- 1Department of Oncology, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 2Department of Oncology, The People’s Hospital of Puer City, Puer, China
- 3Department of Gastroenterology, The 920th Hospital of the Joint Logistics Support Force, PLA, Kunming, China
- 4Department of Prevention and Health Care, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 5The Medical Department, 3D Medicines Inc., Shanghai, China
Gastric cancer is one of the most common cancers, while the current treatment options for gastric cancer are relatively scarce due to insufficient understanding of molecular characteristics and subtypes of gastric cancer. Different gene rearrangements of anaplastic lymphocyte kinase (ALK) have been reported in several types of cancer, especially in NSCLC. The first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib, second-generation (ceritinib, alectinib, and brigatinib) and third-generation (lorlatinib) ALK-TKIs have been widely used for NSCLC patients with ALK rearrangement. However, little was reported about ALK mutation in gastric cancer (GC). Here we identified a novel form of ALK fusion, a case of GC with RAB10-ALK fusion, and this is the first report of ALK fusion in gastric cancer.
Introduction
In recent years, ALK tyrosine kinase inhibitor (TKI) therapy has received great attention in solid tumors such as non-small cell lung cancer. However, few was reported about gene rearrangement of ALK in GC. Here we firstly present a case of GC with RAB10-ALK fusion, which is the first report of ALK fusion in GC.
Case Presentation
A 66-year-old male patient who is a minority (Lisu nationality) in Yunnan, China, was admitted to the hospital due to abdominal pain. The patient has no history of smoking and drinks alcohol occasionally. He has a history of hemorrhoids for many years without special treatment, has no history of infectious diseases such as hepatitis, tuberculosis, malaria, has no history of hypertension, and has no history of surgery. Both his parents are dead; the cause of death is unknown. He has no family history of infectious diseases and genetic diseases. The patient has symptoms of dry mouth, bitter mouth, no nausea, no acid reflux, no melena, mucus pus and blood in the stool, no fever. The mental state is fair, the physical strength is decreased, the appetite is poor, and the sleep is poor. There was no swelling and tenderness of superficial lymph nodes throughout the body.
Examination after admission showed a significant increase in serum biomarkers, including carcinoembryonic antigen, carbohydrate antigen, carbohydrate antigen CA199, carbohydrate antigen CA125, Cytokeratin-19-fragment CYFRA21-1. Gastroscopy revealed unevenness, ulcers, congestion, and edema at the lesser curvature and antrum of stomach (Figure 1A). Magnetic resonance examination displayed a large amount of fluid in the abdominal cavity and multiple lymph nodes adjacent to the abdominal aorta and enlarged (Figure 1B). Pathological examination revealed poorly differentiated adenocarcinoma of the stomach (signet ring cell carcinoma) (Figure 1C). In addition, the needle biopsy specimen was subjected to next generation sequencing (NGS) analysis in a laboratory accredited by the College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendment (CLIA) (3D Medicines Inc., Shanghai, China), covering whole exon regions of 733 cancer related genes; as a result, fusion of RAB10-ALK with frequency of 15.23% and ALK amplification with frequency of 16% were detected in the stomach tissue of the patient (Figures 2A, B). In addition, several other genetic variants were also found, including KDM6A, TP53 mutation, and increased copy number of gene FGF19, BTK, IRS2, FGF3, EMSY(C11orf30), FGF4. Furthermore, immunocytochemistry (IHC) and fluorescence in situ hybridization (FISH) were also performed to verify the above mutation (Figures 3A–E). Immunohistochemical staining (Ventana Medical Systems, Tucson, AZ) showed an increased signal of ALK expression (Figure 3C), and ALK amplification was also verified by FISH (Figure 3E). However, the results of FISH excluded the common ALK fusion form EML4-ALK fusion (Figures 3D, E).
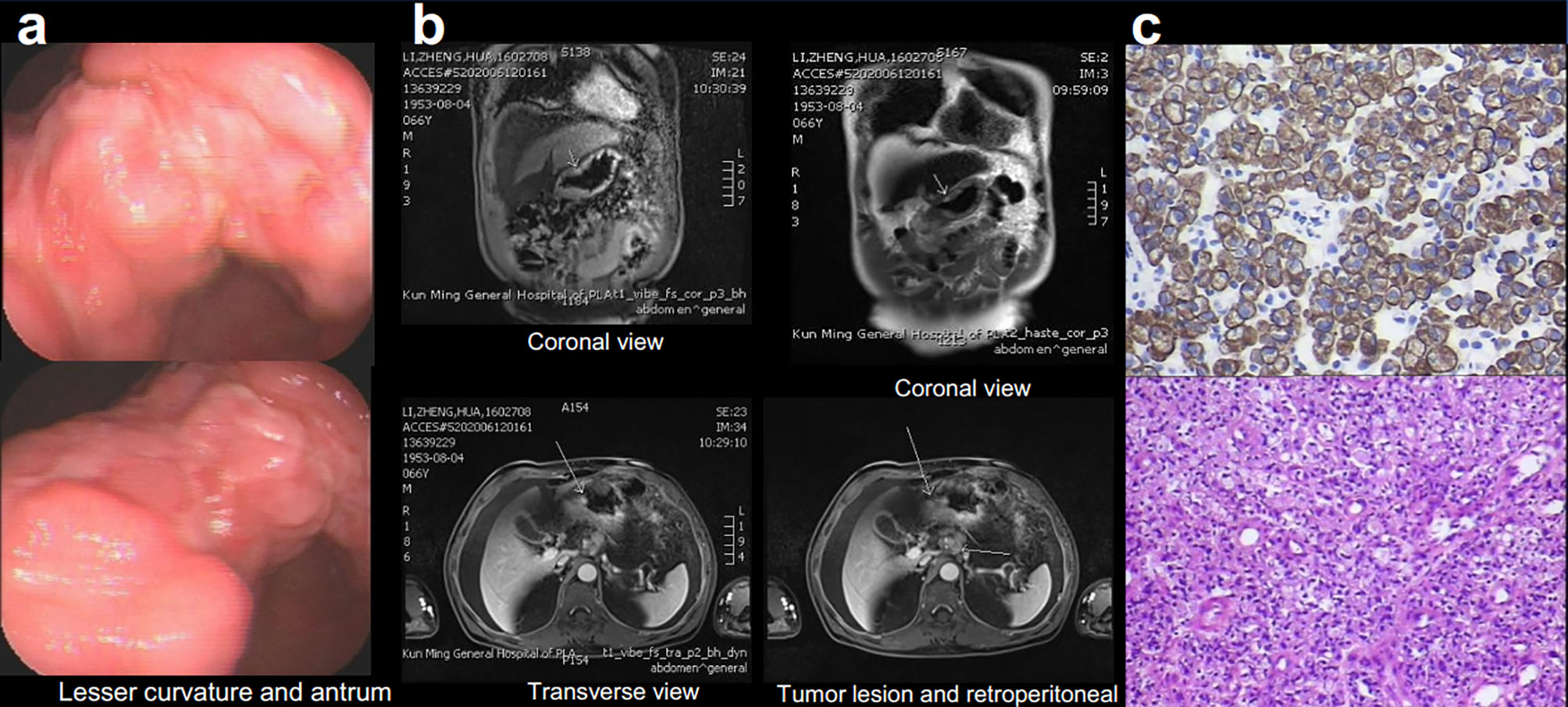
Figure 1 Gastroscopy (A), Magnetic Resonance Imaging (MRI) (B) displayed the tumor lesion, pathological diagnosis (C), Immunohistochemical (IHC) staining for cytokeratin (CK) 6 and hematoxylin–eosin staining (HE).
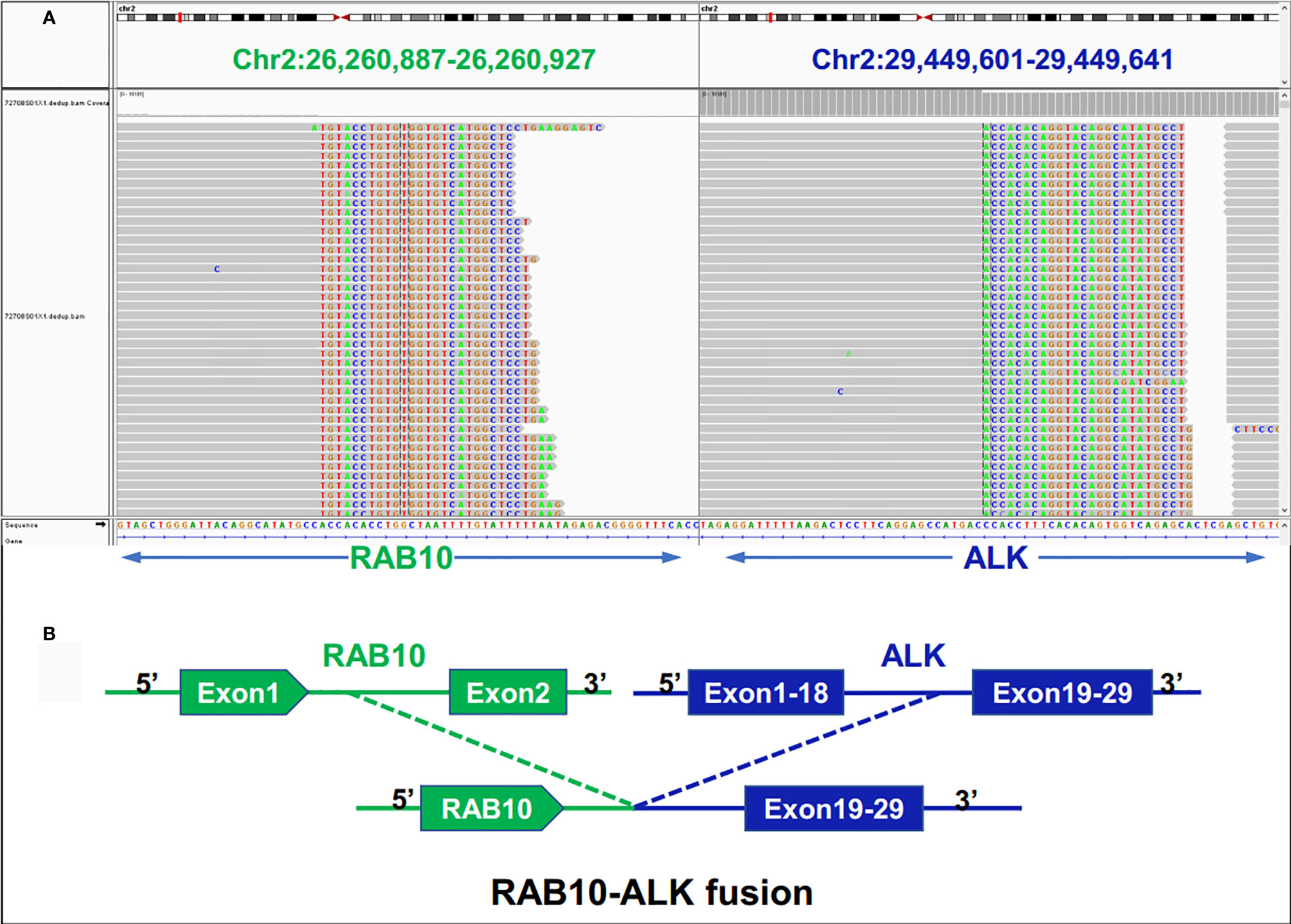
Figure 2 Next-generation sequencing findings for the tumor tissue samples of the patient with gastric cancer. (A) RAB10-ALK fusion was validated manually using the Integrated Genomics Viewer (IGV); (B) a novel intergenic region between RAB10 and ALK exon 20 fusion variant was identified.
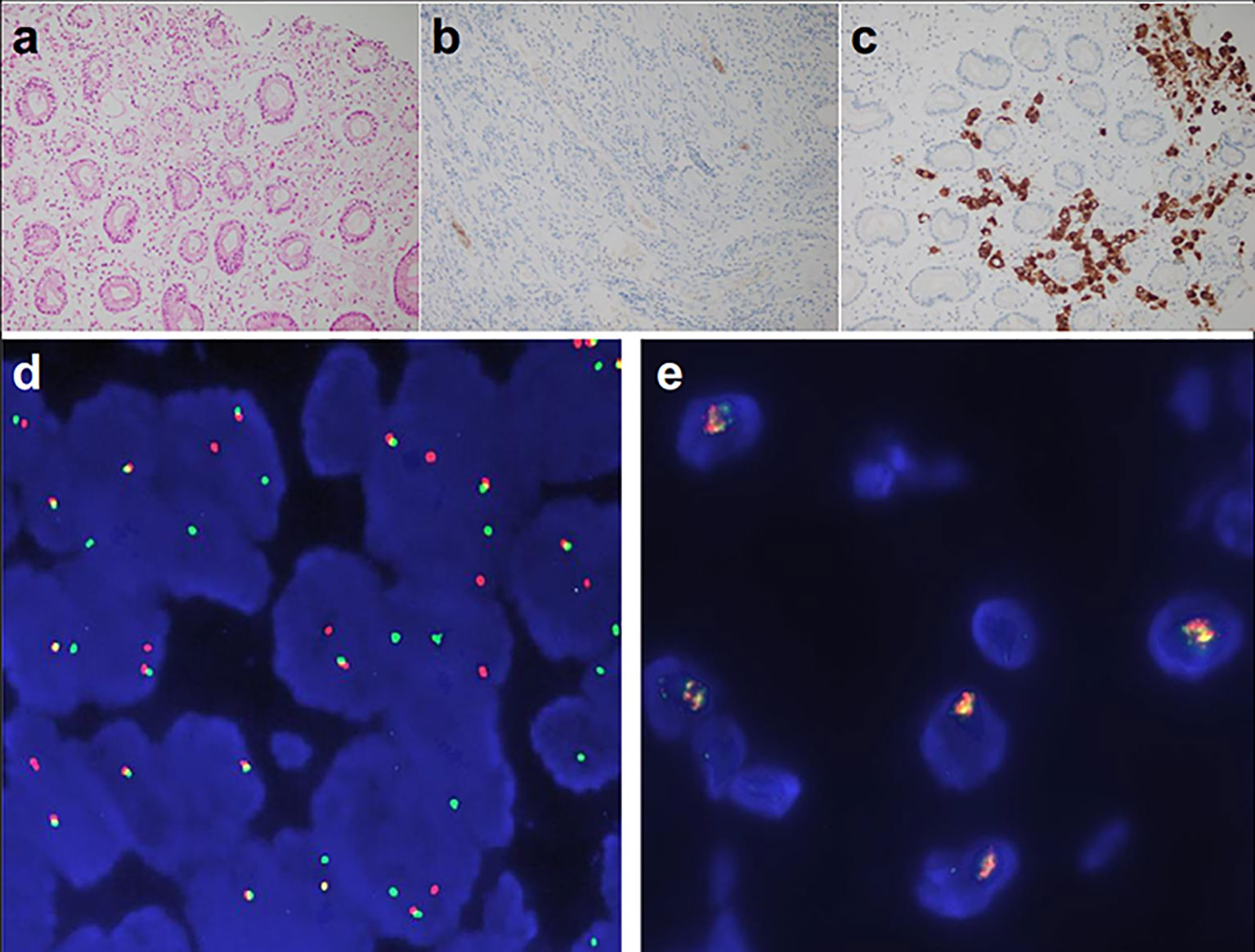
Figure 3 ALK detected by Ventana-D5F3 IHC assay and FISH. (A) HE staining (200×); (B) ALK negative control 200×; (C) ALK IHC staining of the patient (200×), (D) EML4-ALK fusion positive control; (E) ALK detection of the patients, amplification of ALK was displayed (16%).
Discussion
ALK is one of the membrane-bound receptor tyrosine kinases, which consist of an extra-cellular ligand binding domain, a single transmembrane domain, and a cytoplasmic tyrosine kinase domain.
In ALK fusions such as EML4-ALK, the amino-terminal fusion partner is fused to the intracellular tyrosine kinase domain of ALK, resulting in activation of downstream signaling. ALK signals activate numerous downstream pathways, including PI3K–Akt activation, MEKK2/3/MEK5/ERK5 pathway, RAS-MAPK, CRKL-C3G-RAP1, JAK-STAT and JUN pathway (1). In fact, almost all the ALK fusion proteins usually retain the cytoplasmic tyrosine kinase domain of ALK at the C-terminus, while the N-terminus is composed of a different protein. Several clinical trials have now confirmed that patients with ALK positive NSCLC can benefit from treatment with ALK-TKIs such as crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib (2). Recent retrospective clinical researches in ALK positive NSCLC suggest different clinical outcomes based on the specific ALK fusion protein. That is, different ALK fusion form can mediate different downstream signaling and may exhibit different sensitivity to ALK tyrosine kinase inhibitors (TKIs) (2). The NGS method has expanded the different fusion partners identified in ALK positive NSCLC to 90, including Potential Fusion Partners RAB10 (3). However, except for EML4-ALK, which is the most prevalent ALK gene rearrangement in the ALK-positive NSCLC patients, there are few in-depth studies on most of other ALK fusions. The currently known evidence about ALK fusion proteins mainly originates from some of clinical cohorts of patients with ALK-positive NSCLC. Here, we firstly report the RAB10-ALK fusion and ALK amplification that are identified in patients with gastric cancer. This is the first report of an ALK fusion case in gastric cancer, and this is a novel type of ALK fusion. Regrettably, the patient refused to use the drug ALK-TKI of cross-indications, such as crizotinib, ceritinib etc.
Different ALK inhibitors have been used to the majority of ALK-positive patients, and all have shown a certain effect in controlling disease progression, especially in NSCLC (4). Well use of different ALK-TKIs will benefit the patient more. As reported, according to the different ALK mutation sites detected by NGS, a female patient with metastatic ALK rearrangement NSCLC received treatment of different ALK-TKIs, and finally the patient survived more than 48 months (5). However, rare cases of ALK mutations have been observed among GC patients, and there is no applications of ALK-TKIs in the GC treatment at present. For the efficacy of ALK-TKIs in NSCLC, crizotinib was also recommended to the patient reported in this case, but he refused because of his own reasons.
GC is one of the most common cancers worldwide. Although the incidence of GC has been steadily declining in the past few decades, due to the current lack of understanding of GC molecular characteristics and subtypes, the current treatment options for gastric cancer patients are relatively monotonous. Previous studies on the gene fusion mutations in gastric or signet ring adenocarcinoma are few, which may be related to the usual use of methods other than NGS in the past. For example, in 42 cases of signet ring cell carcinoma of the gastrointestinal tract, two different ALK antibody based IHC did not detect the “possibly positive” cases with ALK translocation detected by FISH (6). With the application of NGS technology and other genomic technologies, GC is currently being studied and typed in more detail at the molecular level. Gene fusions were also found in gastric cancer through NGS technology. Some researchers conducted whole-genome sequencing on 32 pairs of gastric signet ring cell carcinoma samples and found frequent CLDN18-ARHGAP26/6 fusion protein (25%), which was associated with a poor prognosis (7). Accordingly, based on the wider application of NGS detection technology, GC can be diagnosed and treated more accurately in the future. ALK rearrangements have been reported in several types of cancer, such as NSCLC, breast cancer, renal cell carcinoma (RCC), diffuse large B-cell lymphoma (DLBCL), serous ovarian carcinoma (SOC), inflammatory myofibroblastic tumor (IMT), renal medulla carcinoma (RMC), colon cancer, and to a lesser extent, esophageal squamous cell carcinoma (ESCC) (8); however, data on ALK rearrangement in GC is rare now.
About 90 different ALK fusion partners have been already identified, such as EML4, TPM-3/-4, TFG, CLTC, PRKAR1A, LMNA, KIF5B, RANBP2, FN1 (3). Although these partner genes have been described for ALK, little data is currently addressed on how different fusion partners affect the efficacy of ALK-TKIs. Therefore, the therapy of ALK positive cancers is currently determined regardless of which fusion partner is present. However, with the development of NGS technology and the advancement of precision medicine, more fusion partners will be identified and clinical evidence will be accumulated; based on these, different clinical strategies will be applied to patients with different ALK fusions. RAB10 is a novel ALK fusion partner that has been associated with cancer. RAB10 is a key regulator of endocytic recycling, belongs to the RAS oncogene superfamily, and it is reported to be an oncogene in HCC and is associated with poor prognosis (9). ALK fusion usually leads to abnormal activation of the ALK kinase domain and induces the activation of downstream signal transduction, leading to the growth of tumor cells. Since RAB10-ALK is a novel fusion mode, whether the domains reserved in RAB10 may contribute to the activation of RAB10-ALK remains unknown. Therefore, it is necessary to further research and verify the function of RAB10-ALK and the response to TKI.
Conclusion
This case provides a new reference for understanding ALK fusion mutations, discovers new molecular characteristics of GC patients, and provides the possibility for the future application of ALK-TKIs in GC patients. NGS can be performed as a routine test to explore more opportunities for treating GC patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HZ and ZW put forward the content of the paper. HZ wrote the manuscript. DX, BL, JL, RL and SZ reviewed literature and clinical data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
HZ is employed by the company 3D Medicines Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patient and his family for consenting to report this case information.
Abbreviations
ALK, anaplastic lymphocyte kinase; CAP, College of American Pathologists; CLIA, Clinical Laboratory Improvement Amendment; FISH, fluorescence in situ hybridization; GC, gastric cancer; IHC, immunocytochemistry; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
References
1. Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol (2016) 27 Suppl 3:iii4–iii15. doi: 10.1093/annonc/mdw301
2. Childress MA, Himmelberg SM, Chen H, Deng W, Lovly CM. ALK Fusion Partners Impact Response to ALK Inhibition: Differential Effects on Sensitivity, Cellular Phenotypes, and Biochemical Properties. Mol Cancer Res (2018) 16(11):1724–36. doi: 10.1158/1541-7786.MCR-18-0171
3. Ou S-H II, Zhu VW, Nagasaka M. Catalog of 5’ Fusion Partners in ALK-positive NSCLC Circa 2020. Jto Clin Res Rep (2020) 1(1):1–10. doi: 10.1016/j.jtocrr.2020.100015
4. Thai AA, Solomon BJ. Treatment of ALK-positive nonsmall cell lung cancer: recent advances. Curr Opin Oncol (2018) 30(2):84–91. doi: 10.1097/CCO.0000000000000431
5. Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med (2016) 374(1):54–61. doi: 10.1056/NEJMoa1508887
6. Alese OB, El-Rayes BF, Sica G, Zhang G, Alexis D, La Rosa FG, et al. Anaplastic lymphoma kinase (ALK) gene alteration in signet ring cell carcinoma of the gastrointestinal tract. Ther Adv Med Oncol (2015) 7(2):56–62. doi: 10.1177/1758834014567117
7. Shu Y, Zhang W, Hou Q, Zhao L, Zhang S, Zhou J, et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat Commun (2018) 9(1):2447. doi: 10.1038/s41467-018-04907-0
8. Ducray SP, Natarajan K, Garland GD, Turner SD, Egger G. The Transcriptional Roles of ALK Fusion Proteins in Tumorigenesis. Cancers (Basel) (2019) 11(8):1074. doi: 10.3390/cancers11081074
Keywords: anaplastic lymphocyte kinase fusion, anaplastic lymphocyte kinase-tyrosine kinase inhibitor, gastric cancer, next generation sequencing, cancer
Citation: Wen Z, Xiong D, Zhang S, Liu J, Li B, Li R and Zhang H (2021) Case Report: RAB10-ALK: A Novel ALK Fusion in a Patient With Gastric Cancer. Front. Oncol. 11:645370. doi: 10.3389/fonc.2021.645370
Received: 23 December 2020; Accepted: 05 January 2021;
Published: 22 February 2021.
Edited by:
Ruowen Zhang, Stony Brook University, United StatesReviewed by:
Rafael Rosell, Catalan Institute of Oncology, SpainSiraj M. Ali, EQRx, Inc., United States
Copyright © 2021 Wen, Xiong, Zhang, Liu, Li, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hushan Zhang, MTUxMTEwMTAwNDFAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work
 Zhengqi Wen1†
Zhengqi Wen1† Hushan Zhang
Hushan Zhang