
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 16 June 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1168321
This article is part of the Research TopicEvolution of Phytochemicals and Phytotherapies in the Treatment and Management of Cancer: Targeted Strategies in Cancer Precision MedicineView all 18 articles
 Muhammad Asif Ali1
Muhammad Asif Ali1 Noohela Khan2
Noohela Khan2 Nabeeha Kaleem1
Nabeeha Kaleem1 Waqas Ahmad1
Waqas Ahmad1 Salem Hussain Alharethi3
Salem Hussain Alharethi3 Bandar Alharbi4
Bandar Alharbi4 Hassan H. Alhassan5
Hassan H. Alhassan5 Maher M. Al-Enazi6
Maher M. Al-Enazi6 Ahmad Faizal Abdull Razis7,8*
Ahmad Faizal Abdull Razis7,8* Babagana Modu8,9
Babagana Modu8,9 Daniela Calina10*
Daniela Calina10* Javad Sharifi-Rad11*
Javad Sharifi-Rad11*Sulforaphane (SFN) is an isothiocyanate with multiple biomedical applications. Sulforaphane can be extracted from the plants of the genus Brassica. However, broccoli sprouts are the chief source of sulforaphane and are 20 to 50 times richer than mature broccoli as they contain 1,153 mg/100 g. SFN is a secondary metabolite that is produced as a result of the hydrolysis of glucoraphanin (a glucosinolate) by the enzyme myrosinase. This review paper aims to summarize and understand the mechanisms behind the anticancer potential of sulforaphane. The data was collected by searching PubMed/MedLine, Scopus, Web of Science, and Google Scholar. This paper concludes that sulforaphane provides cancer protection through the alteration of various epigenetic and non-epigenetic pathways. It is a potent anticancer phytochemical that is safe to consume with minimal side effects. However, there is still a need for further research regarding SFN and the development of a standard dose.
Cancer is one of the most prevalent and fatal diseases throughout the globe. As per the World Health Organization, in 2020, cancer is the sole reason for the death of more than 10 million individuals (1). For the treatment of cancer, various medical treatments are applied, including chemotherapies and radiotherapies. Besides their potential benefits, such treatments also impose detrimental effects on various organs of the body (2). The consumption of phytochemicals must be incorporated into our diet to benefit from their anticancer properties. Since the beginning, cruciferous vegetables are known for their anticancer properties. Unfortunately, these are never ingested in sufficient amounts that can alter the health biomarkers. Cruciferous vegetables are rich in several nutrients and bioactive components, especially isothiocyanates that provide various health benefits (3). The isothiocyanates are the hydrolytic metabolites of glucosinolates, and examples include allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), phenethyl isothiocyanate (PEITC), and sulforaphane (SFN) (4, 5). The most important biological active isothiocyanate is sulforaphane, or simply SFN, which is potent in providing numerous health benefits by modulating epigenetic and non-epigenetic mechanisms. It did not receive much attention until 1992, even though it was first identified and isolated in 1959 (6). This updated review aimed to summarize and highlight the mechanisms behind the anticancer potential of sulforaphane.
This comprehensive review aims to provide updated information on the anticancer mechanisms of sulforaphane. The most relevant and recent data regarding the anticancer activity of sulforaphane were obtained through an online search of the following scientific databases—PubMed/MedLine, Scopus, Web of Science, and Google Scholar—using the next MeSH terms: “Brassica/chemistry”, “Chemoprevention”, “Isothiocyanates/metabolism”, “Isothiocyanates/pharmacology”, “Isothiocyanates/therapeutic use”, “Neoplasms/drug therapy”, “Neoplasms/prevention & control”, “Biological Products/therapeutic use”. The chemical structure has been validated using Pubchem and the taxonomy of the plant according to WorldFloraOnline (7, 8). The most representative data regarding the anticancer activities of sulforaphane are summarized in a schematic diagram and tables.
The dietary sources of SFN include mainly the plants (especially cruciferous vegetables) of the genus Brassica such as broccoli, brussels sprouts, kale, and cauliflower (3). As per a recent review, broccoli sprouts are the chief source of sulforaphane and are 20 to 50 times richer than mature broccoli as they contain 1,153 mg/100 g, whereas the concentration of SFN in mature broccoli is 44–171 mg/100 g. Sulforaphane is significantly effective as it is readily available in blood because of its high bioavailability (80%). The higher bioavailability is due to its lighter molecular weight in comparison to other polyphenols (6, 9). Recently, the trend of nanoencapsulation of various bioactive components to gain maximum benefits is increased. Since the last century, a lot of progress has been made in the field of nanotechnology. Advancement in research has made it possible for the development of polymeric biodegradable nanoparticles for better results and controlled/targeted delivery of drugs. Longhai Piao et al. likewise worked on the nanoencapsulation of sulforaphane using a triblock copolymer PCL-PEG-PCL, i.e., poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone). The results revealed that the PCL-PEG-PCL-encapsulated SFN enhanced the cancer cell apoptosis more efficiently than the free sulforaphane in the in vitro trial. Additionally, the study validated that the SFN encapsulation with triblock copolymer was safe and can be used for effective and targeted sulforaphane delivery to cancerous cells (10). A study on the effect of basil seed gum-encapsulated broccoli sprout extract disclosed that encapsulation helped in the controlled release of sulforaphane, which is more effective in the treatment of cancer than free sulforaphane. Furthermore, the data also revealed that the release of SFN from the micelles was more in intestinal-stimulated conditions compared to the gastric-stimulated condition (11). Similarly, the results of an investigation conducted by Lucía Yepes-Molina et al. showed that the encapsulated sulforaphane had more stability. Additionally, in the in vitro trial, the investigators discovered that the SFN-rich membrane vesicles were potent in reducing the inflammatory markers on HL-60 cell linings. Another group of investigators worked on the encapsulation of sulforaphane in broccoli membrane vesicles (BM vesicles). The study evaluated the capability and potential of encapsulated sulforaphane on SK-MEL-28 (melanoma) cell linings. The in vitro analysis revealed that BM vesicles containing SFN had high absorption and metabolism in cancerous cells. Furthermore, encapsulated sulforaphane efficiently reduced cancer and its markers (12).
Sulforaphane is chemically a product of a 4-(methylsulfonyl) butyl group that is attached to a nitrogen atom (6, 13, 14). SFN is a secondary metabolite produced as a result of the hydrolysis of glucoraphanin (a glucosinolate) by the enzyme myrosinase (6, 9, 15). Because sulforaphane has gained a lot of attention due to its potential against numerous diseases, especially cancer, scientists are interested in developing its derivatives which could be an important discovery in the world of medicine for cancer treatment. The group of Prachi Heda et al. had made some progress in this regard by synthesizing sulforaphane derivatives including SFN-glutathione, SFN-cysteine-glycine, SFN-cysteine, SFN-N-acetylcysteine, PEITC, and sulforaphane. Out of all these SFN derivatives, phenethyl isothiocyanate was found to be the most effective due to its high bioavailability, gastric absorption, and blood–brain barrier permeability. PEITC was involved in the inhibition of human S-adenosylmethionine decarboxylase (a lyase), p38alpha (a transferase), heme-oxygenase 1 (an oxidoreductase), and human cytochrome P450 (an oxidoreductase) activation. Furthermore, the downregulation of these enzymes exhibited a reduction in cancerous cell proliferation and angiogenesis and other cancer-progressing mechanisms (16). A review paper also unveiled the similar anticancer properties of sulforaphane and its derivatives, including sulforaphane and phenethyl isothiocyanate (17). Another in vitro study also disclosed that benzyl sulforaphane (a derivative of SFN) is more efficient in preventing and treating liver carcinoma by restraining the Akt/MAPK pathways and triggering the Nrf2/ARE signaling pathways (18). Similarly, Kun Hu et al. derived a series of SFN derivatives and evaluated the potential of these derivatives in an in vitro study on five different cell linings isolated from liver hepatocellular carcinoma (HepG2), human breast adenocarcinoma (MCF-7), human lung adenocarcinoma (A549), human neuroblastoma (SH-SY5Y), and human colon cancer (HCT-116). The data revealed that the sulforaphane derivative synthesized by introducing the benzyl group at the side chain of sulforaphane (benzyl sulforaphane) showed enhanced anticancer activity than any other derivative and SFN itself. The benzyl derivative of sulforaphane triggered cell cycle arrest apoptosis and Nrf2 activation more efficiently than SFN (19).
Since the discovery of sulforaphane, many scientific studies outlining its health benefits have been published; it is a biologically active phytochemical that has many biological properties, such as anti-inflammatory, anticancer, cardioprotective, antioxidative, cytoprotective, DNA protective, and antimicrobial properties. Additionally, it is also a potent immune booster and detoxifier (9, 14). Table 1 presents all the beneficial effects of sulforaphane proven in various research articles. Although SFN shows various properties, the most important property is its potential against cancer. SFN protects against cancer by inhibiting cancer cell proliferation, arresting the cell cycle, and enhancing the process of apoptosis (24–26). Sulforaphane provides cancer protection via the alteration of several epigenetic and non-epigenetic mechanisms; this was demonstrated in the case of many types of cancer. Sulforaphane stalls the activity of the enzyme histone deacetylase (HDAC) in cancerous cells. The inhibition of histone deacetylase is important in cancer prevention as it enhances several mechanisms such as apoptosis and cell cycle arrest (50–54). Furthermore, SFN also halts histone phosphorylation by enhancing the phosphatases, especially PP1β and PP2α (25, 55, 56). The epigenetic modulation of sulforaphane on breast cancer was assessed in another study. The results disclosed that there was a significant reduction in histone deacetylase activity and the cell proliferation marker (Ki-67) (57). Another study revealed that SFN downgraded processes like cell proliferation, the activity of histone deacetylase, and cell growth. Furthermore, it reduced the expression of various receptors such as estrogen receptor-α and human epidermal growth factor receptor-2 in cancerous cell lining (58) (Figure 1).
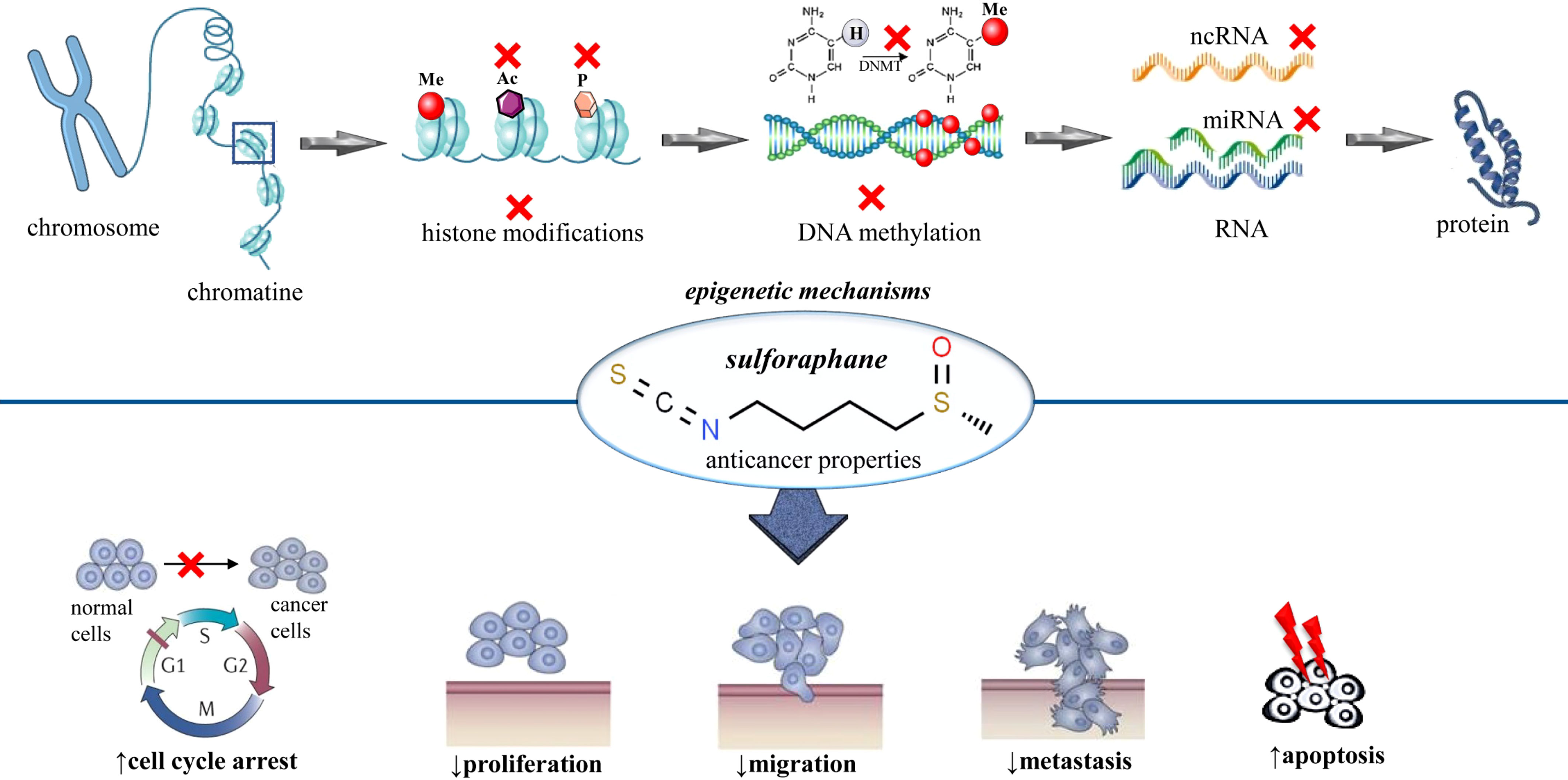
Figure 1 Summarized scheme regarding epigenetic modifications of sulforaphane in cancer. At the cellular level, sulforaphane shows anticancer activity by altering the epigenetic mechanisms. These epigenetic mechanisms involve histone acetylation, histone phosphorylation, DNA methylation, and modulation of noncoding RNAs. x, inhibition; Ac, acetylation; P, phosphorylation; Me, methylation; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; DNMT, DNA methyltransferase.
DNA methyl transferases (DNMTs) are a family of enzymes that catalyze the transfer of the methyl group to the DNA. The suppression of these enzymes enhances the process of apoptosis and cell cycle arrest. SNF actively downregulates the function of DNMTs (especially DNMT1 and DNMT3B) and protects against cancer (25, 54, 59, 60) (Figure 1). Noncoding RNA is a molecule that does not need to be translated into protein to perform its functions. There are several different types of ncRNA, but miRNA (microRNA) and lncRNA (long noncoding RNA) are of great importance. These ncRNAs are usually involved in the regulation of numerous cell functions such as apoptosis, proliferation, and differentiation. The upregulation or downregulation of ncRNA plays a vital role in controlling the progression of cancer. Sulforaphane is one of those bioactive components that can suppress the growth of cancerous cells by regulating these ncRNAs. Various studies validated that SFN exhibits anticancer properties by halting as well as by enhancing the expression of a wide range of miRNAs and lncRNAs (25, 60–63). A study conducted by Scott R. Baier et al. likewise unveiled the off-target effects of SFN. The results were in favor of the hypothesis, i.e., SFN not only targets the cancer suppressor genes but also stimulates the transcriptional activity of long terminal repeats. The study also confirmed that the consumption of broccoli sprouts causes the inhibition of histone acetylation (64) (Figure 1).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that regulates the cellular defense mechanism (65). The activation of Nrf2 channels the cascade of antioxidants and exerts the anticancer effect by causing a reduction in cell transformation and development. SFN decreases cancer proliferation by enhancing the expression of Nrf2. Additionally, sulforaphane suppresses the expression of NF-KB that ultimately downregulates the inflammatory markers (25, 29, 31–34, 66, 67). The group of Julie E. Bauman studied the chemopreventive potential of sulforaphane by using preclinical models. The results of an in vitro model revealed that sulforaphane enhances the activation of Nrf2 signaling in normal mucosal epithelial cells and head and neck squamous cell carcinoma cell lines. The SFN extract was found to be highly effective in blocking the activity of inactivation of an oncogenic factor pSTAT3. However, in the outcomes of the in vivo model, SFN notably decreased the size and incidence of 4-nitroquinoline-1-oxide (a carcinogen) which induced oral cancer in mice (68). Several studies confirmed that sulforaphane provides cancer protection by inhibiting the proliferation of cancerous cells and downgrading the process of angiogenesis. To investigate this potential, Prayag et al. conducted an in vitro study. The results disclosed that sulforaphane notably increased the antioxidant cascade and enhanced the activity of natural cell killers by increasing the expression of MHC class I chain-related proteins A and B (69).
Hormesis is a phenomenon characterized by a biphasic dose or concentration–response, where low doses or concentrations of a compound can stimulate a beneficial effect, while high doses or concentrations can lead to inhibitory effects (70).
This means that, at low doses, a compound may have a stimulatory effect, while at high doses, it may have an inhibitory effect. The review of Calabrese et al. explained this hormetic effect of sulforaphane and revealed that SFN induces hormetic dose responses in studies through the upregulation of the Nrf2/ARE pathway. This biphasic response is well integrated, concentration dependent, and specific to targeted cell types. The hormetic response of sulforaphane can decrease the incidence and severity of various human-related pathologies, and it has also been found to be involved in the enhancement of stem cell proliferation. Similar hormesis-based chemoprotection has been reported with other dietary supplements such as curcumin, ginkgo biloba, ginseng, green tea, and resveratrol. The activation of the Nrf2 (nuclear factor erythroid-derived 2)/ARE (antioxidant response elements) pathway is likely a principal and underlying mechanism of hormesis, which can limit age-related damage, numerous disease processes progression, and induction of toxicities due to chemicals and radiations (70).
Sulforaphane can also be used with conventional cancer treatments to achieve an enhanced effect. The group of Małgorzata Milczarek shed light on the development of new combinations by conducting an in vitro study. The data unveiled that the combination of sulforaphane and fluorouracil is more effective in enhancing the process of apoptosis than fluorouracil alone. In the in vitro analysis, it was confirmed that combination arrests the cancerous cell cycle in the S-phase in the colon cell linings, especially HT-29 and Caco-2 (71). Similarly, the combination of sulforaphane with gefitinib (a potent drug used for the treatment of lung cancer) also showed a marked increase in the inhibition of cell proliferation and expression of numerous factors in comparison to both agents alone (72). Recently, another study disclosed that a combinatory regimen of SFN with allyl isothiocyanate is more effective in cancer prevention compared to a single treatment. The synergism between these two compounds enhanced the arrest of the cell cycle and the programmed cell death. In addition to this, the investigators found that the combined therapy also caused an increase in the downregulation of cell migration (73). Similarly, another research supported the notion that both luteolin and SFN together provide enhanced anti-inflammatory properties than alone. The dose-dependent inhibition of NO by sulforaphane was 51% and by luteolin was 46%, respectively. However, the combined therapy demonstrated inhibited nitric oxide production by 56%, proving that the combination led to an increase in the reduction of inflammatory markers, especially the decrease in nitric oxide production (74).
Broccoli is rich in glucoraphanin which is a precursor of sulforaphane. To study the effect of sulforaphane, a randomized control trial on the prevention of prostate cancer was designed by a group of investigators. The study showed that sulforaphane significantly reduced the progression of prostate cancer and disease severity after the broccoli soup intervention for at least 1 year by altering the gene expression (75). Melanoma is a form of skin cancer that originates from melanocytes (pigment-producing cells). Several studies inferred that the presence of atypical nevi is one of the key risk factors for melanoma. Therefore, in melanoma patients, Shawn Tahata et al. explored the effect of the oral administration of broccoli sprout extract at three different dose levels (50, 100, and 200 µmol). The study inferred that sulforaphane significantly reduced the levels of proinflammatory cytokines in plasma and increased the tumor suppressor decorin in tissues, thus preventing the risk of melanoma (76). Another randomized double-blinded study disclosed that sulforaphane is highly effective in reducing serum prostate-specific antigen (PSA) levels. Serum PSA levels are usually high in men suffering from prostate cancer after radical prostatectomy. The study inferred that the administration of 60 mg SFN in the form of a tablet significantly reduced the PSA progression after at least 3 months of treatment (77). A recent study suggested that sulforaphane is highly beneficial in preventing ulcerative disease by inhibiting the growth of Helicobacter pylori. As per the group of Akinori Yanaka, the consumption of broccoli sprouts consecutively for 2 months can downgrade the colonization of the bacterium. This poses a cancer-preventive effect because of the reduction in oxidative stress caused by H. pylori (78). Additionally, several ongoing and completed clinical trials aim to investigate the chemoprotective and preventive effects of sulforaphane. The investigations also revealed that sulforaphane can help prevent and reduce the risk of cancer via modulating the gene expression by downregulating and upregulating various pathways in cells. However, there is still a need for further investigation on the sulforaphane cancer protective/preventive potential and mechanisms (57, 79–84). Table 2 shows a summary of the representative data regarding the clinical studies with sulphoraphane.
Conventional chemotherapeutic drugs such as paclitaxel, fluorouracil, tamoxifen, and raloxifene are a few commonly used chemo-preventive medications; but, besides their beneficial effect, such chemotherapy also poses several harmful effects (85). For this reason, over the past few years, the use of traditional medicinal plants for the treatment of various diseases has caught the attention of scientists. Medicinal plants are rich in bioactive components and phytochemicals and protect against many diseases without causing detrimental effects on the body’s organs. Such plants are highly advantageous for the innovation of numerous drugs with enormous benefits and lower undesirable effects. Isothiocyanates, especially sulforaphane, are highly effective against cancer and have been used as a medicine in China for thousands of years (86–88). Numerous studies proved that SFN can be used at various doses and in combination with other compounds without showing toxicity. A randomized controlled trial was designed to assess the safety and therapeutic effect of sulforaphane-rich drinks on thyroid hormonal status and thyroid autoimmune status. The results of the 12-week study concluded that the beverage containing on average of 40 µmol of SFN and 600 µmol of glucoraphanin per day was safe to consume (89).
Natural bioactive compounds currently have numerous applications in cancer chemotherapy. Furthermore, the vast spectrum of natural compounds provides important compounds for therapeutic refinement through molecular modifications. Many anticancer compounds are either natural products or substances derived from natural products. The conjugation of toxic natural products with monoclonal antibodies or with macromolecular carriers can lead to more precisely targeted therapies. As only a small percentage of higher plants have been systematically investigated, research on natural compounds as chemotherapeutic agents arouses further interest as well as multidisciplinary scientific collaborations. The results of preclinical studies showed that sulforaphane provides cancer protection through the alteration of various epigenetic and non-epigenetic pathways. The therapeutic limitations of the use of sulforaphane in cancer are represented by the complete lack of knowledge of the potential anticancer effects at the cellular level and the effects on the immune system, the lack of translational studies to establish the effective doses in humans, and the interactions with conventional chemotherapy medication. However, considering everything together, the results of preclinical and clinical studies point to new therapeutic perspectives towards the possible development of new sulforaphane-based anticancer drugs.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer J For Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol (2022) 7(7):627–47. doi: 10.1016/S2468-1253(22)00044-9
3. Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev (2013) 71:709–26. doi: 10.1111/nure.12060
4. Fofaria NM, Ranjan A, Kim S-H, Srivastava SK. Mechanisms of the anticancer effects of isothiocyanates. In: The enzymes. Elsevier (2015).
5. Jaiswal AK. Nutritional composition and antioxidant properties of fruits and vegetables. Academic Press (2020).
6. Kamal MM, Akter S, Lin C-N, Nazzal S. Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch Of Pharmacal Res (2020) 43:371–84. doi: 10.1007/s12272-020-01225-2
7. WFO. Wfo the world flora online (2021). Available at: http://www.worldfloraonline.org/.
8. PubChem. Pubchem (2022). Available at: https://pubchem.ncbi.nlm.nih.gov/.
9. Houghton CA. Sulforaphane: its “coming of age” as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxid Med Cell Longevity (2019) 2019. doi: 10.1155/2019/2716870
10. Kheiri Manjili H, Sharafi A, Attari E, Danafar H. Pharmacokinetics and In vitro and In vivo delivery of sulforaphane by pcl–Peg–Pcl copolymeric-based micelles. Artif Cells Nanomed And Biotechnol (2017) 45:1728–39. doi: 10.1080/21691401.2017.1282501
11. Azarashkan Z, Farahani S, Abedinia A, Akbarmivehie M, Motamedzadegan A, Heidarbeigi J, Hayaloğlu AA. Co-encapsulation of broccoli sprout extract nanoliposomes into basil seed gum: effects on in vitro antioxidant, antibacterial and anti-Listeria activities in ricotta cheese. Int J Food Microbiol (2022) 376:109761. doi: 10.1016/j.ijfoodmicro.2022.109761
12. Yepes-Molina L, Carvajal M. Nanoencapsulation of sulforaphane in broccoli membrane vesicles and their In vitro antiproliferative activity. Pharm Biol (2021) 59:1488–502. doi: 10.1080/13880209.2021.1992450
14. de Oliveira MR. Sulforaphane and its modulation of brain redox status: the mitochondria as a target. In: Oxidative stress and dietary antioxidants in neurological diseases. Elsevier (2020).
15. Parker JK. Introduction to aroma compounds in foods. In: Flavour development, analysis and perception in food and beverages. Elsevier (2015).
16. Heda P, Ravishankar S, Shankar A, Chaganti S, Rajan D, Parekh R, et al. Identifying promising anticancer sulforaphane derivatives using QSAR, docking, and ADME studies. J Student Res (2021) 10. doi: 10.47611/jsrhs.v10i4.2247
17. Kaboli PJ, Khoshkbejari MA, Mohammadi M, Abiri A, Mokhtarian R, Vazifemand R, et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer–contradictory effects and future perspectives. Biomed Pharmacother (2020) 121:109635. doi: 10.1016/j.biopha.2019.109635
18. Ren J, Yuan L, Wang Y, Chen G, Hu K. Benzyl sulforaphane is superior to sulforaphane in inhibiting the Akt/Mapk and activating the Nrf2/Are signalling pathways in Hepg2 cells. J Pharm And Pharmacol (2018) 70:1643–53. doi: 10.1111/jphp.13015
19. Hu K, Qi Y-J, Zhao J, Jiang H-F, Chen X, Ren J. Synthesis and biological evaluation of sulforaphane derivatives as potential antitumor agents. Eur J medicinal Chem (2013) 64:529–39. doi: 10.1016/j.ejmech.2013.03.045
20. Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem (2001) 276:32008–15. doi: 10.1074/jbc.M104794200
21. Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase ii enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiology-Lung Cell And Physiol (2007) 292:L33–9. doi: 10.1152/ajplung.00170.2006
22. Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical 1. Acta Pharmacologica Sin (2007) 28:1343–54. doi: 10.1111/j.1745-7254.2007.00679.x
23. Mangla B, Javed S, Sultan MH, Kumar P, Kohli K, Najmi A, et al. Sulforaphane: a review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother Res (2021) 35:5440–58. doi: 10.1002/ptr.7176
24. Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res (2014) 159:207–23. doi: 10.1007/978-3-642-38007-5_12
25. Su X, Jiang X, Meng L, Dong X, Shen Y, Xin Y. Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway. Oxid Med And Cell Longevity (2018) 2018. doi: 10.1155/2018/5438179
26. Pradhan N, Kar S, Parbin S, Sengupta D, Deb M, Das L, et al. Epigenetic dietary interventions for prevention of cancer. In: Epigenetics of cancer prevention. Elsevier (2019).
27. Myzak MC, Karplus PA, Chung F-L, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res (2004) 64:5767–74. doi: 10.1158/0008-5472.CAN-04-1326
28. Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in bph-1, lncap and pc-3 prostate epithelial cells. Carcinogenesis (2006) 27:811–9. doi: 10.1093/carcin/bgi265
29. Dickinson SE, Rusche JJ, Bec SL, Horn DJ, Janda J, Rim SH, et al. The effect of sulforaphane on histone deacetylase activity in keratinocytes: differences between in vitro and in vivo analyses. Mol Carcinogenesis (2015) 54:1513–20. doi: 10.1002/mc.22224
30. Ganai SA. Histone deacetylase inhibitor sulforaphane: the phytochemical with vibrant activity against prostate cancer. Biomed Pharmacother (2016) 81:250–7. doi: 10.1016/j.biopha.2016.04.022
31. Morimitsu Y, Nakagawa Y, Hayashi K, Fujii H, Kumagai T, Nakamura Y, et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Of Biol Chem (2002) 277:3456–63. doi: 10.1074/jbc.M110244200
32. Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicologic Pathol (2012) 64:503–8. doi: 10.1016/j.etp.2010.11.005
33. Houghton CA, Fassett RG, Coombes JS. Sulforaphane and other nutrigenomic Nrf2 activators: can the clinician’s expectation be matched by the reality? Oxid Med Cell Longevity (2016) 2016. doi: 10.1155/2016/7857186
34. Uddin MS, Al Mamun A, Jakaria M, Thangapandiyan S, Ahmad J, Rahman MA, et al. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci Total Environ (2020) 707:135624. doi: 10.1016/j.scitotenv.2019.135624
35. Lee Y-J, Lee S-H. Sulforaphane induces antioxidative and antiproliferative responses by generating reactive oxygen species in human bronchial epithelial beas-2b cells. J Korean Med Sci (2011) 26:1474–82. doi: 10.3346/jkms.2011.26.11.1474
36. Mukherjee S, Gangopadhyay H, Das DK. Retraction: broccoli: a unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J Agric And Food Chem (2012) 60:2768–8. doi: 10.1021/jf3008585
37. Mukherjee S, Gangopadhyay H, Das DK. Broccoli: a unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J Agric And Food Chem (2008) 56:609–17. doi: 10.1021/jf0728146
38. Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Modulation of phase II enzymes by sulforaphane: implications for its cardioprotective potential. J Agric Food Chem (2009) 57:5615–22. doi: 10.1021/jf900549c
39. Devi JR, Thangam EB. Studies on antioxidant and antimicrobial activities of purified sulforaphane from brassica oleraceae var. rubra. J Pharm Res (2012) 5:3582–4. doi: 10.7314/apjcp.2012.13.5.2095
40. Romeo L, Iori R, Rollin P, Bramanti P, Mazzon E. Isothiocyanates: an overview of their antimicrobial activity against human infections. Molecules (2018) 23:624. doi: 10.3390/molecules23030624
41. Abukhabta S, Khalil Ghawi S, Karatzas KA, Charalampopoulos D, Mcdougall G, Allwood JW, et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur J Nutr (2021) 60:1263–76. doi: 10.1007/s00394-020-02322-0
42. Janczewski Ł. Sulforaphane and its bifunctional analogs: synthesis and biological activity. Molecules (2022) 27:1750. doi: 10.3390/molecules27051750
43. Arner ES, Holmgren A. The thioredoxin system in cancer. In: Seminars in cancer biology. Elsevier (2006). p. 420–6.
44. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res (2008) 58:262–70. doi: 10.1016/j.phrs.2008.09.003
45. Li C, Thompson MA, Tamayo AT, Zuo Z, Lee J, Vega F, et al. Over-expression of thioredoxin-1 mediates growth, survival, and chemoresistance and is a druggable target in diffuse Large b-cell lymphoma. Oncotarget (2012) 3:314–26. doi: 10.18632/oncotarget.463
46. Gonzalez-Donquiles C, Alonso-Molero J, Fernandez-Villa T, Vilorio-Marqués L, Molina A, Martín V. The NRF2 transcription factor plays a dual role in colorectal cancer: a systematic review. PloS One (2017) 12:e0177549. doi: 10.1371/journal.pone.0177549
47. Bian M, Fan R, Zhao S, Liu W. Targeting the thioredoxin system as a strategy for cancer therapy. J Medicinal Chem (2019) 62:7309–21. doi: 10.1021/acs.jmedchem.8b01595
48. Jia J-J, Geng W-S, Wang Z-Q, Chen L, Zeng X-S. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol (2019) 84:453–70. doi: 10.1007/s00280-019-03869-4
49. Kumar H, Kumar RM, Bhattacharjee D, Somanna P, Jain V. Role of Nrf2 signaling cascade in breast cancer: strategies and treatment. Front Pharmacol (2022) 13:720076. doi: 10.3389/fphar.2022.720076
50. Ho E, Clarke JD, Dashwood RH. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr (2009) 139:2393–6. doi: 10.3945/jn.109.113332
51. Kim H-J, Bae S-C. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Trans Res (2011) 3:166.
52. Atwell LL, Beaver LM, Shannon J, Williams DE, Dashwood RH, Ho E. Epigenetic regulation by sulforaphane: opportunities for breast and prostate cancer chemoprevention. Curr Pharmacol Rep (2015) 1:102–11. doi: 10.1007/s40495-014-0002-x
53. Jiang L-L, Zhou S-J, Zhang X-M, Chen H-Q, Liu W. Sulforaphane suppresses In vitro and In vivo lung tumorigenesis through downregulation of hdac activity. Biomed Pharmacother (2016) 78:74–80. doi: 10.1016/j.biopha.2015.11.007
54. Dos Santos PWDS, Machado ART, De Grandis RA, Ribeiro DL, Tuttis K, Morselli M, et al. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells. Food Chem Toxicol (2020) 136:111047. doi: 10.1016/j.fct.2019.111047
55. Most D, Ferguson L, Harris RA. Molecular basis of alcoholism. In: Handbook of clinical neurology (2014).
56. Abbaoui B, Telu KH, Lucas CR, Thomas-Ahner JM, Schwartz SJ, Clinton SK, et al. The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer. J Proteomics (2017) 156:94–103. doi: 10.1016/j.jprot.2017.01.013
57. Atwell LL, Zhang Z, Mori M, Farris PE, Vetto JT, Naik AM, et al. Sulforaphane bioavailability and chemopreventive activity in women scheduled for breast biopsy. Cancer Prev Res (2015) 8:1184–91. doi: 10.1158/1940-6207.CAPR-15-0119
58. Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type–specific apoptosis in human breast cancer cell lines. Mol Cancer Ther (2007) 6:1013–21. doi: 10.1158/1535-7163.MCT-06-0494
59. Pop S, Enciu AM, Tarcomnicu I, Gille E, Tanase C. Phytochemicals in cancer prevention: modulating epigenetic alterations of dna methylation. Phytochem Rev (2019) 18:1005–24. doi: 10.1007/s11101-019-09627-x
60. Hyun TK. A recent overview on sulforaphane as a dietary epigenetic modulator. EXCLI J (2020) 19:131–4. doi: 10.17179/excli2019-2039
62. Rafiei H, Ashrafizadeh M, Ahmadi Z. Micrornas as novel targets of sulforaphane in cancer therapy: the beginning of a new tale? Phytother Res (2020) 34:721–8. doi: 10.1002/ptr.6572
63. Kalhori MR, Khodayari H, Khodayari S, Vesovic M, Jackson G, Farzaei MH, et al. Regulation of long non-coding rnas by plant secondary metabolites: a novel anticancer therapeutic approach. Cancers (2021) 13:1274. doi: 10.3390/cancers13061274
64. Baier SR, Zbasnik R, Schlegel V, Zempleni J. Off-target effects of sulforaphane include the derepression of long terminal repeats through histone acetylation events. J Nutr Biochem (2014) 25:665–8. doi: 10.1016/j.jnutbio.2014.02.007
65. Sharifi-Rad J, Seidel V, Izabela M, Monserrat-Mequida M, Sureda A, Ormazabal V, et al. Phenolic compounds as Nrf2 inhibitors: potential applications in cancer therapy. Cell Commun Signal (2023) 21:89. doi: 10.1186/s12964-023-01109-0
66. Mao L, Yang T, Li X, Lei X, Sun Y, Zhao Y, et al. Protective effects of sulforaphane in experimental vascular cognitive impairment: contribution of the Nrf2 pathway. J Of Cereb Blood Flow Metab (2019) 39:352–66. doi: 10.1177/0271678X18764083
67. Esfandyari S, Aleyasin A, Noroozi Z, Taheri M, Khodarahmian M, Eslami M, et al. The protective effect of sulforaphane against oxidative stress through activation of Nrf2/ARE pathway in human granulosa cells. Cell J (Yakhteh) (2021) 23:692. doi: 10.22074/cellj.2021.7393
68. Bauman JE, Zang Y, Sen M, Li C, Wang L, Egner PA, et al. Prevention of carcinogen-induced oral cancer by sulforaphane. Cancer Prev Res (2016) 9:547–57. doi: 10.1158/1940-6207.CAPR-15-0290
69. Amin PJ, Shankar BS. Sulforaphane induces ROS mediated induction of NKG2D ligands in human cancer cell lines and enhances susceptibility to NK cell mediated lysis. Life Sci (2015) 126:19–27. doi: 10.1016/j.lfs.2015.01.026
70. Calabrese EJ, Kozumbo WJ. The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharmacol Res (2021) 163:105283. doi: 10.1016/j.phrs.2020.105283
71. Milczarek M, Mielczarek L, Lubelska K, Dąbrowska A, Chilmonczyk Z, Matosiuk D, et al. In vitro evaluation of sulforaphane and a natural analog as potent inducers of 5-fluorouracil anticancer activity. Molecules (2018) 23:3040. doi: 10.3390/molecules23113040
72. Wang F, Wang W, Li J, Zhang J, Wang X, Wang M. Sulforaphane reverses gefitinib tolerance in human lung cancer cells via modulation of sonic hedgehog signaling. Oncol Lett (2018) 15:109–14. doi: 10.3892/ol.2017.7293
73. Rakariyatham K, Yang X, Gao Z, Song M, Han Y, Chen X, et al. Synergistic chemopreventive effect of allyl isothiocyanate and sulforaphane on non-small cell lung carcinoma cells. Food Funct (2019) 10:893–902. doi: 10.1039/C8FO01914B
74. Rakariyatham K, Wu X, Tang Z, Han Y, Wang Q, Xiao H. Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct (2018) 9:5115–23. doi: 10.1039/C8FO01352G
75. Traka MH, Melchini A, Coode-Bate J, Al Kadhi O, Saha S, Defernez M, et al. Transcriptional changes in prostate of men on active surveillance after a 12-Mo glucoraphanin-rich broccoli intervention–results from the effect of sulforaphane on prostate cancer prevention (Escape) randomized controlled trial. Am J Of Clin Nutr (2019) 109:1133–44. doi: 10.1093/ajcn/nqz012
76. Tahata S, Singh SV, Lin Y, Hahm E-R, Beumer JH, Christner SM, et al. Evaluation of biodistribution of sulforaphane after administration of oral broccoli sprout extract in melanoma patients with multiple atypical nevievaluation of sulforaphane in patients with atypical nevi. Cancer Prev Res (2018) 11:429–38. doi: 10.1158/1940-6207.CAPR-17-0268
77. Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, et al. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (2015) 8:712–9. doi: 10.1158/1940-6207.CAPR-14-0459
78. Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in helicobacter pylori–infected mice and humans. Cancer Prev Res (2009) 2:353–60. doi: 10.1158/1940-6207.CAPR-08-0192
79. Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M, Graff JN, et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investigational New Drugs (2015) 33:480–9. doi: 10.1007/s10637-014-0189-z
80. ClinicalTrials.gov. Effects of sulforaphane on normal prostate tissue (PHASE) (2016). Available at: https://clinicaltrials.gov/ct2/show/study/NCT00946309?term=sulforaphane&cond=cancer&draw=2&rank=10 (Accessed 30 April 2023).
81. ClinicalTrials.gov. Study to evaluate the effect of sulforaphane in broccoli sprout extract on breast tissue (2018). Available at: https://clinicaltrials.gov/ct2/show/study/NCT00982319?term=sulforaphane&cond=cancer&draw=2&rank=7 (Accessed 30 April 2023).
82. ClinicalTrials.gov. Accumulation of dietary bioactives and prostate cancer (ADaPT) (2020). Available at: https://clinicaltrials.gov/ct2/show/study/NCT04046653?term=sulforaphane&cond=cancer&draw=2&rank=9 (Accessed 30 April 2023).
83. Zhang Z, Garzotto M, Davis EW, Mori M, Stoller WA, Farris PE, et al. Sulforaphane bioavailability and chemopreventive activity in men presenting for biopsy of the prostate gland: a randomized controlled trial. Nutr And Cancer (2020) 72:74–87. doi: 10.1080/01635581.2019.1619783
84. Yuan J. Randomized clinical trial of lung cancer chemoprevention with sulforaphane in former smokers. US Natl Library Of Med: Bethesda Md USA (2022).
85. Watanabe T, Oba T, Tanimoto K, Shibata T, Kamijo S, Ito KI. Tamoxifen resistance alters sensitivity to 5-fluorouracil in a subset of estrogen receptor-positive breast cancer. PloS One (2021) 16:E0252822. doi: 10.1371/journal.pone.0252822
86. Li M, Gao J, Tang Y, Liu M, Wu S, Qu K, et al. Traditional herbal medicine-derived sulforaphene lfs-01 reverses colitis in mice by selectively altering the gut microbiota and promoting intestinal gamma-delta T cells. Front Pharmacol (2018) 8:959. doi: 10.3389/fphar.2017.00959
87. Wang H, Wang F, Wu S, Liu Z, Li T, Mao L, et al. Traditional herbal medicine-derived sulforaphene promotes mitophagic cell death in lymphoma cells through Crm1-mediated P62/Sqstm1 accumulation and ampk activation. Chemico-Biological Interact (2018) 281:11–23. doi: 10.1016/j.cbi.2017.12.017
88. Wu G, Yan Y, Zhou Y, Duan Y, Zeng S, Wang X, et al. Sulforaphane: expected to become a novel antitumor compound. Oncol Res (2020) 28:439. doi: 10.3727/096504020X15828892654385
Keywords: sulforaphane, isothiocyanate, anticancer mechanisms, cytotoxicity, apoptosis
Citation: Asif Ali M, Khan N, Kaleem N, Ahmad W, Alharethi SH, Alharbi B, Alhassan HH, Al-Enazi MM, Razis AFA, Modu B, Calina D and Sharifi-Rad J (2023) Anticancer properties of sulforaphane: current insights at the molecular level. Front. Oncol. 13:1168321. doi: 10.3389/fonc.2023.1168321
Received: 17 February 2023; Accepted: 16 May 2023;
Published: 16 June 2023.
Edited by:
Visweswara Rao Pasupuleti, Reva University, IndiaReviewed by:
Frederick E. Williams, University of Toledo, United StatesCopyright © 2023 Asif Ali, Khan, Kaleem, Ahmad, Alharethi, Alharbi, Alhassan, Al-Enazi, Razis, Modu, Calina and Sharifi-Rad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Faizal Abdull Razis, bWFkZmFpemFsQHVwbS5lZHUubXk=; Daniela Calina, Y2FsaW5hZGFuaWVsYUBnbWFpbC5jb20=; Javad Sharifi-Rad, amF2YWQuc2hhcmlmaXJhZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.