
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health , 02 September 2022
Sec. Oral Epidemiology
Volume 3 - 2022 | https://doi.org/10.3389/froh.2022.960732
This article is part of the Research Topic The Intersection of Periodontitis and Systemic Health View all 5 articles
Parts of this article's content have been modified or rectified in:
Erratum: In vitro activity of anti-rheumatic drugs on release of pro-inflammatory cytokines from oral cells in interaction with microorganisms
Periodontitis patients suffering concomitantly from rheumatoid arthritis (RA) often present with less inflamed periodontal tissues due to the ongoing anti-rheumatic therapy. This in vitro study was aimed to analyze whether anti-inflammatory drugs used in the therapy of RA can modulate the release of IL-8 and IL-1β by professional and non-professional immune cells stimulated with microorganisms. Periodontal ligament (PDL) fibroblasts, monocytic MONO-MAC-6-cells, and gingival keratinocytes were exposed to ibuprofen, prednisolone, and methotrexate with and without lysates of Fusobacterium nucleatum or Candida albicans. Supernatants were obtained and the levels of interleukin(IL)-8 and IL-1β (only MONO-MAC-6) were quantified. The addition of F. nucleatum lysate resulted in the strongest release of proinflammatory cytokines by PDL fibroblast and MONO-MAC-6 cells, while the modification by the tested anti-rheumatic drugs was only minor. After stimulation of the MONO-MAC-cells with F. nucleatum, prednisolone increased the release of IL-8, whereas methotrexate decreased the level. Anti-inflammatory drugs increased the adherence of C. albicans to epithelial cells. In patients with RA, the reduction of the microbial load in subgingival biofilm (biofilm removal) is of major importance; however, the intake of inflammatory drugs may interfere with the inflammatory response.
Periodontitis is one of the most common diseases worldwide with a prevalence of 11% for its severe form [1]. It is a chronic disease resulting from the inflammatory response to a dysbiotic microbiota in the subgingival biofilm that eventually leads to the destruction of teeth-surrounding tissues [2]. Fusobacterium nucleatum, a common opportunistic bacteria, acts as a bridging bacterium between symbiotes and pathogens and is generally thought to contribute to bacterial pathogenicity [3]. The inflammasome activation is crucial in the pathogenesis of periodontal disease and it includes the maturation of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 [4]. IL-8 (IL-8/CXCL9) is a member of the CXC chemokine family, it attracts neutrophils and stimulates the release of neutrophil granules [5]. The expression of IL-8 increases with the severity of periodontal disease [6]. Meanwhile, it is well known that periodontitis does not only locally affect the periodontium. There are many associations with systemic pathologies where periodontitis may influence the progression or therapy of the systemic disease and vice versa. Diabetes mellitus was among the first systemic conditions showing a clear correlation with attachment loss [7]. Another disease that stepped into the focus of research is rheumatoid arthritis (RA). A recent large analysis of health insurance data including more than 500,000 participants in South Korea showed a higher prevalence of periodontitis in individuals with RA (19.6%) than without (16.6%) and also more cases with RA (6.2%) in patients with periodontitis than in those without (5.2%) [8].
RA is a systemic inflammatory disease characterized by joint stiffness, pain, and swelling. Antibodies against rheumatoid factor and citrullinated proteins are detectable in most patients [9]. Numerous inflammatory cells infiltrate the interstitium between cartilage and bone. Concomitantly, higher concentrations of IL-1, IL-6, IL-8, IL-10, and MCP-1 are observed which may be associated with neutrophil autophagy—a phenomenon that might play a role in the pathogenesis of RA [10, 11]. In the 70s and 80s, two inflammatory mediators were discovered to be relevant for bone and tissue destruction in the course of RA: IL-1 and tumor necrosis factor (TNF). While IL-1 is locally active, TNF plays a more prominent part systemically. The treatment of RA is scheduled to control the inflammation and lower disease activity by reducing Il-1 and TNF release [12]. Basically, disease-modifying anti-rheumatic drugs (DMARDs) and nonsteroidal anti-inflammatory drugs (NSAIDs) are applied [9]. Here, we focused on three widely prescribed drugs that belong to different classes; methotrexate, one of the DMARDs, decreases inflammation and counteracts tolerance of TNF inhibitors [12, 13]; ibuprofen, a NSAID, is able to limit local inflammation through reversible inhibition of the cyclooxygenase enzymes COX-1 and COX-2 [13]; and finally prednisolone, a glucocorticoid that suppresses the migration of polymorphonuclear leukocytes and reverses increased capillary permeability [14].
Porphyromonas gingivalis, a major pathogen in periodontitis, has been widely discussed as a possible link between the two diseases since its peptidyl arginine deiminase can citrullinate peptides, and in arthritis patients, increased antibodies against P. gingivalis have been found [15, 16]. However, in a recent murine model, other bacteria have come into focus. Complex analyses of the microbiota show F. nucleatum to be enriched in periodontitis vs. periodontal health [17]. In a murine model, it has been demonstrated that inoculation with F. nucleatum or Aggregatibacter actinomycetemcomitans alone spurred the onset and progression of arthritis [18]. And it was mostly bacterial DNA from F. nucleatum that was detected in the synovial fluid of arthritis patients [19].
In our recent studies on the association of RA with periodontal disease, tooth loss was not associated with the typical signs of periodontitis such as high probing pocket depth and bleeding on probing [16, 20, 21]. This might be a result of the longtime intake of anti-rheumatic drugs. Therefore, the question arose whether this medication might interfere with the periodontal inflammatory response. Further, as Candida albicans was highly present in the subgingival biofilm samples of RA patients (which was not the case in periodontally healthy and periodontitis patients without underlying systemic disease), we also included C. albicans in this study.
The research questions to be answered in the present in vitro study were (i) if anti-inflammatory drugs used in the therapy of RA patients can modulate the release of IL-8 and IL-1β by professional and non-professional immune cells being of relevance in the oral cavity and (ii) how the interaction of microorganisms with these cells is being influenced by anti-inflammatory drugs.
Three different cells were used in the assays: periodontal ligament (PDL) fibroblasts; MONO-MAC-6-cells (DSMZ no. ACC 124), a monocytic cell line of human origin; and telomerase-inactivated gingival keratinocyte (TIGK) cells (ATCC-CRL-3397). The PDL fibroblasts originated from three different donors. PDL fibroblasts were collected from extracted teeth. Before, patients had agreed for using their cells for research purposes and signed a written consent. This procedure is in accordance with the guidelines set by the Cantonal ethical committee KEK. As the obtained biomaterials were categorized as “irreversibly anonymized,” no previous approval was necessary. The method was used as described before [22].
The cell cultivation media were DMEM for PDL fibroblasts and RPMI 1640 medium for MONO-MAC-6 cells, both supplemented with 10% fetal bovine serum (FBS; all Invitrogen; Carlsbad, CA, USA). For TIGK cells, Keratinocyte Growth Medium (KGM-Gold; Lonza, Basel, Switzerland) was used.
The following drugs and concentrations (final) were used in the assays.
• ibuprofen (Brufen 600 mg, BGP Products GmbH, Baar, CH): 2, 10, and 50 μg/ml
• prednisolone (Spricort 20, Spirig Health Care AG, Egerkingen, CH): 20, 100, and 500 ng/ml
• methotrexate (Methotrexat Farmos 10 mg, Orion Pharma AG, Zug, CH): 1, 5, and 25 μg/ml.
The concentrations were chosen based on reported concentrations in plasma or tissue [23–25]. Cytotoxicity was tested using trypan blue exclusion tests for the applied concentrations.
When starting experiments, a dilution series of 1:4 of the drugs in phosphate-buffered saline (PBS) was made. The first tube contained 10-fold of the highest concentrations. The respective negative control was PBS.
Lysates of F. nucleatum ATCC 25586 and C. albicans ATCC 76615 were prepared. A suspension OD600 = 1 in PBS was exposed to 20 min ultrasonication with a power of 280 W; thereafter, the mixture was centrifuged at 10,000 g at 20°C for 10 min. The supernatant was obtained and filtrated by using a pore size of 0.4 μm. This corresponded to a multiplicity of infection (MOI) of ~20:1 (bacteria:cells). Of note is that cells were stimulated rather with bacterial components than entire bacterial cells.
In experiments with TIGK cells, microbial cells of C. albicans ATCC 76615 were suspended in PBS (OD 600 nm = 0.2) resulting in a MOI of ≈5:1 (fungi:cells).
MONO-MAC-6-cells were centrifuged for 5 min at 250 g. The supernatant was removed and the cells were washed two times with PBS. Thereafter, they were adjusted to a density of 106/ml in RPMI 1610 media with 0.5% FBS. The cell suspension was mixed with microbial lysate and one of the drug solutions in a ratio 8:1:1 of which 1 ml was then pipetted per well in a 24-well plate. Each of the drug-microbes-cell suspensions was pipetted to a well plate. The plates were incubated for 18 h before the suspension was transferred to 1.5 ml tubes and centrifuged at 10,000 g for 5 min at 20°C. The supernatants were obtained and stored at −80°C until assayed by ELISA.
Before the experiments, PDL fibroblasts were transferred to 24-wells plates and grown to confluence. The cell cultivation medium was removed. The cells were washed two times with PBS and 1 ml of one of the drug-microbes-cell cultivation media (ratio 1:1:8) was added. Each of the drug-microbes suspension was pipetted to a well plate with seeded PDL fibroblasts (As before, the FBS concentration was reduced to 0.5% in the cell cultivation media). Cells were seeded at 3 × 105/cm2. No more than five passages were used for the experiments. The further procedure followed those of the MONO-MAC-6-cells. All experiments were conducted in triplicates and in three independent repetitions.
TIGK cells were adjusted to 105/ml cell cultivation media and every 1 ml was transferred to a well of a 24-well plate 24 h before starting the experiments. On the day of the experiment, cells were checked for confluency. Then, they were washed two times, before 1 ml of one of the drug-microbes-cell cultivation media (ratio 1:1:8) was added. Each of the drug-microbes suspension was pipetted to a well plate with seeded TIGK cells. After an incubation time of 6 h, media were removed and treated as described before (centrifuging, storing of the supernatants at −80°C).
Then, the cells were washed two times with PBS to remove non-adherent C. albicans cells. Thereafter, 1 ml ice-cold dH2O/well was added. The plates were left in place for 15 min, after intensive mixing, and the suspension was plated on agar plates to determine the colony forming units of adhered (incl. invasive) C. albicans.
In preliminary tests, the suitable biomarkers were selected. All cells released IL-8. In addition, from supernatants obtained after interaction with MONO-MAC-6-cells, the levels of IL-1β were assessed.
Commercially available ELISA kits (R&D Systems, Minnesota, MN, USA) were used according to the manufacturer's instructions. The detection levels were 1 pg/ml for both IL-8 and IL-1β.
Data were compared by using software SPSS 24.0 (IBM, Armonk, NY, USA). One-way ANOVA with post-hoc Bonferroni was applied. Beforehand, the assumption of normally distributed data was confirmed using skewness and kurtosis statistics. In post-hoc analyses, only comparisons of the drug with the respective control of the microbes or at defined drug concentrations with the cells without microbes were considered. The level of statistical significance was set at p < 0.05.
Without stimulation, the cells were released in the mean of 42.47 ± 24.29 pg/ml IL-8 after 18 h of incubation. Prednisolone and ibuprofen in the three applied concentrations had no influence on IL-8 secretion. However, in presence of 1, 5, and 25 μg/ml methotrexate, the IL-8 levels were higher than in the controls (p = 0.001, p = 0.020, p < 0.001; Figure 1A).
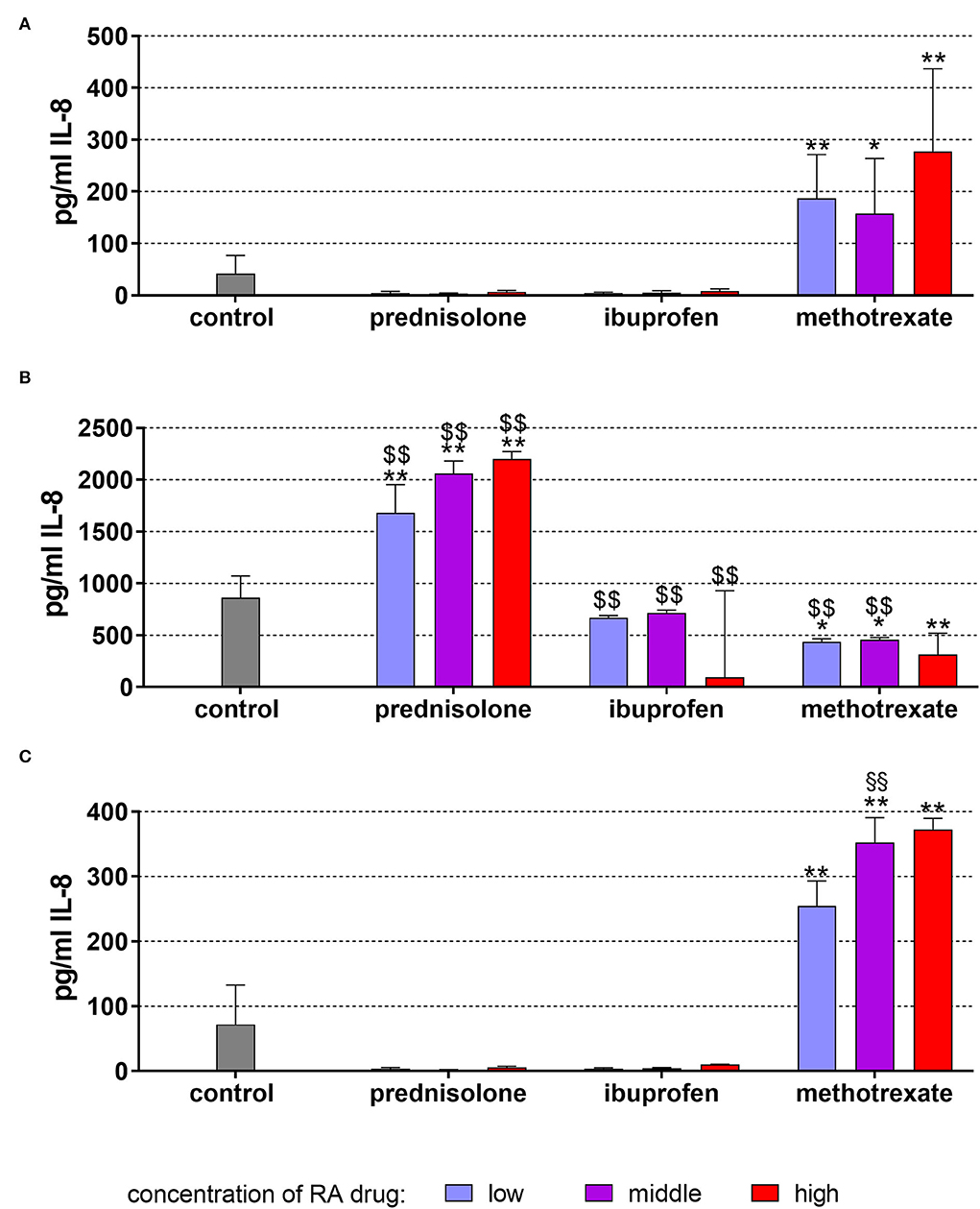
Figure 1. Released interleukin (IL)-8 by monocytic MONO-MAC-6 cells after 18 h of exposure of 20 ng/ml (low), 100 ng/ml (middle), 500 ng/ml (high) prednisolone, 2 μg/ml (low), 10 μg/ml (middle), 50 μg/ml (high) ibuprofen, 1 μg/ml (low), 5 μg/ml (middle), and 25 μg/ml (high) methotrexate (A) w/o microbial compounds, (B) with Fusobacterium nucleatum lysate, and (C) Candida albicans lysate. The columns represent the mean and the bars represent standard deviation. *p < 0.05 vs. control, **p < 0.01 vs. control. $$p < 0.01 vs. respective group w/o microbial compounds.
The exposure to F. nucleatum lysate increased the level to 862.73 ± 209.00 pg/ml (p < 0.001). Higher levels of IL-8 vs. non-bacteria exposed controls were also detected in the presence of the drugs. Compared with F. nucleatum without drugs, ibuprofen did not change the level of IL-8, prednisolone increased the level further up to 2,201.46 ± 70.60 pg/ml (500 ng/ml p < 0.001). In contrast, methotrexate decreased the IL-8 release, but the levels were always still higher than without F. nucleatum (Figure 1B).
C. albicans lysate, however, did not significantly change the released amount of IL-8 (73.23±60.53 pg/ml). Methotrexate augmented the secretion (each p < 0.001), and at 5 μg/ml methothrexate, the IL-8 amount was higher with C. albicans lysate vs. cells without microbes (p = 0.006; Figure 1C).
The level of IL-1β released from MONO-MAC-6 cells without stimulation was in the mean of 2.70 ± 2.65 pg/ml after 18 h of incubation. This relatively low level was not changed further by any of the applied drugs (Figure 2A).
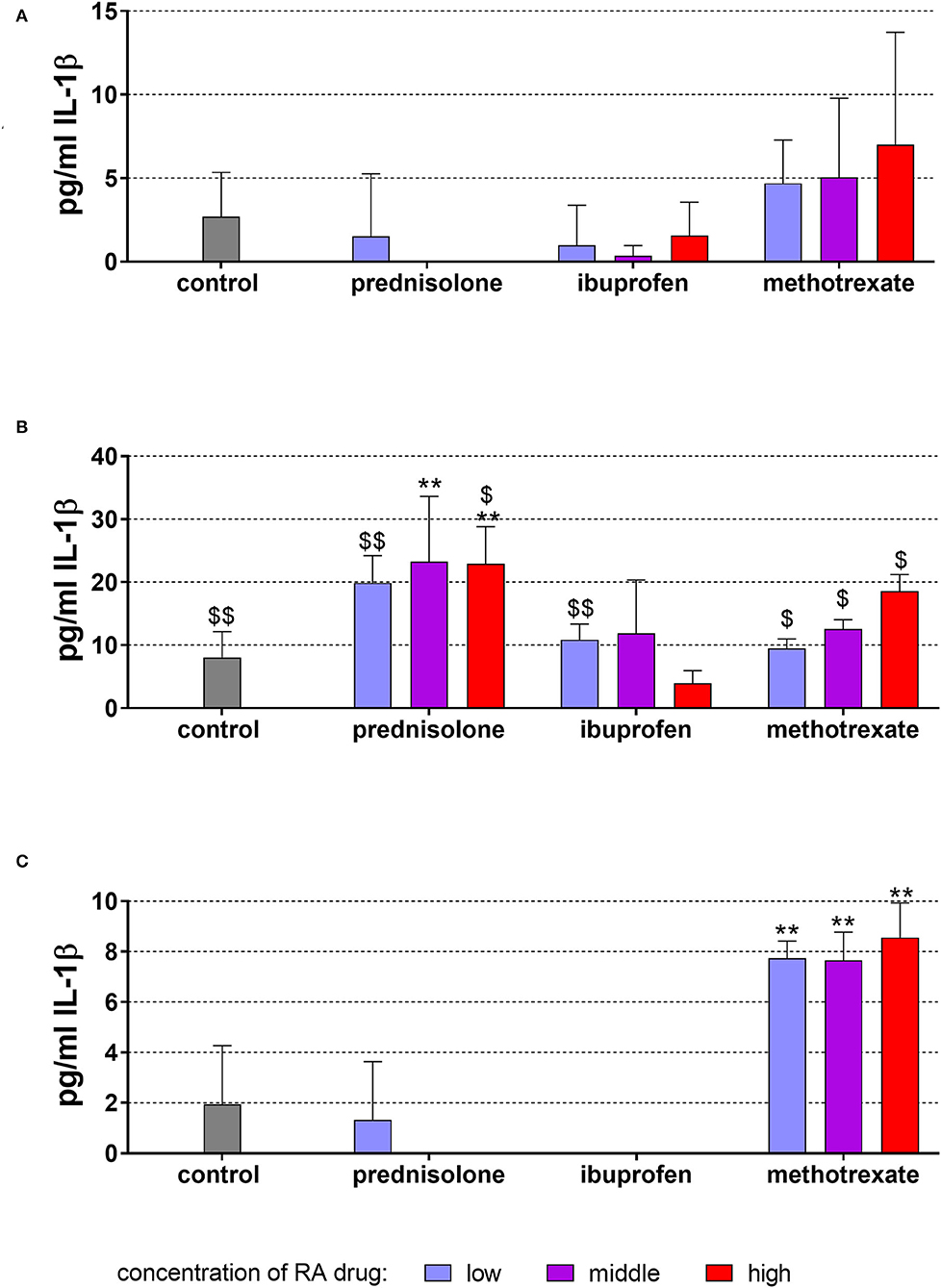
Figure 2. Released interleukin (IL)-1β by monocytic MONO-MAC-6 cells after 18 h of exposure of of 20 ng/ml (low), 100 ng/ml (middle), 500 ng/ml (high) prednisolone, 2 μg/ml (low), 10 μg/ml (middle), 50 μg/ml (high) ibuprofen, 1 μg/ml (low), 5 μg/ml (middle), and 25 μg/ml (high) methotrexate (A) w/o microbial compounds, (B) with Fusobacterium nucleatum lysate, and (C) Candida albicans lysate. The columns represent the mean and the bars represent standard deviation. *p < 0.05 vs. control, **p < 0.01 vs. control. $/$$p < 0.05/p < 0.01 vs. respective group w/o microbial compounds.
The exposure to F. nucleatum lysate increased the levels of IL-1β to 8.06 ± 4.10 pg/ml vs. unstimulated control (p < 0.001) (Figure 2B). Also in the presence of the RA drugs, the level of released IL-1β was most higher in the supernatants of the respective cells without microbes. Prednisolone in concentrations of 100 and 500 ng/ml elevated the amount of released IL-1β (p = 0.004, p = 0.006).
C. albicans lysate did not significantly change the released amount of IL-1β. Elevated levels of IL-1β were found in the presence of methotrexate in all three concentrations (each p < 0.001) (Figure 2C).
Without stimulation, the cells released on average 3.17 ± 2.66 pg/ml IL-8 after 18 h of incubation. The addition of drugs did not statistically significantly modify the release of IL-8 (Figure 3A).
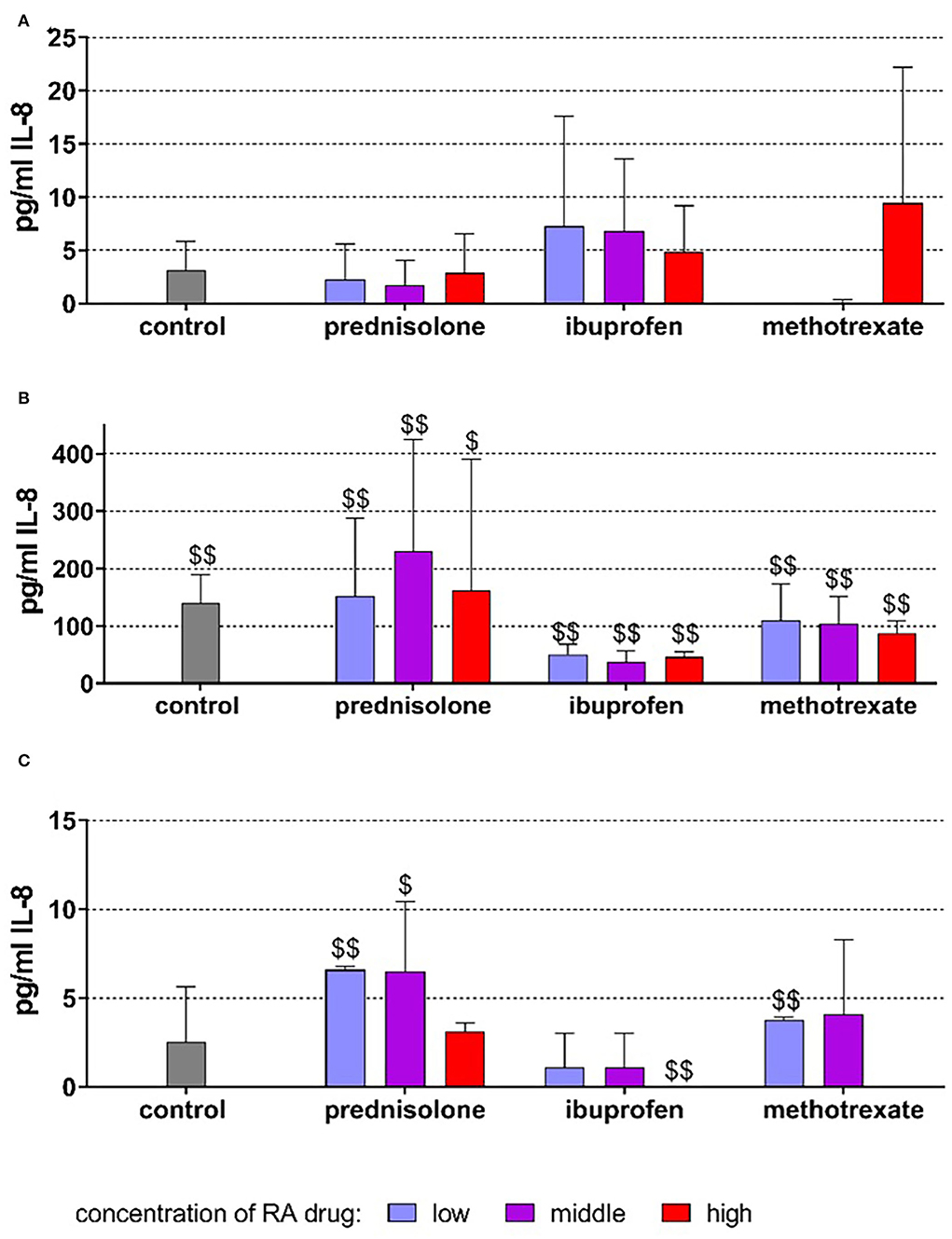
Figure 3. Released interleukin (IL)-8 by PDL fibroblasts after 18 h of exposure of 20 ng/ml (low), 100 ng/ml (middle), 500 ng/ml (high) prednisolone, 2 μg/ml (low), 10 μg/ml (middle), 50 μg/ml (high) ibuprofen, 1 μg/ml (low), 5 μg/ml (middle), and 25 μg/ml (high) methotrexate (A) w/o microbial compounds, (B) with Fusobacterium nucleatum lysate, and (C) Candida albicans lysate. The columns represent the mean and bars represent standard deviation. $/$$p < 0.05/p < 0.01 vs. respective group w/o microbial compound.
The exposure to F. nucleatum lysate increased the level to 140.33 ± 49.18 pg/ml (p < 0.001). The higher levels of IL-8 vs. non-bacteria exposed controls were also detected in the presence of the drugs (Figure 3B). C. albicans did not significantly change the released amount of IL-8 (2.52 ± 3.14 pg/ml). However, at low concentrations of prednisolone (p = 0.003) and methotrexate (p = 0.001), the amount of IL-8 was higher, and at 50 μg/ml, ibuprofen (p = 0.002) was lower than in cells without microbes (Figure 3C).
In these experiments, not microbial lysate but C. albicans cells were added. In the mean of 4.42 ± 0.67, log10 C. albicans cells attached to TIGK cells per well. In presence of RA drugs, always higher numbers were counted [0.31 log10 (100 ng/ml prednisolone) to 1.01 log10 (50 μg/ml ibuprofen)]. The differences were statistically significant for 2 μg/ml and 50 μg/ml ibuprofen (p = 0.013, p < 0.001) as well as for 25 μg/ml methotrexate (p = 0.048; Figure 4A).
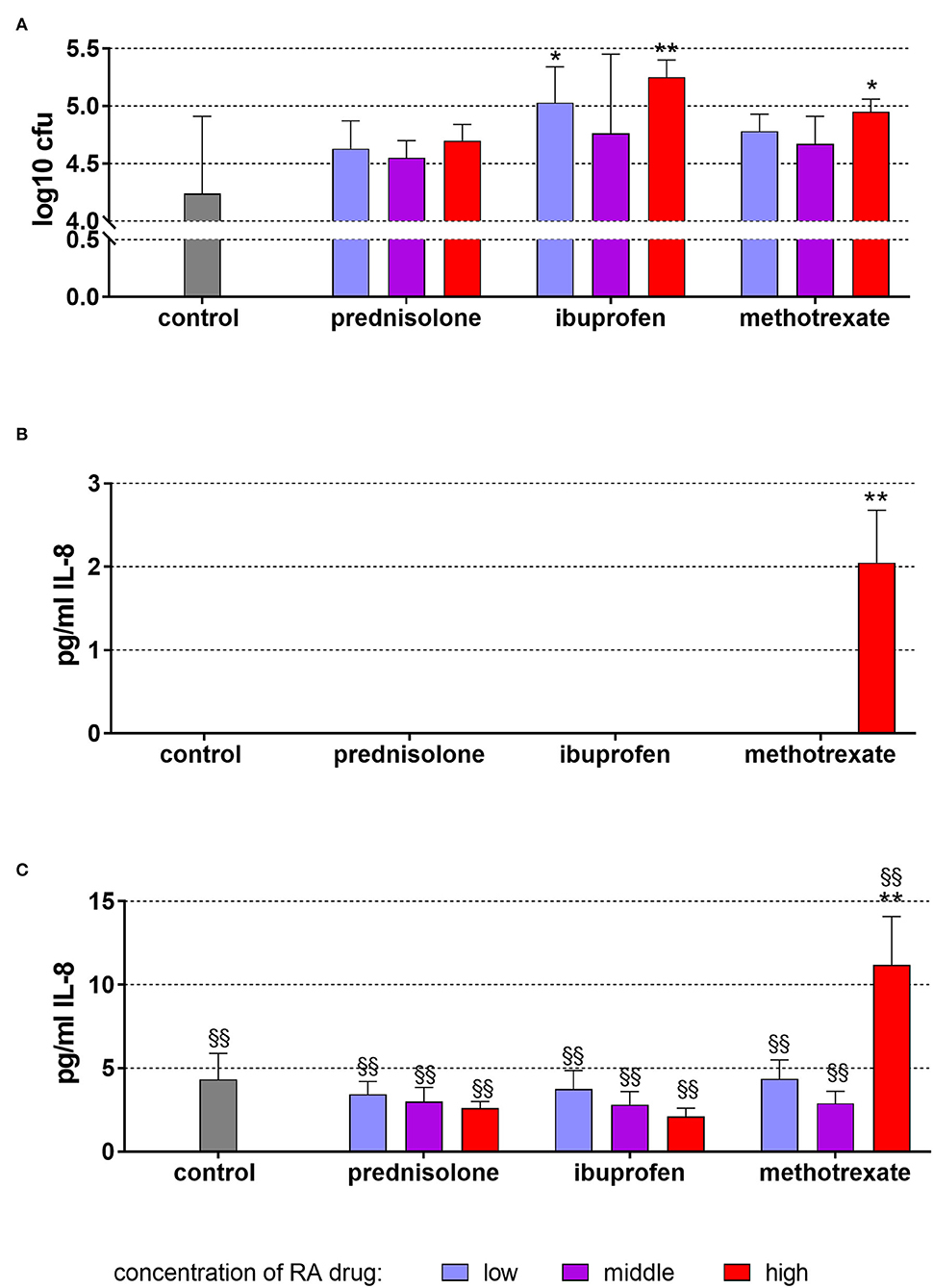
Figure 4. Attached (incl. invaded) Candida albicans to TIGK cells (A) and released IL-8 by TIGK cells after 6 h of exposure of 20 ng/ml (low), 100 ng/ml (middle), 500 ng/ml (high) prednisolone, 2 μg/ml (low), 10 μg/ml (middle), 50 μg/ml (high) ibuprofen, 1 μg/ml (low), 5 μg/ml (middle), and 25 μg/ml (high) methotrexate (B) w/o microbial compounds, and with (C) Candida albicans. The columns represent the mean and the bars represent standard deviation. */**p < 0.05/p < 0.01 vs. control, $/$$p < 0.05/p < 0.01 vs. respective group w/o microbial compound.
Without microbial stimulus, there was only IL-8 detectable after the addition of 25 μg/ml methotrexate and 6 h of incubation (Figure 4B). The exposure to C. albicans increased the IL-8 levels without dependency on added RA drugs (Figure 4C). Also here, higher IL-8 levels were measured after 25 μg/ml methotrexate vs. C. albicans only (p < 0.001).
In this study, the influence of anti-rheumatic drugs with and without microbial stimulus on oral cells was analyzed. The addition of F. nucleatum lysate stimulated most strongly the release of proinflammatory cytokines by PDL fibroblast and monocytic cells. In contrast, the modification by the tested anti-rheumatic drugs was mostly minor.
Among the oral bacteria, Porphyromonas gingivalis is discussed as the most important link between rheumatoid arthritis and periodontitis [26]. P. gingivalis possesses a peptidyl-arginine deiminase that is able to citrullinate bacterial and host proteins [27]. Recently, it has been shown that the outer membrane vesicles of P. gingivalis may contain about 50 citrullinated proteins [28]. In our first experiments, P. gingivalis ATCC 33277 lysate (obtained by suspending bacterial cells, exposing them to ultrasonication, centrifugation, and filtrating the supernatant) was used. But in supernatants of PDL fibroblasts exposed to P. gingivalis without and with anti-rheumatic drugs, IL-8 was never measurable. This can be explained by the content of active gingipains which can cleave and degrade IL-8 [29]. Therefore, in the following experiments, F. nucleatum was used. Compared to periodontal health, the genus Fusobacterium is elevated in gingivitis [30] and it was most increased among all genera in periodontitis [31]. F. nucleatum is a strong inducer of inflammatory cytokines in gingival fibroblasts [32]. This was confirmed in the present study with regard to PDL fibroblasts and, in particular, monocytic cells.
When exposed to non-professional immune cells, that is, PDL-fibroblasts, F. nucleatum without the addition of anti-rheumatic drugs led to an increased release of IL-8. This increase, however, was smaller than in MONO-MAC-6-cells. C. albicans did not affect IL-8 expression. In the assays, we used C. albicans in the logarithmic growth phase (blastospores). In gingival fibroblasts, germinated C. albicans and not blastospores stimulated the release of IL-8 [33]. Among the three different included cell types, MONO-MAC-6 cells represent professional immune cells. These cells are potent cytokine producers except for interferon γ [34]. They release IL-1β and high levels of IL-8, in particular, after stimulation with F. nucleatum lysate. In contrast to F. nucleatum, C. albicans did not stimulate the release of IL-8. Our preparation method of C. albicans may suggest that the used lysate contained cell wall compartments. One cell wall component, the β-glucan has been shown to increase (together with LPS) the release of the anti-inflammatory cytokine IL-10 and do not affect the release of pro-inflammatory cytokines by human monocytes [35]. This may explain the missing influence of C. albicans on the release of pro-inflammatory mediators in our study. Supernatants from a Candida-biofilm did neither change IL-8 mRNA expression in blood cells nor the release of the cytokine into the supernatant [36]. Combined with mixed bacteria, however, there was an increase up to 86-fold of mRNA expression together with a high release [36]. It might be a limitation of the study that we did not stimulate the cells with a lysate obtained from a multi-species mixture.
MONO-MAC-6 cells released high levels of IL-1β after stimulation with F. nucleatum lysate, however, not after stimulation with C. albicans.
Glucocorticoids are widely used in the therapy of RA patients; they reduce inflammatory cell counts in tissues and decrease levels of inflammatory cytokines such as TNFα, IL-1 β, IL-6, and IL-17 [37]. Clinically, in RA patients, administration of prednisolone decreased synovial gene expression and protein level of IL-8 but not of IL-1β [38, 39]. In the present in vitro study, prednisolone, in general, did not influence the release of proinflammatory cytokines by any cells. Only together with F. nucleatum, prednisolone augmented the release of IL-8 and to a minor degree of IL-1β by MONO-MAC-6-cells.
Our results did not find an influence of ibuprofen on the release of IL-8 or IL-1β. This contrasts with a study on bovine fibroblast-like synoviocytes where ibuprofen lowered the secretion of IL-1β after lipopolysaccharide stimulation [40]. Clinically, in orthodontic patients, the use of ibuprofen did not influence the increase of IL-1β level in the gingival crevicular fluid after placement of elastomeric separators [41]. In the past, adjunctive ibuprofen was discussed in periodontal therapy. A 2-week administration lowered clinical signs of periodontal inflammation, but only as a short-term effect [42].
Methotrexate interacts with folate pathways, adenosine, leukotrienes, and cytokines, except for RA patients, it is used for chronic sarcoidosis patients [43]. In RA patients, the increased number of neutrophils can be decreased by methotrexate [44]. An increase in IL-1 expression and protein release by methotrexate has been reported in the monocytic U937 cell line which was correlated with activation of the JUN and FOS pathways [45]. Also in the present in vitro study, methothrexate increased the release without microbial stimulus; however, the release level was lowered after F. nucleatum stimulation. Thus, an ongoing inflammation induced or stimulated by bacteria associated with periodontal disease might be inhibited. In treatment-naïve RA patients, systemic inflammatory variables decreased after a 12-week intake of methotrexate alone or in combination with an anti-TNF-drug; however, there was no influence on periodontal inflammation which was quantified by the periodontal inflamed surface area (PISA) [46]. Clinically, inflammation and severity of periodontitis seem to be associated with the type of RA drugs. RA patients receiving methotrexate and rituximab (an antibody targeting B-cells) or leflunomide (an immunosuppressant) presented fewer sites with bleeding on probing than those receiving methotrexate and TNFα inhibitor [47]. In a recent evaluation of the antimicrobial activity of DMARDs against oral pathogens, methotrexate was the only DMARD to exert antimicrobial activity, and only one against F. nucleatum and Viridans streptococci [48]. With periodontitis, the level of IL-1β in GCF is controversially reported in patients with RA in comparison with those without, some studies reported higher levels in RA [16, 49], others lower levels in RA [50], and another study again did not find a difference [51]. All the RA patients were under respective RA treatment, details on the used drugs related to IL-1β levels were not reported. In a prospective study including RA patients, the levels of IL-1β and IL-8 decreased in the gingival crevicular fluid after TNFα-therapy [52].
The intake of anti-rheumatic drugs is designated to decrease RA disease activity. An analysis of salivary IL-β determined higher levels in periodontitis and RA patients than in periodontally and systemically healthy controls. The RA patients were under treatment with several DMRADs; subgrouping resulted in lower IL-1β levels in patients who received anti-TNFα therapy in comparison to the other RA patients [53].
In our recent analysis of microbiota in subgingival biofilm by using cultivation, surprisingly often yeasts were detected in RA patients which was not the case in periodontitis patients without RA [20]. Hence, we included C. albicans in that in vitro study. Methotrexate and ibuprofen increased the adhesion of C. albicans to gingival epithelial cells. Which mechanism, however, traffics the higher adhesion of C. albicans to host cells remains unclear. In C. albicans, Als3 functions as the adhesin to E-cadherin on oral epithelial cells and mediates endocytosis [54].
This study has several limitations. Only isolated cells were included. The in vivo immune response, however, is a result of concerted action of different cell types. Further, the model organism was F. nucleatum, but dental biofilm consists of hundreds of bacterial species. Then, the levels of cytokines were determined only after one single time point, whereas periodontal inflammation is a time-dependent process. Finally, lysates and not the whole bacteria were used.
Taken together, the release of inflammatory cytokines by professional and non-professional immune cells was mainly induced by bacterial stimuli. Among the tested anti-inflammatory drugs, methotrexate and prednisolone may interfere with the interaction of microorganisms with monocytic cells. Prednisolone increases the release of IL-8 after stimulation with F. nucleatum, whereas methotrexate decreased the level. Anti-inflammatory drugs increased the adherence of C. albicans to epithelial cells. Also, in patients with RA, the reduction of the microbial load in subgingival biofilm (biofilm removal) is of major importance to decrease the inflammatory response, however, the intake of inflammatory drugs may lead to untypical clinical signs of periodontitis in RA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent was obtained from all participants for their participation in this study.
SE and ASt: conceptualization, methodology, and writing. ASt, CS, and GZ: experiments. SE and ASc: interpretation. SE: statistical analyses and supervision. All authors: review and editing. All authors have agreed to the published version of the manuscript.
The study was entirely funded by the Department of Periodontology, University of Bern, Bern, Switzerland. Open access funding was provided by the University of Bern.
The excellent technical support by Fabiola Costanzo (Dept. of Periodontology, University of Bern) is highly appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. (2014) 93:1045–53. doi: 10.1177/0022034514552491
2. Van Dyke TE, Bartold PM, Reynolds EC. The nexus between periodontal inflammation and dysbiosis. Front Immunol. (2020) 11:511. doi: 10.3389/fimmu.2020.00511
3. Llama-Palacios A, Potupa O, Sanchez MC, Figuero E, Herrera D, Sanz M. Proteomic analysis of Fusobacterium nucleatum growth in biofilm versus planktonic state. Mol Oral Microbiol. (2020) 35:168–80. doi: 10.1111/omi.12303
4. Marchesan JT, Girnary MS, Moss K, Monaghan ET, Egnatz GJ, Jiao Y, et al. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol. (2020) 82:93–114. doi: 10.1111/prd.12269
5. Palomino DC, Marti LC. Chemokines and immunity. Einstein. (2015) 13:469–73. doi: 10.1590/S1679-45082015RB3438
6. Finoti LS, Nepomuceno R, Pigossi SC, Corbi SC, Secolin R, Scarel-Caminaga RM. Association between interleukin-8 levels and chronic periodontal disease: a PRISMA-compliant systematic review and meta-analysis. Medicine. (2017) 96:e6932. doi: 10.1097/MD.0000000000006932
7. Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I Risk indicators for attachment loss. J Periodontol. (1994) 65:260–7. doi: 10.1902/jop.1994.65.3.260
8. Lee KH, Choi YY. Rheumatoid arthritis and periodontitis in adults: using the Korean National Health Insurance Service-National Sample Cohort. J Periodontol. (2020) 91:1186–93. doi: 10.1002/JPER.19-0311
9. Sparks J. A. (2019). Rheumatoid arthritis. Ann. Intern. Med. 170:ITC1–16. doi: 10.7326/AITC201901010
10. An Q, Yan W, Zhao Y, Yu K. Enhanced neutrophil autophagy and increased concentrations of IL-6, IL-8, IL-10 and MCP-1 in rheumatoid arthritis. Int Immunopharmacol. (2018) 65:119–28. doi: 10.1016/j.intimp.2018.09.011
11. Dai Y, Hu S. Recent insights into the role of autophagy in the pathogenesis of rheumatoid arthritis. Rheumatology. (2016) 55:403–10. doi: 10.1093/rheumatology/kev337
12. Dayer JM. The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. Joint Bone Spine. (2002) 69:123–32. doi: 10.1016/S1297-319X(02)00363-9
13. Akram M, Daniyal M, Sultana S, Owais A, Akhtar N, Zahid R, et al. Traditional and modern management strategies for rheumatoid arthritis. Clin Chim Acta. (2021) 512:142–55. doi: 10.1016/j.cca.2020.11.003
14. Keysser G. Safety aspects of the treatment with glucocorticoids for rheumatoid arthritis. Z Rheumatol. (2021) 80:295–304. doi: 10.1007/s00393-021-00972-x
15. Bender P, Burgin WB, Sculean A, Eick S. Serum antibody levels against Porphyromonas gingivalis in patients with and without rheumatoid arthritis - a systematic review and meta-analysis. Clin Oral Investig. (2017) 21:33–42. doi: 10.1007/s00784-016-1938-5
16. Bender P, Egger A, Westermann M, Taudte N, Sculean A, Potempa J, et al. Expression of human and Porphyromonas gingivalis glutaminyl cyclases in periodontitis and rheumatoid arthritis-A pilot study. Arch Oral Biol. (2019) 97:223–30. doi: 10.1016/j.archoralbio.2018.10.022
17. Abusleme L, Hoare A, Hong BY, Diaz PI. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. (2021) 86:57–78. doi: 10.1111/prd.12362
18. Ebbers M, Lubcke PM, Volzke J, Kriebel K, Hieke C, Engelmann R, et al. Interplay between P. gingivalis, F nucleatum and A actinomycetemcomitans in murine alveolar bone loss, arthritis onset and progression. Sci Rep. (2018) 8:15129. doi: 10.1038/s41598-018-33129-z
19. Temoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. (2012) 18:117–21. doi: 10.1097/RHU.0b013e3182500c95
20. Maldonado A, Pirracchio L, Imber JC, Burgin W, Moller B, Sculean A, et al. Citrullination in periodontium is associated with Porphyromonas gingivalis. Arch Oral Biol. (2020) 114:104695. doi: 10.1016/j.archoralbio.2020.104695
21. Laugisch O, Wong A, Sroka A, Kantyka T, Koziel J, Neuhaus K, et al. Citrullination in the periodontium–a possible link between periodontitis and rheumatoid arthritis. Clin Oral Investig. (2016) 20:675–83. doi: 10.1007/s00784-015-1556-7
22. Eick S, Goltz S, Nietzsche S, Jentsch H, Pfister W. Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int. (2011) 42:687–700.
23. Bischoff KB. Physiologically based pharmacokinetic modelling. In: Drinking Water and Health, Vol. 8. Pharmacokinetics in Risk Assessment (Washington, D.C.: National Academy of Science) (1987).
24. Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. (2009) 17:275–342. doi: 10.1007/s10787-009-0016-x
25. Magee MH, Blum RA, Lates CD, Jusko WJ. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. (2001) 41:1180–94. doi: 10.1177/00912700122012733
26. Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. (2017) 13:606–20. doi: 10.1038/nrrheum.2017.132
27. Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. (2010) 62:2662–72. doi: 10.1002/art.27552
28. Larsen DN, Mikkelsen CE, Kierkegaard M, Bereta GP, Nowakowska Z, Kaczmarek JZ, et al. Citrullinome of porphyromonas gingivalis outer membrane vesicles: confident identification of citrullinated peptides. Mol Cell Proteomics. (2020) 19:167–80. doi: 10.1074/mcp.RA119.001700
29. Mikolajczyk-Pawlinska J, Travis J, Potempa J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. (1998) 440:282–6. doi: 10.1016/S0014-5793(98)01461-6
30. Nowicki EM, Shroff R, Singleton JA, Renaud DE, Wallace D, Drury J, et al. Microbiota and metatranscriptome changes accompanying the onset of gingivitis. MBio. (2018) 9: e00518–75. doi: 10.1128/mBio.00575-18
31. López-Martínez J, Chueca N, Padial-Molina M, Fernandez-Caballero JA, García F, O'valle F, et al. Bacteria associated with periodontal disease are also increased in health. Med Oral Patol Oral Cir Bucal. (2020) 25:e745–51. doi: 10.4317/medoral.23766
32. Jang JY, Song IS, Baek KJ, Choi Y, Ji S. Immunologic characteristics of human gingival fibroblasts in response to oral bacteria. J Periodontal Res. (2017) 52:447–57. doi: 10.1111/jre.12410
33. Dongari-Bagtzoglou A, Wen K, Lamster IB. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol Immunol. (1999) 14:364–70. doi: 10.1034/j.1399-302X.1999.140606.x
34. Neustock P, Brand JM, Kruse A, Kirchner H. Cytokine production of the human monocytic cell line Mono Mac 6 in comparison to mature monocytes in peripheral blood mononuclear cells. Immunobiology. (1993) 188:293–302. doi: 10.1016/S0171-2985(11)80237-8
35. Leonhardt J, Gross S, Marx C, Siwczak F, Stengel S, Bruns T, et al. Candida albicans β-glucan differentiates human monocytes into a specific subset of macrophages. Front Immunol. (2018) 9:2818. doi: 10.3389/fimmu.2018.02818
36. Bhardwaj RG, Ellepolla A, Drobiova H, Karched M. Biofilm growth and IL-8 & TNF-alpha-inducing properties of Candida albicans in the presence of oral gram-positive and gram-negative bacteria. BMC Microbiol. (2020) 20:156. doi: 10.1186/s12866-020-01834-3
37. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. (2016) 42: 33–46, vii. doi: 10.1016/j.rdc.2015.08.006
38. Youssef PP, Haynes DR, Triantafillou S, Parker A, Gamble JR, Roberts-Thomson PJ, et al. Effects of pulse methylprednisolone on inflammatory mediators in peripheral blood, synovial fluid, and synovial membrane in rheumatoid arthritis. Arthritis Rheum. (1997) 40:1400–8. doi: 10.1002/art.1780400807
39. Gerlag DM, Boyle DL, Rosengren S, Nash T, Tak PP, Firestein GS. Real-time quantitative PCR to detect changes in synovial gene expression in rheumatoid arthritis after corticosteroid treatment. Ann Rheum Dis. (2007) 66:545–7. doi: 10.1136/ard.2006.059790
40. Maghsoudi H, Hallajzadeh J, Rezaeipour M. Evaluation of the effect of polyphenol of escin compared with ibuprofen and dexamethasone in synoviocyte model for osteoarthritis: an in vitro study. Clin Rheumatol. (2018) 37:2471–8. doi: 10.1007/s10067-018-4097-z
41. Kaya Y, Alkan Ö, Kömüroglu AU, Keskin S. Effects of ibuprofen and low-level laser therapy on orthodontic pain by means of the analysis of interleukin 1-beta and substance P levels in the gingival crevicular fluid. J Orofac Orthop. (2021) 82:143–52. doi: 10.1007/s00056-020-00254-2
42. Taiyeb Ali TB, Waite IM. The effect of systemic ibuprofen on gingival inflammation in humans. J Clin Periodontol. (1993) 20:723–8. doi: 10.1111/j.1600-051X.1993.tb00697.x
43. Maksimovic V, Pavlovic-Popovic Z, Vukmirovic S, Cvejic J, Mooranian A, Al-Salami H, et al. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol Biol Rep. (2020) 47:4699–708. doi: 10.1007/s11033-020-05481-9
44. Perpétuo IP, Caetano-Lopes J, Rodrigues AM, Campanilho-Marques R, Ponte C, Canhão H, et al. Methotrexate and low-dose prednisolone downregulate osteoclast function by decreasing receptor activator of nuclear factor-κβ expression in monocytes from patients with early rheumatoid arthritis. RMD Open. (2017) 3:e000365. doi: 10.1136/rmdopen-2016-000365
45. Olsen NJ, Spurlock CF 3rd, Aune TM. Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis Res Ther. (2014) 16, R17. doi: 10.1186/ar4444
46. De Smit MJ, Westra J, Posthumus MD, Springer G, Van Winkelhoff AJ, Vissink A, et al. Effect of anti-rheumatic treatment on the periodontal condition of rheumatoid arthritis patients. Int J Environ Res Public Health. (2021) 18:2529. doi: 10.3390/ijerph18052529
47. Ziebolz D, Rupprecht A, Schmickler J, Bothmann L, Krämer J, Patschan D, et al. Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: Results from a cross-sectional study. J Periodontol. (2018) 89:1310–7. doi: 10.1002/JPER.17-0616
48. Kussmann M, Obermueller M, Spettel K, Winkler S, Aletaha D. In vitro evaluation of disease-modifying antirheumatic drugs against rheumatoid arthritis associated pathogens of the oral microflora. RMD Open. (2021) 7:e001737. doi: 10.1136/rmdopen-2021-001737
49. Xiao F, Li C, Lin Y, Peng Z, Xu X, Wen Y, et al. Increased risk of periodontitis occurrence in patients with rheumatoid arthritis and its association with the levels of IL-1β and TNF-α in gingival crevicular fluid. Ann Palliat Med. (2021) 10:9078–87. doi: 10.21037/apm-21-1782
50. Miranda LA, Islabão AG, Fischer RG, Figueredo CM, Oppermann RV, Gustafsson A. Decreased interleukin-1beta and elastase in the gingival crevicular fluid of individuals undergoing anti-inflammatory treatment for rheumatoid arthritis. J Periodontol. (2007) 78:1612–9. doi: 10.1902/jop.2007.060520
51. Cetinkaya B, Guzeldemir E, Ogus E, Bulut S. Proinflammatory and anti-inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. J Periodontol. (2013) 84:84–93. doi: 10.1902/jop.2012.110467
52. Üstün K, Erciyas K, Kisacik B, Sezer U, Pehlivan Y, Öztuzcu S, et al. Host modulation in rheumatoid arthritis patients with TNF blockers significantly decreases biochemical parameters in periodontitis. Inflammation. (2013) 36:1171–7. doi: 10.1007/s10753-013-9652-9
53. Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR 3rd, Ebersole JL, Miller CS. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. (2010) 37:1068–74. doi: 10.1111/j.1600-051X.2010.01625.x
Keywords: periodontitis, rheumatoid arthritis, anti-inflammatory drugs, proinflammatory cytokines, oral cells
Citation: Stähli A, Scherler C, Zappalà G, Sculean A and Eick S (2022) In vitro activity of anti-rheumatic drugs on release of pro-inflammatory cytokines from oral cells in interaction with microorganisms. Front. Oral. Health 3:960732. doi: 10.3389/froh.2022.960732
Received: 03 June 2022; Accepted: 04 August 2022;
Published: 02 September 2022.
Edited by:
João Botelho, Egas Moniz Interdisciplinary Research Center, PortugalReviewed by:
Oleh Andrukhov, University Dental Clinic Vienna, AustriaCopyright © 2022 Stähli, Scherler, Zappalà, Sculean and Eick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra Stähli, YWxleGFuZHJhLnN0YWVobGlAdW5pYmUuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.