- 1Department of Hematology, West China Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 2Department of Nursing, West China Second University Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 3Cancer Center, West China Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 4Head & Neck Oncology Ward, Cancer Center, West China Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 5Department of Operating Room, West China Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 6West China School of Nursing, Sichuan University, Chengdu, China
- 7School of Nursing–Camden, Rutgers, The State University of New Jersey, Camden, NJ, United States
Background: Many breast cancer survivors face long-term postoperative challenges as a result of developing lymphedema symptoms and chronic lymphedema. The-Optimal-Lymph-Flow (TOLF) program is an intervention based on physiological-cognitive-behavioral principles that teaches patients self-management strategies to activate lymphatic system and promote lymph flow to decrease lymphatic pain, reduce the risk and severity of lymphedema.
Objective: The purpose of this pilot clinical trial was to evaluate the use of TOLF program as an early intervention on improving lymphedema symptom experience (i.e., symptom number, symptom severity, symptom distress, and the impact of symptoms on patients’ activities of daily living) and optimizing lymph fluid levels (measured by the arm volume differences) among breast cancer survivors.
Methods: This study is a parallel, randomized clinical trial. A total of 92 breast cancer patients were randomly assigned to either the TOLF intervention group or the control group focusing on promoting arm mobility. Data were collected at baseline and end of the trial at the 3-month post intervention. The Breast Cancer and Lymphedema Symptom Experience Index was used to measure lymphedema symptom experience. Anthropometric measurements were used for circumferential arm measurements. Generalized linear mixed-effects models were used to evaluate the trial outcomes.
Results: Significant improvements of lymphedema symptom experience were found in patients in the TOLF intervention group in comparison with patients in control group: the number of lymphedema symptoms (P<0.001) and the severity of lymphedema symptoms (P<0.001) as well as the impact of symptoms on patients’ daily living function (P<0.001). Patients in both groups showed improvements in all study outcomes over the 3 months, whereas those in the TOLF group gained greater benefits in reducing the number and severity of lymphedema symptoms. Moreover, the TOLF group had significantly fewer patients with ≥5% arm volume differences ([5/45] vs [13/43], P=0.035) at the study endpoint.
Conclusions: Findings of the study demonstrated positive outcomes of relieving lymphedema symptom experience, optimizing arm circumference and halting the progression of lymphedema status in breast cancer survivors receiving TOLF intervention during early postoperative time. Given its feasibility, acceptability, and effectiveness, this program may be incorporated in routine breast cancer care.
Clinical Trial Registration: http://www.chictr.org.cn/index.aspx, identifier ChiCTR1800016713.
Introduction
Breast cancer is the most common malignancy in women worldwide, with 2.3 million new cases diagnosed annually, and due to improvements in early detection and treatment, the population of breast cancer survivors is constantly expanding (1). Lymphedema is a prevalent and debilitating complication of breast cancer treatment affecting more than 1 in 5 breast cancer survivors (2, 3), leading to chronic ipsilateral arm swelling coupled with multiple distressing symptoms (e.g., swelling, pain, heaviness, tightness, firmness, numbness, stiffness, or impaired arm mobility) due to abnormal fluid accumulation defined as lymphedema symptoms (4, 5). It is well documented that lymphedema and associated symptoms are perceived as constant reminders of cancer and exert tremendous limitations on patients’ daily living function and quality of life (6, 7).
In fact, the latent stage of lymphedema may exist months or years before the overt lymphedema occurs (2). Without timely detection and intervention in the early stage, lymphedema can progress into a chronic condition that no surgical or medical interventions can cure (8, 9). Lymphedema symptoms, experienced by over 50% of women treated for breast cancer, are significant predictors of lymphedema (10, 11). The experience of lymphedema symptoms for breast cancer patients without a diagnosis of lymphedema is a cardinal sign of an early stage of lymphedema because these symptoms often precede changes in arm girth and a lymphedema diagnosis (12–14). Breast cancer survivors who reported 5 or more lymphedema symptoms are more likely to develop lymphedema (15). Effective management of lymphedema symptoms can decrease the risk of developing lymphedema (16–19). Thus, managing lymphedema symptoms is imperative to prevent lymphedema, maintain a normal arm volume, as well as improve activities of daily living (ADLs).
Very limited studies have been designed to build patients’ self-management skills to manage lymphedema symptoms, which are major predictors for lymphedema, impaired physical function, psychological distress, and poor quality of life (11, 20, 21). Majority of previous randomized controlled trials (RCT) were devoted to lymphedema treatment administered by professional therapists (22, 23). However, cost and time to attend therapist-administered treatments remain wide-spread challenges for patients. The-Optimal-Lymph-Flow (TOLF) program is an intervention based on physiological-cognitive-behavioral principles that teaches patients self-management strategies to activate lymphatic system and promote lymph flow to decrease lymphatic pain, reduce the risk and severity of lymphedema (24, 25). TOLF features a web- and mobile-based mHealth system that includes information about lymphedema knowledge and self-care strategies to deliver safe, easy, and feasible digital therapy of lymphatic exercises (i.e., muscle tightening–breathing, muscle tightening–pumping exercises, large muscle exercises) to promote lymph flow and drainage, limb mobility exercises to enhance shoulder and arm function, and general instructions to encourage healthy weight and proper sleep (16, 18, 24, 25). The core TOLF intervention is the 8-minute TOLF lymphatic exercises (16). Patients can learn and follow all the therapeutic exercises through avatar video simulations by logging onto the TOLF system anywhere and anytime with a computer, laptop, or any mobile phones or tablets (17, 25). The effectiveness of TOLF intervention has been demonstrated in several trials (17–19, 25). However, TOLF has not been tested as an early intervention to mitigate lymphedema symptoms, optimize ADLs, and prevent lymphedema. This pilot randomized clinical trial (RCT) was designed to evaluate the use of TOLF program as an early intervention for patients at risk for lymphedema on improving lymphedema symptom experience (i.e., symptom number, symptom severity, symptom distress, and the impact of symptoms on patients’ ADLs and optimizing lymph fluid levels among breast cancer survivors.
Materials and methods
Study design
This was a prospective RCT conducted from January 2019 to June 2020. Potential participants were screened for the risk of lymphedema at 1 month after surgical treatment and those reported 5 or more lymphedema symptoms were deemed as at-risk patients; subsequently and randomly assigned to either the TOLF intervention group or arm mobility control group to assess the effects of TOLF program on lymphedema symptom experience and lymph fluid levels. This trial was registered at the Chinese Clinical Trial Registry (ChiCTR1800016713). The protocol was in accordance with the CONSORT-EHEALTH checklist (26) (Supplementary Material).
Participants and setting
The study was conducted in West China Hospital, a 4300-bed tertiary teaching hospital affiliated with Sichuan university and the leading medical center in the south-western China. Breast cancer patients were introduced to the trial when attending their routine follow-up visits at 1 month after the surgical treatment. Patients were included if they (1) were nonpregnant females aged 18 to 80 years (2); underwent surgical treatment for breast cancer for the first time (3); reported at least 5 lymphedema symptoms (4); had not been diagnosed with lymphedema (5); had access to a smartphone, tablet device or computer/laptop with Internet access; and (6) willing to follow the self-management strategies. The exclusion criteria were as follows (1): patients who had a delayed healing of the incisions (2); the presence of local and distant metastasis (3); patients with history of surgery or trauma on the affected axilla or arm (4); patients with severe mental illness or cognitive impairments (5); patients who had documented advanced cardiac or renal diseases; or (6) with non-breast-cancer-related lymphedema. Each participant signed the written study consent.
Randomization
This study used computer-generated random numbers for randomization with a 1:1 ratio. The random numbers were concealed and administered by the project coordinator. Eligible patients were randomly allocated to either the TOLF intervention group or the arm mobility control group. The interventionists were blinded to group assignment. Participants, outcome assessors, and statistician were unaware which treatment was the intervention of interest and which one was the comparator.
Intervention
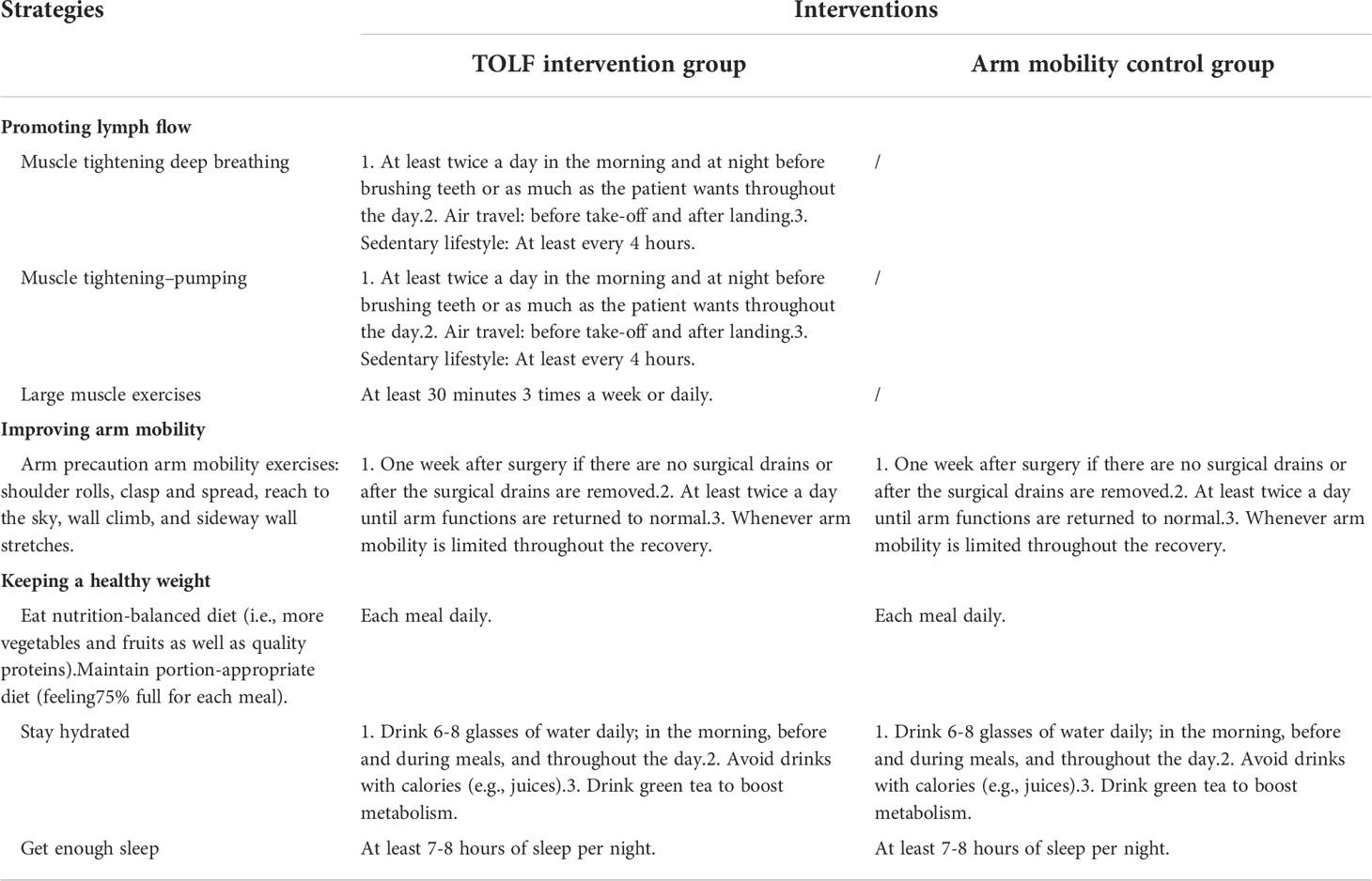
The intervention strategies for the two groups are presented in Table 1. Patients assigned to the TOLF intervention group were granted full access to the web- and mobile-based TOLF platform to learn about all the involved contents. They had the access to the Lymphedema Knowledge module to learn about the lymphatic system, lymphedema basics (e.g., definition, prevalence, diagnosis, symptoms, treatment and etc.), self-care skills, and general instructions about keeping a healthy weight. Patients also had the access to the Therapeutic Lymphatic Exercises module, which provided 8 avatar videos with step-by-step instructions to perform lymphatic exercises to promote lymph flow and optimize shoulder and arm mobility.
Patients assigned to the control group had the same access to the Lymphedema Knowledge module, but they had no access to the core Therapeutic Lymphatic Exercises module. Instead, they were only granted access to the Arm Mobility Exercises to promote arm mobility. The National Comprehensive Cancer Net-work (NCCN) clinical practice guidelines in oncology (27) recommended continued full use of the extremity and range-of-motion exercises (i.e., arm mobility exercises) to reduce the risk of lymphedema for all breast cancer survivors. In the current study, patients assigned to the control group had access to 4 avatar videos for improving arm mobility via the TOLF mHealth system.
During the first in-person research visit, the researchers spent approximately 30~45 minutes introducing the use of the TOLF program and demonstrating each module of the system to participants. It took about 10 minutes for patients in intervention group to learn the 8 avatar videos and about 5 minutes to perform a set of TOLF daily exercises each time. Participants in the control group took about 5 minutes to learn the 4 videos displaying the arm mobility exercises and it took 3 minutes to perform a set of the exercises each time. Patients were required to perform the assigned exercises at least twice a day during the 3-month study period.
Data collection and measures
Patients’ demographic and clinical information were assessed prior to intervention at the first research visit. All outcome data were collected at baseline and at month 3 after the intervention by online questionnaires and anthropometric measurements. An evaluation module base on the mHealth system was set up. Researchers informed the participants the time of each assessment, and participants could log on to the platform to complete the online evaluation. Primary outcome of the study focused on lymphedema symptom experience measured by the Breast Cancer and Lymphedema Symptom Experience Index (BCLE-SEI); lymphedema symptom experience included the dimensions of the number of symptoms, symptom severity, and symptom distress (28). Secondary outcomes included individuals’ ADLs (measured using a subscale of the BCLE-SEI) (28) and arm volume difference (measured in-person using circumferential arm measurements) (15).
The demographic and health information were collected to include age, time since the diagnosis of breast cancer, level of education, marital status, employment status, living status, dominant hand, household incomes, affected arm, types of surgery and treatment.
The BCLE-SEI was used to measure the number of symptoms, symptom severity, symptom distress, and the impaired ADLs (28). The BCLE-SEI is a valid and reliable self-report instrument that consists of two parts, respectively evaluating the occurrence of and distress from lymphedema-related symptoms (28). Part I of the scale evaluates the occurrence (i.e., number and severity of lymphedema symptoms) of the 24 lymphedema symptoms [i.e., impaired limb mobility in shoulder, arm, elbow, wrist, and fingers, arm swelling, breast swelling, chest wall swelling, heaviness, firmness, tightness, stiffness, numbness, tenderness, pain/aching/soreness, redness, blistering, burning, stabbing, tingling (pain and needles), hotness, blistering, limb fatigue, and limb weakness]. Each symptom is rated on a Likert-type scale from 0 (no presence of a given symptom) to 4 (greatest severity of a given symptom) by symptom severity and can also be treated as a dichotomous variable with “0” indicating the absence of a given symptom, and “1”to “4” indicating the presence of a given symptom (28). Part II of the scale assesses symptom distress, that is, the negative impact and suffering evoked by an individual’s experience of lymphedema-related symptoms, including ADLs, social impact, sleep disturbance, sexuality, emotional/psychological distress, and self-perception (28). In addition to the total score of symptom distress, we also focused on the symptom distress on performing ADLs, because having difficulties in performing ADLs is an important indicator for impaired physical function and quality of life, and lymphedema symptom experience is directly associated with individuals’ ability to performing ADLs (6). The internal consistency was demonstrated with a Cronbach’s α of 0.967 for the overall BCLE-SEI; the test-retest reliability and its structure validity were also confirmed to be acceptable (29).
The arm circumferences of both arms were measured using a well-established protocol for arm circumference measurements: at 4-cm intervals consecutive measurements beginning at the wrist and ending at the shoulder to ensure accuracy (15). The arm volume was calculated using the formula where C1 and C2 represent circumferences of the two adjacent measurement locations and D is the distance between C1 and C2 (30). An interarm volume difference of ≥10% is a widely accepted diagnostic criterion for breast cancer-related lymphedema, while 5% difference in interarm volume causes symptoms and impairments in ADLs (12, 13). Therefore, this study used the interarm volume difference of ≥5% as the threshold for minimal arm volume differences.
Sample size
Sample size calculation was based on the results (δ= 4.1, σ = 6.1) of our previous study exploring the effect of TOLF exercise on the number of lymphedema symptoms (16). The formula for comparing the difference between two means (two-sample t-test) was used: and resulted in that a minimum sample size of 36 patients per group were needed with an α of 0.05 and a β of 0.8. Thus, we aimed to recruit a final sample of no less than 90 participants considering a 20% potential dropout rate.
Statistical analysis
SPSS version 21.0 (Statistical Package for the Social Sciences; IBM Corp., Armonk, NY) was used for statistical analysis. Continuous variables were summarized using means with standard deviations (SDs) or medians with inter-quartile ranges (IQRs), depending on the variable distribution. Categorical variables were summarized using frequencies with proportions. Baseline characteristics of the intervention and control groups were compared using independent t test or Mann-Whitney U test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. For the study outcomes of symptom number, symptom severity, symptom distress, and ADLs, Mann-Whitney U test was performed to test the if there were any differences between the two groups. For comparing the proportion of patients presenting arm volume differences ≥5%, Fisher’s exact test was employed. In addition, generalized linear mixed-effects models were conducted to test between-group differences in the change of symptom experience, ADLs, and arm volume differences over the study period. Separate models were estimated for each outcome while adjusting for baseline characteristics that were notably different between groups and incorporated fixed effects for time (baseline vs. 3-month), treatment group (intervention vs. control), and a time by group interaction term, as well as random effects for individuals to account for the repeated measures. The level of statistical significance was set at 0.05, and all statistical tests were 2-sided.
Results
Study participants
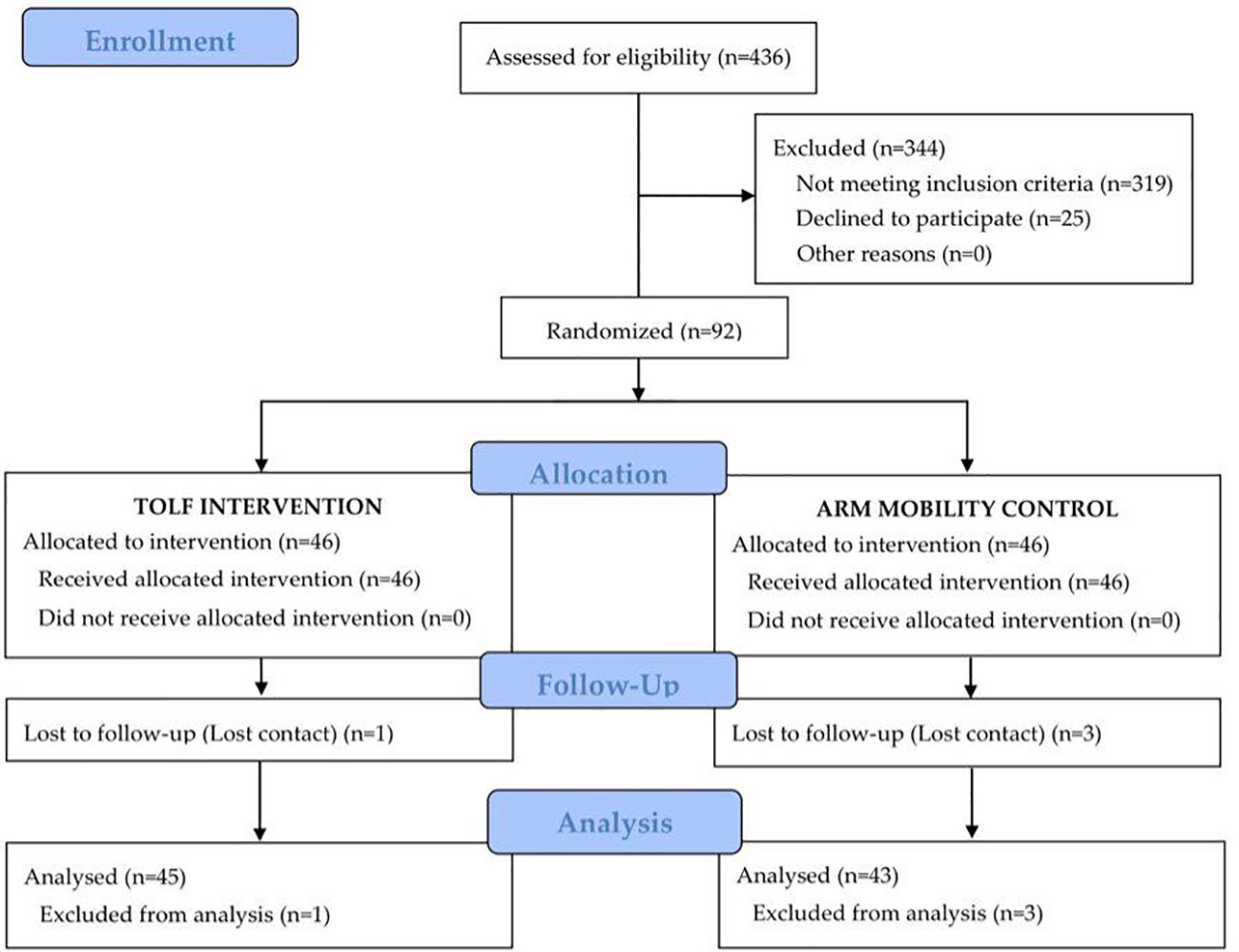
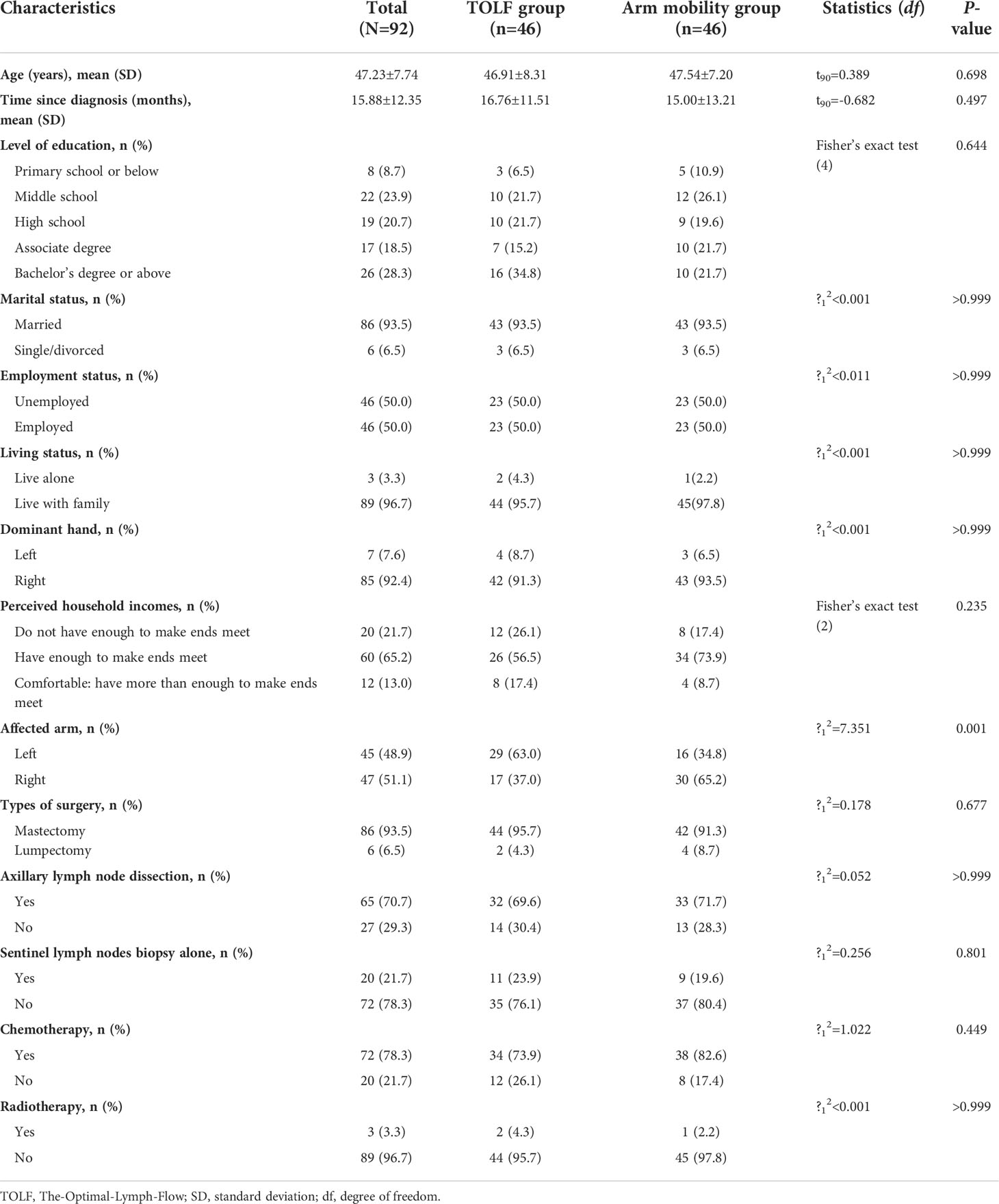
During the recruitment period, 436 patients were assessed for eligibility, 92 met the eligibility criteria and were randomly assigned at a 1:1 ratio to either the TOLF intervention group or the arm mobility control group. All participants learned to use the mHealth system within 45 minutes and were able to perform lymphatic exercises or arm mobility exercises using the avatar videos based on the assigned treatment groups. At the end of the 3-month intervention, one patient in the intervention group and 3 patients in the control group were lost to follow-up. The participant flowchart is presented in Figure 1. Baseline characteristics of all participants are shown in Table 2. There were no significant differences in the baseline characteristics between the two groups except for the affected arm (P=0.001).
Symptom experience and ADLs
At baseline, there were no significant differences in terms of the median scores of the number of symptoms (P=0.271), symptom severity (P=0.117), symptom distress (P=0.710), or ADLs (P=0.706) between the two groups (Table 3). At the endpoint of the 3-month intervention, the TOLF group had significantly lower median scores for the number of symptoms (MedTOLF=5.00, IQR=2.00-7.00 vs MedArm Mobility=10.00, IQR=6.00-14.00; P<0.001), symptom severity (MedTOLF=5.00, IQR=2.00-7.00 vs MedArm Mobility=12.00, IQR=6.00-17.00; P<0.001) and ADLs (MedTOLF=4.00, IQR=4.00-6.00 vs MedArm Mobility=6.00, IQR=4.00-9.00; P=0.001).
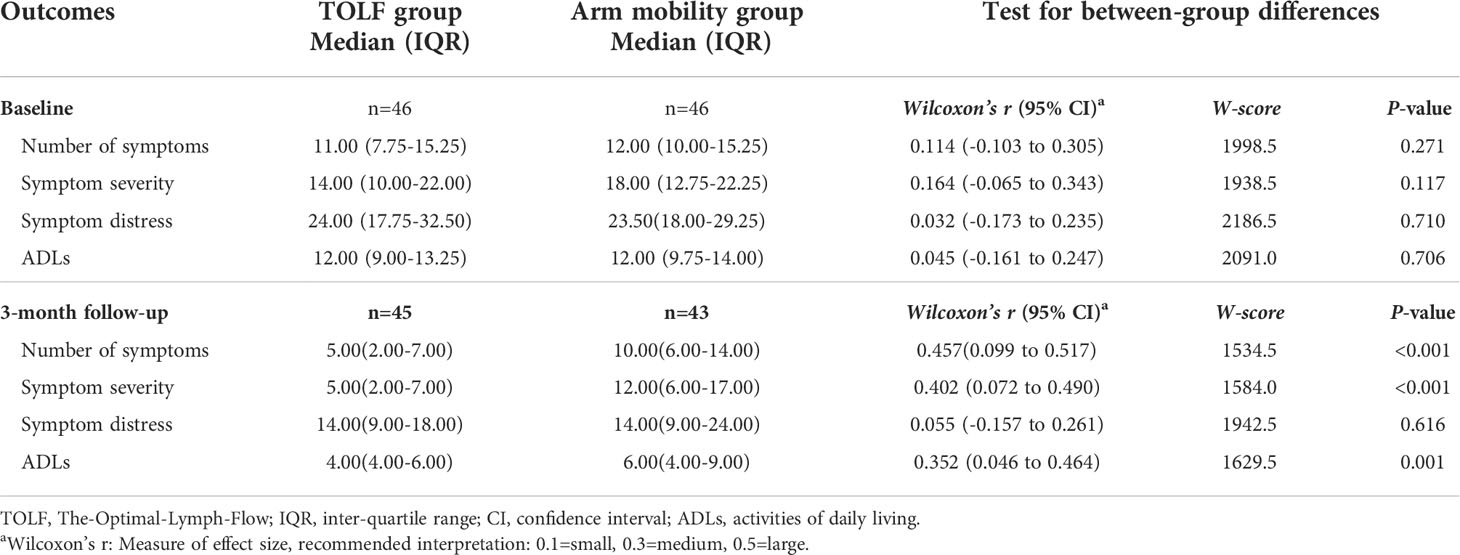
Table 3 Comparison of symptom experience and ADLs between the TOLF intervention group and arm mobility control group at baseline and study endpoint.
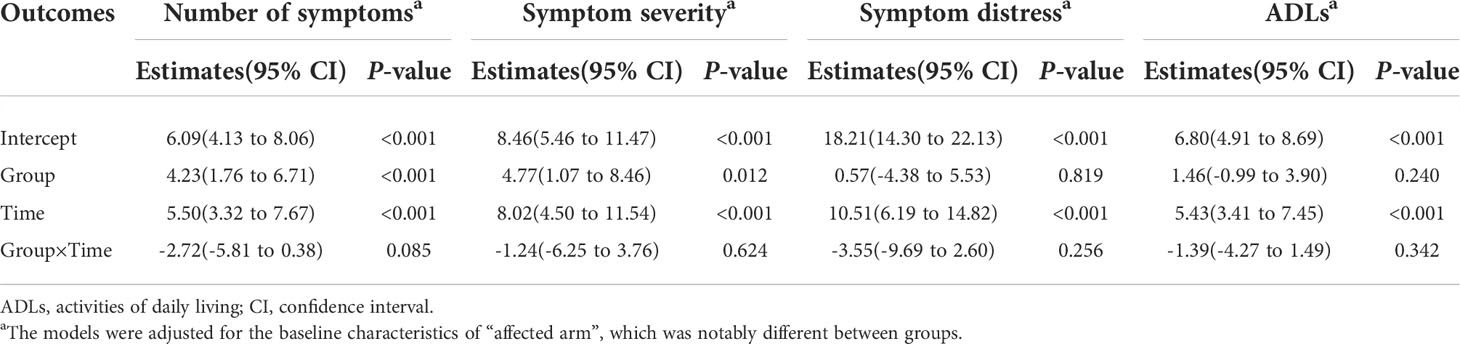
Generalized linear mixed-effects models were used to predict the symptom experience (number of symptoms, symptom severity, and symptom distress) and ADLs across the two measurement time points and to determine group differences in the changes over the study. As shown in Table 4, there was a significant improvement in symptom experience and ADLs for both groups from baseline to the endpoint. There was no significant time by group interaction effect across all study outcomes. In addition, significant between-group differences in the changes of number of symptoms (P<0.001) and symptom severity (P=0.012) throughout the course of the study were detected.
Arm volume differences
At baseline prior to the intervention, there was no significant difference between the two groups in terms of ≥5% arm volume differences (Table 5). At the study endpoint of 3 months, the TOLF group had significantly fewer participants with ≥5% arm volume differences (11.1% [5/45] vs 30.2% [13/43], P=0.035). There was a 12.8% reduction (from 23.9% [11/46] to 11.1% [5/45]) in the proportion of patients with ≥5% arm volume differences from baseline to post-intervention in the TOLF group, while there was a 1.9% increase (from 28.3% [13/46] to 30.2% [13/43]) in the proportion of patients with ≥5% arm volume differences in the arm mobility group. Moreover, the proportion of new cases with ≥5% arm volume differences in the arm mobility group was larger than that in the TOLF group (20.0% [6/30] vs 5.9% [2/34]; Table 5).
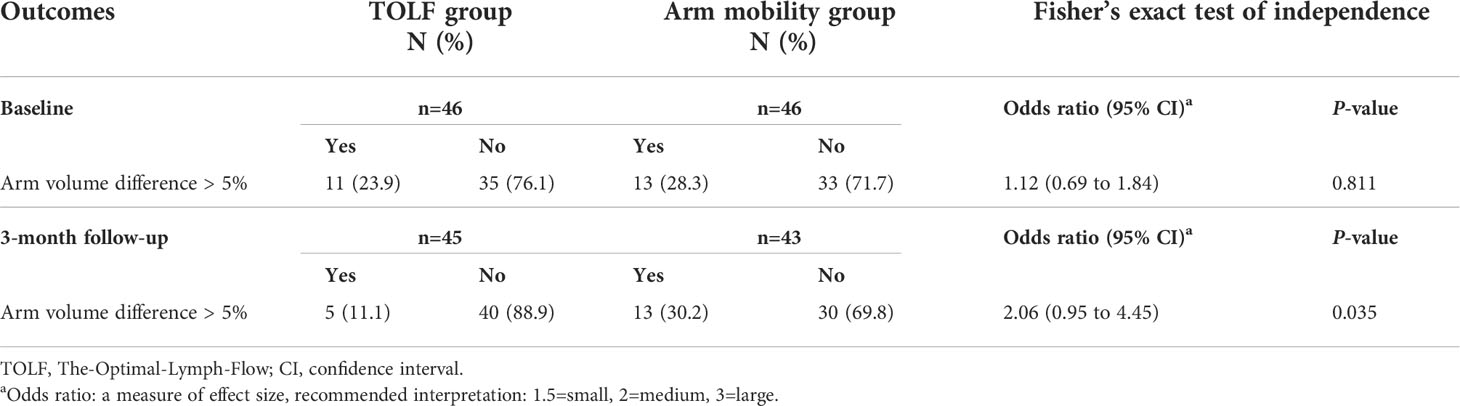
Table 5 Comparison of arm volume differences between the TOLF intervention group and arm mobility control group at baseline and study endpoint.
As shown in Table 6, there were significant differences between the two groups throughout the course of the study in changes of arm volume differences ≥5% (P=0.034). There was no significant time by group interaction effect (P=0.209, P=0.868).

Table 6 Results of the binomial generalized linear mixed-effects models with a logic link function for arm volume differences.
Discussion
A total of 88 at-risk breast cancer patients have completed the 3-month intervention using the TOLF mHealth system in the current study. Significant benefits of the early postoperative TOLF intervention for improving lymphedema symptom experience and optimizing arm volume status were identified. Prior studies have recognized the TOLF intervention as a safe, feasible, and efficacious replacement or complement therapy to manage lymphedema symptoms for breast cancer survivors (16, 18, 25). Extending previous research findings, the present RCT further confirmed the preventive effects of TOLF program in breast cancer survivors who are at risk of developing lymphedema in the early postoperative period (1-month post-surgery).
The study findings revealed that the number and the severity of lymphedema symptoms as well as the impact of these symptoms on patients’ ADLs have been significantly improved by the TOLF intervention in comparison with arm mobility exercise control. Meanwhile, participants in both groups showed improvements in symptom experience and ADLs over the 3 months, whereas those received the TOLF intervention gained greater benefits in reducing the number and severity of lymphedema symptoms. The results suggest that TOLF program should be a promising choice for lymphedema symptom management and prevention of subclinical lymphedema progression. Compared with arm mobility exercises, the TOLF intervention added a set of exercises that were designed to activate lymphatic system involving muscle-tightening deep breathing, muscle-tightening pumping, and large muscle exercises. The muscle-tightening deep breathing and muscle-tightening pumping exercises combine muscle tightening, stretching and pumping movements coordinated with synchronized deep breathing to mimic the physiological process of lymph propulsion, which could render a synergistic effect to stimulate lymph fluid removal in both the affected arm as well as the whole body (19). Large muscle exercises such as walking, jogging and bicycling induce not only musculoskeletal contractions, but also breathing alterations, arterial pulsations, skin tensions, and postural changes (31, 32). These physiological adaptions also help to facilitate lymphatic systems and promote lymph flow throughout the overall body (19, 25). As a result, the therapeutic lymphatic exercises could benefit patients in alleviating lymph fluid accumulation-related symptoms and preventing subclinical lymphedema. On the other side, the set of exercises gradually contribute to relieving the armpit and chest wall tightness caused by the adhesion of the pectoralis major and axillary tissue after surgically incised skin wounds sealed, promoting the formation of loose connective tissue and decreasing tethering of scar tissue. Thus, the exercises also help to reduce distressing symptoms that are related to the impaired arm mobility caused by scar adherence from operative incisions (19).
Findings of our study demonstrated that the TOLF intervention was effective in decreasing lymph fluid levels, indicated by the changes in arm volume differences over the study period. It is important to note that a 12.8% reduction in the proportion of patients with ≥5% arm volume differences from baseline to postintervention was observed in the TOLF intervention group, whereas a 1.9% increase in the arm mobility control group was detected in this study. Moreover, the proportion of new cases with ≥5% arm volume differences in the arm mobility group was higher than that in the TOLF group (20.0% vs 5.9%). Our findings are consistent with a previous study (19), which revealed that 97% of 134 breast cancer patients who received TOLF lymphatic exercises maintained or decreased their preoperative arm volumes at 12 months after surgery, and there was a 12% reduction in proportions of patients with ≥5% arm volume differences in the TOLF group (17). The findings suggest that the TOLF intervention should be more effective in reducing arm volume than the arm mobility control. Muscle-tightening deep breathing and muscle-tightening pumping performed by patients in the TOLF group could facilitate lymph fluid flow and drain via repeated arm muscle tightening, stretching, and pumping movements. Combined with large muscle exercises, the TOLF intervention helps to stimulate the whole-body lymphatic system function more efficiently and further enhance the muscle milking and pumping actions (16). Taken together, application of TOLF lymphatic exercises for breast cancer survivors at risk of developing lymphedema should be effective in preventing or reversing the subclinical stage of breast cancer-related lymphedema and avoiding the increase in arm circumference and volume.
To our knowledge, this is the first study that examined the preventive effects of the TOLF intervention among breast cancer survivors at risk of developing lymphedema in the early postoperative period. Managing lymphedema symptoms is critical to reduce the risk of lymphedema. Breast cancer survivors who report more lymphedema symptoms are more likely to eventually develop lymphedema (21). TOLF intervention focusing on self-management risk reduction strategies in activating lymphatic system to manage lymphedema symptoms, shows great promise for prevention of the progression of latent lymphedema and this intervention holds a number of strengths. First, the low-cost and technologically driven delivery model of the TOLF intervention make it relatively easy to implement and dissemination in clinical practice or at home. Second, the web- and mobile-based TOLF system offered clear instructions about how exercise should be done and how often it should be done to ensure that patients would like to initiate and adhere to the prescribed therapeutic exercise regimen. Third, the TOLF intervention consists of a series of relatively low-intensity exercises and requires low physical demands, as thus the intervention adds little burden on patients and can be appropriate for these vulnerable survivors who underwent surgical operations just one month ago. Finally, the underlying premise of TOLF program is to empower, rather than inhibit, how breast cancer survivors live their lives by emphasizing “what to do,” rather than “what to avoid.” Therefore, the TOLF intervention can be thought as a pragmatic, accessible, acceptable, and well-tolerated self-care strategy for the management and prevention of lymphedema and associated symptoms.
Although our sample size had adequate power for the trial, the comparatively small sample size and single-site trial are limitations of this study. Another limitation of our trial lies in the lack of real-time monitoring of exercise implementation and quality. Future research that includes a larger sample and takes advantage of wearable devices to support real-time monitoring is warranted to verify our study findings. A strength of the study is the comparison of the TOLF intervention versus arm mobility control, which provides evidence for optimal exercise decision-making in breast cancer survivors in the early postoperative period. Meanwhile, the use of technologically driven digital therapy not only enhanced the fidelity, transparency, and reproducibility of the intervention but also improved the patients’ ability to learn and perform the assigned exercise therapy given.
In conclusion, the results of this RCT showed significant benefits of the early postoperative TOLF intervention for managing lymphedema symptoms, improving ADLs, and optimizing arm volume status among breast cancer survivors at risk of developing lymphedema. These findings suggest that the TOLF intervention should be considered as a non-pharmacological, educational, and behavioural strategy for lymphedema prevention and lymphedema symptom management in the early postoperative period.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Biomedical Ethics Committee of the West China Hospital, Sichuan University (Approval number: 2019/24). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: MRF, XF, and XD. Methodology: MRF, XF, YL and XD. Intervention: XD, YS and AZ. Investigation: XD, LF, HC, XZ, YS and AZ. Data curation: XD, YS and AZ. Writing—original draft preparation: XD and YL. Writing—review and editing: MRF, YL and XF. Supervision: XF and MF. Funding acquisition: XF.
Funding
This research was funded by Sichuan Province Science and Technology Development Funds (grant number 2019YFS0295).
Acknowledgments
We thank all the patients who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1015387/full#supplementary-material
References
1. World Health Organization. Available at: https://www.iarc.who.int/news-events/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/ (Accessed Dec 15, 2020).
2. Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. JNCI J Natl Cancer Inst (2001) 93(2):96. doi: 10.1093/jnci/93.2.96
3. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14(6):500–15. doi: 10.1016/s1470-2045(13)70076-7
4. Armer JM, Stewart BR, Shook RP. 30-month post-breast cancer treatment lymphoedema. J Lymphoedema (2009) 4(1):14–8. doi: 10.12968/bjon.2009.18.3.39045
5. Bell RJ, Robinson PJ, Ba Rallon R, Fradkin P, Davis SR. Lymphedema: experience of a cohort of women with breast cancer followed for 4 years after diagnosis in Victoria, Australia. Support Care Cancer (2013) 21(7):2017–24. doi: 10.1007/s00520-013-1763-1
6. Park JH, Merriman J, Brody A, Fletcher J, Yu G, Ko E, et al. Limb volume changes and activities of daily living: A prospective study. Lymphatic Res Biol (2021) 19(3):261–8. doi: 10.1089/lrb.2020.0077
7. Xu S, Liu Y, Zhang T, Zheng J, Lin W, Cai J, et al. The global, regional, and national burden and trends of breast cancer from 1990 to 2019: Results from the global burden of disease study 2019. Front Oncol (2021) 11:689562. doi: 10.3389/fonc.2021.689562
8. Armer JM, Ostby PL, Ginex PK, Beck M, Morgan RL. ONS guidelines for cancer treatment–related lymphedema. Oncol Nurs Forum (2020) 47(5):518–38. doi: 10.1188/20.ONF.518-538
9. Committee E. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology (2016) 49(4):170–84.
10. Avis NE. Quality of life among younger women with breast cancer. Breast (2005) 23(15):3322–30. doi: 10.1016/S0960-9776(19)30194-8
11. Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage (2009) 38(6):849–59. doi: 10.1016/j.jpainsymman.2009.04.030
12. Fu MR, Aouizerat BE, Yu G, Conley Y, Axelrod D, Guth AA, et al. Model-based patterns of lymphedema symptomatology: Phenotypic and biomarker characterization. Curr Breast Cancer Rep (2021) 13(1):1–18. doi: 10.1007/s12609-020-00397-6
13. Fu MR, Conley YP, Axelrod D, Guth AA, Yu G, Fletcher J, et al. Precision assessment of heterogeneity of lymphedema phenotype, genotypes and risk prediction. Breast (2016) 29:231–40. doi: 10.1016/j.breast.2016.06.023
14. Stanton A, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: Lymphatic pump failure and predisposing factors. Lymphatic Res Biol (2009) 7(1):29. doi: 10.1089/lrb.2008.1026
15. Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology (2009) 42(4):161–75. doi: 10.1016/j.jri.2009.07.005
16. Fu MR, McTernan ML, Qiu JM, Ko E, Yazicioglu S, Axelrod D, et al. The effects of kinect-enhanced lymphatic exercise intervention on lymphatic pain, swelling, and lymph fluid level. Integr Cancer Ther (2021) 20:15347354211026757. doi: 10.1177/15347354211026757
17. Fu MR, Axelrod D, Guth AA, Scagliola J, Rampertaap K, El-Shammaa N, et al. A web- and mobile-based intervention for women treated for breast cancer to manage chronic pain and symptoms related to lymphedema: Results of a randomized clinical trial. JMIR Cancer (2022) 8(1):e29485. doi: 10.2196/29485
18. Liu F, Li F, Fu MR, Zhao Q, Wang Y, Pang D, et al. Self-management strategies for risk reduction of subclinical and mild stage of breast cancer-related lymphedema: A longitudinal, quasi-experimental study. Cancer Nurs (2021) 44(6):E493–502. doi: 10.1097/NCC.0000000000000919
19. Fu MR, Axelrod D, Guth AA, Cartwright F, Qiu Z, Goldberg JD, et al. Proactive approach to lymphedema risk reduction: A prospective study. Ann Surg Oncol (2014) 21(11):3481–9. doi: 10.1245/s10434-014-3761-z
20. Fu MR, Axelrod D, Guth A, McTernan ML, Qiu JM, Zhou Z, et al. The effects of obesity on lymphatic pain and swelling in breast cancer patients. Biomedicines (2021) 9(7):818. doi: 10.3390/biomedicines9070818
21. Fu MR, Axelrod D, Cleland CM, Qiu Z, Haber J. Symptom report in detecting breast cancer-related lymphedema. Breast Cancer: Targets Ther (2015) 20157(default):345–52. doi: 10.2147/BCTT.S87854
22. Li BR, Shou Y, Zhang BM, Liu P, Yuan L, Xu SW, et al. Clinical progress on moxibustion in preventing and treating adverse effects of surgery or chemoradiotherapy for breast cancer. J Acupunct Tuina Sci (2018) 16(2):120–6. doi: 10.1007/s11726-018-1035-1
23. Ezzo J, Manheimer E, Mcneely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrance Db Syst Rev (2015) 5:CD003475. doi: 10.1002/14651858.CD003475.pub2
24. Fu MR, Axelrod D, Guth AA, Rampertaap K, El-Shammaa N, Hiotis K, et al. mHealth self-care interventions: managing symptoms following breast cancer treatment. Mhealth (2016) 2:28. doi: 10.21037/mhealth.2016.07.03
25. Fu MR, Axelrod D, Guth AA, Wang Y, Scagliola J, Hiotis K, et al. Usability and feasibility of health IT interventions to enhance self-care for lymphedema symptom management in breast cancer survivors. Internet Interv (2016) 5:56–64. doi: 10.1016/j.invent.2016.08.001
26. Eysenbach G, The C-EGMD, Ritterband L, Christensen H, Bewick B. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res (2011) 13(4):e126. doi: 10.2196/jmir.1923
27. Denlinger CS, Sanft T, Baker KS, Broderick G, Demark-Wahnefried W, Friedman DL, et al. Survivorship, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw (2018) 16(10):1216–47. doi: 10.6004/jnccn.2018.0078
28. Fu MR, Chen CM, Haber J, Guth AA, Axelrod D. The effect of providing information about lymphedema on the cognitive and symptom outcomes of breast cancer survivors. Ann Surg Oncol (2010) 17(7):1847. doi: 10.1245/s10434-010-0941-3
29. Shi S, Lu Q, Fu MR, Ouyang Q, Liu C, Lv J, et al. Psychometric properties of the breast cancer and lymphedema symptom experience index: The Chinese version. Eur J Oncol Nurs (2016) 20:10–6. doi: 10.1016/j.ejon.2015.05.002
30. Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, Prieto Merino D, Mayoral dO, Cerezo Tellez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (2010) 340:140. doi: 10.1136/bmj.b5396
31. Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol (2015) 6(1):1–32. doi: 10.1002/cphy.c140080
Keywords: breast cancer survivors, lymphedema, lymphatic system, early intervention, lymphatic exercises, mobile health, randomized controlled trial
Citation: Du X, Li Y, Fu L, Chen H, Zhang X, Shui Y, Zhang A, Feng X and Fu MR (2022) Strategies in activating lymphatic system to promote lymph flow on lymphedema symptoms in breast cancer survivors: A randomized controlled trial. Front. Oncol. 12:1015387. doi: 10.3389/fonc.2022.1015387
Received: 09 August 2022; Accepted: 05 October 2022;
Published: 24 October 2022.
Edited by:
Simona Maria Fragomeni, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Luigi Losco, University of Salerno, ItalyDesiree Van Den Bongard, Amsterdam University Medical Center, Netherlands
Copyright © 2022 Du, Li, Fu, Chen, Zhang, Shui, Zhang, Feng and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianqiong Feng, ZmVuZ3hpYW5xaW9uZzY2QDEyNi5jb20=; Mei Rosemary Fu, bWVpLnIuZnVAcnV0Z2Vycy5lZHU=; bWY2N0BueXUuZWR1
†These authors have contributed equally to this work and share first authorship
 Xinwen Du
Xinwen Du Yuan Li
Yuan Li Lan Fu3
Lan Fu3 Mei Rosemary Fu
Mei Rosemary Fu