- 1Leishmaniasis Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 2Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 3Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 6Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran
- 7Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran
- 8School of Medicine, Behbahan Faculty of Medical Sciences, Behbahan, Iran
- 9School of Medicine, Razi Hospital, Ilam University of Medical Sciences, Ilam, Iran
Helicobacter pylori (H. pylori) infection is a typical microbial agent that interferes with the complex mechanisms of gastric homeostasis by disrupting the balance between the host gastric microbiota and mucosa-related factors, ultimately leading to inflammatory changes, dysbiosis, and gastric cancer (GC). We searched this field on the basis of PubMed, Google Scholar, Web of Science, and Scopus databases. Most studies show that H. pylori inhibits the colonization of other bacteria, resulting in a less variety of bacteria in the gastrointestinal (GI) tract. When comparing the patients with H. pylori–positive and H. pylori–negative GC, the composition of the gastric microbiome changes with increasing abundance of H. pylori (where present) in the gastritis stage, whereas, as the gastric carcinogenesis cascade progresses to GC, oral and intestinal-type pathogenic microbial strains predominate. H. pylori infection induces a premalignant milieu of atrophy and intestinal metaplasia, and the resulting change in gastric microbiota appears to play an important role in gastric carcinogenesis. The effect of H. pylori–induced GC on GI microbiota is discussed in this review.
1 Introduction
Helicobacter pylori (H. pylori) infection causes chronic gastritis, which can progress to severe gastroduodenal pathologies, including peptic ulcer, gastric cancer (GC), and gastric mucosa–associated lymphoid tissue (MALT) lymphoma (1). H. pylori is usually transmitted in childhood and persists for life if untreated. The infection affects around half of the population in the world, but prevalence varies according to location and sanitation standards (2). H. pylori has unique properties to colonize gastric epithelium in an acidic environment. The pathophysiology of H. pylori infection is dependent on complex bacterial virulence mechanisms and their interaction with the host immune system and environmental factors, resulting in distinct gastritis phenotypes that determine possible progression to different gastroduodenal pathologies (3). The causative role of H. pylori infection in GC development presents the opportunity for preventive screen-and-treat strategies (4). Invasive, endoscopy-based, and non-invasive methods, including breath, stool, and serological tests, are used in the diagnosis of H. pylori infection. Their use depends on the specific individual patient history and local availability (5). H. pylori treatment consists of a strong acid suppressant in various combinations with antibiotics and/or bismuth (6). The dramatic increase in resistance to key antibiotics used in H. pylori eradication demands antibiotic susceptibility testing, surveillance of resistance, and antibiotic stewardship (7).
GC, which refers to the occurrence of cancer in the stomach cell line, is one of the leading causes of cancer-related deaths and ranks as the fifth most common cancer globally, posing a significant global health challenge (8). GC, being multifactorial cancer, has risk factors that include a family history of GC in first-degree relatives, dietary habits, gender, age, race, H. pylori infection, nutritional status, and a history of invasive diseases such as lymphoma- or gastric-related procedures (9, 10). Studies have suggested the potential role of H. pylori and other bacterial genera in the progression of GC (11, 12). H. pylori, as a destructive member of the gastric microbiota, raises global health concerns due to its association with GC (13). This bacterium recruits neutrophils and lymphocytes to the gastric mucus, stimulating the production of reactive oxygen species (ROS) and inflammatory cytokines through the action of the CagA (cytotoxin-associated gene A) protein. This process gradually stimulates cell proliferation, leading to the development of GC and alterations in the composition of the gastrointestinal (GI) tract microbiota (14–18).
This review represents, to the best of our knowledge, the first comprehensive analysis of the interaction between GC and H. pylori, focusing on their impact on the microbiota. The current study highlights variations in microbiota alterations among individuals with GC, with or without H. pylori infection. Investigating the correlation between the presence of H. pylori and microbiota changes in patients with GC is crucial for developing more effective therapies for H. pylori infection in individuals with GC who are H. pylori–positive. Distinct differences exist between individuals with GC who harbor H. pylori and those who do not. Through multi-omics studies that analyze changes in microbial profiles and metabolite alterations, a diverse range of compositions in the GI tract microbiota has been observed in patients with GC depending on the presence or absence of H. pylori. In fact, individuals with H. pylori–positive GC exhibit very low variation in gastric microbiota and significant changes in GC microbiota, including Firmicutes, Proteobacteria, Bacteroides, Streptococcus, Lactobacillus, Escherichia, and Shigella. Eradicating H. pylori eventually restores the disrupted microbiota in patients with GC (19). Conversely, patients with GC without H. pylori are closely linked to high microbial diversity in the gastric microbiota, a significant increase in Haemophilus and Streptococcus, and an elevation in the abundance of metabolites (20–23). All these changes may be due to the inhibitory effects of H. pylori on the colonization of other microorganisms (24, 25). In addition, the four most dominant strains of gut microbiota in individuals with GC with H. pylori strains, including Enterococcus, Escherichia-Shigella, Bacteroides, and Lactobacillus, contribute to the progression of GC by increasing damage to cancerous tissue (26–28), the levels of tumor necrosis factor–alpha (TNF-α) (29), and unfavorable metabolites (30). Although the relationship between microbiota and metabolites in individuals with GC with H. pylori has not been thoroughly described, it is evident that the secondary metabolites produced by H. pylori elevate the metabolism of citric acid and carbohydrates in the gastric tissue of patients with GC (31), exacerbate inflammation in the gastric tissue by stimulating the activation of C-type lectin receptors (32), and inhibit the interferon-alpha (IFN-α) signaling pathway to evade the immune system (33). The importance of analyzing the differences in metabolic activity between GC cases with and without H. pylori reflects their potential to serve as candidate markers for distinguishing which patients with GC harbor H. pylori or not (20). In addition to alterations in microbiota and metabolic changes, studies indicate histopathological changes such as peptic ulcers and epithelial changes in the gastric mucosa of patients with GC with H. pylori. As mentioned regarding the metabolic changes, these histopathological changes are suitable indicators to determine the presence of H. pylori in GC cases. These histopathological changes were not present in the gastric tissue of patients with GC who were not colonized by H. pylori (34). The primary aim of this study was to review the effect of H. pylori–induced GC on GI microbiota.
2 Search strategy
We collected original and review articles in this field by searching through PubMed, Google Scholar, Web of Science, and Scopus databases for English language literature published up to 2024. The search was conducted on the basis of “Gastric or stomach cancer,” “Helicobacter pylori–induced gastric cancer,” “H. pylori eradication” AND “Gut microbiota,” or “Microbiome” as keywords. Studies that reported the role of H. pylori–induced GC on GI microbiota and changes in gastric microbiota following successful H. pylori eradication were enrolled.
3 Gastrointestinal microbiota in patients with gastric cancer
Microbial diversity and composition change in GC (35). Studies suggest that the diversity and composition of the gastric microbiota differ among patients at different histological stages of GC (11, 36). This issue emphasizes that the imbalance of the gastric microbiota is dynamic. The GC microbiome appears to improve with oral and intestinal bacterial taxa (37). Bacterial genera such as Lactococcus, Bacillus, Prevotella, Veillonella, Leptotrichia (38), Achromobacter, Citrobacter, Rhodococcus, Phyllobacterium (11), Peptostreptococcus, Parvimonas, Slackia, and Dialister (39), which commonly colonize the oral cavity, are enriched in the GC microbiota. Reports indicate diverse geographic regions where bacterial species of intestinal commensals, including Lactobacillus (11, 38, 39), Streptococcaceae (39, 40), Staphylococcus (38, 41), Clostridium (11, 38), and Fusobacterium (38, 39), are consistently enriched in GC. There are controversial results regarding the abundance of Streptococcus and Prevotella between GC and non-cancer patients. Studies indicate that they are both increased (38, 42) and decreased (11, 43) in the GC microbiota. The depletion of Neisseria, Comamonadaceae, Acinetobacter (39), Vogesella, and Helicobacter (11, 40) was identified in the GC microbiota. Neisseria (11) was a genus that showed a decrease in the microbiota of patients with GC from regions with low GC risk. However, Veillonella and Leptotrichia increased in relative abundance in the microbiota of patients with GC from areas with high GC risk (11, 44). Gunathilake et al. showed enrichment of H. pylori, Propionibacterium acnes, and Prevotella copri and a decrease in the abundance Lactococcus lactis in the gastric microbiota of patients with GC (45). In a study from Portugal, Ferreira et al. demonstrated that there was a significant decrease in Helicobacter, Neisseria, Streptococcus, and Prevotella and an increase in abundance Lactobacillus, Citrobacter, Clostridium, Achromobacter, and Rhodococcus in cancer versus non-cancer. The profile of the GC mucosal microbiota obtained from the 16S rRNA gene has shown metabolic activities and biochemistry such as carbohydrate metabolism, carbohydrate digestion, absorption (11, 38, 39), membrane transport (11, 43), and nucleotide/purine metabolism (39, 43) to be significantly increased in GC by enhancing the counts of nitrate-reducing bacteria. Consequently, the functions of nitrate reductase (NR) and nitrite reductase (NiR) are significantly enriched in the microbiota of GC subjects, aligning with Correa’s hypothesis (11, 43, 46).
4 Role of H. pylori–induced gastric cancer on gastrointestinal microbiota
During the different stages of GC, the diversity and composition of the bacterial microbiome vary significantly. The microbial complex shows a strong correlation with precancerous lesion stages such as atrophic gastritis (AG) and dysplasia. GC and precancerous lesions can be identified by harboring distinguishable bacterial taxa. Furthermore, the microbial structure changes on the basis of the site in patients with GC; for example, Proteobacteria are abundant in the gastric mucosa, whereas Firmicutes have been found abundantly in gastric juice (36). The reduction in gastric mucosal microbiota diversity, due to the widespread colonization of H. pylori, must be considered a determining factor in the association between gastric precancerous lesions and the gastric microbiota. A microbial model derived from H. pylori–positive gastric biopsies and stool samples serves as a critical predictor of precancerous lesions. This is supported by reports of lower bacterial taxa diversity in gastric biopsies from H. pylori–infected individuals compared to those from H. pylori–negative participants. Among H. pylori–infected individuals, there was an increased abundance (from 0.91% to 68.22%) of Epsilonbacteraeota (the fifth validly described class of the phylum Proteobacteria) and decreased levels of Firmicutes (27.55% to 8.18%) and Proteobacteria (36.53% to 13.97%). Moreover, the ratio of Epsilonbacteraeota remained unchanged in stool and gastric juice samples from the H. pylori–positive groups. Consequently, H. pylori is associated with differences in gastric mucosal bacterial diversity between H. pylori–positive and H. pylori–negative samples, underscoring the role of GI bacteria in the development of gastric precancerous lesions (47).
Exploring the potential mechanisms and dysbiosis of GI microbial composition GC involving H. pylori infection has revealed that richness indexes increase after the eradication of H. pylori infection, with approximately 18 microbial taxa altered in the gastric tract sample groups. Additionally, the dysbiotic microbiota in gastric mucosal biopsies correlate with advanced AG, intestinal metaplasia (IM), and dysplasia, and this dysbiosis may be reversed by eradicating H. pylori. Notably, a study observed the coexistence of Helicobacter, Fusobacterial, Neisseria, Prevotella, Veillonella, and Rothia in cases where H. pylori was absent in healthy superficial gastritis. It can be concluded that dysbiosis of microbial diversity contributes to carcinogenesis (19).
In addition to the remarkable diversification and increased interaction of GI bacterial composition following infection with resistant H. pylori, consideration must be given to the metabolic pathways and enrichment of infectious diseases. This aligns with the findings of the study by Liu et al. (2022), wherein energy metabolism, bacterial secretion systems, lipopolysaccharide synthesis, protein folding, and associated processing are enriched in H. pylori–positive groups. Furthermore, the imbalance in gastric mucosal microbiota manifests in the inhibition of beneficial bacterial growth, such as Lactobacillus. Patients with refractory H. pylori infections may be at higher risk of developing GC compared to other groups (48).
Conversely, virulent H. pylori strains may be crucial for gastric colonization, but not sufficient for the development of GC and ulcers. Distinct microbial communities exist not only in the lower GI tract of H. pylori–infected patients but also that in non-infected individuals. Bacteroides and Bifidobacterium colonize the gut tract of H. pylori–positive patients with lower frequency. Notably, H. pylori–infected patients experiencing stomachache exhibit a lower abundance of Bifidobacterium species, which may be directly associated with gastric ulcers and cancer (49).
The gastric microbial composition profile of patients provides insight into the dysbiotic cancer-associated microbiota. Typically, gastric carcinoma is triggered by H. pylori infection, which reduces acid secretion, allowing for the growth of a gastric microbiome with a different composition. This diversification exacerbates the invasion of bacteria into the gastric mucosa and leads to malignancy. By measuring alpha-diversity (α-diversity) using the Shannon index, it has been found that H. pylori influences patients with gastric carcinoma by decreasing the microbial population and enhancing the composition of other bacterial genera, especially intestinal commensals, in comparison to chronic cases. Overall, the gastric microbiota is dominated by five phyla: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Fusobacteria.
However, whereas the mentioned phyla colonize both GC and chronic gastritis, patients with gastric carcinoma exhibit an over-presentation of Actinobacteria and Firmicutes, along with a lower abundance of Bacteroidetes and Fusobacteria (11). H. pylori alters the overall structure and composition of the microbiota in the specific stomach microenvironment of GC. In patients with both histopathologically H. pylori–positive and H. pylori–negative statuses, there is a tendency for microbial diversity reduction (lower in H. pylori–positive and higher in H. pylori–negative cases). Dominant phyla in the gastric microbiota of H. pylori–positive groups in normal and peri-tumoral tissues are Proteobacteria and Firmicutes in high proportions. Bacterial composition decreases in the peritumoral and tumoral microhabitats (12). It is noteworthy that certain known oral microbiomes, such as Parvimonas micra, Parvimonas stomatis, Fusobacterium nucleatum, and Gemella, are likely associated with colorectal cancer and may contribute to GC. Reports indicate an abundance of oral microbiomes in GC. The differences in bacterial composition and interactions play a pivotal role in determining the total microbiota assemblage at each stage of gastric carcinoma. Significant changes in microbial diversity are observed in the richness of microbiota between GC, superficial gastritis, and IM, validating the presence of microbial dysbiosis in gastric carcinoma. The lack of compatibility may stem from various background factors, such as gender, age, ethnicity, and the involvement of H. pylori. Consequently, there are fewer interactions among gastric microbes at all stages, with notably more interactions between gastric microbes in H. pylori–negative samples than in H. pylori–positive groups. The interactions of H. pylori with gastric microbes are studied as co-occurring interactions with Methylobacillus, Prevotella, and Arthrobacter, along with co-excluding interactions with Firmicutes (Ruminococcus, Bacillales, and Lactobacillus) (39).
Gastric mucosa–associated lymphoid tissue (MALT) lymphoma is correlated with both the presence and absence of H. pylori. Additionally, the microbiota in patients with MALT lymphoma is observed even in the absence of H. pylori. The microbial composition in the gastric mucosal flora of patients with H. pylori–negative MALT lymphoma significantly decreases. This might suggest that the balance of bacteria in the gastric mucosa is disrupted in patients with MALT lymphoma without the presence of H. pylori. The genera Burkholderia and Sphingomonas are identified abundantly in patients with MALT lymphoma compared to those in control groups. Therefore, Burkholderia and Sphingomonas genera may contribute to the progression of MALT lymphoma. In contrast, the enrichment of Prevotella and Veillonella is lower (50). The weighted principal coordinate analysis demonstrates that the colonization of H. pylori increasingly alters the structure of five genera of microbiota (Proteobacteria, Bacteroides, Fusobacteria, Actinobacteria, and Firmicutes); however, it has little impact on the proportion of other members. Thus, alterations in the GC microbiota by increasing bacterial quantity and diversifying the microbial population could promote cancer-related activities (51). The composition of the gastric microbiota in patients with GC infected with H. pylori is shown in Table 1.
5 Impact of H. pylori eradication on gastrointestinal microbiota
5.1 Impact of H. pylori eradication on the gastric microbiome
For many years, H. pylori eradication has been utilized; however, the impact of this eradication on the normal stomach microbiota remains unknown. Eradicating H. pylori reduces the risk of GC, with this effect becoming more pronounced with age. Currently, eradication is targeted at preventing the development of GC (53). The acid-suppressive effects of proton pump inhibitors (PPIs) and the bactericidal activity of antibiotics form the basis of H. pylori eradication therapy. Antibiotics directly and powerfully affect all bacteria in the stomach (54). The strong acid-inhibitory action of PPIs can rapidly raise the stomach’s pH, limiting the influence of gastric acid on eradicating transient bacteria, which is not conducive to digestion and results in various fluctuations in substrate levels (55). Sung et al. reported that a 1-week combined treatment of omeprazole, amoxicillin, and clarithromycin (OAC) effectively eliminated H. pylori, leading to a significant increase in stomach bacterial diversity after 1 year. In the absence of H. pylori, there was a notable shift in bacterial co-occurrence, along with a distinct cluster of oral microorganisms. Levels of Haemophilus, Neisseria, and Actinobacillus were significantly reduced following OAC therapy (56). Additionally, according to Mao et al., stomach microflora diversity and relative quantities were greatly reduced following H. pylori infection. However, after successful eradication, the stomach microbiota might be partially restored to an H. pylori–negative state (57). Mao et al. also noted that, after H. pylori infection, there was a significant decrease in the diversity and relative quantity of stomach microflora. However, following successful eradication, the stomach microbiota may be partially restored to an H. pylori–negative condition (58).
H. pylori exhibits an inverse relationship with the diversity of stomach microbiota. Following successful eradication of H. pylori, the phylum and genus composition of stomach flora can be restored to levels comparable to those of H. pylori–negative patients, leading to an increase in the bacterial diversity index (59). H. pylori has an inverse relationship with the diversity of stomach microbiota. Following successful H. pylori eradication, the phylum and genus composition of the stomach flora can be restored to levels equivalent to H. pylori–negative patients, and the bacterial diversity index rises (60). Research conducted in China and Hong Kong revealed that only H. pylori–related taxa were significantly decreased following eradication. After eradication, Firmicutes, Bacteroidetes, Actinobacteria, Cyanobacteria, and Fusobacteria emerged as the most abundant taxa. These observations indicate that H. pylori serves as the primary disruptor of stomach commensal homoeostasis (19, 60). Notably, a significant increase in the relative abundance of Anaerofustis was observed 6 months after eradication, potentially due to the anti-inflammatory and antimicrobial properties of butyrate-producing bacteria. This increase may contribute to restoring the delicate balance between the human host and the perturbed microbiome (61). The long-term study underscores the potential role of stomach bacteria in the formation and maintenance of precancerous gastric lesions in the absence of H. pylori. These findings suggest that they could serve as therapeutic targets for the prevention of gastric carcinogenesis.
5.2 Impact of H. pylori eradication on the gut microbiome
The literature on the changes in the gut microbiota caused by H. pylori eradication is best categorized as those that investigate immediate, short-term, and long-term impacts. The term “immediate effects” refers to those observed within 2 weeks after the treatment’s completion (62). In a study of 70 patients who underwent bismuth-based triple treatment for 14 days, it was discovered that, on day 14, α-diversity had reduced, and the Bacteroides-to-Firmicutes ratio had fallen from 0.98 to 0.3417 (63). The short-term effects of eradication treatment are those measured within 2–3 months of therapy completion (62). Short-term trials investigated triple therapy with PPI, amoxicillin, and clarithromycin, as well as bismuth-based quadruple therapy for 7 days. Three months following eradication treatment, bacterial diversity was consistently changed. Firmicutes were less common in individuals who had triple treatment, but Proteobacteria were more prevalent. Proteobacteria relative abundance rose in bismuth-treated individuals, but Bacteroidetes and Actinobacteria relative abundance decreased (63–65). Jakobsson et al. revealed that short-term antibiotic treatment for H. pylori eradication delivered a profound insult to the GI flora and resulted in a perturbed oral and colonic microbiome observed one week after treatment and persisting up to four years later. Short-term and long-term changes in gut microbiota after H. pylori eradication are reviewed in Figure 1 (65).
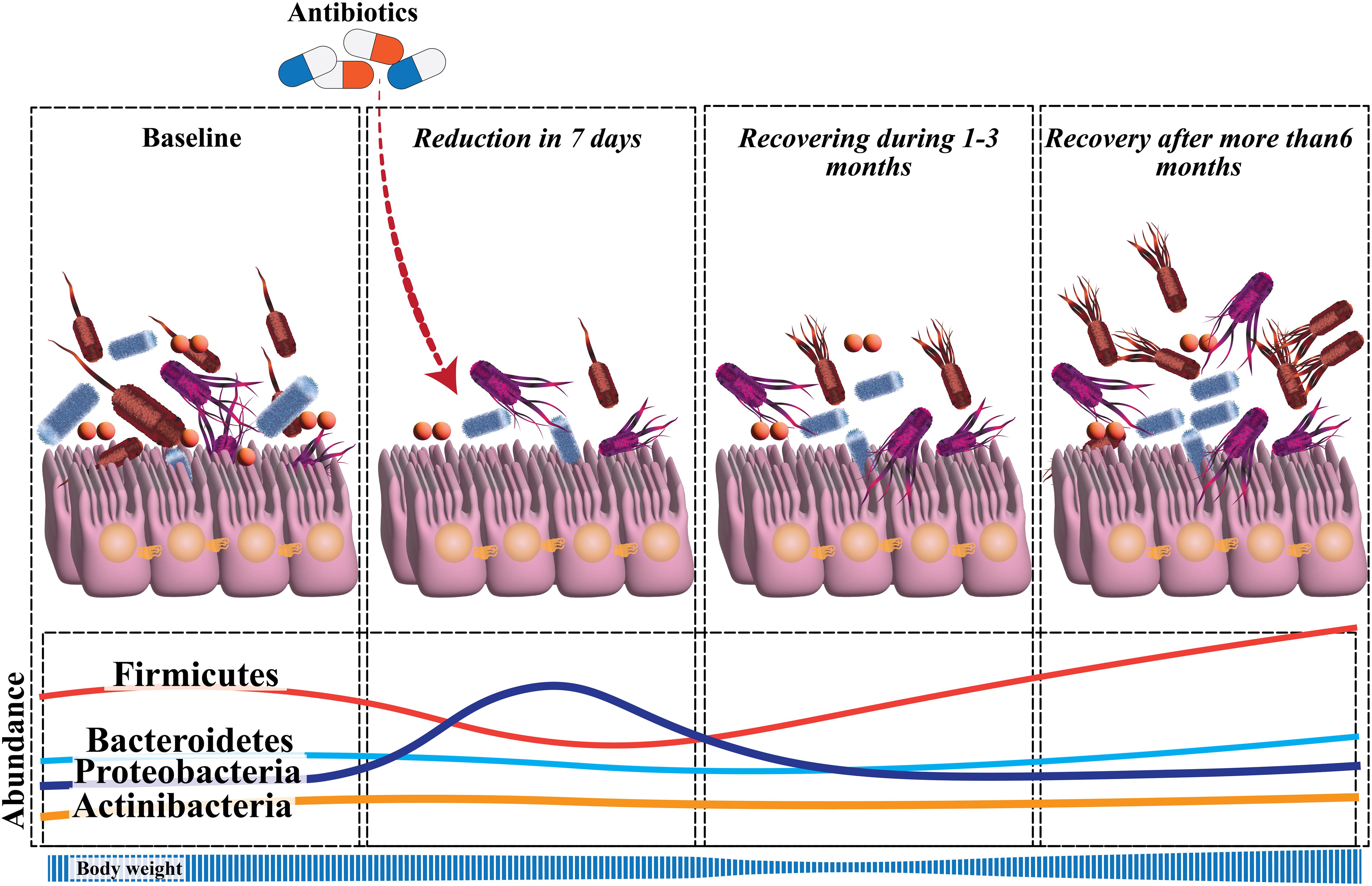
Figure 1. Significant perturbation of the diversity and composition of gut microbiota develops soon after H. pylori eradication. The microbial diversity recovers during the follow-up, but there is not yet sufficient data to confirm the changes in alpha-diversity that occur at the long-term follow-up. There is a reduction in Actinobacteria, relative to baseline, throughout the follow-up. Proteobacteria have a higher relative abundance at the short-term follow-up, which then returns to normal. Only during the long-term follow-up, a reduction in Bacteroidetes and a rise in Firmicutes were evident.
The findings of a study, which indicated that the diversity of microbiota tends to decrease in the short term following eradication before returning to baseline, were consistent with the results of other investigations (63, 66, 67). Long-term studies focus on assessing the effects of eradication therapy on the gut microbiota 6 months or more after treatment. Descriptive studies have examined the long-term impacts of eradication treatment on the gut flora. By 1 year, the α-diversity and β-diversity of the microbiota, along with the relative abundance of all phyla, had returned to pre-treatment levels; however, notable alterations were observed at the genus level (61, 65, 68). More than half of the studies on the impact of H. pylori on the gut microbiota have entailed sub-analyses of the effects of eradication therapy on the gut microbiota (63, 66, 69). A recent comprehensive analysis of 24 studies investigating the influence of H. pylori eradication on the gut microbiota revealed that the majority of studies have shown a significant decrease in the α-diversity of the gut microbiota shortly after eradication, with no further changes reported beyond 6 months after H. pylori eradication. Additionally, Proteobacteria abundance increased during short-term follow-ups, whereas Lactobacillus abundance decreased; Enterobacteriaceae and Enterococcus abundance increased during short-term and intermediate follow-ups (70).
Recent research examining the long-term impacts of H. pylori eradication has revealed that the diversity of the gut microbiota was restored to a baseline state over the 2 years following eradication, with minimal differences in the relative abundances of microbial species at the genus level before and after eradication. However, there were slight variations in taxonomic diversity before and after eradication (71). The interaction between H. pylori and the GI microbiota is depicted in Figure 2. Additionally, according to Tao et al., the model of α-diversity shifts during H. pylori infection, and eradication therapy is illustrated in Figure 3 (72). Future research should focus on investigating the microbiome over time, from pre-eradication to post-eradication and during follow-up, in relation to the development of lesions.
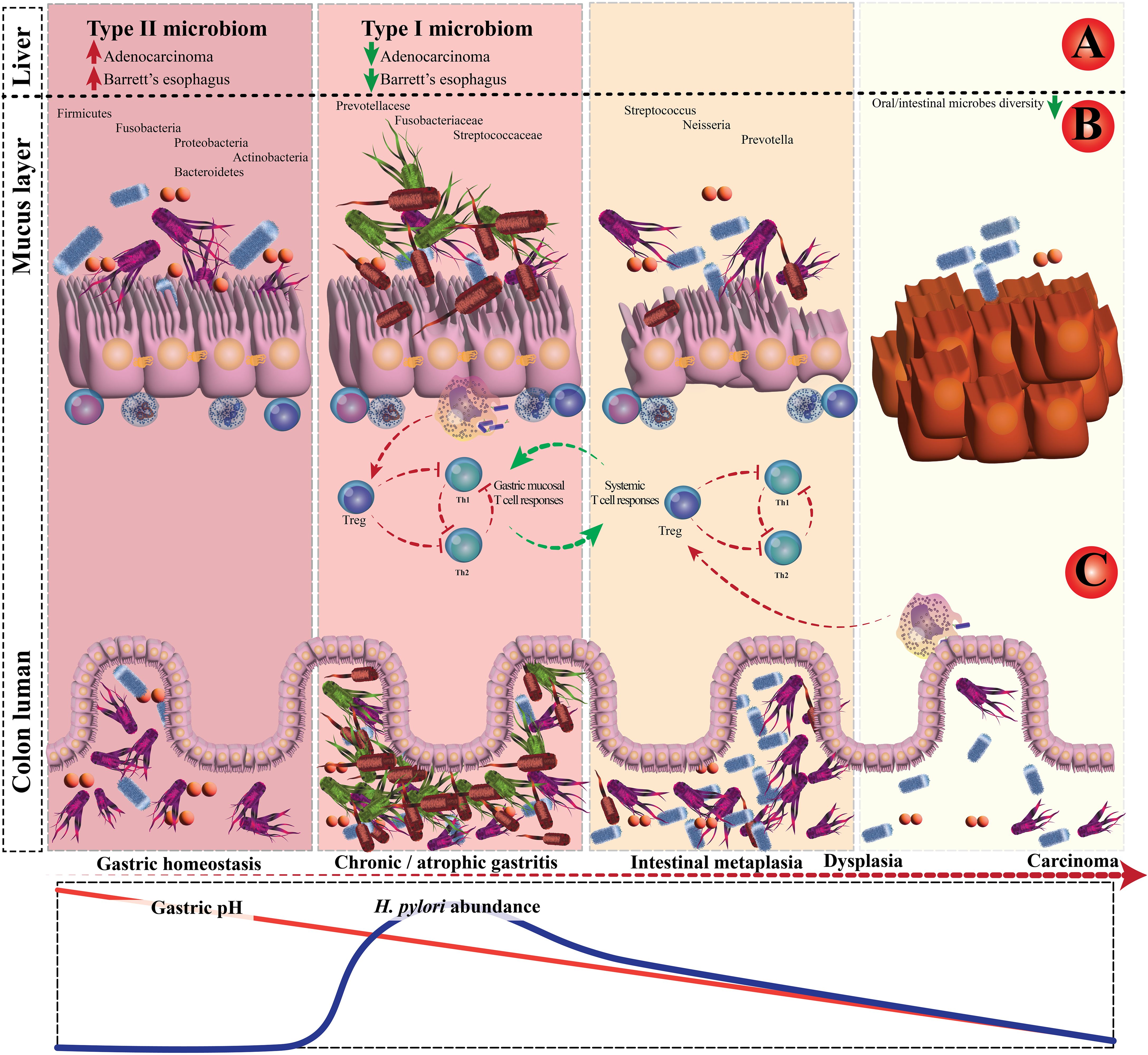
Figure 2. The interplay between Helicobacter pylori and gastrointestinal microbiota. (A) Case-control and epidemiology studies demonstrated that H. pylori infection is inversely associated with Barrett’s esophagus and esophageal adenocarcinoma. (B) Schematic plot presentation of the influence of H. pylori on gastric and colonic microbiota. (C) In chronic H. pylori infections, the H. pylori–experienced dendritic cells retain a semi-mature phenotype.
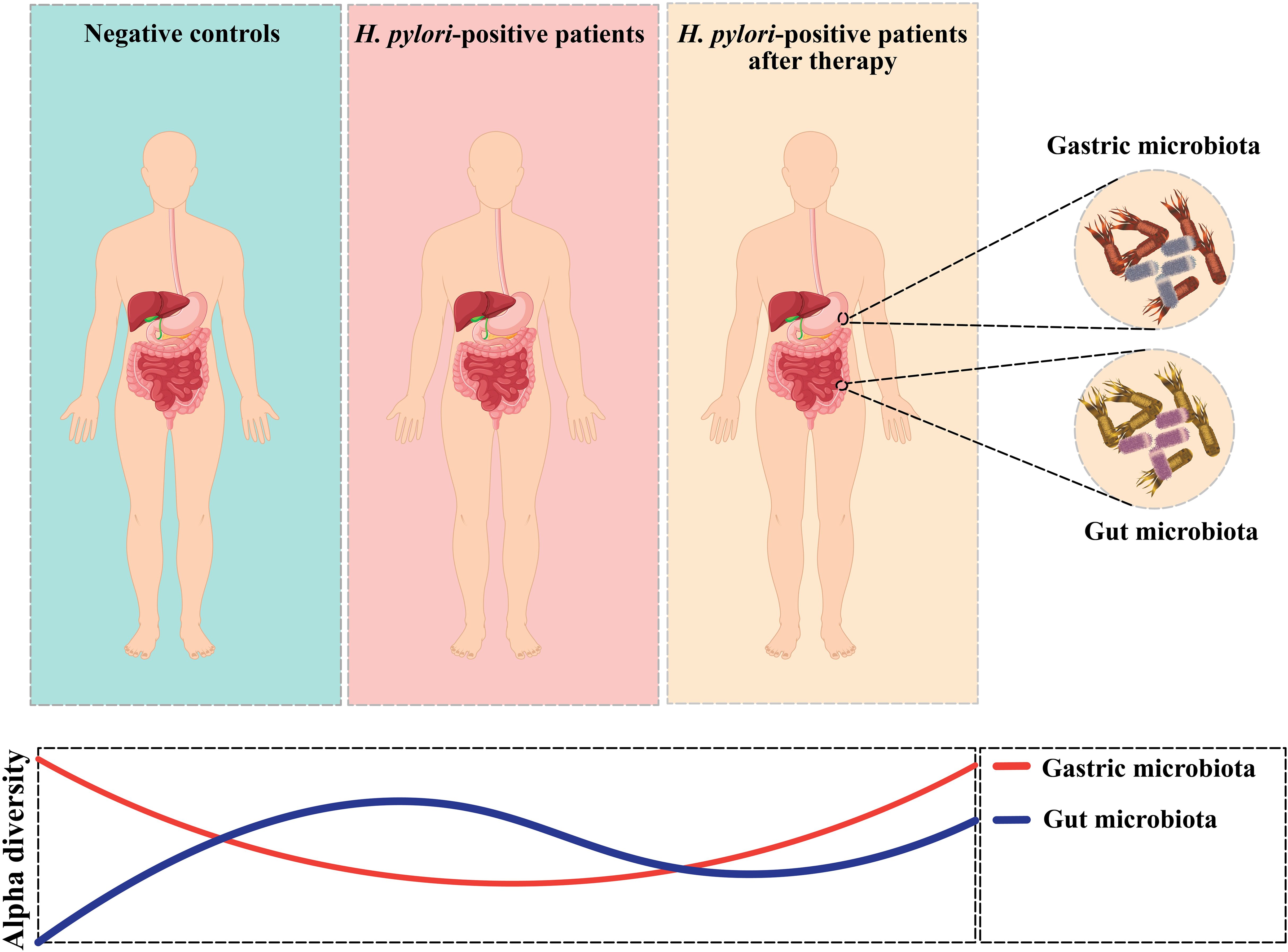
Figure 3. Alpha-diversity shifts in gastric microbiota and Alpha-diversity shifts in gut microbiota.
6 Effect of H. pylori eradication on gastric cancer prevention
Several studies demonstrate that individuals who tested positive for H. pylori were three to six times more likely to develop GC in comparison to uninfected controls. So, it suggested that screening for and eliminating H. pylori is a cost-efficient method for averting GC in individuals in their middle ages (73–75). The recognition of this bacterium as a disease-causing agent prompted certain authors to advocate for diverse programs aimed at eradicating the infection within the population, as a means of curtailing the progression of the disease (76). Many studies of randomized clinical trials (RCTs) showed that eradicating H. pylori leads to a decrease in GC incidence among healthy and undergone endoscopic resection of early GC (Table 2).
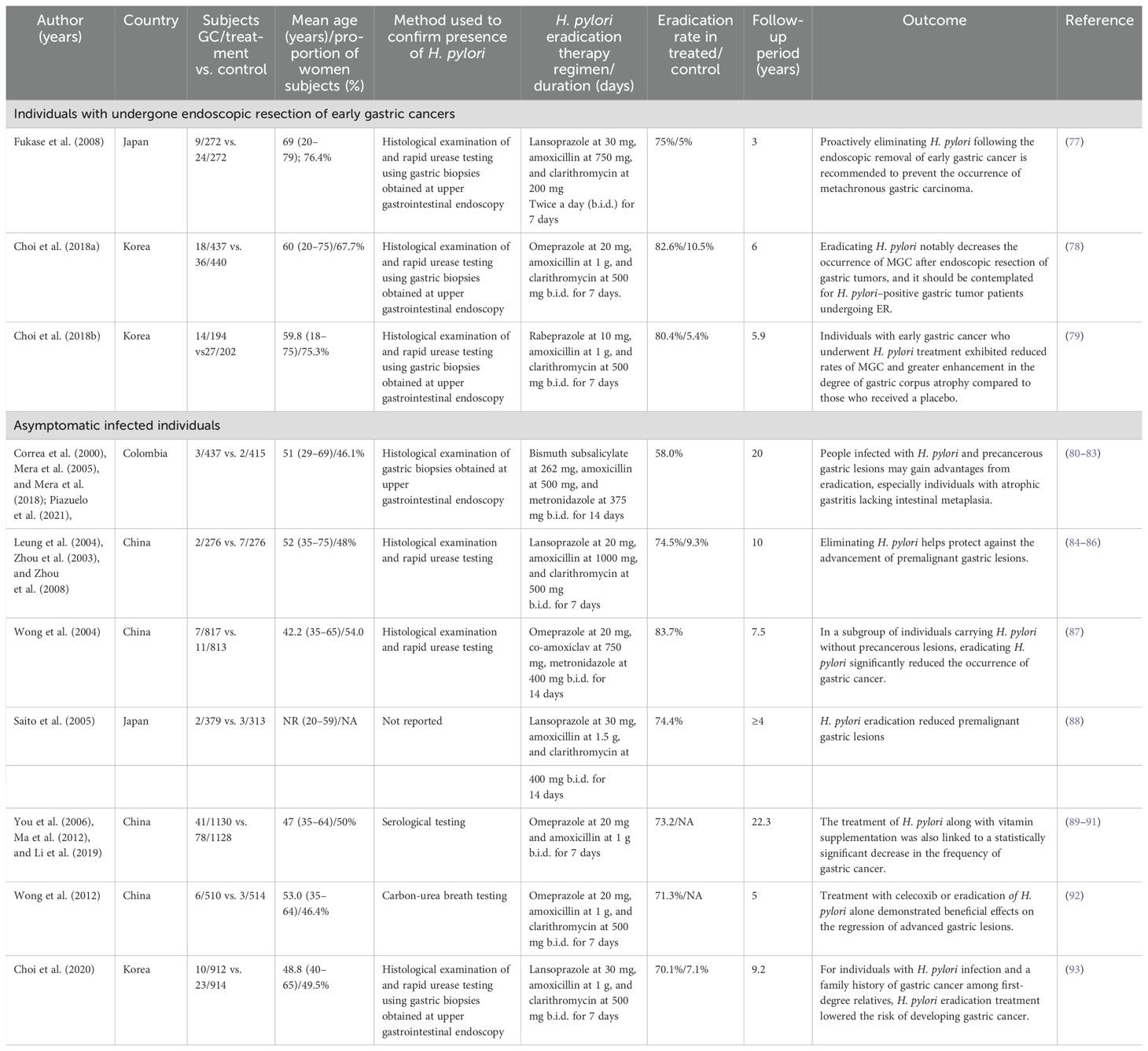
Table 2. Characteristics of randomized controlled trials of H. pylori eradication on individuals with asymptomatic infected gastric cancers and undergone endoscopic resection of early gastric cancers.
7 Conclusion and outlook
Infection with H. pylori, a bacterial carcinogen, stands as the primary cause of GC, claiming hundreds of thousands of lives annually. H. pylori infection significantly contributes to gastric microbial dysbiosis, potentially playing a role in carcinogenesis. Successful eradication of H. pylori may restore the gastric microbiota to a state resembling that of uninfected individuals, thereby exhibiting beneficial effects on the gut microbiota. The current study has underscored variations in microbiota changes among individuals with GC with or without H. pylori. Examining the interplay between H. pylori infection and microbiota changes in patients with GC aids in refining therapy for H. pylori infection in individuals with GC and concurrent H. pylori presence. In the future, it is imperative to comprehensively observe changes in intestinal flora from multiple perspectives through more scientific and rational research methods. This approach will enable a thorough and clear understanding of the causes and outcomes of the relationship between GC and intestinal flora, moving beyond mere correlation analysis.
Author contributions
MH: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. TM: Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing. MBe: Writing – original draft, Writing – review & editing. SK: Writing – original draft, Writing – review & editing. MBa: Writing – original draft, Writing – review & editing. RG: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from Behbahan Faculty of Medical Science (grant number 4152).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
H. pylori, Helicobacter pylori; GC, Gastric cancer; ROS, Reactive oxygen species; GI, Gastrointestinal; TNF-α, Tumor necrosis factor–alpha; E. coli Escherichia coli; B. fragilis, Bacteroides fragilis; HpNGC, H. pylori–negative gastric cancer; Dys, Dysplasia; IM, Intestinal metaplasia; MALT, Mucosa-associated lymphoid tissue; PPIs, Proton pump inhibitors; OAC, Omeprazole, amoxicillin, and clarithromycin; RCT, Randomized clinical trial.
References
1. Choi WT, Lauwers GY, Slavik T. Inflammatory disorders of the stomach. Morson Dawson’s Gastrointestinal Pathol. (2024), 135–94.
2. Yuan C, Adeloye D, Luk TT, Huang L, He Y, Xu Y, et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2022) 6:185–94. doi: 10.1016/S2352-4642(21)00400-4
3. Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. (2022) 53:33–50. doi: 10.1007/s42770-021-00675-0
4. Zhou X, Li Z, Du Y. Prevention and screening of gastric cancer by helicobacter pylori management: synthesis of existing data. Cancer Screening Prev. (2023) 2:260–9. doi: 10.14218/CSP.2023.00010
5. Mărginean CO, Meliț LE, Săsăran MO. Traditional and modern diagnostic approaches in diagnosing pediatric Helicobacter pylori infection. Children. (2022) 9:994. doi: 10.3390/children9070994
6. Huang T-T, Cao Y-X, Cao L. Novel therapeutic regimens against Helicobacter pylori: an updated systematic review. Front Microbiol. (2024) 15:1418129. doi: 10.3389/fmicb.2024.1418129
7. Shiotani A, Roy P, Lu H, Graham DY. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Ther Adv Gastroenterology. (2021) 14:17562848211064080. doi: 10.1177/17562848211064080
8. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
9. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA: Cancer J Clin. (2021) 71:264–79. doi: 10.3322/caac.21657
10. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England). (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
11. Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, MaChado JC, et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. (2018) 67:226–36. doi: 10.1136/gutjnl-2017-314205
12. Liu X, Shao L, Liu X, Ji F, Mei Y, Cheng Y, et al. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine. (2019) 40:336–48. doi: 10.1016/j.ebiom.2018.12.034
13. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
14. Liatsos C, Papaefthymiou A, Kyriakos N, Galanopoulos M, Doulberis M, Giakoumis M, et al. Helicobacter pylori, gastric microbiota and gastric cancer relationship: Unrolling the tangle. World J gastrointestinal Oncol. (2022) 14:959–72. doi: 10.4251/wjgo.v14.i5.959
15. Wroblewski LE, Peek RM Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. (2010) 23:713–39. doi: 10.1128/CMR.00011-10
16. Bakhti SZ, Latifi-Navid S. Interplay and cooperation of Helicobacter pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol. (2021) 21:258. doi: 10.1186/s12866-021-02315-x
17. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Letters. (2014) 345:196–202. doi: 10.1016/j.canlet.2013.08.016
18. Rivas-Ortiz CI, Lopez-Vidal Y, Arredondo-Hernandez LJR, Castillo-Rojas G. Genetic alterations in gastric cancer associated with helicobacter pylori infection. Front Med. (2017) 4. doi: 10.3389/fmed.2017.00047
19. Guo Y, Zhang Y, Gerhard M, Gao J-J, Mejias-Luque R, Zhang L, et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut. (2020) 69:1598–607. doi: 10.1136/gutjnl-2019-319696
20. Shang F, Cao Y, Wan L, Ren Z, Wang X, Huang M, et al. Comparison of Helicobacter pylori positive and negative gastric cancer via multi-omics analysis. mBio. (2023):e0153123. doi: 10.1128/mbio.01531-23
21. Kadeerhan G, Gerhard M, Gao J-J, Mejías-Luque R, Zhang L, Vieth M, et al. Microbiota alteration at different stages in gastric lesion progression: a population-based study in Linqu, China. Am J Cancer Res. (2021) 11 2:561–75.
22. Gantuya B, El Serag HB, Matsumoto T, Ajami NJ, Uchida T, Oyuntsetseg K, et al. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Alimentary Pharmacol Ther. (2020) 51:770–80. doi: 10.1111/apt.15675
23. Gao J-J, Zhang Y, Gerhard M, Mejias-Luque R, Zhang L, Vieth M, et al. Association between gut microbiota and helicobacter pylori-related gastric lesions in a high-risk population of gastric cancer. Front Cell Infection Microbiol. (2018) 8. doi: 10.3389/fcimb.2018.00202
24. Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PloS One. (2008) 3:e2836. doi: 10.1371/journal.pone.0002836
25. Chen C-C, Liou J-M, Lee Y-C, Hong T-C, El-Omar EM, Wu M-S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. (2021) 13:1909459. doi: 10.1080/19490976.2021.1909459
26. Zhang C, Hu A, Li J, Zhang F, Zhong P, Li Y, et al. Combined non-invasive prediction and new biomarkers of oral and fecal microbiota in patients with gastric and colorectal cancer. Front Cell Infection Microbiol. (2022) 12. doi: 10.3389/fcimb.2022.830684
27. Yang Y, Ji R, Zhao X, Cao X, Wang Q, Jiang Q, et al. Alterations in gastric mucosal microbiota in gastric carcinogenesis: A systematic review and meta-analysis. Front Med. (2021) 8. doi: 10.3389/fmed.2021.754959
28. Liu H, Liu X-S. Response: Commentary: Preoperative status of gut microbiota predicts postoperative delirium in patients with gastric cancer. Front Psychiatry. (2022) 13. doi: 10.3389/fpsyt.2022.991290
29. Liu X, Meng J, Zhu J, Huang M, Wen B, Guo R, et al. Comprehensive understandings into complete reconstruction of precatalysts: synthesis, applications, and characterizations. Advanced Materials. (2021) 33:2007344. doi: 10.1002/adma.202007344
30. Sonveaux P, Copetti T, De Saedeleer CJ, Végran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PloS One. (2012) 7:e33418. doi: 10.1371/journal.pone.0033418
31. Liu D, Zhu J, Ma X, Zhang L, Wu Y, Zhu W, et al. Transcriptomic and metabolomic profiling in helicobacter pylori–induced gastric cancer identified prognosis- and immunotherapy-relevant gene signatures. Front Cell Dev Biol. (2021) 9. doi: 10.3389/fcell.2021.769409
32. Nagata M, Toyonaga K, Ishikawa E, Haji S, Okahashi N, Takahashi M, et al. Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors. J Exp Med. (2021) 218. doi: 10.1084/jem.20200815
33. Morey P, Pfannkuch L, Pang E, Boccellato F, Sigal M, Imai-Matsushima A, et al. Helicobacter pylori depletes cholesterol in gastric glands to prevent interferon gamma signaling and escape the inflammatory response. Gastroenterology. (2018) 154:1391–404.e9. doi: 10.1053/j.gastro.2017.12.008
34. Kumar A, Bansal R, Pathak VP, Kishore S, Karya PK. Histopathological changes in gastric mucosa colonized by H. pylori. Indian J Pathol Microbiol. (2006) 49:352–6.
35. Dong L, Yin J, Zhao J, Ma S-R, Wang H-R, Wang M, et al. Microbial similarity and preference for specific sites in healthy oral cavity and esophagus. Front Microbiol. (2018) 9:1603. doi: 10.3389/fmicb.2018.01603
36. Sun Q-H, Zhang J, Shi Y-Y, Zhang J, Fu W-W, Ding S-G. Microbiome changes in the gastric mucosa and gastric juice in different histological stages of Helicobacter pylori-negative gastric cancers. World J Gastroenterology. (2022) 28:365. doi: 10.3748/wjg.v28.i3.365
37. Zhang X, Li C, Cao W, Zhang Z. Alterations of gastric microbiota in gastric cancer and precancerous stages. Front Cell infection Microbiol. (2021) 11:69. doi: 10.3389/fcimb.2021.559148
38. Castaño-Rodríguez N, Goh K-L, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. (2017) 7:1–9. doi: 10.1038/s41598-017-16289-2
39. Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. (2018) 67:1024–32. doi: 10.1136/gutjnl-2017-314281
40. Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, et al. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter. (2014) 19:407–16. doi: 10.1111/hel.2014.19.issue-6
41. Jo HJ, Kim J, Kim N, Park JH, Nam RH, Seok YJ, et al. Analysis of gastric microbiota by pyrosequencing: minor role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. Helicobacter. (2016) 21:364–74. doi: 10.1111/hel.2016.21.issue-5
42. Wang Z, Gao X, Zeng R, Wu Q, Sun H, Wu W, et al. Changes of the gastric mucosal microbiome associated with histological stages of gastric carcinogenesis. Front Microbiol. (2020) 11:997. doi: 10.3389/fmicb.2020.00997
43. Wu Z-F, Zou K, Wu G-N, Jin Z-J, Xiang C-J, Xu S, et al. A comparison of tumor-associated and non-tumor-associated gastric microbiota in gastric cancer patients. Digestive Dis Sci. (2021) 66:1673–82. doi: 10.1007/s10620-020-06415-y
44. Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep. (2016) 6:1–10. doi: 10.1038/srep18594
45. Gunathilake MN, Lee J, Choi IJ, Kim Y-I, Ahn Y, Park C, et al. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: a case-control study. Sci Rep. (2019) 9:1–11. doi: 10.1038/s41598-019-50054-x
46. Wang L, Xin Y, Zhou J, Tian Z, Liu C, Yu X, et al. Gastric mucosa-associated microbial signatures of early gastric cancer. Front Microbiol. (2020), 1548. doi: 10.3389/fmicb.2020.01548
47. Liu D, Chen S, Gou Y, Yu W, Zhou H, Zhang R, et al. Gastrointestinal microbiota changes in patients with gastric precancerous lesions. Front Cell Infection Microbiol. (2021) 11:749207. doi: 10.3389/fcimb.2021.749207
48. Liu D, Wang J, Xie Y. Refractory Helicobacter pylori infection and the gastric microbiota. Front Cell Infection Microbiol. (2022), 1303. doi: 10.3389/fcimb.2022.976710
49. Devi TB, Devadas K, George M, Gandhimathi A, Chouhan D, Retnakumar R, et al. Low Bifidobacterium abundance in the lower gut microbiota is associated with Helicobacter pylori-related gastric ulcer and gastric cancer. Front Microbiol. (2021) 12:631140. doi: 10.3389/fmicb.2021.631140
50. Tanaka T, Matsuno Y, Torisu T, Shibata H, Hirano A, Umeno J, et al. Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine. (2021) 100. doi: 10.1097/MD.0000000000027287
51. Wang L, Zhou J, Xin Y, Geng C, Tian Z, Yu X, et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol hepatology. (2016) 28:261. doi: 10.1097/MEG.0000000000000542
52. Park CH, Lee AR, Lee YR, Eun CS, Lee SK, Han DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. (2019) 24:e12547. doi: 10.1111/hel.2019.24.issue-1
53. Malfertheiner P, Megraud F, O’morain C, Gisbert J, Kuipers E, Axon A, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. (2017) 66:6–30.
54. Rolig AS, Cech C, Ahler E, Carter JE, Ottemann KM. The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota. Infection immunity. (2013) 81:1382–9. doi: 10.1128/IAI.00044-13
55. Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, et al. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J gastroenterology. (2019) 25:2706. doi: 10.3748/wjg.v25.i22.2706
56. Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. (2000) 119:7–14. doi: 10.1053/gast.2000.8550
57. Nakamura M, Matsui H, Serizawa H, Murayama S, Yamaguchi T, Takahashi T, et al. Coinfection of Helicobacter pylori and Neisseria subflava is closely associated with lymph follicle formation in human stomach. Wiley Online Library. J Gastroenterol (2006).
58. Mao L-Q, Zhou Y-L, Wang S-S, Chen L, Hu Y, Yu L-M, et al. Impact of Helicobacter pylori eradication on the gastric microbiome. Gut pathogens. (2021) 13:1–13. doi: 10.1186/s13099-021-00460-2
59. Liou J-M, Chen C-C, Chang C-M, Fang Y-J, Bair M-J, Chen P-Y, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Diseases. (2019) 19:1109–20. doi: 10.1016/S1473-3099(19)30272-5
60. Li TH, Qin Y, Sham PC, Lau K, Chu K-M, Leung WK. Alterations in gastric microbiota after H. pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep. (2017) 7:1–8. doi: 10.1038/srep44935
61. Yap TW-C, Gan H-M, Lee Y-P, Leow AH-R, Azmi AN, Francois F, et al. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PloS One. (2016) 11:e0151893. doi: 10.1371/journal.pone.0151893
62. O’Connor A, Liou J, Gisbert J, O’Morain C. The Effect of Helicobacter pylori antibiotic therapy on the microbiome.
63. Chen L, Xu W, Lee A, He J, Huang B, Zheng W, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine. (2018) 35:87–96. doi: 10.1016/j.ebiom.2018.08.028
64. Oh B, Kim BS, Kim JW, Kim JS, Koh SJ, Kim BG, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. (2016) 21:165–74. doi: 10.1111/hel.2016.21.issue-3
65. Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PloS One. (2010) 5:e9836. doi: 10.1371/journal.pone.0009836
66. He C, Peng C, Wang H, Ouyang Y, Zhu Z, Shu X, et al. The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter. (2019) 24:e12590. doi: 10.1111/hel.2019.24.issue-4
67. Yanagi H, Tsuda A, Matsushima M, Takahashi S, Ozawa G, Koga Y, et al. Changes in the gut microbiota composition and the plasma ghrelin level in patients with Helicobacter pylori-infected patients with eradication therapy. BMJ Open Gastroenterology. (2017) 4:e000182.
68. Hsu P, Pan C, Kao J, Tsay F, Peng N, Kao S, et al. Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter. (2018) 23:e12498. doi: 10.1111/hel.2018.23.issue-4
69. Martín-Núñez GM, Cornejo-Pareja I, Coin-Aragüez L, Roca-Rodríguez M, Muñoz-Garach A, Clemente-Postigo M, et al. H. pylori eradication with antibiotic treatment causes changes in glucose homeostasis related to modifications in the gut microbiota. PloS One. (2019) 14:e0213548.
70. Huang R, Ju Z, Zhou P-K. A gut dysbiotic microbiota-based hypothesis of human-to-human transmission of non-communicable diseases. Sci Total Environment. (2020) 745:141030. doi: 10.1016/j.scitotenv.2020.141030
71. Gudra D, Pupola D, Skenders G, Leja M, Radovica-Spalvina I, Gorskis H, et al. Lack of significant differences between gastrointestinal tract microbial population structure of Helicobacter pylori-infected subjects before and 2 years after a single eradication event. Helicobacter. (2020) 25:e12748. doi: 10.1111/hel.12748
72. Tao ZH, Han JX, Fang JY. Helicobacter pylori infection and eradication: Exploring their impacts on the gastrointestinal microbiota. Helicobacter. (2020) 25:e12754. doi: 10.1111/hel.12754
73. Nomura A, Stemmermann GN, Chyou P-H, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. New Engl J Med. (1991) 325:1132–6. doi: 10.1056/NEJM199110173251604
74. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. New Engl J Med. (1991) 325:1127–31. doi: 10.1056/NEJM199110173251603
75. Forman D, Newell D, Fullerton F, Yarnell J, Stacey A, Wald N, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br Med J. (1991) 302:1302–5. doi: 10.1136/bmj.302.6788.1302
76. Graham D, Shiotani A. The time to eradicate gastric cancer is now. Gut. (2005) 54:735–8. doi: 10.1136/gut.2004.056549
77. Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. (2008) 372:392–7. doi: 10.1016/S0140-6736(08)61159-9
78. Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang H-J, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointestinal endoscopy. (2018) 88:475–85.e2. doi: 10.1016/j.gie.2018.05.009
79. Choi IJ, Kook M-C, Kim Y-I, Cho S-J, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. New Engl J Med. (2018) 378:1085–95. doi: 10.1056/NEJMoa1708423
80. Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Institute. (2000) 92:1881–8. doi: 10.1093/jnci/92.23.1881
81. Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. (2005) 54:1536–40. doi: 10.1136/gut.2005.072009
82. Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. (2018) 67:1239–46. doi: 10.1136/gutjnl-2016-311685
83. Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, et al. The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology. (2021) 160:1106–17.e3. doi: 10.1053/j.gastro.2020.11.017
84. Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin Med J. (2003) 116:11–4.
85. Leung W, Lin S, Ching J, To K, Ng E, Chan F, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. (2004) 53:1244–9. doi: 10.1136/gut.2003.034629
86. Zhou L. S1606 ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China. Gastroenterology. (2008) 4:A–233.
87. Wong BC-Y, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. Jama. (2004) 291:187–94. doi: 10.1001/jama.291.2.187
88. Saito D, Boku N, Fujioka T, Fukuda Y, Matsushima Y, Sakaki N, et al. Impact of H-pylori eradication on gastric cancer prevention: Endoscopic results of the Japanese intervention trial (JITHP-study). A Randomized multi-center trial. Gastroenterology. (2005).
89. You W-C, Brown LM, Zhang L, Li J-Y, Jin M-L, Chang Y-S, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Institute. (2006) 98:974–83. doi: 10.1093/jnci/djj264
90. Ma J-L, Zhang L, Brown LM, Li J-Y, Shen L, Pan K-F, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Institute. (2012) 104:488–92. doi: 10.1093/jnci/djs003
91. Li W-Q, Zhang J-Y, Ma J-L, Li Z-X, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. (2019) 366. doi: 10.1136/bmj.l5016
92. Wong BC, Zhang L, Ma J-L, Pan K-F, Li J-Y, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. (2012) 61:812–8. doi: 10.1136/gutjnl-2011-300154
Keywords: Helicobacter pylori, gastric cancer, bacterial interactions, gastrointestinal microbiota, mucos associated lymphoid tissue
Citation: Heidary M, Akrami S, Madanipour T, Shakib NH, Mahdizade Ari M, Beig M, Khoshnood S, Ghanavati R and Bazdar M (2025) Effect of Helicobacter pylori–induced gastric cancer on gastrointestinal microbiota: a narrative review. Front. Oncol. 14:1495596. doi: 10.3389/fonc.2024.1495596
Received: 12 September 2024; Accepted: 12 December 2024;
Published: 10 January 2025.
Edited by:
Zhaofeng Liang, Jiangsu University, ChinaReviewed by:
Ira Ekmekciu, St Josef Hospital, GermanyBantayehu Addis Tegegne, Debre Markos University, Ethiopia
Copyright © 2025 Heidary, Akrami, Madanipour, Shakib, Mahdizade Ari, Beig, Khoshnood, Ghanavati and Bazdar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roya Ghanavati, Ui5HaGFuYXZhdGlAYmVodW1zLmFjLmly; UWFuYXZhdGkuckBnbWFpbC5jb20=; Monireh Bazdar, RHIubWJhemRhckBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Mohsen Heidary
Mohsen Heidary Sousan Akrami
Sousan Akrami Tohid Madanipour
Tohid Madanipour Nafiseh Hosseinzadeh Shakib
Nafiseh Hosseinzadeh Shakib Marzie Mahdizade Ari
Marzie Mahdizade Ari Masoumeh Beig
Masoumeh Beig Saeed Khoshnood
Saeed Khoshnood Roya Ghanavati
Roya Ghanavati Monireh Bazdar9*
Monireh Bazdar9*