- 1Department of Laboratory, Jinjiang Municipal Hospital, Quanzhou, China
- 2Department of Neurology, Jinjiang Municipal Hospital, Quanzhou, China
Background: Vitamin D is known to have a potential impact on cognitive function and mental health. This study aims to assess the association between dietary vitamin D intake and cognitive performance, as well as depression, in an elderly U.S. population.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 were analyzed. A total of 1,344 elderly participants were categorized into three tertiles based on their dietary vitamin D intake (D2 + D3). Cognitive function was measured using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test, Digit Symbol Substitution Test (DSST), and Animal Fluency Test, while depression was assessed through the Patient Health Questionnaire-9 (PHQ-9). Adjustments were made for confounding variables, including age, sex, race, education, physical activity level, and other dietary factors.
Results: After adjustment for confounders, individuals in the 3rd tertile of vitamin D intake (≥4.9 mcg/day) had significantly reduced odds of low performance on the CERAD test (OR: 0.77, 95% CI: 0.57–0.98; p = 0.031) and Animal Fluency test (OR: 0.63, 95% CI: 0.49–0.85; p = 0.013) compared to the 1st tertile of intake (≤2.4 mcg/day). Similarly, participants in the 3rd tertile of vitamin D intake had lower odds of depression (PHQ-9 score > 4) after adjustment (OR: 0.68, 95% CI: 0.48–0.99; p = 0.046).
Conclusion: Our findings suggest that dietary vitamin D intake is associated with improved cognitive function and depressive symptoms in elderly individuals. However, further longitudinal studies are needed to establish causality and explore the underlying mechanisms.
Introduction
The global prevalence of dementia is rapidly increasing, with an estimated 55 million people affected in 2020, a number projected to triple by 2050 due to population aging (1). Similarly, depression, which affects approximately 10–20% of older adults globally, is expected to rise as life expectancy increases and mental health challenges become more prevalent among the elderly (2). These alarming trends emphasize the urgent need for identifying modifiable risk factors and developing cost-effective interventions such as dietary interventions to diminish the burden of these debilitating conditions.
Vitamin D, a fat-soluble vitamin primarily obtained from limited dietary sources and sunlight exposure, plays a pivotal role in numerous physiological processes, including calcium homeostasis, bone health, and immune modulation (3). Beyond its traditional functions, emerging evidence has highlighted its potential role in cognitive and mental health (4). Decline of cognitive function and depression are significant public health concerns, particularly among older adults (5), who are most vulnerable to deficiencies in vitamin D due to reduced skin synthesis, dietary insufficiencies, and lifestyle changes associated with aging (6).
The brain expresses vitamin D receptors (VDR) and enzymes involved in its metabolism (7), suggesting its active role in neural function. Vitamin D has been implicated in neuroprotection, modulation of neuroinflammation, regulation of neurotrophic factors, and maintenance of neurotransmitter homeostasis (8, 9). While these associations are biologically plausible, findings from observational and interventional studies remain inconsistent, warranting further exploration (10–13). Moreover, cognitive function and depression share a bidirectional relationship, where impairment in one domain can exacerbate issues in the other, creating a complex and intertwined dynamic. Cognitive decline may lead to feelings of hopelessness and depression due to a loss of independence and functionality, while depression can adversely affect cognitive performance through mechanisms such as increased inflammation, increased cortisol levels, altered neurotransmitter activity, and impaired neurogenesis (14, 15). A cohort of 8,268 participants found that higher depressive symptoms were associated with poorer memory and verbal fluency. Furthermore, a linear decline in memory was linked to an acceleration of depressive symptoms over time (16). Other studies have supported these findings (17–20). Despite the recognition of this intricate interplay, the precise pathways connecting these conditions remain incompletely understood.
A growing body of research has explored the roles of vitamin D in cognitive function and depression among older adults. A systematic review and meta-analysis of observational studies found that lower vitamin D levels were associated with a higher risk of depression in adults (21). However, a study in Netherland found no significant association between vitamin D levels and the course of depression or remission rates (22). A cross-sectional analysis on American elderly found that vitamin D intake was positively associated with cognitive performance (23). However, a systematic review concluded that vitamin D supplementation did not provide clear cognitive benefits, with findings varying across different studies (24). Despite numerous studies examining the effects of vitamin D on depression and cognitive function separately, comprehensive analyses focusing on its association with both outcomes simultaneously in older adults remain limited. Performing a study that address both outcomes could strengthen the understanding of dual role of vitamin D in mental health among older adults.
This study aims to address this gap by examining the relationship between dietary vitamin D intake and cognitive and mental function among elderly Americans using the National Health and Nutrition Examination Survey (NHANES) 2013–2014 data. Cognitive function is assessed using standardized tests, including the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test, the Animal Fluency test, and the Digit Symbol Substitution Test (DSST), while mental health outcomes are evaluated through the Patient Health Questionnaire-9 (PHQ-9). By shedding light on whether vitamin D influences cognitive or mental health or both, this study aims to unravel this intricate association and clarify role of vitamin D in these domains. The findings from this research could inform public health policies, dietary recommendations, and future interventional studies aimed at promoting cognitive resilience and mental well-being in older adults.
Materials and methods
Study design and population
This cross-sectional study utilizes data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014, a nationally representative survey conducted by the Centers for Disease Control and Prevention (CDC).1 For this analysis, we included participants aged 60 years and older who completed dietary assessments, cognitive function tests, and mental health evaluations. Participants with missing data on key variables were excluded. A total of 1,344 survey participants who had sufficient information on vitamin D intake, cognitive function, and mental health were included in our analysis. The NHANES protocol was reviewed and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Written informed consent was obtained from all participants.
Dietary vitamin D intake assessment
Dietary vitamin D intake was assessed using two 24-h dietary recall interviews. The first recall was conducted in person, and the second was collected via telephone. Dietary intake data were processed using the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (FNDDS) to calculate total daily vitamin D intake. Supplement use was not included in this analysis due to varying composition, dosage, and bioavailability of supplements, making it difficult to standardize their effects. Details of NHANES dietary analyses are reported in the following link: https://wwwn.cdc.gov/nchs/nhanes/tutorials/dietaryanalyses.aspx.
Cognitive function assessment
Cognitive function was evaluated using three standardized tests: Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word Learning Test, Digit Symbol Substitution Test (DSST), and Animal Fluency Test. CERAD assesses immediate and delayed memory by asking participants to recall a list of 10 words over three trials and again after a delay (typically during which other tasks are completed). The total score of immediate recall is the sum of correctly recalled words across all three trials, ranging from 0 to 30. The delayed recall score ranges from 0 to 10 based on the number of words correctly recalled. The Animal Fluency Test measures verbal fluency and executive function by asking participants to name as many animals as possible within 60 s. The score is the total number of unique animals correctly named. DSST evaluates processing speed, attention, and working memory by requiring participants to match symbols to numbers according to a provided key within 120 s. The score is the total number of correct matches completed within the time limit, ranging from 0 to a maximum score (typically around 133, depending on the test version). In all tests, higher scores indicate better cognitive function (25). Scores from these tests were analyzed individually and as a composite measure of overall cognitive function. Based on earlier published research, we established the lowest tertile of the scores as the cut-off value (26, 27).
Mental health assessment
Mental health status was assessed using the Patient Health Questionnaire-9 (PHQ-9), a validated tool for screening depression severity. The PHQ-9 includes nine items that evaluate the frequency of depressive symptoms over the past 2 weeks, with scores ranging from 0 (no symptoms) to 27 (severe depression). Scores 0–4 show minimal or no depression and scores>4 are related to depressive disorder, ranging from mild depression to severe depression (28).
Covariates
Following assessing previous related researches, demographic, socioeconomic, and lifestyle factors were considered as covariates to account for potential confounding (23, 28). These variables included age, sex, race/ethnicity, educational level, marital status, income, body mass index, serum 25OHD2 + 25OHD3, energy intake, protein intake, carbohydrate intake and fat intake. The total metabolic equivalent (MET) score was determined by summing the MET values from five activity categories: vigorous recreational activities (MET = 8), vigorous occupational activities (MET = 8), moderate recreational activities (MET = 4), moderate occupational activities (MET = 4), and walking or bicycling for transportation (MET = 4). The MET score for physical activity was calculated as follows: Physical activity (MET score) = sum of [days per week of each activity × duration of each activity (minutes) × corresponding MET value]. The final MET scores were then grouped into four levels of activity: inactive (MET minutes/week <250), somewhat active (250 ≤ MET minutes/week <500), active (500 ≤ MET minutes/week <1,000), and very active (MET minutes/week ≥1,000) (29).
Statistical analysis
Data were analyzed using the NHANES 2013–2014 dataset, accounting for the complex sampling design by applying appropriate survey weights, strata, and primary sampling units to ensure nationally representative estimates. Continuous variables were described as means and standard deviations, while categorical variables were presented as frequencies and percentages. Differences across dietary vitamin D intake tertiles were assessed using one-way ANOVA for continuous variables and chi-square tests for categorical variables. Significant differences between tertiles were identified with post hoc LSD tests.
To examine the association between dietary vitamin D (D2 + D3) intake and cognitive performance, weighted logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for low cognitive performance on the CERAD, Animal Fluency, and DSST tests. Similarly, the relationship between dietary vitamin D intake and depressive symptoms (PHQ-9 items) was analyzed using weighted logistic regression. The crude model and adjusted models controlling for potential confounders (age, sex, race, educational level, physical activity level, marital status, income, BMI, serum 25OHD2 + 25OHD3 levels, and macronutrient intake) were fitted.
All analyses were conducted using SPSS software (V22; SPSS Inc., Chicago, IL), and p-values <0.05 were considered statistically significant. Statistically significant associations were highlighted in bold within the tables.
Results
Participant characteristics by tertiles of vitamin D intake
A total of 1,344 participants in the NHANES 2013–2014 study were categorized into tertiles based on their dietary vitamin D intake (D2 + D3). The distribution of gender, age, BMI, and dietary intake significantly varied across tertiles. The proportion of males increased with higher tertiles (40% in the 1st tertile to 56.1% in the 3rd tertile, p < 0.001). Participants in the highest tertile were older (70.28 ± 6.99 years) compared to those in the lowest tertile (68.87 ± 6.52 years, p = 0.006). BMI was significantly lower in the 3rd tertile compared to the 1st (28.56 ± 6 vs. 29.39 ± 6.35 kg/m2, p = 0.016). Energy, protein, carbohydrate, and fat intakes were markedly higher in the 3rd tertile compared to the 1st tertile (all p < 0.001). Serum vitamin D levels, however, showed no significant difference between tertiles (p = 0.11) (Table 1).
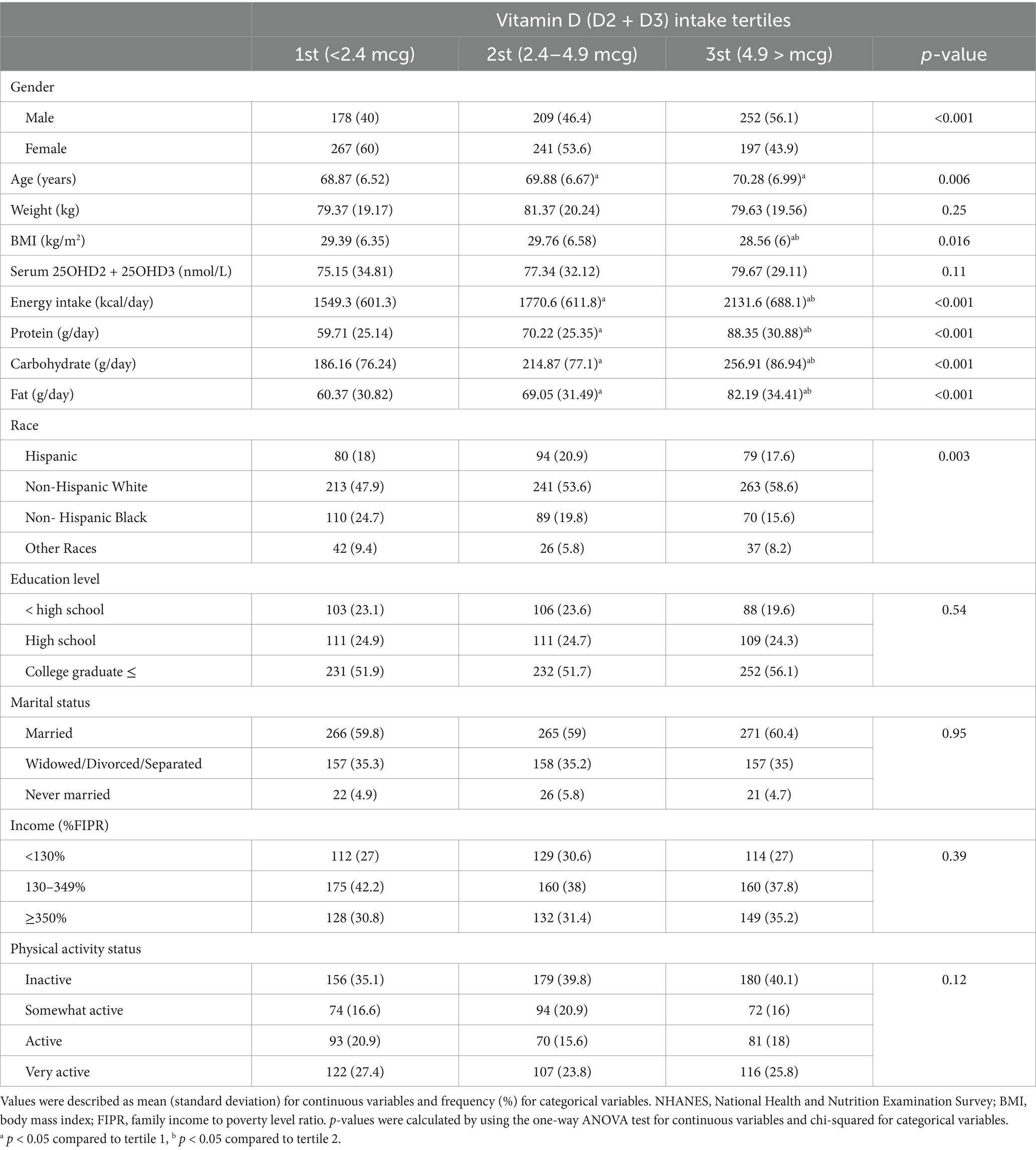
Table 1. Characteristics of participants in different quartiles of VD (D2 + D3) intake, NHANES 2013–2014.
Association between vitamin D intake and brain function
After adjustment for confounders, individuals in the 3rd tertile of vitamin D intake had significantly reduced odds of low performance on the CERAD test (OR: 0.77, 95% CI: 0.57–0.98; p = 0.031) and Animal Fluency test (OR: 0.63, 95% CI: 0.49–0.85; p = 0.013) compared to the 1st tertile of vitamin D intake. No significant association was observed between vitamin D intake and low performance on the DSST test in the crude and adjusted models (Table 2).
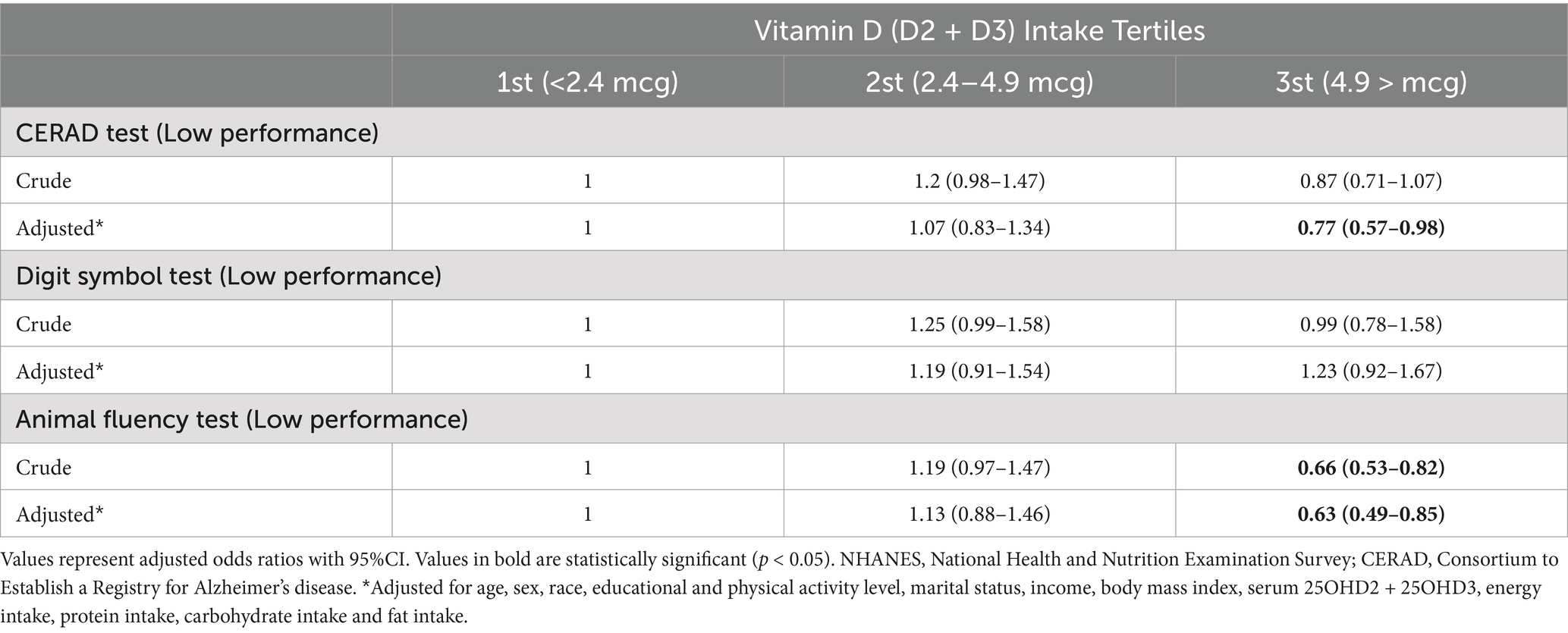
Table 2. Weighted ORs (95%CI) for association of low cognitive performance (CERAD test, Animal Fluency test and DSST) across dietary VD intake, NHANES 2013–2014 (N = 1,344).
Association between vitamin D intake and depression symptoms
Dietary vitamin D intake showed an inverse association with specific depression symptoms and overall depressive status. Individuals in the 3rd (OR: 0.45, 95% CI: 0.26–0.76; p = 0.006) and 2st tertiles (OR: 0.58, 95% CI: 0.36–0.89; p = 0.011) had significantly reduced odds of experiencing thoughts of being better off dead compared to the 1st tertile, even after adjustment. Additionally, participants in the 3rd tertile had lower odds of depression (PHQ-9 score > 4), even after adjustment (OR: 0.68, 95% CI: 0.48–0.99; p = 0.046). However, there was a significant positive association between higher dietary vitamin D intake (3rd title) and the likelihood of trouble sleeping or sleeping too much (OR: 1.36, 95% CI: 1.11–1.66; p = 0.033), following adjustment for confounding factors. Other depression-related symptoms, such as, appetite disturbances, energy levels, and feelings of worthlessness, did not show significant associations after adjustment (Table 3).
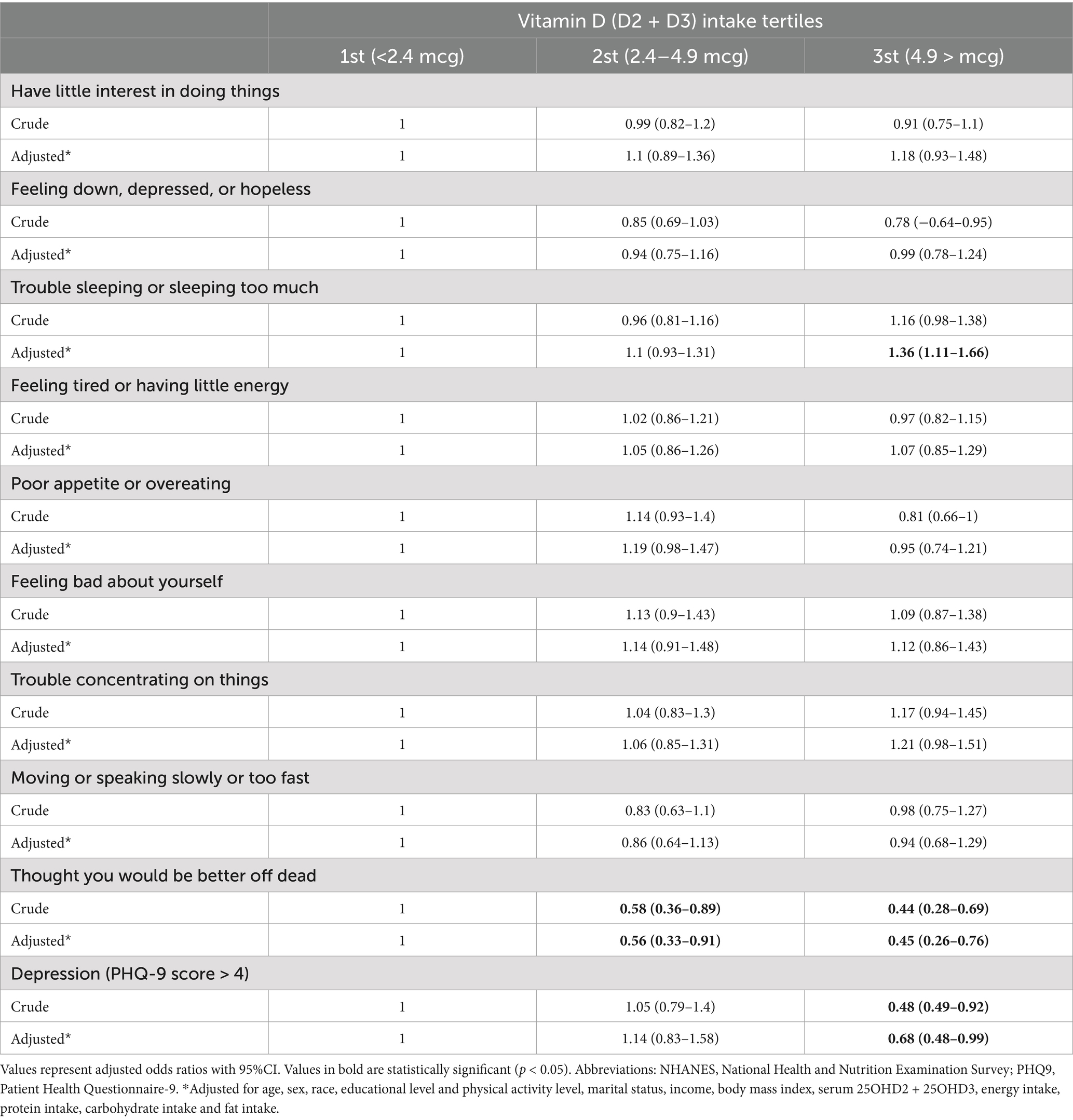
Table 3. Weighted ORs (95%CI) for association of depression (PHQ9 items) across dietary VD intake, NHANES 2013–2014 (N = 1,344).
Discussion
Our study investigates the association between dietary vitamin D intake and cognitive and mental function among elderly Americans, utilizing data from the NHANES 2013–2014 cycle. Our results indicated that dietary intake of vitamin D, independent of vitamin D serum level, is related to brain function and mental health. However, it is important to note that, due to the observational design of our study, the associations observed between dietary vitamin D intake and cognitive function are correlative rather than causal. Moreover, it is important to acknowledge that serum vitamin D levels do not always directly reflect dietary intake, as they are influenced by multiple factors, including sun exposure, supplementation, variations in hepatic and renal conversion of vitamin D to its active forms, and genetic variations such as polymorphisms in genes related to vitamin D transport (e.g., GC encoding vitamin D binding protein) and metabolism (e.g., CYP2R1, CYP27B1) (30–33). While our findings suggest a potential role of dietary vitamin D in cognitive and mental health, this association may be mediated through alternative mechanisms beyond serum levels. Vitamin D-rich foods, such as fatty fish, fortified dairy products, and egg yolks, are integral components of dietary patterns that support cognitive health (34). For example, the Mediterranean diet, abundant in vitamin D sources, has been associated with a lower risk of cognitive decline and depression (35). This association may result from synergistic interactions among nutrients. A clinical trial study suggested that combined intake of these vitamin D and omega-3 may offer superior protection against depression, anxiety, and sleep compared to individual nutrients alone (36). Moreover, emerging research indicates that vitamin D influences the gut microbiota, which in turn affects brain health (37, 38). Vitamin D receptors are present in the gut, and vitamin D deficiency has been linked to dysbiosis, an imbalance in gut microbial communities (39). Such imbalances have been associated with mood disorders and cognitive decline (40). Therefore, vitamin D intake may modulate gut microbiota composition, thereby impacting mental health and cognitive function, independent of vitamin D serum level, warranting further investigation into these pathways.
BMI, age, gender, intakes of macronutrients, and race were significantly different among tertiles of vitamin D intake. The possible effect of these confounders on the final results was minimized by adjusting in the final regression model. BMI is a well-known factor that can influence both cognitive function and mental health outcomes, with lower BMI often associated with improved cognitive performance and a reduced risk of depression (41). However, extreme BMI values, whether low or high, may also signal underlying health issues, such as frailty or metabolic disturbances, that could independently affect cognitive and mental health outcomes (41). By adjusting for BMI in our models, we aimed to isolate the effects of vitamin D intake on cognitive and mental health outcomes. However, differences in BMI could still carry important biological implications. Therefore, future studies should explore these associations further by investigating how changes in BMI over time or extreme values may directly influence cognitive and mental health outcomes, independent of other dietary as well as lifestyle factors.
The cognitive domains influenced by dietary vitamin D intake in this study included verbal fluency and episodic memory, as assessed by the Animal Fluency test and CERAD test, respectively. Individuals in the highest tertile of vitamin D intake showed significantly better performance in the Animal Fluency test, indicating enhanced verbal fluency, which reflects executive function and lexical access. Similarly, a reduced likelihood of poor performance in the CERAD test among those with higher vitamin D intake suggests improved episodic memory, a domain crucial for recalling specific events or experiences. However, in the domain of processing speed and attention, as measured by DSST, no significant association was observed between vitamin D intake and test performance after adjusting for confounders. This highlights that while vitamin D intake may positively influence certain cognitive domains, its effects may not extend universally across all aspects of cognition. Further studies are needed to clarify these domain-specific effects and underlying mechanisms. In terms of depression, the protective effect of vitamin D intake appears specific to more severe depressive symptoms, such as suicidal ideation, while its association with milder symptoms or other domains of depression was not significant. However, we found that higher tertile of vitamin D intake was associated with trouble sleeping. A clinical trial study found that vitamin D status was negatively associated with melatonin secretion (42). On the other hand, various studies suggested beneficial effect of vitamin D in sleep disorders (43, 44). Our study method cannot show causal relationships. Therefore, additional longitudinal studies are needed to investigate the relationship between vitamin D and sleep disorders.
Our findings align with prior studies linking vitamin D status to cognitive and mental health. For example, Shu et al. highlighted the association between higher serum vitamin D levels and improved cognitive function in older American adults, with depressive symptoms mediating this effect in populations with diabetes (28). Similarly, another study observed a positive relationship between dietary vitamin D intake and cognitive function in elderly American populations, suggesting that sufficient vitamin D consumption could mitigate age-related cognitive decline. However, this study focused exclusively on cognitive function without exploring depression as a potential mediator (23). Controversially, a longitudinal study in Swedish men with an 18-year follow-up reported no significant relationship between plasma 25-hydroxyvitamin D [25(OH)D] concentrations, dietary vitamin D intake, or genetic predisposition for vitamin D synthesis and the risk of all-cause dementia, Alzheimer’s disease, vascular dementia, or cognitive decline (13). The discrepancy between our findings and those of Olsson et al. (13) may be attributed to several important differences in study design, population characteristics, and assessment methods. Firstly, Olsson et al. (13) used a 7-day dietary record and assessed cognitive function using the Mini-Mental State Examination (MMSE), whereas we used different dietary assessment tools and employed more comprehensive cognitive function tests. Additionally, the study by Olsson et al. (13) was conducted on a cohort of Swedish older men, whereas the NHANES cohort represents a more ethnically and racially diverse population. This diversity may lead to differing dietary patterns, vitamin D sources, and other cultural factors influencing cognitive function. Moreover, the adjustment models used in both studies differ. Olsson et al. (13) did not adjust for the effect of vitamin D serum levels or income, which could influence cognitive outcomes. In addition, Olsson et al. (13) reported Hazard Ratios (HR) to estimate the risk of dementia, which accounts for time-to-event data, while our study used OR. Unlike HR, which measures the risk of an event occurring over time, OR provides a measure of association between exposure and outcome at a specific point in time, which may lead to different interpretations of the relationship between vitamin D intake and cognitive outcomes. Other studies identified that serum vitamin D level was associated with cognitive function in a Dutch, Brazilian, and American elderly cohort (11, 12, 45). Unlike studies focused on serum levels, our work specifically assesses the practical impact of food-based vitamin D intake on cognitive and mental health outcomes. The serum vitamin D level is more dependent on sun exposure than dietary intake (46). Therefore, our finding regarding the association between dietary vitamin D intake and brain and mental health in American elderly population, independent of sun exposure, may highlight the potential for dietary strategies to promote cognitive and mental health.
Given the observational nature of our study, the proposed mechanisms through which dietary vitamin D intake may influence cognitive and mental health remain speculative. Dietary vitamin D intake can influence cognitive and mental health through various molecular mechanisms. Vitamin D activates receptors in the brain, such as vitamin D receptors (VDRs) (7), which regulate gene expression related to neurogenesis, synaptic plasticity, and neuronal survival (7, 47). It promotes the production of neurotrophic factors like brain derived neurotrophic factors (BDNF) and neural growth factor (NGF), essential for cognitive function and neuroprotection (48). Additionally, vitamin D helps maintain calcium homeostasis for synaptic transmission and plasticity (49), modulates neurotransmitter systems like serotonin and dopamine (50), and reduces oxidative stress and neuroinflammation by inhibiting nuclear factor-kappa-B (NF-κB) activation (51). It also supports the integrity of the blood–brain barrier (52) and influences epigenetic mechanisms, such as DNA methylation and histone modifications, which affect genes involved in brain health (53).
The strengths of our study include its use of a nationally representative dataset, rigorous adjustment for confounders, and dual focus on cognitive and mental health outcomes. However, certain limitations should be acknowledged. First, utilizing data from a decade ago presents certain limitations. While more recent NHANES data cycles are available, the 2013–2014 dataset was selected due to its specific inclusion of variables pertinent to our research focus on dietary vitamin D intake and cognitive function. Moreover, a recent study investigated the trend of vitamin D intake using NHANES survey cycles 2001–2018 and found that despite an increasing trend in dietary vitamin D intake from 2007–2008 to 2017–2018 among the elderly population, dietary vitamin D intake has decreased from 4.83 in 2013–2014 mcg to 4.67 mcg in 2017–2018. However, this decrease was not statistically significant (54). As a result, it is difficult to definitively comment on how the current pattern of vitamin D intake in the elderly American population differs from that in 2013–2014, and future studies should address this issue. Second, the cross-sectional design precludes causal inference, necessitating longitudinal studies to confirm our findings. While we observed significant associations between dietary vitamin D intake and cognitive outcomes, we cannot infer that vitamin D intake directly causes changes in cognitive function or depression. The observed associations may reflect reverse causality, where cognitive decline or depression could lead to altered dietary patterns, including reduced vitamin D intake, rather than vice versa. Third, dietary vitamin D intake was assessed using a two-day 24-h dietary recall, which may not fully capture long-term intake patterns. Although this method is commonly used in large-scale studies like NHANES, it is subject to recall bias and day-to-day variations in dietary intake. Fourth, in this study low cognitive performance defined as the lowest tertile of test scores based on previous research (26, 27). While this approach is common, variations in cutoff points may affect the classification of cognitive impairment and, in turn, the interpretation of findings. Fifth, the study is geographically restricted to the elderly population in the U.S., which may limit the generalizability of the findings to other regions or populations with different environmental or dietary factors. Sixth, adjusting based on sunlight exposure was not possible as NHANES does not provide direct data on sunlight exposure. Seventh, serum vitamin D levels do not always directly reflect dietary intake, as they are influenced by multiple factors such as sun exposure, supplementation, metabolism, and genetic variations, which may confound the observed associations. However, we tried to minimize the effect of vitamin D serum level on the association between vitamin D intake and brain and mental function by adjustment in the regression model.
Conclusion
Our study suggests that higher dietary vitamin D intake is associated with improved cognitive performance, particularly in tests related to animal fluency and memory, as well as lower levels of depression in elderly individuals. These findings indicate that adequate dietary intake of vitamin D may be linked to better cognitive function and mental well-being in older adults. However, due to the cross-sectional nature of the study, we cannot draw causal inferences. The observed associations may be influenced by unmeasured confounders or reverse causality. Future longitudinal and experimental studies are necessary to confirm these findings and explore the potential mechanisms underlying the relationship between dietary vitamin D intake and cognitive health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes.
Ethics statement
The studies involving humans were approved by The National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HC: Conceptualization, Formal analysis, Writing – review & editing. XP: Data curation, Methodology, Writing – original draft. YH: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Jinjiang Municipal Hospital (Shanghai Sixth People’s Hospital Fujian) Science and Technology Program (No. 2023JC02) and Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2024Y9472).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Shin, JH. Dementia epidemiology fact sheet 2022. Ann Rehabil Med. (2022) 46:53–9. doi: 10.5535/arm.22027
2. Uomoto, KE. Increasing identification and follow-up of older adult depression in primary care. J Prim Care Community Health. (2023) 14:21501319231152758. doi: 10.1177/21501319231152758
3. Rebelos, E, Tentolouris, N, and Jude, E. The role of vitamin D in health and disease: a narrative review on the mechanisms linking vitamin D with disease and the effects of supplementation. Drugs. (2023) 83:665–85. doi: 10.1007/s40265-023-01875-8
4. Yang, T, Wang, H, Xiong, Y, Chen, C, Duan, K, Jia, J, et al. Vitamin D supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: a 12-month randomized controlled trial. J Alzheimers Dis. (2020) 78:1509–18. doi: 10.3233/JAD-200926
5. Loza, SB, Foss, MP, Silva, AB, Tafur-Lafuente, M, Lima, NKC, Donadi, EA, et al. Depression and cognitive decline as indicators of mental health in older adults. Arch Gerontol Geriatr Plus. (2024) 1:100066. doi: 10.1016/j.aggp.2024.100066
6. Giustina, A, Bouillon, R, Dawson-Hughes, B, Ebeling, PR, Lazaretti-Castro, M, Lips, P, et al. Vitamin D in the older population: a consensus statement. Endocrine. (2023) 79:31–44. doi: 10.1007/s12020-022-03208-3
7. Lasoń, W, Jantas, D, Leśkiewicz, M, Regulska, M, and Basta-Kaim, A. The vitamin D receptor as a potential target for the treatment of age-related neurodegenerative diseases such as Alzheimer's and Parkinson's diseases: a narrative review. Cells. (2023) 12:660. doi: 10.3390/cells12040660
8. Cui, X, and Eyles, DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. (2022) 14:4353. doi: 10.3390/nu14204353
9. Sailike, B, Onzhanova, Z, Akbay, B, Tokay, T, and Molnár, F. Vitamin D in central nervous system: implications for neurological disorders. Int J Mol Sci. (2024) 25:7809. doi: 10.3390/ijms25147809
10. Annweiler, C, Schott, AM, Rolland, Y, Blain, H, Herrmann, FR, and Beauchet, O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. (2010) 75:1810–6. doi: 10.1212/WNL.0b013e3181fd6352
11. Brouwer-Brolsma, EM, Van De Rest, O, Tieland, M, Van Der Zwaluw, NL, Steegenga, WT, Adam, JJ, et al. Serum 25-hydroxyvitamin D is associated with cognitive executive function in Dutch prefrail and frail elderly: a cross-sectional study exploring the associations of 25-hydroxyvitamin D with glucose metabolism, cognitive performance and depression. J Am Med Dir Assoc. (2013) 14:852.e9–852.e17. doi: 10.1016/j.jamda.2013.06.010
12. Da Rosa, MI, Beck, WO, Colonetti, T, Budni, J, Falchetti, ACB, Colonetti, L, et al. Association of vitamin D and vitamin B(12) with cognitive impairment in elderly aged 80 years or older: a cross-sectional study. J Hum Nutr Diet. (2019) 32:518–24. doi: 10.1111/jhn.12636
13. Olsson, E, Byberg, L, Karlström, B, Cederholm, T, Melhus, H, Sjögren, P, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr. (2017) 105:936–43. doi: 10.3945/ajcn.116.141531
14. Dobielska, M, Bartosik, NK, Zyzik, KA, Kowalczyk, E, and Karbownik, MS. Mechanisms of cognitive impairment in depression. May probiotics help? Front Psych. (2022) 13:904426. doi: 10.3389/fpsyt.2022.904426
15. Guo, Y, Pai, M, Xue, B, and Lu, W. Bidirectional association between depressive symptoms and mild cognitive impairment over 20 years: evidence from the health and retirement study in the United States. J Affect Disord. (2023) 338:449–58. doi: 10.1016/j.jad.2023.06.046
16. Yin, J, John, A, and Cadar, D. Bidirectional associations of depressive symptoms and cognitive function over time. JAMA Netw Open. (2024) 7:e2416305. doi: 10.1001/jamanetworkopen.2024.16305
17. Gatz, JL, Tyas, SL, St John, P, and Montgomery, P. Do depressive symptoms predict Alzheimer's disease and dementia? J Gerontol A Biol Sci Med Sci. (2005) 60:744–7. doi: 10.1093/gerona/60.6.744
18. Wilson, RS, Barnes, LL, Mendes De Leon, CF, Aggarwal, NT, Schneider, JS, Bach, J, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. (2002) 59:364–70. doi: 10.1212/WNL.59.3.364
19. Wilson, RS, Mendes De Leon, CF, Bennett, DA, Bienias, JL, and Evans, DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. (2004) 75:126–9.
20. Yin, J, Lassale, C, Steptoe, A, and Cadar, D. Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: the English longitudinal study of ageing. Int J Epidemiol. (2019) 48:1937–48. doi: 10.1093/ije/dyz085
21. Anglin, RES, Samaan, Z, Walter, SD, and Mcdonald, SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
22. Van Den Berg, KS, Marijnissen, RM, Van Den Brink, RH, Naarding, P, Comijs, HC, and Oude Voshaar, RC. Vitamin D deficiency, depression course and mortality: longitudinal results from the Netherlands study on depression in older persons (NESDO). J Psychosom Res. (2016) 83:50–6. doi: 10.1016/j.jpsychores.2016.03.004
23. Wang, R, Wang, W, Hu, P, Zhang, R, Dong, X, and Zhang, D. Association of Dietary Vitamin D Intake, serum 13(OH)D(3), 25(OH)D(2) with cognitive performance in the elderly. Nutrients. (2021) 13:3089. doi: 10.3390/nu13093089
24. Beauchet, O, Cooper-Brown, LA, and Allali, G. Vitamin D supplementation and cognition in adults: a systematic review of randomized controlled trials. CNS Drugs. (2021) 35:1249–64. doi: 10.1007/s40263-021-00876-z
25. Han, S, Gao, Y, and Gan, D. The combined associations of depression and cognitive impairment with functional disability and mortality in older adults: a population-based study from the NHANES 2011-2014. Front Aging Neurosci. (2023) 15:1121190. doi: 10.3389/fnagi.2023.1121190
26. Chen, SP, Bhattacharya, J, and Pershing, S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. (2017) 135:963–70. doi: 10.1001/jamaophthalmol.2017.2838
27. Li, T, Hu, Z, Qiao, L, Wu, Y, and Ye, T. Chronic kidney disease and cognitive performance: NHANES 2011–2014. BMC Geriatr. (2024) 24:351. doi: 10.1186/s12877-024-04917-2
28. Shu, C, Zheng, C, Du, X, and Luo, D. Exploring the role of vitamin D in cognitive function: mediation by depression with diabetes modulation in older U.S. adults, a NHANES weighted analysis. Front Nutr. (2024) 11:1356071. doi: 10.3389/fnut.2024.1356071
29. Park, J, Park, Y, Lee, Y, Lee, J, Lee, S, Shin, C, et al. Comparative analysis of energy intake and physical activity according to household type and presence of metabolic syndrome in middle-aged men based on data from the 7th Korea national health and nutrition examination survey (KNHANES) (2016-2018). Phys Act Nutr. (2021) 25:1–9. doi: 10.20463/pan.2021.0020
30. Faghfouri, AH, Bagheri, M, Mehdizadeh, A, Ayremlou, P, and Zarrin, R. Vitamin D level and vitamin D receptor gene polymorphisms in Iranian Azeri Turkish patients with autoimmune thyroid diseases. Acta Med Iran. (2018) 56:508–15.
31. Krasniqi, E, Boshnjaku, A, Wagner, KH, and Wessner, B. Association between polymorphisms in vitamin D pathway-related genes, vitamin D status, muscle mass and function: a systematic review. Nutrients. (2021) 13:3109. doi: 10.3390/nu13093109
32. Williams, S, Malatesta, K, and Norris, K. Vitamin D and chronic kidney disease. Ethn Dis. (2009) 19:S5.
33. Zarrin, R, Bagheri, M, Mehdizadeh, A, Ayremlou, P, and Faghfouri, AH. The association of FokI and ApaI polymorphisms in vitamin D receptor gene with autoimmune thyroid diseases in the northwest of Iran. Med J Islam Repub Iran. (2018) 32:18–21. doi: 10.14196/mjiri.32.4
34. Chuang, SY, Lo, YL, Wu, SY, Wang, PN, and Pan, WH. Dietary patterns and foods associated with cognitive function in Taiwanese older adults: the cross-sectional and longitudinal studies. J Am Med Dir Assoc. (2019) 20:544–550.e4. doi: 10.1016/j.jamda.2018.10.017
35. Fekete, M, Varga, P, Ungvari, Z, Fekete, JT, Buda, A, Szappanos, Á, et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: a meta-analysis. Geroscience. (2025). [Online ahead of print]. doi: 10.1007/s11357-024-01488-3
36. Rajabi-Naeeni, M, Dolatian, M, Qorbani, M, and Vaezi, AA. Effect of omega-3 and vitamin D co-supplementation on psychological distress in reproductive-aged women with pre-diabetes and hypovitaminosis D: a randomized controlled trial. Brain Behav. (2021) 11:e2342. doi: 10.1002/brb3.2342
37. Akimbekov, NS, Digel, I, Sherelkhan, DK, Lutfor, AB, and Razzaque, MS. Vitamin D and the host-gut microbiome: a brief overview. Acta Histochem Cytochem. (2020) 53:33–42. doi: 10.1267/ahc.20011
38. Clapp, M, Aurora, N, Herrera, L, Bhatia, M, Wilen, E, and Wakefield, S. Gut microbiota's effect on mental health: the gut-brain axis. Clin Pract. (2017) 7:987. doi: 10.4081/cp.2017.987
39. Aggeletopoulou, I, Marangos, M, Assimakopoulos, SF, Mouzaki, A, Thomopoulos, K, and Triantos, C. Vitamin D and microbiome: molecular interaction in inflammatory bowel disease pathogenesis. Am J Pathol. (2023) 193:656–68. doi: 10.1016/j.ajpath.2023.02.004
40. Jia, M, Fan, Y, Ma, Q, Yang, D, Wang, Y, He, X, et al. Gut microbiota dysbiosis promotes cognitive impairment via bile acid metabolism in major depressive disorder. Transl Psychiatry. (2024) 14:503. doi: 10.1038/s41398-024-03211-4
41. Han, K, Jia, W, Wang, S, Cao, W, Song, Y, Wang, J, et al. Synergistic impact of body mass index and cognitive function on all-cause mortality in older adults: a Nationwide longitudinal study. Front Endocrinol (Lausanne). (2021) 12:620261. doi: 10.3389/fendo.2021.620261
42. Golan, D, Staun-Ram, E, Glass-Marmor, L, Lavi, I, Rozenberg, O, Dishon, S, et al. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun. (2013) 32:180–5. doi: 10.1016/j.bbi.2013.04.010
43. Abboud, M. Vitamin D supplementation and sleep: a systematic review and Meta-analysis of intervention studies. Nutrients. (2022) 14:1076. doi: 10.3390/nu14051076
44. Schiza, S, Bouloukaki, I, Kaditis, A, Lombardi, C, and Bonsignore, MR. Vitamin D deficiency: a forgotten aspect in sleep disorders? A critical update. Sleep Med. (2024) 121:77–84. doi: 10.1016/j.sleep.2024.06.023
45. Llewellyn, DJ, Lang, IA, Langa, KM, and Melzer, D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. (2011) 66:59–65. doi: 10.1093/gerona/glq185
46. Raymond-Lezman, JR, and Riskin, SI. Benefits and risks of sun exposure to maintain adequate vitamin D levels. Cureus. (2023) 15:e38578. doi: 10.7759/cureus.38578
47. Gómez-Oliva, R, Geribaldi-Doldán, N, Domínguez-García, S, Carrascal, L, Verástegui, C, Nunez-Abades, P, et al. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: a role for Wnt/β-catenin signaling. Aging (Albany NY). (2020) 12:13824–44. doi: 10.18632/aging.103510
48. Wang, W, Li, Y, and Meng, X. Vitamin D and neurodegenerative diseases. Heliyon. (2023) 9:e12877. doi: 10.1016/j.heliyon.2023.e12877
49. Ye, X, Zhou, Q, Ren, P, Xiang, W, and Xiao, L. The synaptic and circuit functions of vitamin D in neurodevelopment disorders. Neuropsychiatr Dis Treat. (2023) 19:1515–30. doi: 10.2147/NDT.S407731
50. Wassif, GA, Alrehely, MS, Alharbi, DM, and Aljohani, AA. The impact of vitamin D on neuropsychiatric disorders. Cureus. (2023) 15:e47716. doi: 10.7759/cureus.47716
51. Ali, A, Shah, SA, Zaman, N, Uddin, MN, Khan, W, Ali, A, et al. Vitamin D exerts neuroprotection via SIRT1/nrf-2/ NF-kB signaling pathways against D-galactose-induced memory impairment in adult mice. Neurochem Int. (2021) 142:104893. doi: 10.1016/j.neuint.2020.104893
52. Sayeed, I, Turan, N, Stein, DG, and Wali, B. Vitamin D deficiency increases blood-brain barrier dysfunction after ischemic stroke in male rats. Exp Neurol. (2019) 312:63–71. doi: 10.1016/j.expneurol.2018.11.005
53. Athanasopoulos, D, Karagiannis, G, and Tsolaki, M. Recent findings in Alzheimer disease and nutrition focusing on epigenetics. Adv Nutr. (2016) 7:917–27. doi: 10.3945/an.116.012229
Keywords: vitamin D, cognitive function, depression, elderly, NHANES
Citation: Chen H, Pang X and Huang Y (2025) Higher dietary vitamin D intake influences brain and mental function in elderly Americans: a cross-sectional analysis. Front. Nutr. 12:1564568. doi: 10.3389/fnut.2025.1564568
Edited by:
Paula Goolkasian, University of North Carolina at Charlotte, United StatesReviewed by:
Gerd Faxén Irving, Karolinska Institutet (KI), SwedenI. Made Dwi Mertha Adnyana, Universitas Hindu Indonesia, Indonesia
Copyright © 2025 Chen, Pang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinhui Huang, WWluaHVpSHVhbmcubmV1cm8xOTkxQGdtYWlsLmNvbQ==
 Huizhen Chen
Huizhen Chen Xing Pang2
Xing Pang2