
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 31 March 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1562258
This article is part of the Research TopicFODMAPs: Advances in Research and Clinical PracticeView all articles
Objective: To examine the potential association between dietary index of gut microbiota (DI-GM) scores and constipation in adult women in the United States.
Methods: This cross-sectional study used data from adult participants in the 2005–2010 US National Health Survey (NHANES). The missing values in the covariables were filled by multiple interpolation. Multivariate logistic regression models were used to determine the odds ratios (OR) and 95% confidence intervals (CI) for the association between DI-GM and constipation. Subgroup analyses were also performed to examine the possible interactions between DI-GM and constipation.
Results: Of the 7,325 subjects, 887 reported constipations, with a prevalence of 12.1%. After adjustment for multivariate modeling, the DI-GM score was significantly associated with constipation (0.92 [95% CI 0.87–0.96]; p = 0.001). Similar results were found for the association of beneficial gut microbiota score with constipation (OR 0.89 [95% CI 0.84 to 0.95]; p = 0. 001). Subgroup analyses revealed that the relationship between DI-GM scores and constipation remained stable (p > 0.05).
Conclusion: DI-GM was negatively associated with the incidence of constipation in the female population. Clinicians should consider the influence of dietary structure on the treatment of constipation in women. Dietary intervention can be an important strategy for the comprehensive treatment of constipation.
Constipation is a chronic disease characterized by difficulty in defecation and a decrease in the number of bowel movements (1). The prevalence of constipation in the general population ranges from 3 to 27% (2), and more attention has been paid to constipation in children and the elderly population in the course of clinical treatment (3, 4). In recent years, with the increase of life pressure borne by women in modern society, the incidence of constipation is on the rise (5, 6), which seriously affects the daily life of the female population and brings a huge medical burden to economic and social development (7).
Dietary factors are often cited as the main cause of constipation, and dietary modifications often influence changes in the intestinal microflora (8). Dietary index of gut microbiota (DI-GM) is an assessment index to evaluate the relationship between gut microbiota and dietary factors. Developed by Kase et al. based on a large body of research literature, it is a dietary pattern that effectively identifies beneficial or unfavorable gut microbiota (9). For example, the consumption of whole grains and bran increases the levels of Bifidobacterium spp. and Lactobacillus spp., which are beneficial to the gut flora, whereas the consumption of red meat-rich foods increases the levels of Ruminococcus, Alistipes, Blautia, and Bilophila genera, which are unfavorable to the gut flora (10). Categorized according to whether a food component has a positive or negative effect on the gut microbiota, and is used to assess the quality of diets associated with the maintenance of normal gut flora (11).
Gut microbiota dysbiosis not only interferes with microbially mediated gut secretion and metabolic dysfunction, leading to constipation, but also interferes with the modulation of bowel movements by the brain-gut-microbe axis (12, 13). The number and distribution of intestinal flora play a very important role in maintaining intestinal function, and increasing the number of beneficial intestinal microbiota is commonly used to treat constipation (14). In recent years, simpler and more effective ways to improve intestinal flora and relieve constipation have been explored, and much attention has been paid to modifying the structure of the intestinal flora by adjusting dietary patterns (15–17). However, among the reported studies on constipation, little is known about the relationship between DI-GM and constipation in female populations. In this study, we examined the relationship between DI-GM and constipation in a female population in the United States using data from the NHANES database. We hypothesized that there would be an association between DI-GM scores and constipation, with higher DI-GM scores being associated with a lower risk of constipation.
The NHANES is a nationally representative survey of the health and nutritional status of the non-institutionalized population of the United States, using stratified, multistage probability cluster sampling (18). Data on constipation were only available for the 2005–2010 NHANES cycles. The NHANES study protocol was approved by the NCHS Research Ethics Review Board. Participants provided written informed consent at enrolment (19). The study conducted at Shang Luo Central Hospital (Shang Luo, China) was deemed exempt by the institutional review board because of the use of publicly available anonymized data. This study adhered to the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
This study collected data from the US National Health Survey (NHANES; 2005–2010). The following exclusion criteria were used to limit the analysis to patients with constipation between the ages of ≥20 years: colorectal cancer, missing data from the bowel questionnaire, missing/unavailable gut microbiota diet, and missing data for other covariates were interpolated using multiple interpolations. Figure 1 shows the flowchart of the subject recruitment process.
Constipation was assessed using the NHANES Bowel Health Questionnaire, based on the texture of the stools and the frequency of bowel movements. The questionnaire asked the participants to rate the texture of their stools and the frequency of their bowel movements. The Bristol Stool Frequency Scale (BSFS) consists of cards with different colored pictures and explanations of seven stool types to measure the consistency of their stools (20). Participants were asked to choose the number closest to the type of stool they usually see. BSFS type 1 (characterized by hard, nutty lumps) or type 2 (sausage-like, but lumpy) was used to diagnose constipation. BSFS types 3 (smooth and soft, such as a sausage or snake), 4 (smooth and soft), and 5 (soft plaques with sharp edges) are used to diagnose normal bowel function. BSFS types 6 (consisting of fluffy crumbs with rough edges and a pasty texture) or 7 (watery with no solid crumbs) were used to diagnose diarrhea. Fewer than three bowel movements per week were classified as constipation, between three and 21 bowel movements per week as normal, and more than 21 bowel movements per week as diarrhea. In this study, participants with stool types I and II and fewer than three bowel movements were classified as constipated and the others as non-constipated (21, 22).
Fourteen foods or nutrients were identified as components of DI-GM in the NHANES database based on the scoring criteria in an article by Zheng et al. (23). DI-GM included avocado, broccoli, chickpeas, coffee, cranberries, fermented dairy products, cottage cheese, green tea (because it was not available in NHANES), soy, and whole grains as beneficial components. In contrast, red meat, processed meat, refined grains, and high-fat diets (approximately 40% energy from fat) are considered harmful. NHANES data from 2005 to 2010 were used for dietary assessment calculations. The methodology for calculating DI-GM, its components, and scoring criteria can be found in the NHANES 2005–2010 data. For beneficial to gut microbiota items, the item was scored as 1 if consumption of the item was ≥ the gender-specific median, and 0 otherwise. For unfavorable gut microbiota items, the item was scored as 0 if consumption was ≥ the sex-specific median or 40% (high fat) and 1 otherwise. The scores were summed to give a total DI-GM score, which ranged from 0 to 13 (including beneficial to gut microbiota [range 0 to 9] and unfavorable to gut microbiota [range 0 to 4]) and was scored on a scale of 0–3, 4, 5, and 6 (24).
Based on previous NHANES research, potential covariates included in the analyses were age, sex, race/ethnicity (categorized as non-Hispanic White, non-Hispanic Black, Mexican American, or other), marital status (categorized as married/cohabitating or single, including never married, separated, widowed, or unmarried), including never married, separated, divorced, or widowed, years of education (less than 9, 9–12, or more than 12), and three levels of household income based on poverty income: low (PIR ≤ 1. 3), medium (PIR, 1.3–3.5), and high (PIR > 3.5) (25). Body mass index (weight (kg)/height (m2), height and weight measured at a mobile health screening center), and physical activity are defined as ‘organized or unorganized sports, fitness or recreational activities (e.g., gym work, cycling, running and all team sports), active travel (e.g., walking or cycling), and any other physical activity in, at or around the workplace, at home or any other physical activity while volunteering’. Physical inactivity was defined as less than 150 min of moderate-intensity physical activity per week (26). Smoking was defined as having smoked ≥100 cigarettes in a lifetime, alcohol consumption as at least 12 drinks per year, hypertension (physician-diagnosed systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or taking antihypertensive medication) (27), stroke (physician-diagnosed) and coronary heart disease (physician-diagnosed). Fasting blood glucose level ≥ 7.0 mmol/L, blood glucose level ≥ 11.1 mmol/L in a 2-h randomized oral glucose tolerance test, or use of diabetes medication/insulin for diagnosis of diabetes (28). Carbohydrate and energy intake was obtained by asking respondents to recall all beverages consumed and all foods eaten in the 24 h before the interview. The U.S. Department of Agriculture Nutrient Database was used to calculate data on dietary nutrient intake (29). The use of antidepressants was classified as ‘yes’ or ‘no’ based on the report of the participant (30).
Continuous variables are expressed as means and corresponding 95% confidence intervals (CIs), and categorical variables are expressed as percentages and 95% CIs. Normally distributed data were analyzed using a one-way analysis of variance (ANOVA), and skewed data were analyzed using the Kruskal-Wallis test. Categorical variables are expressed as proportions (%), and continuous variables are expressed as mean (standard deviation [SD]) or median (interquartile range [IQR]), as appropriate. Differences between groups were assessed using one-way ANOVA (for normally distributed data), the Kruskal-Wallis test (for skewed data), and the chi-squared test (for categorical variables). Multivariate logistic regression models were used to determine the odds ratios (OR) and corresponding 95% CIs for the association between the DI-GM scores and constipation. Model 1 was adjusted for sociodemographic characteristics (age, sex, marital status, race/ethnicity, education, household income, physical activity, body mass index (BMI), smoking status, and alcohol consumption status). Model 2 was adjusted for the factors in Model 1 plus hypertension, cardiovascular disease (CVD), stroke, diabetes, antidepressant use. Model 3 was adjusted for the factors in Model 2 plus energy intake, and carbohydrate intake. To assess the stability of the relationship between DI-GM scores and constipation in the population, multiple imputation by chained equations (MICE) and repeated main analyses. To account for missing baseline data, we used multiple imputation based on 5 imputed datasets. and subgroup analyses were performed according to age, physical activity, body mass index, and diabetes status. Heterogeneity and interactions between subgroups were assessed using logistic regression models and likelihood ratio tests. Statistical power was not calculated a priori as the sample size was based entirely on the available data. Analyses were performed using R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria)1 and Free Statistical Software (version 2.0; Beijing Free Clinical Medical Technology). In all analyses, a two-sided p < 0.05 was considered a statistically significant difference.
A total of 17,132 US adults aged ≥20 years, exclusions included: male participants (8303); patients with colorectal cancer (n = 62); missing Bowel Health Questionnaire data (n = 1,312); missing dietary data (n = 130). Consequently, 7,325 subjects were included in the final analysis (Figure 1).
Table 1 summarizes the baseline characteristics of study participants. Of the 7,235 female participants, 887 (12.1%) were diagnosed with chronic constipation, and the mean age (± SD) of the subjects was 48.7 (± 18.0) years. The prevalence of chronic constipation was higher among non-Hispanic White participants, married, middle-income, nonsmokers, alcohol drinkers, more educated, those who exercised ≥150 min/week, those who did not have a chronic disease, and those with lower DI-GM.
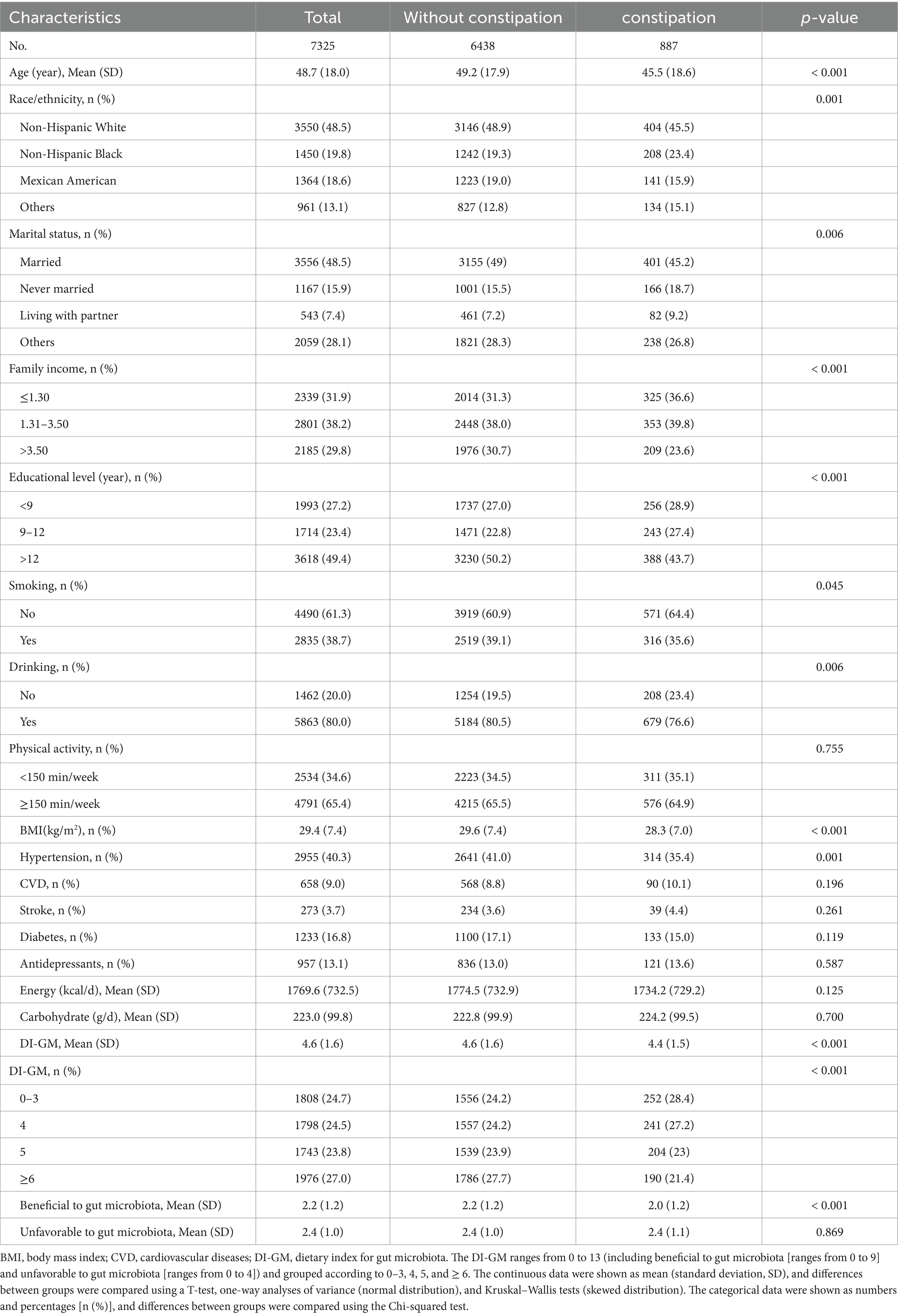
Table 1. General characteristics of the participants from the national health and nutrition examination survey 2005–2010 cycles.
As shown in Table 2, after adjusting for age, race/ethnicity, marital status, education level, household income, smoking status, alcohol consumption, physical activity, BMI, CVD, hypertension, stroke, diabetes mellitus, antidepressant medications, energy intake, and carbohydrate intake, Constipation prevalence decreased by 8% per 1 point increase in DI-GM (0.92 [95% CI 0.87–0.96]; p = 0.001). After grouping by DI-GM, compared with the control group, the prevalence of constipation was higher in the DI-GM ≥ 6 group in the unadjusted model (OR 0.66 [95% CI (0.54–0.8)]; p < 0. 001); in the adjusted model 1, the DI-GM ≥ 6 group was associated with a prevalence of constipation (OR 0.77 [95% CI 0.62–0.95]; p = 0. 014); in adjusted model 2, the DI-GM ≥6 group was associated with the prevalence of constipation (OR 0.77 [95% CI 0.62 to 0.95]; p = 0. 017). In adjusted model 3, the DI-GM ≥6 group was associated with the prevalence of constipation (OR 0.72 [95% CI 0.58 to 0.9]; p = 0. 003). In addition, the prevalence of constipation was significantly reduced with an increase in beneficial gut microbiota (OR = 0.89 [95% CI 0.84 to 0.95]; p = 0. 001), whereas an increase in unfavorable gut microbiota was not associated with the prevalence of constipation (OR 0.94 [95% CI 0.86 to 1.02]; p = 0.112).
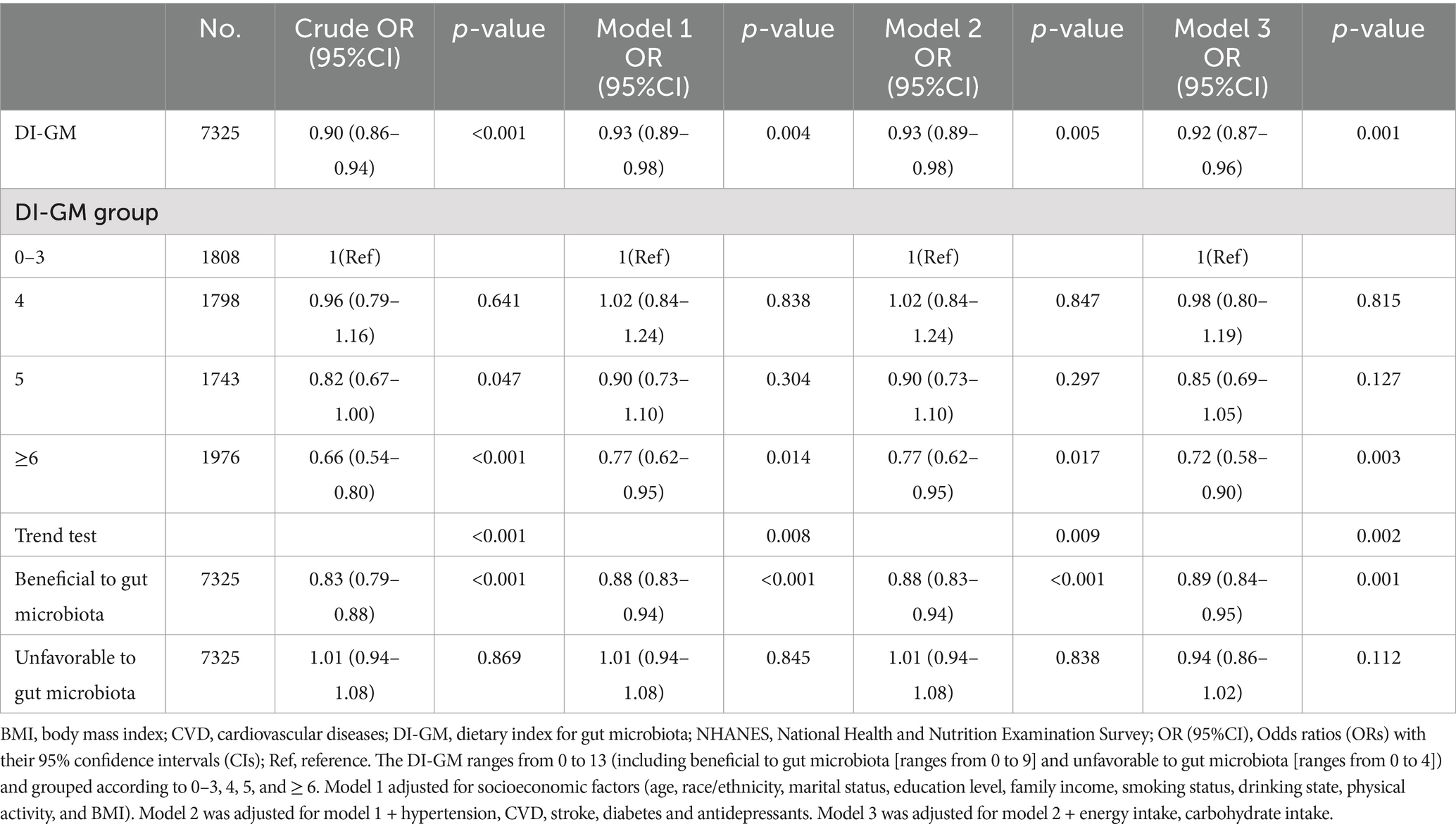
Table 2. Relationship between DI-GM and constipation among US adult women participants in NHANES 2005–2010.
Subgroup analyses showed stable results between DI-GM scores and constipation (in subgroups including beneficial gut flora and unfavorable gut flora) in female participants, adjusted for age, BMI, physical activity, and diabetes, none of which were found to interact (Figure 2).
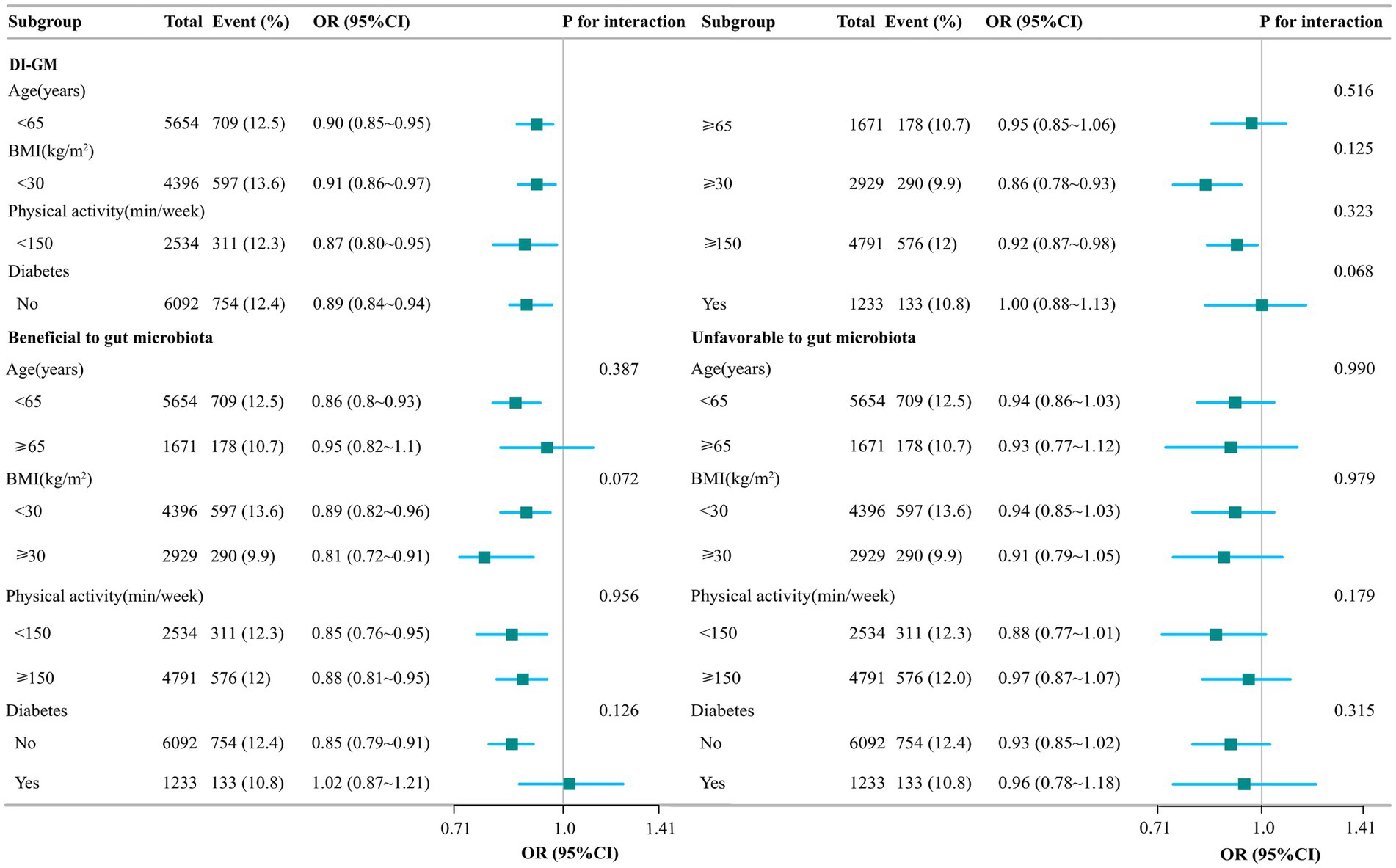
Figure 2. Stratified analysis of the association between DI-GM and constipation. Model adjusted for age, gender, race/ethnicity, marital status, education level, family income, smoking status, drinking state, physical activity, BMI, hypertension, CVD, stroke, diabetes, antidepressants, energy intake, and carbohydrate intake. The DI-GM ranges from 0 to 13 (including beneficial to gut microbiota [ranges from 0 to 9] and unfavorable to gut microbiota [ranges from 0 to 4]).
In this study, we found that as DI-GM scores increased, the risk of constipation decreased in women, with a 28% reduction in the DI-GM ≥ 6 group compared to the DI-GM 0–3 group (OR 0.72 [95% CI 0.58–0.9]; p = 0.003). The beneficial gut microbiota analysis yielded similar results, with an 11% reduction in the risk of constipation (OR 0.89 [95% CI 0.84 to 0.95]; p = 0. 001). Subgroup analyses showed that the association between DI-GM score and constipation remained stable.
Diet and constipation have a long history in modern society and in the female population, where different habits lead to different dietary patterns (31–33). Different foods play an important role in the development and treatment of constipation (34). Foods are beneficial not only because they are rich in dietary fiber, vitamins, polyphenols, and other active ingredients (e.g., avocados are rich in dietary fiber, chickpeas are rich in proteins and vitamins, and coffee beans contain biologically active ingredients) (35–37), but also because of the effect of the composition of the food on the intestinal flora and population. Soy, whole grains, and fermented dairy products promote the proliferation of gut flora such as Lactobacillus and Bifidobacterium. Conversely, unfavorable components of food (e.g., red meat, refined flour, and processed meats) predispose individuals to intestinal inflammation and disruption of the gut flora (38). Different dietary patterns also have different effects on the risk of developing constipation. For example, Mediterranean and high-fiber diets are associated with a lower risk of constipation than Western and ketogenic diets (39). The higher the dietary pattern of food components with beneficial intestinal flora, the higher the DIGM score and the lower the risk of constipation.
The intestinal flora is a complex ecosystem that plays an important role in maintaining intestinal function and the ecological barrier of the body (40). Previous studies have demonstrated a strong association between the development of chronic constipation and disturbances in gut microbiota composition and function, as well as related metabolic dysregulation (41, 42). Dysbiosis of the intestinal flora is characterized by a decrease in the abundance of Bifidobacterium spp., Lactobacillus spp., Prevotella spp., and butyrate-producing genera and has been demonstrated in patients with chronic constipation (13). Metabolites of intestinal biota, short-chain fatty acids, and methane alter intestinal pH, 5-hydroxytryptamine release, mucin secretion, and depolarization of intestinal smooth muscle ion channels (43). The effects of intestinal colonizing bacteria and their metabolites on intestinal function are reciprocal, regulating intestinal peristalsis, transport, secretion, and osmolality through the brain-gut-microbiota axis by secreting catecholamines and serotonin (44). Increasing the species and number of beneficial intestinal flora and improving the biota and metabolism of intestinal colonizing bacteria is an important approach to treating patients with constipation. Given the correlation between diet, intestinal flora, and constipation, remodeling the structure of the intestinal flora by adjusting dietary patterns is an effective strategy for relieving or treating constipation (45). Increasing dietary fiber intake in patients with constipation is the most commonly used method. On the one hand, dietary fiber has a significant effect on altering intestinal flora, and high-fiber diets can improve the number and distribution of beneficial private and Bacteroides bacteria and maintain the diversity of intestinal flora. In contrast, dietary fiber attenuates the inflammatory response, reduces intestinal inflammation, decreases intestinal mucosal damage, and inhibits local and systemic inflammatory responses (46, 47). Probiotics are often used in the prevention and treatment of constipation; they not only further break down and digest food to provide the necessary energy for intestinal cells but also effectively stimulate intestinal peristalsis and promote defecation. Moreover, the beneficial bacteria in probiotics can compete for the survival space of harmful intestinal flora, inhibit the overgrowth of harmful bacteria, and maintain the stability of the intestinal flora (45, 48). Our study showed that an increase in DI-GM helps to reduce the risk of constipation and that a good dietary pattern has a positive effect on the maintenance of intestinal flora homeostasis.
This study has several limitations. First, the initial ‘DI-GM’ was constructed using 14 food items and the specific type of tea consumption was not recorded in the NHANES data, and thus its specific parameters could not be obtained; in the future, alternative food items for tea consumption could be sought according to the NHANES dietary categories to enhance the convincing nature of the data. Second, constipation was identified based on reduced stool frequency and stool type characteristics, in addition to other symptoms such as incomplete stools and straining to pass stools. This information could not be provided because of the lack of content in the Bowel Health Questionnaire, and further bowel questionnaire items and information collection will be conducted in the future to define constipation according to the Rome VI criteria. Third, the NHANES 24-h diet, constipation, and other covariate data were self-reported and may have recall bias. Future sensitivity analyses and propensity score matching should be used to rule out the influence of residual confounders on the results. Finally, the cross-sectional design of this study was unable to determine a causal relationship between DI-GM and constipation. Therefore, further sample size expansion, cohort studies, and randomized controlled trials are needed to clarify the relationship between GI-GM and constipation.
DI-GM was negatively associated with the incidence of constipation in the female population. Clinicians should consider the influence of dietary structure on the treatment of constipation in women. Dietary intervention can be an important strategy for the comprehensive treatment of constipation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WL: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft. GF: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LL: Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. QD: Data curation, Formal analysis, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We thank Huanxian Liu (Department of Neurology, First Medical Center of Chinese PLA General Hospital, Beijing, China) for his helpful review and comments regarding the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ANOVA, a one-way analysis of variance; BMI, body mass index; BSFS, Bristol Stool Frequency Scale; CVD, cardiovascular diseases; DI-GM, dietary index for gut microbiota; IQR, interquartile range; MICE, Multiple imputation by chained equations; NHANES, National Health and Nutrition Examination Survey; OR, (95%CI) Odds ratios (ORs) with their 95% confidence intervals (CIs); SD, standard deviation; STROBE, Strengthening of the Reporting of Observational Studies in Epidemiology.
1. Rao, SSC, Rattanakovit, K, and Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. (2016) 13:295–305. doi: 10.1038/nrgastro.2016.53
2. Serra, J, Pohl, D, Azpiroz, F, Chiarioni, G, Ducrotté, P, Gourcerol, G, et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil. (2020) 32:e13762. doi: 10.1111/nmo.13762
3. Kang, SJ, Cho, YS, Lee, TH, Kim, SE, Han Seung Ryu, HS, Kim, JW, et al. Medical Management of Constipation in elderly patients: systematic review. J Neurogastroenterol Motil. (2021) 27:495–512. doi: 10.5056/jnm20210
4. Van der Zande, JMJ, and Lu, PL. Management of the child with refractory constipation. Aliment Pharmacol Ther. (2024) 60:S42–53. doi: 10.1111/apt.17847
5. Barberio, B, Judge, C, Savarino, EV, and Ford, AC. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:638–48. doi: 10.1016/S2468-1253(21)00111-4
6. Ghosh, DK, Sarkar, DK, Nath, M, Ullah, P, Khondaker, MFA, Chowdhury, SAM, et al. Symptoms and prevalence of constipation among adult population of Bangladesh. Euroasian J Hepatogastroenterol. (2023) 13:45–9. doi: 10.5005/jp-journals-10018-1393
7. Ota, T, Kuratani, S, Masaki, H, Ishizaki, S, Seki, H, and Takebe, T. Impact of chronic constipation symptoms on work productivity and daily activity: a large-scale internet survey. JGH Open. (2024) 8:e70042. doi: 10.1002/jgh3.70042
8. Dasriya, VL, Samtiya, M, Ranveer, S, Dhillon, HS, Devi, N, Sharma, V, et al. Modulation of gut-microbiota through probiotics and dietary interventions to improve host health. J Sci Food Agric. (2024) 104:6359–75. doi: 10.1002/jsfa.13370
9. Kase, BE, Liese, AD, Zhang, J, Murphy, EA, Zhao, L, and Steck, SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
10. Ross, FC, Patangia, D, Grimaud, G, Aonghus Lavelle, A, Dempsey, EM, Ross, RP, et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. (2024) 22:671–86. doi: 10.1038/s41579-024-01068-4
11. Zhang, Z, Bi, C, Wu, R, and Qu, M. Association of the newly proposed dietary index for gut microbiota and constipation: a cross-sectional study from NHANES. Front Nutr. (2025) 12:1529373. doi: 10.3389/fnut.2025.1529373
12. Pan, R, Wang, L, Xu, X, Chen, Y, Wang, HJ, Wang, G, et al. Crosstalk between the gut microbiome and colonic motility in chronic constipation: potential mechanisms and microbiota modulation. Nutrients. (2022) 14:3704. doi: 10.3390/nu14183704
13. Xu, X, Wang, Y, Long, Y, and Cheng, Y. Chronic constipation and gut microbiota: current research insights and therapeutic implications. Postgrad Med J. (2024) 100:890–7. doi: 10.1093/postmj/qgae112
14. Yoo, S, Jung, SC, Kwak, K, and Kim, JS. The role of prebiotics in modulating gut microbiota: implications for human health. Int J Mol Sci. (2024) 25:4834. doi: 10.3390/ijms25094834
15. Beam, A, Clinger, E, and Hao, L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. (2021) 13:2795. doi: 10.3390/nu13082795
16. Van der Schoot, A, Drysdale, C, Whelan, K, and Dimidi, E. The effect of Fiber supplementation on chronic constipation in adults: an updated systematic review and Meta-analysis of randomized controlled trials. Am J Clin Nutr. (2022) 116:953–69. doi: 10.1093/ajcn/nqac184
17. Cao, J, Wang, K, Li, N, Liping Zhang, LP, Qin, L, He, YY, et al. Soluble dietary fiber and cellulose from Saccharina japonica by-product ameliorate Loperamide-induced constipation via modulating enteric neurotransmitters, short-chain fatty acids and gut microbiota. Int J Biol Macromol. (2023) 226:1319–31. doi: 10.1016/j.ijbiomac.2022.11.243
18. CDC. About NHANES. National Health and nutrition examination survey. (2024). Available online at: https://www.cdc.gov/nchs/nhanes/about/index.html (Accessed December 31, 2024)
19. Bradway, D. Guidance on secondary analysis of existing data sets | Office of the Vice President for research. (2015). Available online at: https://ovpr.uconn.edu/services/rics/irb/researcher-guide/secondary-analysis-of-data-sets/ (Accessed December 31, 2024)
20. Aziz, I, Whitehead, WE, Palsson, OS, Törnblom, H, and Simrén, M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev Gastroenterol Hepatol. (2020) 14:39–46. doi: 10.1080/17474124.2020.1708718
21. Du, W, Yang, S, Zhou, H, Wu, YJ, Cai, Y, Meng, H, et al. The association between constipation and stroke based on the NHANES and Mendelian randomization study. Front Neurosci. (2023) 17:1276032. doi: 10.3389/fnins.2023.1276032
22. Xiang, N, Xu, L, Qian, H, and Zhang, D. Multiple obesity indices suggest a close relationship between obesity and constipation: evidence from NHANES. BMC Public Health. (2024) 24:1273. doi: 10.1186/s12889-024-18647-y
23. Zheng, Y, Hou, J, Guo, S, and Song, J. The association between the dietary index for gut microbiota and metabolic dysfunction-associated fatty liver disease: a cross-sectional study. Diabetol Metab Syndr. (2025) 17:17. doi: 10.1186/s13098-025-01589-9
24. Wang, L, Wu, J, Jiang, Z, Wang, C, Lin, F, Zhong, Y, et al. Dietary index for gut microbiota and its protective role against kidney stones: evidence of diabetes as a mediator from NHANES cross-sectional data. Front Nutri. (2025) 12:1532313. doi: 10.3389/fnut.2025.1532313
25. Vilar-Gomez, E, Nephew, LD, Vuppalanchi, R, Gawrieh, S, Mladenovic, A, Pike, F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. (2022) 75:1491–506. doi: 10.1002/hep.32207
26. Yun, L, Vanderloo, LM, Berry, TR, Cheung, AEL, Reilly, NO, Rhodes, RE, et al. Political orientation and public attributions for the causes and solutions of physical inactivity in Canada: implications for policy support. Front Public Health. (2019) 7:153. doi: 10.3389/fpubh.2019.00153
27. Wu, M, Si, J, Liu, Y, Kang, L, and Xu, B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. (2023) 45:2233712. doi: 10.1080/10641963.2023.2233712
28. Chen, Y, Guan, M, Wang, R, and Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999-2018. Front Endocrinol (Lausanne). (2023) 14:1298345. doi: 10.3389/fendo.2023.1298345
29. Xiang, L, Wu, M, Wang, Y, Liu, S, Lin, Q, Luo, G, et al. Inverse J-shaped relationship of dietary carbohydrate intake with serum klotho in NHANES 2007-2016. Nutrients. (2023) 15:3956. doi: 10.3390/nu15183956
30. Rajha, HE, Abdelaal, R, Charfi, K, Alemadi, AO, Sheraim, ASA, Maadid, MAA, et al. Examining depression, antidepressants use, and class and their potential associations with osteoporosis and fractures in adult women: results from ten NHANES cohorts. J Affect Disord. (2025) 369:1223–32. doi: 10.1016/j.jad.2024.10.114
31. Peppas, G, Alexiou, VG, Mourtzoukou, E, and Falagas, ME. Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroenterol. (2008) 8:5. doi: 10.1186/1471-230X-8-5
32. Bouchoucha, M, Fysekidis, M, Deutsch, D, Bejou, B, Sabate, JM, and Benamouzig, R. Biopsychosocial model and perceived constipation severity according to the constipation phenotype. Dig Dis Sci. (2021) 66:3588–96. doi: 10.1007/s10620-020-06654-z
33. Quetant, A, and Eilbert, W. Woman with constipation and abdominal pain. J Am Coll Emerg Physicians Open. (2021) 2:e12509. doi: 10.1002/emp2.12509
34. Bellini, M, Tonarelli, S, Barracca, F, Rettura, F, Pancetti, A, Ceccarelli, L, et al. Chronic constipation: is a nutritional approach reasonable? Nutrients. (2021) 13:3386. doi: 10.3390/nu13103386
35. Okobi, OE, Odoma, VA, Okunromade, O, Oluwasanmi, OL, Itua, B, Ndubuisi, C, et al. Effect of avocado consumption on risk factors of cardiovascular diseases: a systematic review and Meta-analysis. Cureus. (2023) 15:e41189. doi: 10.7759/cureus.41189
36. Ruiz-Zambrano, NL, Pérez-Carrillo, E, Serna-Saldívar, SO, and Tejada-Ortigoza, V. Effect of thermal, nonthermal, and combined treatments on functional and nutritional properties of chickpeas. Crit Rev Food Sci Nutr. (2024) 64:11356–74. doi: 10.1080/10408398.2023.2237577
37. Rai, SP, Ansari, AH, Singh, D, and Singh, S. Coffee, antioxidants, and brain inflammation. Prog Brain Res. (2024) 289:123–50. doi: 10.1016/bs.pbr.2024.06.005
38. García-Montero, C, Fraile-Martínez, O, Gómez-Lahoz, AM, Pekarek, L, Castellanos, AJ, Noguerales-Fraguas, F, et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. Implications for Health and Disease. Nutrients. (2021) 13:699. doi: 10.3390/nu13020699
39. Rollet, M, Bohn, T, and Vahid, F. Association between dietary factors and constipation in adults living in Luxembourg and taking part in the ORISCAV-LUX 2 survey. Nutrients. (2021) 14:122. doi: 10.3390/nu14010122
40. Wang, J, Zhu, N, Su, X, Gao, Y, and Yang, R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. (2023) 12:793. doi: 10.3390/cells12050793
41. Yang, L, Wang, Y, Zhang, Y, Li, WW, Jiang, S, Qian, DW, et al. Gut microbiota: a new avenue to reveal pathological mechanisms of constipation. Appl Microbiol Biotechnol. (2022) 106:6899–6913. doi: 10.1007/s00253-022-12197-2
42. Debnath, N, Kumar, R, Kumar, A, Mehta, PY, and Yadav, AK. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol Genet Eng Rev. (2021) 37:105–153. doi: 10.1080/02648725.2021.1989847
43. Tian, Y, Zuo, L, Guo, Q, Jun Li, J, Hu, ZY, Zhao, K, et al. Potential role of fecal microbiota in patients with constipation. Ther Adv Gastroenterol. (2020) 13:1756284820968423. doi: 10.1177/1756284820968423
44. Chang, L, Wei, Y, and Hashimoto, K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. (2022) 182:44–56. doi: 10.1016/j.brainresbull.2022.02.004
45. Rau, S, Gregg, A, Yaceczko, S, and Limketkai, B. Prebiotics and probiotics for gastrointestinal disorders. Nutrients. (2024) 16:778. doi: 10.3390/nu16060778
46. Gill, SK, Rossi, M, Bajka, B, and Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. (2021) 18:101–16. doi: 10.1038/s41575-020-00375-4
47. Barber, TM, Kabisch, S, Pfeiffer, AFH, and Weickert, MO. The health benefits of dietary fibre. Nutrients. (2020) 12:3209. doi: 10.3390/nu12103209
Keywords: female populations, DI-GM, NHANES, constipation, gut microbiota, dietary index
Citation: Lu W, Feng G, Liu L and Ding Q (2025) Association between dietary index of gut microbiota and constipation in a female population: a cross-sectional study. Front. Nutr. 12:1562258. doi: 10.3389/fnut.2025.1562258
Received: 17 January 2025; Accepted: 14 March 2025;
Published: 31 March 2025.
Edited by:
Marta Stelmach-Mardas, Poznan University of Medical Sciences, PolandReviewed by:
Andrea Deledda, Azienda Ospedaliero-Universitaria Cagliari, ItalyCopyright © 2025 Lu, Feng, Liu and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangfeng Lu, bHV3YW5nZmVuZ3NsQDEyNi5jb20=; Qi Ding, ZHhxcTEyMDVAMTYzLmNvbQ==
†ORCID: Wangfeng Lu, http://orcid.org/0000-0001-9750-7788
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.