- Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
Hepatocellular carcinoma (HCC) is one of the most common lethal cancers worldwide. Natural killer cells (NK cells) play a key role in liver immunosurveillance, but in the tumor microenvironment, NK cells are readily depleted, as evidenced by down-regulation of activating receptors, reduced cytokine secretion, and attenuated killing function. The up-regulation of inhibitory receptors, such as PD-1, TIM-3, and LAG-3, further exacerbates the depletion of NK cells. Combined blockade strategies targeting these immunosuppressive mechanisms, such as the combination of PD-1 inhibitors with other inhibitory pathways (eg. TIM-3 and LAG-3), have shown potential to reverse NK cell exhaustion in preclinical studies. This article explores the promise of these innovative strategies in HCC immunotherapy, providing new therapeutic directions for optimizing NK cell function and improving drug sensitivity.
1 Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, accounting for about 90% of all primary liver cancer cases (1–3). Statistics show that HCC causes about 850,000 new cases and 800,000 deaths each year, and the extremely high mortality rate highlights a major threat to global public health (2, 4–6). HCC is often asymptomatic in the early stages, resulting in most patients being diagnosed at a later stage, with a poor prognosis and difficult to treat (7–10). The treatments for HCC include surgery, localized ablation, liver transplantation, chemotherapy, radiotherapy, and immunotherapy, etc (11–15). However, due to the complex structure of the liver and the heterogeneity of HCC, there are significant limitations in the practical application of these methods (16, 17). Immunotherapy, especially immune checkpoint inhibitors, is effective in some patients with HCC, but the overall response rate is low and drug resistance is easy to (18). Therefore, the development of new strategies that can augment the effect of the existing immunotherapeutic treatments is urgently needed.
Natural Killer Cells (NK cells) are an important component of the innate immune system, capable of rapidly recognizing and clearing virus-infected and tumor cells without relying on antigen presentation (19). In the hepatobiliary system of healthy adults, NK cells exhibit a unique behavioral pattern, with a high activity and frequency, occupying 22.6% of the total number of Intrahepatic lymphocytes (IHLs) (20). Studies have shown that NK cells are effective in inhibiting hepatocellular carcinoma development and progression in the liver microenvironment. However, immunosuppressive factors in the tumor microenvironment, such as TGF-β and IL-10, often impair the anti-tumor function of NK cells, leading to their functional depletion (19, 21). NK cell exhaustion refers to the gradual loss of NK cell function due to prolonged exposure to tumor antigens and inhibitory signals in the tumor microenvironment, and is characterized by downregulation of activation receptors, decreased cytokine secretion, and decreased killing capacity. It is also accompanied by upregulation of inhibitory receptors such as PD-1, TIM-3, and LAG-3 (Figure 1C) (22–24). These changes enable tumor cells to evade immune surveillance and promote growth and metastasis.
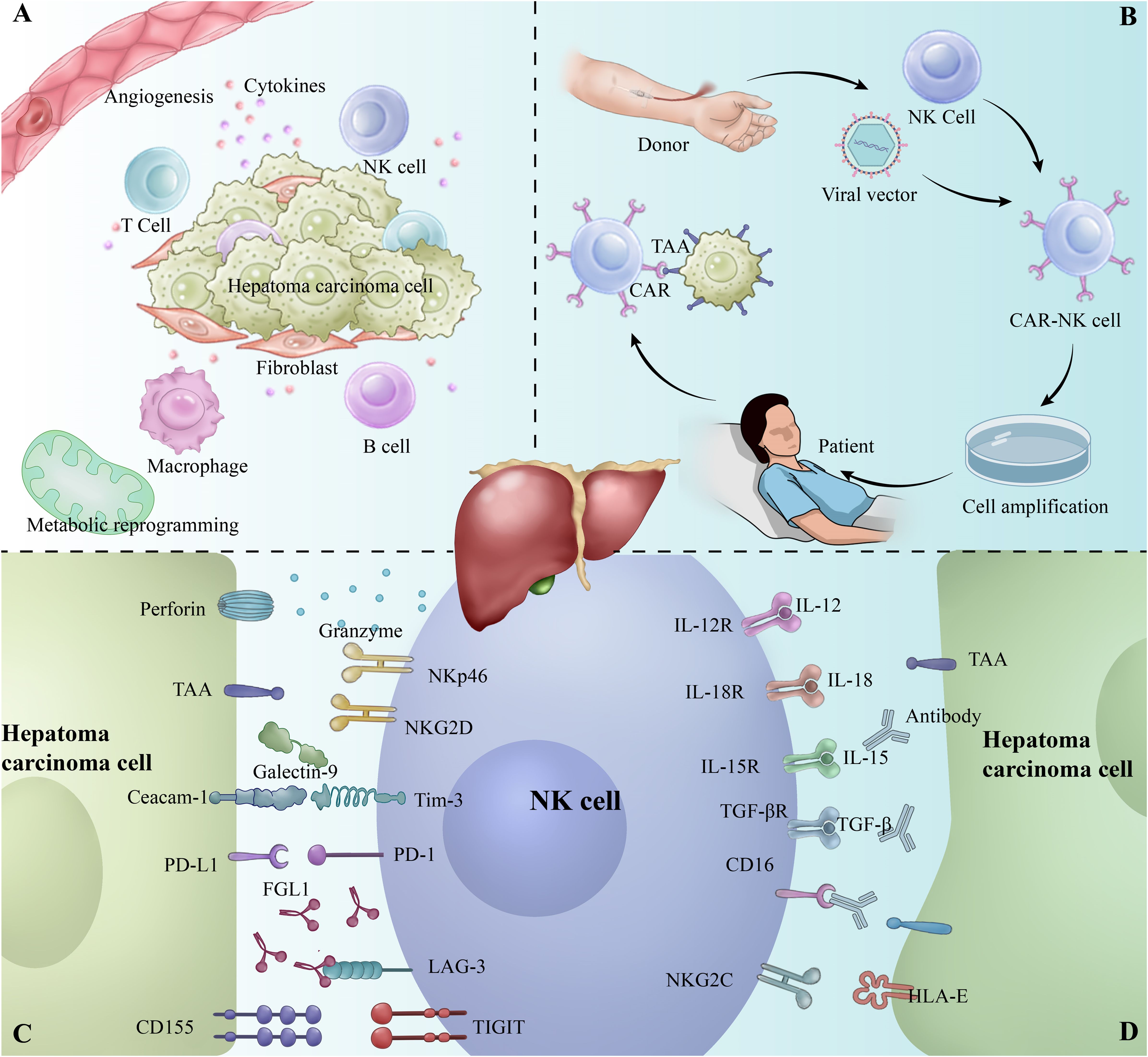
Figure 1. (A) Tumor microenvironment of hepatocellular carcinoma (HCC). (B) Workflow of CAR-NK therapy. (C) Overview of inhibitory receptors involved in the interaction between NK cells and tumor cells. (D) Overview of activating receptors involved in the interaction between NK cells and tumor cells.
Understanding for these mechanisms of immunosuppression provides new directions for the optimization of immunotherapy. By combined blockade of multiple inhibitory pathways such as PD-1, TIM-3, LAG-3, and others, researchers are working to reverse NK cell exhaustion (25, 26). Emerging therapies such as immune checkpoint inhibitors, chimeric antigen receptor for NK cells (CAR-NK) therapies, and immunomodulatory drugs (e.g., IL-15 and IL-2) show great potential to enhance the antitumor activity of NK cells and improve drug sensitivity (27–30).
2 Biology of NK cells
2.1 Generation, differentiation and function of NK cells
NK cells (natural killer cells) are important effector cells of the immune system responsible for anti-tumor and anti-viral resistance, derived from bone marrow hematopoietic stem cells and matured through a multi-step differentiation process (31, 32). Human NK cells are classified into two major subpopulations based on the expression of CD16 and CD56. The majority (85%-95%) of NK cells in peripheral blood are of the CD56-CD16+ subpopulation, displaying a developmentally mature phenotype and possessing a high degree of cytotoxicity (33, 34). Unlike T cells, which depend on antigen presentation, NK cells are capable of lysing without the need for antigen presentation, and are able to pass through lysates containing granzyme B and perforin. Cell particles containing granzyme B and perforin cross the immune synapse to mediate sequential killing of infected or malignant cells (35).
In contrast, the CD56+CD16- subpopulation has fewer NK cells and exhibits an immature phenotype. Although the latter have low cytotoxicity when not activated, they are capable of producing large amounts of cytokines and exerting potent immunomodulatory effects when stimulated by pro-inflammatory cytokines (e.g., IL-15) (36, 37). The two subpopulations complement each other functionally, with the former being primarily responsible for the direct killing effect, and the latter playing an important role in immune response via cytokine secretion.
Upon maturation, NK cells have a potent killing capacity, although this cytotoxicity is not associated with major histocompatibility complex (MHC) expression because NK cells do not express somatically rearranged antigen receptors (38, 39). Instead, NK cells regulate NK cell activity through a balance of their activating (e.g., NKG2D, NKp46) and inhibitory (e.g., KIR family) receptors, thereby killing or causing tolerance in target cells (40, 41).
CD16 (low affinity IgG Fc region receptor III, FcγRIII), the most potent activating receptor expressed by NK cells and the only receptor that can activate NK cells on its own, can assist antibody-mediated immune responses through the antibody-dependent cell-mediated cytotoxicity (ADCC) pathway (42–44). The Fc portion of the antibody was engineered to increase its affinity for CD16a and enhance the ADCC effect. For example, replacing four amino acids in the Fc region to become a GASDALIE mutant significantly enhanced the affinity of Fc for CD16a, while the affinity for CD32b remained almost unchanged (45).
NKG2D protein is an important activating cell surface receptor protein, which is mainly expressed on cytotoxic immune cells, such as NK cells, CD8+ T cells, etc (46, 47). The first known NKG2D receptor is MICA with MICB, which is expressed in a wide variety of tumors (liver cancer, breast cancer, lung cancer) and organ transplant recipient MIC is expressed in tissue cells (48–51). MICA exhibits a very low tumor mutation burden, suggesting that its expression is not significantly affected by DNA editing mechanisms, so NKG2DL overexpression may be a potent strategy for anti-tumor progression (52, 53).
2.2 NK cells in the liver
NK cells are distributed in high concentrations in the liver, accounting for 50% of hepatic innate immune cells, making the liver one of the major sites of NK cell residency (31, 54, 55). This feature arises from the fact that embryonic hepatic hematopoietic stem cells are divided into two fractions, one of which continues to remain in the adult liver, generating the characteristic tissue-resident NK cells (LrNK) (56).
Compared to peripheral blood, NK cells in the liver differ in both effector molecule expression and cellular activity. The CD56+CD16- subpopulation is predominant in the liver (33). LrNK cells have a suppressive function in the immune tolerance microenvironment of the liver, particularly in inhibiting the antiviral response of T cells through the PD-1/PD-L1 pathway. For example, Zhou et al. found that exogenous transfusion of LrNK cells to normal or LrNK cell-deficient mice suppressed antiviral T-cell responses in the liver and was dependent on the PD-1-PD-L1 axis. In contrast, NK (cNK) cells circulating in the peripheral blood promoted T-cell responses (57).
3 Immunosuppressive factors and NK cell exhaustion mechanisms in the HCC tumor microenvironment
The tumor microenvironment (TME) of HCC consists of immune cells, immunosuppressive cells, and mesenchymal stromal cells with hypoxia, angiogenesis, metabolic reprogramming, inflammation, and immunosuppression (Figure 1A) (58, 59). The TME of HCC is characterized by the secretion of a variety of immunosuppressive factors, such as interleukin (IL)-6, IL-10, and transforming growth factor-β (TGF-β), prostaglandin E2 (PGE2) to directly inhibit NK cell activity and promote NK cell exhaustion (60–64).
TGF-β is known to be a potent immunosuppressive factor, which impairs tumor cell recognition by NK cells by down-regulating the expression of activation receptors on the surface of NK cells, such as NKG2D (65, 66). In addition, TGF-β further impairs its anti-tumor effect by inhibiting IFN-γ production and ADCC in NK cells (67, 68). IL-10, on the other hand, impairs its killing activity by inhibiting NK cell proliferation and cytokine secretion (e.g., IFN-γ and TNF-α) (69).
In addition, IL-6, as another important immunosuppressive factor, further promotes immunosuppression in the tumor microenvironment through a complex signaling mechanism. Studies have shown that in intrahepatic cholangiocarcinoma (ICC) cells, IL-6 induces the expression of cyclic RNA (circRNA) GGNBP2 (cGGNBP2). cGGNBP2 encodes a protein, cGGNBP2-184aa, which forms a positive feedback loop that sustainably activates the STAT3 signaling pathway, thereby promoting tumor cell proliferation and metastasis (70). This sustained STAT3 activation indirectly inhibits NK cell function by regulating other immune cells in the tumor microenvironment, further promoting NK cell exhaustion.
These inhibitory factors not only act directly on NK cells, but also indirectly promote NK cell exhaustion by modulating the function of other immune cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (71, 72). For example, this multilayered inhibitory mechanism causes NK cells to gradually lose their function in the TME, which facilitates immune escape from the tumor.
In the TME of HCC, the complex interaction of NK cells with other immune cells forms a suppressive network that further exacerbates NK cell functional exhaustion. Immunosuppressive cells such as dendritic cells (DCs) and regulatory T cells further diminish NK cell activity through secretion of inhibitory factors or direct cell-to-cell contact (73). DCs often exhibit abnormal function in HCC, which are unable to efficiently activate NK cells, but instead may further inhibit NK cell activity through the high expression of PD-L1 on the surface (74). Meanwhile, the increase of Tregs is also one of the important reasons for the suppression of NK cell function in TME. Tregs directly inhibit the activation and function of NK cells through cellular indirect contact and secretion of TGF-β and IL-10 (75).
4 Mechanisms of activation of exhaustion signaling pathways
NK cell exhaustion is closely associated with the activation of specific signaling pathways, of which the PD-1/PD-L1 pathway is one of the most important (Figure 1D). PD-1 is an inhibitory receptor that is highly expressed in the depleted state of NK cells, whereas its ligand, PD-L1, also exhibits a significant up-regulation in tumor cells and tumor-associated immune cells (76). When PD-1 binds to PD-L1, it inhibits the killing function and cytokine secretion of NK cells by down-regulating the activation signals in NK cells (77, 78). In addition, the PD-1/PD-L1 signaling pathway inhibits the activation of the Akt and mTOR pathways in NK cells, leading to metabolic dysfunction and further weakening its anti-tumor effects (79).
However, PD-1/PD-L1 is not the only signaling pathway driving NK cell exhaustion. Other inhibitory receptors such as TIM, TIGIT and LAG-3 are likewise significantly up-regulated in NK cells in the depleted state, forming a co-inhibitory network (26, 80).
4.1 TIM-3
TIM-3 is highly expressed in the depleted state of NK cells and acts as a co-inhibitory receptor involved in the modulation of type I immune responses. The immunomodulatory mechanism of TIM-3 is dependent on its binding to several ligands, such as Galectin-9, phosphatidylserine (PtdSer), HMGB1 and CEACAM-1 (81). PtdSer acts as an “eat-me” signal that promotes the clearance of apoptotic cells by binding to TIM-3. HMGB1, a damage-associated molecular pattern (DAMP), regulates the innate immune response by suppressing the inflammatory response when it binds to TIM-3. Binding of CEACAM-1 is thought to be closely related to inhibitory signaling by TIM-3.
These ligands, including CEACAM-1, Galectin-9, PtdSer, and HMGB1, bind to different regions of TIM-3, respectively, triggering intracellular inhibitory signals (82). For example, upon binding of TIM-3 to Galectin-9, the Y256 and Y263 sites in its cytoplasmic domain are phosphorylated, leading to dissociation of the articulator BAT3 from TIM-3, which in turn inhibits TCR signaling and reduces NK cell immune response, especially reducing the secretion of key cytokines such as IFN-γ (82). Phosphorylation of the Y256 and Y263 sites is not only a critical step in TIM-3-regulated signaling, but also promotes the activation of other inhibitory signals by preventing the binding of BAT3 to TIM-3.
4.2 TIGIT
The co-inhibitory receptor, TIGIT, blocks the direct interaction of NK cells with tumor cells by binding to the ligands CD155 and CD112, which are highly expressed in antigen-presenting cells (APCs) and tumor cells, diminishing their killing ability and further inhibiting cytokine secretion by NK cells, such as TNF-α and IFN-γ (83, 84). In addition, TIGIT interferes with tumor recognition by NK cells through competitive inhibition of CD226. Although TIGIT shares the same ligand as CD226, it binds to CD155 and CD112 with higher affinity, thereby inhibiting CD226-mediated activation signaling. This competitive mechanism further exacerbates the depleted state of NK cells (84).
4.3 LAG-3
LAG-3 is a structurally similar inhibitory receptor to CD4 that inhibits NK cell activation mainly through binding to MHC class II molecules (85). LAG-3 is highly expressed not only in T cells but also upregulated on NK cells, and this upregulation leads to suppression of both innate and adaptive immune functions in tumor patients.
LAG-3 hinders effective recognition and clearance of tumor cells by NK cells through interaction with FGL1 (fibrinogen-like protein), which is highly expressed in hepatocytes and tumor cells. In addition, LAG-3 regulates downstream molecules (e.g., SHP-1 and SHP-2) through inhibitory signaling motifs (e.g., FXXL motifs and KIEELE motifs) in its intracellular structural domains, further blocking the activation signaling pathway of NK cells by dephosphorylating activating signaling molecules (26, 86).
Co-upregulation of these inhibitory receptors is particularly evident in response to chronic antigenic stimulation, especially in NKG2C+ NK cells, where the expression of LAG-3 and PD-1 rises progressively over time (87).
5 Immunotherapy and drug sensitization in the restoration of NK cell function
5.1 Immune checkpoint inhibitors (PD-1/PD-L1)
Immune checkpoint inhibitors (ICIs), especially PD-1/PD-L1 inhibitors, have demonstrated significant clinical efficacy in the treatment of a variety of solid tumors, such as lung, breast, advanced hepatocellular, and pancreatic cancers (6, 88–92). These inhibitors work by blocking the binding of PD-1 to its ligand PD-L1, restoring the anti-tumor activity of NK cells and T cells, and increasing their cytotoxicity and secretion of immune factors such as IFN-γ and TNF-α. In different types of tumors, including HCC, lung cancer, and melanoma, NK cells have shown variable responses to PD-1/PD-L1 inhibitors, influenced by the tumor microenvironment and the extent of PD-1 expression on NK cells. It has been found that not all of the PD-1 in NK cells is derived from endogenous expression, and that NK cells also acquire PD-1 and other inhibitory substances from the membrane of the tumor cells through SLAM receptor-mediated trogocytosis. NK cells can also acquire inhibitory molecules such as PD-1 from the membrane of tumor cells through SLAM receptor-mediated trogocytosis (93). This process results in the suppression of the anti-tumor function of NK cells, which can be reversed by PD-1 inhibitors.
5.2 Joint innovative applications of immunotherapy
Although PD-1/PD-L1 inhibitors show good single-agent efficacy in some tumors, single-agent efficacy is typically lower in metastatic tumors of the hepatobiliary system, and patients experience initial resistance or subsequent decreased drug sensitivity. Therefore, investigators are exploring further enhancement of the anti-tumor effects of NK cells through combination therapies (6, 94–96). The combined blockade of PD-1/PD-L1 inhibitors with other inhibitory pathways has shown promising potential in reversing immune exhaustion during hepatocellular carcinoma treatment. TIM-3, is highly expressed in the TME of HCC, especially on tumor-infiltrating NK cells (e.g., cNK and LrNK cells). Studies have shown that TIM-3, through binding to its ligand phosphatidylserine (PtdSer), induces inhibition of downstream signaling pathways such as PI3K/mTOR, which in turn leads to dysregulation of NK cells and tumor evasion of the immune (6). Through gene ablation, antibody blockade, or lentiviral-mediated TIM-3 disruption experiments, the researchers succeeded in restoring NK cells’ cytokine secretion (e.g., IFN-γ, TNF-α) and cytotoxicity, significantly inhibiting HCC growth (88).
IL-15 and IL-2, two of the most widely studied cytokines, are able to enhance anti-tumor efficacy by stimulating NK cell proliferation and enhancing their effector functions. IL-15 significantly increases NK cell cytotoxicity by activating the downstream JAK/STAT signaling pathway through binding to IL-15Rα (97, 98). IL-2 is also capable of enhancing NK cell activation through the CD25 receptor, but is less used due to its tendency to induce proliferation of Tregs. Currently, researchers are developing modified versions of IL-2, such as mutant IL-2 with selective activation of NK cells, to avoid the side effects of Tregs (99).
6 Conclusion and future
With the development of technologies such as single-cell sequencing, we have the opportunity to further explore the TME and reveal the complex immune cell interactions therein. Although the presence of NK cells in the TME has long been recognized, it remains challenging to effectively manipulate NK cells for therapeutic purposes. NK cell exhaustion in HCC is closely associated with the upregulation of immune checkpoints. Strategies to restore NK cell function, such as immune checkpoint inhibition and cytokine therapy, have shown promise in clinical studies. Notably, CAR-NK cell therapy, with its broad anti-tumor activity and low immune rejection (Figure 1B) (102). It has demonstrated success in hematologic cancers and offers new hope for treating solid tumors like HCC (27, 100, 101). Moving forward, the combination of CAR-NK therapy with other immunotherapies, along with advances in single-cell technologies, will drive further progress in HCC immunotherapy.
Author contributions
YH: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. HL: Formal analysis, Writing – original draft. JL: Formal analysis, Writing – original draft. HW: Formal Analysis, Writing – original draft. AL: Visualization, Writing – original draft. YL: Formal analysis, Writing – original draft. BX: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82260573), National Major Special Science and Technology Project (2017ZX10203207), High-level innovation team and outstanding scholar program in Guangxi Colleges and Universities, “139” projects for training high-level medical science talents from Guangxi (G201903001), The Key Research and Development Project of Guangxi (AB20297009, AA18221001, AB18050020), The Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumor, Ministry of Education/Guangxi, Independent Research Project (GKE2017-ZZ02, GKE2018-KF02, GKE2019-ZZ07), Development and application of medical and health appropriate technology in Guangxi (S2019039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Author correction: hepatocellular carcinoma. Nat Rev Dis Primers. (2024) 10:10. doi: 10.1038/s41572-024-00500-6
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
3. Zhang S, Jiang C, Jiang L, Chen H, Huang J, Gao X, et al. Construction of a diagnostic model for hepatitis B-related hepatocellular carcinoma using machine learning and artificial neural networks and revealing the correlation by immunoassay. Tumour Virus Res. (2023) 16:200271. doi: 10.1016/j.tvr.2023.200271
4. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
6. Yan Y, Su L, Huang S, He Q, Lu J, Luo H, et al. Circadian rhythms and breast cancer: unraveling the biological clock's role in tumor microenvironment and ageing. Front Immunol. (2024) 15:1444426. doi: 10.3389/fimmu.2024.1444426
7. Su L, Luo H, Yan Y, Yang Z, Lu J, Xu D, et al. Exploiting gender-based biomarkers and drug targets: advancing personalized therapeutic strategies in hepatocellular carcinoma. Front Pharmacol. (2024) 15:1433540. doi: 10.3389/fphar.2024.1433540
8. Zhang S, Jiang C, Jiang L, Chen H, Huang J, Zhang J, et al. Uncovering the immune microenvironment and molecular subtypes of hepatitis B-related liver cirrhosis and developing stable a diagnostic differential model by machine learning and artificial neural networks. Front Mol Biosci. (2023) 10:1275897. doi: 10.3389/fmolb.2023.1275897
9. Su K, Shen Q, Tong J, Gu T, Xu K, Li H, et al. Construction and validation of a nomogram for HBV-related hepatocellular carcinoma: A large, multicenter study. Ann Hepatol. (2023) 28:101109. doi: 10.1016/j.aohep.2023.101109
10. Su K, Liu Y, Wang P, He K, Wang F, Chi H, et al. Heat-shock protein 90α is a potential prognostic and predictive biomarker in hepatocellular carcinoma: a large-scale and multicenter study. Hepatol Int. (2022) 16:1208–19. doi: 10.1007/s12072-022-10391-y
11. Su K, Wang F, Li X, Chi H, Zhang J, He K, et al. Effect of external beam radiation therapy versus transcatheter arterial chemoembolization for non-diffuse hepatocellular carcinoma (≥ 5 cm): a multicenter experience over a ten-year period. Front Immunol. (2023) 14:1265959. doi: 10.3389/fimmu.2023.1265959
12. Jiang X, Wang P, Su K, Li H, Chi H, Wang F, et al. Camrelizumab combined with transcatheter arterial chemoembolization and sorafenib or lenvatinib for unresectable hepatocellular carcinoma: A multicenter, retrospective study. Ann Hepatol. (2024) 30:101578. doi: 10.1016/j.aohep.2024.101578
13. Li H, Wu Z, Chen J, Su K, Guo L, Xu K, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. (2023) 23:1537–49. doi: 10.1007/s10238-022-00972-4
14. Li H, Guo L, Su K, Li C, Jiang Y, Wang P, et al. Construction and validation of TACE therapeutic efficacy by ALR score and nomogram: A large, multicenter study. J Hepatocell Carcinoma. (2023) 10:1009–17. doi: 10.2147/JHC.S414926
15. Su K, Guo L, Ma W, Wang J, Xie Y, Rao M, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: A propensity score matching study. Front Immunol. (2022) 13:972503. doi: 10.3389/fimmu.2022.972503
16. Zhang Q, Lou Y, Yang J, Wang J, Feng J, Zhao Y, et al. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut. (2019) 68:2019–31. doi: 10.1136/gutjnl-2019-318912
17. Chen K, Shuen TWH, Chow PKH. The association between tumour heterogeneity and immune evasion mechanisms in hepatocellular carcinoma and its clinical implications. Br J Cancer. (2024) 131:420–9. doi: 10.1038/s41416-024-02684-w
18. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
19. Zhang H, Yang L, Wang T, Li Z. NK cell-based tumor immunotherapy. Bioact Mater. (2024) 31:63–86. doi: 10.1016/j.bioactmat.2023.08.001
20. Chen Y, Tian Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol Immunol. (2021) 18:57–72. doi: 10.1038/s41423-020-00561-z
21. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. (2021) 18:85–100. doi: 10.1038/s41571-020-0426-7
22. Liu K, Cui JJ, Zhan Y, Ouyang QY, Lu QS, Yang DH, et al. Reprogramming the tumor microenvironment by genome editing for precision cancer therapy. Mol Cancer. (2022) 21:98. doi: 10.1186/s12943-022-01561-5
23. Jia H, Yang H, Xiong H, Luo KQ. NK cell exhaustion in the tumor microenvironment. Front Immunol. (2023) 14:1303605. doi: 10.3389/fimmu.2023.1303605
24. Bernard PL, Delconte R, Pastor S, Laletin V, Costa Da Silva C, Goubard A, et al. Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity. J Immunother Cancer. (2022) 10:e004244. doi: 10.1136/jitc-2021-004244
25. Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. (2023) 16:101. doi: 10.1186/s13045-023-01499-1
26. Joller N, Anderson AC, Kuchroo VK. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity. (2024) 57:206–22. doi: 10.1016/j.immuni.2024.01.010
27. Li Y, Basar R, Wang G, Liu E, Moyes JS, Li L, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med. (2022) 28:2133–44. doi: 10.1038/s41591-022-02003-x
28. Zhang H, Lv G, Liu S, Liu D, Wu X-Z. The artificial intelligence watcher predicts cancer risk by facial features. Traditional Med Res. (2022) 7:1. doi: 10.53388/TMR20211227255
29. Liao K, Gong L, Yang Y, He Y, Wang F, Huang Y, et al. A comprehensive review of research progress in Chinese medicines for primary liver cancer treatment. Traditional Med Res. (2022) 7:10. doi: 10.53388/TMR20220207263
30. Man W, Rui S, Huajian C, Xiaohan L, Toru Y, Masaki T, et al. Influence of hydrogel and porous scaffold on the magnetic thermal property and anticancer effect of Fe3O4 nanoparticles. Microstructures. (2023) 3:2023042. doi: 10.20517/microstructures.2023.46
31. He S, Su L, Hu H, Liu H, Xiong J, Gong X, et al. Immunoregulatory functions and therapeutic potential of natural killer cell-derived extracellular vesicles in chronic diseases. Front Immunol. (2023) 14:1328094. doi: 10.3389/fimmu.2023.1328094
32. Santoni A, Carlino C, Gismondi A. Uterine NK cell development, migration and function. Reprod BioMed Online. (2008) 16:202–10. doi: 10.1016/S1472-6483(10)60575-5
33. Stabile H, Fionda C, Gismondi A, Santoni A. Role of distinct natural killer cell subsets in anticancer response. Front Immunol. (2017) 8:293. doi: 10.3389/fimmu.2017.00293
34. Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. (2006) 214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x
35. Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandstrom N, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. (2019) 216:2113–27. doi: 10.1084/jem.20181454
36. Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. (2017) 127:4042–58. doi: 10.1172/JCI90387
37. Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. (1986) 136:4480–6. doi: 10.4049/jimmunol.136.12.4480
38. Dai K, Wu Y, She S, Zhang Q. Advancement of chimeric antigen receptor-natural killer cells targeting hepatocellular carcinoma. World J Gastrointest Oncol. (2021) 13:2029–37. doi: 10.4251/wjgo.v13.i12.2029
39. Xu Z, Huang X. Cellular immunotherapy for hematological Malignancy: recent progress and future perspectives. Cancer Biol Med. (2021) 18:966–80. doi: 10.20892/j.issn.2095-3941.2020.0801
40. Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv. (2020) 4:5868–76. doi: 10.1182/bloodadvances.2020002547
41. Lanier LL. NK cell recognition. Annu Rev Immunol. (2005) 23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526
42. Lenz P, Gessner JE, Sautes C, Schmidt RE, Fc gamma-receptor III. (CD 16) is involved in NK-B cell interaction. Immunobiology. (1996) 196:387–98. doi: 10.1016/S0171-2985(96)80061-1
43. Sanchez CE, Dowlati EP, Geiger AE, Chaudhry K, Tovar MA, Bollard CM, et al. NK cell adoptive immunotherapy of cancer: evaluating recognition strategies and overcoming limitations. Transplant Cell Ther. (2021) 27:21–35. doi: 10.1016/j.bbmt.2020.09.030
44. Chen Y, Lu D, Churov A, Fu R. Research progress on NK cell receptors and their signaling pathways. Mediators Inflammation. (2020) 2020:6437057. doi: 10.1155/2020/6437057
45. Ahmed AA, Keremane SR, Vielmetter J, Bjorkman PJ. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcgammaRIIIa. J Struct Biol. (2016) 194:78–89. doi: 10.1016/j.jsb.2016.02.001
46. Wei C, Xia K, Xie Y, Ye S, Ding Y, Liu Z, et al. Combination of 4-1BB and DAP10 promotes proliferation and persistence of NKG2D(bbz) CAR-T cells. Front Oncol. (2022) 12:893124. doi: 10.3389/fonc.2022.893124
47. Verhaar ER, van Keizerswaard WJC, Knoflook A, Balligand T, Ploegh HL. Nanobody-based CAR NK cells for possible immunotherapy of MICA(+) tumors. PNAS Nexus. (2024) 3:pgae184. doi: 10.1093/pnasnexus/pgae184
48. Seliger B, Koehl U. Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front Immunol. (2022) 13:910595. doi: 10.3389/fimmu.2022.910595
49. Evon DM, Sarkar S, Amador J, Lok AS, Sterling RK, Stewart PW, et al. Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: The PROP UP study. J Hepatol. (2019) 71:486–97. doi: 10.1016/j.jhep.2019.04.016
50. Seller A, Tegeler CM, Mauermann J, Schreiber T, Hagelstein I, Liebel K, et al. Soluble NKG2DLs are elevated in breast cancer patients and associate with disease outcome. Int J Mol Sci. (2024) 25:4126. doi: 10.3390/ijms25074126
51. Boden E, Andreasson J, Hirdman G, Malmsjo M, Lindstedt S. Quantitative proteomics indicate radical removal of non-small cell lung cancer and predict outcome. Biomedicines. (2022) 10:2738. doi: 10.3390/biomedicines10112738
52. Fuertes MB, Domaica CI, Zwirner NW. Leveraging NKG2D ligands in immuno-oncology. Front Immunol. (2021) 12:713158. doi: 10.3389/fimmu.2021.713158
53. Torres N, Regge MV, Secchiari F, Friedrich AD, Spallanzani RG, Raffo Iraolagoitia XL, et al. Restoration of antitumor immunity through anti-MICA antibodies elicited with a chimeric protein. J Immunother Cancer. (2020) 8:e000233. doi: 10.1136/jitc-2019-000233
54. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery. (2020) 19:200–18. doi: 10.1038/s41573-019-0052-1
55. Chi H, Xie X, Yan Y, Peng G, Strohmer DF, Lai G, et al. Natural killer cell-related prognosis signature characterizes immune landscape and predicts prognosis of HNSCC. Front Immunol. (2022) 13:1018685. doi: 10.3389/fimmu.2022.1018685
56. Dong Y, Wan Z, Gao X, Yang G, Liu L. Reprogramming immune cells for enhanced cancer immunotherapy: targets and strategies. Front Immunol. (2021) 12:609762. doi: 10.3389/fimmu.2021.609762
57. Zhou J, Peng H, Li K, Qu K, Wang B, Wu Y, et al. Liver-resident NK cells control antiviral activity of hepatic T cells via the PD-1-PD-L1 axis. Immunity. (2019) 50:403–417 e4. doi: 10.1016/j.immuni.2018.12.024
58. Xu W, Cheng Y, Guo Y, Yao W, Qian H. Targeting tumor associated macrophages in hepatocellular carcinoma. Biochem Pharmacol. (2022) 199:114990. doi: 10.1016/j.bcp.2022.114990
59. Tang J, Zhang S, Jiang L, Liu J, Xu J, Jiang C, et al. Causal relationship between immune cells and hepatocellular carcinoma: a Mendelian randomisation study. J Cancer. (2024) 15:4219–31. doi: 10.7150/jca.96744
60. Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. (2022) 22:557–75. doi: 10.1038/s41568-022-00491-0
61. Bottino C, Walzer T, Santoni A, Castriconi R. Editorial: TGF-β as a key regulator of NK and ILCs development and functions. Front Immunol. (2020) 11:631712. doi: 10.3389/fimmu.2020.631712
62. Wang D, Sun Z, Zhu X, Zheng X, Zhou Y, Lu Y, et al. GARP-mediated active TGF-β1 induces bone marrow NK cell dysfunction in AML patients with early relapse post-allo-HSCT. Blood. (2022) 140:2788–804. doi: 10.1182/blood.2022015474
63. Neo SY, Tong L, Chong J, Liu Y, Jing X, Oliveira MMS, et al. Tumor-associated NK cells drive MDSC-mediated tumor immune tolerance through the IL-6/STAT3 axis. Sci Transl Med. (2024) 16:eadi2952. doi: 10.1126/scitranslmed.adi2952
64. Lee YE, Go GY, Koh EY, Yoon HN, Seo M, Hong SM, et al. Synergistic therapeutic combination with a CAF inhibitor enhances CAR-NK-mediated cytotoxicity via reduction of CAF-released IL-6. J Immunother Cancer. (2023) 11:e006130. doi: 10.1136/jitc-2022-006130
65. Herault A, Mak J, de la Cruz-Chuh J, Dillon MA, Ellerman D, Go M, et al. NKG2D-bispecific enhances NK and CD8+ T cell antitumor immunity. Cancer Immunol Immunother. (2024) 73:209. doi: 10.1007/s00262-024-03795-2
66. Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. (2009) 69:7775–83. doi: 10.1158/0008-5472.CAN-09-2123
67. Nayyar G, Chu Y, Cairo MS. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front Oncol. (2019) 9:51. doi: 10.3389/fonc.2019.00051
68. Zhang K, Zhang M, Luo Z, Wen Z, Yan X. The dichotomous role of TGF-beta in controlling liver cancer cell survival and proliferation. J Genet Genomics. (2020) 47:497–512. doi: 10.1016/j.jgg.2020.09.005
69. Gaggero S, Witt K, Carlsten M, Mitra S. Cytokines orchestrating the natural killer-myeloid cell crosstalk in the tumor microenvironment: implications for natural killer cell-based cancer immunotherapy. Front Immunol. (2020) 11:621225. doi: 10.3389/fimmu.2020.621225
70. Li H, Lan T, Liu H, Liu C, Dai J, Xu L, et al. IL-6-induced cGGNBP2 encodes a protein to promote cell growth and metastasis in intrahepatic cholangiocarcinoma. Hepatology. (2022) 75:1402–19. doi: 10.1002/hep.32232
71. Valentin-Torres A, Day C, Taggart JM, Williams N, Stubblefield SR, Roobrouck VD, et al. Multipotent adult progenitor cells induce regulatory T cells and promote their suppressive phenotype via TGFbeta and monocyte-dependent mechanisms. Sci Rep. (2021) 11:13549. doi: 10.1038/s41598-021-93025-x
72. Chi H, Zhao S, Yang J, Gao X, Peng G, Zhang J, et al. T-cell exhaustion signatures characterize the immune landscape and predict HCC prognosis via integrating single-cell RNA-seq and bulk RNA-sequencing. Front Immunol. (2023) 14:1137025. doi: 10.3389/fimmu.2023.1137025
73. Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. (2004) 25:47–52. doi: 10.1016/j.it.2003.10.012
74. Hu X, Jia X, Xu C, Wei Y, Wang Z, Liu G, et al. Downregulation of NK cell activities in Apolipoprotein C-III-induced hyperlipidemia resulting from lipid-induced metabolic reprogramming and crosstalk with lipid-laden dendritic cells. Metabolism. (2021) 120:154800. doi: 10.1016/j.metabol.2021.154800
75. Tiwari A, Trivedi R, Lin SY. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J BioMed Sci. (2022) 29:83. doi: 10.1186/s12929-022-00866-3
76. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. (2015) 125:3384–91. doi: 10.1172/JCI80011
77. Stenger TD, Miller JS. Therapeutic approaches to enhance natural killer cell cytotoxicity. Front Immunol. (2024) 15:1356666. doi: 10.3389/fimmu.2024.1356666
78. Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. (2020) 11:167. doi: 10.3389/fimmu.2020.00167
79. Zhang N, Yang X, Piao M, Xun Z, Wang Y, Ning C, et al. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. biomark Res. (2024) 12:26. doi: 10.1186/s40364-023-00535-z
80. Dixon KO, Lahore GF, Kuchroo VK. Beyond T cell exhaustion: TIM-3 regulation of myeloid cells. Sci Immunol. (2024) 9:eadf2223. doi: 10.1126/sciimmunol.adf2223
81. Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. (2021) 595:101–6. doi: 10.1038/s41586-021-03626-9
82. Ganjalikhani Hakemi M, Jafarinia M, Azizi M, Rezaeepoor M, Isayev O, Bazhin AV. The role of TIM-3 in hepatocellular carcinoma: A promising target for immunotherapy? Front Oncol. (2020) 10:601661. doi: 10.3389/fonc.2020.601661
83. Chu X, Tian W, Wang Z, Zhang J, Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials. Mol Cancer. (2023) 22:93. doi: 10.1186/s12943-023-01800-3
84. Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. (2020) 8:e000957. doi: 10.1136/jitc-2020-000957
85. Schnell A, Bod L, Madi A, Kuchroo VK. The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. (2020) 30:285–99. doi: 10.1038/s41422-020-0277-x
86. Jiang J, Huang H, Chen R, Lin Y, Ling Q. Immunotherapy for hepatocellular carcinoma recurrence after liver transplantation, can we harness the power of immune checkpoint inhibitors? Front Immunol. (2023) 14:1092401. doi: 10.3389/fimmu.2023.1092401
87. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
88. Yan Y, Li S, Su L, Tang X, Chen X, Gu X, et al. Mitochondrial inhibitors: a new horizon in breast cancer therapy. Front Pharmacol. (2024) 15:1421905. doi: 10.3389/fphar.2024.1421905
89. Chi H, Su L, Yan Y, Gu X, Su K, Li H, et al. Illuminating the immunological landscape: mitochondrial gene defects in pancreatic cancer through a multiomics lens. Front Immunol. (2024) 15:1375143. doi: 10.3389/fimmu.2024.1375143
90. Liang H, Lu Q, Yang J, Yu G. Supramolecular biomaterials for cancer immunotherapy. Res (Wash D C). (2023) 6:0211. doi: 10.34133/research.0211
91. Jiang X, Zhang Y, Wang H, Wang Z, Hu S, Cao C, et al. In-depth metaproteomics analysis of oral microbiome for lung cancer. Res (Wash D C). (2022) 2022:9781578. doi: 10.34133/2022/9781578
92. Ji X, Tian X, Feng S, Zhang L, Wang J, Guo R, et al. Intermittent F-actin perturbations by magnetic fields inhibit breast cancer metastasis. Res (Wash D C). (2023) 6:0080. doi: 10.34133/research.0080
93. Ramezani F, Panahi Meymandi AR, Akbari B, Tamtaji OR, Mirzaei H, Brown CE, et al. Outsmarting trogocytosis to boost CAR NK/T cell therapy. Mol Cancer. (2023) 22:183. doi: 10.1186/s12943-023-01894-9
94. Meng F, Li L, Lu F, Yue J, Liu Z, Zhang W, et al. Overexpression of TIGIT in NK and T cells contributes to tumor immune escape in myelodysplastic syndromes. Front Oncol. (2020) 10:1595. doi: 10.3389/fonc.2020.01595
95. William C, Wangmo C, Ranjan A. Unravelling the application of machine learning in cancer biomarker discovery. Cancer Insight. (2023) 2:15. doi: 10.58567/ci02010001
96. Zeng S, Tang Q, Xiao M, Tong X, Yang T, Yin D, et al. Cell membrane-coated nanomaterials for cancer therapy. Mater Today Bio. (2023) 20:100633. doi: 10.1016/j.mtbio.2023.100633
97. Rautela J, Huntington ND. IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol. (2017) 44:1–6. doi: 10.1016/j.coi.2016.10.004
98. Kim WS, Kim MJ, Kim DO, Byun JE, Huy H, Song HY, et al. Suppressor of cytokine signaling 2 negatively regulates NK cell differentiation by inhibiting JAK2 activity. Sci Rep. (2017) 7:46153. doi: 10.1038/srep46153
99. Chen Z, Tong L, Neo SY, Li S, Gao J, Schlisio S, et al. CD25(bright) NK cells display superior function and metabolic activity under regulatory T cell-mediated suppression. Oncoimmunology. (2023) 12:2175517. doi: 10.1080/2162402X.2023.2175517
100. Du S, Yan J, Xue Y, Zhong Y, Dong Y. Adoptive cell therapy for cancer treatment. Explor (Beijing). (2023) 3:20210058. doi: 10.1002/EXP.20210058
101. Jana D, Zhao Y. Strategies for enhancing cancer chemodynamic therapy performance. Explor (Beijing). (2022) 2:20210238. doi: 10.1002/EXP.20210238
Keywords: hepatocellular carcinoma, NK cell exhaustion, immunotherapy, immune checkpoint blockade, drug sensitivity, tumor microenvironment, oncology drug innovation
Citation: Huang Y, Liao H, Luo J, Wei H, Li A, Lu Y and Xiang B (2025) Reversing NK cell exhaustion: a novel strategy combining immune checkpoint blockade with drug sensitivity enhancement in the treatment of hepatocellular carcinoma. Front. Oncol. 14:1502270. doi: 10.3389/fonc.2024.1502270
Received: 26 September 2024; Accepted: 20 December 2024;
Published: 21 January 2025.
Edited by:
Zhiwen Luo, Fudan University, ChinaReviewed by:
Matthew McMillin, Baylor College of Medicine, United StatesJun Zhu, Fourth Military Medical University, China
Kaige Chen, Wake Forest University, United States
Ke Xu, Chongqing General Hospital, China
Copyright © 2025 Huang, Liao, Luo, Wei, Li, Lu and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangde Xiang, eGlhbmdiYW5nZGVAZ3htdS5lZHUuY24=
 Yuxiang Huang
Yuxiang Huang Hengjian Liao
Hengjian Liao Yujie Lu
Yujie Lu