
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 20 March 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1560913
This article is part of the Research TopicImpact of Oxidation on Nutrition: Source, Absorption and Health EffectsView all 6 articles
 Yong Huang1†
Yong Huang1† Linfeng Wang1†
Linfeng Wang1† Gaojie Zhang1†
Gaojie Zhang1† Yueqiang Peng2
Yueqiang Peng2 Qiao Xu3
Qiao Xu3 Ziling Wei4
Ziling Wei4 Jiang Yu1
Jiang Yu1 Huayang Zhang5
Huayang Zhang5 Yao Zhang1*‡
Yao Zhang1*‡ Jiayu Liu1*‡
Jiayu Liu1*‡Background: The relationship between dietary oxidative balance score (DOBS) and diabetes-related renal events remains unclear.
Methods: In this study, the associations between serum micronutrients and diabetic nephropathy (DN) in participants matched by propensity score (PSM) were retrospectively analyzed. And next, a cross-sectional analysis was performed with the National Health and Nutritional Examination Survey (NHANES) database. Weighted multivariate adjusted logistic regression models, dose–response curves, subgroup analysis, and mediation analysis were the main methods of this study. Finally, sensitivity analyses were performed by PSM and multiple imputation (MI).
Results: Retrospective findings suggest that single antioxidants may not be representative of an individual’s overall antioxidant levels. The results of the cross-sectional study indicated that the higher the DOBS, the greater the beneficial effects on DN [Q4 vs. Q1: OR (95% CI): 0.78 (0.63, 0.96), p for trend = 0.008] and renal function in DN [Q4 vs. Q1: β (95% CI): 5.395 (1.590, 9.199), p for trend = 0.004]. The above correlations were linear negative correlation (p for nonlinear = 0.989) and linear positive correlation (p for nonlinear = 0.593) respectively. Chronic inflammation mediated the above associations to some extent. The results of sensitivity analysis were consistent with the original analysis.
Conclusion: Higher dietary antioxidant exposure has a positive effect on DN and renal function in DN, mediated partially by chronic inflammation.
The global health burden of diabetes on individuals, families, and nations continues to escalate. According to the 2021 International Diabetes Federation (IDF) Diabetes Atlas, approximately 537 million (10.5%) adults aged 20–79 worldwide currently live with diabetes, a figure projected to rise to 783 million by 2045 (1). Among its most severe complications, diabetic nephropathy (DN) is defined as the coexistence of diabetes and chronic kidney disease (CKD) in the absence of other identifiable causes of renal injury. CKD itself is characterized by kidney damage persisting for ≥3 months, evidenced by reduced glomerular filtration rate (GFR < 60 mL/min/1.73m2) or increased albuminuria (2). Epidemiological studies indicate that 20–40% of individuals with diabetes develop DN (3), which now constitutes a leading etiology of end-stage renal disease (ESRD), affecting approximately 40% of type 2 and 30% of type 1 diabetes patients (4, 5).
Oxidative stress (OS), a pivotal pathophysiological mechanism in DN, arises from dynamic imbalances between pro-oxidant factors and antioxidant defense systems (6). While preclinical evidence demonstrates that antioxidants mitigate diabetes and its complications by suppressing OS and reducing free radical production (7), their clinical utility in DN management remains controversial. Existing clinical trials report conflicting conclusions regarding the association between antioxidant interventions and DN progression (8–10), potentially attributable to the predominant focus on isolated antioxidant agents. This observation prompted our hypothesis: the biological effects of single antioxidants may inadequately reflect an individual’s holistic antioxidant capacity.
To address this research gap, we introduced an innovative analytical framework. The Dietary Oxidative Balance Score (DOBS), a novel dietary assessment tool, systematically quantifies the dynamic equilibrium between pro-oxidant and antioxidant components in diets, with established associations to biological aging and periodontal health (11, 12). Although prior studies have explored DN correlations using the Composite Dietary Antioxidant Index (CDAI) (13), DOBS encompassing a broader spectrum of dietary constituents remains uninvestigated in the context of DN and its renal function outcomes.
This study employs a three-phase analytical framework: First, a retrospective analysis preliminarily explores associations between serum trace elements and DN-related renal dysfunction. Second, cross-sectional analyses elucidate dose–response relationships between DOBS and the prevalence of DN and its renal function. Finally, mediation analyses probe the potential role of chronic inflammation in these associations.
This study employed a two-phase analytical approach. Initially, a retrospective analysis was conducted utilizing data from the Physical Examination Center of our institution, spanning January 2023 to December 2023. Subsequently, a cross-sectional analysis was performed using data from the National Health and Nutritional Examination Survey (NHANES) database.
The retrospective component of this investigation received ethical approval from the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University on June 11, 2024 (Ethics Approval Number: 2024–032-01; ChiCTR2400086297). For the NHANES component, as the database had already obtained approval from the National Center for Health Statistics (NCHS) Research Ethics Review Board, no additional ethical clearance was required from our institution. All participants had signed an informed consent form.
The exclusion criteria were defined as follows: (1) absence of microelement testing; (2) comorbidities involving impaired renal function due to primary or secondary renal diseases; (3) pregnancy or presence of other specific types of diabetes mellitus; and (4) comorbidities with severe cardiovascular or cerebrovascular diseases, hyper- or hypothyroidism, or malignant neoplasms. Participants were stratified into a DN group and a non-DN group based on predefined thresholds for FBG ≥ 126 mg/dL and UACR ≥30 mg/g. To ensure comparability, baseline characteristics between the two groups were balanced using 1:1 propensity score matching (PSM). Following this process, a total of 124 participants were enrolled in each group (Figure 1).
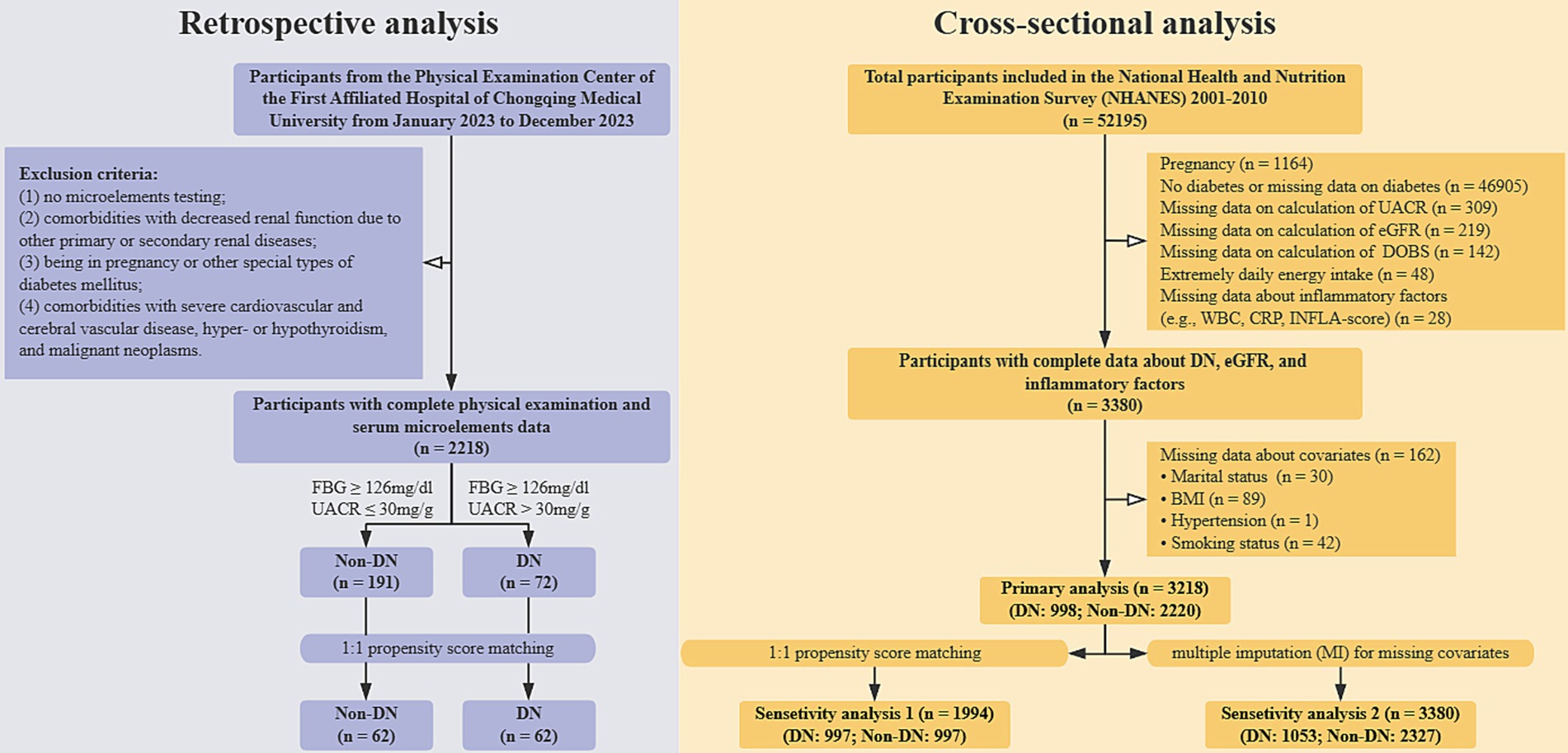
Figure 1. The general flowchart of this study. DOBS, Dietary oxidative balance score; DN, Diabetic nephropathy; UACR, Urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; WBC, White blood cell; CRP, C-reactive protein; INFLA-score, Low-grade inflammation score; BMI, Body mass index.
A total of 5 cycles of data from NHANES 2001–2010 were incorporated. The specific strategies used to exclude participants were as follows: (1) Pregnancy status (n = 1,164); (2) Non-diabetes or missing data on diagnosis of diabetes (n = 46,905); (3) Missing data on the calculation of UACR (n = 309); (4) Missing data on calculation of estimated glomerular filtration rate (eGFR) (n = 219); (5) Missing data on intake of dietary antioxidants or pro-oxidants (n = 142); (6) Extremely energy intake (< 500 kcal/day or > 5,000 kcal/day) (n = 48); (14) (7) Missing data on inflammatory factors (n = 28); (8) Missing data on covariates (n = 162). Ultimately, a total of 3,218 participants were enrolled in this study (Figure 1).
Data from 5 cycles of the NHANES spanning 2001 to 2010 were included in this study. The following exclusion criteria were applied sequentially: (1) pregnancy (n = 1,164); (2) absence of diabetes or missing diabetes diagnosis data (n = 46,905); (3) missing UACR data (n = 309); (4) missing estimated glomerular filtration rate (eGFR) data (n = 219); (5) missing dietary antioxidant or pro-oxidant intake data (n = 142); (6) extreme energy intake (< 500 kcal/day or > 5,000 kcal/day) (n = 48); (7) missing inflammatory factor data (n = 28); and (8) missing covariate data (n = 162). After applying these exclusion criteria, a final cohort of 3,218 participants was included in the analysis (Figure 1).
Dietary intake was assessed using 24-h dietary recall records. Each participant was eligible for two interview sessions. For participants with two complete dietary recall interviews, the mean value of the two sessions was calculated to represent their dietary intake. In cases where only one interview was completed, the data from the first session were used. The DOBS was calculated based on the intake of 14 dietary antioxidants and 2 dietary pro-oxidants (12), as detailed in Supplementary Tables 1, 2. The individual scores for these 16 components were summed to derive the DOBS, with higher scores indicating greater antioxidant levels.
In this study, criteria for the diagnosis of diabetes were as follows: (1) FBG ≥ 126 mg/dL; (2) glycosylated hemoglobin ≥6.5%; (3) doctor told you have diabetes; (4) 2-h oral glucose tolerance test (OGTT) ≥ 200 mg/dL; (5) oral hypoglycemic drugs or use insulin. Those who met any of these criteria were considered to be diabetic. Diabetic patients with a UACR >30 mg/g were recognized as DN (15). Then, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was utilized to calculate eGFR, which can be completed using the “CKDEpi.creat” package of the R software (16). It took into account the participant’s serum creatinine (Scr), age, gender and race (17). The details were as follows:
Based on previous studies (13, 15), variance inflation factors (VIF) < 10 were considered to be free of multicollinearity (18), the following covariates (age, gender, race, marital status, BMI, hypertension and smoking status) were finally included in this study. Hypertension was recognized as having a systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 80 mmHg or being on antihypertensive medications. Smoking status was categorized as non-smoker, previous smoker or current smoker.
To investigate the potential mediating role of inflammation in the relationship between exposure and outcome, this study incorporated several laboratory indicators as markers of inflammation. These included white blood cell count (WBC), lymphocyte count (L), monocyte count (M), neutrophil count (N), and C-reactive protein (CRP). Additionally, a composite indicator, the low-grade inflammation score (INFLA-score), was utilized. The specific calculation methodology for the INFLA-score is detailed in Supplementary Table 1. Briefly, the scores for each of the four parameters were summed to derive the INFLA-score, which ranges from −16 to 16. Higher INFLA-scores indicate elevated levels of low-grade inflammation (19).
In the retrospective analysis, continuous variables were summarized as mean ± standard deviation (SD) for normally distributed data or median (interquartile range) for non-normally distributed data. Categorical variables were expressed as percentages. Statistical analyses included the independent samples t-test for normally distributed continuous variables, the Mann–Whitney U test for non-normally distributed continuous variables, and the chi-square test for categorical variables. Correlation analyses were performed using Pearson’s or Spearman’s methods, depending on data distribution. Both univariate and multivariate logistic regression analyses were conducted to assess associations between variables.
Considering the complex sampling design of NHANES and in order to make the sample nationally representative, the specific rules for weight use were as follows: (1) As mentioned before, if the participants had two complete dietary data, the dietary two-day sample weight (WTDR2D) was used as a weight, otherwise the dietary day one sample weight (WTDRD1) was used as a weight. (2) Data from NHANES 2001–2010 for a total of 5 cycles were included, so the final weight used was either WTDRD1 * 1/5 or WTDR2D * 1/5.
In the cross-sectional analysis, participants’ baseline characteristics were first described. The relationship between the DOBS and DN, as well as eGFR in DN, was examined using multivariable-adjusted regression models and stratified analyses. To explore the dose–response relationship between DOBS and outcomes, restricted cubic spline (RCS) analysis and threshold effects analysis were employed. Additionally, an exploratory mediation analysis was conducted using the “mediation” package in R software to investigate the potential mediating role of inflammation in the relationship between DOBS and outcomes. To ensure the robustness of the findings, two sensitivity analyses were performed. First, 1:1 PSM was conducted using the “MatchIt” package in R software. Second, multiple imputation (MI) was applied using the “mice” package in R software to address missing data. New datasets were generated through these methods, and the aforementioned analyses were repeated to verify result stability (Figure 1).
All statistical analyses in this study were performed with the help of R software (version 4.2.1), SPSS 26.0 and EmpowerStat software (version 4.1). The criterion for statistical significance was p < 0.05.
Supplementary Table 3 demonstrated the clinical baseline data for the two groups of participants after PSM. Regarding serum microelements, only serum selenium was considered to be an independent protective factor for DN [Odds ratio (OR) (95% CI), p: 0.764 (0.638, 0.917), 0.004] (Supplementary Table 4). In addition, Supplementary Table 5 exhibited the correlation between serum microelements and renal function in DN, and it could be found that serum iron, zinc, and selenium were positively correlated with renal function in DN (All r > 0 and p < 0.05).
Table 1 demonstrates the weighted baseline characteristics of the participants after DOBS categorization through quartiles. It could be found that the higher the DOBS, the lower the prevalence of DN and the higher the eGFR in DN (p < 0.001 and p = 0.008, respectively). Additionally, it was interesting to note that this study also observed a greater reduction in WBC, N, CRP, and INFLA-score in the higher DOBS group (All p < 0.001). A total of 1994 participants (997 with DN and 997 without DN) were included after PSM. A schematic diagram of the balance of the dataset before and after PSM was shown in Supplementary Figure 1. Supplementary Table 6 shows the specific details of the weighted baseline characteristics of the participants after PSM, which were consistent with the primary analysis.
The relationships between DOBS and DN and eGFR in DN were demonstrated in Tables 2, 3, respectively. Under the fully adjusted model (Model 3), increased DOBS was associated with reduced prevalence of DN [OR (95% CI), p: 0.98 (0.97, 1.00), 0.006; p for trend = 0.008] and improved eGFR in DN [β (95% CI), p: 0.254 (0.047, 0.461), 0.016; p for trend = 0.004]. Further, to assess whether missing covariates affected the research results, 5 new datasets were obtained in this study through MI. The above analysis was repeated and the effect values were combined. The detailed results were exhibited in Supplementary Table 7. Moreover, the correlations between DOBS and DN and eGFR in DN under different grouping conditions before and after PSM were shown in Supplementary Tables 8, 9, respectively. It could be seen that the association between DOBS and the two outcomes remained stable across subgroups (All p for interaction >0.05).
Figure 2 demonstrates the results of RCS in this study. Taking the DOBS values at OR = 1 and β = 0 as reference values, the relationships between DOBS and DN (p for nonlinear = 0.989) and eGFR in DN (p for nonlinear = 0.593) could be considered as linear. Furthermore, the results of the threshold effects analysis similarly indicated that the effects of DOBS with DN (log likelihood ratio = 0.095) and eGFR in DN (log likelihood ratio = 0.106) both were appropriate to be explained by a single-line model (Supplementary Table 10). These results were consistent across sensitivity analyses.
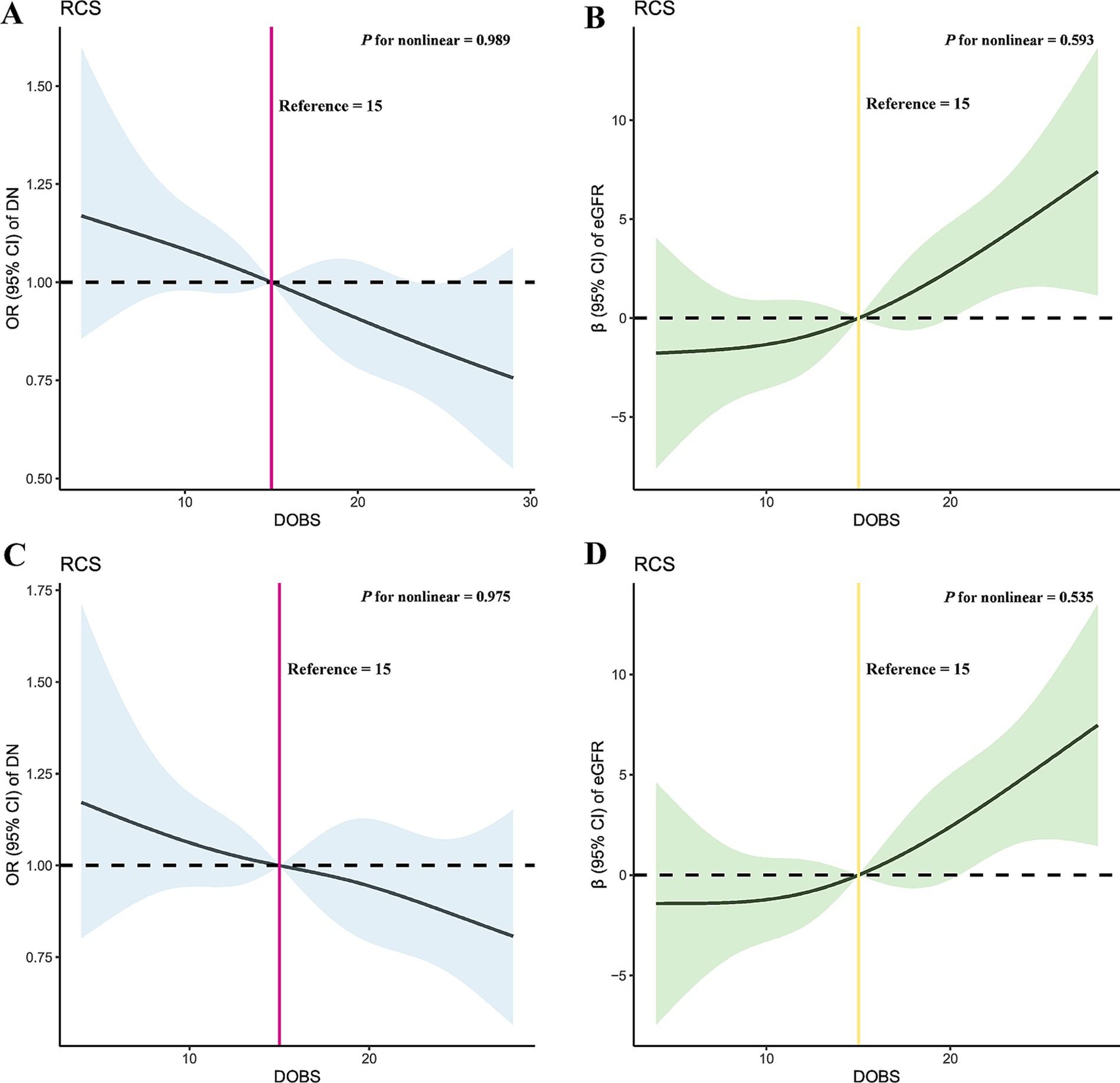
Figure 2. Dose–response relationship between DOBS and DN and eGFR in DN before and after PSM through RCS. (A) RCS of DOBS and DN before PSM; (B) RCS of DOBS and eGFR in DN before PSM; (C) RCS of DOBS and DN after PSM; (D) RCS of DOBS and eGFR in DN after PSM. DOBS, Dietary oxidative balance score; DN, Diabetic nephropathy; eGFR, estimated glomerular filtration rate; RCS, Restricted cubic splines; PSM, Propensity score matching; OR, Odds ratio.
Under the fully adjusted model (model 3), WBC (β, p: −0.022, < 0.001), N (β, p: −0.020, < 0.001), CRP (β, p: −0.012, < 0.001) and INFLA-score (β, p: −0.049, 0.002) were further employed for exploratory mediation analysis between DOBS and DN (Figure 3). In addition, CRP (β, p: −0.020, 0.004) was further employed for exploratory mediation analysis between DOBS and eGFR in DN (Figure 3). The results of the exploratory mediation analysis presented in Figure 4 indicate that CRP and INFLA-score mediated the association between DOBS and DN to some extent, with the mediation proportions of 6.19 and 6.00%, respectively.

Figure 3. The relationship between DOBS and inflammation-mediating factors. DOBS, Dietary oxidative balance score; WBC, White blood cell; L, Lymphocyte; M, Monocyte; N, Neutrophil; CRP, C-reactive protein; INFLA-score, Low-grade inflammation score.
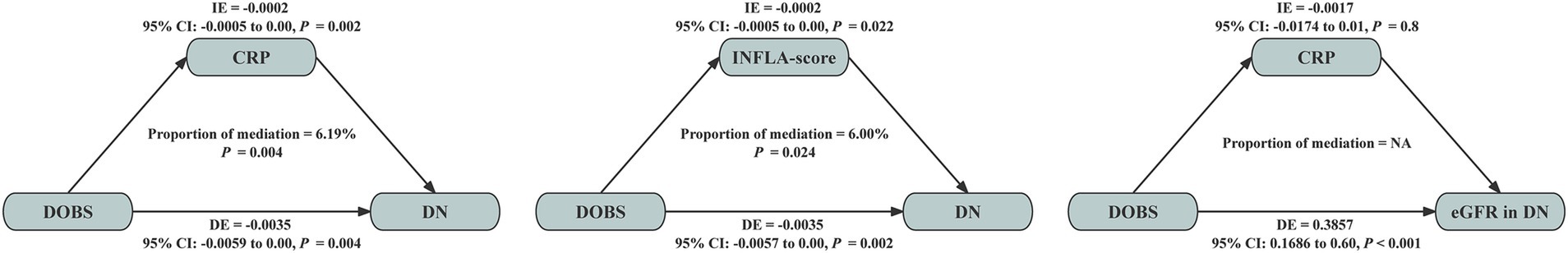
Figure 4. Mediation analyses with inflammatory factors between the association of DOBS and DN and eGFR in DN. DOBS, Dietary oxidative balance score; DN, Diabetic nephropathy; CRP, C-reactive protein; INFLA-score, Low-grade inflammation score; IE, Indirect effect; DE, Direct effect.
In a preliminary retrospective analysis, this study identified a protective effect of serum selenium against DN, which contrasts with previous findings (20). Given this discrepancy, we hypothesized that a single antioxidant may not adequately represent an individual’s overall antioxidant status. Furthermore, dietary micronutrient intake is the primary source of serum micronutrients. Motivated by these considerations, this study introduced the DOBS as a comprehensive index to assess the balance between dietary oxidative and antioxidant components.
To our knowledge, this is the first study to investigate the relationship between the DOBS and DN, as well as renal function in DN. In the cross-sectional analysis, higher DOBS levels were associated with a reduced prevalence of DN and improved renal function in DN, as demonstrated by the fully adjusted model. Specifically, DOBS exhibited a linear negative association with DN prevalence and a linear positive association with renal function. Stratified analyses confirmed that these relationships remained stable across various subgroups. The consistency of these findings was further validated in new datasets generated through PSM and MI, underscoring the robustness of the results. Additionally, mediation analyses revealed that CRP and the INFLA-score partially mediated the association between DOBS and DN.
Emerging evidence indicates that the pathogenesis of DN involves convergent pathways where diverse initiating factors ultimately activate common downstream effectors. This pathophysiological cascade prominently features sustained overproduction of pro-inflammatory cytokines [e.g., interleukin 6 (IL-6), tumor nucrosis factor (TNF-α)] and fibrogenic mediators [e.g., transforming growth factor-β (TGF-β)]. Crucially, oxidative stress serves as both a principal instigator and amplifier in this process, driving disease progression through reactive oxygen species (ROS)-mediated activation of nuclear factor κB (NF-κB) signaling pathway (21). Previous studies have highlighted the beneficial effects of dietary antioxidants on diabetes and its complications. For instance, astaxanthin has been shown to protect pancreatic β-cells and various organs, including the kidneys, from oxidative damage associated with diabetes (22, 23). Additionally, a study by Noonin et al. demonstrated that curcumin mitigates high glucose-induced renal cell secretions, such as intracellular ROS and TGF-β, thereby inhibiting the stimulatory effects on renal fibroblasts (24). A review by Gerardo et al. further summarized the positive impacts of various dietary antioxidants on OS and antioxidant responsiveness in DN (25). Consistent with the above findings, the present study utilized DOBS, a comprehensive dietary antioxidant index, and found that higher DOBS implies greater antioxidant capacity, which in turn has a greater beneficial effect on DN and renal function in DN.
Additionally, this study revealed that chronic inflammation partially mediates the association between the Dietary Oxidative Balance Score (DOBS) and diabetic nephropathy (DN). Inflammation plays a critical role in various pathological processes in DN, including renal tubular fibrosis, inflammatory cell infiltration, extracellular matrix accumulation, and podocyte autophagy. Key inflammatory pathways, such as PI3K/AKT, JAK/STAT3, TLR/NF-κB, TNF-α, TGF-β1, can exacerbate structural damage in the kidneys of DN patients, ultimately contributing to renal insufficiency (26).
Numerous studies have investigated the effects of individual micronutrients on health outcomes. For instance, a randomized controlled trial demonstrated that selenium supplementation increased total antioxidant capacity (TAC) in patients with DN (27). Additionally, a selenium-deficient diet was shown to induce renal OS and injury through TGF-β1 in both normal and diabetic rats (28). Furthermore, higher dietary calcium intake has been associated with a reduced risk of type 2 diabetes (29). Regarding vitamin C, studies have indicated that type 2 diabetics with more severe DN exhibit lower vitamin C levels (30). Vitamin C may exert beneficial effects on DN by modulating blood glucose levels, regulating the expression of oxidative enzymes, and influencing inflammatory proteins in diabetic kidneys, thereby reducing both hyperglycemia and inflammatory responses (31). Similarly, dietary supplementation with pyridoxamine, a structural analog of vitamin B6, has been found to reduce advanced glycation end products (AGEs), thereby inhibiting the activation of the NF-κB and Rho/ROCK pathways, which are implicated in fibrosis and inflammatory responses (32). In contrast to these single-nutrient studies, this study introduces a comprehensive indicator by integrating 14 antioxidants and 2 pro-oxidants into the DOBS. This approach may more accurately reflect the body’s overall antioxidant status compared to individual micronutrient measurements. However, the specific mechanisms underlying these effects warrant further exploration and elucidation in future studies.
The present study has some advantages. First, this study drew inspiration from the retrospective analysis and then applied the index DOBS, which may be able to better reflect dietary antioxidant levels. Second, the robustness of the results of the present study was demonstrated by multiple sensitivity analysis methods. Finally, the role played by chronic inflammation between DOBS and DN was preliminarily explored.
This study similarly has some limitations. (1) The hospital physical examination center did not directly contain data on the dietary microelements of the participants, so serum microelements were used. And the sample size needs to be further expanded. (2) The diagnosis for DN was based on diabetes and UACR, and there was not enough time for its observation (> 3 months). (3) Due to the limited clinical information contained in NHANES, the present study was unable to further categorize DN according to the type of diabetes. (4) Some of the variables included in this study were derived from the questionnaire provided by NHANES, which introduces some recall bias. (5) The mean BMI of the patients included in this study was 32.6, which is in the class I obesity range. Considering that obesity itself may affect the systemic inflammatory state by altering adipokines and gastrointestinal hormone secretion, and that weight loss has been shown to lead to remission of diabetes mellitus (33), this could potentially impact the results of this study. (6) The NHANES data used in this study came from the U.S. population, which makes the generalization of the findings regionally restrictive.
In summary, this study demonstrates that diets rich in antioxidants exert beneficial effects on DN and renal function in DN, with chronic inflammation potentially playing a mediating role. However, these findings and the underlying mechanisms require further validation and exploration in future research.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The retrospective analysis part of this study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and the ethical number was 2024-032-01 (ChiCTR2400086297). NHANES was the primary sources of data analyzed in this study, and no additional ethical review was required from the investigator’s institution. NHANES had been approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. All participants had signed an informed consent form.
YH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. GZ: Data curation, Formal analysis, Writing – review & editing. YP: Resources, Software, Writing – original draft. QX: Validation, Visualization, Writing – review & editing. ZW: Validation, Visualization, Writing – review & editing. JY: Validation, Visualization, Writing – review & editing. HZ: Validation, Visualization, Writing – review & editing. YZ: Conceptualization, Writing – review & editing. JL: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Chongqing Graduate Student Scientific Research Innovation Project (CYS240289).
All participants, staff, and institutions that contributed to the NHANES database are gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1560913/full#supplementary-material
1. Sun, H, Saeedi, P, Karuranga, S, Pinkepank, M, Ogurtsova, K, Duncan, BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Eckardt, KU, Coresh, J, Devuyst, O, Johnson, RJ, Köttgen, A, Levey, AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. (2013) 382:158–69. doi: 10.1016/S0140-6736(13)60439-0
3. van Raalte, DH, Bjornstad, P, Cherney, DZI, de Boer, IH, Fioretto, P, Gordin, D, et al. Combination therapy for kidney disease in people with diabetes mellitus. Nat Rev Nephrol. (2024) 20:433–46. doi: 10.1038/s41581-024-00827-z
4. Jager, KJ, Kovesdy, C, Langham, R, Rosenberg, M, Jha, V, and Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. (2019) 96:1048–50. doi: 10.1016/j.kint.2019.07.012
5. Alicic, RZ, Rooney, MT, and Tuttle, KR. Diabetic kidney disease: challenges, Progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/CJN.11491116
6. Chae, SY, Kim, Y, and Park, CW. Oxidative stress induced by lipotoxicity and renal hypoxia in diabetic kidney disease and possible therapeutic interventions: targeting the lipid metabolism and hypoxia. Antioxidants. (2023) 12:2083. doi: 10.3390/antiox12122083
7. Tan, BL, Norhaizan, ME, Liew, WPP, and Sulaiman, RH. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. (2018) 9:1162. doi: 10.3389/fphar.2018.01162
8. Kandhare, AD, Mukherjee, A, and Bodhankar, SL. Antioxidant for treatment of diabetic nephropathy: a systematic review and meta-analysis. Chem Biol Interact. (2017) 278:212–21. doi: 10.1016/j.cbi.2017.10.031
9. Bolignano, D, Cernaro, V, Gembillo, G, Baggetta, R, Buemi, M, and D’Arrigo, G. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis. PLoS One. (2017) 12:e0178699. doi: 10.1371/journal.pone.0178699
10. Zhong, O, Hu, J, Wang, J, Tan, Y, Hu, L, and Lei, X. Antioxidant for treatment of diabetic complications: a meta-analysis and systematic review. J Biochem Mol Toxicol. (2022) 36:e23038. doi: 10.1002/jbt.23038
11. Wang, X, Sarker, SK, Cheng, L, Dang, K, Hu, J, Pan, S, et al. Association of dietary inflammatory potential, dietary oxidative balance score and biological aging. Clin Nutr. (2024) 43:1–10. doi: 10.1016/j.clnu.2023.11.007
12. Cao, R, Li, A, Geng, F, and Pan, Y. Associations of dietary antioxidant intake with periodontal health among US adults: an exploratory mediation analysis via mitochondrial function. J Clin Periodontol. (2024) 51:702–11. doi: 10.1111/jcpe.13960
13. Zhang, J, Chen, Y, Zou, L, Jin, L, Yang, B, Shu, Y, et al. Dose-response relationship between dietary antioxidant intake and diabetic kidney disease in the US adults with diabetes. Acta Diabetol. (2023) 60:1365–75. doi: 10.1007/s00592-023-02125-9
14. Shivappa, N, Wirth, MD, Hurley, TG, and Hébert, JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and nutrition examination Survey-1999-2002. Mol Nutr Food Res. (2017) 61:630. doi: 10.1002/mnfr.201600630
15. Liu, F, Nie, J, Deng, MG, Yang, H, Feng, Q, Yang, Y, et al. Dietary flavonoid intake is associated with a lower risk of diabetic nephropathy in US adults: data from NHANES 2007-2008, 2009-2010, and 2017-2018. Food Funct. (2023) 14:4183–90. doi: 10.1039/D3FO00242J
16. Inker, LA, Schmid, CH, Tighiouart, H, Eckfeldt, JH, Feldman, HI, Greene, T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
17. Stevens, LA, and Levey, AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. (2009) 20:2305–13. doi: 10.1681/ASN.2009020171
18. Mela, CF, and Kopalle, PK. The impact of collinearity on regression analysis: the asymmetric effect of negative and positive correlations. Appl Econ. (2002) 34:667–77. doi: 10.1080/00036840110058482
19. Cen, M, Song, L, Fu, X, Gao, X, Zuo, Q, and Wu, J. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: findings from the UK biobank. Brain Behav Immun. (2024) 116:1–9. doi: 10.1016/j.bbi.2023.11.019
20. Luo, YY, Zhao, J, Han, XY, Zhou, XH, Wu, J, and Ji, LN. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J. (2015) 128:3276–82. doi: 10.4103/0366-6999.171357
21. Tuttle, KR, Agarwal, R, Alpers, CE, Bakris, GL, Brosius, FC, Kolkhof, P, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. (2022) 102:248–60. doi: 10.1016/j.kint.2022.05.012
22. Kanwugu, ON, Glukhareva, TV, Danilova, IG, and Kovaleva, EG. Natural antioxidants in diabetes treatment and management: prospects of astaxanthin. Crit Rev Food Sci Nutr. (2022) 62:5005–28. doi: 10.1080/10408398.2021.1881434
23. Alugoju, P, Krishna Swamy, VKD, Anthikapalli, NVA, and Tencomnao, T. Health benefits of astaxanthin against age-related diseases of multiple organs: a comprehensive review. Crit Rev Food Sci Nutr. (2023) 63:10709–74. doi: 10.1080/10408398.2022.2084600
24. Noonin, C, and Thongboonkerd, V. Curcumin prevents high glucose-induced stimulatory effects of renal cell secretome on fibroblast activation via mitigating intracellular free radicals and TGF-β secretion. Biomed Pharmacother. (2024) 174:116536. doi: 10.1016/j.biopha.2024.116536
25. Gerardo Yanowsky-Escatell, F, Andrade-Sierra, J, Pazarín-Villaseñor, L, Santana-Arciniega, C, De Jesús, T-VE, Samuel Chávez-Iñiguez, J, et al. The role of dietary antioxidants on oxidative stress in diabetic nephropathy. Iran J Kidney Dis. (2020) 14:81–94.
26. Zhao, L, Hu, H, Zhang, L, Liu, Z, Huang, Y, Liu, Q, et al. Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions. MedComm. (2020) 5:e516:2024. doi: 10.1002/mco2.516
27. Bahmani, F, Kia, M, Soleimani, A, Mohammadi, AA, and Asemi, Z. The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial - expression of concern. Br J Nutr. (2022) 127:155. doi: 10.1017/S000711452100204X
28. Reddi, AS, and Bollineni, JS. Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int. (2001) 59:1342–53. doi: 10.1046/j.1523-1755.2001.0590041342.x
29. Shah, IU, Sameen, A, Manzoor, MF, Ahmed, Z, Gao, J, Farooq, U, et al. Association of dietary calcium, magnesium, and vitamin D with type 2 diabetes among US adults: national health and nutrition examination survey 2007-2014-a cross-sectional study. Food Sci Nutr. (2021) 9:1480–90. doi: 10.1002/fsn3.2118
30. Chou, ST, and Tseng, ST. Oxidative stress markers in type 2 diabetes patients with diabetic nephropathy. Clin Exp Nephrol. (2017) 21:283–92. doi: 10.1007/s10157-016-1283-7
31. Park, NY, Park, SK, and Lim, Y. Long-term dietary antioxidant cocktail supplementation effectively reduces renal inflammation in diabetic mice. Br J Nutr. (2011) 106:1514–21. doi: 10.1017/S0007114511001929
32. Chiazza, F, Cento, AS, Collotta, D, Nigro, D, Rosa, G, Baratta, F, et al. Protective effects of Pyridoxamine supplementation in the early stages of diet-induced kidney dysfunction. Biomed Res Int. (2017) 2017:1–12. doi: 10.1155/2017/2682861
33. Kehagias, D, Lampropoulos, C, Georgopoulos, N, Habeos, I, Kalavrizioti, D, Vamvakas, SS, et al. Diabetes remission after LRYGBP with and without fundus resection: a randomized clinical trial. Obes Surg. (2023) 33:3373–82. doi: 10.1007/s11695-023-06857-z
ALB - Albumin
ALT - Alanine aminotransferase
AST - Aspartate aminotransferase
ALP - Alkaline phosphatase
AGEs - Advanced glycation end products
BMI - Body mass index
CDAI - Composite dietary antioxidant index
CKD - Chronic kidney disease
CKD-EPI - Chronic Kidney Disease Epidemiology Collaboration
CRP - C-reactive protein
DOBS - Dietary oxidative balance score
DN - Diabetic nephropathy
DBP - Diastolic blood pressure
DE - Direct effect
eGFR - estimated glomerular filtration rate
ESRD - End-stage renal disease
FBG - Fasting blood glucose
GFR - Glomerular filtration rate
G/L - Granulocyte-to-lymphocyte ratio
HDL-C - High-density lipoprotein cholesterol
IDF - International Diabetes Federation
INFLA-score - Low-grade inflammation score
IE - Indirect effect
LDL-C - Low-density lipoprotein cholesterol
L - Lymphocyte
M - Monocyte
MI - Multiple imputation
NHANES - National Health and Nutritional Examination Survey
NCHS - National Center for Health Statistics
N - Neutrophil
NF-κB - Nuclear factor-κB
OS - Oxidative stress
OR - Odds ratio
PSM - Propensity score matching
RCS - Restricted cubic spline
Ref - Reference
STROBE - Strengthening the Reporting of Observational Studies in Epidemiology
Scr - Serum creatinine
SBP - Systolic blood pressure
TC - Total cholesterol
TG - Triglyceride
TGF-β - transforming growth factor-β
TAC - Total antioxidant capacity
UACR - Urine albumin-to-creatinine ratio
VIF - Variance inflation factors
WBC - White blood cell
WTDRD1 - Dietary day one sample weight
WTDR2D - Dietary two-day sample weight.
Keywords: dietary oxidative balance score, diabetic nephropathy, renal function, chronic inflammation, NHANES
Citation: Huang Y, Wang L, Zhang G, Peng Y, Xu Q, Wei Z, Yu J, Zhang H, Zhang Y and Liu J (2025) Effects of dietary oxidative balance score on diabetic nephropathy and renal function: insights from retrospective and cross-sectional studies. Front. Nutr. 12:1560913. doi: 10.3389/fnut.2025.1560913
Received: 15 January 2025; Accepted: 11 March 2025;
Published: 20 March 2025.
Edited by:
Malgorzata Rozanowska, Cardiff University, United KingdomReviewed by:
Muniyappan Madesh, Yangzhou University, ChinaCopyright © 2025 Huang, Wang, Zhang, Peng, Xu, Wei, Yu, Zhang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Zhang, emhhbmd5YW83NDA3QDEyNi5jb20=; Jiayu Liu, dXJvbG9naXN0bGl1MjAyMkAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Yao Zhang, https://orcid.org/0000-0001-8878-891X
Jiayu Liu, https://orcid.org/0000-0002-3874-3075
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.