
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 07 March 2025
Sec. Nutritional Immunology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1552358
This article is part of the Research Topic Efficacy of probiotic-enriched foods on digestive health and overall well-being View all 7 articles
Objective: Probiotic supplementation has gained attention for its potential to modulate inflammatory and oxidative stress biomarkers, particularly in metabolic disorders. This meta-analysis evaluates the effects of probiotics on C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), malondialdehyde (MDA), total antioxidant capacity (TAC), glutathione (GSH), and nitric oxide (NO) in patients with diabetes.
Methods: A Meta-Research was conducted on 15 meta-analyses of unique 33 randomized controlled trials (RCTs) published between 2015 and 2022, involving 26 to 136 participants aged 26 to 66 years. Data were synthesized using standardized mean differences (SMD), with sensitivity analysis using a random-effect model.
Results: Probiotic supplementation significantly reduced CRP (SMD = −0.79, 95% CI: −1.19, −0.38), TNF-α (SMD = −1.35, 95% CI: −2.05, −0.66), and MDA levels (WMD: -0.82, 95% CI: −1.16, −0.47). Probiotics increased GSH (SMD = 1.00, 95% CI: 0.41, 1.59), TAC (SMD = 0.48, 95% CI: 0.27, 0.69), and NO (SMD = 0.60, 95% CI: 0.30, 0.91). Result on IL-6 was not significant (SMD = −0.29, 95% CI: −0.66, 0.09). Sensitivity analyses confirmed robustness.
Conclusion: Probiotics significantly improved inflammatory and oxidative stress biomarkers in patients with diabetes, with variations influenced by population and dosage. Future studies should explore novel probiotic strains and longer interventions.
Diabetes mellitus (DM) is a chronic metabolic disorder that has reached epidemic proportions worldwide, affecting approximately 537 million adults as of 2021 (1). This rate is increasing and imposing a significant burden on healthcare systems globally. One of the major challenges in diabetes management is addressing the complications arising from chronic low-grade inflammation and oxidative stress, both of which play critical roles in the progression of diabetes-related complications such as nephropathy and cardiovascular diseases (2). Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and antioxidant defenses, is a hallmark of diabetes (3). Inflammation and oxidative stress are linked, with elevations in one often exacerbating the other in a mutually reinforcing cycle (4). Addressing these interconnected pathways has become a crucial aspect of diabetes care.
Probiotics, defined as live microorganisms that provide health benefits when consumed in adequate amounts, have emerged as a promising intervention in diabetes management (1, 5). The primary mechanisms of action of probiotics involve improving gut microbiota composition, strengthening intestinal barrier integrity, and reducing systemic endotoxemia by lowering lipopolysaccharide (LPS) translocation (6). These mechanisms ultimately lead to decreased activation of inflammatory pathways and enhanced antioxidant defenses. Probiotics also stimulate the production of short-chain fatty acids (SCFAs), which can modulate immune responses and improve glucose metabolism (7, 8).
Despite the growing interest in probiotics, existing research on their effects on oxidative stress and inflammatory markers in patients with diabetes has yielded inconsistent results. Regarding inflammation, while some meta-analysis studies have reported no significant effect of probiotics on C-reactive protein (CRP) levels in patients with diabetes (9, 10), others have demonstrated a significant reduction (11, 12). This could be attributed to differences in statistical analyses and the heterogeneity of studies included in the referenced meta-analyses. This also applies to antioxidant markers such as glutathione (GSH), where the study results remain inconsistent (13, 14). In the current umbrella review, we aimed to provide a comprehensive evaluation of all existing meta-analyses on the effects of probiotics in diabetic patients. To ensure robustness, we systematically assessed all studies included in these meta-analyses to determine whether they met our predefined inclusion and exclusion criteria. Studies that fulfilled these criteria were incorporated into our analysis. Additionally, we identified studies from our systematic search that were not part of the included meta-analyses but met the eligibility criteria and incorporated them as well. This meticulous approach minimized the likelihood of missing relevant studies. By comparing our findings with previous research, performing comprehensive statistical analyses, and evaluating the evidence using the GRADE framework, we sought to provide a definitive conclusion on the efficacy of probiotics in modulating inflammation among diabetic patients. This integrated approach enhances the reliability of our results and contributes to a deeper understanding of the potential role of probiotics in diabetes management.
This meta-analysis aims to evaluate the effects of probiotic supplementation on key biomarkers of oxidative stress (malondialdehyde (MDA), total antioxidant capacity (TAC), GSH, and nitric oxide [NO]) and inflammation (interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and CRP) in individuals with diabetes. By synthesizing evidence from clinical trial studies conducted exclusively on patients with diabetes, this study seeks to provide a robust assessment of the therapeutic potential of probiotics in mitigating oxidative and inflammatory complications in this high-risk population.
This umbrella review and meta-analysis aimed to evaluate the effects of probiotics on inflammatory markers and oxidative stress in diabetic patients by assessing existing meta-analyses and systematic reviews, as well as conducting a comprehensive meta-analysis of randomized controlled trials (RCTs). This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15). The study protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023229865.
To identify relevant studies, a comprehensive search was conducted in the following databases: PubMed, Scopus, Web of Science, EMBASE, and Cochrane Central Library, up to November 2024. The search terms included a combination of Medical Subject Headings (MeSH) and keywords: (“Probiotics” OR “Saccharomyces” OR “Lactobacillus” OR “Bifidobacterium”) AND (“Oxidative Stress” OR “Total Antioxidant Capacity” OR “TAC” OR “Antioxidant” OR “Oxidant” OR “Reactive oxygen species” OR “Malondialdehyde” OR “MDA OR “Glutathione” OR “GSH” OR “Nitric Oxide”).
To increase sensitivity, wildcard terms (e.g., “*”) were used. The search was restricted to English-language publications. Additionally, the reference lists of the included meta-analysis studies were reviewed to identify any RCTs that met the predefined inclusion and exclusion criteria. Studies meeting these criteria were incorporated into the analysis to ensure a comprehensive assessment and minimize the risk of missing relevant trials. In addition to peer-reviewed articles, we conducted a comprehensive search for gray literature and unpublished studies by exploring relevant conference proceedings, theses, dissertations, and clinical trial registries to minimize publication bias and ensure a thorough inclusion of all pertinent data.
The following PICOS criteria were applied for study selection: Population (P): Adults aged ≥18 years with diabetes mellitus; Intervention (I): Probiotic supplementation; Comparison (C): Placebo or control group; Outcomes (O): Oxidative stress biomarkers including MDA, TAC, GSH, and NO, and inflammatory markers such as IL-6, TNF-α, and CRP; Study design (S): Systematic review, meta-analysis, as well as RCT studies, providing effect sizes and corresponding confidence intervals (CI) for each outcome. Exclusion criteria included in vitro or in vivo studies, observational studies, case reports, quasi-experimental studies, and meta-analysis studies lacking sufficient data for effect size calculation.
The methodological quality of the included meta-analysis studies was assessed independently by two reviewers using the Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 checklist (16). AMSTAR2 evaluates both critical and non-critical domains, such as protocol registration, risk of bias assessment, and adherence to statistical best practices in meta-analyses. Reviews with high-quality methodology were considered more reliable, while those with significant flaws were excluded. Discrepancies were resolved through discussion or consultation with a senior author. Studies scoring ≥7 were categorized as high-quality.
The methodological quality of the included RCTs was evaluated using the Cochrane Risk of Bias Tool. This tool assesses seven domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each domain was classified as low, high, or unclear risk, with an overall risk of bias assigned to each study to ensure a rigorous quality assessment (17). Discrepancies were resolved through discussion or consultation with a senior author.
Two independent reviewers screened titles and abstracts, followed by full-text evaluation of eligible studies. Extracted data from meta-analysis and RCT studies included: study characteristics (author, publication year, location), participant details (sample size, age, health status), intervention specifics (probiotic type, dosage, and duration), and outcomes (effect sizes (ESs) with 95% confidence intervals [CIs] for MDA, GSH, TAC, NO, IL-6, TNF-α, and CRP). Disagreements were resolved through discussion with a third reviewer.
Data were synthesized using effect sizes (SMD) and corresponding 95% confidence intervals. Random-effects models were applied for pooling data. Heterogeneity was assessed using the Cochran’s Q test and I2 statistic (I2 > 50%, p < 0.1). Subgroup analyses were performed to explore heterogeneity based on variables such as sample size, probiotic type, intervention duration, and population characteristics. Sensitivity analyses assessed the robustness of the findings by excluding individual studies. To address heterogeneity beyond subgroup and sensitivity analyses, we conducted meta-regression analyses to explore potential sources of variability among studies. Specifically, we examined the effects of moderator variables such as intervention duration and sample size on the observed outcomes. Publication bias was evaluated using funnel plots (for markers with >10 included studies) and statistical tests [Begg’s (18) and Egger’s tests (19)]. If bias was detected, a trim-and-fill method was applied. All statistical analyses were conducted using STATA software version 16.0 (StataCorp, College Station, TX, United States), with a significance threshold of p < 0.05.
The quality of the evidence was evaluated using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) framework (20). This framework takes into account several factors to determine the overall confidence in the effect estimates, including risk of bias, inconsistency, indirectness, imprecision, and publication bias. The risk of bias in each included study was assessed using the Cochrane Risk of Bias tool. Studies with a high risk of bias were downgraded in terms of evidence quality. Heterogeneity across the studies was assessed using the I2 statistic. Significant heterogeneity (I2 > 50%) indicated inconsistency in study results, which could lead to a downgrading of the evidence. Indirectness was considered by evaluating whether the study populations, interventions, and outcomes were directly applicable to the research question. Studies with populations or interventions that did not align closely with the review’s focus were downgraded for indirectness. The precision of the effect estimates was assessed by the width of the confidence intervals (CIs). Wide CIs, suggesting greater uncertainty in the estimates, led to a downgrade in the quality of evidence. To evaluate publication bias, funnel plots and statistical tests were used. If publication bias was detected, the evidence quality was downgraded.
After these assessments, the quality of evidence for each outcome was categorized as high, moderate, low, or very low, helping to clarify the strength of the evidence supporting the effects of probiotics on inflammatory and oxidative stress biomarkers in diabetic patients.
The flow diagram of the study selection process for meta-analysis studies is illustrated in Supplementary Figure S1. A systematic search of electronic databases resulted in a total number of 709 articles. After removing the duplicate articles (n = 94), 615 articles were screened by reading their titles and abstracts, leading to 18 articles whose full texts were evaluated. Ultimately, 15 meta-analyses were included in the systematic review (9–14, 21–29).
Study population were patients with type 2 and 1-diabetes mellitus (T2DM and T1DM), diabetic nephropathy, gestational diabetes mellitus (GDM), and prediabetes. Lactobacillus, Bifidobacterium, and Streptococcus being the most commonly used strains. The quality of the included studies, assessed using tools such as the Cochrane Risk of Bias Tool and the Jadad scale, revealed that most studies were of high quality (Supplementary Table S1). The quality of the included studies was assessed based on a series of quality criteria (Q1–Q16) defined by the AMSTAR 2 checklist and categorized as high or moderate (Supplementary Table S2). Most studies in this review were assessed as having moderate quality (12 of 15 included studies). Among these, the studies by Ardeshirlarijan et al. (24), Zheng et al. (26), Naseri et al. (23) were classified as high quality, meeting most of the criteria with minimal biases. Notably, the study by Naseri et al. (23) exhibited the highest quality, fulfilling all assessment criteria.
The systematic review of meta-analyses revealed that probiotics, particularly Lactobacillus, Bifidobacterium, and Streptococcus, significantly reduced inflammatory markers like CRP (9 of 11 studies) and TNF-α (3 of 4 studies), as well as oxidative stress markers such as MDA (9 of 10 studies), while improving antioxidant levels including GSH (7 of 10 studies) and TAC (7 of 9 studies) in diabetic populations. However, the effects on NO and IL-6 were inconsistent, with several studies reporting no significant changes (7 of 11 and 3 of 5 studies, respectively) (Supplementary Table S1).
Initially, 978 records were identified through systematic database searches. Additionally, 102 unique RCTs cited within 15 meta-analysis studies were added after the initial search to ensure comprehensive inclusion. Following the removal of duplicate articles and exclusion of studies that did not meet the inclusion criteria, a total of 33 RCTs were included in the analysis (30–62) (Figure 1). The characteristics of the included RCTs are summarized in Table 1.
In the risk of bias assessment, most studies had low risk regarding random sequence generation and allocation concealment. However, study by Ismail et al. (43) did not provide sufficient information on these aspects. Selective reporting posed a high risk in several studies. Only three studies did not follow double blinding procedure in their study protocol (37, 50, 57). Finally, most studies had low risk of incomplete outcome data (Table 2).
Probiotic supplementation significantly reduced CRP levels in patients with diabetes (Figure 2). Substantial heterogeneity was observed. Several factors were identified as contributing to the high heterogeneity of the study, including age, sample size, health condition, and baseline BMI (Table 3). Subgroup analysis revealed the most substantial effects in patients with diabetic nephropathy, T2DM, as well as intervention duration ≥10 weeks, multi-strain probiotic, baseline BMI ≥30 kg/m2, and age < 55 years (Table 3). Sensitivity analysis confirmed the robustness of the overall findings (p < 0.05). Meta-regression analysis demonstrated no linear relationship between effect size and sample size or intervention duration (p > 0.05). Unlike Begg’s test (p > 0.05), there were significant small-study effects when performing Egger’s test (p = 0.033). According to the funnel plot, publication bias was evident (Supplementary Figure S2). Then, trim and fill analysis was performed with 34 studies (Six imputed studies, SMD = −1.09, 95% CI: −1.55, −0.64; p < 0.05).
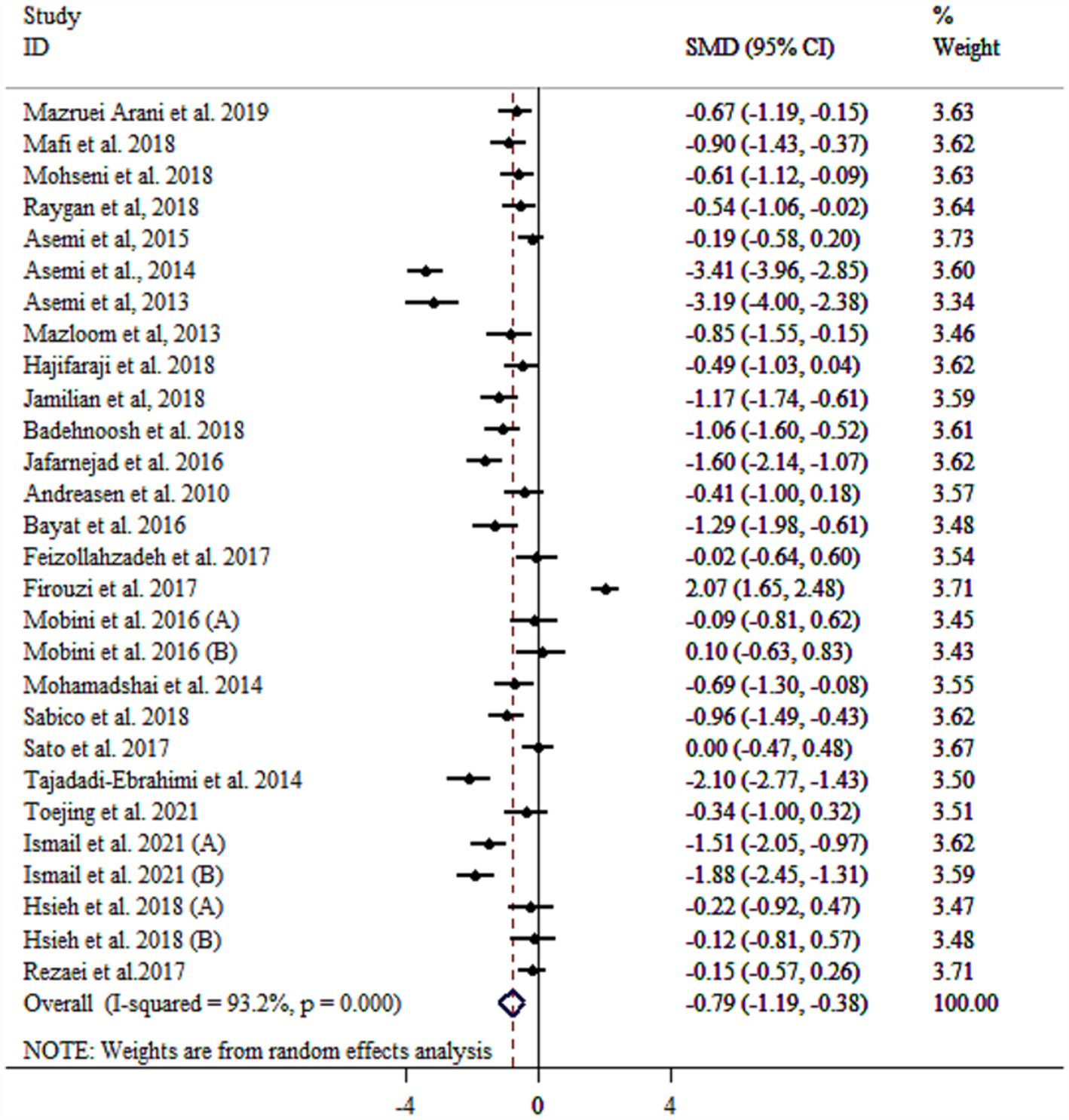
Figure 2. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on CRP.

Table 3. Subgroup analyses for the effects of probiotics supplementation on inflammation and oxidative stress biomarkers.
IL-6 level did not significantly decrease following probiotic supplementation with substantial heterogeneity (Figure 3). Subgrouping by gender, sample size, and duration reduced heterogeneity between studies (Table 3). However, removing the study by Mazloom et al. (48), using sensitivity analysis made the overall results statistically significant (SMD = −0.38, 95% CI: −0.74, −0.02; p < 0.05). Subgroup analyses showed significant decreases in IL-6 in a sample size of ≥60 with mean age of <50 years (p < 0.05), as well as studies administered single-strain probiotic (Table 3). Meta-regression analysis showed that effect size did not have a linear relationship with sample size and intervention duration (p > 0.05). Non-significant outcomes from Egger’s and Begg’s tests validate the reliability of the meta-analysis results (p > 0.05). A visual inspection of the funnel plot revealed that the distribution of studies was symmetrical (Supplementary Figure S3).
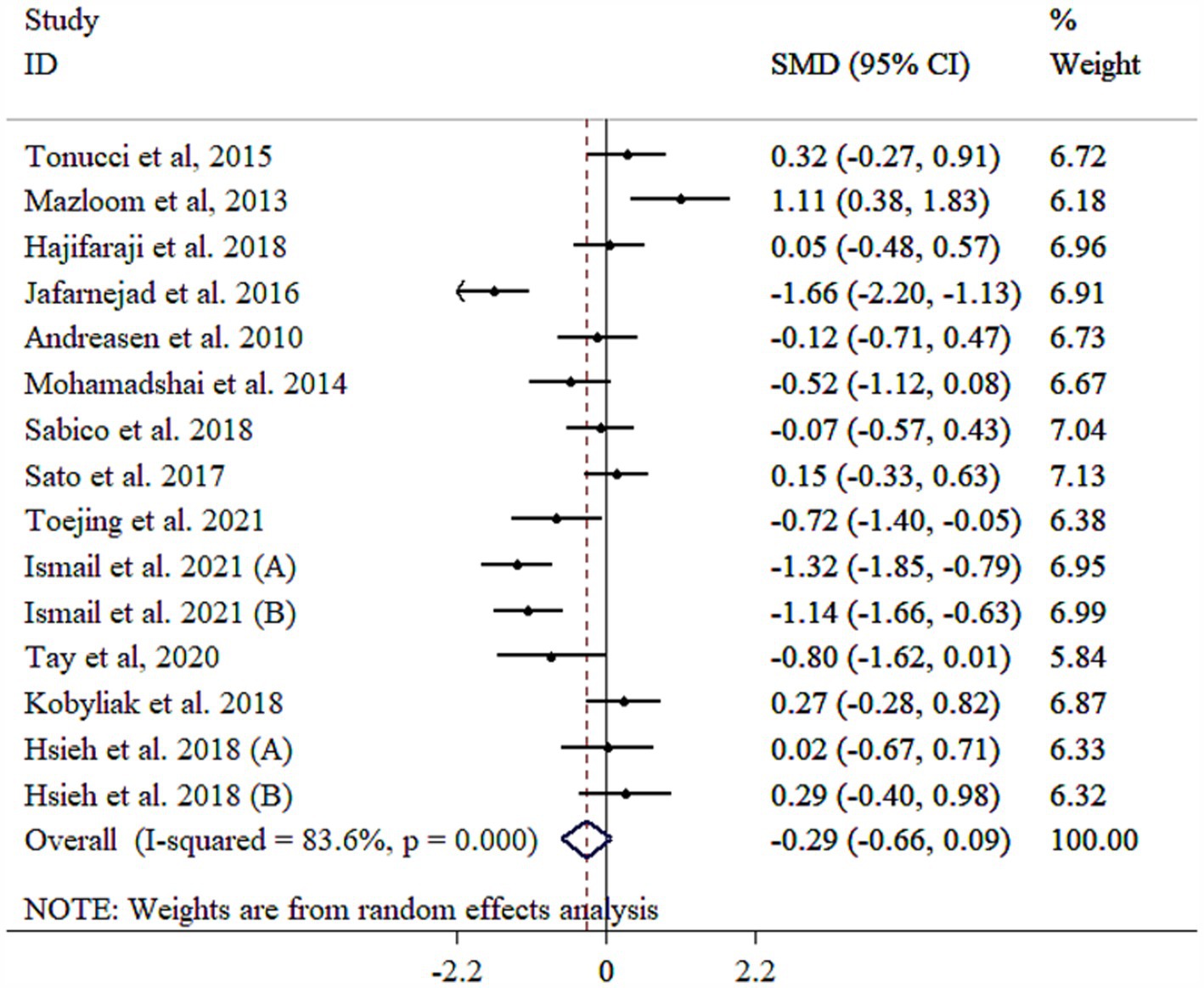
Figure 3. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on IL-6.
Probiotic supplements significantly reduced TNF-α (Figure 4). Heterogeneity among the included studies was high that was reduced following subgroup analysis based on mean age (Table 3). Subgroup analysis revealed the most substantial effects in patients with diabetic nephropathy, intervention duration <10 weeks, single-strain probiotic, baseline BMI <30 kg/m2, sample size of <60, and mean age of ≥55 years (Table 3). Sensitivity analysis confirmed that the pooled results were stable and not influenced by any single study (p < 0.05). Meta-regression analysis revealed that effect size did not have a linear relationship with sample size and intervention duration (p > 0.05). Egger’s test, unlike Begg’s test (p > 0.05), indicated evidence of publication bias (p = 0.001). Publication bias was also detected through visual inspection of the funnel plot (Supplementary Figure S4). Nevertheless, the results remained significant after conducting a trim and fill analysis with 20 studies (Four imputed studies, SMD = −2.19, 95% CI: −3.05, −1.34; p < 0.05).
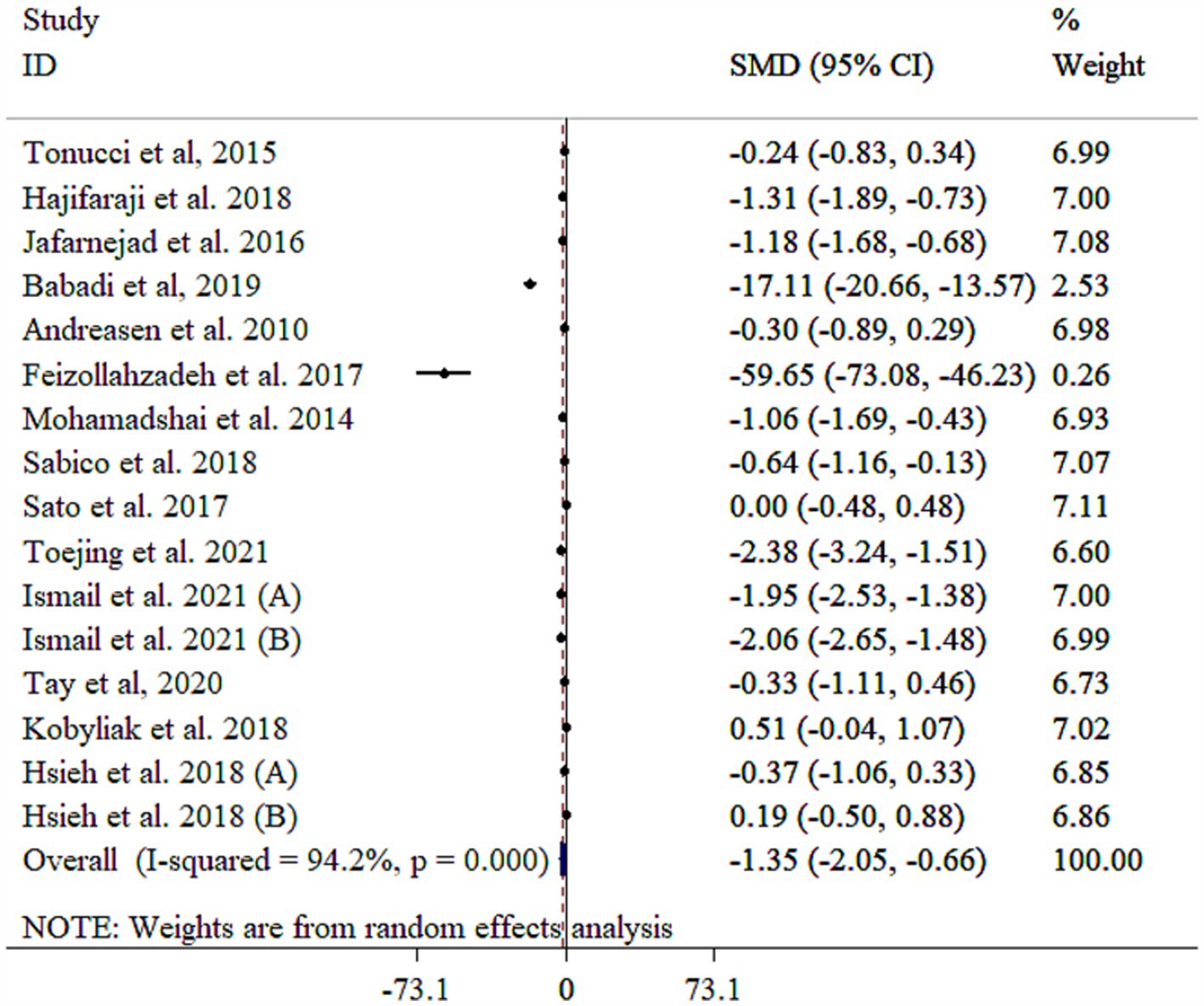
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on TNF-a.
The analysis revealed that probiotics significantly reduced MDA levels, although with substantial heterogeneity (Figure 5). A subgroup analysis identified the study population as the primary source of this heterogeneity (Table 3). The subgroup analysis indicated consistent effects across various populations and study protocols, with the largest reductions observed in single-strain probiotics, diabetic nephropathy patients, both genders, those aged ≥55 years, interventions lasting <10 weeks, studies with a sample size <60, and individuals with a baseline BMI ≥30 kg/m2 (Table 3). Sensitivity analysis confirmed the robustness of these findings (p < 0.05). Meta-regression analysis showed no significant influence of sample size or intervention duration on effect size (p > 0.05). While Egger’s (p = 0.038) and Begg’s (p = 0.025) tests indicated potential publication bias, the funnel plot (Supplementary Figure S5) displayed a symmetric distribution, suggesting no significant bias.
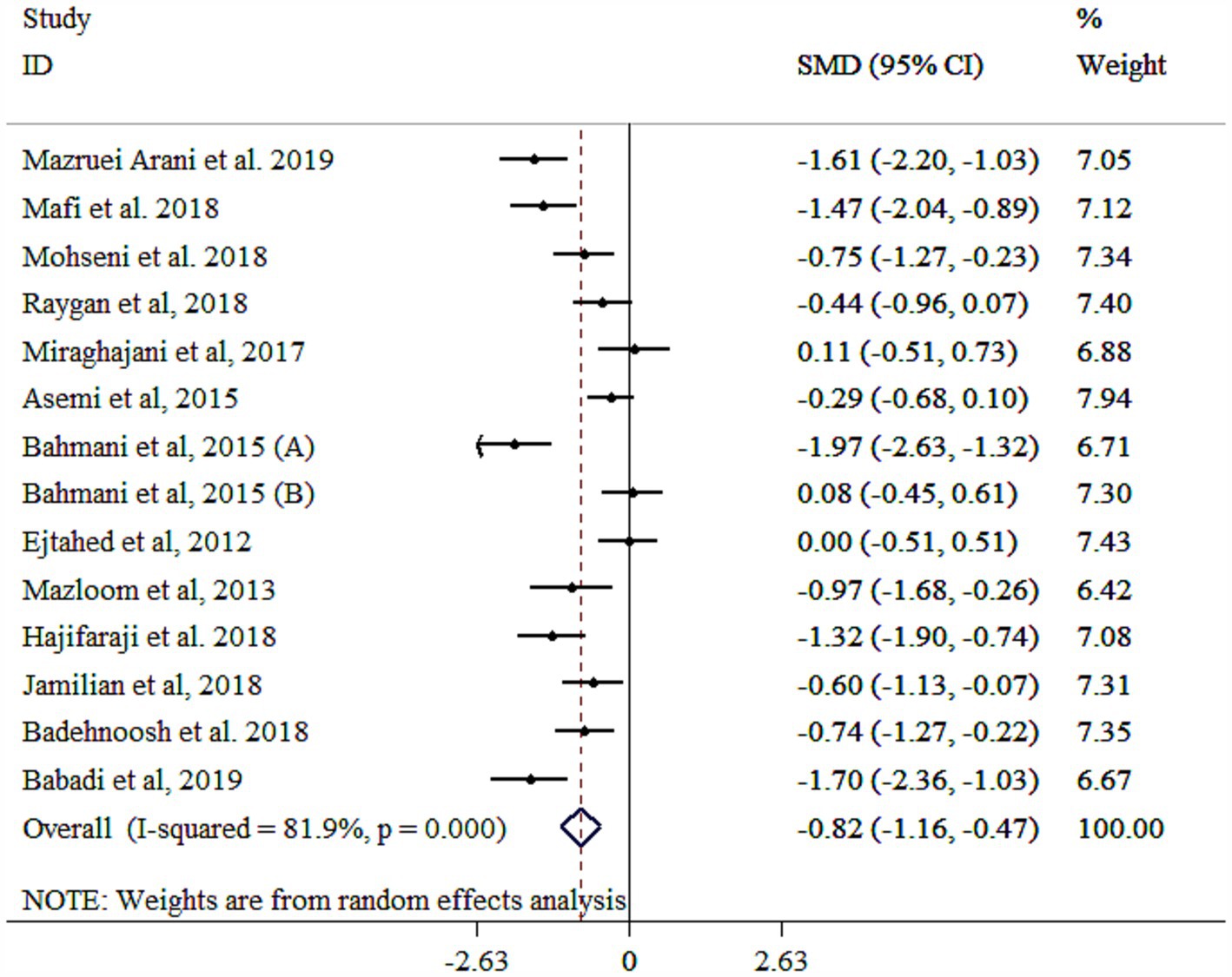
Figure 5. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on MDA.
A significant increase in GSH levels was observed following probiotic supplementation (Figure 6), although high between-study heterogeneity was observed. Subgroup analyses reduced heterogeneity when stratified by gender, study population, and intervention duration (Table 3). Greater effects were noted in studies with longer interventions (≥10 weeks), single-strain probiotics, higher sample sizes (≥60), baseline BMI <30 kg/m2, participants with T2DM, mean age < 55 years, and female participants (Table 3). Sensitivity analysis confirmed the reliability of the findings (p < 0.05). Meta-regression did not identify significant moderators of effect size (p > 0.05). While Egger’s test (p = 0.006) indicated potential publication bias, Begg’s test did not detect bias (p > 0.05). Funnel plot analysis revealed asymmetry (Supplementary Figure S6). However, the trim-and-fill method validated the significant effect of probiotics on GSH levels with 19 studies (Four imputed studies, SMD = 1.22, 95% CI: 0.76, 1.44; p < 0.05).

Figure 6. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on GSH.
Probiotic supplementation significantly increased NO levels, though substantial heterogeneity was observed (Figure 7). Subgroup analyses identified gender, study population, sample size, and baseline BMI as key contributors to this heterogeneity (Table 3). Larger effects were observed in younger participants (<55 years), multi-strain probiotics, longer interventions (≥10 weeks), smaller sample sizes (<60), individuals with T2DM, baseline BMI <30 kg/m2, and both genders (Table 3). The robustness of findings was approved by sensitivity analysis (p < 0.05). Moreover, sample size and study duration did not influence the final results according to meta-regression analysis (p > 0.05). Publication bias assessments provided mixed results, with Egger’s test showing no bias (p > 0.05) and Begg’s test indicating potential bias (p = 0.025). A moderate asymmetry was detected in the funnel plot (Supplementary Figure S7). However, trim-and-fill analysis yielded an adjusted significant effect with 11 studies (One imputed study, SMD = 0.52, 95% CI: 0.21, 0.84; p < 0.05).
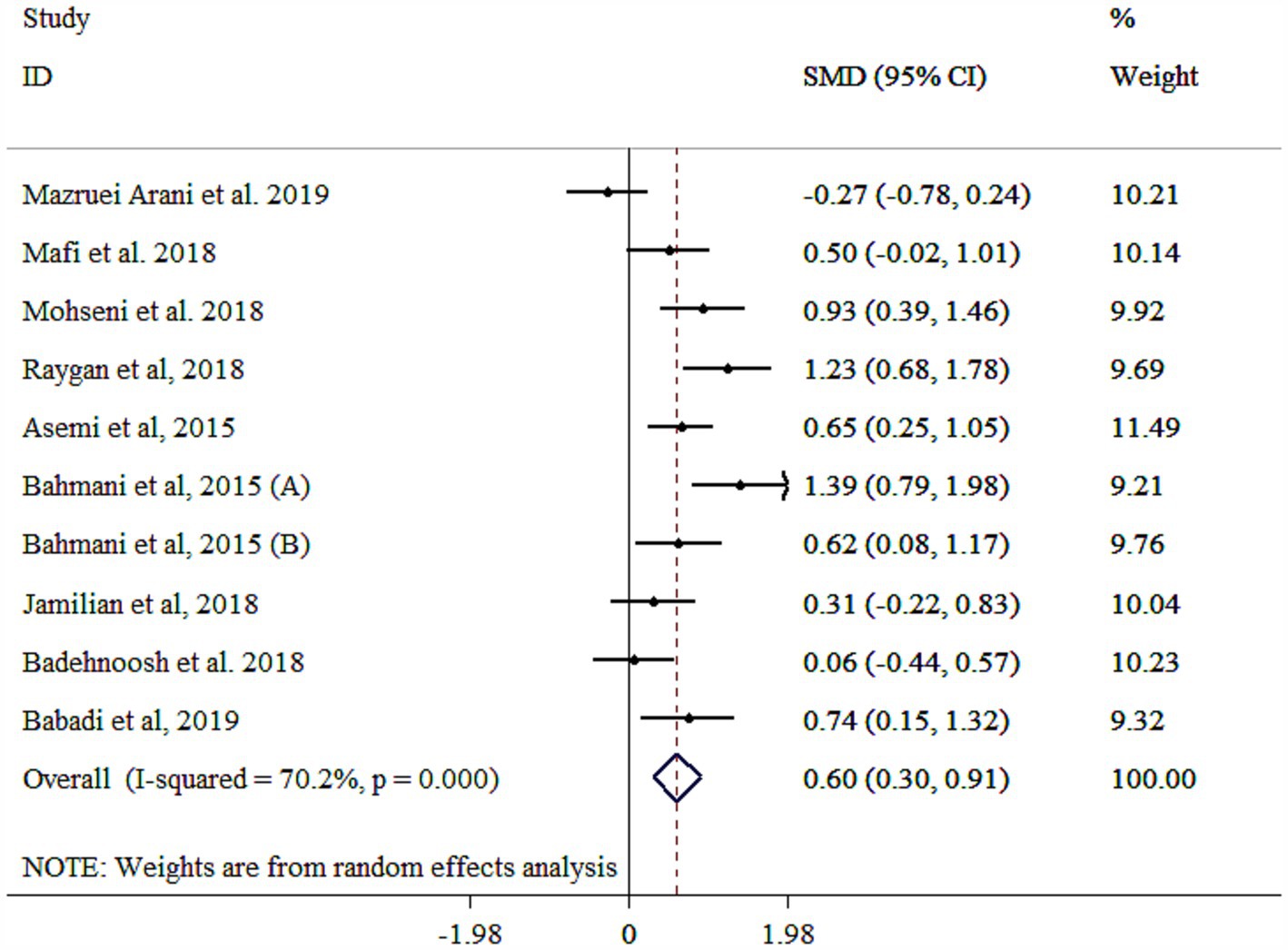
Figure 7. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on NO.
Probiotic supplementation significantly improved TAC levels (Figure 8), with significant heterogeneity across studies. Subgroup analyses identified age, gender, study population, BMI, and sample size as primary sources of heterogeneity (Table 3). Greater effects were observed in female participants, those with BMI <30 kg/m2, mean age ≥ 55 years, and individuals with GDM, particularly in studies using multi-strain probiotics, larger sample sizes (≥60), and shorter intervention durations (<10 weeks) (Table 3). Sensitivity analysis confirmed the robustness of the findings (p < 0.05). Meta-regression analysis showed no significant linear association between effect size and sample size or study duration (p > 0.05). While Egger’s (p = 0.043) and Begg’s (p = 0.019) tests suggested potential publication bias, the funnel plot displayed no evidence of asymmetry (Supplementary Figure S8).
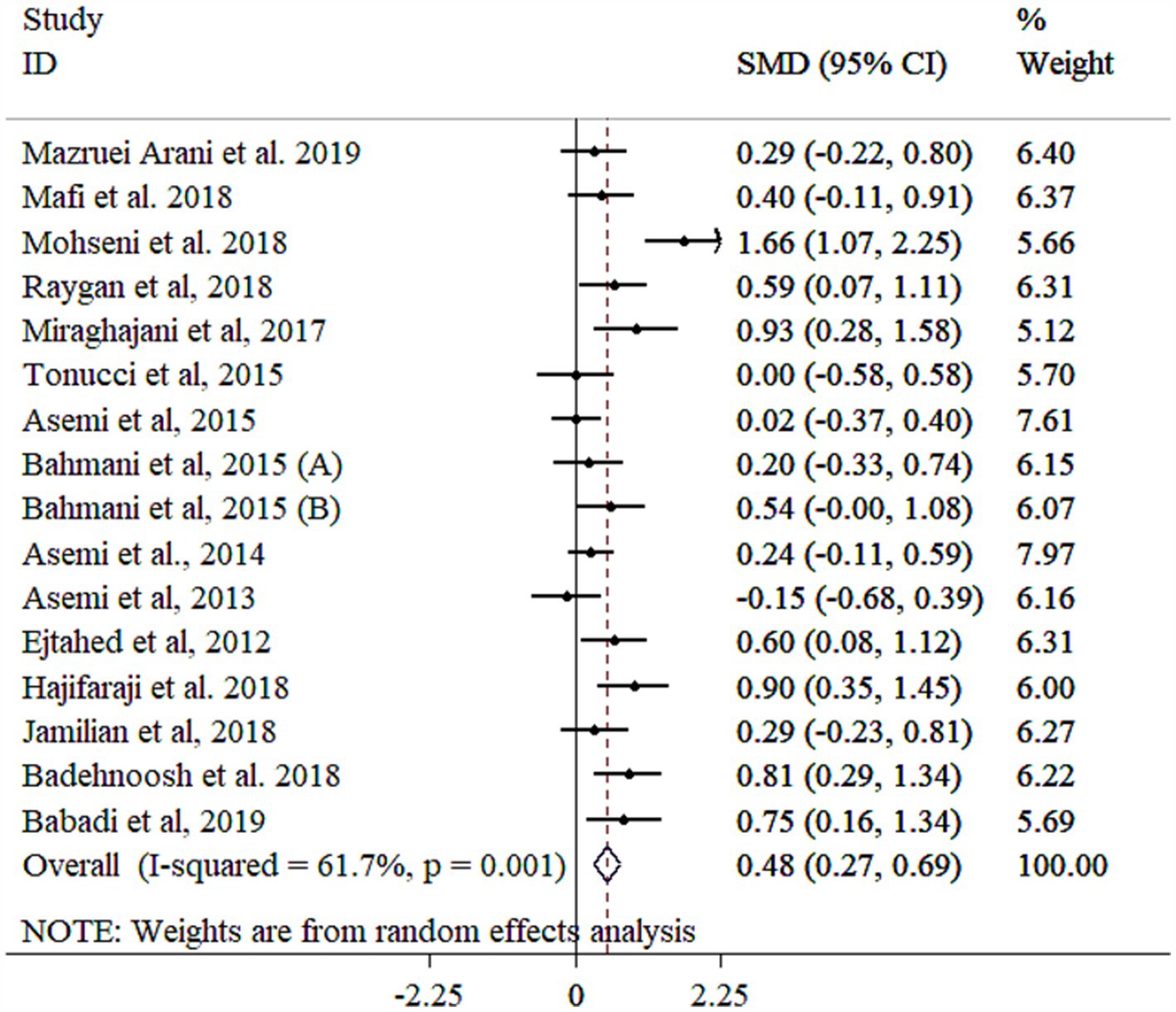
Figure 8. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of probiotics supplementation on TAC.
Table 4 summarizes the findings of the meta-analysis and evaluates the quality of evidence using the GRADE framework for the effect of probiotic supplementation on oxidative stress and inflammatory biomarkers in patients with diabetes. Evidence was frequently downgraded due to serious risk of bias and imprecision, while inconsistency, indirectness, and publication bias were generally not major concerns. Moderate-quality evidence supported significant effects on MDA, GSH, and NO, while low-quality evidence was found for CRP, TNF-α, IL-6, and TAC.
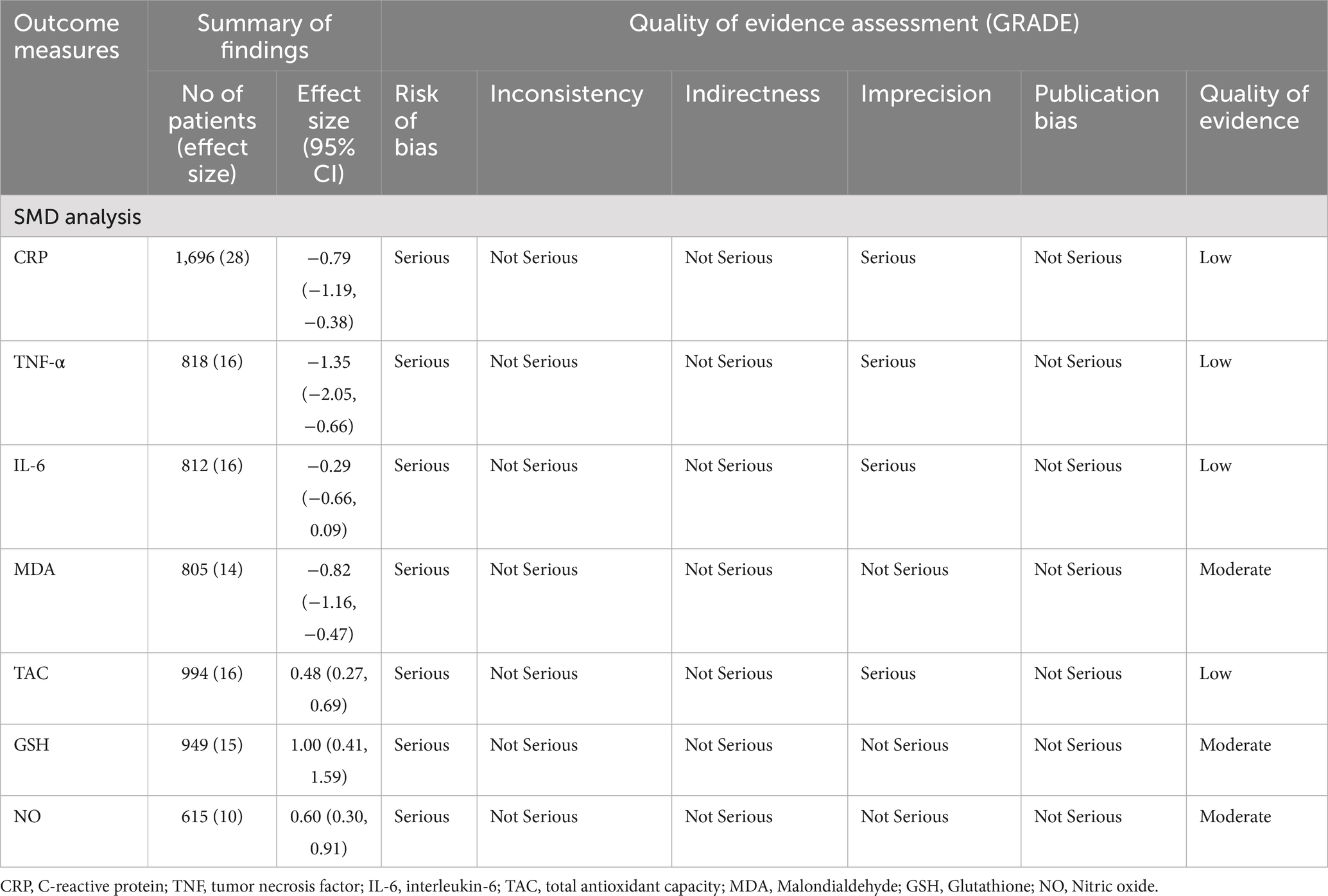
Table 4. Summary of findings and quality of evidence the probiotics supplementation on oxidative stress and inflammatory biomarkers.
This study comprehensively evaluated the effects of probiotics on inflammatory and oxidative stress biomarkers in diabetic populations by summarizing the results of previous meta-analysis and systematic review studies, as well as performing an updated meta-analysis on RCTs. Meta-research of meta-analysis studies revealed that probiotics, particularly strains like Lactobacillus, Bifidobacterium, and Streptococcus, emerged as promising interventions for reducing inflammation and oxidative stress, as evidenced by the significant reductions in markers like CRP, TNF-α, and MDA, alongside improvements in GSH and TAC levels. However, the effects on NO and IL-6 were inconsistent, with several studies reporting no significant changes.
Our meta-analysis results also demonstrated that probiotics had a significant improving effect on inflammatory and oxidative stress markers. However, contrary to most previous meta-analyses, our study revealed a significant increase in NO following probiotic supplementation, which was further confirmed through sensitivity analysis. Consistent with the majority of prior studies, our meta-analysis did not report a significant effect on IL-6. Nonetheless, sensitivity analysis indicated that probiotics could significantly reduce IL-6 levels. In all our pooled analyses, high heterogeneity, stemming from differences in methodology, various probiotic strains, and diverse study populations, reduced the certainty of the findings. Our result must be interpreted with caution due to high heterogeneity. Although subgroup analysis and meta-regression were used to identify the factors contributing to heterogeneity, the low methodological quality of most RCTs conducted so far underscores the need for higher-quality studies with larger sample sizes to enable definitive conclusions in this area.
The conflicting results of meta-analysis studies can be attributed to various factors. First, none of the meta-analysis studies were as comprehensive as ours and had missed some articles. Additionally, certain methodological flaws in these studies could have influenced their results. Some studies used WMD analysis (9, 12–14, 21–23, 25–29). Since various kits and methods with differing sensitivities have been used for measuring biochemical factors, failing to standardize the effect size based on the standard deviation and reporting raw mean differences cannot provide an accurate estimate of the impact of probiotics on biochemical markers (63, 64).
Regarding the subgroup analysis results, although both age groups benefited from probiotic supplementation in improving inflammation and antioxidant status, the effects appear to be more pronounced in individuals under 55 years of age. The observed greater efficacy of probiotic supplementation in individuals under 55 years of age, compared to older adults, may be attributed to several factors. Younger individuals typically have a more diverse and resilient gut microbiota, which can enhance the colonization and activity of administered probiotics, leading to more pronounced anti-inflammatory and antioxidant effects. In contrast, aging is associated with a gradual decline in immune function which may reduce the responsiveness of body to probiotics. Additionally, older adults often experience a natural decrease in gut microbiota diversity and stability, potentially diminishing the effectiveness of probiotic interventions (65). Therefore, the age-related differences in gut microbiota composition and immune system functionality likely contribute to the enhanced benefits of probiotics observed in the younger population. Regarding gender, the effects of probiotics do not appear to be dependent on sex, as both males and females benefit from the positive impacts of probiotics. However, a previous review highlighted differences in the responses of women and men to probiotics, possibly due to variations may be linked to differences in gut microbiota composition (66). Regarding study population, the largest reductions were observed in populations with diabetes-related conditions, such as GDM and diabetic nephropathy. These populations typically exhibit higher baseline levels of inflammation (67, 68), providing a greater scope for improvement. Regarding the duration of supplementation, probiotics did not show significant effects on certain biomarkers, including NO, IL-6, and CRP, in short-term interventions (<10 weeks). However, long-term supplementation (≥10 weeks) significantly improved all markers except IL-6. This finding suggests that probiotics are more effective with prolonged supplementation. Due to the diversity of probiotics and the varying doses studied, subgroup analysis based on these factors was not feasible. However, subgroup analysis based on single and multi-strain probiotics showed that multi-strain probiotic supplements do not necessarily have more pronounced beneficial effects than single-strain ones. Both types of supplements can significantly improve inflammation and oxidative stress, consistent with previous findings (69).
The molecular mechanisms by which probiotics exert their effects on inflammatory and oxidative stress markers are multifaceted and increasingly well-understood. Probiotics modulate systemic inflammation primarily through their influence on the gut microbiota, restoring dysbiosis and strengthening the intestinal epithelial barrier (70). This prevents translocation of lipopolysaccharide (LPS) from gram-negative bacteria, thereby reducing activation of Toll-like receptor 4 (TLR4) and downstream nuclear factor-kappa-B (NF-κB) signaling, a key driver of pro-inflammatory cytokine production such as CRP, TNF-α, and IL-6 (71–73). Novel insights suggest that probiotics also induce epigenetic modifications, including histone deacetylation and microRNA regulation, to suppress the transcription of pro-inflammatory genes (74). Moreover, probiotics have also been shown to regulate the NLRP3 inflammasome, which plays a pivotal role in the activation of caspase-1 and the release of interleukin-1-beta (IL-1β) (75). When IL-1β binds to its receptor, it activates intracellular signaling pathways, like the NF-κB pathway (76). In oxidative stress regulation, probiotics enhance the antioxidant defense systems by modulating redox-sensitive signaling pathways. For instance, they activate nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant gene expression, leading to an increase in TAC (77, 78). Recent studies highlight that certain probiotic strains produce bioactive metabolites, such as exopolysaccharides and indole derivatives, which directly scavenge reactive oxygen species (ROS) and mitigate lipid peroxidation, thereby lowering MDA levels (79, 80). Moreover, probiotics have been shown to modulate NO metabolism by influencing endothelial nitric oxide synthase (eNOS) activity (81). This novel mechanism involves increasing the availability of arginine, the substrate for NO synthesis (82), while concurrently reducing asymmetric dimethylarginine (ADMA), an eNOS inhibitor (83). These emerging molecular insights underscore the multifaceted and innovative roles of probiotics in reducing inflammation and oxidative stress, highlighting their therapeutic potential in managing chronic conditions characterized by these pathological processes.
In terms of clinical mechanisms, reducing inflammatory markers such as CRP and TNF-α can significantly alleviate systemic inflammation, thereby decreasing the risk of complications like vascular dysfunction and insulin resistance (84, 85). Lower levels of MDA, a marker of oxidative stress, indicate reduced cellular and tissue damage, particularly to endothelial cells and blood vessels, which are often impaired in diabetic and cardiovascular conditions (86). Increasing GSH and TAC enhances the antioxidant defenses of body, helping to neutralize free radicals and mitigate oxidative stress, thereby preserving cellular integrity and improving endothelial function (87). Elevated NO levels promote vasodilation, improving blood flow and reducing vascular resistance, which can lower blood pressure and reduce cardiovascular strain (88). Moderate-quality evidence supported significant effects on MDA, GSH, and NO, while low-quality evidence was found for CRP, TNF-α, IL-6, and TAC. Therefore, probiotics cannot yet be definitively recommended as a therapeutic approach for improving inflammation and oxidative stress in diabetic patients, and higher-quality studies need to be conducted.
This meta-analysis possesses several strengths, including rigorous methodology and the application of a various statistical analyses to capture a valid result. Consistent findings across diverse populations further reinforce the reliability of the results. However, some limitations warrant attention. First, subgroup analysis could not be performed on probiotic type and dosage due to variations in administered interventions. Therefore, strain-specific effects of probiotic on each biomarker were not be investigated. However, we performed subgroup analysis based on multi strain/single strain. Second, the high heterogeneity in most of analyses decreased the validity of findings. However, we tried to investigate the possible sources of it by performing subgroup analysis and meta-regression. Third, most included studies were short-term, limiting insights into the sustained effects of probiotics on inflammation and oxidative stress markers. However, subgroup analysis and meta-regression based on intervention duration could determine the impact of duration on effect sizes. Fourth, most of the RCTs conducted to date have methodological limitations, resulting in lower certainty of evidence. Therefore, additional high-quality studies are needed in this field to strengthen the findings and improve the reliability of the conclusions. Additionally, future studies should focus on strain-specific effects of probiotics on each inflammatory and oxidative stress biomarkers. This approach would provide more precise insights into how different probiotic strains contribute to modulating inflammation and oxidative stress in various populations, ultimately enhancing personalized therapeutic strategies.
This meta-analysis highlights the significant potential of probiotics in improving inflammatory and oxidative stress markers like CRP, TNF-α, and MDA, while boosting antioxidant defenses such as GSH, TAC, and NO in patients with diabetes mellitus. However, the non-significant effect on IL-6 suggests variability in strain-specific actions. Moderate-quality evidence supported significant effects on MDA, GSH, and NO, while low-quality evidence was found for CRP, TNF-α, IL-6, and TAC. Therefore, probiotics cannot yet be definitively recommended as a therapeutic approach for improving inflammation and oxidative stress in diabetic patients, and higher-quality studies with a strain-specific approach need to be conducted.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
XC: Conceptualization, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. LiY: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JY: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. CX: Conceptualization, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. LvY: Formal analysis, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1552358/full#supplementary-material
1. Chen, L , Deng, H , Cui, H , Fang, J , Zuo, Z , Deng, J, et al. . Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. (2017) 9:7204–18. doi: 10.18632/oncotarget.23208
2. Furman, D , Campisi, J , Verdin, E , Carrera-Bastos, P , Targ, S , Franceschi, C, et al. . Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. doi: 10.1038/s41591-019-0675-0
3. Brusselle, G , and Bracke, K . Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2014) 11:S322–8. doi: 10.1513/AnnalsATS.201403-118AW
4. Gudkov, AV , and Komarova, EA . p53 and the carcinogenicity of chronic inflammation. Cold Spring Harb Perspect Med. (2016) 6:61. doi: 10.1101/cshperspect.a026161
5. Zarezadeh, M , Musazadeh, V , Ghalichi, F , Kavyani, Z , Nasernia, R , Parang, M, et al. . Effects of probiotics supplementation on blood pressure: An umbrella meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2023) 33:275–86. doi: 10.1016/j.numecd.2022.09.005
6. Basso, FG , Pansani, TN , Turrioni, AP , Soares, DG , de Souza Costa, CA , and Hebling, J . Tumor necrosis factor-α and interleukin (IL)-1β, IL-6, and IL-8 impair in vitro migration and induce apoptosis of gingival fibroblasts and epithelial cells. Delaying Wound Healing J Periodontol. (2016) 87:990–6. doi: 10.1902/jop.2016.150713
7. Mann, ER , Lam, YK , and Uhlig, HH . Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
8. du, Y , He, C , An, Y , Huang, Y , Zhang, H , Fu, W, et al. . The role of short chain fatty acids in inflammation and body health. Int J Mol Sci. (2024) 25:7379. doi: 10.3390/ijms25137379
9. Samah, S , Ramasamy, K , Lim, SM , and Neoh, CF . Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2016) 118:172–82. doi: 10.1016/j.diabres.2016.06.014
10. Kasińska, MA , and Drzewoski, J . Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. (2015) 125:803–13. doi: 10.20452/pamw.3156
11. Tabrizi, R , Ostadmohammadi, V , Lankarani, KB , Akbari, M , Akbari, H , Vakili, S, et al. . The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Pharmacol. (2019) 852:254–64. doi: 10.1016/j.ejphar.2019.04.003
12. AbdelQadir, YH , Hamdallah, A , Sibaey, EA , Hussein, AS , Abdelaziz, M , Abdelazim, A, et al. . Efficacy of probiotic supplementation in patients with diabetic nephropathy: A systematic review and meta-analysis. Clin Nutr ESPEN. (2020) 40:57–67. doi: 10.1016/j.clnesp.2020.06.019
13. Bohlouli, J , Namjoo, I , Borzoo-Isfahani, M , Hojjati Kermani, MA , Balouch Zehi, Z , and Moravejolahkami, AR . Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: A systematic review and meta-analysis of clinical trials. Heliyon. (2021) 7:e05925. doi: 10.1016/j.heliyon.2021.e05925
14. Chen, Y , Yue, R , Zhang, B , Li, Z , Shui, J , and Huang, X . Effects of probiotics on blood glucose, biomarkers of inflammation and oxidative stress in pregnant women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Med Clin (Barc). (2020) 154:199–206. doi: 10.1016/j.medcli.2019.05.041
15. Takkouche, B , and Norman, G . PRISMA statement. Epidemiology. (2011) 22:128. doi: 10.1097/EDE.0b013e3181fe7999
16. Shea, BJ , Reeves, B , Wells, G , Thuku, M , Hamel, C , Moran, J, et al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017):j4008. doi: 10.1136/bmj.j4008
17. Higgins, JP , Altman, DG , Gøtzsche, PC , Jüni, P , Moher, D , Oxman, AD, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Begg, CB , and Mazumdar, M . Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
19. Egger, M , Smith, GD , Schneider, M , and Minder, C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
20. Guyatt, GH , Oxman, AD , Vist, GE , Kunz, R , Falck-Ytter, Y , Alonso-Coello, P, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
21. Hasain, Z , Che Roos, N , Rahmat, F , Mustapa, M , Raja Ali, R , and Mokhtar, N . Diet and pre-intervention washout modifies the effects of probiotics on gestational diabetes mellitus: a comprehensive systematic review and meta-analysis of randomized controlled trials. Nutrients. (2021) 13:3045. doi: 10.3390/nu13093045
22. Kocsis, T , Molnár, B , Németh, D , Hegyi, P , Szakács, Z , Bálint, A, et al. . Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:11787. doi: 10.1038/s41598-020-68440-1
23. Naseri, K , Saadati, S , Ghaemi, F , Ashtary-Larky, D , Asbaghi, O , Sadeghi, A, et al. . The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Eur J Nutr. (2023) 62:543–61. doi: 10.1007/s00394-022-03012-9
24. Ardeshirlarijani, E , Tabatabaei-Malazy, O , Mohseni, S , Qorbani, M , Larijani, B , and Baradar Jalili, R . Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. DARU J Pharm Sci. (2019) 27:827–37. doi: 10.1007/s40199-019-00302-2
25. Yao, K , Zeng, L , He, Q , Wang, W , Lei, J , and Zou, X . Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit. (2017) 23:3044–53. doi: 10.12659/MSM.902600
26. Zheng, HJ , Guo, J , Jia, Q , Huang, YS , Huang, WJ , Zhang, W, et al. . The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2019) 142:303–13. doi: 10.1016/j.phrs.2019.02.016
27. Chan, KY , Wong, MMH , Pang, SSH , and Lo, KKH . Dietary supplementation for gestational diabetes prevention and management: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. (2021) 303:1381–91. doi: 10.1007/s00404-021-06023-9
28. Wang, H , Wang, D , Song, H , Zou, D , Feng, X , Ma, X, et al. . The effects of probiotic supplementation on renal function, inflammation, and oxidative stress in diabetic nephropathy: A systematic review and meta-analysis of randomized controlled trials. Mater Express. (2021) 11:1122–31. doi: 10.1166/mex.2021.1888
29. Dai, Y , Quan, J , Xiong, L , Luo, Y , and Yi, B . Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: a systematic review and meta-analysis. Ren Fail. (2022) 44:862–80. doi: 10.1080/0886022X.2022.2079522
30. Andreasen, AS , Larsen, N , Pedersen-Skovsgaard, T , Berg, RMG , Møller, K , Svendsen, KD, et al. . Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. (2010) 104:1831–8. doi: 10.1017/S0007114510002874
31. Asemi, Z , Alizadeh, SA , Ahmad, K , Goli, M , and Esmaillzadeh, A . Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin Nutr. (2016) 35:819–25. doi: 10.1016/j.clnu.2015.07.009
32. Asemi, Z , Khorrami-Rad, A , Alizadeh, SA , Shakeri, H , and Esmaillzadeh, A . Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. (2014) 33:198–203. doi: 10.1016/j.clnu.2013.05.015
33. Asemi, Z , Zare, Z , Shakeri, H , Sabihi, SS , and Esmaillzadeh, A . Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. (2013) 63:1–9. doi: 10.1159/000349922
34. Babadi, M , Khorshidi, A , Aghadavood, E , Samimi, M , Kavossian, E , Bahmani, F, et al. . The effects of probiotic supplementation on genetic and metabolic profiles in patients with gestational diabetes mellitus: a randomized, double-blind. Placebo-Controlled Trial Probiotics Antimicrob Proteins. (2019) 11:1227–35. doi: 10.1007/s12602-018-9490-z
35. Badehnoosh, B , Karamali, M , Zarrati, M , Jamilian, M , Bahmani, F , Tajabadi-Ebrahimi, M, et al. . The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J Matern Fetal Neonatal Med. (2018) 31:1128–36. doi: 10.1080/14767058.2017.1310193
36. Bahmani, F , Tajadadi-Ebrahimi, M , Kolahdooz, F , Mazouchi, M , Hadaegh, H , Jamal, AS, et al. . The consumption of Synbiotic bread containing Lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind. Placebo-Controlled Trial J Am Coll Nutr. (2016) 35:506–13. doi: 10.1080/07315724.2015.1032443
37. Bayat, A , Azizi-Soleiman, F , Heidari-Beni, M , Feizi, A , Iraj, B , Ghiasvand, R, et al. . Effect of Cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int J Prev Med. (2016) 7:30. doi: 10.4103/2008-7802.175455
38. Ejtahed, HS , Mohtadi-Nia, J , Homayouni-Rad, A , Niafar, M , Asghari-Jafarabadi, M , and Mofid, V . Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. (2012) 28:539–43. doi: 10.1016/j.nut.2011.08.013
39. Feizollahzadeh, S , Ghiasvand, R , Rezaei, A , Khanahmad, H , sadeghi, A , and Hariri, M . Effect of probiotic soy Milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins. (2017) 9:41–7. doi: 10.1007/s12602-016-9233-y
40. Firouzi, S , Majid, HA , Ismail, A , Kamaruddin, NA , and Barakatun-Nisak, MY . Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. (2017) 56:1535–50. doi: 10.1007/s00394-016-1199-8
41. Hajifaraji, M , Jahanjou, F , Abbasalizadeh, F , Aghamohammadzadeh, N , Abbasi, MM , and Dolatkhah, N . Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J Clin Nutr. (2018) 27:581–91. doi: 10.6133/apjcn.082017.03
42. Hsieh, MC , Tsai, WH , Jheng, YP , Su, SL , Wang, SY , Lin, CC, et al. . The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. (2018) 8:16791. doi: 10.1038/s41598-018-35014-1
43. Ismail, A , Darwish, O , Tayel, D , Elneily, D , and Elshaarawy, G . Impact of probiotic intake on the glycemic control, lipid profile and inflammatory markers among patients with type 2 diabetes mellitus. Clinical Diabetol. (2021) 10:468–75. doi: 10.5603/DK.a2021.0037
44. Jafarnejad, S , Saremi, S , Jafarnejad, F , and Arab, A . Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J Nutr Metab. (2016) 2016:5190846. doi: 10.1155/2016/5190846
45. Jamilian, M , Amirani, E , and Asemi, Z . The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin Nutr. (2019) 38:2098–105. doi: 10.1016/j.clnu.2018.10.028
46. Kobyliak, N , Falalyeyeva, T , Mykhalchyshyn, G , Kyriienko, D , and Komissarenko, I . Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. (2018) 12:617–24. doi: 10.1016/j.dsx.2018.04.015
47. Mafi, A , Namazi, G , Soleimani, A , Bahmani, F , Aghadavod, E , and Asemi, Z . Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. (2018) 9:4763–70. doi: 10.1039/C8FO00888D
48. Mafi, A , Namazi, G , Soleimani, A , Bahmani, F , Aghadavod, E , and Asemi, Z . Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. (2018) 9:4763–70. doi: 10.1039/C8FO00888D
49. Mazruei Arani, N , Emam-Djomeh, Z , Tavakolipour, H , Sharafati-Chaleshtori, R , Soleimani, A , and Asemi, Z . The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind. Controlled Trial Probiotics Antimicrob Proteins. (2019) 11:1195–201. doi: 10.1007/s12602-018-9468-x
50. Miraghajani, M , Zaghian, N , Mirlohi, M , Feizi, A , and Ghiasvand, R . The impact of probiotic soy Milk consumption on oxidative stress among type 2 diabetic kidney disease patients: A randomized controlled clinical trial. J Ren Nutr. (2017) 27:317–24. doi: 10.1053/j.jrn.2017.04.004
51. Mobini, R , Tremaroli, V , Ståhlman, M , Karlsson, F , Levin, M , Ljungberg, M, et al. . Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab. (2017) 19:579–89. doi: 10.1111/dom.12861
52. Mohamadshahi, M , Veissi, M , Haidari, F , Javid, AZ , Mohammadi, F , and Shirbeigi, E . Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J Res Med Sci. (2014) 19:531–6. doi: 10.1016/j.clnesp.2023.01.014
53. Mohseni, S , Bayani, M , Bahmani, F , Tajabadi-Ebrahimi, M , Bayani, MA , Jafari, P, et al. . The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev. (2018) 34:2970. doi: 10.1002/dmrr.2970
54. Raygan, F , Rezavandi, Z , Bahmani, F , Ostadmohammadi, V , Mansournia, MA , Tajabadi-Ebrahimi, M, et al. . The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. (2018) 10:51. doi: 10.1186/s13098-018-0353-2
55. Rezaei, M , Sanagoo, A , Jouybari, L , Behnampoo, N , and Kavosi, A . The effect of probiotic yogurt on blood glucose and cardiovascular biomarkers in patients with type II diabetes: A randomized controlled trial. Evidence Based Care. (2017) 6:26–35. doi: 10.22038/ebcj.2016.7984
56. Sabico, S , al-Mashharawi, A , al-Daghri, NM , Wani, K , Amer, OE , Hussain, DS, et al. . Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin Nutr. (2019) 38:1561–9. doi: 10.1016/j.clnu.2018.08.009
57. Sato, J , Kanazawa, A , Azuma, K , Ikeda, F , Goto, H , Komiya, K, et al. . Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci Rep. (2017) 7:12115. doi: 10.1038/s41598-017-12535-9
58. Soleimani, A , Zarrati Mojarrad, M , Bahmani, F , Taghizadeh, M , Ramezani, M , Tajabadi-Ebrahimi, M, et al. . Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. (2017) 91:435–42. doi: 10.1016/j.kint.2016.09.040
59. Tajadadi-Ebrahimi, M , Bahmani, F , Shakeri, H , Hadaegh, H , Hijijafari, M , Abedi, F, et al. . Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. (2014) 65:34–41. doi: 10.1159/000365153
60. Tay, A , Pringle, H , Penning, E , Plank, LD , and Murphy, R . PROFAST: A randomized trial assessing the effects of intermittent fasting and Lacticaseibacillus rhamnosus probiotic among people with prediabetes. Nutrients. (2020) 12:530. doi: 10.3390/nu12113530
61. Toejing, P , Khampithum, N , Sirilun, S , Chaiyasut, C , and Lailerd, N . Influence of Lactobacillus paracasei HII01 supplementation on Glycemia and inflammatory biomarkers in type 2 diabetes: A randomized clinical trial. Food Secur. (2021) 10:455. doi: 10.3390/foods10071455
62. Tonucci, LB , Olbrich dos Santos, KM , Licursi de Oliveira, L , Rocha Ribeiro, SM , and Duarte Martino, HS . Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. (2017) 36:85–92. doi: 10.1016/j.clnu.2015.11.011
63. Andrade, C , and Difference, M . Standardized mean Difference (SMD), and their use in Meta-analysis: as simple as it gets. J Clin Psychiatry. (2020) 81:681. doi: 10.4088/JCP.20f13681
64. Jing, Y , and Lin, L . Comparisons of the mean differences and standardized mean differences for continuous outcome measures on the same scale. JBI Evid Synth. (2024) 22:394–405. doi: 10.11124/JBIES-23-00368
65. Ragonnaud, E , and Biragyn, A . Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. (2021) 18:2. doi: 10.1186/s12979-020-00213-w
66. Chandrasekaran, P , Weiskirchen, S , and Weiskirchen, R . Effects of probiotics on gut microbiota: An overview. Int J Mol Sci. (2024) 25:6022. doi: 10.3390/ijms25116022
67. Donate-Correa, J , Luis-Rodríguez, D , Martín-Núñez, E , Tagua, VG , Hernández-Carballo, C , Ferri, C, et al. . Inflammatory targets in diabetic nephropathy. J Clin Med. (2020) 9:458. doi: 10.3390/jcm9020458
68. Saucedo, R , Ortega-Camarillo, C , Ferreira-Hermosillo, A , Díaz-Velázquez, MF , Meixueiro-Calderón, C , and Valencia-Ortega, J . Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants. (2023) 12:812. doi: 10.3390/antiox12101812
69. Cristofori, F , Dargenio, VN , Dargenio, C , Miniello, VL , Barone, M , and Francavilla, R . Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front Immunol. (2021) 12:578386. doi: 10.3389/fimmu.2021.578386
70. Hemarajata, P , and Versalovic, J . Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294
71. Page, MJ , Kell, DB , and Pretorius, E . The role of lipopolysaccharide-induced cell Signalling in chronic inflammation. Chronic Stress. (2022) 6:24705470221076390. doi: 10.1177/24705470221076390
72. Han, C , Ding, Z , Shi, H , Qian, W , Hou, X , and Lin, R . The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem. (2016) 38:2464–78. doi: 10.1159/000445597
73. Faghfouri, AH , Afrakoti, LGMP , Kavyani, Z , Nogourani, ZS , Musazadeh, V , Jafarlou, M, et al. . The role of probiotic supplementation in inflammatory biomarkers in adults: an umbrella meta-analysis of randomized controlled trials. Inflammopharmacology. (2023) 31:2253–68. doi: 10.1007/s10787-023-01332-8
74. Woo, V , and Alenghat, T . Epigenetic regulation by gut microbiota. Gut Microbes. (2022) 14:2022407. doi: 10.1080/19490976.2021.2022407
75. Kasti, AN , Synodinou, KD , Pyrousis, IA , Nikolaki, MD , and Triantafyllou, KD . Probiotics regulating inflammation via NLRP3 Inflammasome modulation: A potential therapeutic approach for COVID-19. Microorganisms. (2021) 9:376. doi: 10.3390/microorganisms9112376
76. McGeough, MD , Pena, CA , Mueller, JL , Pociask, DA , Broderick, L , Hoffman, HM, et al. . Cutting edge: IL-6 is a marker of inflammation with no direct role in inflammasome-mediated mouse models. J Immunol. (2012) 189:2707–11. doi: 10.4049/jimmunol.1101737
77. Karaca, B , Yilmaz, M , and Gursoy, UK . Targeting Nrf2 with probiotics and Postbiotics in the treatment of periodontitis. Biomol Ther. (2022) 12:729. doi: 10.3390/biom12050729
78. Aboulgheit, A , Karbasiafshar, C , Zhang, Z , Sabra, M , Shi, G , Tucker, A, et al. . Lactobacillus plantarum probiotic induces Nrf2-mediated antioxidant signaling and eNOS expression resulting in improvement of myocardial diastolic function. Am J Physiol Heart Circ Physiol. (2021) 321:H839–h849. doi: 10.1152/ajpheart.00278.2021
79. Averina, OV , Poluektova, EU , Marsova, MV , and Danilenko, VN . Biomarkers and utility of the antioxidant potential of probiotic lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines. (2021) 9:1340. doi: 10.3390/biomedicines9101340
80. Abdel-Wahab, BA , Abd el-Kareem, H , Alzamami, A , Fahmy, C , Elesawy, B , Mostafa Mahmoud, M, et al. . Novel exopolysaccharide from marine Bacillus subtilis with broad potential biological activities: insights into antioxidant, anti-inflammatory, cytotoxicity, and anti-alzheimer activity. Meta. (2022) 12:715. doi: 10.3390/metabo12080715
81. Mahdavi-Roshan, M , Salari, A , Kheirkhah, J , and Ghorbani, Z . The effects of probiotics on inflammation, endothelial dysfunction, and atherosclerosis progression: a mechanistic overview. Heart Lung Circul. (2022) 31:e45–71. doi: 10.1016/j.hlc.2021.09.006
82. Jäger, R , Zaragoza, J , Purpura, M , Iametti, S , Marengo, M , Tinsley, GM, et al. . Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter. Crossover Study Probiotics Antimicrob Proteins. (2020) 12:1330–9. doi: 10.1007/s12602-020-09656-5
83. Pourrajab, B , Naderi, N , Janani, L , Hajahmadi, M , Mofid, V , Dehnad, A, et al. . The impact of probiotic yogurt versus ordinary yogurt on serum sTWEAK, sCD163, ADMA, LCAT and BUN in patients with chronic heart failure: a randomized, triple-blind, controlled trial. J Sci Food Agric. (2022) 102:6024–35. doi: 10.1002/jsfa.11955
84. Stanimirovic, J , Radovanovic, J , Banjac, K , Obradovic, M , Essack, M , Zafirovic, S, et al. . Role of C-reactive protein in diabetic inflammation. Mediat Inflamm. (2022) 2022:3706508.
85. Akash, MSH , Rehman, K , and Liaqat, A . Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. (2018) 119:105–10. doi: 10.1002/jcb.26174
86. An, Y , Xu, BT , Wan, SR , Ma, XM , Long, Y , Xu, Y, et al. . The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc Diabetol. (2023) 22:237. doi: 10.1186/s12933-023-01965-7
87. Tandon, R , and Tandon, A . Unraveling the multifaceted role of glutathione in Sepsis: A comprehensive review. Cureus. (2024) 16:e56896. doi: 10.7759/cureus.56896
Keywords: probiotics, oxidative stress, inflammation, metabolic disorders, biomarkers
Citation: Chen X, Yan L, Yang J, Xu C and Yang L (2025) The impact of probiotics on oxidative stress and inflammatory markers in patients with diabetes: a meta-research of meta-analysis studies. Front. Nutr. 12:1552358. doi: 10.3389/fnut.2025.1552358
Received: 27 December 2024; Accepted: 28 January 2025;
Published: 07 March 2025.
Edited by:
Calinoiu Florina Lavinia, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Nurpudji Astuti Taslim, Hasanuddin University, IndonesiaCopyright © 2025 Chen, Yan, Yang, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Chen, Y2ljaWNoZW4xMDI3QDEyNi5jb20=; Lv Yang, eWw3Mzk5NTVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.