
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 07 April 2025
Sec. Surgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1502014
This article is part of the Research TopicRecent Advances and New Challenges in Minimally Invasive Surgery and Chemotherapy for Colorectal Cancer-volume 2View all 5 articles
Colorectal cancer (CRC) remains a formidable global health challenge, ranking among the most prevalent malignancies and a principal contributor to cancer-associated mortality. While traditional open surgery has historically been the cornerstone of CRC treatment, the advent of minimally invasive techniques, particularly robotic-assisted colorectal surgery (RACS), has garnered significant momentum owing to technological advancements in the field. Robotic platforms, exemplified by the da Vinci Surgical System, offer superior three-dimensional visualization, enhanced dexterity, and heightened precision, yielding improved perioperative outcomes, particularly in anatomically intricate regions such as the pelvis. This review provides a critical appraisal of the current landscape of RACS, emphasizing its superiority over conventional open and laparoscopic approaches. The increased control and precision afforded by robotic surgery have been shown to optimize outcomes in complex procedures such as total mesorectal excision, with evidence indicating reduced intraoperative blood loss, shortened hospital stays, and improved functional recovery. Nonetheless, challenges persist, including absence of haptic feedback, prohibitive costs, and steep learning curve associated with robotic systems. Despite these limitations, RACS has demonstrated considerable promise in sphincter-preserving and function-preserving procedures, ultimately enhancing postoperative quality of life. Beyond the surgical field, this review also investigates the integration of robotic surgery within multidisciplinary treatment strategies for CRC, particularly in the context of locally advanced rectal cancer. The combination of robotic techniques with total neoadjuvant therapy and immunotherapy—especially in tumors characterized by mismatch repair deficiency or high microsatellite instability has shown notable clinical efficacy. Furthermore, emerging personalized therapeutic approaches, including immunotherapies and targeted chemotherapeutic agents, emphasize the transformative potential of RACS in delivering superior oncologic outcomes. Looking towards the future, innovations in robotic platforms, including intraoperative imaging, artificial intelligence, and augmented reality, herald new possibilities for further enhancing the precision and efficacy of colorectal surgeries. The standardization of RACS protocols, alongside ongoing training and robust clinical research, will be critical to fully realizing the benefits of these advancements across diverse clinical settings. By incorporating cutting-edge technologies and personalized treatment methods, robotic-assisted surgery is prepared to become a cornerstone in future of CRC management, with the potential to significantly improve both survival outcomes and patient quality of life.
Colorectal cancer (CRC) remains a significant global health burden, ranking among the most prevalent malignancies and contributing to a substantial number of cancer-related deaths (1). According to the International Agency for Research on Cancer, CRC accounts for more than 1.9 million new cases annually, with an estimated 900,000 fatalities, representing 9.3% of all cancer-associated mortalities (2). Traditionally, open colorectal resection has been the cornerstone of surgical intervention for CRC (3). Although effective in achieving tumor resection and improving survival outcomes, open surgery is accompanied by notable limitations, including extensive surgical trauma, increased complication rates, and prolonged postoperative recovery (4).
Over the past few decades, advances in medical technology have catalyzed the widespread adoption of minimally invasive surgery (MIS) (5). Laparoscopic surgery, as the foundation of MIS, has gained considerable traction in the management of CRC (6). Compared to conventional open approaches, laparoscopic techniques offer several distinct advantages, such as reduced intraoperative trauma, diminished postoperative pain, accelerated recovery, and improved cosmetic outcomes (7). However, despite these benefits, laparoscopic surgery has inherent drawbacks, including a two-dimensional (2D) visual field, restricted instrument dexterity, and a steep learning curve, all of which can compromise surgical accuracy and hinder its broader implementation in clinical practice (8, 9).
To address these limitations, robot-assisted surgical systems have emerged as a transformative innovation. The da Vinci Surgical System, for instance, has revolutionized the surgical landscape by introducing robot-assisted surgery (RAS) into clinical practice, offering enhanced capabilities such as high-definition three-dimensional (3D) visualization, instruments with seven degrees of freedom, and tremor suppression, significantly augmenting surgical precision (10). In the context of CRC surgery, RAS holds promise for improving lymph node dissection quality, reducing perioperative complications, and optimizing functional outcomes, particularly in sphincter-preserving procedures (11). (Figure 1).
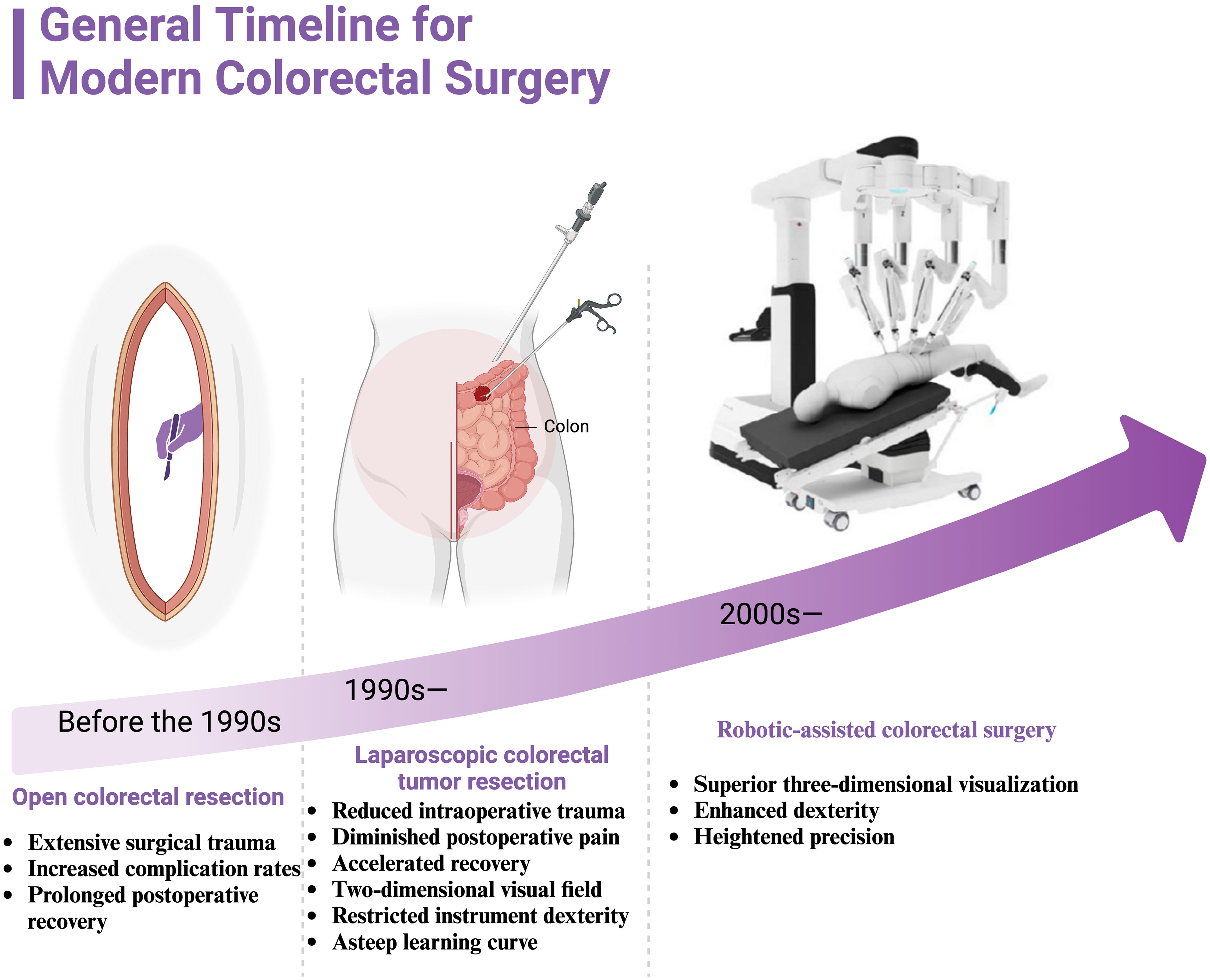
Figure 1. General timeline for modern colorectal surgery. It illustrates the progression of surgical techniques for colorectal cancer treatment from the pre-1990s era of open colorectal resection, through the development of laparoscopic colorectal tumor resection in the 1990s, to the introduction of robotic-assisted colorectal surgery in the 2000s. Before the 1990s, open colorectal resection was the standard procedure, characterized by extensive surgical trauma, higher complication rates, and prolonged postoperative recovery. In the 1990s, laparoscopic colorectal tumor resection emerged, offering advantages such as reduced intraoperative trauma, diminished postoperative pain, and accelerated recovery. However, challenges included the limitations of a two-dimensional visual field, restricted instrument dexterity, and a steep learning curve for surgeons. Since the 2000s, robotic-assisted colorectal surgery has provided superior three-dimensional visualization, enhanced dexterity, and heightened precision, marking a significant advancement in the field of minimally invasive surgery.
As a narrative review, our objective is to synthesize the current body of evidence on robotic-assisted colorectal surgery and its integration into multidisciplinary treatment strategies for CRC. This format allows for a comprehensive examination of both established and emerging data, enabling us to provide a critical overview without restricting the scope to predefined methodological criteria, as might be required in a systematic review. By presenting the literature in this narrative format, we aim to highlight the nuanced clinical insights, evolving technologies, and multidisciplinary considerations that shape the use of robotics in CRC management.
The landscape of robotic-assisted colorectal surgery has witnessed remarkable growth in recent years, primarily fueled by continuous innovations in robotic technology (12). These advancements have significantly enhanced the precision and efficacy of surgical interventions, especially in intricate or technically demanding cases (13). Central to this paradigm shift is the advent of high-definition, three-dimensional imaging and fully articulating robotic instruments, which afford surgeons unprecedented dexterity and spatial awareness (14). These tools enable the execution of complex procedures with heightened control, mitigating the limitations inherent to traditional open or laparoscopic techniques.
Recent studies emphasize the increasing utilization of robotic platforms in colorectal surgeries that have historically posed considerable challenges (15–17). Notably, robotic systems are now routinely employed for lateral lymph node dissections and multi-visceral resections involving adjacent organs, both critical for addressing locally advanced malignancies (18–21). This evolution mirrors growing confidence within the surgical community regarding the capabilities of robotic systems in managing complex colorectal pathologies (22). Such advancements are contributing to the broader adoption of robotic-assisted surgery across diverse colorectal cancer cases, particularly within specialized, high-volume centers where the requisite infrastructure and expertise are readily available (23).
Robotic-assisted colorectal surgery offers a range of advantages over conventional open and laparoscopic approaches, while also presenting unique challenges (24, 25). Laparoscopic surgery, long regarded as the cornerstone of minimally invasive colorectal surgery, is limited by two-dimensional imaging and a constrained range of motion due to rigid, non-articulating instruments (26, 27). These limitations can impede precise dissection, particularly within confined anatomical spaces such as the pelvis (28). Open surgery, while providing direct tactile feedback and a broader operative field, is associated with increased morbidity, including greater postoperative pain, prolonged hospitalization, and slower recovery (29).
Robotic systems bridge these gaps by offering the enhanced dexterity of wristed instruments and superior visualization through high-definition, three-dimensional imaging (30). This capability facilitates more refined dissections in anatomically complex regions, such as during total mesorectal excision (TME) for rectal cancer (31, 32). Comparative studies demonstrate that robotic-assisted surgery can lead to reduced intraoperative blood loss, lower rates of conversion to open surgery, and shorter hospital stays relative to both laparoscopic and open procedures (33). Nonetheless, robotic surgery is not without drawbacks, including extended operative times and higher upfront costs related to the acquisition and maintenance of robotic platforms (34). While long-term oncological outcomes remain comparable between robotic and conventional methods, further research is necessary to determine whether robotic systems offer superior long-term survival or recurrence rates for patients with colorectal cancer (35, 36).
Despite the clear advantages of robotic-assisted colorectal surgery, several technical and operational challenges persist. A major limitation is the absence of haptic feedback, which is integral for surgeons to gauge tissue tension and resistance during dissection (37). The lack of tactile sensation can impair the precision of critical maneuvers, particularly when working near delicate structures. Additionally, the considerable size of robotic systems and the complexity of docking procedures can prolong operative times, particularly in centers where robotic surgery has not yet become routine (38). (Figure 2A).
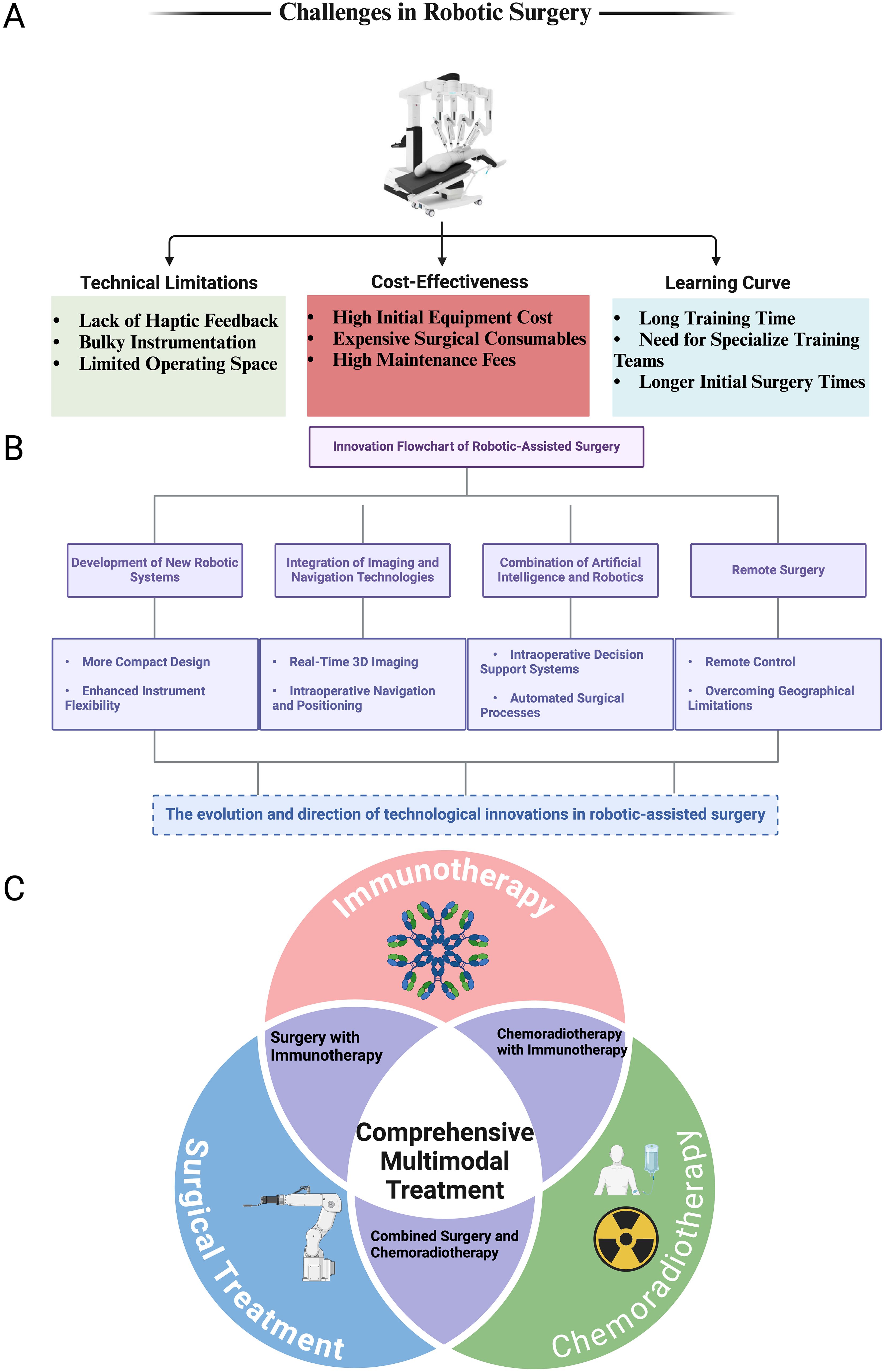
Figure 2. Challenges, innovations, and multimodal roles of robotic-assisted surgery in colorectal cancer treatment. (A) Challenges in Robotic Surgery: This panel illustrates the three primary challenges associated with robotic-assisted surgery: Technical Limitations: Including the lack of haptic feedback, bulky instrumentation, and limited operating space. Cost-Effectiveness: Highlighting the high initial equipment costs, expensive surgical consumables, and high maintenance fees. Learning Curve: Emphasizing the long training times, the need for specialized training teams, and longer initial surgery times for surgeons adopting this technology. (B) Innovation Flowchart of Robotic-Assisted Surgery: This panel presents the technological advancements driving the evolution of robotic surgery: Development of New Robotic Systems: Focused on creating more compact designs and enhancing instrument flexibility. Integration of Imaging and Navigation Technologies: Including real-time 3D imaging and intraoperative navigation and positioning. Combination of Artificial Intelligence and Robotics: Introducing intraoperative decision support systems and automated surgical processes. Remote Surgery: Enabling remote control and overcoming geographical limitations for surgical interventions. (C) Comprehensive Multimodal Treatment: A Venn diagram representing the role of robotic-assisted surgery in the context of multimodal treatment strategies for colorectal cancer. The three intersecting circles represent: Surgical Treatment, Chemoradiotherapy, and Immunotherapy. The overlap between these treatment modalities highlights the integration of robotic surgery in Surgery with Immunotherapy, Combined Surgery and Chemoradiotherapy. The center of the diagram emphasizes the importance of Comprehensive Multimodal Treatment in optimizing patient outcomes for colorectal cancer treatment.
The high cost of robotic platforms, both in terms of initial investment and ongoing maintenance, also represents a significant barrier, especially for smaller hospitals or those in resource-limited settings (39, 40). The cost-effectiveness of robotic surgery remains a subject of ongoing debate, particularly in scenarios where laparoscopic techniques can achieve comparable outcomes at a fraction of the cost (41). Furthermore, the learning curve associated with robotic-assisted surgery is steep (42). Mastery of these systems requires specialized training and substantial experience, which can delay widespread adoption and potentially lead to variability in outcomes during the initial phase of implementation. Addressing these challenges through enhanced system designs, cost-reduction initiatives, and the development of more comprehensive training programs will be critical to improving the accessibility and efficacy of robotic-assisted colorectal surgery.
Total Neoadjuvant Therapy (TNT) has emerged as a pivotal strategy in the management of locally advanced rectal cancer (LARC), integrating both preoperative chemoradiotherapy and systemic chemotherapy prior to surgical resection (43–45). The primary objective of TNT is to optimize oncological outcomes by enhancing tumor downstaging, increasing the rates of pathological complete response (pCR), and improving both disease-free survival (DFS) and overall survival (OS) (46). In contrast to traditional treatment methods, where adjuvant chemotherapy follows surgical intervention, TNT prioritizes systemic chemotherapy in conjunction with neoadjuvant radiotherapy before surgery (47). This approach increases the likelihood of achieving negative surgical margins and addresses micrometastatic disease early in the treatment course.
Administering systemic chemotherapy prior to surgery confers multiple advantages, particularly in controlling micrometastatic disease at an earlier stage, which may reduce the risk of distant metastasis (48). Moreover, completing chemotherapy preoperatively ensures that patients—especially those at heightened risk of postoperative complications or those less likely to tolerate adjuvant therapy due to delayed recovery—receive the full therapeutic benefit. Clinical trials have consistently demonstrated TNT’s efficacy in increasing pCR rates, a surrogate marker strongly correlated with improved long-term outcomes, thereby cementing its clinical value (49, 50).
In the evolving treatment landscape of rectal cancer, the integration of immunotherapy, particularly immune checkpoint inhibitors (ICIs), represents a significant advancement, especially for tumors characterized by mismatch repair deficiency (dMMR) or high microsatellite instability (MSI-H) (51). These tumors, known for their high mutational burden, exhibit enhanced immunogenicity, making them highly susceptible to T-cell-mediated cytotoxicity via immunotherapy (52, 53). For patients with dMMR/MSI-H rectal cancer, ICIs have demonstrated remarkable clinical efficacy, often yielding profound radiologic and clinical responses, with some cases obviating the need for conventional chemoradiotherapy or surgery (54).
Agents such as pembrolizumab and nivolumab have shown high response rates in dMMR/MSI-H patients, enabling treatment de-escalation and significantly improving quality of life by sparing patients from the morbidity associated with traditional, more aggressive treatments (51). These responses are supported by the immune system’s enhanced ability to recognize and eliminate cancer cells in dMMR/MSI-H tumors, driven by their inherent genomic instability. This paradigm shift has fundamentally altered the therapeutic approach for this subset of patients.
Nonetheless, successful immunotherapy hinges on precise patient selection. dMMR or MSI-H status must be confirmed through comprehensive genomic profiling, as patients without these molecular features—namely those with microsatellite-stable (MSS) tumors—derive minimal benefit from ICIs (55). Ongoing research is exploring combinatorial strategies, such as pairing immunotherapy with chemotherapy or radiotherapy in MSS tumors, with the aim of broadening the applicability of ICIs and improving outcomes for a wider patient cohort (56).
Given the rapid advancements in rectal cancer treatment, the imperative for individualized therapeutic strategies has never been more critical (57). While both TNT and immunotherapy represent significant progress, their optimal implementation demands careful tailoring to each patient’s unique clinical and molecular characteristics. Personalizing treatment necessitates consideration of several factors, including tumor genetics, patient comorbidities, and the tumor’s responsiveness to initial therapeutic interventions (58).
In elderly or frail patients, for instance, the intensity of TNT may require modification to reduce treatment-related toxicity while maintaining therapeutic efficacy (59). Conversely, younger or more resilient patients may be candidates for aggressive multimodal approaches aimed at maximizing oncological control (60). The role of multidisciplinary teams—comprising surgical oncologists, medical oncologists, radiation oncologists, and genetic counselors—is indispensable in crafting these individualized treatment regimens, which strive to balance optimal oncological outcomes with quality-of-life considerations.
Advancements in surgical technology, particularly robotic-assisted techniques, further enhance the capacity for personalized care. This is especially pertinent for patients with low rectal tumors, where function-preserving procedures are a priority (61, 62). The integration of these technologies with personalized preoperative and postoperative strategies enables improved functional outcomes while maintaining rigorous cancer control.
Recent advances in robot-assisted colorectal surgery (RACS) have marked the dawn of a new era in surgical precision and patient safety, with next-generation robotic platforms like the da Vinci Xi system at the forefront of these developments (63). These systems offer superior dexterity, enhanced stability, and refined tactile feedback, features that are particularly vital for executing intricate colorectal procedures in anatomically restrictive regions such as the pelvis (64). The expanded range of motion and more intuitive user interface afforded by these platforms have not only improved surgical precision but also enabled surgeons to navigate challenging anatomical planes with greater ease, thereby reducing the technical complexity inherent in operations such as low anterior resection and TME.
Among the most transformative innovations is the integration of intraoperative fluorescence imaging, a method that allows real-time visualization of tissue perfusion, enhancing the surgeon’s ability to delineate resection margins with unparalleled accuracy (65, 66). This technology is pivotal in reducing the risk of anastomotic leaks—one of the most feared complications in colorectal surgery. Additionally, the advent of 3D imaging and augmented reality (AR)-based navigation systems has significantly augmented the surgeon’s capacity to visualize and manipulate within the operative field (67), particularly in the dense and complex anatomy of the pelvis. These tools have refined the precision of resection planes, minimized collateral damage to adjacent tissues, and improved oncological clearance, especially in minimally invasive surgical contexts. (Figure 2B).
With the increasing adoption of RACS across diverse clinical settings, variability in surgical techniques and patient outcomes remains a pressing concern. In response, considerable efforts have been made to standardize the practice of robotic-assisted colorectal surgery (9, 68). Professional organizations and surgical societies have developed comprehensive guidelines designed to harmonize key aspects of robotic surgery, including preoperative planning, patient selection, and intraoperative procedures (69). These guidelines seek to ensure the consistent application of critical steps—such as optimal patient positioning, standardized port placement, and robotic docking techniques—across institutions, thereby enhancing the reproducibility and quality of surgical outcomes.
In tandem with these procedural guidelines, the establishment of rigorous training and accreditation frameworks has further bolstered the standardization of RACS. Simulation-based training programs offer a safe and controlled environment where surgeons can refine their technical skills before transitioning to live surgery (70). Competency evaluations, coupled with proctored surgeries, form an integral part of certification processes, ensuring that only those surgeons who have demonstrated both technical proficiency and adherence to standardized protocols are entrusted with performing robotic procedures independently. Furthermore, continued medical education (CME) requirements ensure that surgeons remain abreast of the latest technological innovations and procedural refinements, fostering a culture of continuous improvement in surgical practice (71).
In colorectal surgery, particularly in the management of malignancies located in the lower rectum, there has been a growing emphasis on techniques that preserve function, aiming to improve outcomes not only in terms of oncological control but also in the quality of life for patients (72). One notable advancement in this regard is the development of sphincter-preserving procedures, such as intersphincteric resection (ISR) (73). ISR allows for the excision of rectal tumors while preserving the sphincter complex, thereby avoiding the need for permanent colostomies and significantly enhancing patient quality of life. The enhanced precision provided by robotic systems is particularly beneficial in these cases, enabling the meticulous dissection required to protect critical structures such as the sphincter complex and pelvic nerves.
Robotic platforms have also revolutionized the performance of TME, which remains the cornerstone of rectal cancer surgery (74). TME demands exacting dissection to maintain the integrity of the mesorectal fascia while ensuring comprehensive oncological resection (75). The fine motor control and superior visualization offered by robotic systems minimize the risk of inadvertent nerve damage, thereby preserving essential pelvic functions such as urinary and sexual function (76). This heightened precision is directly correlated with improved postoperative functional outcomes, translating into both enhanced cancer cure rates and better overall quality of life for patients following surgery.
Therefore, the integration of state-of-the-art robotic technologies into colorectal surgery, alongside concerted efforts to standardize surgical protocols and embrace function-preserving techniques, heralds a paradigm shift in the treatment of colorectal diseases. These advancements not only offer superior oncological outcomes but also foster improved functional recovery, addressing critical issues related to postoperative morbidity and long-term quality of life.
MIS has become integral to the treatment of colorectal cancer, with robotic-assisted techniques representing a considerable leap forward in surgical innovation. The primary oncological benchmarks used to assess the efficacy of these methods include survival rates and local recurrence rates, both of which are pivotal in determining long-term disease control (77).
In terms of survival outcomes, current evidence suggests that RACS achieves comparable, if not superior, OS and DFS rates when measured against conventional laparoscopic techniques (78). The heightened precision afforded by robotic systems facilitates more accurate dissection and resection, potentially enhancing oncological clearance, especially in challenging rectal cancer cases where meticulous pelvic dissection is crucial. Moreover, robotic platforms have demonstrated superior accuracy in performing TME, a procedure essential for reducing local recurrence rates—an important indicator of surgical success (79). The reduction in positive circumferential resection margins (CRM) observed in some robotic-assisted procedures further emphasizes the potential of these technologies to improve oncological outcomes (80).
While achieving oncological control is paramount in colorectal cancer management, postoperative functional outcomes are increasingly recognized as essential to optimizing patient quality of life (81, 82). Factors such as postoperative recovery, bowel function, and patient-reported outcomes serve as critical measures of the success of MIS in preserving physiological function while ensuring therapeutic efficacy (83).
Robotic-assisted surgery has been consistently associated with shorter hospital stays, reduced postoperative pain, and a quicker return to routine activities, reflecting its superiority in immediate recovery compared to traditional open surgery (84). These advantages are largely attributable to smaller incisions, reduced intraoperative blood loss, and the enhanced precision of robotic systems, all of which contribute to minimizing surgical trauma. Furthermore, the preservation of long-term bowel function is a crucial consideration. Robotic platforms, with their ability to enable precise nerve-sparing techniques, play a pivotal role in maintaining autonomic nerve integrity, thereby reducing the risk of postoperative complications such as urinary incontinence, fecal incontinence, and sexual dysfunction (72, 85, 86). These considerations are particularly relevant in procedures such as low anterior resection and TME, where preserving pelvic nerve function through accurate mesorectal fascia dissection is critical.
Patient-reported quality of life (QoL) following robotic-assisted colorectal surgery consistently shows improvements across key domains, including physical health, mental well-being, and social functioning (87, 88). The minimally invasive character of robotic procedures, combined with their function-preserving capabilities, results in fewer disruptions to daily life and enhanced long-term satisfaction. Notably, robotic systems enable sphincter-preserving procedures, which markedly improve quality of life by avoiding the need for permanent colostomies and preserving bowel continuity (89).
The evolution of minimally invasive techniques in colorectal surgery has led to the development of various approaches, each with distinct advantages and limitations. Robotic-assisted surgery, laparoscopic surgery, and transanal total mesorectal excision (taTME) are three key methods currently employed in clinical practice (90).
Laparoscopic colorectal surgery has long been established as a standard minimally invasive approach, offering reduced postoperative pain, shorter hospital stays, and quicker recovery compared to open surgery (91). However, laparoscopic techniques are often constrained by limited dexterity and two-dimensional visualization, which can impede precision, particularly in anatomically complex regions such as the pelvis (92). In contrast, robotic-assisted surgery overcomes these limitations by offering enhanced 3D visualization, superior dexterity via wristed instruments, and improved ergonomics for the surgeon (93). These advantages translate into greater precision, particularly in deep pelvic dissections, and potentially superior oncological and functional outcomes.
TaTME is a relatively novel approach that has garnered attention for its ability to achieve high-quality mesorectal excision in patients with mid-to-low rectal cancer (94). The transanal approach offers enhanced visualization of the distal rectum, facilitating more precise dissection and allowing for highly accurate resections. While taTME holds promise, particularly for difficult-to-reach tumors, it also presents challenges, including a steep learning curve and concerns regarding increased recurrence rates in some studies (95, 96). Compared to both laparoscopic and robotic techniques, taTME may offer unique advantages in select cases, but its risk-to-benefit ratio must be carefully evaluated in light of these concerns (97).
In summary, the landscape of minimally invasive colorectal surgery has evolved considerably, with robotic-assisted techniques offering distinct advantages in both oncological and functional outcomes. The comparison of surgical approaches emphasizes the importance of individualized treatment planning, wherein the choice of technique is driven by patient-specific factors, tumor characteristics, and the expertise of the surgical team. As the field continues to advance, ongoing research and refinement of these techniques will further clarify the optimal strategies for achieving both curative and functional success in colorectal cancer surgery.
Despite the numerous advantages offered by RACS, complications remain an inevitable risk, as with any complex surgical intervention. These complications are typically classified into intraoperative and postoperative challenges, each presenting distinct obstacles that may hinder optimal surgical outcomes.
Intraoperatively, the steep learning curve associated with robotic surgery presents a significant challenge, even for seasoned surgeons (98, 99). Issues such as suboptimal port placement, technical malfunctions, and difficulty in navigating intricate anatomical regions can lead to prolonged operative times and increased intraoperative blood loss. Additionally, the dependence on advanced technology introduces the potential for system failures, which may necessitate conversion to open or laparoscopic techniques, thereby diminishing the anticipated benefits of a minimally invasive approach. Pelvic surgery, particularly in the context of rectal cancer, is especially fraught with technical difficulties due to the constrained operative field, heightening the risk of vascular and nerve injury, which can have profound postoperative implications (100).
Postoperatively, patients undergoing RACS are susceptible to a range of complications, some of which are unique to the robotic platform. Anastomotic leaks, one of the most dreaded complications in colorectal surgery, carry serious implications for morbidity and mortality (101). Other postoperative concerns include infections, particularly deep surgical site infections, delayed bowel function recovery (ileus), and thromboembolic events (102). Although robotic techniques have demonstrated a reduction in wound infections and shorter hospitalizations, these benefits do not entirely negate the possibility of postoperative complications, which demand vigilant surveillance and timely intervention to prevent escalation.
The prevention of complications in RACS hinges on meticulous preoperative planning and precise intraoperative management (103). Preoperative risk assessment is critical in identifying patients who may be more vulnerable to complications, thus enabling the customization of surgical strategies. This process typically involves comprehensive imaging to assess tumor location and complexity, along with thorough evaluations of patient comorbidities, which can significantly influence intraoperative decisions and postoperative recovery.
Intraoperatively, the prevention of complications is closely linked to the deployment of advanced monitoring technologies and strict adherence to well-established surgical protocols (104). For instance, the integration of intraoperative imaging methods, such as fluorescence-guided surgery, enables real-time visualization of tissue perfusion, facilitating the accurate assessment of anastomotic viability and reducing the likelihood of leaks (105, 106). Furthermore, intraoperative neurophysiological monitoring serves a vital role in safeguarding pelvic nerves during dissection, which is crucial for preserving postoperative urinary and sexual function (107). The precision inherent in robotic platforms enhances the implementation of these preventive strategies, thereby contributing to superior clinical outcomes.
Additionally, strict adherence to standardized protocols—encompassing optimal port placement, efficient robotic docking, and stringent adherence to oncological principles such as ensuring negative circumferential resection margins—can substantially reducing the risk of intraoperative complications (108). Proactive measures, including the use of hemostatic agents and preemptive fluid management strategies, further reduce intraoperative risk and enhance surgical safety (109, 110).
When complications do arise, prompt detection and timely intervention are imperative to limit their impact on patient outcomes. Early identification of postoperative complications, such as anastomotic leaks or infections, requires a high level of clinical vigilance (111). This is often facilitated by the adoption of enhanced recovery after surgery (ERAS) protocols, which emphasize close postoperative monitoring, early mobilization, and active patient participation in their recovery process (112). The use of standardized postoperative care pathways allows for early detection of warning signs, enabling swift diagnostic imaging and laboratory investigations to confirm the presence of complications and guide appropriate therapeutic interventions.
The management of complications in RACS is further optimized through a multidisciplinary approach, in which surgeons collaborate with anesthesiologists, radiologists, and specialized nursing teams to provide comprehensive patient care. For instance, the management of anastomotic leaks may necessitate both surgical and non-surgical interventions, ranging from percutaneous drainage and antibiotic therapy to reoperation, depending on the severity of the complication (113, 114). Similarly, the management of thromboembolic events requires coordinated efforts between the surgical and hematology teams to ensure timely anticoagulation, while carefully balancing the risk of bleeding (115–117). This multidisciplinary approach ensures that complications are addressed holistically, encompassing not only immediate clinical needs but also the long-term implications for patient recovery and quality of life.
The landscape of chemotherapeutic agents is progressively advancing to selectively target molecular pathways integral to the pathogenesis of colorectal cancer, including those regulating angiogenesis, programmed cell death (apoptosis), and the tumor microenvironment (118, 119). Notably, inhibitors of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) have demonstrated significant efficacy when employed in conjunction with robotic-assisted surgical techniques, particularly in advanced or recurrent cases where conventional chemotherapy alone proves inadequate (120). This synergistic approach augments the precision of tumor excision, offering the potential to enhance both oncological outcomes and overall patient survival (121).
Immunotherapy, particularly immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), has emerged as a cornerstone of colorectal cancer treatment (122). These inhibitors restore the immune system’s capacity to recognize and eliminate cancer cells, especially in patients with MSI-H tumors, which exhibit heightened sensitivity to immunotherapy (123). The integration of robotic-assisted surgery, facilitating meticulous tumor excision, in conjunction with immunotherapy may reduce recurrence rates and improve long-term survival outcomes. (Figure 2C).
The advent of personalized medicine has ushered in a paradigm shift in the management of colorectal cancer (124). Advances in genomic sequencing now permit the identification of critical mutations—such as those in KRAS, NRAS, and BRAF—enabling the customization of therapeutic strategies (125). The incorporation of this molecular insight into robotic-assisted surgery allows for highly tailored surgical interventions, informed by the tumor’s unique genetic profile. For instance, patients harboring specific mutations may benefit from more extensive resections, while others may be candidates for minimally invasive procedures that prioritize functional preservation (126).
Pharmacogenomics further complements personalized medicine by elucidating how individual genetic variability modulates responses to therapeutic agents (127, 128). This knowledge enables clinicians to optimize drug dosages and reduce adverse effects, thereby enhancing the efficacy of both chemotherapeutic and immunotherapeutic regimens. Such personalized approaches markedly improve survival outcomes and quality of life by reducing treatment-related toxicity.
Clinical trials remain indispensable in the evolution of robotic-assisted colorectal surgery and treatment paradigms. Ongoing studies are evaluating the combination of robotic surgery with neoadjuvant immunotherapy, investigating whether such regimens can downstage tumors and facilitate less invasive surgical interventions (35, 129). Additionally, research on oligometastatic colorectal cancer is exploring the potential for robotic precision to achieve complete resection of metastatic lesions, potentially prolonging disease-free survival (130).
Further investigation is dedicated to refining perioperative care through ERAS protocols, specifically adapted for robotic-assisted procedures. These efforts are crucial in minimizing complication rates, shortening hospital stays, and accelerating postoperative recovery, thereby improving patient outcomes in the colorectal cancer cohort.
Robotic-assisted colorectal surgery has emerged as a pivotal innovation in the contemporary management of colorectal cancer, offering unmatched precision and flexibility, particularly in anatomically complex areas such as the lower rectum. Numerous studies consistently affirm the advantages of robotic platforms, highlighting their superior control and enhanced visualization, which significantly enhance the surgeon’s ability to navigate the intricacies of confined pelvic spaces. When compared with conventional laparoscopic techniques, robotic-assisted approaches have demonstrated reduced intraoperative blood loss, shortened hospital stays, and expedited patient recovery, all while maintaining the oncological rigor required to secure clear resection margins and low recurrence rates (131). The corresponding improvements in postoperative quality of life further emphasize the superiority of robotic-assisted methods, especially for complex colorectal cancer surgeries (132).
The efficacy of robotic surgery is further magnified when embedded within a multidisciplinary treatment framework (133). This comprehensive approach, uniting the expertise of oncologists, radiologists, pathologists, and surgeons, ensures that all facets of a patient’s disease are thoroughly addressed. Such a collaborative strategy enhances decision-making, reduces perioperative risks, and contributes to better survival outcomes. This teamwork is particularly indispensable in the management of colorectal cancer, where personalized treatment plans tailored to the patient’s unique disease characteristics can optimize both immediate and long-term outcomes.
To fully harness the benefits of robotic-assisted colorectal surgery, the development and widespread adoption of standardized protocols are crucial. The current variability in surgical techniques and perioperative management across institutions emphasizes the need for universally accepted, evidence-based guidelines (134). Standardizing practices will ensure consistent delivery of high-quality care, reducing discrepancies in outcomes and enhancing patient safety. Moreover, ensuring uniform and comprehensive training in robotic techniques is crucial to ensuring that surgeons across institutions are proficient in the use of these advanced systems (135).
Ongoing innovation and research are critical to further refining robotic surgery. Technological advancements, such as enhanced haptic feedback, machine learning-assisted decision support, and the integration of artificial intelligence, hold tremendous potential to improve the precision and efficiency of robotic procedures. Furthermore, long-term oncological studies are essential to conclusively determine the superiority of robotic-assisted surgery over alternative approaches, particularly concerning survival outcomes, recurrence rates, and sustained quality of life.
Looking to the future, several areas merit focused investigation to strengthen the role of robotics in colorectal cancer surgery. Advanced intraoperative imaging, including fluorescence-based visualization and artificial intelligence-driven data analytics, may enable surgeons to refine resection margins while identifying critical structures in real time, thereby reducing complications and improving oncological control (136, 137). Augmented reality applications promise an additional layer of precision, allowing overlays that highlight blood vessels, nerves, and tumor borders, which can guide more precise dissections (138). As genomic profiling of colorectal cancer continues to elucidate tumor-specific characteristics, integrating these insights with the dexterity of robotic platforms could permit targeted resections that maintain organ function. Simultaneously, combining robotic surgery with cutting-edge immunotherapies—such as checkpoint inhibitors—and highly selective chemotherapeutic agents has the potential to address residual disease more effectively, shortening recovery times and prolonging survival. Finally, the establishment of uniform training curricula and certification standards across institutions will help ensure equitable access to these technologies and uphold consistently high levels of surgical care.
EC: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Investigation, Validation, Visualization, Writing – original draft. WZ: Investigation, Validation, Writing – original draft.
The author(s) declare that no financial support was received for the research and/or publication of this article.
Figures were created with biorender.com (www.biorender.com).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRC: Colorectal Cancer
RACS: robotic-assisted colorectal surgery
TME: total mesorectal excision
TNT: total neoadjuvant therapy
dMMR: mismatch repair deficiency
MSI-H: high microsatellite instability
MIS: minimally invasive surgery
RAS: robot-assisted surgery
3D: three-dimensional
LARC: locally advanced rectal cancer
pCR: pathological complete response
DFS: disease-free survival
OS: overall survival
ICIs: immune checkpoint inhibitors
MSS: microsatellite-stable
AR: augmented reality
CME: continued medical education
ISR: intersphincteric resection
CRM: circumferential resection margins
QoL: quality of life
TaTME: transanal total mesorectal excision
ERAS: enhanced recovery after surgery
VEGF: vascular endothelial growth factor
EGFR: epidermal growth factor receptor
PD-1: programmed cell death protein 1
CTLA-4: cytotoxic T-lymphocyte-associated antigen 4
1. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, Pas M, Klerk E-d, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. New Engl J Med. (2015) 372:1324–32. doi: 10.1056/NEJMoa1414882
4. Jiang W-Z, Xu J-M, Xing J-D, Qiu H-Z, Wang Z-Q, Kang L, et al. Short-term outcomes of laparoscopy-assisted vs open surgery for patients with low rectal cancer: the lasre randomized clinical trial. JAMA Oncol. (2022) 8:1607–15. doi: 10.1001/jamaoncol.2022.4079
5. Linghu EQ. New direction for surgery: super minimally invasive surgery. World J Gastroenterol. (2024) 30:1676–9. doi: 10.3748/wjg.v30.i12.1676
6. Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe J, Suwa Y, et al. Long-term results of a randomized study comparing open surgery and laparoscopic surgery in elderly colorectal cancer patients (Eld lap study). Surg Endosc. (2021) 35:5686–97. doi: 10.1007/s00464-020-08026-0
7. Krieg A, Kolbe EW, Kaspari M, Krieg S, Loosen SH, Roderburg C, et al. Trends and outcomes in colorectal cancer surgery: A multicenter cross-sectional study of minimally invasive versus open techniques in Germany. Surg Endosc. (2024) 38(11):6338–46. doi: 10.1007/s00464-024-11210-1
8. Liu G, Zhang S, Zhang Y, Fu X, Liu X. Robotic surgery in rectal cancer: potential, challenges, and opportunities. Curr Treat Options Oncol. (2022) 23:961–79. doi: 10.1007/s11864-022-00984-y
9. Gómez Ruiz M, Lainez Escribano M, Cagigas Fernández C, Cristobal Poch L, Santarrufina Martínez S. Robotic surgery for colorectal cancer. Ann Gastroenterol Surg. (2020) 4:646–51. doi: 10.1002/ags3.12401
10. Sterk MFM, Crolla R, Verseveld M, Dekker JWT, van der Schelling GP, Verhoef C, et al. Uptake of robot-assisted colon cancer surgery in the Netherlands. Surg Endosc. (2023) 37:8196–203. doi: 10.1007/s00464-023-10383-5
11. Dhanani NH, Olavarria OA, Bernardi K, Lyons NB, Holihan JL, Loor M, et al. The evidence behind robot-assisted abdominopelvic surgery: A systematic review. Ann Internal Med. (2021) 174:1110–7. doi: 10.7326/M20-7006
12. Chatterjee S, Das S, Ganguly K, Mandal D. Advancements in robotic surgery: innovations, challenges and future prospects. J Robotic Surg. (2024) 18:28. doi: 10.1007/s11701-023-01801-w
13. Wu H, Xue D, Deng M, Guo R, Li H. Progress, challenges, and future perspectives of robot-assisted natural orifice specimen extraction surgery for colorectal cancer: A review. BMC Surg. (2024) 24:255. doi: 10.1186/s12893-024-02538-5
14. Wang Y, Cao D, Chen SL, Li YM, Zheng YW, Ohkohchi N. Current trends in three-dimensional visualization and real-time navigation as well as robot-assisted technologies in hepatobiliary surgery. World J Gastrointest Surg. (2021) 13:904–22. doi: 10.4240/wjgs.v13.i9.904
15. Laredo JA, Torres-Small S, Patel D, Byerly S, Filiberto DM, Wood EH. Recent patterns in minimally invasive colectomies: where are we now? Laparoscopic Endoscopic Robotic Surg. (2024) 8(1):23–7. doi: 10.1016/j.lers.2024.09.003
16. Piozzi GN, Subramaniam S, Di Giuseppe DR, Duhoky R, Khan JS. Robotic colorectal surgery training: portsmouth perspective. Ann Coloproctol. (2024) 40:350–62. doi: 10.3393/ac.2024.00444.0063
17. Gauci C, Ravindran P, Pillinger S, Lynch AC. Robotic surgery for multi-visceral resection in locally advanced colorectal cancer: techniques, benefits and future directions. Laparoscopic Endoscopic Robotic Surg. (2023) 6:123–6. doi: 10.1016/j.lers.2023.11.001
18. Alipouriani A, Gorgun E. Robotic rectal cancer surgery: current controversies. Curr Surg Rep. (2024) 12:122–8. doi: 10.1007/s40137-024-00397-w
19. Kim HJ, Choi G-S. Single-port robotic low anterior resection with lateral pelvic node dissection in locally advanced rectal cancer. Dis Colon Rectum. (2021) 64:e718. doi: 10.1097/DCR.0000000000002170
20. Solbakken AM, Sellevold S, Spasojevic M, Julsrud L, Emblemsvåg H-L, Reims HM, et al. Navigation-assisted surgery for locally advanced primary and recurrent rectal cancer. Ann Surg Oncol. (2023) 30:7602–11. doi: 10.1245/s10434-023-13964-9
21. Bae JH, Song J, Yoo RN, Kim JH, Kye B-H, Lee IK, et al. Robotic lateral pelvic lymph node dissection could harvest more lateral pelvic lymph nodes over laparoscopic approach for mid-to-low rectal cancer: A multi-institutional retrospective cohort study. Biomedicines. (2023) 11:1556. doi: 10.3390/biomedicines11061556
22. Ravendran K, Abiola E, Balagumar K, Raja AZ, Flaih M, Vaja SP, et al. A review of robotic surgery in colorectal surgery. Cureus. (2023) 15:e37337. doi: 10.7759/cureus.37337
23. Schootman M, Hendren S, Ratnapradipa K, Stringer L, Davidson NO. Adoption of robotic technology for treating colorectal cancer. Dis Colon Rectum. (2016) 59:1011–8. doi: 10.1097/DCR.0000000000000688
24. Grass F, Hahnloser D. On-demand robotics—the best of both worlds for robotic-assisted laparoscopic surgery. Surgery. (2024) 176(5):1534–7. doi: 10.1016/j.surg.2024.07.051
25. Rouanet P, Bertrand MM, Jarlier M, Mourregot A, Traore D, Taoum C, et al. Robotic versus laparoscopic total mesorectal excision for sphincter-saving surgery: results of a single-center series of 400 consecutive patients and perspectives. Ann Surg Oncol. (2018) 25:3572–9. doi: 10.1245/s10434-018-6738-5
26. Köckerling F. Robotic vs. Standard laparoscopic technique–what is better? Front Surg. (2014) 1:15.
27. Trinh BB, Jackson NR, Hauch AT, Hu T, Kandil E. Robotic versus laparoscopic colorectal surgery. JSLS: J Soc Laparoendoscopic Surgeons. (2014) 18(4). doi: 10.4293/JSLS.2014.00187
28. Han J. Can robotic surgery lead the way in the treatment of rectal cancer? Ann Coloproctol. (2024) 40:87.
29. Morino M, Allaix ME, Giraudo G, Corno F, Garrone C. Laparoscopic versus open surgery for extraperitoneal rectal cancer: A prospective comparative study. Surg Endoscopy And Other Interventional Techniques. (2005) 19:1460–7. doi: 10.1007/s00464-004-2001-1
30. Cepolina F, Razzoli R. Review of robotic surgery platforms and end effectors. J Robotic Surg. (2024) 18:74. doi: 10.1007/s11701-023-01781-x
31. Larach JT, Kong J, Flynn J, Wright T, Mohan H, Waters PS, et al. Impact of the approach on conversion to open surgery during minimally invasive restorative total mesorectal excision for rectal cancer. Int J Colorectal Dis. (2023) 38:83. doi: 10.1007/s00384-023-04382-0
32. Wang Y, Zhao G-H, Yang H, Lin J. A pooled analysis of robotic versus laparoscopic surgery for total mesorectal excision for rectal cancer. Surg Laparoscopy Endoscopy Percutaneous Techniques. (2016) 26:259–64. doi: 10.1097/SLE.0000000000000263
33. Violante T, Ferrari D, Mathis KL, D’Angelo A-LD, Dozois EJ, Merchea A, et al. Robotic-assisted surgery conversion: the sooner, the better? Insights from a single-center study. J Gastrointest Surg. (2024) 28(7):1158–60. doi: 10.1016/j.gassur.2024.04.003
34. Childers CP, Maggard-Gibbons M. Estimation of the acquisition and operating costs for robotic surgery. Jama. (2018) 320:835–6. doi: 10.1001/jama.2018.9219
35. Shin JK, Kim HC, Lee WY, Yun SH, Cho YB, Huh JW, et al. Is robotic surgery beneficial for rectal cancer patients with unfavorable characteristic after neoadjuvant chemoradiotherapy? Ann Surg Oncol. (2024) 31:3203–11.
36. DiBrito SR, Manisundaram N, Kim Y, Peacock O, Hu CY, Bednarski B, et al. Perioperative and oncological outcomes following robotic en bloc multivisceral resection for colorectal cancer. Colorectal Dis. (2024) 26(5):949–57. doi: 10.1111/codi.16964
37. Awad MM, Raynor MC, Padmanabhan-Kabana M, Schumacher LY, Blatnik JA. Evaluation of forces applied to tissues during robotic-assisted surgical tasks using a novel force feedback technology. Surg Endoscopy. (2024) 38(10):6193–202. doi: 10.1007/s00464-024-11131-z
38. Cofran L, Cohen T, Alfred M, Kanji F, Choi E, Savage S, et al. Barriers to safety and efficiency in robotic surgery docking. Surg Endosc. (2022) 36:206–15. doi: 10.1007/s00464-020-08258-0
39. Mehta A, Cheng Ng J, Andrew Awuah W, Huang H, Kalmanovich J, Agrawal A, et al. Embracing robotic surgery in low- and middle-income countries: potential benefits, challenges, and scope in the future. Ann Med Surg. (2022) 84:104803. doi: 10.1016/j.amsu.2022.104803
40. Lawrie L, Gillies K, Duncan E, Davies L, Beard D, Campbell MK. Barriers and enablers to the effective implementation of robotic assisted surgery. PloS One. (2022) 17:e0273696. doi: 10.1371/journal.pone.0273696
41. Guerrero-Ortiz MA, Sánchez-Velazquez P, Burdío F, Gimeno M, Podda M, Pellino G, et al. Cost-effectiveness of robotic vs laparoscopic distal pancreatectomy. Results from the national prospective trial robocostes. Surg Endosc. (2024) 38(11):6270–81. doi: 10.1007/s00464-024-11109-x
42. Horesh N, Anteby R, Shiber M, Zager Y, Khaikin M. Learning curve of robotic-assisted low anterior resection for low and mid rectal cancer. J Laparoendosc Adv Surg Tech A. (2024) 34(12):1051–5. doi: 10.1089/lap.2024.0221
43. Choi GS, Kim HJ. The role of lateral pelvic lymph node dissection in advanced rectal cancer: A review of current evidence and outcomes. Ann Coloproctol. (2024) 40:363–74. doi: 10.3393/ac.2024.00521.0074
44. Alden SL, Lee V, Narang AK, Meyer J, Gearhart SL, Christenson ES. Circulating tumor DNA to predict radiographic and pathologic response to total neoadjuvant therapy in locally advanced rectal cancer. Oncologist. (2024) 29:e414–e8. doi: 10.1093/oncolo/oyad336
45. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the cao/aro/aio-12 randomized clinical trial. JAMA Oncol. (2022) 8:e215445. doi: 10.1001/jamaoncol.2021.5445
46. Burgio V, Ronzoni M, Spanu D, Passoni P, Elmore U, Albarello L, et al. Induction chemotherapy plus concomitant oxaliplatin-based chemoradiotherapy for locally advanced rectal cancer: A real world experience at san raffaele hospital. J Clin Oncol. (2024) 42:144–. doi: 10.1200/JCO.2024.42.3_suppl.144
47. Jung KU, Kim HO, Kim H, Lee D, Cheong C. Unveiling the profound advantages of total neoadjuvant therapy in rectal cancer: A trailblazing exploration. Ann Surg Treat Res. (2023) 105:341–52. doi: 10.4174/astr.2023.105.6.341
48. Body A, Prenen H, Latham S, Lam M, Tipping-Smith S, Raghunath A, et al. The role of neoadjuvant chemotherapy in locally advanced colon cancer. Cancer Manag Res. (2021) 13:2567–79. doi: 10.2147/cmar.S262870
49. Liu S, Wang X, Zhuang Y, Bai S, Wu X, Ye Y, et al. Total neoadjuvant treatment to increase the clinical complete response rate for distal locally advanced rectal cancer (Tess): A study protocol of a prospective, open-label, multicenter, single-arm, phase 2 trial. Cancer Med. (2023) 12:13352–60. doi: 10.1002/cam4.6034
50. Ando K, Kagawa Y, Uemura M, Watanabe J, Miyo M, Emi Y, et al. A multicenter single-arm phase ii study examining the efficacy of tnt for locally advanced rectal cancer: ensemble-2. J Clin Oncol. (2024) 42:3601–. doi: 10.1200/JCO.2024.42.16_suppl.3601
51. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
52. Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. (2021) 27:1236–41. doi: 10.1158/1078-0432.Ccr-20-3054
53. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
54. Zhang Q, Li J, Shen L, Li Y, Wang X. Opportunities and challenges of immunotherapy for dmmr/msi-H colorectal cancer. Cancer Biol Med. (2023) 20:706–12. doi: 10.20892/j.issn.2095-3941.2023.0240
55. Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip J-M. Deficient mismatch repair/microsatellite unstable colorectal cancer: diagnosis, prognosis and treatment. Eur J Cancer. (2022) 175:136–57. doi: 10.1016/j.ejca.2022.07.020
56. Ding K, Mou P, Wang Z, Liu S, Liu J, Lu H, et al. The next bastion to be conquered in immunotherapy: microsatellite stable colorectal cancer. Front Immunol. (2023) 14:1298524. doi: 10.3389/fimmu.2023.1298524
57. Kusumaningrum AE, Makaba S, Ali E, Singh M, Fenjan MN, Rasulova I, et al. A perspective on emerging therapies in metastatic colorectal cancer: focusing on molecular medicine and drug resistance. Cell Biochem Funct. (2024) 42:e3906. doi: 10.1002/cbf.3906
58. Riedl JM, Moik F, Esterl T, Kostmann SM, Gerger A, Jost PJ. Molecular diagnostics tailoring personalized cancer therapy-an oncologist’s view. Virchows Arch. (2024) 484:169–79. doi: 10.1007/s00428-023-03702-7
59. Awawda M, Taha T, Salman S, Billan S, Hijab A. The evolving treatment paradigm of locally advanced rectal cancer: A narrative review. J Gastrointest Oncol. (2022) 13:2033–47. doi: 10.21037/jgo-22-13
60. Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. (2015) 150:402–9. doi: 10.1001/jamasurg.2014.3572
61. Wang C, Liu X, Wang W, Miao Z, Li X, Liu D, et al. Treatment options for distal rectal cancer in the era of organ preservation. Curr Treat Options Oncol. (2024) 25:434–52. doi: 10.1007/s11864-024-01194-4
62. Arp DT, Appelt AL, Jensen LH, Havelund BM, Nissen HD, Risumlund SL, et al. Treatment planning for patients with low rectal cancer in a multicenter prospective organ preservation study. Phys Med. (2024) 118:103206. doi: 10.1016/j.ejmp.2023.103206
63. Marchegiani F, Siragusa L, Zadoroznyj A, Laterza V, Mangana O, Schena CA, et al. New robotic platforms in general surgery: what’s the current clinical scenario? Med (Kaunas). (2023) 59(7). doi: 10.3390/medicina59071264
64. Quezada-Diaz FF, Smith JJ. Options for low rectal cancer: robotic total mesorectal excision. Clin Colon Rectal Surg. (2021) 34:311–6. doi: 10.1055/s-0041-1726449
65. Lauwerends LJ, van Driel PBAA, Baatenburg de Jong RJ, Hardillo JAU, Koljenovic S, Puppels G, et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. (2021) 22:e186–e95. doi: 10.1016/S1470-2045(20)30600-8
66. Liu YZ, Shah SK, Sanders CM, Nwaiwu CA, Dechert AF, Mehrotra S, et al. Utility and usability of laser speckle contrast imaging (Lsci) for displaying real-time tissue perfusion/blood flow in robot-assisted surgery (Ras): comparison to indocyanine green (Icg) and use in laparoscopic surgery. Surg Endosc. (2023) 37:4803–11. doi: 10.1007/s00464-022-09590-3
67. Lastrucci A, Wandael Y, Barra A, Ricci R, Maccioni G, Pirrera A, et al. Exploring augmented reality integration in diagnostic imaging: myth or reality? Diagn (Basel). (2024) 14(13). doi: 10.3390/diagnostics14131333
68. Wright JP, Albert MR. A current review of robotic colorectal surgery. Ann Laparoscopic Endoscopic Surg. (2020) 5. doi: 10.21037/ales.2019.12.01
69. Bittermann C, Berlet M, Wilhelm D. Principles of robot-assisted colorectal surgery. Eur Surg. (2024). doi: 10.1007/s10353-024-00838-x
70. Guerrero-Antolino P, Gutiérrez-Sánchez C, Millán-Scheiding M. Assessment tools for minimally invasive surgery simulation programmes: A narrative review. Ann Laparoscopic Endoscopic Surg. (2024) 9. doi: 10.21037/ales-23-66
71. Lee JY, Mucksavage P, Sundaram CP, McDougall EM. Best practices for robotic surgery training and credentialing. J Urol. (2011) 185:1191–7.
72. Varela C, Kim NK. Surgical treatment of low-lying rectal cancer: updates. Ann Coloproctol. (2021) 37:395–424. doi: 10.3393/ac.2021.00927.0132
73. Kim JC, Lee JL, Kim CW, Kim JR, Kim J, Park SH. Technical, functional, and oncological validity of robot-assisted total-intersphincteric resection (T-isr) for lower rectal cancer. Eur J Surg Oncol. (2023) 49:188–95. doi: 10.1016/j.ejso.2022.07.010
74. Burghgraef TA, Crolla R, Verheijen PM, Fahim M, van Geloven A, Leijtens JWA, et al. Robot-assisted total mesorectal excision versus laparoscopic total mesorectal excision: A retrospective propensity score-matched cohort analysis in experienced centers. Dis Colon Rectum. (2022) 65:218–27. doi: 10.1097/dcr.0000000000002031
75. Cho MS, Bae HW, Kim NK. Essential knowledge and technical tips for total mesorectal excision and related procedures for rectal cancer. Ann Coloproctol. (2024) 40:384–411. doi: 10.3393/ac.2024.00388.0055
76. Kim NK, Kim YW, Cho MS. Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: expert technical tips for robotic surgery. Surg Oncol. (2015) 24:172–80. doi: 10.1016/j.suronc.2015.06.012
77. Nors J, Iversen LH, Erichsen R, Gotschalck KA, Andersen CL. Incidence of recurrence and time to recurrence in stage I to iii colorectal cancer: A nationwide danish cohort study. JAMA Oncol. (2024) 10:54–62. doi: 10.1001/jamaoncol.2023.5098
78. Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (Real): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. (2022) 7:991–1004. doi: 10.1016/s2468-1253(22)00248-5
79. Evans KM, Sahawneh JM, Ferrara M. Rectal cancer surgery: is robotic surgery supported by solid evidence? Ann Laparoscopic Endoscopic Surg. (2023) 8. doi: 10.21037/ales-22-76
80. Wu H, Guo R, Li H. Short-term and long-term efficacy in robot-assisted treatment for mid and low rectal cancer: A systematic review and meta-analysis. Int J Colorectal Dis. (2023) 39:7. doi: 10.1007/s00384-023-04579-3
81. Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection - a matched-controlled study. Dis Colon Rectum. (2017) 60:1032–40. doi: 10.1097/dcr.0000000000000862
82. Chong RC, Ong MW, Tan KY. Managing elderly with colorectal cancer. J Gastrointest Oncol. (2019) 10:1266–73. doi: 10.21037/jgo.2019.09.04
83. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (Eras®) society recommendations: 2018. World J Surg. (2019) 43:659–95. doi: 10.1007/s00268-018-4844-y
84. Choi JH, Diab AR, Tsay K, Kuruvilla D, Ganam S, Saad A, et al. The evidence behind robot-assisted abdominopelvic surgery: A meta-analysis of randomized controlled trials. Surg Endosc. (2024) 38:2371–82. doi: 10.1007/s00464-024-10773-3
85. Knol J, Keller DS. Total mesorectal excision technique-past, present, and future. Clin Colon Rectal Surg. (2020) 33:134–43. doi: 10.1055/s-0039-3402776
86. Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol. (2011) 8:51–7. doi: 10.1038/nrurol.2010.206
87. Martins RS, Fatimi AS, Mahmud O, Jahangir A, Mahar MU, Aamir SR, et al. Multidimensional quality of life after robotic versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. World J Surg. (2023) 47:1310–9. doi: 10.1007/s00268-023-06936-3
88. Li A, Stanislaus CT, Steffens D, McBride KE, Leslie S, Thanigasalam R, et al. Prospective cohort study investigating quality of life outcomes following multi-speciality robotic-assisted surgery. J Minim Access Surg. (2024) 20:37–46. doi: 10.4103/jmas.jmas_253_22
89. Pappou EP, Temple LK, Patil S, Smith JJ, Wei IH, Nash GM, et al. Quality of Life and Function after Rectal Cancer Surgery with and without Sphincter Preservation. Front Oncol. (2022) 12:944843. doi: 10.3389/fonc.2022.944843
90. Burghgraef TA, Hol JC, Rutgers ML, Crolla R, van Geloven AAW, Hompes R, et al. Laparoscopic versus robot-assisted versus transanal low anterior resection: 3-year oncologic results for a population-based cohort in experienced centers. Ann Surg Oncol. (2022) 29:1910–20. doi: 10.1245/s10434-021-10805-5
91. Parker JM, Feldmann TF, Cologne KG. Advances in laparoscopic colorectal surgery. Surg Clin North Am. (2017) 97:547–60. doi: 10.1016/j.suc.2017.01.005
92. Flynn J, Larach JT, Kong JCH, Waters PS, Warrier SK, Heriot A. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: A systematic review. Colorectal Dis. (2021) 23:2806–20. doi: 10.1111/codi.15843
93. Wong SW, Crowe P. Visualisation ergonomics and robotic surgery. J Robot Surg. (2023) 17:1873–8. doi: 10.1007/s11701-023-01618-7
94. Liu H, Zeng Z, Zhang H, Wu M, Ma D, Wang Q, et al. Morbidity, mortality, and pathologic outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer short-term outcomes from a multicenter randomized controlled trial. Ann Surg. (2023) 277:1–6. doi: 10.1097/sla.0000000000005523
95. Wasmuth HH, Faerden AE, Myklebust T, Pfeffer F, Norderval S, Riis R, et al. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. (2020) 107:121–30. doi: 10.1002/bjs.11459
96. Vignali A, Elmore U, Milone M, Rosati R. Transanal total mesorectal excision (Tatme): current status and future perspectives. Updates Surg. (2019) 71:29–37. doi: 10.1007/s13304-019-00630-7
97. Rutgers MLW, Bemelman WA, Khan JS, Hompes R. The role of transanal total mesorectal excision. Surg Oncol. (2022) 43:101695. doi: 10.1016/j.suronc.2021.101695
98. Wong SW, Crowe P. Factors affecting the learning curve in robotic colorectal surgery. J Robot Surg. (2022) 16:1249–56. doi: 10.1007/s11701-022-01373-1
99. Soomro NA, Hashimoto DA, Porteous AJ, Ridley CJA, Marsh WJ, Ditto R, et al. Systematic review of learning curves in robot-assisted surgery. BJS Open. (2020) 4:27–44. doi: 10.1002/bjs5.50235
100. Moszkowicz D, Alsaid B, Bessede T, Penna C, Nordlinger B, Benoît G, et al. Where does pelvic nerve injury occur during rectal surgery for cancer? Colorectal Dis. (2011) 13:1326–34. doi: 10.1111/j.1463-1318.2010.02384.x
101. Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. (2022) 40:101708. doi: 10.1016/j.suronc.2022.101708
102. Mazzotta E, Villalobos-Hernandez EC, Fiorda-Diaz J, Harzman A, Christofi FL. Postoperative ileus and postoperative gastrointestinal tract dysfunction: pathogenic mechanisms and novel treatment strategies beyond colorectal enhanced recovery after surgery protocols. Front Pharmacol. (2020) 11:583422. doi: 10.3389/fphar.2020.583422
103. Barkhordarian S, Dardik A. Preoperative assessment and management to prevent complications during high-risk vascular surgery. Crit Care Med. (2004) 32:S174–85. doi: 10.1097/01.ccm.0000115625.30405.12
104. Chung KC, Kotsis SV. Complications in surgery: root cause analysis and preventive measures. Plast Reconstr Surg. (2012) 129:1421–7. doi: 10.1097/PRS.0b013e31824ecda0
105. Uppal JS, Meng E, Caycedo-Marulanda A. Current applications of indocyanine green fluorescence in colorectal surgery: A narrative review. Ann Laparoscopic Endoscopic Surg. (2023) 8. doi: 10.21037/ales-22-84
106. Diana M. Fluorescence-guided surgery applied to the digestive system: the cybernetic eye to see the invisible. Cirugía Española (English Edition). (2018) 96:65–8. doi: 10.1016/j.cireng.2017.10.002
107. Lee Y-J, van den Berg NS, Orosco RK, Rosenthal EL, Sorger JM. A narrative review of fluorescence imaging in robotic-assisted surgery. Laparoscopic Surg. (2021) 5. doi: 10.21037/ls-20-98
108. Chang C, Steinberg Z, Shah A, Gundeti MS. Patient positioning and port placement for robot-assisted surgery. J Endourol. (2014) 28:631–8. doi: 10.1089/end.2013.0733
109. Behrens AM, Sikorski MJ, Kofinas P. Hemostatic strategies for traumatic and surgical bleeding. J BioMed Mater Res A. (2014) 102:4182–94. doi: 10.1002/jbm.a.35052
110. Navarro LH, Bloomstone JA, Auler JO Jr., Cannesson M, Rocca GD, Gan TJ, et al. Perioperative fluid therapy: A statement from the international fluid optimization group. Perioper Med (Lond). (2015) 4:3. doi: 10.1186/s13741-015-0014-z
111. Tsai Y-Y, Chen WT-L. Management of anastomotic leakage after rectal surgery: A review article. J Gastrointest Oncol. (2019) 10:1229–37. doi: 10.21037/jgo.2019.07.07
112. Tazreean R, Nelson G, Twomey R. Early Mobilization in Enhanced Recovery after Surgery pathways: Current Evidence and Recent Advancements. J Comp Eff Res. (2022) 11:121–9. doi: 10.2217/cer-2021-0258
113. Thomas MS, Margolin DA. Management of colorectal anastomotic leak. Clin Colon Rectal Surg. (2016) 29:138–44. doi: 10.1055/s-0036-1580630
114. Keller DS, Talboom K, van Helsdingen CPM, Hompes R. Treatment modalities for anastomotic leakage in rectal cancer surgery. Clin Colon Rectal Surg. (2021) 34:431–8. doi: 10.1055/s-0041-1736465
115. Levy JH, Connors JM, Steiner ME, Douketis J, Spyropoulos AC. Management of oral anticoagulants prior to emergency surgery or with major bleeding: A survey of perioperative practices in north america: communication from the scientific and standardization committees on perioperative and critical care haemostasis and thrombosis of the international society on thrombosis and haemostasis. Res Pract Thromb Haemost. (2020) 4:562–8. doi: 10.1002/rth2.12320
116. Levesque AA, Lewin AR, Rimsans J, Sylvester KW, Coakley L, Melanson F, et al. Development of multidisciplinary anticoagulation management guidelines for patients receiving durable mechanical circulatory support. Clin Appl Thromb Hemost. (2019) 25:1076029619837362. doi: 10.1177/1076029619837362
117. Muntz JE, Michota FA. Prevention and management of venous thromboembolism in the surgical patient: options by surgery type and individual patient risk factors. Am J Surg. (2010) 199:S11–20. doi: 10.1016/j.amjsurg.2009.10.007
118. Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. (2020) 5:22. doi: 10.1038/s41392-020-0116-z
119. Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer. (2012) 11:1–13. doi: 10.1016/j.clcc.2011.05.005
120. Haraldsdottir S, Bekaii-Saab T. Integrating anti-egfr therapies in metastatic colorectal cancer. J Gastrointest Oncol. (2013) 4:285–98.
121. Liu B, Zhou H, Tan L, Siu KTH, Guan X-Y. Exploring treatment options in cancer: tumor treatment strategies. Signal Transduction Targeted Ther. (2024) 9:175. doi: 10.1038/s41392-024-01856-7
122. Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death -1 (Pd-1) and ligand (Pd-L1): A new era in cancer active immunotherapy. Pharmacol Ther. (2019) 194:84–106. doi: 10.1016/j.pharmthera.2018.09.008
123. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. (2019) 16:361–75. doi: 10.1038/s41575-019-0126-x
124. Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. (2022) 72:372–401. doi: 10.3322/caac.21728
125. El Zaitouni S, Laraqui A, Ghaouti M, Benzekri A, Kettani F, Bajjou T, et al. Kras, nras and braf mutational profile of colorectal cancer in a series of moroccan patients. Cancer Control. (2024) 31:10732748241262179. doi: 10.1177/10732748241262179
126. Ford SJ, Gronchi A. Indications for surgery in advanced/metastatic gist. Eur J Cancer. (2016) 63:154–67. doi: 10.1016/j.ejca.2016.05.019
127. Magarbeh L, Gorbovskaya I, Le Foll B, Jhirad R, Müller DJ. Reviewing pharmacogenetics to advance precision medicine for opioids. Biomed Pharmacother. (2021) 142:112060. doi: 10.1016/j.biopha.2021.112060
128. Zhou Y, Peng S, Wang H, Cai X, Wang Q. Review of personalized medicine and pharmacogenomics of anti-cancer compounds and natural products. Genes. (2024) 15:468. doi: 10.3390/genes15040468
129. Park SY, Lee SM, Park JS, Kim HJ, Choi GS. Robot Surgery Shows Similar Long-Term Oncologic Outcomes as Laparoscopic Surgery for Mid/Lower Rectal Cancer but Is Beneficial to Ypt3/4 after Preoperative Chemoradiation. Dis Colon Rectum. (2021) 64:812–21. doi: 10.1097/dcr.0000000000001978
130. Germani MM, Borelli B, Boraschi P, Antoniotti C, Ugolini C, Urbani L, et al. The management of colorectal liver metastases amenable of surgical resection: how to shape treatment strategies according to clinical, radiological, pathological and molecular features. Cancer Treat Rev. (2022) 106:102382. doi: 10.1016/j.ctrv.2022.102382
131. Farah E, Abreu AA, Rail B, Salgado J, Karagkounis G, Zeh HJ, et al. Perioperative outcomes of robotic and laparoscopic surgery for colorectal cancer: A propensity score-matched analysis. World J Surg Oncol. (2023) 21:272. doi: 10.1186/s12957-023-03138-y
132. Kawka M, Fong Y, Gall TMH. Laparoscopic versus robotic abdominal and pelvic surgery: A systematic review of randomised controlled trials. Surg Endosc. (2023) 37:6672–81. doi: 10.1007/s00464-023-10275-8
133. Marcus HJ, Ramirez PT, Khan DZ, Layard Horsfall H, Hanrahan JG, Williams SC, et al. The ideal framework for surgical robotics: development, comparative evaluation and long-term monitoring. Nat Med. (2024) 30:61–75. doi: 10.1038/s41591-023-02732-7
134. Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. (2020) 3:e1918911–e. doi: 10.1001/jamanetworkopen.2019.18911
135. Stefanidis D, Huffman EM, Collins JW, Martino MA, Satava RM, Levy JS. Expert consensus recommendations for robotic surgery credentialing. Ann Surg. (2022) 276:88–93. doi: 10.1097/sla.0000000000004531
136. Guni A, Varma P, Zhang J, Fehervari M, Ashrafian H. Artificial intelligence in surgery: the future is now. Eur Surg Res. (2024) 276(1):88–93. doi: 10.1159/000536393
137. Andras I, Mazzone E, van Leeuwen FWB, De Naeyer G, van Oosterom MN, Beato S, et al. Artificial intelligence and robotics: A combination that is changing the operating room. World J Urol. (2020) 38:2359–66. doi: 10.1007/s00345-019-03037-6
Keywords: robotic-assisted colorectal surgery, multidisciplinary treatment strategies, colorectal cancer, narrative review, clinical efficacy
Citation: Chen E, Chen L and Zhang W (2025) Robotic-assisted colorectal surgery in colorectal cancer management: a narrative review of clinical efficacy and multidisciplinary integration. Front. Oncol. 15:1502014. doi: 10.3389/fonc.2025.1502014
Received: 26 September 2024; Accepted: 20 March 2025;
Published: 07 April 2025.
Edited by:
Nobu Oshima, Kyoto University Graduate School of Medicine, JapanReviewed by:
Andrea Marco Tamburini, San Raffaele Hospital (IRCCS), ItalyCopyright © 2025 Chen, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Engeng Chen, Y2hlbmVuZ2VuZ0B6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.