
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 08 July 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1374594
This article is part of the Research TopicTreatment Response and Resistance to Targeted Therapies for NSCLCView all 16 articles
 Akihiro Nishiyama1*
Akihiro Nishiyama1* Shigeki Sato1
Shigeki Sato1 Hiroyuki Sakaguchi1
Hiroyuki Sakaguchi1 Hiroshi Kotani1
Hiroshi Kotani1 Kaname Yamashita1
Kaname Yamashita1 Koushiro Ohtsubo1
Koushiro Ohtsubo1 Shigeki Nanjo2
Shigeki Nanjo2 Seiji Yano2
Seiji Yano2 Keishi Mizuguchi3
Keishi Mizuguchi3 Hiroko Ikeda3
Hiroko Ikeda3 Shinji Takeuchi1*
Shinji Takeuchi1*We report a case of limited effectiveness of dabrafenib and trametinib in a 59-year-old man with poorly differentiated lung carcinoma and a rare BRAF K601E mutation. The patient, unresponsive to chemotherapy and immunotherapy, received these targeted agents as second-line treatment. Despite a notable initial response, tumor regression lasted only 52 days. A subsequent liquid biopsy revealed additional alterations (BRAF amplification, KIT amplification, TP53 S241F), indicating a complex resistance mechanism. This case underscores the challenges in treating BRAF K601E-mutant lung carcinoma, emphasizing the need for advanced molecular diagnostics, personalized approaches, and further research into more effective therapies for unique genetic profiles.
BRAF mutations are recognized as tumor-agnostic driver mutations that play pivotal roles in the pathogenesis of various cancers (1). Among the more than 50 identified BRAF mutations, the V600E variant is the most prevalent, accounting for approximately 63% of all BRAF mutations (1). In non-small cell lung cancer (NSCLC), these mutations occur in approximately 3% of cases, with one-third involving the V600E mutations. Targeted therapy, utilizing a BRAF inhibitor such as dabrafenib in combination with a MEK inhibitor like trametinib, is approved for melanoma and NSCLCs with the BRAF V600E mutation. However, the treatment landscape for non-V600E mutations, such as K601E, is unclear (2). These non-V600E mutations are often associated with distinct clinical behaviors and responses to therapy in NSCLC, necessitating further exploration of effective treatment strategies.
A 59-year-old man visited Kanazawa University Hospital with swelling in the left neck and chest stiffness. Imaging studies, including PET-CT, revealed a nodule in the upper lobe of the left lung and multiple lesions in the lymph nodes, skin, and bone. Biopsy of the skin lesions of the precordium revealed poorly differentiated carcinoma (Figure 1A), with negative immunostaining for TTF-1 and positive immunostaining for p40 and p16 (Figures 1B-D). Despite p16 positivity suggesting human papillomavirus-associated oropharyngeal cancer, no oropharyngeal findings were observed. The patient was diagnosed with poorly differentiated lung carcinoma, with programmed cell death ligand (PD-L1) expression levels ranging from 50% to 74%. Before genomic testing, the patient received carboplatin, nab-paclitaxel, and pembrolizumab as the first-line treatment. However, due to worsening pain from the growth of cutaneous metastases and deterioration of performance status, this treatment was discontinued after two courses (Supplementary Figure 1A, B). Genomic testing (Oncomine Dx Target Test Multi-CDx system®) of precordium skin metastasis (70% tumor cell content) revealed a mutation of BRAF K601E with a variant allele frequency of 17.4% (Figure 1E). Subsequently, dabrafenib (300 mg/day) and trametinib (2 mg/day) were administered as the second-line treatments. One month later, significant tumor reduction was observed (Figure 2), but by 1.5 months, several cutaneous metastases, including the precordium lesion, had enlarged, indicating limited treatment efficacy (Supplementary Figures 1C, 2). The patient then received docetaxel and ramucirumab as third-line treatments, which were discontinued in 8 days because of severe side effects, such as disorientation or gastrointestinal bleeding.
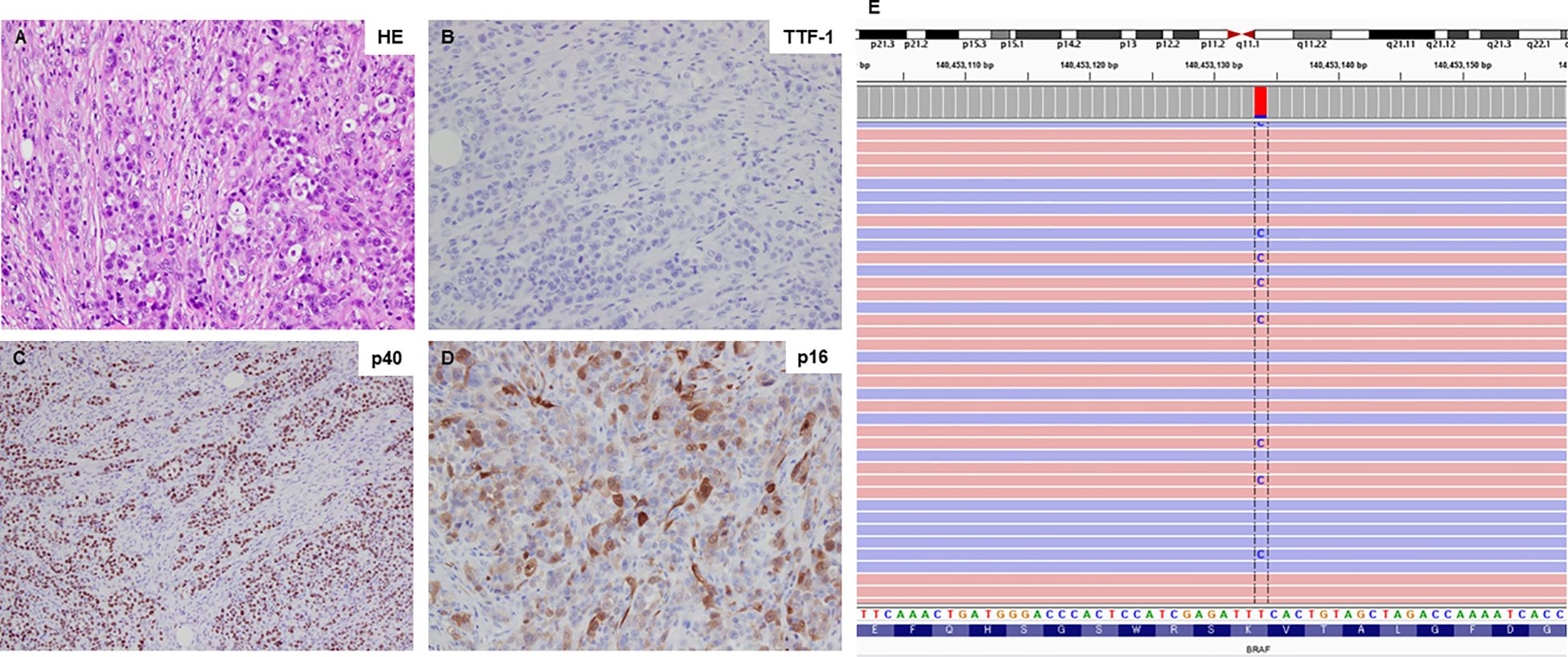
Figure 1 Histological findings and BRAF K601E sequence data. (A) Hematoxylin-eosin staining of the primary tumor sample (20×). (B-D) The primary tumor sample was negative for TTF-1 expression but positive for p40 and p16 expression (all shown at 20×). (E) Next-generation sequencing data illustrating the BRAF K601E mutation, visualized using the Integrative Genomics Viewer (IGV).
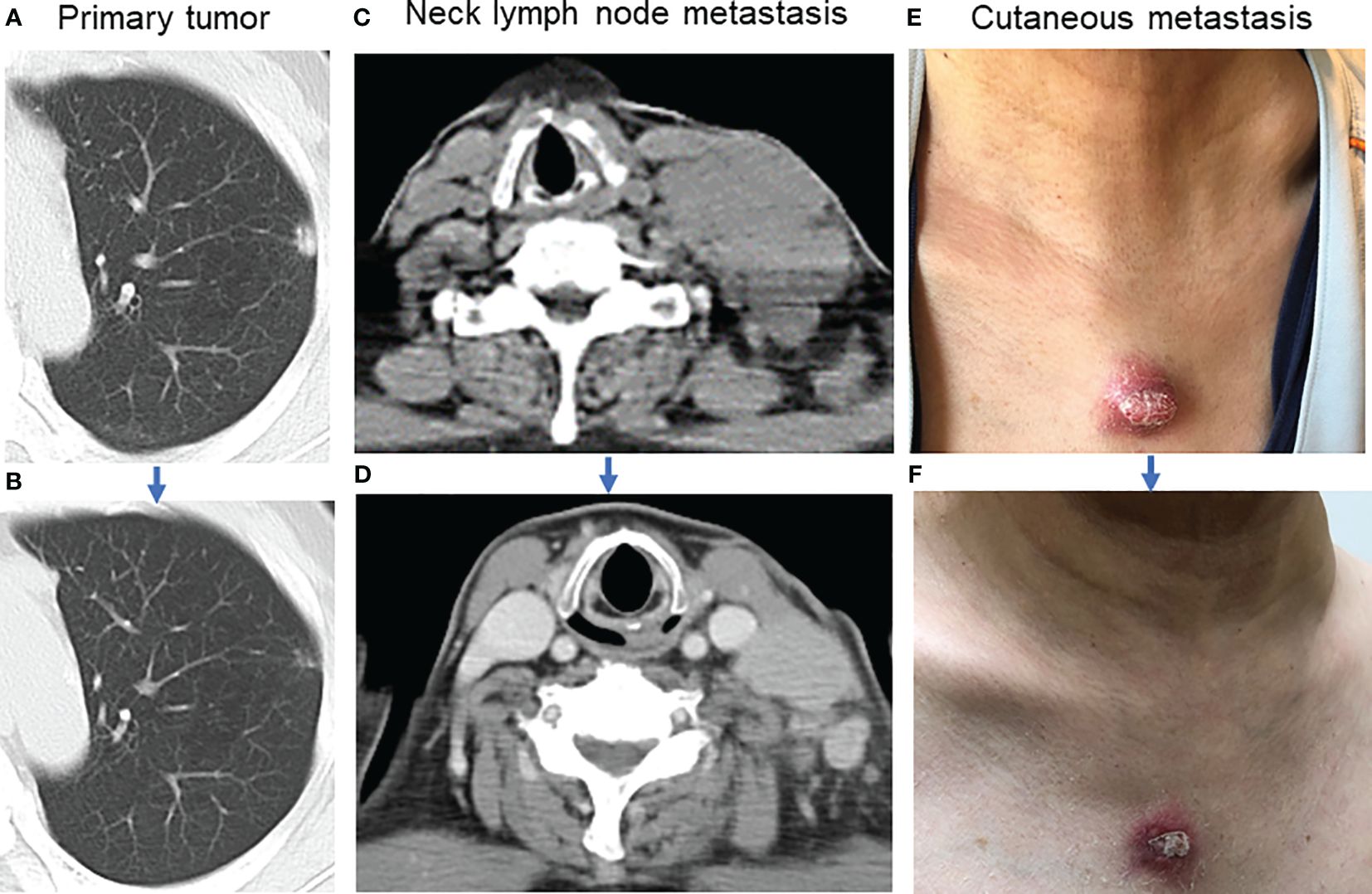
Figure 2 Tumor shrinkage after dabrafenib and trametinib. (A) Image of the primary tumor located in the left upper lobe of the lung before combination therapy. (B) The primary tumor almost disappeared one month after combination therapy. (C) Lymph node metastasis in the left neck before combination therapy. (D) Left neck lymph node metastasis decreased one month after combination therapy. (E) The appearance of the precordium cutaneous metastasis before combination therapy. (F) Precordium cutaneous metastasis shrank one month after combination therapy.
Given the aggressive nature of his disease, he agreed to a rechallenge with dabrafenib and trametinib without a washout period. One month after the rechallenge, a liquid biopsy (Guardant360®) detected BRAF K601E, BRAF amplification, KIT amplification, and TP53 S241F mutations (Table 1). However, the treatment was ineffective, and he passed away 1.5 months after the initiation of the re-challenge.
This case report underscores the complexities and challenges of treating poorly differentiated lung carcinoma with the BRAF K601E mutation, highlighting the nuanced nature of cancer therapies, particularly in cases involving less common mutations. The initial rapid tumor regression observed with dabrafenib and trametinib treatment, followed by the development of resistance within two months, illustrates the dynamic interplay of molecular mechanisms in cancer treatment.
The resistance mechanism in our patient can be partly attributed to the nature of RAF inhibitors. These inhibitors are classified as αC-helix-IN (CI) or αC-helix-OUT (CO). CO inhibitors, such as vemurafenib and dabrafenib, are known to effectively target the monomeric form of BRAF V600E. However, their efficacy is significantly reduced against non-V600E mutations, such as K601E, which tend to form dimers. This dimerization can hinder the binding of CO inhibitors to the second protomer, leading to reduced efficacy (3, 4). These molecular dynamics could explain the rapid development of resistance in our patient, where the initial inhibition of monomeric BRAF K601E by dabrafenib was overcome by the emergence of dimeric forms. Interestingly, some patients with a higher variant allele frequency of BRAF K601E than ours exhibited a longer response to dabrafenib and trametinib (5, 6).
Additional genetic alterations identified in post-treatment liquid biopsy—BRAF amplification, KIT amplification, and the TP53 S241F mutation—further complicate the response to treatment. Coexisting genetic changes can interact with the primary BRAF mutations to promote resistance. For example, BRAF amplification can increase the expression of mutant proteins, thereby reducing efficacy (7). Similarly, KIT amplification (8) and the TP53 S241F mutation (9, 10) can activate additional oncogenic pathways or alter apoptosis, contributing to the observed resistance. In our study, both BRAF and KIT showed medium levels of amplification, suggesting that these alterations may not robustly drive resistance mechanisms owing to their moderate intensity. Additionally, targeted BRAF therapy may induce alterations in the MAPK pathway, such as the emergence of NRAS Q61K mutations (11, 12). However, these alterations were not detected in the present case. Understanding these complex interactions is crucial for developing effective treatment strategies for such patients.
The findings from this case emphasize the need for personalized treatment strategies for NSCLC, particularly for patients with rare or atypical BRAF mutations. The development of newer CI inhibitors or dual-action inhibitors that can target the monomeric and dimeric forms of BRAF could offer more effective treatment solutions (3, 4). Numerous ongoing clinical trials are investigating potential treatments for BRAF non-V600E mutations, including BRAF fusions (Supplementary Table 1). Additionally, integrating genomic profiling into routine clinical practice can help guide treatment choices and identify potential resistance mechanisms early during treatment.
In conclusion, this case of poorly differentiated lung carcinoma with the BRAF K601E mutation demonstrates the challenges of treating complex cancer cases. A multifaceted approach that includes advanced molecular diagnostics, personalized therapy, and continuous monitoring is essential for improving patient outcomes. This case serves as a call for further research into the molecular underpinnings of BRAF-mutant lung carcinomas and highlights the need for clinical trials to explore novel therapeutic agents targeting specific molecular pathways in patients with non-V600E BRAF mutations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AN: Writing – original draft, Writing – review & editing. SS: Writing – review & editing. HS: Writing – review & editing. HK: Writing – review & editing. KY: Writing – review & editing. KO: Writing – review & editing. SN: Writing – review & editing. SY: Writing – review & editing. KM: Writing – review & editing. HI: Writing – review & editing. ST: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We extend our deepest gratitude to the patient and his family for their generosity in providing informed consent for the use of personal medical data in this study. We would also like to express our appreciation to SRL Co., Ltd. for their assistance in providing BRAF K601E sequence data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1374594/full#supplementary-material
Supplementary Figure 1 | Changes in scout images for confirmation of therapeutic effect. (A) Scout image acquired before the initiation of first-line treatment. (B) Scout image captured after completion of two courses of the first-line treatment. (C) Scout image acquired 1.5 months after initiation of combination therapy.
Supplementary Figure 2 | Progression of cutaneous metastases during combination therapy. (A) Appearance of the precordium cutaneous metastasis one month after initiation of combination therapy. (B) Notable enlargement of the precordium cutaneous metastasis 1.5 months after initiation of combination therapy. (C) Appearance of shoulder skin metastases one month after initiation of combination therapy. (D) Significant enlargement of shoulder skin metastases 1.5 months into combination therapy.
Supplementary Table 1 | Ongoing clinical trials exploring potential treatments for Non-V600E BRAF mutations and BRAF fusions.
1. Owsley J, Stein MK, Porter J, K In G, Salem M, O’Day S, et al. Prevalence of class I-III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp Biol Med. (2021) 246:31–9. doi: 10.1177/1535370220959657
2. Menzer C, Menzies AM, Carlino MS, Reijers I, Groen EJ, Eigentler T, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol. (2019) 37:3142–51. doi: 10.1200/JCO.19.00489
3. Karoulia Z, Wu Y, Ahmed TA, Xin Q, Bollard J, Krepler C, et al. An integrated model of RAF inhibitor action predicts inhibitor activity against oncogenic BRAF signaling. Cancer Cell. (2016) 30:485–98. doi: 10.1016/j.ccell.2016.06.024
4. Adamopoulos C, Ahmed TA, Tucker MR, M.U. Ung P, Xiao M, Karoulia Z, et al. Exploiting allosteric properties of RAF and MEK inhibitors to target therapy-resistant tumors driven by oncogenic BRAF signaling. Cancer Discovery. (2021) 11:1716–35. doi: 10.1158/2159-8290.c.6549364
5. Su PL, Kin CY, Chen YL, Chen WL, Lin CC, Su WC. Durable response to combined dabrafenib and trametinib in a patient with BRAF K601E mutation-positive lung adenocarcinoma: A case report. JTO Clin Res Rep. (2021) 2:100202. doi: 10.1016/j.jtocrr.2021.100202
6. Rogiers A, Thomas D, Borght SV, van den Oord JJ, Bechter O, Dewaele M, et al. Dabrafenib plus trametinib in BRAF K601E-mutant melanoma. Br J Dermatol. (2019) 180:421–2. doi: 10.1111/bjd.17250
7. Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J and Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. (2010) 3. doi: 10.1126/scisignal.2001148
8. Lovly CM, Shaw AT. Molecular pathways: Resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. (2014) 20:2249–56. doi: 10.1158/1078-0432.CCR-13-1610
9. Surojit S, Raymond P, Fred B, Rago C, Diaz LA, Kinzler KW, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci USA. (2009) 106:3964–9. doi: 10.1073/pnas.0813333106
10. Kron A, Alidousty C, Scheffler M, Merkelbach-Bruse S, Seidel D, Riedel R, et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann Oncol. (2018) 29:2068–75. doi: 10.1093/annonc/mdy333
11. Abravanel DL, Nishino M, Sholl LM, Ambrogio C, Awad MM. An acquired NRAS Q61K mutation in BRAF V600E-mutant lung adenocarcinoma resistant to dabrafenib plus trametinib. J Thorac Oncol. (2018) 13:e131–3. doi: 10.1016/j.jtho.2018.03.026
Keywords: BRAF K601E mutation, lung carcinoma, dabrafenib, trametinib, resistance to targeted therapy, liquid biopsy
Citation: Nishiyama A, Sato S, Sakaguchi H, Kotani H, Yamashita K, Ohtsubo K, Nanjo S, Yano S, Mizuguchi K, Ikeda H and Takeuchi S (2024) Challenges in the treatment of BRAF K601E-mutated lung carcinoma: a case report of rapid response and resistance to dabrafenib and trametinib. Front. Oncol. 14:1374594. doi: 10.3389/fonc.2024.1374594
Received: 22 January 2024; Accepted: 26 June 2024;
Published: 08 July 2024.
Edited by:
Shiyou Wei, Sichuan University, ChinaReviewed by:
Po-Lan Su, National Cheng Kung University, TaiwanCopyright © 2024 Nishiyama, Sato, Sakaguchi, Kotani, Yamashita, Ohtsubo, Nanjo, Yano, Mizuguchi, Ikeda and Takeuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Nishiyama, YW4wNTEwQHN0YWZmLmthbmF6YXdhLXUuYWMuanA=; Shinji Takeuchi, dGFrZXVjaGlAc3RhZmYua2FuYXphd2EtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.