
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 07 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1545542
Oncolytic viruses represent a distinct class of viruses that selectively infect and destroy tumor cells while sparing normal cells. Despite their potential, oncolytic viruses encounter several challenges as standalone therapies. Consequently, the combination of oncolytic viruses with other therapeutic modalities has emerged as a prominent research focus. This paper summarizes the tumor-killing mechanisms of oncolytic viruses, explores their integration with radiotherapy, chemotherapy, immune checkpoint inhibitors, CAR-T, and CAR-NK therapies, and provides an overview of related clinical trials. By synthesizing these advancements, this study seeks to offer valuable insights for the clinical translation of oncolytic virus combination therapies.
Oncolytic viruses offer a novel and promising approach to cancer therapy. They selectively infect and destroy tumor cells, sparing normal cells in the process (1). As the number of oncolytic viruses approved by the Food and Drug Administration (FDA) for clinical use continues to grow, interest in this therapeutic strategy has markedly increased. Oncolytic viruses can be administered as monotherapy or combined with radiotherapy, chemotherapy, immunotherapy, or cell-based therapies, presenting promising prospects for cancer treatment. Currently, Several oncolytic viruses are employed, such as adenovirus (Ad) (2), herpes simplex virus (HSV) (3), vaccinia virus (VV) (4), reovirus (5), poliovirus (6), coxsackie virus (CV) (7), Newcastle disease virus (NDV) (8), vesicular stomatitis virus (VSV) (9), myxoma virus (10) and some Senteroviruses (11).
Research on oncolytic viruses began in the early twentieth century, revealing that certain wild-type or naturally attenuated viral strains could effectively treat cancer (12). Over recent decades, significant advancements had been achieved in cancer therapy with oncolytic viruses, and the milestones are summarized in Figure 1.
The antitumor effects of oncolytic viruses extend beyond receptor expression, potential mutations or transcriptional resistance. Furthermore, these viruses can stimulate non-autoantigen responses and amplify antitumor immune (13, 14).
Preclinical and clinical evidence demonstrates that oncolytic viruses can inhibit tumor growth through via multiple mechanisms. Nonetheless, several challenges limit their clinical translation. However, the clinical application of oncolytic viruses is hindered by challenges such as safety concerns, immune evasion, large-scale production, and clinical trials design (1, 15). Integrating oncolytic viruses with complementary therapies may enhance their efficacy and address existing challenges.
Oncolytic viruses proliferate rapidly within tumor cells competing for biomolecules and energy, ultimately leading to host cell damage. Oncolytic virus releases progeny viruses after lysis of tumor cells, which infect nearby tumor cells and gradually metastasize to distal tumor cells until cleared by the host immune system (16). Viral replication of whin tumor cells alters their metabolic profile, inhibiting DNA repair, disrupts cell cycle regulation, and promotes apoptosis.
Finally, tumor cells destroyed by oncolytic viruses also release damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), which intensify the immune response against the surrounding tumor cells (Figure 2).
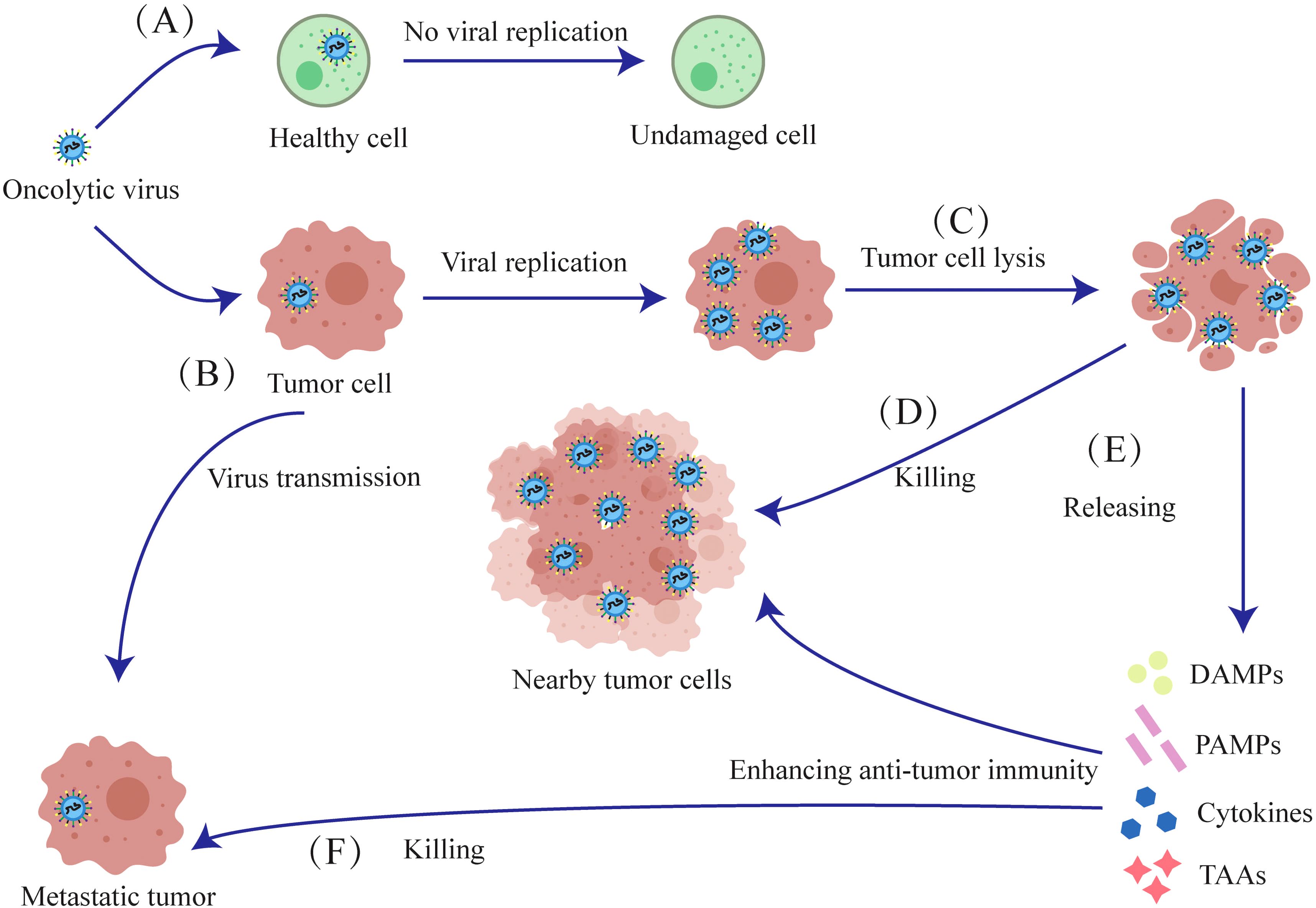
Figure 2. Mechanisms of oncolytic viruses. (A) The oncolytic virus has no destructive effect on normal cells. (B) The oncolytic virus selectively infects tumor cells, initiating viral replication within them. (C) Oncolytic viruses proliferate and lyse tumor cells. (D) The released oncolytic viruses then infect nearby tumor cells and lyse them. (E) Tumor cells lysed by oncolytic viruses release Cytokines, TAAs, DAMPs and PAMPs, which induce anti-tumor immunity against proximal and metastatic tumors. (F) After lysing tumor cells to release antigens and cytokines, oncolytic viruses further infect and kill metastatic tumors.
While oncolytic virus generally spare healthy cells, excessive oncolytic activity may result in off-target effects or heightened toxicity, potentially causing damage to normal tissues (17). The efficacy of oncolytic viruses is affected by factors such as virus type, injection dose, tumor cells sensitivity, and genetic modifications (18, 19).
Oncolytic viruses can elicit specific B-cell and T-cell responses against tumor antigens, and potentially prevent long-term tumor recurrence. B lymphocytes primarily secrete antibodies and eliminate tumor cells through antibody-mediated mechanisms. Cytotoxic T lymphocytes (CTLs) directly recognize and destroy tumor cells, while helper T cells regulate and amplify the immune response. Innate immune cells, such as macrophages, natural killer (NK) cells and dendritic cells (DCs) can also directly kill tumor cells and secrete cytokines that amplify adaptive immune responses or sensitize tumor cells to viruses (20, 21).
Oncolytic viruses induce the release of toll-like receptor (TLR) ligands, PAMPs and DAMPs from the infected tumor cells, which then activate antigen-presenting cells (APCs), NK cells and T cells. TLR ligands can counteract tumor-induced immunosuppression by modulating cytokines (22). Furthermore, DCs expressing the Major Histocompatibility Complex-1(MHC-1) and MHC-2 receptors respectively activate the CD8+ and CD4+T cells by presenting antigens. NK cells and activated CD8+T cells synergistically release perforin, granzyme and cytokines that directly kill tumor cells (Figure 3).
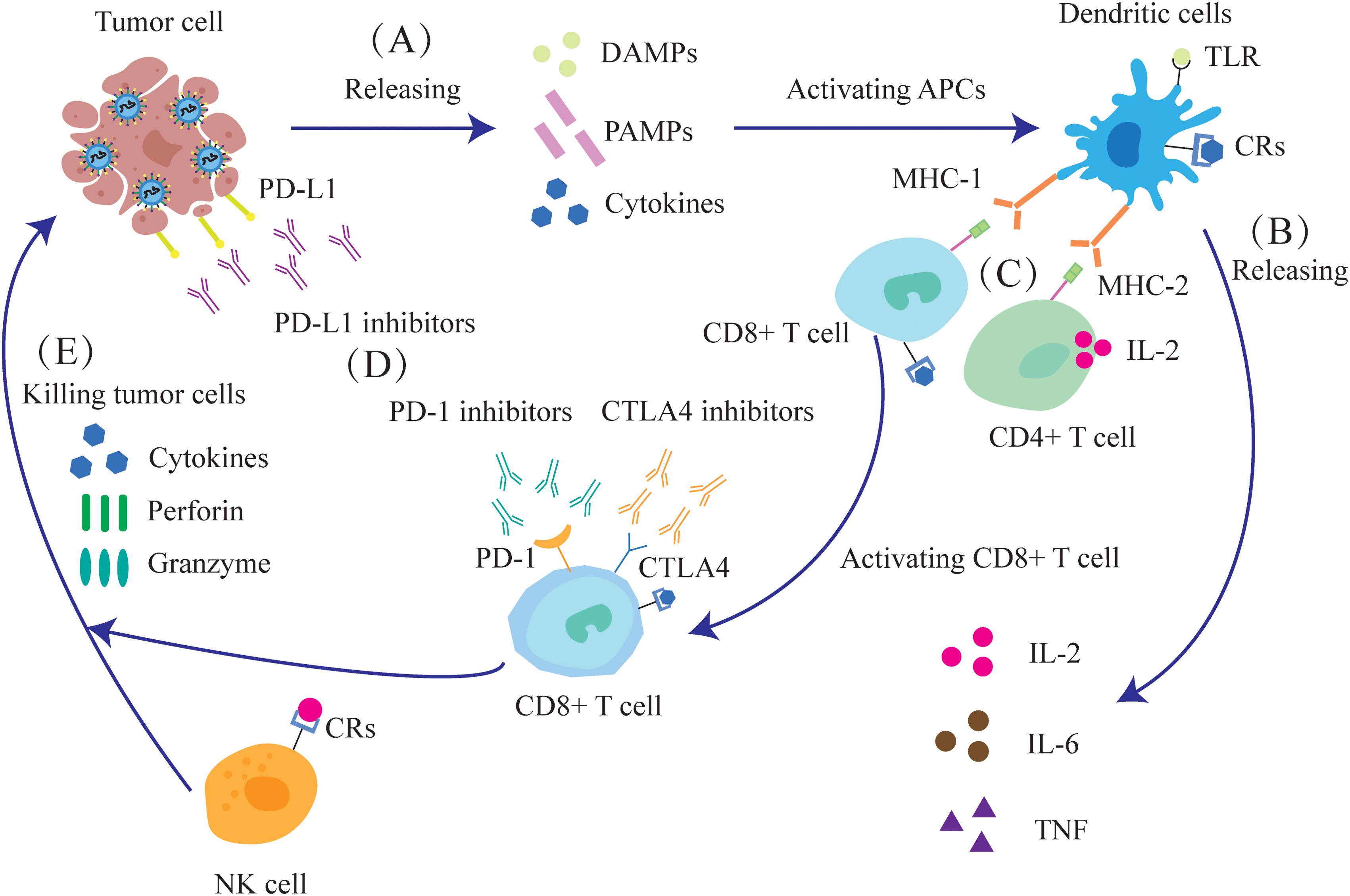
Figure 3. Oncolytic viruses can enhance the anti-tumor immune response and improve the effect of immunotherapy. (A) The destruction of tumor cells releases DAMPs, PAMPs and cytokines. (B) APCs, such as DCs, are activated and release IL-2, IL-6, and tumor necrosis factor (TNF). (C) After dendritic cells mature, they activate CD4+ and CD8+ T cells through MHC. (D) Oncolytic virus infection leads to increased expression of the immune checkpoint molecules PD-1, PD-L1 and CTLA-4, as well as enhanced sensitivity of tumor cells to immune checkpoint inhibitors (ICIs). (E) NK cells, together with activated CD8+T cells, can release cytokines, perforin and granzyme to kill tumor cells.
Oncolytic viruses elicit a host immune response that suppresses the tumor-killing effects, but this can be mitigated by combining oncolytic virus with low-dose chemotherapy or transforming growth factor beta (TGF-β) (23, 24). These agents can transiently suppress immune response, thus ensuring survival of the virus particles till they reach the tumor site. However, the precise safe dosage of these drugs still required further investigation.
While anti-viral immunity impairs efficacy, tumor-killing responses can be enhanced by immune stimulation. Tumor regression can be enhanced by increasing their therapeutic dose, immunostimulants immunostimulants as adjuvants (25, 26), or engineering recombinant viruses to express immunostimulatory genes or modified promoter elements (27). Interestingly, a virus that is oncogenic in one host may exert oncolytic properties against various tumors in another host (28).
The expression of cytotoxic proteins can also boost the efficacy of oncolytic viruses. For instance, adenovirus death protein (ADP) is a glycoprotein that effectively lyses cells and releases viral particles during advanced stages of infection. Consequently, viruses overexpressing ADP can spread more rapidly and efficiently within tumors (29).
In a Phase I clinical trial, patients with malignant pleural mesothelioma received intrathoracic oncolytic virus therapy, which reduced tumor cell density and increase the density of multiple immune cells (30).
Recombinant oncolytic virus strains are develop by incorporating target genes or expression elements in the viral genome. These engineered viruses produce supplementary proteins in the host tumor cells, which enhance the anti-tumor efficacy of oncolytic viruses or confer additional therapeutic attributes (31).
The oncolytic vaccinia virus represents a promising recombinant strain. Studies have shown that vaccinia virus, when armed with IL-2, IL-15 or HBD2 is highly effective at recognizing and killing tumor cells (32–34). Notably, recombinant vaccinia virus strains expressing bacterial flagellin have demonstrated oncolytic effects in solid tumor models (35).
Newcastle disease virus (NDV) is the longest-used oncolytic virus in clinical trials and has a well-established safety record as a monotherapy, attributed to its robust induction of antiviral responses in non-transformed mammalian cells (36). NDV can also be recombined in many ways, for instance, an NDV strain expressing matrix metalloproteinase (MMP) 8 enhances viral accumulation in tumors, thereby improving oncolytic efficacy. Additionally, NDV strains have been engineered to promote the release of IFNγ from virus-infected melanomas cells (37, 38).
In addition, many oncolytic viruses have been successfully reprogrammed, and it is believed that more successful novel oncolytic viruses will be designed for preclinical and clinical trials in the future.
The tumor microenvironment (TME) is a complex network of tumor and stromal cells that fosters the growth and survival of cancer cells. Depending on cytokine profile and infiltration of immune cells, the TME can be categorized as immunologically “cold” or “hot”.
A cold TME is defined by elevated levels of inhibitory cytokines and immune checkpoint molecules, along with high infiltration of immunosuppressive cells, which hinder the immune system from accurately identifying and killing tumor cells (39). Conversely, a hot TME facilitates detection and elimination of tumor cells via immune effector cells, pro-inflammatory cytokines, and immunostimulatory molecules.
Since hot tumors respond better to immunotherapy, transforming the TME from “cold” to “hot” can increase the effectiveness of oncolytic viruses by enhancing immune recognition and destruction of tumor cells (40).
Oncolytic viruses can transform the TME from “cold” to “hot”, and evoke an adaptive antitumor immune response by releasing tumor-associated antigens (TAAs), PAMPS, and DAMPS. These immunostimulatory molecules recruit antigen-presenting cells (APCs) to the tumor site (41), resulting in antitumor and antiviral responses (Figure 4). Thus, oncolytic viruses are a promising immunotherapeutic agent for tumor control.
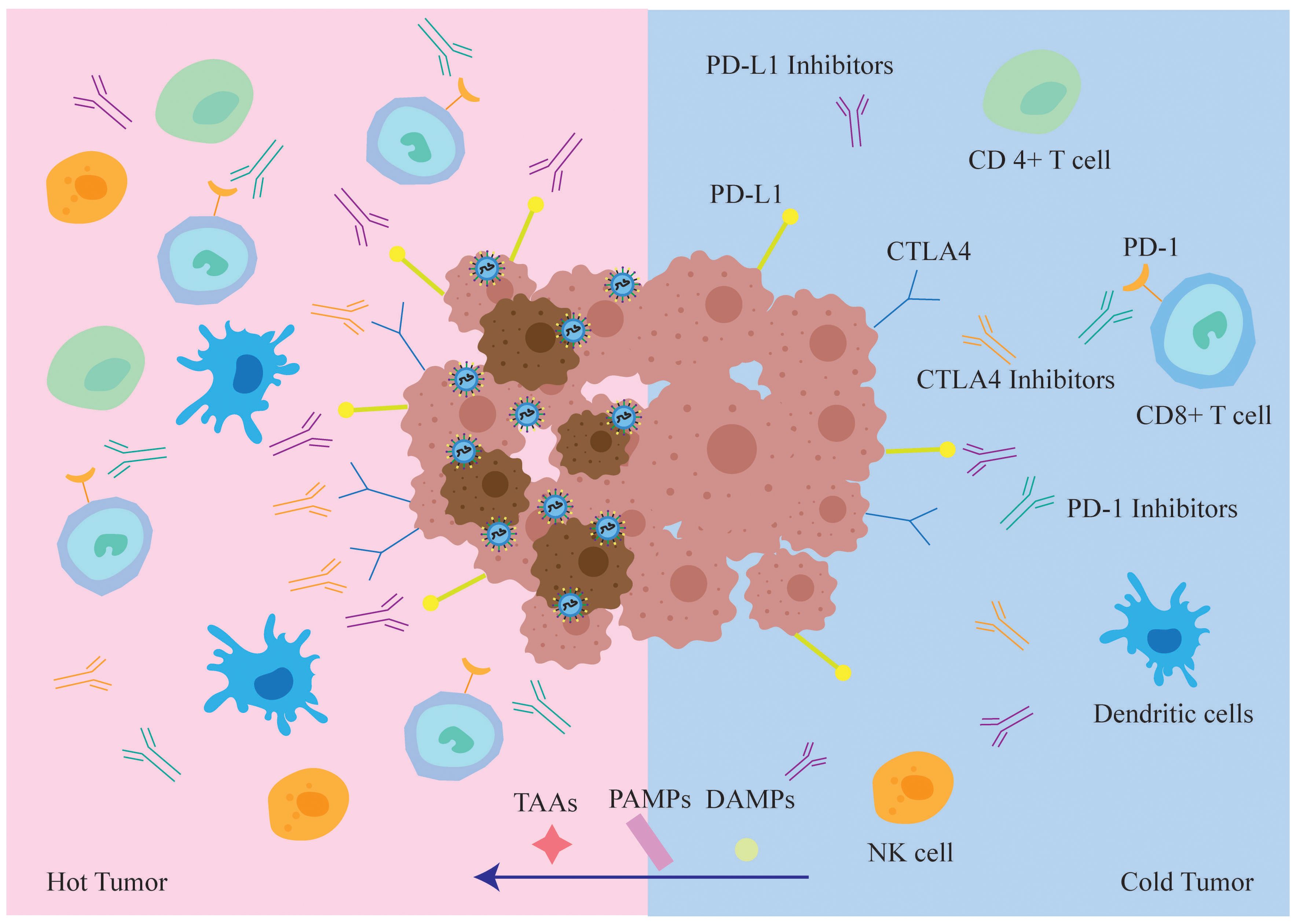
Figure 4. In immune “hot” TME, tumor cells infected with oncolytic virus are more easily recognized and cleared by the immune system. While oncolytic virus lyses tumor cells, it also draws various types of immune cells into the tumor microenvironment, transforming “cold” tumors into “hot” tumors and increasing the sensitivity of tumor cells to the immune checkpoint inhibitors ICIs. At the same time, TAA, PAMPs and DAMPs will also enter the “hot” tumor, TAA enhances the recognition and killing of tumor cells by the host immune system, whereas PAMPs and DAMPs enhance anti-tumor cellular immune responses.
Evidence indicates that viruses can transport genes encoding immunostimulatory molecules, such as cytokines, chemokines, and co-stimulatory receptors, to the TME, thereby enhancing antitumor immunity (42).
Viral particles and gene products released upon tumor cell lysis can also activate the immune system. Furthermore, oncolytic viruses can inhibit tumor angiogenesis, reduce tumor blood supply, and induce tumor hypoxia and nutrient deficiency by inactivating pro-angiogenic factors secreted by tumor cells. Virus-infected cells often trigger an inflammatory response that can lead to tissue damage. Oncolytic viruses can modulate the tumor microenvironment by regulating the production and release of inflammatory factors, reducing surrounding tissue damage, and thereby enhancing therapeutic efficacy.
Oncolytic adenoviruses engineered to locally express inflammatory cytokines IL-12 and PD-L1 blocking antibodies can repolarize TME, enhance CD8 T cell activity, and can kill tumor cells by altering the tumor microenvironment (43, 44).
Oncolytic viruses, as a novel anticancer approach, function by selectively infecting and lysing tumor cells. Despite their potential, the clinical efficacy of oncolytic virus monotherapy is significantly constrained by several factors:
Oncolytic virus infection depends on specific viral receptors, the expression of which varies widely among tumor types and patients. This heterogeneity markedly impacts the efficacy of oncolytic viruses, complicating efforts to achieve consistent outcomes across tumors. Moreover, the high mutation rates of solid tumors frequently undermine the long-term effectiveness of oncolytic virus monotherapy (45).
Infection of tumor cells by oncolytic viruses activates host immune responses, resulting in viral clearance and diminished tumor-killing efficacy. Tumors suppress immune responses via mechanisms like elevated PD-L1 expression or recruitment of regulatory T cells (Tregs), further reducing oncolytic virus efficacy (43, 46, 47).
While effective at lysing local tumors, oncolytic viruses show limited efficacy in controlling metastatic tumors. Monotherapy frequently fails to comprehensively target metastatic lesions.
Increasing the viral dose may improve therapeutic efficacy but also heightens the risk of adverse effects, including inflammatory responses, organ failure, and, in severe cases, death (17). Monotherapy struggles to balance the need for therapeutic efficacy with ensuring patient safety.
Given the limitations of oncolytic virus monotherapy, combination therapy has become a pivotal approach in cancer treatment. Integrating multiple therapeutic approaches achieves synergistic effects, effectively killing tumor cells while addressing the limitations of monotherapy.
For example, radiotherapy and chemotherapy disrupt tumor barriers, enhancing viral penetration; immune checkpoint inhibitors modulate host immune responses, reducing the clearance of oncolytic viruses. Furthermore, the combination of oncolytic viruses with CAR-T or CAR-NK cells not only improves the tumor microenvironment but also releases viral particles and cytokines that further activate and enhance the functions of CAR-T and CAR-NK cells, ultimately improving the overall therapeutic efficacy.
Although radiotherapy is routinely used for local tumors and early-stage disease, it has several disadvantages, including off-target effects on normal cells and the development of radio-resistance in the tumor cells, which compromise therapeutic outcomes. Furthermore, radiotherapy is largely ineffective against metastatic growth. Similarly, chemotherapeutic agents are typically non-selective, affecting normal tissues and resulting in systemic toxicity and side effects that can significantly impact patients’ quality of life. In addition, tumor cells frequently mutate, generating drug-resistant clones that diminish or nullify the therapeutic effects of chemotherapeutic drugs.
On the other hand, oncolytic viruses selectively infect and lyse tumor cells. Furthermore, while radiotherapy and chemotherapy are typically localized, oncolytic viruses can target metastatic tumors due to their ability to infect tumor cells. Thus, combining oncolytic viruses with chemotherapy or radiotherapy can provide both local and systemic disease control (48, 49).
Numerous preclinical studies have investigated the combination of oncolytic virus with radiotherapy and chemotherapy. Recent studies have shown that the combination of temozolomide or vincristine with oncolytic viruses can significantly kill mouse tumors (50, 51). At the same time, oncolytic viruses have been shown to enhance the efficacy of mitomycin and hydroxycamptothecin (52).
In clinical trials, H101, an oncolytic virus approved by China’s State Drug Administration, has been widely tested in combination with chemoradiotherapy. A number of clinical studies demonstrate the significant efficacy of oncolytic viruses in treating various tumors when combined with chemoradiotherapy (53–56).
Researchers have also combined a novel telomerase oncolytic virus with radiotherapy to treat patients with esophageal cancer, integrating recombinant technology with combination therapy (57).
Additionally, a prospective randomized phase 2 trial combining oncolytic viruses with radiotherapy reported a significant reduction in positivity in local biopsies (58). Reovirus has also demonstrated efficacy in clinical patients when combined with radiotherapy and chemotherapy (59, 60).
Numerous clinical studies on the combination of oncolytic viruses with chemoradiotherapy have reported favorable therapeutic outcomes with minimal side effects. Some clinical trials of oncolytic virus combined with chemoradiotherapy have been listed in Table 1.
The expression of immune checkpoint molecules on immune cells will inhibit their function preventing the body from generating an effective antitumor immune response. These checkpoints can be exploited by tumors to evade immune surveillance. can be exploited by tumors to evade immune surveillance. Immune checkpoint inhibitors (ICIs), also referred to as immune system modulators, target immune checkpoints to enhance the immune response or to relieve immune suppression. Commonly used ICIs include Nivolumab, Ipilimumab, Pembrolizumab and Atezolizumab.
ICIs re-engage T cell anti-tumor activity of T cells by reversing the immunosuppressive tumor microenvironment. Oncolytic viruses can stimulate immune response and promote immune cell infiltration, while ICIs amplify this effect by reducing inhibitory signals, thereby enhancing immune response and therapeutic effect. The response to ICIs depends heavily on the TME, where “hot” tumors respond better to treatment, whereas “cold” tumors are less responsive. Therefore, improving TME is a key strategy for enhancing treatment efficacy (61).
At present, the combination of oncolytic viruses and ICIs has demonstrated significant anti-tumor effects in preclinical studies across various tumor types. Recent studies have further validated this approach (62–64).
Numerous clinical trials have been conducted on combination therapies. At present, a variety of ICIs combined with oncolytic viruses are currently being evaluated in clinical trials.
Nivolumab, a PD-1 inhibitor, was the first approved immunotherapy drug in China. Nivolumab has been combined with various recombinant oncolytic viruses in multiple tumor types, demonstrating sufficient safety and significant tumor regression (65–67).
Ipilimumab, a monoclonal antibody targeting CTLA-4, enhances the immune system’s ability to kill cancer cells by inhibiting an immunosuppressive checkpoint. HF10 is a biologically selected replicating oncolytic virus derived from herpes simplex virus type 1 (HSV-1). So far, numerous clinical trials have evaluated the combination of HF10 and Ipilimumab demonstrating remarkable efficacy (68–70).
Pembrolizumab is the only PD-1 inhibitor globally and in China that has received first-line three indications and single-agent indications for advanced non-small cell lung cancer (NSCLC). Numerous clinical trials combining Pembrolizumab with oncolytic viruses have shown sustained responses, with clinical benefits observed even in refractory patients (71–73).
Atezolizumab, a PD-L1 inhibitor, was approved by the US FDA in 2016. Atezolizumab binds to PD-L1 on tumor cells and block its interaction with PD-1 on T cells and antigen presenting cells, thereby relieving immunosuppression and enhancing T cell-mediated tumor cell destruction. At present, clinical studies have proved that Atezolizumab combined with oncolytic virus is very effective and safe (74, 75).
Of course, in addition to the above major classes of immune checkpoint inhibitors, other ICIs such as the PD-1 antibody Camrelizumab and the PD-L1 antibodies Durvalumab and Avelumab also show promising research prospects, with several ongoing preclinical and clinical trials. The results of the experiment are also being expected.
Therefore, combining oncolytic virus with ICIs may enhance anti-tumor immune responses. Some clinical trials of oncolytic viruses combined with immune checkpoint inhibitors are listed in Table 2.
T cells are genetically engineered to express a chimeric antigen receptor (CAR) transgene and become chimeric antigen receptor T cells (CAR-T cells). CAR proteins are comprised of three components: the extracellular antigen-recognition domain of the single-chain fragment variable region (scFv), a transmembrane domain, and the intracellular CD3ζ domain (76). The design of CAR-T cells is complex and has evolved through five generations.
Oncolytic viruses activate CAR-T cells and also guide them to the infected tumor cells. In turn, the activated CAR-T cells release cytokines, such as IL-2, IFN-γ and TNF-α, which enhance replication and infectivity of oncolytic viruses, thereby amplifying the overall therapeutic effect (Figure 5).

Figure 5. The anti-tumor mechanism of oncolytic viruses and CAR-T cells. (A) T cells are genetically modified to express CAR transgenes. (B) CAR-T cells can release IL-2, IFN-γ, and TNF-α after activation. (C) These molecules can intensify the inflammatory response in tumor cells, thereby increasing their susceptibility to viral infection. (D) CAR-T cells can release perforin to kill tumor cells. (E) Oncolytic viruses can release viral particles after they replicate and lyse tumor cells. (F) These viral particles can stimulate and activate CAR-T cells to produce effects.
CAR-T cell therapy is a new cellular immunotherapy technique that integrates synthetic receptors into T cells, enabling them to recognize and kill tumor cells using homologous targeting ligands (77, 78). However, it was soon discovered that CAR-T therapy has limitations and side effects such as systemic toxicity and neurotoxicity, and the therapeutic effect may not be durable. At the same time, the complex construction process of CAR-T cells presents significant challenges for their application (79, 80).
Experimental data demonstrate the substantial clinical efficacy of CAR-T cells in treating hematologic malignancies (81). However, significant challenges remain in using CAR-T cells to treat advanced solid tumors. These challenges are primarily due to the low transport efficiency of CAR-T cells to TME, issues with antigen recognition, and structural differences between the structure of other solid tumors and hematologic tumors. Single therapy with CAR-T cells may not overcome these problems smoothly (82). However, with the FDA approval of the first oncolytic virus, T-VEC,the combination of oncolytic virus and CAR-T cells has officially opened the prelude. Recombinant oncolytic viruses may suppress local immunity, resulting in better therapeutic efficacy and persistence of CAR-T cells (83).
Oncolytic virus serve as reliable adjuvant for CAR-T cell therapy in solid tumors (84). Preclinical experiments have shown that oncolytic viruses loaded with IL-7 and CXCL11, in combination with CAR-T cells, significantly enhance tumor cell killing (85, 86).
At the same time, an interesting study in which oncolytic viruses expressing PD-L1 blocking micro-antibodies with CAR-T cells successfully controlled solid tumor growth (87). Another study combined CAR-T cells with oncolytic viruses equipped with the chemokine RANTES and the cytokine IL15, which improved the survival rate of tumor-bearing mice (88). Additionally, innovative strategies have been developed to encapsulate oncolytic viruses within CAR-T cells for treating solid tumors, yielding promising results (89).
Current studies suggest that the combination of oncolytic virus and CAR-T cells enhances therapeutic efficacy by enabling the virus to replicate and destroy tumor cells, stimulate immunity, and modulate the tumor’s immunosuppressive microenvironment, thus promoting CAR-T cell survival and activity.
Despite encouraging preclinical results, only one clinical trial investigating the combination of CAR-T cells and oncolytic viruses is ongoing, and further clinical trials are expected to follow. This may be due to the immunosuppressive tumor microenvironment, T cell depletion, or the absence of suitable antigen targets.
The summary of clinical trials of oncolytic virus combined with CAR-T cells for cancer treatment is shown in Table 3.
The slow progress of clinical trials combining CAR-T cells and oncolytic virus may be attributed to the systemic toxicity and neurotoxicity caused by CAR-T cells, which compromise their safety. In recent years, numerous patient deaths in CAR-T cells clinical trials have led to the emergency suspension of the experiment by FDA. In this way, attention has shifted to the carrier function of CAR T cells, using them to deliver oncolytic viruses to tumors to produce the effect of killing tumor cells. Studies have shown that CAR-T cells can deliver low doses of oncolytic viruses without affecting T cells quantity or function. CAR-T cells can be used as a carrier to release the virus to multiple tumor targets, thus further enhancing the role of killing tumor cells (90).
Due to concerns about the safety of CAR-T cells, attention has shifted to NK cells that express a low level of PD-1, which promote the migration of dendritic cells and cause less immunosuppression. Genetically modified NK cells express CAR transgenes are termed Chimeric Antigen Receptor NK cells (CAR-NK cells).CAR-NK cells are engineered to recognize and annihilate cancer cells (Figure 6). The viral particles released from the lysed tumor cells can stimulate CAR-NK cells in a manner similar to the CAR-T cells.
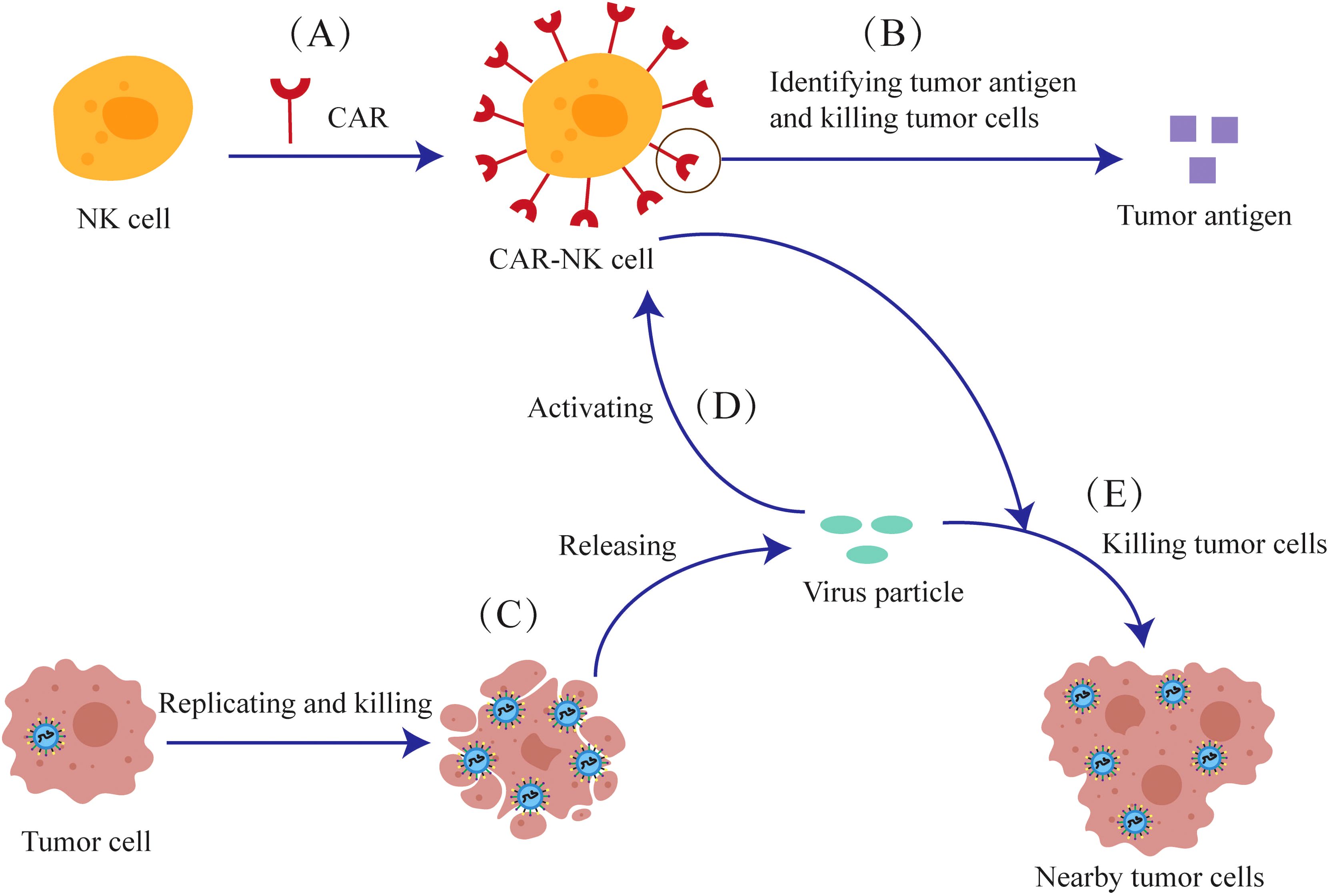
Figure 6. The anti-tumor mechanism of the combination of oncolytic viruses and CAR-NK cells. (A) NK cells are genetically modified to express CAR-Transgenes. (B) The CAR expressed on CAR-NK cells can recognize tumor antigens and kill tumor cells. (C) Oncolytic viruses can release viral particles after they replicate and lyse tumor cells (D) Viral particles can also activate CAR-NK cells (E) CAR-NK cells can kill nearby tumor cells together with viral particles.
CAR-NK cells are easier to construct than CAR-T cells and avoid issues such as alloreactivity, cytokine release syndrome, and graft-versus-host reactions. As long as NK cells are used, CAR-NK can be mass-produced. CAR-NK therapy is also significantly safer and does not induce strong neurotoxicity. At the same time, the survival cycle of NK cells is much shorter than that of T cells, and any off-target effects are rapidly cleared (91, 92).
As mentioned above, the shorter lifespan of NK cells necessitates multiple drug injections for CAR-NK therapy. But once the drug is injected multiple times, it will inevitably reduce safety. The continuous stimulation of immunity by oncolytic viruses helps extend the duration of CAR-NK cells without repeatedly injecting drugs.
At present, relevant preclinical studies have confirmed this view. EGFR-CAR constructs have been designed and transduced into NK cells to generate CAR-NK cells. The combination of second-generation EGFR-CAR NK cells and oncolytic herpes simplex virus to in treating breast cancer brain metastases in mouse models demonstrated significant tumor cell killing and improved survival (93). Another study showed that oncolytic viruses expressing IL15/IL15Rα, when combined with EGFR-CAR NK cells, induced a strong anti-tumor response in glioblastoma treatment (94).
In recent years, preclinical trials combining CAR-NK cells and oncolytic viruses have shown encouraging results. There are more and more clinical trials on CAR-NK cells, but the safety and efficacy of CAR-NK cells for clinical use remain subjects of debate. At present, no clinical trials have been conducted combining oncolytic viruses with CAR-NK cells.
In recent years, oncolytic viruses have received widespread attention as a promising cancer therapy. Meanwhile, genetic modification of oncolytic viruses can substantially enhance their anti-tumor efficacy.
This article reviews the factors influencing the anti-tumor efficacy of oncolytic viruses and their application in combination with other therapies, while also listing ongoing or completed clinical trials. The results showed that the combination of oncolytic viruses with other treatments was more effective than monotherapy. In the future, the design of more oncolytic viruses and their use in combination with other therapeutic approaches are expected to further enhance clinical efficacy and safety. In the future, We expect that oncolytic viruses will increasingly be combined with CAR-T cells and CAR-NK cells in clinical trials.
For the genetically modified part of the oncolytic viruses, utilizing better-targeted nanomaterials as a carrier to deliver the virus may enhance its efficacy, representing an innovative and more effective treatment strategy when combined with nanomaterials. At present, a team has started similar experiments, but further studies are needed to evaluate their effects (95).
WD: Writing – original draft. JN: Writing – original draft. LZ: Writing – review & editing. PZ: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Supported by the Scientific and Technological Innovation Major Base of Guangxi (No.2022-36-Z05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mondal M, Guo J, He P, Zhou D. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother. (2020) 16:2389–402. doi: 10.1080/21645515.2020.1723363
2. Gállego Pérez-Larraya J, Garcia-Moure M, Labiano S, Patiño-García A, Dobbs J, Gonzalez-Huarriz M, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N Engl J Med. (2022) 386:2471–81. doi: 10.1056/NEJMoa2202028
3. Kawamura Y, Hua L, Gurtner A, Wong E, Kiyokawa J, Shah N, et al. Histone deacetylase inhibitors enhance oncolytic herpes simplex virus therapy for Malignant meningioma. BioMed Pharmacother. (2022) 155:113843. doi: 10.1016/j.biopha.2022.113843
4. Yang Z, Gray M, Winter L. Why do poxviruses still matter? Cell Biosci. (2021) 11:96. doi: 10.1186/s13578-021-00610-8
5. Seyed-Khorrami SM, Soleimanjahi H, Soudi S, Habibian A. MSCs loaded with oncolytic reovirus: migration and in vivo virus delivery potential for evaluating anti-cancer effect in tumor-bearing C57BL/6 mice. Cancer Cell Int. (2021) 21:244. doi: 10.1186/s12935-021-01848-5
6. Ghajar-Rahimi G, Kang KD, Totsch SK, Gary S, Rocco A, Blitz S, et al. Clinical advances in oncolytic virotherapy for pediatric brain tumors. Pharmacol Ther. (2022) 239:108193. doi: 10.1016/j.pharmthera.2022.108193
7. Lu D, Huang Y, Kong Y, Tao T, Zhu X. Gut microecology: Why our microbes could be key to our health. BioMed Pharmacother. (2020) 131:110784. doi: 10.1016/j.biopha.2020.110784
8. Chan LC, Kalyanasundram J, Leong SW, Masarudin MJ, Veerakumarasivam A, Yusoff K, et al. Persistent Newcastle disease virus infection in bladder cancer cells is associated with putative pro-survival and anti-viral transcriptomic changes. BMC Cancer. (2021) 21:625. doi: 10.1186/s12885-021-08345-y
9. Sarwar A, Hashim L, Faisal MS, Haider MZ, Ahmed Z, Ahmed TF, et al. Advances in viral oncolytics for treatment of multiple myeloma - a focused review. Expert Rev Hematol. (2021) 14:1071–83. doi: 10.1080/17474086.2021.1972802
10. Villa NY, Rahman MM, Mamola J, Sharik ME, de Matos AL, Kilbourne J, et al. Transplantation of autologous bone marrow pre-loaded ex vivo with oncolytic myxoma virus is efficacious against drug-resistant Vk*MYC mouse myeloma. Oncotarget. (2022) 13:490–504. doi: 10.18632/oncotarget.28205
11. Kolyasnikova NM, Pestov NB, Sanchez-Pimentel JP, Barlev NA, Ishmukhametov AA. Anti-cancer virotherapy in Russia: lessons from the past, current challenges and prospects for the future. Curr Pharm Biotechnol. (2023) 24:266–78. doi: 10.2174/1389201023666220516121813
12. Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. (2007) 15:651–9. doi: 10.1038/sj.mt.6300108
13. Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. (2018) 6:140. doi: 10.1186/s40425-018-0458-z
14. Zhang S, Rabkin SD. The discovery and development of oncolytic viruses: are they the future of cancer immunotherapy? Expert Opin Drug Discovery. (2021) 16:391–410. doi: 10.1080/17460441.2021.1850689
15. Lemay CG, Keller BA, Edge RE, Abei M, Bell JC. Oncolytic viruses: the best is yet to come. Curr Cancer Drug Targets. (2018) 18:109–23. doi: 10.2174/1568009617666170206111609
16. Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. (2018) 18:498–513. doi: 10.1038/s41577-018-0014-6
17. Buijs PR, Verhagen JH, van Eijck CH, van den Hoogen BG. Oncolytic viruses: From bench to bedside with a focus on safety. Hum Vaccin Immunother. (2015) 11:1573–84. doi: 10.1080/21645515.2015.1037058
18. Seymour LW, Fisher KD. Oncolytic viruses: finally delivering. Br J Cancer. (2016) 114:357–61. doi: 10.1038/bjc.2015.481
19. Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: A review. JAMA Oncol. (2017) 3:841–9. doi: 10.1001/jamaoncol.2016.2064
20. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
21. Marelli G, Howells A, Lemoine NR, Wang Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front Immunol. (2018) 9:866. doi: 10.3389/fimmu.2018.00866
22. Jhawar SR, Thandoni A, Bommareddy PK, Hassan S, Kohlhapp FJ, Goyal S, et al. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front Oncol. (2017)7:202. doi: 10.3389/fonc.2017.00202
23. Groeneveldt C, van Hall T, van der Burg SH, Ten Dijke P, van Montfoort N. Immunotherapeutic potential of TGF-β Inhibition and oncolytic viruses. Trends Immunol. (2020) 41:406–20. doi: 10.1016/j.it.2020.03.003
24. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. (2013) 19:329–36. doi: 10.1038/nm.3089
25. Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, et al. Trial Watch:: Oncolytic viruses for cancer therapy. Oncoimmunology. (2014) 3:e28694. doi: 10.4161/onci.28694
26. John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong anti-tumor immunity against established cancer. Cancer Res. (2012) 72:1651–60. doi: 10.1158/0008-5472.CAN-11-2788
27. Guo ZS, Naik A, O’Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. (2005) 65:9991–8. doi: 10.1158/0008-5472.CAN-05-1630
28. Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz). (2008) 56 Suppl 1:3s–59s. doi: 10.1007/s00005-008-0047-9
29. Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. (2005) 12:141–61. doi: 10.1038/sj.cgt.7700771
30. Chintala NK, Choe JK, McGee E, Bellis R, Saini JK, Banerjee S, et al. Correlative analysis from a phase I clinical trial of intrapleural administration of oncolytic vaccinia virus (Olvi-vec) in patients with Malignant pleural mesothelioma. Front Immunol. (2023) 14:1112960. doi: 10.3389/fimmu.2023.1112960
31. Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. (2008) 6:529–40. doi: 10.1038/nrmicro1927
32. Liu L, Li H, Xu Q, Wu Y, Chen D, Yu F. Anti-tumor activity of recombinant oncolytic vaccinia virus with human IL2. Open Med (Wars). (2022) 17:1084–91. doi: 10.1515/med-2022-0496
33. Shakiba Y, Vorobyev PO, Yusubalieva GM, Kochetkov DV, Zajtseva KV, Valikhov MP, et al. Oncolytic therapy with recombinant vaccinia viruses targeting the interleukin-15 pathway elicits a synergistic response. Mol Ther Oncolytics. (2023) 29:158–68. doi: 10.1016/j.omto.2023.05.002
34. Sun T, Luo Y, Wang M, Xie T, Yan H. Recombinant oncolytic vaccinia viruses expressing human β-defensin 2 enhance anti-tumor immunity. Mol Ther Oncolytics. (2019) 13:49–57. doi: 10.1016/j.omto.2019.03.010
35. Shakiba Y, Vorobyev PO, Naumenko VA, Kochetkov DV, Zajtseva KV, Valikhov MP, et al. Oncolytic efficacy of a recombinant vaccinia virus strain expressing bacterial flagellin in solid tumor models. Viruses. (2023) 15:828. doi: 10.3390/v15040828
36. Santry LA, VanVloten JP, Matuszewska K, Bridle BW, Petrik JJ, Wootton SK. Recombinant newcastle disease virus as an oncolytic therapy for ovarian and prostate cancers. Mol Ther. (2016) 24:S29. doi: 10.1016/S1525-0016(16)32875-1
37. Guo T, Liu X, Zhang Z, Luo Y, Li T, Li L, et al. A recombinant Newcastle disease virus expressing MMP8 promotes oncolytic efficacy. Chin Chem Lett. (2021) 32:962–6. doi: 10.1016/j.cclet.2021.05.001
38. Numpadit S, Ito C, Nakaya T, Hagiwara K. Investigation of oncolytic effect of recombinant Newcastle disease virus in primary and metastatic oral melanoma. Med Oncol. (2023) 40:138. doi: 10.1007/s12032-023-02002-z
39. Li Z, Ma R, Ma S, Tian L, Lu T, Zhang J, et al. ILC1s control leukemia stem cell fate and limit development of AML. Nat Immunol. (2022) 23:718–30. doi: 10.1038/s41590-022-01198-y
40. Melcher A, Harrington K, Vile R. Oncolytic virotherapy as immunotherapy. Science. (2021) 374:1325–6. doi: 10.1126/science.abk3436
41. Filley AC, Dey M. Immune system, friend or foe of oncolytic virotherapy? Front Oncol. (2017) 7:106. doi: 10.3389/fonc.2017.00106
42. Aurelian L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco Targets Ther. (2016) 9:2627–37. doi: 10.2147/OTT.S63049
43. de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. Armed oncolytic viruses: A kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. (2018) 41:28–39. doi: 10.1016/j.cytogfr.2018.03.006
44. Wang D, Porter CE, Lim B, Rosewell Shaw A, Robertson CS, Woods ML, et al. Ultralow-dose binary oncolytic/helper-dependent adenovirus promotes anti-tumor activity in preclinical and clinical studies. Sci Adv. (2023) 9:eade6790. doi: 10.1126/sciadv.ade6790
45. Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics. (2019) 15:234–47. doi: 10.1016/j.omto.2019.10.007
46. Volovat SR, Scripcariu DV, Vasilache IA, Stolniceanu CR, Volovat C, Augustin IG, et al. Oncolytic virotherapy: A new paradigm in cancer immunotherapy. Int J Mol Sci. (2024) 25:1180. doi: 10.3390/ijms25021180
47. Goradel NH, Alizadeh A, Hosseinzadeh S, Taghipour M, Ghesmati Z, Arashkia A, et al. Oncolytic virotherapy as promising immunotherapy against cancer: mechanisms of resistance to oncolytic viruses. Future Oncol. (2022) 18:245–59. doi: 10.2217/fon-2021-0802
48. Chowaniec H, Ślubowska A, Mroczek M, Borowczyk M, Braszka M, Dworacki G, et al. New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy. Front Immunol. (2024) 15:1375433. doi: 10.3389/fimmu.2024.1375433
49. Simpson GR, Relph K, Harrington K, Melcher A, Pandha H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother. (2016) 5:1–13. doi: 10.2147/OV.S66083
50. Vasileva N, Ageenko A, Byvakina A, Sen'kova A, Kochneva G, Mishinov S, et al. The recombinant oncolytic virus VV-GMCSF-lact and chemotherapy drugs against human glioma. Int J Mol Sci. (2024) 25:4244. doi: 10.3390/ijms25084244
51. Anjum S, Naseer F, Ahmad T, Liaquat A, Abduh MS, Kousar K. Co-delivery of oncolytic virus and chemotherapeutic modality: Vincristine against prostate cancer treatment: A potent viro-chemotherapeutic approach. J Med Virol. (2024) 96:e29748. doi: 10.1002/jmv.29748
52. Zhang J, He Q, Mao D, Wang C, Huang L, Wang M, et al. Efficacy and adverse reaction management of oncolytic viral intervention combined with chemotherapy in patients with liver metastasis of gastrointestinal Malignancy. Front Oncol. (2023) 13:1159802. doi: 10.3389/fonc.2023.1159802
53. Zhang X, Wang Y, Lv X, Wang F, Zhou Q, Zhang F, et al. Intratumoral injection of oncolytic virus (H101) in combination with concurrent chemoradiotherapy for locally advanced cervical cancer. Int J Gynecol Cancer. (2023) 33(7):1051–6. doi: 10.1136/ijgc-2022-003914
54. He Q, Liu Y, Zou Q, Guan YS. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World J Gastroenterol. (2011) 17:2353–5. doi: 10.3748/wjg.v17.i18.2353
55. Zhang R, Cui Y, Guan X, Jiang X. A recombinant human adenovirus type 5 (H101) combined with chemotherapy for advanced gastric carcinoma: A retrospective cohort study. Front Oncol. (2021) 11:752504. doi: 10.3389/fonc.2021.752504
56. Lu W, Zheng S, Li X, Huang J, Zheng X, Li Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. (2004) 10:3634–8. doi: 10.3748/wjg.v10.i24.3634
57. Tanabe S, Kojima T, Tazawa H, Noma K, Katsui K, Hori K, et al. 554P Phase I clinical trial of OBP-301, a novel telomerase-specific oncolytic virus, in combination with radiotherapy in esophageal cancer patients. Ann Oncol. (2021) 5:612. doi: 10.1016/j.annonc.2021.08.1076
58. Freytag SO, Stricker H, Lu M, Elshaikh M, Aref I, Pradhan D, et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2014) 89:268–76. doi: 10.1016/j.ijrobp.2014.02.034
59. Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. (2010) 16:3067–77. doi: 10.1158/1078-0432.CCR-10-0054
60. Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced Malignancies. Clin Cancer Res. (2012) 18:2080–9. doi: 10.1158/1078-0432.CCR-11-2181
61. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. (2018) 15:422–42. doi: 10.1038/s41571-018-0003-5
62. Kurokawa C, Agrawal S, Mitra A, Galvani E, Burke S, Varshine A, et al. Mediation of anti-tumor activity by AZD4820 oncolytic vaccinia virus encoding IL-12. Mol Ther Oncol. (2024) 32:200758. doi: 10.1016/j.omton.2023.200758
63. Mei S, Peng S, Vong EG, Zhan J. A dual-functional oncolytic adenovirus ZD55-aPD-L1 scFv armed with PD-L1 inhibitor potentiates its anti-tumor activity. Int Immunopharmacol. (2024) 128:111579. doi: 10.1016/j.intimp.2024.111579
64. Jhawar SR, Wang SJ, Thandoni A, Bommareddy PK, Newman JH, Marzo AL, et al. Combination oncolytic virus, radiation therapy, and immune checkpoint inhibitor treatment in anti-PD-1-refractory cancer. J Immunother Cancer. (2023) 11:e006780. doi: 10.1136/jitc-2023-006780
65. Middleton MR, Aroldi F, Sacco J, Milhem MM, Curti Brendan D, Vanderwalde AM, et al. An open-label, single-arm, phase II clinical trial of RP1, an enhanced potency oncolytic herpes virus, combined with nivolumab in four solid tumor types: Initial results from the skin cancer cohorts. J Clin Oncol. (2020) 38:22050. doi: 10.1200/JCO.2020.38.15_suppl.e22050
66. Harb WA, Rosen LS, Wang D, Fakih M, Mahadevan D, Clemens W, et al. A phase I study of enadenotucirev (EnAd), an oncolytic Ad11/Ad3 chimeric group B adenovirus, in combination with nivolumab in tumors of epithelial origin. J Clin Oncol. (2017) 35:3115. doi: 10.1200/JCO.2017.35.15_suppl.TPS3115
67. Harrington KJ, Sacco JJ, Olsson-Brown AC, Chan TY, Nenclares P, Leslie I, et al. A phase 1 trial of RP2, a first-in-class, enhanced potency oncolytic HSV expressing an anti-CTLA-4 antibody as a single agent and combined with nivolumab in patients with advanced solid tumors. J Clin Oncol. (2022) 40:2704. doi: 10.1200/JCO.2022.40.16_suppl.TPS2704
68. Isei T, Yokota K, Uhara H. Topline results from phase II of combination treatment with canerpaturev (HF10), an oncolytic viral immunotherapy, and ipilimumab in patients with unresectable or metastatic melanoma after anti-PD-1 therapy. Ann Oncol. (2018) 29:452–3. doi: 10.1093/annonc/mdy289.023
69. Yokota K, Isei T, Uhara H, Fujisawa Y, Takenouchi T, Kiyohara Y, et al. Final results from phase II of combination with canerpaturev (formerly HF10), an oncolytic viral immunotherapy, and ipilimumab in unresectable or metastatic melanoma in second-or later line treatment. Ann Oncol. (2019) 30:557. doi: 10.1093/annonc/mdz255.053
70. Andtbacka R, Ross MI, Agarwala SS, Taylor MH, Vetto JT, Neves RL, et al. Final results of a phase II multicenter trial of HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB-IV unresectable or metastatic melanoma. J Clin Oncol. (2017) 35:9510. doi: 10.1200/JCO.2017.35.15_suppl.9510
71. Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase ib study. Clin Cancer Res. (2020) 26:71–81. doi: 10.1158/1078-0432.CCR-19-2078
72. Guan J, Sun K, Guerrero CA, Zheng J, Xu Y, Mathur S, et al. A phase 2 study of in situ oncolytic virus therapy and stereotactic body radiation therapy followed by pembrolizumab in metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. (2024) 118:1531–40. doi: 10.1016/j.ijrobp.2023.08.044
73. Nassiri F, Patil V, Yefet LS, Singh O, Liu J, Dang RMA, et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat Med. (2023) 29:1370–8. doi: 10.1038/s41591-023-02347-y
74. Randolph Hecht J, Chan A, Martin M, Mach N, Hurvitz SA, Rottey S, et al. Phase Ib study of talimogene laherparepvec (T-VEC) injection into liver metastases (LMs) in combination with intravenous (IV) atezolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC) or colorectal cancer (CRC). J Clin Oncol. (2019) 37:725. doi: 10.1200/JCO.2019.37.4_suppl.TPS725
75. Manso L, Villagrasa P, Chic N. Changes in T cell clonality in AWARE-1 study, a window-of-opportunity study with atezolizumab and the oncolytic virus pelareorep in early breast cancer. J ImmunoTherapy Cancer. (2020) 8:855. doi: 10.1136/jitc-2020-SITC2020.0806
76. Styczyński J. A brief history of CAR-T cells: from laboratory to the bedside. Acta Haematol Pol. (2020) 51:2–5. doi: 10.2478/ahp-2020-0002
77. Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu Rev Med. (2016) 67:165–83. doi: 10.1146/annurev-med-051914-021702
78. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11:69. doi: 10.1038/s41408-021-00459-7
79. Qualls D, Salles G. Optimizing CAR T cell therapy in lymphoma. Hematol Oncol. (2021) 39 Suppl 1:104–12. doi: 10.1002/hon.2844
80. Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR-T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. (2017) 9:1183–97. doi: 10.15252/emmm.201607485
81. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
82. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. (2016) 13:394. doi: 10.1038/nrclinonc.2016.65
83. Ajina A, Maher J. Prospects for combined use of oncolytic viruses and CAR-T-cells. J Immunother Cancer. (2017) 5:90. doi: 10.1186/s40425-017-0294-6
84. Stilpeanu RI, Secara BS, Cretu-Stancu M, Bucur O. Oncolytic viruses as reliable adjuvants in CAR-T cell therapy for solid tumors. Int J Mol Sci. (2024) 25:11127. doi: 10.3390/ijms252011127
85. Huang J, Zheng M, Zhang Z, Tang X, Chen Y, Peng A, et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother. (2021) 70:2453–65. doi: 10.1007/s00262-021-02856-0
86. Wang G, Zhang Z, Zhong K, Wang Z, Yang N, Tang X, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. (2023) 31:134–53. doi: 10.1016/j.ymthe.2022.08.021
87. Tanoue K, Rosewell Shaw A, Watanabe N, Porter C, Rana B, Gottschalk S, et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances anti-tumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. (2017) 77:2040–51. doi: 10.1158/0008-5472.CAN-16-1577
88. Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. (2014) 74:5195–205. doi: 10.1158/0008-5472.CAN-14-0697
89. Evgin L, Kottke T, Tonne J, Thompson J, Huff AL, van Vloten J, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. (2022) 14:eabn2231. doi: 10.1126/scitranslmed.abn2231
90. VanSeggelen H, Tantalo DG, Afsahi A, Hammill JA, Bramson JL. Chimeric antigen receptor-engineered T cells as oncolytic virus carriers. Mol Ther Oncolytics. (2015) 2:15014. doi: 10.1038/mto.2015.14
91. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. (2020) 382:545–53. doi: 10.1056/NEJMoa1910607
92. Ma R, Li Z, Chiocca EA, Caligiuri MA, Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. (2023) 9:122–39. doi: 10.1016/j.trecan.2022.10.003
93. Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. (2016) 7:27764–77. doi: 10.18632/oncotarget.8526
94. Ma R, Lu T, Li Z, Teng KY, Mansour AG, Yu M, et al. An oncolytic virus expressing IL15/IL15Rα Combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. (2021) 81:3635–48. doi: 10.1158/0008-5472.CAN-21-0035
Keywords: oncolytic virus, tumor therapy, combined treatment, clinical trials, immunity
Citation: Du W, Na J, Zhong L and Zhang P (2025) Advances in preclinical and clinical studies of oncolytic virus combination therapy. Front. Oncol. 15:1545542. doi: 10.3389/fonc.2025.1545542
Received: 15 December 2024; Accepted: 09 January 2025;
Published: 07 February 2025.
Edited by:
Wenxue Ma, University of California, San Diego, United StatesCopyright © 2025 Du, Na, Zhong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Zhong, emhvbmdsaXBpbmdAZ3htdS5lZHUuY24=; Pumin Zhang, cHpoYW5nYmNtQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.