- 1Department of Periodontics, Stomatological Hospital of Xiamen Medical College & Xiamen Key Laboratory of Stomatological Disease Diagnosis and Treatment, Xiamen, China
- 2Department of Oral Medicine, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing, China
Introduction: Almost all cases of malignant acanthosis nigricans with oral manifestations occurred in older age groups. Here, we report a case of malignant acanthosis nigricans in a young female presented with chief complaints of oral mucosal hyperplasia, who had previously been diagnosed with allergies.
Case presentation: A 30-year-old female developed oral hyperplasia and rash following seafood consumption, with subsequent resolution of the rash but persistent oral lesions and the appearance of pigmentation on her hands, neck, and axillae. Clinical examination revealed papillomatosis, hyperemia, and velvety hyperplasia in the oral cavity. Biopsy results confirmed papillary hyperplasia. Despite the patient’s belief in good health, she was advised to undergo further systemic examinations. Elevated serum tumor markers and histologic analysis of an endoscopic biopsy confirmed gastric cancer with duodenal infiltration, leading to the diagnosis of malignant acanthosis nigricans. Unfortunately, the patient passed away due to heart failure during chemotherapy treatment.
Conclusions: The majority of patients with malignant acanthosis nigricans present with oral lesions before the underlying malignancy is detected, emphasizing the importance of timely comprehensive systemic examination. Furthermore, our case suggests that age may not be a restrictive factor for the development of malignant acanthosis nigricans, and the presence of a rash can potentially obscure the cutaneous manifestations associated with this condition.
1 Introduction
Acanthosis nigricans is a rare mucocutaneous disorder characterized by skin hyperpigmentation and thickening in specific regions, such as the neck, axillae, and groin (1). Additionally, some patients exhibit filiform growths on the oral mucosa (2), which bear resemblance to lesions induced by human papillomavirus.
There were several different classifications for acanthosis nigricans, with malignant acanthosis nigricans included in each of them (3–5). Malignant acanthosis nigricans is considered a paraneoplastic syndrome, associated with cancers of the stomach, ovary, bladder, breast, liver, and kidney (6). Mucous membrane involvement is indicative of the malignant form.
Here, we report a case of a patient presenting with filiform papillomas on oral mucosa who was ultimately diagnosed with malignant acanthosis nigricans. While malignant acanthosis nigricans has mainly been reported in older populations (7), the patient in this case was only 30 years old. She had confidence in her physical health and denied all relevant systemic symptoms at her initial visit.
2 Case report
A 30-year-old woman presented to the Oral Medicine Department of Peking University School and Hospital of Stomatology with a complaint of 4-month hyperplasia in the mouth. She recalled that the oral manifestation and skin eruptions occurred after consuming seafood and had previously been diagnosed with atopic dermatitis at other institutions. The rash resolved after treatment, whereas pigmentation appeared on the hands, neck, and axillae. However, there was no improvement in the oral lesions. The patient underwent laboratory tests (blood routine, blood biochemistry, erythrocyte sedimentation rate, ANA spectrum, and T-cell subtype), and the results were all within normal ranges. The patient denied weight loss, a history of cancer, drug use, diabetes, unconventional sexual contact, venereal disease, and other endocrine disorders.
Oral examination results identified papillomatosis on the lips, hard palate mucosa, and gingiva with apparent hyperemia. Velvety hyperplasia was observed on the buccal mucosa and the dorsum of the tongue. Dermatological examination showed hyperpigmentation on the neck, axillae, and hands (Figure 1). Biopsies were performed on the lower lip and axillae, revealing papillary hyperplasia (Supplementary Figure S1).
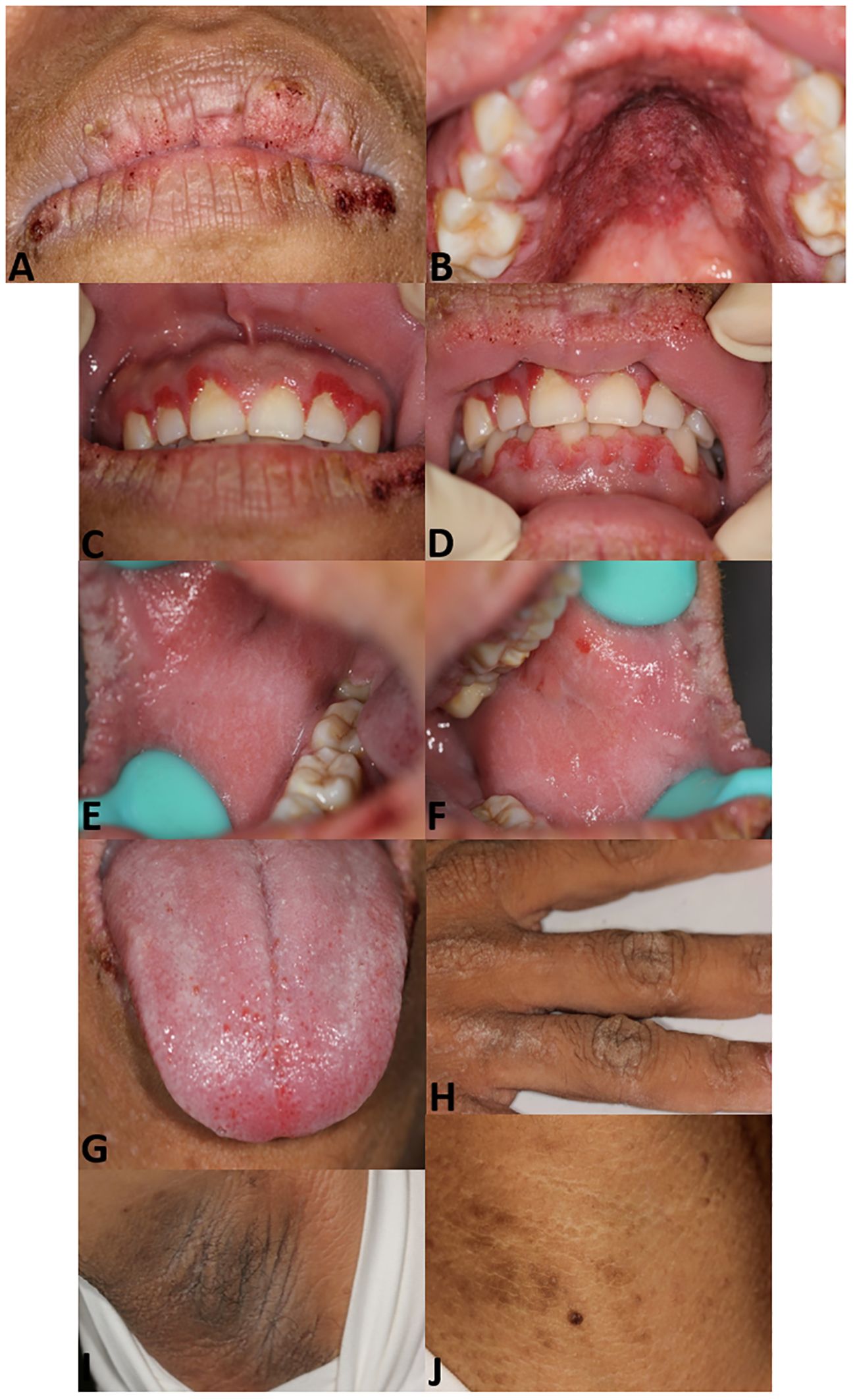
Figure 1. Patient images. Papillomatosis with scab on lips (A). Papillomatosis on hard palate mucosa with hyperemia (B). Papillomatosis on gingiva (C, D). Velvety hyperplasia on right (E) and left buccal mucosa (F). Velvety verrucous hyperplasia on the dorsum of the tongue and swelling of lingual papilla (G). Hyperpigmentation on the hand (H), axillae (I), and neck (J).
The patient underwent a systemic examination for underlying malignancy. Enhanced computed tomography results showed gastric wall thickening in the angle and enlarged lymph nodes in the lesser curvature, suggesting a possibility of gastric cancer (Supplementary Figure S2). Gastroscope examination results showed ulcers and papillary hyperplasia on the gastric wall and esophagus (Figures 2A, B). Histologic analysis of endoscopic biopsy confirmed gastric carcinoma with duodenal infiltration (Figures 2C, D). Immunohistochemistry results showed cytokeratin-7 (CK7) (+++), cytokeratin-20 (CK20) (+++), mucin-5 (MUC5) (+++), and caudal type homeobox 2 (CDX2) (+++), indicating primary gastric adenocarcinoma (poorly differentiated). Levels of serum tumor markers, including carcinoembryonic antigen (11.25 ng/mL, normal range: <5.0 ng/mL), carbohydrate antigen 19-9 (>1,000 U/mL, normal range: <37.0 U/mL), carbohydrate antigen 125 (165.30 U/mL, normal range: <35.0 U/mL), carbohydrate antigen 72-4 (57.87 U/mL, normal range: <6.9 U/mL), and tissue polypeptide antigen (2236.00 U/L, normal range: <120 U/L), were significantly elevated.
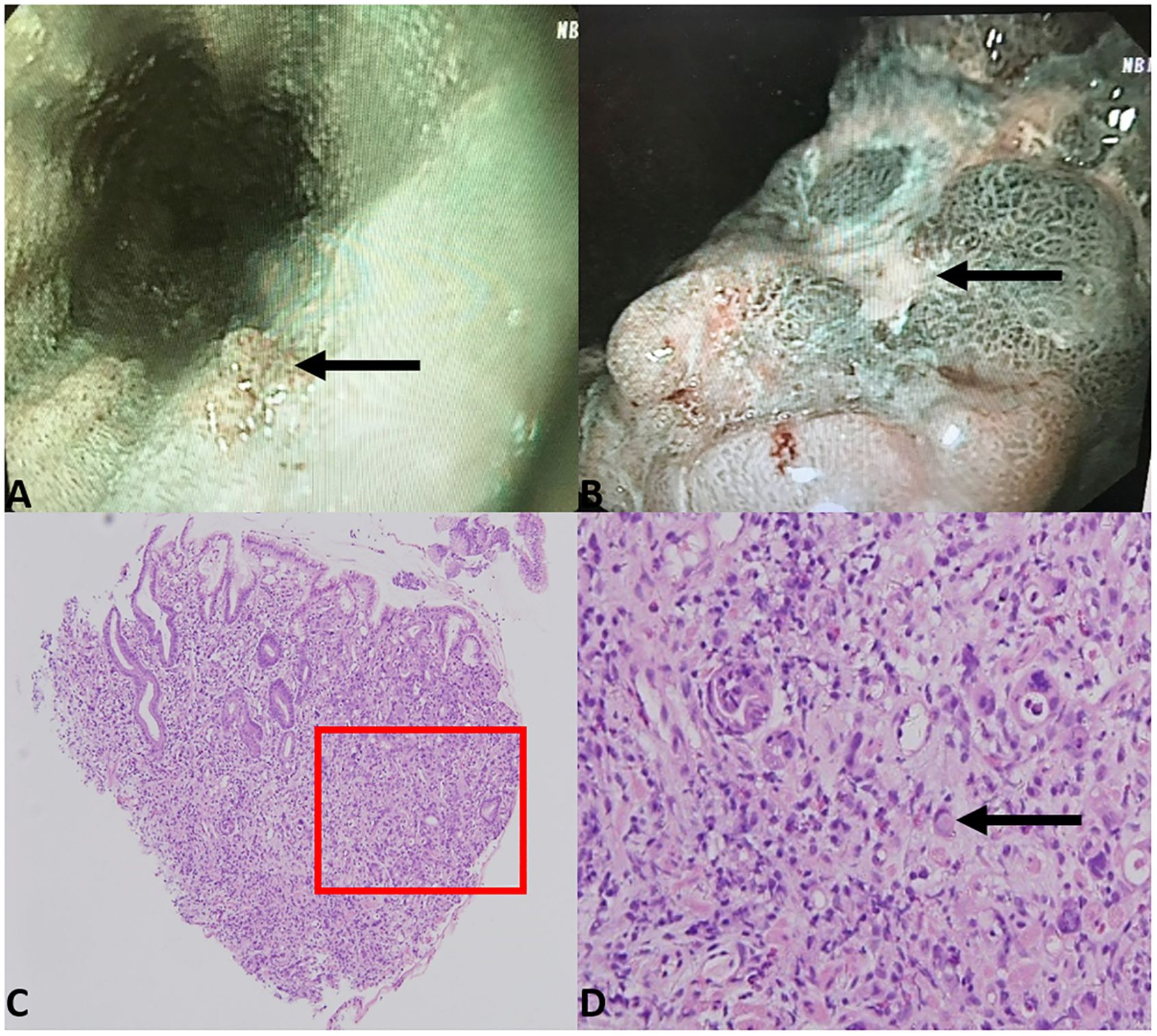
Figure 2. Gastroscope examination results showed papillary hyperplasia of the lower part of the esophagus (A) and rugged gastric angle posterior wall with ulcer (B). Biopsy of the gastric angle revealed atypical tumor cells infiltrating the lamina propria of the gastric mucosa, with Lauren classification indicating a mixed type (C) 40× magnification). Localized signet ring cell carcinoma was observed (D) 200× magnification).
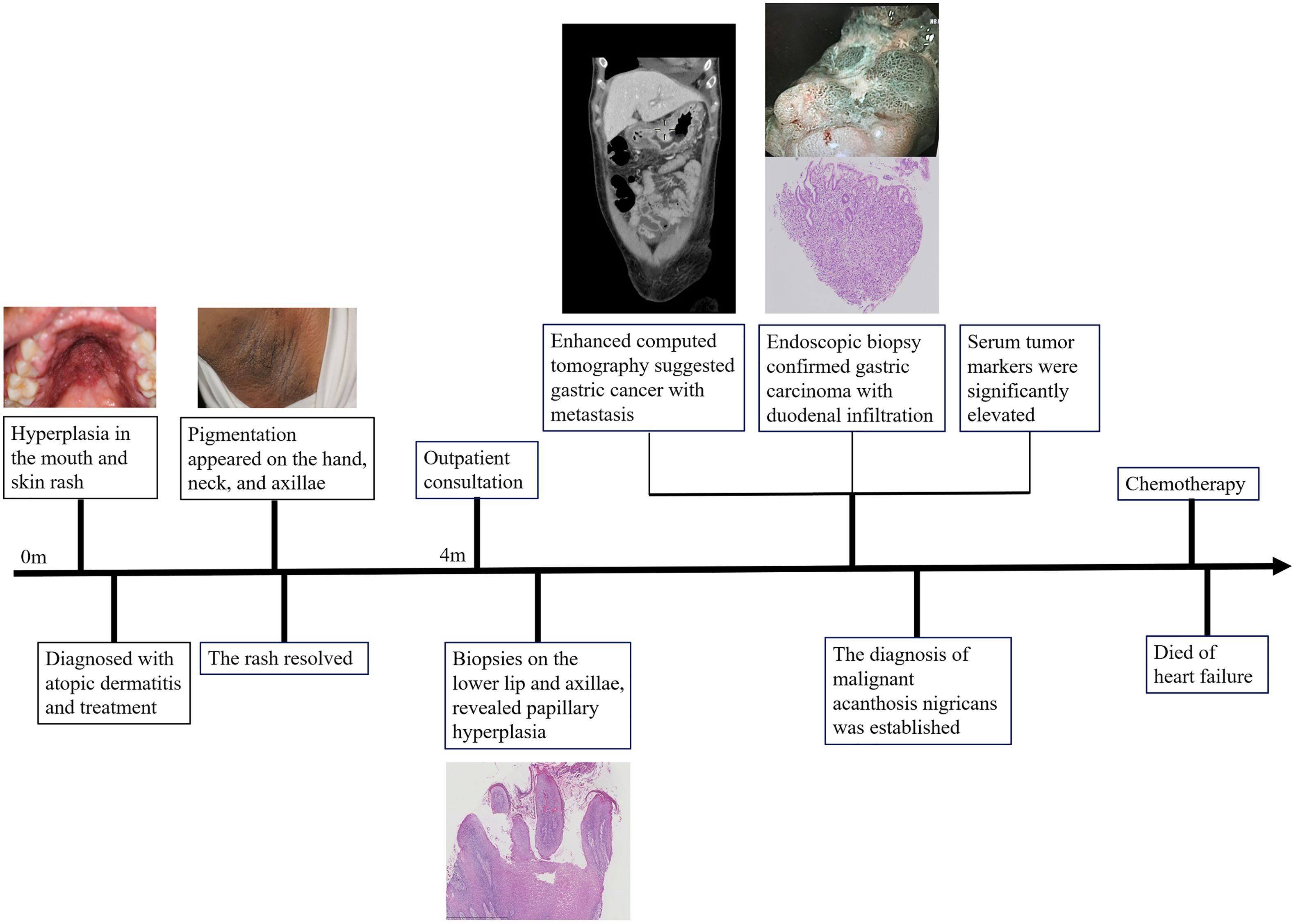
The diagnosis of malignant acanthosis nigricans was established. The patient received chemotherapy for the metastatic gastric carcinoma. Unfortunately, the patient died of heart failure during the treatment period. The timeline of the patient’s condition, medical visits, and outcome is presented in Figure 3.
3 Discussion
Malignant acanthosis nigricans in young populations is rare. In this report, we present a case of malignant acanthosis nigricans with multiple papillomatous and velvety oral lesions in a young female. Upon systemic examination, gastric cancer was detected. To the best of our knowledge, this is the first reported case of a young individual with malignant acanthosis nigricans presenting primarily with oral manifestations.
The differential diagnosis of acanthosis nigricans on oral mucosa holds particular importance for patient prognosis. Similar oral changes may occur in inflammatory papillary hyperplasia and HPV infection. Inflammatory papillary hyperplasia primarily presents as multiple small fibrous papillary growths, usually caused by wearing dentures and candidiasis, visible mainly on the denture-bearing hard palate (8). HPV-13 and HPV-32 infection can lead to multifocal, verrucous papules of the lips, buccal mucosa, and tongue (9), whereas HPV-6 and HPV-11 infections manifest as nodular or stalky masses with papillary surfaces (10). HPV tests help exclude these diseases.
Some skin disorders and syndromes also present with oral papillary lesions. Cowden syndrome (11) and ectrodactyly–ectodermal dysplasia–clefting syndrome (12) showed no evidence of skin hyperpigmentation. Linear epidermal nevus (13) and sebaceous nevus syndrome (14) can present both oral and cutaneous lesions resembling our case. The main point of differential diagnosis is that verrucous papules or pigmentation in these diseases appear in a linear configuration along skin tension lines. Focal dermal hypoplasia (15) is characterized by developmental disorders in multiple tissues and organs. Compared to this case, the above syndrome exhibits relatively small-range oral lesions, limited areas of skin lesions, and lighter pigmentation, with a tendency for family history. Specific genetic tests help make a correct diagnosis.
Extensive areas of papillary growth in oral mucosa and skin pigmentation in folds suggested that the patient might have acanthosis nigricans. Given that the benign and malignant forms of acanthosis nigricans can hardly be distinguished by mucocutaneous examination alone (2), we advised a thorough examination despite the young patient’s lack of discomfort, pain, or weight loss. This led to the discovery of gastric adenocarcinoma.
It is worth mentioning that Krawczyk (16) previously reported a case of papular rash with excessive keratosis, skin discoloration, and pruritus occurring simultaneously. Our patient also presented with a rash and was diagnosed with “allergy”. The presence of rash with excessive keratosis, oral papillary growth, and other manifestations should be carefully evaluated by clinicians to avoid overlooking the symptoms of malignant acanthosis nigricans.
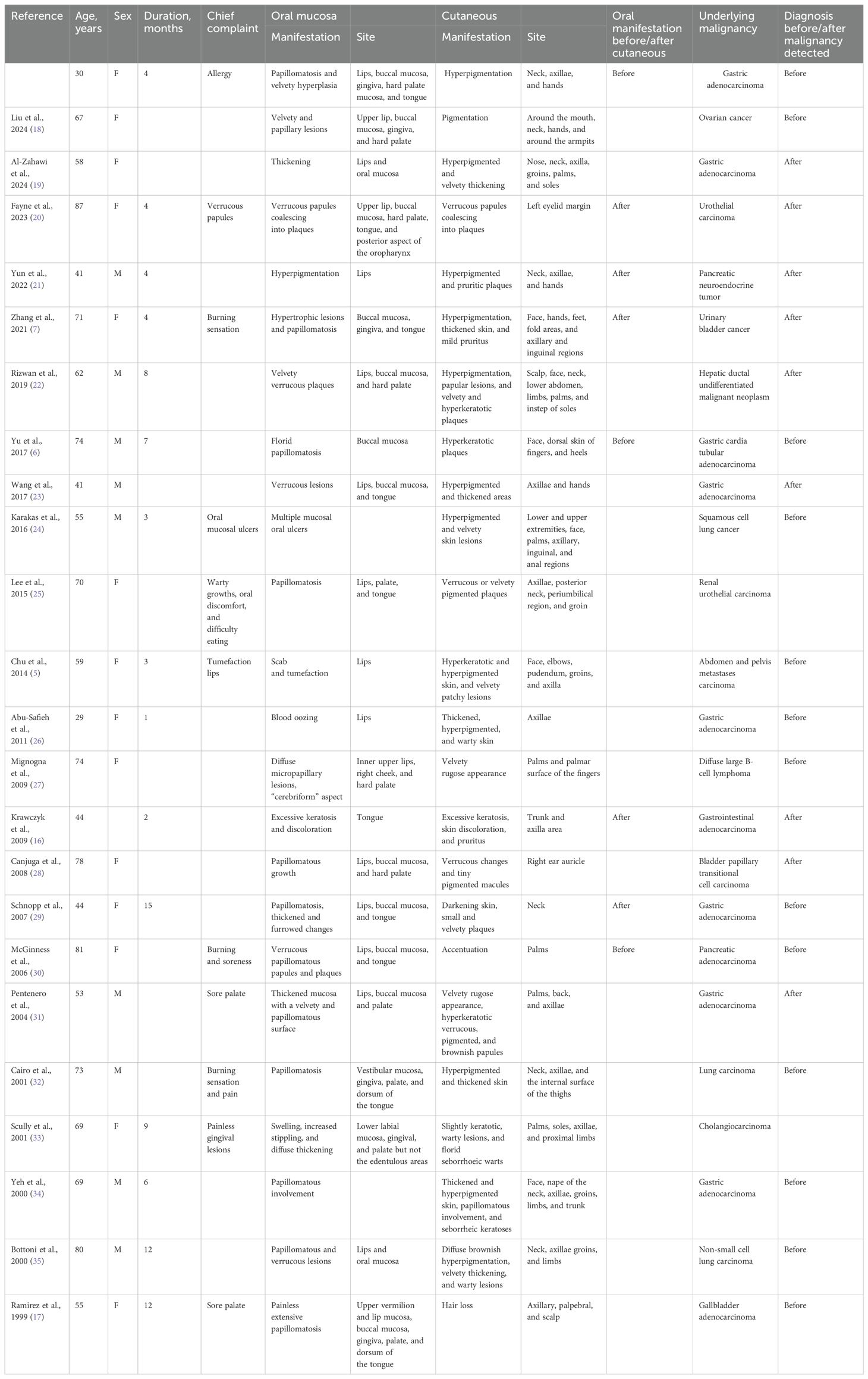
Ramirez-Amador documented 12 cases of malignant acanthosis nigricans with oral manifestations prior to 1999 (17). Our literature review identified 23 additional cases since then. Nearly all reported cases involved patients older than 40 years. Papillomatosis emerged as the most prevalent oral manifestation, observed in 72.2% of cases, primarily affecting the lips. Among these malignancies, 42% were gastric cancers. Notably, 59.1% of patients exhibited oral lesions preceding the diagnosis of the malignancy. We present the cases in Table 1 and summarize them in Supplementary Figure S3.
In conclusion, a significant percentage of malignant acanthosis nigricans patients exhibited oral lesions prior to the detection of the underlying malignancy. Therefore, clinicians, especially oral healthcare professionals, should remain vigilant. Detailed differential diagnosis is necessary, and a comprehensive systemic examination is required to facilitate early diagnosis of malignancies and improve patient prognosis. Furthermore, in contrast to previous cases, our patient was only 30 years old and considered herself to be in a healthy condition. This suggests that age may not be a restrictive factor for malignant acanthosis nigricans. On the other hand, the rash may coexist with skin and mucosal lesions, warranting that differentiation from allergies and clinical observations of mucosal manifestations after the regression of the rash are important.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZL: Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft. WC: Data curation, Investigation, Writing – original draft. YL: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Fujian Provincial Health Technology Project (2022QNB027) and Program for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-23A05). The funders had no role in the study design, data collection and analysis, and decision on publication or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1459148/full#supplementary-material
References
1. Das A, Datta D, Kassir M, Wollina U, Galadari H, Lotti T, et al. Acanthosis nigricans: A review. J Cosmet Dermatol. (2020) 19:1857–65. doi: 10.1111/jocd.13544
2. Kutlubay Z, Engin B, Bairamov O, Tüzün Y. Acanthosis nigricans: A fold (intertriginous) dermatosis. Clin Dermatol. (2015) 33:466–70. doi: 10.1016/j.clindermatol.2015.04.010
3. Sinha S, Schwartz RA. Juvenile acanthosis nigricans. J Am Acad Dermatol. (2007) 57:502–8. doi: 10.1016/j.jaad.2006.08.016
4. Eberting CL, Javor E, Gorden P, Turner ML, Cowen EW. Insulin resistance, acanthosis nigricans, and hypertriglyceridemia. J Am Acad Dermatol. (2005) 52:341–4. doi: 10.1016/j.jaad.2004.10.867
5. Chu HW, Li JM, Chen GF, Ma JY. Oral Malignant acanthosis nigricans associated with endometrial adenocarcinoma. Int J Oral Sci. (2014) 6:247–9. doi: 10.1038/ijos.2014.1
6. Yu Q, Li XL, Ji G, Wang Y, Gong Y, Xu H, et al. Malignant acanthosis nigricans: an early diagnostic clue for gastric adenocarcinoma. World J Surg Oncol. (2017) 15:208. doi: 10.1186/s12957-017-1274-5
7. Zhang R, Jiang M, Lei W, Wang A. Malignant acanthosis nigricans with recurrent bladder cancer: A case report and review of literature. Onco Targets Ther. (2021) 14:951–7. doi: 10.2147/ott.S290124
8. Gual-Vaqués P, Jané-Salas E, Egido-Moreno S, Ayuso-Montero R, Marí-Roig A, López-López J. Inflammatory papillary hyperplasia: A systematic review. Med Oral Patol Oral Cir Bucal. (2017) 22:e36–42. doi: 10.4317/medoral.21405
9. Said AK, Leao JC, Fedele S, Porter SR. Focal epithelial hyperplasia - an update. J Oral Pathol Med. (2013) 42:435–42. doi: 10.1111/jop.12009
10. Lacey CJ, Woodhall SC, Wikstrom A, Ross J. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. (2013) 27:e263–70. doi: 10.1111/j.1468-3083.2012.04493.x
11. Elo JA, Sun HH, Laudenbach JM, Singh HM. Multiple oral mucosal hamartomas in a 34-year old female. Head Neck Pathol. (2017) 11:393–8. doi: 10.1007/s12105-016-0777-7
12. Mansur AT, Aydingöz IE, Kocaayan N. A case of EEC syndrome with peri/intraoral papillomatosis and widespread freckling. J Dermatol. (2006) 33:225–6. doi: 10.1111/j.1346-8138.2006.00052.x
13. Haberland-Carrodeguas C, Allen CM, Lovas JG, Hicks J, Flaitz CM, Carlos R, et al. Review of linear epidermal nevus with oral mucosal involvement–series of five new cases. Oral Dis. (2008) 14:131–7. doi: 10.1111/j.1601-0825.2006.01355.x
14. Warnke PH, Russo PA, Schimmelpenning GW, Happle R, Härle F, Hauschild A, et al. Linear intraoral lesions in the sebaceous nevus syndrome. J Am Acad Dermatol. (2005) 52:62–4. doi: 10.1016/j.jaad.2004.05.046
15. Wright JT, Puranik CP, Farrington F. Oral phenotype and variation in focal dermal hypoplasia. Am J Med Genet C Semin Med Genet. (2016) 172c:52–8. doi: 10.1002/ajmg.c.31478
16. Krawczyk M, Mykała-Cieśla J, Kołodziej-Jaskuła A. Acanthosis nigricans as a paraneoplastic syndrome. Case reports and review of literature. Pol Arch Med Wewn. (2009) 119:180–3. doi: 10.20452/pamw.642
17. Ramirez-Amador V, Esquivel-Pedraza L, Caballero-Mendoza E, Berumen-Campos J, Orozco-Topete R, Angeles-Angeles A. Oral manifestations as a hallmark of Malignant acanthosis nigricans. J Oral Pathol Med. (1999) 28:278–81. doi: 10.1111/j.1600-0714.1999.tb02039.x
18. Liu Y, Wu X, Chen S, Meng W. Oral Malignant acanthosis nigricans: an early diagnostic sign for ovarian carcinoma: A case report. Clin Cosmet Investig Dermatol. (2024) 17:359–63. doi: 10.2147/ccid.S447977
19. Al-Zahawi S, Sadeghi Y, Azhari V, Mahmoudi H, Daneshpazhooh M. Bullous pemphigoid, Malignant acanthosis nigricans, and erysipeloid carcinoma in a woman with gastric adenocarcinoma. Clin Case Rep. (2024) 12:e8351. doi: 10.1002/ccr3.8351
20. Fayne R, Mervak JE. Correlated improvement of mucosal Malignant acanthosis nigricans and metastatic urothelial carcinoma with oncologic therapy. JAAD Case Rep. (2023) 37:35–7. doi: 10.1016/j.jdcr.2023.05.003
21. Yun JSW, McCormack C, Goh M, Chiang C. Acanthosis nigricans in a patient with metastatic insulinoma post peptide receptor radionuclide therapy. Endocrinol Diabetes Metab Case Rep. (2022) 2022. doi: 10.1530/edm-21-0150
22. Rizwan M, Iftikhar N, Sarfraz T, Ullah O. Malignant acanthosis nigricans: an indicator of internal Malignancy. J Coll Physicians Surg Pak. (2019) 29:888–90. doi: 10.29271/jcpsp.2019.09.888
23. Wang L, Long H, Wen H, Liu Z, Ling T. Image Gallery: Generalized mucosal and cutaneous papillomatosis, a unique sign of Malignant acanthosis nigricans. Br J Dermatol. (2017) 176:e99. doi: 10.1111/bjd.15396
24. Karakas Y, Esin E, Lacin S, Ceyhan K, Heper AO, Yalcin S. A case of acanthosis nigricans as a paraneoplastic syndrome with squamous cell lung cancer. Onco Targets Ther. (2016) 9:4815–20. doi: 10.2147/ott.S95020
25. Lee HC, Ker KJ, Chong WS. Oral Malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. (2015) 151:1381–3. doi: 10.1001/jamadermatol.2015.2139
26. Abu-Safieh Y, Khelfa S. Acanthosis nigricans: A presentation of gastric adenocarcinoma. Arab J Gastroenterol. (2011) 12:156–7. doi: 10.1016/j.ajg.2011.04.001
27. Mignogna MD, Fortuna G, Falleti J, Leuci S. Gastric diffuse large B-cell lymphoma (DLBCL) exhibiting oral acanthosis nigricans and tripe palms. Dig Liver Dis. (2009) 41:766–8. doi: 10.1016/j.dld.2009.02.049
28. Canjuga I, Mravak-Stipetić M, Kopić V, Galić J. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. (2008) 16:91–5.
29. Schnopp C, Baumstark J. Images in clinical medicine. Oral acanthosis nigricans N Engl J Med. (2007) 357:e10. doi: 10.1056/NEJMicm062917
30. McGinness J, Greer K. Malignant acanthosis nigricans and tripe palms associated with pancreatic adenocarcinoma. Cutis. (2006) 78:37–40.
31. Pentenero M, Carrozzo M, Pagano M, Gandolfo S. Oral acanthosis nigricans, tripe palms and sign of leser-trélat in a patient with gastric adenocarcinoma. Int J Dermatol. (2004) 43:530–2. doi: 10.1111/j.1365-4632.2004.02159.x
32. Cairo F, Rubino I, Rotundo R, Prato GP, Ficarra G. Oral Acanthosis nigricans as a marker of internal Malignancy. A case report. J Periodontol. (2001) 72:1271–5. doi: 10.1902/jop.2000.72.9.1271
33. Scully C, Barrett WA, Gilkes J, Rees M, Sarner M, Southcott RJ. Oral acanthosis nigricans, the sign of Leser-Trélat and cholangiocarcinoma. Br J Dermatol. (2001) 145:506–7. doi: 10.1046/j.1365-2133.2001.04393.x
34. Yeh JS, Munn SE, Plunkett TA, Harper PG, Hopster DJ, du Vivier AW. Coexistence of acanthosis nigricans and the sign of Leser-Trélat in a patient with gastric adenocarcinoma: a case report and literature review. J Am Acad Dermatol. (2000) 42:357–62. doi: 10.1016/s0190-9622(00)90112-9
Keywords: malignant acanthosis nigricans, malignancy, papillomatosis, velvety hyperplasia, gastric cancer, case report
Citation: Liu Z, Cao W and Liu Y (2024) Malignant acanthosis nigricans with oral manifestations in a young female: a case report and literature review. Front. Oncol. 14:1459148. doi: 10.3389/fonc.2024.1459148
Received: 03 July 2024; Accepted: 02 September 2024;
Published: 24 September 2024.
Edited by:
Hem Chandra Jha, Indian Institute of Technology Indore, IndiaReviewed by:
M. Badawy Abdel-Naser, New York University, United StatesWalter Belda, University of São Paulo, Brazil
Marcela Saeb Lima, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico
Copyright © 2024 Liu, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, c2hhbm5vbjEzNTc5QDE2My5jb20=
 Zijian Liu
Zijian Liu Wuling Cao
Wuling Cao Yang Liu2*
Yang Liu2*