- 1Department of Radiotherapy and Chemotherapy, Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine, Hebei, Cangzhou, China
- 2Department of Radiotherapy and Chemotherapy, Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine East Ward, Hebei, Cangzhou, China
The present study aims to investigate the therapeutic value of third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) combined with cranial radiotherapy (RT) in patients with EGFR-positive non-small cell lung cancer (NSCLC) and brain metastases (BMs).
Methodology: This is a retrospective study that involved 213 patients with EGFR-NSCLC and BMs, with the patients divided into two groups: the upfront cranial RT (ucRT) group (n = 96) and the non-ucRT group (n = 117). All patients were administered with osimertinib, and those in the ucRT group also underwent RT. The overall survival (OS), progression-free survival (PFS) and intracranial PFS (IPFS) of the two groups were compared.
Results: The ucRT group manifested a markedly higher IPFS than the non-ucRT group (29.65 months vs 21.8 months; P < 0.0001). The subgroup analysis revealed that patients with oligometastases (OLOGO-BMs; 1–3 BMs) demonstrated a notably longer OS (44.5 months vs 37.3 months; P < 0.0001), PFS (32.3 months vs 20.8 months; P = 0.6884) and IPFS (37.8 months vs 22.1 months; P < 0.0001) in the ucRT group than in the non-ucRT group. However, for patients with multiple BMs, there was no significant difference in OS (27.3 months vs 34.4 months; P = 0.0710) and PFS (13.7 months vs 13.2 months; P = 0.0516) between the ucRT group and the non-ucRT group; the ucRT group exhibited a higher IPFS (26.4 months vs 21.35 months; P = 0.0028). Cox’s multivariate analysis of patients with OLOGO-BM indicated that the use of ucRT was linked to a better OS (heart rate [HR] = 0.392; 95% confidence interval [CI]: 0.178–0.863; P = 0.020) and PFS (HR = 0.558; 95% CI: 0.316–0.986; P = 0.044).
Conclusion: Upfront cerebral cranial stereotactic radiosurgery can improve outcomes in EGFR-positive patients with NSCLC and OLOGO-BM. However, for patients with multiple BMs, the preferable strategy may be pre-treatment with EGFR-TKIs.
1 Introduction
Brain metastases (BMs) are the most common and debilitating complications linked to non-small cell lung cancer (NSCLC) occurring in an estimated 20%–40% of all diagnosed cases (1). The prevalence is even more alarming in patients who test positive for mutations in the epidermal growth factor receptor (EGFR) gene, with rates soaring between 44% and 63% (2). During the era when chemotherapy was the mainstay of treatment, the effectiveness of chemotherapeutic agents was markedly limited, as these drugs struggled to penetrate the blood–brain barrier. This shortcoming led to a dire prognosis for patients with BMs (3).
The landscape of treatment, however, has been radically transformed by the advent of targeted therapies, particularly small-molecule EGFR tyrosine kinase inhibitors (EGFR-TKIs). These have emerged as the front-line treatment for advanced NSCLC in patients with EGFR mutations, demonstrating far superior blood–brain barrier penetration compared with traditional chemotherapy (4). For instance, a phase II trial revealed that patients with NSCLC and BMs treated with erlotinib as a first-line regimen experienced median overall survival (OS) rates of 15.9–22.9 months and progression-free survival (PFS) rate of 5.8–14.5 months (5). Further clinical trials have demonstrated similar or even improved outcomes, including impressive intracranial PFS (IPFS) (6, 7).
Historically, treatment options for BM were mainly confined to whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS) and surgical resection. Emerging evidence suggests a synergistic therapeutic effect between cranial radiotherapy (RT) and EGFR-TKI. The former appears to facilitate the penetration of the latter through the blood–brain barrier, thereby increasing its concentration in the cerebrospinal fluid and amplifying RT’s antitumour effects (8). Numerous studies confirm the potential of combining cranial RT and EGFR-TKI for managing BM, although the findings are not universally consistent (9). However, this enhanced survival often comes at the cost of detrimental side effects, such as memory loss and cognitive decline. These are mainly due to the cranial RT, thereby igniting ongoing debates over its role and timing in patients who are EGFR-positive (10).
Recent advances in third-generation EGFR-TKIs have shown particular promise for patients with NSCLC and BM, owing to their superior penetration through the blood–brain barrier (11). Studies such as the FLAURA and AENEAS trials have shown extended PFS and IPFS with the use of osimertinib and almonertinib, respectively (12, 13). While the efficacy of these third-generation inhibitors is evident, the role of cranial RT in conjunction with these advanced drugs remains relatively unclear. Although there is evidence supporting the enhanced effectiveness of combining first or second-generation EGFR-TKIs with cranial RT (14), questions linger regarding whether such a synergistic benefit extends to third-generation EGFR-TKIs.
Given the scarcity of literature addressing this specific combination in patients with NSCLC and both EGFR-positivity and BMs, the authors of the present paper conducted a retrospective study to explore the clinical significance and optimal timing of cranial RT in this unique patient cohort.
2 Materials and methods
2.1 Patient selection
The medical records of patients with NSCLC carrying EGFR mutations and diagnosed with BMs admitted to Cangzhou Hospital of Integrative Traditional Chinese and Western Medicine (TCM-WM) of Hebei between January 2018 and December 2022 were meticulously collated. The inclusion criteria were as follows: patients (1) with histopathologically verified lung adenocarcinoma; (2) with BMs, ascertained by magnetic resonance imaging (MRI); (3) with either exon 19 deletion (19DEL) or exon 21 points mutation (21L858R mutation), confirmed by genetic testing; and (4) who received osimertinib as an initial treatment regimen.
The exclusion criteria were as follows: patients (1) who previously used EGFR-TKI without receiving osimertinib during cranial RT or within one week after RT; (2) who underwent surgical resection at an initial BM diagnosis; and (3) with other serious illnesses that could affect their survival or cognitive function, such as stroke, Parkinson’s disease or severe liver or kidney dysfunction.
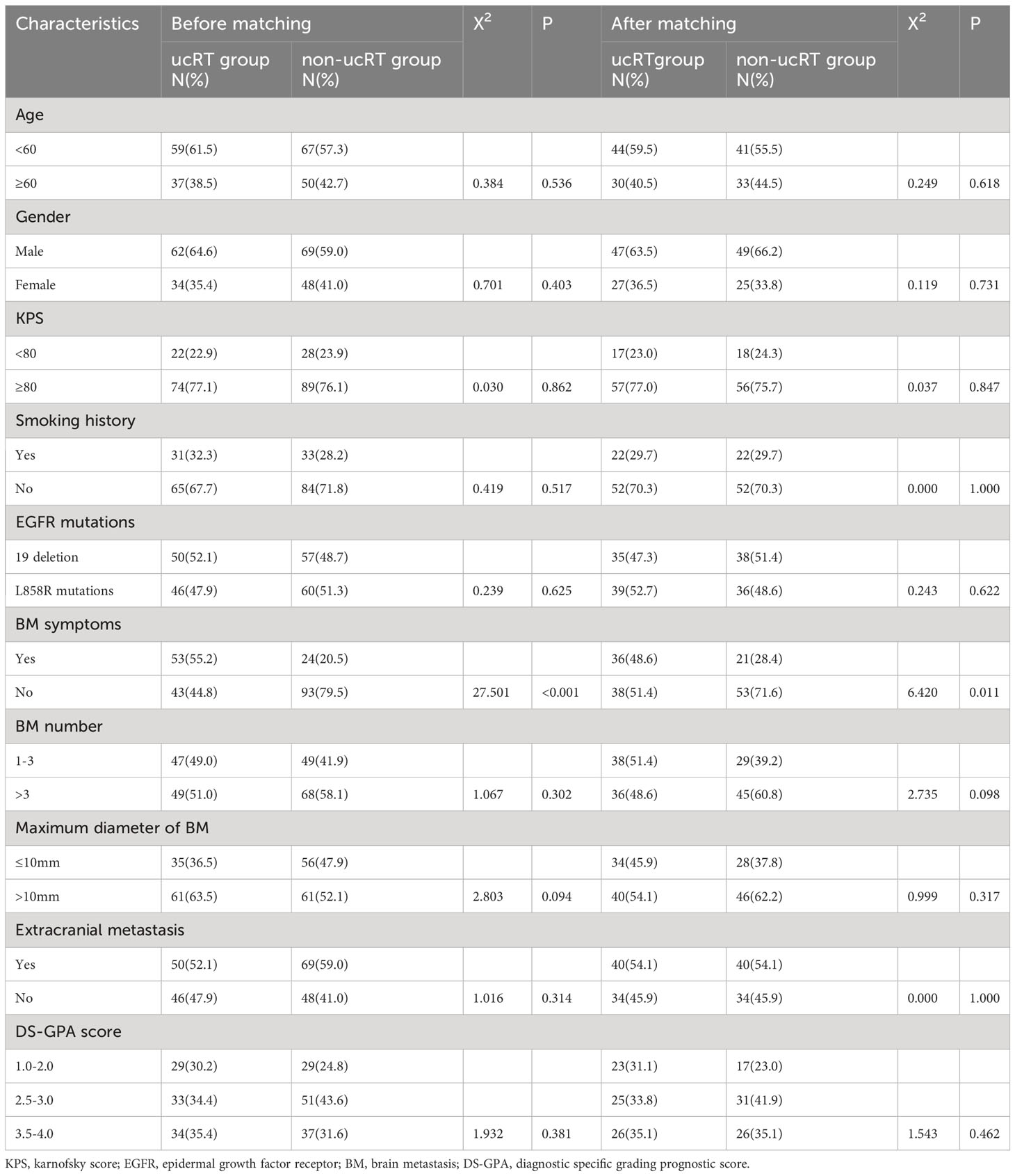
A total of 213 patients fitting these criteria were recruited for the study, and their baseline characteristics, namely age, gender, Karnofsky performance score (KPS), smoking history, EGFR mutation type, number of BMs, maximum BM diameter, BM symptoms and extracranial metastases, were documented. All the patients were evaluated using the diagnostic-specific grading prognostic score (DS-GPA) for BMs (15). The data collection cut-off date was 1 December 2022. The study received approval from the ethics committee of Cangzhou Hospital of Integrative TCM-WM of Hebei, and all patient-involved procedures complied with the principles of the Declaration of Helsinki. Informed consent was obtained from all the patients.
2.2 Treatment protocols
Patients were assigned to either the upfront cranial RT group (ucRT) or the non-RT group based on cranial RT application timing. The ucRT cohort comprised patients receiving cranial RT concurrent with a third-generation EGFR-TKI or patients commencing third-generation EGFR-TKI therapy after undergoing cranial RT (with an interval not exceeding one week). The term ‘upfront’ refers to treatment given as the first-line therapy for patients with BMs before the occurrence of any evidence of intracranial or systemic progression. The non-ucRT group included patients receiving osimertinib as their sole first-line treatment; all the patients were administered osimertinib at a standard daily dose of 80 mg. To reduce neurotoxicity and preserve cognitive function, cranial RT involved hippocampal-avoidance WBRT (HA-WBRT, 30 Gy/10 f; hippocampi doses: Dmax ≤ 17 Gy, Dmean ≤ 10 Gy and D40 ≤ 9 Gy) and and SRS (dose determined in line with the Rt Oncology Group 90-05 and 95-08 institutional guidelines) (16, 17). The former was predominantly used in patients with multiple BMs (BM number > 3) and patients not eligible for SRS (n = 36), whereas SRS was primarily utilised in patients with 1–3 BMs (n = 38).
The RT dose and plan were recorded as follows: Among the 213 patients in this study, 96 had oligometastatic brain disease (ucRT group: n = 47; non-ucRT group: n = 49). All 47 patients in the ucRT group underwent SRS treatment, with the following clinical data:
In patients with 1 BM (n = 18), the median BM diameter was 0.85 cm (0.7–3 cm), with a mean of 1.3 cm. The brain metastasis volume was <1 cm3 in 12 patients, and 1.2 cm3, 5.9 cm3, 9.5 cm3, 10.4 cm3, 10.8 cm3 and 15.8 cm3 in the remaining 6 patients, respectively. The SRS dose fractionation scheme was: 21–23 Gy/1 F in the 12 patients with a BM diameter of <1 cm3, 18 Gy/1 F in the 1.2 cm3 lesion, 27 Gy/3 F in the 5.9 cm3 lesion and 30 Gy/5 F in the rest.
In patients with 2 BMs (n = 23), the median BM maximum diameter was 1.5 cm (0.6–2.9 cm), with a mean of 1.6 cm. The brain metastasis volume and the corresponding SRS dose fractionation scheme were: <1 cm3 in 19 lesions (20–23 Gy/1 F), 1–5 cm3 in 14 lesions (18–20 Gy/1 F or 27–30 Gy/3–5 F), 5–10 cm3 in 10 lesions (27–30 Gy/3–5 F) and >10 cm3 in 3 lesions (30 Gy/5 F).
In patients with 3 BMs (n = 6), the median BM maximum diameter was 1.2 cm (0.6–2.8 cm), with a mean of 1.5 cm. The brain metastasis volume and the corresponding SRS dose fractionation scheme were: <1 cm3 in 10 lesions (22–23 Gy/1 F), 1–5 cm3 in 3 lesions (20 Gy/1 F, or 27 Gy/3 F), 5–10 cm3 in 2 lesions (27–30 Gy/3–5 F), and >10 cm3 in 3 lesions (30 Gy/5 F).
2.3 Follow-up and response assessment
For the ucRT group, systemic and intracranial response assessments were undertaken 1 month after RT completion and every 2–3 months subsequently. For the non-ucRT group, tumour response was routinely evaluated every 2–3 months after the commencement of the osimertinib treatment. The cut-off date for the follow-up was December 2022. In cases of suspected intracranial or systemic progression, cranial MRI or computed tomography were swiftly performed. Treatment response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumours version 1.1.
2.4 Study endpoints
The primary endpoints comprised OS, PFS and IPFS. The first, OS, was defined as the duration from the initiation of osimertinib treatment to either the patient’s death or the last known follow-up; PFS referred to the period from the commencement of osimertinib treatment to systemic progression, death from any cause or the last known follow-up; and IPFS referred to the time from the onset of osimertinib treatment to intracranial tumour progression, death from any cause or the last known follow-up. All the patients were subject to data review at the final follow-up visit.
2.5 Statistical analysis
Data analysis was executed using the SPSS software 27.0 and GraphPad Prism software 8.0. The baseline characteristics of patients in both groups were contrasted using the chi-square test or Fisher’s exact test, and OS, PFS and IPFS were calculated using the Kaplan–Meier method. Differences in survival curves between the two groups were assessed using the log-rank test. The association of various factors with survival was analysed using the Cox proportional risk model. In the univariate model, variables linked with survival (P < 0.10) were incorporated into the multivariate model, with a two-sided P-value of <0.05 denoting statistical significance.
To account for potential selection bias arising from the retrospective nonrandomised study design, differences in OS, PFS and IPFS between the two groups were analysed using inverse probability of treatment weighting (IPTW). The presence or absence of BM symptoms was not included as a covariate in the IPTW, as this factor represents an obvious indication for determining whether to perform cranial RT. All other baseline characteristics were incorporated into the covariates of IPTW.
In the present propensity score matching (PSM) process, the authors utilised a 1:1 nearest-neighbour-matching algorithm with a calliper width of 0.02 to align patients from the ucRT (unconsolidated RT) group with those from the non-ucRT group. This strict matching criterion was employed to ensure a high degree of similarity between the matched pairs in terms of their propensity scores. Despite the authors’ efforts to closely match patients from both groups, the inherent variability within the patient cohorts meant that not all individuals could be paired. This discrepancy in the final matched numbers reflects the stringent calliper setting, which prioritises the quality of the match over the quantity.
3 Results
3.1 Characteristics of the patient cohort
This study involved a cohort of 213 patients, 96 of whom were placed in the ucRT group, while 117 were allocated to the non-ucRT group. Following IPTW, 74 patients were placed in each group. The baseline attributes of the patients in both groups are outlined Table 1. It can be seen that there were no significant disparities between the two groups in terms of age, sex, smoking history, KPS, EGFR mutation type, number of BMs and DS-GPA score. Notably, the ucRT group had a higher prevalence of patients with symptomatic BM (P < 0.05). The median follow-up time was 18.6 months (range: 3.2–48.5 months) for the ucRT group and 16.4 months (range: 2.7–46.3 months) for the non-ucRT group.
3.2 Survival analysis
For the entire cohort, the median OS was 36.4 months. The ucRT and non-ucRT cohorts had a median OS of 37.6 and 36.2 months, respectively (P = 0.0716; Figure 1A). In terms of PFS, the median for all patients was 16.2 months, with the ucRT and non-ucRT groups demonstrating 17.35 and 14.4 months, respectively (P = 0.6884; Figure 1B). As for IPFS, the overall median was 27.3 months, with the ucRT and non-ucRT groups reporting 29.65 and 21.8 months, respectively (P < 0.0001; Figure 1C).
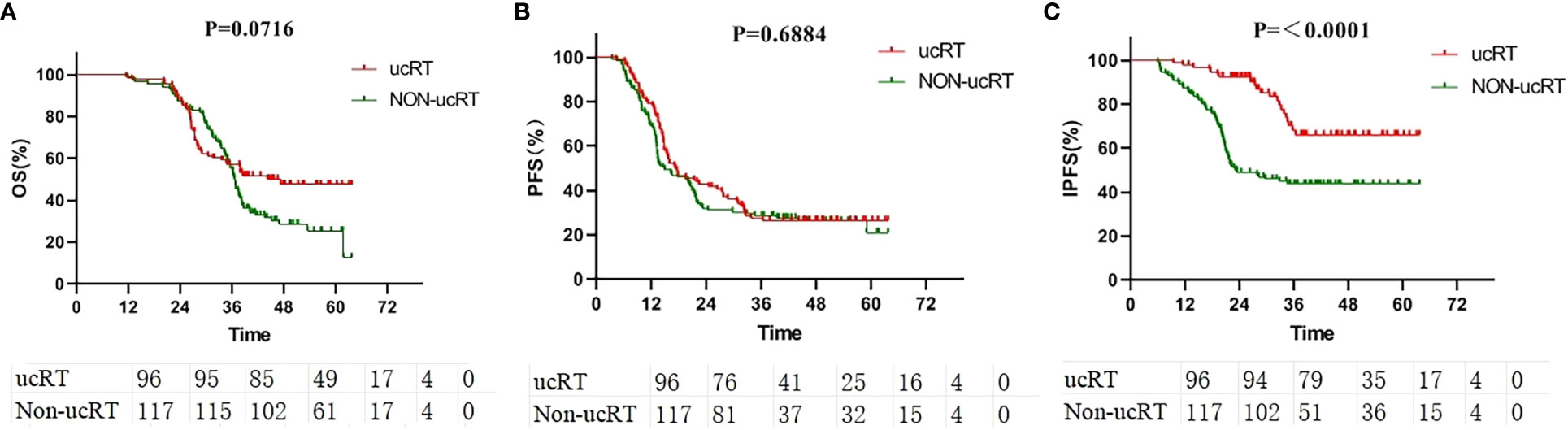
Figure 1 (A) Comparison of OS in the ucRT and non-ucRT groups. (B) Comparison of PFS in the ucRT and non-ucRT groups. (C) Comparison of IPFS in the ucRT and non-ucRT groups.
3.3 Survival analysis in the inverse probability of the treatment weighting cohort
All the patients underwent IPTW, after which survival analysis was conducted on 74 patients in each group. Here, age, gender, smoking history, KPS, EGFR mutation type, number of BMs, maximum BM diameter and extracranial metastasis status were balanced. After IPTW, there were no significant deviations in OS and PFS between the two groups (P = 0.1481 and P = 0.4643; Figures 2A, B). However, IPFS was markedly higher in the ucRT group than in the non-ucRT group (P < 0.0001; Figure 2C).
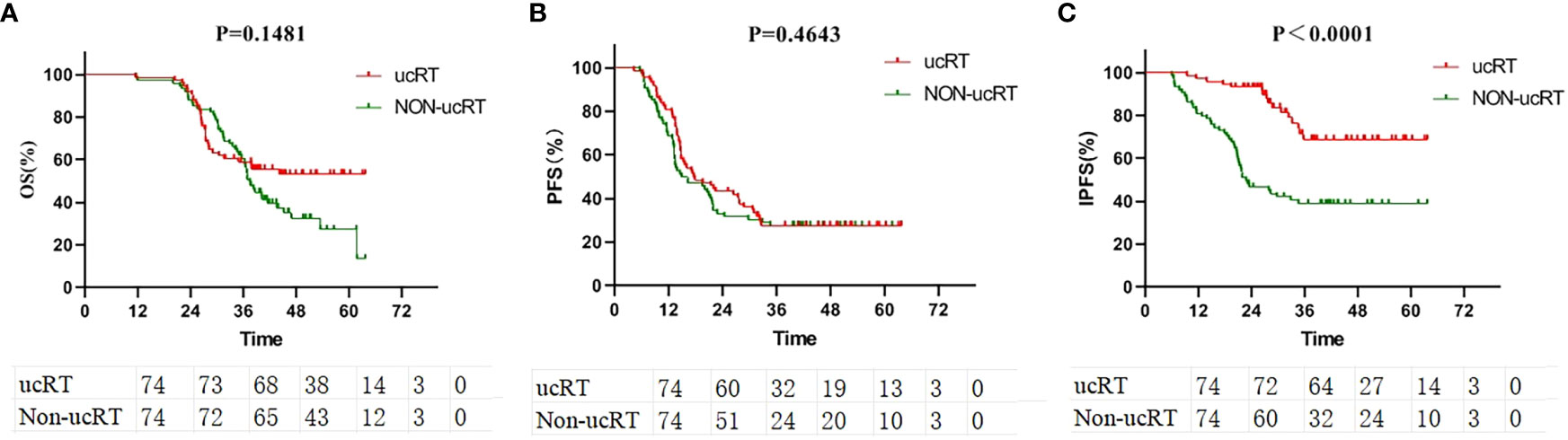
Figure 2 (A) Comparison of OS between ucRT and non-ucRT groups after IPTW. (B) Comparison of PFS between ucRT and non-ucRT groups after IPTW. (C) Comparison of IPFS in ucRT and non-ucRT groups after IPTW.
3.4 Subgroup survival analysis stratified by the brain metastases number
The patients were segregated into the multiple-BMs subgroup and the oligometastases (OLOGO-BMs) subgroup based on the number of BMs, with OLOGO-BM characterised as 1–3 BM lesions with a maximum diameter of ≤3.5 cm (maximum diameter of ≤3.0 cm in the ucRT group and a maximum diameter of ≤3.5 cm in the non-ucRT group) (18). The remaining cases were classified as having multiple BMs. Survival analysis was then performed on patients from both groups; in the multiple-BMs subgroup before IPTW the ucRT and non-ucRT groups demonstrated a median OS of 27.3 and 34.35 months, respectively (P = 0.0710; Figure 3A); a median PFS of 13.7 and 13.15 months, respectively (P = 0.0516; Figure 3B); and a median IPFS of 26.4 and 21.35 months, respectively (P = 0.0028; Figure 3C). Meanwhile, in the OLOGO-BMs subgroup, the ucRT and non-ucRT groups had a median OS of 44.5 and 37.3 months, respectively (P < 0.0001; Figure 3D); a median PFS of 32.3 and 20.8 months, respectively (P = 0.0011; Figure 3E); and a median IPFS of 37.8 and 22.1 months, respectively (P < 0.0001; Figure 3F). Furthermore, the Cox multifactorial analysis of OS and PFS in the OLOGO-BMs subgroup demonstrated that ucRT was linked with better OS (heart rate [HR] = 0.392; 95% confidence interval [CI]: 0.178–0.863; P = 0.020) and PFS (HR = 0.558; 95% CI: 0.316–0.986; P = 0.044) than seen in the non-ucRT group (Supplementary Tables 1, 2). These findings validate the effectiveness of ucRT in patients with OLOGO-BM.
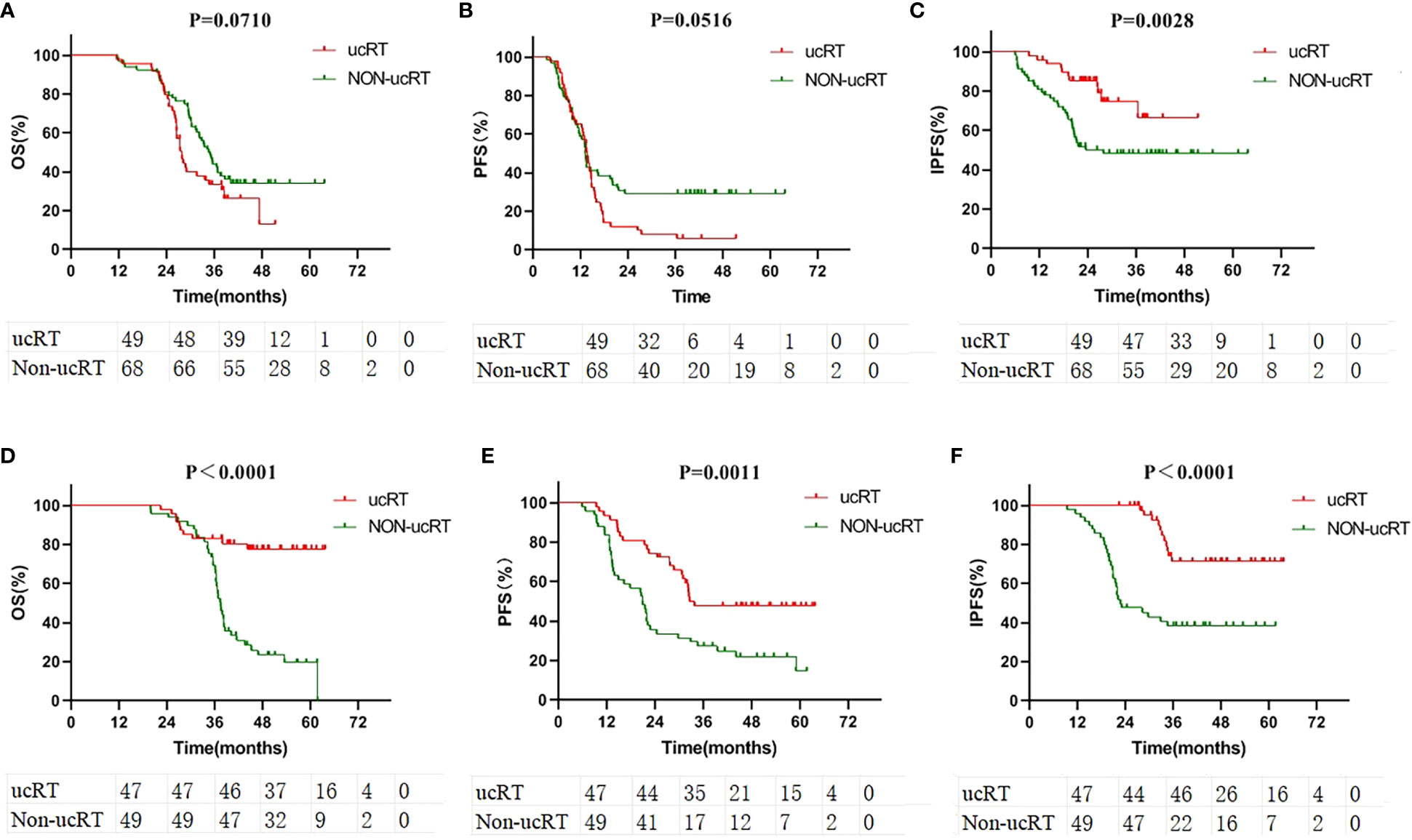
Figure 3 (A) Comparison of OS in the subgroup of patients with multiple brain metastases. (B) Comparison of PFS in the subgroup of patients with multiple brain metastases. (C) Comparison of IPFS in the subgroup of patients with multiple brain metastases. (D) Comparison of OS in the subgroup of patients with oligometastases. (E) Comparison of PFS in the subgroup of patients with oligometastases. (F) Comparison of IPFS in the subgroup of patients with oligometastases.
Following IPTW, 38 patients in the ucRT group and 29 patients in the non-ucRT group were successfully matched in the OLOGO-BM subgroup. In the multiple-BMs subgroup, 36 patients in the ucRT group were successfully matched with 45 patients in the non-ucRT group. The discrepancies in covariates between the two groups are outlined in Table 2. In the OLOGO-BM subgroup, all 38 patients in the ucRT group received SRS. Conversely, in the multiple-BMs subgroup, all 36 patients in the ucRT group underwent HA-WBRT. There were no significant post-IPTW disparities in OS and PFS between the two groups in the multiple-BMs subgroup (27.3 months and 34.9 months; P = 0.0890; Figure 4A; 13.7 months and 13.2 months; P = 0.1356; Figure 4B). Notably, IPFS was significantly higher in the ucRT group than in the non-ucRT group, with medians of 26.35 and 21.0 months, respectively (P = 0.0009; Figure 4C). In the OLOGO-BM subgroup, post-IPTW, the ucRT group reported a significantly higher OS, PFS and IPFS compared with the non-ucRT group, with a median OS of 44.45 and 37.5 months, respectively (P < 0.0001; Figure 4D); a median PFS of 31.95 and 20.8 months, respectively (P = 0.0104; Figure 4E); and a median IPFS of 37.7 and 24.3 months, respectively (P = 0.0003; Figure 4F). As can be seen from the forest map of OS and PFS of patients with oligocerebral metastasis, extracranial metastases and patients with maximum BM diameter > 10mm can benefit from non-ucRT in terms of OS. Patients with age < 60 and L858R mutation may have prolonged PFS from non-ucRT (Figure 5).
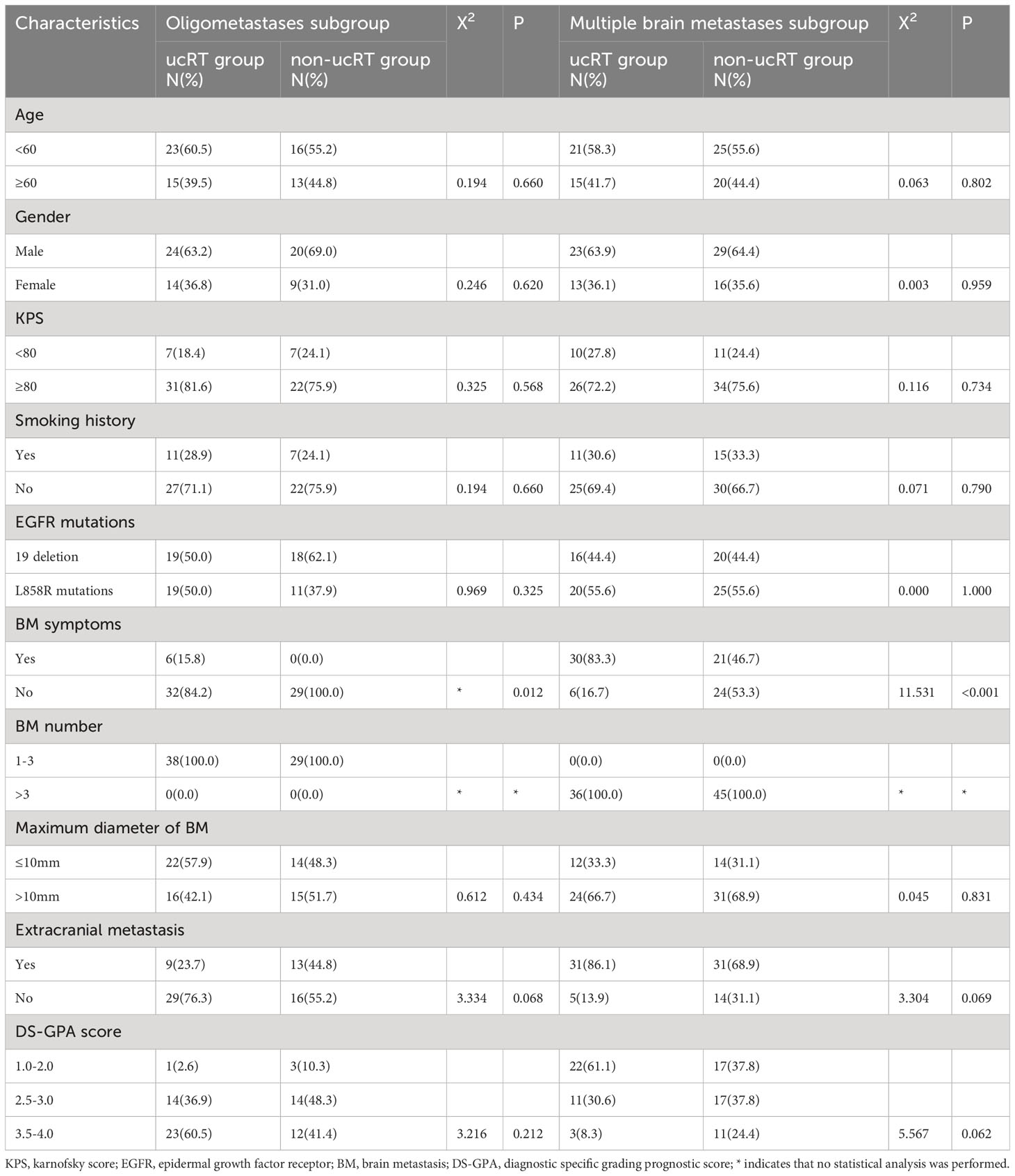
Table 2 Comparison of general clinical information of patients in the subgroup of oligometastases and the subgroup of multiple brain metastases after IPTW.
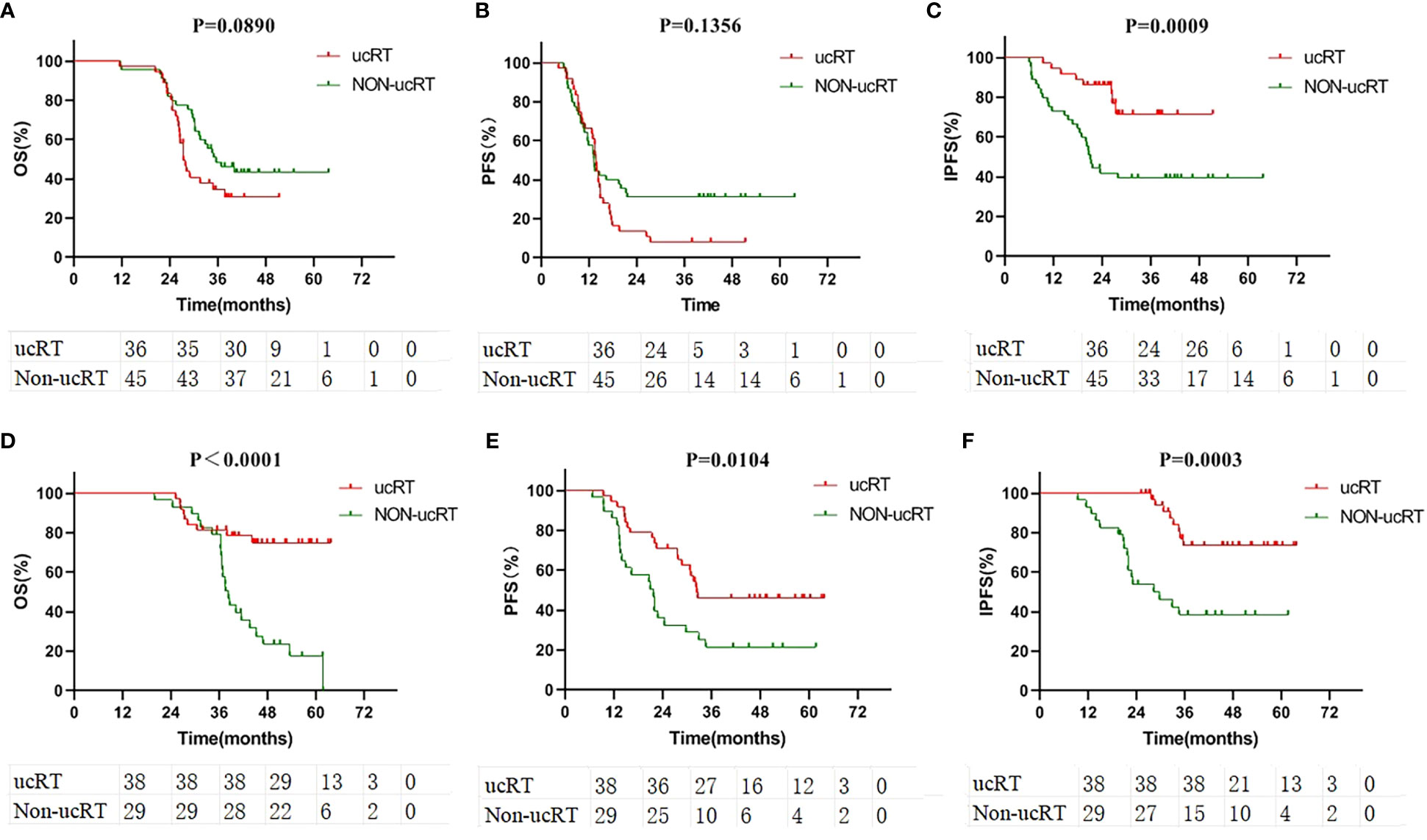
Figure 4 (A) Comparison of OS in the subgroup of patients with multiple brain metastases after IPTW. (B) Comparison of PFS in the subgroup of patients with multiple brain metastases after IPTW. (C) Comparison of IPFS in the subgroup of patients with multiple brain metastases after IPTW. (D) Comparison of OS in the subgroup of patients with oligometastases after IPTW. (E) Comparison of PFS in the subgroup of patients with oligometastases after IPTW. (F) Comparison of IPFS in the subgroup of patients with oligometastases after IPTW.
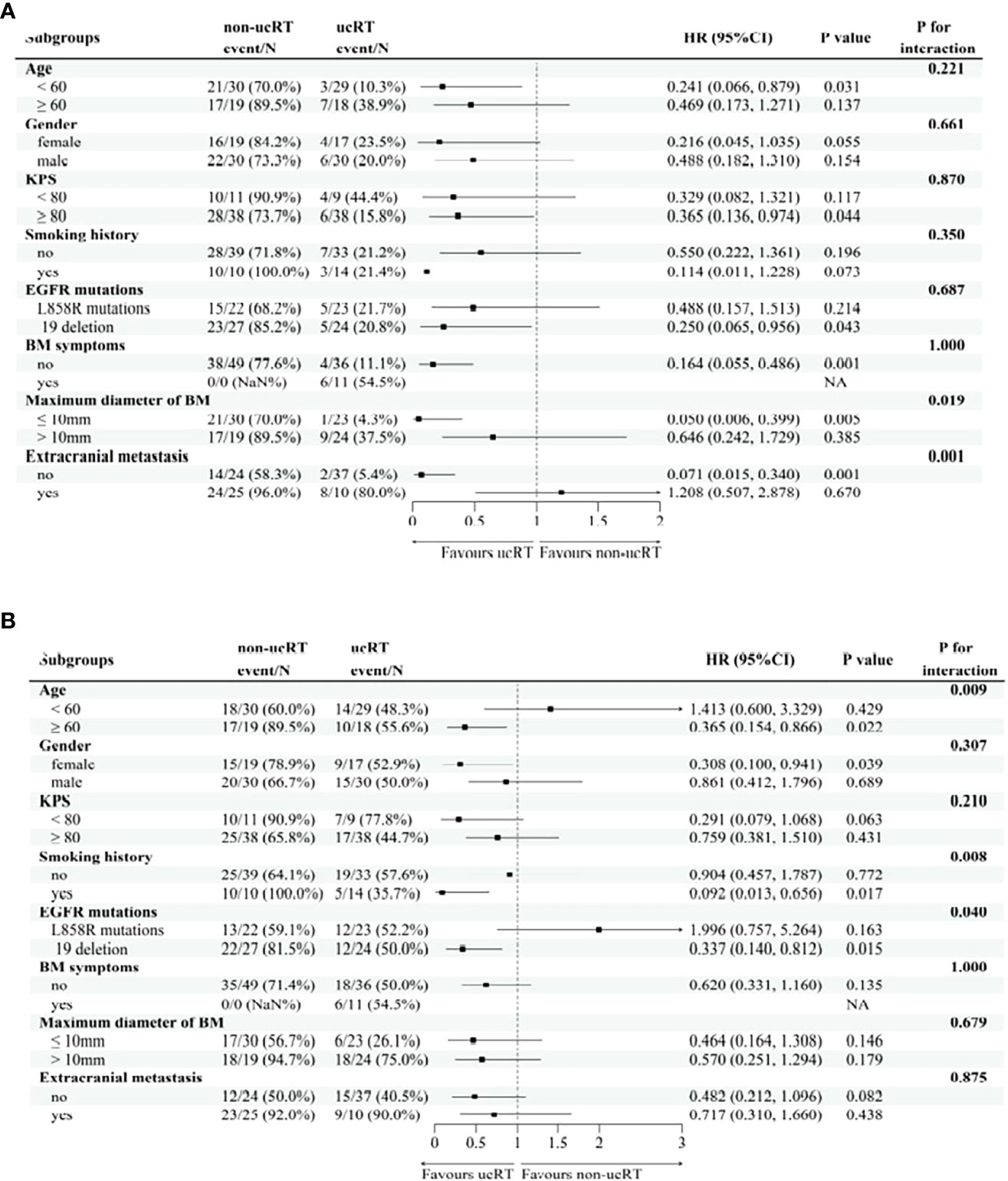
Figure 5 (A) the subgroup of forest map for Overall Survival in patients with oligocerebral metastasis. (B) the subgroup of forest map for Progression-Free Survival in patients with oligocerebral metastasis.
3.5 Failure modes
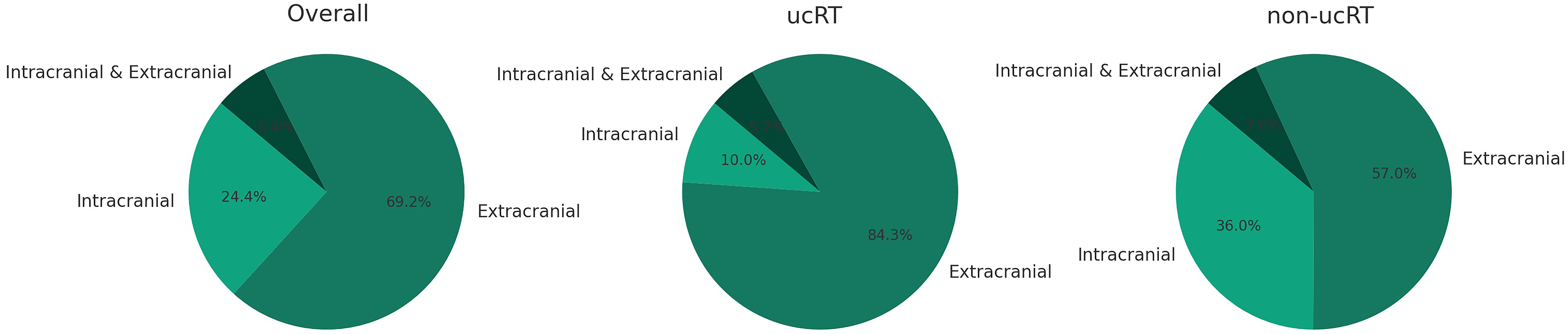
Throughout the follow-up period, 156 patients (73.2%) registered either intracranial or extracranial tumour progression. Of these, the ucRT group exhibited a predominant first site of progression extracranially, whereas the non-ucRT group showed a significant trend towards intracranial progression as the initial site (P < 0.001; Figure 6).
3.6 Adverse events
The EGFR-TKI-related adverse reactions: The incidence of adverse reactions in the ucRT group and the non-ucRT group was 75.0% and 73.5%, respectively (P = 0.804). Among them, diarrhoea was the most common (45.8% vs 44.4%, P = 0.839), followed by rashes (44.8% vs 43.6%, P = 0.860), paronychia (32.3% vs 30.8%, P = 0.812), dry skin (31.3% vs 29.9%, P = 0.833), etc. Most were grade 1–2 (97.9% vs 98.3%, P = 1.000; Supplementary Table 3). Adverse reactions related to brain RT: The incidence of adverse reactions was 51.3% and 85.7% in the OLOGO-BM group and multiple-BMs groups, respectively (P < 0.001). The most common adverse reactions were dizziness (31.9% vs 53.1%, P = 0.036), headache (27.7% vs 51.1%, P = 0.019), radiation dermatitis (17.0% vs 47.3%, P = 0.002) and neurocognitive dysfunction (32% vs 85.7%, P < 0.001). The incidence of grade 3 and above adverse reactions was 2.1% and 4.1% (P = 1.000; Supplementary Table 4).
4 Discussion
As a first-line treatment for stage IV NSCLC with EGFR mutations, osimertinib has substantially extended both OS and PFS compared with first-generation EGFR-TKIs (19). Specifically, in the context of EGFR-positive NSCLC with concurrent BM, third-generation EGFR-TKIs have exhibited remarkable efficacy. One clinical study comparing osimertinib to gefitinib/erlotinib revealed a strikingly extended PFS (15.2 months vs 9.6 months) (20). Additionally, the FLAURA clinical trial demonstrated significant enhancements in IPFS for the osimertinib group as opposed to the gefitinib/erlotinib group (21). Similar outcomes were reported in domestic studies involving other third-generation EGFR-TKIs, such as almonertinib and furmonertinib (13, 22).
While RT remains an essential treatment option for patients with BM, particularly those manifesting symptoms, the introduction of highly effective third-generation EGFR-TKIs has stirred debate concerning the role and optimal timing of cranial RT. Several studies have tried to evaluate the effectiveness of combining first/second-generation EGFR-TKIs with cranial RT versus monotherapy but have yielded inconclusive results (9, 23–25). These disparate findings point to the ongoing ambiguity around the ideal timing for cranial RT in the era of potent EGFR-TKIs.
The present analysis was inspired by previous research aiming to discern whether a synergy exists between pre-emptive cranial RT and third-generation EGFR-TKIs in prolonging survival. Although existing studies suggest that the addition of cranial RT to third-generation EGFR-TKI treatments does not dramatically extend OS and PFS, these results should be interpreted cautiously. Most of the patients in these studies were administered third-generation EGFR-TKIs as a second-line treatment or beyond, which may have introduced bias (26–28).
In the present study, which exclusively involves patients treated with osimertinib as a first-line option, the authors found no significant differences in OS and PFS between the ucRT group and the non-ucRT group. However, IPFS was notably higher in the ucRT group than in the non-ucRT group, especially among patients with limited BM (1–3 lesions) (29, 30). The subgroup analysis further revealed that ucRT could be associated with improved OS and PFS, especially in patients with OLOGO-BM. This improvement may be attributed to the heightened radiosensitivity of EGFR-positive NSCLC cells and the ability of cranial RT to efficiently clear intracranial tumours while potentially facilitating better EGFR-TKI penetration through the blood–brain barrier.
It is important to note that the present study is retrospective and carries inherent limitations, such as a risk of selection bias, a lack of exploration of the role of cranial RT before and after intracranial progression during osimertinib treatment and a failure to assess the adverse effects of different RT modalities. Furthermore, inconsistencies in follow-up treatment choices among treating physicians may have also affected the outcomes. Nonetheless, the present findings contribute valuable insights into a heavily debated and clinically significant issue, warranting further prospective trials for confirmation.
5 Conclusion
For patients with EGFR-positive NSCLC and BM, stratification according to BM status can guide treatment selection. For patients with OLOGO-BM, ucSRS may improve OS, PFS and IPFS more effectively than ucEGFR-TKI, advocating for early cranial SRS for eligible patients who can tolerate SRS. Conversely, for patients with multiple BMs, ucRT may not be as effective as upfront EGFR-TKI in improving OS and PFS and may increase neurotoxicity and reduce patients’ quality of life. Thus, upfront EGFR-TKI treatment might be the optimal choice for patients with multiple BMs. These conclusions warrant further validation through prospective studies with larger sample sizes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Cangzhou Hospital of Integrative TCM-WM of Hebei. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JZ: Conceptualization, Formal analysis, Resources, Writing – original draft. YZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. YS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. LX: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft. HL: Data curation, Resources, Validation, Writing – original draft. XY: Data curation, Investigation, Writing – original draft. KF: Investigation, Software, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1259880/full#supplementary-material
Abbreviations
EGFR, Epidermal Growth Factor Receptor; TKIs, tyrosine kinase inhibitors; OS, Overall Survival; PFS, Progression-Free Survival; IPFS, Intracranial Progression-Free Survival; BM, Brain metastases; NSCLC, non-small cell lung cancer; ORR, objective response rate; WBRT, whole-brain radiation therapy; HA-WBRT, hippocampal-avoidance whole-brain radiation therapy; SRS, stereotactic radiosurgery; RT, radiation therapy; MRI, magnetic resonance imaging; KPS, Karnofsky performance score; DS-GPA, diagnostic-specific grading prognostic score; CT, Computed Tomography; RECIST, Response Evaluation Criteria in Solid Tumors; IPTW, Inverse Probability of Treatment Weighting; KPS, Karnofsky Performance Status.
References
1. Barnholtz JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol (2004) 22(14):2865–72. doi: 10.1200/JCO.2004.12.149
2. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer (2015) 88(1):108–11. doi: 10.1016/j.lungcan.2015.01.020
3. Wilhelm I, Molnar J, Fazakas C, Haskó J, Krizbai IA. Role of the blood brain barrier in the formation of brain metastases. Int J Mol Sci (2013) 14(1):1383–411. doi: 10.3390/ijms14011383
4. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose geftinib. J Clin Oncol (2006) 24(27):4517–20. doi: 10.1200/JCO.2006.06.6126
5. Park SJ, Kim HT, Lee DH, Kim SW, Suh C, Lee JS, et al. Effificacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer (2012) 77:556–60. doi: 10.1016/j.lungcan.2012.05.092
6. Iuchi T, Shingyoji M, Sakaida, Hatano K, Nagano O, Itakura M, et al. Phase II trial of gefifitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer (2013) 82(2):282–7. doi: 10.1016/j.lungcan.2013.08.016
7. Schuler M, Wu YL, Hirsh V, O'Byrne K, Yamamoto N, Mok T, et al. First-line Afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol (2016) 11(3):380–90. doi: 10.1016/j.jtho.2015.11.014
8. Zeng YD, Liao H, Qin T, Zhang L, Wei WD, Liang JZ, et al. Blood-brain barrier permeability of geftinib in patients with brain metastases from non small-cell lung cancer before and during whole brain radiation therapy. Oncotarget (2015) 6(10):8366–76. doi: 10.18632/oncotarget.3187
9. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: A retrospective multi-institutional analysis. J Clin Oncol (2017) 35(10):1070–7. doi: 10.1200/JCO.2016.69.7144
10. Tsai PF, Yang CC, Chuang CC, Huang TY, Wu YM, Pai PC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: A prospective study. Radiat Oncol (2015) 10:253. doi: 10.1186/s13014-015-0562-x
11. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res (2016) 22(20):5130–40. doi: 10.1158/1078-0432.CCR-16-0399
12. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
13. Lu S, Dong XR, Jian H, Chen J, Chen G, Sun Y, et al. AENEAS: A randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastatic non-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol (2022) 40(27):3162–71. doi: 10.1200/JCO.21.02641
14. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiotherapy in patients with EGFR-mutant NSCLC that develop brain metastases. Radiat Oncol (2016) 95(2):673–9. doi: 10.1016/j.ijrobp.2016.01.037
15. Zhu LH, Fan XW, Sun L, Ni TT, Li YQ, Wu CY. New prognostic system specific for epidermal growth factor receptor-mutated lung cancer brain metastasis. Front In Oncol (2023) 13:1093084. doi: 10.3389/fonc.2023.1093084
16. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys (2000) 47(2):291–8. doi: 10.1016/s0360-3016(99)00507-6
17. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet (2004) 363(9422):1665–72. doi: 10.1016/S0140-6736(04)16250-8
18. Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol (2010) 40(2):107–11. doi: 10.1093/jjco/hyp167
19. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EQFR-mutated advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
20. Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC FLAURA Japanese subset. Jpn J Clin Oncol (2019) 49(1):29–36. doi: 10.1093/jjco/hyy179
21. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol (2018) 36(33):3290–7. doi: 10.1200/JCO.2018.78.3118
22. Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, et al. Central nervous system efficacy of furmonertinib (AST2818) versus gefitinib as first-line treatment for EGFR-mutated NSCLC: results from the FURLONG study. J Thorac Oncol (2022) 17(11):1297–305. doi: 10.1016/j.jtho.2022.07.1143
23. Li CH, Guo JD, Zhao L, Hu F, Nie W, Wang H, et al. Upfront whole brain radiotherapy for multiple brain metastases in patients with EGFR-mutant lung adenocarcinoma. Cancer Manag Res (2019) 23(11):3433–43. doi: 10.2147/CMAR.S196881
24. Saida Y, Watanabe S, Abe T, Shoji S, Nozaki K, Ichikawa K, et al. Efficacy of EGFR-TKIs with or without upfront brain radiotherapy for EGFR-mutant NSCLC patients with central nervous system metastases. Thorac Cancer (2019) 10(11):2106–16. doi: 10.1111/1759-7714.13189
25. Miyawaki E, Kenmotsu H, Mori K, Harada H, Mitsuya K, Mamesaya N, et al. Optimal sequence of local and EGFR-TKI therapy for EGFR-mutant non-small cell lung cancer with brain metastases stratified by number of brain metastases. Int J Radiat Oncol Biol Phys (2019) 104(3):604–13. doi: 10.1016/j.ijrobp.2019.02.051
26. Zhai XY, Li WH, Jia WX, Jia WX, Jing W, Tian YR, et al. Therapeutic effect of osimertinib plus cranial radiotherapy compared to osimertinib alone in NSCLC patients with eEGFR-activating mutations and brain metastases: a retrospective study. Radiat Oncol (2021) 16(1):233. doi: 10.1186/s13014-021-01955-7
27. Xie LJ, Nagpal S, Wakelee HA, Li G, Soltys SG, Neal JW, et al. Osimertinib for EGFR-mutant lung cancer with brain metastases: results from a single-center retrospective study. Oncologist (2019) 24(6):836–43. doi: 10.1634/theoncologist.2018-0264
28. Yu F, Ni JJ, Zeng WQ, Zhou Y, Guo TT, Zeng Y, et al. Clinical value of upfront cranial radiation therapy in osimertinib-treated epidermal growth factor receptor–mutant non-small cell lung cancer with brain metastases. Int J Radiat Oncol Biol Phys (2021) 111(3):804–15. doi: 10.1016/j.ijrobp.2021.05.125
29. Xie B, Sun LY, Chen YJ, Zhou J, Zheng JH, Zhang WM. Epidermal growth factor receptor gene mutations in non-small-cell lung cancer cells are associated with increased radiosensitivity in vitro. Cancer Manag Res (2018) 10:3551–60. doi: 10.2147/CMAR.S165831
Keywords: non-small cell lung cancer, brain metastases, epidermal growth factor receptor, tyrosine kinase inhibitor, craniocerebral radiotherapy
Citation: Zhou J, Zhou Y, Sun Y, Xiao L, Lu H, Yin X and Fan K (2024) The efficacy of upfront craniocerebral radiotherapy and epidermal growth factor receptor-tyrosine kinase inhibitors in patients with epidermal growth factor receptor-positive non-small cell lung cancer with brain metastases. Front. Oncol. 13:1259880. doi: 10.3389/fonc.2023.1259880
Received: 17 July 2023; Accepted: 15 December 2023;
Published: 19 January 2024.
Edited by:
John Varlotto, Edwards Comprehensive Cancer Center, United StatesReviewed by:
Ningbo Liu, Tianjin Medical University, ChinaHsin-Hua Lee, Kaohsiung Medical University, Taiwan
Bing Xia, Hangzhou Cancer Hospital, China
Copyright © 2024 Zhou, Zhou, Sun, Xiao, Lu, Yin and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunchuan Sun, c3VuX3l1bmNodWFuQDE2My5jb20=
 Jianxi Zhou1
Jianxi Zhou1 Yunchuan Sun
Yunchuan Sun