
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 26 March 2025
Sec. Nutritional Immunology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1561119
This article is part of the Research TopicDietary Bioactive Compounds as Coadjuvants in Antiviral Preventive and Curative StrategiesView all 3 articles
Marine polysaccharides, particularly those derived from red, brown, and green algae, have shown promising antiviral activity. Among them, sulfated polysaccharides are particularly notable due to their broad-spectrum antiviral properties. These include direct viral destruction, inhibition of virus adsorption, disruption of viral transcription and replication, and the stimulation of the host’s antiviral immunity. With low toxicity, minimal drug resistance, and excellent biocompatibility, these polysaccharides represent promising candidates for the development of antiviral medications. For instance, carrageenan, a polysaccharide from red algae, and fucoidan, a polymer from brown algae, have both been proven to effectively inhibit viral infections. Sulfated polysaccharides from green algae, such as those found in Ulva species, also exhibit antiviral properties, including activity against the Japanese encephalitis virus. These polysaccharides function by blocking the attachment of viruses to host cells or interfering with various stages of the viral life cycle. Moreover, marine polysaccharides have been shown to enhance host immune responses, thereby aiding in viral clearance. Although these findings highlight the antiviral potential of marine polysaccharides, most studies have been conducted in vitro or in animal models. Further clinical trials are necessary to validate their effectiveness and safety for therapeutic use.
Algae are photosynthetic organisms widely distributed in nature. Based on their size and structure, they can be categorized into microalgae and macroalgae. Microalgae usually exist as single cells or in small groups, whereas algae exhibit multicellular structures and mainly include the categories of green, brown and red algae (1). At present, researcher have found antiviral, anti-tumor, anti-inflammatory, antibacterial, immune regulation, hypoglycemic, hypolipidemic and other active substances in algae. Among them, obtaining new antiviral drugs from algae has attracted extensive attention (2–10). In recent years, antiviral substances derived from algae have primarily focused on seaweed polysaccharides, proteins, small molecules, and algae extracts (Table 1). Among these, the sulfated polysaccharides from algae have been the subject of the most extensive research (Figure 1), which showcases the structural classification of sulfated polysaccharides derived from red, green, and brown algae (11). These polysaccharides can inhibit viral invasion and replication by interfering with the binding of viruses to receptors on the surface of host cells, making them a strong candidate for the development of natural antiviral drugs (12).
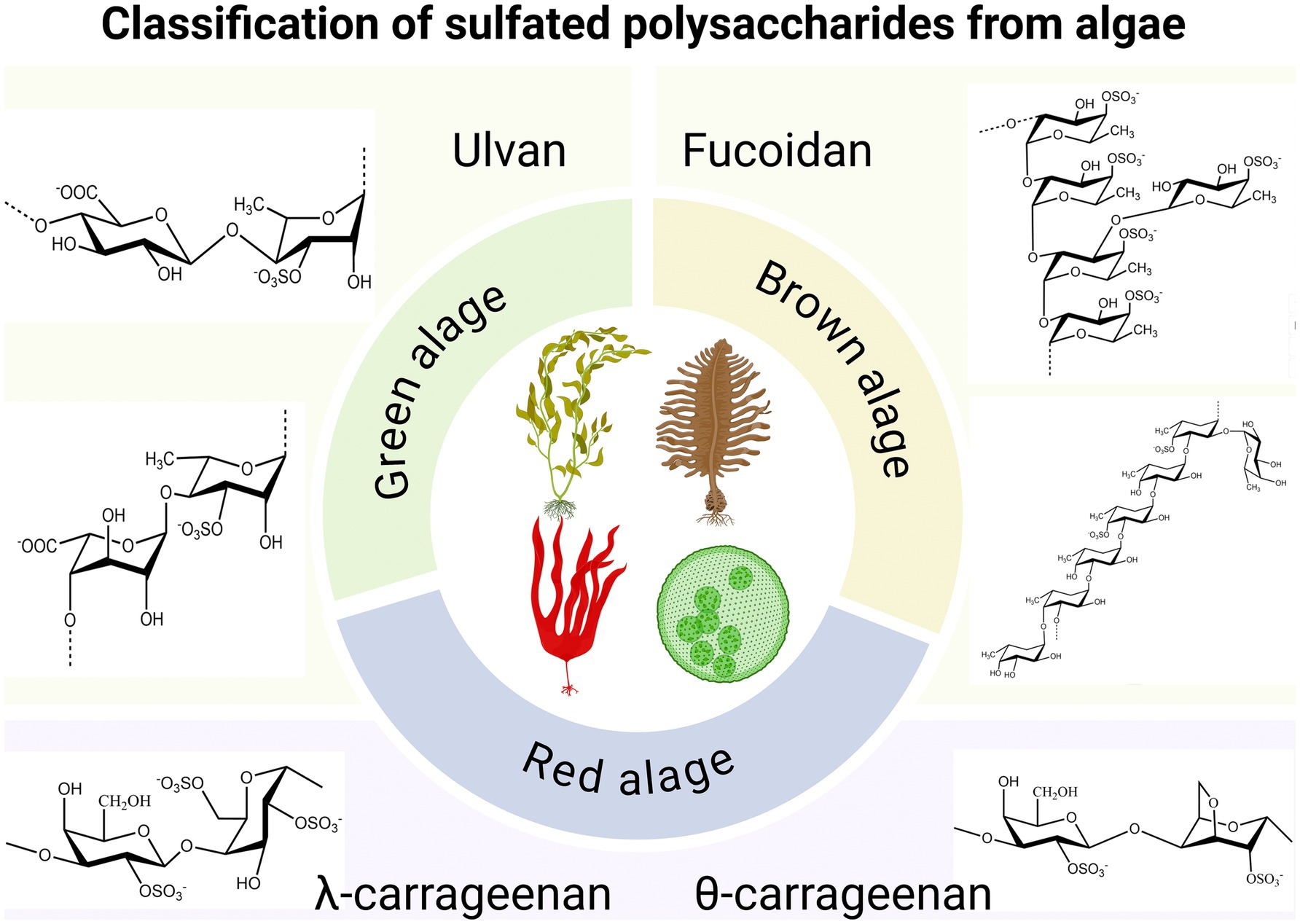
Figure 1. Classification of sulfated polysaccharides from algae. Created with BioRender.com.
Viruses, which are pathogenic microorganisms that pose significant threats to human health and life, are classified in the Baltimore classification system (Figure 2). About 60% of infectious diseases are caused by viruses (13). They are unique in that they can only reproduce inside other organisms’ cells. Viruses are generally assumed to be inactive since their genetic material is mostly a type of DNA or RNA, which has a protein shell and relatively simple structure (14). The primary method for preventing viral diseases is vaccination. However, vaccines for some viruses are still not available (15). In addition, some of the virus replication processes are within the cellular metabolic pathway and it is therefore not simple to eradicate virus particles (16). The antiviral drugs used are toxic and cannot completely eliminate the virus, and some even cause serious virus resistance (17). Therefore, it is an extremely urgent task to find and develop new natural antiviral substances with different mechanisms and low toxicity. This paper aims to provide a comprehensive review of the antiviral potential of marine sulfated polysaccharides, particularly those derived from green, brown, and red algae. The focus is on the structure–function relationship of these polysaccharides, including the key monosaccharides and sulfation patterns that contribute to their antiviral activity. By examining the molecular mechanisms through which these polysaccharides exert their effects, we seek to enhance the understanding of their therapeutic potential, highlighting their promise as natural antiviral agents with low toxicity and minimal drug resistance, while also emphasizing the need for further clinical studies to validate their efficacy in human applications.

Figure 2. The Baltimore classification of viruses. Created with BioRender.com.
This study uses Google Scholar, PubMed Pro, and Web of Science to search for the literature of algal polysaccharides. Advanced search includes keywords in the keywords, abstract, and title of the articles. The keywords used are “antiviral - algal polysaccharide,” “algal polysaccharide,” “activity - algal polysaccharide,” and “structural composition - algal polysaccharide” with particular focus on antiviral mechanisms of algal polysaccharides. Non-English articles are excluded to ensure consistency in language. Additionally, further exploration of terms such as “algal polysaccharide antiviral mechanism,” “immune response modulation by algal polysaccharides,” and “mechanism of green algal polysaccharides against viral infection” is undertaken to capture a broader range of relevant research on the antiviral properties and immune-modulating effects of algal polysaccharides.
Sulfated polysaccharides is a name given to naturally and semi-synthetic acid polysaccharides formed by substituting the hydroxyl group on the monosaccharide in the macromolecular chain with a sulfate group (18). Antiviral activity is thought to be one of the most significant biological activities of sulfated polysaccharides (Table 2), whether they are synthesized by direct extraction or by chemical modification (19). The selection of an appropriate extraction method for sulfated polysaccharides depends on the desired structural integrity and biological activity of the extracted compounds. For instance, carrageenan, derived from red algae such as Kappaphycus alvarezii and Eucheuma denticulatum, is typically extracted using hot water extraction, alkali treatment, and enzymatic hydrolysis (20). Fucoidan, a sulfated polysaccharide from brown algae, is commonly extracted from Fucus vesiculosus, Laminaria japonica, and Sargassum spp. using acid extraction, enzymatic hydrolysis, and ultrasound-assisted extraction (21). Ulvan, originating from green algae such as Ulva lactuca and Enteromorpha prolifera, is usually obtained through hot water extraction, acid or alkali treatment, and microwave-assisted extraction. Currently, commonly employed extraction techniques include hot water extraction, microwave-assisted extraction, and ultrasound-assisted extraction (22).
Sulfated polysaccharides were identified to effectively inhibit some viruses, including influenza A virus (IAV), human immunodeficiency virus (HIV), dengue virus (DENV), herpes simplex virus (HSV) and hepatitis B virus (HBV) (23–25). In addition, sulfated polysaccharides are revealed to be of low cytotoxicity and drug resistance, show good biocompatibility with zero side effects, and are reported to activate immune functions (26–28). Hence, it provides a point of departure for designing new food supplements and antiviral medicines.
Extracting sulfated polysaccharides from algae has become an important method for obtaining natural sulfated polysaccharides (29, 30). The content of natural sulfated polysaccharides in algae is extremely rich (12). Common sulfated polysaccharides include fucoidan, which is derived from kelp and brown algae, as well as carrageenan and agarose. Additionally, sulfated mannan, sulfated polysaccharides from green algae (particularly Ulva pertusa), and various sulfated polysaccharides extracted from Spirulina are also notable. These naturally occurring polysaccharides exhibit varying degrees of antiviral activity, as summarized in Table 3.
The red algae that contain sulfated polysaccharides mainly include Chondrus, Euchema, Furcellaria, Gigartina, Hypnea, and Iridae. The major cell wall component of red algae is a class of sulfated linear polysaccharides, also called carrageenan, which accounts for over 30%, and even up to 75%, of the dry weight of the algae (31–35). It has a repetitive disaccharide structure in carrageenan, with alternating 4-linked 3,6-anhydro-α-D-galactopyranose or 4-linked α-D-galactopyranose and 3-linked β-D-galactopyranose, and are substituted with sulfates at various positions (36, 37). The lambda-carrageenan is composed of D-galactose units, which have nearly three equatorial sulfates. The iota-and kappa-carrageenan are made up of an equal amount of alternating galactose and 3,6-anhydrogalactose kappa-with a sulfate group while iota-contains two disaccharides in the axial position (38).
Based on their structural characteristics, such as sulfation patterns and the presence of 3,6-anhydro bridges in α-linked galactose residues, carrageenans are classified into various types designated by Greek letters: kappa (κ)-, iota (ι)-, lambda (λ)-, nu (ν)-, mu (μ)-, and theta (θ)-carrageenan (39, 40). In addition to alternating sugar units and substituted sulfates, carrageenans also contain other carbohydrate residues like glucose, uronic xylose, and acid (41, 42). Various unique physical and chemical properties are related to the structure and composition of different types of carrageenans (43). According to their unique and different properties, carrageenans are widely used in food, cosmetics and pharmaceutical industries (42). Different types of carrageenans also exhibit many biological characteristics, such as antiviral, antithrombotic, anticancer and immunomodulatory activities (44). The κ-, ι-and λ-carrageenans are three kinds of carrageenans with deeper research and greater economic development value, which have better inhibition effects on different viruses (45). Different types of carrageenans or modified carrageenans isolated from various algae have not been detected to be toxic. In many cases, carrageenan treatment does not have harmful effects on metabolic activity or cell morphological, and no irritation or toxicity has been observed in the in vivo experiments, all of which prove that carrageenan is a non-toxic additive and has been approved by the European Union for use as a food additive (46, 47).
Research has shown that, ι-and λ-carrageenan exhibit effective inhibitory effects on dengue virus type 2 and 3 in Vero and HepG2 cells, with effective concentration of 0.14–4.1 μg/mL (48). This inhibitory effect on the virus is achieved by reducing the number of plaques to suppress virus production, and does not depend on the multiplicity of the multiplicity of infection (MOI) in the range of 0.001 to 1. λ- Carrageenan has a dual interference and inhibitory effect on virus adsorption and nucleocapsid internalization into cytoplasm. A study has shown that the 2 kDa κ-carrageenan oligosaccharide derived from carrageenan polysaccharides effectively inhibits the replication of H1N1 influenza virus in Madin-Darby Canine Kidney (MDCK) cell, with a selectivity index greater than 25.0 (49). In addition, 2 kDa κ-carrageenan oligosaccharide better inhibited the replication of influenza A virus than the higher molecular weight κ-carrageenan oligosaccharide, and showed a dose dependent inhibitory effect on IAV proliferation. The anti-virus effect of κ-carrageenan oligosaccharide may directly inactivate virus particles after pretreatment of MDCK cells. Unlike carrageenan polysaccharide, κ-carrageenan oligosaccharide can also prevent the expression of IAV mRNA and protein after internalization into cells, but it does not interfere with virus adsorption.
Alginate, a prominent acidic polysaccharide found extensively in the cell walls of brown algae, consists of poly-D-glucuronic acid, the central skeleton of poly-D-mannuronic acid, the alternating residues of D-couric acid and D-mannuronic acid (50). Alginate is widely utilized in biomedical science and engineering for its antiviral properties (49). Fucoidan is a sulfated polysaccharide characterized by fucose skeleton (51), and is the most widely studied and applied fucose in brown algae (52). It was first separated by Kylin in 1913 (53). Early studies on its structure showed that the main chain of fucoidans from brown algae in the order Fucales (family Fucaceae) is composed of alternating 1 → 3- and 1 → 4-linked α-L-fucosyl residues (54–56). Studies have shown that the fucoidan SHAP-1 and SHAP-2 separated and purified from Sargassum have anti HSV activity. Both fucoidan SHAP-1 and SHAP-2 are composed of galactose and fucose. The skeleton of the two fucoidan consists of α-(1 → 3)-linked L-Fucp residues, primarily sulfated at the C-2 and C-4 positions. The side chains include terminally linked α-L-Fucp and α-D-Galp residues, with (1 → 2)-, (1 → 6)-, and (1 → 2,6)-linked β-D-Galp residues mainly attached to the O-4 position of the main chain residues. In addition, studies have shown that SHAP-1 and SHAP-2 can exert anti HSV activity by preventing HSV-2 from adsorbing onto the host cell membrane in the early stage of infection. It can be concluded that the antiviral mechanism of fucoidan is to block the adsorption of HSV-2 virus particles on host cells (57). The aqueous alcohol extract of Sargassum showed antiviral activity against the highly prevalent human enterovirus Echovirus 9. Through qualitative determination of the type of secondary metabolites in the extract and evaluation of cytotoxicity, the quinones, proanthocyanidins, catechins, polar triterpenes, hydrolyzable tannins, etc. were found to strongly inhibit the replication of Echovirus 9 (58). Fucoidan exhibits a range of biological activities, including in vitro resistance to various RNA and DNA viruses, particularly to important human pathogens such as HSV-1, HSV-2, cytomegalovirus, dengue virus and HIV (59). Fucose is a compound with complex structure, which has antiviral activity due to its direct interaction with envelope virus (60). Dinesh et al.’s experiment showed that the fucoidan components have inhibitory activity against HIV-1 in vitro (61). Moulard et al. have proved that fucose exerts its antiviral effects by binding to HIV-1, thereby blocking the initial entry stage of the virus (62). These macromolecules may shield the positively charged amino acids in the viral envelope glycoprotein gp120 or strongly bind with specific sulfate groups, thereby inhibiting HIV-1 activity (62, 63). Akamatsu et al. isolated a novel fucosan polysaccharide named MC26 from the marine brown alga species Sargassum. MC26 demonstrated potent activity against the influenza virus while exhibiting low cytotoxicity (64). Mandal et al. extracted fucoidan sulfate from brown seaweed, and found that the fucoidan can effectively combat HSV-1 and HSV-2, while showing no cytotoxic effects in Vero cell cultures. Copolysaccharides mainly inhibit the formation of virus induced syncytium by suppressing virus-cell interactions, thereby proving their antiviral activity (65, 66).
A fucoidan (KW) extracted from the brown alga Kjellmaniella crassifolia, showed excellent activity against influenza IAV (67). KW demonstrated a broad-spectrum antiviral effect against IAV and a low tendency to induce viral drug resistance. It effectively inhibited IAV infection with low toxicity in vitro (68). KW effectively inactivated virus particles, prevented virus transmission after attachment, and inhibited the activity of virus neuraminidase binding, thereby blocking virus release (69). In terms of host regulation, KW effectively inhibited the activation of pathways including PKCα, NF-κB and EGFR, and inhibited viral endocytosis (70). Intranasal administration of KW to mice markedly enhanced the survival rate and lowered the viral titer following IAV infection (71).
In recent decades, Sargassum fusiforme polysaccharide (SFP) has attracted wide attention due to its antioxidant, immune regulation, anti-tumor, anti-aging and hypoglycemic effects (72–75). Some studies have found that SFPs with different molecular weights can combine with viruses and show good antiviral activity in vitro. After treatment with SFP, the expression of virus gene and protein decreased significantly, and the 9 kDa molecular weight SFP-3 showed the best antiviral effect (44, 76). Surprisingly, SFP in chickens showed effects in reducing immune suppression, inhibiting shedding, and minimizing organ damage caused by viral infections. To further improve the antiviral effect of SFP, there are studies using nanoparticle technology to prepare hydrophobic SFP into three kinds of nanomicelles (SFP-C12M, SFP-C14M, and SFP-C16M), which can enhance the antiviral effect of SFP. Compared with SFP, all three types of micelles showed better antiviral effect, with the SFP-C12M being the most stable and the SFP-C16M showing the best antiviral effect, which also exhibited activity during the virus replication stage. Subsequent giant unilamellar vesicle exposure experiment found that the antiviral activity of nano micelles may act on the phospholipid membrane of ALV-L virus, and offer a novel approach for developing antiviral drugs (77, 78).
Ulvan is a significant water-soluble polysaccharide present in green seaweeds belonging to the Ulvales order, such as Enteromorpha and Ulva lactuca. The main components are xylose, rhamnose, sulfate, glucuronic acid and iduronic acid (79). Lahaye et al. showed that both natural and chemically modified formulations of Ulva lactuca contain many oligosaccharide repeating units, with the main repeating disaccharide unit being in the form of 3-sulfate composed of iduronic acid or glucuronic acid. This demonstrates that the structure of Ulva lactuca has great complexity and variability (80). Ulvan, accounts for 8–29% of the dry weight of green seaweed and is the most prevalent polysaccharide found in its cell walls. Studies conducted both in vitro and in vivo have demonstrated that ulvan possesses antibacterial, anticoagulant, immunomodulatory properties and antiviral (80–85). Studies have shown that Ulva lactuca can effectively suppress the measles virus (MeV) by limiting the formation of syncytia, and inhibit HSV virus by inhibiting DNA replication and transcription, and downregulating HSV protein synthesis. The edible blue-green algae Nostoc flagelliforme contains the acidic polymer known as Nostoc flagelliforme polysaccharide (NSF). Kanekiyo et al. discovered that NSF can effectively inhibit a number of enveloped viruses. Studies have confirmed that NSF can achieve anti-herpes effect by inhibiting the combination of virus and host cells, and Nostoc flagelliforme polysaccharide can be used as a candidate drug for anti-herpes (86).
The mosquito-borne flavivirus known as the Japanese encephalitis virus (JEV) kills many people in Southeast Asia each year and produces a significant number of encephalitis cases (87, 88). But effective drugs to inhibit JEV are still lacking, so the development of cheap and easily available antiviral drugs with low side effects is urgently needed (89). One of the experiments documented treatment impact on JEV infection with the aid of U. lactuca sulfated polysaccharide extract. Chiu et al. found sulfated polysaccharides in Ulva lactuca had antiviral and anti-inflammatory properties toward Vero cells infected by JEV and mixed primary glial cells upon treatment in vitro and inspected antiviral action under electron microscopy. Then, the antiviral effect of a sulfated polysaccharide extract of Ulva lactuca was then investigated in C3H/HeN mice infected with JEV (89). The results are also similar to other sulfated polysaccharides, which are more suitable as preventive agents rather than therapeutic agents and can also interfere with the binding of viruses to cells. However, examination of the mixture by virus binding assay combined with transmission electron microscopy revealed that the extracts could bind to JEV as a 110 nm sized complex, indicating that the Ulva sulfated polysaccharide extracts could adsorb JEV particles, thereby preventing the virus from entering cells. Unfortunately, this sulfate polysaccharide appears to be selective toward the Flaviviridae and does not bind to dengue viruses and West Nile viruses of the same genus. In the JEV model of murine infection, the antiviral effect of carrageenan was more remarkable. Encephalitis appeared 5 days after infection and all mice died shortly after indicating that carrageenan treatment not only delayed the onset time but also improved the survival rate of C3H/HeN mice (90). In the examination of host brain indicators, it was found that carrageenan was able to reduce the mRNA and protein expression levels of JEV, and significantly decreased the proinflammatory cytokines such as iNOS and TNF α (91–93). Sulfated polysaccharides have been shown to inhibit viral infections through multiple mechanisms, including blocking viral receptors and interfering with intracellular replication steps. These polysaccharides can prevent viral attachment by competitively binding to cell surface receptors or viral glycoproteins, thereby reducing viral entry. Additionally, they may disrupt key stages of viral replication, such as inhibiting viral RNA synthesis or interfering with viral protein processing, ultimately limiting viral propagation (94).
Sulfated polysaccharides from algae have unique structures that exhibit antiviral properties. They operate at various stages of the viral life cycle either by directly inactivating virions before infection or by suppressing replication of the virus in host cells. Polysaccharide-rich algae thus play a huge role in antiviral drug discovery and development. The major steps in the viral life cycle—attachment, penetration, uncoating, biosynthesis, assembly, and release—vary from species to species (Figure 3) (95). Besides disrupting the life cycle of the virus and suppressing the replication of viruses, algal polysaccharides also stimulate the host’s antiviral immune response and accelerate virus clearance. Thus, algal polysaccharides can inhibit virus life cycles at different stages of a virus’s life cycle or can directly inactivate virus particles before virus infection. Typically, the mode of antiviral action is associated with specific structural characteristics of polysaccharides and particular viral serotypes (66).
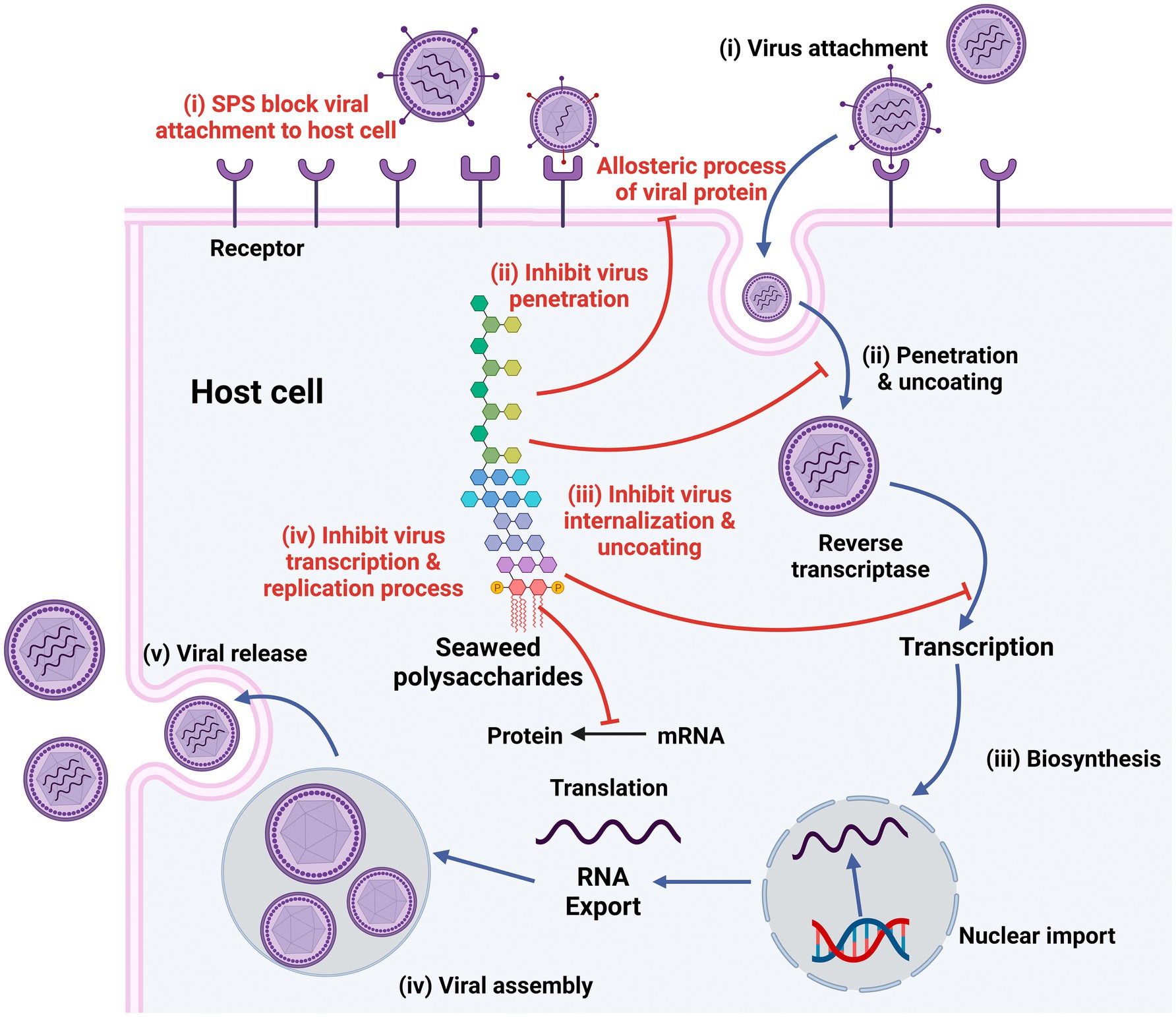
Figure 3. Stages in life cycle of virus (Black) and mechanism of antiviral actions of sulfated polysaccharides (SPs; Red). Created with BioRender.com.
Due to the negative charge, sulfated polysaccharides can act directly on virus particle surfaces, thus inhibiting virus infectivity or killing the virus. Sulfated polysaccharides from marine sources can have the capacity to bind with spike glycoprotein, preventing SARS-CoV-2 from entering host cells (96). By targeting viral envelope glycoproteins, sulfated alginate polysaccharides of Laminaria japonica were shown to suppress HSV-1 at low concentrations by preventing the infection process and blocking the virus from detecting surface receptors on the host cell (97). Numerous studies further demonstrate that one of the modes of action of carrageenan is that it exerts a direct virus-killing effect on some enveloped viruses, which means that the virus loses its infecting ability, hence reducing virus reproduction. λ-carrageenan has the ability to bind strongly to herpes simplex virus, thereby inactivating HSV particles and inhibiting the replication of HSV. Furthermore, in low doses, the red algal polysaccharide carrageenan can directly inactivate HSV-2 (7, 98). The direct killing effect of the virus maybe because carrageenan exerts some sort of stable virus particle-carrageenan complex where the binding itself is irreversible. These sulfated polysaccharides occupy the location on the viral envelope where the virus must connect to the host cell, making it unable to carry out its following infection process (66). Namely, the polysaccharide of carrageenan can kill viruses directly and then prevent virus infection.
For infection to occur, viruses must adsorb and penetrate target cells. The first step in the viral invasion process is the interaction with the host cell surface through electrostatic forces. The unstable reversible binding is then changed into stable irreversible adsorption in order to carry out the subsequent steps. Natural and synthesized sulfated polysaccharides have high polyanion characteristics. They can make the adsorption of viruses or host cellular surface proteins hinder through electrostatic contact with their cells, consequently blocking the binding site of viruses and host cellular receptors (99). Figure 4 depicts the SARS-CoV-2 entrance and replication cycle, along with putative inhibitory sites for marine-derived metabolites. The virus connects to the ACE2 receptor, then penetrates the cell and releases its RNA. The RNA generates viral proteins, which form new virus particles. These particles are processed in the endoplasmic reticulum-Golgi pathway and then released to infect new cells, particularly those that express ACE2 receptors in organs such as the heart, lungs, intestines and kidneys (100–102). Marine-derived natural compounds, such as seaweed polysaccharides, exhibit antiviral activity through two main mechanisms: inhibition of viral entry and replication. The S glycoprotein is a primary antigen on the virus surface, mediating necessary attachment and membrane fusion for cellular entry. Therefore, antiviral compounds can block this process by inhibiting the attachment of S protein to ACE2 receptors in a dose-dependent manner. Additionally, viral proteases like 3CLpro and PLpro, as well as RdRp, facilitate viral replication and transcription. By targeting key viral proteins such as N and M, these compounds can further suppress viral replication and block the spread of infection (103, 104). Furthermore, increased furin expression enhances MERS-CoV pseudovirion infection, while furin siRNA silencing reduces furin expression and subsequently viral entry. Fucoidans inhibit coronaviruses by targeting both the viral spike protein and the host cell furin, thereby interfering with viral infection (105, 106).

Figure 4. SARS-CoV-2 entry and replication cycle, with potential inhibition sites where seaweed polysaccharides may exert antiviral activity. Created with BioRender.com.
Carrageenan is capable of masking the positive charge on the host cell surface by its negative sulfate group charge, thus interfering with the process of virus adsorption. Mazumder et al. isolated high molecular weight sulfated galactose from red algae, which can prevent the initial attachment of viruses to host cells and demonstrates activity against herpes HSV-1 and HSV-2 (107). Then, Kalucci et al. verified that λ-carrageenan might obstruct the virus’s ability to adhere to host cell surfaces (90, 108). Alginate glucan produced from brown algae has been shown to have strong antiviral properties against a variety of viruses, including the human HSV and HIV. Fucoidan acts by inhibition of viral particles binding to host cells, thus activating its antiviral activity (109). Moreover, sulfated polymannurate (SPMG) is a new sulfated marine polysaccharide derived from brown algae that can prevent the penetration and adsorption of HIV-1 by competitively sharing a gp120 common binding site with sCD4 or masking its gp120 docking site on the T lymphocyte surface with sCD4 (110). Sulfated fucoidan polysaccharide cotyledons obtained from nodular seaweed have been reported to give a very significant inhibition against the early stages of HBV, HCV and HIV-1 infection but show no effect on the late stages of the same virus infection (19). Alginate has a direct interaction with the envelope glycoprotein on the surface of dengue virus DEN2 (66). Furthermore, by blocking the virus’s contact with host cells, the acidic polysaccharide Nostoflan, which is extracted from the edible blue-green algae Nostoc flagellate, also showed a good suppression of HSV-1 production (86). In summary, the adsorption step of virus infection could be interfered with by marine polysaccharides originated from various sources to prevent virus infection.
After internalizing into host cells, algal polysaccharides, particularly low molecular weight algal oligosaccharides, can impede transcription and replication in addition to blocking the viral invasion process. The sulfated polysaccharide chains have some identical binding sites with some RNA template primers-related enzymes, leading to their competitive inhibition. However, by acting on similar targets inside intracellular targets, sulfated polysaccharides directly affect replication-related enzymes and prevent transcription and replication. Thus, Talarico et al. demonstrated that by interfering with possible host cell targets, ι-carrageenan may prevent DENV multiplication in mosquito cells (111). Furthermore, carrageenan oligosaccharides with tiny molecular weights can enter host cells to prevent transcription and viral multiplication. Low molecular weight κ-carrageenan oligosaccharides have been found by Wang et al. to successfully suppress influenza A H1N1 virus multiplication in vitro and in vivo (49). Additionally, certain marine polysaccharides derived from brown algae prevent viruses from replicating in host cells. At a dose of 0.5–1.0 mg/mL, Queiroz et al. discovered that the fucans isolated from Fusarium vesiculatum demonstrated a notable inhibitory impact on HIV reverse transcriptase in vitro. Furthermore, alginate derivative 911 can not only suppress HIV-1 reverse transcriptase activity but also inhibit virus adsorption. Conclusively, marine polysaccharides could interfere with viral replicase or other potential targets in the host cell to inhibit viral transcription and replication (112–114).
Proceeding from the pathogenesis of the disease, pathogenic treatment for viral infection should focus on the virus directly or on the process of its adsorption and penetration into cells, as well as on innate immunity activation, antioxidant defense system strengthening, immune cytokine production, and indirect antiviral effects (115). Many pharmacological experiments have demonstrated that sulfated polysaccharide is a potent immune regulator in an attempt to maintain the integrity of body balance via a series of immunomodulatory regulation activities against natural killer cells (NK cells), macrophages, T/B lymphocytes, and other immune cells through the promotion of cytokine release and antibody production, which may indirectly activate the complement system, inhibit virus replication, and accelerate the virus clearance process (26, 111). In macrophages, they promote immune responses by upregulating the production of nitric oxide, interleukin-6, prostaglandin E2, interleukin-1β, and tumor necrosis factor-α. Additionally, they enhance the expression of key enzymes such as inducible nitric oxide synthase and cyclooxygenase-2, further amplifying the inflammatory response and immune defense mechanisms. The study’s findings demonstrated that giving mice carrageenan for 8 h causes them to produce type I interferon, as well as significantly enhances the NK cell activities and lymphocyte proliferation rate. In addition, the oligosaccharide of carrageenan may stimulate macrophages and NK cells activity, improving IL-2 and TNF-α (116, 117). These findings suggest that the antiviral effect of carrageenan is closely linked to its ability to strengthen the host immune system, indirectly inhibiting viral replication and facilitating virus clearance.
Marine polysaccharides, particularly those derived from red, brown, and green algae, have demonstrated significant antiviral properties. These sulfated polysaccharides exhibit broad-spectrum antiviral activities, including direct inactivation of viruses, inhibition of viral adsorption, suppression of viral transcription and replication, and enhancement of the host’s immune response. Notably, they possess low toxicity, minimal drug resistance, and excellent biocompatibility, making them promising candidates for the development of antiviral medications. For instance, carrageenan from red algae and fucoidan from brown algae have been proven effective against viruses such as influenza, HIV, and herpes simplex virus. Similarly, sulfated polysaccharides from green algae, like those found in Ulva species, exhibit antiviral properties against diseases such as the Japanese encephalitis virus. These polysaccharides function by inhibiting the attachment of viruses to host cells or by interfering with various stages of the viral life cycle. Furthermore, marine polysaccharides can boost the host’s immune responses and promote viral clearance.
While these findings underscore the antiviral potential of marine polysaccharides, most studies have been conducted in vitro or in animal models. Further clinical trials are necessary to validate their effectiveness and safety for therapeutic use. In addition, the structural complexity of marine polysaccharides poses difficulties in the standardization of extracts and formulations, which makes it challenging to establish consistent therapeutic applications. Although various antiviral mechanisms have been proposed, a comprehensive understanding of the precise molecular interactions between polysaccharides and viral components is still lacking. In addition, the natural origin of these compounds poses regulatory challenges, as they may not meet current standards required for drug approval. By addressing these challenges, marine polysaccharides could be a valuable addition to antiviral therapies, providing new solutions for the treatment of various viral infections.
XD: Software, Writing – original draft, Writing – review & editing. YQ: Software, Writing – original draft, Writing – review & editing. NJ: Writing – original draft, Writing – review & editing. YW: Software, Writing – review & editing. QN: Writing – review & editing. JW: Writing – review & editing. CZ: Supervision, Writing – original draft, Writing – review & editing. YZ: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The project was funded by FuXiaQuan National Independent Innovation Demonstration Zone Collaborative Innovation Platform Project (2023FX0001), Fujian Province Key Laboratory for the Development of Bioactive Material from Marine Algae (2022KF11), the Natural Science Foundation of Liaoning Province (2023-MS-189) and 345 Talent Program of Shengjing Hospital (M1417).
Figures were created with Biorender (https://app.biorender.com/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Matin, M, Koszarska, M, Atanasov, AG, Król-Szmajda, K, Jóźwik, A, Stelmasiak, A, et al. Bioactive potential of algae and algae-derived compounds: focus on anti-inflammatory, antimicrobial, and antioxidant effects. Molecules. (2024) 29:4695. doi: 10.3390/molecules29194695
2. Zhao, C, Lai, S, Wu, D, Liu, D, Zou, X, Ismail, A, et al. miRNAs as regulators of antidiabetic effects of fucoidans. eFood. (2019) 1:2–11. doi: 10.2991/efood.k.190822.001
3. Zhao, C, Lin, G, Wu, D, Liu, D, You, L, Högger, P, et al. The algal polysaccharide ulvan suppresses growth of hepatoma cells. Food Front. (2020) 1:83–101. doi: 10.1002/fft2.13
4. Qiu, Y, Chen, Z, Zhu, Y, Wen, J, Wen, Y, Liu, Y, et al. Green algal polysaccharides and derivatives as potential therapeutics for metabolic diseases. Food Biosci. (2024) 62:105310. doi: 10.1016/j.fbio.2024.105310
5. Gamal-Eldeen, AM, Ahmed, EF, and Abo-Zeid, MA. In vitro cancer chemopreventive properties of polysaccharide extract from the brown alga, Sargassum latifolium. Food Chem Toxicol. (2009) 47:1378–84. doi: 10.1016/j.fct.2009.03.016
6. Ananthi, S, Raghavendran, HR, Sunil, AG, Gayathri, V, Ramakrishnan, G, and Vasanthi, HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga). Food Chem Toxicol. (2010) 48:187–92. doi: 10.1016/j.fct.2009.09.036
7. Harden, EA, Falshaw, R, Carnachan, SM, Kern, ER, and Prichard, MN. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir Res. (2009) 83:282–9. doi: 10.1016/j.antiviral.2009.06.007
8. Jung, W, Choi, I, Oh, S, Park, S, Seo, S, Lee, S, et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem Toxicol. (2008) 47:293–7. doi: 10.1016/j.fct.2008.11.012
9. Mayer, AM, Rodríguez, AD, Berlinck, RG, and Hamann, MT. Marine pharmacology in 2005–6: marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim Biophys Acta-Gen Subj. (2009) 1790:283–308. doi: 10.1016/j.bbagen.2009.03.011
10. Mao, W, Li, H, Li, Y, Zhang, H, Qi, X, Sun, H, et al. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int J Biol Macromol. (2008) 44:70–4. doi: 10.1016/j.ijbiomac.2008.10.003
11. Jiao, G, Yu, G, Zhang, J, and Ewart, HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. (2011) 9:196–223. doi: 10.3390/md9020196
12. Ngo, DH, and Kim, SK. Sulfated polysaccharides as bioactive agents from marine algae. Int J Biol Macromol. (2013) 62:70–5. doi: 10.1016/j.ijbiomac.2013.08.036
13. Parvez, MK, and Parveen, S. Evolution and emergence of pathogenic viruses: past, present, and future. Intervirology. (2017) 60:1–7. doi: 10.1159/000478729
14. Payne, S. Introduction to animal viruses. Viruses. (2017) 1–11. doi: 10.1016/B978-0-12-803109-4.00001-5
15. Kwon, D. HIV: how close are we to a vaccine — or a cure? Nature. (2024). doi: 10.1038/d41586-024-02840-5
16. Eisenreich, W, Rudel, T, Heesemann, J, and Goebel, W. How viral and intracellular bacterial pathogens reprogram the metabolism of host cells to allow their intracellular replication. Front Cell Infect Microbiol. (2019) 9:42. doi: 10.3389/fcimb.2019.00042
17. Kausar, S, Khan, FS, Rehman, MIMU, Akram, M, Riaz, M, Rasool, G, et al. A review: mechanism of action of antiviral drugs. Int J Immunopathol Pharmacol. (2021) 35:205873842110026. doi: 10.1177/20587384211002621
18. Berezhnaya, YD, Kazachenko, AS, Malyar, YN, and Borovkova, VS. Sulfation of various polysaccharide structures: different methods and perspectives. Chemistry. (2024) 6:640–65. doi: 10.3390/chemistry6040038
19. Mukherjee, S, Ghosh, K, Hahn, F, Wangen, C, Strojan, H, Müller, R, et al. Chemically sulfated polysaccharides from natural sources: assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int J Biol Macromol. (2019) 136:521–30. doi: 10.1016/j.ijbiomac.2019.05.005
20. Xie, JH, Shen, MY, Nie, SP, Zhao, Q, Li, C, and Xie, MY. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydr Polym. (2014) 101:479–83. doi: 10.1016/j.carbpol.2013.09.075
21. Xie, JH, Xie, MY, Shen, MY, Nie, SP, Li, C, and Wang, YX. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J Sci Food Agric. (2010) 90:1353–60. doi: 10.1002/jsfa.3935
22. Xie, JH, Shen, MY, Xie, MY, Nie, SP, and Chen, Y. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr Polym. (2012) 89:177–84. doi: 10.1016/j.carbpol.2012.02.068
23. Liu, C, Chen, H, Chen, K, Gao, Y, Gao, S, Liu, X, et al. Sulfated modification can enhance antiviral activities of Achyranthes bidentata polysaccharide against porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Int J Biol Macromol. (2012) 52:21–4. doi: 10.1016/j.ijbiomac.2012.09.020
24. Sansone, C, Brunet, C, Noonan, DM, and Albini, A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants. (2020) 9:392. doi: 10.3390/antiox9050392
25. Sharma, A, Shahid, A, Banerjee, R, and Kumar, KJ. Emerging insights into the structure-activity relationship of water-soluble polysaccharides in antiviral therapy. Eur J Med Chem Rep. (2023) 10:100122. doi: 10.1016/j.ejmcr.2023.100122
26. Huang, L, Shen, M, Morris, GA, and Xie, J. Sulfated polysaccharides: immunomodulation and signaling mechanisms. Trends Food Sci Technol. (2019) 92:1–11. doi: 10.1016/j.tifs.2019.08.008
27. Jegadeshwari, B, and Rajarm, R. A critical review on pharmacological properties of sulfated polysaccharides from marine macroalgae. Carbohydr Polym. (2024) 344:122488. doi: 10.1016/j.carbpol.2024.122488
28. Lu, W, Yang, Z, Chen, J, Wang, D, and Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr Polym. (2021) 272:118526. doi: 10.1016/j.carbpol.2021.118526
29. Silva, TH, Alves, A, Popa, EG, Reys, LL, Gomes, ME, Sousa, RA, et al. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter. (2012) 2:278–89. doi: 10.4161/biom.22947
30. Wang, L, Wang, X, Wu, H, and Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar Drugs. (2014) 12:4984–5020. doi: 10.3390/md12094984
31. McCandless, EL, and Craigie, JS. Sulfated polysaccharides in red and brown algae. Annu Rev Plant Physiol. (1979) 30:41–53. doi: 10.1146/annurev.pp.30.060179.000353
32. Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J Appl Phycol. (2001) 13:173–84. doi: 10.1023/a:1011142124213
33. Ghanbarzadeh, M, Golmoradizadeh, A, and Homaei, A. Carrageenans and carrageenases: versatile polysaccharides and promising marine enzymes. Phytochem Rev. (2018) 17:535–71. doi: 10.1007/s11101-018-9548-2
34. Rupert, R, Rodrigues, KF, Thien, VY, and Yong, WTL. Carrageenan from Kappaphycus alvarezii (Rhodophyta, Solieriaceae): metabolism, structure, production, and application. Front Recent Dev Plant Sci. (2022) 13:859635. doi: 10.3389/fpls.2022.859635
35. Jiao, G, Yu, G, Wang, W, Zhao, X, Zhang, J, and Ewart, SH. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J Ocean Univ China. (2012) 11:205–12. doi: 10.1007/s11802-012-1906-x
36. Cunha, L, and Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar Drugs. (2016) 14:42. doi: 10.3390/md14030042
37. Bai, RG, and Tuvikene, R. Potential antiviral properties of industrially important marine algal polysaccharides and their significance in fighting a future viral pandemic. Viruses. (2021) 13:1817. doi: 10.3390/v13091817
38. De Sf-Tischer, PC, Talarico, LB, Noseda, MD, Guimarães, SMPB, Damonte, EB, and Duarte, MER. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr Polym. (2006) 63:459–65. doi: 10.1016/j.carbpol.2005.09.020
39. Knutsen, SH, Myslabodski, DE, Larsen, B, and Usov, AI. A modified system of nomenclature for red algal galactans. Bot Mar. (1994) 37:163–73. doi: 10.1515/botm.1994.37.2.163
40. Álvarez-Viñas, M, Souto, S, Flórez-Fernández, N, Torres, MD, Bandín, I, and Domínguez, H. Antiviral activity of carrageenans and processing implications. Mar Drugs. (2021) 19:437. doi: 10.3390/md19080437
41. Van De Velde, F, Knutsen, S, Usov, A, Rollema, H, and Cerezo, A. 1H and 13C high-resolution NMR spectroscopy of carrageenans: application in research and industry. Trends Food Sci Technol. (2002) 13:73–92. doi: 10.1016/s0924-2244(02)00066-3
42. Campo, VL, Kawano, DF, Da Silva, DB, and Carvalho, I. Carrageenans: biological properties, chemical modifications and structural analysis – a review. Carbohydr Polym. (2009) 77:167–80. doi: 10.1016/j.carbpol.2009.01.020
43. Qureshi, D, Nayak, SK, Maji, S, Kim, D, Banerjee, I, and Pal, K. Carrageenan: a wonder polymer from marine algae for potential drug delivery applications. Curr Pharm Des. (2019) 25:1172–86. doi: 10.2174/1381612825666190425190754
44. Li, L, Ni, R, Shao, Y, and Mao, S. Carrageenan and its applications in drug delivery. Carbohydr Polym. (2013) 103:1–11. doi: 10.1016/j.carbpol.2013.12.008
45. Li, J, Pan, A, Xie, M, Zhang, P, and Gu, X. Characterization of a thermostable κ-carrageenase from a hot spring bacterium and plant protection activity of the oligosaccharide enzymolysis product. J Sci Food Agric. (2018) 99:1812–9. doi: 10.1002/jsfa.9374
46. Carlucci, MJ, Ciancia, M, Matulewicz, MC, Cerezo, AS, and Damonte, EB. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir Res. (1999) 43:93–102. doi: 10.1016/s0166-3542(99)00038-8
47. Pujol, CA, Scolaro, LA, Ciancia, M, Matulewicz, MC, Cerezo, AS, and Damonte, EB. Antiviral activity of a carrageenan from Gigartina skottsbergii against intraperitoneal murine herpes simplex virus infection. Planta Med. (2006) 72:121–5. doi: 10.1055/s-2005-373168
48. Talarico, LB, and Damonte, EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology. (2007) 363:473–85. doi: 10.1016/j.virol.2007.01.043
49. Wang, W, Zhang, P, Hao, C, Zhang, X, Cui, Z, et al. In vitro inhibitory effect of carrageenan oligosaccharide on influenza a H1N1 virus. Antivir Res. (2011) 92:237–46. doi: 10.1016/j.antiviral.2011.08.010
50. Mabeau, S, and Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J Exp Bot. (1987) 38:1573–80. doi: 10.1093/jxb/38.9.1573
51. Du, B, Zhao, Q, Cheng, C, Wang, H, Liu, Y, Zhu, F, et al. A critical review on extraction, characteristics, physicochemical activities, potential health benefits, and industrial applications of fucoidan. eFood. (2022) 3:e19. doi: 10.1002/efd2.19
52. Anjana, K, and Arunkumar, K. Brown algae biomass for fucoxanthin, fucoidan and alginate; update review on structure, biosynthesis, biological activities and extraction valorisation. Int J Biol Macromol. (2024) 280:135632. doi: 10.1016/j.ijbiomac.2024.135632
53. Kylin, H. Zur Biochemie der Meeresalgen. Physiol Chem. (1913) 83:171–97. doi: 10.1515/bchm2.1913.83.3.171
54. Usov, AI, and Bilan, MI. Fucoidans — sulfated polysaccharides of brown algae. Russ Chem Rev. (2009) 78:785–99. doi: 10.1070/rc2009v078n08abeh004063
55. Holtkamp, AD, Kelly, S, Ulber, R, and Lang, S. Fucoidans and fucoidanases—focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol. (2008) 82:1–11. doi: 10.1007/s00253-008-1790-x
56. Kusaykin, M, Bakunina, I, Sova, V, Ermakova, S, Kuznetsova, T, Besednova, N, et al. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol J. (2008) 3:904–15. doi: 10.1002/biot.200700054
57. Sun, Q, Li, Y, Ni, L, Li, Y, Cui, Y, Jiang, S, et al. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr Polym. (2019) 229:115487. doi: 10.1016/j.carbpol.2019.115487
58. Ponce, NMA, Pujol, CA, Damonte, EB, Flores, ML, and Stortz, CA. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr Res. (2003) 338:153–65. doi: 10.1016/s0008-6215(02)00403-2
59. Hidari, KI, Takahashi, N, Arihara, M, Nagaoka, M, Morita, K, and Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. (2008) 376:91–5. doi: 10.1016/j.bbrc.2008.08.100
60. Ghosh, T, Chattopadhyay, K, Marschall, M, Karmakar, P, Mandal, P, and Ray, B. Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology. (2008) 19:2–15. doi: 10.1093/glycob/cwn092
61. Dinesh, S, Menon, T, Hanna, LE, Suresh, V, Sathuvan, M, and Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int J Biol Macromol. (2015) 82:83–8. doi: 10.1016/j.ijbiomac.2015.09.078
62. Moulard, M, Lortat-Jacob, H, Mondor, I, Roca, G, Wyatt, R, Sodroski, J, et al. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp 120. J Virol. (2000) 74:1948–60. doi: 10.1128/jvi.74.4.1948-1960.2000
63. De Parseval, A, Bobardt, MD, Chatterji, A, Chatterji, U, Elder, JH, David, G, et al. A highly conserved arginine in gp 120 governs HIV-1 binding to both syndecans and CCR5 via sulfated motifs. J Biol Chem. (2005) 280:39493–504. doi: 10.1074/jbc.m504233200
64. Akamatsu, E, Shimanaga, M, and Kamei, Y. Isolation of an anti-influenza virus substance, MC26 from a marine brown alga, Sargassum piluliferum, and its antiviral activity against influenza virus. Coastal Bioenvironment-Saga University. (2023) 1:1348–7175.
65. Mandal, P, Pujol, CA, Carlucci, MJ, Chattopadhyay, K, Damonte, EB, and Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry. (2008) 69:2193–9. doi: 10.1016/j.phytochem.2008.05.004
66. Damonte, E, Matulewicz, M, and Cerezo, A. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem. (2004) 11:2399–419. doi: 10.2174/0929867043364504
67. Eierhoff, T, Hrincius, ER, Rescher, U, Ludwig, S, and Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza a viruses (IAV) into host cells. PLoS Pathog. (2010) 6:e1001099. doi: 10.1371/journal.ppat.1001099
68. Synytsya, A, Bleha, R, Synytsya, A, Pohl, R, Hayashi, K, Yoshinaga, K, et al. Mekabu fucoidan: structural complexity and defensive effects against avian influenza a viruses. Carbohydr Polym. (2014) 111:633–44. doi: 10.1016/j.carbpol.2014.05.032
69. Wang, W, Wu, J, Zhang, X, Hao, C, Zhao, X, Jiao, G, et al. Inhibition of influenza a virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci Rep. (2017) 7:40760. doi: 10.1038/srep40760
70. Ohuchi, M, Asaoka, N, Sakai, T, and Ohuchi, R. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect. (2006) 8:1287–93. doi: 10.1016/j.micinf.2005.12.008
71. Kumar, N, Xin, Z, Liang, Y, Ly, H, and Liang, Y. NF-κB signaling differentially regulates influenza virus RNA synthesis. J Virol. (2008) 82:9880–9. doi: 10.1128/jvi.00909-08
72. Hu, P, Li, Z, Chen, M, Sun, Z, Ling, Y, Jiang, J, et al. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr Polym. (2015) 139:150–8. doi: 10.1016/j.carbpol.2015.12.019
73. Ji, D, You, L, Ren, Y, Wen, L, Zheng, G, and Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J Funct Foods. (2017) 36:332–40. doi: 10.1016/j.jff.2017.06.051
74. Park, S, Hwang, E, Shin, Y, Lee, D, Yang, J, Park, J, et al. Immunostimulatory effect of enzyme-modified Hizikia fusiforme in a mouse model in vitro and ex vivo. Mar Biotechnol. (2017) 19:65–75. doi: 10.1007/s10126-017-9727-y
75. Fan, S, Zhang, J, Nie, W, Zhou, W, Jin, L, Chen, X, et al. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma Hep G2 cells. Food Chem Toxicol. (2017) 102:53–62. doi: 10.1016/j.fct.2017.01.020
76. Sun, Y, Chen, X, Zhang, L, Liu, H, Liu, S, Yu, H, et al. The antiviral property of Sargassum fusiforme polysaccharide for avian leukosis virus subgroup J in vitro and in vivo. Int J Biol Macromol. (2019) 138:70–8. doi: 10.1016/j.ijbiomac.2019.07.073
77. Gong, J, Chen, M, Zheng, Y, Wang, S, and Wang, Y. Polymeric micelles drug delivery system in oncology. J Control Release. (2012) 159:312–23. doi: 10.1016/j.jconrel.2011.12.012
78. Sun, Y, Chen, X, Liu, H, Liu, S, Yu, H, Wang, X, et al. Preparation of new Sargassum fusiforme polysaccharide long-chain alkyl group nanomicelles and their antiviral properties against ALV-J. Molecules. (2021) 26:3265. doi: 10.3390/molecules26113265
79. Lahaye, M, and Ray, B. Cell-wall polysaccharides from the marine green alga “Ulva rigida” (Ulvales, Chlorophyta) — NMR analysis of ulvan oligosaccharides. Carbohydr Res. (1996) 283:161–73. doi: 10.1016/0008-6215(95)00407-6
80. Lahaye, M, Robic, A, and Biopolymères, IA. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. (2007) 8:1765–74. doi: 10.1021/bm061185q
81. Ngo, D, Wijesekara, I, Vo, T, Van Ta, Q, and Kim, S. Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res Int. (2010) 44:523–9. doi: 10.1016/j.foodres.2010.12.030
82. Wijesekara, I, Pangestuti, R, and Kim, S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym. (2010) 84:14–21. doi: 10.1016/j.carbpol.2010.10.062
83. Hardouin, K, Bedoux, G, Burlot, A, Donnay-Moreno, C, Bergé, J, Nyvall-Collén, P, et al. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. (2016) 16:233–9. doi: 10.1016/j.algal.2016.03.013
84. Berri, M, Olivier, M, Holbert, S, Dupont, J, Demais, H, Goff, ML, et al. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res. (2017) 28:39–47. doi: 10.1016/j.algal.2017.10.008
85. Song, L, Chen, X, Liu, X, Zhang, F, Hu, L, Yue, Y, et al. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar Drugs. (2015) 14:4. doi: 10.3390/md14010004
86. Kanekiyo, K, Hayashi, K, Takenaka, H, Lee, J, and Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol Pharm Bull. (2007) 30:1573–5. doi: 10.1248/bpb.30.1573
87. Hoke, CH, Nisalak, A, Sangawhipa, N, Jatanasen, S, Laorakapongse, T, Innis, BL, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. (1988) 319:608–14. doi: 10.1056/nejm198809083191004
88. Howley, PM, and Knipe, DM. Fields virology: Emerging viruses Lippincott Williams & Wilkins (2020).
89. Chiu, Y, Chan, Y, Li, T, and Wu, C. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar Biotechnol. (2011) 14:468–78. doi: 10.1007/s10126-011-9428-x
90. Carlucci, M, Scolaro, L, and Damonte, E. Inhibitory action of natural carrageenans on herpes simplex virus infection of mouse astrocytes. Chemotherapy. (1999) 45:429–36. doi: 10.1159/000007236
91. Griffin, DE. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Invest. (1997) 100:2948–51. doi: 10.1172/jci119847
92. Woolfson, AD, Malcolm, RK, and Gallagher, R. Drug delivery by the intravaginal route. Crit Rev Ther Drug Carrier Syst. (2000) 17:47. doi: 10.1615/critrevtherdrugcarriersyst.v17.i5.30
93. Zlotnik, A, and Yoshie, O. Chemokines. Immunity. (2000) 12:121–7. doi: 10.1016/s1074-7613(00)80165-x
94. Ray, B, Ali, I, Jana, S, Mukherjee, S, Pal, S, Ray, S, et al. Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses. (2021) 14:14. doi: 10.3390/v14010035
95. Hans, N, Malik, A, and Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour Technol Rep. (2020) 13:100623. doi: 10.1016/j.biteb.2020.100623
96. Song, S, Peng, H, Wang, Q, Liu, Z, Dong, X, Wen, C, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. (2020) 11:7415–20. doi: 10.1039/d0fo02017f
97. Saha, S, Navid, MH, Bandyopadhyay, SS, Schnitzler, P, and Ray, B. Sulfated polysaccharides from Laminaria angustata: structural features and in vitro antiviral activities. Carbohydr Polym. (2011) 87:123–30. doi: 10.1016/j.carbpol.2011.07.026
98. Carlucci, M, Scolaro, L, and Damonte, E. Herpes simplex virus type 1 variants arising after selection with an antiviral carrageenan: lack of correlation between drug susceptibility and syn phenotype. J Med Virol. (2002) 68:92–8. doi: 10.1002/jmv.10174
99. Chen, L, and Huang, G. The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol. (2018) 115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056
100. Okechukwu, QN, Adepoju, FO, Kanwugu, ON, Adadi, P, Serrano-Aroca, Á, Uversky, VN, et al. Marine-derived bioactive metabolites as a potential therapeutic intervention in managing viral diseases: insights from the SARS-CoV-2 in silico and pre-clinical studies. Pharmaceuticals. (2024) 17:328. doi: 10.3390/ph17030328
101. Emrani, J, Ahmed, M, Jeffers-Francis, L, Teleha, JC, Mowa, N, Newman, RH, et al. SARS-CoV-2, infection, transmission, transcription, translation, proteins, and treatment: a review. Int J Biol Macromol. (2021) 193:1249–73. doi: 10.1016/j.ijbiomac.2021.10.172
102. Hathaway, D, Pandav, K, Patel, M, Riva-Moscoso, A, Singh, BM, Patel, A, et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. (2020) 52:478–95. doi: 10.3947/ic.2020.52.4.478
103. Xian, Y, Zhang, J, Bian, Z, Zhou, H, Zhang, Z, Lin, Z, et al. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin B. (2020) 10:1163–74. doi: 10.1016/j.apsb.2020.06.002
104. Oliyaei, N, Moosavi-Nasab, M, and Mazloomi, SM. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. Biotech. (2022) 12:154. doi: 10.1007/s13205-022-03210-6
105. Millet, JK, and Whittaker, GR. Host cell entry of middle east respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci. (2014) 111:15214–9. doi: 10.1073/pnas.1407087111
106. Yang, CW, Hsu, HY, Lee, YZ, Jan, JT, Chang, SY, Lin, YL, et al. Natural fucoidans inhibit coronaviruses by targeting viral spike protein and host cell furin. Biochem Pharmacol. (2023) 215:115688. doi: 10.1016/j.bcp.2023.115688
107. Mazumder, S, Ghosal, PK, Pujol, CA, Carlucci, MJ, Damonte, EB, and Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int J Biol Macromol. (2002) 31:87–95. doi: 10.1016/s0141-8130(02)00070-3
108. Carlucci, MJ, Pujol, CA, Ciancia, M, Noseda, MD, Matulewicz, MC, Damonte, EB, et al. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int J Biol Macromol. (1997) 20:97–105. doi: 10.1016/s0141-8130(96)01145-2
109. Trinchero, J, Ponce, NMA, Córdoba, OL, Flores, ML, Pampuro, S, Stortz, CA, et al. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother Res. (2008) 23:707–12. doi: 10.1002/ptr.2723
110. Miao, B, Geng, M, Li, J, Li, F, Chen, H, Guan, H, et al. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem Pharmacol. (2004) 68:641–9. doi: 10.1016/j.bcp.2004.04.009
111. Sozzani, S, Bosisio, D, Scarsi, M, and Tincani, A. Type I interferons in systemic autoimmunity. Autoimmunity. (2010) 43:196–203. doi: 10.3109/08916930903510872
112. Xin, X, Ding, H, Geng, M, Liang, P, Li, Y, and Guan, H. Studies of the anti-AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin J Mar Drugs. (2000) 19:4–8.
113. Queiroz, K, Medeiros, V, Queiroz, L, Abreu, L, Rocha, H, Ferreira, C, et al. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother. (2008) 62:303–7. doi: 10.1016/j.biopha.2008.03.006
114. Talarico, LB, Noseda, MD, Ducatti, DRB, Duarte, MER, and Damonte, EB. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J Gen Virol. (2011) 92:1332–42. doi: 10.1099/vir.0.028522-0
115. Besednova, N, Makarenkova, I, Zvyagintseva, T, Imbs, T, Somova, L, and Zaporozhets, T. Antiviral action and pathogenetic targets for seaweed sulfated polysaccharides in herpesvirus infections. Biomed Khim. (2016) 62:217–27. doi: 10.18097/pbmc20166203217
116. Zhou, G, Sun, Y, Xin, H, Zhang, Y, Li, Z, and Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol Res. (2004) 50:47–53. doi: 10.1016/j.phrs.2003.12.002
117. Yuan, H, Song, J, Li, X, Li, N, and Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. (2006) 243:228–34. doi: 10.1016/j.canlet.2005.11.032
118. Mori, T, O’Keefe, BR, Sowder, RC, Bringans, S, Gardella, R, Berg, S, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. (2004) 280:9345–53. doi: 10.1074/jbc.m411122200
119. Emau, P, Tian, B, O’Keefe, B, Mori, T, McMahon, J, Palmer, K, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J Med Primatol. (2007) 36:244–53. doi: 10.1111/j.1600-0684.2007.00242.x
120. Férir, G, Palmer, KE, and Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology. (2011) 417:253–8. doi: 10.1016/j.virol.2011.07.004
121. Xiong, S, Fan, J, and Kitazato, K. The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biotechnol. (2010) 86:805–12. doi: 10.1007/s00253-010-2470-1
122. Balzarini, J, Van Laethem, K, Peumans, WJ, Van Damme, EJ, Bolmstedt, A, Gago, F, et al. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp 120 envelopes. J Virol. (2006) 80:8411–21. doi: 10.1128/jvi.00369-06
123. Alexandre, KB, Gray, ES, Lambson, BE, Moore, PL, Choge, IA, Mlisana, K, et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp 120 and sensitivity to the lectins, griffithsin, cyanovirin-N and scytovirin. Virology. (2010) 402:187–96. doi: 10.1016/j.virol.2010.03.021
124. Santoyo, S, Jaime, L, Plaza, M, Herrero, M, Rodriguez-Meizoso, I, Ibañez, E, et al. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J Appl Phycol. (2011) 24:731–41. doi: 10.1007/s10811-011-9692-1
125. Santoyo, S, Plaza, M, Jaime, L, Ibañez, E, Reglero, G, and Señorans, FJ. Pressurized liquid extraction as an alternative process to obtain antiviral agents from the edible microalga Chlorella vulgaris. J Agric Food Chem. (2010) 58:8522–7. doi: 10.1021/jf100369h
126. Lee, W, Kim, M, Lee, S, Jung, H, and Oh, J. Prophylactic efficacy of orally administered Bacillus poly-γ-glutamic acid, a non-LPS TLR4 ligand, against norovirus infection in mice. Sci Rep. (2018) 8:26935. doi: 10.1038/s41598-018-26935-y
127. Kim, M, Lee, J, Cho, H, Jung, H, Lee, W, Seo, HY, et al. Antiviral efficacy of orally delivered neoagarohexaose, a nonconventional TLR4 agonist, against norovirus infection in mice. Biomaterials. (2020) 263:120391. doi: 10.1016/j.biomaterials.2020.120391
128. Lee, DY, Lin, X, Paskaleva, EE, Liu, Y, Puttamadappa, SS, Thornber, C, et al. Palmitic acid is a novel CD4 fusion inhibitor that blocks HIV entry and infection. AIDS Res Hum Retrovir. (2009) 25:1231–41. doi: 10.1089/aid.2009.0019
129. Shih, S, Tsai, K, Li, Y, Chueh, C, and Chan, E. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J Med Virol. (2003) 70:119–25. doi: 10.1002/jmv.10363
130. Reynolds, D, Huesemann, M, Edmundson, S, Sims, A, Hurst, B, Cady, S, et al. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: a review of antiviral potential throughout pathogenesis. Algal Res. (2021) 57:102331. doi: 10.1016/j.algal.2021.102331
131. Paskaleva, EE, Lin, X, Li, W, Cotter, R, Klein, MT, Roberge, E, et al. Inhibition of highly productive HIV-1 infection in T cells, primary human macrophages, microglia, and astrocytes by Sargassum fusiforme. AIDS Res Ther. (2006) 3:15. doi: 10.1186/1742-6405-3-15
132. Paskaleva, EE, Lin, X, Duus, K, McSharry, JJ, Veille, JL, Thornber, C, et al. Sargassum fusiforme fraction is a potent and specific inhibitor of HIV-1 fusion and reverse transcriptase. Virol J. (2008) 5:8. doi: 10.1186/1743-422x-5-8
133. Resh, MD. A myristoyl switch regulates membrane binding of HIV-1 gag. Proc Natl Acad Sci. (2004) 101:417–8. doi: 10.1073/pnas.0308043101
134. De Souza, LM, Sassaki, GL, Romanos, MTV, and Barreto-Bergter, E. Structural characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from southeastern Brazilian coast. Mar Drugs. (2012) 10:918–31. doi: 10.3390/md10040918
135. Chirasuwan, N, Chaiklahan, R, Kittakoop, P, Chanasattru, W, Ruengjitchatchawalya, M, Tanticharoen, M, et al. Anti-HSV-1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Sci Asia. (2009) 35:137–41. doi: 10.2306/scienceasia1513-1874.2009.35.137
136. Wang, H, Li, Y, Shen, W, Rui, W, Ma, X, and Cen, Y. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot Mar. (2007) 50:185–90. doi: 10.1515/bot.2007.022
137. Plouguerné, E, De Souza, L, Sassaki, G, Cavalcanti, J, Romanos, MV, Da Gama, B, et al. Antiviral sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar Drugs. (2013) 11:4628–40. doi: 10.3390/md11114628
138. Ueno, M, Nogawa, M, Siddiqui, R, Watashi, K, Wakita, T, Kato, N, et al. Acidic polysaccharides isolated from marine algae inhibit the early step of viral infection. Int J Biol Macromol. (2018) 124:282–90. doi: 10.1016/j.ijbiomac.2018.11.152
139. Mendes, GS, Duarte, ME, Colodi, FG, Noseda, MD, Ferreira, LG, Berté, SD, et al. Structure and anti-metapneumovirus activity of sulfated galactans from the red seaweed Cryptonemia seminervis. Carbohydr Polym. (2013) 101:313–23. doi: 10.1016/j.carbpol.2013.09.026
140. Thuy, TTT, Ly, BM, Van, TTT, Van Quang, N, Tu, HC, Zheng, Y, et al. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr Polym. (2014) 115:122–8. doi: 10.1016/j.carbpol.2014.08.068
141. Pujol, CA, Ray, S, Ray, B, and Damonte, EB. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int J Biol Macromol. (2012) 51:412–6. doi: 10.1016/j.ijbiomac.2012.05.028
142. Mycroft-West, CJ, Su, D, Pagani, I, Rudd, TR, Elli, S, Guimond, SE, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost. (2020) 120:1700–15. doi: 10.1055/s-0040-1721319
143. Ciejka, J, Botwina, P, Nowakowska, M, Szczubiałka, K, and Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS One. (2019) 14:e0214646. doi: 10.1371/journal.pone.0214646
144. Morokutti-Kurz, M, Graf, C, and Prieschl-Grassauer, E. Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int J Gen Med. (2017) 10:53–60. doi: 10.2147/ijgm.s120665
145. Faccin-Galhardi, LC, Ray, S, Lopes, N, Ali, I, Espada, SF, Santos, JPD, et al. Assessment of antiherpetic activity of nonsulfated and sulfated polysaccharides from Azadirachta indica. Int J Biol Macromol. (2019) 137:54–61. doi: 10.1016/j.ijbiomac.2019.06.129
146. Wang, S, Liu, Z, Qin, L, and He, M. Structural characterization of the marine sulfated polysaccharide PAE with antiviral activity. Chin J Mar Drugs. (2019) 38:17–22.
147. Kim, M, Yim, JH, Kim, SY, Kim, HS, Lee, WG, Kim, SJ, et al. In vitro inhibition of influenza a virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir Res. (2012) 93:253–9. doi: 10.1016/j.antiviral.2011.12.006
148. Huang, X, Wang, D, Hu, Y, Lu, Y, Guo, Z, Kong, X, et al. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int J Biol Macromol. (2007) 42:166–71. doi: 10.1016/j.ijbiomac.2007.10.019
149. Chen, Y, Yang, Y, Yuan, W, Wang, Z, Ming, K, Zeng, L, et al. Effects of bush sophora root polysaccharide and its sulfate on DHAV-1 replication. Carbohydr Polym. (2018) 197:508–14. doi: 10.1016/j.carbpol.2018.06.039
150. Wang, H, Zhang, N, Li, H, and Yu, L. Effect of sulfated modification on Codonopsis pilosula polysaccharide against HSV-I. China Sciencepap. (2015) 10:706–9.
151. Liu, Y, He, K, Yang, M, and Pu, Q. Antiviral activity against HSV-2 of sulfated polysaccharide from Cyathula Officinalis Kuan in vitro. Chin J Appl Environ Biol. (2004) 10:46–50.
152. Hayashi, K, Hayashi, T, and Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res Hum Retrovir. (1996) 12:1463–71. doi: 10.1089/aid.1996.12.1463
153. Baba, M, Snoeck, R, Pauwels, R, and De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. (1988) 32:1742–5. doi: 10.1128/aac.32.11.1742
154. Lomartire, S, and Gonçalves, AMM. Antiviral activity and mechanisms of seaweeds bioactive compounds on enveloped viruses—a review. Mar Drugs. (2022) 20:385. doi: 10.3390/md20060385
155. Chi, Y, Zhang, M, Wang, X, Fu, X, Guan, H, and Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int J Biol Macromol. (2020) 157:75–82. doi: 10.1016/j.ijbiomac.2020.04.187
156. Yamada, T, Ogamo, A, Saito, T, Uchiyama, H, and Nakagawa, Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydr Polym. (2000) 41:115–20. doi: 10.1016/s0144-8617(99)00083-1
157. Bouhlal, R, Haslin, C, Chermann, J, Colliec-Jouault, S, Sinquin, C, Simon, G, et al. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophyta, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar Drugs. (2011) 9:1187–209. doi: 10.3390/md9071187
Keywords: marine polysaccharides, sulfated polysaccharides, virus, antiviral mechanisms, algae
Citation: Dong X, Qiu Y, Jia N, Wu Y, Nie Q, Wen J, Zhao C and Zhai Y (2025) Recent advances of edible marine algae-derived sulfated polysaccharides in antiviral treatments: challenges vs. opportunities. Front. Nutr. 12:1561119. doi: 10.3389/fnut.2025.1561119
Received: 16 January 2025; Accepted: 12 March 2025;
Published: 26 March 2025.
Edited by:
Miguel Angel Prieto Lage, University of Vigo, SpainReviewed by:
Pauline Donn, University of Yaounde I, CameroonCopyright © 2025 Dong, Qiu, Jia, Wu, Nie, Wen, Zhao and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongzhen Zhai, emhhaXl6QHNqLWhvc3BpdGFsLm9yZw==; Chao Zhao, emhjaGFvQGxpdmUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.