
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 26 March 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1549948
This article is part of the Research TopicNutrition, Inflammation and Oxidative Stress in Obstetrics and GynecologyView all 12 articles
Background: Composite dietary antioxidant index (CDAI) has been found protective to women’s health. However, the association between CDAI level and the risk of endometriosis in women is unclear.
Methods: A total of 4,153 women from the National Health and Nutrition Examination Survey (NHANES) 2001–2006 were included in this cross-sectional study. We evaluated the association between CDAI level and the risk of endometriosis using three logistic regression models and restricted cubic spline. Stratified and sensitivity analyses were also performed.
Results: Logistic regression analysis found that CDAI level was inversely associated with the development of endometriosis. The associated odds ratio (OR) for each SD increase in CDAI was 0.98 [95%CI: 0.96–0.99]. After dividing the CDAI level into four quartiles, we found that compared with the CDAI level in Q1 (−1.89, −1.79), the ORs [95%CI] associated with endometriosis in Q2 (−1.79, −0.69), Q3 (−0.69, 1.42) and Q4 (1.42, 47.92) were 0.94 [0.87, 1.03], 0.95 [0.88, 1.04] and 0.83 [0.77, 0.91], respectively, with p trend<0.001. Restricted cubic spline showed a negative dose–response relationship between CDAI level and endometriosis risk. In addition, the protective effect of CDAI on endometriosis was more obvious in women aged 30–39 years (OR = 0.83, 95% CI = 0.69–0.99), gave relatively more births (OR = 0.82, 95% CI = 071–0.93), lower family income (OR = 0.69, 95% CI = 0.54–0.88), Non-Hispanic Black (OR = 0.72, 95% CI = 0.58–0.89), less educated (OR = 0.69, 95% CI = 0.52–0.91), smoker (OR = 0.74, 95% CI = 0.61–0.89), alcohol drinker (OR = 0.86, 95% CI = 0.77–0.97), overweight or obese (OR = 0.76, 95% CI = 0.60–0.97), and hypertensive (OR = 0.72, 95% CI = 0.60–0.87).
Conclusion: Our findings may provide valuable insights into the primary prevention of endometriosis in women and further prospective studies are warranted.
Endometriosis is defined as the presence of endometrial-like glands and stroma outside the uterus. It is estimated that this disease affects approximately 10% of women (1, 2) and is prone to serious complications, including dysmenorrhea and chronic pelvic pain (3). In addition, endometriosis can also lead to adverse prognoses, such as infertility, ovarian cancer, cardiovascular disease, and skin melanoma (4–6). The onset of endometriosis may be related to hormonal, neural and immune factors, lifestyle, diet, etc. In studies examining the association between diet and endometriosis, consumption of fruits and vegetables, fish oil, dairy products rich in calcium and vitamin D, and omega-3 fatty acids has been suggested to potentially reduce the risk of endometriosis (7–10). Conversely, intake of products high in trans-unsaturated fatty acids, general fat consumption, and intake of beef and other red meats, as well as alcohol, have been associated with an increased risk of endometriosis (7, 11, 12). However, no definitive correlations have been established between these foods and the risk of endometriosis. Further research is warranted to fully elucidate the impact of diet on the risk of developing endometriosis and to inform preventive strategies targeting this modifiable risk factor.
A large amount of evidence shows that vitamin and mineral supplements have a protective effect on women’s health (13–15). Antioxidants (such as vitamin A or carotenoids, vitamins C and E) can prevent oxidative stress in cells, thereby reducing cell damage and reducing the occurrence of diseases (16). A prospective cohort study of 34,492 postmenopausal women by Yochum et al. found that vitamin E in food had a protective effect on death caused by stroke (17). Osganian et al. conducted a prospective study and showed that women who took vitamin C supplements had a lower risk of coronary heart disease (18). However, a systematic review from the United States Preventive Services Task Force (USPSTF) found that supplementation of a single nutrient did not have a significant impact on preventing chronic diseases (cardiovascular disease, cancer, etc.) in adults (19). Therefore, a large number of studies suggest that people can supplement a variety of complex nutrients to maintain health (20, 21).
The composite dietary antioxidant index (CDAI) is a comprehensive score used to assess the total antioxidant capacity (TAC) of an individual’s diet. It is based on various dietary vitamins and minerals with antioxidant effects, including vitamins A, C, E and carotene, as well as the minerals selenium and zinc (22, 23). Previous studies have found that CDAI, as a new dietary health score indicator, is closely related to women’s health. A cross-sectional study by Shen et al. showed that there is a nonlinear negative correlation between CDAI and infertility in American women (24). Moreover, Li et al. performed a cross-sectional study on young American women and suggested that CDAI was negatively correlated with migraine attacks, and a higher CDAI may be a protective factor for preventing migraine attacks (24). Rumiris et al. conducted a randomized, double-blind, placebo-controlled daily antioxidant supplementation trial, and the results showed that compared with iron and folic acid supplementation alone, antioxidant supplementation in pregnant women with low antioxidant status was associated with better maternal and perinatal outcomes (25). Another cross-sectional study by Zhao et al. on Americans found that CDAI was negatively correlated with depression in overweight and obese adults, especially among women (26). However, the relationship between CDAI and endometriosis in women remains unclear.
Therefore, this study aimed to explore the relationship between CDAI and endometriosis in women using data from the National Health and Nutrition Examination Survey (NHANES), a large-scale and representative U.S. population survey. The results of this study may provide valuable insights into the role of CDAI levels in the occurrence of endometriosis in women, thereby providing important reference value for the primary prevention of endometriosis in female population worldwide.
NHANES was initiated by the National Center for Health Statistics (NCHS) to construct a nationally representative health data of the American population. The survey uses a complex, multi-stage, stratified probability sampling, cross-sectional design to collect information on approximately 5,000 people each year. The survey began sampling in 1999 and releases data every 2 years. The research design of NHANES has been approved by the NCHS Research Ethics Review Board, and the study obtained informed consent from all participants. The NHANES survey data, detailed survey operation manual, informed consent form, and manuals for each sampling period are publicly available on the NHANES website.1
Since some years of the NHANES survey did not include assessments of endometriosis history or diet, we included data from subjects from 2001 to 2006, with a total of 31,509 people participating in the survey. The inclusion criteria for the subjects in this study were: (a) Women aged 20–54 years old; (b) With information about endometriosis diagnosis; (c) With 24-h dietary recall on the first and second day. After excluding samples that did not meet the above inclusion criteria, this study included 4,153 female subjects. The specific exclusion process is shown in Figure 1.
Our study outcome was the history of endometriosis diagnosis, which was assessed using the NHANES Reproductive Health Questionnaire (RHQ), which assesses women’s reproductive health issues, including menstrual history, pregnancy history, breastfeeding, use of oral contraceptives and hormone replacement therapy, and other related conditions. We used the RHQ question “Has a doctor or other health professional ever told that had endometriosis? (Endometriosis is a disease in which the tissue that forms the lining of the uterus/womb attaches to other places, such as the ovaries, fallopian tubes, or abdominal cavity)” to collect whether the subjects had endometriosis. Participants who answered “yes” to this question was considered to be diagnosed with endometriosis.
The dietary assessment data of the subjects was collected from the dietary section of NHANES, which assessed the subjects’ 24-h dietary recall data for two non-consecutive days and collected information on the participants’ food intake. The dietary recall interview on the first day of the subject was conducted at the Mobile Examination Center (MEC), and the dietary recall on the second day was conducted by telephone 3 to 10 days after the completion of the first recall interview. We calculated the average daily dietary intake based on the dietary recall data of these 2 days, and calculated the CDAI level of all subjects based on the study of Wright et al. (27). Specifically, the CDAI calculation is based on the sum of the average daily standardized intake of zinc, selenium, carotenoids, vitamin A, vitamin C, and vitamin E. The standardized intake is calculated by subtracting the mean value from the intake of the food and dividing it by the standard deviation. The specific calculation formula of CDAI is:
To reduce potential confounding bias in the analysis, we selected the following covariates based on previous relevant studies and clinical significance: age distribution (years) (20–29, 30–39, 40–49, 50–54), race (Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, other race-including multi-racial), education level (<=HS graduate, HS graduate, some college or associate degree, college graduate or above), marital status (married, widowed, divorced, separated, never married, living with partner), ratio of family income to poverty (<1.3, 1.3–3.5, > = 3.5), BMI (kg/m2) (< 24.9, 25–29.9, 30–34.9, ≥35), drinking (yes or no), smoking—cigarette use (never, past, current smoker), number of live births (0, 1, 2, > = 3), hypertension (yes or no), diabetes (yes or no), coronary heart disease (yes or no), antihypertensive medication use (yes or no), antidiabetic medication use (yes or no), anticholesterol medication use (yes or no), and female hormone use (yes or no). Supplementary Table 1 provides a detailed description of these covariate assessments.
First, descriptive statistics were performed. Categorical variables were described as frequency and percentage, and the chi-square test or Fisher’s exact test was used to compare the endometriosis patient group with the non-patient group. Numerical variables that conformed to the normal distribution were described as mean (± standard deviation [SD]), and the independent sample t test was used for inter-group comparison. Numerical variables that were not normally distributed were described as median [first quartile (P25) and third quartile (P75)], and the Wilcoxon test was used for group comparison. Second, three logistic regression models were used to explore the relationship between CDAI and endometriosis. Model A only adjusted for age; Model B adjusted for age, race, education level, marital status, the ratio of family income to poverty, number of live births and BMI; Model C further adjusted for alcohol use, smoking—cigarette use, hypertension, diabetes, congestive heart failure, and coronary heart disease on the basis of Model B. In addition, we also used the restricted cubic spline (RCS) method to explore the dose–response relationship between CDAI level and the risk of endometriosis. We further conducted subgroup analysis for age distribution (years), number of live births, ratio of family income to poverty, race, education level, marital status, smoking cigarette use, alcohol use, BMI (kg/m2), coronary heart disease, hypertension, diabetes. We used product interaction terms to measure whether there was an interaction effect between CDAI and each covariate. Finally, we also conducted several sensitivity analyses to evaluate the robustness of the research results. Specifically, we first excluded participants who had previously used female hormones, and verified the relationship between CDAI and endometriosis based on the above three logistic regression models. In addition, we excluded participants who used antihypertensive drugs, hypoglycemic drugs, and lipid-lowering drugs, respectively, and also took the above steps to verify the findings of this study. Finally, we excluded individuals with any missing values for the variables involved in this study. All p values reported in this study were two-sided tests, and the significance level was set at p < 0.05. We used recommended NHANES weight parameters to calculate weighted characteristics. All statistical analyses were performed using R software (version 4.4.1).
A total of 4,153 adult women were included in this study, with a mean age of 35.6 ± 10.0 years, 21.9% Mexican American, 4.1% other Hispanic, 48.0% Non-Hispanic White, and 21.3% Non-Hispanic Black. Among them, 288 were diagnosed with endometriosis. The median CDAI level (P25, P75) of the endometriosis patient group was −1.28 [−1.81, 0.42], which was lower (p = 0.006) than that of non-endometriosis patients. Compared with the non-endometriosis group, the intake of vitamin E (p = 0.047), vitamin A (p = 0.010), beta-carotene (p = 0.003), vitamin C (p = 0.004), and selenium (p = 0.014) was significantly lower in the endometriosis group. In addition, the two groups have different differences in age (p < 0.001), education level (p = 0.002), and family income (p < 0.001). There were significant differences in, marital status (p < 0.001), drinking (p = 0.018), smoking history (p = 0.001), suffering from hypertension (p < 0.001), and taking estrogen drugs (p < 0.001; Table 1).
Table 2 illustrates the association between CDAI level and risk of endometriosis in women using a multivariate logistic regression model. In model A, we observed that continuous CDAI level was negatively associated with the occurrence of endometriosis, and the associated odds ratio (OR) for each SD increase in CDAI was 0.98 [95% CI: 0.96–0.99]. After dividing CDAI levels into quartiles, it was found that compared with CDAI levels in Q1 (−1.89, −1.79), Q2 (−1.79, −0.69), Q3 (−0.69, 1.42) and Q4 (1.42, 47.92) were associated with endometriosis with ORs of 0.94 [0.87, 1.03], 0.95 [0.88, 1.04] and 0.83 [0.77, 0.91], respectively, with a p trend<0.001.
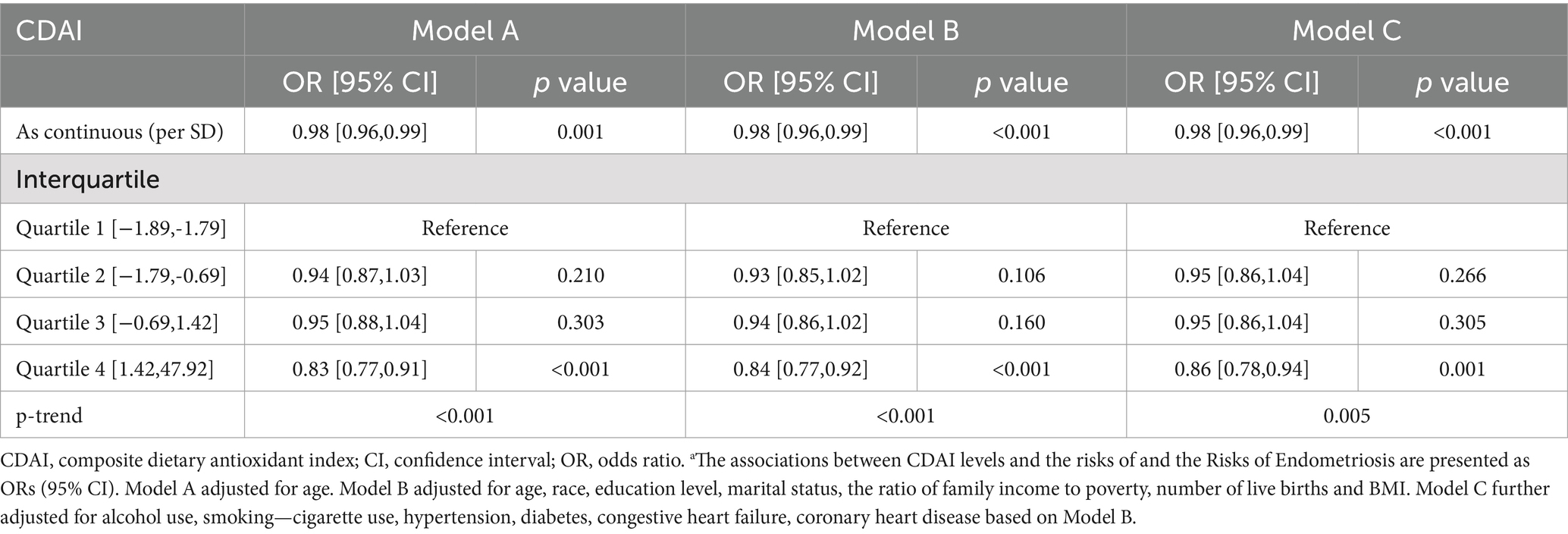
Table 2. Associations between CDAI Levels and the Risks of Endometriosis in Women aged 20–54 years a.
In model B, higher CDAI levels were associated with a lower risk of endometriosis (OR = 0.98, 95% CI: 0.96–0.99) after adjusting for age, race, education level, marital status, the ratio of family income to poverty, number of live births and BMI. Similarly, compared with Q1 level, the OR of CDAI level in Q2, Q3 and Q4 were 0.93 [0.85, 1.02], 0.94 [0.86, 1.02] and 0.84 [0.77, 0.92], respectively, with p trend<0.001.
In addition, after additional adjustment for alcohol use, smoking-cigarette use, hypertension, diabetes, congestive heart failure, coronary heart disease based on model B, we obtained similar findings in model C, with the OR of Q4 being 0.86 [95% CI: 0.78, 0.94] compared with Q1. In addition, the results of the RCS curve showed that there was a negative dose–response relationship between CDAI level and the risk of endometriosis (Figure 2).
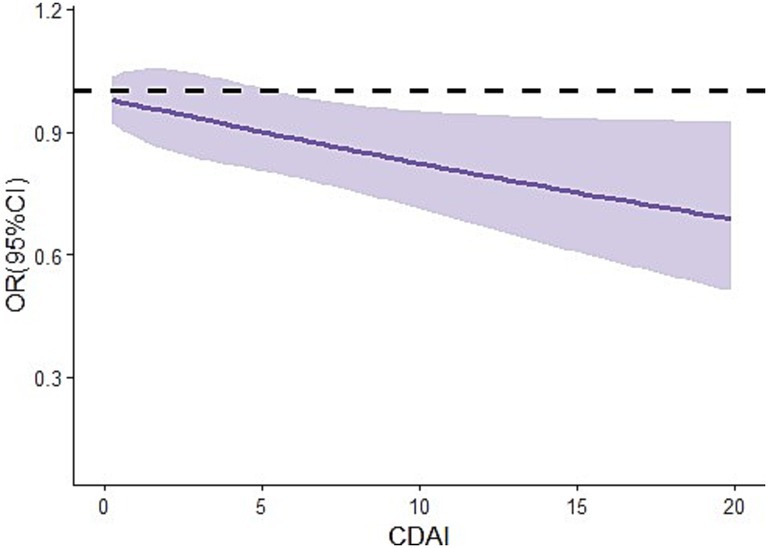
Figure 2. Dose–response relationship between CDAI levels and the risks of endometriosis in women aged 20–54 years. CI, confidence interval; OR, odds ratio; CDAI, composite dietary anti-oxidant index.
The results of subgroup analysis based on different categorical variables showed that compared with low CDAI, high CDAI was significantly associated with a lower risk of endometriosis in the following subgroups: 30–39 years (OR = 0.83, 95% CI: 0.69–0.99), number of live births = 2 (OR = 0.79, 95% CI: 0.64–0.98) or ≥ 3 (OR = 0.82, 95% CI: 0.71–0.93), ratio of family income to poverty<1.3 (OR = 0.69, 95% CI: 0.54–0.88), Non-Hispanic Black (OR = 0.72, 95% CI: 0.58–0.89), education level ≤ HS graduate (OR = 0.69, 95% CI: 0.52–0.91), current smoker (OR = 0.74, 95% CI: 0.76–0.93), past alcohol drinker (OR = 0.86, 95% CI: 0.77–0.97), BMI 25–29.9 kg/m2 (OR = 0.80, 95% CI: 0.67–0.97) or ≥ 35 kg/m2 (OR = 0.76, 95% CI: 0.60–0.97), no coronary heart disease (OR = 0.86, 95% CI: 0.78–0.95), with hypertension (OR = 0.72, 95% CI: 0.60–0.87), no diabetes (OR = 0.86, 95% CI: 0.78–0.94; Table 3).
After excluding participants who had ever used female hormones, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs respectively, high levels of CDAI still had a significant protective effect on women with endometriosis (Supplementary Tables 2–5), indicating that the effect of CDAIs on the risk of endometriosis is not interfered with by the use of these drugs. In addition, after excluding individuals with missing covariates, the association between CDAI and the risk of endometriosis still holds (Supplementary Table 6), which is consistent with the main research results and illustrates the robustness of the results of this study.
Our study is the first to investigate the relationship between CDAI and the risk of endometriosis in a nationally representative adult female population in the United States. First, we observed that the risk of endometriosis was lower in women with higher CDAI levels, and this was still robust after controlling for all covariates. In addition, there was a negative dose–response relationship between the level of CDAI and the risk of endometriosis. We further found that the protective effect of high CDAI levels on endometriosis were more pronounced in females aged 30–39 years, gave relatively more births, lower family income, Non-Hispanic Black, less educated, current smoker, past alcohol drinker, overweight or obese, no coronary heart disease, with hypertension, no diabetes.
We found that CDAI is an important protective factor for the risk of endometriosis, indicating that an antioxidant diet can play an important role in the primary prevention of endometriosis. Although there is limited evidence that CDAI is associated with the risk of endometriosis. Some studies have found that dietary factors are associated with the risk of endometriosis. A case–control study conducted by Parazzini et al. found that consuming more green vegetables and fresh fruits can reduce the risk of endometriosis in women (7), which was verified in another literature review of 11 studies (28). A diet rich in green vegetables and fruits is rich in vitamin C, carotenoids, folic acid, and lycopene, and these micronutrients may help inhibit cell proliferation (29). It is worth noting that an antioxidant diet also has a protective effect on women who already have endometriosis. An observational study by Krabbenborg et al. on 157 patients with endometriosis showed that an antioxidant diet can improve the symptoms of endometriosis and improve the quality of life of patients (30). Supplementing relevant antioxidant diets can inhibit endometriosis-related symptoms in patients with endometriosis, which may be related to the anti-inflammatory, antioxidant, antiproliferative and immunomodulatory effects of antioxidant diets (31). In addition, Mier-Cabrera et al. found that after patients with endometriosis adopted a high-antioxidant diet, their antioxidant markers in peripheral blood were improved (32). Additionally, an antioxidant-rich diet confers protective effects against complications associated with endometriosis. For instance, antioxidants have been demonstrated to modulate oxidative stress and cellular signaling pathways in ovarian clear cell carcinoma, which may be of significant importance for the prevention and treatment of ovarian cancer (33). Antioxidants can also inhibit the development and progression of cervical cancer (34). Moreover, antioxidants provide protection against infertility in women, which is a common complication of endometriosis (35).
Important components of CDAI include vitamins A, C, E, carotene and important trace elements (zinc, selenium). These dietary elements play an important role in the occurrence and development of endometriosis. Pierzchalski et al. found that all-trans retinoic acid (ATRA) can promote hormonal changes (36) and inhibit endometriosis. In addition, some studies have found that ATRA can also reduce the level of IL-6, which is involved in the pathogenesis of endometriosis (37), including the migration and invasion of endometriotic cells (38). Studies have shown that vitamin A has the effect of preventing dioxin-induced tissue damage (39, 40). A population-based study by Zhou et al. found that women with endometriosis had lower vitamin A intake than women without endometriosis (41). Further, β-carotene is an important source of vitamin A (42). A large number of studies have found that β-carotene can prevent lipid peroxidation in cells and reduce free radical damage to DNA (43, 44). β-Carotene is also thought to enhance NK cell function (45). It is reported that approximately 50% of the vitamin A in the diet of the American population comes from β-carotene (46). These studies all suggest that vitamin A and carotene may protect against the development of endometriosis.
Vitamin C and E are two common antioxidants. Vitamin C, as a cofactor for a variety of essential enzymes, is involved in the synthesis of catecholamines and vasopressin (47) and in the hydroxylation of collagen (48, 49). Studies have demonstrated that vitamin C can inhibit the adhesion and growth of endometrial cells, thereby alleviating the symptoms of endometriosis (50). Vitamin E has anti-angiogenic and anti-inflammatory effects (51, 52). Vitamin E has been shown to reduce levels of free radicals and reactive oxygen species (ROS), thereby attenuating oxidative stress and inflammatory responses, which in turn alleviates pain associated with endometriosis. Additionally, vitamin E may modulate immune responses and decrease levels of inflammatory markers (53). Since both vitamins are involved in antioxidant processes, which are also the basis of the pathogenesis of endometriosis, these two vitamins may have some protective value against the development of endometriosis. Although vitamin C is primarily considered a micronutrient with a protective effect against endometriosis (54–56), some studies have shown that vitamin E is not associated with the risk of this disease (55). It is worth noting that some studies have found that the two vitamins have a synergistic effect, and their combined use may have a better antioxidant effect and be more beneficial for disease prevention (57).
Trace elements play an important role in the occurrence and development of endometriosis, especially zinc and selenium in CDAI. Zinc is involved in the composition of proteins in various construction, enzymatic and catalytic processes in cells, and helps maintain the body’s homeostasis; it also exists in the form of ions and acts as a signaling molecule (58). Studies have found that the concentration of MMP is positively correlated with the severity of endometriosis (59), and zinc deficiency may affect MMP, and estrogen levels may also affect MMP. In addition, zinc constitutes an important component of ZEB1 and ZEB2 molecules, which are involved in epithelial-mesenchymal transition (EMT) in the process of endometriosis and are associated with the severity of endometriosis. However, no studies have found that zinc deficiency may impair the expression of ZEB1 and ZEB2. In the studies of Messala et al. and Lai et al., the blood zinc levels of patients with endometriosis were measured to be 22% lower than those of healthy control women (60) and 43% lower (61), respectively, which is consistent with the findings of our study.
On the other hand, selenium is an important component of selenoproteins in the human body, which can function both as enzymes and as non-enzyme proteins (62). In endometriosis, selenium is positively correlated with glutathione peroxidase (63), which is an important component for regulating cellular oxidative stress. Therefore, there is a certain relationship between selenium and the occurrence of endometriosis. Singh et al. observed that lower selenium concentrations in follicular fluid were associated with an increased risk of endometriosis-related infertility compared with tubal infertility. In a study of patients with endometriosis, Guerrero et al. found that when patients took vitamin E, C, selenium and zinc at the same time, the severity of the disease decreased. In contrast, the lower the oral antioxidant nutrient intake of patients, the more severe the disease (64). Zinc and selenium are essential trace elements with significant immunomodulatory effects. Sahdeo et al. demonstrated that zinc, by forming complexes with various bioactive molecules, exhibits anti-inflammatory and antioxidant properties; zinc can exert anti-inflammatory effects by inhibiting the TLR4/NF-κB signaling pathway (65). Selenium, as an antioxidant, can modulate immune responses and reduce oxidative stress, thereby alleviating inflammation (65). It should be noted that other non-nutritive antioxidants can also play significant roles in the development of endometriosis. Curcumin can reduce the production of pro-inflammatory cytokines (such as TNF-α, IL-1, and IL-6) by inhibiting the NF-κB, JAK/STAT, and MAPK signaling pathways (66). Moreover, curcumin can activate endogenous antioxidant enzymes (such as superoxide dismutase [SOD] and glutathione peroxidase [GPx]), further enhancing its anti-inflammatory effects (65). Pázmándi et al. showed that gingerol can reduce inflammatory responses by inhibiting the NF-κB and PI3K/Akt/mTOR signaling pathways (67).
Finally, the association between CDAI and the risk of endometriosis is heterogeneous in different subgroups, which may be related to the different susceptibility of different groups to endometriosis. Most patients with endometriosis develop disease before the age of 45 (68). We found that the protective effect of CDAI on endometriosis is most significant among people aged 30–39, indicating that relatively younger individuals are more likely to benefit from the protection of an antioxidant diet. In addition, previous studies have found that black women have a lower incidence of endometriosis than white women (69). It was found that non-pregnant women have a higher risk of endometriosis, so the protective effect of antioxidant diet on these women might be smaller than that of women who have been pregnant (50, 70). Women who have been pregnant exhibit a lower risk of endometriosis due to the hormonal environment and immune regulation during pregnancy (71). In addition, women with higher education levels were reported to have a higher risk of endometriosis (72). However, we found a protective effect of an antioxidant diet against the development of endometriosis only in relatively low-educated women. No study has found a clear link between marital status and the risk of endometriosis (73). Our study found that CDAI has a protective effect on endometriosis in women with different marital statuses, indicating that this effect has nothing to do with whether they are single or not. The results of a meta-analysis showed that the risk of endometriosis between smoking and endometriosis was not significant (74), but our study only found a protective effect of CDAI on endometriosis among current smokers, indicating that there may be a certain interaction between these factors, which is worthy of further exploration. Another study found that there is a certain positive correlation between drinking alcohol and the occurrence of endometriosis (75). We found that the protective effect of CDAI on endometriosis was only among women with a history of drinking. Tang et al. found that compared with women of normal weight, obese women have a higher incidence of endometriosis (76). Our study found that the protective effect of CDAI is more obvious among women with high BMI or obesity. Overall, women diagnosed with endometriosis are more likely to develop coronary heart disease (77), hypertension, and hypercholesterolemia (78). A diagnosis of endometriosis is associated with an increased risk of developing type 2 diabetes in non-obese women and in women without a history of infertility or gestational diabetes (79). On the other hand, women with hypertension and hypercholesterolemia are also more likely to develop endometriosis (78), indicating that there is a certain interaction between endometriosis and the above diseases. This study found that the protective effect of CDAI on endometriosis is more obvious in women with hypertension, but without diabetes and coronary heart disease, the specific mechanism remains to be explored. However, this study has certain limitations. First, we used a cross-sectional design, so we cannot make clear causal inferences. Second, this study only included vitamin A, vitamin C, vitamin E, carotenoids, zinc and selenium in dietary components to calculate the level of CDAI, but did not consider additional nutrient supplements. The calculation of the Composite Dietary Antioxidant Index (CDAI) is based on two 24-h dietary recalls, which may not reflect the long-term dietary habits of the participants. Future studies could further evaluate the relationship between long-term dietary habits and endometriosis. In addition, only American female population was used in this study, so our findings cannot be extrapolated to all women in the world. Therefore, more research is needed, especially longitudinal and ethnically diverse studies to verify our findings.
Our study firstly explores the relationship between CDAI and the risk of endometriosis based on a large nationally representative female sample of childbearing age. We found that women with higher CDAI levels tend to have a lower incidence of endometriosis, and there is a negative dose–response relationship between CDAI levels and the risk of endometriosis. In addition, we also explored the differences in the protective effect of CDAI on the occurrence of endometriosis in different subgroups. A protective effect of CDAI on the risk of endometriosis was observed in our study, but it should be noted that, based on the cross-sectional design, causality cannot be fully established; it remains unclear whether an antioxidant-rich diet reduces the risk of endometriosis or if women with endometriosis simply have poorer diets. Prospective or interventional studies are required to confirm the causal nature of this relationship. In total, our results provide an important reference for the primary prevention of endometriosis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found at: https://www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YYu: Conceptualization, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. JS: Methodology, Software, Visualization, Writing – review & editing. DW: Formal analysis, Investigation, Writing – review & editing. MX: Validation, Writing – review & editing. YYa: Supervision, Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We appreciate NCHS for its research design and data sharing, as well as all the NHANES investigators and participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1549948/full#supplementary-material
1. Ghiasi, M, Kulkarni, MT, and Missmer, SA. Is endometriosis more common and more severe than it was 30 years ago? J Minim Invasive Gynecol. (2020) 27:452–61. doi: 10.1016/j.jmig.2019.11.018
2. Giudice, LC, and Kao, LCJL. Theories of. Pathogenesis. (2004) 364:1789–99. doi: 10.1016/S0140-6736(04)17403-5
3. Holt, VL, and Weiss, NS. Recommendations for the Design of Epidemiologic Studies of endometriosis. Epidemiology. (2000) 11:654–9. doi: 10.1097/00001648-200011000-00007
4. Fourquet, J, Gao, X, Zavala, D, Orengo, JC, Abac, S, Ruiz, A, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril. (2010) 93:2424–8. doi: 10.1016/j.fertnstert.2009.09.017
5. Kvaskoff, M, Mu, F, Terry, KL, Harris, HR, Poole, EM, Farland, L, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. (2015) 21:500–16. doi: 10.1093/humupd/dmv013
6. Moradi, M, Parker, M, Sneddon, A, Lopez, V, and Ellwood, D. Impact of endometriosis on Women's lives: a qualitative study. BMC Womens Health. (2014) 14:123. doi: 10.1186/1472-6874-14-123
7. Parazzini, F, Chiaffarino, F, Surace, M, Chatenoud, L, Cipriani, S, Chiantera, V, et al. Selected food intake and risk of endometriosis. Hum Reprod. (2004) 19:1755–9. doi: 10.1093/humrep/deh395
8. Britton, JA, Westhoff, C, Howe, G, and Gammon, MD. Diet and benign ovarian tumors (United States). Cancer Causes Control. (2000) 11:389–401. doi: 10.1023/a:1008921710400
9. Harris, HR, Chavarro, JE, Malspeis, S, Willett, WC, and Missmer, SA. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol. (2013) 177:420–30. doi: 10.1093/aje/kws247
10. Savaris, AL, and do Amaral, VF. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. (2011) 158:314–8. doi: 10.1016/j.ejogrb.2011.05.014
11. Missmer, SA, Chavarro, JE, Malspeis, S, Bertone-Johnson, ER, Hornstein, MD, Spiegelman, D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. (2010) 25:1528–35. doi: 10.1093/humrep/deq044
12. Matalliotakis, IM, Cakmak, H, Fragouli, YG, Goumenou, AG, Mahutte, NG, and Arici, A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet. (2008) 277:389–93. doi: 10.1007/s00404-007-0479-1
13. Bartley, KA, Underwood, BA, and Deckelbaum, RJ. A life cycle micronutrient perspective for Women's health. Am J Clin Nutr. (2005) 81:1188s–93s. doi: 10.1093/ajcn/81.5.1188
14. Lampe, JW, Huang, Y, Neuhouser, ML, Tinker, LF, Song, X, Schoeller, DA, et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am J Clin Nutr. (2017) 105:466–75. doi: 10.3945/ajcn.116.144840
15. Liu, C, Kuang, X, Li, K, Guo, X, Deng, Q, and Li, D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: a systematic review and Meta-analysis of randomized controlled trials. Food Funct. (2020) 11:10817–27. doi: 10.1039/d0fo00787k
16. Pisoschi, AM, Pop, A, Iordache, F, Stanca, L, Predoi, G, and Serban, AI. Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur J Med Chem. (2021) 209:112891. doi: 10.1016/j.ejmech.2020.112891
17. Yochum, LA, Folsom, AR, and Kushi, LH. Intake of antioxidant vitamins and risk of death from stroke in postmenopausal women. Am J Clin Nutr. (2000) 72:476–83. doi: 10.1093/ajcn/72.2.476
18. Osganian, SK, Stampfer, MJ, Rimm, E, Spiegelman, D, Hu, FB, Manson, JE, et al. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol. (2003) 42:246–52. doi: 10.1016/s0735-1097(03)00575-8
19. O'Connor, EA, Evans, CV, Ivlev, I, Rushkin, MC, Thomas, RG, Martin, A, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and Cancer: updated evidence report and systematic review for the us preventive services task force. JAMA. (2022) 327:2334–47. doi: 10.1001/jama.2021.15650
20. McBurney, MI, Blumberg, JB, Costello, RB, Eggersdorfer, M, Erdman, JW Jr, Harris, WS, et al. Beyond nutrient deficiency-opportunities to improve nutritional status and promote health modernizing Dris and supplementation recommendations. Nutrients. (2021) 13:844. doi: 10.3390/nu13061844
21. Rautiainen, S, Manson, JE, Lichtenstein, AH, and Sesso, HD. Dietary supplements and disease prevention - a global overview. Nat Rev Endocrinol. (2016) 12:407–20. doi: 10.1038/nrendo.2016.54
22. Yu, YC, Paragomi, P, Wang, R, Jin, A, Schoen, RE, Sheng, LT, et al. Composite dietary antioxidant index and the risk of colorectal Cancer: findings from the Singapore Chinese health study. Int J Cancer. (2022) 150:1599–608. doi: 10.1002/ijc.33925
23. Zhao, L, Sun, Y, Cao, R, Wu, X, Huang, T, and Peng, W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. (2022) 10:988727. doi: 10.3389/fpubh.2022.988727
24. Shen, Y, Tan, Z, Duan, Z, Chen, J, Yang, Z, and Lin, X. Association between the composite dietary antioxidant index and infertility: the National Health and nutrition examination survey 2013-2020. BMC Public Health. (2024) 24:2376. doi: 10.1186/s12889-024-19933-5
25. Rumiris, D, Purwosunu, Y, Wibowo, N, Farina, A, and Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy. (2006) 25:241–53. doi: 10.1080/10641950600913016
26. Zhao, L, Zhang, X, Guo, S, Han, K, Sun, Y, Li, X, et al. Relationship between composite dietary antioxidant index and depression among overweight and obese adults. J Affect Disord. (2023) 341:358–65. doi: 10.1016/j.jad.2023.08.140
27. Wright, ME, Mayne, ST, Stolzenberg-Solomon, RZ, Li, Z, Pietinen, P, Taylor, PR, et al. Development of a comprehensive dietary antioxidant index and application to lung Cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
28. Parazzini, F, Viganò, P, Candiani, M, and Fedele, L. Diet and endometriosis risk: a literature review. Reprod Biomed Online. (2013) 26:323–36. doi: 10.1016/j.rbmo.2012.12.011
29. Bosetti, C, Altieri, A, and La Vecchia, C. Diet and environmental carcinogenesis in breast/Gynaecological cancers. Curr Opin Obstet Gynecol. (2002) 14:13–8. doi: 10.1097/00001703-200202000-00003
30. Krabbenborg, I, de Roos, N, van der Grinten, P, and Nap, A. Diet quality and perceived effects of dietary changes in Dutch endometriosis patients: an observational study. Reprod Biomed Online. (2021) 43:952–61. doi: 10.1016/j.rbmo.2021.07.011
31. Huijs, E, and Nap, A. The effects of nutrients on symptoms in women with endometriosis: a systematic review. Reprod Biomed Online. (2020) 41:317–28. doi: 10.1016/j.rbmo.2020.04.014
32. Mier-Cabrera, J, Aburto-Soto, T, Burrola-Méndez, S, Jiménez-Zamudio, L, Tolentino, MC, Casanueva, E, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. (2009) 7:54. doi: 10.1186/1477-7827-7-54
33. Amano, T, Murakami, A, Murakami, T, and Chano, T. Antioxidants and therapeutic targets in ovarian clear cell carcinoma. Antioxidants (Basel). (2021) 10:187. doi: 10.3390/antiox10020187
34. Ono, A, Koshiyama, M, Nakagawa, M, Watanabe, Y, Ikuta, E, Seki, K, et al. The preventive effect of dietary antioxidants on cervical Cancer development. Medicina (Kaunas). (2020) 56:604. doi: 10.3390/medicina56110604
35. Vašková, J, Klepcová, Z, Špaková, I, Urdzík, P, Štofilová, J, Bertková, I, et al. The importance of natural antioxidants in female reproduction. Antioxidants (Basel). (2023) 12:907. doi: 10.3390/antiox12040907
36. Pierzchalski, K, Taylor, RN, Nezhat, C, Jones, JW, Napoli, JL, Yang, G, et al. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod. (2014) 91:84. doi: 10.1095/biolreprod.114.119677
37. Li, S, Fu, X, Wu, T, Yang, L, Hu, C, and Wu, R. Role of Interleukin-6 and its receptor in endometriosis. Med Sci Monit. (2017) 23:3801–7. doi: 10.12659/msm.905226
38. Li, L, Gao, H, Pan, L, Zhao, Y, Liang, Z, Zhang, Q, et al. All-trans retinoic acid inhibits epithelial-to-mesenchymal transition (Emt) through the Down-regulation of Il-6 in endometriosis. Ann Palliat Med. (2021) 10:11348–61. doi: 10.21037/apm-21-2175
39. Hickenbottom, SJ, Follett, JR, Lin, Y, Dueker, SR, Burri, BJ, Neidlinger, TR, et al. Variability in conversion of Beta-carotene to vitamin a in men as measured by using a double-tracer study design. Am J Clin Nutr. (2002) 75:900–7. doi: 10.1093/ajcn/75.5.900
40. Thorp, VJ. Effect of Oral contraceptive agents on vitamin and mineral requirements. J Am Diet Assoc. (1980) 76:581–4. doi: 10.1016/S0002-8223(21)39279-3
41. Zhou, X, Shen, W, Zhu, J, Chen, Y, and Zhang, J. Association between the oxidative balance score and endometriosis: a population-based study. Int J Women's Health. (2024) 16:1293–301. doi: 10.2147/IJWH.S466189
42. Strobel, M, Tinz, J, and Biesalski, HK. The importance of Beta-carotene as a source of vitamin a with special regard to pregnant and breastfeeding women. Eur J Nutr. (2007) 46 Suppl 1:I1–I20. doi: 10.1007/s00394-007-1001-z
43. Manda, K, and Bhatia, AL. Pre-Administration of Beta-Carotene Protects Tissue Glutathione and Lipid Peroxidation Status Following Exposure to gamma radiation. J Environ Biol. (2003) 24:369–72.
44. Omenn, GS. Chemoprevention of lung Cancer: the rise and demise of Beta-carotene. Annu Rev Public Health. (1998) 19:73–99. doi: 10.1146/annurev.publhealth.19.1.73
45. Carlos, TF, Riondel, J, Mathieu, J, Guiraud, P, Mestries, JC, and Favier, A. Beta-carotene enhances natural killer cell activity in Athymic mice. In Vivo. (1997) 11:87–91.
46. Block, G. Nutrient sources of Provitamin a carotenoids in the American diet. Am J Epidemiol. (1994) 139:290–3. doi: 10.1093/oxfordjournals.aje.a116996
47. Gazvani, R, and Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction (Cambridge, England). (2002) 123:217–26. doi: 10.1530/rep.0.1230217
48. Iborra, A, Palacio, JR, Ulcova-Gallova, Z, and Martínez, P. Autoimmune response in women with endometriosis. Am J Reprod Immunol. (2000) 44:236–41. doi: 10.1111/j.8755-8920.2000.440408.x
49. Oosterlynck, DJ, Meuleman, C, Waer, M, and Koninckx, PR. Transforming growth factor-Beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. (1994) 83:287–92.
50. Li, X, Xie, Z, Qiu, H, Xie, X, and Liu, L. Exploring the causal associations between diet-derived circulating antioxidants and the risk of endometriosis: a Mendelian randomization study. Front Nutr. (2024) 11:1453147. doi: 10.3389/fnut.2024.1453147
51. Gleicher, N, el-Roeiy, A, Confino, E, and Friberg, J. Is endometriosis an autoimmune disease? Obstet Gynecol. (1987) 70:115–22.
52. Sinaii, N, Cleary, SD, Ballweg, ML, Nieman, LK, and Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. (2002) 17:2715–24. doi: 10.1093/humrep/17.10.2715
53. Showell, MG, Mackenzie-Proctor, R, Jordan, V, and Hart, RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. (2020) 8:Cd007807. doi: 10.1002/14651858.CD007807.pub4
54. Darling, AM, Chavarro, JE, Malspeis, S, Harris, HR, and Missmer, SA. A prospective cohort study of vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr. (2013) 5:17–26. doi: 10.5301/je.5000151
55. Roshanzadeh, G, Jahanian Sadatmahalleh, S, Moini, A, Mottaghi, A, and Rostami, F. The relationship between dietary micronutrients and endometriosis: a case-control study. Int J Reprod Biomed. (2023) 21:333–42. doi: 10.18502/ijrm.v21i4.13272
56. Schink, M, Konturek, PC, Herbert, SL, Renner, SP, Burghaus, S, Blum, S, et al. Different nutrient intake and prevalence of gastrointestinal comorbidities in women with endometriosis. J Physiol Pharmacol. (2019) 70:9. doi: 10.26402/jpp.2019.2.09
57. Lu, X, Wu, Z, Wang, M, and Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: a randomized controlled study. J Int Med Res. (2018) 46:4624–33. doi: 10.1177/0300060518786918
58. Maret, W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. (2013) 4:82–91. doi: 10.3945/an.112.003038
59. Malvezzi, H, Aguiar, VG, Paz, CC, Tanus-Santos, JE, Penna, IA, and Navarro, PA. Increased circulating Mmp-2 levels in infertile patients with moderate and severe pelvic endometriosis. Reprod Sci. (2013) 20:557–62. doi: 10.1177/1933719112459234
60. Messalli, EM, Schettino, MT, Mainini, G, Ercolano, S, Fuschillo, G, Falcone, F, et al. The possible role of zinc in the Etiopathogenesis of endometriosis. Clin Exp Obstet Gynecol. (2014) 41:541–6. doi: 10.12891/ceog19332014
61. Lai, GL, Yeh, CC, Yeh, CY, Chen, RY, Fu, CL, Chen, CH, et al. Decreased zinc and increased Lead blood levels are associated with endometriosis in Asian women. Reprod Toxicol. (2017) 74:77–84. doi: 10.1016/j.reprotox.2017.09.001
62. Hariharan, S, and Dharmaraj, S. Selenium and Selenoproteins: It's role in regulation of inflammation. Inflammopharmacology. (2020) 28:667–95. doi: 10.1007/s10787-020-00690-x
63. Singh, AK, Chattopadhyay, R, Chakravarty, B, and Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing Ivf. Reprod Toxicol. (2013) 42:116–24. doi: 10.1016/j.reprotox.2013.08.005
64. Hernández Guerrero, CA, Bujalil Montenegro, L, De la Jara, DJ, Mier Cabrera, J, and Bouchán, VP. Endometriosis and deficient intake of antioxidants molecules related to peripheral and peritoneal oxidative stress. Ginecol Obstetr Mexico. (2006) 74:20–8.
65. Prasad, S, and Lall, R. Zinc-curcumin based complexes in health and diseases: an approach in Chemopreventive and therapeutic improvement. J Trace Elem Med Biol. (2022) 73:127023. doi: 10.1016/j.jtemb.2022.127023
66. Kahkhaie, KR, Mirhosseini, A, Aliabadi, A, Mohammadi, A, Mousavi, MJ, Haftcheshmeh, SM, et al. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology. (2019) 27:885–900. doi: 10.1007/s10787-019-00607-3
67. Pázmándi, K, Szöllősi, AG, and Fekete, T. The "root" causes behind the anti-inflammatory actions of ginger compounds in immune cells. Front Immunol. (2024) 15:1400956. doi: 10.3389/fimmu.2024.1400956
68. Haas, D, Chvatal, R, Reichert, B, Renner, S, Shebl, O, Binder, H, et al. Endometriosis: a premenopausal disease? Age pattern in 42, 079 patients with endometriosis. Arch Gynecol Obstet. (2012) 286:667–70. doi: 10.1007/s00404-012-2361-z
69. Bougie, O, Yap, MI, Sikora, L, Flaxman, T, and Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and Meta-analysis. BJOG. (2019) 126:1104–15. doi: 10.1111/1471-0528.15692
70. Oszajca, K, and Adamus, A. Diet in prevention and treatment of endometriosis: current state of knowledge. Curr Nutr Rep. (2024) 13:49–58. doi: 10.1007/s13668-024-00518-y
71. Vendittelli, F, Barasinski, C, Rivière, O, Bourdel, N, and Fritel, X. Endometriosis and risk of adverse pregnancy outcomes: a retrospective multicenter cohort study. Fertil Steril. (2025) 123:137–47. doi: 10.1016/j.fertnstert.2024.07.037
72. Hemmings, R, Rivard, M, Olive, DL, Poliquin-Fleury, J, Gagné, D, Hugo, P, et al. Evaluation of risk factors associated with endometriosis. Fertil Steril. (2004) 81:1513–21. doi: 10.1016/j.fertnstert.2003.10.038
73. Eljamay, SM, Elhassadi, JE, Haleim, NR, and Eljamay, FM. Endometriosis and its relationship to marital status. N Afr J Sci Publishing. (2023) 1:7–12.
74. Bravi, F, Parazzini, F, Cipriani, S, Chiaffarino, F, Ricci, E, Chiantera, V, et al. Tobacco smoking and risk of endometriosis: a systematic review and Meta-analysis. BMJ Open. (2014) 4:e006325. doi: 10.1136/bmjopen-2014-006325
75. Parazzini, F, Cipriani, S, Bravi, F, Pelucchi, C, Chiaffarino, F, Ricci, E, et al. A Metaanalysis on alcohol consumption and risk of endometriosis. Am J Obstet Gynecol. (2013) 209:106.e1–106.e10. doi: 10.1016/j.ajog.2013.05.039
76. Tang, Y, Zhao, M, Lin, L, Gao, Y, Chen, GQ, Chen, S, et al. Is body mass index associated with the incidence of endometriosis and the severity of Dysmenorrhoea: a case-control study in China? BMJ Open. (2020) 10:e037095. doi: 10.1136/bmjopen-2020-037095
77. Mu, F, Rich-Edwards, J, Rimm, EB, Spiegelman, D, and Missmer, SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. (2016) 9:257–64. doi: 10.1161/CIRCOUTCOMES.115.002224
78. Mu, F, Rich-Edwards, J, Rimm, EB, Spiegelman, D, Forman, JP, and Missmer, SA. Association between endometriosis and hypercholesterolemia or. Hypertension. (2017) 70:59–65. doi: 10.1161/HYPERTENSIONAHA.117.09056
Keywords: composite dietary antioxidant index, endometriosis, women, NHANES, crosssectional
Citation: Yu Y, Sun J, Wang D, Xing M and Yang Y (2025) Association between the composite dietary antioxidant index and risk of endometriosis in women: a national population-based study. Front. Nutr. 12:1549948. doi: 10.3389/fnut.2025.1549948
Received: 22 December 2024; Accepted: 05 March 2025;
Published: 26 March 2025.
Edited by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandReviewed by:
Denisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), MexicoCopyright © 2025 Yu, Sun, Wang, Xing and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqi Yang, cmFua2V5eWFuZzFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.