
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 03 April 2024
Sec. Cancer Genetics
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1339955
We report a case of recurrent retroperitoneal leiomyosarcoma in a male who achieved a rapid and robust but transient clinical response to low-dose iodine-125 brachytherapy. A FANCD2 frameshift mutation was detected by gene sequencing in the cancerous tissue.
Retroperitoneal sarcoma (RPS) is a rare disease, and surgery is the mainstay of its treatment (1). Retroperitoneal leiomyosarcoma (RLMS) is a type of RPS. Recurrence after RPS resection, which occurs in more than half of patients, is primarily related to tumor biology and is associated with a significant decrease in overall survival (2). Treatment for recurrent RPS is individualized through multidisciplinary discussions (3). Research strategies are under development to expand genomic screening to guide treatment and improve the outcomes of patients with sarcoma (4, 5). Alterations in DNA damage repair (DDR) pathway genes have been associated with sarcoma development (6). Nacev et al. (7) characterized genomic alterations in a large cohort of 2,138 patients with 45 sarcoma subtypes by leveraging an institution-wide tumor genomic profiling initiative. They found that the frequency of DDR gene alterations was 10% in LMS. One of the altered genes across subtypes was FANCA (0.6%). Chudasama (8) observed deleterious aberrations in LMS were observed in multiple components of the homologous recombination repair (HRR) pathway, with high frequencies in PTEN (57%), BRCA2 (53%), ATM (22%). Additionally, aberrations were also found in members of the Fanconi anemia complementation groups, specifically FANCA (27%) and FANCD2 (10%).The most promising approaches for advanced leiomyosarcoma (LMS) include those targeting the DDR pathway (9). Research demonstrates FANCD2 plays a role in its resistance to treatment in alveolar rhabdomyosarcoma and a specific inhibition of FANCD2 leading to increased sensitivity to radiation in the PAX3-FOXO1 fusion gene cell line (10). However, there is still a lack of research on the effects of FANCD2 deletion and genetic mutations on tumor sensitivity to radiotherapy. In this study, we present the clinical journey of a recurrent RLMS patient with a FANCD2 gene mutation who underwent multiple radioactive iodine-125 (I-125) brachytherapy. This case highlights the potential of clinical molecular diagnostics in improving the management of radiation therapy for recurrent RLMS.
A 78-year-old male was admitted to our hospital in May 2015 with a history of three surgeries for retroperitoneal leiomyoma between 2001 and 2013. He had a recurrence 17 months after the last operation. A computed tomography (CT) scan revealed a tumor in the left upper abdomen measuring 12.9 × 6.9 × 6.6 cm. The patient experienced abdominal pain with a numeric rating scale (NRS) for pain score above 5. He patient refused further surgery and chemotherapy. To shrink tumors and relieve pain, I-125 radioactive seed implantation was performed under CT guidance, implanting 30 seeds once every 14 days. Ninety seeds (0.5 mCi) were implanted into the tumor bed, with a D90 [dose received by 90% of the gross tumor volume (GTV)] of 64.8 Gy. A drainage tube was provisionally installed, and 800 mL of reddish liquid was removed. A complete response of the left upper abdomen tumor was achieved after four months (Figure 1), and the NRS for pain score decreased to zero. However, a new metastatic lesion appeared in the left iliac fossa. The repeated local tumor recurrences were controlled by repeated implantations of 30–59 I-125 radioactive seeds with a D90 range of 15–65 Gy (Figures 2, 3). The specific treatment details are shown in Table 1. The patient had no serious complications during or after the seed implantations and was discharged from hospital 1-2 days after the seed implantations every time.
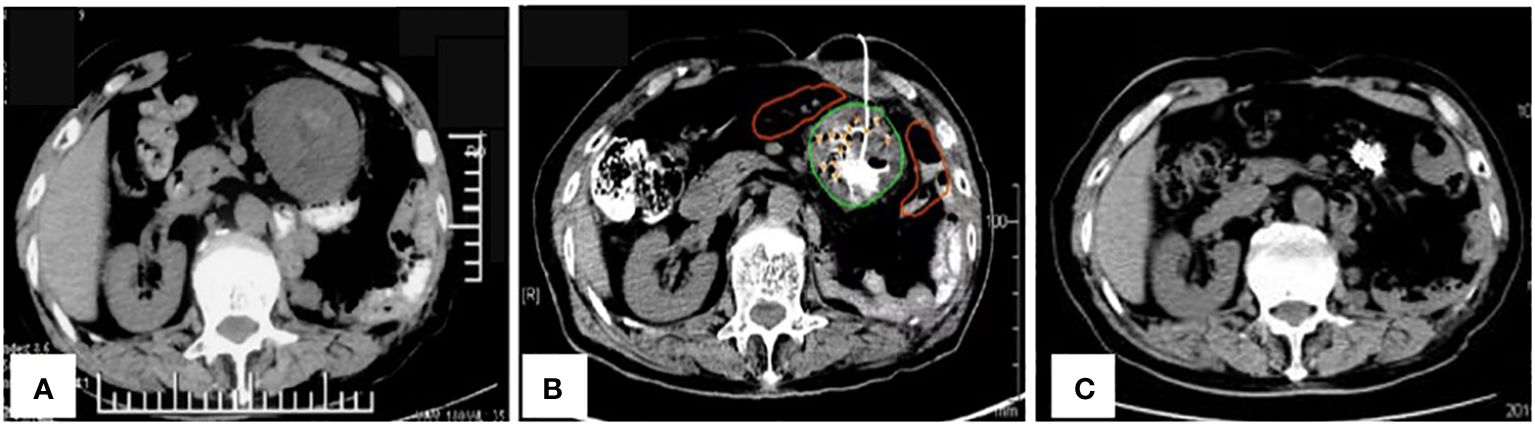
Figure 1 (A) Pre-operative imaging showed a recurrence in the left upper abdomen; (B) Placement of a drainage tube during the operation; (C) A complete response of the tumors in the left upper abdomen after six months and three iodine-125 seed implantation sessions.
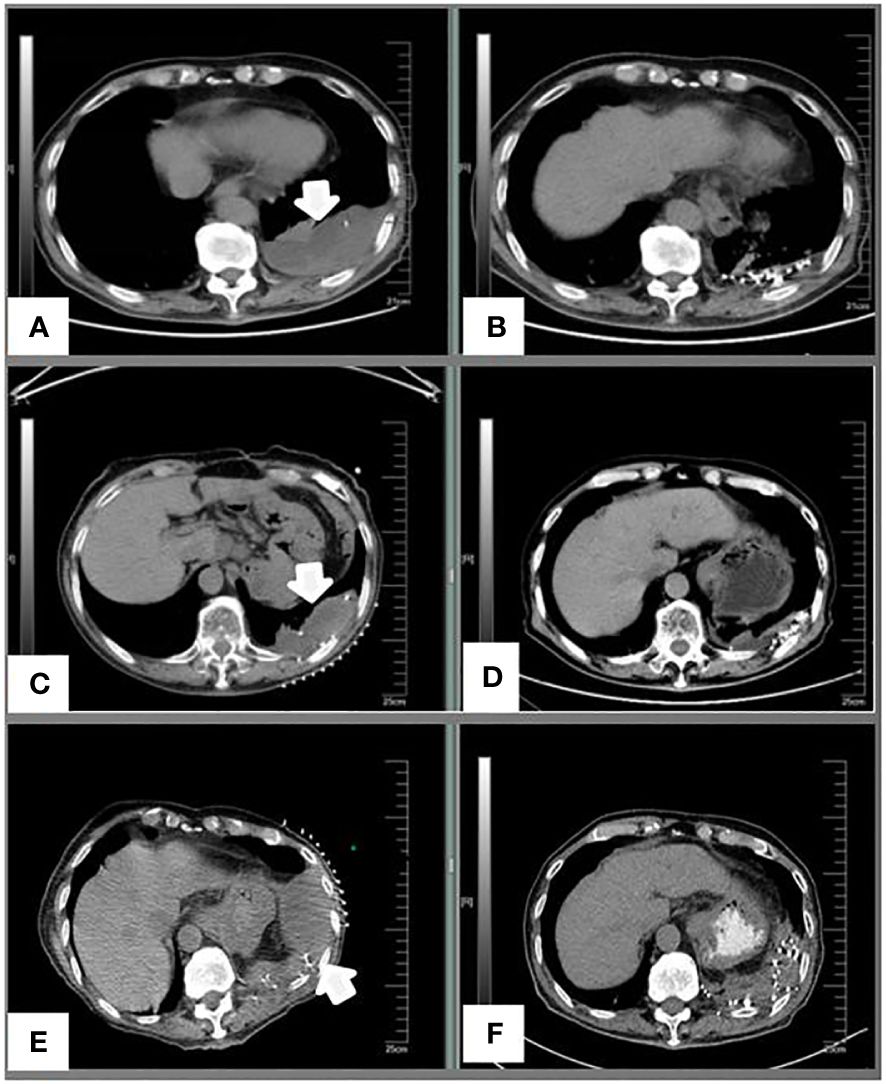
Figure 2 (A) A preoperative image of the left pleural metastases in September 2016. (B) Five months after I-125 seed implantation; (C) Progression of the left pleural metastases before treatment in January 2018. (D) Three months after I-125 seed implantation; (E) Re-progression of the left pleural metastases before treatment in November 2018. (F) Three months after I-125 seed implantation.
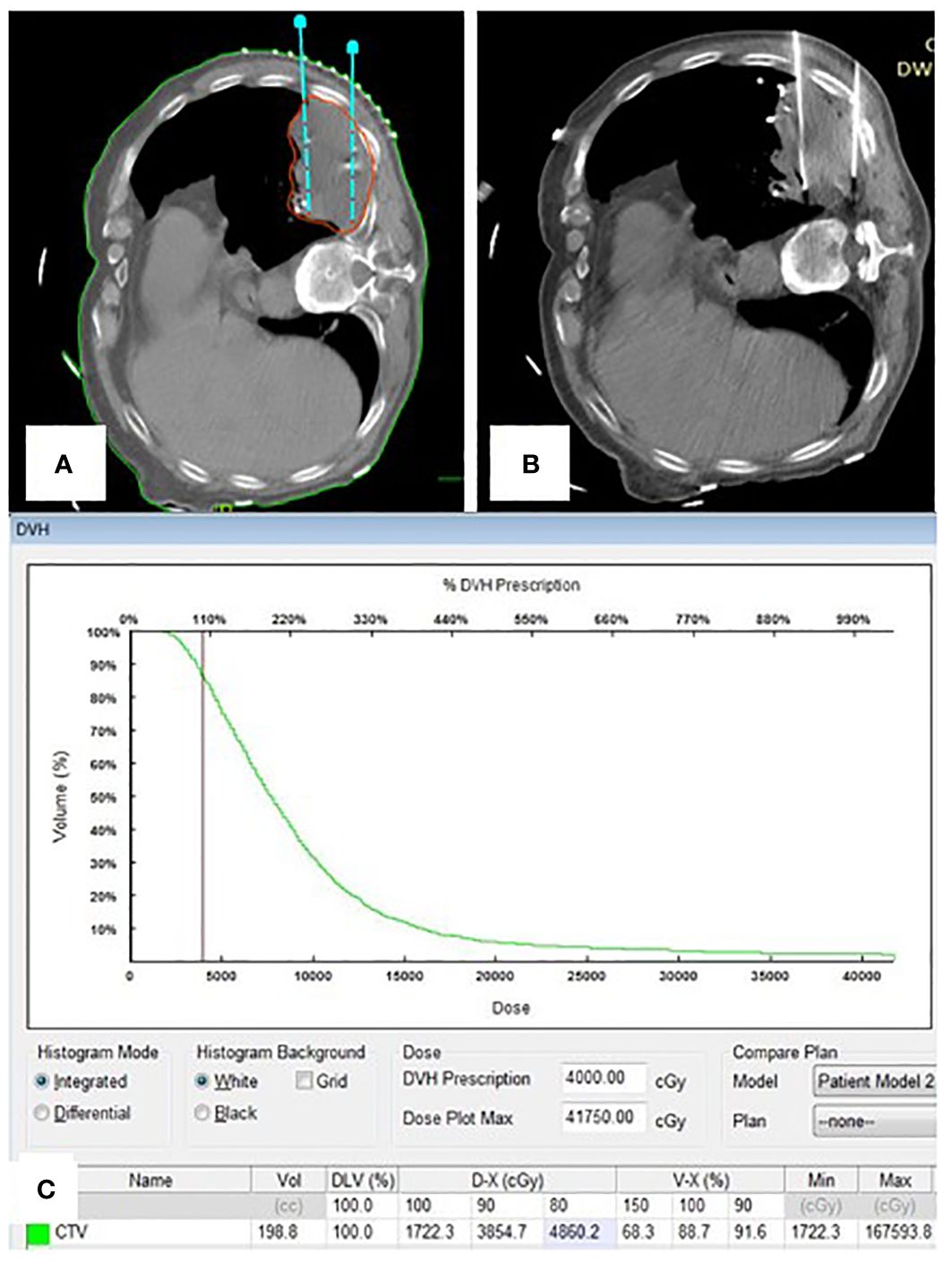
Figure 3 (A) Portion of pre-plan images; (B) Portion of real-time CT guided 125I seed implantation; (C) Dose-volume histogram of 125I seeds postoperative.
In January 2018, a left pleural progression (Figure 2) biopsy was performed for genetic assessment during seed implantation. The pathology results indicated a low-grade malignant leiomyosarcoma, consistent with the diagnosis. Targeted DNA and RNA sequencing of a panel of 422 genes revealed an FANCD2 frameshift mutation involving c.451_452delAA (p.K151fs) and c.3917delT (p.L1306). The patient refused systemic treatment. Subsequently, the patient’s mental condition deteriorated, refusing to re-enter the hospital for further medical treatments. The patient died from a self-administered drug overdose in September 2019, with an overall survival of 18 years. The patient underwent over 20 I-125 radioactive seed implantations without causing radiation-associated damage and lived for over four years after being deemed unsuitable for surgical resection.
The RPS recurrence intervals gradually shorten with the increase in the number of recurrences, and patients often die due to the local influence of recurrent tumors (11, 12). Therefore, reducing the local burden of recurrent RPS is critical to patient survival. Radiation treatment is generally considered beneficial for controlling local tumors. However, due to large volume of RPS tumors and their proximity to surrounding normal tissues, the risk of radiation-associated toxicity is substantial, reaching grade ≥3 toxicity in >30% of the cases (13, 14). Radioactive seed implantation represents a solution to mitigate healthy tissue damage. I-125 implantation is advantageous as brachytherapy inflicts less radiation damage to adjacent structures such as the bowel and genitourinary tract (15). Good local control, the goal of I-125 implantation for RPS, has been achieved (16). The I-125 seeds deliver continuous low-dose radiation, making them possibly more effective than daily pulsed high-dose irradiation in treating the hypoxic portion of large, slow-growing necrotic tumors (17). In the case of this patient with recurrent RLMS and such tumors, the repeated implantation of I-125 seeds for continuous radiotherapy proved beneficial. For certain tumors, repeated I-125 seed implantation could deliver better curative effects than external radiation therapy (18).
The literature indicates that the average dose for recurrent retroperitoneal sarcoma was reported to be 160 Gy (17). However, this research found that a D90 range of 15–65 Gy also yielded excellent results. The extraordinary response to low-dose radiation in this case might be attributed to the patient’s unique genetic background, the FANCD2 gene mutation. The Fanconi anemia pathway has been shown to be triggered during DNA replication to repair DNA damage (19). FANCD2 is the focal center of the Fanconi anemia signaling system that repairs DNA damage and safeguards genomic stability (20). The FANCD2 gene has 1,451 nucleotides and 44 exons, is located on chromosome 3p25.3, and has a mutation frequency of roughly 3% (21). Patients with Fanconi anemia are sensitive to ionizing radiation (22). Knocking down FANCD2 gene expression increases cancer cell sensitivity to gamma rays (23). Some studies have shown that silencing FANCD2 could greatly improve ionizing radiation sensitivity in vitro (24, 25). This study was the first to show the effect of FANCD2 mutations on the clinical sensitivity of RLMS to radiotherapy. The effectiveness of low-dose radiotherapy in sarcoma has been previously reported in a case in which SS18-POU5F1 sarcoma had a quick, robust, but transient clinical response to low-dose radiation, while it showed no response to various systemic therapies, including immune checkpoint inhibitors, angiogenesis inhibitors, and chemotherapy (26). From sarcoma’s clinical molecular diagnostics standpoint, the good response to low-dose radiation is an intriguing finding. This would be a fruitful area for further work.
This case report had several limitations. First, systemic treatment was limited, especially in combination with immunotherapy. Second, the local CR helped prolong the recurrence interval. The difficulty in avoiding radiation-induced complications in the peritoneal cavity with an increasing number of seeds limited our ability to find an optimum dose. The multiple local progressions might be related to insufficient local doses. Third, the patient died from a self-administered drug overdose.
We reported repeated I-125 seed implantations applied in recurrent RLMS with excellent results that might be attributed to the patient’s unique genetic background, the FANCD2 gene mutation. RLMS displays significant clinical and biologic heterogeneity. The future of RLMS treatment is contingent upon a greater understanding of tumor biology and the continued development of molecular markers. Future studies should investigate FANCD2 gene mutations in RLMS, as they might render it vulnerable to radiation.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Medical Ethics Committee of Hebei general Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from Postoperative puncture specimens of patients. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XL: Writing – original draft. JZ: Writing – review & editing, Data curation. XD: Writing – review & editing, Data curation. GC: Writing – review & editing, Supervision. HZ: Writing – review & editing. JW: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Porpiglia AS, Reddy SS, Farma JM. Retroperitoneal sarcomas. Surg Clin North Am. (2016) 96:993–1001. doi: 10.1016/j.suc.2016.05.009
2. Chouliaras K, Senehi R, Ethun CG, Poultsides G, Tran T, Grignol V, et al. Recurrence patterns after resection of retroperitoneal sarcomas: an eight-institution study from the US Sarcoma Collaborative. J Surg Oncol. (2019) 120:340–7. doi: 10.1002/jso.25606
3. Tseng WW, Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Ford SJ, et al. Management of locally recurrent retroperitoneal sarcoma in the adult: an updated consensus approach from the transatlantic Australasian retroperitoneal sarcoma working group. Ann Surg Oncol. (2022) 29:7335–48. doi: 10.1245/s10434-022-11864-y
4. Dufresne A, Brahmi M, Karanian M, Blay JY, et al. Using biology to guide the treatment of sarcomas and aggressive connective-tissue tumours. Nat Rev Clin Oncol. (2018) 15:443–58. doi: 10.1038/s41571-018-0012-4
5. Wang Z, Shi N, Naing A, Janku F, Subbiah V, Araujo DM, et al. Survival of patients with metastatic leiomyosarcoma: the MD Anderson Clinical Center for targeted therapy experience. Cancer Med. (2016) 5:3437–44. doi: 10.1002/cam4.956
6. Ballinger ML, Goode DL, Ray-Coquard I, James PA, Mitchell G, Niedermayr E, et al. Monogenic and polygenic determinants of sarcoma risk: an international genetic study. Lancet Oncol. (2016) 17:1261–71. doi: 10.1016/S1470-2045(16)30147-4
7. Nacev BA, Sanchez-Vega F, Smith SA, Antonescu CR, Rosenbaum E, Shi H, et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nat Commun. (2022) 13:3405. doi: 10.1038/s41467-022-30453-x
8. Chudasama P, Mughal SS, Sanders MA, Hübschmann D, Chung I, Deeg KI, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. (2018) 9:144. doi: 10.1038/s41467-017-02602-0
9. Lacuna K, Bose S, Ingham M, Schwartz G. Therapeutic advances in leiomyosarcoma. Front Oncol. (2023) 13:1149106. doi: 10.3389/fonc.2023.1149106
10. Singh M, Leasure JM, Chronowski C, Geier B, Bondra K, Duan W, et al. FANCD2 is a potential therapeutic target and biomarker in alveolar rhabdomyosarcoma harboring the PAX3-FOXO1 fusion gene. Clin Cancer Res Off J Am Assoc Cancer Res. (2014) 20:3884–95. doi: 10.1158/1078-0432.CCR-13-0556
11. MacNeill AJ, Miceli R, Strauss DC, Bonvalot S, Hohenberger P, Van Coevorden F, et al. Post-relapse outcomes after primary extended resection of retroperitoneal sarcoma: A report from the Trans-Atlantic RPS Working Group. Cancer. (2017) 123:1971–8. doi: 10.1002/cncr.30572
12. Gronchi A, Miceli R, Allard MA, Callegaro D, Le Péchoux C, Fiore M, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann Surg Oncol. (2015) 22:1447–54. doi: 10.1245/s10434-014-4130-7
13. Cosper PF, Olsen J, DeWees T, Van Tine BA, Hawkins W, Michalski J, et al. Intensity modulated radiation therapy and surgery for management of retroperitoneal sarcomas: A single-institution experience. Radiat Oncol. (2017) 12:198. doi: 10.1186/s13014-017-0920-y
14. Roeder F, Ulrich A, Habl G, Uhl M, Saleh-Ebrahimi L, Huber PE, et al. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis. BMC Cancer. (2014) 14:617. doi: 10.1186/1471-2407-14-617
15. Classen J, Hehr T, Lamprecht U, Zumbrägel A, Bamberg M, Budach W. Hyperfractionated 192Ir brachytherapy for recurrent retroperitoneal sarcoma: A technique for delivery of local tumor boost dose. Strahlenther Onkol. (2003) 179:118–22. doi: 10.1007/s00066-003-0998-z
16. Yang B, Guo WH, Lan T, Yuan F, Liu GJ, Zan RY, et al. CT-guided 125I seed implantation for inoperable retroperitoneal sarcoma: A technique for delivery of local tumor brachytherapy. Exp Ther Med. (2016) 12:3843–50. doi: 10.3892/etm.2016.3897
17. Kumar PP, Good RR. Interstitial 125I implantation in the retreatment of retroperitoneal soft tissue sarcoma. Rep case Acta Radiol Oncol. (1986) 25:37–9. doi: 10.3109/02841868609136375
18. Li Y, Wang Y, Liu B, Li Z, Wang W. (125) I brachytherapy seeds implantation for inoperable low-grade leiomyosarcoma of inferior vena cava. Korean J Radiol. (2013) 14:278–82. doi: 10.3348/kjr.2013.14.2.278
19. Kuhnert VM, Kachnic LA, Li L, Purschke M, Gheorghiu L, Lee R, et al. FANCD2-deficient human fibroblasts are hypersensitive to ionising radiation at oxygen concentrations of 0% and 3% but not under normoxic conditions. Int J Radiat Biol. (2009) 85:523–31. doi: 10.1080/09553000902883810
20. Nepal M, Che R, Ma C, Zhang J, Fei P. FANCD2 and DNA damage. Int J Mol Sci. (2017) 18:1804. doi: 10.3390/ijms18081804
21. Kee Y, D’Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. (2012) 122:3799–806. doi: 10.1172/JCI58321
22. Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. (2006) 107:4223–33. doi: 10.1182/blood-2005-10-4240
23. Lyakhovich A, Surralles J. FANCD2 depletion sensitizes cancer cells repopulation ability in vitro. Cancer Lett. (2007) 256:186–95. doi: 10.1016/j.canlet.2007.06.006
24. Feng H-J, Bao Y-L, Liang Z-P, Zhao F-P, Xu S-E, Xu W, et al. Silencing of FANCD2 enhances the radiosensitivity of Recurrent cervical lymph node-derived head and neck squamous cell carcinoma HSC-4 cells. Int J Oncol. (2017) 50:1241–50. doi: 10.3892/ijo.2017.3902
25. Li Y, Zhang Y, Yang Q, Zhou X, Guo Y, Ding F, et al. Silencing of FANCI promotes DNA damage and sensitizes ovarian cancer cells to carboplatin. Curr Cancer Drug Targets. (2022) 22:591–602. doi: 10.2174/1568009622666220331091709
Keywords: retroperitoneal leiomyosarcoma, FANCD2, brachytherapy, radioactive iodine-125, low dose
Citation: Liu Xl, Zhao J, Di Xm, Cao G, Zhang H and Wang J (2024) Case report: Highly response to low-dose brachytherapy in recurrent retroperitoneal leiomyosarcoma with FANCD2 frameshift mutation: a unique case study. Front. Oncol. 14:1339955. doi: 10.3389/fonc.2024.1339955
Received: 20 November 2023; Accepted: 12 March 2024;
Published: 03 April 2024.
Edited by:
Junjie Wang, Peking University Third Hospital, ChinaReviewed by:
Rony Benson, Marsleeva Medicty, IndiaCopyright © 2024 Liu, Zhao, Di, Cao, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Zhang, aG9uZ3Rhb3poYW5nbWRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.