scutellaria baicalensis is renowned for its high flavonoid content, containing four primary flavones: baicalin, baicalein, wogonoside and wogonin (125, 126). Baicalein and wogonin were found to activate the TREK-2 potassium channel, potentially contributing to neuroprotection. In COS-7 cells expressing TREK-2, both compounds increased channel opening frequency without affecting conductance or open time. Baicalein provided continuous activation, while wogonin activated TREK-2 transiently. These findings suggest that baicalein and wogonin may help regulate resting membrane potential (RMP) under pathological conditions, supporting their neuroprotective effects (77).
Sinomenium acutum, traditionally used for rheumatic arthritis and neuralgia, contains sinomenine, an active compound with immunosuppressive, anti-inflammatory, and analgesic properties that effectively alleviates both neuropathic and inflammatory pain (127, 128). Sinomenine, the active ingredient in Sinomenium acutum, shows analgesic effects in a formalin-induced inflammatory pain model in mice. Intraperitoneal administration of sinomenine reduced pain behaviors and suppressed c-Fos expression in the spinal cord. In DRG neurons, sinomenine increased the spike threshold, reduced firing frequency, and inhibited Nav currents dose-dependently, suggesting that its peripheral analgesic effect involves inhibition of Na+ channels (78).
Sophorae radix, derived from the roots of Sophora species, is traditionally used for pain relief and as an anti-inflammatory agent in conditions like arthritis (129). Cav3.2 T-type Ca2+ channels are known for their role in pain signaling. Sophora flavanone G from Sophorae Radix and hop-derived analogues, (2S)-6-PNG and (2S)-8-PNG, are effective T-channel blockers. (2R/S)-6-PNG showed significant effects in reducing mechanical and visceral pain, as well as neuropathic allodynia in mice, without noticeable side effects on motor or cardiovascular function (79).
Puerarin has shown analgesic effects in neuropathic pain models. It alleviates paclitaxel-induced pain by blocking Nav channels, especially TTX-R Nav1.8 channels, in a β1 subunit-dependent manner. In CCI model, puerarin also reduced pain by downregulating P2X3 receptor expression in DRG neurons, increasing pain thresholds. These findings highlight puerarin's potential in managing neuropathic pain through modulation of Nav1.8 and P2X3 channels in sensory neurons (80, 81).
Paeoniflorin, derived from the roots of Paeonia lactiflora, is widely used for pain relief and to treat conditions such as gynecological disorders, liver disease, neuroinflammation and rheumatoid arthritis (130, 131). Its anti-inflammatory, immunoregulatory, and antioxidant properties enhance its role in managing autoimmune diseases (132). Paeoniflorin acts as an analgesic by modulating ion channels involved in pain transmission, particularly through its stable binding to TRPV1, which directly suppresses the response of DRG neurons to capsaicin (82). It also inhibits L-type voltage-dependent calcium currents in a concentration-dependent manner and shifts the inactivation curve to more negative potentials in NG108-15 cells, indicating its role in modulating neuronal excitability and neuroendocrine functions (83). These channels regulate neuronal excitability and Ca2+ influx, which are crucial for nociceptive signal propagation. By inhibiting these channels, paeoniflorin effectively reduces pain perception and mitigates hyperalgesia, making it a potent agent for managing chronic and neuropathic pain.
Grayanoids, derived from the dried roots of Rhododendron molle, are traditionally used for pain relief and modulate voltage-gated Na+ channels. They exhibit strong anti-nociceptive effects in pain models, such as the acetic acid-induced writhing, hot-plate, and formalin tests, with voltage-gated Na+ channels serving as key targets for their analgesic and toxic effects (133).
Artemisia annua, part of the Asteraceae family, has long been used in traditional medicine for malaria and fever treatment (134). Artemisia species and artemisinin exhibit various pharmacological effects, including antibacterial, antifungal, antioxidant and anti-inflammatory properties (135, 136). Artemisinin and its derivatives have shown significant pain-relieving effects by modulating ion channels like the P2X4 receptor in the DRG, associated with neuropathic pain. It demonstrated stronger antinociceptive effects, while artemisinin exhibited notable anti-inflammatory properties, reducing key pro-inflammatory cytokines (84, 137).
Frankincense and myrrh are traditional resins used to relieve pain. Frankincense, from Boswellia carterii, may help regulate immune function, while myrrh, from Commiphora myrrha, has anti-inflammatory and antimicrobial effects (138, 139). Frankincense and myrrh, traditionally used together for synergistic pain relief, were studied for their mechanisms in neuropathic pain using mouse models. In a CCI model, a water extract of frankincense and myrrh effectively alleviated thermal hypersensitivity and mechanical allodynia (85). The studies highlighted the role of the TRPV1 receptor and the TLR4/MyD88 pathway in neuropathic pain. A water extract of frankincense and myrrh treatment reduced TRPV1 expression at both mRNA and protein levels and decreased calcium response in DRG neurons, while also inhibiting neuroinflammatory TLR4/MyD88 signaling in the spinal cord. These findings suggest that a water extract of frankincense and myrrh alleviates neuropathic pain by modulating TRPV1 and reducing neuroinflammation through the TLR4/MyD88 pathway, offering a potential approach for neuropathic pain treatment targeting ion channels and inflammatory signaling (86).
The leaves of Camellia sinensis, commonly consumed as green and black tea, contain polyphenols like epigallocatechin-3-gallate, a primary compound in green tea that has shown promise in preclinical studies for neuropathic pain treatment due to its anti-inflammatory and antioxidant effects, and has also demonstrated the ability to reduce bone cancer pain (140). In neuropathic pain caused by peripheral nerve injury, epigallocatechin-3-gallate and its derivatives were tested for analgesic effects. They effectively reduced thermal hyperalgesia long-term by inhibiting fatty acid synthase and lowering inflammatory protein levels, making it a promising candidate for neuropathic pain treatment in preclinical development (141). Epigallocatechin gallate inhibited ASIC3 currents effectively at low concentrations and reduced acid-induced pain behaviors in mice, highlighting its potential as a structural basis for developing pain-targeted drugs that modulate ASIC3 channels (87).
Citrus plants that have compounds including narirutin, naringenin, limonene, diosmetin, and newly studied TRPM3 blockers like Isosakuranetin, show promising potential for neuropathic pain management by targeting pain-related ion channels. Narirutin and Naringenin inhibit Nav1.7 and Nav1.8 Na+ channels, respectively, to reduce pain signaling. Limonene modulates TRPA1 channels, inducing pain topically but inhibiting pain systemically. Diosmetin acts as a TRPV1 antagonist, effectively reducing heat- and capsaicin-induced pain. Isosakuranetin, identified as a potent TRPM3 blocker, and hesperetin decrease responses to noxious heat and chemical pain in mice. Together, these compounds offer novel mechanisms for pain relief, targeting TRPV1, TRPA1, Nav1.7, Nav1.8, and TRPM3 channels, and highlight the potential for citrus-derived compounds in developing selective and effective analgesics (88, 89, 142–144).
Ephedrine, derived from Ephedra sinica, E. intermedia, or E. equisetina, is known for its anti-inflammatory properties which contribute to its analgesic effects (145–147). These medicines are commonly used to treat pain conditions, primarily due to their anti-inflammatory actions. Interestingly, ephedra herb extracts not only activate the TRPV1 channel, a key mediator of pain sensation, but also induce its desensitization. This desensitization, observed in vivo, reduces capsaicin-induced pain by suppressing TRPV1 activity in peripheral sensory neurons, highlighting a dual mechanism where initial activation of TRPV1 is followed by a loss of channel sensitivity, ultimately leading to analgesia. These findings suggest ephedra herb extracts that may exert its pain-relieving effects through a combination of anti-inflammatory properties and modulation of TRPV1 signaling (90).
The extracts and chemical components of the genus Hericium, a group of medicinal mushrooms traditionally used in herbal medicine, is actively progressing, revealing various pharmacological activities such as anticancer, antioxidant, anti-inflammatory, and nerve growth-promoting properties. Notably, H. erinaceus has gained attention for its potential to treat Alzheimer's disease, cancer, inflammation, depression, and nerve injury, highlighting its diverse health-promoting effects (148, 149). Erinacine-S, a small active component derived from H. erinaceus inhibits P2R-mediated Ca2+ signaling and reduces neuropathic pain and neuroinflammation in cell and mouse models through the modulation of P2X4 and P2X7. Ethanol extracts and erinacine-S showed potential for treating neuropathic pain (91).
Magnolol, a polyphenolic compound from the bark of Magnolia officinalis, exhibits multiple pharmacological effects. It has anti-inflammatory properties by inhibiting NF-κB, antioxidative effects useful for skin disorders, and anticancer effects in thyroid, bladder, and glioblastoma cells (150–152). Magnolol inhibited Nav and Kv channels. These inhibitory effects on Nav and Kv channels may contribute to magnolol's neuroprotective properties (92). Honokiol and magnolol, two active compounds from the bark of Magnolia officinalis, were tested for pain relief in mice. While they did not reduce pain in the tail-flick, hot-plate, or neurogenic phase of the formalin test, both compounds significantly reduced pain in the inflammatory phase of the formalin-induced response. They decreased formalin-induced c-Fos expression in the spinal cord's dorsal horn without affecting motor coordination or memory. These findings suggest that honokiol and magnolol may effectively treat inflammatory pain without causing motor or cognitive side effects (93). Magnolin, the major tetrahydrofurofuranoid lignan from Magnolia denudata, significantly alleviated paclitaxel-induced CIPN, which is characterized by sensory disturbances and neuropathic pain, through the suppression of ERK phosphorylation in the DRG (153).
The leaves of Mentha arvensis are rich in menthol. Menthol, known for activating the TRPM8 channel to produce a cooling sensation, is widely used in topical analgesics (154). A previous study tested whether menthol also blocks Nav, which are critical in pain sensation. Results showed that menthol inhibits Nav1.8, Nav1.9, and TTX-S channels in a concentration-, voltage-, and frequency-dependent manner, promoting inactivation and reducing high-frequency neuronal firing. Low concentrations of menthol provided pain relief in mice, suggesting that its analgesic effect involves selective Na+ channel blockade (94). However, high doses of menthol increase neuron excitability by inhibiting leak K+ channels, likely K2P channels, in dural afferent neurons. This inhibition leads to membrane depolarization and lowers the threshold for AP generation, which may explain menthol's pronociceptive effect at high concentrations (155). That means, menthol's dual role in pain modulation, with lower doses providing analgesia and higher doses enhancing pain responses.
Vitexin, a C-glycosylated flavone (5, 7, 4-trihydroxyflavone-8-glucoside), is a primary bioactive compound in the traditional herb Crataegus pinnatifida (156). Vitexin reduces mechanical and thermal hyperalgesia and inhibits pain-like behaviors in various inflammatory pain models in mice. Its antinociceptive effects against inflammatory pain may be partially mediated by targeting the TRPV1 channel, reducing oxidative stress, and modulating cytokine production (95).
Lycium barbarum, commonly known as goji berry or wolfberry, has been widely used in medicine and contains bioactive compounds like polysaccharides, carotenoids, and betaine (157). The therapeutic effects of Lycium barbarum polysaccharides and capsaicin were investigated in a dextran sulfate sodium (DSS)-induced colitis model in rats. The treatments, administered via gavage, significantly reduced oxidative stress, inflammatory responses, and pain signaling. Specifically, both Lycium barbarum polysaccharides and capsaicin downregulated the expression of TRPV1 and TRPA1 ion channels in the colon, which are closely associated with pain and inflammation (96).
Inula britannica, traditional medicine for arthritis and back pain, was studied for its pain-relieving effects. The flower essential oil and its major component, patuletin, demonstrated significant antinociceptive effects in male mice across various pain models (tail-flick, writhing, formalin-induced, and glutamate-induced tests). Essential oil effects were reduced by opioid antagonists and blocked by methylene blue and glibenclamide, suggesting the involvement of opioid receptors and activation of the NO-cyclic GMP-protein kinase G/ATP-sensitive potassium channel signaling pathway (12, 122).
Garcinia mangostana (mangosteen) has fruit-derived products used in traditional medicine for treating infections and reducing fever (158, 159). α-Mangostin, a primary xanthone from mangosteen pericarps, exhibits antioxidant, anti-inflammatory, and analgesic effects, likely by modulating ion channels in nociceptive neurons. α-Mangostin enhances K+ conductance, activates TREK/TRAAK channels, inhibits TRPV1 currents, and partially suppresses TTX-S Nav channels, which reduces neuronal excitability and pain. This demonstrates that α-Mangostin exerts multi-target analgesic effects by modulating pain-related ion channels expressed in DRG neurons. Also, molecular docking and in silico ADME analyses support its stable interactions with these channels and its potential as a safe, multi-target analgesic agent without crossing the blood-brain barrier (97).
Pellitorine, from the fruits of Tetradium daniellii, as the first TRPV1 antagonist derived from the Evodia species. Through bioactivity-guided extraction and isolation, pellitorine blocks capsaicin-induced Ca2+ uptake. While other isolated compounds (e.g., N-isobutyl-4,5-epoxy-2E-decadienamide) showed no TRPV1 activity, pellitorine emerged as a competitive inhibitor. This compound, structurally analogous to capsaicin, may help inhibit inflammation-related pain (98). It also alleviated cold allodynia in an oxaliplatin-induced model (160).
The flowers and fruits from Rhododendron molle G. Don, a traditional medicinal herb, are well-known for their pain-relieving properties. Rhodojaponin III, the primary active and toxic component extracted from this plant, has been investigated for its antinociceptive effects, underlying mechanisms, and subacute toxicity. Rhodojaponin III showed significant pain-relieving effects in nociceptive pain models, including hot plate, tail-immersion, acetic acid writhing, and formalin tests, as well as reduction in hyperalgesia in a CCI model. Molecular docking and electrophysiological studies revealed that Rhodojaponin III mildly inhibits Nav1.7, Nav1.8, and Nav1.5 (99).
Pain management remains a complex and persistent challenge in both clinical and research settings, primarily due to the multifaceted nature of pain signaling pathways and the diverse origins of pain conditions. Opioids, NSAIDs, and gabapentin are commonly used pharmacological therapies, widely recognized for their effectiveness in pain relief. However, their uses often come with significant side effects. Opioids are associated with risks of addiction and tolerance (3, 6); NSAIDs can lead to gastrointestinal irritation, cardiovascular events, and renal dysfunction (7, 9); Gabapentin, while effective for neuropathic pain, may cause dizziness, sedation, and dependency in some cases (5, 10, 11). These adverse effects not only limit the long-term usability of these medications but also highlight the necessary need for alternative therapeutic strategies. These challenges underscore the importance of exploring alternative therapeutic strategy that can effectively manage pain while offering improved safety profiles.
Medicinal herbs may offer a promising solution to this issue. These herbs have been performed for their analgesic properties in traditional medicine, and recent advancements in scientific research have begun to unravel their molecular mechanisms of action (Figure 1). Plant-based compounds derived from a single herb often include multiple extracts, each contributing to analgesic effects. Even a single phytochemical adopts a multi-targeted approach, rather than targeting a single ion channel as seen in conventional single-target therapies (68, 82, 83, 89, 97). For instance, α-mangostin applied to DRG neurons significantly inhibited TRPV1 currents in the micromolar (µM) range and TTX-S Nav currents in the millimolar (mM) range. Furthermore, α-mangostin activated K2P channels in the µM range, hyperpolarizing the RMP of DRG neurons (97). This highlights that a single molecule like α-mangostin can exert broad effects by simultaneously modulating various ion channels associated with pain transmission, providing comprehensive and potent pain relief. Since pain involves complex pathophysiological mechanisms, targeting a single pathway is insufficient to address and treat it fully. From this perspective, the diverse analgesic mechanisms of natural compounds make them a promising option for treating multifaceted pain conditions.
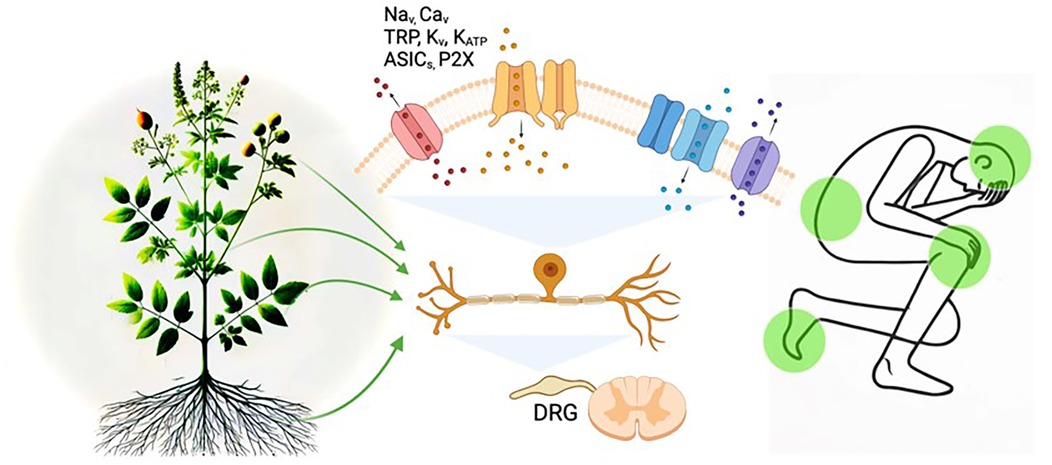
Figure 1. Diagram of mechanisms linking plant-derived medicines, ion channels, and pain modulation. Illustrating the potential mechanisms of pain modulation by plant-derived compounds through sensory neuronal ion channels. The image depicts how bioactive compounds from plants may target ion channels, such as Na+, Ca2+, TRP, Kv, KATP ASICs, and P2X, in dorsal root ganglion (DRG) neurons to alleviate pain signals. The integration of natural products and ion channel regulation in pain pathways is highlighted.
Interestingly, different parts of a plant, such as fruits, flowers, stems, leaves, and roots, are rich in varying levels of phytochemicals with analgesic properties, making them promising candidates for pain management applications. Fruits and flowers, include flavonoids, phenolic acids, essential oils, and alkaloids (161, 162). Stems and leaves commonly harbor tannins and terpenoids (163, 164), and roots frequently contain saponins and polysaccharides (67, 165). Understanding these specific phytochemical profiles allows for more targeted research and application in pain management. Moving forward, the botanical and pharmaceutical industries are likely to emphasize the systematic utilization of these plant components, optimizing extraction and formulation methods to maximize their therapeutic potential in analgesics.
Despite their potential, only a small fraction of herb-related compounds has successfully been translated into clinical practice. Among these, certain herbal medicines have demonstrated clinical efficacy as analgesics. For example, curcumin, widely used for managing pain and inflammation, is safely administered at 400–600 mg of standardized powder up to three times daily or 1–3 g of dried powdered root (166, 167). Similarly, clinical trials recommend 100–250 mg of Boswellia carterii extract daily for at least 4 weeks to improve pain, stiffness, and joint function (168). Menthol, commonly used to alleviate cold symptoms such as nasal congestion and nighttime cough, is safely applied topically in ointments (5–10 ml) to the chest and neck, making it suitable for individuals aged 2 years and older (169). However, several factors limit the broader clinical application of these compounds, with one significant challenge being the lack of standardization for active compounds. The multi-component and complex nature of herbal extracts make it difficult to standardize specific active ingredients and clearly define their effects (170–173).
Integrating medicinal herbs into recent clinical pain management protocols remains a formidable challenge. Key areas requiring further research include the standardization of dosages, ensuring the consistency and quality of herbal extracts, and optimizing delivery methods to maximize therapeutic benefits. Moreover, large-scale clinical trials are essential to validate the efficacy and safety of these herbal compounds across diverse patient populations. Addressing these issues could pave the way for the successful clinical translation of medicinal herbs, providing safer and more effective alternatives for pain management.
SEK: Conceptualization, Funding acquisition, Investigation, Visualization, Writing – original draft. GC: Funding acquisition, Supervision, Writing – review & editing. SKK: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (RS-2024-00352528 to SEK) (RS-2024-00352547, RS-2023-00302281 to GC) (RS-2023-00262810 to SKK).
Figures created in BioRender. Kim S. (2025). https://BioRender.com/u95a991.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. Figures in this manuscript were partially created using the AIbased tool DALL·E. Figures created in BioRender. Kim S. (2025). https://BioRender.com/u95a991.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Merskey H, Spear FG. The concept of pain. J Psychosom Res. (1967) 11:59–67. doi: 10.1016/0022-3999(67)90057-8
2. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
3. Volkow ND, McLellan AT. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med. (2016) 374:1253–63. doi: 10.1056/NEJMra1507771
4. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
5. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. (2017) 18:247–54. doi: 10.1016/j.jpain.2016.10.021
6. Humphreys K, Shover CL, Andrews CM, Bohnert ASB, Brandeau ML, Caulkins JP, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford-lancet commission. Lancet. (2022) 399:555–604. doi: 10.1016/S0140-6736(21)02252-2
7. Sisignano M, Geisslinger G. Rethinking the use of NSAIDs in early acute pain. Trends Pharmacol Sci. (2023) 44:193–5. doi: 10.1016/j.tips.2023.01.001
8. Woolf CJ. Capturing novel non-opioid pain targets. Biol Psychiatry. (2020) 87:74–81. doi: 10.1016/j.biopsych.2019.06.017
9. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. (2020) 180:114147. doi: 10.1016/j.bcp.2020.114147
10. Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. (2012) 11:52–64. doi: 10.2174/187152312803476255
11. Corriere MA, Daniel LL, Dickson AL, Nepal P, Hall K, Plummer WD, et al. Concurrent gabapentin and opioid use and risk of mortality in medicare recipients with non-cancer pain. Clin Pharmacol Ther. (2023) 114:1050–7. doi: 10.1002/cpt.3019
12. Zarei M, Mohammadi S, Komaki A. Antinociceptive activity of Inula britannica L. and patuletin: in vivo and possible mechanisms studies. J Ethnopharmacol. (2018) 219:351–8. doi: 10.1016/j.jep.2018.03.021
13. Rouwette T, Avenali L, Sondermann J, Narayanan P, Gomez-Varela D, Schmidt M. Modulation of nociceptive ion channels and receptors via protein-protein interactions: implications for pain relief. Channels (Austin). (2015) 9:175–85. doi: 10.1080/19336950.2015.1051270
14. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. (2010) 120:3760–72. doi: 10.1172/JCI42843
15. Luo J, Walters ET, Carlton SM, Hu H. Targeting pain-evoking transient receptor potential channels for the treatment of pain. Curr Neuropharmacol. (2013) 11:652–63. doi: 10.2174/1570159X113119990040
16. Chung G, Kim SK. Therapeutics for chemotherapy-induced peripheral neuropathy: approaches with natural compounds from traditional eastern medicine. Pharmaceutics. (2022) 14(7):1407. doi: 10.3390/pharmaceutics14071407
17. Bi Z, Zheng Y, Yuan J, Bian Z. The efficacy and potential mechanisms of Chinese herbal medicine on irritable bowel syndrome. Curr Pharm Des. (2017) 23:5163–72. doi: 10.2174/1381612823666170822101606
18. Lee H, Jo HG, Lee D. Oral administration of East Asian herbal medicine for peripheral neuropathy: a protocol for systematic review and meta-analysis with using association rule analysis to identify core herb pattern. Medicine (Baltimore). (2021) 100:e27644. doi: 10.1097/MD.0000000000027644
19. Jo HG, Seo J, Lee D. Clinical evidence construction of East Asian herbal medicine for inflammatory pain in rheumatoid arthritis based on integrative data mining approach. Pharmacol Res. (2022) 185:106460. doi: 10.1016/j.phrs.2022.106460
20. Bauer BA, Tilburt JC, Sood A, Li GX, Wang SH. Complementary and alternative medicine therapies for chronic pain. Chin J Integr Med. (2016) 22:403–11. doi: 10.1007/s11655-016-2258-y
21. Chen L, Michalsen A. Management of chronic pain using complementary and integrative medicine. Br Med J. (2017) 357:j1284. doi: 10.1136/bmj.j1284
22. Gagnier JJ, van Tulder MW, Berman B, Bombardier C. Herbal medicine for low back pain: a cochrane review. Spine (Phila Pa 1976). (2007) 32:82–92. doi: 10.1097/01.brs.0000249525.70011.fe
23. Cortright DN, Szallasi A. TRP channels and pain. Curr Pharm Des. (2009) 15:1736–49. doi: 10.2174/138161209788186308
24. Skerratt SE, West CW. Ion channel therapeutics for pain. Channels (Austin). (2015) 9:344–51. doi: 10.1080/19336950.2015.1075105
25. Jayakar S, Shim J, Jo S, Bean BP, Singeç I, Woolf CJ. Developing nociceptor-selective treatments for acute and chronic pain. Sci Transl Med. (2021) 13:eabj9837. doi: 10.1126/scitranslmed.abj9837
26. Markman JD, Dworkin RH. Ion channel targets and treatment efficacy in neuropathic pain. J Pain. (2006) 7:S38–47. doi: 10.1016/j.jpain.2005.09.008
27. Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD. The role of voltage-gated sodium channels in pain signaling. Physiol Rev. (2019) 99:1079–151. doi: 10.1152/physrev.00052.2017
28. Barbieri R, Nizzari M, Zanardi I, Pusch M, Gavazzo P. Voltage-gated sodium channel dysfunctions in neurological disorders. Life (Basel). (2023) 13(5):1191. doi: 10.3390/life13051191
29. Israel MR, Tanaka BS, Castro J, Thongyoo P, Robinson SD, Zhao P, et al. Na(V) 1.6 regulates excitability of mechanosensitive sensory neurons. J Physiol. (2019) 597:3751–68. doi: 10.1113/JP278148
30. Cardoso FC, Lewis RJ. Sodium channels and pain: from toxins to therapies. Br J Pharmacol. (2018) 175:2138–57. doi: 10.1111/bph.13962
31. Gomez K, Stratton HJ, Duran P, Loya S, Tang C, Calderon-Rivera A, et al. Identification and targeting of a unique Na(V)1.7 domain driving chronic pain. Proc Natl Acad Sci U S A. (2023) 120:e2217800120. doi: 10.1073/pnas.2217800120
32. Catterall WA. Voltage gated sodium and calcium channels: discovery, structure, function, and pharmacology. Channels (Austin). (2023) 17:2281714. doi: 10.1080/19336950.2023.2281714
33. Jurkovicova-Tarabova B, Lacinova L. Structure, function and regulation of Ca(V) 2.2 N-type calcium channels. Gen Physiol Biophys. (2019) 38:101–10. doi: 10.4149/gpb_2019004
34. Roca-Lapirot O, Radwani H, Aby F, Nagy F, Landry M, Fossat P. Calcium signalling through L-type calcium channels: role in pathophysiology of spinal nociceptive transmission. Br J Pharmacol. (2018) 175:2362–74. doi: 10.1111/bph.13747
35. Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. (2006) 7:S13–30. doi: 10.1016/j.jpain.2005.09.007
36. Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. (2015) 67:821–70. doi: 10.1124/pr.114.009654
37. Patel R, Montagut-Bordas C, Dickenson AH. Calcium channel modulation as a target in chronic pain control. Br J Pharmacol. (2018) 175:2173–84. doi: 10.1111/bph.13789
38. Sekiguchi F, Tsubota M, Kawabata A. Involvement of voltage-gated calcium channels in inflammation and inflammatory pain. Biol Pharm Bull. (2018) 41:1127–34. doi: 10.1248/bpb.b18-00054
39. Harding EK, Zamponi GW. Central and peripheral contributions of T-type calcium channels in pain. Mol Brain. (2022) 15:39. doi: 10.1186/s13041-022-00923-w
40. Qian C, Fan Y, Zong L, Miao C, Ji LL, Wan L, et al. Opening K(ATP) channels induces inflammatory tolerance and prevents chronic pain. Brain Behav Immun. (2023) 107:76–86. doi: 10.1016/j.bbi.2022.09.017
41. Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. (2004) 500:203–19. doi: 10.1016/j.ejphar.2004.07.026
42. Tsantoulas C. Emerging potassium channel targets for the treatment of pain. Curr Opin Support Palliat Care. (2015) 9:147–54. doi: 10.1097/SPC.0000000000000131
43. Gao N, Li M, Wang W, Liu Z, Guo Y. The dual role of TRPV1 in peripheral neuropathic pain: pain switches caused by its sensitization or desensitization. Front Mol Neurosci. (2024) 17:1400118. doi: 10.3389/fnmol.2024.1400118
44. Do N, Zuo D, Kim M, Kim M, Ha HJ, Blumberg PM, et al. Discovery of dual TRPA1 and TRPV1 antagonists as novel therapeutic agents for pain. Pharmaceuticals (Basel). (2024) 17(9):1209. doi: 10.3390/ph17091209
45. González-Ramírez R, Chen Y, Liedtke WB, Morales-Lázaro SL. Frontiers in neuroscience TRP channels and pain. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis (2017). p. 125–47.
46. Hung CY, Tan CH. TRP channels in nociception and pathological pain. Adv Exp Med Biol. (2018) 1099:13–27. doi: 10.1007/978-981-13-1756-9_2
47. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. (2013) 29:355–84. doi: 10.1146/annurev-cellbio-101011-155833
48. Lee CH, Chen CC. Roles of ASICs in nociception and proprioception. Adv Exp Med Biol. (2018) 1099:37–47. doi: 10.1007/978-981-13-1756-9_4
49. Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. (2010) 128:549–58. doi: 10.1016/j.pharmthera.2010.08.006
50. Hung CH, Chin Y, Fong YO, Lee CH, Han DS, Lin JH, et al. Acidosis-related pain and its receptors as targets for chronic pain. Pharmacol Ther. (2023) 247:108444. doi: 10.1016/j.pharmthera.2023.108444
51. Martínez-Rojas VA, Barragán-Iglesias P, Rocha-González HI, Murbartián J, Granados-Soto V. Role of TRPV1 and ASIC3 in formalin-induced secondary allodynia and hyperalgesia. Pharmacol Rep. (2014) 66:964–71. doi: 10.1016/j.pharep.2014.06.011
52. North RA. P2x3 receptors and peripheral pain mechanisms. J Physiol. (2004) 554:301–8. doi: 10.1113/jphysiol.2003.048587
53. Hu SQ, Hu JL, Zou FL, Liu JP, Luo HL, Hu DX, et al. P2x7 receptor in inflammation and pain. Brain Res Bull. (2022) 187:199–209. doi: 10.1016/j.brainresbull.2022.07.006
54. Zhang WJ, Zhu ZM, Liu ZX. The role of P2X4 receptor in neuropathic pain and its pharmacological properties. Pharmacol Res. (2020) 158:104875. doi: 10.1016/j.phrs.2020.104875
55. Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2x4 receptors and neuropathic pain. Front Cell Neurosci. (2013) 7:191. doi: 10.3389/fncel.2013.00191
56. Burnstock G. Purinergic mechanisms and pain. Adv Pharmacol. (2016) 75:91–137. doi: 10.1016/bs.apha.2015.09.001
57. Ren W, Yuan L, Li J, Huang XJ, Chen S, Zou DJ, et al. Ethanolic extract of Aconiti brachypodi radix attenuates nociceptive pain probably via inhibition of voltage-dependent Na+ channel. Afr J Tradit Complement Altern Med. (2012) 9(4):574–83. doi: 10.4314/ajtcam.v9i4.15
58. Yu S, Xu L, Wei PK, Qin ZF, Li J, Peng HD. Study on analgesic effect of traditional Chinese medicine. Chin J Integr Med. (2008) 14:151–6. doi: 10.1007/s11655-008-0151-z
59. Wang CF, Gerner P, Wang SY, Wang GK. Bulleyaconitine A isolated from Aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. Anesthesiology. (2007) 107:82–90. doi: 10.1097/01.anes.0000267502.18605.ad
60. Yang X, Dai Y, Ji Z, Zhang X, Fu W, Han C, et al. Allium macrostemon bunge. Exerts analgesic activity by inhibiting NaV1.7 channel. J Ethnopharmacol. (2021) 281:114495. doi: 10.1016/j.jep.2021.114495
61. Zhu C, Wang M, Guo J, Su SL, Yu G, Yang Y, et al. Angelica Dahurica extracts attenuate CFA-induced inflammatory pain via TRPV1 in mice. Evid Based Complement Alternat Med. (2022) 2022:4684830. doi: 10.1155/2022/4684830
62. Chen X, Sun W, Gianaris NG, Riley AM, Cummins TR, Fehrenbacher JC, et al. Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J Biol Chem. (2014) 289:9600–10. doi: 10.1074/jbc.M113.536862
63. Zhang A, Gao Y, Zhong X, Xu C, Li G, Liu S, et al. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. (2010) 1313:215–21. doi: 10.1016/j.brainres.2009.11.067
64. Li R, Zhao C, Yao M, Song Y, Wu Y, Wen A. Analgesic effect of coumarins from radix angelicae pubescentis is mediated by inflammatory factors and TRPV1 in a spared nerve injury model of neuropathic pain. J Ethnopharmacol. (2017) 195:81–8. doi: 10.1016/j.jep.2016.11.046
65. Su X, Wu B, Zhang W, Ji YH, Wang Q, Tan ZY. Inhibitory effects of columbianadin on nociceptive behaviors in a neuropathic pain model, and on voltage-gated calcium currents in dorsal root ganglion neurons in mice. Front Pharmacol. (2019) 10:1522. doi: 10.3389/fphar.2019.01522
66. Wang ZJ, Tabakoff B, Levinson SR, Heinbockel T. Inhibition of Nav1.7 channels by methyl eugenol as a mechanism underlying its antinociceptive and anesthetic actions. Acta Pharmacol Sin. (2015) 36:791–9. doi: 10.1038/aps.2015.26
67. Xu Y, Yu Y, Wang Q, Li W, Zhang S, Liao X, et al. Active components of Bupleurum chinense and Angelica biserrata showed analgesic effects in formalin induced pain by acting on Nav1.7. J Ethnopharmacol. (2021) 269:113736. doi: 10.1016/j.jep.2020.113736
68. Yan S, Huang Y, Xiao Q, Su Z, Xia L, Xie J, et al. Regulation of transient receptor potential channels by traditional Chinese medicines and their active ingredients. Front Pharmacol. (2022) 13:1039412. doi: 10.3389/fphar.2022.1039412
69. Xu Y, Sun J, Li W, Zhang S, Yang L, Teng Y, et al. Analgesic effect of the main components of Corydalis yanhusuo (yanhusuo in Chinese) is caused by inhibition of voltage gated sodium channels. J Ethnopharmacol. (2021) 280:114457. doi: 10.1016/j.jep.2021.114457
70. Zhang X, Chen Q, Wang Y, Peng W, Cai H. Effects of curcumin on ion channels and transporters. Front Physiol. (2014) 5:94. doi: 10.3389/fphys.2014.00094
71. Zhao S, Yang J, Han X, Gong Y, Rao S, Wu B, et al. Effects of nanoparticle-encapsulated curcumin on HIV-gp120-associated neuropathic pain induced by the P2X(3) receptor in dorsal root ganglia. Brain Res Bull. (2017) 135:53–61. doi: 10.1016/j.brainresbull.2017.09.011
72. Nguelefack TB, Dutra RC, Paszcuk AF, Andrade EL, Tapondjou LA, Calixto JB. Antinociceptive activities of the methanol extract of the bulbs of Dioscorea bulbifera L. Var sativa in mice is dependent of NO-cGMP-ATP-sensitive-K(+) channel activation. J Ethnopharmacol. (2010) 128:567–74. doi: 10.1016/j.jep.2010.01.061
73. Rhim H, Kim H, Lee DY, Oh TH, Nah SY. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur J Pharmacol. (2002) 436:151–8. doi: 10.1016/S0014-2999(01)01613-2
74. Lee JH, Choi SH, Lee BH, Hwang SH, Kim HJ, Rhee J, et al. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α. Neurosci Lett. (2013) 548:143–8. doi: 10.1016/j.neulet.2013.05.048
75. Zhao Q, Zhang X, Long S, Wang S, Yu H, Zhou Y, et al. Licochalcone mediates the pain relief by targeting the voltage-gated sodium channel. Mol Pharmacol. (2023) 104:133–43. doi: 10.1124/molpharm.122.000658
76. Yu M, Ye F, Ma C, Jin X, Ji H, Wang D, et al. Ligustrazine mitigates chronic venous disease-induced pain hyperalgesia through desensitization of inflammation-associated TRPA1 activity in DRG. J Ethnopharmacol. (2022) 298:115667. doi: 10.1016/j.jep.2022.115667
77. Kim EJ, Kang D, Han J. Baicalein and wogonin are activators of rat TREK-2 two-pore domain K+ channel. Acta Physiol (Oxf). (2011) 202:185–92. doi: 10.1111/j.1748-1716.2011.02263.x
78. Lee JY, Yoon SY, Won J, Kim HB, Kang Y, Oh SB. Sinomenine produces peripheral analgesic effects via inhibition of voltage-gated sodium currents. Neuroscience. (2017) 358:28–36. doi: 10.1016/j.neuroscience.2017.06.024
79. Sekiguchi F, Fujita T, Deguchi T, Yamaoka S, Tomochika K, Tsubota M, et al. Blockade of T-type calcium channels by 6-prenylnaringenin, a hop component, alleviates neuropathic and visceral pain in mice. Neuropharmacology. (2018) 138:232–44. doi: 10.1016/j.neuropharm.2018.06.020
80. Xu C, Xu W, Xu H, Xiong W, Gao Y, Li G, et al. Role of puerarin in the signalling of neuropathic pain mediated by P2X3 receptor of dorsal root ganglion neurons. Brain Res Bull. (2012) 87:37–43. doi: 10.1016/j.brainresbull.2011.10.007
81. Zhang XL, Cao XY, Lai RC, Xie MX, Zeng WA. Puerarin relieves paclitaxel-induced neuropathic pain: the role of na(v)1.8 β1 subunit of sensory neurons. Front Pharmacol. (2018) 9:1510. doi: 10.3389/fphar.2018.01510
82. Ruan Y, Ling J, Ye F, Cheng N, Wu F, Tang Z, et al. Paeoniflorin alleviates CFA-induced inflammatory pain by inhibiting TRPV1 and succinate/SUCNR1-HIF-1α/NLPR3 pathway. Int Immunopharmacol. (2021) 101:108364. doi: 10.1016/j.intimp.2021.108364
83. Tsai TY, Wu SN, Liu YC, Wu AZ, Tsai YC. Inhibitory action of L-type Ca2+ current by paeoniflorin, a major constituent of peony root, in NG108-15 neuronal cells. Eur J Pharmacol. (2005) 523:16–24. doi: 10.1016/j.ejphar.2005.08.042
84. Ying M, Liu H, Zhang T, Jiang C, Gong Y, Wu B, et al. Effect of artemisinin on neuropathic pain mediated by P2X(4) receptor in dorsal root ganglia. Neurochem Int. (2017) 108:27–33. doi: 10.1016/j.neuint.2017.02.004
85. Hu D, Wang C, Li F, Su S, Yang N, Yang Y, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast. (2017) 2017:3710821. doi: 10.1155/2017/3710821
86. Liao Y, Guo C, Wen A, Bai M, Ran Z, Hu J, et al. Frankincense-myrrh treatment alleviates neuropathic pain via the inhibition of neuroglia activation mediated by the TLR4/MyD88 pathway and TRPV1 signaling. Phytomedicine. (2023) 108:154540. doi: 10.1016/j.phymed.2022.154540
87. Yan XG, Li WG, Qi X, Zhu JJ, Huang C, Han SL, et al. Subtype-selective inhibition of acid-sensing ion channel 3 by a natural flavonoid. CNS Neurosci Ther. (2019) 25:47–56. doi: 10.1111/cns.12979
88. Straub I, Krügel U, Mohr F, Teichert J, Rizun O, Konrad M, et al. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol. (2013) 84:736–50. doi: 10.1124/mol.113.086843
89. Zhou LD, Lu YN, Liu Q, Shi XM, Tian J. Modulation of TRPV1 function by Citrus reticulata (tangerine) fruit extract for the treatment of sensitive skin. J Cosmet Dermatol. (2023) 22:1369–76. doi: 10.1111/jocd.15578
90. Nakamori S, Takahashi J, Hyuga S, Tanaka-Kagawa T, Jinno H, Hyuga M, et al. Ephedra herb extract activates/desensitizes transient receptor potential vanilloid 1 and reduces capsaicin-induced pain. J Nat Med. (2017) 71:105–13. doi: 10.1007/s11418-016-1034-9
91. Yang PP, Chueh SH, Shie HL, Chen CC, Lee LY, Chen WP, et al. Effects of hericium erinaceus mycelium extracts on the functional activity of purinoceptors and neuropathic pain in mice with L5 spinal nerve ligation. Evid Based Complement Alternat Med. (2020) 2020:2890194. doi: 10.1155/2020/2890194
92. Gong CL, Wong KL, Cheng KS, Kuo CS, Chao CC, Tsai MF, et al. Inhibitory effects of magnolol on voltage-gated na+ and K+ channels of NG108-15 cells. Eur J Pharmacol. (2012) 682:73–8. doi: 10.1016/j.ejphar.2012.02.013
93. Lin YR, Chen HH, Ko CH, Chan MH. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. (2007) 81:1071–8. doi: 10.1016/j.lfs.2007.08.014
94. Gaudioso C, Hao J, Martin-Eauclaire MF, Gabriac M, Delmas P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain. (2012) 153:473–84. doi: 10.1016/j.pain.2011.11.014
95. Borghi SM, Carvalho TT, Staurengo-Ferrari L, Hohmann MS, Pinge-Filho P, Casagrande R, et al. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J Nat Prod. (2013) 76:1141–9. doi: 10.1021/np400222v
96. Chen YS, Lian YZ, Chen WC, Chang CC, Tinkov AA, Skalny AV, et al. Lycium barbarum polysaccharides and capsaicin inhibit oxidative stress, inflammatory responses, and pain signaling in rats with dextran sulfate sodium-induced colitis. Int J Mol Sci. (2022) 23(5):2423. doi: 10.3390/ijms23052423
97. Kim SE, Yin MZ, Roh JW, Kim HJ, Choi SW, Wainger BJ, et al. Multi-target modulation of ion channels underlying the analgesic effects of α-mangostin in dorsal root ganglion neurons. Phytomedicine. (2023) 115:154791. doi: 10.1016/j.phymed.2023.154791
98. Oláh Z, Rédei D, Pecze L, Vizler C, Jósvay K, Forgó P, et al. Pellitorine, an extract of Tetradium daniellii, is an antagonist of the ion channel TRPV1. Phytomedicine. (2017) 34:44–9. doi: 10.1016/j.phymed.2017.06.006
99. Yang J, Yang Q, Zhao J, Sun S, Liu M, Wang Y, et al. Evaluation of rhodojaponin III from Rhododendron molle G. Don on oral antinociceptive activity, mechanism of action, and subacute toxicity in rodents. J Ethnopharmacol. (2022) 294:115347. doi: 10.1016/j.jep.2022.115347
100. Chan YT, Wang N, Feng Y. The toxicology and detoxification of Aconitum: traditional and modern views. Chin Med. (2021) 16:61. doi: 10.1186/s13020-021-00472-9
101. Zhao P, Tian Y, Geng Y, Zeng C, Ma X, Kang J, et al. Aconitine and its derivatives: bioactivities, structure-activity relationships and preliminary molecular mechanisms. Front Chem. (2024) 12:1339364. doi: 10.3389/fchem.2024.1339364
102. Tanimura Y, Yoshida M, Ishiuchi K, Ohsawa M, Makino T. Neoline is the active ingredient of processed aconite root against murine peripheral neuropathic pain model, and its pharmacokinetics in rats. J Ethnopharmacol. (2019) 241:111859. doi: 10.1016/j.jep.2019.111859
103. Suzuki T, Miyamoto K, Yokoyama N, Sugi M, Kagioka A, Kitao Y, et al. Processed aconite root and its active ingredient neoline may alleviate oxaliplatin-induced peripheral neuropathic pain. J Ethnopharmacol. (2016) 186:44–52. doi: 10.1016/j.jep.2016.03.056
104. Zhu HQ, Xu J, Shen KF, Pang RP, Wei XH, Liu XG. Bulleyaconitine A depresses neuropathic pain and potentiation at C-fiber synapses in spinal dorsal horn induced by paclitaxel in rats. Exp Neurol. (2015) 273:263–72. doi: 10.1016/j.expneurol.2015.09.006
105. Xie MX, Zhu HQ, Pang RP, Wen BT, Liu XG. Mechanisms for therapeutic effect of bulleyaconitine A on chronic pain. Mol Pain. (2018) 14:1744806918797243. doi: 10.1177/1744806918797243
106. Friese J, Gleitz J, Gutser UT, Heubach JF, Matthiesen T, Wilffert B, et al. Aconitum sp. Alkaloids: the modulation of voltage-dependent na+ channels, toxicity and antinociceptive properties. Eur J Pharmacol. (1997) 337:165–74. doi: 10.1016/S0014-2999(97)01268-5
107. Hook IL. Danggui to Angelica sinensis root: are potential benefits to European women lost in translation? A review. J Ethnopharmacol. (2014) 152:1–13. doi: 10.1016/j.jep.2013.12.018
108. Lu Y, Wu H, Yu X, Zhang X, Luo H, Tang L, et al. Traditional Chinese medicine of Angelicae pubescentis radix: a review of phytochemistry, pharmacology and pharmacokinetics. Front Pharmacol. (2020) 11:335. doi: 10.3389/fphar.2020.00335
109. Chen YF, Tsai HY, Wu TS. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. (1995) 61:2–8. doi: 10.1055/s-2006-957987
110. Li X, Wang J, Gao L. Anti-inflammatory and analgesic activity of R.A.P. (radix Angelicae pubescentis) ethanol extracts. Afr J Tradit Complement Altern Med. (2013) 10:422–6. doi: 10.4314/ajtcam.v10i6.2
111. Yano S, Suzuki Y, Yuzurihara M, Kase Y, Takeda S, Watanabe S, et al. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur J Pharmacol. (2006) 553:99–103. doi: 10.1016/j.ejphar.2006.09.020
112. Zhou X, Cheng H, Xu D, Yin Q, Cheng L, Wang L, et al. Attenuation of neuropathic pain by saikosaponin a in a rat model of chronic constriction injury. Neurochem Res. (2014) 39(11):2136–42. doi: 10.1007/s11064-014-1407-y
113. Li P, Gong Y, Zu N, Li Y, Wang B, Shimizu F. Therapeutic mechanism of saikosaponin-d in anti-Thy1 mAb 1-22-3-induced rat model of glomerulonephritis. Nephron Exp Nephrol. (2005) 101:e111–8. doi: 10.1159/000087437
114. Chae HK, Kim W, Kim SK. Phytochemicals of Cinnamomi Cortex: cinnamic acid, but not cinnamaldehyde, attenuates oxaliplatin-induced cold and mechanical hypersensitivity in rats. Nutrients. (2019) 11(2):432. doi: 10.3390/nu11020432
115. Kim C, Lee JH, Kim W, Li D, Kim Y, Lee K, et al. The suppressive effects of Cinnamomi Cortex and its phytocompound coumarin on oxaliplatin-induced neuropathic cold allodynia in rats. Molecules. (2016) 21(9):1253. doi: 10.3390/molecules21091253
116. Sui F, Yang N, Zhang C, Du X, Li L, Weng X, et al. Effects of ingredients from Chinese herbs with nature of cold or hot on expression of TRPV1 and TRPM8. Zhongguo Zhong Yao Za Zhi. (2010) 35(12):1594–8. doi: 10.4268/cjcmm20101220
117. Namer B, Seifert F, Handwerker HO, Maihöfner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. (2005) 16:955–9. doi: 10.1097/00001756-200506210-00015
118. Kang DW, Moon JY, Choi JG, Kang SY, Ryu Y, Park JB, et al. Antinociceptive profile of levo-tetrahydropalmatine in acute and chronic pain mice models: role of spinal sigma-1 receptor. Sci Rep. (2016) 6:37850. doi: 10.1038/srep37850
119. Sun J, Liu X, Zhao S, Zhang S, Yang L, Zhang J, et al. Prediction and verification of potential lead analgesic and antiarrhythmic components in Corydalis yanhusuo W. T. Wang based on voltage-gated sodium channel proteins. Int J Biol Macromol. (2022) 216:537–46. doi: 10.1016/j.ijbiomac.2022.07.024
120. Babu A, Prasanth KG, Balaji B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm Biol. (2015) 53:838–48. doi: 10.3109/13880209.2014.943247
121. Hasriadi PW, Wasana D, Vajragupta O, Rojsitthisak P, Towiwat P. Mechanistic insight into the effects of curcumin on neuroinflammation-driven chronic pain. Pharmaceuticals (Basel). (2021) 14(8):777. doi: 10.3390/ph14080777
122. Goyal S, Goyal S, Goins AE, Alles SRA. Plant-derived natural products targeting ion channels for pain. Neurobiol Pain. (2023) 13:100128. doi: 10.1016/j.ynpai.2023.100128
123. Yang R, Wang LQ, Yuan BC, Liu Y. The pharmacological activities of licorice. Planta Med. (2015) 81(18):1654–69. doi: 10.1055/s-0035-1557893
124. Wang L, Zhang J, Hong Y, Feng Y, Chen M, Wang Y. Phytochemical and pharmacological review of da chuanxiong formula: a famous herb pair composed of chuanxiong rhizoma and gastrodiae rhizoma for headache. Evid Based Complement Alternat Med. (2013) 2013:425369. doi: 10.1155/2013/425369
125. Huang HC, Wang HR, Hsieh LM. Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol. (1994) 251:91–3. doi: 10.1016/0014-2999(94)90447-2
126. Lin YL, Lin RJ, Shen KP, Dai ZK, Chen IJ, Wu JR, et al. Baicalein, isolated from Scutellaria baicalensis, protects against endothelin-1-induced pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression. J Ethnopharmacol. (2011) 138:373–81. doi: 10.1016/j.jep.2011.09.014
127. Zhu Q, Sun Y, Zhu J, Fang T, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci Rep. (2014) 4:7270. doi: 10.1038/srep07270
128. Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol. (2012) 50:1053–61. doi: 10.3109/13880209.2012.656847
129. Zeng FF, Chen ZH, Luo FH, Liu CJ, Yang X, Zhang FX, et al. Sophorae tonkinensis radix et rhizoma: a comprehensive review of the ethnopharmacology, phytochemistry, pharmacology, pharmacokinetics, toxicology and detoxification strategy. J Ethnopharmacol. (2024) 337:118784. doi: 10.1016/j.jep.2024.118784
130. Wang J, Song C, Gao D, Wei S, Sun W, Guo Y, et al. Effects of Paeonia lactiflora extract on estrogen receptor β, TPH2, and SERT in rats with PMS anxiety. Biomed Res Int. (2020) 2020:4690504. doi: 10.1155/2020/4690504
131. Zhang L, Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther. (2020) 207:107452. doi: 10.1016/j.pharmthera.2019.107452
132. Lu Y, Yin L, Yang W, Wu Z, Niu J. Antioxidant effects of paeoniflorin and relevant molecular mechanisms as related to a variety of diseases: a review. Biomed Pharmacother. (2024) 176:116772. doi: 10.1016/j.biopha.2024.116772
133. Yang J, Zhao J, Zhang J. The efficacy and toxicity of grayanoids as analgesics: a systematic review. J Ethnopharmacol. (2022) 298:115581. doi: 10.1016/j.jep.2022.115581
134. Mahdian Dehkordi F, Kaboutari J, Zendehdel M, Javdani M. The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems. Korean J Pain. (2019) 32:160–7. doi: 10.3344/kjp.2019.32.3.160
135. Feng X, Cao S, Qiu F, Zhang B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol Ther. (2020) 216:107650. doi: 10.1016/j.pharmthera.2020.107650
136. Ali M, Iqbal R, Safdar M, Murtaza S, Mustafa G, Sajjad M, et al. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol Biol Rep. (2021) 48:7703–10. doi: 10.1007/s11033-021-06777-0
137. Favero FF, Basting RT, de Freitas AS, Dias Rabelo LDS, Nonato FR, Zafred RRT, et al. Artemisinin and deoxyartemisinin isolated from Artemisia annua L. promote distinct antinociceptive and anti-inflammatory effects in an animal model. Biomed Pharmacother. (2024) 178:117299. doi: 10.1016/j.biopha.2024.117299
138. Massoud AM, El Ebiary FH, Abou-Gamra MM, Mohamed GF, Shaker SM. Evaluation of schistosomicidal activity of myrrh extract: parasitological and histological study. J Egypt Soc Parasitol. (2004) 34(3 Suppl):1051–76.15658062
139. Shalaby MA, Hammouda AA. Analgesic, anti-inflammatory and anti-hyperlipidemic activities of Commiphora molmol extract (myrrh). J Intercult Ethnopharmacol. (2014) 3:56–62. doi: 10.5455/jice.20140130015014
140. Bimonte S, Cascella M, Schiavone V, Mehrabi-Kermani F, Cuomo A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Des Devel Ther. (2017) 11:2737–42. doi: 10.2147/DDDT.S142475
141. Xifró X, Vidal-Sancho L, Boadas-Vaello P, Turrado C, Alberch J, Puig T, et al. Novel epigallocatechin-3-gallate (EGCG) derivative as a new therapeutic strategy for reducing neuropathic pain after chronic constriction nerve injury in mice. PLoS One. (2015) 10:e0123122. doi: 10.1371/journal.pone.0123122
142. Adamante G, de Almeida AS, Rigo FK, da Silva Silveira E, Coelho YO, De Prá SD, et al. Diosmetin as a novel transient receptor potential vanilloid 1 antagonist with antinociceptive activity in mice. Life Sci. (2019) 216:215–26. doi: 10.1016/j.lfs.2018.11.029
143. Kaimoto T, Hatakeyama Y, Takahashi K, Imagawa T, Tominaga M, Ohta T. Involvement of transient receptor potential A1 channel in algesic and analgesic actions of the organic compound limonene. Eur J Pain. (2016) 20:1155–65. doi: 10.1002/ejp.840
144. Zhou Y, Cai S, Moutal A, Yu J, Gómez K, Madura CL, et al. The natural flavonoid naringenin elicits analgesia through inhibition of NaV1.8 voltage-gated sodium channels. ACS Chem Neurosci. (2019) 10:4834–46. doi: 10.1021/acschemneuro.9b00547
145. Hyuga S, Hyuga M, Oshima N, Maruyama T, Kamakura H, Yamashita T, et al. Ephedrine alkaloids-free ephedra herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J Nat Med. (2016) 70:571–83. doi: 10.1007/s11418-016-0979-z
146. Kasahara Y, Hikino H, Tsurufuji S, Watanabe M, Ohuchi K. Antiinflammatory actions of ephedrines in acute inflammations1. Planta Med. (1985) 51:325–31. doi: 10.1055/s-2007-969503
147. Yeom MJ, Lee HC, Kim GH, Lee HJ, Shim I, Oh SK, et al. Anti-arthritic effects of Ephedra sinica STAPF herb-acupuncture: inhibition of lipopolysaccharide-induced inflammation and adjuvant-induced polyarthritis. J Pharmacol Sci. (2006) 100:41–50. doi: 10.1254/jphs.FP0050637
148. Tan YF, Mo JS, Wang YK, Zhang W, Jiang YP, Xu KP, et al. The ethnopharmacology, phytochemistry and pharmacology of the genus hericium. J Ethnopharmacol. (2024) 319:117353. doi: 10.1016/j.jep.2023.117353
149. Wang M, Gao Y, Xu D, Konishi T, Gao Q. Hericium erinaceus (yamabushitake): a unique resource for developing functional foods and medicines. Food Funct. (2014) 5:3055–64. doi: 10.1039/C4FO00511B
150. Mainardi T, Kapoor S, Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J Allergy Clin Immunol. (2009) 123:283–94; quiz 295–6. doi: 10.1016/j.jaci.2008.12.023
151. Shen JL, Man KM, Huang PH, Chen WC, Chen DC, Cheng YW, et al. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules. (2010) 15:6452–65. doi: 10.3390/molecules15096452
152. Teng CM, Yu SM, Chen CC, Huang YL, Huang TF. EDRF-release and ca+(+)-channel blockade by magnolol, an antiplatelet agent isolated from Chinese herb Magnolia officinalis, in rat thoracic aorta. Life Sci. (1990) 47:1153–61. doi: 10.1016/0024-3205(90)90176-R
153. Kim N, Chung G, Son SR, Park JH, Lee YH, Park KT, et al. Magnolin inhibits paclitaxel-induced cold allodynia and ERK1/2 activation in mice. Plants (Basel). (2023) 12(12):2283. doi: 10.3390/plants12122283
154. Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. (2013) 154:2169–77. doi: 10.1016/j.pain.2013.06.043
155. Lee S, Jang IS. Menthol excites dural afferent neurons by inhibiting leak K(+) conductance in rats. Neurosci Lett. (2023) 813:137427. doi: 10.1016/j.neulet.2023.137427
156. Zhu Q, Mao LN, Liu CP, Sun YH, Jiang B, Zhang W, et al. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci Rep. (2016) 6:19266. doi: 10.1038/srep19266
157. Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. (2013) 54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023
158. Wang MH, Zhang KJ, Gu QL, Bi XL, Wang JX. Pharmacology of mangostins and their derivatives: a comprehensive review. Chin J Nat Med. (2017) 5(2):81–93. doi: 10.1016/S1875-5364(17)30024-9
159. Chen G, Li Y, Wang W, Deng L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: a review. Expert Opin Ther Pat. (2018) 28:415–27. doi: 10.1080/13543776.2018.1455829
160. Lee JH, Kim N, Park S, Kim SK. Analgesic effects of medicinal plants and phytochemicals on chemotherapy-induced neuropathic pain through glial modulation. Pharmacol Res Perspect. (2021) 9(6):e00819. doi: 10.1002/prp2.819
161. Hajhashemi V, Ghannadi A, Pezeshkian SK. Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J Ethnopharmacol. (2002) 82:83–7. doi: 10.1016/S0378-8741(02)00137-X
162. Abdollahi M, Karimpour H, Monsef-Esfehani HR. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacol Res. (2003) 48:31–5. doi: 10.1016/S1043-6618(03)00206-8
163. Piazza S, Fumagalli M, Martinelli G, Pozzoli C, Maranta N, Angarano M, et al. Hydrolyzable tannins in the management of Th1, Th2 and Th17 inflammatory-related diseases. Molecules. (2022) 27(21):7593. doi: 10.3390/molecules27217593
164. Devi M, Bamrah PK, Goyal R, Choudhary M, Chopra H. Insights on the emerging therapeutic potential of terpenoids as anti-inflammatory agents: a scoping review. J Bio-X Res. (2024) 7:0006. doi: 10.34133/jbioxresearch.0006
165. Li W, Zhang Y, Zhao S, Zhao X, Xie J. Efficient enrichment and characterization of triterpenoid saponins from Platycodon grandiflorus roots. J Chromatogr A. (2024) 1735:465332. doi: 10.1016/j.chroma.2024.465332
166. Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. (2019) 24(16):2930. doi: 10.3390/molecules24162930
167. Eke-Okoro UJ, Raffa RB, Pergolizzi JV Jr., Breve F, Taylor R Jr. Curcumin in turmeric: basic and clinical evidence for a potential role in analgesia. J Clin Pharm Ther. (2018) 43:460–6. doi: 10.1111/jcpt.12703
168. Yu G, Xiang W, Zhang T, Zeng L, Yang K, Li J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther. (2020) 20:225. doi: 10.1186/s12906-020-02985-6
169. DeGeorge KC, Ring DJ, Dalrymple SN. Treatment of the common cold. Am Fam Physician. (2019) 100(5):281–9.31478634
170. Barkat MA, Goyal A, Barkat HA, Salauddin M, Pottoo FH, Anwer ET. Herbal medicine: clinical perspective and regulatory status. Comb Chem High Throughput Screen. (2021) 24:1573–82. doi: 10.2174/1386207323999201110192942
171. Singh DB, Pathak RK, Rai D. From traditional herbal medicine to rational drug discovery: strategies, challenges, and future perspectives. Rev Bras Farmacogn. (2022) 32:147–59. doi: 10.1007/s43450-022-00235-z
172. Bilia AR, Mukherjee PK, Andrade-Cetto A, Katiyar CK, Bachar SC, Matsabisa MG, et al. Editorial: drug development of herbal medicines: regulatory perspectives. Front Pharmacol. (2022) 13:989934. doi: 10.3389/fphar.2022.989934
Keywords: traditional medicine, phytochemicals, ion channels, analgesic, pain
Citation: Kim SE, Chung G and Kim SK (2025) Phytochemical-based therapeutics from traditional eastern medicine: analgesic effects and ion channel modulation. Front. Pain Res. 6:1537154. doi: 10.3389/fpain.2025.1537154
Received: 3 December 2024; Accepted: 9 January 2025;
Published: 31 January 2025.
Edited by:
Aubin Moutal, Saint Louis University, United StatesReviewed by:
Paramita Basu, University of Pittsburgh, United StatesCopyright: © 2025 Kim, Chung and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geehoon Chung, Z2VlaG9vbi5jaHVuZ0BraHUuYWMua3I=; Sun Kwang Kim, c2traW03N0BraHUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.