
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 May 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.862224
This article is part of the Research Topic Understanding the Link Between Obesity and Breast Cancer View all 8 articles
Purpose: To evaluate the influence of obesity on clinicopathological characteristics of breast cancer; to explore the effect of obesity on the prognosis and performance of endocrine therapy in breast cancer patients.
Methods: Patients with luminal/HER2-negative early breast cancer were included and categorized into the non-obese (BMI<28kg/m2) and obese (BMI≥28kg/m2) groups according to body mass index (BMI). Clinicopathological characteristics and treatment modalities were compared between groups. Interaction of adjuvant endocrine therapy with obesity was analyzed.
Results: A total of 2,875 patients were included: 2,598 non-obese and 277 obese. A higher rate of patients with comorbidities (OR: 2.83, 95%CI 2.13-3.74, P<0.001) or PR-positive tumor (OR: 1.63, 95%CI 1.03-2.58, P=0.037) were identified in the obese group. Obesity was not associated with disease recurrence (P=0.839) or overall survival (P=0.140) in the whole population. Subgroup analysis did show an association with worse relapse-free survival (RFS, HR 3.48, 95%CI 1.31-9.22, P=0.012) and overall survival (OS, HR 4.67, 95%CI 1.28-16.95, P=0.019) in luminal A breast cancer. These results could not be reproduced in the luminal B subtype with a RFS (HR 0.78, 95%CI 0.41-1.49, P=0.454) or OS (HR 1.17, 95%CI 0.50-2.74, P=0.727). Furthermore, obesity did not impact endocrine therapy effectiveness in Tamoxifen or the aromatase inhibitor group (RFS: interact P=0.381; OS: interact P=0.888).
Conclusions: The impact of obesity on prognosis interacted with luminal subtype status in Chinese breast cancer patients which was not related with endocrine treatment modality.
Breast cancer is the most commonly diagnosed cancer among women (1), with luminal tumors accounting for 70% of all cases (2). Obesity has been widely recognized as a risk factor for postmenopausal breast cancer but rather protective for premenopausal women from developing breast cancer (3–5). Meanwhile, obesity is associated with impaired survival, regardless of menopausal status (3, 4). Substantial evidence has suggested that obese women with early stage disease have an increased risk of recurrence (6–9) and reduced survival (6–12) compared to normal-weight women with early-stage disease. Studies have also identified obesity as a risk factor for contralateral breast cancer, as well as second primary cancers (13, 14). Furthermore, the association between obesity and outcomes of breast cancer varied by tumor estrogen receptor (ER) status (15). The prospective POSH study, which enrolled 2,956 patients aged ≤40 at breast cancer diagnosis, revealed that obesity is an independent prognostic factor for ER-positive but not ER-negative breast cancer (15). Regarding molecular subtypes, the association between obesity and prognosis was still in controversy (15–17). Increasing body mass index (BMI) was found to be associated with inferior outcomes in luminal/human epidermal growth factor receptor 2 (HER2)-negative disease but not other subtypes (7, 16, 18).
Obesity has been associated with increased local and circulating estrogen production due to high expression of the aromatase in the breast and peripheral fat (19, 20). Aromatase inhibitors (AIs) can inhibit the conversion of androgens to estrogens in adipose tissue, indicating AIs may be less effective in obese postmenopausal patients (21). Retrospective analysis from ATAC and ABCSG-12 trials found that obese patients treated with anastrozole had worse prognosis than those without obesity. This was not the case in the tamoxifen treatment group (22, 23).
Therefore, the objectives of our study are to explore whether obesity is prognostic and predictive in Chinese breast cancer survivors. We also searched for potential clinicopathological features, for instance, a luminal subtype that might influence the impact of obesity on survival and performance of endocrine therapy in breast cancer patients.
Patients included in the study underwent radical surgery in the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University between January 2009 and June 2018. The inclusion criteria were female patients diagnosed with invasive luminal/HER2-negative invasive breast cancer. Luminal-like was defined as ER and/or progesterone receptor (PR) positive. The exclusion criteria included: missing values for BMI, missing information for adjuvant therapy, receiving neoadjuvant treatment, having previous malignancy in medical history, and having stage IV of disease. This study was performed in accordance with the terms of the Declaration of Helsinki and approved by the Ethical Committee Review Board of Institution.
Clinicopathological information was retrieved from Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB), including BMI, age, menopausal status, comorbidities evaluated by Charlson Comorbidity Index (CCI), type of surgery, histopathological type, histological grade, expression of ER, PR, Ki-67, HER2 status, tumor size, lymph node involvement, and adjuvant treatment. Included patients were less than 60 years of age, less than 60 years of age and amenorrheic for at least 36 months, or with prior bilateral oophorectomy who were considered postmenopausal.
BMI at baseline, collected at the first day after hospitalization for surgery, was calculated as weight in kilograms divided by the square of the height in meters and was used in the classification of obesity (BMI ≥ 28 kg/m2) and non-obesity (BMI < 28 kg/m2) (24). Hormone receptor (HR) positivity was defined if there was at least 1% staining in tumor nuclei. ER expression cut-off was set at 50% since the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer considered tumor cell HR staining≥50% as highly endocrine-responsive tumors (25). Status of HER2 was determined according to the 2018 ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guideline for HER2 testing (26). Invasive luminal/HER2-negative breast cancers were classified into two subtypes: luminal A and luminal B/HER2-negative according to the 2015 St. Gallen Consensus (27). Luminal A is defined as HR-positive, HER2-negative, PR ≥ 20%, and Ki-67 < 14%. Luminal B/HER2-negative is defined as HR-positive, HER2-negative, and at least one of the following: Ki-67 ≥ 14% or PR negative or < 20%.
Adjuvant treatment was decided for each patient through multidisciplinary discussion. Patients would receive treatment reminders from a center-specific smart-phone based application (28) and from breast cancer-specific nurses. Patient’s adherence to treatment was evaluated through regular prescription records. Patients were considered non-adherent if they received treatment inconsistent with multidisciplinary team recommendation or if they spontaneously stopped treatment regardless of physician recommendation. Follow-up was accomplished annually by breast cancer specific nurses and assistants in our center through medical records reviews and/or regular phone calls. Follow-up information regarding recurrence and survival status were updated to June 2020. Relapse-free survival (RFS) and overall survival (OS) were primary outcomes. RFS was defined as the interval between surgery and ipsilateral ductal carcinoma in situ (DCIS), ipsilateral invasive breast tumor recurrence, locoregional invasive recurrence, distant recurrence, and death from any cause. OS was defined as the interval between surgery and death from any cause (29).
Categorical variables were compared using Chi-square tests or Fisher’s exact tests to find out the difference of clinicopathological characteristics and receipt of adjuvant treatment between obese and non-obese patients. Multivariate logistic regression was applied to analyze the association between obesity and clinicopathological characteristics. Kaplan–Meier curve and log-rank test were performed to compare disease outcomes between obese and non-obese patients stratified by luminal subtypes. Subgroup analysis and forest plots were conducted using stratified Mantel-Haenszel test to estimate hazard ratio (HR) with 95%CI (confidence interval). Cox proportional hazards regression model was applied to determine the association between obesity and survival outcomes in different subgroups. Two-tailed P value <0.05 was considered statistically significant. All statistical analyses were carried out using SPSS version 26 (SPSS, Inc., Chicago, IL, USA).
A total of 2,875 luminal breast cancer patients were included (Figure 1). Clinicopathological characteristics and adjuvant treatments according to BMI groups were listed in Tables 1, 2. The mean age of the study population was 56.41 ± 12.71 years old and 66.6% of patients were aged ≥50 years old. There were 919 (32.0%) patients with at least one comorbidity by CCI, for instance diabetes, chronic kidney diseases, peripheral vascular disease, etc. Only 7.6% of patients had lymphovascular invasion. A total of 1,851 (64.4%) patients had tumors ≤ 2.0 cm. Exactly 1,882 (65.5%) patients presented with node negative disease. There were 1,743 (60.6%) and 665 (23.1%) with grade I-II and III tumors, respectively, leaving the remaining 467 (16.2%) patients histologic grade unknown. Invasive ductal carcinoma (IDC) was diagnosed in 2,407 (83.7%) patients. The proportions of patients with ER ≥ 50%, PR-positive, and Ki−67 ≥ 14% were 91.5%, 85.2%, and 54.4% respectively. Moreover, 926 (32.2%), and 1,949 (67.8%) patients had luminal A, and luminal B/HER2-negative tumors. The clinicopathological features by luminal subtype was presented in Supplementary Table S1.
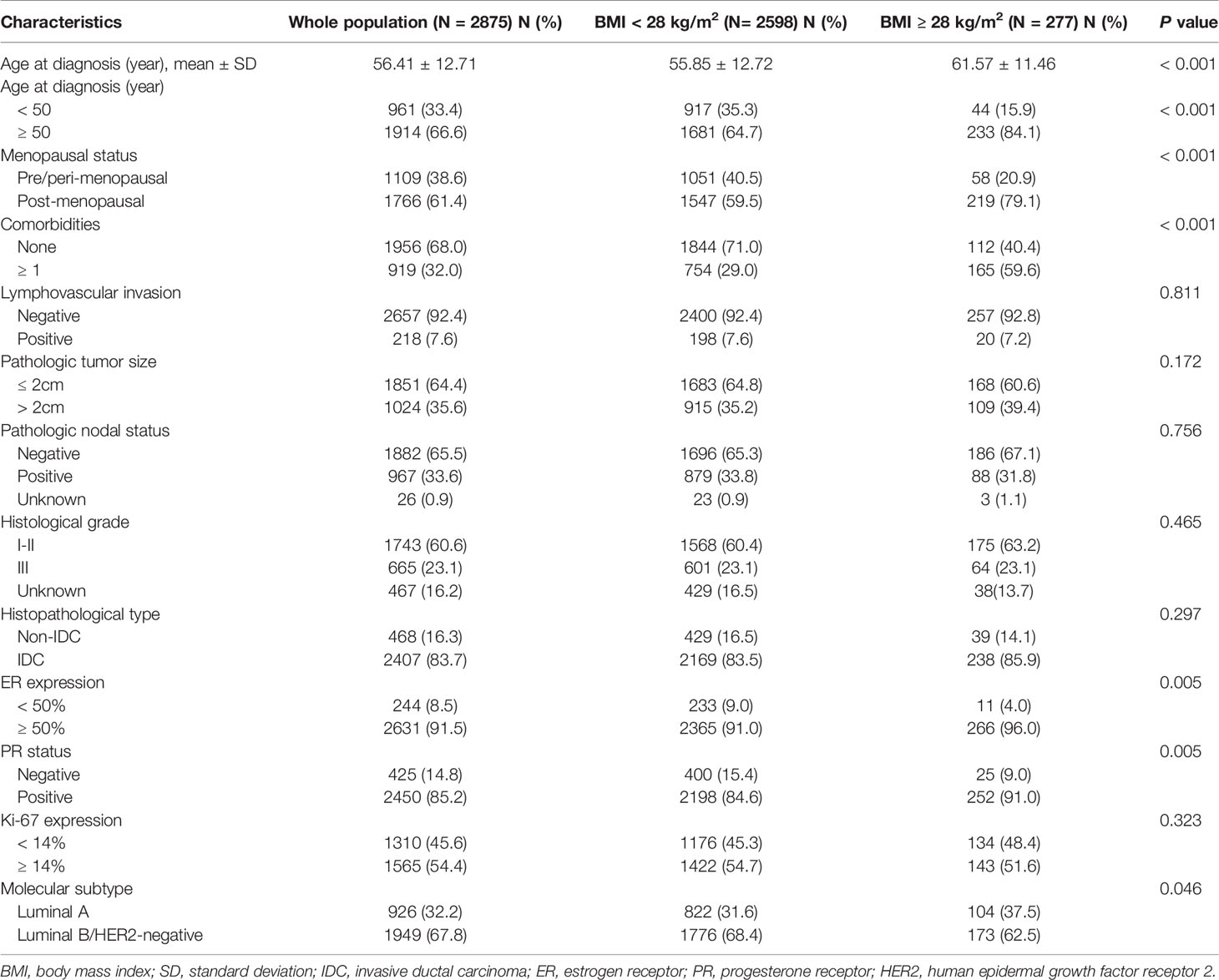
Table 1 Distribution of clinicopathological characteristics in the whole population and different BMI groups.
In terms of surgical approach, 1,029 (35.8%) patients received breast-conserving surgery (BCS) and 1,709 (59.4%) patients received sentinel lymph node biopsy (SLNB). Regarding adjuvant treatment, the proportions of patients who underwent adjuvant chemotherapy, radiotherapy, endocrine therapy, and ovarian function suppression (OFS) treatment were 56.1%, 52.9%, 95.2%, and 6.1%, respectively. A total of 2,738 patients received adjuvant endocrine therapy. According to the first endocrine therapy agent received, 1,872 patients received AI treatment (including 118 patients who received OFS simultaneously) and 866 patients received selective estrogen receptor modulators (SERM) treatment (including 56 patients who received OFS simultaneously). A total of 174 patients received OFS.
The median BMI of the whole population was 23.15 (21.30-25.39) kg/m2: 2598 (90.37%) with BMI < 28 kg/m2 and the remaining 277 (9.63%) with BMI ≥ 28 kg/m2. Univariate analysis found that age, menopausal status, comorbidities, ER expression, PR positivity, and luminal subtypes were significantly different between two BMI groups (all P < 0.05, Table 1). Lymphovascular invasion, tumor size, node involvement, grade 3 tumors, histologic type, and Ki-67 percentage were not different between the non-obese and obese patients (P > 0.05).
Multivariate analysis demonstrated that only comorbidities and PR positivity were independently associated with obesity (Table 3). Obese patients were more likely to be diagnosed with concomitant comorbidities [odds ratio (OR) 2.83, 95%CI 2.13-3.74, P < 0.001] and slightly more PR-positive tumors (OR 1.63, 95%CI 1.03-2.58, P = 0.037). It also revealed the trend for obese patients to be older than non-obese patients (OR 1.71, 95%CI 0.97-3.02, P = 0.066).
After a median follow-up of 50 (range 4-127) months, 170 RFS events, and 75 deaths have been recorded. The 5-year RFS and OS were 88.2% and 93.6% in the whole population. Pathological tumor size, node status, histological grade, PR expression, and adjuvant endocrine therapy were independent predictors for RFS in luminal patients, while luminal subtype was not independently associated with survival (P=0.075, Supplementary Table S2). There was no significant difference between non-obese and obese patients in terms of RFS (5-year RFS 88.0% vs 90.3%, P = 0.839) and OS (5-year OS 93.6% vs 93.4%, P = 0.140; Figures 2A, B; Supplementary Table S2).
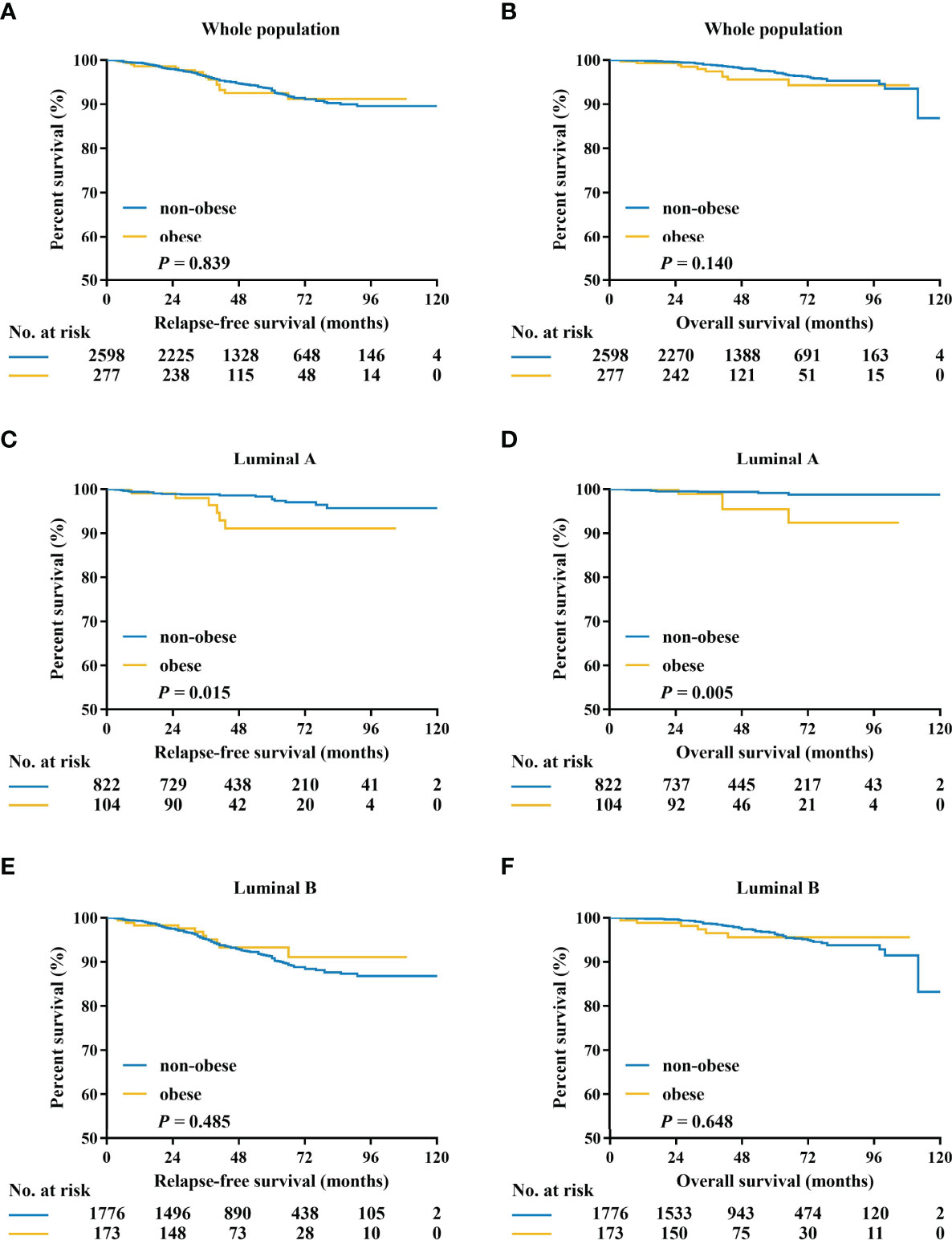
Figure 2 Kaplan–Meier estimates of relapse-free survival and overall survival in patients with HR-positive/HER2-negative tumors (A, B), luminal A tumors (C, D), and luminal B tumors (E, F). HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Exploratory subgroup analysis was then done to evaluate prognosis difference according to different clinicopathological subtypes between the obese and non-obese BMI groups (Figure 3). As shown in the forest plot, obesity had a different impact on prognosis for the two luminal subtypes (interact P = 0.022 for RFS, interact P = 0.056 for OS; Figures 3A, B). For patients with luminal A disease, those obese reported a significant impaired RFS (5-year RFS: 91.1% vs 94.6%, P = 0.015; Figure 2C) and OS (5-year OS: 90.2% vs 98.5%, P = 0.005; Figure 2D) compared to non-obese patients. Multivariate analysis demonstrated that obesity was an independent risk factor for luminal A patients, with an adjusted HR of 3.48 (95%CI 1.31-9.22) for RFS and 4.67 (95%CI 1.28-16.95) for OS. Meanwhile, obesity had no significant impact on RFS (univariate P = 0.485; Figure 2E; multivariate HR 0.78, 95%CI 0.41-1.49, P = 0.454; Supplementary Table S4) or OS (univariate P = 0.648; Figure 2F; multivariate HR 1.17, 95%CI 0.50-2.74, P = 0.727; Supplementary Table S5) in luminal B population.
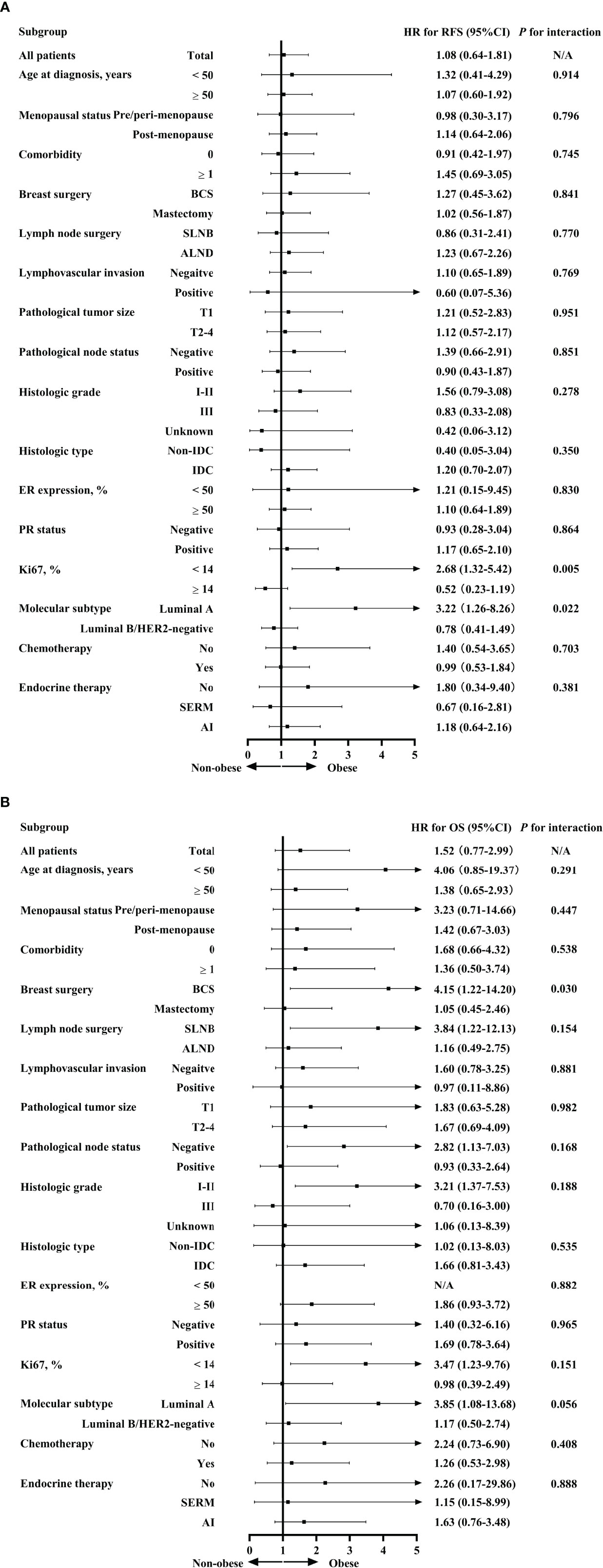
Figure 3 Interaction between BMI and clinicopathological characteristics in predicting RFS (A) and OS (B). BMI, body mass index; RFS, relapse-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; BCS, breast conservative surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; IDC, invasive ductal carcinoma; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; SERM, selective estrogen receptor modulators; AI, aromatase inhibitors.
Apart from luminal subtype, no other clinicopathological feature had interaction with obesity in prognosis prediction (Figure 3). For instance, when stratified by menopausal status, obesity was not associated with survival in either pre/peri-menopausal patients or post-menopausal patients in terms of RFS (interact P = 0.796) or OS (interact P = 0.447).
The adherence to endocrine therapy was 91.8% (1,719/1,872) for AI and 92.5% (801/866) for SERM (P=0.549). Kaplan–Meier curves of RFS and OS according to obesity status in patients treated with SERM or AI are listed in Figure 4. There was no interaction between obesity and endocrine treatment modality in predicting RFS (interact P = 0.381) and OS (interact P = 0.888). In the subgroup of patients on SERM treatment (with or without OFS), the obese patients showed no significant differences in 5-year RFS (87.0% vs 85.3%, P = 0.625) or 5-year OS (90.9% vs 95.9%, P = 0.703) compared with the non-obese (Figures 4A, B). Regarding patients receiving AI treatment (with or without OFS), there was no difference in OS or RFS between obese and non-obese patients (5-year RFS: 92.2% vs 90.1%, P = 0.680, Figure 4C; 5-year OS: 94.9% vs 92.9%, P = 0.253; Figure 4D). Patients receiving OFS showed similar RFS and OS between the two BMI groups (data not shown).
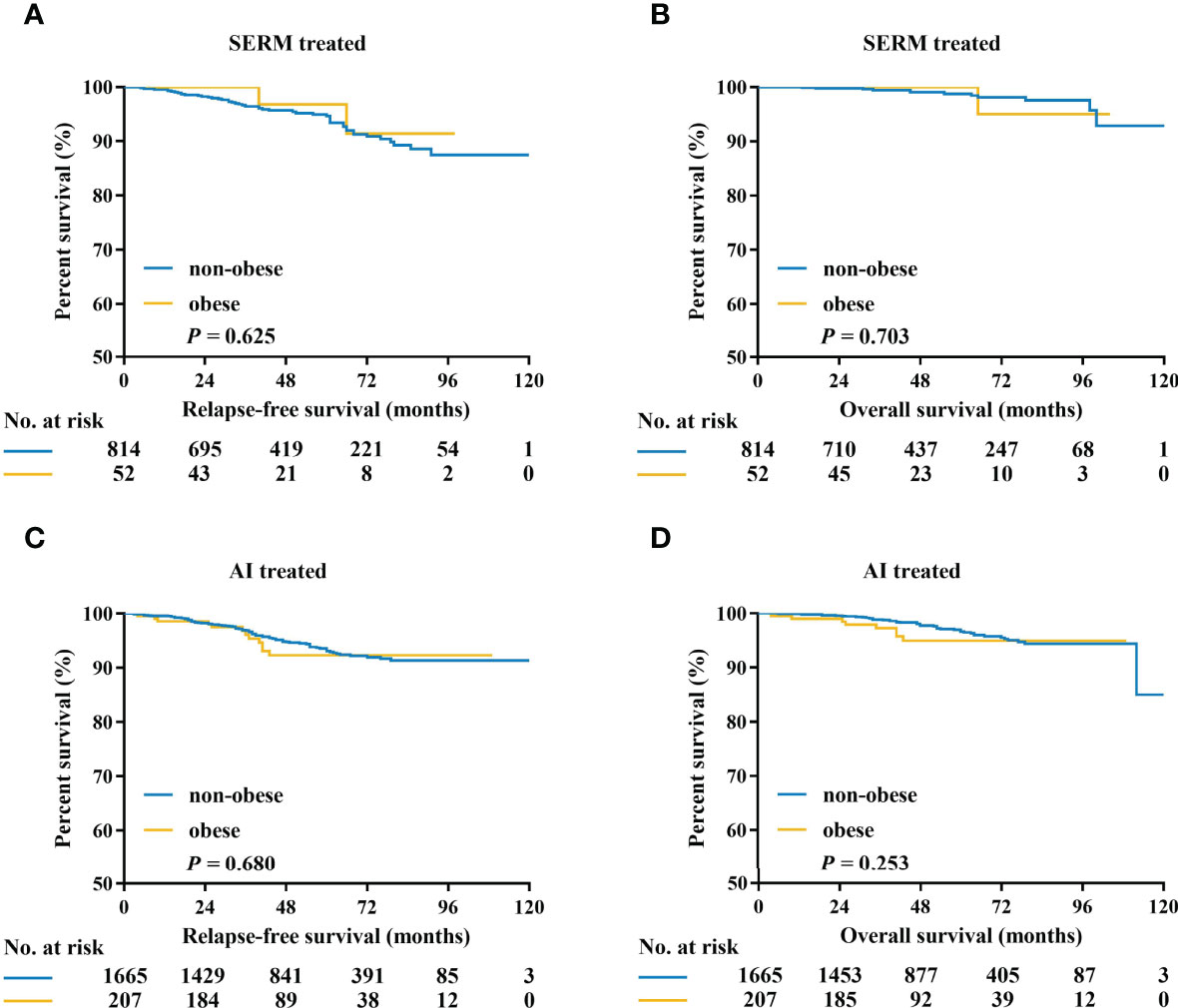
Figure 4 Kaplan–Meier estimates of relapse-free survival and overall survival in patients treated with SERM (A, B), and AI (C, D). SERM, selective estrogen receptor modulators; AI, aromatase inhibitors.
Our current study included 2,875 Chinese luminal breast cancer patients. We have found that obesity was related to the patient’s comorbidity and PR status. We also concluded that obese patients with luminal A breast cancer have significantly shorter OS and RFS. This relation was not significant in luminal B breast cancer. Our study is one of the few to investigate the impact of obesity on prognosis of breast cancer patients according to luminal subtypes in the Chinese population, which may provide an insight into how obesity adversely influences clinical outcomes.
Nearly one-tenth of our study population were obese, which was relatively lower than the Western population (30). In our study, we found that obese patients tended to be elder and more likely to have a medical history of comorbidities compared to non-obese patients, which is consistent with previous studies (6–8, 17, 31). Interestingly, our cohort found that obese patients had a slightly higher proportion of PR-positive tumors compared to non-obese patients, which was similar to the findings of Ladoire et al. (31). A preclinical study with obese rats with breast cancer induced by high-fat diet and 1-methyl-1-nitrosourea demonstrated that obesity enhanced the tumor expression of PR. Obesity-induced PR expression in tumors was thought to be an adaption to extreme metabolic changes and loss of sex hormone production during menopause (32). Additionally, previous studies reported that obesity was related with more advanced and biologically adverse tumors, such as larger tumor size, more lymph node involvement, higher proportion of ER-negative, triple negative tumors, grade III, or high Ki-67 index, and this could not be reproduced in our study (16, 33, 34), which may be explained by our study only including the luminal population. Molecular subtypes were similarly distributed in obese and non-obese patients in our study. Whereas in the PAM50 testing dated from the CALGB 9741 study, obese breast cancer patients had a significantly larger share of luminal B breast cancers (35), possibly explained by ethnic background difference or different molecular subtype detecting methods. In terms of adjuvant systemic therapy, obese patients were less likely to receive chemotherapy, which may be contributed to the elder age and the relatively worse baseline performance status (36, 37). Moreover, obese patients would also be more likely to receive insufficient dosage of treatment according to actual body surface area, which would also impact the patient’s disease outcome (38).
With a growing body of evidence exploring the correlation between obesity and clinical outcomes, controversy still exists on the relation between obesity and prognosis in specific molecular subtypes of breast cancer (7, 17, 19, 39). In our current study, we did not find the prognostic value of obesity in the overall luminal/HER2-negative population or in the subgroup of luminal B tumors. Meanwhile, we demonstrated a significant inverse relationship between BMI and disease outcomes in patients with luminal A breast cancer, which was in accordance with previously published literature based on PAM50 subtypes (40, 41). In an analysis based on the LACE and Pathways studies, obesity was adversely correlated with outcomes only among those with luminal A cancers, basically driven by class II/III obesity (BMI ≥ 35 kg/m2) (40). Similarly, Kwan et al. also found that the association between BMI and disease outcome was the strongest for luminal A tumors (41). Though obesity has been shown to be related with less favorable characteristics (9, 42, 43), the statistical significance of survival outcome still existed in luminal A patients after adjusting for other clinicopathological factors. Possible explanations for such difference between luminal A and luminal B tumors might be as follows. Firstly, increased endogenous estrogen with obesity would fuel the growth of ER-positive tumors but the effect would be less obvious for luminal B tumors which were also hormone receptor-positive but to a lesser extent (40). In addition, luminal B disease had a more aggressive nature compared to luminal A disease with increased proliferation and less hormone receptor expression, which would overlap and mask the effect of obesity. In addition, we showed in the univariate model that nodal status was associated with RFS in Luminal A patients (Supplementary Table S3). However, node status was no longer significantly related to prognosis in Luminal A patients in the multivariate model (Table 4), possibly due to the effect of chemotherapy, which suggests that standard adjuvant chemotherapy brings substantial benefit for node positive Luminal patients.
It is widely recognized that obesity has great influence on the endocrine system both in pre- and post-menopausal breast cancer patients (20, 21). Retrospective analysis from the ATAC trial found that tamoxifen was equally effective across all BMI levels, while anastrozole was significantly less effective in obese patients compared to normal weight patients (23). Similarly, results from the ABCSG-12 trial, exclusively including premenopausal patients treated with OFS, showed a worse disease outcome for obese patients treated with AI but not for tamoxifen cohorts (24). Speculation has arisen whether the constant dosage of anastrozole is sufficient for the obese to suppress the estrogen to a relatively low extent. Anastrozole was found to be inferior to tamoxifen for overweight or obese premenopausal patients in the ABCSG-12 trial and equal to tamoxifen for obese postmenopausal patients in the ATAC trial (23, 24). Other large phase III trials did not find any influence of obesity on letrozole (34) or exemestane (44) performance, possibly due to the different potencies of estrogen level suppression ability (45). In our study, we did not observe an effect of obesity on AI or tamoxifen treatment. Studies of large sample size are needed and essential to compare the efficacy of different types of AI for obese patients.
There are certainly several limitations in this study. Firstly, it is a retrospective study from a single institution with possible selection bias. Secondly, we did not take the effect of weight change after diagnosis into account for the lack of relevant information. Weight gain following breast cancer diagnosis has been reported to be associated with worse survival (46, 47). In addition, we did not include the lifestyle covariates like physical activity and diet preference into survival analysis, which have been related with breast cancer outcomes (48–50). Finally, it is important to carry out a comprehensive analysis of additional metabolic parameters other than obesity, for instance, metabolic syndrome, insulin, c-peptide, blood glucose, etc. on patients’ outcomes (51–53), so as to provide a more precise view regarding patient metabolism and cancer survival.
In conclusion, the adverse impact of obesity on luminal-like patients’ survival announced in previous studies could not be reproduced with our cohort of the Chinese luminal-like population. Our study found that obesity was associated with slightly more PR positive tumor and worse clinical outcomes in luminal A subtype but not for luminal B tumor. In patients treated with adjuvant endocrine therapy, obesity had no significant impact on patients’ outcome, regardless of endocrine treatment regimen received, which deserves further clinical evaluation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Data collection and analysis were performed by SZ. YT, SZ, and WC wrote the first draft of the manuscript. XC made substantial contributions to the conception of the work and revised the manuscript. KS substantively revised the manuscript. All authors have contributed to read and approved the final manuscript for submission.
The authors appreciate the financial supports by the National Natural Science Foundation of China (Grant Number: 81772797, 82072897), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007); and Shanghai Sailing Program (21YF1427400). All these financial sponsors had no role in the study design, collection, analysis or interpretation of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.862224/full#supplementary-material
AI, Aromatase inhibitor; ASCO, American Society of Clinical Oncology; BCS, Breast-conserving surgery; BMI, Body mass index; CAP, College of American Pathologists; CI, Confidence interval; DCIS, Ductal carcinoma in situ; ER, Estrogen receptor; HER2, Human epidermal growth factor receptor 2; HR, Hormone receptor; HR, Hazard ratio; IDC, Invasive ductal carcinoma; OFS, Ovarian function suppression; OR, Odds ratio; OS, Overall survival; PR, Progesterone receptor; RFS, Recurrence-free survival; SERM, Selective estrogen receptor modulator; SLNB, Sentinel lymph node biopsy.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
2. Ignatiadis M, Sotiriou C. Luminal Breast Cancer: From Biology to Treatment. Nat Rev Clin Oncol (2013) 10:494–506. doi: 10.1038/nrclinonc.2013.124
3. Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body Mass Index and Survival in Women With Breast Cancer-Systematic Literature Review and Meta-Analysis of 82 Follow-Up Studies. Ann Oncol (2014) 25(10):1901–14. doi: 10.1093/annonc/mdu042
4. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and Adverse Breast Cancer Risk and Outcome: Mechanistic Insights and Strategies for Intervention. CA Cancer J Clin (2017) 67(5):378–97. doi: 10.3322/caac.21405
5. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide Burden of Cancer Attributable to Diabetes and High Body-Mass Index: A Comparative Risk Assessment. Lancet Diabetes Endocrinol (2018) 6:e6–e15. doi: 10.1016/s2213-8587(18)30150-5
6. Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL, et al. Obesity and Outcomes in Premenopausal and Postmenopausal Breast Cancer. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res Cosponsored by Am Soc Prev Oncol (2005) 14:1686–91. doi: 10.1158/1055-9965.Epi-05-0042
7. Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, Diabetes, and Survival Outcomes in a Large Cohort of Early-Stage Breast Cancer Patients. Ann Oncol Off J Eur Soc Med Oncol (2013) 24:2506–14. doi: 10.1093/annonc/mdt224
8. Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of Obesity on Prognosis After Early-Stage Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:25–31. doi: 10.1200/jco.2010.29.7614
9. Kamineni A, Anderson ML, White E, Taplin SH, Porter P, Ballard-Barbash R, et al. Body Mass Index, Tumor Characteristics, and Prognosis Following Diagnosis of Early-Stage Breast Cancer in a Mammographically Screened Population. Cancer Causes Control CCC (2013) 24:305–12. doi: 10.1007/s10552-012-0115-7
10. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality From Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
11. Niraula S, Ocana A, Ennis M, Goodwin PJ. Body Size and Breast Cancer Prognosis in Relation to Hormone Receptor and Menopausal Status: A Meta-Analysis. Breast Cancer Res Treat (2012) 134:769–81. doi: 10.1007/s10549-012-2073-x
12. Protani M, Coory M, Martin JH. Effect of Obesity on Survival of Women With Breast Cancer: Systematic Review and Meta-Analysis. Breast Cancer Res Treat (2010) 123:627–35. doi: 10.1007/s10549-010-0990-0
13. Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship Between Potentially Modifiable Lifestyle Factors and Risk of Second Primary Contralateral Breast Cancer Among Women Diagnosed With Estrogen Receptor-Positive Invasive Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27:5312–8. doi: 10.1200/jco.2009.23.1597
14. Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, Tamoxifen Use, and Outcomes in Women With Estrogen Receptor- Positive Early-Stage Breast Cancer. J Natl Cancer Institute (2003) 95:1467–76. doi: 10.1093/jnci/djg060
15. Copson ER, Cutress RI, Maishman T, Eccles BK, Gerty S, Stanton L, et al. Obesity and the Outcome of Young Breast Cancer Patients in the UK: The POSH Study. Ann Oncol: Off J Eur Soc Med Oncol (2015) 26:101–12. doi: 10.1093/annonc/mdu509
16. Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, et al. Obesity at Diagnosis Is Associated With Inferior Outcomes in Hormone Receptor-Positive Operable Breast Cancer. Cancer (2012) 118:5937–46. doi: 10.1002/cncr.27527
17. Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wöckel A. Endocrine Therapy in Obese Patients With Primary Breast Cancer: Another Piece of Evidence in an Unfinished Puzzle. Breast Cancer Res Treat (2012) 131:925–31. doi: 10.1007/s10549-011-1874-7
18. Jeon YW, Kang SH, Park MH, Lim W, Cho SH, Suh YJ. Relationship Between Body Mass Index and the Expression of Hormone Receptors or Human Epidermal Growth Factor Receptor 2 With Respect to Breast Cancer Survival. BMC Cancer (2015) 15:865. doi: 10.1186/s12885-015-1879-4
19. Lorincz AM, Sukumar S. Molecular Links Between Obesity and Breast Cancer Endocr Relat Cancer. (2006) 13:279.
20. Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, Breast Cancer and Obesity: A Complex Interaction. Trends Endocrinol Metabolism: TEM (2012) 23:83–9. doi: 10.1016/j.tem.2011.10.003
21. Goodwin PJ. Obesity and Endocrine Therapy: Host Factors and Breast Cancer Outcome. Breast (Edinburgh Scotland) (2013) 22 (Suppl 2):S44–7. doi: 10.1016/j.breast.2013.07.008
22. Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of Body Mass Index on Recurrences in Tamoxifen and Anastrozole Treated Women: An Exploratory Analysis From the ATAC Trial. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28:3411–5. doi: 10.1200/jco.2009.27.2021
23. Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, et al. Impact of Body Mass Index on the Efficacy of Endocrine Therapy in Premenopausal Patients With Breast Cancer: An Analysis of the Prospective ABCSG-12 Trial. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:2653–9. doi: 10.1200/jco.2010.33.2585
24. Zhou BF. Predictive Values of Body Mass Index and Waist Circumference for Risk Factors of Certain Related Diseases in Chinese Adults–Study on Optimal Cut-Off Points of Body Mass Index and Waist Circumference in Chinese Adults. Biomed Environ Sci BES (2002) 15:83–96.
25. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Thresholds for Therapies: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol (2009) 20(8):1319–29. doi: 10.1093/annonc/mdp322
26. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. (2018) 36:2105–22. doi: 10.1200/jco.2018.77.8738
27. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring Therapies–Improving the Management of Early Breast Cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol: Off J Eur Soc Med Oncol (2015) 26:1533–46. doi: 10.1093/annonc/mdv221
28. Yu J, Wu J, Huang O, Chen X, Shen K. A Smartphone-Based App to Improve Adjuvant Treatment Adherence to Multidisciplinary Decisions in Patients With Early-Stage Breast Cancer: Observational Study. J Med Internet Res 16 (2021) 23(9):e27576. doi: 10.2196/27576
29. Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for Time-to-Event End Point Definitions in Breast Cancer Trials: Results of the DATECAN Initiative (Definition for the Assessment of Time-To-Event Endpoints in CANcer Trials)†. Ann Oncol Off J Eur Soc Med Oncol (2015) 26:873–9. doi: 10.1093/annonc/mdv106
31. Ladoire S, Dalban C, Roché H, Spielmann M, Fumoleau P, Levy C, et al. Effect of Obesity on Disease-Free and Overall Survival in Node-Positive Breast Cancer Patients in a Large French Population: A Pooled Analysis of Two Randomised Trials. Eur J Cancer (Oxford Engl (2014) 1990) 50:506–16. doi: 10.1016/j.ejca.2013.11.013
32. Giles ED, Wellberg EA, Astling DP, Anderson SM, Thor AD, Jindal S, et al. Obesity and Overfeeding Affecting Both Tumor and Systemic Metabolism Activates the Progesterone Receptor to Contribute to Postmenopausal Breast Cancer. Cancer Res (2012) 72:6490–501. doi: 10.1158/0008-5472.Can-12-1653
33. Kwan ML, Kroenke CH, Sweeney C, Bernard PS, Weltzien EK, Castillo A, et al. Association of High Obesity With PAM50 Breast Cancer Intrinsic Subtypes and Gene Expression. BMC Cancer (2015) 15:278. doi: 10.1186/s12885-015-1263-4
34. Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thürlimann B, et al. Obesity and Risk of Recurrence or Death After Adjuvant Endocrine Therapy With Letrozole or Tamoxifen in the Breast International Group 1-98 Trial. J Clin Oncol (2012) 30:3967–75. doi: 10.1200/jco.2011.40.8666
35. Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, Gradishar W, et al. Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741 (Alliance). J Natl Cancer Institute (2015) 107(9). doi: 10.1093/jnci/djv179
36. Colleoni M, Li S, Gelber RD, Price KN, Coates AS, Castiglione-Gertsch M, et al. Relation Between Chemotherapy Dose, Oestrogen Receptor Expression, and Body-Mass Index. Lancet (2005) 366:1108–10. doi: 10.1016/s0140-6736(05)67110-3
37. Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Herderon IC, et al. Relationship Between Toxicity and Obesity in Women Receiving Adjuvant Chemotherapy for Breast Cancer: Results From Cancer and Leukemia Group B Study 8541. J Clin Oncol Off J Am Soc Clin Oncol (1996) 14:3000–8. doi: 10.1200/jco.1996.14.11.3000
38. de Azambuja E, McCaskill-Stevens W, Francis P, et al. The Effect of Body Mass Index on Overall and Disease-Free Survival in Node-Positive Breast Cancer Patients Treated With Docetaxel and Doxorubicin-Containing Adjuvant Chemotherapy: The Experience of the BIG 02-98 Trial. Breast Cancer Res Treat (2010) 119:145–53. doi: 10.1007/s10549-009-0512-0
39. Robinson PJ, Bell RJ, Davis SR. Obesity is Associated With a Poorer Prognosis in Women With Hormone Receptor Positive Breast Cancer. Maturitas (2014) 79:279–86. doi: 10.1016/j.maturitas.2014.07.004
40. Cespedes Feliciano EM, Kwan ML, Kushi LH, Chen WY, Weltzien EK, Castillo AL, et al. Body Mass Index, PAM50 Subtype, Recurrence, and Survival Among Patients With Nonmetastatic Breast Cancer. Cancer (2017) 123:2535–42. doi: 10.1002/cncr.30637
41. Kwan ML, Quesenberry CP Jr., Caan BJ. RE: Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741. J Natl Cancer Institute (2016) 108. doi: 10.1093/jnci/djv333
42. Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of Body Mass Index to Tumor Markers and Survival Among Young Women With Invasive Ductal Breast Carcinoma. Cancer (2001) 92:720–9. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t
43. Blair CK, Wiggins CL, Nibbe AM, Storlie CB, Prossnitz ER, Royce M, et al. Obesity and Survival Among a Cohort of Breast Cancer Patients Is Partially Mediated by Tumor Characteristics. NPJ Breast Cancer (2019) 5:33. doi: 10.1038/s41523-019-0128-4
44. Seynaeve C, Hille E, Hasenburg A, Rea D, Markopoulos C, Hozumi Y, et al. Abstract S2-3: The Impact of Body Mass Index (BMI) on the Efficacy of Adjuvant Endocrine Therapy in Postmenopausal Hormone Sensitive Breast Cancer (BC) Patients; Exploratory Analysis From the TEAM Study. Cancer Res (2010) 70:S2–3. doi: 10.1158/0008-5472.SABCS10-S2-3
45. Folkerd EJ, Dixon JM, Renshaw L, A'Hern RP, Dowsett M. Suppression of Plasma Estrogen Levels by Letrozole and Anastrozole Is Related to Body Mass Index in Patients With Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30:2977–80. doi: 10.1200/jco.2012.42.0273
46. Bradshaw PT, Ibrahim JG, Stevens J, Cleveland R, Abrahamson PE, Satia JA, et al. Postdiagnosis Change in Bodyweight and Survival After Breast Cancer Diagnosis. Epidemiol (Cambridge Mass.) (2012) 23:320–7. doi: 10.1097/EDE.0b013e31824596a1
47. Chen X, Lu W, Zheng W, Gu K, Chen Z, Zheng Y, et al. Obesity and Weight Change in Relation to Breast Cancer Survival. Breast Cancer Res Treat (2010) 122:823–33. doi: 10.1007/s10549-009-0708-3
48. Schmid D, Leitzmann MF. Association Between Physical Activity and Mortality Among Breast Cancer and Colorectal Cancer Survivors: A Systematic Review and Meta-Analysis. Ann Oncol: Off J Eur Soc Med Oncol (2014) 25:1293–311. doi: 10.1093/annonc/mdu012
49. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical Activity, Risk of Death and Recurrence in Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Epidemiological Studies. Acta Oncol (Stockholm Sweden) (2015) 54:635–54. doi: 10.3109/0284186x.2014.998275
50. Saxe GA, Rock CL, Wicha MS, Schottenfeld D. Diet and Risk for Breast Cancer Recurrence and Survival. Breast Cancer Res Treat (1999) 53:241–53. doi: 10.1023/a:1006190820231
51. Buono G, Crispo A, Giuliano M, De Angelis C, Schettini F, Forestieri V, et al. Combined Effect of Obesity and Diabetes on Early Breast Cancer Outcome: A Prospective Observational Study. Oncotarget (2017) 8(70):115709–17. doi: 10.18632/oncotarget.22977
52. Buono G, Crispo A, Giuliano M, De Angelis C, Schettini F, Forestieri V, et al. Metabolic Syndrome and Early Stage Breast Cancer Outcome: Results From a Prospective Observational Study. Breast Cancer Res Treat (2020) 182(2):401–9. doi: 10.1007/s10549-020-05701-7
Keywords: breast cancer, obese, luminal subtype, prognosis, aromatase inhibitor
Citation: Tong Y, Zhu S, Chen W, Chen X and Shen K (2022) Association of Obesity and Luminal Subtypes in Prognosis and Adjuvant Endocrine Treatment Effectiveness Prediction in Chinese Breast Cancer Patients. Front. Oncol. 12:862224. doi: 10.3389/fonc.2022.862224
Received: 25 January 2022; Accepted: 08 April 2022;
Published: 05 May 2022.
Edited by:
Emily Jane Gallagher, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Laura G Estevez, MD Anderson Cancer Center Madrid, SpainCopyright © 2022 Tong, Zhu, Chen, Chen and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosong Chen, Y2hlbnhpYW9zb25nMDE1NkBob3RtYWlsLmNvbQ==; Kunwei Shen, a3dzaGVuQG1lZG1haWwuY29tLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.