
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 October 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1265726
This article is part of the Research Topic HPV infection and cervical, vagina, and vulvar cancers View all 14 articles
A correction has been applied to this article in:
Corrigendum: Associations of multi-human papillomavirus infections with expression of p16 in a cohort of women who underwent colposcopy: a retrospective study of 5165 patients
 Yulong Zhang1†
Yulong Zhang1† Haibo Li2†
Haibo Li2† Xiaowen Li1†
Xiaowen Li1† Zelong Li1
Zelong Li1 Qianru You1
Qianru You1 Hanwen Liu1
Hanwen Liu1 Zhiyan Zhao3
Zhiyan Zhao3 Yanzhao Su1*
Yanzhao Su1* Xiangqin Zheng1*
Xiangqin Zheng1* Yusha Chen4
Yusha Chen4 Jiancui Chen4
Jiancui Chen4 Huan Yi1*
Huan Yi1*Objective: Investigate HPV types in cervical specimens, their correlation with p16 expression in lesions, and diagnostic value for cervical lesions. Enhance clinical diagnosis reliability.
Methods: Retrospective cross-sectional study at Fujian Maternity and Child Health Hospital’s Cervical Disease Center (Jun 2019-Dec 2021). Patients with abnormal cervical screening underwent colposcopy and conization. Pathological diagnosis based on colposcopy, cervical biopsy, ECC, and conization. Analyzed HPV genotyping (18 HR-HPV, 5 LR-HPV) and p16 expression correlation. Statistical analysis used R software.
Results: he expression of p16 is significantly associated with the infection of high-risk HPV types, such as 16, 33, 52, and 58, with an increased risk of 1.4 times or higher (OR=1.91, 3.14, 1.40, and 1.78, respectively). The risk of p16 expression increased 4-fold for multiple high-risk HPV types [adjusted OR (95% CI) = 4 (2.92~5.5), P-value <0.001]. Compared to the p16(-) group, the p16(+) group had a higher association with cervical lesions worse than HSIL (High-grade Squamous Intraepithelial Lesions).In the group with multiple Human Papillomavirus Infections with types 16, 33, 52, and 58, the risk of cervical lesions worse than HSIL increased by up to 660-fold compared to the negative group (adjusted OR=660.62, 95% CI: 91.39~4775.53, P<0.001), indicating that this combination of HPV types posed the greatest risk for cervical lesions above HSIL.
Conclusions: p16 plays a crucial role in cervical lesion progression, linked to high-risk HPV. Combining p16 with HPV screening improves cervical cancer detection. Studying multiple HPV infections will enhance prevention and management.
Cervical cancer is one of the most common gynecological malignancies worldwide, with the highest incidence among malignant neoplasms of the female reproductive system, only second to breast cancer (1). At present, cervical cancer causes up to 30,000 deaths of women in China every year, which poses a huge threat to women’s health in the country (2).
The development of cervical cancer is a long-term and continuous process of tumor progression, which includes cytological abnormalities, low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and finally, carcinogenesis. This process requires the involvement of multiple pathogenic factors, multiple oncogenes, and occurs through a series of steps (3, 4). One crucial factor in the development of cervical cancer is persistent infection with human papillomavirus (HPV). The World Health Organization (WHO) has listed cervical cancer as the first most common cancer caused by HPV infection (5).
Currently, HPV-DNA detection is the primary screening method for cervical cancer in China. However, it has its limitations, including high sensitivity and low specificity due to the influence of various factors in both the host and the virus (6). Especially for precancerous lesions, HPV-DNA testing is only a qualitative test, which cannot classify the severity of lesions nor distinguish between transient and persistent infections. As a result, it cannot guarantee the accuracy of cancer diagnoses (6, 7). There was also research revealed the importance of the HPV mRNA test to define how severe is a cervical lesion, more research is needed to prove (8).
To improve the accuracy of cervical cancer detection and prognosis, researchers have been investigating the role of p16, a tumor suppressor gene involved in the progression of uterine cervical lesions (9). The p16 protein, produced by this gene, has been found to inhibit the cell cycle, thereby negatively regulating cell growth, and controlling cell hyperproliferation. Dysfunctional pathways resulting from aberrant p16 protein expression may induce cervical intraepithelial neoplasia (CIN) and influence the occurrence and development of cervical cancer (10–12).
However, while the significance of p16 in cervical cancer progression has been studied, there is still a lack of research on its interaction with different HPV infection genotypes (13). As a result, the relationship between p16 expression and cervical lesions remains unclear, and the potential value of combining HPV detection with p16 testing in differentiating cervical lesions needs further exploration.
In this study, we conducted a retrospective analysis of patients with cervical lesions using histopathology as the standard for diagnoses (14). All patients underwent HPV typing and p16 expression testing. The main objective was to evaluate the diagnostic significance of HPV typing and p16 detection alone or in combination for cervical lesions, aiming to provide a more reliable clinical diagnosis method. This approach would help avoid overtreatment and reduce the rate of misdiagnosis in patients with mild lesions confirmed by postoperative pathology (14).
This cross-sectional study included patients who underwent colposcopy and conization due to abnormal cervical cancer screening results at the cervical disease center of Fujian Maternity and Child Health Hospital from June 2019 to December 2021. Cervical cancer screening involved ThinPrep Cytology Test (TCT) and/or HPV genotyping. Abnormal cytology results were defined as Atypical Squamous Cells of Undetermined Significance (ASC-US), Low-grade Squamous Intraepithelial Lesion (LSIL), High-grade Squamous Intraepithelial Lesion (HSIL), Atypical Glandular Cells (AGC), Endocervical Adenocarcinoma in situ (AIS), Squamous Cell Carcinoma (SCC), and Adenocarcinoma. The interval between cervical cancer screening and histological examination was less than 3 months. Clinical information, including age, gravidity, parity, HPV genotypes, and cervical pathology, was extracted from the department’s medical records (Figure 1).
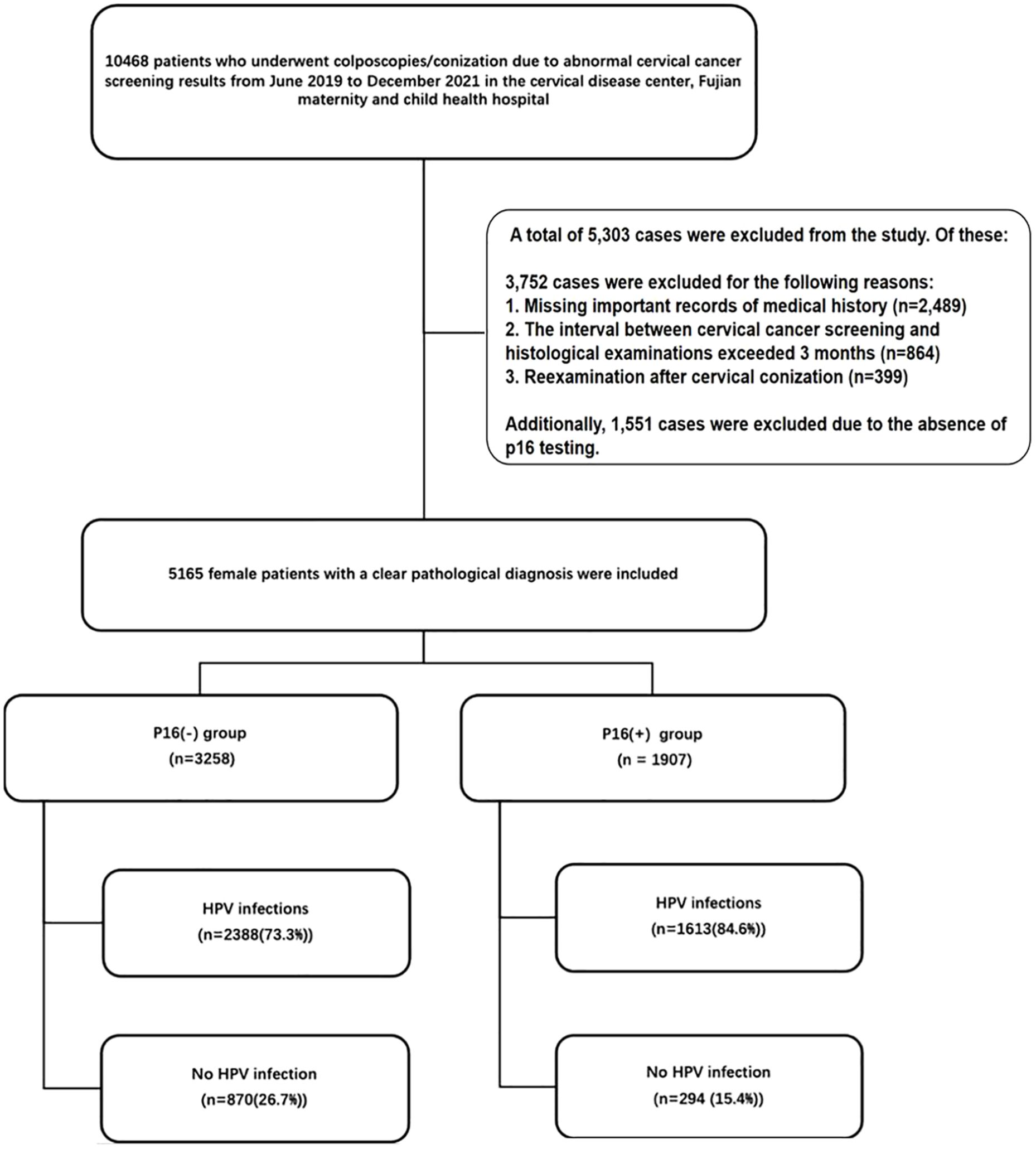
Figure 1 Out of a total of 10,468 patients who underwent colposcopies/conization due to abnormal cervical cancer screening results from June 2019 to December 2021, 5,303 cases were excluded. Of these, 3,752 cases were excluded for the following reasons: 1. Missing important records of medical history (n=2,489). 2. The interval between cervical cancer screening and histological examinations exceeded 3 months (n=864). 3. Reexamination after cervical conization (n=399). Additionally, 1,551 cases were excluded due to the absence of p16 testing. A total of 5,165 female patients with clear pathological diagnoses were included in the final analysis. HPV, human papillomavirus.
The study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2023KY038). Due to the retrospective nature of the study, informed consent was exempted.
PCR-RDB HPV genotyping (Yaneng Biotech) was performed to identify 18 genotypes of high-risk HPV (HR-HPV): HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and 83, as well as 5 types of low-risk HPV (LR-HPV): HPV-6, 11, 42, 43, and 81.
Colposcopy referrals were based on the ASCCP guidelines (10). All patients underwent colposcopy and cervical biopsy. Additionally, patients with HPV-16 and 18 infections, AGC/AIS/HSIL cytology, and type 3 cervical transformation zone underwent endocervical curettage (ECC). Cervical cone resection was performed in cases with liquid-based cytology results indicating HSIL, AGC-FN (atypical glandular cell, favor neoplastic), AIS, or cervical pathological biopsy and ECC results indicating CIN2-3 (cervical intraepithelial neoplasia 2-3). Two blinded senior pathologists independently performed the pathological evaluation of cervical biopsies, ECC, and conization tissues. Standard haematoxylin-eosin stain was used in this study, standard H&E protocol allows visualization of tissue morphology by imparting blue-stained nuclei and pink-stained cytoplasm/connective tissue. It is the routine stain for histopathology, providing an overview of tissue architecture and cytology.
The final pathological diagnosis was determined using the most severe result among evaluations of cervical biopsies, ECC, and conization tissues. The histologic endpoints were defined according to the 2014 WHO classification of tumors of the female reproductive organs (4th Edition) (11) and Lower Anogenital Squamous Terminology (LAST) recommendations as follows (12): Normal cervix; LSIL, which includes CIN1 and p16 negative CIN2; HSIL, including p16 positive CIN2 and CIN3; AIS; invasive cervical cancer. Furthermore, HSIL, AIS, and invasive cervical cancer were classified as HSIL+.
The Leisegang D-10625, Model1DS Ur Nr 55764, Colposcope from Berlin, Germany, was used for cervix examination. After exposing the cervix using an appropriately sized Cusco’s speculum, the vulva, vagina, and cervix were examined before the application of 3% acetic acid solution for each patient. Colposcopic abnormalities were classified as normal, abnormal, or unsatisfactory. Biopsies were taken from abnormal areas using punch cervical biopsy tissue forceps. The cervical specimens were processed in the histopathology laboratory, and a histopathologist blinded to the HPV status of the participants performed the diagnosis. Immunocytochemical staining was performed using the P16/Ki67 double staining kit on each cervical specimens. Experimental operations were strictly in accordance with the kit instructions and the technical instructions for double staining of cervical cells, and two experienced pathologists conducted and interpreted the double staining of cervical epithelial cell.
Categorical variables were presented as frequencies (percentages), and statistical analyses were performed using R software and its packages (Open Access, Version 4.0.2). Descriptive statistics showed mean ± standard deviation for continuous variables, while frequency and percentage were used for categorical variables. The statistical differences among p16 status for clinical characteristics were tested with t-tests for continuous variables and Chi-square tests for categorical variables. Univariate and multivariate logistic regression analyses, adjusting for age, gravidity, parity, and pregnancy, were used to determine the association between multiple HPV infections and cervical lesions. Two-tailed P-values less than 0.05 were considered statistically significant.
The analysis included a total of 5165 female patients with definitive pathological diagnoses. Among them, there were 3258 cases with p16(-) and 1907 cases with p16(+). The mean age of patients with p16(+) was significantly older than that of patients with p16(-) (42.5 ± 11.1 vs. 39.4 ± 10.9, p<0.001). The prevalence of HPV infection was 73.3% (n=2388) in patients with p16(-), whereas it was 84.6% (n=1613) in patients with p16(+) (P<0.001). P16(+) was associated with the infection of high-risk HPV types 16, 33, 52, 56, 58, and low-risk HPV type 81 (P<0.05) (Table 1). Figure 2 shows the intersections of the HPV genotype. HPV genotypes 16, 18, 52, 51 and 33 had the most frequent infections, and there was coinfection (Figure 2).
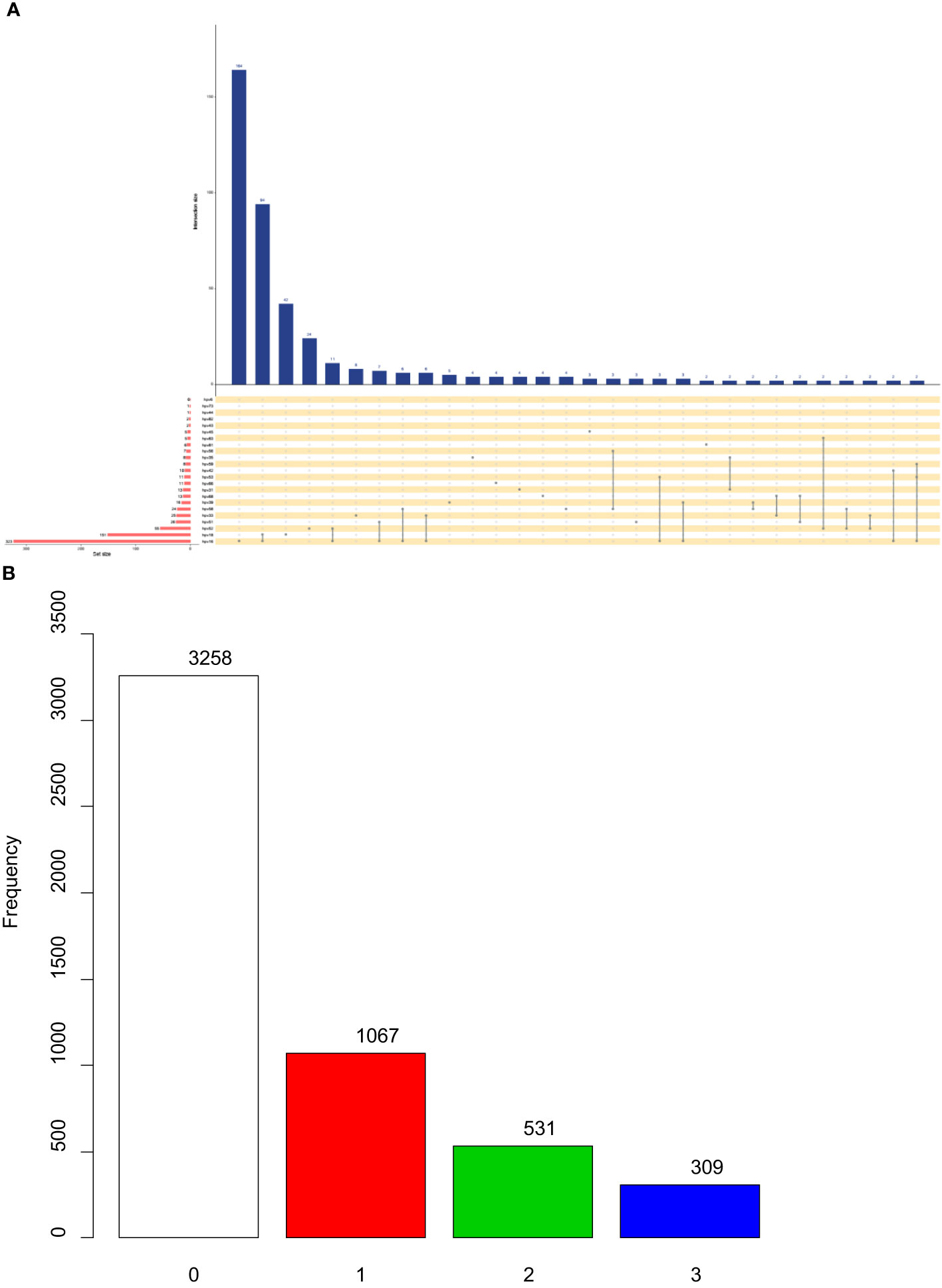
Figure 2 Upset plots of the intersections of HPV genotype (A) and different expression level of p16 (B). Each row corresponds to a set of infection genotype(s), and the bar chart on the left demonstrates the size of each set. Each column corresponds to a possible intersection: the filled-in cells show which set is a part of an intersection.
Each row corresponds to a set of infection genotype(s), and the bar chart on the left demonstrates the size of each set. Each column corresponds to a possible intersection: the filled-in cells show which set is a part of an intersection.
Figure 3 depicts the relationship between different HPV genotypes and p16 expression. In the crude models, high-risk HPV types 16, 33, 52, 56, 58, and 81 showed a significant correlation with p16 expression, whereas a negative relationship was observed for HPV type 18. After adjusting for confounding factors, the results remained consistent with the univariate analysis. Infection with high-risk HPV types increased the risk of p16(+) by approximately 1.4 times or higher (OR=1.91, 3.14, 1.40, and 1.78, respectively).
Models adjusted for age, gravidity, parity, and cervical histology.
Table 2 presents the results of univariate and multivariate logistic regression analyses of multiple HPV infections and p16 expression. The highest incidence of p16(+) was identified in individuals infected with HPV33+ and multiple high-risk HPV infections (MH-HPV+). Moreover, there was an increased risk of p16(+) for HPV genotypes 16, 33, 52, and 58 alone, as well as for multiple high-risk HPV infections.
Specifically, the risk of p16(+) increased 4.38-fold when infected with HPV33 alone [adjusted OR (95% CI) = 4.38 (2.617.36), P < 0.001], and 4-fold when infected with multiple high-risk HPV genotypes [adjusted OR (95% CI) = 4 (2.925.5), P < 0.001].
Table 3 presents the comparison of lesions more severe than HSIL between the p16(-) and p16(+) groups. Across all groups, our study found that compared to the p16(-) group, the p16(+) group had a higher association with cervical lesions worse than HSIL.
In the negative+ group, the risk of cervical lesions above HSIL was 43.06-fold higher than that of the negative group [adjusted OR=43.06, 95% CI: 28.8264.33, P<0.001]. In the SH+ group, the risk of cervical lesions above HSIL was 93.58-fold higher than that of the SH group [adjusted OR=93.58, 95% CI: 64.47135.85, P<0.001]. The MH+ group demonstrated the highest risk increase, with p16(+) patients having a 660-fold higher risk of cervical lesions above HSIL compared to the negative group [adjusted OR=660.62, 95% CI: 91.394775.53, P<0.001]. This group represented the most significant risk for cervical lesions above HSIL. In the other+ group, the risk of cervical lesions above HSIL was 41.54-fold higher than that of the other group [adjusted OR=41.54, 95% CI: 28.9659.59, P<0.001]. Similarly, in the MO+ group, the risk of lesions above HSIL was 80.91-fold higher than that of the other group [adjusted OR=80.91, 95% CI: 53.28~122.87, P<0.001].
Cervical cancer is unique as it is the only type of cancer with a clear etiology and complete tertiary prevention measures. The two most common ways of screening for cervical cancer are cervical cytology and HPV detection (13). Cytology is based on microscopic morphology and has limitations, such as complex grading, subjectivity, and variable diagnostic repeatability, leading to insufficient sensitivity. On the other hand, HPV tests have high sensitivity but lower specificity due to potential transient infections being missed, and they cannot reflect the extent or severity of HPV-induced lesions.
Countries with established cervical cancer screening programs are increasingly adopting HPV primary screening as the preferred method (14, 15). Early detection through improved screening methods can significantly improve survival rates for cervical cancer patients. Abnormal expression of p16 is closely related to HPV-16 and HPV-18 infections, and its expression increases with the progression of CIN and cervical cancer (16–18). Patients with p16-negative HPV-associated cervical cancer tend to have worse prognoses (19). Combining TCT with dual staining of p16/Ki67 has shown high sensitivity and specificity in detecting HSIL, making it an effective screening method (20).
Multiple infections are common in healthy women (15.8%) but less prevalent in cervical cancer patients (3%-4%), and the relationship between multiple infection and pathogenicity requires further study (18).
In our study, 5165 female patients with definite pathological diagnoses were included, with 1907 exhibiting positive p16 expression and 3258 showing negative p16 expression. P16 expression correlated positively with high-risk HPV types, including HPV-16, HPV-33, HPV-52, and HPV-58, with an increased risk compared to p16(-) cases (9).
The p16 gene, located on chromosome 9, encodes the p16 protein, which inhibits cell proliferation by preventing cells from entering the S phase (21, 22). Variations in the p16 gene and inactivation of its proteins are common in various malignant tumors, including cervical cancer (23).
Persistent infection with high-risk HPV is associated with cervical intraepithelial neoplasia and cervical cancer (24, 25). HPV can exist in free or integrated form, and persistent infection may lead to gene instability and lesion escalation (26, 27). The E7 gene of HPV inactivates the pRb protein, promoting cell cycle progression and potentially leading to feedback overexpression of p16 (22). Thus, the overexpression of p16 in tumor cells is linked to HPV infection (28).
Over 80% of patients with HPV infections experience transient infections, while 4% to 10% develop persistent HPV infections, leading to cervical lesions and potentially cancer (29). Among the 200 identified HPV types, HPV-16 and HPV-18 are the most common and pathogenic types (30). Multiple HPV infections are more common in LSIL and HSIL patients, with longer durations of infection increasing the risk of cervical lesions (30).
Positive p16 protein expression is correlated with increasing cervical lesion levels, making it a predictor of cervical lesion escalation (31, 32). Combining HPV with p16 testing can enhance cervical cancer detection and risk assessment (33). P16 expression has been proposed as a new indicator for cervical cancer screening (19).
The current study’s limitations include its retrospective cross-sectional design, which may introduce selection bias, and the potential impact of residual confounding factors. Multicenter prospective cohort studies are needed to validate the findings. Another potential limitation of this study is that heavy methylation of the p16 gene promoter region can lead to silencing and decreased expression of p16, resulting in false negative results by immunohistochemistry. In the latter study, it will be important to understand the potential confounding effects of high p16 methylation when interpreting p16 immunohistochemistry results in cervical specimens.
In conclusion, p16 expression is crucial in cervical lesion progression and is associated with high-risk HPV genotypes (HPV-16, 33, 52, and 58). Incorporating p16 testing into HPV screening can enhance cervical cancer detection. Further research on multiple HPV infections’ role in cervical lesion development will improve cervical cancer prevention and management.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The paper was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2022YJ002). The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was waived due to the retrospective nature of the study.
YZ: Writing – original draft, Writing – review & editing. HBL: Writing – original draft, Writing – review & editing. XL: Data curation, Methodology, Writing – original draft, Writing – review & editing. ZL: Data curation, Writing – original draft, Writing – review & editing. QY: Data curation, Investigation, Writing – original draft. HWL: Data curation, Investigation, Writing – review & editing. ZZ: Data curation, Investigation, Writing – original draft. HY: Writing – review & editing. YS: Writing – review & editing. XZ: Writing – review & editing. YC: Writing – review & editing. JC: Writing – review & editing.
This study was supported by grants from Fujian Medical University Education and Teaching Reform Research Project (No. J21055), Young and Middle-Aged Key Talents Training Project in Fujian Province (2019-ZQN-23), Fujian Maternity and Child Health Hospital (YCXQ 18-17) and the Natural Science Foundation of Fujian Province, China (2020J01331).
The authors are grateful for the support provided by Liyu Dai.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health (2020) 8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z, et al. Cancer incidence and mortality: A cohort study in China, 2008-2013. Int J Cancer (2017) 141(7):1315–23. doi: 10.1002/ijc.30825
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
4. Cabibi D, Napolitano C, Giannone AG, Micciulla MC, Porcasi R, Lo Coco R, et al. Predictive role of the p16 immunostaining pattern in atypical cervical biopsies with less common high risk HPV genotypes. Diagnostics (Basel) (2021) 11(11):1947. doi: 10.3390/diagnostics11111947
5. Cohen E, Coviello C, Menaker S, Martinez-Duarte E, Gomez C, et al. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck (2020) 42(8):2021–9. doi: 10.1002/hed.26134
6. Yu L, Chen X, Liu X, Fei L, Ma H, Tian T, et al. Significance of triple detection of p16/ki-67 dual-staining, liquid-based cytology and HR HPV testing in screening of cervical cancer: A retrospective study. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.915418
7. Cuzick J, Adcock R, Carozzi F, Gillio-Tos A, De Marco L, Del Mistro A, et al. Combined use of cytology, p16 immunostaining and genotyping for triage of women positive for high-risk human papillomavirus at primary screening. Int J Cancer (2020) 147(7):1864–73. doi: 10.1002/ijc.32973
8. Frega A, Pavone M, Sesti F, Leone C, Bianchi P, Cozza G, et al. Sensitivity and specificity values of high-risk HPV DNA, p16/ki-67 and HPV mRNA in young women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL). Eur Rev Med Pharmacol Sci (2019) 23(24):10672–7.
9. Solomon D, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, et al. The 2001 bethesda systemTerminology for reporting results of cervical cytology. JAMA (2002) 287(16):2114–9. doi: 10.1001/jama.287.16.2114
10. Nayar R, Chhieng DC, Crothers B, Darragh TM, Davey DD, Eisenhut C, et al. Moving forward—the 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol (2020) 9(4):291–303. doi: 10.1016/j.jasc.2020.05.002
11. Bruni L, Albero G, ICO Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in the world: Summary report. (2016).
12. Tan SC, Ismail MP, Duski DR, Othman NH, Ankathil R. Prevalence and type distribution of human papillomavirus (HPV) in Malaysian women with and without cervical cancer: an updated estimate. Bioscience Rep (2018) 38(2). doi: 10.1042/BSR20171268
13. Perkins R, Guido RL, Saraiya M, Sawaya F. Summary of current guidelines for cervical cancer screening and management of abnormal test results: 2016–2020. J Womens Health (Larchmt) (2021) 30(1):5–13. doi: 10.1089/jwh.2020.8918
14. Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US preventive services task force. JAMA (2018) 320(7):687–705. doi: 10.1001/jama.2018.10400
15. Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM, et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol (2011) 12(9):880–90. doi: 10.1016/S1470-2045(11)70188-7
16. Ying ZJ. Analysis of the expression of HPV- derived E7 protein in cervical lesions and evaluation of its clinical significance. Chin J Pract Gynecol Obstetrics (2018) 34(06):654–7. H.D.W.X.H.R.M.D.-y.F.H.D. doi: 10.1038/s41598-020-78345-8
17. Jieqiong CAO, Zhao Y. Research progress in correlation between p16, E6/E7 proteins and cervical cancer and precancerous lesions. J Clin Pathological Res (2020) 40(02):443–7. doi: 10.3389/fmicb.2019.03116
18. Lin H, Moh JS, Ou YC, Shen SY, Tsai YM, ChangChien CC, et al. A simple method for the detection and genotyping of high-risk human papillomavirus using seminested polymerase chain reaction and reverse hybridization. Gynecologic Oncol (2005) 96(1):84–91. doi: 10.1016/j.ygyno.2004.09.043
19. da Mata S, Ferreira J, Nicolás I, Esteves S, Esteves G, Lérias S, et al. P16 and HPV genotype significance in HPV-associated cervical cancer—A large cohort of two tertiary referral centers. Int J Mol Sci (2021) 22(5):2294. doi: 10.3390/ijms22052294
20. Qian Q-P, Zhang X, Ding B, Jiang SW, Li ZM, Ren ML, et al. Performance of P16/Ki67 dual staining in triaging hr-HPV-positive population during cervical Cancer screening in the younger women. Clinica Chimica Acta (2018) 483:281–5. doi: 10.1016/j.cca.2018.05.023
21. Cai S, Han K. Research on expression and importance of p53, p16 and VEGF-C in cervical cancer. J Gynécologie Obstétrique Biologie la Reprod (2015) 44(7):639–45. doi: 10.1016/j.jgyn.2014.07.012
22. de Freitas AC, Coimbra EC, Leitão M. Molecular targets of HPV oncoproteins: Potential biomarkers for cervical carcinogenesis. Biochim Biophys Acta (BBA) - Rev Cancer (2014) 1845(2):91–103. doi: 10.1016/j.bbcan.2013.12.004
23. Tsakogiannis D, Moschonas GD, Bella E, Kyriakopoulou Z, Amoutzias GD, Dimitriou TG, et al. Association of p16 (CDKN2A) polymorphisms with the development of HPV16-related precancerous lesions and cervical cancer in the Greek population. J Med Virol (2018) 90(5):965–71. doi: 10.1002/jmv.24996
24. Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstetrics Gynecol (2015) 125(2). doi: 10.1097/AOG.0000000000000669
25. Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol (1999) 189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
26. Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M, et al. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes (2010) 40(1):1–13. doi: 10.1007/s11262-009-0412-8
27. Hopman AH, Smedts F, Dignef W, Ummelen M, Sonke G, Mravunac M, et al. Transition of high-grade cervical intraepithelial neoplasia to micro-invasive carcinoma is characterized by integration of HPV 16/18 and numerical chromosome abnormalities. J Pathol (2004) 202(1):23–33. doi: 10.1002/path.1490
28. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck (2012) 34(4):459–61. doi: 10.1002/hed.21974
29. Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine (2013) 31:F1–F31. doi: 10.1016/j.vaccine.2013.10.002
30. De Brot L, Pellegrini B, Moretti ST, Carraro DM, Soares FA, Rocha RM, et al. Infections with multiple high-risk HPV types are associated with high-grade and persistent low-grade intraepithelial lesions of the cervix. Cancer Cytopathol (2017) 125(2):138–43. doi: 10.1002/cncy.21789
31. Bergeron C. Détection de la p16 dans les lésions précancéreuses du col utérin. Annales Pathologie (2010) 30(5, Supplement 1):105–6. doi: 10.1016/j.annpat.2010.07.034
32. Hidalgo JV, Pellegrini B, Moretti ST, Carraro DM, Soares FA, Rocha RM, et al. AgNOR, p16 and human papillomavirus in low-grade squamous intra-epithelial lesions of the uterine cervix. Preliminary report. Biotech Histochem (2012) 87(4):257–64. doi: 10.3109/10520295.2011.637514
Keywords: HPV, cervical cancer, p16, cervical lesions, retrospective study
Citation: Zhang Y, Li H, Li X, Li Z, You Q, Liu H, Zhao Z, Su Y, Zheng X, Chen Y, Chen J and Yi H (2023) Associations of multi-human papillomavirus infections with expression of p16 in a cohort of women who underwent colposcopy: a retrospective study of 5165 patients. Front. Oncol. 13:1265726. doi: 10.3389/fonc.2023.1265726
Received: 23 July 2023; Accepted: 20 September 2023;
Published: 26 October 2023.
Edited by:
Songlin Zhang, University of Texas Health Science Center at Houston, United StatesReviewed by:
Mirella Fortunato, Azienda Sanitaria Ospedaliera S.Croce e Carle Cuneo, ItalyCopyright © 2023 Zhang, Li, Li, Li, You, Liu, Zhao, Su, Zheng, Chen, Chen and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanzhao Su, c3V5YW56aGFvNjA5QDE2My5jb20=; Xiangqin Zheng, Wmhlbmd4cTEyMTVAMTYzLmNvbQ==; Huan Yi, eWlodWFuZ3Nya0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.