- 1Faculty of Biology, Biology Department, Technion-Israel Institute of Technology, Haifa, Israel
- 2Cancer Center, HaEmek Medical Center, Afula, Israel
- 3Department of Oncology, Galilee Medical Center, Nahariya, Israel
- 4Azrielly Faculty of Medicine, Bar Ilan University, Zafed, Israel
- 5Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
The use of medical cannabis (MC) to treat cancer-related symptoms is rising. However, there is a lack of long-term trials to assess the benefits and safety of MC treatment in this population. In this work, we followed up prospectively and longitudinally on the effectiveness and safety of MC treatment. Oncology patients reported on multiple symptoms before and after MC treatment initiation at one-, three-, and 6-month follow-ups. Oncologists reported on the patients' disease characteristics. Intention-to-treat models were used to assess changes in outcomes from baseline. MC treatment was initiated by 324 patients and 212, 158 and 126 reported at follow-ups. Most outcome measures improved significantly during MC treatment for most patients (p < 0.005). Specifically, at 6 months, total cancer symptoms burden declined from baseline by a median of 18%, from 122 (82–157) at baseline to 89 (45–138) at endpoint (−18.98; 95%CI= −26.95 to −11.00; p < 0.001). Reported adverse effects were common but mostly non-serious and remained stable during MC treatment. The results of this study suggest that MC treatment is generally safe for oncology patients and can potentially reduce the burden of associated symptoms with no serious MC-related adverse effects.
Introduction
Many comorbidities are associated with oncology diseases. Cancer-associated symptoms include pain (1), anxiety (2), depression (3), insomnia (4), decreased quality of life (5), increased disability (6) and negative effects on sexuality (7). These symptoms are some of the most fundamental causes of oncology patients suffering and disability while undergoing therapies, and some may even lead to worsened prognosis (3). Though well-known and documented, there is no optimal treatment for all of these comorbidities as of yet (8). Traditionally, cancer-related pain is mainly treated by opioid analgesics. In a recent Cochrane collaboration review of opioids for cancer pain, which thoroughly assessed 152 studies with 13,524 patients, treatment success was reported by 95% of patients, but most did not assess pain reduction adequately. Moreover, Wiffen et al. (9) concluded that the quality of evidence in favor of opioid treatment is poor, as the studies on which the treatment decision was based were old and with small sample size, and adverse events rates ranged from 11 to 77% (9). That is probably one of the reasons why most oncologists perceive opioid treatment as hazardous and alternative therapies are required.
A promising substitute for opioid-based medication is medical cannabis (MC). However, there is a knowledge gap in the study of cannabis, especially for treating cancer-related pain, and results are controversial. Thus far, only a few randomized controlled trials (10–16) and even fewer cohorts (17–19) investigated the effects of cannabinoids on cancer-related pain and scantly also on other cancer symptoms. Consequently, these findings led to a weak recommendation for utilizing cannabinoids for cancer pain treatment (20). However, although these studies were randomized controlled trials, most of them consisted of a small sample size and additional studies are needed (21). A more recent meta-analysis showed no favorable effect for Nabiximols in cancer pain (22). Nevertheless, a recent study showed that most cancer patients requested MC treatment from their oncologist (23).
The adverse effects (AEs) from cannabinoids for cancer treatment are generally well tolerated by the patients and categorized as mild to moderate. The most frequent AEs are memory impairment, drowsiness, nausea, vomiting and xerostomia (dry mouth). Cannabinoid treatment for cancer-related pain is generally recognized as safe (24). Nonetheless, drug-drug interactions should be taken into account. Recent retrospective and prospective studies showed decreased response rates when immunotherapy was administered concomitantly with an MC treatment (25, 26). Although a previous prospective analysis was conducted on cancer patients following 6–8 weeks of treatment (17), it lacked the added value of repeated observation by multiple follow-up points, the utilization of validated questionnaires, specific MC treatment characterization and longer follow-up. Thus, we conducted a multi-center, prospective, 6-month longitudinal study that followed up on the effectiveness and safety parameters of MC treatment for cancer-associated symptoms. The approach of real-world evidence undertaken in the study provides prospective and structured data collection, and allows the data mining of many patients from real-world data, as is especially important for cancer patients that commonly suffer from associated comorbidities. Similar approaches have proven very useful in assessing the effectiveness of medical treatments in the fields of trauma, cancer, cardiology, stroke and arthritis (27). Similarly, worldwide opioid registries have assisted in reducing treatment-related mortality (28) and in assessing the treatment's long-term effectiveness (29).
Methods
Israeli Medical Cannabis Regulations
The Israel Ministry of Health (IMOH) regulations allow issuing an MC license to treat palliative phase cancer patients and cancer patients with antineoplastic treatment-related adverse effects (30). Licenses for MC use are issued by specific oncologists that received a personalized mandate from the IMOH. The issuing oncologist then prescribes the MC dose (grams per month), route of administration and the cannabidiol (CBD) and (-)-Δ9-trans-tetrahydrocannabinol (THC) concentrations (30), based on the IMOH guidelines. Two routes of MC consumption are approved: inflorescences (for smoking or inhaling) and/or oil extracts (for sublingual use). The initial dose is 20 gr/month regardless of the route of administration. At the time this study was conducted, MC was purchased at pharmacies and was non-reimbursable. The official contraindications for MC in Israel were pregnancy, lactation, and previous psychotic diagnosis or family history of psychotic illness.
Study Design
This multi-center, prospective, long-term study was conducted between January 2019 and September 2021. The institutional Ethics Committee of Haemek Medical Center (#0137-18-EMC) and Galil Medical Center (#0010-19-NHR) approved the study. This was a pure observational study with no interventional component whatsoever, so registration at the Clinical Trials Register was not required. Importantly, no recognizable information on participating patients is published anywhere in this research paper.
Hebrew-speaking patients aged >18 years licensed for MC for treating any form of cancer-related symptoms for the first time were eligible to participate in the study. After explaining the study procedures, oncologists who agreed to participate (all are co-authors in the current study) and regularly issue MC licenses, obtained written informed consents from eligible patients. Copies of the consent forms along with the patients' contact information were sent to the study coordination center. To avoid any possible influence of the collected data on the physicians' decisions regarding the clinical management of their patients, prescribing physicians had no access to data collected on individual patients.
Patients were instructed to complete the study questionnaires at baseline, before MC treatment initiation (T0; up to a few days before), and at three follow-up times: one (T1), three (T3), and six (T6) months following treatment initiation. The questionnaire consisted of 174 questions at baseline and a variable number of about 220 at each follow-up. Questions were presented in a dynamic format customized to individual responses, where responses to a particular question determined the subsequent questions asked. To reduce the study burden, patients were given a choice to skip questions. Hence, each patient completed a unique set of questions and each question received a different number of responses. Data was collected online by the secured survey technology Qualtrics® (Provo, Utah, version 12018) (31). Whenever patients had difficulties using the web platform, the questionnaires could be completed by phone with the assistance of the study coordinator. No financial compensation was offered to participating patients. The STROBE statement checklist for cohort studies is presented in the Supplementary Table 1.
Study Questionnaires
Oncologist reported information included cancer diagnosis, classification of malignant tumors (TNM), cancer treatment protocol and the Eastern Cooperative Oncology Group (ECOG) Performance Status score (32). Patient-reported information included demographics, analgesics consumption, MC treatment characteristics as well as Hebrew validated oncology-related questionnaires, including (1) the study's primary outcome measure of the total sum of Memorial Symptom Assessment Scale (MSAS) of cancer symptoms burden (33); (2) average weekly pain intensity on a 0–10 numerical pain scale (NPS) and the weekly average worst and least pain intensities; (3) The short-form McGill Pain Questionnaire (SF-MPQ) (34); (4) Quality of life - EuroQol (EQ5) (35); (5) Pittsburgh Sleep Quality Index (PSQI) (36); (6) Beck Depression Inventory-II (BDI-II) (37); (7) Pain Catastrophizing Scale (PCS) (38); (8) General Anxiety Disorder (GAD-7) (39); and (9) the Female sexual function index (FSFI) (40), males received a modified version. Using a predetermined list (41), patients were requested to report on adverse effects they could attribute directly to MC use at each follow-up time-point. AEs were later classified as serious or non-serious, according to the FDA's definition (42).
Phytocannabinoids Dose Assessment
Since the IMOH reform (43), MC cultivators in Israel are required to accurately indicate the THC and CBD concentrations in their products (30). We calculated the monthly doses of THC and CBD only for patients who reported fully on their MC treatment regimen, according to the products they reported consuming, based on their total and specific monthly doses. For example, if a patient reported consuming 10 g of THC10/CBD10 product and another 10 g of THC20/CBD4 product, the patient's calculated monthly consumption is 6,000 mg and 2,800 mg of THC and CBD, respectively. Notably, MC cultivators in Israel are not required to report on phytocannabinoids other than THC and CBD or terpenoids concentrations in their MC products.
Statistical Analysis
R software (V.4.0.4) with lme4 (44), atable (45), and tidyverse (46) packages were used to analyze changes in outcome measures by linear mixed-effect regression models to assess the duration effect of the treatment (47). Due to the heterogeneity of the study characteristics, only intention to treat (ITT) analyses were conducted. Due to the prospective, longitudinal data collection design, each of the time points had a different sample size and was analyzed with the corresponding baseline information. In cases that had no differences in the measures between time points, the text relates to T0 data. Chi-square or Kruskal-Wallis rank tests were conducted to establish the similarity of demographic data between the three follow-ups. For the effect of size and confidence interval (CI), a Cohen's d test was utilized. The Shapiro-Wilk test of normality demonstrated non-normal distribution for all measures. Thus, data are presented as median (IQR, Q1–Q3, i.e., quartiles 25 and 75). Differences were considered significant at the p < 0.05 level. Incidences are presented as numbers and percentages of patients. The required minimum sample size was calculated for the study by G*Power statistical analysis (48), accounting for the following: four time-point repeated measures analysis, within factor analysis, medium effect size (0.25), α ≤ 0.05, power of 0.80 and 14 observables (all measured parameters). Based on these, a sample size of 56 patients was determined as appropriate. Notably, due to the exploratory nature of the study and many potential subgroup analyses, no maximum sample size objective was determined.
Results
Sample
A total of 404 patients enrolled in the study following the acceptance of an MC license and obtaining pharmacy prescriptions. Of those, 80 (20%) were not eligible for further analyses due to the below-mentioned reasons (Figure 1, CONSORT flow diagram). Of the remaining 324 patients that initiated MC treatment and completed the baseline (T0) questionnaires, follow-up questionnaires were completed by 212 (at T1), 158 (T3), and 126 (T6) patients. The following reasons led to the decline in the number of participants over time: lost to follow-up [55 patients (17%)], ceased MC treatment due to ineffectiveness [24 patients (7%)], and due to MC-related AEs [36 patients (11%)], and due to no further need [e.g., chemotherapy-induced AEs stopped; 14 patients (4%)]. All of the patients that stopped MC treatment for the abovementioned reasons were alive at the 6-month follow-up period. Notably, 69 patients (21%) passed away during the follow-up period. About 98% of the patients provided data online and the rest by telephone calls.
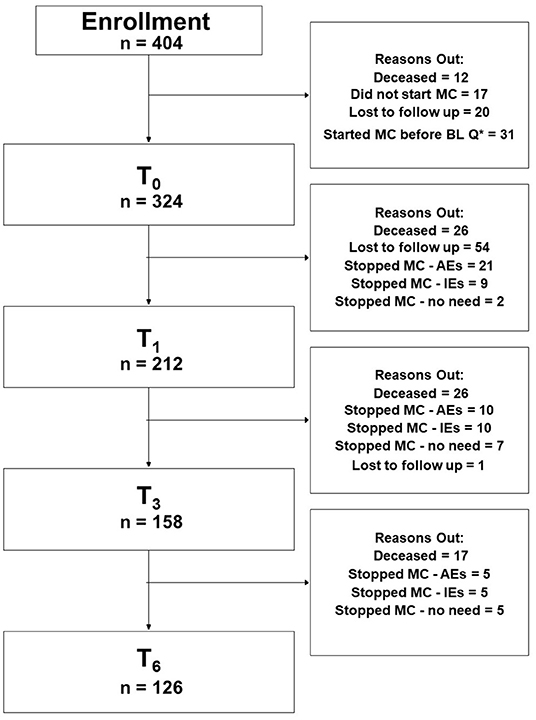
Figure 1. Consort flowchart diagram. MC, medical cannabis; Q*, questionnaire; BL, baseline; AEs, adverse effects; IEs, ineffectiveness; T0, baseline time point (prior to MC treatment initiation); T1, 1-month follow-up; T3, 3-month follow-up; T6, 6-month follow-up; PP analyses refer to patients that answered the study questionnaires at baseline and at all follow-up time points.
Sensitivity Analyses for Eligibility Criteria
Baseline demographic characteristics did not differ between eligible (n = 324) and non-eligible (n = 80) patients for age, gender, comorbidities, or overall analgesics consumption (Supplementary Table 2). Mentionable, eligible patients were more likely to have breast or colon cancer diagnoses than non-eligible patients.
Baseline Demographics and Cancer Characteristics
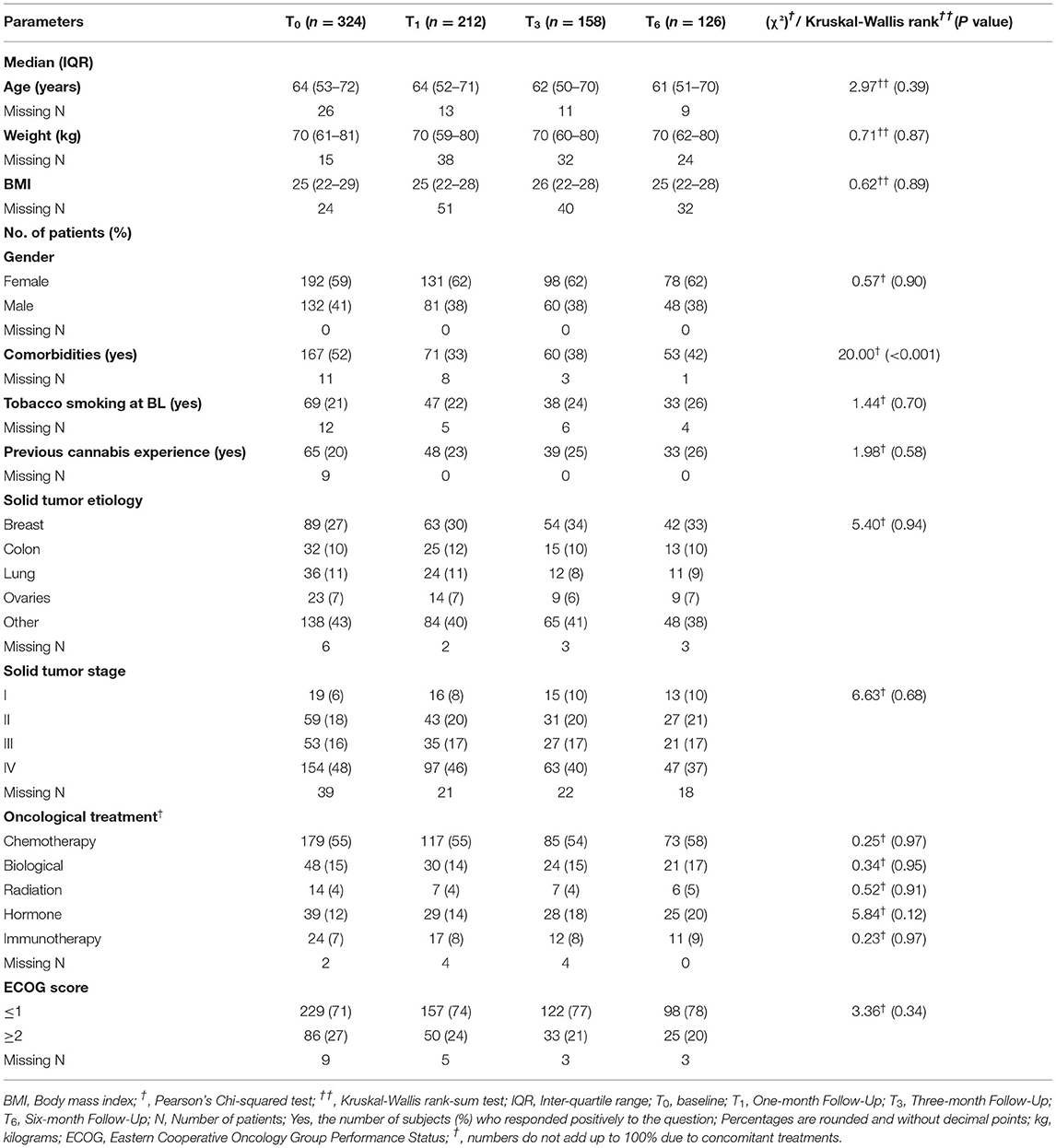
Patients in the sample were on average 64 (49–68) years old and the majority (59% n = 192) were females. Previous exposure to cannabis was reported by 20% (n = 65) (Table 1). Oncology diagnoses were diverse, with breast cancer being the most frequent diagnosis (n = 89, 27%), followed by colon, lung and ovarian cancers (n = 32, 10%, n = 36, 11%, and n = 23, 7%; respectively). Most patients (n = 154, 48%) were categorized as stage IV cancer. Chemotherapy was the most prevalent current treatment protocol (n = 179, 55%). Most patients (n = 229, 71%) were scored by their oncologist as not disabled (scored ≤ 1 based on Eastern Cooperative Oncology Group (ECOG) Performance Status score) (Table 1). Most demographic measures did not change during the 6 months follow-up in the ITT cohort. Notably, the rate of patients with comorbidities decreased from baseline (χ2(3) = 20.00, p < 0.001). More elaborated oncology diagnoses are presented in Supplementary Table 3.
MC Treatment Characteristics
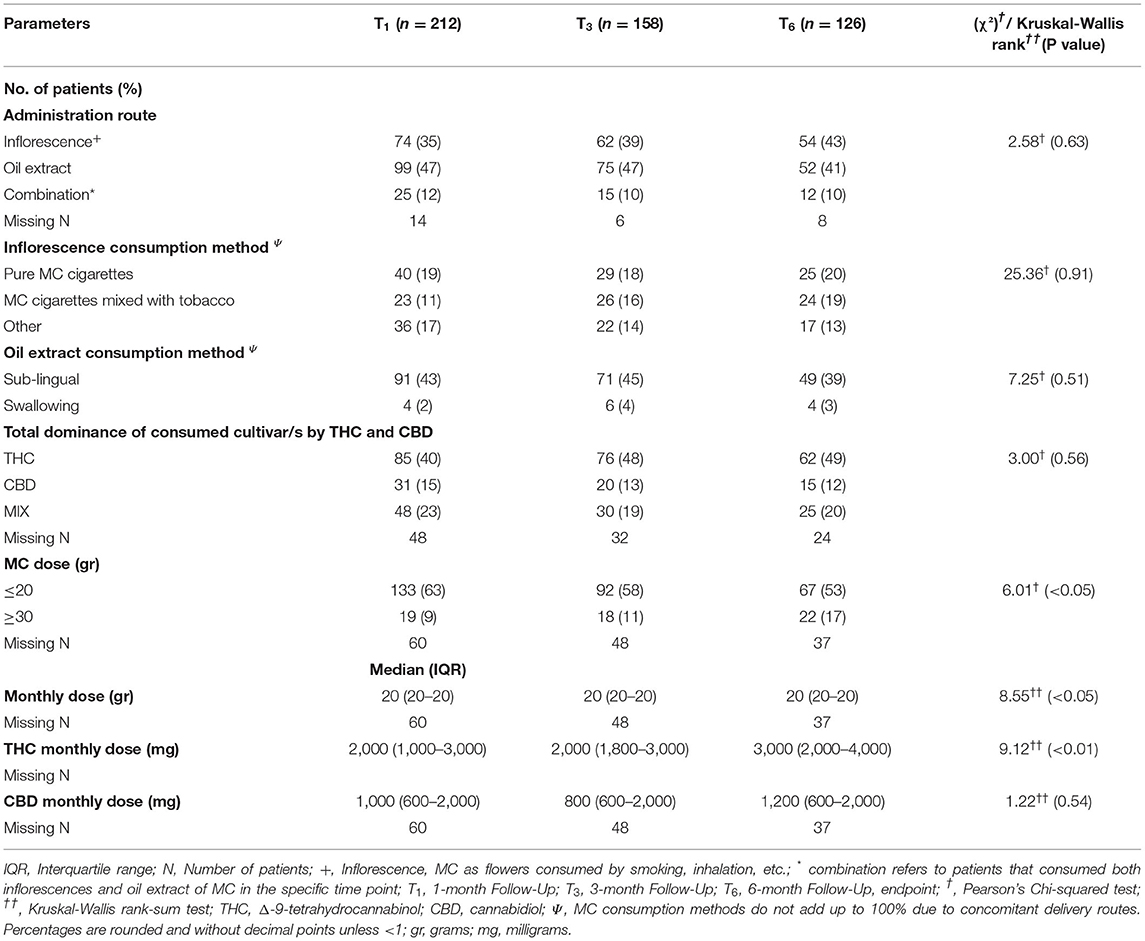
Most MC treatment measures did not differ significantly during the six-month treatment. At the endpoint, MC oil extract was the most common route of administration (n = 52, 41%), consumed mostly sublingually. Although total monthly MC dose remained stable at a median (IQR) of 20 (20) gr, there was a significant increase that can be observed by the Mean ± SD from 21 ± 6.4 gr at T1 to 23 ± 6.3 gr at T6 (χ2(2)=8.55, p < 0.05). THC-rich cultivars were consumed more frequently, with monthly doses of THC increasing from 2,000 (1,000–3,500) mg at T1 to 3,000 (2,000–4,000) mg at endpoint (χ2(2) = 3.12, p < 0.01). CBD monthly doses did not change significantly during the study (Table 2).
Pain Measures
Patients have been suffering from pain for 4 (2–4) months at T0. All pain measures improved from T0 at all the follow–up time points, as revealed by means of linear mixed regression model analyses. Mentionable are the following significant changes between T0 and T6 for patients that reached the endpoint: average weekly pain intensity reduced by a median of 20% and IQR of 0 to 50% from 7 (3–9) to 5 (3–7) (−0.98; 95%CI = −1.43 to −0.54; p < 0.001); least pain intensity declined by a median of 25% and IQR of 0% to 56% from 6 (3–8) to 5 (2–7) (−0.81; 95%CI= −1.40 to −0.21; p < 0.01) and worst pain intensity by a median of 20% and IQR of 0 to 43% from 8 (6–10) to 6 (4–8) (−1.78; 95%CI = −2.31 to −1.26; p < 0.001). The total SF–MPQ score dropped by a median of 7% and IQR of −17 to 45% from 23 (15–30) to 20 (10–27) (−4.74; 95%CI = −6.80 to −2.68; p < 0.001). Within the SF–MPQ, the affective pain components showed a reduction by a median of 20% and IQR of 0 to 56% from 6 (4–8) to 4 (2–7) (−2.08; 95% CI = −2.75 to −1.40; p < 0.001) and the sensory pain components by a median of 0% and IQR of −32 to 37% from 17 (10–22) to 16 (8–22) (−2.79; 95%CI = −4.36 to −1.23; p < 0.001). The full spectrum of responses for all pain measures is presented in Figure 2; the numbers and percentage of patients reporting positive (i.e., pain decrease), no change or negative (i.e., pain increase) responses at each time point are indicated. While most patients reported some degree of pain intensities decrease, about 20% of patients reported either no change in their pain intensity from baseline or on pain intensity increase. Mentionable, the rates of no change from baseline were higher (35–40%) for the sensory and affective pain intensities. Focusing on the most clinically important measures, the cumulative treatment response rates of average weekly pain intensity and total McGill score are presented in Figure 3. Notably, 36 and 33% of the cohort reported on ≥30% average pain intensity and total SF–MPQ score reduction at T6, respectively.
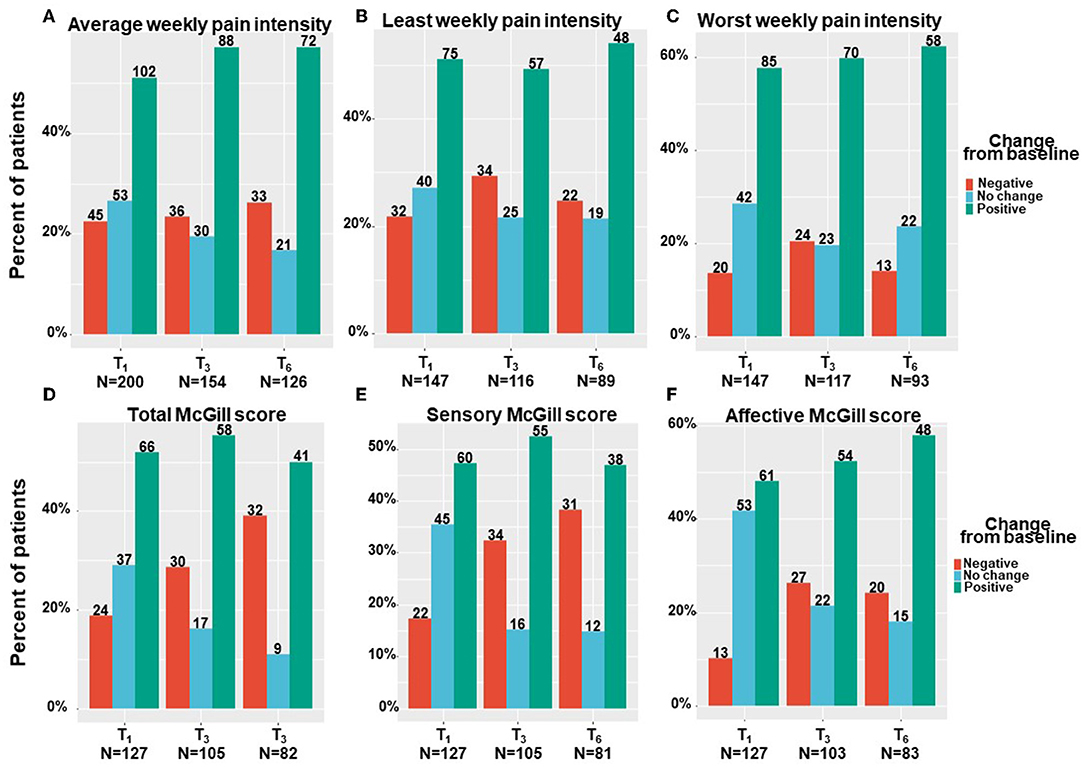
Figure 2. Pain measures change from baseline per time point. T1, 1-month Follow-Up; T3, 3-month Follow-Up; T6, 6-month Follow-Up: Negative indicate patients that reported on higher pain intensity at a follow-up compared to baseline; Positive indicate patients that reported on lower pain intensity at a follow-up compared to baseline; No change indicate patients that reported on the same pain intensity at a follow-up compared to baseline; Numbers of patients are based on patients that reported fully on the measures at baseline and at the corresponding follow-up time point.
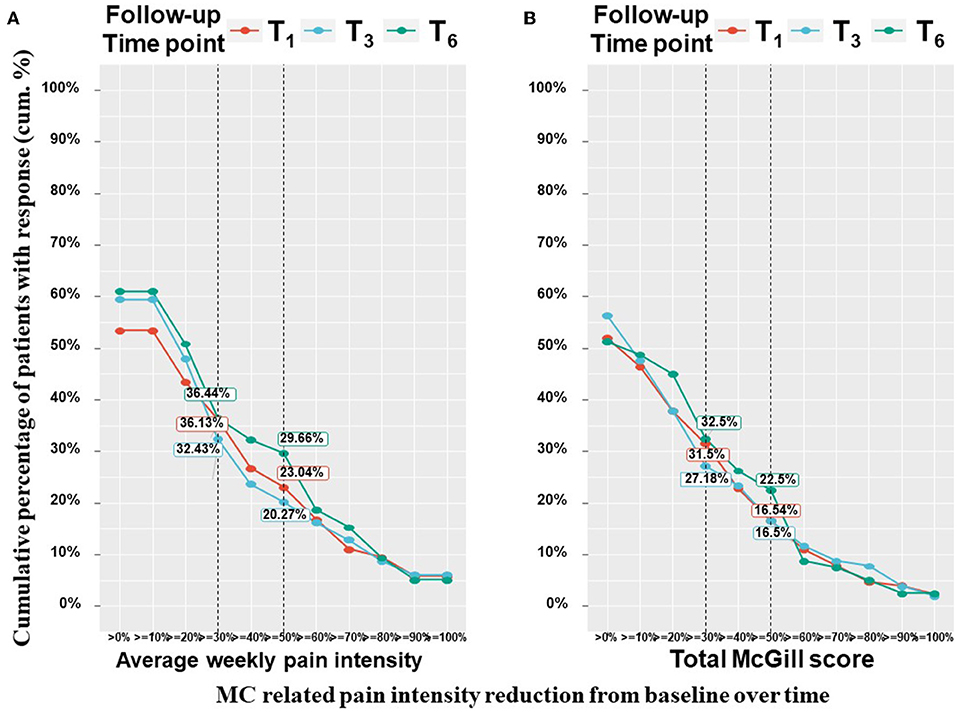
Figure 3. Pain measures cumulative treatment response rates per time point. T1, 1-month Follow-Up; T3, 3-month Follow-Up; T6, 6-month Follow-Up; MC, medical cannabis; The percentages on the Y-axis indicates the cumulative percentage of patients with a response; Every point on the X-axis represent the percentage of MC related pain intensity reduction from baseline, no change values or an increase of pain intensities are not represented in this figure; These figure displays percentages from the entire cohort (n = 212 at T1, n = 158 at T3, n = 126 at T6) but only positive (pain decrease) reports are visible.
Analgesics Consumption
Of the patients in the cohort that reported fully on their analgesic medications' consumption at T0 and T6 (n = 126), 40% of those (n = 30) who had been using analgesic medications (over-the-counter, non-steroidal anti-inflammatory drugs, opioids, anticonvulsants, and antidepressants) at T0 (n = 74), were no longer using them. Conversely, ten patients (20%) initiated analgesic medications at T6 while not consuming any at T0. Specifically, very few patients that survived to T6 consumed opioids at T0. When translated into morphine equivalent dose, Median (IQR) was 0 (0-0) at both time points.
Cancer Symptom Burden
The study's primary outcome measure, the cancer symptom burden (i.e., MSAS total score), decreased significantly from T0 to T6 by means of linear mixed regression model analyses. Cancer symptom burden decreased by a median of 18% and IQR of−22% worsening to 57% reduction from 122 (82–157) to 89 (45–138) (−18.98; 95%CI= −26.95 to −11.00; p < 0.001). The subscales of the MSAS questionnaire also improved significantly. Specifically, MSAS general distress index decreased by a median of 22% and IQR of −5% worsening to 54% reduction from 52 (34–66) to 34 (18–57) (−10.29; 95%CI= −13.50 to −7.08; p < 0.001), physiological index decreased by a median of 18% and IQR of −10% worsening to 60% reduction from 36 (24–52) to 23 (12–44) (−8.24; 95%CI= −11.05 to −5.43; p < 0.001) and the psychological index decreased by a median of 18% and IQR of −21% worsening to 51% reduction from 31 (17–41) to 22 (11–36) (−5.81; 95%CI= −7.98 to −3.64; p < 0.001). Figure 4A demonstrates the numbers and percentage of patients reporting positive change (e.g., total cancer symptom burden decrease), no change or negative (e.g., total cancer symptom burden increase) change at each time point. Notably, most patients (about 60%) reported a positive effect. Figure 4B demonstrates the cumulative treatment response rates of the total cancer symptom burden. Notably, almost 40% of the cohort reported on ≥30% total cancer symptom burden at T6.
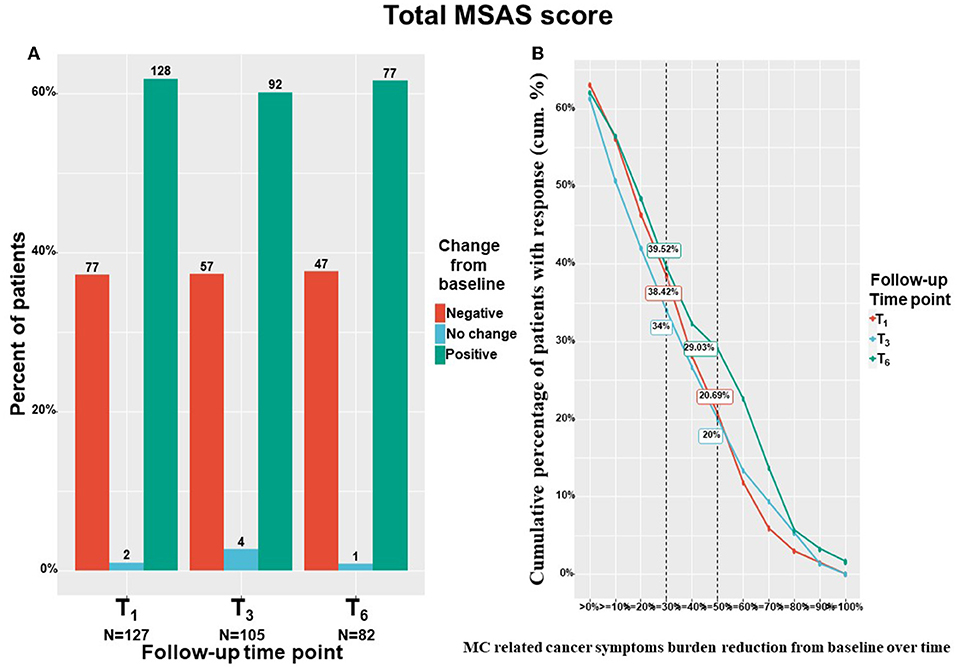
Figure 4. Total cancer symptom burden change from baseline. T1, 1-month Follow-Up; T3, 3-month Follow-Up; T6, 6-month Follow-Up: MSAS, Memorial Symptom Assessment Scale; (A) Negative indicate patients that reported on higher pain intensity at a follow-up compared to baseline; Positive indicate patients that reported on lower pain intensity at a follow-up compared to baseline; No change indicate patients that reported on the same pain intensity at a follow-up compared to baseline; MC, medical cannabis; The percentages on the Y-axis indicates the cumulative percentage of patients with response; For (B), every point on the X-axis represent the percentage of MC related pain intensity reduction from baseline, no change values or an increase of pain intensities are not represented in this figure; Numbers of patients are based on patients that reported fully on the measures at baseline and at the corresponding follow-up time point; (B) displays percentages from the entire cohort (n = 212 at T1, n = 158 at T3, n = 126 at T6) but only positive (MSAS score improvement) reports are visible.
Cancer Comorbid-Related Symptoms
By means of linear mixed regression model analyses, a significant decrease was found in anxiety levels, which decreased by a median of 22% and IQR of −14% worsening to 64% improvement from 9 (3–14) at T0 to 6 (2–11) at T6 (−2.35; 95%CI= −3.31 to −1.40; p < 0.001). Depression severity also decreased by a median of 12% and IQR of −24% worsening to 30% improvement from 19 (11–24) at T0 to 15 (10–22) at T6 (−1.97; 95%CI= −3.26 to −0.68; p < 0.001). Pain catastrophizing scores reduced by a median of 18% and IQR of −10% worsening to 45% improvement, from 30 (19–39) at T0 to 24 (12–33) at T6 (−5.44; 95%CI= −7.73 to −3.15; p < 0.001). Sleep disturbance scores decreased by 16% and IQR of 0% worsening to 43% improvement from 12 (7–15) at T0 to 8 (6–12) at T6 (−3.07; 95%CI = −3.95 to −2.18; p < 0.001). Finally, the quality–of–life score decreased (i.e., improved) significantly from T0 to T6 by a median of 14% and IQR of −18% worsening to 40% improvement reduction from 4 (3–5) to 4 (2–5) (−0.55; 95%CI= −0.83 to −0.27; p < 0.001). According to the IQR range, unlike most comorbid cancer–related symptoms, where the lower limit of the IQR percentage of change response was negative, the IQR sleep disturbance scores showed only positive changes (0–43%). Figure 5 demonstrates the numbers and percentage of patients reporting positive change (e.g., comorbid symptoms decrease), no change, or negative change (e.g., comorbid symptoms increase) at each time point. Notably, most patients (about 60%) reported a positive effect.
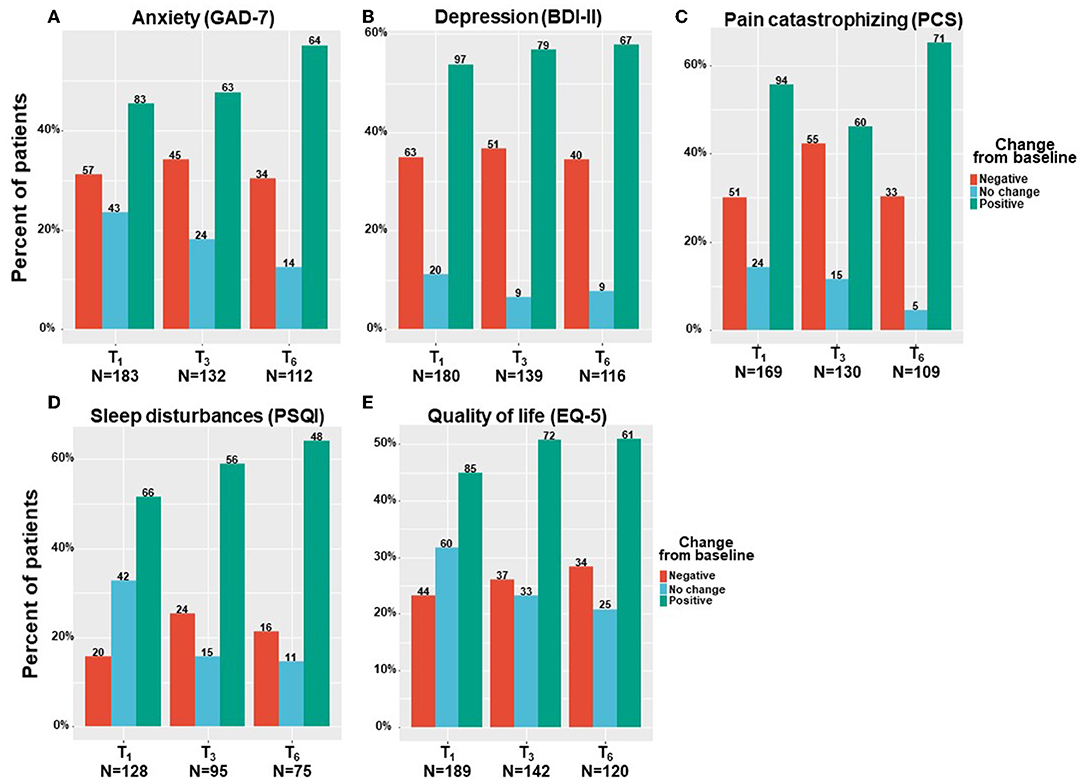
Figure 5. Cancer comorbid symptoms change from baseline. T1, 6-month Follow-Up; T3, 6-month Follow-Up; T6, 6-month Follow-Up: GAD-7, general anxiety disorder; BDI, Beck depression inventory; PCS, pain catastrophizing scale; PSQI, Pittsburgh sleep quality index; EQ-5, Euro-QoL questionnaire; Negative indicates patients that reported on higher comorbid symptoms at a follow-up compared to baseline; Positive indicate patients that reported on lower comorbid symptoms at a follow-up compared to baseline; No change indicate patients that reported on the same comorbid symptoms at a follow-up compared to baseline; Numbers of patients are based on patients that reported fully on the measures at baseline and at the corresponding follow-up time point.
Sexuality Problems
As described in the methods section, sexuality problems were assessed with specific and different validated questionnaires for females and males. After adjusting for the higher proportion of females in the sample, the response rate to the sexuality questionnaires between the genders was similar. Notably, the response rate to these questionnaires was very low (12–17%).
We found that males mainly reported on absolute improvement in their sexuality problems following MC treatment (Figure 6A), with scores increased by a median of 6% and IQR of 0% with no change to 29% improvement, from 7 (5–18) at T0 to 5 (5–26) at T6 (−2.39; 95%CI= −10.65 to 5.86; p = 0.52). On the contrary, females reported mainly on absolute worsening in their sexuality problems following MC treatment (Figure 6B), with scores reduced by a median of −2% and IQR of −51% worsening to 8% improvement, from 12 (5–39) at T0 to 21 (5–55) at T6 (6.99; 95%CI = −2.14 to 16.13; p < 0.001). Nonetheless, these changes during treatment were not significant for both sexes.
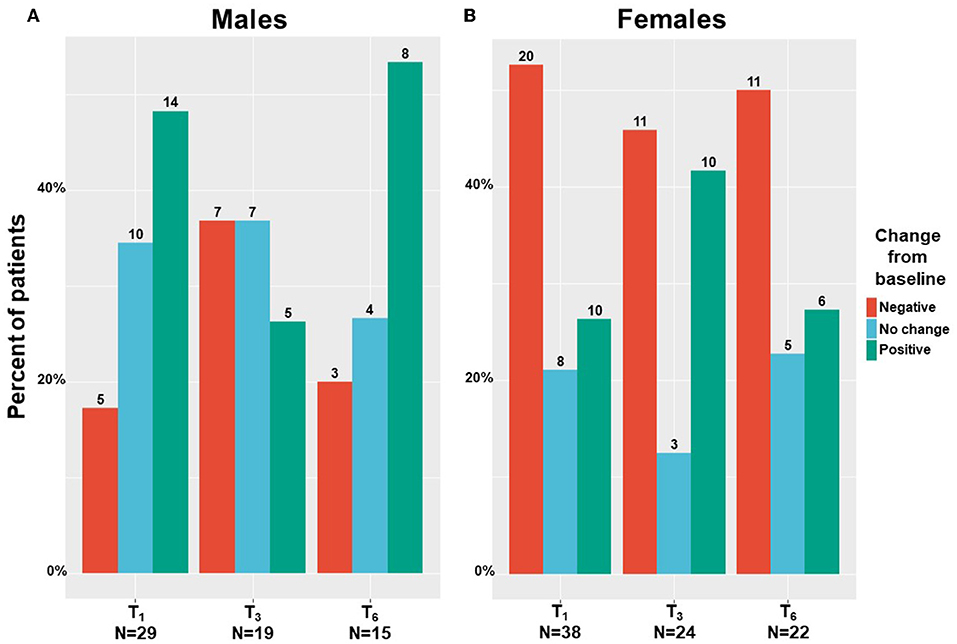
Figure 6. Sexuality problems change during medical cannabis between sexes. T1, 1-month Follow-Up; T3, 3-month Follow-Up; T6, 6-month Follow-Up; numbers on bars represent the number of patients that reported.
Weight Characteristics
Weight and body mass index (BMI) remained unchanged on average, but their ranges had a small change that was statistically significant, from 70 (60–80) kg and 25 (23–29) at T0 to 70 (62–80) kg and 25 (22–8) at T6 (-0.96; 95%CI= −1.83 to −0.10; p < 0.05 and −0.42; 95%CI = −0.73 to −0.10; p < 0.01, respectively).
Medical Cannabis Treatment Safety
Overall, by means of generalized linear mixed regression model analyses, we found 20%-30% of patients reported on AEs with no significant change across treatment duration, from T1 to T6 (0.47; 95%CI = −0.29 to 1.24; p = 0.22). These AEs were mainly non-serious according to FDA definition (42) and did not cause MC treatment discontinuation. The AEs of the affected systems in descending order of report rates were: central nervous systems (10–17%), gastrointestinal (7–10%), psychological (5–10%), ophthalmic (3–5%), musculoskeletal (3–6%), cardiovascular (1–3%) and auditory (2–4%), all showed no significant change across treatment duration (Figure 7). A total of 36 (11%) patients discontinued MC treatment due to MC-related AEs. The specifics of the AE were unknown for eight of them, the remainder were fatigue (n = 5), dizziness (n = 4), hallucinations (n = 4), bad taste (n = 3), drowsiness (n = 2), and abdominal pain, anxiety, cough, fainting, heat waves, hypotension, nausea, palpitations, restlessness and shortness of breath (one each). Cessation of treatment was made by patients alone, so no account for the severity of AEs could be done. We were able to verify that patients lost to follow-up did not pass away during the study period.
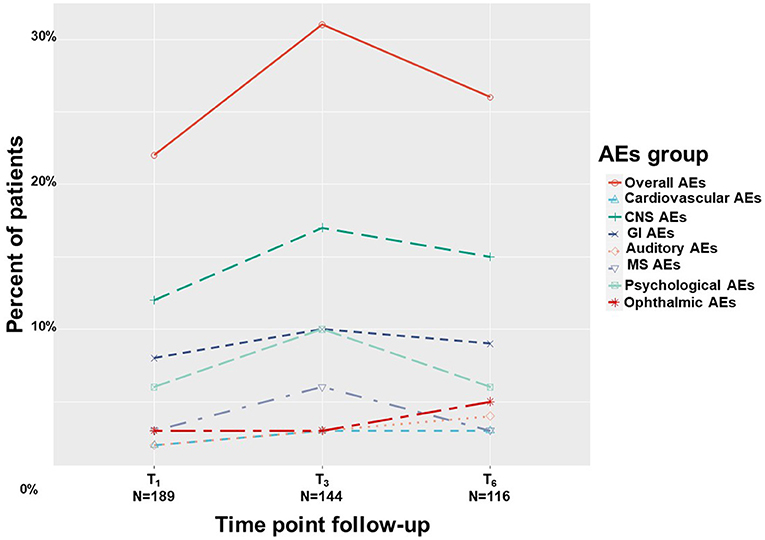
Figure 7. Medical cannabis-related adverse effects. T1, 1-month Follow-Up; T3, 3-month Follow-Up; T6, 6-month Follow-Up; AEs, adverse effects; Overall, at least one AE report; CNS, central nervous system; GI, gastrointestinal; MS, musculoskeletal.
Notably, 69 (21%) of the patients that initiated MC treatment passed away, while 255 patients survived during the 6-months follow-up period. Several significant differences were found between these groups, presented in Supplementary Table 4.
Hospitalizations
During an overall period of 9 months, the 3 months preceding T0, and between T0 to either T1, T3, or T6, there were n = 80, 25%, n = 14, 7%, n = 15, 10% and n = 25, 20%, hospitalizations due to surgeries, respectively, and n = 98, 30%, n = 26, 12%, n = 21, 13% and n = 14, 11%, hospitalizations due to other reasons, respectively. Most surgeries were performed in the 3 months preceding MC treatment initiation and included mostly solid tumor removals. At the follow-ups, most hospitalizations were also for the reason of solid tumor removal. Other reasons for hospitalizations included cancer, treatment diagnosis and oncology treatment complications. Notably, none of the hospitalizations were considered directly related to the MC treatment.
Discussion
There is a growing interest in studies on the effectiveness of an MC treatment for oncology patients (69). The main finding of the current study is that most cancer comorbid symptoms improved significantly during 6 months of MC treatment. The change in all pain measures was small. However, as placebo effect is mostly achieved within the first 4 to 12 weeks of treatment (70), it is likely that the changes from baseline found after 6 months are not the result of placebo.
Additionally, we found that MC treatment in cancer patients was well tolerated and safe. These findings align with a few previously published prospective studies on cancer patients (17, 71). The added value of the current analysis is the utilization of validated tools, precise MC treatment measurement, multiple follow-up time points and rigorous follow-up on reasons for dropout from the study. These allowed us to assess the real-life effectiveness and safety of MC treatment for palliative oncology patients.
In a previous report by our group (72), we reported the short-term effects of MC treatment. Some of the reported measures did not improve significantly (i.e., weekly least and worst pain intensities, pain catastrophizing, depression, quality of life and anxiety). We presumed that these parameters may require additional treatment duration for effects to be apparent due to their inherent nature. Indeed, most of the examined parameters were revealed as significant after 6 months. Another support for the long-term benefits of MC treatment is corroborated by a recent retrospective analysis that compared cancer patients with MC treatment to without, which demonstrated symptom relief to those with treatment (49). We presented additional, more comprehensive perspectives of the effects of MC on these measures, including comparisons between rates of patients reporting positive (e.g., improvement), no change or negative (e.g., worsening) rates at each time point, as well as cumulative treatment response that was indorsed by Farrar et al. (50) to make data from clinical studies more understandable. We demonstrated MC treatment was helpful for many oncology patients; however, additional studies are needed to better characterize those patients who could benefit from it. Nonetheless, although more than 50% of the patients reported a reduction in pain intensity, and while 40% of the patients discontinued analgesic medications, 2025% reported on pain intensity increase and 20% reported initiation of an analgesic medication following 6 months of MC treatment. These findings suggest either MC treatment was not equally effective in pain intensity reduction in all patients, some patients might have developed tolerance, or the progression of the oncological disease could not be managed by the previously stable analgesic treatment regimen.
Many of the measures that improved are associated with improved quality of life, which may suggest some of the effect of MC treatment on pain intensity was indirect, as previously discussed (51–53). Studies have shown that quality of life in patients that suffer from a severe illness such as cancer plays an important role in treatment adherence and success (54–56). Furthermore, the multifactorial effect on chronic pain comorbidities by measures such as quality of life, disability, sleep, anxiety and others were all previously suggested to indirectly affect the observed reduction in pain intensity (57–59).
As the prevalence of cancer diagnoses in our study was similar to previous studies conducted in Israel (17, 71) and to the general cancer prevalence in the public, it can be assumed that the current study findings could be generalized to oncology patients in Israel.
Medical cannabis has been previously reported as a possible remedy for cachexia and appetite loss (60–63). The current study found that the patients' weight and BMI did not change on average during the 6-month follow-ups, but the range did decrease significantly. As a substantial portion of the cohort was diagnosed with progressed cancer, a weight decline is expected with disease progression.
In the current study, almost half of the sample stopped all analgesic medications following 6 months of MC treatment. One explanation for this could be that MC constituted a substitution analgesic (64, 65). Indeed, previous prospective studies have demonstrated similar findings in chronic non-cancer pain cohorts (57, 58), and in a survey of gynecologic cancer patients, almost half reported that they decreased opioids following MC initiation (66). Another explanation is that the disease of patients that survived the 6 months of MC treatment was milder; they had fewer comorbidities and might also be cancer-free by the endpoint.
In the extended period of 6 months, there were mostly non-serious AEs with no significant change from those at the one-month checkpoint, the most frequent being dizziness and tiredness. This finding aligns with previous studies (57, 71), suggesting these AEs can be attributed to the MC treatment and not to the disease itself. While earlier results of Aviram et al. (57) reported a decrease in MC-related AEs during treatment for chronic non-cancer pain, in the current study of palliative oncology patients, the AEs remained stable during the 6 months of the study. This finding may be attributed to the relatively stable MC treatment dose (20 gr for most patients) in the current study, contrary to a significant dose increase in Aviram's research and a different frequent administration route. Notably, it may be suggested that in the 6 months of MC treatment, patients did not need to increase the dose for MC treatment to be as effective as at the beginning of the treatment. Notwithstanding, in the current study, the monthly dose of THC increased during the 6 months treatment. Similar long-term safety was previously demonstrated in cancer patients (67). It is plausible that THC dose increase in the current study was not associated with elevation of AEs rates because it is unrelated or that patients that started consuming MC products with higher THC concentration are those less susceptible to its effects, as was demonstrated in a study on the sex differences of MC related AEs (68). Nonetheless, we found that THC monthly dose increased significantly in the 6 months of MC treatment from a Median (IQR) of 2,000 mg (1,000–3,000) to 3,000 mg (2,000–4,000). This increase can be explained by the increase in the rate of patients consuming THC-rich MC from 40 to 49%. It is possible patients developed tolerance to THC or that oncologists tread more cautiously at the beginning of the treatment (start low, go slow), starting the dose adjustment from lower THC concentrations and increasing as the treatment progress according to the suggested treatment protocol (73).
Limitations
This study has a few limitations. First, no control or placebo groups were assigned, and it is hard to isolate a placebo response from a “true” drug effect. Hence, a prudent interpretation of the results is needed. Second, though only validated questionnaires were utilized and patient responses were kept anonymous from their physician, self-report bias may have still occurred. Third, although advanced statistical approaches for missing data imputation were used, they do not entirely protect the results from this shortcoming (47). Fourth, although the current study presents accurate AEs reports, it is possible that an additional unknown number of patients who were lost to follow-up might have discontinued MC use due to AEs. Finally, due to the sizeable dropout rate of 61% at the endpoint, some survival bias can be seen in most measures, because as time progressed patients showed higher response rates for most measures.
Conclusions
In conclusion, this prospective, comprehensive and large-scale cohort demonstrated an overall mild to modest long-term statistical improvement of all investigated measures including pain, associated symptoms and, importantly, reduction in opioid (and other analgesics) use. It seems that MC treatment is safe for oncology patients, but its efficacy and clinical relevance may be limited. Oncologists should carefully consider the possible benefits of MC treatment to their patients before prescribing it.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional Ethics Committee of Haemek Medical Center (#0137-18-EMC) and Galil Medical Center (#0010-19-NHR) approved the study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JA, GL, GB-S, and DM: conceptualization. JA, AO, IC, AL, MAA, GB-S, LA, DG, NM, AK, and AS: data curation. JA and YV: formal analysis and validation. DM: funding acquisition and resources. JA and DM: investigation. JA, GL, GB-S, YV, and DM: methodology. GL and DM: project administration and supervision. JA, YV, SP, and DM: visualization. JA: writing—original draft. JA, GL, YV, AO, SP, IC, AL, MAA, GB-S LA, DG, NM, AK, AS, and DM: writing—review & editing. All authors participated in data collection, discussed the results, and commented on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the Evelyn Gruss Lipper Charitable Foundation. This sponsor had no role or influence on the research or this submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2022.861037/full#supplementary-material
References
1. Portenoy RK, Ebtesam A. Cancer pain syndromes. Hematol Oncol Clin North America. (2018) 32:371–86. doi: 10.1016/j.hoc.2018.01.002
2. Curran L, Sharpe L, Butow P. Anxiety in the context of cancer: a systematic review and development of an integrated model. Clin Psychol Rev. (2017) 56:40–54. doi: 10.1016/j.cpr.2017.06.003
3. Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. (2017) 52:58–70. doi: 10.1016/j.ctrv.2016.11.004
4. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. (2001) 19:895–908. doi: 10.1200/JCO.2001.19.3.895
5. Allart-Vorelli P, Porro B, Baguet F, Michel A, Cousson-Gélie F. Haematological cancer and quality of life: a systematic literature review. Blood Cancer J. (2015) 5:e305–e305. doi: 10.1038/bcj.2015.29
6. Neo J, Fettes L, Gao W, Higginson IJ, Maddocks M. Disability in activities of daily living among adults with cancer: a systematic review and meta-analysis. Cancer Treat Rev. (2017) 61:94–106. doi: 10.1016/j.ctrv.2017.10.006
7. Rhoten BA. Head and neck cancer and sexuality. Cancer Nurs. (2016) 39:313–20. doi: 10.1097/NCC.0000000000000289
8. Levit L, Balogh, E, Nass, S, Ganz, PA,. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis Committee on Improving the Quality of Cancer Care. Washington, DC: National Academies Press. (2013). Available online at: http://www.nap.edu. doi: 10.17226/18359
9. Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain - an overview of cochrane reviews (Review). Cochrane Datab System Rev. (2017) 7:1–23. doi: 10.1002/14651858.CD012592
10. Noyes R, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. J Clin Pharma. (1975) 18:84–9. doi: 10.1002/cpt197518184
11. Noyes R, Brunk S, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharma. (1975) 15:139–43. doi: 10.1002/j.1552-4604.1975.tb02348.x
12. Jochimsen PR, Lawton RL, VerSteeg K, Noyes R. Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Int J Clin Pharmacol Ther. (1978) 24:223–27. doi: 10.1002/cpt1978242223
13. Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Int J Clin Pharmacol Ther. (1978) 23:397–401. doi: 10.1002/cpt1978234397
14. Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. (2012) 13:438–49. doi: 10.1016/j.jpain.2012.01.003
15. Fallon MT, Lux EA, Mcquade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. (2017) 11:119–33. doi: 10.1177/2049463717710042
16. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. (2014) 47:166–73. doi: 10.1016/j.jpainsymman.2013.02.018
17. Bar-Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E. The medical necessity for medicinal cannabis: prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. In: Evidence-Based Complementary and Alternative Medicine : ECAM 2013. (2013). p. 510392. doi: 10.1155/2013/510392
18. Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal thc spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. (2013) 46:207–18. doi: 10.1016/j.jpainsymman.2012.07.014
19. Anderson S, Zylla D, McGriff D, Arneson T. Impact of medical cannabis on patient-reported symptoms for patients with cancer enrolled in minnesota's medical cannabis program. J Oncol Pract. (2019) 15:e338–45. doi: 10.1200/JOP.18.00562
20. Brown M, Farquhar-Smith P. Cannabinoids and cancer pain: a new hope or a false dawn? Eur J Internal Med. (2018) 49:30–6. doi: 10.1016/j.ejim.2018.01.020
21. Häuser W, Welsch P, Klose P, Radbruch L, Fitzcharles MA. Efficacy, Tolerability and Safety of Cannabis-Based Medicines for Cancer Pain : A Systematic Review with Meta-Analysis of Randomised Controlled Trials. (Berlin, Germany: Schmerz). (2019). doi: 10.1007/s00482-019-0373-3
22. Boland EG, Bennett MI, Allgar V, Boland JW. Cannabinoids for adult cancer related pain: systematic review and meta-analysis. BMJ Support. Palliative Care. (2020) 10:14–24. doi: 10.1136/bmjspcare-2019-002032
23. Coyne Z, Cowzer D, Hennessy M, Linehan A, Hennessy BT, Grogan W, et al. Cannabis and cancer: examining the use and perceived benefits in an irish cancer cohort. J Clin Oncol. (2020) 38:e24178–e24178. doi: 10.1200/JCO.2020.38.15_suppl.e24178
24. Darkovska-Serafimovska M, Serafimovska T, Arsova-Sarafinovska Z, Stefanoski S, Keskovski Z, Balkanov T. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J Pain Res. (2018) 11:837. doi: 10.2147/JPR.S160556
25. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. (2019) 24:549–54. doi: 10.1634/theoncologist.2018-0383
26. Bar-Sela G, Cohen I, Campisi-Pinto S, Lewitus GM, Oz-Ari L, Jehassi A, et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers. (2020) 12:2447. doi: 10.3390/cancers12092447
27. Gilklich RE, Dreyer N, Leavy M. Registries evaluating patient outcomes, agency for healthcare, research and quality (AHRQ). in Agency for Healthcare Research and Quality. Rockville, MD. (2010). Available online at: http://www.ahrq.gov (accessed May 12, 2022).
28. Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. (2008) 94:151–57. doi: 10.1016/j.drugalcdep.2007.11.003
29. Portenoy RK, Farrar JT, Backonja M, Cleeland CS, Yang K, Friedman M, et al. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin J Pain. (2007) 23:287–99. doi: 10.1097/AJP.0b013e31802b582f
30. Landshaft Y, Albo B, Mechoulam R, Afek A. The Updated Green Book (2019): The Official Guide to Clinical Care in Medical Cannabis (IMCP-GCP). IMOH Website. (2019). Available online at: https://www.health.gov.il/hozer/mmk154_2016.pdf (accessed May 12, 2022).
31. Qualtrics LLC. Qualtrics (Version 12018). Provo, Utah, USA. (2015). Available online at: http://Www.Qualtrics.Com (accessed May 12, 2022).
32. Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer. (1990) 65:1864–66. doi: 10.1002/1097-0142(19900415)65:8<1864::AID-CNCR2820650832>3.0.CO;2-U
33. Webber K, Davies AN. Validity of the memorial symptom assessment scale-short form psychological subscales in advanced cancer patients. J Pain Symptom Manage. (2011) 42:761–67. doi: 10.1016/j.jpainsymman.2011.02.007
34. Melzack R. The short-form mcgill pain questionnaire. Pain. (1987) 30:191–97. doi: 10.1016/0304-3959(87)91074-8
35. Brooks R, EuroQol Group. EuroQol: the current state of play. Health Policy. (1996) 37:53–72. doi: 10.1016/0168-8510(96)00822-6
36. Shochat T, Tzischinsky O, Oksenberg A, Peled R. Validation of the pittsburgh sleep quality index hebrew translation (PSQI-H) in a sleep clinic sample. Israel Med Assoc J. (2007) 9:853–56.
37. Beck A, Robert T, Steer A, Brown GK. Beck Depression Inventory-II. San Antonio. (1996) 78:490–98. doi: 10.1037/t00742-000
38. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7:524. doi: 10.1037/1040-3590.7.4.524
39. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–97. doi: 10.1001/archinte.166.10.1092
40. Rosen C, Brown J, Heiman S, Leiblum C, Meston R, Shabsigh D. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marit Ther. (2000) 26:191–208. doi: 10.1080/009262300278597
41. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. (2017) 20:E755–96. doi: 10.36076/ppj.20.5.E755
42. Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the food and drug administration, 1998-2005?. Arch Internal Med. (2007) 167:1752–59. doi: 10.1001/archinte.167.16.1752
43. Sznitman SR. Trends in medical cannabis licensure, israel, 2013 – 2018. Drug Alcohol Rev. (2020) 39:763–7. doi: 10.1111/dar.13116
44. Bates D, Maechler M, Bolker B, Walker S. Lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.7. In: Comprehensive R Archive Network (CRAN). (2014) Available online at: CRANR-projectorg/package=lme4.
45. Ströbel A, Haynes A. R Package Atable: Create Tables for Reporting Clinical Trials. Version 0.1.5. Comprehensive R Archive Network (CRAN). In: Comprehensive R Archive Network (CRAN). (2019). doi: 10.32614/RJ-2019-001 Available online at: https://cran.r-project.org/web/packages/atable/atable.pdf (accessed May 12, 2022).
46. Wickham H. Tidyverse: Easily Install and Load'Tidyverse Packages. Version 1.3.0. In: Comprehensive R Archive Network (CRAN). (2019). Available online at: https://cran.r-project.org/web/packages/tidyverse/tidyverse.pdf (accessed May 12, 2022).
47. Gewandter JS, McDermott MP, McKeown A, Smith SM, Williams MR, Hunsinger M, et al. Reporting of missing data and methods used to accommodate them in recent analgesic clinical trials: ACTTION systematic review and recommendations. Pain. (2014) 155:1871–77. doi: 10.1016/j.pain.2014.06.018
48. Faul F, Erdfelder E, Albert-Georg L, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
49. Pawasarat IM, Schultz EM, Frisby JC, Mehta S, Angelo MA, Hardy SS, et al. The efficacy of medical marijuana in the treatment of cancer-related pain. J Palliat Med. (2020) 23:809–16. doi: 10.1089/jpm.2019.0374
50. Farrar JT, Dworkin RH, Max MB. Use of the cumulative proportion of responders analysis graph to present pain data over a range of cut-off points : making clinical trial data more understandable. J Pain Symptom Manage. (2006) 31:369–77. doi: 10.1016/j.jpainsymman.2005.08.018
51. Schlag AK, Zafar R, Nutt D. Medical cannabis and epilepsy in the UK – a qualitative analysis of the carers' perspective: ‘we're asking for quality of life for our children.' Drug Sci Policy Law. (2021) 7:205032452110349. doi: 10.1177/20503245211034930
52. Nutt DJ, Phillips LD, Barnes MP, Brander B, Curran HV, Fayaz A, et al. A multicriteria decision analysis comparing pharmacotherapy for chronic neuropathic pain, including cannabinoids and cannabis-based medical products. Cannabis Cannab Res. (2021) 1–19. doi: 10.1089/can.2020.0129
53. Aviram J, Lewitus GM, Vysotski Y, Berman P, Shapira A, Procaccia S, et al. Sex differences in medical cannabis related adverse effects. Pain. (2021) 163:975–83. doi: 10.1097/j.pain.0000000000002463
54. Bottomley A. The cancer patient and quality of life. Oncologist. (2002) 7:120–25. doi: 10.1634/theoncologist.7-2-120
55. Kaplan RM. The significance of quality of life in health care. Quality Life Res. (2003) 12:3–16. doi: 10.1023/A:1023547632545
56. Sitlinger A, Zafar S. Health-related quality of life: the impact on morbidity and mortality. Physiol Behav. (2011) 176:139–48. doi: 10.1016/j.soc.2018.05.008
57. Aviram J, Pud D, Gershoni T, Schiff Keren B, Ogintz M, Vulfsons S, et al. Medical cannabis treatment for chronic pain: outcomes and prediction of response. Eur J Pain. (2020) 25:359–74. doi: 10.1002/ejp.1675
58. Haroutounian S, Meidan R, Davidson E. The effect of medicinal cannabis on pain and quality of life outcomes in chronic pain: a prospective open-label study. Clin J Pain. (2016) 32:1036–43. doi: 10.1097/AJP.0000000000000364
59. Yassin M, Garti A, Robinson D. Effect of medicinal cannabis therapy (MCT) on severity of chronic low back pain, sciatica and lumbar range of motion. Int J Anesthesiol Pain Med. (2016) 2:1–6. doi: 10.33140/JAPM/01/02/00006
60. Gorter RW. Cancer cachexia and cannabinoids. Complem Med Res. (1999) 6:21–2. doi: 10.1159/000057152
61. Wang J, Wang Y, Tong M, Pan H, Li D. Medical cannabinoids for cancer cachexia: a systematic review and meta-analysis. BioMed Res Int. (2019) 1–6. doi: 10.1155/2019/2864384
62. Whiting PF, Wolff RF, Deshpande S, Nisio MD, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. J Am Med Assoc. (2015) 313:2456–73. doi: 10.1001/jama.2015.6358
63. Rosager EV, Møller C, Sjögren M. Treatment studies with cannabinoids in anorexia nervosa: a systematic review. Eat Weight Diso. (2020) 26:407–15. doi: 10.1007/s40519-020-00891-x
64. Stith SS, Vigil JM, Adams IM, Reeve AP. Effects of legal access to cannabis on scheduled ii–v drug prescriptions. J Am Med Dir Assoc. (2017) 19:59–64. doi: 10.1016/j.jamda.2017.07.017
65. Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain. (2018) 19:37. doi: 10.1186/s10194-018-0862-2
66. Blake EA, Ross M, Ihenacho U, Figueroa L, Silverstein E, Flink D, et al. Non-prescription cannabis use for symptom management amongst women with gynecologic malignancies. Gynecol Oncol Rep. (2019) 30:100497. doi: 10.1016/j.gore.2019.100497
67. Vigano A, Aprikian S, Kasvis P, Bacis V, Al Harrasi A, Aubin NM, et al. Safety and effectiveness of medical cannabis as a complementary option for supportive cancer care: results from the cannabis pilot project. J Clin Oncol. 38:12106–12106. doi: 10.1200/JCO.2020.38.15_suppl.12106
68. Aviram J, Lewitus GM, Vysotski Y, Yellin B, Berman P, Shapira A, et al. Prolonged medical cannabis treatment is associated with quality of life improvement and reduction of analgesic medication consumption in chronic pain patients. Front Pharmacol. (2021) 12:1–14. doi: 10.3389/fphar.2021.613805
69. Goyal S, Kubendran S, Kogan M, Rao YJ. High expectations : the landscape of clinical trials of medical marijuana in oncology. Complement Ther Med. (2020) 49:1–7. doi: 10.1016/j.ctim.2020.102336
70. Enck P, Klosterhalfen S. Placebos and the placebo effect in drug trials. In: J Barrett and C Page eds. Concepts and Principles of Pharmacology. Handbook of Experimental Pharmacology. Cham: Springer. (2019). doi: 10.1007/164_2019_269
71. Schleider LB, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, et al. Prospective Analysis of Safety and Efficacy of Medical Cannabis in Large Unselected Population of Patients with Cancer. Eur J Intern Med. (2018) 49:37–43. doi: 10.1016/j.ejim.2018.01.023
72. Aviram J, Lewitus GM, Vysotski Y, Uribayev A, Procaccia S, Cohen I, et al. Short-term medical cannabis treatment regimens produced beneficial effects among palliative cancer patients. Pharmaceuticals. (2020) 13:1–16. doi: 10.3390/ph13120435
Keywords: medical use, cannabis, phytocannabinoids, oncology, cancer, prospective
Citation: Aviram J, Lewitus GM, Vysotski Y, Amna MA, Ouryvaev A, Procaccia S, Cohen I, Leibovici A, Akria L, Goncharov D, Mativ N, Kauffman A, Shai A, Bar-Sela G and Meiri D (2022) The Effectiveness and Safety of Medical Cannabis for Treating Cancer Related Symptoms in Oncology Patients. Front. Pain Res. 3:861037. doi: 10.3389/fpain.2022.861037
Received: 24 January 2022; Accepted: 25 April 2022;
Published: 20 May 2022.
Edited by:
Haggai Sharon, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Luigi Cardia, University of Messina, ItalyAnne Katrin Schlag, Imperial College London, United Kingdom
Copyright © 2022 Aviram, Lewitus, Vysotski, Amna, Ouryvaev, Procaccia, Cohen, Leibovici, Akria, Goncharov, Mativ, Kauffman, Shai, Bar-Sela and Meiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Meiri, ZG1laXJpQHRlY2huaW9uLmFjLmls; Gil Bar-Sela, Z2lsX2JhQGNsYWxpdC5vcmcuaWw=
 Joshua Aviram
Joshua Aviram Gil M. Lewitus1
Gil M. Lewitus1 Idan Cohen
Idan Cohen Gil Bar-Sela
Gil Bar-Sela David Meiri
David Meiri