- 1Department of Bioconvergence, Hoseo University, Asan, Republic of Korea
- 2Department of Food and Nutrition, Obesity/Diabetes Research Center, Hoseo University, Asan, Republic of Korea
Editorial on the Research Topic
Precision nutrition and nutrients: making the promise a reality
1 Introduction
Precision nutrition offers a transformative approach that transcends generic dietary guidelines, providing individualized strategies based on genetic, metabolic, and environmental variability (1). Unlike traditional nutrition frameworks, precision nutrition optimizes health outcomes by addressing specific genetic variations of individuals that influence nutrient metabolism, such as single-nucleotide polymorphisms (SNPs) linked to differential responses to vitamins, lipids, and other nutrients to influence metabolic disease risk (2–4).
In addition to genetics, the gut microbiome modulates the diet-health relationship (5, 6). Although not directly addressed in this Research Topic, the microbiome's importance in nutrient absorption and metabolism is undeniable (7, 8). Genetic predisposition interacts with lifestyle behaviors including stress, sleep, physical activity, dietary intake, and environmental exposures, emphasizing the need for integrative frameworks in precision nutrition (9, 10).
This Research Topic features five studies exploring genetic and methodological advancements in precision nutrition, including SNPs influencing carotenoid bioavailability, gene-lifestyle interactions in lipoprotein metabolism, and machine-learning applications in predicting glycemic responses. Collectively, these contributions demonstrate the growing potential of precision nutrition for disease prevention and public health strategies.
2 Overview of the Research Topic
The featured studies encompass diverse approaches to advancing precision nutrition (Von Holle et al.; Paoletti et al.; Liu et al.; Park et al.; Hur et al.).
One study included in this Research Topic substantially expands the understanding of novel SNPs (Von Holle et al.) in the regulation of carotenoids, particularly lutein, and zeaxanthin. It also includes a comprehensive review of genome-based personalized nutrition technologies (Park et al.). Other studies address amino acid bioavailability and gene-lifestyle interactions in lipoprotein metabolism (Hur et al.; Paoletti et al.; Liu et al.), collectively highlighting the role of genetic variations in tailoring dietary interventions. These findings form a robust framework for translating precision nutrition research into actionable clinical applications.
3 Theme integration
3.1 Genetic basis of precision nutrition
Advances in understanding the genetic factors influencing nutrient metabolism have laid the foundation for precision nutrition. Previous studies have shown that polymorphisms in the BCO1 gene are associated with the metabolism of carotenoids, particularly beta-carotene (11). The current investigation, however, identifies novel SNPs (rs6564851-C and rs6420424-A) located upstream of the BCO1 gene, providing new insights into the genetic regulation of carotenoid metabolism (Von Holle et al.). These specific SNPs were found to significantly impact circulating levels of lutein and zeaxanthin, further elucidating how genetic variations influence the nutrient responses of individuals (Von Holle et al.). Additionally, the patent review in this Research Topic highlights the expanding importance of genome-based personalized nutrition technologies (Park et al.). It explores how genetic information is increasingly integrated into personalized dietary recommendations, particularly those tailored to disease prevention and management (Park et al.). While earlier reviews primarily focused on genetic insights without emphasizing their application (12), this study emphasizes the translation of genetic discoveries into commercial and clinical settings, illustrating the practical implementation of precision nutrition approaches. This study advances the field by describing how genetic discoveries are translated into clinical and commercial applications.
3.2 Dietary interventions and nutrient bioavailability
The investigation into amino acid bioavailability, using the indicator amino acid oxidation method, builds on earlier research that focused primarily on static measures of amino acid content to assess protein quality (13). The metabolic availability assessment of amino acids provides a more refined understanding of protein quality by accounting for factors beyond simple digestibility, particularly for amino acids susceptible to heat and processing modifications (Paoletti et al.). Furthermore, the exploration of ketogenic therapy in treating brain diseases represents an expansion of precision nutrition approaches, demonstrating how specific dietary interventions can be tailored for therapeutic outcomes. This therapeutic strategy shows promise across various neurological conditions, with emerging evidence supporting its efficacy through altered patient metabolism and ketone production (Liu et al.). The novelty of these findings lies in the adaptation of specific dietary interventions to optimize therapeutic outcomes through precise metabolic assessment and dietary modification. Together, these studies illustrate the growing promise of precision nutrition to improve health outcomes through integrating cutting-edge methodologies in nutrient bioavailability assessment and personalized dietary interventions.
3.3 Lifestyle and polygenic interactions
The critical role of individual genetic variants, such as CETP_rs708272, in modulating HDL-C levels and cardiovascular risk is well established (14, 15). However, this Research Topic presents new research identifying additional SNPs, including ZPR1_rs3741297, BUD13_rs180327, and ALDH1A2_rs588136, that interact with environmental factors, such as dietary energy intake and sulfur-containing microbial diets, that modulate HDL-C levels (Hur et al.) in contrast to previous studies that predominantly focused on the effects of single-gene variants (16). This research introduces a more elegant and comprehensive approach by interjecting multiple genetic variants and their interactions with lifestyle factors. This multifactorial perspective is novel in its application to precision nutrition, highlighting the importance of integrating genetic predisposition with environmental influences to optimize dietary interventions. It advances a significant advance in the understanding of gene-environment interactions, paving the way for more personalized dietary recommendations aimed at improving lipoprotein metabolism and overall health.
4 Path forward: precision nutrition and genetic predispositions
The future of precision nutrition hinges on integrating multi-omics data with advanced computational tools, as illustrated in Figure 1. While genome-wide association studies continue to reveal genetic variants affecting nutrient metabolism, metabolomic profiling enhances our understanding of individual responses (7). Machine learning algorithms have proven valuable in predicting metabolic responses, facilitating the transition from theoretical research to practical applications.
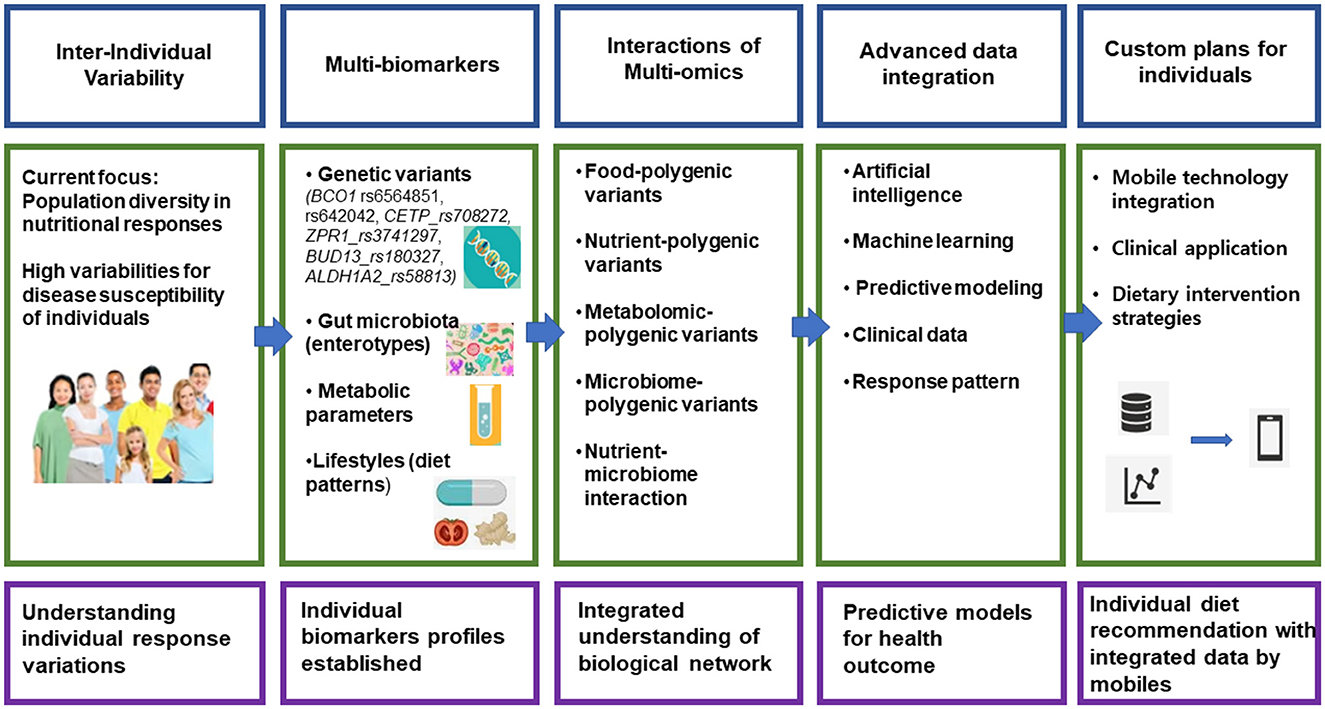
Figure 1. Framework for implementation of precision nutrition. This image illustrates a comprehensive framework for developing personalized nutrition recommendations, flowing from left to right across five key stages. It begins with understanding individual variability in how people respond to nutrition, then moves through collecting multiple biomarkers (including genetic variants and gut microbiota), analyzing various “-omics” interactions (like food-polygenic variants), using advanced data integration with artificial intelligence and machine learning, and finally delivering customized nutrition plans through mobile technology. The bottom row provides a concise summary of each stage's goal, ultimately leading to personalized diet recommendations delivered via mobile devices.
Translating genetic discoveries into actionable dietary recommendations remains crucial. While polygenic risk scores combined with lifestyle factors provide a framework for personalized dietary guidance (17), current research is limited by its predominant focus on European populations (18, 19). Beyond expanding population diversity, emerging evidence highlights specific genetic variants, such as missense mutations and those in UTR regions, as potential targets for nutrient-based interventions, offering innovative approaches to disease prevention and management (4, 20).
Recent technological advances, particularly in mobile applications, are democratizing access to precision nutrition tools. These developments, combined with emerging systems biology approaches integrating microbiome, proteomics, and metabolomics data, promise more nuanced dietary interventions (21, 22). This comprehensive approach will enable the development of more effective, personalized nutrition strategies suitable for clinical implementation.
5 Conclusion
The studies in this Research Topic demonstrate significant advances in precision nutrition, from genetic variant identification to dietary optimization. While challenges remain in integrating multi-omics data and scaling personalized approaches, these findings provide a clear path forward. The potential to optimize health outcomes through interventions tailored to genetic, metabolic, and lifestyle factors represents a promising direction toward personalized nutritional guidance that considers individual requirements while promoting broader public health.
Author contributions
SP: Conceptualization, Writing – original draft, Writing – review & editing.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Han T, Wei W, Jiang W, Geng Y, Liu Z, Yang R, et al. The future landscape and framework of precision nutrition. Engineering. (2024) 42:15–25. doi: 10.1016/j.eng.2024.01.020
2. Livingstone KM, Ramos-Lopez O, Pérusse L, Kato H, Ordovas JM, Martínez JA. Precision nutrition: a review of current approaches and future endeavors. Trends Food Sci Technol. (2022) 128:253–64. doi: 10.1016/j.tifs.2022.08.017
3. Gkouskou KK, Grammatikopoulou MG, Lazou E, Vasilogiannakopoulou T, Sanoudou D, Eliopoulos AG, et al. genomics perspective of personalized prevention and management of obesity. Hum Genomics. (2024) 18:4. doi: 10.1186/s40246-024-00570-3
4. Liu M, Park S. The role of PNPLA3_rs738409 gene variant, lifestyle factors, and bioactive compounds in nonalcoholic fatty liver disease: a population-based and molecular approach towards healthy nutrition. Nutrients. (2024) 16:1239. doi: 10.3390/nu16081239
5. Leeming ER, Louca P, Gibson R, Menni C, Spector TD, Le Roy CI. The complexities of the diet-microbiome relationship: advances and perspectives. Genome Med. (2021) 13:10. doi: 10.1186/s13073-020-00813-7
6. Park S, Li C, Wu X, Zhang T. Gut microbiota alterations and their functional differences in depression according to enterotypes in asian individuals. Int J Mol Sci. (2023) 24:13389. doi: 10.3390/ijms241713329
7. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. (2015) 163:1079–94. doi: 10.1016/j.cell.2015.11.001
8. Wilson ML, Davies IG, Waraksa W, Khayyatzadeh SS, Al-Asmakh M, Mazidi M. The impact of microbial composition on postprandial glycaemia and lipidaemia: a systematic review of current evidence. Nutrients. (2021) 13:3887. doi: 10.3390/nu13113887
9. Reddon H, Guéant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci. (2016) 130:1571–97. doi: 10.1042/CS20160221
10. Choi Y, Kwon HK, Park S. Polygenic variants linked to oxidative stress and the antioxidant system are associated with type 2 diabetes risk and interact with lifestyle factors. Antioxidants. (2023) 12:1280. doi: 10.3390/antiox12061280
11. Yabuta S, Urata M, Wai Kun RY, Masaki M, Shidoji Y. Common SNP rs6564851 in the BCO1 gene affects the circulating levels of β-carotene and the daily intake of carotenoids in healthy Japanese women. PLoS ONE. (2016) 11:e0168857. doi: 10.1371/journal.pone.0168857
12. Lee S, Kim S-A, Hong J, Kim Y, Hong G, Baik S, et al. Identification of genetic variants related to metabolic syndrome by next-generation sequencing. Diabetol Metab Syndr. (2022) 14:119. doi: 10.1186/s13098-022-00893-y
13. Rafii M, Pencharz PB, Ball RO, Tomlinson C, Elango R, Courtney-Martin G. Bioavailable methionine assessed using the indicator amino acid oxidation method is greater when cooked chickpeas and steamed rice are combined in healthy young men. J Nutr. (2020) 150:1834–44. doi: 10.1093/jn/nxaa086
14. Liu M, Jin HS, Park S. Protein and fat intake interacts with the haplotype of PTPN11_rs11066325, RPH3A_rs886477, and OAS3_rs2072134 to modulate serum HDL concentrations in middle-aged people. Clin Nutr. (2020) 39:942–9. doi: 10.1016/j.clnu.2019.03.039
15. Yoo MG, Yun JH, Koo SK, Lee HJ. The effect of the association between CETP variant type and alcohol consumption on cholesterol level differs according to the ALDH2 variant type. Sci Rep. (2022) 12:15129. doi: 10.1038/s41598-022-19171-y
16. Murray MF, Khoury MJ, Abul-Husn NS. Addressing the routine failure to clinically identify monogenic cases of common disease. Genome Med. (2022) 14:60. doi: 10.1186/s13073-022-01062-6
17. Park S, Yang HJ, Kim MJ, Hur HJ, Kim SH, Kim MS. Interactions between polygenic risk scores, dietary pattern, and menarche age with the obesity risk in a large hospital-based cohort. Nutrients. (2021) 13:3772. doi: 10.3390/nu13113772
18. Hüls A, Wright MN, Bogl LH, Kaprio J, Lissner L, Molnár D, et al. Polygenic risk for obesity and its interaction with lifestyle and sociodemographic factors in European children and adolescents. Int J Obes. (2021) 45:1321–30. doi: 10.1038/s41366-021-00795-5
19. Han HY, Masip G, Meng T, Nielsen DE. Interactions between polygenic risk of obesity and dietary factors on anthropometric outcomes: a systematic review and meta-analysis of observational studies. J Nutr. (2024) 154:3521–43. doi: 10.1016/j.tjnut.2024.10.014
20. Mehta NH, Huey SL, Kuriyan R, Peña-Rosas JP, Finkelstein JL, Kashyap S, et al. Potential mechanisms of precision nutrition-based interventions for managing obesity. Adv Nutr. (2024) 15:100186. doi: 10.1016/j.advnut.2024.100186
21. Sen P, Orešič M. Integrating omics data in genome-scale metabolic modeling: a methodological perspective for precision medicine. Metabolites. (2023) 13:855. doi: 10.3390/metabo13070855
22. Yuan H, Jung ES, Chae SW, Jung SJ, Daily JW, Park S. Biomarkers for health functional foods in metabolic dysfunction-associated steatotic liver disorder (MASLD) prevention: an integrative analysis of network pharmacology, gut microbiota, and multi-omics. Nutrients. (2024) 16:3061. doi: 10.3390/nu16183061
Keywords: precision nutrition, genetic variants, amino acid bioavailability, gene-lifestyle interaction, ketogenic diet
Citation: Park S (2025) Editorial: Precision nutrition and nutrients: making the promise a reality. Front. Nutr. 12:1553149. doi: 10.3389/fnut.2025.1553149
Received: 30 December 2024; Accepted: 15 January 2025;
Published: 27 January 2025.
Edited and reviewed by: Annalisa Terranegra, Sidra Medicine, Qatar
Copyright © 2025 Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunmin Park, c21wYXJrQGhvc2VvLmVkdQ==
 Sunmin Park
Sunmin Park