
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health , 02 November 2022
Sec. Oral Epidemiology
Volume 3 - 2022 | https://doi.org/10.3389/froh.2022.938405
This article is part of the Research Topic Impact of Uncontrolled Diabetes on Oral Disease Progression and Healing View all 7 articles
Purpose: Dental caries is a significant public health issue affecting both the diabetic and nondiabetic populations. However, the problem and associated factors of dental caries among diabetics and nondiabetics patients are not well-known in Ethiopia. This study aims to compare the prevalence of dental caries and associated factors among diabetic and nondiabetic patients at the Outpatient Department of Bichena Primary Hospital in Northwest, Ethiopia.
Methods: Institutional based, comparative cross-sectional study was conducted from October 7 to December 6, 2019, among 200 diabetes and 400 nondiabetic adult patients. A consecutive sampling technique was implemented to recruit study participants. Data were collected by a pretested structured questionnaire and analysis was performed in Statistical Package for Social Science version 20. Bivariable and multivariable logistic regressions were employed and variables with a p-value < 0.05 were declared statistically significant.
Results: A total of 582 patients were involved in the study with a response rate of 97.0%. The prevalence of dental caries was 67.9% [95% confidence interval (CI): 63.2%–72.8%] and 79.6% (95% CI: 74.0%–85.70%) in nondiabetic and diabetic group, respectively. Females gender [adjusted odds ratio (AOR) = 1.79, 95% CI: 1.15–2.77], poor oral hygiene (AOR = 2.95, 95% CI: 1.71–5.11), lack of regular teeth cleaning habits (AOR = 3.26, 95% CI: 2.13–4.97), feeling dry mouth (AOR = 2.31, 95% CI: 1.11–4.81), sugared tea drinking (AOR = 2.00, 95% CI: 1.18–3.38), inadequate oral health knowledge (AOR = 3.51, 95% CI: 2.19–5.62), and khat chewing (AOR = 2.14, 95% CI: 1.24–3.71) were significantly associated factors with high prevalence of dental caries.
Conclusion: The prevalence of caries was significantly higher among diabetics than nondiabetics. Oral health education with preventive measures such as improving teeth cleaning practice, reducing sugary foods and drinks intake, and improving oral hygiene practice of patients should be mainstreamed along with diabetic follow-up care.
Dental caries is the localized destruction of susceptible dental hard tissues by acidic by-products from the bacterial fermentation of dietary carbohydrates (1). Dental caries is the most common chronic illness in the world (2, 3), which affects almost half of the world's population (44%) (4, 5). It is also the most prevalent oral condition, with 2.3 billion individuals affected, according to the 2015 Global Burdens of Disease Study (6).
People are at risk of dental caries throughout their lifetimes (1, 5); in particular, diabetic people are at a higher risk of developing caries because of hypo salivation and high salivary glucose levels (7). There is a high correlation between high blood glucose levels and high salivary glucose levels in diabetic patients (8).
Low-income countries have the highest dental caries burden, most of which go untreated (5, 9). The consequences of untreated dental caries can be pain, chewing problems, broken teeth, tooth abscesses, infection, and tooth loss. All of these consequences may lead to recurrent antibiotic prescriptions, distress, and sleep disturbance (10, 11). It also affects eating, learning, communication skills, work performance, recreational activities, growth, and development, and has a long-term psychological effect on the affected patients (12, 13).
Dental caries is becoming more prevalent in Africa (14), with rates reaching 86.63% in Egypt (15), 83.7% in Uganda (16), and 67.9% in Eritrea (17). In Ethiopia, the prevalence of dental caries among adults ranged from 31.5% to 78.2% (18, 19). There is also a difference across Ethiopia, with the uppermost prevalence reported in the Tigray region (46.59%) and the bottom prevalence reported in Addis Ababa (34.20%). The overall prevalence of dental caries in Ethiopia was reported to be 40.98% (20). Dental caries prevalence among diabetic patients has been reported to be 78.9% in India (21), 84.49% in Pakistan (22), and 67% in China (23).
Previous studies reported that age, sex, body mass index, educational level, socioeconomic status, poor oral hygiene, poor tooth cleaning habits, and increasing consumption of sugary food have an impact on dental caries experience (15, 24–26), whereas Diabetic patients, possible risk factors include age, sex, socioeconomic status, feeling of dry mouth during meals (xerostomia), poor dietary control, and inadequate knowledge about their increased risk for oral health illness (22, 27).
Dental caries is a preventable disease (28). World Health Organization (WHO) recommends eating a healthy diet, attending regular dental check-ups, cleaning teeth twice a day with fluoride toothpaste, and avoiding sweetened foods and drinks for dental caries prevention (2, 4, 5).
Dental caries prevalence continues to increase in the African region due to growing consumption of free sugars and inadequate exposure to fluoride (2). Similarly, a study conducted in Ethiopia reported a 78.2% prevalence of dental caries among adults (19). Although research studies in several countries show a high prevalence of dental caries in diabetic patients, there is no research on dental caries in diabetic patients in Ethiopia. In addition, studies conducted in other areas of the country had study populations from dental clinics only. Further, the study includes factors like socioeconomic status using wealth index, oral hygiene status using oral hygiene index-simplified, and body mass index, which are keys for dental caries development. These risk factors are not properly measured using their indexes in previous studies. Therefore, this study aimed to assess the prevalence of dental caries and its associated factors among diabetic patients and nondiabetic patients in Bichena Primary Hospital, 2019.
An Institutional based comparative cross-sectional study was conducted from October 7, 2019, to December 6, 2019. This study was conducted at Bichena primary hospital outpatient department, located about 265 km away from Addis Ababa, in Bichena town, East Gojjam, Amhara, Ethiopia. It has been functional since April 2015. The catchment population of the hospital reaches 450,000 people. The hospital provides different inpatient and outpatient services including dental health services and follow-up health services to the population in the surrounding area of Enemay woreda and the nearby districts including Enarj Eawga woreda, Debay Tilat Gin woreda, and Shebel Berenta Woreda.
All diabetic and nondiabetic adult patients, who were utilizing health services at Bichena Primary Hospital outpatient department in the last 1 year.
For diabetic patients: Those diabetic patients who were available during the data collection time during routine working hours.
For nondiabetics: Those patients who were available during the data collection time during working hours in the outpatient department.
Age greater than or equal to 18 years old.
Known diabetic patients.
Those individuals with random blood sugar levels less than 200 mg/dl or fasting blood sugar less than 126 mg/dl for nondiabetic patients.
Those who are unable to respond to interviews like acute psychosis patients.
Those who have any physical disorders not permitting oral examination like temporomandibular joint disorders.
Individuals suffering from systemic illness, pregnant mothers.
Unwillingness to participate.
The outcome variable was dental caries among diabetic and nondiabetic adult patients. On the contrary, the explanatory variables were age, sex, body mass index, religion, educational status, marital status, occupation, residence, wealth index, oral hygiene status, tooth brushing habit, feeling dry mouth, history of dental visits, alcohol consumption, sugared foods/drinks, cigarette smoking, and khat chewing.
Dental caries: the presence of tooth decay, or missing or filled teeth at the time of the oral examination.
Sound tooth: a tooth is recorded as sound if it shows no evidence of treated or untreated clinical caries.
Clinical caries: defined as a cavity diagnosed by visual examination/probing of the mouth.
Filled tooth due to caries: a tooth is considered filled when it has one or more permanent restorations due to the presence and/or history of tooth decay.
Missed tooth due to caries: a tooth is considered missed when it has been permanently extracted because of caries. Excluding permanent teeth missing due to any other reason like absent congenitally, extracted for orthodontic reasons or because of periodontal disease, trauma.
Decayed, missing and filled teeth (DMFT)-index per person: the average number of permanent teeth per person that are decayed (D), Missing (M), and Filled (F) teeth because of caries.
Diabetes mellitus: diagnosed in participants with fasting blood glucose levels greater than or equal to 126 mg/dl (7.0 mmol/L), or with normal glucose but under treatment (diet or medical) for diabetes. Fasting is defined as no caloric intake for at least 8 h.
Nondiabetic patients: diagnosed in participants with fasting blood sugar levels less than 126 mg/dl or random blood sugar less than 200 mg/dl and who are not with diet or medical treatment.
Simplified oral hygiene index (OHI-S): a method by which the extent of debris (soft foreign material loosely attached to the tooth) and the extent of calculus (hardening foreign material firmly attached to the tooth) on six surfaces of six preselected teeth is estimated (29).
Oral hygiene status: oral hygiene will be classified into good, fair, and poor using the OHI-S scores as follows: Good (0–1.2 OHI-S score), Fair (1.3–3.0 OHI-S score), and Poor (3.1–6.0 OHI-S score) (29).
Adequate knowledge: respondents were considered to be knowledgeable if he/she is correctly answered greater than five of the total knowledge assessing questions (30).
Inadequate knowledge: respondents were considered inadequately knowledgeable if he/she was correctly answered less than or equal to five of the total knowledge assessing questions.
The sample size of the study was determined by using factors significantly associated with dental caries by considering the following assumptions: two-sided confidence level (95%), power 80%, the ratio of exposures to no exposures 1:2, considering experiencing tooth pain as significant determinants of dental caries and the proportion in the exposed group was 75.3%, the proportion in the unexposed group was 62.8% with an adjusted odds ratio (AOR) of 1.78 (31). Using EPI-info version 6, StatCalc toolbar auto-calculator software the calculated sample size was 600 adult patients (200 for diabetes and 400 for nondiabetic patients) including a 10% nonresponse rate. A consecutive sampling technique was used to select study participants until the required sample size was reached.
Data were collected using a pretested structured interviewer-administered questionnaire and oral examination. The questionnaire was developed from WHO oral health survey with modifications (32). The questionnaire was prepared in English, translated into the local language Amharic, and back into English to check its consistency. Oral examination was performed by trained nurses for dental caries and oral hygiene status. The agreement of the examiners in the detection of dental caries was analyzed using Cohen's kappa statistics for inter-and intraexaminer reliability giving values ranging from 0.87 to 0.96 and 0.96 to 1.0, respectively.
Dental caries was evaluated using the WHO oral health assessment form for adults by assessing DMFT index (32). Oral hygiene status was also assessed using the oral hygiene index-simplified by examination of dental debris and calculus on specific preselected surfaces of teeth (29). According to their time of feeding, RBS (random blood sugar) or FBS (fasting blood sugar) was determined by the data collector.
Body weight was measured using a digital weighing scale (Adult Scale ASTOR) to the nearest 0.1 kg with the participants barefooted and wearing light clothes. Height was measured using Adult Scale ASTOR to the nearest 0.1 cm with the shoes and any hats of the study participant removed. The weighing scales were checked and adjusted at zero level between each measurement and were tested for repeatability of the measures. Participants were standing upright with the head, shoulder, buttock, lower limb, and heel of the foot touching the vertical stand. Body mass index was calculated as weight in kilograms over height in meters squared and was categorized as less than 18.50 (below normal weight), 18.50–24.99 (normal weight), greater than or equal to 25.00 (overweight).
The household wealth index (socioeconomic status) was assessed based on ownership of selected household items. These variables were collected using Ethiopian Demographic and Health Survey 2016 household characteristics questionnaire. The items include: Electricity, Radio, Television, Computer, Refrigerator, Chair, Bed with cotton/spring mattress, Electric mitad, Mobile telephone, a means of transportation, Bicycle, Motorcycle, Animal-drawn cart, Car or Truck, Bajaj, What is your monthly Income, Bank account, Owns a house, agricultural land, Number of members per sleeping room, Cows/bulls, Horses/donkeys/mules, Goats/Sheep, Chickens, Beehives, Source of drinking water, Type of toilet facility, Type of cooking fuel, and Type of flooring.
The data quality was maintained through careful design and pretesting of the questionnaire. Two days of training were given to data collectors on the objectives of the study, how to interview, how to collect data from patients, and maintain confidentiality. Then after providing the training, a pretest was conducted on 5% of the sample size prior to the actual data collection period at Debrework primary hospital. The principal investigator was closely monitoring the data collection process to ensure the completeness of questions. In addition to the above, data were rechecked during data entry before analysis, to prevent missing important data. Incomplete questionnaires were discarded from the analysis.
The collected data were cleared, coded, and entered in Epidata version 3.1 and exported to Statistical Package for Social Science (SPSS) version 20.0 (IBM SPSS Statistics for Windows, version 20 IBM Corp., Armonk, NY, USA)) for further analysis. Descriptive (frequency distribution tables, mean and standard deviation), as well as inferential analysis, was performed. Categorization was done for continuous variables using information from different pieces of literature. Cohen's kappa coefficients were used to assess inter- and intraexaminer agreements. Principal component analysis was done to construct the household wealth index by asking for all assets they have. Communality value >0.5, Kaiser–Meyer–Olkin (KMO) (sampling adequacy) with 0.87, and complex structure factor (eigenvalue) greater than 1 were considered.
Bivariate and multivariable were done to identify significantly associated variables with the dependent variable. Model fitness was checked using the Hosmer–Lemeshow test with (p-value < 0.05). A backward stepwise logistic regression model was used during multivariable logistic regression to control confounding effects. From the bivariate analysis, variables with p < 0.25 were considered for multivariable analysis. From the multivariable logistic regression analysis, variables with a significance level of p < 0.05 were taken as statistically significant and independently associated with dental caries.
A letter of ethical approval was obtained from the Ethical Review Board (ERB) of Debre Markos University, College of Health Sciences (HSC/R/C/Ser/Co/253/11/12). Furthermore, before the data collection, a formal permission letter was obtained from Bichena Primary Hospital to the outpatient department. In addition, written informed consent was obtained from respondents to confirm their willingness to participate in the study after explaining the objective of the study. The respondents noticed that they had the right to refuse or terminate at any point during the interview. The information provided by each respondent was kept confidential. Those identified as having dental caries and fasting blood sugar greater than 125 mg/dl were referred to the dental and diabetics outpatient department for better treatment and investigation.
Five hundred eighty-two patients (386 nondiabetics and 196 diabetics) participated (with a response rate of 97.0%). The age distribution of the study participants in the diabetic group was with normal age group pattern (18- to 70-year old) and the mean age of the group was 40.5 (±13.7) years. The nondiabetic group was with normal age group pattern (20–75 years old) and the mean age of the group was 47.3 (±12.7) years (Table 1).
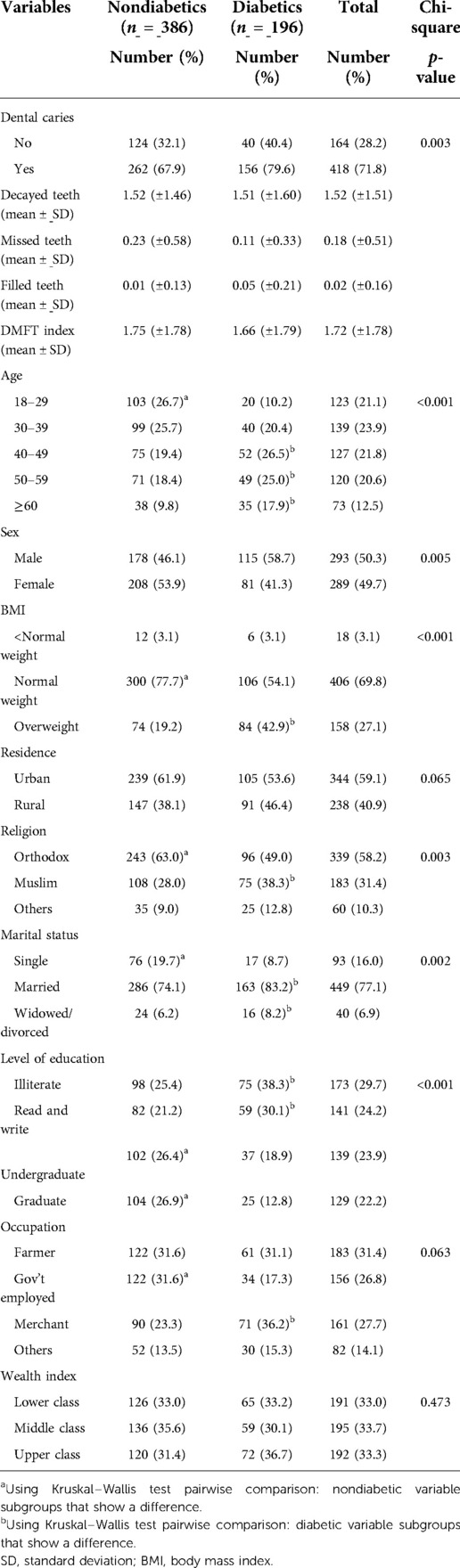
Table 1. Sociodemographic and clinical characteristics of study participants attending the outpatient department in Bichena Primary Hospital (BPH) in northwest Ethiopia, 2019 (n = 582).
This study showed that 72 (18.7%) of nondiabetics and 85 (43.4%) of diabetics were khat chewers. Similarly, 15 (3.88%) of diabetics and 10 (5.10%) of nondiabetics were cigarette smokers (Table 2).
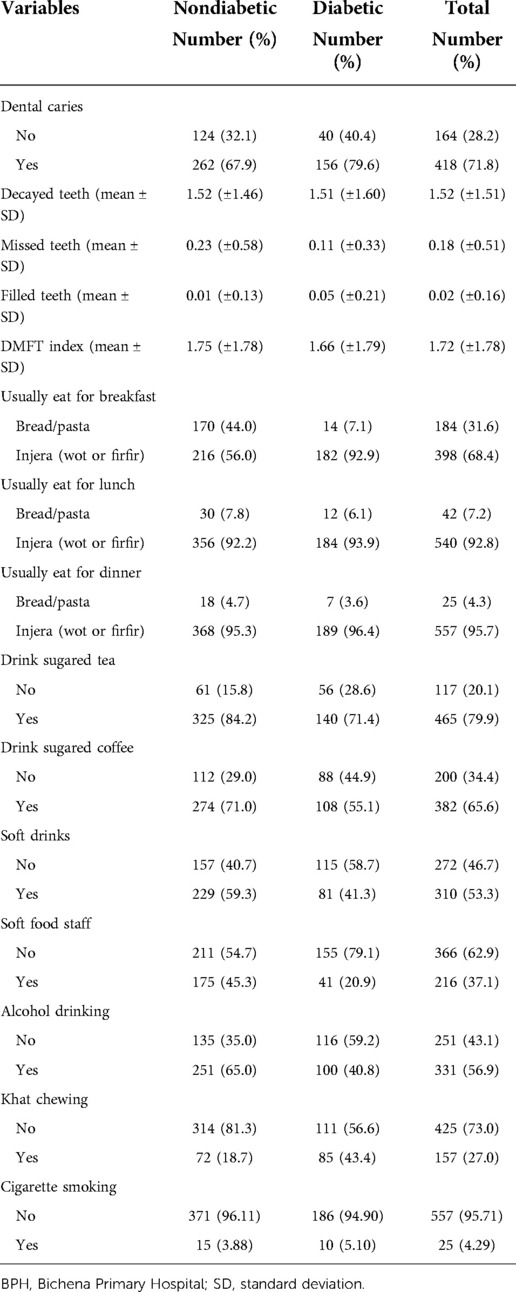
Table 2. Lifestyle characteristics and food consumption patterns of study participants attending the outpatient department in BPH, northwest Ethiopia, 2019.
Assessment of the responses to the ten questions related to oral health knowledge showed that 245 (63.50%) nondiabetics and 88 (44.90%) diabetics had adequate knowledge about dental caries. Additionally, 180 (46.60%) nondiabetics and 56 (28.90%) diabetic patients reported that they had teeth cleaning practices (Table 3).
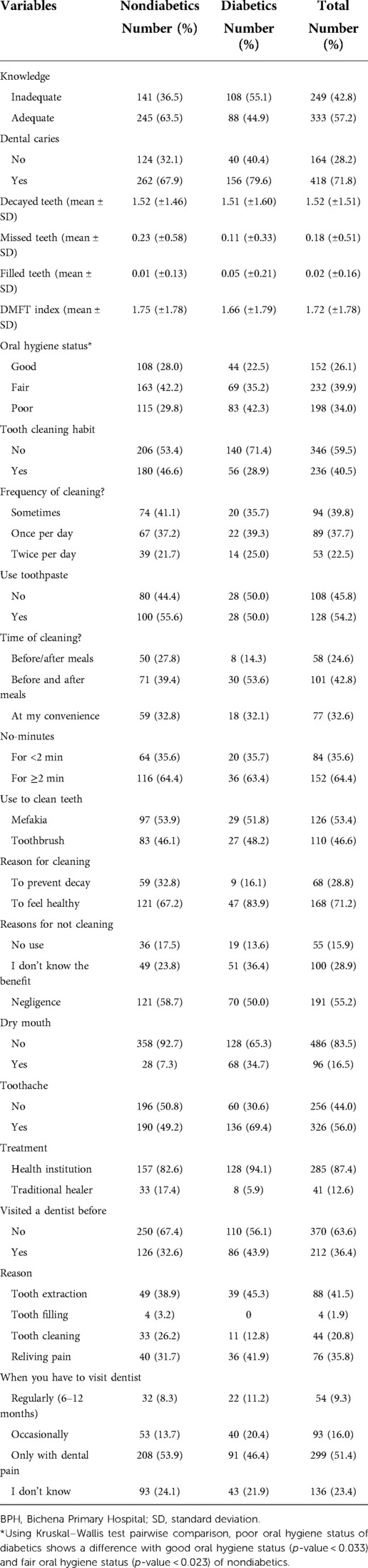
Table 3. Knowledge and practice on oral health of study participants attending the outpatient department in BPH, northwest Ethiopia, 2019.
The fair oral hygiene status of study participants covers 42.20% of nondiabetics. Poor oral hygiene status was reported in 115 (29.8%) nondiabetics. The oral hygiene status of diabetic patients shows that 69 (35.20%) and 83 (42.3%) of study participants had fair and poor oral hygiene status respectively (Table 3).
Of the 582 study participants' teeth examined, 418 (71.8%) were found to have at least one tooth decayed, missed, or filled due to dental caries. The prevalence of dental caries in nondiabetic group was 67.9 [95% confidence interval (CI): 63.2%–72.8%] and for diabetics 79.6% (95% CI: 74.0%–85.70%). The prevalence of dental caries in nondiabetics and diabetics shows a significant difference (chi-square = 8.81: p-value = 0.003) (Figure 1).

Figure 1. Prevalence of dental caries among nondiabetic and diabetic patients attending the outpatient department in BPH, northwest Ethiopia, 2019. BPH, Bichena Primary Hospital.
During binary logistic regression analysis; sex, body mass index, level of education, simplified oral hygiene index, teeth cleaning habit, frequency of dental visit, feeling of dry mouth during meals, oral health-related knowledge, sugared tea drinking, sugared coffee drinking, alcohol drinking and khat chewing candidate (p-value < 0.25) for multiple logistic regression among non-diabetes.
Whereas, the multiple logistic regressions revealed that sex (AOR = 1.98, 95% CI: 1.21–3.24), poor oral hygiene (AOR = 2.75, 95% CI: 1.41–5.36), lack of regular teeth cleaning habits (AOR = 3.86, 95% CI: 2.35–6.34), inadequate oral health-related knowledge (AOR = 3.31, 95% CI: 1.90–5.76), and khat chewing (AOR = 2.25, 95% CI: 1.11–4.57) were significantly associated with dental caries among nondiabetic (Table 4).
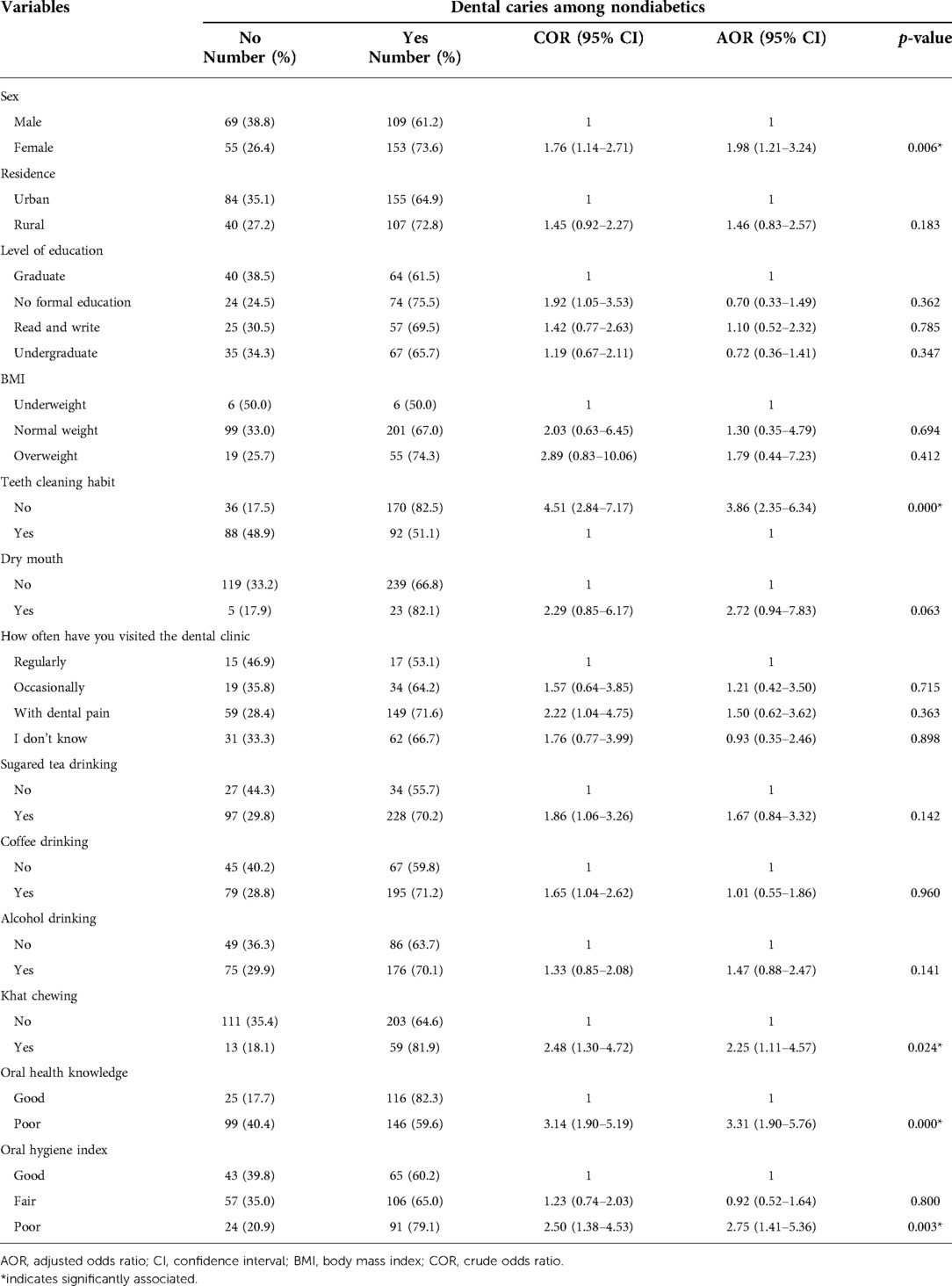
Table 4. Associated factors of dental caries among nondiabetics from study participants attending the outpatient department in Bichena Primary Hospital in Northwest Ethiopia, 2019.
During binary logistic regression analysis, potential dental caries associated factors that showed a p-value < 0.25 were; wealth index, level of education, simplified oral hygiene index, teeth cleaning habit, visiting dental clinic before, frequency of dental visit, feeling of dry mouth during meals, toothache, oral health-related knowledge, sugared tea drinking, sugared coffee drinking and khat chewing candidate for multiple logistic regression among diabetes.
Whereas, the multiple logistic regression revealed that fair simplified oral hygiene index (AOR = 3.05, 95% CI: 1.04–8.97), poor oral hygiene (AOR = 5.13, 95% CI: 1.77–14.91), feeling dry mouth during meals (AOR = 3.16, 95% CI: 1.11–8.98), inadequate oral health-related knowledge (AOR = 5.24, 95% CI: 2.12–12.96), visiting dental clinic before (AOR = 2.66, 95% CI: 1.02–6.92), and toothache (AOR = 2.43, 95% CI: 1.01, 5.79) were significantly associated with dental caries among diabetic patients (Table 5).
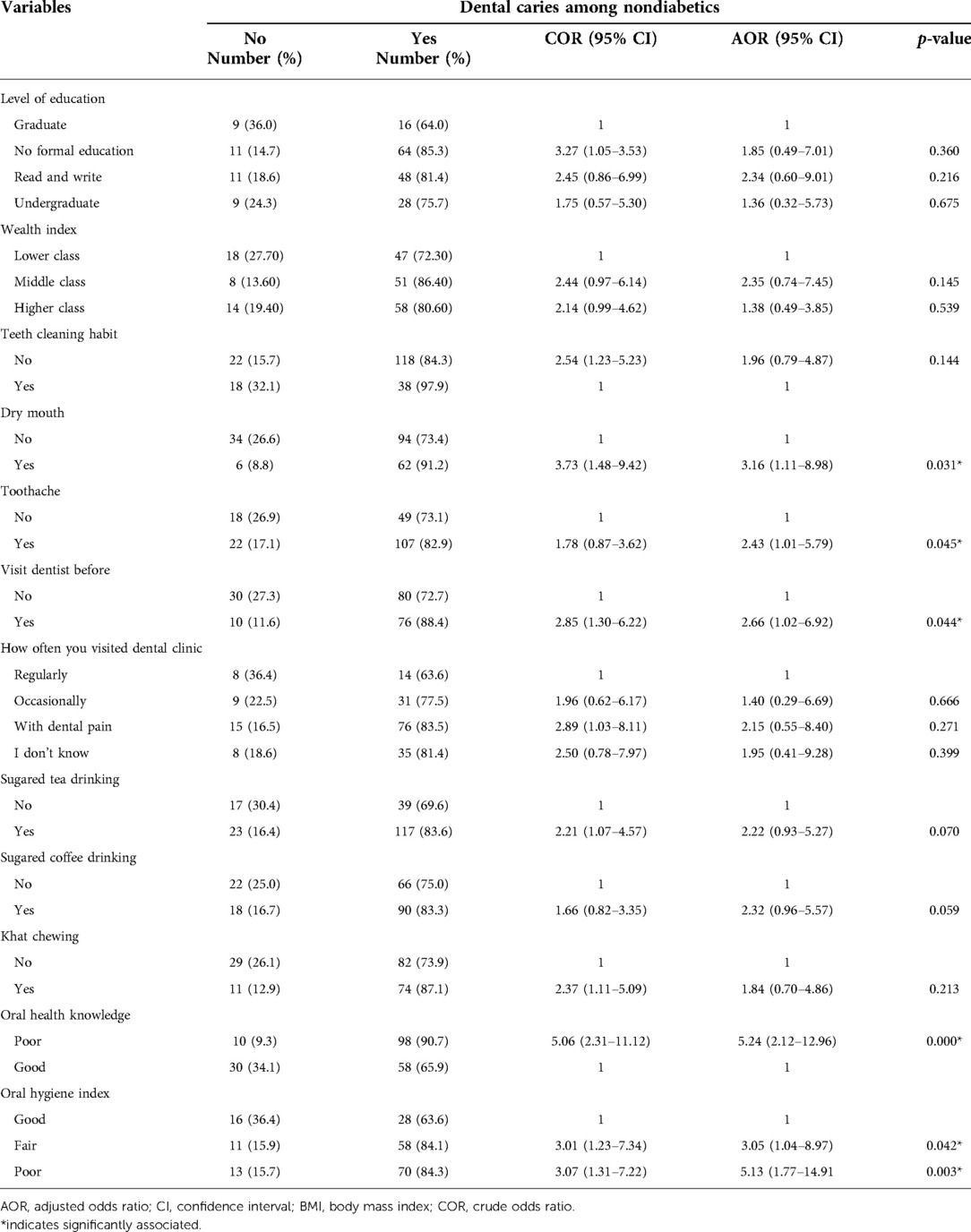
Table 5. Associated factors of dental caries among diabetics from study participants attending the Outpatient Department in Bichena Primary Hospital, northwest Ethiopia, 2019.
During binary logistic regression analysis; sex, residence, body mass index, level of education, simplified oral hygiene index, teeth cleaning habit, frequency of dental visit, visiting dental clinic before, feeling of dry mouth during meals, oral health-related knowledge, sugared tea drinking, sugared coffee drinking, toothache and khat chewing candidate (p-value < 0.25) for multiple logistic regression.
Whereas, the multiple logistic regression revealed that being female (AOR = 1.79, 95% CI: 1.15–2.77), having poor oral hygiene status (AOR = 2.95, 95% CI: 1.71–5.11), not cleaning teeth AOR = 3.26, 95% CI: 2.13–4.97), inadequate oral health-related knowledge (AOR = 3.51, 95% CI: 2.19–5.62), feeling of dry mouth during meals (AOR = 2.31, 95% CI: 1.11–4.81), sugared tea drinking (AOR = 2.00, 95% CI: 1.18–3.38), and khat chewing (AOR = 2.14, 95% CI: 1.24–3.71) were found significantly linked with dental caries (Table 6).
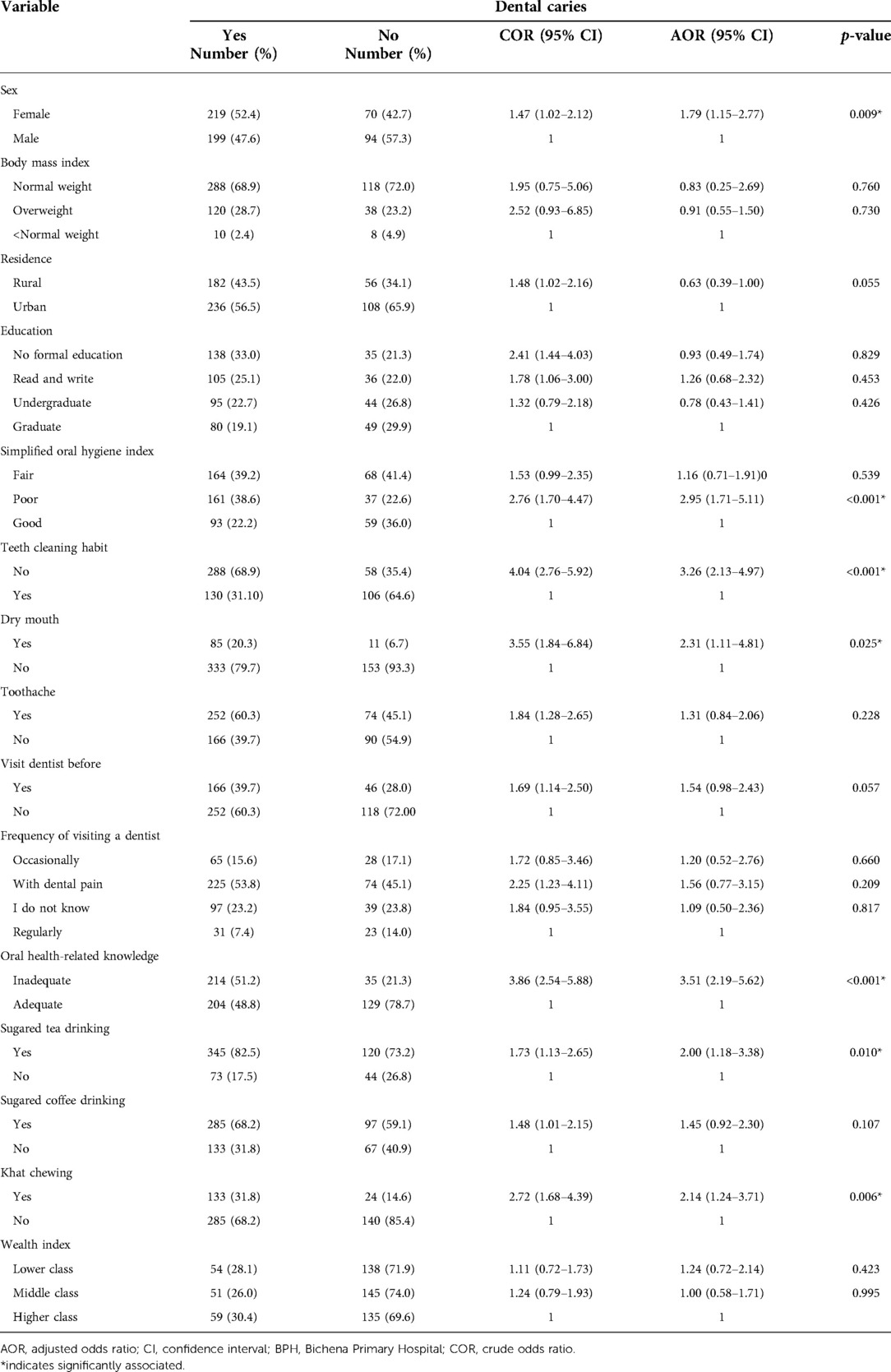
Table 6. Associated factors of dental caries among study participants attending the outpatient department in BPH, Northwest Ethiopia, 2019.
In this study, the prevalence of dental caries was 79.6% in diabetics and 67.9% in nondiabetics. This demonstrates that the prevalence of dental caries varies significantly between groups. This statistically significant difference is supported by studies conducted in Chennai, India (27), Jammu and Kashmir in India (7), in Karachi, Pakistan (33). The justification for this may be a higher content of salivary glucose in diabetic patients (8) as well as a lack of awareness among diabetic patients about oral complications. On the other hand, some studies from Uruguay (34) and Pakistan (35) reported no significant difference. The possible reason for these discrepancies might be due to the poor metabolic control of diabetics (36).
In contrast, a study from Tumkur, South India, reported a higher prevalence of dental caries among nondiabetics (37). The possible explanation for this discrepancy may be good awareness of diabetic patients on diet management practices, like sugar-free diets (37). Another reason might be the difference in frequency of visiting a dentist, which facilitates early detection and treatment (38).
The prevalence of dental caries among diabetics (79.6%) was in line with the study reported from Asmara, Eritrea, 79% (39), and from New Delhi, India (78.9%) (21). However, it is less than that of a study done in Pakistan (83.85%) (22). On the other hand, the prevalence of dental caries among diabetics is higher than a study in southwest Cameroon at 19.5% (29), Sheshdeh in Iran at 43% (40), in Gujarat, India, at 73.3% (36), in Shandong, China, 67% (23). The possible explanation for this difference may be the difference in population characteristics, diet, duration of diabetes, and treatment (23).
The prevalence of dental caries among nondiabetics was comparable with the study conducted in Asmara, Eritrea, 67.9% (17), and Brazil 68.5% (41). Whereas this is less than a study conducted in Bahir Dar, Ethiopia, 75.0% (42), in Debre Tabor, Ethiopia, 78.2% (19), Egypt 86.63% (15), in San Luis Potosi, Mexico, 76.5% (43). The reason for this may be age group differences, and population study differences, such as those visiting only dental clinics may increase the prevalence of dental caries. But, this prevalence of dental caries among nondiabetics is greater than in Adama, Ethiopia, with 35.10% (18) Finote Selam, Ethiopia, with 48.50% (44), Aksum, Ethiopia, with 35.4% (31), Gondar, Ethiopia, with 23.64% (45), in Butajira, Ethiopia, with 60% (46), Shashamane, Ethiopia, with 64.6% (47), and Port Harcourt, Nigeria, with 35.1% (48).
This study identifies the female gender as an independent predictor of the prevalence of dental caries. The prevalence of dental caries was significantly higher among those who were females compared to those who were males. This finding is consistent with the previous studies (16, 24, 43, 48). This is contradicted by a study in Adama, Ethiopia, which say males are more at risk (18). The reason for higher prevalence might be the early eruption of permanent teeth in females (49). So, being exposed to influent factors for a long time can raise the decay prevalence of permanent teeth among females more than males. Another reason could be hormonal fluctuation during puberty and salivary flow rate difference between females and males (50). On the other hand, a study conducted in Butajira reported no significant difference between males and females in dental caries experience (46).
Additionally, this study stated that not cleaning teeth was associated with the prevalence of caries. Other authors have reported similar findings in Finote Selam, Dessie, Russia (13, 24, 44). This may be due to the fact that toothbrushing removes away food debris from the mouth.
Likewise, this study reveals that xerostomia (feeling of dry mouth during meals) was a significant factor associated with dental caries. This finding is in agreement with the findings reported in Uganda and Pakistan (16, 33). This may be due to decreased saliva flow rate, which is a natural defense of oral health.
Furthermore, those who had khat chewing habits were found significantly associated with tooth decay. The study in Butajira town and the West Wollega zone in Ethiopia supports this finding (46, 51). On the other hand, this finding is in contrast to a study reported in Jimma, Ethiopia (26). The possible explanation for this may be khat chewer's sugary food intake like soft drinks and sugar with khat, which could have enhanced the association.
Furthermore, this study finding reveals that respondents who had poor knowledge related to dental caries had a significant association with dental caries as compared to those who had good knowledge about dental caries, supported by a study done in Ethiopia (19).
Moreover, this study found that poor oral hygiene status was also significantly associated with the prevalence of dental caries. This finding is consistent with studies reported in India, Cameroon, Iran, Spain, and India (29, 40, 43). Poor oral hygiene status means the oral with debris, which may provide nutrients and time for the bacteria to produce acid and finally tooth decay.
Finally, those who take tea with sugar were significantly associated with the occurrence of dental caries. Similar findings were reported in recent studies done in Mekelle town, Ethiopia (25), and Debre Birhan town, Ethiopia (52). It is well-recognized that sugar plays an important role in caries development. A sugary diet allows the bacteria to attach easily to the dental surface. The bacteria then convert into acids that cause demineralization of the hard tissue of the teeth.
In this study, the prevalence of dental caries was assessed among three socioeconomic groups. Socioeconomic status comparison using chi-square shows no association between diabetic and nondiabetic groups with a chi-square p-value = 0.473 (Table 1). Similarly, the multiple logistic regression analysis does not provide statistically significant evidence that supports the risk of socioeconomic status on dental caries prevalence (Table 6).
This study has its strengths. The first strength was study participants selected a consecutive sampling method and this reduces the selection bias. The second was questionnaire was adapted from WHO oral health survey fifth edition with some modifications. In addition, these studies were conducted in outpatient department patients including dental clinics. Further, the study includes factors like socioeconomic status using wealth index, oral hygiene status using oral hygiene index-simplified, and body mass index which are keys for dental caries development. And these risk factors are properly measured using their indexes.
This study also has limitations that should be taken into account when interpreting results. The first limitation is related to its design. Cross-sectional study design measures cause and effect at the same time. Therefore, it cannot establish temporal associations. Hence it was impossible to know the true causal order of disease. Dental caries were detected using clinical diagnosis only. It was not supported with radiological examination due to a lack of the instrument. This might affect the actual prevalence of the problem. In addition, the amount and duration of intake of sweet food items and drinks were not assessed. The other limitation of the study was not considering the metabolically controlled level of diabetics due to the lack of Glycosylated hemoglobin (HbA1c) instruments. Further, we recommend that future studies use the pulpal involvement ulcer due to root fragments fistula and abscess (PUFA) index to determine clinical conditions resulting from untreated dental caries.
The prevalence of caries was significantly higher among diabetics than nondiabetics. Oral health education with preventive measures such as improving teeth cleaning practice, reducing sugary foods and drinks intake, and improving oral hygiene practice of patients should be mainstreamed along with diabetic follow-up care.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Review Board (ERB) of Debre Markos University, College of Health Sciences. The patients/participants provided their written informed consent to participate in this study.
AS, GDK, and GA participated in the conception and design of the study. AS and MT participated in data collection and wrote sections of the manuscript. AS performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank Debre Markos University, Bichena Primary Hospital staff members, data collectors, supervisors, and study participants' co-operation for data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AOR, adjusted odds ratio; BPH, Bichena Primary Hospital; CI, confidence interval; DMFT, decayed, missed and filled teeth; FBS, fasting blood sugar; KMO, Kaiser–Meyer–Olkin; mg/dl, milligram per deciliter; RBS, random blood sugar; OHI-S, simplified oral hygiene index; SPSS, Statistical Package for Social Science; WHO, World Health Organization
1. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. (2007) 369(9555):51–9. doi: 10.1016/S0140-6736(07)60031-2
2. World Health Organization. Promoting oral health in Africa: Prevention and control of oral diseases and noma as part of essential noncommunicable disease interventions. Brazzaville, Republic of Congo: World Health Organization (2016). 126 p.
3. Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. (2017) 44:39–51. doi: 10.1111/jcpe.12685
4. Benzian H, Williams D. The challenge of oral disease: a call for global action. The oral health atlas. 2nd ed. Geneva: FDI World Dental Federation (2015).
5. World Health Organization. Sugars and dental caries. Geneva, Switzerland: World Health Organization (2017).
6. Bernabé E, Marcenes W, Kassebaum NJ, Hernandez CR, Bailey J, Hay SI, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. (2020) 99(4):362–73. doi: 10.1177/0022034520908533
7. Singh I SP, Singh A, Singh T, Kour R. Diabetes an inducing factor for dental caries: a case control analysis in Jammu. J Int Soc Prevent Communit Dent. (2016) 6(2):125–9. doi: 10.4103/2231-0762.178748
8. Azizi A, Modaberi A. The correlation of blood glucose with salivary glucose level in diabetic patients. J Islamic Dent Assoc. (2014) 25(4):274–77.
9. Nurelhuda N, Lee H, Bridge G. Oral health in the Arab world: The silent epidemic of dental caries. In: Laher I, editor. Handbook of Healthcare in the Arab World. Cham, Switzerland: Springer (2021). https://scholar.google.com/scholar?q=Prevalence+of+dental+caries+and+associated+factors+among+Aksum+Primary+School+students,+Aksum+Town,+Ethiopia+2019:+A+cross-sectional&hl=en&as_sdt=0,5
10. Martins-Júnior P, Vieira-Andrade R, Corrêa-Faria P, Oliveira-Ferreira F, Marques L, Ramos-Jorge M. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. (2013) 47(3):211–8. doi: 10.1159/000345534
11. Lamster IB. Diabetes and oral health—current concepts regarding periodontal disease and dental carie. US Endocrinol. (2012) 8(2):93–7. doi: 10.17925/USE.2012.08.02.93
12. Yadav K, Prakash S. Dental caries: a review. Asian J Biomed Pharm Sci. (2016) 6(53):73–80. doi: 10.15272/ajbps.v6i53.773
13. Teshome A, Muche A, Girma B. Prevalence of dental caries and associated factors in east Africa, 2000–2020: systematic review and meta-analysis. Front Public Health. (2021) 9:1–15. doi: 10.3389/fpubh.2021.645091
14. Abid A, Maatouk F, Berrezouga L, Azodo C, Uti O, E-Shamy H, et al. Prevalence and severity of oral diseases in the Africa and Middle East region. Adv Dent Res. (2015) 27(1):10–7. doi: 10.1177/0022034515582062
15. Abbass MMS, AbuBakr N, Radwan IA, Rady D, Moshy SE, Ramadan M, et al. The potential impact of age, gender, body mass index, socioeconomic status and dietary habits on the prevalence of dental caries among Egyptian adults: a cross-sectional study. F1000Res. (2019) 8:2–18. doi: 10.12688/f1000research.17892.1
16. Kalanzi D, Mayanja-Kizza H, Nakanjako D, Mwesigwa CL, Ssenyonga R, Amaechi BT. Prevalence and factors associated with dental caries in patients attending an HIV care clinic in Uganda: a cross sectional study. BMC Oral Health. (2019) 19(1):1–8. doi: 10.1186/s12903-019-0847-9
17. Abdelhamid N, Bahta H, Mohammed S, Dhanni C, Elfatih AM. Prevalence of dental caries and evaluation of mean DMFT index among secondary school students in Asmara, Eritrea. Afr J Oral Health. (2019) 8(2):1–7. https://ajoh.oauife.edu.ng/index.php/ajoh/article/view/37
18. Meyrema AK, Kedir TR. Prevalence of oral health care and problems among Rift Valley University health sciences students in Adama, South east, Ethiopia. Afr J Oral Health. (2018) 8(1):16–23. doi: 10.4314/ajoh.v8i1.178496
19. Tafere Y, Chanie S, Dessie T, Gedamu H. Assessment of prevalence of dental caries and the associated factors among patients attending dental clinic in Debre Tabor general hospital: a hospital-based crosssectional study. BMC Oral Health. (2018) 18(1):1–7. doi: 10.1186/s12903-018-0581-8
20. Zewdu T, Abu D, Agajie M, Sahilu T. Dental caries and associated factors in Ethiopia: systematic review and meta-analysis. Environ Health Prev Med. (2021) 26(1):1–11. doi: 10.1186/s12199-021-00943-3
21. Rawal I, Ghosh S, Hameed SS, Shivashankar R, Ajay VS, Patel SA, et al. Association between poor oral health and diabetes among Indian adult population: potential for integration with NCDs. BMC Oral Health. (2019) 19(1):1–10. doi: 10.1186/s12903-019-0884-4
22. Khahro MM, Shaikh Q, Baloch M, Channa SA, Surwaich A. Frequency of dental caries among patients with type-II diabetes mellitus. Prof Med J. (2019) 26(6):865–9. doi: 10.29309/TPMJ/2019.26.06.2579
23. Wang Y, Xing L, Yu H, Zhao L. Prevalence of dental caries in children and adolescents with type 1 diabetes: a systematic review and meta-analysis. BMC Oral Health. (2019) 19(1):1–9. doi: 10.1186/s12903-019-0903-5
24. Drachev SN, Brenn T, Trovik TA. Dental caries experience and determinants in young adults of the Northern State Medical University, Arkhangelsk, Northwest Russia: a cross-sectional study. BMC Oral Health. (2017) 17(1):136. doi: 10.1186/s12903-017-0426-x
25. Sehdev B, Muruts L, Ganji KK. Prevalence of tooth decay and associated factors among Ethiopian patients. Pesqui Bras Odontopediatria Clin Integr. (2020) 20:e4835. doi: 10.1590/pboci.2020.053
26. Shenkute D, Asfaw T. Streptococcus mutans dental carries among patients attending Jimma University specialized hospital, Ethiopia. Int J Res Stud Biosci. (2019) 7(2):49–55. doi: 10.20431/2349-0365.0702005.
27. Seethalakshmi C, Jagat Reddy RC, Asifa N, Prabhu S. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a cross-sectional study. J Clin Diagn Res. (2016) 10(3):ZC12–4. doi: 10.7860/JCDR/2016/16310.7351
28. Schwendicke F, Frencken J, Innes N. Caries excavation: Evolution of treating cavitated carious lesions. Nijmegen, Netherlands: Karger Medical and Scientific Publishers (2018).
29. Bissong M, Azodo CC, Agbor MA, Nkuo-Akenji T, Fon PN. Oral health status of diabetes mellitus patients in Southwest Cameroon. Odontostomatol Trop. (2015) 38(150):49–57.26934773
30. Gualie YT, Tayachew AT. Assessment of knowledge, attitude, and practice toward oral hygiene among governmental secondary school students in Debre Tabor Town, Amhara Region, North Central Ethiopia 2018: Institutional based cross sectional survey. Int J Oral Health Sci. (2019) 8:92–8. doi: 10.4103/ijohs.ijohs_37_18
31. Zeru T, Muruts L, Zeru M, Gebremariam B, Girmay A, Bahre D. Prevalence of dental caries and associated factors among Aksum Primary School students, Aksum Town, Ethiopia 2019: A cross-sectional. J Dental Oral Health. (2019) 5:130–5.
33. Moosa Y, Shahzad M, Shaikh AA, Matloob SA, Khalid M. Influence of diabetes mellitus on oral health. Pak Oral Dent J. (2018) 38:67–70.
34. Techera A, Villamonte G, Pardo L, Jordi MDCL. Comparison of the oral health status of diabetic and non-diabetic Uruguayan children aged 8–12. Odontoestomatología. (2018) 20(32):84–91. doi: 10.22592/ode2018n32a11
35. Khan I, Shan T, Manzoor MA. Comparison of dental caries status of type-2 diabetics with nondiabetics. Pak Armed Forces Med J. (2019) 69(4):854–6.
36. Malvania EA, Sheth SA, Sharma AS, Mansuri S, Shaikh F, Sahani S. Dental caries prevalence among type II diabetic and nondiabetic adults attending a hospital. J Int Soc Prevent Communit Dent. (2016) 6:S232–6. doi: 10.4103/2231-0762.197202
37. Bharateesh JV, Ahmed M, Kokila G. Diabetes and oral health: a case-control study. Int J Prev Med. (2012) 3:806–9.23189233
38. Ferizi LJ, Dragidella F, Spahiu L, And AB, Kotori V. The influence of type 1 diabetes mellitus on dental caries and salivary composition. Int J Dent. (2018) 2018:1–7. doi: 10.1155/2018/5780916
39. Bahru Y, Abdu SS. A study of dental problems in diabetic patients. Ethiop Med J. (1992) 30(2):95–103.1606949
40. Moshaverinia M, Lavaee F, Moshaverinia S, Gholami F. The prevalence of dental caries in diabetic patients of Sheshdeh Qarebolaq. Researchgate. (2013) 2(1):11–20.
41. Costa SM, Vasconcelos M, Hadded JPA, Abreu MHN. The severity of dental caries in adults aged 35 to 44 years residing in the metropolitan area of a large city in Brazil: a cross-sectional study. BMC Oral Health. (2012) 12(25):1–11.22214223
42. Mulu W, Demilie T, Yimer M, Meshesha K, Abera B. Dental caries and associated factors among primary school children in Bahir Dar city: a cross-sectional study. BMC Res Notes. (2014) 7(1):1–7.24382056
43. García-Cortés JO, Loyola-Rodriguez JP, Loyola-Leyva A, Navarrete-Hernández JDJ, Márquez-Rodríguez S, Fernández-Barrera MA, et al. Socio-behavioral factors associated to caries prevalence and DMFT index in adolescents and young adults in a developing country. West Indian Med J. (2017) 10:1–22.
44. Teshome A, Yitayeh A, Gizachew M. Prevalence of dental caries and associated factors among Finote Selam primary school students aged 12-20 years, Finote Selam town, Ethiopia. OHDM. (2016) 15(1):1–6.
45. Teshome A, Andualem G, Derese K. Dental caries and associated factors among patients attending the university of gondar comprehensive hospital dental clinic, North West Ethiopia: a hospital-based cross-sectional study. Clin Cosmet Investig Dent. (2020) 12:191. doi: 10.2147/CCIDE.S247179
46. Bogale B, Engida F, Hanlon C, Prince MJ, Gallagher JE. Dental caries experience in adults: a cross-sectional community survey within Ethiopia. Res Sq. (2020) 21(1):1–30.
47. Geleto A, Sinba E, Ali MM. Dental caries and associated factors among patients visiting Shashamane Comprehensive Specialized Hospital. PLoS One. (2022) 17(3):e0265000. doi: 10.1371/journal.pone.0265000
48. Umanah AU, Braimoh OB. Oral hygiene practices and factors influencing the choice of oral hygiene materials among undergraduate students at the University of Port Harcourt, Rivers State, Nigeria. J Dent Allied Sci. (2017) 6(1):1–5.
49. Brito ACM, Bezerra IM, Cavalcante DDFB, Pereira AC, Vieira V, Montezuma MF, et al. Dental caries experience and associated factors in 12-year-old-children: a population based-study. Braz Oral Res. (2020) 34:1–10.
50. Youssefi MA, Afroughi S. Prevalence and associated factors of dental caries in primary schoolchildren: an Iranian setting. Int J Dent. (2020) 2020:1–7.
51. Guracho TT, Atomssa EM, Megersa OA, Tolossa T. Determinants of dental caries among adolescent patients attending Hospitals in West Wollega Zone, Western Ethiopia: a case-control study. PLoS One. (2021) 16(12):e0260427. doi: 10.1371/journal.pone.0260427
Keywords: dental caries, diabetics, nondiabetics, patients, Ethiopia
Citation: Shiferaw A, Alem G, Tsehay M and Kibret GD (2022) Dental caries and associated factors among diabetic and nondiabetic adult patients attending Bichena Primary Hospital’s Outpatient Department. Front. Oral. Health 3:938405. doi: 10.3389/froh.2022.938405
Received: 7 May 2022; Accepted: 21 September 2022;
Published: 2 November 2022.
Edited by:
Vijay Prakash Mathur, All India Institute of Medical Sciences, IndiaReviewed by:
Charu Mohan Marya, Sudha Rustagi College of Dental Sciences and Research, India© 2022 Shiferaw, Alem, Tsehay and Kibret. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anley Shiferaw anleyshiferaw9@gmail.com
Specialty Section: This article was submitted to Oral Epidemiology, a section of the journal Frontiers in Oral Health
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.