- 1School of Clinical Medicine, Tsinghua University, Beijing, China
- 2Department of Urology, Beijing Tsinghua Changung Hospital, Beijing, China
- 3School of Medical, Tsinghua University, Beijing, China
- 4Department of Urology, The First Affiliated Hospital of Kunming Medical University, Kunming, China
The immune checkpoint inhibitor (ICI) is a promising strategy for treating cancer. However, the efficiency of ICI monotherapy is limited, which could be mainly attributed to the tumor microenvironment of the “cold” tumor. Prostate cancer, a type of “cold” cancer, is the most common cancer affecting men’s health. Radiotherapy is regarded as one of the most effective prostate cancer treatments. In the era of immune therapy, the enhanced antigen presentation and immune cell infiltration caused by radiotherapy might boost the therapeutic efficacy of ICI. Here, the rationale of radiotherapy combined with ICI was reviewed. Also, the scheme of radiotherapy combined with immune checkpoint blockades was suggested as a potential option to improve the outcome of patients with prostate cancer.
Introduction
The immune checkpoint inhibitor (ICI) is believed to be promising for tumor patients (1). It could restore the antitumor function of cytotoxic T cells by blocking immune checkpoints, like PD-1 and CTLA-4 (2). However, ICI monotherapy is approved and useful for some cancers (e.g., breast, lung, kidney, bladder) (3) but has a very limited role in other cancers, with an ORR rate of around 3%–5% in prostate cancer (4), around 10%–13% in pancreatic cancer (5, 6), etc. Several clinical trials have explored the use of single-agent immune checkpoint inhibitors for the treatment of metastatic castration-resistant prostate cancer (mCRPC). The benefit seems to be restricted to a subset of patients (7). In a retrospective series of 11 patients with mCRPC characterized by MSI-H/dMMR who were treated with anti–PD-1/PD-L1 inhibitors alone or in combination with another CPI, the ORR was 50%; 54.5% of the patients demonstrated a PSA drop of higher than 50%, and four of these patients had a PSA decline of more than 99% (8). In 2017, the FDA approved pembrolizumab for the treatment of unresectable and metastatic tumors with an MSI-high (MSI-H) status or dMMR after progression on standard lines of treatment (9). Low incidence of TMB is one of the most important factors for ICI therapy failure (10). Behind this perspective, the true mechanism of ICI therapy is based on the antitumor function of CD8+ cytotoxic T lymphocytes. To fight against tumor, the human immune system must first recognize tumor cells with neo-antigens; then, T cells need to move to the tumor sight and contact with tumor cells to provide their antitumor effect. However, in the meantime, tumors would develop multiple mechanisms (mainly including T-cell priming dysfunction and T-cell homing dysfunction) to escape the hunt from tumor-reactive T cells (11). These tumors are called “Cold” tumors, tumors with a specific tumor microenvironment which causes the failure of ICI therapy (12).
“Cold” tumors have a limited infiltration of cytotoxic T lymphocytes (CTLs), a high activity of suppressor immune cells, and a poor response to ICI therapy. “Hot” tumors, on the opposite, are tumors that respond better to ICI therapy due to high infiltration of T cells and low activity of suppressor immune cells (Figure 1). “Cold” tumors are also called infiltrated excluded, non-inflamed, or non-immune reactive tumors (13). It is considered that prostate cancer is one of the “Cold” tumors (14).
Prostate cancer is the second most common cancers among men, after melanoma (15, 16). After definitive therapy, high-risk prostate tumors may have a recurrence and metastatic rate of around 30% (17). Although androgen deprivation therapy (ADT) is a standard therapy for prostate cancer in advanced stages, the tumor would still progress eventually. Around 70%–80% of prostate cancer would metastasize into the bone, which worsens the prognosis for prostate cancer patients (16). The immunologic landscapes of prostate cancer allow limited infiltration of immune cells into the tumor microenvironment (TME). The dysfunction of the immune system prevents it from initiating an effective response against tumors (18). Consequently, prostate cancer has a poor rate of response to ICI therapy (19).
Radiotherapy increases the levels of immune checkpoints (PD-1, CTLA-4) locally in the tumor by enhancing the activation and infiltration of immune cells into the TME and the level of PD-L1 expression on the surface of tumor cells (20). Moreover, immune check inhibitors can restore the antitumor activity of T cells by binding PD-1, CTLA, and PD-L1 and lower the immune escape of tumor cells (21). Consequently, radiotherapy and immune checkpoint inhibitor therapy are mechanistically complementary, and their combination may provide favorable outcomes in “Cold” tumor treatments. To improve the therapeutic effect of immune checkpoint blockade in prostate cancer, in this paper, radiotherapy is suggested as a preferable option for combined immune checkpoint inhibitor therapy to achieve a better therapeutic effect in patients with prostate cancer.
Mechanisms of the “cold” tumor resisting ICI
The primary characteristic of a “cold” tumor is the absence of T-cell infiltration, which leads to low response rates of ICI therapy. Either defective T-cell priming or defective T-cell homing to the tumor bed is the reason for the failure of T-cell infiltration.
T-cell priming dysfunction
Lack of tumor antigens and defects in tumor antigen presentation machinery are two main reasons for defective T-cell priming.
Lack of tumor antigens is the most direct cause of T-cell priming disorders. There are two categories of targeted tumor antigens: non-mutated self-antigens and neoantigens (22). Self-antigens, such as tumor-associated antigens (TAAs) and cancer/testis antigens (CTAs), are overexpressed non-mutated proteins in tumor cells. Neoantigens, also called tumor-specific antigens (TSAs), are specific mutated proteins in tumor cells. The recognition of tumor neoantigens by the immune system might promote T-cell priming and infiltration to the tumor region, which may result in antitumor responses (23).
This leads to tumor mutation burden (TMB), the total amount of non-synonymous single-nucleotide mutations in a tumor, a potential predictor for the response of ICI therapy. It is generally believed that tumors with a higher TMB would carry a higher neoantigen load that could be recognized by the immune system, making greater T-cell infiltration (24). Multiomics network analysis has further proved that higher mutation or neoantigen burden was positively correlated with higher CTL infiltration in various solid tumors, whereas lower mutational or neoantigen burden might be correlated with the lack of CTL infiltration and the failure of ICI therapy (25). However, kidney cancer presents with low TMB incidence, which showed 40% of response rate to ICI therapy (26). In gastric cancer, no difference in response rate was observed between TMB-H (16.7%) and TMB-L (16.2%) tumors treated with ICI (27). This evidence suggests that there are many other factors determining ICI response or resistance.
Damage to the machinery responsible for processing and presenting tumor antigens is another important cause of T-cell priming or homing defects in the tumor bed (APM). Normally, antigen-presenting cells (APCs) could express the tumor neoantigen peptide–MHC class I complex on its surface after recognition. However, impairs in the APM pathway, like downregulation of MHC-I molecule expression, could lead to the failure in presenting antigen peptide-MHC class I complexes even though tumor-specific antigens were present. During antigen processing and presentation, transporters associated with antigen processing (TAP) transport cytosolic cleaved antigens to the endoplasmic reticulum to bind with MHC molecules. Therefore, the deletion of TAP could also cause antigen presentation process failure, which further affects the priming of T cells (28). Beta-2-microglobulin (B2M), the invariant chain of the MHC, is critical for the successful folding and transport of MHC-I to the cell surface (29). Knocking down the B2M gene in human carcinoma cell lines led to the absence of MHC-I molecules expressed on their surface and the failure of tumor-specific T-cell recognition and cytotoxicity (30). These findings suggest that defects in the APM pathway would interfere with T-cell priming and the effectiveness of ICI therapy despite the presence of tumor antigens.
Dysfunction of dendritic cells (DCs) could also impair T-cell priming. DC activation requires pattern recognition receptors (PRRs), which are found on the surface of DCs and include pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (31). This activation enables DCs to present a tumor antigen peptide-MHC class I complex to T cells when contracting with them. DCs could also express secondary signaling necessary for T-cell activation, such as B7 (32). Tumor cells could impair the function of DCs by trapping “danger signals”. For example, stanniocalcin 1 (STC1) could trap DAMPs, leading to the failure of DC activation and T-cell activation. This eventually contributes to tumor immune escape (33). Furthermore, BATF3 DCs are the primary source of CXC-chemokine ligand 9 and 10 (CXCL9 and CXCL10), two key chemokines required for the recruitment of CD8+ T cells to tumors. In the absence of BATF3 DCs, CD8+ T cells would not migrate to the tumor region (34). However, a number of tumor types, including prostate cancer, did not express CXCL9 and CXCL10, which prevented CD8+ effector T cells from activating (35).
Differentiation and recruitment of DCs require various factors like Fms-like tyrosine kinase 3 ligand (FLT3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (36). Deficiency of these factors could result in a reduced number of DCs in secondary lymphoid organs and attenuated T-cell immune responses (37). To sum up, impaired DC activation, a lack of DCs, and the overexpression of co-suppressive signals could all lead to T-cell activation failure as DC-T cell crosstalk plays a vital role in naïve T-cell priming.
T-cell homing dysfunction
Recent evidence has shown that the activation of tumor cell oncogenic pathways is associated with the immune-suppressive TME of “cold tumor”, which causes the potential for ICI therapy resistance. When the WNT pathway was activated, CCL4 expression is downregulated, resulting in decreased recruitment of BATF3 DCs to the TME (38). Also, the WNT pathway activation would cause decreased expression of CXCL9 and CXCL10, making CTLs not being able to recruit to the tumor region. Furthermore, Spranger et al. have found a negative correlation between CD8A expression and activation of the WNT signaling pathway via human metastatic carcinoma sample analysis (25). Direct injection of BATF3 DCs into the tumor region restored CXCL9 and CXCL10 expression and T-cell infiltration in WNT signaling pathway-positive tumors (39). Activation of the PI3K/AKT pathway could decrease autophagic activity by reducing the lipidation of the autophagosome protein LC3, which inhibits T-cell homing to the cancer site (40). A TCGA dataset analysis also revealed that the expression of T-cell effector molecules (like IFN-γ, granzyme B) was negatively correlated with the activation of the PI3K/AKT pathway in various carcinomas (40). Oncogenic K-RAS mutations might also contribute to tumor-promoting inflammation through the production of inhibitory cytokines (IL-6, IL-8, etc.), the activation of NLRP3 inflammasome, and the release of chemokines (CCL5, CCL9, etc.) (41). In addition, oncogenic MYC signaling could overexpress CD47 and PD-L1 on tumor cells. As a immune checkpoint, CD47 can attach to signal regulatory proteins α(SIRPα) on the surface of macrophages in the tumor microenvironment and deliver “don’t eat me” messages in order to evade immune surveillance (42, 43). Consequently, CD47 becomes an attractive target for immunotherapy. PD-L1 and CD47 are both highly expressed in tumor cells and can be simultaneously regulated by MYC. A high expression of MYC in tumor cells can regulate the tumor microenvironment by acting on innate immune cells and adaptive immune cells as well as cytokines, and activation of MYC can upregulate the expression of CD47 and PD-L1 leading to immunosuppression and tumor proliferation (MYC can directly act on the promoters of CD47 and PD-L1 and then regulate their mRNA and protein expression levels) (44).
The immunosuppressive cells at tumor sites could also prevent T-cell homing in “cold tumors”. For example, CXCL12, generated by cancer-associated fibroblasts (CAF), could inhibit T-cell infiltration in the tumor region (45). Moreover, CTLs could not reach the edge of “cold” tumors because they could be trapped within the stroma of the tumor or in the peri-tumoral tissue because of the unique immunosuppressive structure built by immunosuppressive cells, like tumor-associated macrophages (TAM), cancer-associated fibroblasts (CAF), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) (45) (Supplementary Figure S1).
Immune landscape of prostate cancer
Prostate cancer is considered to be a “cold” tumor as the tumor mutation burden is low, causing a lower number of T-cell infiltrations (46). Prostate cancer also presents a lot of hypoxic zones, which seriously affect the antitumor function of T cells by a variety of mechanisms including the depletion of essential nutrients, abnormal angiogenesis, increased expression of adenosine, acidic pH, immunosuppressive transforming growth factor β (TGF β), and upregulation of PD-L1 (47, 48). Moreover, hypoxic zones could attract immature myeloid cells into the tumor region and turn into myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages, making the TME of prostate cancer even more immunosuppressed (47). In fact, the immune cells that are part of the prostate tumor microenvironment are frequently characterized by an anergic and immunosuppressive phenotype and include regulatory T cells (Tregs), M2-polarized tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) (49).
Furthermore, the expression of major histocompatibility complex (MHC) class I, a molecule presenting antigenic protein fragments to cytotoxic T cells, is lost or diminished in prostate cancer (50, 51). Also, PTEN, whose existence could improve the immunosuppressive tumor microenvironment, is frequently downregulated (52), leading to a decrease in the response to immunotherapy in prostate cancer. Single-cell analysis revealed that CD4+ FOXP3+ CD25+ T cells and CD8+ FOXP3+ CD25+ T cells are the main T-cell types in prostate cancer. However, FOXP3+ T cells are immunosuppressive regulatory T cells that both inhibit naive T-cell proliferation and produce inhibitory cytokines, like IL-6 and IL-8 (53, 54).
Radiotherapy in prostate cancer
Radiotherapy is considered to be one of the most effective therapies for prostate cancer. There are mainly two techniques applied to deliver radiations to the prostate cancer area, namely, brachytherapy (the use of seeds placed in the body) and external beam (projecting energy through the skin) (55).
Brachytherapy means the direct placement of radioactive sources into the prostate gland under the guidance of transrectal ultrasound. There are two dose rates, low dose rate and high dose rate. The low dose rate refers to the permanent implantation of seeds in the prostate tissue so that the seeds project radioactivity gradually. This dose is mainly used in clinics (56). The high dose rate refers to a radiation therapy whose dosage is high enough to possibly lead to leakage to surrounding organs from the prostate tissues. As a result, the high dose rates are rarely applied in the clinic. The advantage of brachytherapy is that the whole process is convenient for patients as it only takes a day or less and no further intervention is needed (57). In addition, the erectile function would not be affected due to brachytherapy (58).
External beam radiation therapy (EBRT) is a commonly used local treatment of tumors in which strong X-ray would emit specifically targeting the prostate tissues (59). Its radiation dose is high (higher than brachytherapy), whereas its radiation would emit less to the surrounding tissues. Radiotherapy could be used in not only patients with localized prostate cancer but also patients with bone metastasis or with metastatic castration-resistant disease (60).
Combination therapy of radiation therapy and androgen deprivation therapy (ADT) is considered to be effective for patients with prostate cancer (61). It is reported that this combination has more advantages than surgical therapy. There are lower risks, such as hemorrhage, myocardial infarction, pulmonary embolism, urine incontinence, and erectile dysfunction, when treating prostate cancer in its early stages (62). This evidence also proved the concept that radiation therapy could be used in company with other therapy in patients with prostate cancer.
Radiotherapy could “heat” prostate cancer
The conventional goal of radiation in the treatment of cancer is to use high-energy particles to cause deadly DNA damage in tumor cells, causing the cancer cells to eventually perish (63). Recently, the ability of radiotherapy to cause an antitumor immune response has drawn large attention as cancer immune therapy has achieved great success in clinics (64). Studies have proved that radiotherapy could cause immunogenic cell death and cellular stress, which could increase the exposure of tumor-associated antigens and damage-associated molecular patterns (DAMPs) (65). Elevated tumor-associated antigens and DAMPs could activate DCs. Furthermore, DCs could present tumor-associated antigens to CD8+ T cells and help recruit CD8+ T cells into the TME to enhance the antitumor responses (66).
Furthermore, the interferon genes (STING) pathway would be activated in the presence of DAMPs. Production of interferon gene pathways, including type I interferons (IFN-α and IFN-β), could further activate antitumor immune responses (67). Both preclinical and clinical studies validated that radiotherapy could increase antitumor immune cells such as CD4+, CD8+ T, cytotoxic NK, and CD8+CD56+ natural killer T (NKT) cells infiltrating into the TME (68, 69). All of these data imply that radiotherapy may transform “cold” cancers with low immunogenicity and immune cell infiltration into “hot” tumors with dense immune cell infiltration.
Meanwhile, elevated interferon activity via radiotherapy could upregulate exhaustion molecules such as PD-1 and PD-L1 (70, 71). Therefore, the combination of radiotherapy plus immune checkpoint inhibitor would be necessary to block the elevated exhaustion molecules, thus avoiding cancer immune escape and enhancing the therapeutic effect of radiotherapy (Supplementary Figure S2).
Clinical studies exploring radiotherapy plus immune checkpoint blockade
There are several clinical trials exploring the therapeutic effect of radiotherapy plus ICI in treating cancers.
A phase 1 study combined anti-PD-1/PD-L1 therapy with stereotactic body radiotherapy (SBRT); finding the combination could be well-tolerable by patients (72). There are five phase 1/2 studies that also showed that anti-PD-1/PD-L1 therapy plus radiotherapy was tolerable for patients with advanced non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), and small cell lung cancer (SCLC) (73–77). These findings indicate that the combination of radiotherapy plus ICI is feasible for cancer treatment.
As this field is in its preliminary stages, most studies have shown encouraging efficiencies in patients treated with combination of radiotherapy and ICI. Most combinations of radiotherapy and ICI are based on stereotactic body radiotherapy (SBRT), which could precisely deliver ablative doses of radiation in image-guided and intensity-modulated manners (78). A phase 2 study in resectable non-small-cell lung cancer showed that patients who received 24-Gy SBRT before durvalumab (PD-1 antibody) treatment demonstrated a higher objective response rate (ORR) (53.5%) than patients who only received durvalumab or SBRT (6.7%) (79); however, long-term prognoses (such as OS, PFS) were not recorded in the study. A phase 3 study found that stage III NSCLC patients undergoing radiotherapy and durvalumab acquired a markedly improved progression-free survival (PFS) (17.2 vs. 5.6 months) and overall survival (OS) (The 12-month overall survival rate was 83.1% in the durvalumab group, as compared with 75.3% in the placebo group. The 24-month overall survival rate was 66.3% in the durvalumab group, as compared with 55.6% in the placebo group.) compared with those who only received radiotherapy (80). Tai et al. showed that combination of Y-90-RE with nivolumab had an optimistic ORR of 31% in patients with advanced hepatocellular carcinoma (HCC) (81). Chiang et al. reported an astonishing 100% ORR in five patients treated with SBRT followed by nivolumab for large unresectable HCC; another case report showed complete pathological response following Y-90-RE and nivolumab bridging therapy prior to partial hepatectomy (82, 83). Another phase I trial that evaluated liver/lung SBRT with ipilimumab reported that 23% of patients experienced clinical benefit which corresponded to an increase in CD8+ T cells and CD8+/CD4+ ratios (84). Although these findings are promising, these trials only recruited limited patients in only one medical center.
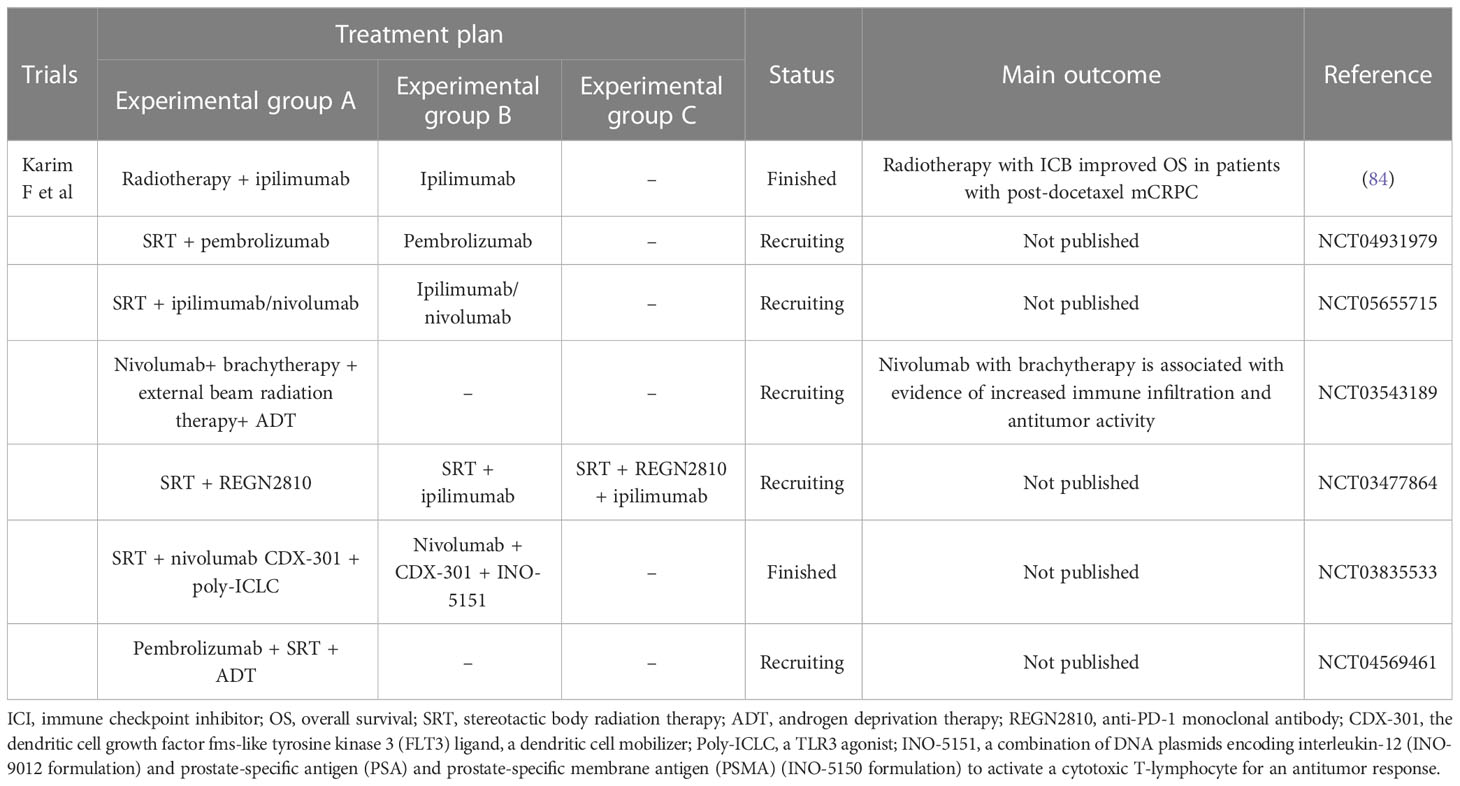
There are also trials that have been carried out to investigate the interaction of radiation and ICI therapy in prostate cancer. Slovin et al. explored the combination of ipilimumab (anti-CTLA-4) and radiotherapy in patients with metastatic castration-resistant prostate cancer, finding that ipilimumab 10 mg/kg plus radiotherapy suggested clinical antitumor activity with disease control and manageable adverse events (85); in addition, the trial suggested that treatment with ipilimumab improved progression-free survival compared with placebo (median 4.0 vs. 3.1 months in the placebo group; p < 0.0001). Two phase 3 studies have evaluated the therapeutic effect of ipilimumab plus radiotherapy in patients with metastatic castration-resistant prostate cancer (86, 87). In a preplanned long-term analysis including 799 patients, ipilimumab plus radiotherapy improved the overall survival of these patients significantly. The OS rates at 3, 4, and 5 years were approximately two to three times higher than that of patients receiving only radiotherapy (87).
PD-L1 expression was observed in serial biopsy samples in a phase 1 trial of nivolumab in combination with external beam radiation therapy and brachytherapy in patients with grade 5 prostate cancer and increased immune infiltration. A strong immune infiltrate was observed in the tissues, with an increase in CD8+ and FOXP3+/CD4+ T cells in the tissues and CD4+ effector T cells in the peripheral blood (88). Although a potentially T-cell-mediated antitumor effect of ipilimumab was observed in 13%–23% of men enrolled in the two trials, these data suggest that only a minority of men with mCRPC derive a benefit from the single-agent ipilimumab. Based on preclinical data showing that radiotherapy could improve the tumor microenvironment and the antitumor effect of CTLA-4, these studies used radiotherapy as a boost for ipilimumab. The long-term analysis shows that OS is improved with ipilimumab plus RT versus placebo plus RT in patients with post-docetaxel mCRPC, and treatment was associated with a fraction of patients with long-term survival. Although these findings might suggest that the combination of radiotherapy plus ICI would bring benefits to patients with advanced-stage prostate cancer, no definitive phase 3 studies are testing this combination of ICI plus radiotherapy in prostate cancer; more clinical trials should be conducted to confirm its therapeutic effect and other details such as dose, volume, fractionation, and sequence. The current research studies are listed in Table 1, which can further prove the efficacy of radiotherapy combined with ICI in prostate cancer. Those studies might also point out patients with which a specific prostate cancer stage could benefit most from using this combination. Furthermore, these studies might also show the association between radiotherapy plus ICI and conventional therapy such as androgen deprivation therapy, answering questions such as whether this strategy is better. Whether this strategy could be used on patients undergoing androgen deprivation therapy and so on.
Perspective
To sum up, radiotherapy combined with immune checkpoint blockade may be a more effective combination to enhance the efficacy of ICI monotherapy in the treatment of prostate cancers, since radiotherapy may boost tumor antigen presentation and CTL infiltration into the cancer region. However, there are currently only a few clinical trials focused on the combination of radiotherapy and immune checkpoint blockade for the treatment of prostate cancer. Further clinical trials are expected in the future to validate its efficiency in treating prostate cancer.
Author contributions
TL, XQ, XL, BX, and JXL conceptualized, wrote, and reviewed the manuscript. JYL, JY, FX, JL, and GY were involved in writing the article.
Funding
This study was supported by the Beijing Guo Yinglu Urology Development Foundation (Grant No. 12022C6035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1210673/full#supplementary-material
References
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
2. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet (2021) 398(10304):1002–14. doi: 10.1016/S0140-6736(21)01206-X
3. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol (2019) 10:168. doi: 10.3389/fimmu.2019.00168
4. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol (2020) 38(5):395–405. doi: 10.1200/JCO.19.01638
5. Bockorny B, Macarulla T, Semenisty V, Borazanci E, Feliu J, Ponz-Sarvise M, et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the COMBAT/KEYNOTE-202 trial. Clin Cancer Res (2021) 27(18):5020–7. doi: 10.1158/1078-0432.CCR-21-0929
6. Padron LJ, Maurer DM, O'Hara MH, O'Reilly EM, Wolff RA, Wainberg ZA, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat Med (2022) 28(6):1167–77. doi: 10.1038/s41591-022-01829-9
7. de Almeida DVP, Fong L, Rettig MB, Autio KA. Immune checkpoint blockade for prostate cancer: niche role or next breakthrough? Am Soc Clin Oncol Educ Book (2020) 40:1–18. doi: 10.1200/EDBK_278853
8. Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol (2019) 5(4):471–8. doi: 10.1001/jamaoncol.2018.5801
9. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res (2019) 25(13):3753–8. doi: 10.1158/1078-0432.CCR-18-4070
10. Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol (2021) 14(1):156. doi: 10.1186/s13045-021-01164-5
11. Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics (2021) 11(11):5365–86. doi: 10.7150/thno.58390
12. Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol (2022) 43(7):523–45. doi: 10.1016/j.it.2022.04.010
13. Mortezaee K, Najafi M. Immune system in cancer radiotherapy: Resistance mechanisms and therapy perspectives. Crit Rev Oncol Hematol (2021) 157:103180. doi: 10.1016/j.critrevonc.2020.103180
14. Mortezaee K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J Biochem Mol Toxicol (2021) 35(4):e22708. doi: 10.1002/jbt.22708
15. Mishra R, Haldar S, Placencio V, Madhav A, Rohena-Rivera K, Agarwal P, et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest. (2018) 128(10):4472–84. doi: 10.1172/JCI99397
16. Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell (2019) 179(5):1177–1190 e13. doi: 10.1016/j.cell.2019.10.029
17. Maughan BL, Pal SK, Gill D, Boucher K, Martin C, Salgia M, et al. Modulation of premetastatic niche by the vascular endothelial growth factor receptor tyrosine kinase inhibitor pazopanib in localized high-risk prostate cancer followed by radical prostatectomy: A phase II randomized trial. Oncologist (2018) 23(12):1413–e151. doi: 10.1634/theoncologist.2018-0652
18. Wu Z, Chen H, Luo W, Zhang H, Li G, Zeng F, et al. The landscape of immune cells infiltrating in prostate cancer. Front Oncol (2020) 10:517637. doi: 10.3389/fonc.2020.517637
19. Redman JM, Steinberg SM, Gulley JL. Quick efficacy seeking trial (QuEST1): a novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J Immunother Cancer. (2018) 6(1):91. doi: 10.1186/s40425-018-0409-8
20. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20(4):203–17. doi: 10.1038/s41568-020-0246-1
21. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol (2017) 8:561. doi: 10.3389/fphar.2017.00561
22. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x
23. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
24. Mouw KW, Goldberg MS, Konstantinopoulos PA, D'Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discovery (2017) 7(7):675–93. doi: 10.1158/2159-8290.CD-17-0226
25. McGrail DJ, Federico L, Li Y, Dai H, Lu Y, Mills GB, et al. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat Commun (2018) 9(1):1317. doi: 10.1038/s41467-018-03730-x
26. Valero C, Lee M, Hoen D, Zehir A, Berger MF, Seshan VE, et al. Response rates to anti-PD-1 immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol (2021) 7(5):739–43. doi: 10.1001/jamaoncol.2020.7684
27. McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol (2021) 32(5):661–72. doi: 10.1016/j.annonc.2021.02.006
28. Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. (2008) 57(11):1719–26. doi: 10.1007/s00262-008-0515-4
29. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
30. Torrejon DY, Abril-Rodriguez G, Champhekar AS, Tsoi J, Campbell KM, Kalbasi A, et al. Overcoming genetically based resistance mechanisms to PD-1 blockade. Cancer Discovery (2020) 10(8):1140–57. doi: 10.1158/2159-8290.CD-19-1409
31. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell (2010) 140(6):805–20. doi: 10.1016/j.cell.2010.01.022
32. Logue EC, Sha WC. CD28-B7 bidirectional signaling: a two-way street to activation. Nat Immunol (2004) 5(11):1103–5. doi: 10.1038/ni1104-1103
33. Lin H, Kryczek I, Li S, Green MD, Ali A, Hamasha R, et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell (2021) 39(4):480–493 e6. doi: 10.1016/j.ccell.2020.12.023
34. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell (2017) 31(5):711–723 e4. doi: 10.1016/j.ccell.2017.04.003
35. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev (2018) 63:40–7. doi: 10.1016/j.ctrv.2017.11.007
36. Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature (2019) 574(7776):45–56. doi: 10.1038/s41586-019-1593-5
37. Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood (2009) 114(4):835–43. doi: 10.1182/blood-2009-02-206318
38. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature (2015) 523(7559):231–5. doi: 10.1038/nature14404
39. Gajos-Michniewicz A, Czyz M. WNT signaling in melanoma. Int J Mol Sci (2020) 21(14):4852. doi: 10.3390/ijms21144852
40. Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discovery (2016) 6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283
41. Hamarsheh S, Gross O, Brummer T, Zeiser R. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun (2020) 11(1):5439. doi: 10.1038/s41467-020-19288-6
42. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell (2009) 138(2):271–85. doi: 10.1016/j.cell.2009.05.046
43. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr., et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell (2009) 138(2):286–99. doi: 10.1016/j.cell.2009.05.045
44. Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science (2016) 352(6282):227–31. doi: 10.1126/science.aac9935
45. Bassani-Sternberg M, Digklia A, Huber F, Wagner D, Sempoux C, Stevenson BJ, et al. A phase Ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma-A proof of antigen discovery feasibility in three patients. Front Immunol (2019) 10:1832. doi: 10.3389/fimmu.2019.01832
46. Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature (2011) 470(7333):214–20. doi: 10.1038/nature09744
47. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene (2017) 36(4):439–45. doi: 10.1038/onc.2016.225
48. Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. (2018) 128(11):5137–49. doi: 10.1172/JCI96268
49. Krueger TE, Thorek DLJ, Meeker AK, Isaacs JT, Brennen WN. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate (2019) 79(3):320–30. doi: 10.1002/pros.23738
50. Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst (1995) 87(4):280–5. doi: 10.1093/jnci/87.4.280
51. Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, et al. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate (1997) 33(4):233–9. doi: 10.1002/(sici)1097-0045(19971201)33:4<233::aid-pros2>3.0.co;2-i
52. Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. (2018) 15(4):222–34. doi: 10.1038/nrurol.2018.9
53. Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol (2006) 177(10):7398–405. doi: 10.4049/jimmunol.177.10.7398
54. Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res (2007) 13(23):6947–58. doi: 10.1158/1078-0432.CCR-07-0842
55. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci (2012) 9(3):193–9. doi: 10.7150/ijms.3635
56. Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst (2000) 92(19):1582–92. doi: 10.1093/jnci/92.19.1582
57. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules (2022) 27(17):5730. doi: 10.3390/molecules27175730
58. Stember DS, Mulhall JP. The concept of erectile function preservation (penile rehabilitation) in the patient after brachytherapy for prostate cancer. Brachytherapy (2012) 11(2):87–96. doi: 10.1016/j.brachy.2012.01.002
59. Breitkreutz DY, Weil MD, Bazalova-Carter M. External beam radiation therapy with kilovoltage x-rays. Phys Med (2020) 79:103–12. doi: 10.1016/j.ejmp.2020.11.001
60. Morris MJ, Fong L, Petrylak DP, Sartor AO, Higano CS, Pagliaro LC, et al. Safety and clinical activity of atezolizumab (atezo) + radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC). Results phase Ib Clin trial (2020) 38(15_suppl):5565–5. doi: 10.1200/JCO.2020.38.15_suppl.5565
61. Spek A, Graser A, Habl G, Muacevic A, Fuerweger C, Seitz M, et al. Single-fraction image-guided robotic radiosurgery efficiently controls local prostate cancer recurrence after radical prostatectomy. BJUI Compass. (2020) 1(4):139–45. doi: 10.1002/bco2.32
62. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature (2011) 480(7378):480–9. doi: 10.1038/nature10673
63. Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. (2018) 11:1179064418761639. doi: 10.1177/1179064418761639
64. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev (2015) 41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011
65. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med (2007) 13(9):1050–9. doi: 10.1038/nm1622
66. Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med (2000) 191(3):423–34. doi: 10.1084/jem.191.3.423
67. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity (2014) 41(5):843–52. doi: 10.1016/j.immuni.2014.10.019
68. Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut (2019) 68(2):335–46. doi: 10.1136/gutjnl-2017-315485
69. Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol (2008) 180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132
70. Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, et al. Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res (2019) 29(10):846–61. doi: 10.1038/s41422-019-0224-x
71. Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol (2011) 186(5):2772–9. doi: 10.4049/jimmunol.1003208
72. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol (2018) 36(16):1611–8. doi: 10.1200/JCO.2017.76.2229
73. Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol (2020) 6(6):848–55. doi: 10.1001/jamaoncol.2019.6731
74. Powell SF, Gold KA, Gitau MM, Sumey CJ, Lohr MM, McGraw SC, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: A phase IB study. J Clin Oncol (2020) 38(21):2427–37. doi: 10.1200/JCO.19.03156
75. Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer. (2019) 133:83–7. doi: 10.1016/j.lungcan.2019.05.001
76. Papadopoulos KP, Johnson ML, Lockhart AC, Moore K, Falchook GS, Formenti SC, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res (2020) 26(5):1025–33. doi: 10.1158/1078-0432.CCR-19-2609
77. Welsh JW, Heymach JV, Guo C, Menon H, Klein K, Cushman TR, et al. Phase 1/2 trial of pembrolizumab and concurrent chemoradiation therapy for limited-stage SCLC. J Thorac Oncol (2020) 15(12):1919–27. doi: 10.1016/j.jtho.2020.08.022
78. Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol (2013) 14(1):e28–37. doi: 10.1016/S1470-2045(12)70510-7
79. Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol (2021) 22(6):824–35. doi: 10.1016/S1470-2045(21)00149-2
80. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
81. Tai WM, Loke KSH, Gogna A, Tan SH, Ng DCE, Hennedige TP, et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209-678. J Clin Oncol (2020) 38(15_suppl):4590–4590. doi: 10.1200/JCO.2020.38.15_suppl.4590
82. Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: A potential synergistic treatment strategy. Front Oncol (2019) 9:1157. doi: 10.3389/fonc.2019.01157
83. Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol (2018) 41(11):1799–802. doi: 10.1007/s00270-018-1993-1
84. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res (2017) 23(6):1388–96. doi: 10.1158/1078-0432.CCR-16-1432
85. Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol (2013) 24(7):1813–21. doi: 10.1093/annonc/mdt107
86. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2014) 15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5
87. Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. (2020) 78(6):822–30. doi: 10.1016/j.eururo.2020.07.032
88. Yuan Z, Fernandez D, Dhillon J, Abraham-Miranda J, Awasthi S, Kim Y, et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis (2021) 24(1):140–9. doi: 10.1038/s41391-020-0254-y
Keywords: radiotherapy, immune checkpoint inhibitor, prostate cancer, cold tumor, toxicity
Citation: Li T, Qian X, Liu J, Xue F, Luo J, Yao G, Yan J, Liu X, Xiao B and Li J (2023) Radiotherapy plus immune checkpoint inhibitor in prostate cancer. Front. Oncol. 13:1210673. doi: 10.3389/fonc.2023.1210673
Received: 23 April 2023; Accepted: 04 July 2023;
Published: 21 July 2023.
Edited by:
Umang Swami, The University of Utah, United StatesReviewed by:
Vinay Mathew Thomas, The University of Utah, United StatesCopyright © 2023 Li, Qian, Liu, Xue, Luo, Yao, Yan, Liu, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Xiao, YmVpZGF4aWFvYm9AMTYzLmNvbQ==; Jianxing Li, bGp4MUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Tianjie Li
Tianjie Li Xinye Qian
Xinye Qian Jinyang Liu
Jinyang Liu Feng Xue1
Feng Xue1 Jing Luo
Jing Luo Jun Yan
Jun Yan