
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 06 February 2023
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1020339
This article is part of the Research Topic Case Reports in Thoracic Oncology: 2022 View all 42 articles
 Manman Cui1
Manman Cui1 Duchang Zhai1
Duchang Zhai1 Yan Liu1
Yan Liu1 Xiuzhi Zhou1
Xiuzhi Zhou1 Tingting Wang1
Tingting Wang1 Lihuan Wang2
Lihuan Wang2 Wu Cai1*
Wu Cai1* Guohua Fan1
Guohua Fan1 Shenghong Ju3
Shenghong Ju3Ewing’s sarcoma is a part of a rare group of malignant neoplasms, whose pathological morphological features are small round cells. Extraskeletal Ewing’s sarcoma is a more uncommon primary tumor. Herein, we report the case of a 66-year-old man who complained of chest tightness. Subsequent chest CT scans revealed an irregular and uneven density mass on the right side of the anterior mediastinum with invasion of the superior vena cava, pericardium and right lung. The patient’s clinical symptoms were improved after performing excision of the mediastinal lesions under cardiopulmonary bypass. Based on histological and immunohistochemical findings, the tumor was diagnosed as extraskeletal Ewing’s sarcoma.
Extraskeletal Ewing’s sarcoma (EES) is a small round cell malignant tumor that grows outside of bone tissue, which arises from the mediastinum is extremely rare. Compared with patients with bone tumors, patients with EES have an older average age of onset. The clinical features and imaging appearances are non-specific, and patients often present with a rapidly growing mass that causes localized pain (1). The diagnosis of this tumor mainly relies on pathological, cytogenetic, immunohistochemical and molecular genetic analysis. We report a patient who presented with chest tightness and had a mediastinal ES, which was not confirmed until postoperative pathological examination was performed.
A 66-year-old man with an anterior mediastinal tumor was admitted to the hospital for surgical treatment. He went to the emergency department of the hospital 1 day prior because of chest tightness, and plain chest CT showed a mass on the right side of the anterior mediastinum that compressed the adjacent right lung, accompanied by pleural effusion (Figures 1A, B). Laboratory examinations revealed elevated levels of troponin, cardiac enzymes, CA125, NSE and cytokeratin, which indicated myocardial injury and abnormal cancer markers. The preoperative contrast-enhanced CT scan showed a giant cystic and solid mass on the right side of the anterior mediastinum, which had moderately uneven enhancement, and the mediastinal lymph nodes were enlarged (Figures 1C, D). The mass on the right side of the anterior mediastinum with invasion of the superior vena cava, pericardium and right lung could be observed from the coronal multiplanar reconstruction images (Figures 1E, F). In summary, we initially considered that the mass was a malignant tumor arising from the thymus.
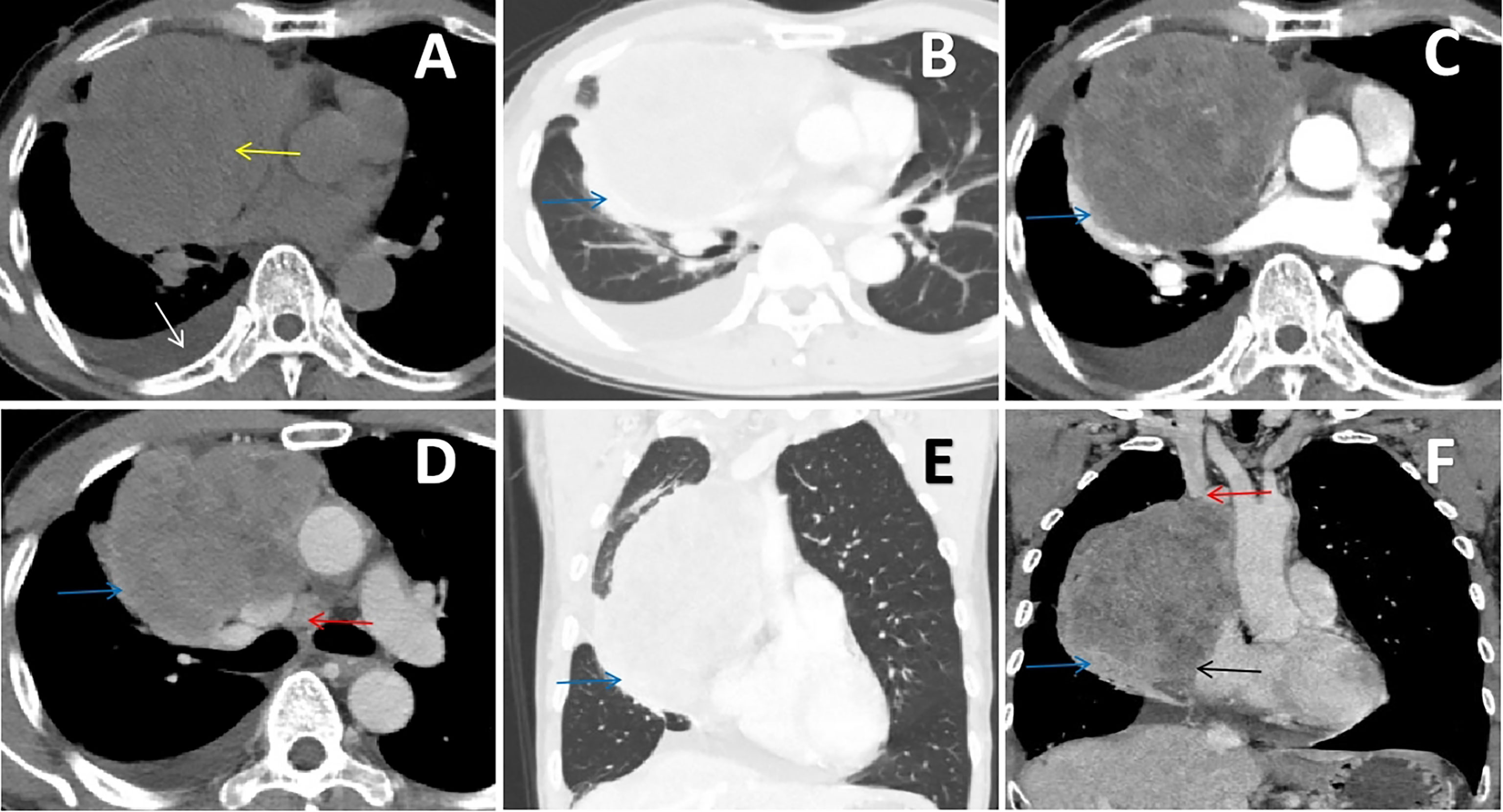
Figure 1 (A, B) Chest CT scan showing an irregular soft tissue density mass (yellow arrow) on the right side of the anterior mediastinum that compressed the adjacent right lung (blue arrow), accompanied by pleural effusion (white arrow). (C, D) Contrast-enhanced chest CT scan showing that the mass had moderately uneven enhancement, which invaded the right lung (blue arrow), and the mediastinal lymph nodes were enlarged (red arrow). (E, F) The coronal multiplanar reconstruction images clearly delineated the irregular and uneven density mass on the right side of the anterior mediastinum with invasion of the superior vena cava (red arrow), pericardium (black arrow) and right lung (blue arrow).
Intraoperative exploration revealed that the tumor had invaded the superior vena cava, pericardium and right middle lung, so the surgeon adopted resection of the mediastinal lesions under cardiopulmonary bypass, partial resection of the superior vena cava with artificial blood vessel replacement and lobectomy of the right middle lung with lymph node dissection. The pathological findings confirmed that it was a small round cell malignant tumor (Figure 2A). The mediastinal lymph nodes submitted for examination showed that metastasis of the tumor had already occurred. The immunohistochemical results demonstrated a Ki‐67 of 90%, positivity for AE1/AE3, P16, CD 99 (Figure 2B), CD56 and synaptophysin, partial positivity for EMA, MDM2 and desmin (Figure 2C), and negativity for vimentin, CK7, CAM5.2, SMA, NSE and S-100. Based on these findings, primary mediastinal ES was diagnosed. The patient’s chest tightness and overall condition improved after the operation, and he was discharged after 1 month of symptomatic and supportive treatments. One month later, the patient was admitted to the hospital again. His blood test showed a significant increase in the heart failure index score. After symptomatic treatments, the symptoms improved. Subsequently, the patient’s chest tightness was not relieved, and he had orthopnea. After consultation with experts, he was considered to have postoperative cachexia of a malignant tumor. There was a risk of respiratory cardiac arrest at any time, and the prognosis was very poor. Four days later, he was transferred to the ICU for treatment. Echocardiography showed that there was a large amount of pericardial effusion, and combined with the history of the tumor invading the pericardium, the effusion was considered to be tumor tissue. Finally, pathological examination of the pericardial effusion confirmed that it had malignant tumor cells (Figure 2D).
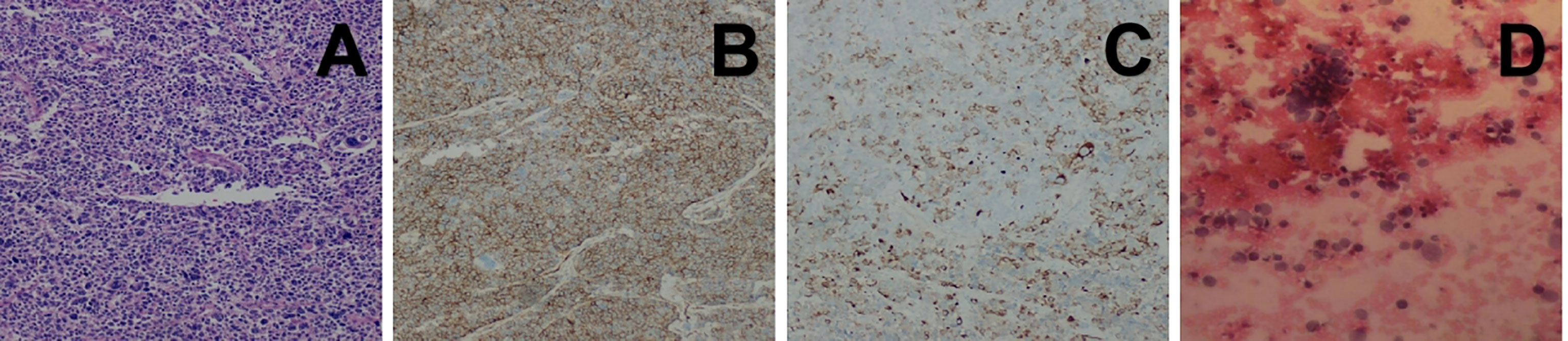
Figure 2 (A) Histopathological image showing tumor cells composed of primitive small round cells (H&E staining). (B, C) Immunohistochemical images showing positivity for CD99 and desmin. (D) Histopathological image showing pericardial effusion, which consisted of poor differentiation of malignant tumors.
Because the circulatory failure caused by pericardial tamponade was difficult to cure, the family members of the patient finally refused further invasive surgery for the patient; only noninvasive ventilators were used to maintain the patient’s vital signs, and he had entered the end stage of clinical life.
EES is a rare entity with high-grade malignancy that commonly involves the soft tissues of the trunk and extremities. It is more common in the lower limbs, paraspinal, retroperitoneum and chest wall and rarely occurs in solid organs. Primary mediastinal EES is extremely rare (2, 3). Therefore, the case we present can increase our understanding of this malignant tumor. Its early clinical symptoms are mostly uncharacteristic; it usually presents as a painless mass that rarely attracts attention, and the late stage manifests as a progressive enlargement of the mass accompanied by pain and the symptoms of invaded adjacent tissues. The histological pattern of EES consists of a large number of small round cells with a uniform shape, which have a high nuclear to cytoplasmic ratio (4). In immunohistochemistry, CD99, FLI-1 and vimentin are almost expressed, and NSE and synaptophysin are expressed to varying degrees. CD99, the product of the MIC gene, is a relatively specific antibody of the ES family. However, CD99 expression is not specific to ES, as it can be expressed by other tumors, such as ependymoma (5). To the best of our knowledge, the translocation t(11;22)(q24;q12) is an important factor for EES diagnosis as well as differential diagnosis. Delattre et al. reported that 85% of cases were associated with the translocation t(11;22)(q24;q12), which leads to the formation of the EWS-FLI-1 fusion gene (6). The imaging findings of EES are often nonspecific, and most CT scans show that it is a soft tissue density mass in different parts. In magnetic resonance imaging (MRI), the mass shows a moderate signal on T1WI and a moderately high signal on T2WI. Sometimes the density or signal is uneven because of haemorrhage and necrosis. It is more common to see cystic degeneration and necrosis, but calcification is rarely observed, and capsules may appear. Enhanced scans can show that a mass is uneven and that the degree of enhancement is different. Since the tumor is invasive, it tends to invade surrounding tissue structures. The CT of the patient we report clearly revealed that the tumor had invaded the superior vena cava, pericardium and lung tissue. Furthermore, enhanced scanning can show the blood supplying arteries of the mass and can also be used to evaluate the feasibility of tumor surgery and determine clinical treatment plans. Since mediastinal ES is relatively rare, it needs to be differentiated from malignant thymoma, mediastinal lymphoma, teratoma, seminoma, endodermal sinus tumor and solitary fibroma. Malignant thymoma should be considered first when a large, irregular and uneven mass appears in the anterior mediastinum, which was the reason for misdiagnosis in the study. Malignant thymomas show similar radiologic findings as EWS (7). However, malignant thymomas are much less malignant than mediastinal ES, such as they usually have no lymph node metastasis and no pleural effusion. In addition, malignant thymomas are usually accompanied by myasthenia gravis. Teratomas are often accompanied by fat, calcification, and bone and soft tissue density masses. The majority of lymphomas in the mediastinum have lymphadenopathy in the neck and other areas of the mediastinum, and the clinical symptoms are more typical. If there is a homogeneous and soft tissue density solid mass with no calcification and fat accompanied by elevated β-HCG levels, then seminoma should be considered. Enhanced scans of mediastinal endodermal sinus tumors show centripetal irregular striped and reticular blood vessels, and the surrounding irregular thick-walled ring is obviously enhanced. In addition, AFP and β-HCG levels are significantly increased in most patients. Large solitary fibromas are heterogeneous, and map-like uneven enhancement and creeping blood vessels can be observed by enhanced scanning. However, the final diagnosis depends on pathology and immunohistochemistry. Adverse prognostic factors consist of large tumors, nonsurgical treatment and regional lymph node metastasis (8). Treatment of EES includes combination therapy with chemotherapy and topical therapy delivered by surgical resection, radiation, or both (9). Covelli et al. confirmed that surgical resection alone can improve the survival rate of EES patients but also proposed that surgery combined with radiotherapy and chemotherapy is the best treatment for EES (10). The patient we report underwent surgery, but he did not receive chemotherapy or radiotherapy for economic reasons. Finally, he had a poor prognosis and entered the end stage of clinical life. Liu et al. reported a case of mediastinal ES that invaded surrounding tissues and was unresectable, but after chemotherapy, the patient’s disease was almost completely in remission with no serious side effects (11).
Most cases of primary EES in the mediastinum appear as masses upon diagnosis with no specific clinical symptoms. On imaging, these tumors are usually large, enveloped, soft tissue density masses that can compress or invade adjacent tissues and easily metastasize, and an enhanced scans can show inhomogeneous moderate or significant enhancement. Imaging can be performed as a diagnostic tool, but the final diagnosis is mainly based on pathology and immunohistochemistry. In terms of treatment, surgical resection combined with chemotherapy, radiotherapy or both is the best solution.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the participant for the publication of any potentially identifiable images or data included in this article.
MC, DZ, XZ, YL, TW, LW, GF, SJ, and WC conceived the idea for the article. MC drafted the manuscript. WC approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Project of State Key Laboratory of Radiation Medicine and Protection, Soochow University (GZK1202136), “National Tutor System” Training Program for Health Youth Key Talents in Suzhou (Qngg2021006、Qngg2021039), Suzhou Health Talent (GSWS2021025), “Image Medical Star” Project of Suzhou Medical Association (2022YX-M05), Pre-research Foundation of Second Affiliated Hospital of Soochow University (SDFEYBS1904), and Project of Nuclear Technology Medical Application Supported by Discipline Construction of Second Affiliated Hospital of Soochow University (XKTJ-HRC20210010). The authors declare that this study received funding from “Technological Innovation” Project of CNNC Medical Industry Co. Ltd. (ZHYLYB2021006). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of the article, or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Abboud A, Masrouha K, Saliba M, Haidar R, Saab R, Khoury N, et al. Extraskeletal Ewing sarcoma: Diagnosis, management and prognosis. Oncol Lett (2021) 21(5):354. doi: 10.3892/ol.2021.12615
2. Reali A, Mortellaro G, Allis S, Trevisiol E, Anglesio SM, Bartoncini S, et al. A case of primary me-diastinal ewing's Sarcoma/Primitive neuroectodermal tumor presenting with initial compression of superi-or vena cava. Ann Thorac Med (2013) 8(2):121–3. doi: 10.4103/1817-1737.109834
3. Halefoglu AM. Extraskeletal ewing's sarcoma presenting as a posterior mediastinal mass. Arch Bronconeumol. (2013) 49(2):82–4. doi: 10.1016/j.arbres.2012.02.020
4. Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett (2007) 254(1):1–10. doi: 10.1016/j.-canlet.2006.12.009
5. Kurdi M, Eberhart C. Immunohistochemical expression of Olig2, CD99, and EMA to differentiate oli-godendroglial-like neoplasms. Folia Neuropathol. (2021) 59(3):284–90. doi: 10.5114/fn.2021.108526
6. Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature (1992) 359(6391):162–5. doi: 10.1038/359162a0
7. Bae SH, Hwang JH, Da Nam B, Kim HJ, Kim KU, Kim DW, et al. Multiple Ewing Sarcoma/Primiti-ve neuroectodermal tumors in the mediastinum: A case report and literature review. Med (Baltim-ore). (2016) 95(7):e2725. doi: 10.1097/MD.0000000000002725
8. Chen Z, Jiao Y, Liu Z, Yang J, Sun J, Wang P. Extraskeletal ewing's sarcoma: Outcomes and CT fe-atures of endoceliac lesions. Transl Cancer Res (2021) 10(9):4065–75. doi: 10.21037/tcr-21-607
9. Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, et al. Bone sarcomas: SMO-EURAC-AN-GENTURIS-ERN PaedCan clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Su-ppl 4):iv79–95. doi: 10.1093/annonc/mdy310
10. Covelli HD, Beekman JF, Kingry RL. Extraskeletal ewing's sarcoma: Prolonged survival with recurr-ence after operation. South Med J (1980) 73(9):1294–5. doi: 10.1097/00007611-198009000-00053
Keywords: Ewing’s sarcoma, extraskeletal, oncology, mediastinum, primary, chest
Citation: Cui M, Zhai D, Liu Y, Zhou X, Wang T, Wang L, Cai W, Fan G and Ju S (2023) Case report: Primary mediastinal Ewing’s sarcoma presenting with chest tightness. Front. Oncol. 12:1020339. doi: 10.3389/fonc.2022.1020339
Received: 16 August 2022; Accepted: 28 December 2022;
Published: 06 February 2023.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanReviewed by:
Alessandro Inserra, Bambino Gesù Children’s Hospital (IRCCS), ItalyCopyright © 2023 Cui, Zhai, Liu, Zhou, Wang, Wang, Cai, Fan and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wu Cai, eHdnNjA4QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.