
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 10 March 2025
Sec. Breast Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1530391
 Nadia Harbeck1†
Nadia Harbeck1† Adam Brufsky2†
Adam Brufsky2† Chloe Grace Rose3†
Chloe Grace Rose3† Beata Korytowsky3†
Beata Korytowsky3† Connie Chen3
Connie Chen3 Krista Tantakoun4†
Krista Tantakoun4† Endri Jazexhi4†
Endri Jazexhi4† Do Hoang Vien Nguyen4†
Do Hoang Vien Nguyen4† Meaghan Bartlett4†
Meaghan Bartlett4† Imtiaz A. Samjoo4*†
Imtiaz A. Samjoo4*† Timothy Pluard5†
Timothy Pluard5†Aim: Since 2021, additional real-world evidence (RWE) has emerged on the effectiveness of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) as first-line treatment of HR-positive/HER2-negative (HR+/HER2−) advanced/metastatic breast cancer (A/MBC), necessitating this updated review.
Methods: MEDLINE®, Embase®, and Cochrane Databases (07/06/2019–01/09/2024), and key congresses (2020–2024) were searched. Studies reporting first-line CDK4/6i use, over 100 participants, and progression-free survival (PFS) and/or overall survival (OS) data were included.
Results: This update included 82 unique studies, 42.7% for palbociclib, 7.3% for ribociclib, and 3.7% for abemaciclib; 46.3% assessed multiple CDK4/6i. In studies including multiple CDK4/6is, median PFS was 23.4–31.0 months for palbociclib, 19.8–44.0 for ribociclib, and 14.0–39.5 for abemaciclib. When reached, median OS was 38.0–58.0 months, 40.4–52.0 months, and 34.4 months, respectively. These real-world PFS and OS results were within the range of single-arm and CDK4/6i versus endocrine therapy (ET) studies, where CDK4/6i demonstrated greater benefits than ET alone.
Conclusion: First-line CDK4/6i RWE demonstrates significant clinical benefits in HR+/HER2− A/MBC. These data are important to guide clinical decision-making, as they include patients who are not adequately represented in clinical trials. Studies with longer follow-up are needed to assess long-term benefits of all three CDK4/6i therapies in HR+/HER2− A/MBC.
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer deaths in women globally (1). According to GLOBOCAN, approximately 2.0 million new cases of BC were diagnosed in 2022 worldwide, accounting for 11.5% of all new cancer cases and 6.8% of all cancer-related deaths (2).
The disease stage and subtype at diagnosis strongly influence survival with BC. Based on Surveillance, Epidemiology, and End Results (SEER) data from 2014–2020, 28% of patients were diagnosed with regional stage BC (i.e., cancer that has spread to regional lymph nodes; advanced BC [ABC]) and 6% were diagnosed with distant BC stage (i.e., cancer has metastasized; metastatic BC [MBC]) (3). The most common BC subtype is hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−), with an age-adjusted rate of 90.0 new cases per 100,000 women. Among those with HR+/HER2− advanced/metastatic breast cancer (A/MBC), the 5-year relative survival rate between 2014–2020 was 86.7% and 31.9% in patients with ABC and MBC, respectively (3).
Therapeutic options for HR+/HER2− A/MBC have expanded with the introduction of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) into clinical practice. Palbociclib (Ibrance®, approved in 2015 in the United States [US]) (4), ribociclib (Kisqali®, approved in 2017 in the US) (5), and abemaciclib (Verzenio™, approved in 2017 in the US) (6) have been approved for use in combination with endocrine therapy (ET), including aromatase inhibitors (AIs) or fulvestrant, or as a single agent (abemaciclib). These approvals are based on the results of several randomized controlled trials (RCTs) (4–6) that met their study endpoint by demonstrating improvement in progression-free survival (PFS) among patients receiving CDK4/6i compared with those receiving ET monotherapy. Since their introduction, CDK4/6i plus ET have become the standard of care for first-line treatment of HR+/HER2− A/MBC, due to their efficacy, safety, and maintenance of quality of life when used as first-line treatment for patients with A/MBC (7–10).
Due to narrow patient eligibility criteria and study endpoints, RCTs are limited in providing a comprehensive understanding of clinical reality in routine practice. Real-world evidence (RWE) not only offers valuable insight into the effectiveness of treatments for HR+/HER2− A/MBC patient subgroups that may be underrepresented in clinical trials (e.g., older adults, those with comorbid or multimorbid conditions, Black, Indigenous, and People of Color [BIPOC] patients) but also reveals emerging patterns of care over extended periods, particularly after market approval. A systematic literature review (SLR) of RWE studies of CDK4/6i in the treatment of HR+/HER2− A/MBC was previously published including publications up to July 6, 2019 (11). At that time there were still limited follow-up data available, and limited real-world data for ribociclib and abemaciclib relative to palbociclib, given it was the first CDK4/6i approved for use in A/MBC. RWE for this class has grown in the years following, and some of the recent data have focused on describing outcomes among the three agents within the CDK4/6i class. The objective of this study was to understand the evolution of evidence around CDK4/6i to help inform clinical decision-making by highlighting the patient experience in the real world. Therefore, an updated SLR was conducted to summarize the effectiveness results of CDK4/6i from first-line RWE studies published since the previous review (11).
This SLR followed the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12) (Appendix A in the Supplementary Material), which have been previously described (11). The search for the previous SLR (11) was performed on July 6, 2019. For this updated review, literature searches using OVID Medline, EMBASE, Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews were conducted on October 7, 2020, June 1, 2021, December 1, 2022, January 6, 2023, October 18, 2023, and January 9, 2024 to capture all data published since the previous SLR search in 2019; results from these searches were pooled for this analysis. Details of the most recent search strategy are presented in Appendix B in the Supplementary Material. The data presented were collected uniformly across all searches. This review was not registered as it was developed a priori.
Updated grey literature searches of prespecified key clinical conferences were also performed to identify abstracts and posters from January 2020 to January 2024. These included the San Antonio Breast Cancer Symposium (SABCS), the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), ESMO BC, ESMO Asia, the Professional Society for Health Economics and Outcomes Research (ISPOR), and ISPOR Europe (EU). Only abstracts from January 2022 to January 2024 were included in the analysis to present only the most up-to-date information available in the literature.
Studies were assessed for eligibility by two independent reviewers using the systematic review software DistillerSR (DistillerSR Inc., Ottawa, Ontario, Canada) according to the predefined Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria (Table 1). Discrepancies between the two independent reviewers during screening were resolved by consensus, with any disputes resolved by a third reviewer.
Studies were included if they reported RWE on patients aged ≥18 years with HR+/HER2− A/MBC receiving treatment with a CDK4/6i. Studies were excluded if published in any language other than English or before 2019. Only studies reporting data on CDK4/6i treatment in the first-line setting were included to focus on the treatment landscape wherein CDK4/6i are standard first-line treatment for HR+/HER2− A/MBC. To enhance the robustness of the review findings and relevance of the literature being summarized, studies with sample sizes of fewer than 100 patients and/or those that did not specify the line of therapy or specific CDK4/6i were excluded. Outcomes of interest included median PFS and/or median overall survival (OS) with corresponding hazard ratios (HRs), where available.
Data from the publications identified in this review were extracted into a standardized form in Microsoft® Excel (Microsoft Corporation, Redmond, WA, US). A single reviewer performed data extraction and was independently assessed for accuracy and completeness by a second reviewer.
During data analysis, the included studies were categorized by study design (i.e., single-arm or comparative). Comparative studies, which assessed multiple treatment arms, were further stratified based on the comparator, distinguishing between ET and other CDK4/6i. Within each study design category, PFS and/or OS were then evaluated according to the type of CDK4/6i assessed (i.e., palbociclib, ribociclib, abemaciclib, and any CDK4/6i regimen), as well as the patient population (i.e., the overall population or specific subgroups). Any CDK4/6i regimen was defined as one that evaluated a CDK4/6i—whether palbociclib, ribociclib, or abemaciclib—but the results were not specific to any single CDK4/6i. Prespecified subgroups of interest, identified a priori in consultation with clinicians and listed in Appendix C in the Supplementary Material, were also included in the analysis. Of note is that the number of studies reporting on specific subgroups may not reflect the total number of included studies as some studies report on multiple subgroups.
The results were organized first to provide an overview of findings from single-arm studies, followed by a detailed analysis of comparative studies. Each section explored outcomes in the overall population and prespecified subgroups, offering a thorough understanding of PFS and/or OS across various CDK4/6i regimens.
Of the included studies, only full-text publications were assessed for quality because conference abstracts often lack sufficient methodological data to assess study quality. Two independent reviewers performed the study quality assessments, resolving discrepancies through consensus. Risk of bias assessment was performed for included studies using the Newcastle-Ottawa scale (NOS) for nonrandomized studies (scores 7–9, 4–6, and <4 are considered low, intermediate, and high risk, respectively) (13). The ISPOR questionnaire (14) and ESMO Guidance for Reporting Oncology real-World Evidence (ESMO-GROW) checklist (15) were also used to determine the risk of bias for the included comparative studies and to assess appropriate reporting and transparency.
A total of 6737 records were identified, of which 4845 were found through database searches and 1892 through grey literature searches. The results of the literature search and study selection processes of each update are shown in Appendix D in the Supplementary Material. After the removal of duplicates, 4836 records were screened at the title and abstract stage, of which 2491 full texts were retrieved and assessed for eligibility. In total, 882 records were included in the SLR. The reasons for exclusion at the full-text stage of each update are summarized in Appendix D in the Supplementary Material.
Among the 882 records included in the SLR, 787 were excluded for reasons such as small sample sizes (<100 patients), unspecified line of therapy or type of CDK4/6i assessed, data on CDK4/6i treatment beyond first-line therapy, and/or lack of reported outcomes of interest (i.e., PFS and/or OS). Consequently, 95 publications (51 full-text articles and 44 conference abstracts/posters) representing 82 unique studies reported effectiveness data in the first-line setting and were included in the qualitative synthesis. Among these, 35 studies (42.7%) focused on palbociclib, 6 (7.3%) featured ribociclib, and 3 (3.7%) assessed abemaciclib. The remaining 38 studies (46.3%) investigated more than one CDK4/6i. A list of included studies is shown in Appendix E in the Supplementary Material.
The majority (n=51 [62.2%]) of the unique studies were single-arm (Figure 1A). The remaining studies were comparative in design (direct comparison or descriptive), with 12 studies comparing CDK4/6i to ET (Figure 1B) and 22 describing or comparing effectiveness studies evaluating multiple CDK4/6i (Figure 1C). Notably, three unique studies—GOIRC-04-2019, REACHAUT, and RIBANNA—were each represented by multiple associated records that led to their inclusion in both the single-arm and comparative design categories. The GOIRC-04-2019 study had two records: one for a single-arm analysis (16) and another for a comparison of multiple CDK4/6i (17). Similarly, the REACHAUT study included a single-arm analysis (18) and a comparison of two ribociclib regimens with different backbone therapies (AI or fulvestrant) (19). The RIBANNA study was represented by three abstracts, two of which compared CDK4/6i with ET (20, 21), whereas the third was a comparison of ribociclib treatment in combination with different ET therapies (AI or fulvestrant) (22).
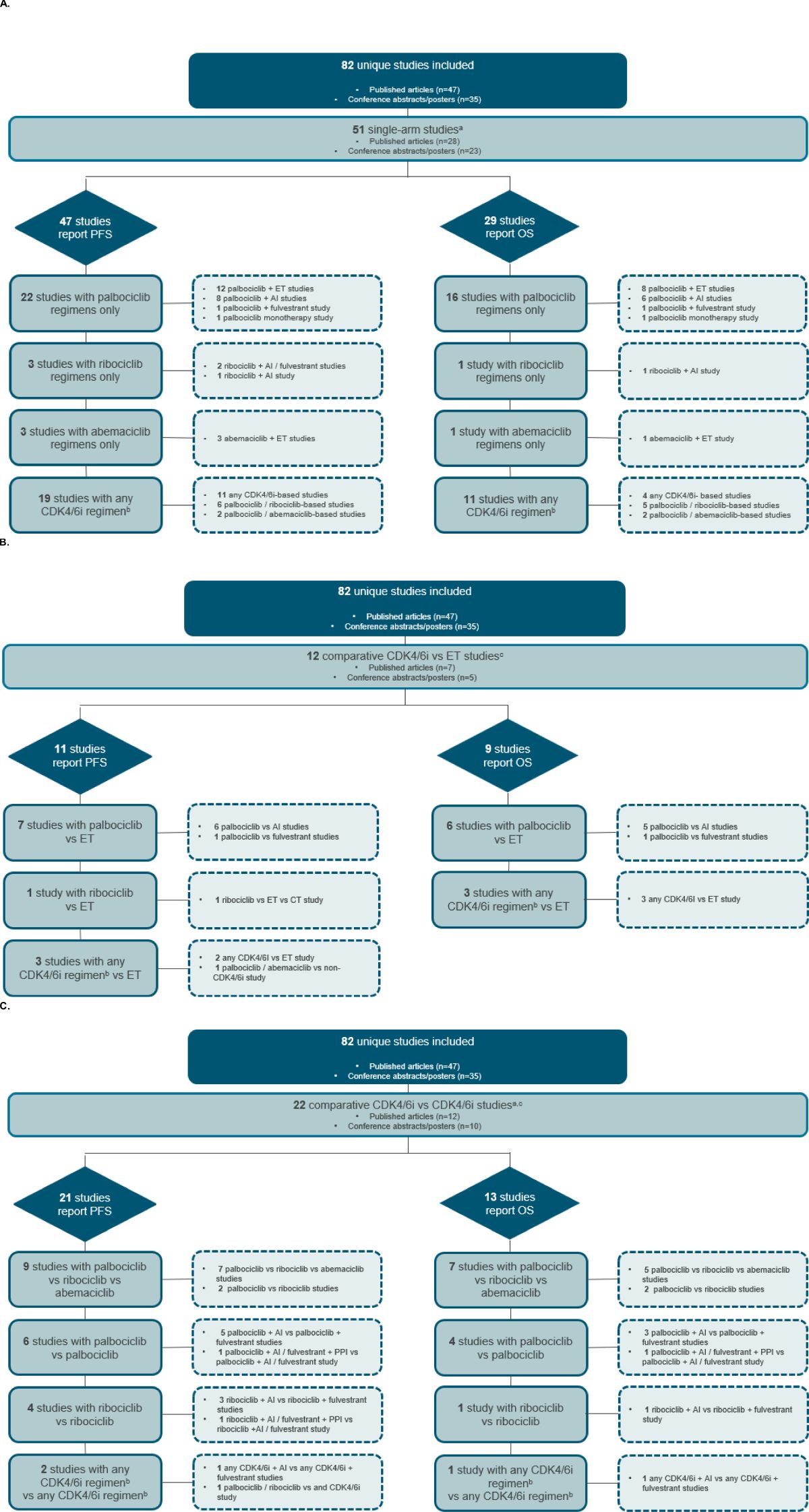
Figure 1. Study attrition diagram for (A) single-arm studies (B) comparative CDK4/6i versus ET studies, and (C) comparative CDK4/6i versus CDK4/6i studies. aThe GOIRC-04-2019 and REACHAUT studies had multiple associated records using single-arm and comparative analyses and are thus counted in both study design categories. bAny CDK4/6i regimen was defined as that in which a CDK4/6i—whether palbociclib, ribociclib, or abemaciclib—was evaluated, but the results were not specific to any single CDK4/6i. cThe RIBANNA study had multiple associated records, including ET and CDK4/6i comparator arms, resulting in its inclusion in both comparative study design categories. AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CT, chemotherapy; ET, endocrine therapy; OS, overall survival; PFS, progression-free survival; PPII, proton pump inhibitor.
Across all study types, the majority (43.9%) were conducted in Europe, with the highest representations from the United Kingdom (n=7), Spain (n=7), and Italy (n=6). Other regions included North America, Latin America, Asia-Pacific, and the Middle East (Figure 2).
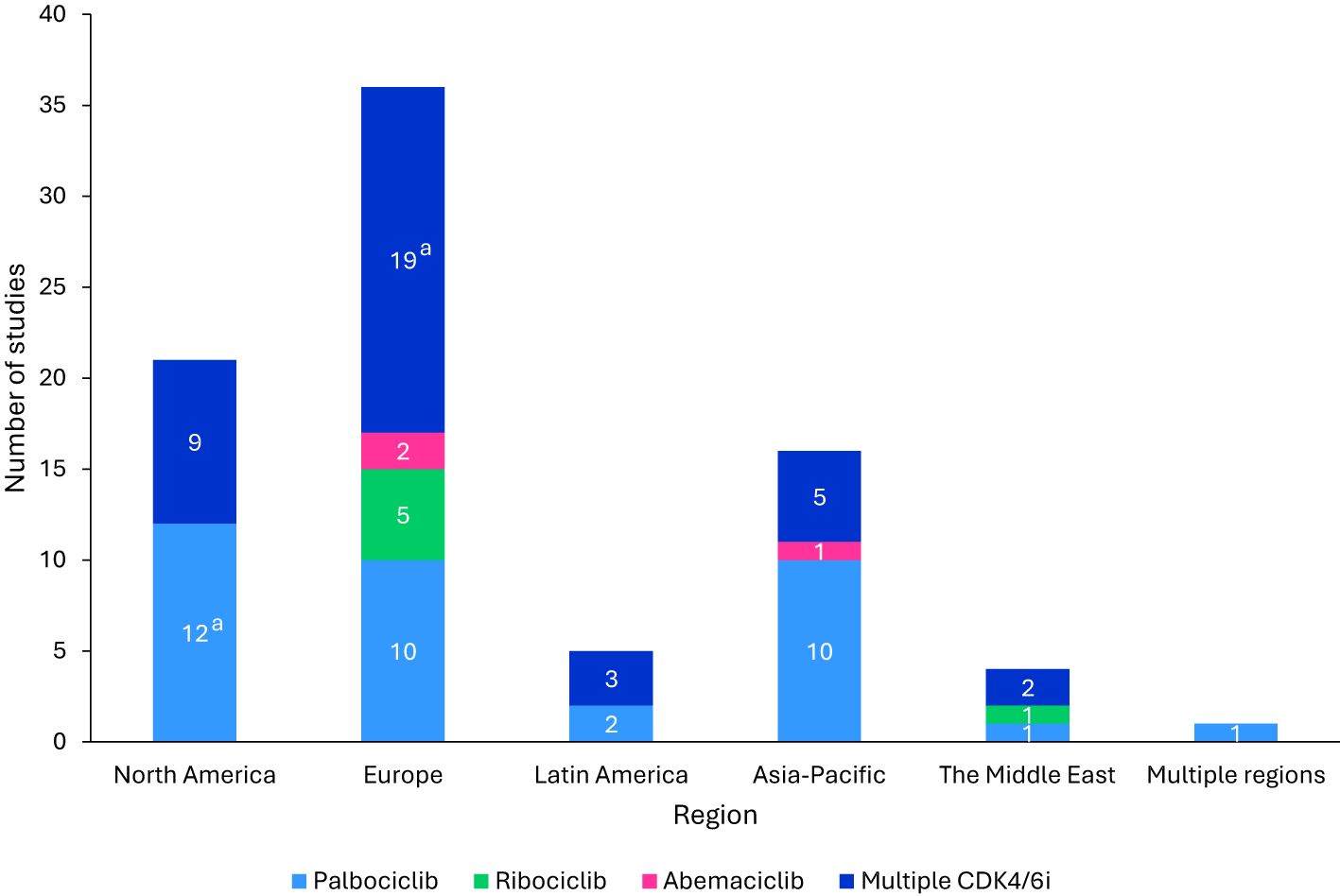
Figure 2. Regional distribution of included studies. aThe GOIRC-04-2019 study had multiple associated records, one evaluating palbociclib in North America and the other evaluating any CDK4/6i regimen in Europe. As such, this study is accounted for in both categories. bStudies were classified as “Multiple CDK4/6i” if two or more specified CDK4/6i were included in the study. CDK4/6i, cyclin-dependent kinase 4/6 inhibitors.
For all included full-text reports, the NOS quality scores ranged from 4–8 points out of 9; 66.7% (34/51) had a score of 4–6, and 33.3% (17/51) had a score of 7 or 8. Comparative studies were assessed using the ISPOR questionnaire and ESMO-GROW checklist, which indicated that the overall credibility of these reports was generally sufficient (87.0% [20/23] reports were identified as being of sufficient credibility). However, it is important to note that studies with an overall rating of sufficient credibility may still have significant limitations.
Among the assessed studies, the main source of biases identified concerned the study design and analyses. Nine studies used stabilized inverse probability of treatment weighting (sIPTW) and 1:1 propensity score matching (PSM) methods to control for differences in baseline demographics and clinical characteristics between treatment groups (23–31). However, the majority of studies did not provide evidence that robust statistical methods, such as sIPTW or PSM, were used to ensure comparability of treatment groups and only reported results descriptively. Moreover, the nonrandomized nature of these studies means confounding factors could affect these results. Data collection methods, including data cleaning and validation processes, were generally consistent among the assessed studies. All records underwent chart abstraction by certified tumor registrars. For two studies, this was followed by a quality control review for transparency and completeness by clinical analytics teams in two studies (28, 32). The remaining studies did not provide adequate details on how the different data sources were assessed.
Full results for the quality assessments are presented in Appendix F in the Supplementary Material.
Of the 51 single-arm studies, PFS data were reported in 47, whereas OS data were reported in 29 (Figure 1A). Data from studies evaluating any CDK4/6i regimen without CDK4/6i-specific results are summarized in Appendix G in the Supplementary Material.
The PFS data for patients receiving palbociclib were reported in 22 single-arm studies (Figure 1A). Of these, 11 studies reported results for the overall population (Supplementary Table 1, Appendix H in the Supplementary Material), three reported results for specific subgroups of patients (Supplementary Tables 1, 2, Appendix I in the Supplementary Material), and the remaining eight reported both overall and subgroup population data.
In 10 single-arm studies evaluating palbociclib plus ET (unspecified) in the overall patient population, median PFS ranged from 12.1 (n=103) (33) to 37.8 months (n=434) (34). The PFS results were similar to those of palbociclib plus AI, reported in seven single-arm studies, with median PFS ranging from 11.8 (35) to 39.0 months (16) in the overall population. However, it should be noted that the study with a median PFS of 11.8 months had only 30 patients in the arm receiving palbociclib plus AI (letrozole) (35). Omitting this study resulted in a median PFS range of 28.7 (n=305) (36) to 39.0 months (n=241) (16). In comparison, median PFS was lower in one single-arm study evaluating palbociclib plus fulvestrant in the overall population, with a median PFS of 19.6 months (n=317) (37). Data for palbociclib monotherapy was reported in a conference abstract from Taiwan (n=53), indicating that median PFS was not reached after a median follow-up of 24.5 months (38) (Supplementary Table 1, Appendix H in the Supplementary Material). Of the nine studies with quality assessment, all were considered intermediate risk (NOS score of 4–6) (32–36, 39–43).
Subgroups based on the types of metastases (e.g., visceral, bone, liver, and so on) were assessed in six single-arm studies. Among patients presenting with visceral metastases, median PFS ranged from 15.3 (n=65) (42) to 27.9 months (n=78) (32), whereas those with no visceral metastases had a higher median PFS, ranging from 27.8 (n=212) (44) to 31.3 months (n=240) (42). For patients with bone-only metastases, the median PFS ranged from 20.0 (n=30) (45) to 44.9 months (n=123) (32). One study found that patients without liver metastases (n=245) had a statistically significant improvement in median PFS (12.7 months) compared with those with liver metastasis (n = 60; 31.3 months) with a HR of 2.17 (1.42-3.31; P < 0.001) (42).
Subgroups based on hormonal status (e.g., progesterone receptor [PR]-positive [+]/PR-negative [−], HER2 status, estrogen receptor [ER]/PR strong/weak) were assessed in four studies. Among patients with PR+ disease, the median PFS ranged from 24.5 (n=74) (42) to 38.0 months (n=127) (41), whereas those with PR− disease had a lower median PFS, ranging from 17.9 (n=75) (42) to 18.0 months (n=23) (41). Where reported, this trend was statistically significantly in favor of those with PR+ disease. Additionally, median PFS was generally similar among patients with HER2-zero (13.0 [n=83] (41) to 23 months (40)) and HER2-low status (19.0 [n=71] (40) to 25.0 months [n=67] (41)). One study also compared patients with strong (n=425) versus weak ER/PR expression (n=92) and found that PFS was statistically significantly higher in those with strong expression (42).
Subgroups based on ET response (e.g., de novo, recurrent, endocrine-resistant/sensitive) were assessed in three studies. Median PFS ranged from 14 (46) to 14.5 months (n=193) (44) for patients who relapsed within 12 months, 27.3 (n=86) (44) to 29.0 months (n=220) (46) for those who relapsed after more than 12 months, and 28.0 (n=233) (46) to 33.6 months (n=109) (44) for de novo patients. One study observed the highest median PFS in patients with no ET (25.4 months; n=126), followed by those with secondary resistance (20.3 months; n=58) and primary resistance (12.7 months; n=38) (42). A statistically significant difference was noted, with patients showing primary ET resistance having a higher risk of progression compared to those with no ET (HR: 1.91, 95% CI: 1.13–3.24; P=0.022). No significant difference was observed for secondary ET resistance (HR: 0.87, 95% CI: 0.52–1.49; P=0.022) or ET-sensitive patients (HR: 0.81, 95% CI: 0.50–1.32; P=0.022) compared to those with no ET (42).
Subgroups based on dose modifications were assessed in three studies. Median PFS was similar for patients with and without dose reductions, ranging from 25.0 (n=80) (41) to 28.0 months (n=377) (47) and 19.0 (n=385) (47) to 22.0 months (n=70) (41), respectively, with no statistically significant difference reported. One study reported dose modifications specifically due to grade 3 afebrile neutropenia and showed a statistically significant lower 24-month PFS rate among patients who required dose modifications (55.3%; n=128) than those who maintained their doses (67.9%; n=174) (34).
Three studies assessed subgroups based on risk factors (e.g., comorbidities, Charleson Comorbidity Index [CCI] score). Median PFS ranged from 12.5 to 23.7 months among patients with various comorbid disorders (e.g., vascular, psychiatric, metabolic, lymphatic, cardiac; n range: 32-495) (48), with increasing presence of risk factors generally correlating with lower median PFS (32, 42); however, no tests for statistical significance were conducted.
Additional prespecified subgroups of interest were assessed in the single-arm studies that reported PFS data for palbociclib-based regimens, which included menopausal status, Eastern Cooperative Oncology Group (ECOG) score, age, race/ethnicity, and palbociclib starting dose. The results of these studies are described in Supplementary Table 1, Appendix I in the Supplementary Material. The PFS results for studies that assessed other subgroups are detailed in Supplementary Table 2, Appendix I in the Supplementary Material.
Overall, in studies with subgroups of interest data, six received a quality assessment, with NOS scores of 5 or 6 (intermediate risk of bias) (32, 34, 40–42, 49).
The PFS data for patients receiving ribociclib were reported in three single-arm studies (Figure 1A). Of these, one study reported results for the overall population (Supplementary Table 2, Appendix H in the Supplementary Material) (50), another study reported subgroup-only results (Supplementary Table 3, Appendix I in the Supplementary Material) (51), and the third reported both overall and subgroup population data (18).
In a conference abstract of the single-arm REACHAUT study, the median PFS was 29.7 months among patients receiving ribociclib plus AI or fulvestrant in the overall patient population (n=283), with a median follow-up duration of 14.4 months (18). In another abstract of a single-arm study evaluating ribociclib plus AI (n=154), median PFS was reported to be 20.6 months, although the duration of follow-up was not provided (Supplementary Table 2, Appendix H in the Supplementary Material) (50).
Two single-arm studies assessing ribociclib in combination with either AI or fulvestrant provided insights into prespecified subgroups of interest. In addition to the overall population, the conference proceeding for the REACHAUT study also evaluated patients with visceral metastases (n=116) and reported a median PFS of 32.7 months (18). The other study compared outcomes between patients who did not require a dose reduction and those who experienced a late dose reduction (51). After a median follow-up time of 18.4 months, the median PFS for patients without a dose reduction (n=46) was 15.6 months, while the median PFS for those with a late dose reduction (n=31) was not reached (51) (Supplementary Table 3, Appendix I in the Supplementary Material). This study was assessed for quality with a NOS score of 5 and judged to be of sufficient credibility (51).
The PFS data for patients receiving abemaciclib in combination with ET (unspecified) in the overall population were reported in three single-arm studies (Figure 1A). Median PFS across these studies ranged from 21.4 (n=63) to 23.0 months (n=69), with 71.6% to 81.1% of patients achieving PFS at 12 months (Supplementary Table 3, Appendix H in the Supplementary Material) (52–54). Notably, none of the studies reported PFS data for specific patient subgroups. Only one of these studies was evaluated for quality (54); it received an NOS score of 4 and was judged to have insufficient credibility.
The OS data for patients receiving palbociclib were reported in 16 single-arm studies (Figure 1A). Of these, six studies reported results for the overall population (Supplementary Table 1, Appendix H in the Supplementary Material), two reported results exclusively for specific subgroups of patients (Supplementary Tables 1, 2, Appendix I in the Supplementary Material), and the remaining eight studies reported both overall and subgroup population data.
In seven single-arm studies evaluating palbociclib plus ET (unspecified) in the overall patient population, median OS ranged from 33.0 (n=1066) (41) to 42 months (n=762) (46) when reached, with one study from Chile reporting a median OS of 111 months (n=67) (55). One of these studies (n=434) also reported a 24-month OS rate of 91.4% (34). Across five single-arm studies evaluating palbociclib plus AI, median OS was not reached where reported; however, the 24-month OS rate ranged from 70.0% (35) to 78.0% (n=242) (32). In comparison, median OS was 44.1 months in one single-arm study evaluating palbociclib plus fulvestrant (n=317) (37) (Supplementary Table 1, Appendix H in the Supplementary Material). Data for palbociclib monotherapy was reported in a conference abstract from Taiwan (n=53), indicating that median OS was not reached after a median follow-up of 24.5 months (Supplementary Table 1, Appendix H in the Supplementary Material) (38). The six studies reporting median OS underwent quality assessment and had NOS scores from 4 to 6 (35, 40–43, 55).
Four single-arm studies included the same subgroups based on hormonal status as described in Section 3.3.1.1.2. Where median OS was reached, it ranged from 39.0 (n=127) (41) to 44.0 months (n=530) (56) for patients with PR+ disease and 28.0 (n=23) (41) to 40.0 months (n=213) (56) for those with PR− disease, with a statistically significant difference favoring PR+ patients. In two studies comparing patients with HER2-zero and HER2-low status, one found a higher OS rate in those with HER2-zero status, while the other reported a longer median OS in those with HER2-low status; however, these results were not statistically significant (Supplementary Table 1, Appendix I in the Supplementary Material (40).
Additional prespecified subgroups of interest were assessed in the single-arm studies that reported OS data for palbociclib-based regimens, which included metastases (e.g., bone, visceral), comorbidities (e.g., disorders, CCI score), dose modification, ECOG score, and ET response (e.g., de novo, recurrent). The results of these studies are described in Supplementary Table 1, Appendix I in the Supplementary Material. The OS results for studies that assessed other subgroups are detailed in Supplementary Table 2, Appendix I in the Supplementary Material.
Overall, in studies with subgroups of interest data, six of these studies were evaluated for quality (32, 34, 40–42, 49); all were identified as having an intermediate risk of bias (NOS scores of 5 or 6).
The OS data for patients in the overall population receiving ribociclib in combination with ET (unspecified) were reported in one single-arm study (Figure 1A). In a conference abstract of the single-arm REACHAUT study (n = 283), the 12-month OS rate was 90.3% after a median follow-up duration of 14.4 months (Supplementary Table 2, Appendix H in the Supplementary Material) (18). No OS data for specific patient subgroups were reported.
The OS data for patients in the overall population receiving abemaciclib in combination with ET (unspecified) were reported in one single-arm study (n = 69; Figure 1A). In a conference abstract that reported effectiveness results from the Slovenian National Institute of Public Health and the Slovenian Cancer Registry, median OS was not reached after a median follow-up of 24 months (Supplementary Table 3, Appendix H in the Supplementary Material) (53). No OS data for specific patient subgroups were reported.
Of the 12 comparative (including direct and descriptive comparison) studies evaluating CDK4/6i versus ET, PFS data were reported in 11 studies, whereas OS data were reported in nine studies (Figure 1B). Of note, although there are comparative studies for ribociclib versus ET, only PFS data were reported. Additionally, no studies evaluated abemaciclib in comparison to ET. Results from studies evaluating any CDK4/6i regimen versus ET without CDK4/6i-specific data are summarized and included in Appendix J in the Supplementary Material.
The PFS data for patients receiving palbociclib versus ET were reported in seven studies (Figure 1B). Of these, three studies reported results for the overall population (Table 2, Figure 3), two focused on specific patient subgroup populations (Supplementary Table 4, Appendix I in the Supplementary Material), and the remaining two reported data for both the overall and subgroup populations.
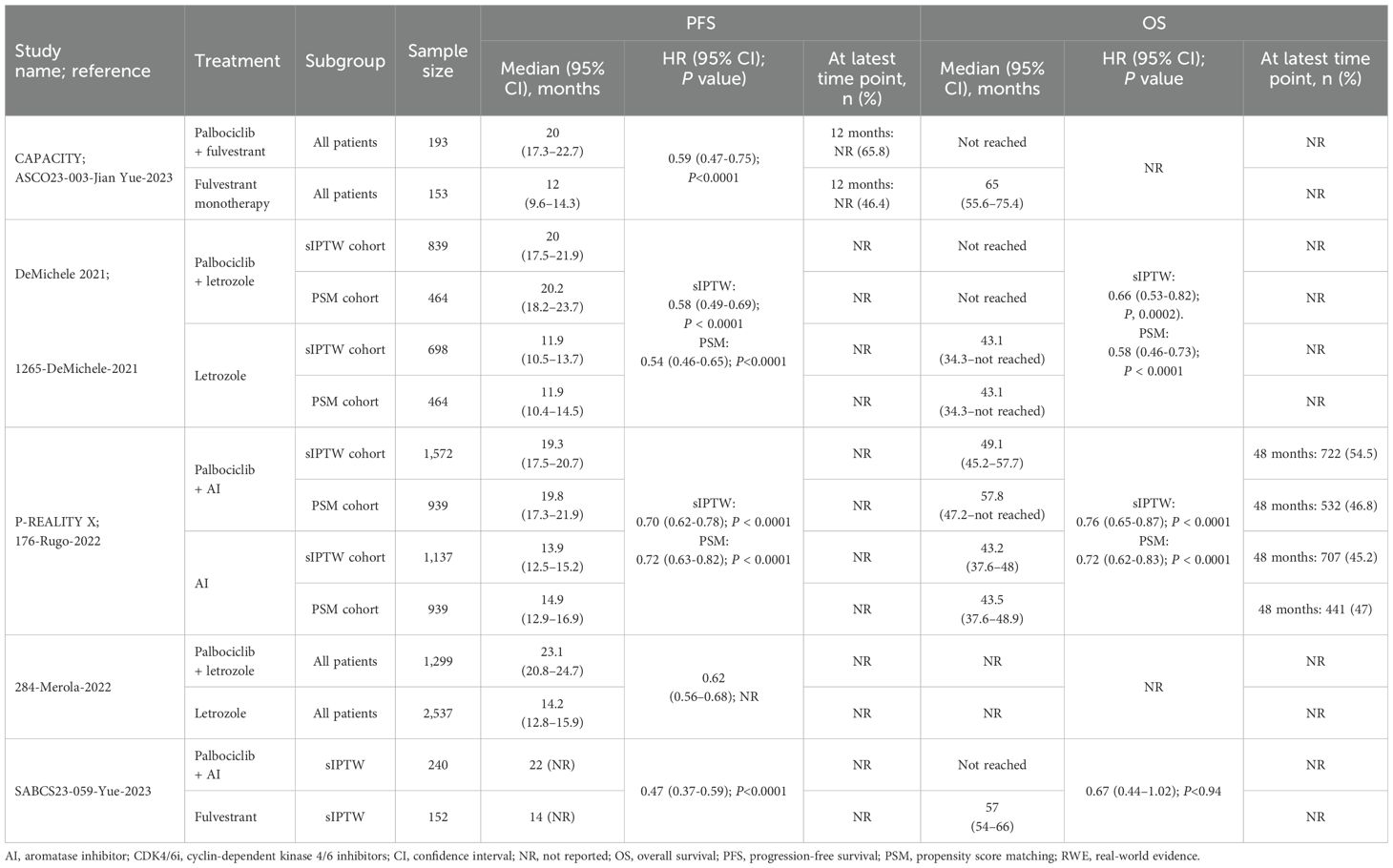
Table 2. Effectiveness outcomes for overall first-line palbociclib in comparative RWE studies versus ET.
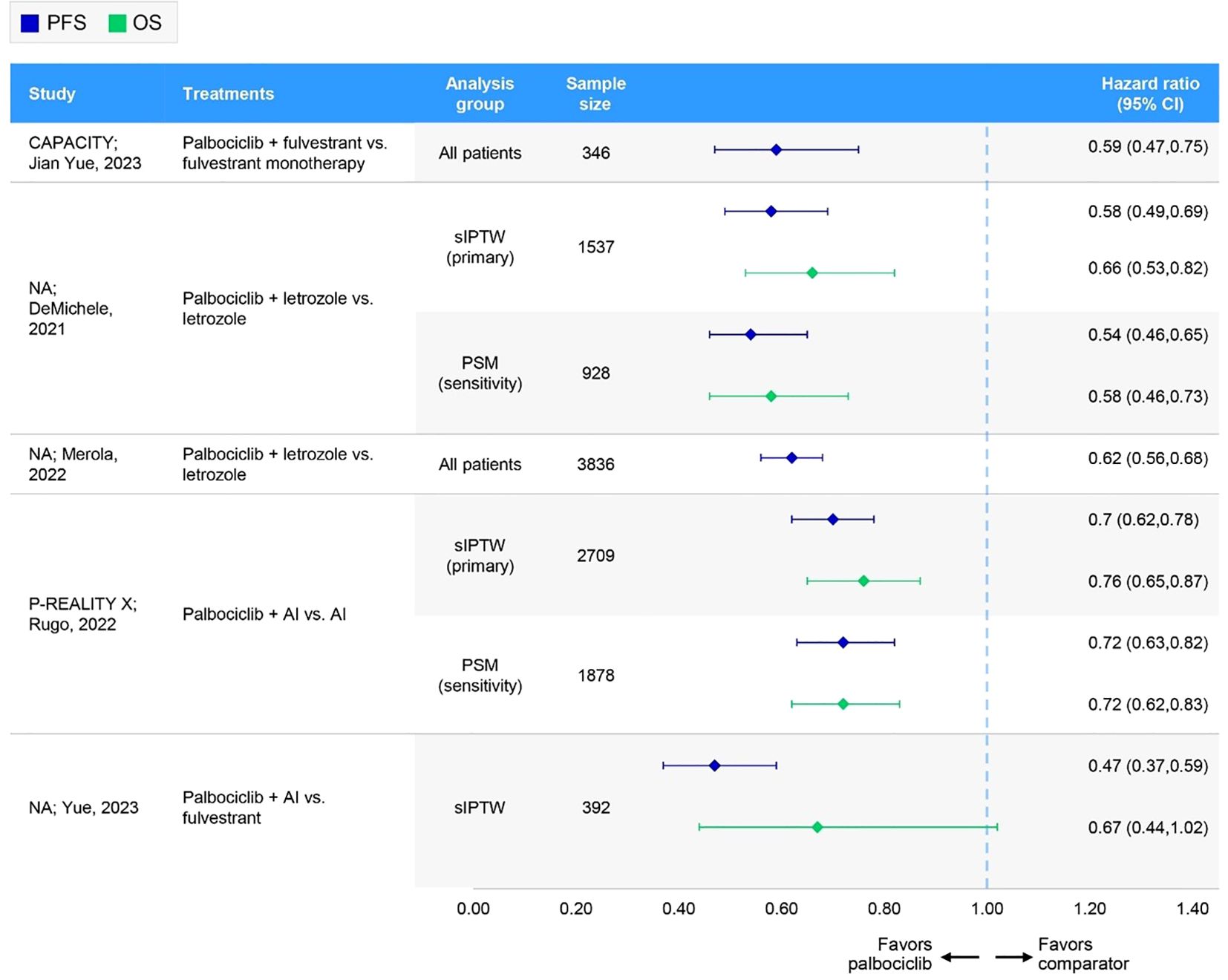
Figure 3. Forest plot of hazard ratios for effectiveness outcomes for overall first-line palbociclib in comparative RWE studies versus ET. AI, aromatase inhibitor; CI, confidence interval; NA, not applicable; OS, overall survival; PFS, progression-free survival; PSM, propensity score matching; sIPTW, stabilized inverse probability of treatment weighting.
In four comparative studies that used sIPTW as the primary analysis of the outcome measure, palbociclib plus AI consistently demonstrated greater PFS benefits relative to control treatment with AI alone, resulting in a median PFS of 19.3 (n=1572) (28) to 23.1 months (n=1229) (31) for palbociclib plus AI and 11.9 (n=698) (27) to 13.9 months (n=1137) (28) for the control. Similarly, the CAPACITY study conference abstract reported improved PFS outcomes with palbociclib plus fulvestrant (n=193) compared with the control arm of fulvestrant alone (n=153), demonstrating a longer median PFS (20.0 months vs. 12.0 months) and a greater proportion of patients experiencing PFS at 12 months (65.8% vs. 46.4%) (57). Overall, palbociclib-based regimens consistently demonstrated statistically significant improvements in PFS compared to ET (Table 2, Figure 3). Of the three studies assessed for quality (27, 28, 31), two had a low risk of bias (NOS scores of 8) (27, 28), while one had an intermediate risk (NOS score of 5) (31). ISPOR questionnaire assessment found all three to be of sufficient overall credibility.
Three separate records of the P-REALITY X study assessed three different subgroups using data from the Flatiron Health database: patients aged 75 years or older (23), those with lung and/or liver metastases (24), and those with cardiovascular disease (58). Another unique study also included subgroups based on lung or liver metastases (24), while a third study assessed patients aged 65 years or older (30). The fourth study assessed African American subgroups (29). All four unique comparative studies demonstrated statistically significant improvements in PFS when treated with palbociclib plus AI versus AI alone (Supplementary Table 4, Appendix I in the Supplementary Material). The four publications assessed for quality had NOS scores of 8 and sufficient credibility according to the ISPOR questionnaire (23, 24, 29, 30).
The PFS data for patients receiving ribociclib plus AI or fulvestrant versus ET and chemotherapy alone were reported in one comparative study (RIBANNA) (Figure 1B).
In a conference abstract for the fifth interim analysis of the RIBANNA study, patients receiving ribociclib in combination with AI or fulvestrant (n=2163) had a median PFS of 32.2 months, similar to the 35.2 months observed with ET monotherapy (n=237). In contrast, patients receiving chemotherapy alone (n=181) had a shorter median PFS of 16.7 months (Table 3) (20).
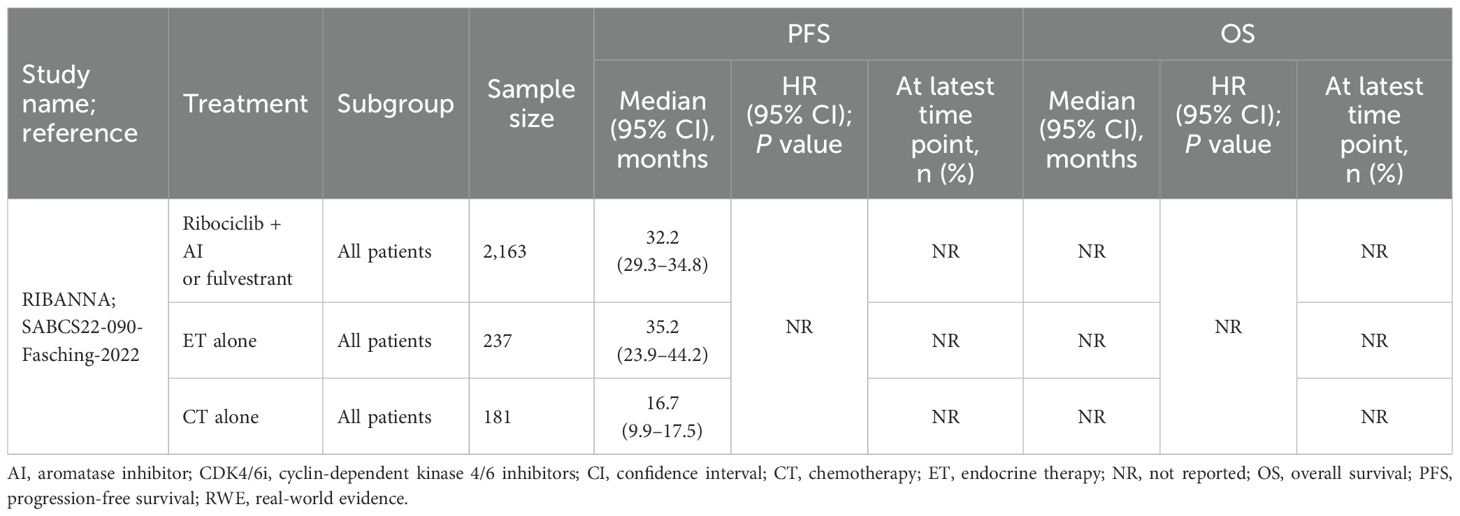
Table 3. Effectiveness outcomes for overall first-line ribociclib in comparative RWE studies versus ET.
In specific subgroup analyses of the RIBANNA study, patients with liver metastases receiving ribociclib plus AI or fulvestrant (n=384) had a median PFS of 16.6 months, which was significantly shorter than the 36.6 months observed in patients without liver metastases (n=1427). Conversely, those receiving ET alone had a median PFS of 10.4 months with liver metastases (n=23) versus 37.6 months without it (n=169). For patients on chemotherapy, the median PFS was 13.5 months in those with liver metastases (n=65) compared with 16.8 months in those without (n=78; Supplementary Table 5, Appendix I in the Supplementary Material) (21).
The OS data for patients receiving palbociclib versus ET were reported in six studies (Figure 1B). Of these, two studies reported results for the overall population (Table 2, Figure 3), two studies focused on specific patient subgroup populations (Supplementary Table 4, Appendix I in the Supplementary Material), and the remaining two studies reported data for both the overall and subgroup populations.
In three comparative studies evaluating palbociclib plus AI versus AI alone, median OS was generally not reached for patients receiving palbociclib (27, 57), with the exception of one study. In P-REALITY X, the median OS was 49.1 and 57.8 months, with 48-month OS rates of 54.5% and 46.8% in two weighted patient groups (i.e., sIPTW [n=1572] and PSM [n=939]) (28). In contrast, the median OS for the control group (AI alone) ranged from 43.1 (n=698) (27) to 57.0 months (n=152) (57) across the studies, with 45.2% and 47.0% of patients experiencing OS at 48 months in the sIPTW and PSM cohorts in P-REALITY X (28). Similarly, the CAPACITY study conference abstract reported that the median OS was not reached with palbociclib plus fulvestrant, whereas the median OS was 65.0 months with fulvestrant alone (57). Overall, palbociclib-based regimens consistently demonstrated statistically significant improvements in OS compared to ET (Table 2, Figure 3). Of the two studies that underwent quality assessment, both were of high quality (NOS score of 8 and sufficient credibility) (27, 28).
The same three comparative studies reporting PFS data for subgroups of patients described in Section 3.4.1.1.2 also reported OS data. Consistent with the PFS results, all studies demonstrated statistically significant improvements in OS with palbociclib plus AI compared with AI alone among older patients (23, 30), those with lung and/or liver metastases (26, 59), patients with cardiovascular disease (58), and African American patients (29) (Supplementary Table 4, Appendix I in the Supplementary Material). Assessed publications were all low risk and had sufficient credibility (23, 24, 29, 30).
Of the 22 comparative studies (including direct and descriptive comparison) evaluating a particular CDK4/6i regimen versus another CDK4/6i regimen, 12 studies directly compared two or more specified CDK4/6 inhibitors. The remaining studies evaluated the same CDK4/6i in combination with different backbone therapies (e.g., palbociclib plus AI vs. palbociclib plus fulvestrant). For completeness, these studies are summarized and included in Appendix K in the Supplementary Material.
The PFS data for patients receiving palbociclib, ribociclib, or abemaciclib were available in nine comparative studies (Figure 1C). Five studies reported results for the overall population (Table 4, Figure 4), while four reported overall and subgroup population data (Supplementary Table 6, Appendix I in the Supplementary Material).
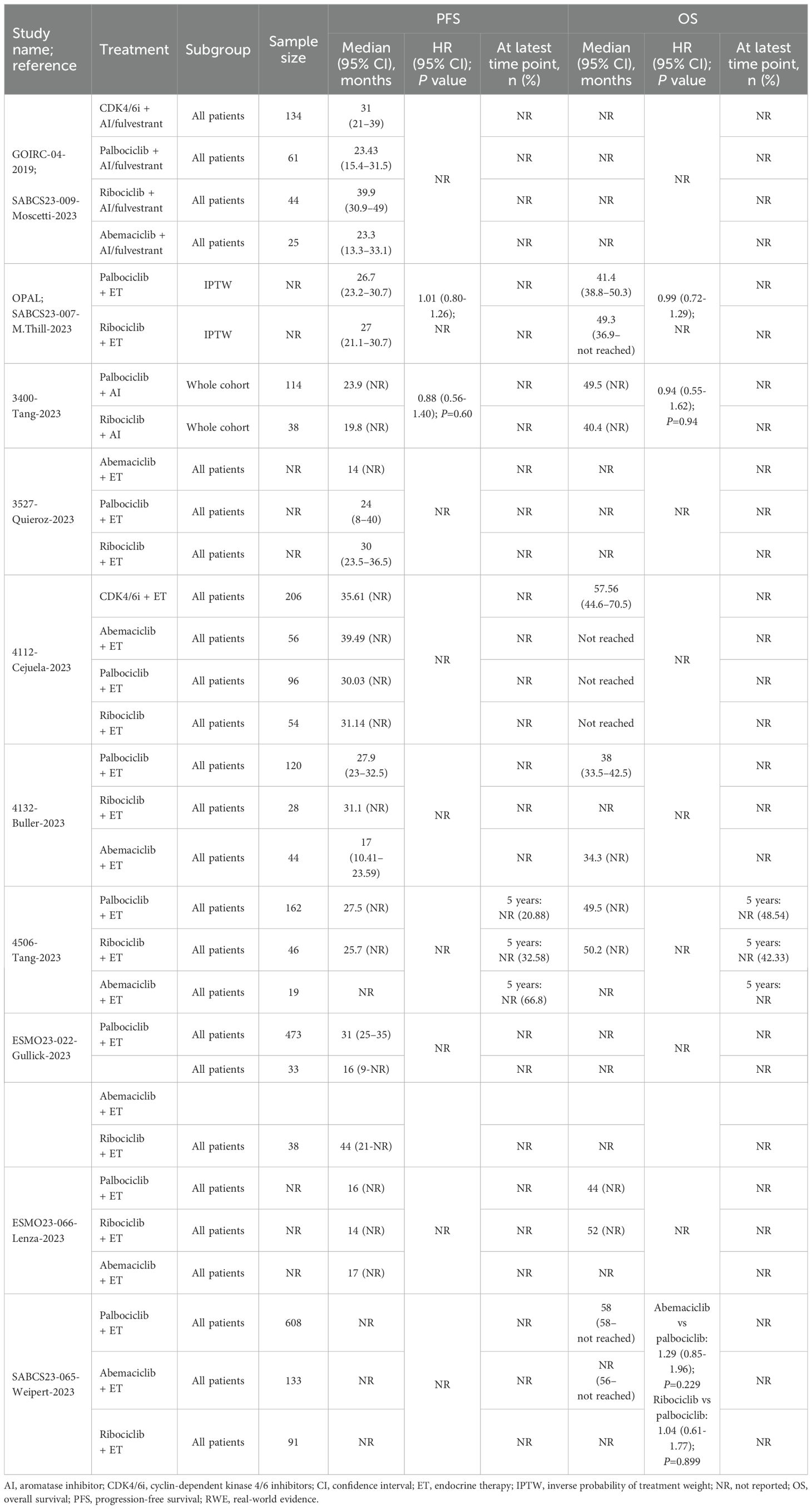
Table 4. Effectiveness outcomes for overall first-line comparative RWE studies assessing two or more specified CDK4/6i.
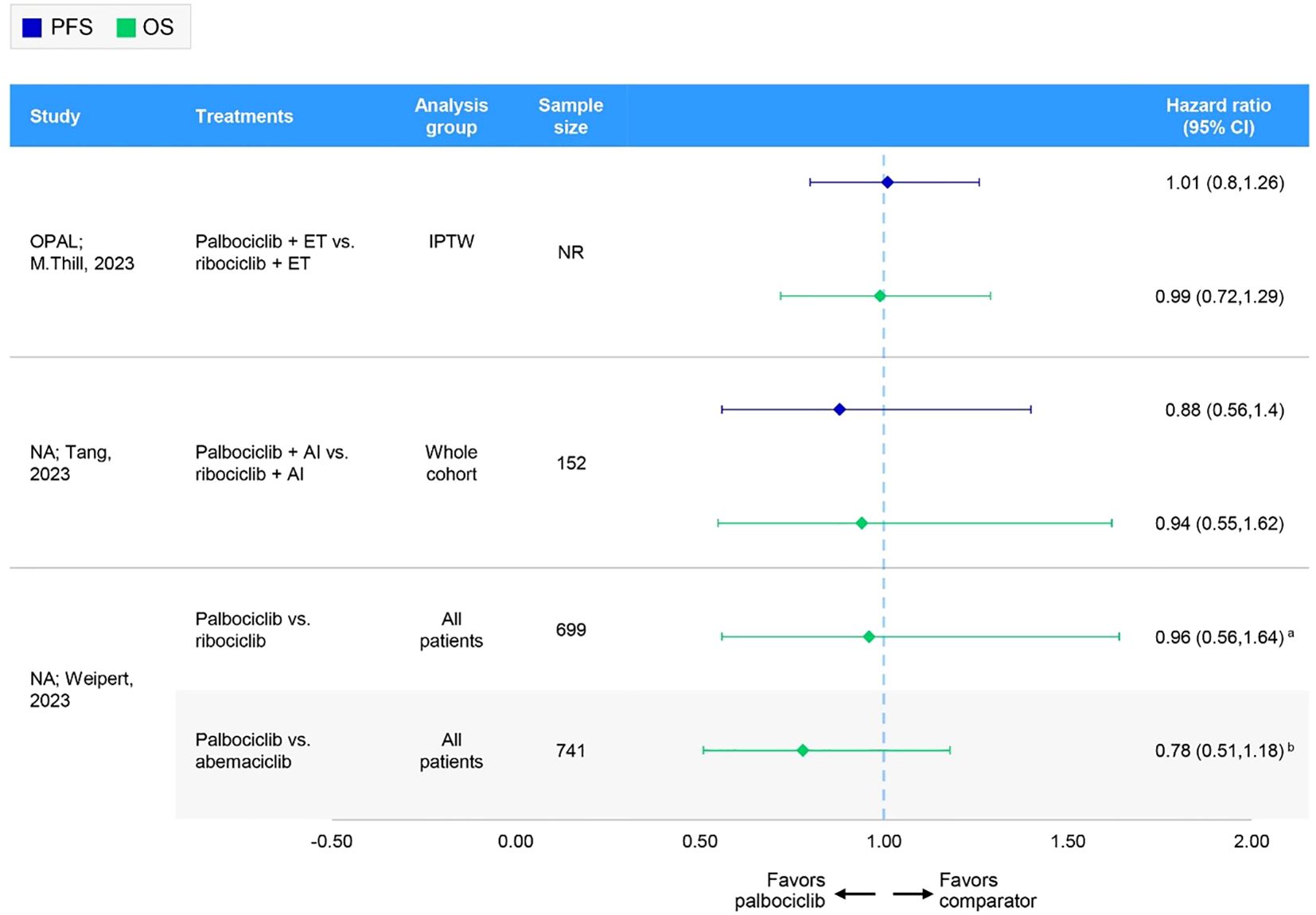
Figure 4. Forest plot of hazard ratios for effectiveness outcomes for overall first-line comparative RWE studies assessing two or more specified CDK4/6i. aHazard ratio has been inverted from that originally published by Weipert et al. for abemaciclib vs palbociclib: 1.29 (95% CI: 0.85-1.96). (60) bHazard ratio has been inverted from that originally published by Weipert et al. for ribociclib vs palbociclib: 1.04 (95% CI: 0.61-1.77) (60). AI, aromatase inhibitor; CI, confidence interval; ET, endocrine therapy; IPTW, inverse probability of treatment weighting; NA, not applicable; NR, not reported; OS, overall survival; PFS, progression-free survival.
In these nine comparative studies, median PFS was comparable across CDK4/6i-based regimens. Specifically, PFS ranged from 16.0 (n=NR) (61) to 31.0 months (n=473) (62) for patients receiving palbociclib, 14.0 (n=NR) (61) to 44.0 months (n=38) (62) for those receiving ribociclib, and 14.0 (n=NR) (63) to 39.5 months (n=56) (64) for abemaciclib. Notably, lower median PFS results for palbociclib and ribociclib were reported in a conference poster reporting on initial data from the Canarian Breast Cancer Group of Spain; however, information regarding the methods used, sample sizes, and follow-up times was largely unavailable (61). Omitting this study resulted in a median PFS range of 23.4 (n=61) (17) to 31.0 months (n=473) (62) for palbociclib and 19.8 (n=38) (65) to 44.0 (n=38) (62) for ribociclib. In one study, the PFS rate at 5 years was 20.9%, 32.6%, and 66.8% among palbociclib-, ribociclib-, and abemaciclib-based regimens, respectively (66). However, it should be noted that the sample sizes varied greatly between treatments, with 162 patients receiving palbociclib, 46 receiving ribociclib, and only 19 patients receiving abemaciclib (66). Two studies, the German OPAL study and a real-world UK study, compared palbociclib and ribociclib and showed no statistically significant difference in PFS (65, 67); no comparisons with abemaciclib were available (Table 4, Figure 4). Quality assessment was performed for four of these studies; three had NOS scores of 7 and were judged of sufficient credibility on the ISPOR questionnaire (63, 64, 66), while the remaining study (68) had a NOS score of 5 and was judged of insufficient quality due to low scores in the credibility, analyses, and interpretation domains of the ISPOR questionnaire.
Subgroups based on ET response (e.g., de novo, recurrent, endocrine-resistant/sensitive) were reported in three studies. Across the different CDK4/6i-based regimens, the median PFS and PFS rates were consistently higher among patients with de novo disease than those with recurrent disease (61, 64, 66). For patients with recurrent disease, median PFS ranged from 8.0 (n = NR) (61) to 20.9 months (n = 88) (66) with palbociclib, 6.0 (n = NR) (61) to 18.9 months (n = 34) (66) with ribociclib, and 12.0 months (n = NR) (61) with abemaciclib. One study included endocrine-resistant subgroups and found median PFS was higher among patients receiving the palbociclib regimen (n = 37; 17.0 months) than those receiving the ribociclib regimen (n = 20; 10.4 months) (64).
Additional prespecified subgroups of interest were assessed in the comparative studies that reported PFS data for palbociclib versus ribociclib versus abemaciclib, which included metastases (e.g., visceral), dose reduction, hormonal status (e.g., ER-positive [ER+], PR−), and age. The results of these studies are described in Supplementary Table 6, Appendix I in the Supplementary Material.
Overall, in studies with subgroups of interest data, the quality assessment of two studies indicated they were of reasonably high quality (both with NOS scores of 7 and judged sufficiently credible per the ISPOR questionnaire) (64, 66).
The OS data for patients receiving palbociclib, ribociclib, or abemaciclib were available in seven comparative studies (Figure 1C). Three studies reported results for the overall population (Table 4, Figure 4), while four reported overall and subgroup population data (Supplementary Table 6, Appendix I in the Supplementary Material).
In these seven comparative studies, the data reflect a broadly similar level of OS benefit across the CDK4/6i class. More specifically, median OS ranged from 38.0 (n=120) (68) to 58.0 months (n=608) (60) among patients receiving a palbociclib regimen, with one study reporting a 5-year OS rate of 48.5% (n=162) (66). Similarly, patients on a ribociclib regimen experienced median OS ranging from 40.4 (n=38) (65) to 52.0 months (n=NR) (61), with a 5-year OS rate of 42.3% (n=46) (66). In comparison, OS results for abemaciclib regimens were often not reached or not reported; however, a comparable median OS of 34.3 months was reported in one study (n=44) (68). In the German registry study, OPAL and a real-world UK study, the comparison between palbociclib and ribociclib indicated no statistically significant difference in OS (65, 67) (Table 4, Figure 4). Where reported, median follow-up ranged from 27.6 (64) to 49.8 months (65) across studies. Quality assessment was performed for three of these studies; two had a NOS score of 7 and were judged to have sufficient credibility on the ISPOR questionnaire (64, 66), whereas the remaining study (68) had a NOS score of 5 and was judged to be of insufficient credibility due to low scores in the credibility, analyses, and interpretation domains of the ISPOR questionnaire.
Subgroups based on ET response, as described in Section 3.5.1.1.2, were reported in three studies. Across the different CDK4/6i-based regimens, median OS and OS rates were consistently higher among patients with de novo disease than those with recurrent disease (61, 65, 66). For patients with recurrent disease, median OS ranged from 22.0 (n=NR) (61) to 48.4 months (n=NR) (65) with palbociclib, 28.0 (n=NR) (61) to 44.6 months (n=34) (66) with ribociclib, and 30.7 months (n=8) (66) with abemaciclib (Supplementary Table 6, Appendix I in the Supplementary Material). Quality assessment of one of these studies indicated it was of reasonably high quality (with a NOS score of 7 and judged sufficiently credible per the ISPOR questionnaire) (66).
Additional prespecified subgroups of interest were assessed in the comparative studies that reported OS data for palbociclib versus ribociclib versus abemaciclib, which included metastases (e.g., visceral), hormonal status (e.g., ER+, PR−), and age. The results of these studies are described in Supplementary Table 6, Appendix I in the Supplementary Material.
As experience treating patients with HR+/HER2− A/MBC in the first-line setting continues to grow, the RWE base should be examined and updated periodically to better understand the patient experience of those treated with a CDK4/6i. Newly identified studies published after an earlier SLR confirm the validity of prior findings and fully inform care and policy development for health care consumers with the latest research (69). Updated reviews also provide the benefit of further guiding future opportunities for research and synthesis as new evidence emerges or new methods develop.
Our previous SLR from 2021 provided a qualitative assessment of 114 eligible RWE studies of approved CDK4/6i in HR+/HER2− A/MBC conducted between 2015 and 2019 across various outcomes related to effectiveness, safety, patient-reported outcomes, and more (11). The overall CDK4/6i evidence base has historically been most established for supporting the real-world effectiveness of palbociclib. However, newer data have emerged for first-line ribociclib and abemaciclib in HR+/HER2− A/MBC, necessitating this updated synthesis of the current RWE landscape of CDK4/6i therapy. Outcomes of interest for this update and the subsequent qualitative synthesis included OS and/or PFS data published since 2019. These two outcomes are widely used in RCTs for evaluating treatment effectiveness and are the primary considerations of oncologists when choosing a specific therapy for patients. Thus, our focused approach provides new insights into the initial use of CDK4/6i in the real world, fleshing out the picture from RCTs, directly informing first-line treatment strategies, and enhancing real-world clinical decision-making for patients with HR+/HER2− A/MBC.
The current synthesis of recently published RWE shows that treating patients with HR+/HER2− A/MBC with CDK4/6i in the first-line setting effectively improves survival outcomes. These results are based on additional data from 82 unique studies spanning almost 5 years since our previously published findings (11). Furthermore, these results corroborate efficacy estimates observed in clinical trials (70). Overall, this updated review captures a greater body of RWE, with the newly included studies encompassing a wider range of study designs (i.e., single-arm and comparative studies), study follow-up times, subgroup population characteristics (e.g., age, racial/ethnic identity, sensitivity to ET, dose reductions, presence of visceral metastases), and additional CDK4/6i effectiveness data spanning several regions in North America, Europe, South America, and Asia. Across the 10 studies comparing different CDK4/6i in the overall population, a total of 1634 patients received palbociclib, 339 patients received ribociclib, and 310 patients received abemaciclib (where reported), with similar effectiveness results. Specifically, the median PFS ranged from 23.4 (17) to 31.0 months (62) for palbociclib, 19.8 (66) to 44.0 months (62) for ribociclib, and 14.0 (63) to 39.5 months (64) for abemaciclib after the exclusion of outlying results from a conference abstract. Furthermore, median OS ranged from 38.0 (68) to 58.0 months (60) for palbociclib, 40.4 (65) to 52.0 months (61) for ribociclib, and 34.4 months in one study for abemaciclib (68). These data highlight an important consideration for assessing longitudinal real-word effectiveness outcomes; estimates may be unreliable if there is a substantial censoring resulting from limited follow up, which needs to be considered as this may lead to an underestimate of survival differences. As for all comparisons, it is important to look at the broader scope of the data; instead of focusing on the median, which is a descriptive statistic reflecting only one point in time, we have focused on the hazard ratios, which take into account the full Kaplan–Meier curves and censoring that are critical to discern the robustness of the outcomes data. These PFS and OS results should be interpreted with caution as the median follow-up times for patients on ribociclib and abemaciclib were consistently shorter than palbociclib (68). Notably, within the included studies for abemaciclib, the available effectiveness data frequently appeared to be lower or not reached. This is likely due to abemaciclib being the most recently approved CDK4/6i; abemaciclib was approved by the US Food and Drug Administration in 2017, and by the European Medicines Agency in 2018 (71, 72), relative to palbociclib approval in 2015 (US) and 2016 (Europe) (73, 74) and ribociclib approval in 2017 (75, 76). There was also a slower initial uptake after approval given reimbursement negotiations as well as greater uptake once the secondary endpoints of survival read out for all CDK4/6i. As a result, the included studies evaluating abemaciclib had comparatively shorter follow-up durations of cohorts. The other key point is the imbalance of patients in each cohort across comparative studies wherein there was a smaller sample of ribociclib and abemaciclib patients at this juncture. However, the vast majority of studies show relatively consistent findings with those observed in single-arm studies and CDK4/6i versus ET comparisons, where CDK4/6i consistently demonstrated greater survival benefits relative to ET alone.
It is critical to note, as indicated by the ISPOR quality assessment, that the comparative studies have significant limitations. For one, the shorter limited follow-up in the abemaciclib and ribociclib cohorts presents an underlying challenge given the later approvals of these CDK4/6i and limited initial uptake. As a result, OS and, in some studies, even PFS, had not reached 50% of events by the time of reporting, making the results unstable until further follow-up. Landmark analyses may be preferred at the earlier time points for comparison purposes, where more patients contributed data. Additionally, most of the studies were descriptive in nature, not controlling for differences in baseline characteristics, such as age or presence of visceral metastases to enable robust comparison of outcomes. Furthermore, the sample size of these studies may preclude the ability to make such comparisons given the relatively limited number of ribociclib and abemaciclib patients as well as in some subgroups. There was also considerable heterogeneity in the clinical variables included and in the level of missing data due to the variety of data sources. It is important to consider this variability, as physician choice between different CDK4/6i is often based on these factors. Lastly, most studies included only select sites, investigators, and patients within a single country, which introduces potential bias and limits the generalizability of the study conclusions. This contrasts with studies that included all eligible patients within an electronic health record database where multiple sites across an entire EHR network are represented. Despite these limitations, 20 studies (87.0%) were identified as being of sufficient credibility due to thorough reporting of study relevance (e.g., population, intervention, and outcomes) and methodology (e.g., statistical analysis and data sources), as well as appropriate descriptions and interpretations of the corresponding results.
Leveraging additional RWE, such as those from comparative studies, may also help guide clinicians towards alternative therapeutic options with comparable effectiveness and safety to CDK4/6i, although the caveats mentioned above need to be considered knowing the selection biases that exist. Despite this, our findings in this updated review were consistent with previously published data based on a different set of studies (11), as well as with efficacy estimates observed in RCTs for these agents (70, 77). Palbociclib with its large body of real-world evidence and the longest follow-up showed generally consistent survival benefit as in RCTs; as more real-world data becomes available for ribociclib and abemaciclib, it will be possible to gain a more complete picture of clinical benefit of CDK4/6i as a class and will also facilitate more robust comparisons between CDK4/6i. Current evidence from comparative studies does not indicate that there is any one CDK4/6i that is better than the other. However, RWE can be used in conjunction with data from RCTs to inform treatment decisions in the clinic, particularly for specific patient populations that may be excluded from RCTs, such as older patients, African-American patients, patients with specific comorbidities or types/numbers of metastases, patients with specific cancer subtypes or genetic signatures, or patients with different treatment-free intervals, to name a few of the subgroups identified in this analysis.
This study has potential limitations. Because the current review only included studies assessing OS and/or PFS, the full effect and benefits of CDK4/6i in real-world settings in terms of therapeutic response, health-related quality of life, and safety were not captured; a targeted review assessing publications across all CDK4/6i found limited safety, quality of life or patient-reported outcome data in the literature (78). A recent SLR assessing evidence from both RCTs and RWE showed that palbociclib was effective, well tolerated, and maintained QoL in older patients with HR+/HER2− A/MBC; clinical benefit profile of palbociclib in real-world settings was consistent with results seen in clinical trials (70). One other recent SLR assessing the impact of palbociclib on patient quality of life found that quality of life is largely maintained while on treatment with palbociclib to ET therapy as assessed in RCTs and RWE (10), but similar large RWE studies have not yet been published for ribociclib or abemaciclib, or for the CDK4/6i class as a whole. Thus, there are future opportunities for synthesis of available RWE evaluating these additional outcomes in populations with HR+/HER2− A/MBC. The follow-up duration of cohorts in the included studies may be insufficient to inform the long-term survival benefits of CDK4/6i use, as some studies had median follow-up times of 18.5 months or less (18, 51, 79, 80), and many studies did not report median follow-up durations. Moreover, the nonrandomized nature of these studies means these results could be affected by confounding factors. It is also important to recognize that there is variability in the rigor and robustness of RWE studies – a majority of studies included in this analysis (59%) has NOS scores of 4 or 5, indicating lower quality of these studies; lack of a comparator arm was the most significant contributor to lower scores. This suggests the need for cautious interpretation when reviewing outcomes and conclusions from these studies, and emphasizes that quality assessment of the published research is an important factor to consider when giving weight to published outcomes.
Finally, the current review only provided a qualitative evaluation of RWE on the effectiveness of CDK4/6i in HR+/HER2− A/MBC. Although inherent to RWE, the inclusion of diverse patient populations, subgroups, and geographical regions results in notable heterogeneity across studies that can affect the generalizability and comparability of findings, it represents patients who may not have been included in clinical trials given strict inclusion/exclusion criteria. Future research may incorporate quantitative analyses to help synthesize data from different sources, account for variations in study design and population, and offer more robust estimates of effectiveness and safety to more accurately guide clinical decision-making.
Consistent with findings from a previously published review, as well as with CDK4/6i clinical trials, the single-arm and comparative RWE studies included in this updated SLR indicate that first-line CDK4/6i are effective treatments for patients with HR+/HER2− A/MBC with the largest available real-world data reported for palbociclib at this time. With increasing use of CDK4/6i in first-line standard of care for HR+/HER2− A/MBC, we can potentially expect more long-term comparative data to become available in bigger RWE studies. With longer follow-up and larger patient cohorts, the current body of evidence can better inform real-world treatment guidelines and clinical decision-making. These data fill important knowledge gaps from randomized clinical trials and may help guide clinical decision-making for broad patient populations and specific subgroups that may particularly benefit from CDK4/6i therapy.
Introduction
● Breast cancer remains the most commonly diagnosed cancer for women.
● The introduction of CDK4/6i changed the treatment landscape for HR+/HER2– A/MBC, resulting in a new standard-of-care.
● The available RWE on the impact of CDK4/6i has increased since the publication of a previous SLR, and now includes real-world data for all three CDK4/6i, although this data is predominantly for palbociclib.
Materials and methods
● Literature published since the previous SLR searches was included in this analysis.
● OVID Medline, EMBASE, Cochrane databases, and key clinical congress proceedings were searched.
● Studies were included if they reported RWE in adult patients with HR+/HER2 – A/MBC who received treatment with a CDK4/6i in the first-line setting.
● Studies were excluded if published before 2019, had fewer than 100 patients, or did not specify the line of therapy or a specific CDK4/6i.
● Outcomes of interest were median PFS and/or median OS.
● Studies were categorized by study design, with further stratification by comparator arm if/when possible.
● Data were reported for overall populations and pre-identified subgroups of interest.
● Risk of bias and credibility were assessed using the Newcastle-Ottawa scale, the ISPOR questionnaire, and the ESMO-GROW checklist.
Results
● Eighty-two unique studies were included in this qualitative synthesis.
● Most studies (43%) evaluating a single CDK inhibitor were palbociclib studies; 46% of the studies assessed more than one CDK inhibitor.
● In single arm studies, CDK4/6i were generally effective at improving survival outcomes in real-world clinical practice both in a broad population and in subgroups of high clinical interest (eg, older patients, patients with visceral or bone metastases, patients with comorbidities).
● When compared to ET monotherapy, palbociclib plus AI demonstrated improved PFS in broad populations and subgroups; limited data was available for ribociclib plus ET versus ET alone, and no comparative studies for abemaciclib were identified in the SLR.
● In the studies comparing CDK4/6i regimens, the impact on PFS and OS were generally comparable across the three CDK4/6i in the overall population.
Discussion
● Since the previous SLR investigating the real-world impact of CDK4/6i was developed, the available pool of RWE has grown.
● This updated synthesis of RWE published since 2019 indicates that CDK4/6i treatment in the first-line setting is effective at improving survival outcomes in patients with HR+/HER2– A/MBC across a wide range of study designs and subgroups of interest; however, the most patients studied had received palbociclib + ET.
● However, these data should be interpreted with caution as there is limited median follow-up time for patients being treated with ribociclib or abemaciclib.
● Additionally, results from RWE studies should be considered in the context of study design, strength of the statistical methods, possible geographical bias, and sample size.
● The studies included in this analysis were largely identified as being of sufficient credibility, and the data are consistent with previously published studies and RCTs.
Conclusions
● Consistent with the previous review and RCTs, the published RWE indicates that CDK4/6i are effective first-line treatments for patients with HR+/HER2– A/MBC.
● Longer-term comparative data from larger RWE studies will add to the current body of evidence and provide additional resources to guide clinical decision-making.
NH: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. CR: Writing – original draft, Writing – review & editing. BK: Writing – original draft, Writing – review & editing. CC: Writing – original draft, Writing – review & editing. KT: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. EJ: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. DN: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. MB: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. IS: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. TP: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Pfizer Inc. The funder had the following involvement in the study: study design, writing of this article, and the decision to submit it for publication.
The authors acknowledge Joanna Bielecki, who developed, conducted, and documented the database searches; she is employed by EVERSANA, Canada. Medical writing and editorial assistance were provided by Radia Kamal from EVERSANA, Canada, and Rachel C. Brown, PhD and Vinay Pasupuleti, PhD from Oxford PharmaGenesis, Inc, Newtown, PA, USA. AI was not used in the development or revision of this manuscript. Medical writing and editorial assistance were funded by Pfizer Inc.
CR, BK, and CC were employed by the company Pfizer Inc. NH, AB, and TP were consultants for the company Pfizer Inc. MB, KT, DN, EJ, and IS were employees of the company EVERSANA, Canada, which was a paid consultant to Pfizer Inc. in connection with the development of this manuscript.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1530391/full#supplementary-material
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Ferlay J EM, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today . Available online at: https://gco.iarc.who.int/today (Accessed July 2024).
3. Health NIo. Cancer stat facts: Female breast cancer . Available online at: https://seer.cancer.gov/statfacts/html/breast.html (Accessed May 2024).
4. Finn RS, Martin M, Rugo HS, Jones S, Im S, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. (2016) 375:1925–36. doi: 10.1056/NEJMoa1607303
5. Hortobagyi GN, Stemmer SM, Burris HA, Yap Y, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. (2022) 386:942–50. doi: 10.1056/NEJMoa2114663
6. Johnston S, Martin M, Di Leo A, Im S, Awada A, Forrester T, et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. (2019) 5:5. doi: 10.1038/s41523-018-0097-z
7. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. (2020) 31:1623–49. doi: 10.1016/j.annonc.2020.09.010
8. McAndrew NP, Finn RS. Clinical review on the management of hormone receptor-positive metastatic breast cancer. JCO Oncol Pract. (2022) 18:319–27. doi: 10.1200/OP.21.00384
9. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. (2016) 34:3069–103. doi: 10.1200/JCO.2016.67.1487
10. Samjoo IA, Hall A, Chen C, Nguyen B, Bartlett M, Smith AL, et al. A systematic review of health-related quality of life outcomes in patients with advanced breast cancer treated with palbociclib. J Comp Eff Res. (2024) 13:e240111. doi: 10.57264/cer-2024-0111
11. Harbeck N, Bartlett M, Spurden D, Hooper B, Zhan L, Rosta E, et al. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: A systematic literature review of real-world evidence studies. Future Oncol. (2021) 17:2107–22. doi: 10.2217/fon-2020-1264
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses . Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 2024).
14. Berger ML, Martin BC, Husereau D, Worley K, Allen JD, Yang W, et al. A questionnaire to assess the relevance and credibility of observational studies to inform health care decision making: An ISPOR-AMCP-NPC good practice task force report. Value Health. (2014) 17:143–56. doi: 10.1016/j.jval.2013.12.011
15. Castelo-Branco L, Pellat A, Martins-Branco D, Valachis A, Derksen JWG, Suijkerbuijk KPM, et al. ESMO guidance for reporting oncology real-world evidence (GROW). Ann Oncol. (2023) 34:1097–112. doi: 10.1016/j.annonc.2023.10.001
16. Izano MA, Teka M, Johanson C, et al. Time to treatment discontinuation and time to next treatment as proxies of real-world progression-free survival in breast cancer patients. Cancer Res. (2022) 82(12_Suppl):4089. doi: 10.1158/1538-7445.AM2022-4089
17. Moscetti L. CDK4/6 inhibitors in advanced breast cancer (ABC). Preliminary results of the CDK4/6i choice and sequence of treatments in a series of 174 patients. GOIRC-04-2019 retro/prospective observational study. Cancer Res. (2024) 84(9_Supplementary):PO1-05-02-PO1-05-02. doi: 10.1158/1538-7445.SABCS23-PO1-05-02
18. Singer CF, Egle D, Greil R, Petru E, Ohler L, Balic M, et al. REACH AUT: Efficacy and safety of first-line (1L) ribociclib (RIB) + endocrine therapy (ET) in HR+, HER2- metastatic breast cancer (MBC) from a real-world (RW) study: 3rd interim analysis. Ann Oncol. (2022) 33:S217. doi: 10.1016/j.annonc.2022.03.214
19. Singer. C. Real-world outcomes with first-line ribociclib + endocrine therapy in patients with metastatic HR+, HER2– breast cancer: Fourth interim analysis of REACH AUT trial. Cancer Res. (2024) 84(9_Supplement):PO3-16-10. doi: 10.1158/1538-7445.SABCS23-PO3-16-10
20. Fasching PA, Brucker C, Decker T, Engel A, Göhler T, Jackisch C, et al. Progression-free survival and patient-reported outcomes in HR+, HER2- ABC patients treated with first-line ribociclib + endocrine therapy (ET) or ET monotherapy or chemotherapy in real world setting: 5th interim analysis of RIBANNA. Cancer Res. (2023) 83(5_Supplement):P4-01-03-P4-01-03. 10.1158/1538-7445.SABCS22-P4-01-03
21. Woeckel A, Brucker C, Decker T, Figueroa M, Roos C, Schmidt M, et al. Real-world efficacy of ribociclib (RIB) + aromatase inhibitor (AI)/fulvestrant (FUL) in subgroups of special interest: 5th interim analysis (IA) of the RIBANNA study. Ann Oncol. (2023) 34(Supplement 2):S366–7. doi: 10.1016/j.annonc.2023.09.617
22. Jackisch C, Brucker C, Decker T, Engel A, Fasching PS, Göhler T, et al. RIBANNA 5th interim analysis: Matched-pair analysis of progression-free survival (PFS) across treatment cohorts and comparison of frontline ribociclib + endocrine therapy PFS data from RIBANNA vs MONALEESA trials, in HR+, HER2– ABC. Cancer Res. (2023) 83:P4–01-01. doi: 10.1158/1538-7445.SABCS22-P4-01-01
23. Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, et al. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front Oncol. (2023) 13:1237751. doi: 10.3389/fonc.2023.1237751
24. Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, et al. Palbociclib combined with an aromatase inhibitor in patients with breast cancer with lung or liver metastases in US clinical practice. Cancers. (2023) 15:5268. doi: 10.3390/cancers15215268
25. Brufsky A, Liu X, Li B, McRoy L, Layman RM. Real-world tumor response of palbociclib plus letrozole versus letrozole for metastatic breast cancer in us clinical practice. Targeted Oncol. (2021) 16:601–11. doi: 10.1007/s11523-021-00826-1
26. Brufsky A, Liu X, Li B, McRoy L, Layman RM. Real-world effectiveness of palbociclib plus letrozole vs letrozole alone for metastatic breast cancer with lung or liver metastases: Flatiron database analysis. Front Oncol. (2022) 12:865292. doi: 10.3389/fonc.2022.865292
27. DeMichele A, Cristofanilli M, Brufsky A, Liu X, Mardekian J, McRoy L, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in us real-world clinical practice. Breast Cancer research: BCR. (2021) 23:37. doi: 10.1186/s13058-021-01409-8
28. Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C, et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast cancer. (2022) 8:114. doi: 10.1038/s41523-022-00479-x
29. Rugo HS, Liu X, Li B, McRoy L, Chen C, Layman RM, et al. Real-world effectiveness of palbociclib plus aromatase inhibitors in African American patients with metastatic breast cancer. Oncologist. (2023) 28:866–74. doi: 10.1093/oncolo/oyad209
30. Rugo HS, Liu X, Li B, McRoy L, Layman RM, Brufsky A. Real-world comparative effectiveness of palbociclib plus letrozole versus letrozole in older patients with metastatic breast cancer. Breast. (2023) 69:375–81. doi: 10.1016/j.breast.2023.03.015
31. Merola D, Young J, Schrag D, Lin KJ, Alwardt S, Schneeweiss S. Effectiveness research in oncology with electronic health record data: A retrospective cohort study emulating the PALOMA-2 trial. Pharmacoepidemiology Drug Safety. (2023) 32:426–34. doi: 10.1002/pds.5565
32. Law JW, Mitra D, Kaplan HG, Alfred T, Brufsky AM, Emir B, et al. Real-world treatment patterns and clinical effectiveness of palbociclib plus an aromatase inhibitor as first-line therapy in advanced/metastatic breast cancer: Analysis from the US syapse learning health network. Curr Oncol (Toronto Ont). (2022) 29:1047–61. doi: 10.3390/curroncol29020089
33. Wang W, Lei W, Fang Z, Jiang R, Wang X. Efficacy, safety, and predictive model of palbociclib in the treatment of HR-positive and HER2-negative metastatic breast cancer. BMC Cancer. (2024) 24:1. doi: 10.1186/s12885-023-11764-8
34. Kim SG, Kim MH, Kim GM, Kim GM, Kim JH, Kim JY, et al. Efficacy of limited dose modifications for palbociclib-related grade 3 neutropenia in hormone receptor-positive metastatic breast cancer. Cancer Res Treat. (2023) 55:1198–209. doi: 10.4143/crt.2022.1543
35. Palmieri C, Musson A, Harper-Wynne C, Wheatley D, Bertelli G, Macpherson IR, et al. A real-world study of the first use of palbociclib for the treatment of advanced breast cancer within the UK national health service as part of the novel Ibrance patient program. Br J Cancer. (2023) 129:852–60. doi: 10.1038/s41416-023-02352-5
36. Kim JY, Shin J, Ahn JS, Park YH, Im YH. Real world experience of second-line treatment strategies after palbociclib and letrozole: Overall survival in metastatic hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer. Cancers. (2023) 15:3431. doi: 10.3390/cancers15133431
37. ClinicalTrials.gov: NCT04460911. Treatment patterns and clinical outcomes among patients with HR+/HER2- MBC receiving palbociclib combination therapy in the US community oncology setting . Available online at: https://clinicaltrials.gov/study/NCT04460911 (Accessed January 2024).
38. Huang K-J, Kuo T-H, Chao T-C, Liu C-Y, Tsai Y-F, Huang C-C, et al. High incidence of palbociclib related neutropenia in asian patients associated with genetic polymorphisms. Cancer Res. (2023) 83:P4–01-31. doi: 10.1158/1538-7445.SABCS22-P4-01-31
39. Cardoso Borges F, Alves da Costa F, Ramos A, Ramos C, Bernado C, Brito C, et al. Real-world effectiveness of palbociclib plus fulvestrant in advanced breast cancer: Results from a population-based cohort study. Breast (Edinburgh Scotland). (2022) 62:135–43. doi: 10.1016/j.breast.2022.02.005
40. Carlino F, Diana A, Ventriglia A, Piccolo A, Mocerino C, Riccardi F, et al. HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: Results of a multicenter, retrospective cohort study. Cancers. (2022) 14:4981. doi: 10.3390/cancers14204981
41. Elnaghi KAEA, Alghanmi HA, Elsamany SA, Almarzoki F, Elsafty M, Jaffal M. Hormonal-receptors-positive and HER2-negative patients with metastatic breast cancer treated with first-line palbociclib and hormonal therapy: Impact of first-cycle neutropenia and dose reduction on therapeutic outcome. Breast J. (2023) 2023:8994954. doi: 10.1155/2023/8994954
42. Kim JY, Oh JM, Park YH, Ahn JS, Im YH. Which clinicopathologic parameters suggest primary resistance to palbociclib in combination with letrozole as the first-line treatment for hormone receptor-positive, HER2-negative advanced breast cancer? Front Oncol. (2021) 11:759150. doi: 10.3389/fonc.2021.759150
43. Lai J-I, Kuo T-H, Huang K-J, Chai LMX, Lee M-H, Liu C-Y, et al. Clinical and genotypic insights into higher prevalence of palbociclib associated neutropenia in Asian patients. oncologist. (2023) 4:e455–e466. doi: 10.1093/oncolo/oyad304
44. Masuda. N. Real-world progression-free survival and overall survival of palbociclib plus endocrine therapy in Japanese patients with HR+/HER2- ABC in the first- or second-line setting: A multicenter observational study. Cancer Res. (2024) 84(9_Supplement):PO3-04-10.
45. Mardani. M. Clinical outcomes of CDK4/6 inhibitors in patients with bone only metastatic breast cancer. Cancer Res. (2024) 84(9_Supplement):PO5-05-05-PO5-05-05.
46. Martinez-Janez N, Ezquerra MB, Henao F, Manso L, Anton A, Zamora P, et al. PalboSpain: Observational analysis of first-line therapy with palbociclib in patients with HR+/HER2- metastatic breast cancer (MBC) in real-life conditions. Cancer Res. (2023) 83:P4-01-28. doi: 10.1158/1538-7445.SABCS22-P4-01-28
47. Anton FM, Bellet-Ezquerra M, Sanchez LMM, Carrasco FH, Torres AA, Morales S, et al. Real-world treatment patterns and outcomes of patients receiving palbociclib plus endocrine therapy in Spain: Subgroup analysis based on age, sites and number of metastatic locations, menopausal status and dose received from PALBOSPAIN study. ESMO Open. (2023) 8:101422. doi: 10.1016/j.esmoop.2023.101422
48. Tripathy D, Blum JL, Karuturi MS, Zhang Z, Gauthier E, Rocque G, et al. Impact of comorbidities on real-world (RW) clinical outcomes of patients (pts) with hormone receptor-positive/human epidermal growth factor 2-negative (HR+/HER2-) advanced breast cancer (ABC) treated with palbociclib and enrolled in POLARIS. Ann Oncol. (2023) 34(Supplement 2):S332. doi: 10.1016/j.annonc.2023.09.551
49. El Badri S, Hills D, Tahir B, Bezecny P, Britton F, Davies M, et al. Palbociclib in combination with aromatase inhibitors in patients >= 75 years with oestrogen receptor-positive, human epidermal growth factor receptor 2 negative advanced breast cancer: A real-world multicentre UK study. Breast. (2021) 60:199–205. doi: 10.1016/j.breast.2021.10.010
50. Hall P, Howell S, Venkitaraman R, Thomson A, Raja F, King J, et al. Socioeconomic outcomes with ribociclib in patients with HR+, HER2- advanced breast cancer (ABC) in UK real-world settings. Breast. (2023) 68:S47. doi: 10.1016/S0960-9776(23)00201-1
51. Kristensen KB, Jensen AB, Thomsen IMN, Berg T, Kodahl AR. Dose modifications of ribociclib and endocrine therapy for treatment of ER+ HER2- metastatic breast cancer. Breast Cancer Res Treat. (2021) 188:799–809. doi: 10.1007/s10549-021-06215-6
52. King JWL, Fakhouri W, Jarvis RS, Badreldin W, Harper G, Bateman L, et al. Abemaciclib for treating patients with HR+, HER2- advanced/metastatic breast cancer in the UK: A real-world study. ESMO Open. (2023) 8:101433. doi: 10.1016/j.esmoop.2023.101433
53. Matos E, Cankar K, Rezun N, Dejanovic K, Auprih M, Ovcaricek T. Efficacy and safety of abemaciclib in the treatment of HR+ HER2-advanced breast cancer: Real world data. Libri Oncologici. (2023) 51:70–1.
54. Nozawa K, Terada M, Onishi M, Ozaki Y, Takano T, Fakhouri W, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2- metastatic breast cancer patients in Japan. Breast Cancer. (2023) 30:657–65. doi: 10.1007/s12282-023-01461-6
55. Walbaum B, Reyes JM, Rodriguez P, Muniz S, Medina L, Ibanez C, et al. Palbociclib in advanced stage hormone receptor-positive breast cancer: Real-world data from a Chilean multicentre registry. ecancermedicalscience. (2023) 17:1636. doi: 10.3332/ecancer.2023.1636
56. Moreno. F. Real-world outcomes of patients receiving first-line palbociclib plus endocrine therapy in Spain: Subgroup analysis based on tumor grade, progesterone receptor, KI-67 and histological subtype from PALBOSPAIN study. Cancer Res. (2024) 84(9_Supplement):PO1-16-008.
57. Yue J, Yuan P, Wang X, Ju J, Gao S-l, Yang Z, et al. Comparative effectiveness of palbociclib plus aromatase inhibitor versus fulvestrant alone as initial endocrine therapy for HR+/HER2- advanced breast cancer in Chinese clinical practice: A real-world study. (2023). doi: 10.1200/JCO.2023.41.16_suppl.e13057
58. Brufsky. A. Real-world effectiveness of palbociclib plus aromatase inhibitors (AI) in metastatic breast cancer patients with cardiovascular diseases. Cancer Res. (2023) 84(9_Supplement):PO1-17-05-PO1-17-05. doi: 10.1158/1538-7445.SABCS23-PO1-17-05
59. Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, et al. Real-world effectiveness of palbociclib (PAL) plus an aromatase inhibitor (AI) vs AI alone in patients who have metastatic breast cancer (MBC) with lung or liver metastases. ESMO Open. (2023) 8:101412. doi: 10.1016/j.esmoop.2023.101412
60. Weipert C, Wander S, Davis AA, Bucheit L, Saha J, Liao J, et al. Real-world (RW) utilization and patient outcomes across three CDK4/6 inhibitors in metastatic breast cancer (MBC). Cancer Res. (2024) 84:PO4–18-02. doi: 10.1158/1538-7445.SABCS23-PO4-18-02
61. Lenza IC, Valenti EL, Alonso MG, Ambite RS, Sosa MH, Quevedo REA, et al. Initial results from the Canarian registry of luminal breast cancer patients treated with first-line CDK 4/6 inhibitors. Ann Oncol. (2023) 34(Supplement 2):S377–8. doi: 10.1016/j.annonc.2023.09.643
62. Gullick G, Owen C, Cook S, Helbrow J, Squires R, Reed HM, et al. UK real-world data (RWD) of cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) use in metastatic breast cancer (MBC). (2023). doi: 10.1016/j.annonc.2023.09.598
63. Queiroz MM, Sacardo KP, Ribeiro MF, Gadotti LL, Saddi R, de Carvalho Oliveira LJ, et al. Real-world treatment outcomes in HR+ HER2- metastatic breast cancer patients treated with CDK4/6 inhibitors: Results from a reference center in Brazil. Cancer Treat Res Commun. (2023) 35:100683. doi: 10.1016/j.ctarc.2023.100683
64. Cejuela M, Gil-Torralvo A, Castilla MA, Domínguez-Cejudo MA, Falcón A, Benavent M, et al. Abemaciclib, palbociclib, and ribociclib in real-world data: A direct comparison of first-line treatment for endocrine-receptor-positive metastatic breast cancer. Int J Mol Sci. (2023) 24:8488. doi: 10.3390/ijms24108488
65. Tang HKC, De Souza K, Ahmad O, Shafiq T, Khan S, Anand A, et al. Palbociclib or ribociclib with aromatase inhibitor in post-menopausal women with ER+/HER2- advanced breast cancer? Real-world overall survival evidence. Clin Oncol. (2023) 35:e420. doi: 10.1016/j.clon.2023.02.040
66. Tang H, Yeo D, De Souza K, Ahmad O, Shafiq T, Ofor O, et al. Clinical impact of CDK4/6 inhibitors in de novo or PR- or very elderly post-menopausal ER+/HER2- advanced breast cancers. Cancers. (2023) 15:5164. doi: 10.3390/cancers15215164
67. Thill. M. Palbociclib versus ribociclib in first-line treatment of patients with hormone-receptor positive HER2 negative advanced breast cancer – real world outcome data from the German registry platform OPAL. Cancer Res. (2024) 84(9_Supplement):PO1-04-12. doi: 10.1158/1538-7445.SABCS23-PO1-04-12
68. Buller W, Pallan L, Chu T, Khoja L. CDK4/6 inhibitors in metastatic breast cancer, a comparison of toxicity and efficacy across agents in a real-world dataset. J Oncol Pharm Pract. (2023) 29:1825–35. doi: 10.1177/10781552231163121
69. Garner P, Hopewell S, Chandler J, MacLehose H, Schunemann HJ, Akl EA, et al. When and how to update systematic reviews: Consensus and checklist. BMJ. (2016) 354:i3507. doi: 10.1136/bmj.i3507
70. Brain E, Chen C, Simon S, Pasupuleti V, Pfitzer KV, Gelmon KA. Palbociclib in older patients with advanced/metastatic breast cancer: A systematic review. Target Oncol. (2024) 19:303–20. doi: 10.1007/s11523-024-01046-z
71. United States Food and Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer . Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer (Accessed August 2024).
72. European Medicines Agency.Verzenios (abemaciclib) . Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios (Accessed August 2024).
73. United States Food and Drug Administration. Palbociclib (Ibrance capsules) . Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance-capsules (Accessed August 2024).
74. European Medicines Agency. Ibrance (palbociclib) . Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/ibrance (Accessed August 2024).
75. United States Food and Drug Administration. Ribociclib (Kisqali) . Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali (Accessed August 2024).
76. European Medicines Agency. Kisqali (ribociclib) . Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/kisqali (Accessed August 2024).
77. Tong F, Lu Y, Ma HF, Shen J. Comparative efficacy and safety of CDK4/6 inhibitors combined with endocrine therapies for HR+/HER2-breast cancer: Systematic review and network meta-analysis. Heliyon. (2024) 10:e31583. doi: 10.1016/j.heliyon.2024.e31583
78. Zhao M, Hanson KA, Zhang Y, Zhou A, Cha-Silva AS. Place in therapy of cyclin-dependent kinase 4/6 inhibitors in breast cancer: A targeted literature review. Target Oncol. (2023) 18:327–58. doi: 10.1007/s11523-023-00957-7
79. Rath S, Elamarthi P, Parab P, Gulia S, Nandhana R, Mokal S, et al. Efficacy and safety of palbociclib and ribociclib in patients with estrogen and/or progesterone receptor positive, HER2 receptor negative metastatic breast cancer in routine clinical practice. PloS One. (2021) 16:e0253722. doi: 10.1371/journal.pone.0253722
80. Zhang L, Song G, Shao B, Xu L, Xiao Y, Wang M, et al. The efficacy and safety of palbociclib combined with endocrine therapy in patients with hormone receptor-positive HER2-negative advanced breast cancer: A multi-center retrospective analysis. Anticancer Drugs. (2021) 33:e635–e43. doi: 10.1097/CAD.0000000000001210
Keywords: CDK4/6 inhibitors, quality assessment, breast, metastasis, real-world evidence, systematic literature review, HR+/HER2−
Citation: Harbeck N, Brufsky A, Rose CG, Korytowsky B, Chen C, Tantakoun K, Jazexhi E, Nguyen DHV, Bartlett M, Samjoo IA and Pluard T (2025) Real-world effectiveness of CDK4/6i in first-line treatment of HR+/HER2− advanced/metastatic breast cancer: updated systematic review. Front. Oncol. 15:1530391. doi: 10.3389/fonc.2025.1530391
Received: 18 November 2024; Accepted: 06 February 2025;
Published: 10 March 2025.
Edited by:
Parvez Khan, University of Nebraska Medical Center, United StatesReviewed by:
Sadia Iqrar, Jamia Hamdard University, IndiaCopyright © 2025 Harbeck, Brufsky, Rose, Korytowsky, Chen, Tantakoun, Jazexhi, Nguyen, Bartlett, Samjoo and Pluard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imtiaz A. Samjoo, SW10aWF6LlNhbWpvb0BFdmVyc2FuYS5jb20=
†ORCID: Nadia Harbeck, orcid.org/0000-0002-9744-7372
Adam Brufsky, orcid.org/0000-0001-8080-7960
Chloe Grace Rose, orcid.org/0009-0003-4327-5493
Beata Korytowsky, orcid.org/0009-0000-4017-939X
Krista Tantakoun, orcid.org/0009-0001-3213-4455
Endri Jazexhi, orcid.org/0009-0001-6151-3323
Do Hoang Vien Nguyen, orcid.org/0009-0008-6022-1792
Meaghan Bartlett, orcid.org/0000-0003-4616-8776
Imtiaz A. Samjoo, orcid.org/0000-0003-1415-8055
Timothy Pluard, orcid.org/0000-0002-3640-3077
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.