
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 April 2025
Sec. Thoracic Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1569517
 Sama I. Sayin1,2
Sama I. Sayin1,2 Ella A. Eklund1,2,3
Ella A. Eklund1,2,3 Kevin X. Ali2,3
Kevin X. Ali2,3 Jozefina J. Dzanan2,3
Jozefina J. Dzanan2,3 Moe Xylander2
Moe Xylander2 Martin Dankis2,3
Martin Dankis2,3 Per Lindahl4,5
Per Lindahl4,5 Volkan I. Sayin2,3
Volkan I. Sayin2,3 Andreas Hallqvist1,6
Andreas Hallqvist1,6 Clotilde Wiel2*
Clotilde Wiel2*Background: Metastatic organotropism in lung cancer significantly influences prognosis, yet current treatment and clinical management guidelines are largely generalized for metastatic disease, regardless of organ site involvement. Notably, up to 30% of non-small cell lung cancer (NSCLC) patients present with brain metastases (BM) at diagnosis, underscoring the need for a more nuanced understanding of metastatic patterns. However, real-world clinical data on metastatic organotropism in well-characterized patient cohorts remain surprisingly scarce. Here, we evaluate patterns of metastasis, clinical characteristics and survival outcomes in patients with lung adenocarcinoma (LUAD), the major histological NSCLC subtype.
Methods: We performed a multi-center retrospective study including 913 stage IV LUAD patients, diagnosed and molecularly assessed in western Sweden between 2016–2021. Our primary study outcome was the distribution of specific metastatic sites and its impact on Overall Survival (OS).
Results: Out of 913 stage IV LUAD patients, 23.4% had BM. These patients exhibited markedly different metastatic patterns compared to those without BM, and median survival was significantly shorter (6 months) than those without BM (7.8 months) (p = 0.021). In addition, more than one metastatic tumor in the brain coincided with worse OS, compared to those with no, or with only one metastatic tumor in the brain. Importantly, OS was also influenced by metastasis in specific extracranial organs, like the pleura and lungs.
Conclusions: Our study highlights the distinct metastatic patterns and survival outcomes associated with BM in stage IV LUAD. These findings emphasize the need for site-specific approaches in managing metastatic disease due to BM’s impact on survival.
Lung cancer is the most prevalent and lethal cancer worldwide, and majority of patients are diagnosed with advanced metastatic disease (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all cases, and among them lung adenocarcinoma (LUAD) is the most abundant histological subtype (1). While recent advances in treatment strategies like targeted- and immunotherapy have greatly improved outcomes for patients with early stage and locally advanced disease, no curative treatments exist till date for metastatic disease, which remains the leading cause of mortality in these patients (2). Identification of novel prognostic factors to further guide clinical management of metastatic disease is therefore crucial and urgent.
It is now well-established that metastatic dissemination of primary tumors throughout the body is not random, and solid tumors metastasize preferentially to certain organs, a process termed metastatic organotropism. While this phenomenon has been studied extensively in animal models (3, 4), few studies until recently have reported metastatic organotropism patterns of lung cancer in the clinic setting. Emerging evidence now suggests that metastatic organotropism in lung cancer patients is strongly related to previously well-established prognostic factors such as age (5, 6), oncogenic driver mutations (7), histological subtypes (8), as well as response to treatment (5, 7, 9).
Importantly, current treatment and clinical management recommendations apply broadly for metastatic disease independent of organ site involvement (10). While this can be followed in practice for metastasis to other organs, metastatic involvement of the brain requires specialized treatment and management strategies in clinical reality, owing to the sensitivity of the anatomical location and the highly selective permeability of the blood-brain barrier to systemic treatment agents (11). Nevertheless, BM is reported at diagnosis in 25-29% of NSCLC patients with metastatic disease and up to 50% will develop BM during the disease course (12).
Importantly, patients with BM have significantly worse prognosis than those with only extracranial metastatic disease (11, 13–15). In addition, BM patients with stable intracranial metastatic disease who have progressive extracranial disease have worse prognosis than those with stable extracranial disease (16). Metastatic involvement of certain extracranial organs but not others have been shown to affect response to immunotherapy among BM patients (17, 18). While preclinical studies have provided possible explanations for these differences through demonstrating unique biological phenotypes of BM compared to primary tumors and their metastases in other organ sites (7, 19), clinical data to aid further stratification within BM patient group to guide clinical management based on extracranial metastatic disease patterns are lacking.
Real-world clinical data on metastatic organotropism in well-characterized patient cohorts are surprisingly scarse, and studies of organotropism in relation to BM and its effect on clinical outcomes are lacking. Here, we report in detail metastatic organotropism in relation to BM and related clinical outcomes in all patients with stage IV/metastatic lung adenocarcinoma (LUAD) in the West Sweden cohort (9, 20). We present a comprehensive report on patterns of metastasis, clinical characteristics and survival outcomes in western Sweden by combining data from the Swedish Lung Cancer Registry (SLCR) with 95% coverage, manual health chart data curation and histopathological analyses.
By combining data about metastatic sites recorded in the SLCR and through data curation from health charts to identify sites of metastasis unreported/not included in original report. The Swedish healthcare system is primarily government-funded and provides universal access to all citizens. Therefore, patients have equal access to diagnostic examinations and treatments.
We conducted a multi-center retrospective study including all consecutive NSCLC patients diagnosed with Stage IV LUAD and having molecular assessment performed between 2016–2021 in western Sweden (n = 913). Patient demographics (including age, gender, Eastern Cooperative Oncology Group (ECOG) performance status and smoking history), cancer stage, sites of metastasis, pathological details (histology, mutation status) and outcome data were retrospectively collected from patient charts and the Swedish Lung Cancer Registry. All patients had CT scans of the thorax and abdomen as part of the routine diagnostic workup. Pleural metastasis is defined as either a visual mass on the CT scan or malignant cells in pleural effusion confirmed by cytological assessment. Clinical staging was based on TNM staging guidelines 7th edition until 2018 and based on TNM staging 8th edition thereafter. Approval from the Swedish Ethical Review Authority (Dnr 2019-04771 and 2021-04987) was obtained prior to study commencement.
The primary outcome of this study was presence of metastasis in given organ sites at diagnosis and overall survival (OS), defined as the interval between the date of diagnostic sample collection and the date of death from any cause. Patients alive or lost to follow-up were censored at the cut-off date or last contact. Median follow-up time was 35 months (95% CI 31.1–38.9) and was estimated using the reverse Kaplan–Meier method. One patient died before final diagnosis and is thus excluded from the OS analysis. We compared OS stratified on metastatic organ involvement for the entire cohort. BM diagnosed within 8 weeks from date of diagnostic sample collection was considered as diagnosed at baseline. Data cut-off date was 2024-09-17.
Clinical characteristics were summarized using descriptive statistics and evaluated with univariate analysis in table form. Distribution of metastatic sites was assessed with Pearson Correlation. Survival was estimated using the Kaplan–Meier method. Log-rank test was used to assess significant differences in OS between groups. Multivariable Cox regression analyses were conducted to compensate for potential confounders. Statistical significance was set at p < 0.05, and no adjustments were made for multiple comparisons. Data analysis was conducted using IBM SPSS Statistics version 27 and R version 3.4.
All consecutively diagnosed patients with LUAD in West Sweden between 2016-2021 with molecular assessment were included in this study. Total 913 patients with stage IV LUAD were included in the study (Figure 1) and clinical characteristics are summarized in Table 1.
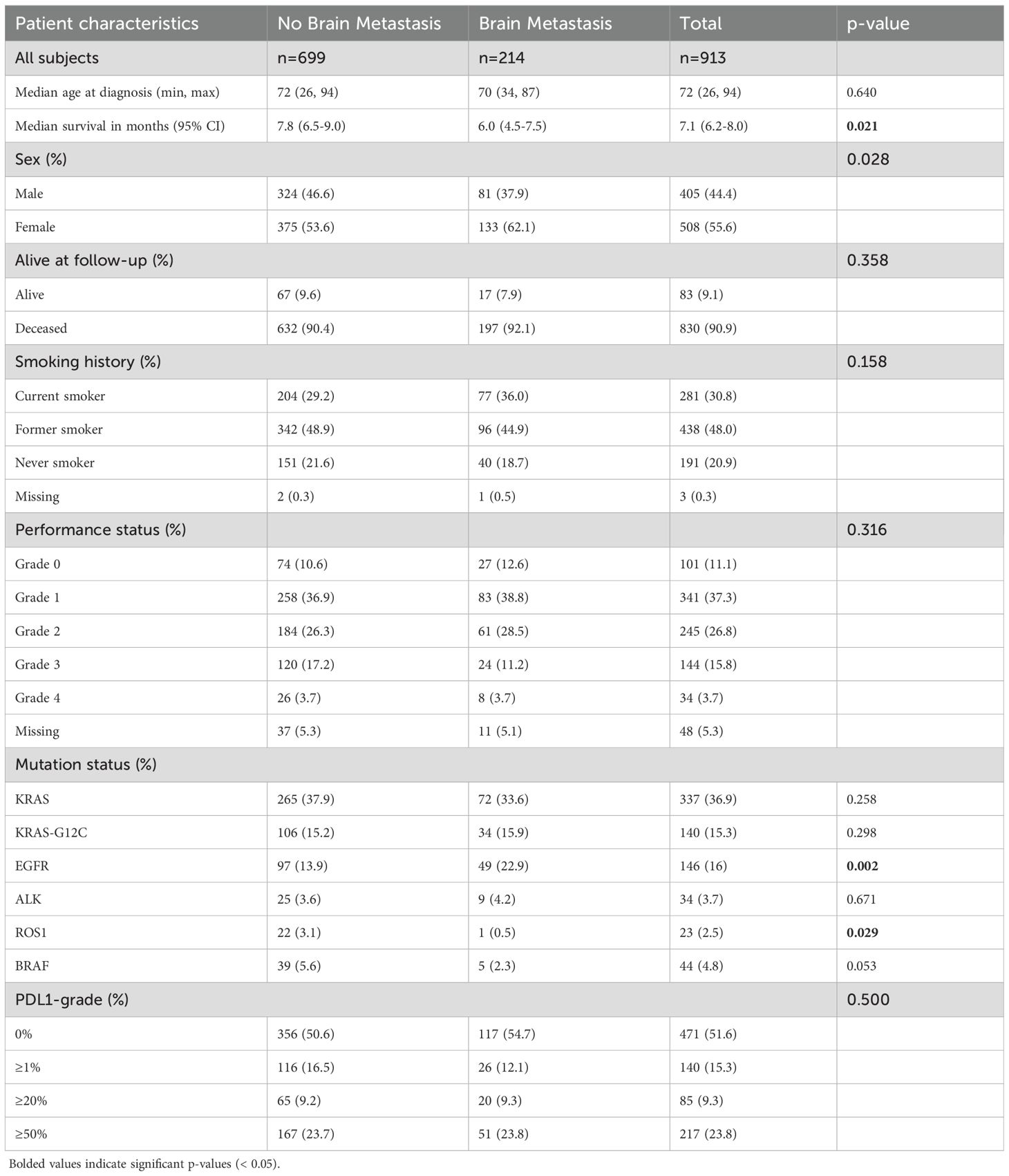
Table 1. Characteristics of the total cohort as well as stratified by presence or absence of brain metastasis at diagnosis.
Of all patients with metastatic disease, those with BM had significantly lower median survival (6 months) than those without BM (7.8 months) (p = 0.021). There were significantly higher proportion of females in the BM group (62.1%) than No BM group (53.6%) (p = 0.028). KRAS was the most frequently mutated gene in primary tumors of both groups, while significantly higher proportion of BM patients had mutations in EGFR (22.9% vs 13.9%; p = 0.002).
First, we mapped the distribution of metastatic sites for each patient with stage IV disease. We identified subgroups by specific organs involved and mapped individual pattern for each patient (Figure 2; Supplementary Figure 1). Most patients had metastasis to the bone (38.6%, n = 352), followed by the lung (27.8%, n = 254), pleura (24.8%, n = 226) and brain (23.4%, n = 214) (Figure 3; Supplementary Figure 2A). Liver was the site with the least number of patients with metastatic involvement (13.8%, n = 94) followed by adrenal gland (16.9%, n = 123). Importantly, up to 54% of patients with pleura metastasis had no other metastatic organ involvement, followed by lung (44.1%) and brain (39.9%) as sites with higher levels of single organ metastasis. In contrast, only 22.7% of all patients with liver- and 15.7% with adrenal metastasis did not involve any other organs (Figure 3).
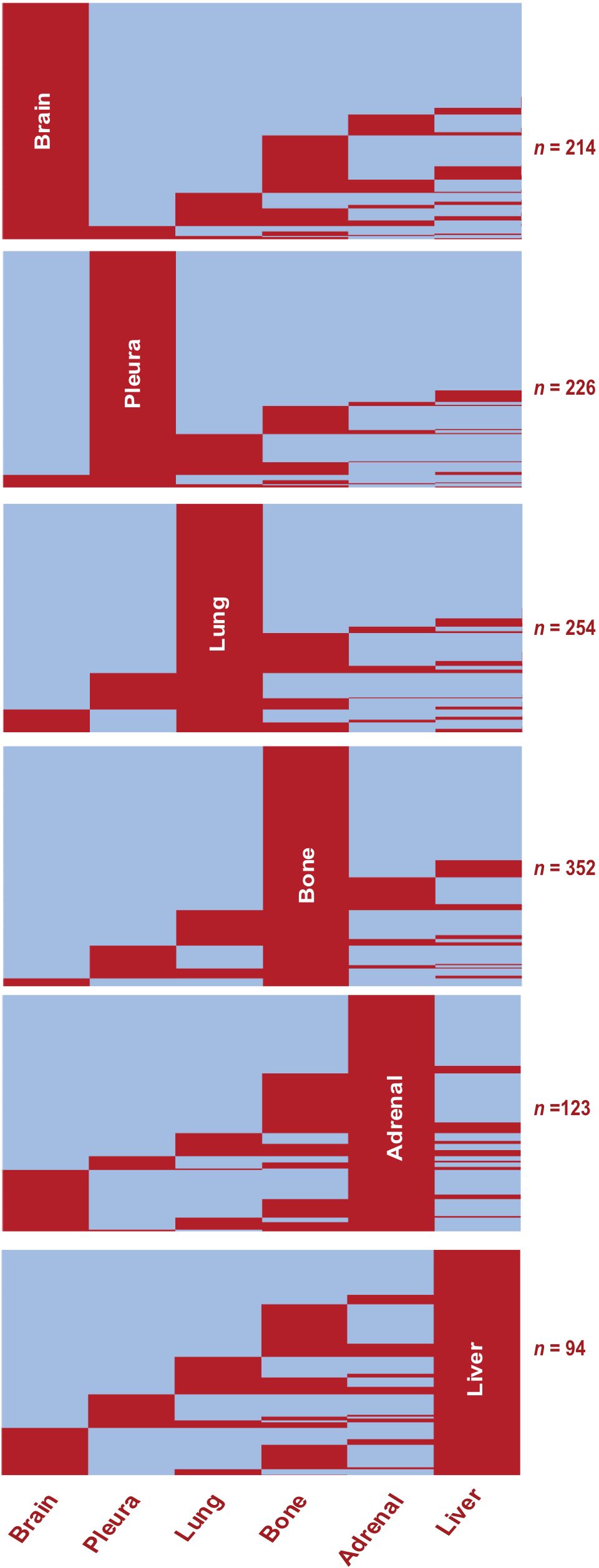
Figure 2. Metastatic Organotropism in Stage IV LUAD. Heatmaps showing presence (red) or absence (blue) of metastasis at given organ sites in the study population (n = 913). Rows represent individual patients. Subgroups show pattern of metastasis among all patients with the given organ site involvement.
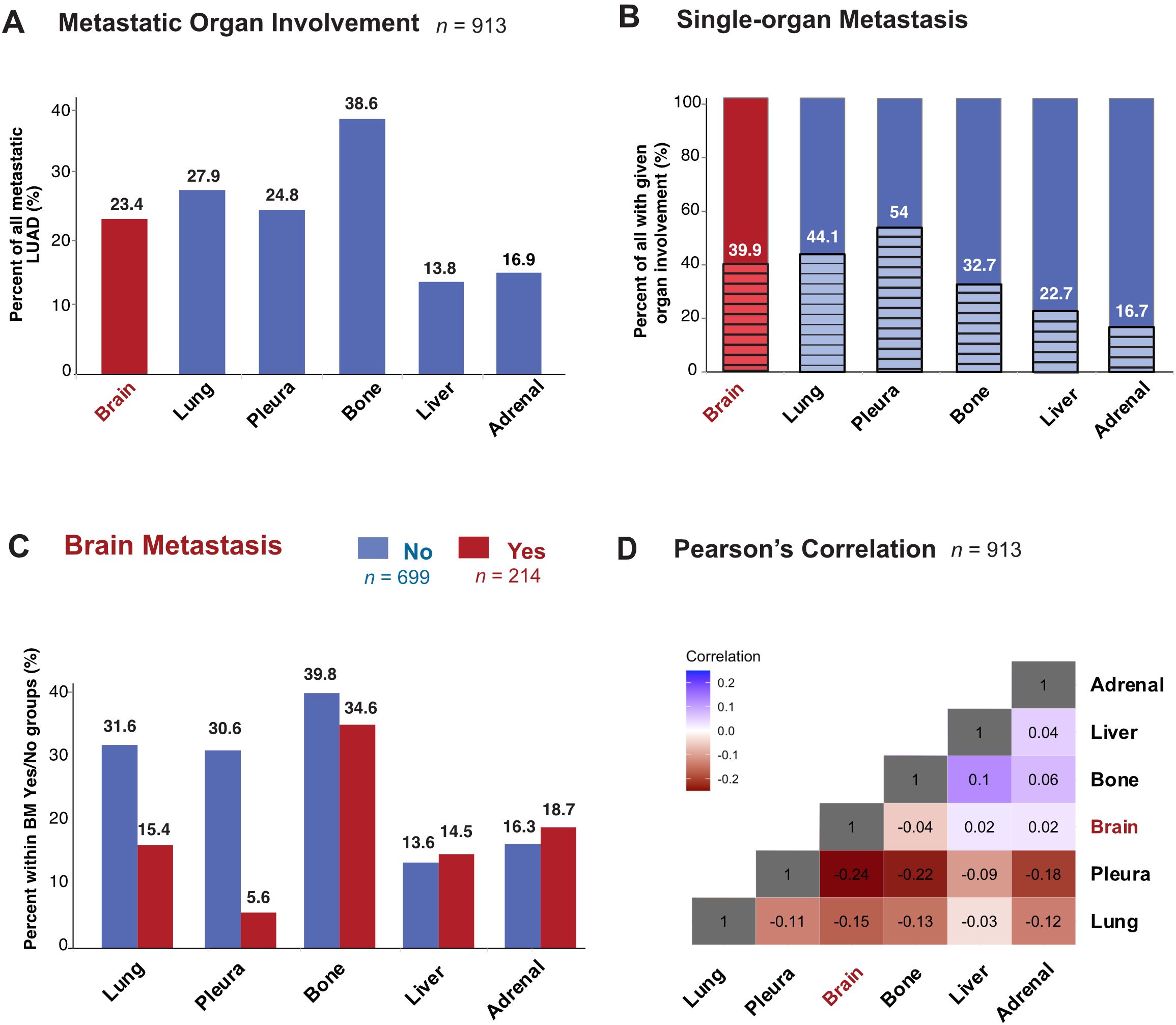
Figure 3. Metastatic Organotropism in relation to BM in stage IV LUAD. (A) Percentage of all patients in the study population with metastatic involvement of given organ site at diagnosis. (B) Percentage of all patients with given organ site involvement with single-organ metastasis (C) Percentage with metastatic involvement of given organ site at diagnosis in subgroups with BM (red) and without BM (blue). (D) Heatmap showing Pearson’s correlation values between pairs of organ sites of metastasis.
Next, to study organotropism of LUAD in relation to the brain, we first sub-grouped patients into those with (n = 214) or without (n = 699) BM (Figure 3). Bone was the organ of metastasis for most patients with (34.6%) or without BM (39.8%). The percentage of patients with metastasis to the liver and adrenal were similar regardless of brain involvement. In contrast to the bone, adrenal and liver, where the proportions were similar between BM or no BM groups, there were large differences in metastasis to the pleura and lung depending on brain involvement status. The most dramatic difference was seen in patients with BM, who unlike those without BM, had only 5.6% metastasis to the pleura, while those without BM 30.6% had metastasis to the pleura. Similarly, in the lung, while metastatic involvement in no BM group was 31.6%, only 15.4% of stage IV LUAD with metastases in the brain also had metastases in the lung. Pearson’s correlation coefficient was accordingly lowest (-0.24) between the pleura and brain, while liver and bone had the highest correlation (0.1) (Figure 3; Supplementary Figure 2B).
We found that among all patients with metastasized LUAD, presence of metastasis in the brain correlated significantly (p = 0.019) with worse OS (6.0 months; 95% CI 4.5-7.5) compared with patients with metastatic disease without brain involvement (7.8 months; 95% CI 6.5-9.1) (Figure 4). Interestingly, having more than one metastatic tumor in the brain corresponded with significantly worse OS (4.7 months; 95% CI 3.0-6.4) compared to those without (7.8 months; 95% CI 6.5-9.1) or with only one metastatic lesion in the brain (8.1 months; 95% CI 5.4-10.9) (Figure 4). Multivariate analysis showed BM as the second most significant variable affecting OS, with less effect than ECOG but similar effect to smoking and greater effect than age at diagnosis on survival outcomes (Figure 4).
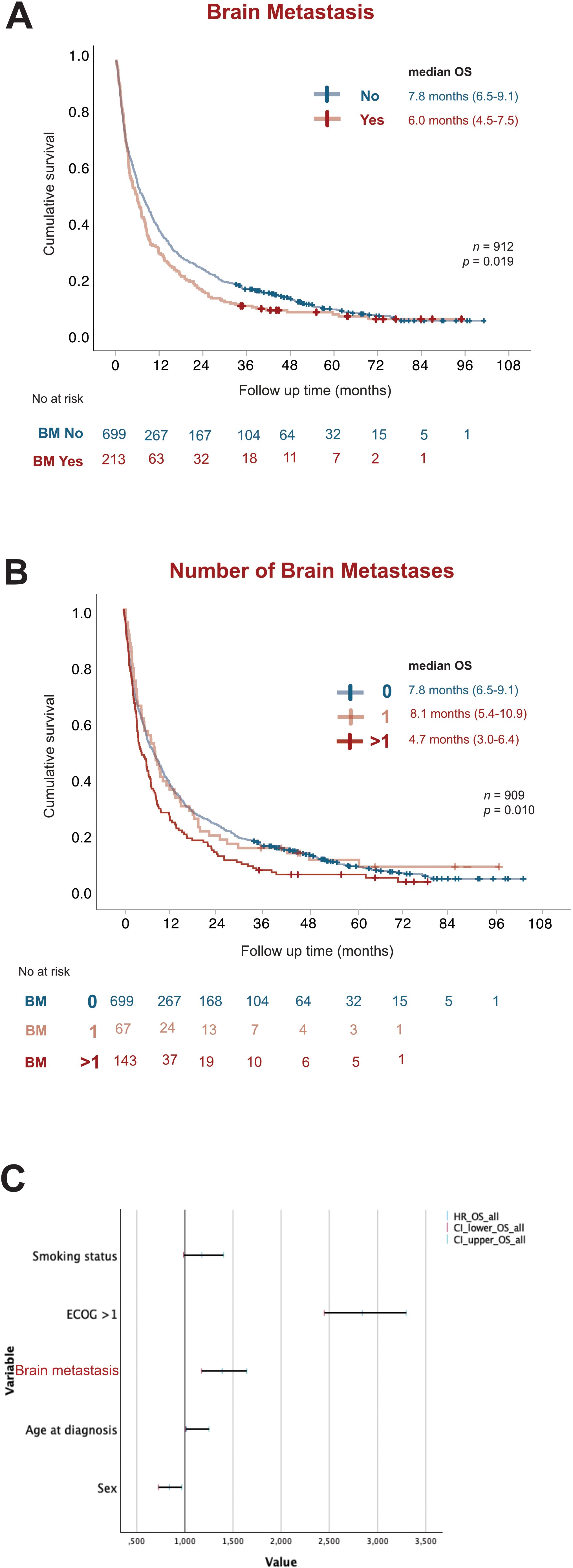
Figure 4. BM worsens prognosis in stage IV LUAD. Kaplan-Meier estimates comparing (A) overall survival (OS) stratified by presence (red) or absence (blue) of BM. (B) OS stratified by total number of BM present at diagnosis as no brain metastasis (blue), 1 BM tumor (light red) and more than 1 BM tumor (dark red). (C) Forest plot of multivariate COX regression analysis for overall survival in the study population. OS, overall survival; NR, Not reached; HR, Hazard Ratio; CI, Confidence of interval.
Next, we analyzed how metastatic involvement of each organ site in relation to BM affected survival outcomes, regardless of other organ involvement. Importantly, metastatic involvement of each organ in relation to the brain had significant effects on OS (Figure 5 and Table 2). For all organs, BM patients had worse OS than those with no BM independent of other organ involvement. Among BM patients, involvement of pleura, lung or liver worsened prognosis independent of other organ involvement, while metastatic involvement of the bone or adrenal gland did not affect survival further among BM patients (Figure 5 and Table 2).
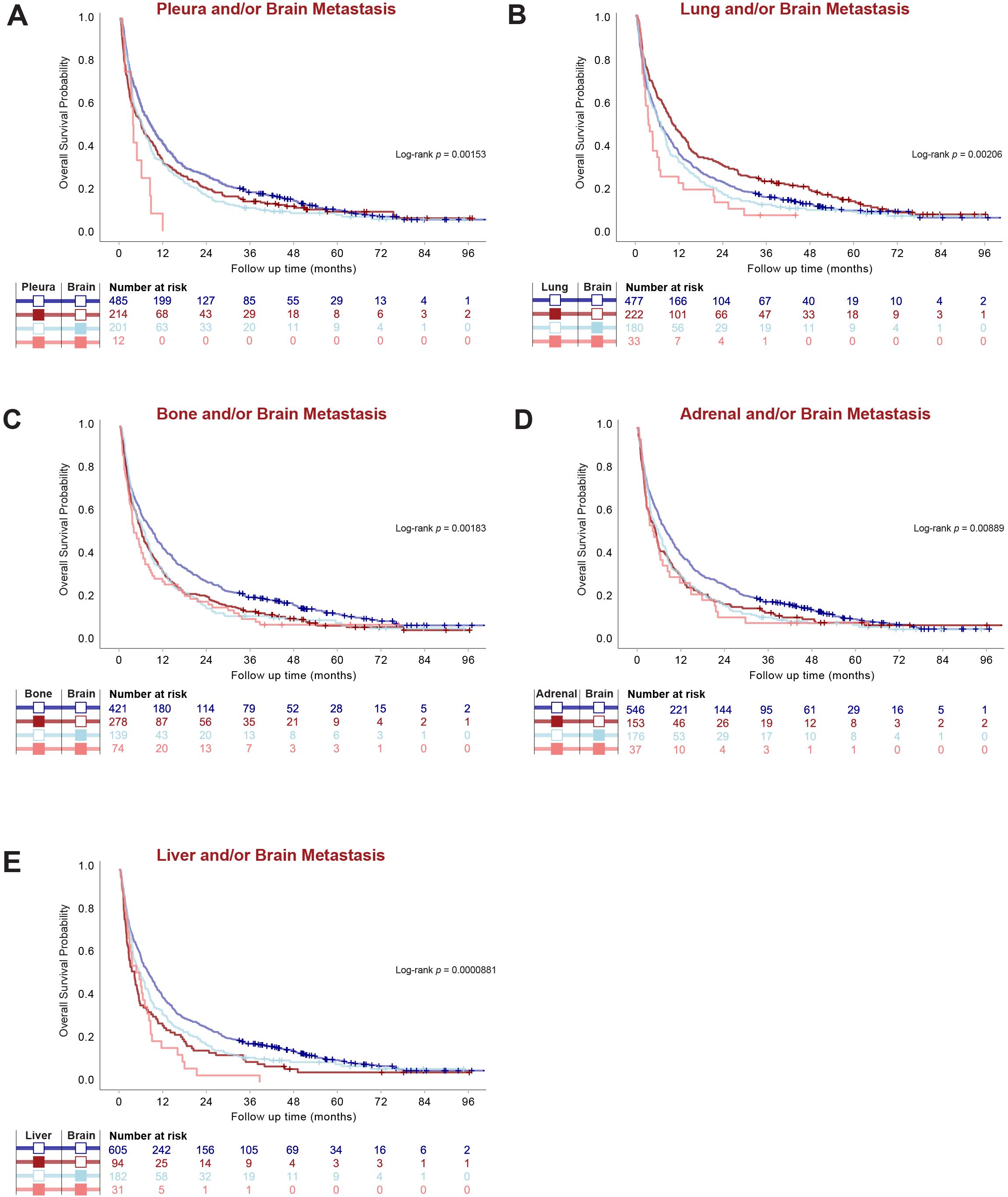
Figure 5. Metastatic Organotropism in relation to BM affects survival outcomes in stage IV LUAD. (A–E) Kaplan-Meier estimates comparing overall survival (OS) by given organ-site involvement in relation to BM regardless of other organ involvement.
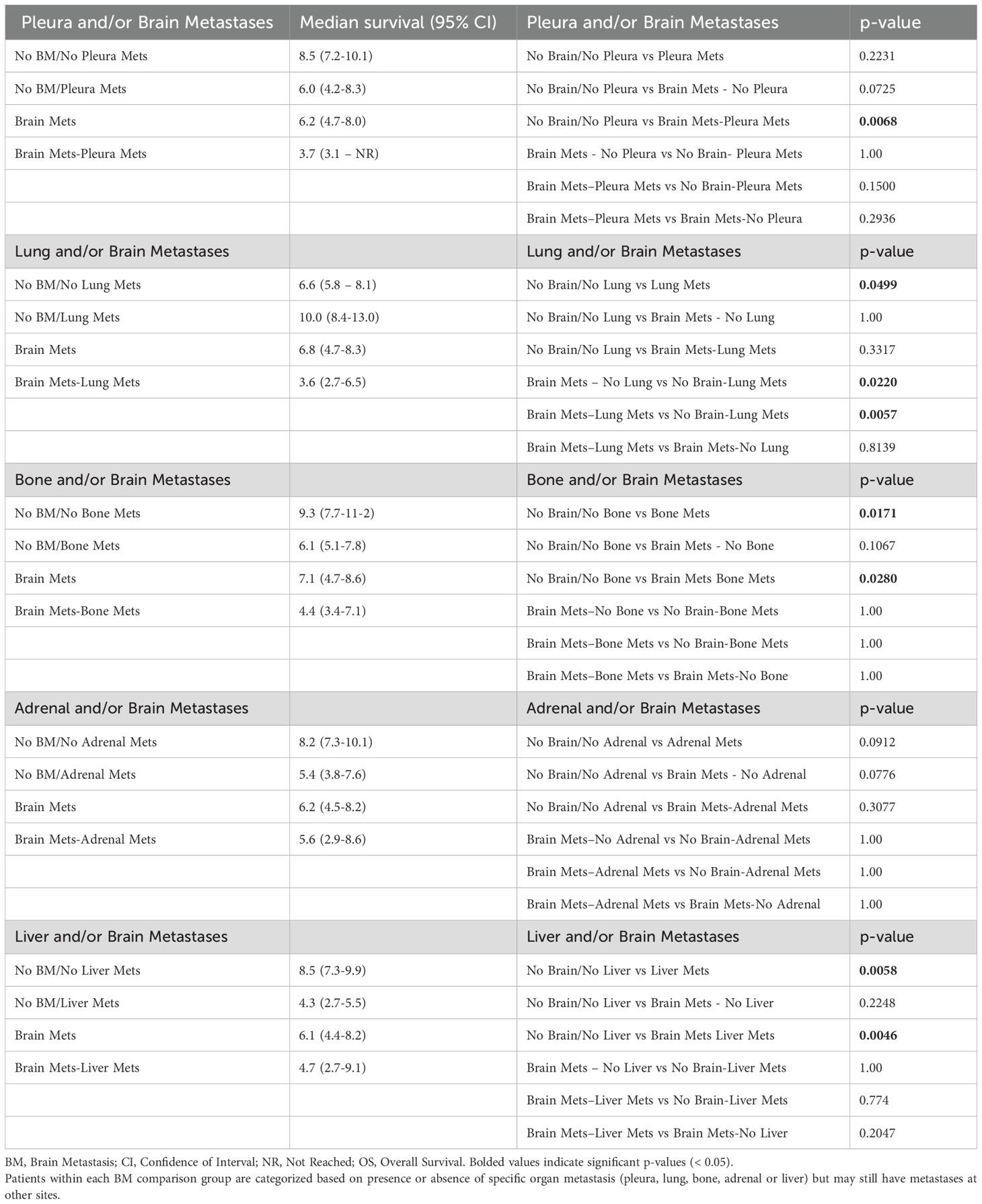
Table 2. Median survival and individual p-values for pairwise OS comparisons presented in Figure 5, regardless of other organ involvement.
BM patients with pleura metastasis, regardless of other organ involvement, had drastically worse OS (3.7 months; 95% CI 3.1-NR) than those without pleura involvement (6.2 months; 95% CI 4.7-8.0). However, pleura metastasis did not affect survival in the absence of BM (p = 0.2231). Interestingly, BM patients with metastasis to the lungs had numerically worse OS (3.6 months; 95% CI 2.7-6.5) than BM patients without lung involvement (6.8 months; 95% CI 4.7-8.3), although metastasis to the lungs in the absence of BM even improved OS (10.0 months; 95% CI 8.4-13.0) (p = 0.0499) (Figure 5 and Table 2).
This multicenter retrospective study provides novel insights into metastatic organotropism in LUAD with a specific focus on BM and their impact on clinical outcomes. Our findings reveal several critical and previously unreported aspects of metastatic patterns and survival in patients with stage IV LUAD, particularly highlighting the distinct organotropism of metastasis in relation to the brain. Notably, we found that patients with BM exhibit significantly altered metastatic patterns compared to those without BM, characterized by a markedly lower prevalence of pleura and lung metastases. Furthermore, our analysis demonstrated that BM is associated with worse OS, and that survival outcomes are further modulated by metastatic involvement of specific extracranial organs, such as the pleura and lungs. These findings provide compelling evidence of the unique biology underlying brain metastases in LUAD and emphasize the critical need for organ site-specific approaches in the management of metastatic disease.
We and others have previously reported higher frequency of BM in females with NSCLC, consistent with the findings in the present study (21, 22). Our results also corroborate previous reports showing that BM is associated with lower median survival (15) and a higher frequency of EGFR mutations (23–26), further emphasizing the clinical and molecular uniqueness of BM in LUAD. To our knowledge, the negative correlation between metastasis to the brain and the pleura or lungs observed in this study has not been reported previously. This unique finding suggests that the presence of BM may actively influence metastatic patterns, potentially limiting involvement of certain organ systems such as the pleura.
The frequency distribution of metastatic sites in our cohort aligns with previous studies by Tamura et al. and Lengel et al., where the highest rates of metastases were observed in the bone, lung, brain, adrenal gland, and liver in descending order (7, 27). Similar to these studies, our data also show that the liver and adrenal gland had the lowest frequency of metastatic involvement and were also the least likely sites of single-organ metastases. These findings could suggest that metastases to these organs may occur later in the metastatic cascade, supporting the hypothesis that they represent more advanced disease stages in LUAD.
In contrast to Tamura et al. (27), who reported that liver and adrenal metastases were associated with poor survival in NSCLC but found no significant survival differences related to lung and pleura involvement when studied independent of BM, our study highlights the critical prognostic impact of pleura and lung metastases in the presence of BM. Specifically, we found that while pleura metastases alone did not affect survival, their coexistence with BM drastically worsened prognosis, further underscoring the importance of organ-specific interactions in metastatic disease.
Rihimäki et al. (28) previously reported metastatic site involvement of the nervous system and respiratory system using data from the Swedish Cancer Registry and the Swedish National Cause of Death Registry. However, our analysis by combining more detailed-level data from the Swedish Lung Cancer Registry and manual curation of health records maps these sites of metastasis with higher resolution and accuracy. This distinction reveals a survival advantage associated with lung metastases in patients without BM, as also reported by Li et al. (5), but highlights a negative prognostic impact of lung metastases in the presence of BM.
This study has several clinical and methodological limitations. First, we only analyzed metastatic site involvement at diagnosis. Metastatic involvement is generally underreported, particularly for sites that become involved later in the disease course or during palliative treatment, as metastatic sites are not routinely mapped without clinical indication. Variability in diagnostic modalities, such as the limited use of PET-CT, which is more sensitive than CT, but not universally performed, may also contribute to underdiagnosis. Additionally, autopsy studies frequently reveal previously undetected metastatic sites, leading to discrepancies in reported frequencies compared to those based on diagnosis alone (29). These factors highlight the need for comprehensive and standardized approaches to map metastatic organotropism across the disease trajectory.
Second, while treatment influences OS, our study focuses on metastatic patterns rather than treatment effects. A more in-depth evaluation of treatment impact would require a separate study. Furthermore, the evolution of treatment options, including the introduction of third-generation EGFR-TKIs like osimertinib, may have influenced survival outcomes. However, as treatment effects were not the focus of this analysis, they were not accounted for, further reinforcing the need for a dedicated study on treatment impact.
This study demonstrates that metastatic organotropism differs significantly among LUAD patients depending on the presence of brain metastases. Notably, metastases to the pleura and lung are rare but drastically worsen prognosis in BM patients, suggesting unique organ-specific interactions that influence survival outcomes. These findings provide novel insights into the biology of metastatic spread in LUAD and underscore the critical need for organ-specific treatment strategies, particularly in the management of brain metastases. Further research is warranted to elucidate the underlying mechanisms driving these patterns and to optimize clinical management for patients with advanced disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by by Swedish Ethical Review Authority (Dnr 2019-04771 and 2021-04987). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because all data are presented in a de-identified form according to the Swedish Ethical Review Authority, no informed consent is required.
SS: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. EE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – review & editing, Writing – original draft. KA: Visualization, Writing – review & editing. JD: Formal Analysis, Writing – review & editing. MX: Visualization, Writing – review & editing. MD: Formal Analysis, Writing – review & editing. PL: Writing – review & editing. VS: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. AH: Funding acquisition, Supervision, Writing – review & editing, Conceptualization. CW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Swedish Research Council (2018-02318 and 2022-00971 to VIS, 2021-03138 to CW), the Swedish Cancer Society (23-3062 to VS, 22-0612FE to CW), the Gothenburg Society of Medicine (2019; 19/889991 to EE), Assar Gabrielsson Research Foundation (to EE, KA, JD, CW, and VS), the Swedish state under the agreement between the Swedish government and the county councils, Department of Oncology, Sahlgrenska University Hospital (to EE and AH), the Swedish Society for Medical Research (2018; S18-034 to VS), the Knut and Alice Wallenberg Foundation, and the Wallenberg Centre for Molecular and Translational Medicine (to VS).
We thank members of the Swedish Lung Cancer Registry and the continuous reporting by Swedish healthcare employees.
Authors EE and AH have received lecturing honoraria from AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1569517/full#supplementary-material
Supplementary Figure 1 | Distribution of most frequent sites of metastasis in stage IV LUAD. Heatmap showing unsupervised hierarchical clustering of sites of metastasis in the entire study population (n = 913). Rows represent individual patients.
Supplementary Figure 2 | (A) Distribution of all sites of metastasis in Stage IV LUAD, including less frequent sites (B) Pearson chart showing correlation coefficient between all organ sites of metastasis.
BM, Brain Metastasis; ECOG, Eastern Cooperative Oncology Group; HR, Hazard Ratio; LUAD, Lung Adenocarcinoma; NSCLC, Non-Small Cell Lung Cancer; OS, Overall Survival; PS, Performance Status.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Dillekas H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. (2019) 8:5574–6. doi: 10.1002/cam4.2474
3. Dunbar KJ, Efe G, Cunningham K, Esquea E, Navaridas R, Rustgi AK. Regulation of metastatic organotropism. Trends Cancer. (2024) 11:216–31. doi: 10.1016/j.trecan.2024.11.012
4. Carrolo M, Miranda JAI, Vilhais G, Quintela A, Sousa MFE, Costa DA, et al. Metastatic organotropism: a brief overview. Front Oncol. (2024) 14:1358786. doi: 10.3389/fonc.2024.1358786
5. Li J, Zhu H, Sun L, Xu W, Wang X. Prognostic value of site-specific metastases in lung cancer: A population based study. J Cancer. (2019) 10:3079–86. doi: 10.7150/jca.30463
6. Gu Y, Zhang J, Zhou Z, Liu D, Zhu H, Wen J, et al. Metastasis patterns and prognosis of octogenarians with NSCLC: A population-based study. Aging Dis. (2020) 11:82–92. doi: 10.14336/AD.2019.0414
7. Lengel HB, Mastrogiacomo B, Connolly JG, Tan KS, Liu Y, Fick CN, et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell. (2023) 41:970–985.e3. doi: 10.1016/j.ccell.2023.03.018
8. Wang X, Wang Z, Pan J, Lu ZY, Xu D, Zhang HJ, et al. Patterns of extrathoracic metastases in different histological types of lung cancer. Front Oncol. (2020) 10:715. doi: 10.3389/fonc.2020.00715
9. Eklund EA, Wiel C, Fagman H, Akyurek LM, Raghavan S, Nyman J, et al. KRAS mutations impact clinical outcome in metastatic non-small cell lung cancer. Cancers (Basel) 14(9). (2022) 14(9):2063. doi: 10.3390/cancers14092063
10. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
11. Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. (2020) 20:4–11. doi: 10.1038/s41568-019-0220-y
12. Fenske DC, Price GL, Hess LM, John WJ, Kim ES. Systematic review of brain metastases in patients with non-small-cell lung cancer in the United States, European Union, and Japan. Clin Lung Cancer. (2017) 18:607–14. doi: 10.1016/j.cllc.2017.04.011
13. Gillespie CS, Mustafa MA, Richardson GE, Alam AM, Lee KS, Hughes DM, et al. Genomic alterations and the incidence of brain metastases in advanced and metastatic NSCLC: A systematic review and meta-analysis. J Thorac Oncol. (2023) 18:1703–13. doi: 10.1016/j.jtho.2023.06.017
14. Sereno M, Hernandez de Cordoba I, Gutierrez-Gutierrez G, Casado E. Brain metastases and lung cancer: molecular biology, natural history, prediction of response and efficacy of immunotherapy. Front Immunol. (2023) 14:1297988. doi: 10.3389/fimmu.2023.1297988
15. Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. (2016) 45:139–62. doi: 10.1016/j.ctrv.2016.03.009
16. Li AY, Gaebe K, Zulfiqar A, Lee G, Jerzak KJ, Sahgal A, et al. Association of brain metastases with survival in patients with limited or stable extracranial disease: A systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e230475. doi: 10.1001/jamanetworkopen.2023.0475
17. Huang Y, Zhu L, Guo T, Chen W, Zhang Z, Li W, et al. Metastatic sites as predictors in advanced NSCLC treated with PD-1 inhibitors: a systematic review and meta-analysis. Hum Vaccin Immunother. (2021) 17:1278–87. doi: 10.1080/21645515.2020.1823779
18. Yang K, Li J, Bai C, Sun Z, Zhao L. Efficacy of immune checkpoint inhibitors in non-small-cell lung cancer patients with different metastatic sites: A systematic review and meta-analysis. Front Oncol. (2020) 10:1098. doi: 10.3389/fonc.2020.01098
19. Yuzhalin AE, Yu D. Brain metastasis organotropism. Cold Spring Harb Perspect Med 10(5). (2020) 10(5):a03742. doi: 10.1101/cshperspect.a037242
20. Eklund EA, Mourad A, Wiel C, Sayin SI, Fagman H, Hallqvist A, et al. Assessing the prognostic value of KRAS mutation combined with tumor size in stage I-II non-small cell lung cancer: a retrospective analysis. Front Oncol. (2024) 14:1396285. doi: 10.3389/fonc.2024.1396285
21. Isaksson J, Berglund A, Louie K, Willen L, Hamidian A, Edsjo A, et al. KRAS G12C mutant non-small cell lung cancer linked to female sex and high risk of CNS metastasis: population-based demographics and survival data from the national swedish lung cancer registry. Clin Lung Cancer. (2023) 24:507–18. doi: 10.1016/j.cllc.2023.05.002
22. Chen S, Hua X, Jia J, Wu Y, Wei S, Xu L, et al. Risk factors for brain metastases in patients with non-small cell lung cancer: a meta-analysis of 43 studies. Ann Palliat Med. (2021) 10:3657–72. doi: 10.21037/apm-20-1722
23. Zhao W, Zhou W, Rong L, Sun M, Lin X, Wang L, et al. Epidermal growth factor receptor mutations and brain metastases in non-small cell lung cancer. Front Oncol. (2022) 12:912505. doi: 10.3389/fonc.2022.912505
24. Shin DY, Na II, Kim CH, Park S, H. Baek and SH. Yang: EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. (2014) 9:195–9. doi: 10.1097/JTO.0000000000000069
25. Kang Y, Jin Y, Li Q, Yuan X. Advances in lung cancer driver genes associated with brain metastasis. Front Oncol. (2020) 10:606300. doi: 10.3389/fonc.2020.606300
26. Bhatt VR, D’Souza SP, Smith LM, Cushman-Vokoun AM, Noronha V, Verma V, et al. Epidermal growth factor receptor mutational status and brain metastases in non-small-cell lung cancer. J Glob Oncol. (2017) 3:208–17. doi: 10.1200/JGO.2016.003392
27. Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. (2015) 3:217–21. doi: 10.3892/mco.2014.410
28. Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. (2014) 86:78–84. doi: 10.1016/j.lungcan.2014.07.020
Keywords: lung cancer, organotropism, brain metastasis, stage IV, LUAD
Citation: Sayin SI, Eklund EA, Ali KX, Dzanan JJ, Xylander M, Dankis M, Lindahl P, Sayin VI, Hallqvist A and Wiel C (2025) Distinct metastatic organotropism shapes prognosis in lung adenocarcinoma with brain metastasis. Front. Oncol. 15:1569517. doi: 10.3389/fonc.2025.1569517
Received: 31 January 2025; Accepted: 18 March 2025;
Published: 04 April 2025.
Edited by:
Weimin Gao, Barrow Neurological Institute (BNI), United StatesReviewed by:
Zeng Jingtong, Yunnan Cancer Hospital, ChinaCopyright © 2025 Sayin, Eklund, Ali, Dzanan, Xylander, Dankis, Lindahl, Sayin, Hallqvist and Wiel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clotilde Wiel, clotilde.wiel@gu.se
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.