- 1Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Key Laboratory of Otorhinolaryngology Head and Neck Surgery (Capital Medical University), Ministry of Education, Beijing, China
- 3Department of Otorhinolaryngology Head and Neck Surgery, Qingdao Municipal Hospital, Qingdao, China
- 4School of Systems Biology, George Mason University, Manassas, VA, United States
- 5Research Centre for Medical Genetics, Moscow, Russia
- 6Department of Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 7Institute of Neuropsychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
Introduction: The gut microbiome (GM) has been implicated in cancer pathogenesis and treatment, including head and neck cancers (HNC). However, the specific microbial compositions influencing HNC and the underlying mechanisms remain largely unknown.
Methods: This study utilized published genome-wide association studies (GWAS) summary data-based two-sample Mendelian randomization (MR) to uncover the GM compositions that exert significant causal effects on HNC. Functional annotation and enrichment analysis were conducted to better understand the significant genetic variables and their connection with HNC. The HNC dataset included 2,281 cases and 314,193 controls. The GM GWAS data of 211 gut taxa (35 families, 20 orders, 16 classes, 9 phyla, and 131 genera) were obtained from the MibioGen consortium, involving 18,340 participants.
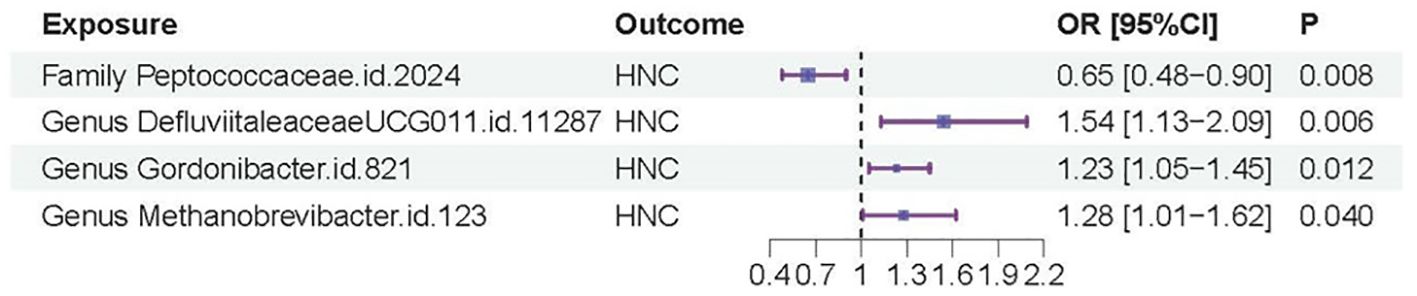
Results: MR analysis revealed four GM compositions exerting causal effects on HNC. Specifically, family Peptococcaceae.id.2024 was significantly associated with a 35% reduced risk of HNC (OR=0.65; 95%CI=0.48-0.90; P=0.0080). In contrast, genus DefluviitaleaceaeUCG-011.id.11287 (OR=1.54; 95%CI=1.13-2.09; P=0.0060), genus Gordonibacter.id.821 (OR=1.23; 95%CI=1.05-1.45; P=0.012), and genus Methanobrevibacter.id.123 (OR=1.28; 95%CI=1.01-1.62; P=0.040) showed a significant association with an increased risk of HNC. These GMs interact with genes and genetic variants involved in signaling pathways, such as GTPase regulation, influencing tumor progression and disease prognosis.
Conclusions: Our study demonstrates, for the first time, the causal influence of specific gut microbiome compositions on HNC, offering significant insights for advancing clinical research and personalized treatments. The identified GMs may serve as potential biomarkers or therapeutic targets, paving the way for innovative approaches in HNC diagnosis, prevention, and therapy.
1 Introduction
Head and neck cancer (HNC) encompasses a range of malignancies that originate in areas such as the oral cavity, throat, larynx, salivary glands, and nasal passages, posing a significant global health issue (1). This disease is notably prevalent, with millions of new cases diagnosed each year. Key risk factors, including tobacco and alcohol consumption, human papillomavirus (HPV) infection, and various environmental exposures, contribute to its widespread occurrence (2). Early detection is critical as it greatly affects the prognosis; HNC diagnosed at an early stage often responds well to treatment, resulting in high survival rates. Conversely, advanced-stage HNC presents more treatment challenges and has a less favorable prognosis (3). Efforts in raising awareness and promoting prevention are essential to reduce the disease’s impact (4).
The human microbiome is a dynamic community that colonizes various organs, with the gut being particularly abundant in microbial species due to its unique structure and role (5). The gut microbiome (GM) includes around 1500 distinct species (6), predominantly belonging to four major bacterial phyla: Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria (7). These bacteria are involved in a multitude of immune and metabolic activities in the gut. Studies reveal that the gut microbiome and its metabolic products have a substantial impact on the host’s physiology, influencing processes such as vitamin synthesis, production of intestinal hormones, maintenance of intestinal barrier integrity, and the digestion and absorption of nutrients (8).
Cancer can disrupt the balance of the GM, affecting immune modulation and metabolite production, which in turn impacts the prognosis and treatment outcomes of various cancer types. Understanding and manipulating the GM may provide novel therapeutic avenues for cancer prevention and treatment, highlighting the importance of considering the microbiome in cancer research and patient care (9–12). Specific microbial compositions and metabolites have been linked to different cancer types including HNC (13), further emphasizing the intricate relationship between the GM and cancer pathogenesis and treatment (14, 15). Exploring the intricate interactions between the gut microbiome and HNC could unveil novel avenues for HNC prevention, early detection, and therapeutic interventions.
Few studies have examined the link between the GM and HNC, highlighting the importance of understanding the connection between the intestinal microbiome and cancer treatments in HNCs (13). Notably, no prior research has investigated the causal effect of GM on HNC at the genetic level. Our hypothesis is that certain GM compositions, if not all, exert a causal effect on the risk of HNC. Identifying these GMs could offer significant insights into their role in cancer progression, which would not only enhance clinical research but also pave the way for more targeted and personalized treatment approaches for HNC.
Mendelian randomization (MR) analysis is a method that evaluates the causal relationship between risk factors and outcomes by using genetic variants associated with the risk factors as instrumental variables (IVs) (16). This approach can provide stronger genetic evidence and enhance the validity of causal inferences. In this study, we utilized the most comprehensive and recent GWAS summary data on human GM to deeply explore the genetic relationships involved.
2 Methods
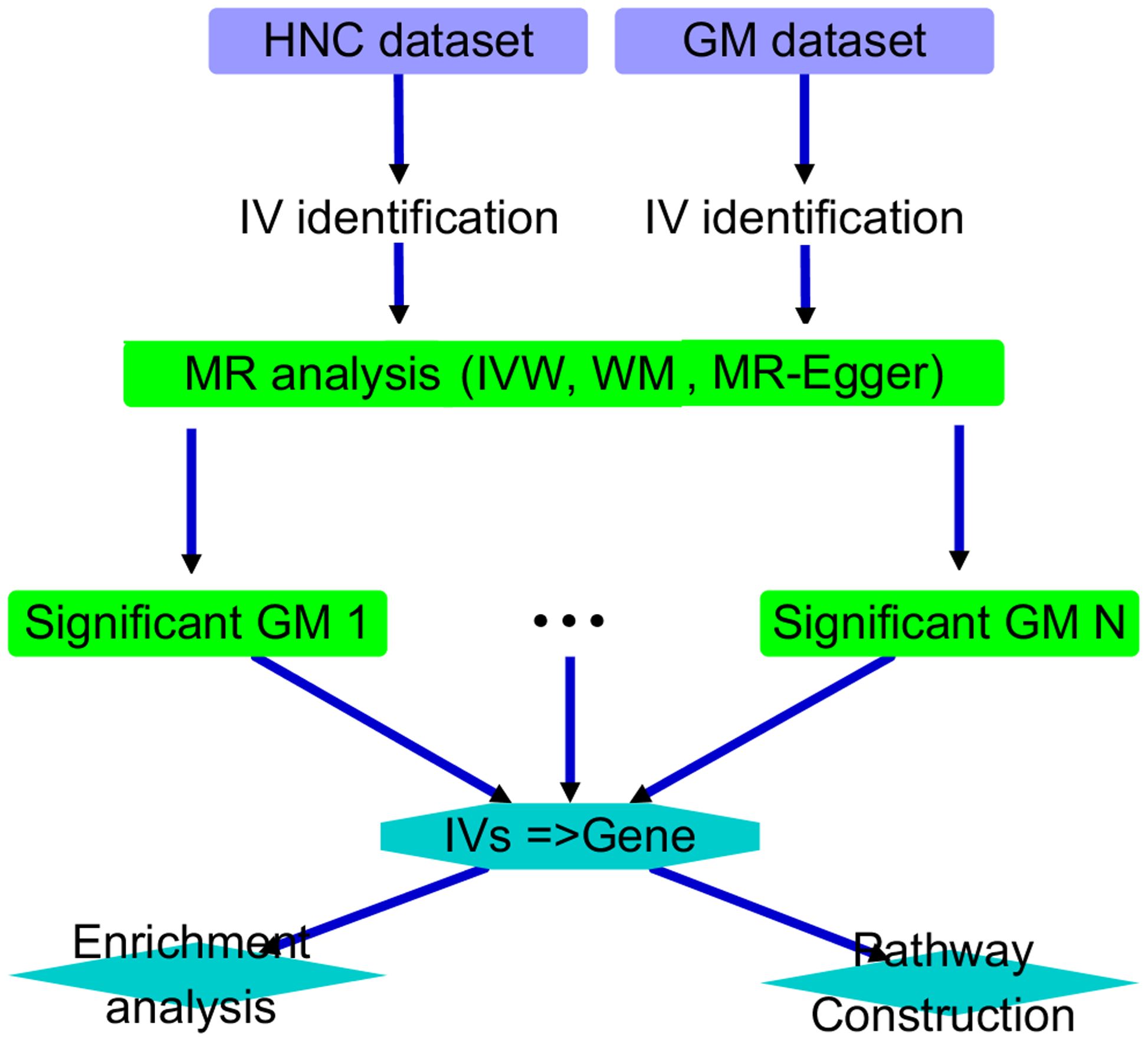
This study was structured as follows. First, we conducted an MR analysis on each of the 211 GM datasets to investigate the causal relationship between the corresponding GM taxa and HNC. We identified GM taxa with significant causal relationships for further analysis and discussion. Specifically, an AI-based literature data mining was performed to explore the connection between their IVs and corresponding genes and HNC. Functional pathways were then constructed accordingly. Please see Figure 1 for the overall workflow of the study.
2.1 Data source
The GWAS summary data utilized in this study were all obtained from publicly available datasets. Inclusion Criteria for HNC Datasets: The data for both HNC cases and controls were obtained from publicly available datasets, specifically the FinnGen R10 database (https://r10.risteys.finngen.fi/) (17). The HNC group comprised 2,281 cases identified using International Classification of Diseases (ICD) codes (phenocode: C3_HEAD_AND_NECK_EXALLC), while the control group included 314,193 individuals without HNC. Due to the reliance on summary-level genetic data from genome-wide association studies (GWAS), detailed clinical and demographic information—such as age, gender, comorbidities, and lifestyle factors—were not available. This limitation is further emphasized by the absence of granular patient-level data. However, the dataset predominantly includes participants of European ancestry, which ensures consistency in genetic background across both cases and controls. The alleles and effects of the two datasets were harmonized before the analysis. The GM GWAS data of 211 gut taxa (35 families, 20 orders, 16 classes, 9 phyla, and 131 genera) were obtained from the MibioGen consortium, involving 18,340 participants (18). Fifteen gut taxa without known names were excluded from the analysis.
2.2 MR analysis
The primary Mendelian randomization (MR) analysis utilized the inverse-variance weighted (IVW) approach, supplemented by the weighted median and MR-Egger methods, as implemented in TwoSampleMR (19). The primary MR analysis aimed to infer causal relationships between gut microbiome compositions and HNC. We utilized several statistical methods as implemented in TwoSampleMR (19): Inverse-Variance Weighted (IVW): This approach combines estimates from multiple single-nucleotide polymorphisms (SNPs) while accounting for their variances, providing a robust estimate of causal effects. Weighted Median: This method estimates causal effects by giving more weight to SNPs with more accurate estimates, allowing for valid causal inference even if some SNPs are invalid instruments. MR-Egger: This method estimates causal effects while allowing for directional pleiotropy. The intercept from the MR-Egger model can indicate the presence of pleiotropy, helping to assess the validity of the instrumental variables used.
For the MR analysis, single nucleotide polymorphisms (SNPs) were chosen as instrumental variables (IVs) based on their strong association with GM taxa from threshold of P < 1×10–5, ensuring that only SNPs with robust associations were included as IVs. To avoid linkage disequilibrium, we applied a clumping threshold of r² < 0.001 within a 10 Mb window, ensuring that the selected SNPs were independent.
To address potential biases due to insufficient IVs, we employed multiple MR methods, including the Inverse-Variance Weighted (IVW) approach, which provides the primary estimate of causal effects. Additionally, Weighted Median and MR-Egger methods were used to account for potential biases such as pleiotropy (where SNPs influence multiple traits). These additional methods offer robust estimates even if some IVs are invalid, helping mitigate the risk of bias from weak or insufficient instruments. Heterogeneity tests (e.g., Cochran’s Q and I² statistics) were also conducted to assess the consistency of the results across the SNPs, with thresholds of P < 0.05 and I2 > 0.25, respectively (20). This study adhered to the STROBE-MR checklist for strengthening the reporting of observational studies utilizing Mendelian Randomization (21).
2.3 Functional enrichment analysis
To gain a deeper understanding of the use of instrumental variables (SNPs) in Mendelian randomization (MR) analysis connecting gut microbiota (GM) and head and neck cancers (HNCs), we performed a functional enrichment analysis on the genes mapped to these IVs using the DAVID platform (https://david.ncifcrf.gov/). The input for this analysis comprised genes corresponding to IVs derived from GM data used in MR analysis. The IVs were mapped to genes using Entrez.elink (https://www.ncbi.nlm.nih.gov/books/NBK25500/#_E-utilities_5._eLink). This analysis involved examining Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The main goal was to explore the molecular functions, biological processes, and cellular components associated with the target genes and their gene products. Additionally, it aimed to investigate their roles in various biological pathways, including those related to metabolism, signaling, and diseases.
2.4 Knowledge-based pathway construction
To better understand the use of instrumental variables (SNPs) in MR analysis linking GM and HNCs, we conducted a thorough data mining analysis using an AI-powered tool from AIC LLC (https://www.gousinfo.com/en/advancedsearch.html). This process involved creating molecular pathways connecting GM and HNCs via genes linked to the chosen SNPs. We hypothesized that these SNPs influence molecular pathways bridging GM and HNCs. To validate this, we reviewed relevant references and statements about the target genes, ensuring quality by excluding unrelated associations. We then constructed molecular pathways connecting GM and HNC based on the identified correlations.
3 Results
3.1 MR analysis result
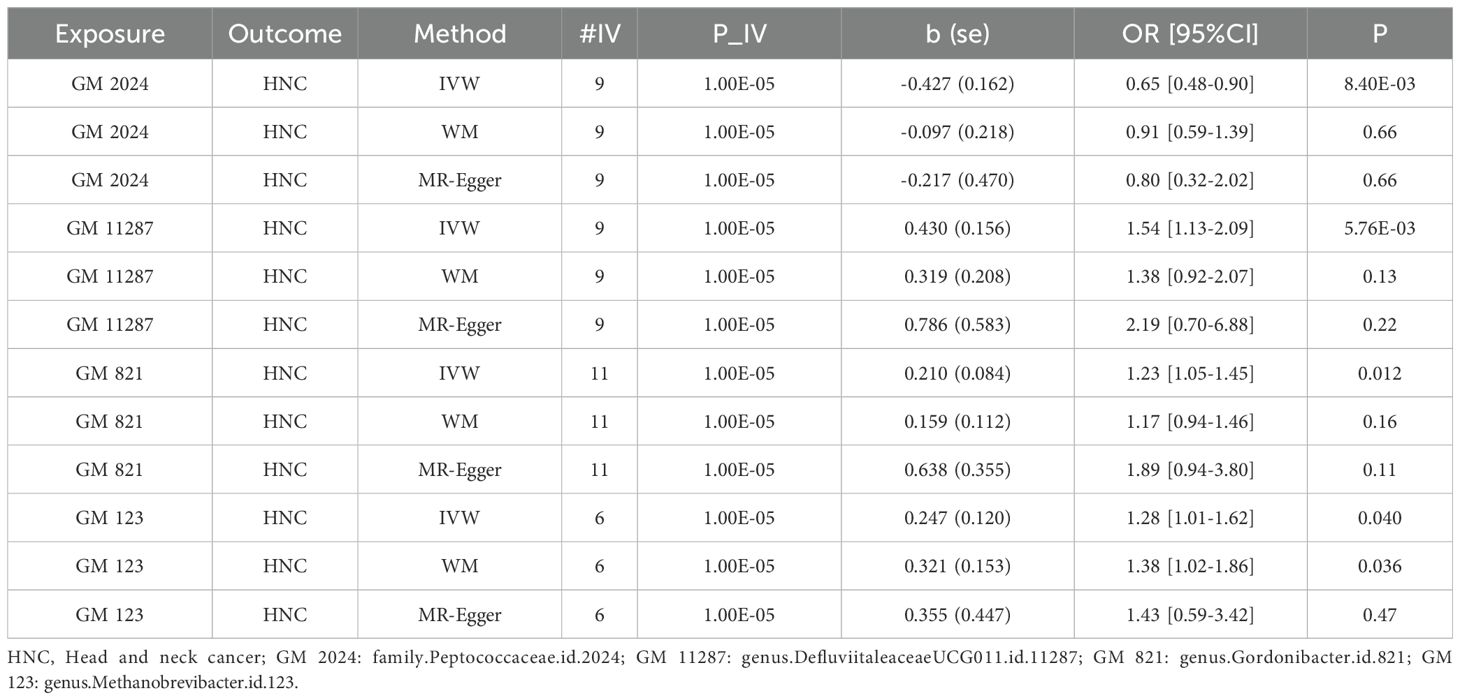
In the MR analysis, five datasets were excluded due to insufficient instrumental variables (IVs). Our results indicate that genus DefluviitaleaceaeUCG011.id.11287, genus Gordonibacter.id.821, and genus Methanobrevibacter.id.123 were linked to an increased disease risk, whereas family Peptococcaceae.id.2024 was associated with a reduced disease risk. The results are presented in Table 1. Here, Methanobrevibacter spp. is a genus of archaea within the Kingdom Euryarchaeota and family Methanobacteriaceae,. Archaea are distinct from bacteria and eukaryotes, possessing unique metabolic pathways, which may contribute to their role in disease pathogenesis.
Table 1 shows that, using the IVW method, the analysis revealed that family.Peptococcaceae.id.2024 was significantly associated with a reduced risk of HNC. The odds ratio (OR) =0.65 with a 95% confidence interval (CI) = 0.48-0.90, and the p-value was 0.0084, indicating a statistically significant protective effect. The WM and MR-Egger methods, however, did not show significant associations. The WM method yielded an OR=0.91 (95% CI= 0.59-1.39) with a p-value of 0.655, while the MR-Egger method produced an OR=0.80 (95% CI=0.32-2.02) with a P=0.66. These methods may not have shown significance due to their lower statistical power compared to the IVW method, especially when the effect size is modest. We also visualize these results in scatter plot as shown in Figure 2A.
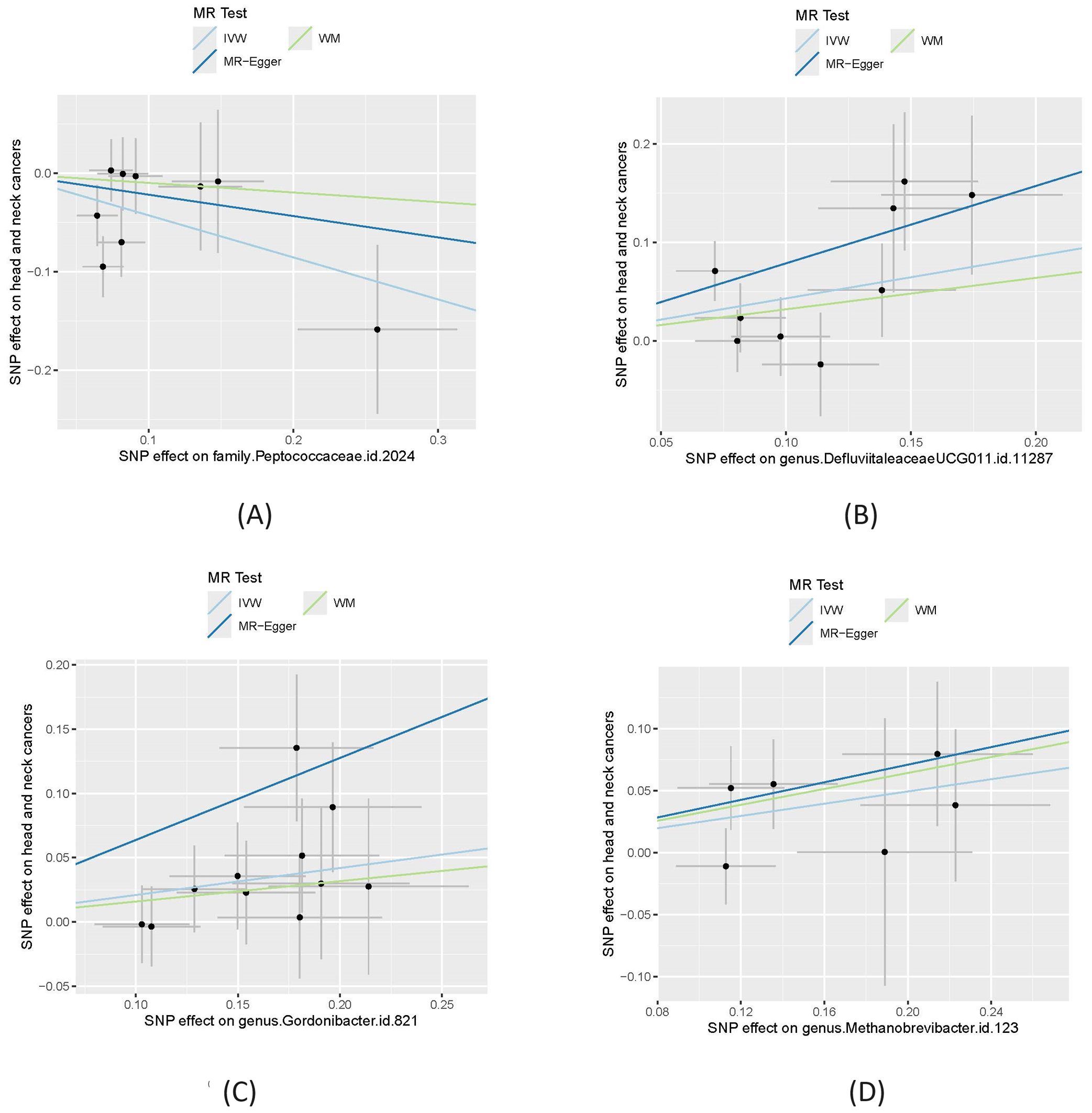
Figure 2. Causal effect of four gut microbiomes on head and neck cancers. (A) Effect of family.Peptococcaceae.id.2024; (B) effect of genus.DefluviitaleaceaeUCG011.id.11287; (C) Effect of genus.Gordonibacter.id.821; (D) Effect of genus.Methanobrevibacter.id.123. In each plot, the lines were the effect sizes (B) of the MR analysis. IVW, inverse variance weighted; WM, weighted median.
Additionally, heterogeneity analysis suggested that the directions of causal effects were consistent across the different techniques. No directional pleiotropy (P > 0.05 and MR-Egger intercept < 0.01) or heterogeneity (P > 0.05) was detected, indicating that the study results are reliable and the lack of significance in the WM and MR-Egger methods does not undermine the overall findings.
In contrast, genus.DefluviitaleaceaeUCG011.id.11287 (OR=1.54; 95%CI=1.13-2.09; P= 0.0058), genus.Gordonibacter.id.821 (OR=1.23; 95%CI=1.05-1.45; P = 0.012), and genus.Methanobrevibacter.id.123 (OR=1.28; 95%CI=1.01-1.62; P=0.040) showed a significant association with an increased risk of HNC, as shown in Figures 2B–D. Similar to family.Peptococcaceae.id.2024, the WM and MR-Egger methods did not show significant associations for most tests due to their lower statistical power compared to the IVW method. However, no directional pleiotropy (P > 0.05, MR-Egger intercept < 0.01) or heterogeneity (P > 0.05) was detected, indicating that the study results are reliable. The lack of significance in the WM and MR-Egger methods does not undermine the overall findings. We present a forest plot illustrating the causal effects of the four GMs on HNC, as determined by the main method (IVW), in Figure 3.
It should be noted that none of the four taxa showed significance after FDR correction (P > 0.05), likely due to the small number of IVs used in our MR analysis, which resulted in relatively larger p-values. Moreover, Leave-One-Out (LOO) sensitivity analysis showed that the results for one of the four gut microbiota (genus.Methanobrevibacter.id.123) were influenced by removing one instrumental variable (rs10202904). This may be due to the fact that only six IVs were used in this analysis. However, for the other three gut microbiota, removing any single IV did not significantly influence the results, suggesting the robustness of the MR analysis outcomes. The LOO plot is presented in Figure 4.
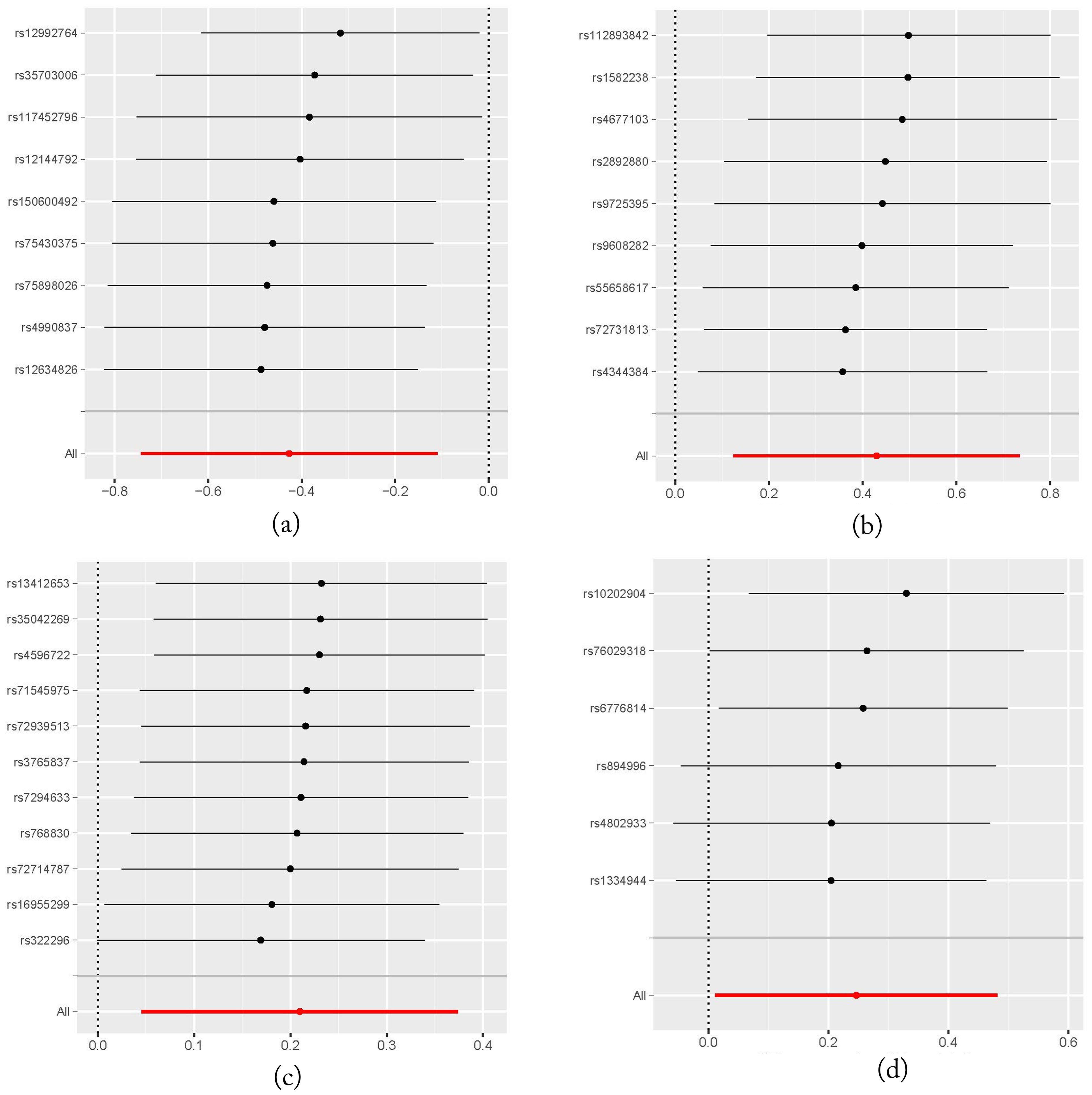
Figure 4. Leave-One-Out plots illustrating the robustness of the MR analysis for four gut microbiota taxa. (A) family.Peptococcaceae.id.2024 on head and neck cancers; (B) genus.DefluviitaleaceaeUCG011.id.11287 on head and neck cancers; (C) genus.Gordonibacter.id.821 on head and neck cancers; (D) genus.Methanobrevibacter.id.123 on head and neck cancers.
3.2 Analysis of IVs with enrichment analysis
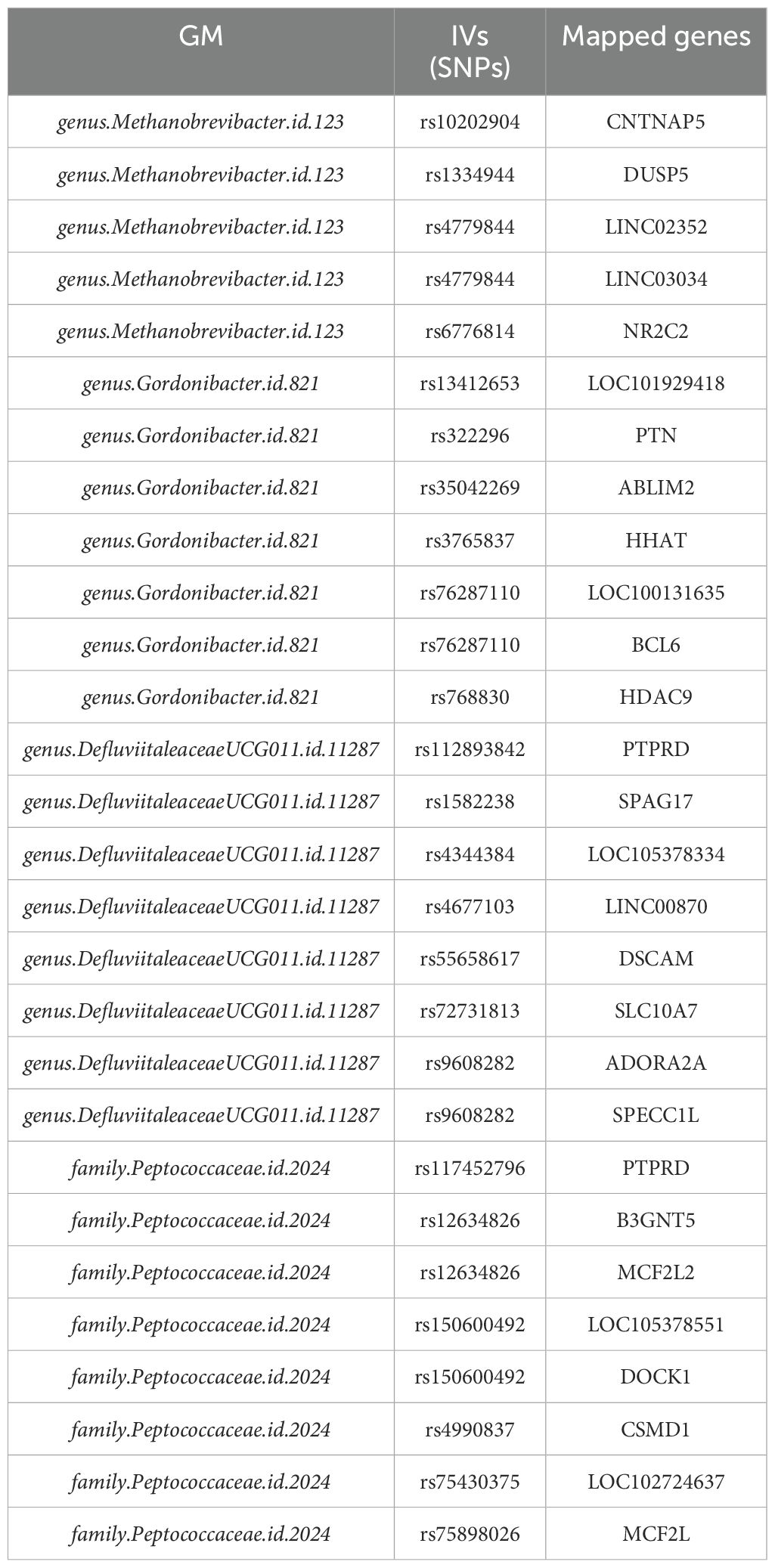
Table 2 lists the IVs with mapped genes for each of the four GM presented in Table 1. Please note, here we only listed the ones with at least one mapped genes, as enrichment analysis only take gene symbols as inputs.
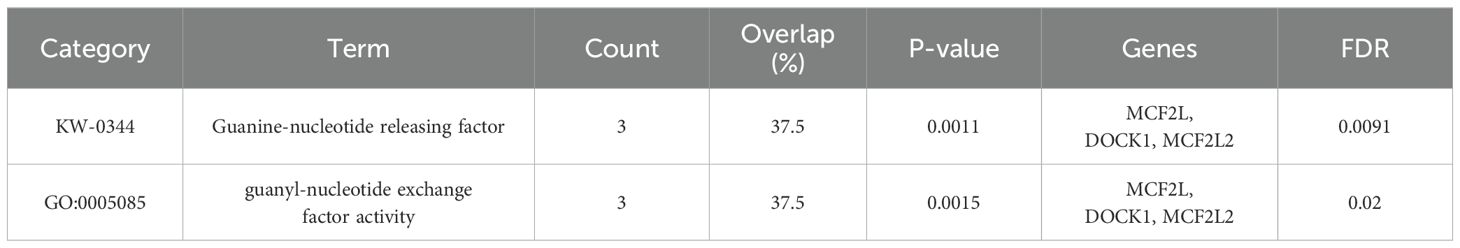
Enrichment analysis revealed that only the genes corresponding to IVs from family.Peptococcaceae.id.2024 were significantly enriched in two pathways: Guanine-nucleotide releasing factor (GNRF) and Guanyl-nucleotide exchange factor (GEF) activity. These pathways are essential for regulating small GTPases, which are crucial molecules in cell growth and migration. In contrast, genes corresponding to IVs from the other three gut microbiota did not show significant enrichment in any pathways, indicating that their genes were not functionally collaborating on specific functions.
3.3 Knowledge-based pathway connecting HNC and four GMs
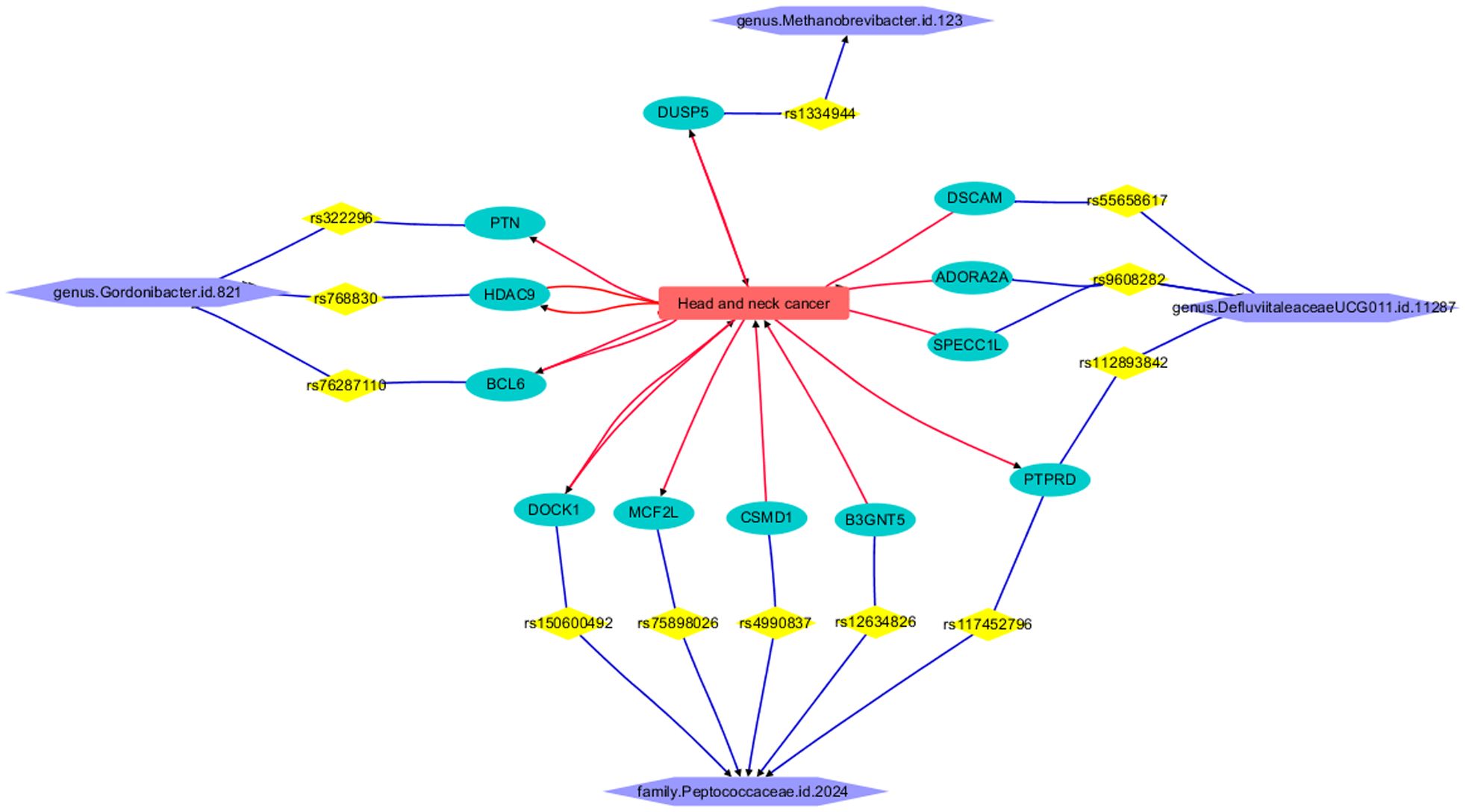
The LDM process revealed that HNC is connected to 12 out of the 27 genes corresponding to GM IVs, forming a functional pathway that links HNC with the four GMs, as illustrated in Figure 5. Each relationship or edge between HNC and the genes was supported by one or more references. The constructed pathway helps depict the mechanism by which genetic liability to the four types of GMs influences the pathological development and progression of HNC. It is important to note that none of the IVs (SNPs) have been reported to have a direct relationship with HNC, nor do the remaining 15 out of the 27 genes corresponding to GM-related IVs. This finding underscores the need for further research on these genes and genetic variants.
We have integrated the findings from our MR analysis, enrichment analysis, and pathway analysis to create the Ring diagram (Circus plot), illustrating the association between the four GM taxa and HNC, as shown in Figure 6.

Figure 6. Ring diagram showing the causal association between four gut microbiota and head and neck cancer: Family Peptococcaceae (reduced risk); Genus Defluviitaleaceae UCG-011 (increased risk); Genus Gordonibacter (increased risk); Genus Methanobrevibacter (increased risk).
4 Discussion
The gut microbiome (GM) has been implicated in cancer pathogenesis and treatment, including head and neck cancers (HNC) (13). However, the specific microbial compositions influencing HNC and the underlying mechanisms remain largely unknown. In this study, we used Mendelian randomization (MR) analysis to explore the causal effect of 211 GM compositions on HNC, identifying four with significant causal effects: family Peptococcaceae (id.2024), genus Defluviitaleaceae UCG-011 (id.11287), genus Gordonibacter (id.821), and genus Methanobrevibacter (id.123). Further analysis of the instrumental variables (SNPs) and the corresponding genes revealed functional pathways and networks linking HNC to these four GMs. These findings enhance our understanding of the relationships between the gut microbiome and HNC.
Our MR analysis identified that genus DefluviitaleaceaeUCG011.id.11287, genus Gordonibacter.id.821, and genus Methanobrevibacter.id.123 were associated with an increased risk of disease, whereas family Peptococcaceae.id.2024 was linked to a decreased risk of disease (Table 1). Despite the WM and MR-Egger methods not showing significance, there was no evidence of directional pleiotropy (P > 0.05 and MR-Egger intercept < 0.01) or heterogeneity (P > 0.05), suggesting that the study results are robust. The absence of significance in the WM and MR-Egger methods does not detract from the overall findings.
Enrichment analysis revealed that three genes (MCF2L, DOCK1, and MCF2L2) corresponding to the IVs from the family Peptococcaceae.id.2024 were significantly enriched in Guanine-nucleotide releasing factor (GNRF) and Guanyl-nucleotide exchange factor (GEF) activity-related pathways (Table 3). These pathways are crucial for regulating small GTPases, which are pivotal molecules in cell growth and migration. GTPases play a significant role in modulating gut microbiota composition and function, with dysregulation potentially leading to health implications. This interplay between GTPases and the GM highlights their importance as potential therapeutic targets (22, 23). Additionally, the GM reciprocally impacts GTPases by modulating their activity and expression, thereby influencing immune responses, metabolism, and signaling pathways bidirectionally to maintain gut homeostasis and health (24, 25).
In HNC, dysregulation of GNRF, particularly Vav2, can lead to the abnormal activation of GTPases, promoting tumor progression through altered signaling pathways, ribosome biogenesis, invasion, immunosuppression, and metastasis (26–29). GNRF’s role in accelerating HNC is further supported by its enhancement of cellular activities and impact on signaling pathways, with potential therapeutic targets identified as DOCK2 and ARHGEF2 (30, 31). Additionally, studies have shown that the GEF activity of proteins such as PLEKHG4, Tiam1, and VAV2 influences cell behavior and therapeutic targets in HNC (32–34). These findings suggest that GTPase regulation is a common influential mechanism in both HNC and the family Peptococcaceae.id.2024.
The functional pathway illustrated in Figure 5 demonstrates the connection of HNC to the genus Gordonibacter.id.821 through three genes: PTN, BCL6, and HDAC9. Elevated levels of Pleiotrophin (PTN) in HNC contribute to aggressive tumor behavior and correlate with poor prognosis (35). The overexpression of BCL6 in HNC fosters tumor growth and metastasis, with BCL6 expression and neutrophil infiltration serving as significant prognostic indicators in HPV-related oropharyngeal cancer (36). Furthermore, the heightened expression of HDAC9 in HNC drives tumor progression, metastasis, and resistance to cisplatin therapy, highlighting its potential as a therapeutic target across various HNC subtypes (37–39).
The gene connecting HNC and genus Methanobrevibacter.id.123 is DUSP5. DUSP5 plays a significant role in HNC progression. Downregulation of DUSP5 promotes tumor growth by dysregulating MAPK signaling pathways, leading to cetuximab resistance and potential influence by lncRNA-ENST00000412010 (40, 41). Conversely, upregulation of DUSP5 suppresses tumor growth, inhibiting proliferation and invasion, indicating its potential as a therapeutic target (42, 43).
Four genes, namely DSCAM, ADORA2A, SPECC1L, and PTPRD, establish a connection between the genus DefluviitaleaceaeUCG011.id.11287 and HNC. Overexpression of DSCAM and ADORA2A in HNC has been correlated with tumor progression and a poorer prognosis, thereby identifying them as potential therapeutic targets (44, 45). SPECC1L facilitates HNC progression by modulating cell pathways. Moreover, the presence of the BCL6-SPECC1L fusion gene in nasopharyngeal carcinoma implies its relevance in this specific subtype (46). Alterations in the PTPRD gene affect tumor suppression and cell growth in HNC, suggesting its involvement in disease progression and its potential therapeutic significance (47).
It’s important to note that some IVs (SNPs) mapped to genes do not have a documented connection with HNC, nor do the IVs themselves. Additionally, most genes corresponding to these IVs were not simultaneously enriched in the same pathways, indicating their diverse functionality. This highlights the need for further research into these genes and genetic variants to elucidate their potential role in HNC.
This study has several limitations. First, all participants were of European origin, which limits the generalizability of our findings to other populations. Second, the relatively small number of HNC cases compared to controls may have reduced the statistical power to detect subtle associations. Third, the reported results regarding the microbiota are based on extremely broad taxonomic levels (family, genus, and phylum), which may result in findings that are too general and lack specificity. The use of 16S rRNA gene sequencing in the MiBioGen consortium’s GWAS data allows for detection only at these broad levels, with no genetic data available at the species level. Additionally, the negative results may carry biases related to sample size and power, warranting cautious interpretation. Furthermore, the analysis may be influenced by contamination from environmental factors and other confounding variables. Our findings are also impacted by the presence of numerous genetic variants with relatively small effect sizes, which can complicate causal inference. Moreover, none of the taxa showed significance after FDR correction, likely due to the small number of instrumental variables (IVs) used in our MR analysis, which limited statistical power and led to relatively larger p-values. Lastly, the potential for a high rate of false positives among the identified variants further underscores the need for careful interpretation of our results.
5 Conclusion
Our study identified four gut microbiota (GMs) with significant causal relationships with HNC at the genetic level. Specifically, genetic predisposition to the family Peptococcaceae.id.2024 was linked to a decreased risk of HNC, while genetic predispositions to the genus DefluviitaleaceaeUCG011.id.11287, genus Gordonibacter.id.821, and genus Methanobrevibacter.id.123 were associated with an increased risk of HNC. These GMs interact with genes and genetic variants involved in signaling pathways, such as GTPase regulation, influencing tumor progression and disease prognosis. Our results indicate a causal effect of certain GM microbial compositions on HNC, providing significant insights for advancing clinical research and tailoring treatment approaches for HNC.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://r10.risteys.finngen.fi/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ML: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MS: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. BH: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AB: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. HC: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. FZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was partially supported by the Beijing Municipal Administration of Hospitals’ Youth Programme (QML20210203) and R&D Program of Beijing Municipal Education Commission (KM202210025014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GM, Gut Microbiome; GO, Gene Ontology; GWAS, Genome-Wide Association Studies; HNC, Head and Neck Cancer; HPV, human papillomavirus; ICD, International Classification of Diseases; IV, Instrumental Variable; IVW, Inverse-Variance Weighted; KEGG, Kyoto Encyclopedia of Genes and Genomes; LOO, Leave-One-Out; MR, Mendelian Randomization; OR, Odds Ratio; CI, Confidence Interval; SNP, Single Nucleotide Polymorphism; WM, Weighted Median.
References
1. Goldschmidt S, Soltero-Rivera M, Quiroz A, Wong K, Rebhun R, Zwingenberger A, et al. The diagnostic yield of preoperative screening for oral cancer in dogs over 15 years, part 1: locoregional screening. J Am Vet Med Assoc. (2023) 261:S14–23. doi: 10.2460/javma.23.05.0299
2. Garcia AB, Infante de la Torre JR, Carbonero RB, Esteve AM, Barragan VV, Vicente JS, et al. Prognostic value of haematological parameters and [(18)F]FDG PET/CT metabolic parameters in head and neck cancer. Rev Esp Med Nucl Imagen Mol (Engl Ed). (2024) 43:31–8. doi: 10.1016/j.remnie.2023.10.002
3. Li H, Zhao L, Song Y, Liu Y, Ni S, Liu S. Prognostic significance of primary tumor surgery in adenoid cystic carcinoma patients with distant metastases at diagnosis: A population-based database analysis in head and neck region. Ear Nose Throat J. (2023) 20:1455613231207262. doi: 10.1177/01455613231207262
4. Chaudhary V, Chaudhary N, Mathews S, Singh RD. Human papilloma virus-associated oral pharyngeal squamous cell carcinoma: prevalence, prevention, and awareness of vaccination in the Indian population. Crit Rev Oncog. (2023) 28:63–72. doi: 10.1615/CritRevOncog.2023048944
5. Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, Chang MW. Microbiome and human health: current understanding, engineering, and enabling technologies. Chem Rev. (2023) 123:31–72. doi: 10.1021/acs.chemrev.2c00431
7. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. (2013) 341:1237439. doi: 10.1126/science.1237439
8. Barlow GM, Mathur R. Type 2 diabetes and the microbiome. J Endocr Soc. (2022) 7:bvac184. doi: 10.1210/jendso/bvac184
9. Miszczuk E, Bajguz A, Kiraga L, Crowley K, Chlopecka M. Phytosterols and the digestive system: A review study from insights into their potential health benefits and safety. Pharm (Basel). (2024) 17(5):557. doi: 10.3390/ph17050557
10. Green GBH, Cox-Holmes AN, Backan O, Valbak O, Potier ACE, Chen D, et al. Exploring gut microbiota alterations with trimethoprim-sulfamethoxazole and dexamethasone in a humanized microbiome mouse model. Microorganisms. (2024) 12(5):1015. doi: 10.3390/microorganisms12051015
11. Caldarelli M, Rio P, Marrone A, Ocarino F, Chiantore M, Candelli M, et al. Gut-brain axis: focus on sex differences in neuroinflammation. Int J Mol Sci. (2024) 25(10):5377. doi: 10.3390/ijms25105377
12. Sidiropoulos T, Dovrolis N, Katifelis H, Michalopoulos NV, Kokoropoulos P, Arkadopoulos N, et al. Dysbiosis signature of fecal microbiota in patients with pancreatic adenocarcinoma and pancreatic intraductal papillary mucinous neoplasms. Biomedicines. (2024) 12(5):1040. doi: 10.3390/biomedicines12051040
13. Makarewicz J, Kazmierczak-Siedlecka K, Sobocki BK, Dobrucki IT, Kalinowski L, Stachowska E. Anti-cancer management of head and neck cancers and oral microbiome-what can we clinically obtain? Front Cell Infect Microbiol. (2024) 14:1329057. doi: 10.3389/fcimb.2024.1329057
14. Zeng J, Yi B, Chang R, Li J, Zhu J, Yu Z, et al. The causal effect of gut microbiota and plasma metabolome on lung cancer and the heterogeneity across subtypes: A mendelian randomization study. J Pers Med. (2024) 14(5):453. doi: 10.3390/jpm14050453
15. Messaritakis I, Koulouris A, Boukla E, Vogiatzoglou K, Lagkouvardos I, Intze E, et al. Exploring gut microbiome composition and circulating microbial DNA fragments in patients with stage II/III colorectal cancer: A comprehensive analysis. Cancers (Basel). (2024) 16(10):1923. doi: 10.3390/cancers16101923
16. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
17. Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
18. Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. (2021) 53:156–65. doi: 10.1038/s41588-020-00763-1
19. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
20. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
21. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
22. Meng Y, Zhang X, Zhai Y, Li Y, Shao Z, Liu S, et al. Identification of the mutual gliding locus as a factor for gut colonization in non-native bee hosts using the ARTP mutagenesis. Microbiome. (2024) 12:93. doi: 10.1186/s40168-024-01813-0
23. Lozano J, Cunha E, Almeida C, Nunes M, Dias R, Vicente E, et al. Analyzing the safety of the parasiticide fungus Mucor circinelloides: first insights on its virulence profile and interactions with the avian gut microbial community. Microbiol Spectr. (2024) 12:e0407823. doi: 10.1128/spectrum.04078-23
24. Munoz-Juan A, Assie A, Esteve-Codina A, Gut M, Benseny-Cases N, Samuel BS, et al. Caenorhabditis elegans endorse bacterial nanocellulose fibers as functional dietary Fiber reducing lipid markers. Carbohydr Polym. (2024) 331:121815. doi: 10.1016/j.carbpol.2024.121815
25. Hong M, Li Z, Liu H, Zheng S, Zhang F, Zhu J, et al. Fusobacterium nucleatum aggravates rheumatoid arthritis through FadA-containing outer membrane vesicles. Cell Host Microbe. (2023) 31:798–810 e7. doi: 10.1016/j.chom.2023.03.018
26. Fernandez-Parejo N, Lorenzo-Martin LF, Garcia-Pedrero JM, Rodrigo JP, Dosil M, Bustelo XR. VAV2 orchestrates the interplay between regenerative proliferation and ribogenesis in both keratinocytes and oral squamous cell carcinoma. Sci Rep. (2024) 14:4060. doi: 10.1038/s41598-024-54808-0
27. Xiao GY, Tan X, Rodriguez BL, Gibbons DL, Wang S, Wu C, et al. EMT activates exocytotic Rabs to coordinate invasion and immunosuppression in lung cancer. Proc Natl Acad Sci U S A. (2023) 120:e2220276120. doi: 10.1073/pnas.2220276120
28. Li X, Lu W, Zhou T, Zhao F, Yang L. Timosaponin AIII suppresses RAP1 signaling pathway to enhance the inhibitory effect of paclitaxel on nasopharyngeal carcinoma. Comput Math Methods Med. (2022) 2022:6756676. doi: 10.1155/2022/6756676
29. Ling Z, Li W, Hu J, Li Y, Deng M, Zhang S, et al. Targeting CCL2-CCR4 axis suppress cell migration of head and neck squamous cell carcinoma. Cell Death Dis. (2022) 13:158. doi: 10.1038/s41419-022-04610-5
30. Namkoong H, Edahiro R, Takano T, Nishihara H, Shirai Y, Sonehara K, et al. DOCK2 is involved in the host genetics and biology of severe COVID-19. Nature. (2022) 609:754–60. doi: 10.1038/s41586-022-05163-5
31. Zhu Y, Liu W, Wang Z, Wang Y, Tan C, Pan Z, et al. ARHGEF2/EDN1 pathway participates in ER stress-related drug resistance of hepatocellular carcinoma by promoting angiogenesis and Malignant proliferation. Cell Death Dis. (2022) 13:652. doi: 10.1038/s41419-022-05099-8
32. Yuan Q, Fan Y, Liu Z, Wang X, Jia M, Dong Y, et al. Pleckstrin homology and RhoGEF domain containing G4 (PLEKHG4) leads to the activation of RhoGTPases promoting the Malignant phenotypes of thyroid cancer. Apoptosis. (2023) 28:1315–31. doi: 10.1007/s10495-023-01861-1
33. Guo H, Ji Y, Zhang B, Huang X. Fibulin-3 sponges Tiam1 to manipulate MMP-7 activity through beta-catenin signaling in oral squamous cell carcinoma. Med Oncol. (2022) 39:154. doi: 10.1007/s12032-022-01746-4
34. Liu W, Miao C, Zhang S, Liu Y, Niu X, Xi Y, et al. VAV2 is required for DNA repair and implicated in cancer radiotherapy resistance. Signal Transduct Target Ther. (2021) 6:322. doi: 10.1038/s41392-021-00735-9
35. Li B, Wong IY, Chan FS, Chan KK, Wong CL, Law TT, et al. Impact of extracapsular extension of lymph node in adenocarcinoma of the stomach. Ann Surg Oncol. (2020) 27:4225–32. doi: 10.1245/s10434-020-08541-3
36. Atique M, Muniz I, Farshadi F, Hier M, Mlynarek A, Macarella M, et al. Genetic mutations associated with inflammatory response caused by HPV integration in oropharyngeal squamous cell carcinoma. Biomedicines. (2023) 12(1):24. doi: 10.3390/biomedicines12010024
37. Milan TM, Eskenazi APE, Bighetti-Trevisan RL, de Almeida LO. Epigenetic modifications control loss of adhesion and aggressiveness of cancer stem cells derived from head and neck squamous cell carcinoma with intrinsic resistance to cisplatin. Arch Oral Biol. (2022) 141:105468. doi: 10.1016/j.archoralbio.2022.105468
38. Rastogi B, Raut SK, Panda NK, Rattan V, Radotra BD, Khullar M. Overexpression of HDAC9 promotes oral squamous cell carcinoma growth, regulates cell cycle progression, and inhibits apoptosis. Mol Cell Biochem. (2016) 415:183–96. doi: 10.1007/s11010-016-2690-5
39. Ratovitski EA. Phospho-DeltaNp63alpha/microRNA network modulates epigenetic regulatory enzymes in squamous cell carcinomas. Cell Cycle. (2014) 13:749–61. doi: 10.4161/cc.27676
40. Boeckx C, Op de Beeck K, Wouters A, Deschoolmeester V, Limame R, Zwaenepoel K, et al. Overcoming cetuximab resistance in HNSCC: the role of AURKB and DUSP proteins. Cancer Lett. (2014) 354:365–77. doi: 10.1016/j.canlet.2014.08.039
41. Cheng Y, Peng H, Lu XQ, Liang CQ, Fan JP, Liu HH. Role of lncRNA-ENST00000412010 in regulating nasopharyngeal cancer cell survival. Rhinology. (2020) 58:588–96. doi: 10.4193/Rhin19.341
42. Zhang Q, Xing Y, Jiang S, Xu C, Zhou X, Zhang R, et al. Integrated analysis identifies DUSP5 as a novel prognostic indicator for thyroid follicular carcinoma. Thorac Cancer. (2020) 11:336–45. doi: 10.1111/1759-7714.13270
43. Boonruang K, Kim I, Kwag C, Ryu J, Baek SJ. Quercetin induces dual specificity phosphatase 5 via serum response factor. BMB Rep. (2023) 56:508–13. doi: 10.5483/BMBRep.2023-0051
44. Qiu Z, Pan XX, You DY. LncRNA DSCAM-AS1 promotes non-small cell lung cancer progression via regulating miR-577/HMGB1 axis. Neoplasma. (2020) 67:871–9. doi: 10.4149/neo_2020_190826N821
45. Mattox AK, Roelands J, Saal TM, Cheng Y, Rinchai D, Hendrickx W, et al. Myeloid cells are enriched in tonsillar crypts, providing insight into the viral tropism of human papillomavirus. Am J Pathol. (2021) 191:1774–86. doi: 10.1016/j.ajpath.2021.06.012
46. Fang SG, Xia TL, Fu JC, Li T, Zhong Q, Han F. BCL6-SPECC1L: A novel fusion gene in nasopharyngeal carcinoma. Technol Cancer Res Treat. (2022) 21:15330338221139981. doi: 10.1177/15330338221139981
Keywords: gut microbiome, head and neck cancer, Mendelian randomization, enrichment analysis, functional annotation
Citation: Lian M, Sun M, Han B, Baranova A, Cao H and Zhang F (2024) Gut microbiome’s causal role in head and neck cancer: findings from mendelian randomization. Front. Oncol. 14:1453202. doi: 10.3389/fonc.2024.1453202
Received: 22 June 2024; Accepted: 28 October 2024;
Published: 19 November 2024.
Edited by:
Dietmar Thurnher, Medical University of Graz, AustriaReviewed by:
Georgia Damoraki, National and Kapodistrian University of Athens, GreeceBhaskar Roy, Chinese Academy of Sciences, China
Copyright © 2024 Lian, Sun, Han, Baranova, Cao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Lian, bGlhbm1lbmcxOTg2MTIyMkAxNjMuY29t
 Meng Lian
Meng Lian Minghong Sun
Minghong Sun Boxuan Han
Boxuan Han Ancha Baranova
Ancha Baranova Hongbao Cao
Hongbao Cao Fuquan Zhang
Fuquan Zhang